Abstract
In France, an epidemic peak of acute diarrhea is observed each winter. Previous results suggested a viral etiology for these winter epidemics. We investigated the role of enteric viruses in acute diarrhea and their molecular diversity. One hundred sixty-one patients with acute diarrhea and 45 healthy patients (controls) from the general population were given a standardized questionnaire between December 1998 and May 1999. Stool specimens were screened for group A and C rotaviruses, human caliciviruses, astroviruses, and adenovirus types 40 and 41 by reverse transcription-PCR and/or enzyme immunoassay. Virologic analysis was positive for 63 cases (39%). Caliciviruses and group A rotaviruses were the most frequent (19 and 17% of cases, respectively). Two control stool specimens were found positive for group A rotavirus, and one was found positive for astrovirus. Molecular characterization of the strains disclosed a cocirculation of P[8],G1, P[8],G4, and P[4],G2 rotaviruses; type 1, 2, 3, 4, and 8 astroviruses; and Sapporo-like and Norwalk-like human caliciviruses. These four types of viruses accounted for an attributable risk of acute diarrhea of 34.7% for the general population, under the assumption of a causal role of these viruses.
Viral gastroenteritis is a common illness in humans of all age groups, with a high morbidity reported worldwide (20). Four types of viruses often have been considered the cause of this disease all over the world: group A rotaviruses, adenovirus types 40 and 41, astroviruses, and human caliciviruses, including Norwalk-like and Sapporo-like viruses (2, 12, 13, 20). However, it was not known to what extent community-acquired gastroenteritis was caused by these viruses, as most studies were conducted in hospitals and nursing homes or in the context of outbreaks.
The recent development of sensitive molecular tools, such as reverse transcription (RT)-PCR, led to improved diagnostic assays, particularly for human caliciviruses, allowing etiologic surveys in the community. Consequently, in a previous study (3), Bon et al. looked for the four most important gastroenteritis viruses in children consulting a pediatrician for gastroenteritis and highlighted the importance of viruses as etiologic agents as well as the high rate of Norwalk-like viruses (second after rotaviruses). Similar results were also reported by Pang et al. for young children in the community (33). To our knowledge, two etiologic surveys in the community have included patients of all age groups, in The Netherlands (7) and in England (35). These authors conducted large-scale physician-based studies and detected viruses in 15 and 22% of the cases, respectively.
In France, where acute diarrhea (AD) has been monitored by the Sentinel network of general practitioners (GPs) since 1991 (5), an epidemic peak is observed each year in winter; it was estimated that about 2% of the French population consulted their GPs for AD during January 1995 (8). The origin of these forms of gastroenteritis remained unknown, although the results of a case-control study by Letrilliart et al. (24) suggested a viral etiology.
Here, we conducted a physician-based case-control survey of AD nested in the French Sentinel surveillance network during the winter of 1998 to 1999. It included subjects from all age groups from 23 December 1998 to 5 May 1999. Both clinical data and stool samples were collected. Virologic analyses were performed, including screening for group A rotaviruses, human caliciviruses, astroviruses, and adenovirus types 40 and 41 and typing of group A rotaviruses, caliciviruses, and astroviruses. We also investigated the geographical distribution of the identified strains. In addition, we examined the prevalence of group C rotaviruses, which have been reported sporadically as human pathogens in children and adults in several parts of the world (18, 22, 28).
MATERIALS AND METHODS
Design, case definition, and selection of controls.
The 103 GPs with the highest rate of participation in the Sentinel network during the previous 12 months were asked to take part in this study. Eighty GPs responded between 23 December 1998 and 5 May 1999. The cases included during this period were patients experiencing AD, defined as losing at least three soft or aqueous stools per 24 h for less than 2 weeks; 90% of these patients were enrolled before 1 March 1999. Controls were defined as people consulting for any other conditions and not reporting any gastrointestinal symptoms during the month preceding the consultation; these patients were enrolled between 27 February and 5 May 1999. To maintain the same level of precision for estimations for each age group, GPs were asked to include one patient from each of the following age groups: 0 to 3, 4 to 15, 16 to 65, and more than 65 years.
During the consultation, a standardized questionnaire was completed by GPs for each patient after the latter had given informed consent. Each patient was also asked to send by express mail a stool sample to a virology laboratory (Dijon, France). The questionnaire concerned the date and location of the consultation and demographic characteristics of the patient. A description of the diarrhea as well as associated symptoms (nausea, vomiting, abdominal pains, fever, and respiratory difficulties) was also registered in the questionnaire.
Laboratory methods.
Stool samples were screened for the presence of group A and C rotaviruses, human caliciviruses, astroviruses, and adenovirus types 40 and 41 by an enzyme immunoassay (EIA) and/or RT-PCR.
