Abstract
The phosphatidylinositol 3-kinase (PI 3-kinase)/Akt signaling pathway is an important mediator of growth factor-dependent survival of mammalian cells. A variety of targets of the Akt protein kinase have been implicated in cell survival, including the protein kinase glycogen synthase kinase 3β (GSK-3β). One of the targets of GSK-3β is translation initiation factor 2B (eIF2B), linking global regulation of protein synthesis to PI 3-kinase/Akt signaling. Because of the central role of protein synthesis, we have investigated the involvement of eIF2B, which is inhibited as a result of GSK-3β phosphorylation, in programmed cell death. We demonstrate that expression of eIF2B mutants lacking the GSK-3β phosphorylation or priming sites is sufficient to protect both Rat-1 and PC12 cells from apoptosis induced by overexpression of GSK-3β, inhibition of PI 3-kinase, or growth factor deprivation. Consistent with these effects on cell survival, expression of nonphosphorylatable eIF2B prevented inhibition of protein synthesis following treatment of cells with the PI 3-kinase inhibitor LY294002. Conversely, cycloheximide induced apoptosis of PC12 and Rat-1 cells, further indicating that protein synthesis was required for cell survival. Inhibition of translation resulting from treatment with cycloheximide led to the release of cytochrome c from mitochondria, similar to the effects of inhibition of PI 3-kinase. Expression of nonphosphorylatable eIF2B prevented cytochrome c release resulting from PI 3-kinase inhibition but did not affect cytochrome c release or apoptosis induced by cycloheximide. Regulation of translation resulting from phosphorylation of eIF2B by GSK-3β thus appears to contribute to the control of cell survival by the PI 3-kinase/Akt signaling pathway, acting upstream of mitochondrial cytochrome c release.
The survival of most mammalian cells is dependent on extracellular signals that suppress programmed cell death. Recent studies have shown that a major signaling pathway by which survival factors prevent apoptosis involves activation of phosphatidylinositol 3-kinase (PI 3-kinase) (46) and its downstream effector, the protein-serine/threonine kinase Akt (for a review, see reference 11). However, the targets of PI 3-kinase/Akt signaling that promote cell survival remain to be fully elucidated. Ultimately, the PI 3-kinase/Akt pathway affects a conserved cell death program in which apoptosis results from activation of a cascade of cysteine proteases termed caspases (for a review, see reference 6). In mammalian cells, the activation of caspases in response to most cell death stimuli is triggered by the release of cytochrome c from mitochondria, which is regulated by members of the Bcl-2 family (6, 22, 23).
Previous studies have shown that Akt acts upstream of mitochondria to prevent cytochrome c release (27). Consistent with this site of action, one of the substrates of Akt that has been implicated in cell survival is the Bcl-2 family member Bad (12, 13). Phosphorylation by Akt inactivates Bad, preventing it from causing cytochrome c release. However, Akt has also been shown to promote the survival of cells in which Bad is not expressed (11) and to prevent the release of cytochrome c from mitochondria by mechanisms that are independent of Bad phosphorylation (27), indicating that Bad is not the only target of Akt responsible for cell survival. Akt has also been reported to inhibit apoptosis by phosphorylating human caspase 9 (7), but the lack of Akt phosphorylation sites in caspase 9 of other species limits the generality of this finding (20, 37). Additional targets of Akt that have been implicated in control of cell survival include the transcription factors Forkhead (5, 29), CREB (17), and NF-κB (26, 32, 35, 38), apoptosis signal-regulating kinase 1 (28), and glycogen synthase kinase 3β (GSK-3β) (36).
GSK-3β is a ubiquitously expressed protein-serine/threonine kinase whose activity is inhibited by Akt phosphorylation (8). A role for GSK-3β in regulating apoptosis downstream of PI 3-kinase/Akt signaling was first demonstrated in Rat-1 fibroblasts and PC12 pheochromocytoma cells (36). Overexpression of active GSK-3β induced apoptosis of these cells, whereas expression of a dominant-negative mutant of GSK-3β prevented apoptosis resulting from inhibition of PI 3-kinase (36). These results implicating GSK-3β in cell survival have subsequently been extended to other cell types, including primary neurons both in culture and in the brains of transgenic mice (1, 9, 24, 31, 33). Inhibition of GSK-3β as a result of phosphorylation by protein kinase A has also been shown to contribute to the promotion of neuronal survival by cyclic AMP (30).
GSK-3β phosphorylates a variety of substrates, including glycogen synthase and other metabolic enzymes, β-catenin, transcription factors, and the translation initiation factor eIF2B (43). Initially identified as a regulator of glycogen synthesis, GSK-3β plays an important physiological role in coupling metabolism and protein synthesis to growth factor stimulation (42, 43). Because of the central role of protein synthesis in maintaining cell functions, we have investigated the involvement of translational regulation in cell survival mediated by the PI 3-kinase/Akt/GSK-3β signaling pathway. Phosphorylation of the ɛ subunit of eIF2B by GSK-3β results in its inactivation and subsequent inhibition of translation initiation (42). The GSK-3β phosphorylation site is conserved and regulates the activity of eIF2B in insects as well as several mammalian species (42, 44). We report here that expression of a mutant eIF2B lacking the GSK-3β phosphorylation site prevents apoptosis induced by overexpression of GSK-3β, inhibition of PI 3-kinase, or growth factor deprivation. Consistent with this, inhibition of translation by cycloheximide was sufficient to induce apoptosis in a number of cell lines. Moreover, inhibition of translation resulting either from treatment with cycloheximide or from phosphorylation of eIF2B by GSK-3β led to the release of cytochrome c from mitochondria, consistent with previously reported effects of Akt on cytochrome c release (27). Translational regulation resulting from phosphorylation of eIF2B by GSK-3β thus appears to play a key role in the control of programmed cell death by the PI 3-kinase/Akt signaling pathway.
MATERIALS AND METHODS
Cell culture.
PC12 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum and 5% horse serum. Rat-1 cells were grown in DMEM supplemented with 10% calf serum.
