Abstract
A highly sensitive, non-probe-based, real-time quantitative reverse transcriptase PCR was developed for viral load measurements in both serum and liver samples from patients with hepatitis C virus (HCV) infection. With synthetic RNA, the linearity of the approach was conserved over a wide range of HCV copy numbers. There was a strong correlation between hepatic and serum viral load measurements (r = 0.689, P = 0.004, n = 15), indicating that the level of viremia reflected the amount of virus present in the liver.
Hepatitis C virus (HCV) is the major causative agent of non-A, non-B viral hepatitis (2, 11). Acute HCV infection is often asymptomatic, and approximately 70% of cases progress to chronic hepatitis. This may lead to progressive liver disease, cirrhosis, liver failure, and hepatocellular carcinoma over 20 to 30 years. Factors associated with disease progression following HCV infection include the viral genotype, alcohol consumption, and the viral load (5). High viral loads are associated with decreased rates of response to interferon therapy (20), and a decrease in the HCV load during the early phase of treatment (2 to 12 weeks) has been shown to predict effective treatment responses (22). The HCV loads in infected patients range from undetectable to 108 copies/ml and are currently measured with either PCR (HCV Amplicor Monitor 2.0; Roche Diagnostic Systems, Branchburg, N.J.) (14, 15) or branched-DNA (VERSANT HCV RNA 3.0 assay; Bayer Diagnostics, Berkeley, Calif.) (1, 4) assays. Recently, a commercial enzyme immunoassay that measures free HCV core antigen has become available (Ortho HCV core antigen ELISA test system; Ortho-Clinical Diagnostics) (17).
Although there are a wealth of information about and numerous assays for viral load detection in serum and plasma, few studies have correlated serum and hepatic viral loads. Moreover, recent developments in real-time PCR have not yet been applied to intrahepatic viral load measurements. Here, we describe a highly sensitive real-time quantitative reverse transcriptase (RT) PCR (QRT-PCR) assay for viral load measurements in both serum and liver samples.
Serum samples and RNA extraction.
Serum samples from 40 chronically HCV-infected patients were stored at −70°C and thawed once immediately prior to use. Liver biopsies obtained at the same time as the serum samples were available for 15 patients, in addition to five other liver biopsies from HCV-positive individuals. Total RNA was extracted from 200 μl of serum as described previously (19). Hepatic RNA was extracted by taking a portion of liver tissue snap-frozen in liquid nitrogen at the time of biopsy. Samples were subsequently weighed and homogenized in 50 μl of Tri-Reagent (Sigma). A further 750 μl of Tri-Reagent was added, followed by 200 μl of chloroform. The sample was centrifuged, and RNA was precipitated with 500 μl of isopropanol. The resultant pellet was washed with 70% ethanol and redissolved in 50 μl of water, and the total RNA yield was determined by spectrophotometric measurement of the optical density at 260 nm.
HCV RNA quantification.
The aim of this study was to develop a simple, real-time QRT-PCR assay for the quantification of HCV RNA in both serum and liver samples with SYBR Green I detection rather than the use of labeled probes. Real-time QRT-PCR assays were performed with a LightCycler (Roche) with both single-round amplification and a novel limited-cycle nested amplification. RNA standards were transcribed with T7 polymerase from amplicons derived from 5′ untranslated region PCR products cloned into pGEM-T Easy (Promega) and treated twice with RQ1 DNase. The RNA was quantified by spectrophotometry. For single-round quantification of serum samples, RNA was reverse transcribed as described previously (7). For limited-cycle nested PCR quantification, an RT-PCR was performed in a one-step reaction with 15 cycles of PCR (the limited-cycle step), followed by a real-time QRT-PCR. HCV loads were measured by real-time PCR with outer primers KY78 and KY80 for single-round reactions (21) or outer primers plus inner primers hep21b and hep22 for limited-cycle nested reactions (19). Reaction mixtures included 2 μl of Fast Start DNA Master SYBR Green I (Roche), 4 mM MgCl2, a 0.5 μM concentration of each primer, and 2 μl of the template in a total volume of 20 μl. Following incubations for 10 min at 95°C, the LightCycler PCR was performed for 40 cycles, each cycle consisting of 15 s at 95°C, annealing at 60°C for 5 s, and extension at 72°C for 10 s. Fluorescence was monitored at 530 nm, and the specificity of the signal was checked by melting curve analysis.