Group A rotaviruses were detected by an EIA with group-specific monoclonal antibodies as previously described (34). Group C rotaviruses were screened by RT-PCR. Briefly, RNA was extracted from 20% stool suspensions in phosphate-buffered saline with a QIA Amp viral RNA kit (Qiagen, Hilden, Germany), and RT-PCR was performed by using primers C1 and C4 as described by Gouvea et al. (14). Samples which were found negative for group A rotaviruses were tested for both group A and group C rotaviruses by combined RT-PCR assays with a pool of primers: Beg 9 and End 9 (15) and con2 and con3 (11) for group A and C1 and C4 for group C. G and P typing of group A rotaviruses was done as described by Gouvea et al. (15) and Gentsch et al. (11) (the letters G and P indicate the gene segments encoding, respectively, the glycoprotein and the phosphoprotein of the rotavirus).
Human caliciviruses were detected by RT-PCR with four primer sets in separate reactions, thus allowing the detection of Norwalk-like viruses (genogroups I and II) and Sapporo-like viruses. The degenerate primer NVp110, described by Le Guyader et al. (23), was used for RT, together with NVp36 (37), SR48-50-52 (1), NI (16), and SR80 (31) for PCR.
Astroviruses and adenovirus types 40 and 41 were detected with an EIA kit (IDEIA Astrovirus [Dako Diagnostics Ltd.] and Adenoclone type 40/41 EIA [Meridian Diagnostics Inc., Cincinnati, Ohio]). For astroviruses, the results were confirmed by amplification of a region located in open reading frame 2 by RT-PCR with primers Mon 244 and Mon 245 by the method described by Noel et al. (30).
Genotyping of astroviruses and caliciviruses was done by direct sequencing of the PCR products with an ABI Prism Big Dye Terminator Cycle Sequencing Ready Reaction kit (PE Biosystems) and an automated sequencer (model 373A DNA sequencing system [PE Biosystems]).
Sequence analysis was done by using the national service Infobiogen (http://www.infobiogen.fr). Sequence alignments were carried out by using Clustal W software. Phylogenetic analysis was performed with the PHYLIP package (version 3.5c). Replicate data sets (n = 100) were generated by bootstrap resampling and analyzed by the neighbor-joining method. The tree was drawn with Drawtree software, and the geographic distribution of strains was investigated with ArcView software (ESRI version 3.2).
Statistical methods.
To estimate the relative risk of AD attributable to viruses, we used binary logistic regression to calculate odds ratios (ORs) and their 95% confidence intervals with LogXact software (CYTEL version 4). We compared categorical data by the chi-square test. Two-tailed tests with a significance level (P value) of <0.050 were used. The proportion of AD in the general population which could actually be attributable to one of the four screened viruses corresponds to the attributable risk and was calculated as follows (36): AR = [(OR − 1) × Pi]/OR; AR is the attributable risk and Pi is the proportion of infections in the sample.
RESULTS
Detection of viruses among patients and controls.
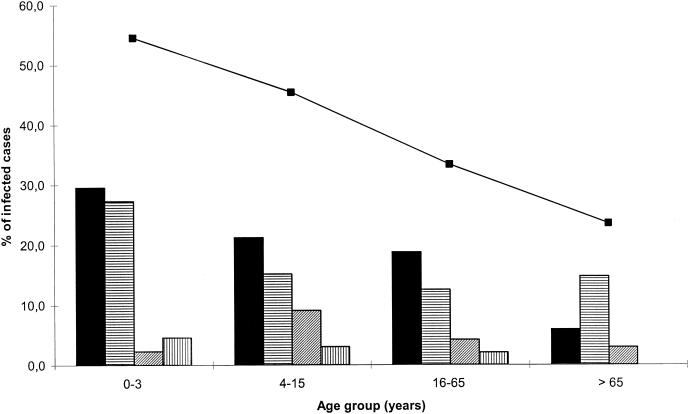
GPs interviewed a total of 200 patients and 45 individuals serving as controls, but stool samples were received only from 161 patients and 45 controls. Patients and controls were almost equally distributed among the four age groups. Viral detection was positive for at least one virus in 63 patients (39.1%), including seven dual infections. As shown in Fig. 1, infections tended to decrease significantly with age, as the proportion of infected patients in each age group decreased by about 10% (the P value for the trend was <0.001): 54.5% in children 0 to 3 years old, 45.5% for patients who were 4 to 15 years old, 32.6% for patients who were 16 to 65 years old, and 22.8% for patients who were more than 65 years old. Caliciviruses were detected in 31 (19.2%) of AD patients, and group A rotaviruses were detected in 28 (17.4%). Astroviruses and adenovirus types 40 and 41 were detected in seven and four patient samples, respectively (4.3 and 2.5%, respectively), but no group C rotavirus was detected. Among these viruses, two rotaviruses and one astrovirus were detected only by RT-PCR.
