Abstract
Isolates from patients with confirmed tuberculosis from London were collected over 2.5 years between 1995 and 1997. Restriction fragment length polymorphism (RFLP) analysis was performed by the international standard technique as part of a multicenter epidemiological study. A total of 2,779 samples representing 2,500 individual patients from 56 laboratories were examined. Analysis of these samples revealed a laboratory cross-contamination rate of between 0.54%, when only presumed cases of cross-contamination were considered, and 0.93%, when presumed and possible cases were counted. Previous studies suggest an extremely wide range of laboratory cross-contamination rates of between 0.1 and 65%. These data indicate that laboratory cross-contamination has not been a common problem in routine practice in the London area, but in several incidents patients did receive full courses of therapy that were probably unnecessary.
Isolation of Mycobacterium tuberculosis is the definitive method for confirming the diagnosis of tuberculosis. Cross-contamination is an inherent problem in culturing mycobacteria due to both the sensitive recovery systems in use and the ability of the bacilli to survive outside the host for extended periods. Viable tubercle bacilli have been recovered from heat-fixed sputum smears and from 0.9% sodium chloride decontamination solutions up to 3 weeks after inoculation (1). False-positive culture results can occur as a result of contamination at many stages, including patient sampling, microscopy, and specimen inoculation (7, 17).
A suspected diagnosis of tuberculosis has significant implications for patients and their contacts and for health care resources. For the patient, it entails a course of potentially toxic chemotherapy. For the contacts, it involves worry and time taken to attend screening. For health services, it further dilutes already stretched resources and personnel.
Our research team has recently completed a multidisciplinary collaborative study to investigate the epidemiology of tuberculosis within the London area using the international standard IS6110 restriction fragment length polymorphism (RFLP) method and secondary typing techniques (12). An important aspect of any analysis of tuberculosis epidemiology is the identification and quantification of laboratory cross-contamination that may falsely increase the number of cases included in clusters. In a recent review, most large studies were shown to have clusters containing strains that were likely to be present through cross-contamination (8). In order to ensure that the clusters produced by the study were as reliable as possible, a thorough review of the data was performed to elucidate which samples could have represented cross-contamination in the laboratory. Laboratories have different procedures to identify laboratory cross-contamination. In this paper, we report on the frequency of cross-contamination where it has not been recognized by laboratories through routine procedures.
MATERIALS AND METHODS
Patient samples.
A total of 2,779 isolates of M. tuberculosis were isolated in London laboratories between 1 July 1995 and 31 December 1997. After multiple isolates from the same patient were eliminated, isolates from 2,500 individual patients were included in the study; 448 isolates had one to four copies of IS6110, and 2,042 isolates had five or more copies. The methods and overall results of the study have been described in detail elsewhere (12).
Molecular epidemiological techniques.
All isolates that had been identified as M. tuberculosis were typed by IS6110 RFLP analysis using the international standard technique (18). All patterns were entered by one researcher into a database using GelCompar software (version 4.0; Applied Maths, Koutrai, Belgium) and then analyzed independently. The isolates were compared using the Dice coefficient with the parameter settings at 1.2% band position tolerance with optimization. A molecular cluster was defined as a series of isolates that had identical banding patterns (100% identity), and this computer similarity was subject to visual verification. Strains that differed by one band were regarded as not belonging to the same molecular cluster.
Epidemiological data collection.
Epidemiological information was gathered in the first instance by a structured pro forma questionnaire, which was used during record review to collect information about cases of culture-confirmed tuberculosis. Two health authorities (in east and southeast London) were able to provide data that were stored in local databases. Additional microbiological data from the PHLS United Kingdom antimicrobial resistance surveillance network (MYCOBNET) database was obtained. Potential links between members of the clusters were determined by reference to these pro forma questionnaires after molecular cluster results were known.
Defining cross-contaminants.