Plasmids and site-directed mutagenesis.
cDNAs encoding wild-type rat GSK-3β, wild-type rat eIF2Bɛ (provided by T. Herbert, University of Dundee, Dundee, United Kingdom), mutant S535A eIF2B, mutant S539A eIF2B, Flag-tagged wild-type and S535A eIF2B, and human tau (provided by K. S. Kosik, Brigham and Women’s Hospital, Boston, Mass.) were transcribed from the cytomegalovirus promoter in pcDNA3. cDNA encoding dominant-negative PI 3-kinase Δp85 (15) was transcribed from the SRα promoter (provided by C. Rudd, Dana-Farber Cancer Institute). Site-directed mutagenesis of rat eIF2Bɛ cDNA was performed with the QuikChange Site-Directed Mutagenesis Kit (Stratagene) to change Ser535 (the GSK-3β phosphorylation site in rat eIF2B) (18, 42) to Ala using the primer 5′-CCCGAGGAGCTGGACGCCCGAGCAGGCTCCCC-3′ and to change Ser539 (the priming site for phosphorylation by GSK-3β) to Ala using the primer 5′-GCCGAGCAGGCGCCCCTCAGCTGGATGAC-3′. The constructs were sequenced to confirm that only the intended point mutations were introduced.
Transfection and apoptosis assays.
A total of 105 cells were plated on poly-l-lysine-treated coverslips in 35-mm-diameter plates 24 h before transfection. Transfections were performed using 4 μl of Lipofectamine Reagent (Life Technologies, Inc.)/plate according to the manufacturer’s instructions. Cells were transfected with 0.5 μg of pcDNA3 or the indicated GSK-3β, eIF2B, or PI 3-kinase expression construct plus 0.5 μg of the green fluorescent protein (GFP) expression construct pEGFP-C1 (Clontech). Two days after transfection, cells were fixed and stained with 0.5 μg of the DNA dye bisbenzimide (Hoechst 33258)/ml. Transfected cells were identified by GFP fluorescence and scored for apoptosis by nuclear morphology as previously described (36). Typically, 100 to 150 cells transfected with each vector were counted per experiment.
Immunoblot analysis.
PC12 cells were transfected on the day after plating. One or two days after transfection, cells were lysed, and 50 or 100 μg of proteins was electrophoresed in sodium dodecyl sulfate–12% polyacrylamide gels and transferred to nitrocellulose membranes. Membranes were incubated with the indicated primary antibodies, followed by incubation with a horseradish peroxidase-conjugated secondary antibody. Proteins were identified using the ECL system (Amersham Life Science Inc.).
Isolation of transfected cells by cell sorting.
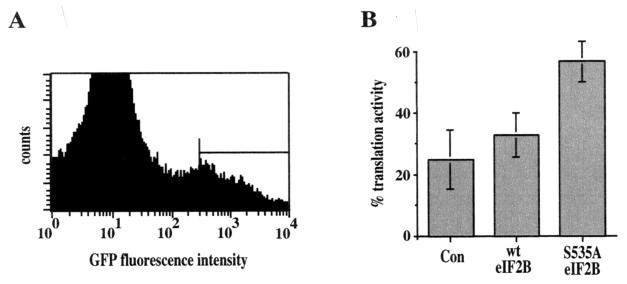
A total of 3 × 106 PC12 cells were plated in 100-mm plates and transfected the following day with 4 μg of control (pcDNA3), wild-type eIF2B, or S535A eIF2B expression plasmids plus 4 μg of the GFP expression plasmid pEGFP-C1 using 80 μl of Lipofectamine Reagent/plate. One or two days after transfection, cells were trypsinized, resuspended in phosphate-buffered saline (PBS), and sorted for GFP fluorescence in a FACScan (Becton Dickinson) flow cytometer. A total of 105 cells expressing high levels of GFP were collected and plated in a 48-well plate for use in assays of protein synthesis.
Analysis of translation.
PC12 or Rat-1 cells were incubated with 10 μCi of [35S]methionine/ml for 2 or 3 h. Cells were collected, protein was precipitated with 10% trichloroacetic acid, and incorporation of [35S]methionine was determined by scintillation counting.
Cytochrome c release.
Cells were fixed in 4% formaldehyde in PBS for 10 min, permeabilized by treatment with 0.5% Triton X-100 in PBS for 5 min on ice, and incubated in blocking buffer (5% goat serum and 1% bovine serum albumin in PBS) for 1 h. Coverslips were first incubated for 1 h with anti-cytochrome c antibody (6H2.B4; PharMingen) diluted 1:100 in blocking buffer and were then incubated for 1 h in the dark with CY3-conjugated anti-mouse immunoglobulin G (Sigma) diluted 1:200 in blocking buffer. Cells with diffuse staining, in contrast to the localized thread-like staining consistent with mitochondrial cytochrome c, were scored as having released cytochrome c from mitochondria (3). Cells were photographed using a Bio-Rad confocal microscope.
RESULTS
Effect of a nonphosphorylatable mutant of eIF2B on cell survival.
GSK-3β has been reported to phosphorylate serine-535 of the ɛ subunit of eIF2B in both mammalian and insect cells, resulting in its inactivation and subsequent inhibition of the initiation of translation (42, 44). We confirmed that eIF2B was phosphorylated at this site in both PC12 and Rat-1 cells by immunoblotting with a phosphospecific peptide antibody (42) (data not shown). To determine whether phosphorylation of eIF2B by GSK-3β plays a role in control of cell survival, we then generated a nonphosphorylatable mutant of rat eIF2B (S535A) in which the serine residue phosphorylated by GSK-3β was changed to alanine. PC12 and Rat-1 cells were cotransfected with plasmids expressing wild-type or S535A eIF2B together with a GSK-3β expression plasmid and a plasmid expressing GFP. Transfected cells were identified by fluorescence microscopy to detect GFP expression and were scored for apoptosis by nuclear morphology after staining with Hoechst dye as previously described (36).
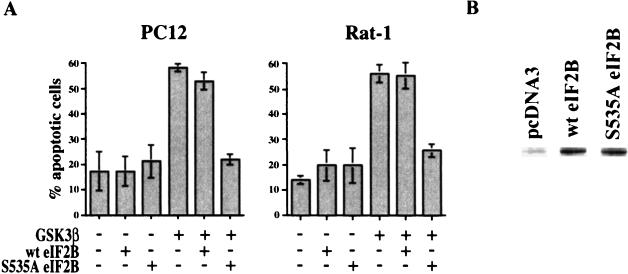
As previously reported (36), expression of GSK-3β induced apoptosis in approximately 60% of the transfected cells (Fig. 1A). A similar level of apoptosis was observed when cells were cotransfected with plasmids expressing GSK-3β and wild-type eIF2B. In contrast, expression of the nonphosphorylatable mutant S535A eIF2B protected cells from apoptosis induced by GSK-3β expression, reducing the level of apoptosis in both Rat-1 and PC12 cells to the background levels obtained in control cultures. The wild-type and mutant eIF2Bs were expressed at similar levels (Fig. 1B), indicating that the activity of the S535A eIF2B mutant in suppressing apoptosis did not result from differential expression compared to the wild-type protein. Inhibition of translation as a result of phosphorylation of eIF2B thus appears to play a critical role in cell death induced by GSK-3β.