FIG. 1.
Distributions of the four viruses among the patients according to age groups. Bars indicate the following viruses: ▪, calicivirus; ▤, rotavirus; ▨, astrovirus; ▥, adenovirus types 40 and 41. The line shows the total percentage of infected patients.
Among the seven dual infections, six involved a calicivirus: three dual infections with a rotavirus, two with adenovirus type 40 or 41, and one with an astrovirus. The last one was a dual infection with both a rotavirus and an astrovirus. Among the youngest patients (0 to 3 years old), caliciviruses were as frequent as rotaviruses (29.5 and 27.3%, respectively) (Fig. 1), and two patients were dually infected by both a rotavirus and a calicivirus. There was no significant difference in the distributions of rotaviruses in the other three age groups (P = 0.9). Viral detection was positive for 3 of the 45 controls (6.7%). Two of them tested positive for group A rotavirus (0 to 3 and more than 65 years old), and the third one (more than 65 years old) tested positive for an astrovirus, which was detected by RT-PCR.
As shown in Table 1, there was a significant association between any of the four viral identifications and AD (OR, 9.0; 95% confidence interval, 2.7 to 47.4). Subgroup analysis showed a significant association between AD and either caliciviruses (OR, 15.2; 95% confidence interval, 2.6 to infinity) or rotaviruses (OR, 4.5; 95% confidence interval, 1.1 to 40.9). Taken together, the four viruses were responsible for an attributable risk of AD of 34.7% of the general population during the period considered.
TABLE 1.
ORs for viral infections in AD
| Virus | No. of:
|
OR | 95% Confidence interval | |
|---|---|---|---|---|
| Patients (n = 161) | Controls (n = 45) | |||
| Calicivirus | 31 | 0 | 15.2 | 2.6-Infinity |
| Rotavirus | 28 | 2 | 4.5 | 1.0-40.9 |
| Astrovirus | 7 | 1 | 2.0 | 0.2-92.8 |
| Adenovirus | 4 | 0 | 1.5 | 0.2-Infinity |
| All four viruses | 63a | 3 | 9.0 | 2.7-47.4 |
Including seven with dual infections.
Clinical characteristics of viral diarrhea.
For the 161 patients who sent in stool samples, complete clinical data were available for only 137, including the description of the diarrhea and associated symptoms, such as nausea or vomiting, fever, abdominal pains, and respiratory difficulties. More patients found positive for at least one virus than patients found negative suffered from nausea or vomiting (P = 0.02) and had aqueous stools (P = 0.045) (Table 2). Among the infected patients, more rotavirus-positive than calicivirus-positive patients had fever (57 versus 28%, respectively; P = 0.045). This was the only significant difference between these two groups. These analyses excluded the seven dual infections and the seven single infections with either an astrovirus (five infections) or an adenovirus (two infections). In general, fewer children less than 4 years old than patients over that age had nausea or vomiting (47 versus 50 to 77%; P = 0.003) and abdominal pains (52 versus 83 to 95%; P < 0.0001).
TABLE 2.
Clinical features in patients with or without a detected virus
| Symptom | No. (%) of patients
|
Pa | |
|---|---|---|---|
| Positive for infection | Negative for infection | ||
| Soft stools | 6 (11) | 19 (24) | 0.045 |
| Aqueous stools | 50 (89) | 59 (75) | 0.045 |
| Nausea or vomiting | 42 (75) | 45 (55) | 0.02 |
| Abdominal pains | 44 (81) | 64 (80) | 0.83 |
| Fever | 23 (42) | 26 (32) | 0.27 |
| Respiratory signs | 12 (21) | 22 (28) | 0.40 |
Determined by the chi-square test.
Genotyping of group A rotaviruses, astroviruses, and caliciviruses.
Genotyping of group A rotaviruses by multiplex RT-PCR showed that 17 (61%) of the 28 rotavirus strains detected were P[8],G1; 4 (14.5%) were P[8],G4 and 4 (14.5%) were P[4],G2. The three remaining strains could not be typed. No geographic pattern was observed for the distribution of the different genotypes, and there was no association between a given genotype and age or severity of the illness.
The genotype of each astrovirus strain was determined by sequence analysis of a 348-bp region of open reading frame 2 (nucleotides 4571 to 4918). The strains exhibited a diversity of genotypes. The seven strains detected in the patients were distributed as follows: type 1, one strain; type 2, one strain; type 3, one strain; type 4, two strains; and type 8, two strains. The strain detected in the controls was also classified as type 8 and had 97.4% nucleotide identity with the two type 8 strains detected in the patients. The three type 8 strains were detected in the same geographic area. No association was observed between a given type and age.
