Abstract
We have characterized a new member of the mammalian PAK family of serine/threonine kinases, PAK5, which is a novel target of the Rho GTPases Cdc42 and Rac. The kinase domain and GTPase-binding domain (GBD) of PAK5 are most closely related in sequence to those of mammalian PAK4. Outside of these domains, however, PAK5 is completely different in sequence from any known mammalian proteins. PAK5 does share considerable sequence homology with the Drosophila MBT protein (for “mushroom body tiny”), however, which is thought to play a role in development of cells in Drosophila brain. Interestingly, PAK5 is highly expressed in mammalian brain and is not expressed in most other tissues. We have found that PAK5, like Cdc42, promotes the induction of filopodia. In N1E-115 neuroblastoma cells, expression of PAK5 also triggered the induction of neurite-like processes, and a dominant-negative PAK5 mutant inhibited neurite outgrowth. Expression of activated PAK1 caused no noticeable changes in these cells. An activated mutant of PAK5 had an even more dramatic effect than wild-type PAK5, indicating that the morphologic changes induced by PAK5 are directly related to its kinase activity. Although PAK5 activates the JNK pathway, dominant-negative JNK did not inhibit neurite outgrowth. In contrast, the induction of neurites by PAK5 was abolished by expression of activated RhoA. Previous work has shown that Cdc42 and Rac promote neurite outgrowth by a pathway that is antagonistic to Rho. Our results suggest, therefore, that PAK5 operates downstream to Cdc42 and Rac and antagonizes Rho in the pathway, leading to neurite development.
The Rho family GTPases, including Cdc42, Rac, and Rho, were first identified as proteins that have key roles in regulating the organization of the actin cytoskeleton in mammalian fibroblasts (18, 35, 38, 39). Microinjection of activated Cdc42 into fibroblasts causes the induction of filopodia, while activated Rac leads to lamellipodium formation, and activated Rho causes the formation of stress fibers. Both Cdc42Hs and Rac have also been shown to have a role in the dissolution of stress fibers (10, 18, 26), which may reflect an antagonism between these two GTPases and Rho (19, 40). While they were initially characterized in fibroblasts, the Rho GTPases have also been shown to regulate the morphologies of other types of cells. For example, the Rho GTPases have been shown to have important roles in the regulation of neurite outgrowth in Caenorhabditis elegans, Drosophila melanogaster, chick, and mammalian primary neurons and cell lines (5, 8, 14, 19, 22, 25, 48, 55). This is probably due at least in part to the fact that filopodia and lamellipodia play key roles in the elongation of neurites (24).
In mammalian neuronal cell lines, Cdc42 and Rac appear to act antagonistically with Rho. Introduction of constitutively active mutants of Cdc42 and Rac into N1E-115 neuroblastoma cells leads to the formation of neurites (41, 52) and the production of filopodia and lamellipodia in developing growth cones (19), whereas introduction of the dominant-negative mutants of Cdc42 and Rac inhibits neurite outgrowth in N1E-115 cells and PC12 cells (8, 19, 41, 52). In contrast, activated RhoAV14 causes neurite retraction in PC12 cells and N1E-115 cells, while inhibition of RhoA stimulates the production of neurites in N1E-115 cells (11, 19, 50, 51, 52). These results suggest that inactivation of RhoA actually leads to the activation of Cdc42 and Rac, thus leading to the production of neurites (19). Consistent with this, N1E-115 cells form neurites when they are grown in the absence of serum but not when they are grown in the presence of serum. This is presumably because components in serum, especially lipophosphatidic acid, activate Rho, which in turn blocks the production of neurites in response to Cdc42 and Rac (19).
While Cdc42 and Rac clearly have important roles in regulating morphologic changes that control growth cone formation and neurite outgrowth, the mechanisms by which the GTPases operate in neuronal cells are still not entirely understood. The identification and characterization of molecular targets for the Rho GTPases is an important step in determining how they control cell morphology in both neuronal and non-neuronal cells. Members of the mammalian p21-activated kinase (PAK) family of serine/threonine kinases constitute a family of kinases that bind to Rac and Cdc42, but not Rho (for reviews, see references 7, 17, and 44). The PAK family members can be placed into two categories based on their amino acid sequences. The first category, referred to here as class A, includes mammalian PAK1, PAK2, and PAK3. Each of these protein kinases has a carboxyl-terminal kinase domain and an amino-terminal regulatory domain. Within the regulatory domain is a GTPase-binding domain (GBD) that binds to activated Cdc42 and Rac. The regulatory domain also contains two or three proline-rich regions that bind to SH3 domain containing proteins, including the adapter protein Nck and the exchange factor PIX (7), and a motif that can bind to G protein βγ subunits (7). The members of this family are all quite similar in sequence, exhibiting 73% overall sequence identity and ca. 92% sequence identity within the kinase domain and GBD (44). Members of this subfamily of PAKs are thought to have important roles in regulating cell morphology and cytoskeletal organization, although they may not specifically mediate cytoskeletal effects that are triggered by Cdc42 and Rac (13, 21, 45, 47).
PAK4 is the first PAK family member to be identified that belongs to a second category of PAKs based on its sequence (1), referred to here as class B. PAK4 contains an amino-terminal GBD and a carboxyl-terminal kinase domain, but it does not bind to PIX or Nck and it does not have a G protein βγ binding motif (1; C. Dan, unpublished results). Furthermore, the GBD and kinase domains of PAK4 have only ca. 50% identity with those of the class A PAKs, and the regulatory domain of PAK4 outside of the GBD is completely different from those of the other PAKs (1). Unlike other PAKs, PAK4 was shown to be a link between Cdc42 and filopodium formation (1). In addition to filopodium formation, PAK4 may also have other functions. For example, a constitutively active PAK4 mutant also leads to the dissolution of stress fibers and focal adhesions, most likely by inhibiting the activity of RhoA (36).
Here we have characterized a new member of the PAK family, PAK5, which interacts with Cdc42 and Rac. Like PAK4, PAK5 falls into the class B category of PAKs based on its predicted amino acid sequence. PAK5 shares ca. 84% sequence identity with PAK4 in its kinase domain, and 80% identity within its GBD motif. PAK5 also shares partial sequence identity with the GBD and kinase domain of another new member of the class B category, PAK6, which is expressed in the testis and prostate (53). Outside of the kinase domain and GBD motifs, PAK5 is completely different from both PAK4 and PAK6. Furthermore, unlike PAK4 and PAK6, PAK5 is expressed primarily in the brain. This is of particular interest because PAK5 is similar to Drosophila MBT protein (for “mushroom body tiny”) (28). MBT is thought to have a role in development, proliferation, or survival of cells in the mushroom body, a structure of the Drosophila brain. Strikingly, expression of PAK5 triggered both filopodium formation and neurite outgrowth in N1E-115 cells, while dominant-negative PAK5 mutants inhibited neurite outgrowth. PAK5 triggered neurite outgrowth much more effectively than PAK4, and activated PAK1 had no effect on neurite outgrowth. Our results suggest that PAK5 is an important mediator in the signaling pathway by which Rho GTPases control the cytoskeletal changes that are necessary for promoting neurite outgrowth.
MATERIALS AND METHODS
Cloning of PAK5.