FIG. 1.
(A) Nonphosphorylatable mutant S535A eIF2B inhibits apoptosis induced by GSK-3β. PC12 and Rat-1 cells were cotransfected with 0.5 μg of pcDNA3 or the indicated GSK-3β, wild-type rat eIF2Bɛ (wt eIF2B), and mutant S535A eIF2B expression constructs plus 0.5 μg of the GFP expression construct pEGFP-C1. Two days after transfection, cells were fixed and stained with Hoechst dye. Transfected cells were identified by GFP fluorescence and scored for apoptosis by nuclear morphology. Data are averaged from three independent experiments. Error bars, standard deviations. (B) A total of 8 × 105 PC12 cells were plated into 60-mm plates and transfected the following day with 4 μg of pcDNA3 or FLAG-tagged wild-type or mutant S535A eIF2B expression constructs using 50 μl of Lipofectamine Reagent/plate. Two days after transfection, cells were lysed and 50 μg of proteins was analyzed by immunoblotting with a monoclonal anti-FLAG M5 antibody (10 μg/ml; Sigma).
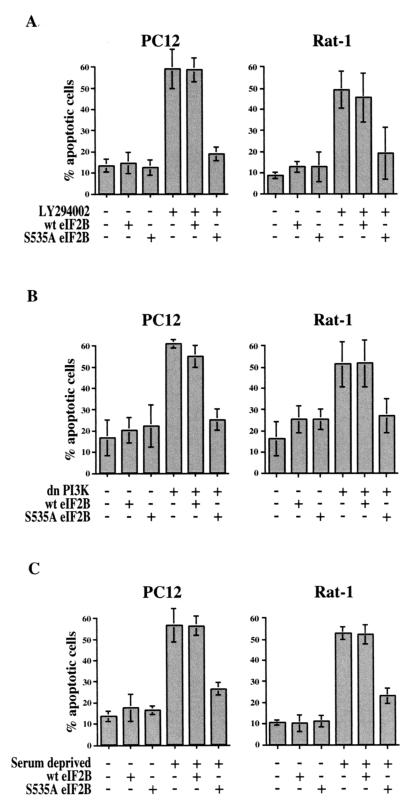
To further test the role of eIF2B in cell survival, we determined whether the mutant S535A eIF2B could protect cells from apoptosis induced by inhibition of PI 3-kinase or by deprivation of survival factors. Transfection with a plasmid expressing S535A eIF2B, but not wild-type eIF2B, effectively prevented apoptosis of both Rat-1 and PC12 cells induced either by treatment with the small molecule PI 3-kinase inhibitor LY294002 (Fig. 2A) or by cotransfection with a plasmid expressing a dominant-negative mutant of PI 3-kinase (Fig. 2B). Likewise, expression of S535A eIF2B substantially inhibited apoptosis induced by withdrawal of serum growth factors (Fig. 2C). Regulation of translation via phosphorylation of eIF2B thus appears to be an important determinant not only of apoptosis induced by overexpression of GSK-3β but also of apoptosis induced by inhibition of PI 3-kinase or by growth factor deprivation.
FIG. 2.
Effect of S535A eIF2B on apoptosis induced by PI 3-kinase inhibition or serum deprivation. Cells were cotransfected with expression plasmids for wild-type or S535A mutant eIF2B or for dominant-negative PI 3-kinase (dnPI3K) plus pEGFP-C1. Two days after transfection, cells were either left untreated, treated with 50 μM LY294002, or deprived of serum for 24 h; then they were fixed and stained with Hoechst dye. Transfected cells were identified by GFP fluorescence and scored for apoptosis by nuclear morphology. Data are averaged from three independent experiments.
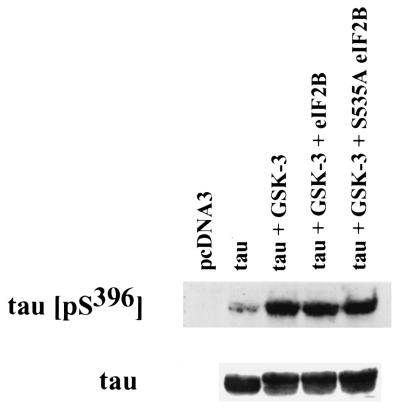
As a control to ensure that cell survival resulting from overexpression of S535A eIF2B did not result from a general inhibition of GSK-3β in transfected cells, we assayed the effect of overexpression of wild-type or mutant eIF2B on GSK-3β phosphorylation of another substrate, tau protein. PC12 cells were transiently transfected with expression plasmids for human tau, GSK-3β, and either wild-type or mutant S535A eIF2B. Phosphorylation of tau was assayed by immunoblotting cell lysates with a phosphospecific antibody for the GSK-3β phosphorylation site in human tau (pSer396). Cotransfection with GSK-3β induced the phosphorylation of tau protein, which was not affected by expression of either wild-type or mutant S535A eIF2B (Fig. 3). It thus appears that expression of the eIF2B constructs does not result in an overall inhibition of intracellular GSK-3β activity.
FIG. 3.
Effect of overexpression of wild-type or mutant eIF2B on GSK-3β phosphorylation of tau. A total of 5 × 106 PC12 cells were plated into 100-mm plates and transfected the following day with 9 μg of DNA using 100 μl of Lipofectamine Reagent/plate. Where indicated, cells were transfected with 1.2 μg of tau expression plasmid, 6 μg of GSK-3β expression plasmid, and 1.2 μg of expression constructs for wild-type or S535A mutant eIF2B, plus pcDNA3 to a total of 9 μg of DNA. One day after transfection, cells were lysed and 100 μg of proteins was analyzed by immunoblotting with a polyclonal anti-tau (pS396) phosphospecific antibody (0.4 μg/ml; BioSource) or with a monoclonal anti-tau antibody (1 μg/ml; BioSource).
Effect of priming site mutant S539A.