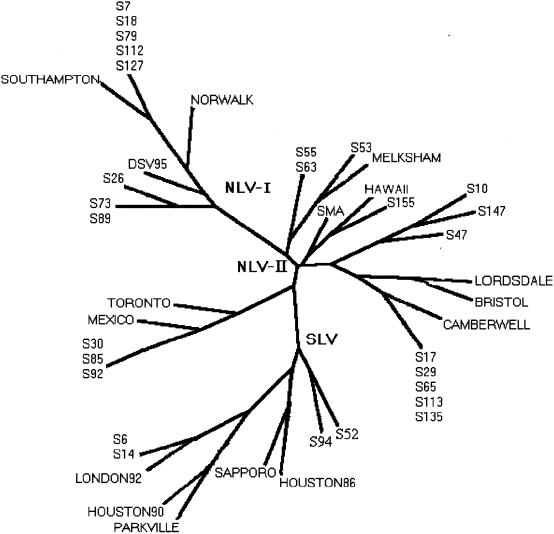
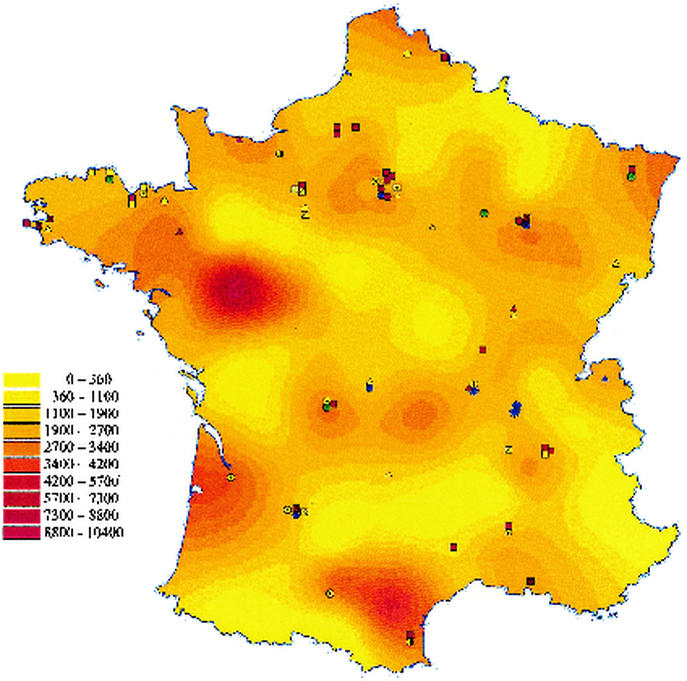
The genotypes of 27 of the 31 calicivirus strains were determined by sequencing of a 76-bp fragment of the polymerase gene used for detection. As for astroviruses, the results showed a great diversity of strains clustered in eight different groups (Fig. 2): 23 of the 27 strains (85%) were Norwalk-like viruses, and the other 4 (15%) were Sapporo-like viruses. Among the 23 Norwalk-like virus strains, five related to Southampton virus (>92% nucleotide identity) were genogroup I strains. Three other genogroup I strains could not be classified into one of the clusters described: two strains were related to strain JPNNV23 (97% identity), and the other strain was related to strain H104-94J (93% identity) (GenBank accession numbers D82331 and AB020553, respectively). The other Norwalk-like virus strains belonged to genogroup II and could be further divided into four genetic groups represented by Lordsdale virus (eight strains), Melksham virus (three strains), Mexico virus (three strains), and Hawaii virus (one strain). The Sapporo-like virus strains could be divided into two groups, the Sapporo/82 virus group (96% identity with Plymouth virus; GenBank accession number X86559) and the London/92 virus group (95% identity with London/92 virus). As most of the strains were located in geographic regions far from one another, it was difficult to propose a geographic pattern of distribution. However, four out of the five Southampton-like viruses were found in the same northwestern region (Côtes d'Armor), the three Melksham-like viruses were found in the southwestern region, and the three Mexico-like viruses were found in the northern region (Fig. 3). Finally, all Sapporo-like viruses were detected in children less than 4 years old, whereas for Norwalk-like viruses, no relationship between a genetic cluster and age was observed.
FIG. 2.
Phylogenetic tree based on a 76-nucleotide region of the polymerase gene of calicivirus strains detected in France from December 1998 to May 1999. Designations consisting of the letter S followed by a number indicate the patient sample number positive for a calicivirus. GenBank accession numbers for Norwalk-like virus (NLV) strains representative of genogroup I strains were M87661 (Norwalk), L07418 (Southampton), and U04469 (Desert Shield [DS]); those for Norwalk-like virus strains representative of genogroup II strains were U07611 (Hawaii), L23831 (Snow Mountain), U22498 (Mexico), U02030 (Toronto), X86557 (Lordsdale), U46500 (Camberwell), X76716 (Bristol), and X81879 (Melksham). GenBank accession numbers for Sapporo-like virus (SLV) strains were S77903 (Sapporo/82), U95643 (Houston/86), U67858 (London/92), U95644 (Houston/90), and U73124 (Parkville).