The EST database was screened by using a BLAST search in order to identify new members of the PAK family. EST clone ts97b05.x1 showed similarity to the kinase domain of mammalian PAK4 from amino acid 483 to the stop codon. To obtain the 5′ end of the corresponding clone, a reverse transcription-PCR (RT-PCR) was carried out with cDNA derived from human testis total RNA as a template. The 5′ primer was a degenerate oligonucleotide corresponding to the sequence APSNFEH within the GBD of PAK4 and the 3′ primer (AGTAGGGAGTGCCAACCAAT) was an oligonucleotide corresponding to a region in ts97b05.x1 that differs from PAK4. The resulting PCR product was cloned and sequenced. Further screening of the database revealed that both the PCR product and ts97b05.x1 were homologous to human mRNA for KIAA1264. To obtain the full-length cDNA in one piece, another PCR was carried out with oligonucleotides corresponding to the 5′ and 3′ ends of KIAA1264. The full-length PCR product was designated PAK5. PAK5 was also later found to be nearly identical to GenBank clone ABO40812.
Plasmids.
cDNAs encoding PAK5, PAK5RD, and PAK5RDΔGBD were subcloned between the ClaI and EcoRI sites on the pCAN-Myc1 expression vector containing a Myc epitope tag. PAK5RD, corresponding to amino acids 1 to 451, is the amino-terminal part of PAK5 without the kinase domain and was generated by PCR with PAK5 cDNA as the template. PAK5RDΔGBD, corresponding to amino acids 31 to 451, lacks both the kinase domain and the GBD and was generated by PCR with PAK5 cDNA as the template. Constitutively active PAK5(S573N) in pCAN-Myc1 was generated by site-directed mutagenesis (Stratagene QuickChange kit) and contains a serine-to-glutamic acid substitution at amino acid 602, which is a putative autophosphorylation site, and a serine-to-asparagine substitution at amino acid 573 within the kinase domain. PAK5(K478M) was also generated by site-directed mutagenesis and contains a lysine-to-methionine substitution at amino acid 478. cDNAs encoding PAK5, PAK5(S573N), and PAK5(K478M) were also subcloned between the HindIII and SacII sites on pEGFP-C3 (Clontech) expression vector to obtain PAK5-enhanced green fluorescent protein (EGFP) vectors. Myc-tagged PAK4 is described elsewhere (1). PAK4(S445N) was described by Qu et al. (36). Hemagglutinin (HA)-tagged JNK, glutathione S-transferase (GST)–c-Jun, and MEKK1Δ were described by Minden et al. (31). Myc-wild-type RhoA vector and bacterial expression vectors containing Rac, Rho, and Cdc42 are gifts from A. Hall. PAK1(T423E) is a gift from J. Chernoff. Dominant-negative JNK has point mutations in which its two phosphorylation sites (Thr-183 and Tyr-185) are converted to Ala and Phe, respectively, and is described elsewhere (9). Cdc42V12, Rac1V12, and RhoV14 are described (30). C3 transferase expression vector was a gift from R. Prywes. The GST-C21 fusion protein has been described by Reid et al. (37) and was a gift of John Collard.
Northern blots.
Northern analysis was performed by using a human multiple tissue RNA blot (Clontech). Hybridization and washes were carried out as recommended by the manufacturer. The probe was a 400-bp fragment from within the PAK5 kinase domain that differs in sequence from PAK4 and all other sequences in the GenBank database. The probe was labeled with [α-32P]dCTP (Amersham) by using a random priming kit (Stratagene).
Overlay assay.
The overlay assay used here was as described earlier (27). Briefly, 293 cells were transfected with 10 μg of empty vector, Myc-PAK4, or Myc-PAK5. PAK4 and PAK5 were then immunopurified by using anti-Myc antibody (9E10; Santa Cruz), and the immunopurified proteins were separated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene difluoride (PVDF) membrane. The membrane was then washed and blocked with phosphate-buffered saline (PBS) containing 1% bovine serum albumin and 100 mM dithiothreitol (DTT). Recombinant GTPases (2 μg) were preloaded with either [γ-32P]GTP or [β-32P]GDP and were incubated for 5 min with the PVDF membrane. The membrane was washed for 5 min and was exposed to a film for 2 h.
Production of GST-C21 fusion protein and Rho GTPase activity assays.
GST-C21 contains the N-terminal 90 amino acids of the Rho effector protein Rhotekin, consisting of the Rho binding domain. Escherichia coli BL21 transformed with the GST-C21 construct was grown at 30°C to an optical density at 600 nm of 0.3. Expression and purification of the fusion protein and the GTPase activity assays were performed as described previously (40). In brief, lysates were prepared from 293 cells that were transfected with Myc-RhoA expression vector together with either empty vector, PAK5, or activated PAK5 vector. Lysates were then incubated with bacterially expressed GST-C21 fusion proteins bound to glutathione-coupled agarose beads. The beads and the bound proteins were washed three times with an excess of lysis buffer and then eluted with Laemmli sample buffer. The bound RhoA proteins were analyzed by Western blotting with antibody against the Myc tag. A portion of the lysate was saved for direct Western blot analysis with anti-Myc antibody.
Preparation of recombinant proteins.
Recombinant GST–c-Jun, GST-RhoV14, GST-Rac1V12, and GST-Cdc42V12 were prepared as described previously (43).
Cell culture and transfection.
All cells were grown at 37°C in 5% CO2. 293 and N1E-115 cells were cultured in Dulbecco modified Eagle medium containing 10% fetal bovine serum (Gibco-BRL). For transfections in 293 cells, a total of 10 μg of DNA was transfected into the cells in 10-cm plates (60% confluent) by using the calcium phosphate precipitation method. For N1E-115 cells, 4 μg of total DNA was transfected into the cells by using FuGENE6 (Roche) in 35-mm wells (seeded at 104 cells/well) containing coverslips that were precoated with 10 μg of mouse laminin (Gibco-BRL) ml−1 for 1 h at 37°C.
Western blots.
Western blot analyses were carried out as described previously (1).
Protein kinase assays.
To assay histone H4 (HH4) phosphorylation by the PAKs, 293 cells were transfected with 10 μg of empty vector, Myc-PAK4, or Myc-PAK5 (wild-type or constitutively active) expression vectors. Cells were harvested in M2 buffer (32) at 48 h after transfection. Equal amounts of the Myc-tagged proteins, as assayed by Western blotting, were then immunopurified from ca. 100 μg of cell extracts with antibody generated against the Myc epitope tag (9E10; Santa Cruz) and protein A-Sepharose. After 2 h of incubation at 4°C, the immunoprecipitates were washed twice in M2 buffer and twice in a buffer containing 20 mM HEPES (pH 7.5) and 10 mM MgCl2 and then incubated with 5 μg of HH4 in a buffer containing 20 mM HEPES (pH 7.5), 10 mM MgCl2, 20 mM β-glycerol phosphate, 10 mM p-nitrophenyl phosphate (PNPP), 1 mM DTT, 50 μM Na3VO4, 20 μM ATP, and 5 μCi of [γ-32P]ATP for 20 min at 30°C. The reaction was terminated with SDS-PAGE sample buffer, followed by SDS-PAGE and autoradiography.