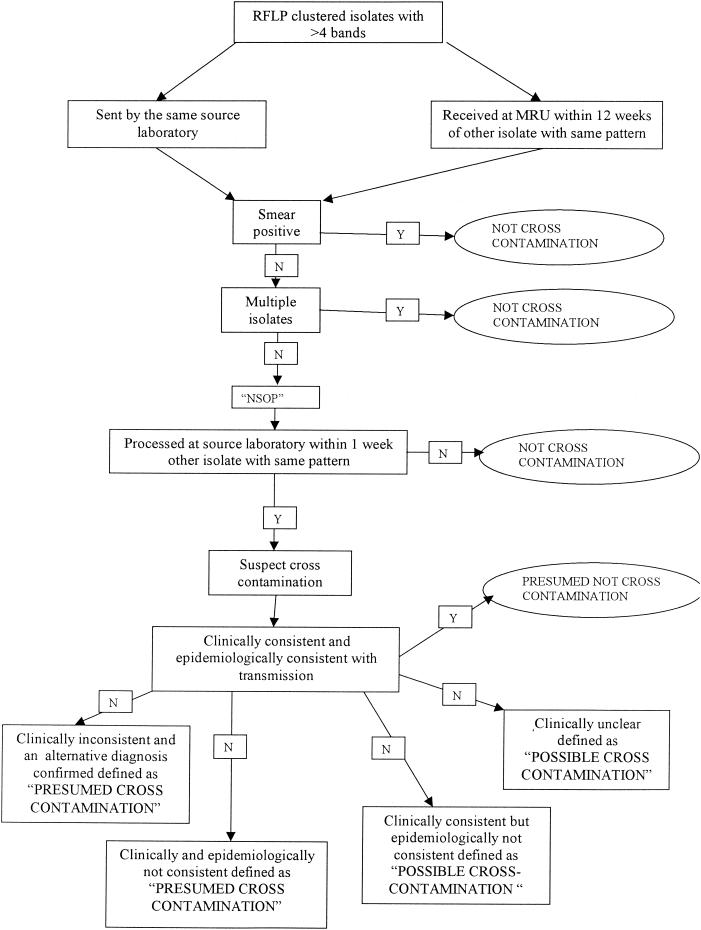
All clustered isolates with greater than four bands on IS6110 RFLP typing sent from the same source laboratory were considered for possible laboratory cross-contaminants and investigated further. Those received at the PHLS Mycobacterial Reference Unit within 12 weeks of each other were also identified for further study as possible cases of cross-contamination. If samples were found to be smear positive or patients had multiple positive cultures, laboratory cross-contamination was excluded. Any isolate obtained from a sample that had been shown to be smear negative and that constituted the only positive sample from that individual was defined as “negative smear one positive” and was checked for the date of processing within the source laboratory. If the sample was processed within 1 week of another with the same RFLP fingerprint, the possibility of laboratory cross-contamination was suspected. One week was chosen because laboratories processing smaller numbers of mycobacterial samples might batch them over two or more days, and hence, samples booked into the laboratory on different dates could be processed on the same day, exposing them to the risk of cross-contamination. More detailed information was collected to determine the likelihood of these being cases of cross-contamination. This included the original study pro forma questionnaire completed for each isolate, the microbiology records obtained by visiting the source laboratory, inspection of the clinical case notes for each patient, consultation with the tuberculosis liaison nurse, and the opinion of the physician in charge of the patient. Strains were defined as presumed cases of cross-contamination if the patient's clinical condition was not consistent with tuberculosis and an alternative diagnosis had been identified. Alternatively, they were defined as presumed cases of cross-contamination if the clinical and epidemiological data were inconsistent with tuberculosis. Possible cases of cross-contamination were defined as those where the clinical data were consistent but the epidemiological data were inconsistent with transmission. Wherever the final diagnosis could not be accurately determined, it was considered to be a possible case of cross-contamination. This diagnostic process is illustrated as a decision tree in Fig. 1.
FIG. 1.
Flowchart illustrating the decision pathway used to identify cases of cross-contamination and to assess their clinical significance. MRU, PHLS Mycobacterium Reference Unit; NSOP, negative smear one positive.
RESULTS
Our first action was to detect and remove double entries. We identified three previously unrecognized cases of double entry of the same patient's details, one that was entered under an anonymous genitourinary medicine number and then reentered under a chest clinic number and two that were entered twice with different dates of birth.
After this initial screening, we found a total of 74 isolates (each from a single patient) that might be suspected to be from laboratory cross-contamination. These included isolates from 35 clusters obtained from 19 separate laboratories. The other members of the clusters were believed to be genuine cases based on the criterion of smear positivity, multiple cultures from the same patient, or clinical opinion.
After analysis of the patient records, 11 (0.54%) were identified as presumed false-positive reports due to laboratory cross-contamination. The detailed assessment of these cases is summarized in Table 1. One case (I) involved a refugee hospitalized after detention at an airport on the day of arrival in the United Kingdom for the first time. The patient was diagnosed clinically as having a case of tuberculosis, although the initial specimen was smear negative. The isolate apparently grown from this specimen was of a type identical to a strain cluster from the local community, consisting of patients who had no links to the country of origin of the refugee. This was defined as a case of presumed laboratory cross-contamination in a patient with presumed tuberculosis.