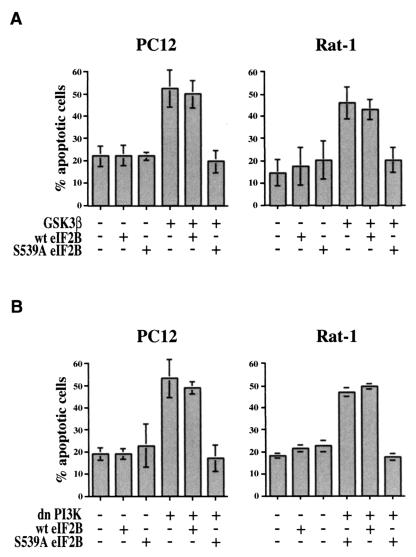
Phosphorylation of many proteins by GSK-3β requires a priming phosphorylation at position +4 relative to the GSK-3β phosphorylation site, which creates a docking site for the binding of GSK-3β to its substrates (10, 19). In particular, phosphorylation of eIF2B serine 535 by GSK-3β requires a priming phosphorylation at serine 539, which may be catalyzed by the protein kinase DYRK (dual-specificity tyrosine-phosphorylated and -regulated kinase) (41, 45). To further substantiate the role of GSK-3β phosphorylation of eIF2B in cell survival, we therefore examined the effect of an eIF2B mutant, S539A, in which the priming serine 539 was changed to alanine. As with the S535A mutant, transfection of PC12 or Rat-1 cells with the S539A mutant of eIF2B effectively inhibited apoptosis induced either by GSK-3β (Fig. 4A) or by dominant-negative PI 3-kinase (Fig. 4B). Since the requirement for a priming phosphorylation at the +4 position is unique to GSK-3, these results further demonstrate a specific role of phosphorylation of eIF2B by GSK-3β in cell survival.
FIG. 4.
Effect of priming site mutant S539A eIF2B on apoptosis induced by GSK-3β or PI 3-kinase inhibition. PC12 and Rat-1 cells were transfected with the indicated expression plasmids as described for Fig. 1. Data are averaged from two to four independent experiments.
Effect of S535A eIF2B on protein synthesis.
To ensure that expression of the mutant S535A eIF2B was sufficient to maintain translation following inhibition of PI 3-kinase, we assayed protein synthesis in transfected cells (Fig. 5). PC12 cells were cotransfected with a GFP expression plasmid and with plasmids expressing either wild-type or mutant S535A eIF2B. Transfected cells expressing GFP were isolated by fluorescence-activated cell sorting, cultured overnight, and assayed for translational activity by [35S]methionine incorporation. In control cells transfected with the GFP expression plasmid alone, inhibition of PI 3-kinase by treatment with LY294002 reduced protein synthesis to approximately 25% of that observed in untreated cells. Expression of wild-type eIF2B had little if any effect, but expression of S535A eIF2B substantially blocked the effect of PI 3-kinase inhibition, maintaining translation at about 60% of the level in untreated cells.
FIG. 5.
Effect of S535A eIF2B on translation following inhibition of PI 3-kinase. A total of 3 × 106 PC12 cells were transfected either with a control (pcDNA3) or with a wild-type eIF2B or S535A eIF2B expression plasmid plus the GFP expression plasmid pEGFP-C1. One or two days after transfection, cells were sorted for GFP fluorescence in a flow cytometer. A total of 105 cells expressing high levels of GFP (indicated by the horizontal line in panel A) were plated in a 48-well plate. The following day, cells were treated with 50 μM LY294002 for 4 h and then labeled with 10 μCi of [35S]methionine/ml for 3 h. Data are presented (B) as the percent [35S]methionine incorporation (percent translation activity) in cells treated with LY294002 compared to untreated controls. Data are averaged from three independent experiments with duplicate samples.
These results indicate that expression of the nonphosphorylatable S535A eIF2B mutant significantly attenuates the inhibition of protein synthesis that would normally result from inhibition of PI 3-kinase, consistent with its ability to interfere with apoptosis. The residual inhibition of protein synthesis by LY294002 that is not blocked by expression of S535A eIF2B may result from inhibition of the protein kinase TOR, which is activated by Akt and stimulates translation via phosphorylation of the ribosomal protein S6 kinase, p70S6K, and of proteins that bind to eIF4E (14). Consistent with this possibility, inhibition of TOR by treatment with rapamycin reduced protein synthesis by about 30% (data not shown), although rapamycin did not induce apoptosis in either PC12 or Rat-1 cells (47).
Induction of apoptosis by cycloheximide.
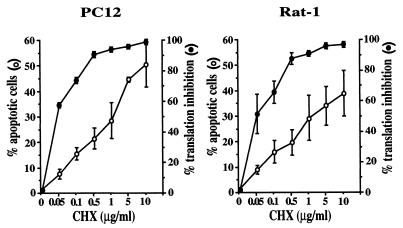
The ability of S535A eIF2B to maintain protein synthesis and to protect cells from apoptosis following inhibition of PI 3-kinase indicates that translational regulation plays an important role in cell survival. We further investigated the role of protein synthesis in apoptosis by treating cells with cycloheximide, a general inhibitor of translation. PC12 and Rat-1 cells were treated with varying doses of cycloheximide, followed by analysis of protein synthesis by [35S]methionine incorporation and of apoptosis by nuclear morphology. As previously reported for other cell types (2, 34, 40), inhibition of protein synthesis by cycloheximide induced apoptosis of both PC12 and Rat-1 cells (Fig. 6), consistent with a requirement for translation in cell survival. Similar induction of apoptosis as a result of inhibiting protein synthesis with cycloheximide was also observed in HeLa, MCF7, U937, and 293T cells (data not shown).
FIG. 6.
Induction of apoptosis by cycloheximide. For assays of protein synthesis (filled circles), 106 PC12 and Rat-1 cells were plated in 100-mm plates, treated the following day with the indicated concentrations of cycloheximide (CHX) for 30 min, labeled with 10 μCi of [35S]methionine/ml for 2 h, and analyzed by trichloroacetic acid precipitation. Data are averaged from three independent experiments, each with duplicate samples. For analysis of apoptosis (open circles), 105 cells were plated on poly-l-lysine-treated coverslips in 35-mm plates and treated with cycloheximide the following day for 16 h. Cells were then stained with Hoechst dye and scored for apoptosis by nuclear morphology. Data are averaged from three independent experiments.
