Abstract
The inactivation of smears that contain Mycobacterium tuberculosis for microscopy before removal of the material from a biosafety cabinet is an important safety factor in preventing the potential transmission of tuberculosis to laboratory workers. The fixing and inactivating properties of heat flaming, 70% ethanol, and 1, 3, and 5% phenol in ethanol for smears containing M. tuberculosis were investigated. Heat flaming failed to inactivate the smear material, whereas 5% phenol in ethanol successfully fixed and inactivated all smears containing M. tuberculosis both from concentrated sputum samples and from culture material.
The potential hazard of laboratory work with Mycobacterium tuberculosis is well recognized. There are numerous records of laboratory-acquired tuberculosis (TB) infections, with aerosols and skin punctures being the most common reported routes of transmission (10, 13, 15, 18, 19, 24). The resurgence of TB in industrialized countries in the past decade has resulted in an increasing number of specimens and cultures being tested in laboratories, thereby enhancing the potential of accidental exposure for laboratory staff.
Stringent safety precautions must be followed for laboratory work with M. tuberculosis (6, 14). There remains one part of the routine laboratory process, however, where the hazard for potential exposure has not been addressed—unstained smears. Specimen and culture manipulations for mycobacteriological analyses, including the preparation of smears, are carried out within a biosafety cabinet (BSC). The smears are usually then heat fixed by passage of the slides through the flame of a gas burner or by placement on a hot plate. After the smears are fixed, they are removed from the BSC to a staining sink or they may be stored, transported to a reference laboratory, or used in proficiency testing panels.
Several studies have shown that heat-fixed smear material, whether fixed by flaming or for 2 h at 65°C on a hot surface, still contains viable bacilli (1, 4; L. R. B. Giacomelli, S. R. Sespede, A. M. W. Barreto, and C. L. Cardoso, Abstr. Clin. Microbiol., abstr. C-129, p. 131, 1999). Our data from this study confirm that M. tuberculosis is still viable after flame fixing of smear material. Additional problems with heat fixing are that the use of a flame inside a BSC is not recommended and that 2 h of fixing on a hot plate is cumbersome and time-consuming when large numbers of slides must be fixed.
When slides containing viable M. tuberculosis smear material are removed from a BSC, laboratory staff can be exposed to infective material if slides are broken, thereby possibly generating aerosols or skin penetration, or the smear material may flake off slides, thus potentially infecting the worker and contaminating the laboratory environment. Staining methods used for acid-fast bacilli (AFB), whether fluorochrome or carbol fuchsin based, are known to kill mycobacteria. However, staining is done over a sink outside the BSC, necessitating the prior removal of unstained smears from the cabinet.
The purpose of this study was to determine a rapid method which would inactivate as well as fix smear preparations of M. tuberculosis within the BSC while still retaining good staining performance. Solutions of 70% ethanol and 1, 3, and 5% phenol in 70% ethanol as well as heat flaming were used to fix smears of sputum concentrates containing high numbers of AFB as well as smears of M. tuberculosis cultures. After the smear material was fixed, it was tested for viability by using the BACTEC 460 culture system (Becton Dickinson, Sparks, Md.). Fixed smears were also stained to check the quantity of AFB present and the quality of the stained smears.
Preliminary reagent fixing tests were carried out in parallel at two separate laboratories: the Laboratories Branch, Ontario Ministry of Health and Long-Term Care, Toronto, Ontario, Canada, and the Florida Department of Health, Bureau of Laboratories, Jacksonville. After the results of these tests were examined, additional tests to confirm the preliminary finding that 5% phenol in ethanol was the optimal reagent, as well as heat fixing tests, were performed at the Ontario laboratory.
MATERIALS AND METHODS
Unused microscope slides were cleaned by soaking in 70% ethanol for 30 min and drying before use. All test procedures, including preparing smear material, making smears, and air drying, fixing, and inoculating samples into BACTEC (BT) vials, were performed inside a BSC. All smear materials were tested for viability both before and after fixing and for staining quality after fixing.
Preliminary reagent fixing tests.
Air-dried smears made from M. tuberculosis-spiked sputum concentrates and from cultures of M. tuberculosis growing on Lowenstein-Jensen (LJ) slants were fixed in all of the test reagents for 5 and 10 min. The following reagents were tested: 70% ethanol and 1, 3, and 5% phenol in 70% ethanol.