To analyze the kinase activity of hemagglutinin (HA)-tagged JNK, 293 cells were transfected with either 10 μg of empty vector or 5 μg of HA-tagged JNK expression vector in the absence or presence of increasing doses of Myc-PAK5 (1, 3, and 5 μg) or expression vectors containing Rac2L61 (2.5 μg) or MEKK1Δ (2.5 μg). The total amount of DNA in each transfection was kept at 10 μg by using empty vector. Cells were harvested 48 h after transfection, and the amount of HA-JNK in cell lysates was normalized by Western blotting. Equal amounts of HA-JNK were then immunopurified from ca. 100 μg of cell lysates by using an anti-HA antibody (12CA5; Boehringer Mannheim) and protein A-Sepharose in M2 buffer at 4°C for 2 h. The protein A-Sepharose beads were washed twice in M2 buffer and twice in a buffer containing 20 mM HEPES (pH 7.5) and 10 mM MgCl2 and then incubated in a buffer containing 20 mM HEPES (pH 7.5), 10 mM MgCl2, 20 mM β-glycerol phosphate, 10 mM PNPP, 1 mM DTT, 50 μM Na3VO4, 20 μM ATP, and 5 μCi of [γ-32P]ATP, together with 2 μg of purified recombinant GST-c-Jun for 20 min at 30°C. The reaction was terminated with SDS-PAGE sample buffer, followed by SDS-PAGE and autoradiography. The phosphorylation of c-Jun was quantitated by phosphorimager analysis.
Immunofluorescence.
After transient transfection, N1E-115 cells were fixed in 3% paraformaldehyde for 20 min, permeabilized in 0.1% Triton X-100, and blocked with 5% goat serum for 20 min at room temperature. Cells were stained for the presence of Myc-PAK5, Myc-PAK5(K478M), Myc-PAK5RD, Myc-PAK5RDΔGBD, or Myc-Cdc42N17 with mouse anti-Myc antibody (9E10) and then incubated with goat anti-mouse immunoglobulin G conjugated with rhodamine (Pierce).
Neurite outgrowth scoring assay.
N1E-115 cells were grown on coverslips precoated with mouse laminin, in 35-mm wells, and transfected with 4 μg of the indicated expression vectors. At 20 h after transfection, the N1E-115 cells were examined by fluorescence microscopy to detect the presence of the green fluorescent cells that contain the transfected plasmids. Transfected cells bearing a neurite-like structure with a length of at least one cell body were counted and scored as the percentage of total transfected cells. The images were taken either with a Nikon Diaphot 300 inverted microscope with epifluorescence attachments and a color charge-coupled device camera with a ×10 objective lens or with an Olympus IX70 Fluoview confocal laser-scanning microscope with a DIC attachment and a 100× objective lens.
RESULTS
Identification of PAK5, a novel member of the PAK family of kinases.
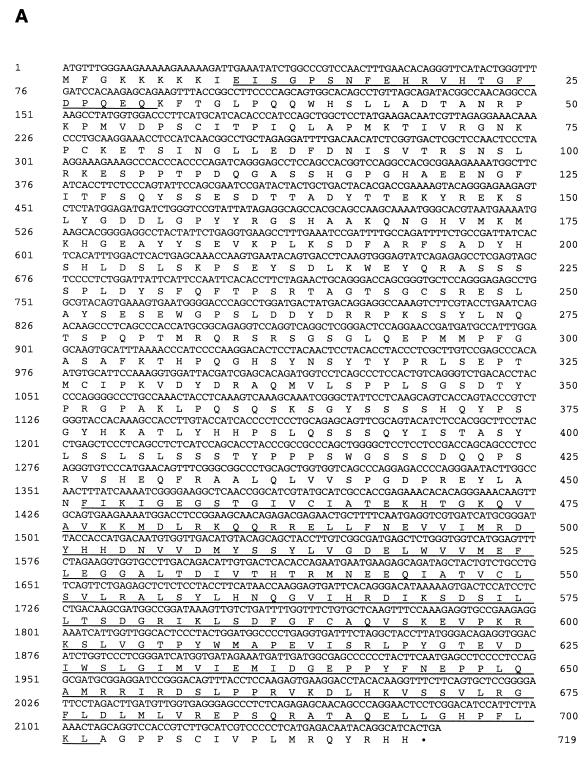
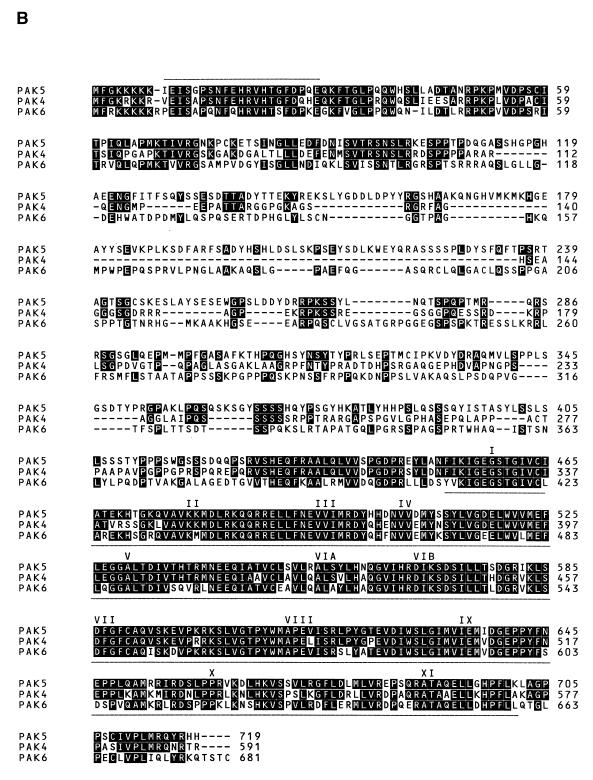
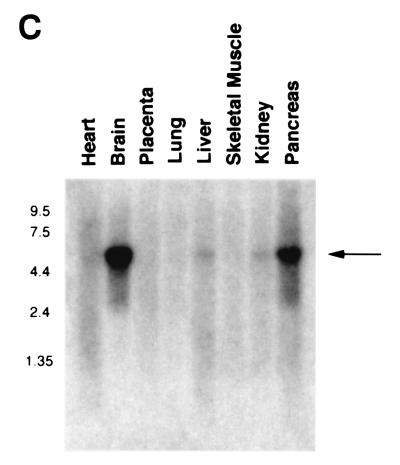
The human PAK5 cDNA was cloned as described in Materials and Methods. The sequence of the full-length PAK5 and the predicted amino acid sequence are shown in Fig. 1A. A comparison between the predicted amino acid sequences of the PAK5 and other members of the class B family of PAKs (PAK4 and PAK6) is shown in Fig. 1B. The percent sequence identities between the PAK5 kinase domain and GBD with the corresponding domains of other members of the PAK family, including human PAK1, PAK4, PAK6, and Drosophila MBT, are shown in Table 1. Outside of the GBD and kinase domain, there is little sequence homology between PAK5 and any other members of the PAK family. Furthermore, no obvious PIX binding sites or Gβγ binding domains similar to those in PAK1 are evident in PAK5. Northern analysis of PAK5 indicates that it is expressed in brain and pancreas. Very little or no PAK5 could be detected in several other human tissues that were examined (Fig. 1C and data not shown).
FIG. 1.
Sequence and expression pattern of PAK5. (A) Sequence of human PAK5. (B) Alignment of PAK5 with PAK4 and PAK6. The overscored region is the GBD. The underlined region is the kinase domain. The 11 subdomains conserved throughout serine/threonine kinases are indicated. (C) A human multiple tissue mRNA Northern blot was probed with a cDNA containing part of the kinase domain of PAK5. A band of ∼5.5 kb is indicated.
TABLE 1.