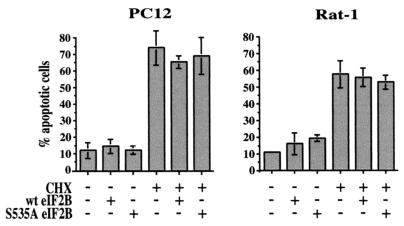
As a control for the specificity of the antiapoptotic effect of the mutant S535A eIF2B, we examined its effect on apoptosis induced by inhibition of protein synthesis with cycloheximide. Both PC12 and Rat-1 cells were transfected with plasmids expressing either wild-type or S535A eIF2B and treated with cycloheximide (Fig. 7). As expected, but in contrast to its effect on apoptosis resulting from inhibition of PI 3-kinase (see Fig. 1 and 2), expression of the mutant S535A eIF2B did not protect cells from apoptosis induced by cycloheximide (Fig. 7). It therefore appears that the eIF2B mutant specifically prevents apoptosis resulting from inhibition of PI 3-kinase signaling.
FIG. 7.
S535A eIF2B does not affect apoptosis induced by cycloheximide. Cells were transfected with a control, wild-type eIF2B, or mutant S535A eIF2B expression plasmid plus the GFP expression plasmid pEGFP-C1. Two days after transfection, cells were treated with 10 μg of cycloheximide (CHX)/ml for 24 h and then stained with Hoechst dye, and GFP-positive cells were scored for apoptosis by nuclear morphology. Data are averaged from three independent experiments.
Effect of inhibition of protein synthesis on release of cytochrome c from mitochondria.
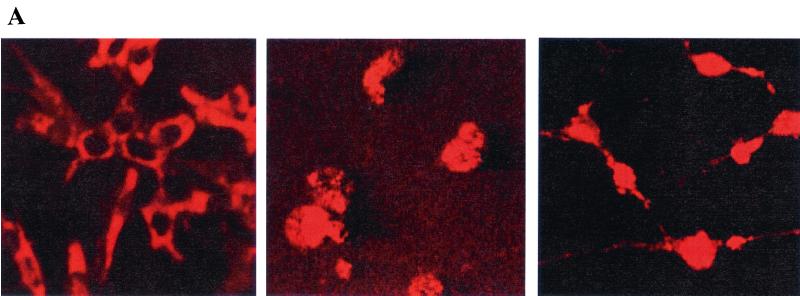
We next sought to determine whether phosphorylation of eIF2B and inhibition of protein synthesis leads to release of cytochrome c from mitochondria, which is known to be inhibited by PI 3-kinase/Akt signaling (27). Cells were analyzed for cytochrome c localization by immunostaining with an anti-cytochrome c antibody as previously described (3). Control cells displayed a discrete cytoplasmic pattern of cytochrome c staining, whereas cells treated with either LY294002 or cycloheximide displayed a diffuse pattern of staining indicating the release of cytochrome c from mitochondria (Fig. 8A). Quantitation of the number of cells with diffuse cytochrome c staining after treatment for varying times indicated that treatment with the PI 3-kinase inhibitor LY294002 and treatment with cycloheximide resulted in the release of cytochrome c with similar kinetics (Fig. 8B). It thus appears that inhibition of protein synthesis with cycloheximide, like inhibition of the PI 3-kinase/Akt signaling pathway, acts to promote cytochrome c release. This effect of cycloheximide on cytochrome c release is consistent with the previous observation that cycloheximide decreases the mitochondrial transmembrane potential in rat hepatocytes (2).
FIG. 8.
Inhibition of translation induces cytochrome c release. (A) A total of 105 PC12 cells were plated on poly-l-lysine-treated coverslips in 35-mm plates and treated the following day with either LY294002 (50 μM; middle panel) or cycloheximide (10 μg/ml; right panel) for 6 h. (Left panel) Control. Cells were analyzed for cytochrome c release by anti-cytochrome c immunostaining, and representative fields were photographed with a confocal microscope. (B) Cells were treated for the indicated times with LY294002 or cycloheximide (CHX) and analyzed for cytochrome c release by immunostaining. Data are averaged from three independent experiments.
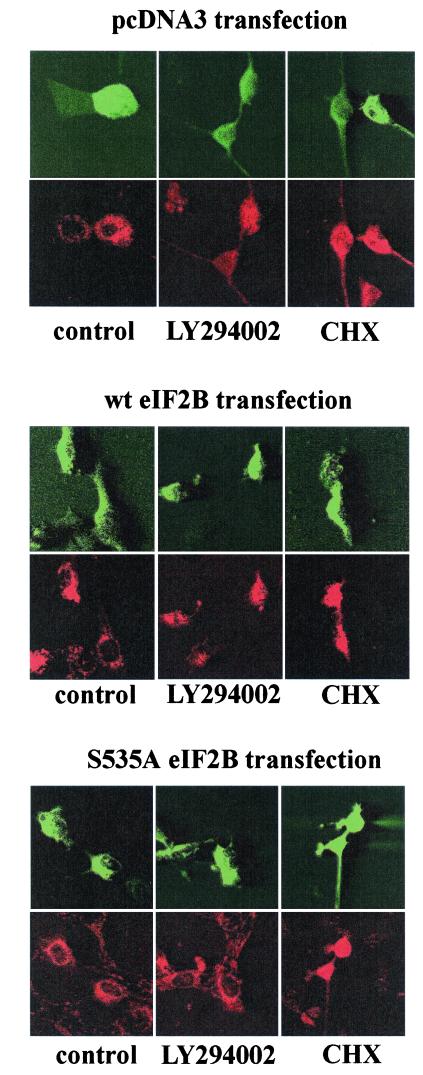
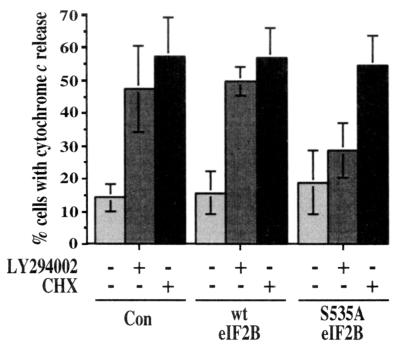
To determine whether inhibition of translation resulting from phosphorylation of eIF2B also acted to induce the release of cytochrome c, cells were transfected with either wild-type eIF2B or the nonphosphorylatable mutant S535A eIF2B and treated with either cycloheximide or the PI 3-kinase inhibitor LY294002. Transfected cells were identified by GFP fluorescence and stained with an anti-cytochrome c antibody to assess cytochrome c release. Fluorescence micrographs of representative cells are shown in Fig. 9, and data from a series of such experiments are presented in Fig. 10. Transfection with a plasmid expressing the mutant S535A eIF2B substantially inhibited cytochrome c release induced by treatment with LY294002 but not that induced by cycloheximide. In contrast, but consistent with its lack of effect on cell survival, transfection with a plasmid expressing wild-type eIF2B had no significant effect on mitochondrial cytochrome c release. It thus appears that inhibition of protein synthesis resulting from phosphorylation of eIF2B acts to induce apoptosis upstream of mitochondria, leading to the release of cytochrome c.