FIG. 3.
Detected viruses. The symbols on the map of France show the following viruses: green—adenovirus types 40 and 41 (open hexagon); blue—astrovirus type 1 (open square), type 2 (open octagon containing solid square), type 3 (open triangle), type 4 (open pentagon), and type 8 (open star); yellow—human calicivirus strains Southampton (open square), H104-94J (bisected open circle), JPNNV23 (black and white circle), Hawaii (open hexagon containing solid square), Lordsdale (open triangle), Melksham (open octagon containing solid square), Mexico (open pentagon), London (open pentagon containing solid circle), and Sapporo (open square containing solid square); yellow box containing multiplication sign—nontyped; red—group A rotaviruses P[8],G1 (open square), P[8],G4 (open triangle), and P[4],G2 (open circle); red box containing multiplication sign—nontyped. The color gradations in the map indicate the incidence rates for AD as reported on the Sentinel network from December 1998 to May 1999, shown as the number of cases per 100,000 inhabitants (color key to left of map).
DISCUSSION
This study reports a physician-based study of AD in France, including 161 patients and 45 controls 0 to more than 65 years old and virologic analyses including group A and C rotaviruses, caliciviruses, astroviruses, and adenovirus types 40 and 41. Our investigation was conducted from December 1998 to May 1999. Here we show that 39% of the patients were infected by at least one virus and that this epidemic was associated not with a single strain but with a large number of strains exhibiting great molecular diversity, as shown by molecular characterization of rotaviruses, caliciviruses, and astroviruses.
Calicivirus genotyping revealed a great diversity of strains, including Sapporo-like and Norwalk-like virus strains. Norwalk-like virus strains were the most prevalent and were found in all age groups, whereas Sapporo-like virus strains were found in children less than 4 years old, as previously reported (6). Among the Norwalk-like viruses, genogroup II strains predominated, and 53% of them (8 of 15) were related to Lordsdale virus. The predominance of genogroup II strains and especially of Lordsdale virus-related strains has been reported in various countries in the last several years (19, 21). However, whereas the cocirculation of different outbreak strains was observed during certain years (21), the diversity of strains evidenced here was not previously reported in the general population of industrialized countries over such a short period. Most of the strains described here were detected in various regions, although others, like the Melksham virus-related strains, seemed to be clustered in space and time, suggesting that they might have been recently introduced into the population. However, such clustered strains might also represent rare strains seldom encountered in our study.
The group A rotaviruses that we detected displayed cocirculation of P[8],G1, P[8],G4, and P[4],G2 strains, with the P[8],G1 strains predominating. It was previously reported in a 3-year study (4) that the predominant strain circulating in France was P[8],G1, followed by P[8],G4. The predominance of P[8],G1 has been reported in many studies (20).
Genotyping of astroviruses in our study also disclosed a great diversity of cocirculating types, because the eight strains detected in seven patients and one control included five different types (1, 2, 3, 4, and 8). As in many worldwide studies, a previous pediatrician-based study conducted from 1995 to 1998 (3) showed that type 1 was predominant (9 out of the 10 strains; unpublished data). The diversity described in the present investigation may have been due to the fact that all age groups were tested, whereas most studies reporting type1 predominance were conducted with young children (9, 26, 29, 32). Here, six of the eight strains detected were found in patients more than 3 years old, and both of the astroviruses detected in young children were not type 1 but were types 4 and 8. The uncommon type 8 was detected in three samples from patients living in the same region. This type has been described in the United Kingdom (GenBank accession number Z66541, showing 93.7 to 94.4% identity with the three type 8 strains described here) and was recently reported in Australia, Egypt, and Mexico (25, 26, 27). An important diversity of astrovirus strains, including type 8, was also reported in Barcelona during the same period as our study (17). The prevalence of type 8 astrovirus may have been previously underestimated, or type 8 may constitute an emerging type in France.
In this report, we showed that group A rotaviruses and caliciviruses were the most frequent, as they were detected in 17.4 and 19.2% of patients, respectively. Until now, few data were available concerning the prevalence of these viruses in nonhospitalized patients in all age groups, even when sensitive methods were used. In their physician-based study, de Wit et al. (7) found both Norwalk-like viruses and rotaviruses in 5% of patients, and these were the most prevalent viruses. Tompkins et al. (35) also found rotaviruses and small round-structured viruses in 7.7 and 6.5% of patients, respectively, consulting a GP. An evident reason for the higher percentages observed in our study is that we investigated a period shorter than 6 months, including the winter epidemic peak (23 December 1998 to 5 May 1999), and not an entire year.