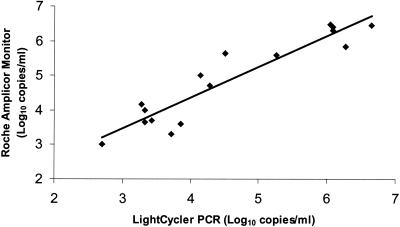
With synthetic HCV RNA as the template, the sensitivity of the single-round real-time QRT-PCR assay was approximately 103 HCV copies/reaction or 5 × 104 copies/ml when the dilution factor was calculated. A wide linear relationship was observed (up to 6.5 × 108 copies/reaction) between the number of PCR cycles needed to detect a fluorescence signal and the number of HCV copies. We measured the serum viral loads of 24 patients chronically infected with various HCV genotypes prevalent in Australia (1a, 1b, 2a, 2b, 2c, and 3a) (6). Three patients had viral loads below the sensitivity of this version of the assay (5 × 104 copies/ml), although they were positive by a qualitative in-house nested RT-PCR assay (19). The remaining 21 patients had viral loads that ranged from 2.8 × 105 to 9.1 × 106 copies/ml. To increase the sensitivity and specificity of the assay, a 15-cycle, one-step RT-PCR was introduced with a subsequent real-time quantitative PCR for the second round. With synthetic RNA, the modified method was able to detect a wide range of HCV copy numbers (from 28 to 2.8 × 106 HCV copies/reaction) while maintaining linearity. The sensitivity of the limited-cycle nested QRT-PCR assay was greater than 28 copies/reaction (or 1,400 copies/ml), which was approximately 40-fold greater than that of the single-round QRT-PCR assay. The sensitivity of this assay compares favorably with those of the previously reported methods using real-time quantitation with TaqMan technology (Roche) and an ABI Prism 7700 sequence detection system (Perkin-Elmer), which have reported sensitivities of 10 copies/reaction (9, 16) and 1,000 copies/reaction (12). The sensitivity of LightCycler (Roche) and SYBR Green I detection has also been reported to be 10 copies/reaction (10). To further validate this version of the assay, the viral loads of 16 HCV-positive serum samples, which ranged from 1 × 103 to 1.2 × 106 copies/ml (as determined with the Amplicor Monitor assay), were shown to correlate closely with results obtained with the nested real-time QRT-PCR assay (r= 0.876, P < 0.0001, n = 16) (Fig. 1).
FIG. 1.
Comparison of HCV load measurements with nested real-time QRT-PCR and Amplicor Monitor assay results. Sixteen HCV-positive serum samples with a wide range of viral loads, measured with the Amplicor Monitor assay and ranging from 1 × 103 to 1.2 × 106 copies/ml, were evaluated with the nested real-time QRT-PCR assay (r = 0.862, P < 0.0001, n = 16).
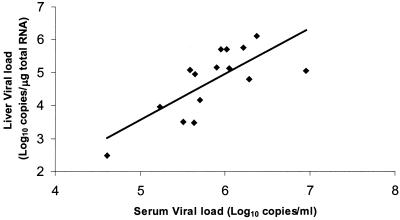
HCV replicates in the liver, yet few studies have attempted to relate viral loads in the liver to clinical outcomes, preferring to examine the predictive value of serum viral loads. The latter could be considered a suitable surrogate for hepatic viral loads if they were shown to correlate, yet few studies have shown this and the results reported to date are not convincing. The limited-cycle nested QRT-PCR assay was therefore used to measure hepatic HCV loads. The amount of liver tissue used for analysis weighed between 0.8 and 8.1 mg (mean = 3.88 ± 2.1 mg), from which 2.1 to 12.9 μg (mean = 6.62 ± 3.1 μg) of RNA was extracted. The yield of total RNA averaged 1.71 × 103 μg/g of liver tissue, a value similar to that found in an earlier study of 1.01 × 103 μg of RNA/g (13). Hepatic viral loads ranged from 3.0 × 102 to 1.3 × 106 HCV RNA molecules/μg of total RNA, and the average was 2.38 × 105 ± 3.55 × 105 copies/μg of RNA. A correlation has been demonstrated between hepatic viral loads, measured in terms of the number of HCV copies per microgram of total RNA, and serum viral loads measured with the Amplicor Monitor assay, although it was weak (r = 0.417, n = 20) (13). Other studies have shown that the HCV RNA load in serum is substantially reflected by the amount of virus in the liver without providing correlation coefficients (3, 8). Here, we report for the first time a statistically significant correlation between hepatic and serum viral load measurements (r = 0.689, P = 0.004, n = 15), indicating that the level of viremia does, in fact, reflect the amount of virus present in the liver (Fig. 2).
FIG. 2.
Correlation between HCV RNA levels in 15 paired serum (number of copies per milliliter) and liver (number of copies per microgram of total RNA) samples (r = 0.689, P = 0.004, n = 15).