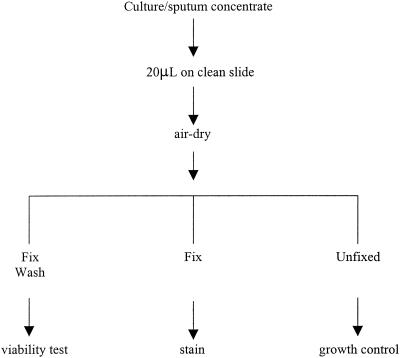
For each smear material, fixing reagent, and fixing time, four identically prepared smears were used. Smears 1 and 2 were fixed and tested for viability, smear 3 was fixed and stained, and smear 4 was left unfixed and tested for viability as a growth control (Fig. 1).
FIG. 1.
Scheme for the treatment of smear material samples.
These tests were performed in parallel at two laboratories.
Heat (flame) fixing test.
Twelve smears each from M. tuberculosis-spiked sputum concentrates and from M. tuberculosis cultures on LJ slants were prepared and air dried. Eleven smears from each smear material were heat fixed by passage three times through the flame of a gas burner and then allowed to cool. Ten smears from each smear material were then tested for viability, and the remaining smear was stained. The remaining two unfixed smears were tested for viability as growth controls.
Confirmation tests.
Three smears each from 22 AFB-positive (3+ or 4+, where 3+ is 1 to 9 AFB per ×100 field and 4+ is more than 9 AFB per ×100 field) clinical sputum concentrates and from 10 isolates of M. tuberculosis growing both on LJ slants and in Mycobacterium Growth Indicator tubes (MGIT) (Becton Dickinson) were prepared and air dried. Smear 1 from each set was fixed and tested for viability, smear 2 was fixed and stained, and smear 3 was left unfixed and tested for viability as a growth control.
All confirmation test smears were fixed for 5 min in 5% phenol in ethanol only.
Preparation of M. tuberculosis-spiked sputum concentrates.
A suspension of M. tuberculosis equivalent to a McFarland no. 1 standard was prepared in 5 ml of sterile saline. Three milliliters of this suspension was centrifuged at 7,600 × g for 15 min, and the supernatant was discarded. The cell button was transferred to a tube containing 3 ml of decontaminated, AFB-negative sputum concentrate material and vortexed well.
Preparation of smears.
Smears from sputum concentrates were prepared by placing 20-μl amounts of material on slides and then air drying the slides.
Smears from LJ cultures were prepared by emulsifying a loopful of culture in 20 μl of sterile saline on a slide and air drying the slides.
Smears from MGIT cultures were prepared by placing 20-μl aliquots of the growth sediment from the tubes on slides and then air drying the slides.
Fixing methods. (i) Reagent tests.
Wash bottles containing 70% ethanol and 1, 3, and 5% phenol in 70% ethanol were prepared. Smears were prepared, air dried, and placed on a rack over a pan inside the BSC. Each of the fixing solutions was applied for 5 and 10 min by flooding the smears with reagent from the wash bottle. The smears for viability testing were then washed thoroughly for 2 min in distilled water to remove the fixing reagent. The smears for staining were stained directly after fixing, without removal of the reagent.
(ii) Heat fixing tests.
Air-dried smears were heat fixed by passage three times through the flame of a gas burner and then allowed to cool.
Viability tests.
Smear material from the fixed and washed slides was scraped into tubes of 0.5 ml of sterile saline by using sterile swabs. The swabs were squeezed against the sides of the tubes to remove the smear material. This material was then inoculated into BT vials supplemented with 0.1 ml of PANTA (Becton Dickinson). The BT vials were incubated at 37°C and monitored for growth twice weekly for 8 weeks by using the BACTEC 460 system. Vials from the confirmation tests were further incubated and tested for growth at 12 weeks. A smear was made from any vial showing growth when the growth index reached >100. A blood agar plate was inoculated with 0.1 ml of culture from any vial showing growth and was incubated for 48 h at 37°C to check for contamination. Smears from positive BT cultures were stained by the Kinyoun method and examined for AFB.
Growth controls.