Controls were included in this study even though they were not matched to patients, because it was far more difficult for GPs to induce control individuals than patients to send in stool samples. Consequently, in this study, only one control was included for four patients, and enrollment began later for controls than for patients. However, when we restricted the patient sample to the 11 patients recruited during the same period as the controls, 4 (36%) were positive, leading to a relative risk of a similar magnitude (OR, 8; 95% confidence interval, 1.5 to 43.4). Therefore, our results do not seem to be due to a bias in the selection of controls.
The present results for the four age groups showed that viral infection was more frequent in children less than 15 years old than in adults. Similar results were obtained in physician-based studies in The Netherlands (21) and the United Kingdom (35). These results may reflect a nonviral origin for an important part of the gastroenteritis cases among adults. The maximum duration of the diarrhea, as given in the case definition for our study (less than 14 days), makes unlikely a chronic illness as a cause of the gastroenteritis.
The importance of caliciviruses as causative agents of acute gastroenteritis in young children was recently emphasized in a pediatrician-based study conducted in Dijon (3) and in a community-based study conducted with children less than 2 years old in Finland (33). Another finding concerns the prevalence of rotavirus infection in patients more than 3 years old, which was found to be stable in all age groups. This result indicates that symptomatic infections caused by rotavirus in children more than 3 years old and in adults are not rare, although subclinical infections may be the most common outcome (20). In our study, only 2 out of 45 controls were infected by a rotavirus, including 1 more than 65 years old. Probably because of the limited number of controls and the recruitment period, which began after the epidemic peak, we did not observe more asymptomatic rotavirus infections.
A viral etiology could not be established for 61% of the cases, despite the use of RT-PCR for caliciviruses, rotaviruses, and astroviruses. The time between sampling and the beginning of clinical symptoms was in most cases less than 7 days and thus may not have accounted for the lack of sensitivity of the virologic methods. In addition to the four viruses tested, other viruses may have been present, such as Aichi virus, suggested to be a novel viral gastroenteritis agent and recently reported in the United Kingdom (10), or other microorganisms may have been present, such as pathogenic bacteria, as previously shown (7, 35).
Our results indicate that, under the assumption of a causal role of group A rotaviruses, caliciviruses, astroviruses, and adenovirus types 40 and 41, these viruses accounted for an attributable risk of AD of 34.7% for the general French population between 23 December 1998 and 5 May 1999; the most important viruses were caliciviruses and group A rotaviruses. The high incidence of caliciviruses in AD provides important clues to the etiology of large-scale epidemics, especially for undiagnosed gastroenteritis.
In conclusion, the finding that so many virus strains cocirculated during a single epidemic at a national level raises questions about their origins, such as contaminated food or environmental reservoirs, as these viruses are known to be very resistant to environmental factors. Their interactions with the environment are currently under investigation in a limited area in France and over a longer period.
Acknowledgments
We thank Laurent Letrilliart and Cécile Viboud for help in writing the article and the Sentinel physicians and their patients for participation in this study.
This work was supported by the Centre Hospitalier Universitaire, Dijon, France, and by grant QLK1-CT-1999-00594 from the European Community.
REFERENCES
- 1.Ando, T., S. S. Monroe, J. R. Gentsch, Q. Jin, D. C. Lewis, and R. I. Glass. 1995. Detection and differentiation of antigenically distinct small round-structured viruses (Norwalk-like viruses) by reverse transcription-PCR and Southern hybridization. J. Clin. Microbiol. 33:64-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blacklow, N. R., and H. B. Greenberg. 1991. Viral gastroenteritis. N. Engl. J. Med. 325:252-264. [DOI] [PubMed] [Google Scholar]
- 3.Bon, F., P. Fascia, M. Dauvergne, D. Tenenbaum, H. Planson, A. M. Petion, P. Pothier, and E. Kohli. 1999. Prevalence of group A rotavirus, human calicivirus, astrovirus, and adenovirus type 40 and 41 infections among children with acute gastroenteritis in Dijon, France. J. Clin. Microbiol. 37:3055-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bon, F., C. Fromantin, S. Aho, P. Pothier, E. Kohli, et al. 2000. G and P genotyping of rotavirus strains circulating in France over a three-year period: detection of G9 and P[6] strains at low frequencies. J. Clin. Microbiol. 38:1681-1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boussard, E., A. Flahault, J. F. Vibert, and A. J. Valleron. 1996. Sentiweb: French communicable disease surveillance on the World Wide Web. Br. Med. J. 313:1381-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiba, S., S. Nakata, K. Numata-Kinoshita, and S. Honma. 2000. Sapporo virus: history and recent findings. J. Infect. Dis. 181(Suppl. 2):S303-S308. [DOI] [PubMed] [Google Scholar]
- 7.de Wit, M. A., M. P. Koopmans, L. M. Kortbeek, N. J. van Leeuwen, A. I. Bartelds, and Y. T. van Duynhoven. 2001. Gastroenteritis in Sentinel general practices, The Netherlands. Emerg. Infect. Dis. 7:82-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flahault, A., P. Garnerin, P. Chauvin, N. Farran, Y. Saidi, C. Diaz, L. Toubiana, J. Drucker, and A. J. Valleron. 1995. Sentinelle traces of an epidemic of acute gastroenteritis in France. Lancet 346:162-163. [DOI] [PubMed] [Google Scholar]
- 9.Gaggero, A., M. O'Ryan, J. S. Noel, R. I. Glass, S. S. Monroe, N. Mamani, V. Prado, and L. F. Avendano. 1998. Prevalence of astrovirus infection among Chilean children with acute gastroenteritis. J. Clin. Microbiol. 36:3691-3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gallimore, C., J. Green, and D. Brown. 2000. Aichi virus, a cause of human gastroenteritis? In PHLS 25th Annual Scientific Conference. Public Health Laboratory Service, London, England.