Comparison between PAK5 and other members of the PAK familya
| PAK5 domain | % Sequence identity
|
|||
|---|---|---|---|---|
| hPAK4 | MBT | hPAK6 | hPAK1 | |
| Kinase | 84 | 80 | 76 | 57 |
| GBD | 86 | 76 | 70 | 57 |
The percent sequence identity between the PAK5 kinase domain and the GBD with the corresponding domains of other members of the PAK family, including human PAK4, Drosophila MBT, human PAK6, and human PAK1, was calculated. Sequence comparisons are based on the predicted amino acid sequences.
PAK5 interacts with GTP-bound Rac and Cdc42Hs.
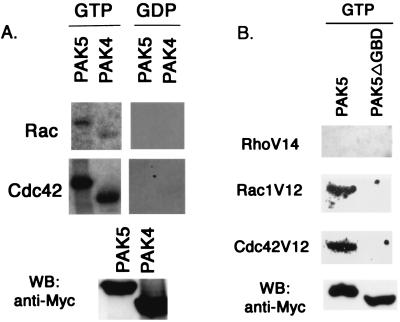
Sequencing analysis indicates that PAK5 has a putative GBD/CRIB motif similar to that of PAK4. To determine whether PAK5 interacts with the GTPases, an overlay assay was used in which filters containing immobilized PAK4 and PAK5 were probed with GTP-loaded Rac1 or Cdc42. We found that PAK5 interacts with both GTP-bound Cdc42 and Rac (Fig. 2A), suggesting that PAK5 is a target for these GTPases. Like PAK4, however, PAK5 appeared to interact more tightly with Cdc42 than Rac. PAK5 did not bind to GTP-loaded RhoA, and a PAK5 mutant lacking the GBD (PAK5DGBD) no longer bound to Cdc42 or Rac (Fig. 2B)
FIG. 2.
PAK4 and PAK5 interact with activated Rac and Cdc42. (A) 293 cells were transfected with equal amounts of expression vectors containing wild-type Myc-tagged PAK4 or PAK5. After transient expression, Myc-PAK4 and Myc-PAK5 were immunoprecipitated from whole-cell lysates by using a mouse anti-Myc antibody and protein A-Sepharose. The immunocomplexes were separated by SDS-PAGE and transferred to a PVDF membrane. The membranes were then probed with purified wild-type Rac or Cdc42 that was preloaded with either [γ-32P]GTP or [β-32P]GDP as described in Materials and Methods (see top two panels). A portion of the lysate was used for Western blot analysis (WB) with anti-Myc antibody to detect the PAK4 and PAK5 expression levels (bottom panel). (B) 293 cells were transfected with equal amounts of expression vectors containing Myc-tagged wild-type PAK5 or Myc-tagged PAK5ΔGBD. After transient transfection, PAK5 and PAK5ΔGBD were immunoprecipitated from whole-cell lysates and transferred to a PVDF membrane as described above, and the membranes were probed with purified RhoV14, Rac1V12, or Cdc42V12 that was preloaded with [γ-32P]GTP as described above. PAK5 and PAK5ΔGBD expression in whole-cell lysates was detected by Western blots (WB) by using anti-Myc antibody, as shown in the bottom panel.
PAK5 autophosphorylates and phosphorylates an exogenous substrate.
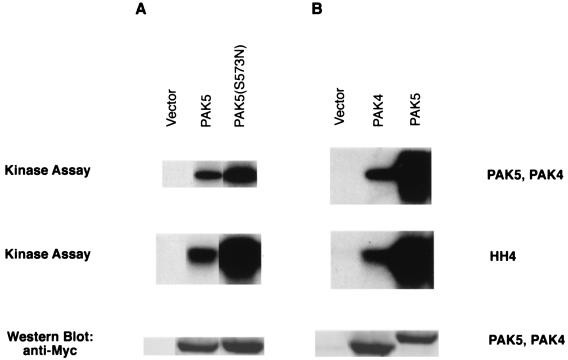
Previously, we have shown that wild-type PAK4 can autophosphorylate and phosphorylate HH4 (1) and that a constitutively active PAK4, PAK4(S445N), has stronger kinase activity than wild-type PAK4 (36). PAK4(S445N) has a serine-to-glutamic acid mutation at the putative autophosphorylation site (amino acid 474) and a serine-to-asparagine mutation at amino acid 445, which is thought to function by stabilizing the catalytic loop (36, 49). This mutant has an elevated level of kinase activity, but its substrate specificity does not appear to be altered and it does not induce nonspecific morphologic changes (36). In order to determine whether PAK5 functions similarly, 293 cells were transfected with Myc-tagged PAK5 expression vector or Myc-tagged PAK5(S573N), which contains mutations analogous to those in PAK4(S445N). Myc-tagged wild-type PAK4 expression vector was used for comparison. Equal amounts of PAK5, PAK5(S573N), and PAK4 (as assessed by Western blotting) were immunopurified from cell lysates and incubated with HH4 in kinase buffer with [γ-32P]ATP. Autophosphorylation and HH4 phosphorylation were analyzed after SDS-PAGE and autoradiography (Fig. 3). The results indicate that both PAK5 and PAK5(S573N) could autophosphorylate and phosphorylate HH4, although the kinase activity of PAK5(S573N) was stronger than that of wild-type PAK5 (Fig. 3A). Wild-type PAK5 had significantly stronger activity than an equivalent amount of PAK4. In fact, the autoradiogram had to be overexposed relative to PAK5 activity in order to detect PAK4 activity (Fig. 3B).
FIG. 3.
PAK5 autophosphorylates and phosphorylates HH4. 293 cells were transfected with equal amounts of either GFP vector or expression vectors containing Myc-tagged PAK4, Myc-tagged PAK5, or Myc-tagged PAK5(S573N). After transient expression, the amounts of Myc-PAK4 and Myc-PAK5 were normalized by Western blots probed with a mouse anti-Myc antibody. Approximately equal amounts of Myc-PAK4, Myc-PAK5, and Myc-PAK5(S573N) were immunoprecipitated from whole-cell lysates by using a mouse anti-Myc antibody and protein A-Sepharose. The immunocomplexes were then incubated with HH4 and [γ-32P]ATP in in vitro kinase buffer. Substrate phosphorylation and autophosphorylation were analyzed after SDS-PAGE (15% gel) and autoradiography. Both the autophosphorylation of PAK4, PAK5, and PAK5(S573N) and the phosphorylation of HH4 are indicated. The gel was exposed for 15 min (A) or 2 h (B). Western blots showing expression of Myc-PAK5 and Myc-PAK4 are shown in the bottom panel (10% gel).
PAK5 activates the JNK pathway.
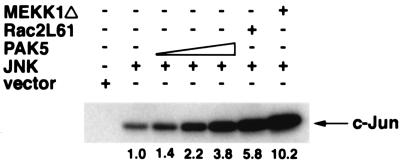
In addition to regulation of the actin cytoskeleton, one of the functions of Cdc42Hs and Rac is to activate the JNK MAP kinase pathway (2, 4, 6, 30, 54). Since PAK5 interacts with Rac and Cdc42, we tested its ability to activate JNK. 293 cells were transfected with a HA-tagged JNK expression vector together with increasing doses of an expression vector containing the PAK5 cDNA. Activated Rac and MEKK1 expression vectors were used as positive controls. After transient expression, JNK was immunopurified from cell lysates, followed by an in vitro kinase assay with GST–c-Jun as a substrate. The results indicate that overexpression of the wild-type PAK5 led to activation of the JNK pathway that was almost as strong as activation by Rac (Fig. 4). In contrast, PAK4 activation of JNK was significantly lower than Rac activation (1). PAK5 did not activate the ERK pathway, and it only inefficiently activated the p38 pathway (data not shown).