FIG. 9.
Effect of S535A eIF2B on cytochrome c release. PC12 cells were transfected with a control (pcDNA3), wild-type eIF2B, or mutant S535A eIF2B expression plasmid plus pEGFP-C1. Two days after transfection, cells were treated with either LY294002 (50 μM) or cycloheximide (CHX; 10 μg/ml) for 6 h. Transfected cells were identified by GFP fluorescence (upper panels) and analyzed for release of cytochrome c using an anti-cytochrome c antibody (lower panels). Confocal images of a representative experiment are shown.
FIG. 10.
Quantitation of effects of S535A eIF2B on cytochrome c release. PC12 cells were transfected with a control (Con), wild-type eIF2B, or S535A eIF2B expression plasmid plus pEGFP-C1, treated with LY294002 or cycloheximide (CHX), and analyzed for cytochrome c release as described for Fig. 9. Data are averaged from four independent experiments.
DISCUSSION
The PI 3-kinase/Akt signaling pathway plays a central role in the survival of most growth factor-dependent mammalian cells. Activation of the Akt protein kinase ultimately serves to inhibit apoptosis by blocking the release of cytochrome c from mitochondria (27), thereby preventing cytochrome c-dependent activation of cytosolic caspases. A variety of substrates of the Akt protein kinase have been implicated in the regulation of cell survival, including the Bcl-2 family member Bad (12, 13), caspase 9 (7), the transcription factors Forkhead (5, 29), CREB (17), and NF-κB (26, 32, 35, 38), and the protein kinase GSK-3β (36). In the present study, we demonstrate that GSK-3β controls cell survival, at least in part, as a result of phosphorylation of translation initiation factor eIF2B and global regulation of protein synthesis.
GSK-3β, the first characterized physiological substrate of Akt, was initially identified as a protein kinase that regulates glycogen synthesis in response to insulin signaling. Subsequent studies have shown that GSK-3β is a ubiquitously expressed protein kinase whose activity is inhibited by Akt phosphorylation in response to stimulation of cells by a variety of growth factors (8). A role for GSK-3β in cell survival was initially indicated by experiments showing that overexpression of active GSK-3β induced apoptosis of PC12 and Rat-1 cells, whereas expression of a dominant-negative mutant of GSK-3β was sufficient to prevent apoptosis following inhibition of PI 3-kinase (36). These results have been confirmed and extended to several other cell types, including primary neurons both in culture and in transgenic mice (1, 4, 9, 24, 30, 31, 33), indicating that GSK-3β is an important target of the PI 3-kinase/Akt pathway in signaling cell survival.
The substrates of GSK-3β include metabolic enzymes, several transcription factors, β-catenin, and the translation initiation factor eIF2B (43). Phosphorylation by GSK-3β inactivates eIF2B, leading to an inhibition of translation in the absence of growth factor stimulation of the PI 3-kinase/Akt pathway. In the present study, we have shown that expression of eIF2B mutants lacking the GSK-3β phosphorylation or priming sites is sufficient to maintain both cell survival and protein synthesis following inhibition of PI 3-kinase signaling. Expression of nonphosphorylatable eIF2B mutants inhibited apoptosis resulting from either overexpression of active GSK-3β, inhibition of PI 3-kinase, or withdrawal of serum growth factors, indicating a critical role for translational regulation in prevention of apoptosis by PI 3-kinase/Akt signaling.
Consistent with such a requirement for translation in cell survival, inhibition of protein synthesis with cycloheximide induced apoptosis of Rat-1 cells, PC12 cells, and several human cell lines, as has been previously reported for human HL60 leukemia cells (34), rat hepatocytes (2), and human T-cell lines (40). A role for translational regulation in cell survival is also consistent with the findings of previous studies indicating that inhibition of translation resulting from phosphorylation of the α subunit of initiation factor eIF2 is involved in apoptosis induced by the double-stranded RNA-activated protein kinase PKR (21, 39).
Previous studies have shown that PI 3-kinase/Akt signaling acts upstream of mitochondria to prevent the release of cytochrome c and subsequent activation of cytosolic caspases (27). Expression of the nonphosphorylatable eIF2B mutant prevented cytochrome c release following inhibition of PI 3-kinase, whereas inhibition of translation with cycloheximide induced cytochrome c release. Regulation of translation resulting from phosphorylation of eIF2B thus appears to affect the apoptotic cascade upstream of mitochondria, consistent with eIF2B serving as a target of PI 3-kinase/Akt signaling in the prevention of apoptosis.
It is clear that multiple targets of Akt are important in regulating cell survival (11), and additional targets of GSK-3β may also play significant roles. In the Wnt signaling pathway, GSK-3β phosphorylates β-catenin, which has been implicated in neuronal apoptosis induced by β-amyloid peptide (48). However, overexpression of either β-catenin or its associated transcription factor TCF does not protect cortical neurons or PC12 cells from apoptosis induced by growth factor deprivation or GSK-3β overexpression (24; M. Pap and G. M. Cooper, unpublished data). As a component of Wnt signaling, GSK-3β may also contribute to regulation of NF-κB transcription factors, with proapoptotic effects in some cells and antiapoptotic effects in others (4, 25). These differences may be related to the fact that the PI 3-kinase/Akt and Wnt pathways regulate GSK-3β by distinct mechanisms and may induce the phosphorylation of different target proteins (16, 19). In contrast to these cell-specific effects, the global regulation of protein synthesis is a common response of cells to growth factor stimulation. Regulation of translation by the PI 3-kinase/Akt/GSK-3β pathway may thus represent a general mechanism of cell survival signaling, coupling mitochondrial integrity and cytochrome c release to the central metabolic activity of protein synthesis.
Acknowledgments
We are grateful to C. G. Proud for the eIF2B phosphospecific antibody, to T. Herbert for eIF2B cDNA, to C. E. Rudd for the dominant-negative PI 3-kinase plasmid, to K. S. Kosik for the human tau expression plasmid, to E. Drouin and C. Powell for help with the flow cytometer, to A. Visegrády for assistance with confocal microscopy, to M. Jurk for helpful discussions, and to U. Hansen and K. McCall for comments on the manuscript.
This work was supported by NIH grant RO1 CA18689.