TABLE 1.
Presumed cases of laboratory cross-contamination detailing symptoms, therapy, and final diagnosis
| Case | Reason investigateda | Antituberculous drugs given | Assessment conclusion |
|---|---|---|---|
| A | CXR—prominent hilum | None | Left atrial myxoma |
| B | Persistent cough | None | Lung carcinoma |
| C | Cough, fever | None | Presumed pneumonia |
| D | CXR—parenchymal shadowing, cough | None | Pneumocystis carinii pneumonia |
| F | Persistent cough, breathlessness | None | Laryngeal tumor |
| G | HIV positive with skin lesions | None | Kaposi's sarcoma |
| H | Elevated inflammatory markers | Chemoprophylaxis | Polymyalgia |
| I | Port of entry screening; symptomatic; CXR—abnormal | Full treatment course | Clinically considered case of pulmonary tuberculosis; no possible epidemiological link to cluster |
| J | Port of entry screening; asymptomatic | None | Not clinically or epidemiologically consistent |
| K | Port of entry screening; asymptomatic | Chemoprophylaxis | Not clinically or epidemiologically consistent |
| L | Sterile pyuria | None | Bacterial urinary tract infection |
CXR, chest X ray; HIV, human immunodeficiency virus.
In a further eight cases (0.32%), it was not possible to state categorically whether clustering was due to cross-contamination, as some of the patients had died or been lost to follow-up or the final diagnosis remained unclear. An example of this is the case (N) of a patient who was given a full course of therapy but whose symptoms persisted posttherapy. Also, there were two cases (M and P) of clinically defined tuberculosis that were smear negative and had isolates that were identical to others processed in the laboratory in parallel. Since there was no clear link between the patients but more distant transmission could not be excluded, these cases were defined as possible cross-contamination. As transmission could not be absolutely excluded, this was defined as a case of possible cross-contamination. The clinical interpretation of these cases is summarized in Table 2. The remaining 55 isolates were considered to represent genuine cases of tuberculosis.
TABLE 2.
Possible cases of laboratory cross-contamination detailing symptoms, therapy, and final diagnosis
| Case | Reason investigateda | Anti-TB drugs | Assessment conclusion |
|---|---|---|---|
| M | CXR—upper zone shadowing, cavitation; clinically consistent | Full course | Presumed case of TB; no obvious epidemiological link to clustered cases possible laboratory contamination between two known cases |
| N | CXR—upper zone shadowing and effusion; pleuritic chest pain | Full course | Treated as case of TB; symptoms and signs persisted posttreatment |
| O | Fine-needle aspirate of persisting lymphadenopathy | Unknown | Unknown—case notes lost |
| P | CXR—upper zone shadowing; clinically consistent | Full course | Presumed case of TB; no obvious epidemiological link to clustered cases: possible laboratory contamination between two known cases |
| Q | CXR—effusion, pneumothorax; breathless, cachexia, persistent cough | Unknown | Known congestive cardiac failure and carcinoma of bronchus; diagnosis of TB uncertain; already transferred abroad for terminal care before culture result |
| R | Unknown | Unknown | Case notes unobtainable |
| S | HIV positive, low CD4 counts, febrile, cough | Full course | Multiple other samples isolated MAIC; probably not TB but possibility of dual infection, hence not denotified |
| T | CXR—pleural effusion; immunocompromised post-renal transplant; past history of TB | Full course | Current diagnosis uncertain; fully treated in view of past history and immunocompromise |
CXR, chest X ray; HIV, human immunodeficiency virus; MAIC, Mycobacterium avium-M. intracellulare complex; TB, tuberculosis.
The overall previously unrecognized laboratory cross-contamination rate was 11 cases out of 2,042 patients (0.54%) when only the presumed cases of cross-contamination were considered. When the possible cases of cross-contamination were also included, 19 out of 2,042 (0.93%) were identified. Cross-contamination incidents were recorded for 11 (19%) of the 56 laboratories involved in the London-wide study. Cross-contamination strains were found in 14 (8.77%) of the clusters identified during the study (12).