FIG. 4.
PAK5 activates the JNK pathway. 293 cells were transfected with either empty vector or 5 μg of expression vector containing HA-tagged JNK (HA-JNK) either alone or with increasing doses of expression vectors containing Myc-tagged PAK5 (1, 3, and 5 μg) or expression vectors containing Rac2L61 (2.5 μg) or MEKK1Δ (2.5 μg). After transient expression, cells were lysed, and the amount of HA-JNK was normalized by Western blots probed with a mouse anti-HA antibody. Equal amounts of HA-JNK were then immunoprecipitated from whole-cell lysates by using a mouse anti-HA antibody and protein A-Sepharose. The immunoprecipitates were then incubated with recombinant GST–c-Jun in the presence of [γ-32P]ATP in in vitro kinase buffer. Substrate phosphorylation was analyzed after SDS-PAGE and autoradiography. The phosphorylation of GST–c-Jun is indicated. The numbers indicate the extent (fold) of activation of JNK by PAK5, Rac2L61, and MEKK1Δ as quantitated by phosphorimager analysis.
Expression of PAK5 leads to the formation of filopodia and neurite processes in N1E-115 cells.
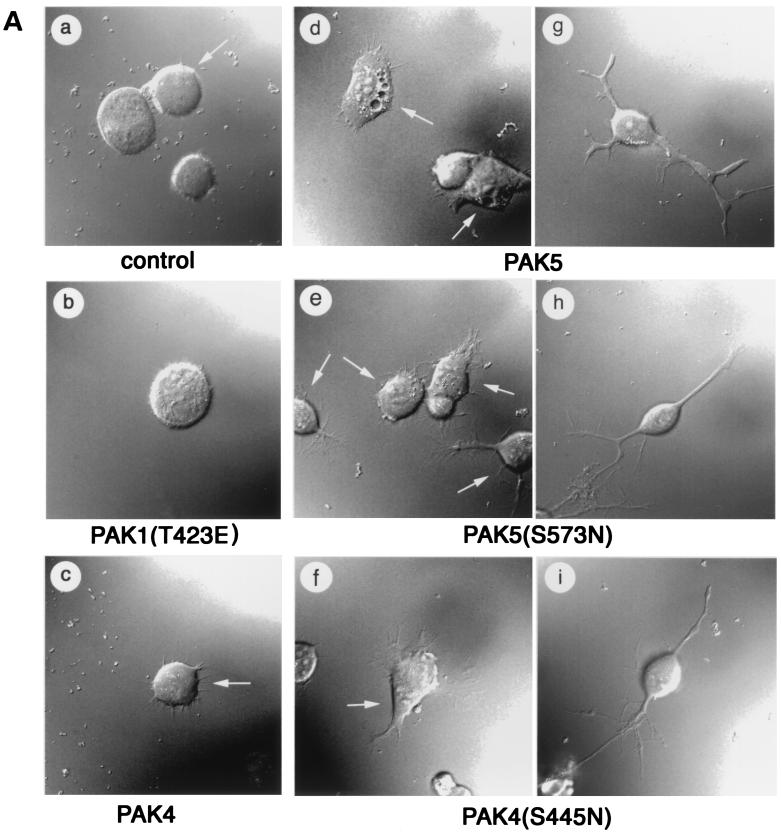
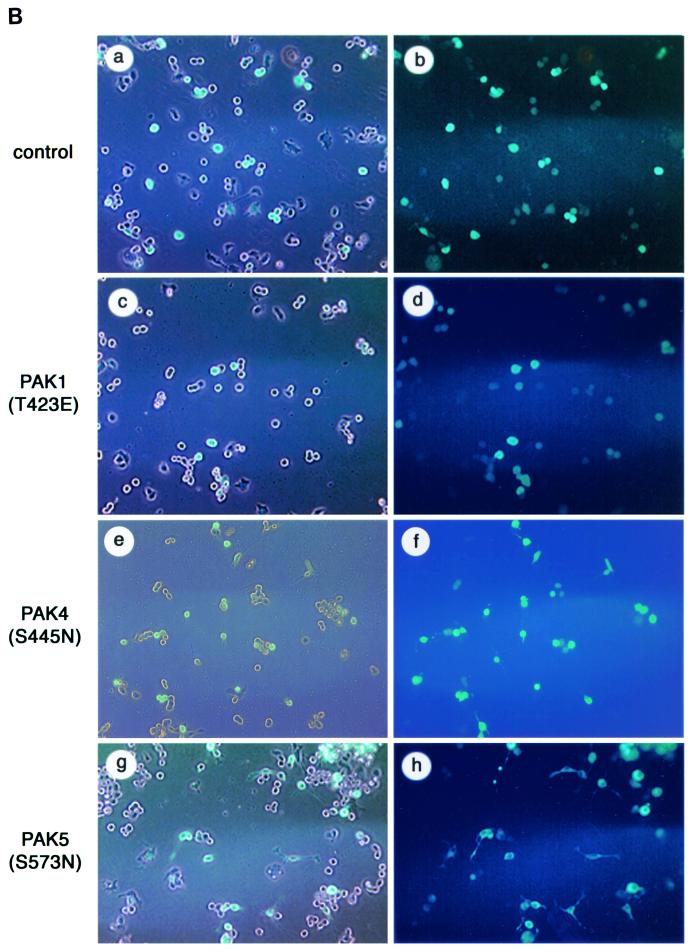
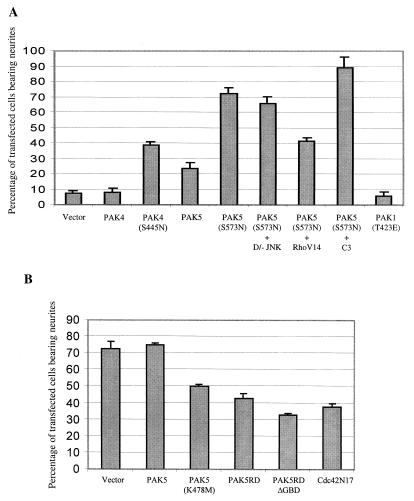
To determine whether expression of PAK5 could promote morphologic changes in neuronal cells, N1E-115 neuroblastoma cells grown in the presence of serum were transiently transfected with either empty vector containing only EGFP or expression vectors containing PAK5 or PAK5(S573N) fused to EGFP. For comparison, cells were transfected with a vector containing wild-type PAK4, activated PAK4(S445N) (36), or activated PAK1(T423E). Cells transfected with EGFP alone appeared similar to nontransfected cells. Most of the cells had rounded morphologies, and ca. 7% of them had neurite-like extensions. In contrast, at least three times as many PAK5-expressing cells had neurite-like processes. Most strikingly, expression of PAK5(S573N) led to the production of long neurite-like processes in ca. 70% of the transfected cells. In contrast to PAK5, expression of wild-type PAK4 did not promote neurite outgrowth in these cells significantly above the basal level, while activated PAK4 triggered neurite outgrowth in only ca. 40% of the transfected cells. Similar results were obtained even when larger amounts of PAK4 plasmid were transfected (data not shown). Both PAK4 and PAK5 triggered filopodium formation in most of the transfected cells. Expression of PAK1(T423E) did not lead to the increased production of either filopodia or neurites in these cells. Representative cells from each condition visualized at ×60 magnification are shown in Fig. 5A. Figure 5B shows fields of cells expressing empty vector, PAK1(T423E), PAK4(S445N), or PAK5(S573N) visualized at a ×10 magnification. The percentages of neurite-bearing cells under the different conditions are summarized in Fig. 6A.