REFERENCES
- 1.Bijur, G. N., P. De Sarno, and R. S. Jope. 2000. Glycogen synthase kinase-3β facilitates staurosporine- and heat shock-induced apoptosis. J. Biol. Chem. 275:7583–7590. [DOI] [PubMed] [Google Scholar]
- 2.Blom, W. M., H. J. G. M. de Bont, I. Meijerman, G. J. Mulder, and J. F. Nagelkerke. 1999. Prevention of cycloheximide-induced apoptosis in hepatocytes by adenosine and by caspase inhibitors. Biochem. Pharmacol. 58:1891–1898. [DOI] [PubMed] [Google Scholar]
- 3.Bossy-Wetzel, E., D. D. Newmeyer, and D. R. Green. 1998. Mitochondrial cytochrome c release in apoptosis occurs upstream of DEVD-specific caspase activation and independently of mitochondrial transmembrane depolarization. EMBO J. 17:37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bournat, J. C., A. M. C. Brown, and A. P. Soler. 2000. Wnt-1 dependent activation of the survival factor NF-κB in PC12 cells. J. Neurosci. Res. 61:21–32. [DOI] [PubMed] [Google Scholar]
- 5.Brunet, A., A. Boni, M. J. Zigmond, M. Z. Lin, P. Juo, L. S. Hu, M. J. Anderson, K. C. Arden, J. Blenis, and M. E. Greenberg. 1999. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96:857–868. [DOI] [PubMed] [Google Scholar]
- 6.Budihardjo, I., H. Oliver, M. Lutter, X. Luo, and X. Wang. 1999. Biochemical pathways of caspase activation during apoptosis. Annu. Rev. Cell Dev. Biol. 15:269–290. [DOI] [PubMed] [Google Scholar]
- 7.Cardone, M. H., N. Roy, H. R. Stennicke, G. S. Salvesen, T. F. Franke, E. Stanbridge, S. Frisch, and J. C. Reed. 1998. Regulation of cell death protease caspase-9 by phosphorylation. Science 282:1318–1321. [DOI] [PubMed] [Google Scholar]
- 8.Cross, D. A. E., D. R. Alessi, P. Cohen, M. Andjelkovich, and B. A. Hemmings. 1995. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378:785–789. [DOI] [PubMed] [Google Scholar]
- 9.Crowder, R. J., and R. S. Freeman. 2000. Glycogen synthase kinase-3β activity is critical for neuronal death caused by inhibiting phosphatidylinositol 3-kinase or Akt but not for death caused by nerve growth factor withdrawal. J. Biol. Chem. 275:34266–34271. [DOI] [PubMed] [Google Scholar]
- 10.Dajani, R., E. Fraser, S. M. Roe, N. Young, V. Good, T. C. Dale, and L. H. Pearl. 2001. Crystal structure of glycogen synthase kinase 3β: structural basis for phosphate-primed substrate specificity and autoinhibition. Cell 105:721–732. [DOI] [PubMed] [Google Scholar]
- 11.Datta, S. R., A. Brunet, and M. E. Greenberg. 1999. Cellular survival: a play in three Akts. Genes Dev. 13:2905–2927. [DOI] [PubMed] [Google Scholar]
- 12.Datta, S. R., H. Dudek, X. Tao, S. Masters, H. Fu, Y. Gotoh, and M. E. Greenberg. 1997. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91:231–241. [DOI] [PubMed] [Google Scholar]
- 13.del Peso, L., M. Gonzales-Garcia, C. Page, R. Herrera, and G. Nunez. 1997. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science 278:687–689. [DOI] [PubMed] [Google Scholar]
- 14.Dennis, P. B., S. Fumagalli, and G. Thomas. 1999. Target of rapamycin (TOR): balancing the opposing forces of protein synthesis and degradation. Curr. Opin. Gen. Dev. 9:49–54. [DOI] [PubMed] [Google Scholar]
- 15.Dhand, R., K. Hara, I. Hiles, B. Bax, I. Gout, G. Panayotou, M. J. Fry, K. Yonezawa, M. Kasuga, and M. D. Waterfield. 1994. PI 3-kinase: structural and functional analysis of intersubunit interactions. EMBO J. 13:511–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding, V. W., R.-H. Chen, and F. McCormick. 2000. Differential regulation of glycogen synthase kinase 3β by insulin and Wnt signaling. J. Biol. Chem. 275:32475–32481. [DOI] [PubMed] [Google Scholar]
- 17.Du, K., and M. Montminy. 1998. CREB is a regulatory target for the protein kinase Akt/PKB. J. Biol. Chem. 273:32377–32379. [DOI] [PubMed] [Google Scholar]
- 18.Flowers, K. M., H. Mellor, R. L. Matts, S. R. Kimball, and L. S. Jefferson. 1996. Cloning and characterization of complementary and genomic DNAs encoding epsilon-subunit of rat translation initiation factor-2B. Biochim. Biophys. Acta 1307:318–324. [DOI] [PubMed] [Google Scholar]
- 19.Frame, S., P. Cohen, and R. M. Biondi. 2001. A common phosphate binding site explains the unique substrate specificity of GSK3 and its inactivation by phosphorylation. Mol. Cell 7:1321–1327. [DOI] [PubMed] [Google Scholar]
- 20.Fujita, E., A. Jinbo, H. Matuzaki, H. Konishi, U. Kikkawa, and T. Momoi. 1999. Akt phosphorylation site found in human caspase-9 is absent in mouse caspase-9. Biochem. Biophys. Res. Commun. 264:550–555. [DOI] [PubMed] [Google Scholar]
- 21.Gil, J., J. Alcami, and M. Esteban. 1999. Induction of apoptosis by double-stranded-RNA-dependent protein kinase (PKR) involves the α subunit of eukaryotic translation initiation factor 2 and NF-κB. Mol. Cell. Biol. 19:4653–4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Green, D. R., and J. C. Reed. 1998. Mitochondria and apoptosis. Science 281:1309–1312. [DOI] [PubMed] [Google Scholar]
- 23.Gross, A., J. M. McConnell, and S. J. Korsmeyer. 1999. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 13:1899–1911. [DOI] [PubMed] [Google Scholar]
- 24.Hetman, M., J. E. Cavanaugh, D. Kimelman, and Z. Xia. 2000. Role of glycogen synthase kinase-3β in neuronal apoptosis induced by trophic withdrawal. J. Neurosci. 20:2567–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoeflich, K. P., J. Luo, E. A. Rubie, M.-S. Tsao, O. Jin. and J. R. Woodgett. 2000. Requirement for glycogen synthase kinase-3β in cell survival and NF-κB activation. Nature 406:86–90. [DOI] [PubMed] [Google Scholar]