One smear from each test set was left unfixed and inoculated into a BT vial, as in the protocol used for the viability tests, as a growth control.
Staining controls.
One smear from each test set was fixed and stained to assess the quantity of AFB and the quality of the stained smears. Smears from sputum concentrates were stained by the auramine-rhodamine method, and smears from cultures were stained by the Kinyoun method.
RESULTS
Preliminary reagent fixing tests.
After being fixed in 70% ethanol, two of four M. tuberculosis-spiked concentrate smears and one of four M. tuberculosis LJ culture smears remained viable after 5 min of fixing (Table 1). One of four M. tuberculosis LJ culture smears was viable after 10 min of fixing.
TABLE 1.
Preliminary reagent fixing test results
| Smear material | No. of smears resulting in viable cultures after fixing for the indicated time (min) in the following reagenta:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 70% Ethanol
|
1% Phenol in ethanol
|
3% Phenol in ethanol
|
5% Phenol in ethanol
|
|||||
| 5 | 10 | 5 | 10 | 5 | 10 | 5 | 10 | |
| M. tuberculosis sputum concentrates | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| M. tuberculosis LJ cultures | 1 | 1 | 0 | 1 | 2 | 2 | 0 | 0 |
For each sample, reagent, and time, four replicates were used. Results from two laboratories are combined.
After being fixed in 1% phenol in ethanol, one of four M. tuberculosis-spiked concentrate smears was viable after 5 min of fixing and one of four M. tuberculosis LJ culture smears was viable after 10 min of fixing (Table 1).
After being fixed in 3% phenol in ethanol, two of four M. tuberculosis LJ culture smears were viable after both 5 and 10 min of fixing (Table 1).
After being fixed in 5% phenol in ethanol, all smears tested were nonviable after 5 and 10 min of fixing (Table 1).
Flame fixing tests.
Materials from flame-fixed smears (10 each for M. tuberculosis-spiked concentrate material and M. tuberculosis LJ culture material) all remained viable after heat fixing.
Confirmation tests.
After being fixed in 5% phenol in ethanol for 5 min, all smears tested (AFB-positive sputum concentrates [n = 22], M. tuberculosis from LJ slants [n = 10], and M. tuberculosis from MGIT cultures [n = 10]) were nonviable.
Controls. (i) Growth controls.
Unfixed growth controls from all tests were viable, indicated by a rising growth index in BT cultures. Smears from these cultures showed AFB, with cording in M. tuberculosis cultures, and tests for contamination were negative.
(ii) Staining controls.
All smears showed good staining and the presence of 3+ or 4+ AFB.
Viability tests.
All BT vials from viability tests showing growth had AFB-positive smears, with M. tuberculosis cultures demonstrating typical cording. Sterility plates showed no growth of contaminants at 48 h. The 22 specimen concentrates used in confirmation tests yielded cultures of M. tuberculosis (13), M. avium complex (4), M. kansasii (1), and M. xenopi (4).
DISCUSSION
The incidence of TB infections in laboratory workers is estimated to be three to nine times greater than that in the general population (8, 13, 20, 22). In the past 20 years, 44% of all reported laboratory-acquired bacterial infections have been due to M. tuberculosis (7). Because of the low infective dose of M. tuberculosis for humans (fewer than 10 bacilli represent a 50% infective dose [9, 11, 23]), all clinical specimens from patients with possible TB must be considered potentially infectious. The presence of multidrug-resistant strains of M. tuberculosis now isolated in laboratories and the increased workload of specimens and cultures being processed in large laboratories have enhanced the potential hazard of TB infections for laboratory workers.
The most common route of transmission of M. tuberculosis is via aerosols, which may be generated at any stage of the laboratory processing of specimens and cultures as well as from work with infected animals (2, 17, 25, 26). Cutaneous injury infections with M. tuberculosis have also been reported (16, 24). There are no documented reports of laboratory-acquired TB infections that can be directly attributed to the manipulation of unstained smears; however, in most instances the actual source of exposure for laboratory-acquired TB infections remains unknown (7).
Mycobacteriology laboratory staff should carry out a risk assessment of all stages of their laboratory operations (12, 23), recognizing that M. tuberculosis is classified as a biosafety level 3 risk agent (6, 14). The preparation of smear material on microscope slides from specimens and cultures, when carried out within a certified BSC and with biosafety level 3 operational precautions (14), should not present a hazard. The removal of smears from the BSC, if the material contains viable bacilli, carries the possible risk of aerosol transmission or cutaneous infection if the slides are broken or if material flakes off the slides.