- 11.Gentsch, J. R., R. I. Glass, P. Woods, V. Gouvea, M. Gorziglia, J. Flores, B. K. Das, and M. K. Bhan. 1992. Identification of group A rotavirus gene 4 types by polymerase chain reaction. J. Clin. Microbiol. 30:1365-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glass, R. I., J. Noel, T. Ando, R. Fankhauser, G. Belliot, A. Mounts, U. D. Parashar, J. S. Bresee, and S. S. Monroe. 2000. The epidemiology of enteric caliciviruses from humans: a reassessment using new diagnostics. J. Infect. Dis. 181(Suppl. 2):S254-S261. [DOI] [PubMed] [Google Scholar]
- 13.Glass, R. I., J. Noel, D. Mitchell, J. E. Herrmann, N. R. Blacklow, L. K. Pickering, P. Dennehy, G. Ruiz-Palacios, M. L. de Guerrero, and S. S. Monroe. 1996. The changing epidemiology of astrovirus-associated gastroenteritis: a review. Arch. Virol. Suppl. 12:287-300. [DOI] [PubMed] [Google Scholar]
- 14.Gouvea, V., J. R. Allen, R. I. Glass, Z. Y. Fang, M. Bremont, J. Cohen, M. A. McCrae, L. J. Saif, P. Sinarachatanant, and E. O. Caul. 1991. Detection of group B and C rotaviruses by polymerase chain reaction. J. Clin. Microbiol. 29:519-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gouvea, V., R. I. Glass, P. Woods, K. Taniguchi, H. F. Clark, B. Forrester, and Z. Y. Fang. 1990. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J. Clin. Microbiol. 28:276-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green, J., C. I. Gallimore, J. P. Norcott, D. Lewis, and D. W. Brown. 1995. Broadly reactive reverse transcriptase polymerase chain reaction for the diagnosis of SRSV-associated gastroenteritis. J. Med. Virol. 47:392-398. [DOI] [PubMed] [Google Scholar]
- 17.Guix, S., S. Caballero, C. Villena, R. Bartolome, C. Latorre, N. Rabella, M. Simo, A. Bosch, and R. M. Pinto. 2002. Molecular epidemiology of astrovirus infection in Barcelona, Spain. J. Clin. Microbiol. 40:133-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang, B., P. H. Dennehy, S. Spangenberger, J. R. Gentsch, and R. I. Glass. 1995. First detection of group C rotavirus in fecal specimens of children with diarrhea in the United States. J. Infect. Dis. 172:45-50. [DOI] [PubMed] [Google Scholar]
- 19.Jiang, X., N. Wilton, W. M. Zhong, T. Farkas, P. W. Huang, E. Barrett, M. Guerrero, G. Ruiz-Palacios, K. Y. Green, J. Green, A. D. Hale, M. K. Estes, L. K. Pickering, and D. O. Matson. 2000. Diagnosis of human caliciviruses by use of enzyme immunoassays. J. Infect. Dis. 181(Suppl. 2):S349-S359. [DOI] [PubMed] [Google Scholar]
- 20.Kapikian, Z. 1997. Viral gastroenteritis, p. 285-343. In K. R. E. Sa (ed.), Viral infections in humans, 4th ed. Plenum Book Company, New York, N.Y.