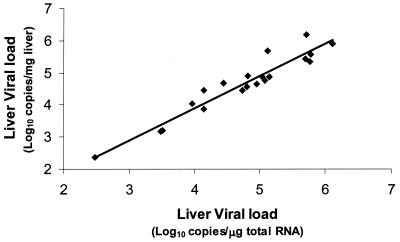
In previous studies, liver viral load measurements have been expressed as the number of copies per milligram of liver tissue or the number of copies per microgram of total RNA. In this study, we show that either unit appears to be suitable, as there was a significant correlation between viral loads measured in terms of the number of copies per milligram of liver tissue and those measured as the number of copies per microgram of total RNA (r = 0.937, P < 0.0001, n = 20) (Fig. 3). These results are consistent with those of De Moliner et al. (3), who also demonstrated a close correlation between the viral load in the liver, expressed as the number of copies per gram of tissue, and the number of copies per microgram of total cellular RNA (r = 0.85) (3). The average number of HCV RNA molecules per gram of liver tissue was 2.1 × 108 ± 3.71 × 108, around 170-fold higher than the RNA levels in serum (1.22 × 106 ± 2.0 × 106 copies/ml). This finding is similar to that of Terrault et al. (18), who showed that the ratio of HCV RNA in liver to that in serum was 103:1. In contrast, McGuiness et al. (13) found that the level in liver tissue was greater by a factor of >104. This difference may relate to the fact that the hepatic viral loads of the patients in the previous study, who predominantly had advanced HCV infection, were around eightfold higher (1.83 × 106 ± 0.75 × 106 copies/μg of RNA) than those recorded in the present study (2.38 × 105 ± 3.55 × 105 copies/μg of RNA).
FIG. 3.
Relationship between HCV RNA levels in 20 liver biopsies expressed in terms of the number of copies per milligram of liver tissue and the number of copies per microgram of total cellular RNA (r = 0.937, P < 0.0001, n = 20).
In conclusion, the real-time QRT-PCR assay is a sensitive, accurate, and reliable method for monitoring of HCV loads in both serum and liver samples.
Acknowledgments
We thank the Virology Diagnostic Laboratory, SEALS, Prince of Wales Hospital, and Roche Diagnostics for their help and Parisa Aslani for statistical analysis.
REFERENCES
- 1.Beld, M., R. Sentjens, S. Rebers, C. Weegink, J. Weel, C. Sol, and R. Boom. 2002. Performance of the new Bayer VERSANT HCV RNA 3.0 assay for quantitation of hepatitis C virus RNA in plasma and serum: conversion to international units and comparison with the Roche COBAS Amplicor HCV monitor, version 2.0, assay. J. Clin. Microbiol. 40:788-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choo, Q. L., G. Kuo, A. J. Weiner, L. R. Overby, D. W. Bradley, and M. Houghton. 1989. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science 244:359-362. [DOI] [PubMed] [Google Scholar]
- 3.De Moliner, L., P. Pontisso, G. L. De Salvo, L. Cavalletto, L. Chemello, and A. Alberti. 1998. Serum and liver HCV RNA levels in patients with chronic hepatitis C: correlation with clinical and histological features. Gut 42:856-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Detmer, J., R. Lagier, J. Flynn, C. Zayati, J. Kolberg, M. Collins, M. Urdea, and R. Sanchez-Pescador. 1996. Accurate quantification of hepatitis C virus (HCV) RNA from all HCV genotypes by using branched-DNA technology. J. Clin. Microbiol. 34:901-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freeman, A. J., G. J. Dore, M. G. Law, M. Thorpe, J. Von Overbeck, A. R. Lloyd, G. Marinos, and J. M. Kaldor. 2001. Estimating progression to cirrhosis in chronic hepatitis C virus infection. Hepatology 34:809-816. [DOI] [PubMed] [Google Scholar]
- 6.Freeman, A. J., A. Zekry, L. R. Whybin, C. E. Harvey, I. A. van Beek, S. L. de Kantzow, W. D. Rawlinson, C. R. Boughton, P. W. Robertson, G. Marinos, and A. R. Lloyd. 2000. Hepatitis C prevalence among Australian injecting drug users in the 1970s and profiles of virus genotypes in the 1970s and 1990s. Med. J. Aust. 172:588-591. [DOI] [PubMed] [Google Scholar]
- 7.Hu, K. Q., C. H. Yu, and J. M. Vierling. 1993. One-step RNA polymerase chain reaction for detection of hepatitis C virus RNA. Hepatology 18:270-274. [PubMed] [Google Scholar]