- 26.Kane, L. P., V. S. Shapiro, D. Stokoe, and A. Weiss. 1999. Induction of NF-κB by the Akt/PKB kinase. Curr. Biol. 9:601–604. [DOI] [PubMed] [Google Scholar]
- 27.Kennedy, S. G., E. S. Kandel, T. K. Cross, and N. Hay. 1999. Akt/protein kinase B inhibits cell death by preventing the release of cytochrome c from mitochondria. Mol. Cell. Biol. 19:5800–5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim, A. H., G. Khursigara, X. Sun, T. F. Franke, and M. V. Chao. 2001. Akt phosphorylates and negatively regulates apoptosis signal-regulating kinase 1. Mol. Cell. Biol. 21:893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kops, G. J. P. L., N. D. de Ruiter, A. M. M. De Vries-Smits, D. R. Powell, J. L. Bos, and B. M. T. Burgering. 1999. Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature 398:630–634. [DOI] [PubMed] [Google Scholar]
- 309.Li, M., X. Wang, M. K. Meintzer, T. Laessig, M. J. Birnbaum, and K. A. Heidenreich. 2000. Cyclic AMP promotes neuronal survival by phosphorylation of glycogen synthase kinase 3β. Mol. Cell. Biol. 20:9356–9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lucas, J. J., F. Hernandez, P. Gomez-Ramos, M. A. Moran, R. Hen, and J. Avila. 2001. Decreased nuclear β-catenin, tau hyperphosphorylation and neurodegeneration in GSK-3β conditional transgenic mice. EMBO J. 20:27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Madrid, L. V., C. Y. Wang, D. C. Guttridge, A. J. Schottelius, A. S. Baldwin, Jr., and M. W. Mayo. 2000. Akt suppresses apoptosis by stimulating the transactivation potential of the RelA/p65 subunit of NF-κB. Mol. Cell. Biol. 20:1626–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maggiwar, S. B., N. Tong, S. Ramirez, H. A. Gelbard, and S. Dewhurst. 1999. HIV-1 Tat-mediated activation of glycogen synthase kinase-3β contributes to Tat-mediated neurotoxicity. J. Neurochem. 73:578–586. [DOI] [PubMed] [Google Scholar]
- 34.Martin, S. J., S. V. Lennon, A. M. Bonham, and T. G. Cotter. 1990. Induction of apoptosis (programmed cell death) in human leukemic HL-60 cells by inhibition of RNA or protein synthesis. J. Immunol. 145:1859–1867. [PubMed] [Google Scholar]
- 35.Ozes, O. N., L. D. Mayo, J. A. Gustin, S. R. Pfeffer, L. M. Pfeffer, and D. B. Donner. 1999. NF-κB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature 401:82–85. [DOI] [PubMed] [Google Scholar]
- 36.Pap, M., and G. M. Cooper. 1998. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-kinase/Akt cell survival pathway. J. Biol. Chem. 273:19929–19932. [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez, J., H.-H. Chen, S.-C. Lin, and Y. Lazebnik. 2000. Caspase phosphorylation, cell death, and species variability. Science 287:1363. [Google Scholar]
- 38.Romashkova, J. A., and S. S. Makarov. 1999. NF-κB is a target of AKT in anti-apoptotic PDGF signalling. Nature 401:86–89. [DOI] [PubMed] [Google Scholar]
- 39.Srivastava, S. P., K. U. Kumar, and R. J. Kaufman. 1998. Phosphorylation of eukaryotic translation initiation factor 2 mediates apoptosis in response to activation of the double-stranded RNA-dependent protein kinase. J. Biol. Chem. 273:2416–2423. [DOI] [PubMed] [Google Scholar]
- 40.Tang, D., J. M. Lahti, J. Grenet, and V. J. Kidd. 1999. Cycloheximide-induced T-cell death is mediated by a Fas-associated death domain-dependent mechanism. J. Biol. Chem. 274:7245–7252. [DOI] [PubMed] [Google Scholar]
- 41.Wang, X., F. E. M. Paulin, L. E. Campbell, E. Gomez, K. O’Brien, N. Morrice, and C. G. Proud. 2001. Eukaryotic initiation factor 2B: identification of multiple phosphorylation sites in the ɛ-subunit and their functions in vivo. EMBO J. 20:4349–4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Welsh, G. I., C. M. Miller, A. J. Loughlin, N. T. Price, and C. G. Proud. 1998. Regulation of eukaryotic initiation factor eIF2B: glycogen synthase kinase-3 phosphorylates a conserved serine which undergoes dephosphorylation in response to insulin. FEBS Lett. 421:125–130. [DOI] [PubMed] [Google Scholar]
- 43.Welsh, G. I., C. Wilson, and C. G. Proud. 1996. GSK3: a SHAGGY frog story. Trends Cell Biol. 6:274–279. [DOI] [PubMed] [Google Scholar]
- 44.Williams, D. D., G. D. Pavitt, and C. G. Proud. 2001. Characterization of the initiation factor eIF2B and its regulation in Drosophila melanogaster. J. Biol. Chem. 276:3733–3742. [DOI] [PubMed] [Google Scholar]
- 45.Woods, Y. L., P. Cohen, W. Becker, R. Jakes, M. Goedert, X. Wang, and C. G. Proud. 2001. The kinase DYRK phosphorylates protein-synthesis initiation factor eIF2Bɛ at Ser539 and the microtubule-associated protein tau at Thr212: potential role for DYRK as a glycogen synthase kinase 3-priming kinase. Biochem. J. 355:609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yao, R., and G. M. Cooper. 1995. Requirement for phosphatidylinositol-3 kinase in the prevention of apoptosis by nerve growth factor. Science 267:2003–2006. [DOI] [PubMed] [Google Scholar]
- 47.Yao, R., and G. M. Cooper. 1996. Growth factor-dependent survival of rodent fibroblasts requires phosphatidylinositol 3-kinase but is independent of pp70S6K activity. Oncogene 13:343–351. [PubMed] [Google Scholar]
- 48.Zhang, Z., H. Hartmann, V. M. Do, D. Abramowski, C. Sturchler-Pierrat, M. Staufenbiel, B. Sommer, M. van de Wetering, H. Clevers, P. Saftig, B. De Strooper, X. He, and B. A. Yankner. 1998. Destabilization of β-catenin by mutations in presenilin-1 potentiates neuronal apoptosis. Nature 395:698–702. [DOI] [PubMed] [Google Scholar]