DISCUSSION
This study was initiated to determine the significance of cross-contamination where the primary laboratory had not identified it. The problem of cross-contamination has always been recognized, but it is less clear how common this problem is. Review of the literature revealed a large variation in reported rates of cross-contamination, ranging from 0.1 to >65% (Table 3). However, the highest rates reported were found in studies that were initiated because of suspicion that incidents of cross-contamination were occurring. This was usually due to isolation rates that were above those normally expected. It is not surprising, therefore, that such studies produce high rates of cross-contamination. If the comparison is limited to large unselected population-based studies, the rates (with one exception) are below 3%. A recent review (7) found a mean false-positive rate of 3.1% from 14 studies of >100 patients. However, it is extremely difficult to compare these studies because they are not standardized in any way. In some cases, the denominator is described as total samples submitted for analysis, while in other cases, the denominator consists of positive samples only. Furthermore, some laboratories will try to identify cases of cross-contamination. Thus, retrospective studies of all isolates will not give a true picture of the extent of cross-contamination. Our study provides an estimate of the risk of cross-contamination that is unrecognized by the reporting laboratory and that is likely to result in inappropriate or unnecessary therapy in our catchment area. Lack of recognition may have occurred because there was no system for identifying cross-contaminants in the host laboratory or because the system failed to detect it.
TABLE 3.
Reported cross-contamination rates for M. tuberculosis
| Rate (%) | No. of cross-contaminants | Sample size | Total or positives only | All samples or incident only | Date | Place (reference) |
|---|---|---|---|---|---|---|
| 0.1 | 1 | 907 | Total | All | 1980 | Africa (1) |
| 0.1 | 3 | 2,305 | Positive | All | 1997 | Chicago (5) |
| 0.2 | 3 | 1,500 | Positive | All | 1992 | Denmark (4) |
| 0.2 | 8 | 3,600 | Positive | All | 1993 | San Francisco (17) |
| 0.3 | 12 | 4,075 | Positive | All | 1996 | Chicago (19) |
| 0.8 | 45 | 5,798 | Total | All | 1980 | Africa (13) |
| 1.5 | Not reported | Not reported | Positive | All | 1995 | Holland (4) |
| 1.8 | 9 | 496 | Positive | All | 1994 | San Francisco (16) |
| 2.29 | 49 | 1,439 | Positive | All | 1994 | Denmark (4) |
| 2.6 | 3 | 117 | Positive | All | 1994 | New York City (2) |
| 3.5 | 9 | 259 | Positive | Incident | 1992 | Arkansas (6) |
| 4 | 8 | 199 | Positive | Incident | 1997 | Denver (8) |
| 7.8 | 24 | 306 | Total | All | 1995 | France (10) |
| 8 | 60 | 750 | Positive | Incident | 1995 | New York City (15) |
| 9 | 11 | 114 | Positive | Incident | 1997 | Wisconsin (3) |
| 12 | 5 | 42 | Positive | Incident | 1997 | Denver (8) |
| 13 | 9 | 70 | Positive | Incident | 1993 | Los Angeles (14) |
| 65.9 | 60 | 91 | Positive | Incident | 1995 | Brazil (9) |
Several factors contribute to laboratory cross-contamination, ranging from simple mislabeling of specimens to laboratory protocols that are not adapted for left-handedness (4, 17). Initial sample processing can result in the transfer of bacilli by aerosol, splash, loop, or pipette, which invariably involves consecutive sample numbers. While it might be better to process samples individually, the widespread practice of alkaline decontamination inevitably means having several samples in a batch in the safety cabinet at the same time, since it is too time-consuming to wait for decontamination of each one separately. Ensuring adequate airflow through the cabinet is of course essential for the safety of the staff, but it also helps to minimize aerosol spread. Subsequent processing of samples with contaminated equipment has been a well-documented source of cross-contamination in several instances (3, 8, 17), particularly involving needle carryover from Bactec460 radiometric analyzers (Becton Dickinson, Sparks, Md.). This appears to be less of a problem recently, which may reflect adjustments in the temperature and maintenance programs for the needles and more widespread use of liquid culture systems using noninvasive growth detection. Another frequently cited problem has been the contamination of common delivery tubes or containers for additives. The effects of such contamination can produce startling cross-contamination levels of up to 60% (9). Another factor is the number of positive samples being processed within the laboratory—the more positive samples, the more opportunities for cross-contamination (13).