- 8.Idrovo, V., P. J. Dailey, L. J. Jeffers, E. Coelholittle, D. Bernstein, M. Bartholomew, L. Alvarez, M. S. Urdea, M. L. Collins, and E. R. Schiff. 1996. Hepatitis C virus RNA quantification in right and left lobes of the liver in patients with chronic hepatitis C. J. Viral Hepatitis 3:239-246. [DOI] [PubMed] [Google Scholar]
- 9.Kishimoto, H., K. Yoshioka, M. Yano, K. Ukai, H. Ito, K. Watanabe, O. Kawamata, and S. Kakumu. 2001. Real-time detection system for quantitation of hepatitis C virus RNA: a comparison with the other three methods. Hepatol. Res. 19:12-21. [DOI] [PubMed] [Google Scholar]
- 10.Komurian-Pradel, F., G. Paranhos-Baccala, M. Sodoyer, P. Chevallier, B. Mandrand, V. Lotteau, and P. Andre. 2001. Quantitation of HCV RNA using real-time PCR and fluorimetry. J. Virol. Methods 95:111-119. [DOI] [PubMed] [Google Scholar]
- 11.Kuo, G., Q. L. Choo, H. J. Alter, G. L. Gitnick, A. G. Redeker, R. H. Purcell, T. Miyamura, J. L. Dienstag, M. J. Alter, C. E. Stevens, et al. 1989. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science 244:362-364. [DOI] [PubMed] [Google Scholar]
- 12.Martell, M., J. Gomez, J. I. Esteban, S. Sauleda, J. Quer, B. Cabot, R. Esteban, and J. Guardia. 1999. High-throughput real-time reverse transcription PCR quantitation of hepatitis C virus RNA. J. Clin. Microbiol. 37:327-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGuinness, P. H., G. A. Bishop, D. M. Painter, R. Chan, and G. W. McCaughan. 1996. Intrahepatic hepatitis C RNA levels do not correlate with degree of liver injury in patients with chronic hepatitis C. Hepatology 23:676-687. [DOI] [PubMed] [Google Scholar]
- 14.Mellor, J., A. Hawkins, and P. Simmonds. 1999. Genotype dependence of hepatitis C virus load measurement in commercially available quantitative assays. J. Clin. Microbiol. 37:2525-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nolte, F. S., C. Thurmond, and M. W. Fried. 1995. Preclinical evaluation of Amplicor hepatitis C virus test for detection of hepatitis C virus RNA. J. Clin. Microbiol. 33:1775-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takeuchi, T., A. Katsume, T. Tanaka, A. Abe, K. Inoue, K. Tsukiyama-Kohara, R. Kawaguchi, S. Tanaka, and M. Kohara. 1999. Real-time detection system for quantification of hepatitis C virus genome. Gastroenterology 116:636-642. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka, E., C. Ohue, K. Aoyagi, K. Yamaguchi, S. Yagi, K. Kiyosawa, and H. J. Alter. 2000. Evaluation of a new enzyme immunoassay for hepatitis C virus (HCV) core antigen with clinical sensitivity approximating that of genomic amplification of HCV RNA. Hepatology 32:388-393. [DOI] [PubMed] [Google Scholar]
- 18.Terrault, N. A., P. J. Dailey, L. Ferrell, M. L. Collins, J. C. Wilber, M. S. Urdea, B. N. Bhandari, and T. L. Wright. 1997. Hepatitis C virus—quantitation and distribution in liver. J. Med. Virol. 51:217-224. [PubMed] [Google Scholar]
- 19.White, P. A., X. Zhai, I. Carter, Y. Zhao, and W. D. Rawlinson. 2000. Simplified hepatitis C virus genotyping using heteroduplex mobility analysis. J. Clin. Microbiol. 38:477-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamada, G., M. Takatani, F. Kishi, M. Takahashi, T. Doi, T. Tsuji, S. Shin, M. Tanno, M. S. Urdea, and J. A. Kolberg. 1995. Efficacy of interferon alfa therapy in chronic hepatitis C patients depends primarily on hepatitis C virus RNA level. Hepatology 22:1351-1354. [PubMed] [Google Scholar]
- 21.Young, K. K., R. M. Resnick, and T. W. Myers. 1993. Detection of hepatitis C virus RNA by a combined reverse transcription-polymerase chain reaction assay. J. Clin. Microbiol. 31:882-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeuzem, S., J. H. Lee, A. Franke, B. Ruster, O. Prummer, G. Herrmann, and W. K. Roth. 1998. Quantification of the initial decline of serum hepatitis C virus RNA and response to interferon alfa. Hepatology 27:1149-1156. [DOI] [PubMed] [Google Scholar]