- 21.Koopmans, M., J. Vinje, E. Duizer, M. de Wit, and Y. van Duijnhoven. 2001. Molecular epidemiology of human enteric caliciviruses in The Netherlands. Novartis Found. Symp. 238:197-214. [DOI] [PubMed] [Google Scholar]
- 22.Kuzuya, M., R. Fujii, M. Hamano, M. Yamada, K. Shinozaki, A. Sasagawa, S. Hasegawa, H. Kawamoto, K. Matsumoto, A. Kawamoto, A. Itagaki, S. Funatsumaru, and S. Urasawa. 1998. Survey of human group C rotaviruses in Japan during the winter of 1992 to 1993. J. Clin. Microbiol. 36:6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le Guyader, F., M. K. Estes, M. E. Hardy, F. H. Neill, J. Green, D. W. Brown, and R. L. Atmar. 1996. Evaluation of a degenerate primer for the PCR detection of human caliciviruses. Arch. Virol. 141:2225-2235. [DOI] [PubMed] [Google Scholar]
- 24.Letrilliart, L., J. C. Desenclos, and A. Flahault. 1997. Risk factors for winter outbreak of acute diarrhoea in France: case-control study. Br. Med. J. 315:1645-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mendez-Toss, M., P. Romero-Guido, M. E. Munguia, E. Mendez, and C. F. Arias. 2000. Molecular analysis of a serotype 8 human astrovirus genome. J. Gen. Virol. 81:2891-2897. [DOI] [PubMed] [Google Scholar]
- 26.Mustafa, H., E. A. Palombo, and R. F. Bishop. 2000. Epidemiology of astrovirus infection in young children hospitalized with acute gastroenteritis in Melbourne, Australia, over a period of four consecutive years, 1995 to 1998. J. Clin. Microbiol. 38:1058-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naficy, A. B., M. R. Rao, J. L. Holmes, R. Abu-Elyazeed, S. J. Savarino, T. F. Wierzba, R. W. Frenck, S. S. Monroe, R. I. Glass, and J. D. Clemens. 2000. Astrovirus diarrhea in Egyptian children. J. Infect. Dis. 182:685-690. [DOI] [PubMed] [Google Scholar]
- 28.Nilsson, M., B. Svenungsson, K. O. Hedlund, I. Uhnoo, A. Lagergren, T. Akre, and L. Svensson. 2000. Incidence and genetic diversity of group C rotavirus among adults. J. Infect. Dis. 182:678-684. [DOI] [PubMed] [Google Scholar]
- 29.Noel, J., and D. Cubitt. 1994. Identification of astrovirus serotypes from children treated at the Hospitals for Sick Children, London 1981-93. Epidemiol. Infect. 113:153-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noel, J., T. W. Lee, J. B. Kurtz, R. I. Glass, and S. S. Monroe. 1995. Typing of human astrovirus from clinical isolates by enzyme immunoassay and nucleotide sequencing. J. Clin. Microbiol. 33:797-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Noel, J. S., B. L. Liu, C. D. Humphrey, E. M. Rodriguez, P. R. Lambden, I. N. Clarke, D. M. Dwyer, T. Ando, R. I. Glass, and S. S. Monroe. 1997. Parkville virus: a novel genetic variant of human calicivirus in the Sapporo virus clade, associated with an outbreak of gastroenteritis in adults. J. Med. Virol. 52:173-178. [PubMed] [Google Scholar]
- 32.Palombo, E. A., and R. F. Bishop. 1996. Annual incidence, serotype distribution, and genetic diversity of human astrovirus isolates from hospitalized children in Melbourne, Australia. J. Clin. Microbiol. 34:1750-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pang, X. L., S. Honma, S. Nakata, and T. Vesikari. 2000. Human caliciviruses in acute gastroenteritis of young children in the community. J. Infect. Dis. 181(Suppl. 2):S288-S294. [DOI] [PubMed] [Google Scholar]
- 34.Pothier, P., and E. Drouet.1987. Development and evaluation of a rapidone-step ELISA for rotavirus detection in stool specimens using monoclonal antibodies. Ann. Inst. Pasteur Virol. 13:285-295.
- 35.Tompkins, D. S., M. J. Hudson, H. R. Smith, R. P. Eglin, J. G. Wheeler, M. M. Brett, R. J. Owen, J. S. Brazier, P. Cumberland, V. King, and P. E. Cook. 1999. A study of infectious intestinal disease in England: microbiological findings in cases and controls. Commun. Dis. Public Health 2:108-113. [PubMed] [Google Scholar]
- 36.Valleron, A. J. 1998. Introduction à la biostatistique. Masson, Paris, France.
- 37.Wang, J., X. Jiang, H. P. Madore, J. Gray, U. Desselberger, T. Ando, Y. Seto, I. Oishi, J. F. Lew, K. Y. Green, et al. 1994. Sequence diversity of small, round-structured viruses in the Norwalk virus group. J. Virol. 68:5982-5990. [DOI] [PMC free article] [PubMed] [Google Scholar]