FIG. 5.
PAK5 induces neurite outgrowth and filopodia. (A) Expression vectors containing EGFP (control) or equal amounts of PAK5, PAK5(S573N), PAK4, PAK4(S445N), or PAK1(T423E) were transfected into N1E-115 cells. Vectors containing PAK4 and PAK5 were either cotransfected with an EGFP vector at a 1:3 EGFP/PAK5 ratio or expressed as EGFP fusions, with similar results. PAK1(T423E) was cotransfected with EGFP. Cells were visualized by fluorescence microscopy at 20 h after transfection. Cells were then photographed with a ×100 objective lens. Where more than one cell is shown, the transfected cells, as observed by fluorescence microscopy, are indicated by an arrow. Cells transfected with empty vector EGFP, PAK1(T423E), or PAK4 are shown in panels a, b, and c. Representative examples of the PAK5-, PAK5(S573N)-, and PAK4(S445N)-expressing cells that had filopodia but not neurites are shown in panels d, e, and f. Representative examples of PAK5-, PAK5(S573N)-, and PAK4(S445N)-expressing cells that had differentiated and extended neurites are shown in panels g, h, and i. (B) Representative fields of cells transfected with empty EGFP vector, PAK1(T423E), PAK4(S445N), or PAK5(S573N) were taken by using a ×10 objective. The left side shows cells that were viewed under visible light and UV light together. The right side shows the same field that was viewed under only UV light.
FIG. 6.
Quantification of the effects of PAK5 on neurite formation. (A) Cells were transfected with expression vectors containing EGFP (control), EGFP-PAK5, EGFP-PAK5(S573N), PAK4, PAK4(S445N), or PAK1(T423E) and EGFP. EGFP-PAK5(S573N) was also transfected with either empty vector or with a threefold excess of dominant-negative JNK, RhoV14, or C3 transferase and then grown in the presence of serum. Cells bearing neurites were counted, and the percentages of transfected cells that had neurites are indicated. Cotransfection of PAK5(S573N) vector with EGFP vector gave similar results as EGFP-PAK5(S573N) (data not shown). (B) Cells were transfected with the indicated expression vectors. At 24 h after transfection the cells were incubated in serum-free medium. After 72 h, immunofluorescence assays were performed with anti-Myc antibody to identify transfected cells. Transfected cells bearing neurites were counted, and the percentages of transfected cells that had neurites are indicated. Approximately 100 transfected cells were counted in each experiment. The results are an average of at least two independent experiments for each condition.
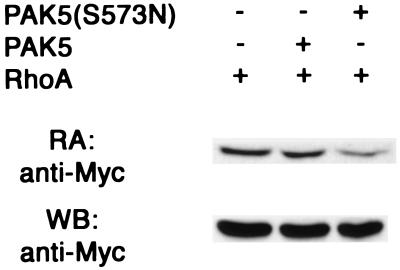
The Cdc42 and Rac pathways appear to function antagonistically with the Rho pathway in N1E-115 cells, where Cdc42 and Rac are required for neurite outgrowth and Rho causes neurite retraction (19, 41, 52). To see whether PAK5 functions antagonistically with Rho, cells were transfected with PAK5(S573N) together with an activated RhoAV14 vector. Expression of RhoAV14 caused a significant reduction in the percentage of PAK5(S573N)-transfected cells bearing neurites (see Fig. 6A). Approximately 90% of cells transfected with PAK5 and the Rho inhibitor C3 transferase (38, 39) had neurites. Dominant-negative JNK had no inhibitory effect on neurite outgrowth (see Fig. 6A), although it effectively blocked MEKK activation of the c-Jun promoter (data not shown). The results indicate that PAK5 triggers neurite outgrowth by a pathway that functions antagonistically to the Rho pathway and that is independent of JNK activation. To see whether PAK5 can actually inhibit RhoA activity, 293 cells were transfected with wild-type RhoA together with either empty vector, wild-type PAK5, or PAK5(S573N), and RhoA activity was assessed by a Rhotekin binding assay. As shown in Fig. 7, activated PAK5 caused a significant reduction in the amount of activated Rho, but had no effect on total RhoA levels.
FIG. 7.
Activated PAK5 inhibits Rho activity. 293 cells were transfected with wild-type Myc-RhoA expression vector together with equal amounts of either empty vector, wild-type EGFP-PAK5, or EGFP-PAK5(S573N) expression vectors (without Myc tags). After transient expression, cell lysates were incubated with GST-Rhotekin glutathione agarose complexes, which bind specifically to GTP-loaded RhoA (40). Complexes were then washed and separated by SDS-PAGE, and the GTP RhoA content was analyzed by Western blot analysis by using anti-Myc antibody (top panel). The total RhoA content in an aliquot of whole-cell lysates is shown in the bottom panel.
PAK5 is necessary for neurite outgrowth in N1E-115 cells.
To see whether PAK5 is necessary for neurite outgrowth, N1E-115 cells were transfected with either empty vector or vectors containing one of three different dominant-negative PAK5 mutants; PAK5(K478M) has a single point mutation within the kinase domain rendering it kinase inactive, PAK5RD lacks the kinase domain, and PAK5RDΔGBD lacks the kinase domain and the GBD so that it cannot titrate the Cdc42 and Rac. After transient transfection, cells were changed to serum-free media to induce neurite outgrowth, and cells were observed 72 h later. While ca. 70% of the empty vector transfected cells had neurites, expression of each of the dominant-negative mutants led to a reduction in the percentage of neurite-bearing cells. The levels of inhibition by the dominant-negative PAK5 mutants were similar to the level of inhibition that resulted from expression of dominant-negative Cdc42N17. Expression of wild-type PAK5 did not cause any inhibition in neurite outgrowth. These results are summarized in Fig. 6B. Taken together, these results indicate that PAK5 is both necessary and sufficient to induce neurite outgrowth in N1E-115 cells.
DISCUSSION
Cytoskeletal organization is a critical part of neuronal development. For example, filopodia and lamellipodia play key roles in the guidance of neuronal growth cones toward attractive cues and away from repulsive cues, and neurite extension occurs when these filopodia and lamellipodia are stabilized. Stabilization of these structures is followed by extension of new filopodia and lamellipodia so that the cycle of axon guidance and growth can continue (24, 33). Because the formation of filopodia and lamellipodia is so important for neurite outgrowth and growth cone guidance, there has been considerable interest in the possible role for the Rho GTPases in these processes. Of particular interest are Cdc42 and Rac, which were first described as proteins that regulate filopodium and lamellipodium formation in fibroblasts. Both Cdc42 and Rac have subsequently been found to play key roles in all aspects of neural development, including growth cone guidance and the extension of axons (24).
The effector proteins that mediate the morphologic changes induced by the Rho GTPases in neuronal cells are not yet clearly defined. The PAK serine/threonine kinases are good candidates because they are direct targets of Cdc42 and Rac. However, PAK1, -2, and -3, which belong to the class A category of PAKs, may not be directly involved in all of the cytoskeletal changes induced by these GTPases, and effector mutants of Cdc42 and Rac that cannot bind to them can still induce filopodia and lamellipodia (13, 21). In contrast, PAK4, which is the founding member of the class B group of PAKs, directly regulates filopodium formation in response to Cdc42. PAK4 has a modified GBD compared with the class A PAKs, and it can even bind to Cdc42 effector mutants that do not bind PAK1, -2, and -3 via this domain (1). Here we describe the characterization of a new member of the class B family of PAK family of PAKs, PAK5. PAK5 is similar to PAK4 in sequence within the GBD and kinase domain, but it is expressed primarily in the brain. PAK5 is therefore an excellent candidate for a GTPase target involved in neurite outgrowth.