The traditional method for fixing AFB smear material is heating. Dried material on slides may be passed several times through the flame of a gas burner, or slides may be placed on a heat block. Several studies, as well as our data, have shown that heat fixing does not kill M. tuberculosis (1, 4; Giacomelli et al., Abstr. Clin. Microbiol.).
The resistance of mycobacteria to disinfectants is considered to be due to the high lipid content of their complex cell wall (3, 5, 21). Ethanol has been shown to be tuberculocidal with sufficient contact time when used to treat suspensions of M. tuberculosis, but it is less effective in the presence of organic material, such as sputum (3, 26). Alcohols are also effective fixing agents. Phenol is a known tuberculocidal agent, even in the presence of concentrated organic material, such as sputum (5, 21, 26). In this study, we tested 70% ethanol as well as three concentrations of phenol in 70% ethanol in order to determine an effective fixing agent that would also rapidly inactivate M. tuberculosis and that could be applied within the confines of a BSC. Smears containing high numbers of M. tuberculosis and other AFB in sputum concentrates, as well as smears prepared from actively growing M. tuberculosis obtained from solid and liquid culture media, were investigated. The methods replicated routine smear preparation work as it is performed in most mycobacteriology laboratories.
Our tests verified that heat fixing smears by passing them through a gas flame does not inactivate M. tuberculosis bacilli. Preliminary fixing test results showed that phenol-ethanol solutions were more successful in inactivating M. tuberculosis smear material. While some smears were inactivated by the lower phenol concentrations, optimal results were obtained with a solution of 5% phenol in ethanol. Smears made from a solid culture medium, such as LJ slants, potentially contain more biomass of M. tuberculosis than do smears made from specimen concentrates. This fact would explain the higher concentration of phenol in ethanol required to inactivate these smears (Table 1). Smears made from concentrated sputum contain a high organic load and therefore require an agent such as phenol for successful inactivation. The confirmation tests showed that the application of 5% phenol in alcohol for 5 min both fixed and inactivated all the smear material tested without affecting subsequent staining quality. A total of 50 smears from both AFB-positive sputum concentrates and M. tuberculosis cultures were successfully inactivated by this method during our tests.
We therefore recommend that all smears that may contain M. tuberculosis be treated with 5% phenol in ethanol for 5 min within a BSC as a rapid and effective fixing and inactivating method. The fixing solution can be applied to air-dried slides placed on a rack over a pan within the cabinet. Smears can then be safely removed from the BSC for staining, storage, or transportation.
When agents, such as M. tuberculosis, that are infectious for humans are processed within laboratories, they present a risk to workers, coworkers, and the community. The recognition of potential hazards by laboratory risk assessments and the institution of protocols to minimize or eliminate the potential for exposure to the agents are effective ways to avoid laboratory-acquired infections.
REFERENCES
- 1.Allen, B. W. 1981. Survival of tubercle bacilli in heat-fixed sputum smears. J. Clin. Pathol. 34:719-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barenfanger, J. 1993. Making your lab safe against multi-drug-resistant Mycobacterium tuberculosis. Clin. Microbiol. Newsl. 15:76-80. [Google Scholar]
- 3.Best, M., S. A. Sattar, V. S. Springthorpe, and M. E. Kennedy. 1990. Efficacies of selected disinfectants against Mycobacterium tuberculosis. J. Clin. Microbiol. 28:2234-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blair, E. B., W. W. Bretherton, and A. H. Tull. 1972. A method to render unstained mycobacterial smears safe for storage or shipment. Appl. Microbiol. 23:826.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Block, S. S. 1991. Disinfection, sterilization and preservation, 4th ed. Lea & Febiger, Publishers, Philadelphia, Pa.
- 6.Centers for Disease Control and Prevention and National Institutes of Health. 1999. Biosafety in microbiological and biomedical laboratories, 4th ed. U.S. Department of Health and Human Services, Washington, D.C.