While good laboratory practice can minimize the risk, cross-contamination remains a possibility in any center, and maintaining levels of suspicion is important to allow prompt detection. In particular, attention should be given to any negative smear one positive sample. Additionally, laboratories using both liquid culture systems and solid slopes (e.g., Bactec and Lowenstein-Jensen) may be alerted by growth in one system only or if <6 colonies are isolated on the slope. Investigation typically shows that cross-contaminated samples were processed concurrently with a consecutively numbered true-positive sample. However, cross-contamination has been discovered from samples processed 15 specimens apart due to needle carryover (3).
Our data suggest the importance of identifying incidents of cross-contamination quickly. Among the cases where cross-contamination was defined as presumed, 3 of the 11 patients had some form of antituberculosis therapy: prophylaxis in two cases and a full treatment course in one. However, among the eight cases of possible cross-contamination, the therapeutic consequences were identified in five cases and all were given a full course of antituberculosis chemotherapy. It is possible that these patients received unnecessary antituberculosis drugs, with all the possibilities of adverse events, for no benefit. Also, while an incorrect diagnosis of tuberculosis is being entertained, the correct diagnosis may not be made. In three cases, we detected presumed or possible cross-contamination when the patient was thought to have tuberculosis. This too can have significant adverse outcome for the patient if the strains differ in their antibiotic susceptibility test results.
The data presented in this report argue strongly for the need to perform some routine test to determine whether isolates from the same laboratory are possible cross-contaminants as soon as possible to prevent unnecessary therapy. Rapid PCR-based methods, such as variable number tandem repeat and spoligotyping, have been proposed for this purpose (11). Isolates with the same profile but no epidemiological link would suggest laboratory cross-contamination, and the clinicians could be advised appropriately.
Acknowledgments
D.B. and colleagues received funding from the NHS Executive London Research and Development Programme. We are also grateful to the European Union for support under grants BMH4-CT97-91202 and SMT4-CT96-2097 (provision of GelCompar software).
The views expressed in this publication are those of the authors and not necessarily those of the NHS Executive or the Department of Health.
We gratefully acknowledge the assistance of Angela Costetsos in performing the data searches and of Malcolm Perkins and Bharat Pankhania. This study would not have been possible without the support of the clinical and laboratory staff from the 56 participating hospitals and the steering group, committee, technicians, and research assistants from the Epidemiology of Tuberculosis in London Study.
REFERENCES
- 1.Aber, V. R., B. W. Allen, D. A. Mitchison, P. Ayuma, E. A. Edwards, and A. B. Keyes. 1980. Quality control in tuberculosis bacteriology. 1. Laboratory studies on isolated positive cultures and the efficiency of direct smear examination. Tubercle 61:123-133. [DOI] [PubMed] [Google Scholar]
- 2.Alland, D., G. E. Kalkut, A. R. Moss, R. A. McAdam, J. A. Hahn, W. Bosworth, E. Drucker, and B. R. Bloom. 1994. Transmission of tuberculosis in New York City. An analysis by DNA fingerprinting and conventional epidemiologic methods. N. Engl. J. Med. 330:1710-1716. [DOI] [PubMed] [Google Scholar]
- 3.Anonymous. 1997. Multiple misdiagnoses of tuberculosis resulting from laboratory error—Wisconsin, 1996. Morb. Mortal. Wkly. Rep. 46:797-801. [PubMed] [Google Scholar]
- 4.Bauer, J., V. O. Thomsen, S. Poulsen, and A. B. Andersen. 1997. False-positive results from cultures of Mycobacterium tuberculosis due to laboratory cross-contamination confirmed by restriction fragment length polymorphism. J. Clin. Microbiol. 35:988-991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhattacharya, M., S. Dietrich, L. Mosher, F. Siddiqui, B. E. Reisberg, W. S. Paul, and J. R. Warren. 1998. Cross-contamination of specimens with Mycobacterium tuberculosis: clinical significance, causes, and prevention. Am. J. Clin. Pathol. 109:324-330. [DOI] [PubMed] [Google Scholar]