While members of the Drosophila PAK family, including Drosophila PAK and MBT, are thought to be involved in neuronal development (12, 34), the roles for mammalian PAKs in neurogenesis are less well defined. We have found that expression of activated PAK5 led to a dramatic increase in the formation of neurite-like extensions in N1E-115 cells. Even in the subset of PAK5-expressing cells in which neurites were not seen, there was an increased production of filopodia, which is an important step in the production of neurite processes. Our results suggest, therefore, that PAK5 may in fact be a major target for Cdc42, and possibly Rac, in neuronal cells, which regulates the formation of filopodia and neurite processes. Previous work has shown that PAK1 can also trigger neurite outgrowth in PC12 cells. However, this required that it be targeted to the membrane by addition of a membrane targeting sequence, and it occurred by a mechanism that did not require its kinase activity or its Cdc42/Rac binding domain (GBD). PAK1 might therefore function as a type of scaffolding protein that recruits other proteins to the membrane, where they can promote neurite outgrowth (8). We have found that activated PAK1 did not promote neurite outgrowth in N1E-115 cells. In contrast, PAK5 clearly triggered both filopodium formation and neurite outgrowth in these cells. This did not require membrane targeting of PAK5, and the extent of neurite outgrowth was directly related to the level of its kinase activity. Our results suggest therefore that PAK5 regulates the morphology of these cells by phosphorylating target proteins that are specifically involved in neurite outgrowth.
PAK5, along with PAK4 (1) and PAK6 (53), fall into the second class of PAKs (class B). Unlike the class A PAKs, which are quite similar to each other throughout their sequences, PAK4, -5, and -6 are similar to each other only within their GBD and kinase domain. Outside of this region, in the presumed regulatory domain, they are almost completely different from each other. While the reasons for these differences are not known, it seems likely that they may mediate interactions with different regulatory proteins and thereby contribute to unique functions of the different PAKs. Another difference between PAK4, -5, and -6 is in their expression patterns. Whereas PAK4 is ubiquitously expressed (1; data not shown), PAK6 is expressed primarily in the prostate and testes (53), and PAK5 is expressed primarily in the brain. The differences in their sequences and expression patterns makes it seem likely that the different PAKs may have different though possibly overlapping functions. To see whether other members of the class B family could function similarly to PAK5, we scored N1E-115 cells for neurite outgrowth after transfection of PAK4. While activated PAK4 triggered filopodium formation, it promoted neurite outgrowth less efficiently than did activated PAK5. Furthermore, unlike wild-type PAK5, wild-type PAK4 did not promote neurite outgrowth significantly above the background levels. Thus, while members of the class B family of PAKs may generally be capable of triggering neurite outgrowth when they are activated, based on its expression pattern and its dramatic effects on the morphologies of N1E-115 cells, PAK5 is the best candidate for a cytoskeletal regulatory protein involved in promoting neurite outgrowth.
The mechanism by which PAK5 triggers neurite outgrowth in N1E-115 cells remains to be elucidated. While the formation of filopodia is clearly an important part of neurite outgrowth, our results indicate that filopodium formation on its own is clearly not sufficient for the production of neurites, since some of the PAK5 (and PAK4) transfected cells had filopodia but not neurites. This suggests that other signaling pathways regulated by PAK5 are also required for neurite outgrowth. Our results suggest that, like Cdc42 and Rac, PAK5 may promote neurite outgrowth by a mechanism that is antagonistic to Rho (19, 41, 52). We have found that overexpression of a constitutively active Rho mutant, RhoV14, blocks neurite production by activated PAK5 and that activated PAK5 can inhibit RhoA activity. We have also found that, like Cdc42 and Rac (2, 4, 6, 30, 54), PAK5 activates the JNK pathway. However, while JNK has been implicated in PC12 cell differentiation (15, 16, 23), it was shown to be dispensable for differentiation of N1E-115 cells (41), and we have found that a kinase-inactive JNK has no inhibitory effect on neurite outgrowth triggered by PAK5. Our results therefore suggest that JNK is part of a parallel PAK5 activated pathway and not part of the pathway leading to morphologic changes.
The Drosophila protein MBT is quite similar to PAK5 in sequence, especially within the kinase domain and GBD motif. Interestingly, disruption of the mbt gene leads to a reduction in the number of cells in the mushroom body of Drosophila brain. MBT was therefore proposed to be involved in the regulation of proliferation or survival of neuronal cells (28). It is interesting that cell death in the developing nervous system can result from improper connections, or lack of connections, between neurons and their targets (42). Since MBT is similar to PAK5 in sequence, an intriguing possibility is that cell death in mbt mutant flies might occur because cells fail to develop axons properly and therefore fail to make their proper connections. Another possibility is that MBT and PAK5 could specifically trigger cell survival pathways. In this regard it is interesting that PAK5 activated JNK, since the JNK pathway has been implicated in the regulation of cell growth in mammalian cells (29), and it is thought to contribute to both survival and apoptosis in different parts of the brain (20).
Future work will involve identifying PAK5 substrates, as well as determining how PAK5 is regulated by upstream activators. Although PAK5 binds to Cdc42 and Rac, the possibility remains that other GTPases can bind to the PAK5 GBD and regulate its activity. Furthermore, extracellular stimuli that regulate PAK5’s kinase activity have not yet been identified. Previous studies have indicated that neurotrophic factors, such as NGF, BDGF, NT-3, and NT-4, and chemoattractants, such as netrin-1, can induce neurite outgrowth or regulate axon guidance (3, 46). Such stimuli will be good candidates for possible activators of the Rho GTPases and PAK5, as well as other cytoskeletal regulatory proteins in mammalian cells.
Acknowledgments
We thank Chloe Bulinsky for help with microscopy, Mike Sheetz for critically reading the manuscript, and A. Hall, R. Prywes, and J. Collard for reagents.
This work was supported by grant R01 CA76342 and an American Scientist Development Grant Award from the American Heart Association to A.M.