- 7.Harding, A., and K. Brandt Byers. 2000. Epidemiology of laboratory-associated infections, p. 35-54. In D. O. Fleming and D. L. Hunt (ed.), Biological safety: principles and practices, 3rd ed. ASM Press, Washington, D.C.
- 8.Harrington, J. M., and H. S. Shannon. 1976. Incidence of tuberculosis, hepatitis, brucellosis and shigellosis in British medical laboratory workers. Br. Med. J. 1:759-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Health Canada. 1996. Guidelines for preventing the transmission of tuberculosis in Canadian Health Care Facilities and other institutional settings. Can. Commun. Dis. Rep. 22(Suppl. 1):1-50. [PubMed] [Google Scholar]
- 10.Kao, A. S., D. A. Ashford, M. M. McNeil, N. G. Warren, and R. C. Good. 1997. Descriptive profile of tuberculin skin testing programs and laboratory-acquired tuberculosis infections in public health laboratories. J. Clin. Microbiol. 35:1847-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knudsen, R. C. 1998. Risk assessment for biological agents in the laboratory. J. Am. Biol. Safety Assoc. 3:99-104. [Google Scholar]
- 12.Knudsen, R. C. 1999. Risk assessment for working with infectious agents in the biological laboratory, p. 1-10. In J. Y. Richmond (ed.), Anthology of biosafety. III. Application of principles. American Biological Safety Association, Mundelein, Ill.
- 13.Kubica, G. P. 1990. Your tuberculosis laboratory: are you really safe from infection? Clin. Microbiol. Newsl. 12:85-87. [Google Scholar]
- 14.Laboratory Centre for Disease Control. 1996. Laboratory biosafety guidelines, 2nd ed. Health Canada, Health Protection Branch, Ottawa, Ontario, Canada.
- 15.Menzies, D., A. Fanning, L. Yuan, and M. Fitzgerald. 1995. Tuberculosis among health care workers. N. Engl. J. Med. 332:92-98. [DOI] [PubMed] [Google Scholar]
- 16.Peerbooms, P. G. H., G. J. J. van Doornum, H. van Deutekom, R. A. Coutinho, and D. van Soolingen. 1995. Laboratory-acquired tuberculosis. Lancet 345:1311-1312. [DOI] [PubMed] [Google Scholar]
- 17.Pike, R. M. 1976. Laboratory-associated infections: summary and analysis of 3291 cases. Health Lab. Sci. 13:105-114. [PubMed] [Google Scholar]
- 18.Pike, R. M. 1978. Past and present hazards of working with infectious agents. Arch. Pathol. Lab. Med. 102:333-336. [PubMed] [Google Scholar]
- 19.Pike, R. M. 1979. Laboratory-associated infections: incidence, fatalities, causes and prevention. Annu. Rev. Microbiol. 33:41-66. [DOI] [PubMed] [Google Scholar]
- 20.Reid, D. D. 1957. Incidence of tuberculosis among workers in medical laboratories. Br. Med. J. 2:10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rutala, W. A., E. C. Cole, N. S. Wannamaker, and D. J. Weber. 1991. Inactivation of Mycobacterium tuberculosis and Mycobacterium bovis by 14 hospital disinfectants. Am. J. Med. 91:267S-271S. [DOI] [PubMed] [Google Scholar]
- 22.Saint-Paul, M., Y. Delplace, C. Tufel, G. B. Cabasson, and A. Cavieneaux. 1972. Tuberculoses professionelles dan les laboratoires de bacteriologie. Arch. Mal. Prof. Med. Trav. 33:305-309. [PubMed] [Google Scholar]
- 23.Sewell, D. L. 1995. Laboratory-associated infections and biosafety. Clin. Microbiol. Rev. 8:389-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma, V. K., B. Kumar, B. D. Radotra, and S. Kaur. 1990. Cutaneous inoculation tuberculosis in laboratory personnel. Int. J. Dermatol. 29:293-294. [DOI] [PubMed] [Google Scholar]
- 25.Shireman, P. K. 1992. Endometrial tuberculosis acquired by a health care worker in a clinical laboratory. Arch. Pathol. Lab. Med. 116:521-523. [PubMed] [Google Scholar]
- 26.Voss, A. 1999. Prevention and control of laboratory-acquired infections, p. 165-166. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. American Society for Microbiology, Washington, D.C.