- 6.Braden, C. R., G. L. Templeton, M. D. Cave, S. Valway, I. M. Onorato, K. G. Castro, D. Moers, Z. Yang, W. W. Stead, and J. H. Bates. 1997. Interpretation of restriction fragment length polymorphism analysis of Mycobacterium tuberculosis isolates from a state with a large rural population. J. Infect. Dis. 175:1446-1452. [DOI] [PubMed] [Google Scholar]
- 7.Burman, W. J., and R. R. Reves. 2000. Review of false-positive cultures for Mycobacterium tuberculosis and recommendations for avoiding unnecessary treatment. Clin. Infect. Dis. 31:1390-1395. [DOI] [PubMed] [Google Scholar]
- 8.Burman, W. J., B. L. Stone, R. R. Reves, M. L. Wilson, Z. Yang, H. El Hajj, J. H. Bates, and M. D. Cave. 1997. The incidence of false-positive cultures for Mycobacterium tuberculosis. Am. J. Respir. Crit. Care Med. 155:321-326. [DOI] [PubMed] [Google Scholar]
- 9.de C. Ramos, M., H. Soini, G. C. Roscanni, M. Jaques, M. C. Villares, and J. M. Musser. 1999. Extensive cross-contamination of specimens with Mycobacterium tuberculosis in a reference laboratory. J. Clin. Microbiol. 37:916-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gutierrez, M. C., V. Vincent, D. Aubert, J. Bizet, O. Gaillot, L. Lebrun, C. Le Pendeven, M. P. Le Pennec, D. Mathieu, C. Offredo, B. Pangon, and C. Pierre-Audigier. 1998. Molecular fingerprinting of Mycobacterium tuberculosis and risk factors for tuberculosis transmission in Paris, France, and surrounding area. J. Clin. Microbiol. 36:486-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kremer, K., D. van Soolingen, R. Frothingham, W. H. Haas, P. W. Hermans, C. Martin, P. Palittapongarnpim, B. B. Plikaytis, L. W. Riley, M. A. Yakrus, J. M. Musser, and J. D. van Embden. 1999. Comparison of methods based on different molecular epidemiological markers for typing of Mycobacterium tuberculosis complex strains: interlaboratory study of discriminatory power and reproducibility. J. Clin. Microbiol. 37:2607-2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maguire, H., J. W. Dale, T. D. McHugh, P. D. Butcher, S. H. Gillespie, A. Costetos, H. Al-Ghusein, R. Holland, A. L. Dickens, L. Marston, P. Wilson, R. Pitman, R. Strahan, F. A. Drobniewski, and D. K. Banerjee. 2002. Molecular epidemiology of tuberculosis in London 1995-7 demonstrating low rate of active transmission. Thorax 57:617-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitchison, D. A., A. B. Keyes, E. A. Edwards, P. Ayuma, S. P. Byfield, and A. J. Nunn. 1980. Quality control in tuberculosis bacteriology. 2. The origin of isolated positive cultures from the sputum of patients in four studies of short course chemotherapy in Africa. Tubercle 61:135-144. [DOI] [PubMed] [Google Scholar]
- 14.Nitta, A. T., P. T. Davidson, M. L. de Koning, and R. J. Kilman. 1996. Misdiagnosis of multidrug-resistant tuberculosis possibly due to laboratory-related errors. JAMA 276:1980-1983. [PubMed] [Google Scholar]
- 15.Nivin, B., K. Kaye, and S. S. Munsiff. 1997. Detection of laboratory cross-contamination of Mycobacterium cultures. Clin. Infect. Dis. 25:943.. [DOI] [PubMed] [Google Scholar]
- 16.Small, P. M., P. C. Hopewell, S. P. Singh, A. Paz, J. Parsonnet, D. C. Ruston, G. F. Schecter, C. L. Daley, and G. K. Schoolnik. 1994. The epidemiology of tuberculosis in San Francisco. A population-based study using conventional and molecular methods. N. Engl. J. Med. 330:1703-1709. [DOI] [PubMed] [Google Scholar]
- 17.Small, P. M., N. B. McClenny, S. P. Singh, G. K. Schoolnik, L. S. Tompkins, and P. A. Mickelsen. 1993. Molecular strain typing of Mycobacterium tuberculosis to confirm cross-contamination in the mycobacteriology laboratory and modification of procedures to minimize occurrence of false-positive cultures. J. Clin. Microbiol. 31:1677-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Embden, J. D., M. D. Cave, J. T. Crawford, J. W. Dale, K. D. Eisenach, B. Gicquel, P. Hermans, C. Martin, R. McAdam, and T. M. Shinnick. 1993. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J. Clin. Microbiol. 31:406-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wurtz, R., P. Demarais, W. Trainor, J. McAuley, F. Kocka, L. Mosher, and S. Dietrich. 1996. Specimen contamination in mycobacteriology laboratory detected by pseudo-outbreak of multidrug-resistant tuberculosis: analysis by routine epidemiology and confirmation by molecular technique. J. Clin. Microbiol. 34:1017-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]