REFERENCES
- 1.Abo, A., J. Qu, M. S. Cammarano, C. Dan, A. Fritsch, V. Baud, B. Belisle, and A. Minden. 1998. PAK4, a novel effector for Cdc42Hs, is implicated in the reorganization of the actin cytoskeleton and in the formation of filopodia. EMBO J. 17:6527–6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagrodia, S., B. Derijard, R. J. Davis, and R. A. Cerione. 1995. Cdc42 and PAK-mediated signaling leads to Jun kinase and p38 mitogen-activated protein kinase activation. J. Biol. Chem. 270:27995–27998. [DOI] [PubMed] [Google Scholar]
- 3.Berninger, B., and M. Poo. 1996. Fast actions of neurotrophic factors. Curr. Opin. Neurobiol. 6:324–330. [DOI] [PubMed] [Google Scholar]
- 4.Brown, J. L., L. Stowers, M. Baer, J. Trejo, S. Coughlin, and J. Chant. 1996. Human Ste20 homologue hPAK1 links GTPases to the JNK MAP kinase pathway. Curr. Biol. 6:598–605. [DOI] [PubMed] [Google Scholar]
- 5.Brown, M. D., B. J. Cornejo, and J. R. Bamburg. 2000. Cdc42 stimulates neurite outgrowth and formation of growth cone filopodia and lamellipodia. J. Neurobiol 43:352. [DOI] [PubMed] [Google Scholar]
- 6.Coso, O. A., M. Chiariello, J. C. Yu, H. Teramoto, P. Crespo, N. Xu, T. Miki, and J. S. Gutkind. 1995. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell 81:1137–1146. [DOI] [PubMed] [Google Scholar]
- 7.Daniels, R. H., and G. M. Bokoch. 1999. p21-activated protein kinase: a crucial component of morphological signaling? Trends Biochem. Sci. 24:350–355. [DOI] [PubMed] [Google Scholar]
- 8.Daniels, R. H., P. S. Hall, and G. M. Bokoch. 1998. Membrane targeting of p21-activated kinase 1 (PAK1) induces neurite outgrowth from PC12 Cells. EMBO J. 17:754–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Derijard, B., M. Hibi, I. H. Wu, T. Barrett, B. Su, T. Deng, M. Karin, and R. J. Davis. 1994. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell 76:1025–1037. [DOI] [PubMed] [Google Scholar]
- 10.Dutartre, H., J. Davoust, J. P. Gorvel, and P. Chavrier. 1996. Cytokinesis arrest and redistribution of actin-cytoskeleton regulatory components in Cells expressing the Rho GTPase CDC42Hs. J. Cell Sci. 109:367–377. [DOI] [PubMed] [Google Scholar]
- 11.Gebbink, M. F., O. Kranenburg, M. Poland, F. P. van Horck, B. Houssa, and W. H. Moolenaar. 1997. Identification of a novel, putative Rho-specific GDP/GTP exchange factor and a RhoA-binding protein: control of neuronal morphology. J. Cell Biol. 137:1603–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hing, H., J. Xiao, N. Harden, L. Lim, and S. L. Zipursky. 1999. Pak functions downstream of Dock to regulate photoreceptor axon guidance in Drosophila. Cell 97:853–863. [DOI] [PubMed] [Google Scholar]
- 13.Joneson, T., M. McDonough, D. Bar-Sagi, and L. Van Aelst. 1996. RAC regulation of actin polymerization and proliferation by a pathway distinct from Jun kinase. Science 274:1374–1376. [DOI] [PubMed] [Google Scholar]
- 14.Kaufmann, N., Z. P. Wills, and D. Van Vactor. 1998. Drosophila Rac1 controls motor axon guidance. Development 125:453–461. [DOI] [PubMed] [Google Scholar]
- 15.Kick, G., G. Messer, G. Plewig, P. Kind, and A. E. Goetz. 1996. Strong and prolonged induction of c-jun and c-fos proto-oncogenes by photodynamic therapy. Br. J. Cancer 74:30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kita, Y., K. D. Kimura, M. Kobayashi, S. Ihara, K. Kaibuchi, S. Kuroda, M. Ui, H. Iba, H. Konishi, U. Kikkawa, S. Nagata, and Y. Fukui. 1998. Microinjection of activated phosphatidylinositol-3-kinase induces process outgrowth in rat PC12 Cells through the Rac-JNK signal transduction pathway. J. Cell Sci. 111:907–915. [DOI] [PubMed] [Google Scholar]
- 17.Knaus, U. G., and G. M. Bokoch. 1998. The p21Rac/Cdc42-activated kinases (PAKs). Int. J. Biochem. Cell Biol. 30:857–862. [DOI] [PubMed] [Google Scholar]
- 18.Kozma, R., S. Ahmed, A. Best, and L. Lim. 1995. The Ras-related protein Cdc42Hs and bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol. Cell. Biol. 15:1942–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kozma, R., S. Sarner, S. Ahmed, and L. Lim. 1997. Rho family GTPases and neuronal growth cone remodelling: relationship between increased complexity induced by Cdc42Hs, Rac1, and acetylcholine and collapse induced by RhoA and lysophosphatidic acid. Mol. Cell. Biol. 17:1201–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuan, C.-Y., D. Yang, D. R. Samanta Roy, R. J. Davis, P. Rakic, and R. A. Flavell. 1999. The Jnk1 and Jnk2 protein kinases are required for regional specific apoptosis during early brain development. Neuron 22:667–676. [DOI] [PubMed] [Google Scholar]
- 21.Lamarche, N., N. Tapon, L. Stowers, P. D. Burbelo, P. Aspenstrom, T. Bridges, J. Chant, and A. Hall. 1996. Rac and Cdc42 induce actin polymerization and G1 cell cycle progression independently of p65PAK and the JNK/SAPK MAP kinase cascade. Cell 87:519–529. [DOI] [PubMed] [Google Scholar]
- 22.Lamoureux, P., Z. F. Altun-Gultekin, C. Lin, J. A. Wagner, and S. R. Heidemann. 1997. Rac is required for growth cone function but not neurite assembly. J. Cell Sci. 110:635–641. [DOI] [PubMed] [Google Scholar]
- 23.Leppa, S., R. Saffrich, W. Ansorge, and D. Bohmann. 1998. Differential regulation of c-Jun by ERK and JNK during PC12 Cell differentiation. EMBO J. 17:4404–4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo, L. 2000. Rho GTPases in neuronal morphogenesis. Nat. Rev. Neurosci. 1:173–180. [DOI] [PubMed] [Google Scholar]
- 25.Luo, L., Y. J. Liao, L. Y. Jan, and Y. N. Jan. 1994. Distinct morphogenetic functions of similar small GTPases: Drosophila Drac1 is involved in axonal outgrowth and myoblast fusion. Genes Dev. 8:1787–1802. [DOI] [PubMed] [Google Scholar]
- 26.Manser, E., H. Y. Huang, T. H. Loo, X. Q. Chen, J. M. Dong, T. Leung, and L. Lim. 1997. Expression of constitutively active alpha-PAK reveals effects of the kinase on actin and focal complexes. Mol. Cell. Biol. 17:1129–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin, G. A., G. Bollag, F. McCormick, and A. Abo. 1995. A novel serine kinase activated by rac1/CDC42Hs-dependent autophosphorylation is related to PAK65 and STE20. EMBO J. 14:1970–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melzig, J., K. H. Rein, U. Schafer, H. Pfister, H. Jackle, M. Heisenberg, and T. Raabe. 1998. A protein related to p21-activated kinase (PAK) that is involved in neurogenesis in the Drosophila adult central nervous system. Curr. Biol. 8:1223–1226. [DOI] [PubMed] [Google Scholar]
- 29.Minden, A., and M. Karin. 1997. Regulation and function of the JNK subgroup of MAP kinases. Biochim. Biophys. Acta 1333:F85–F104. [DOI] [PubMed] [Google Scholar]
- 30.Minden, A., A. Lin, F. X. Claret, A. Abo, and M. Karin. 1995. Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases Rac and Cdc42Hs. Cell 81:1147–1157. [DOI] [PubMed] [Google Scholar]
- 31.Minden, A., A. Lin, M. McMahon, C. Lange-Carter, B. Derijard, R. J. Davis, G. L. Johnson, and M. Karin. 1994. Differential activation of ERK and JNK mitogen-activated protein kinases by Raf-1 and MEKK. Science 266:1719–1723. [DOI] [PubMed] [Google Scholar]
- 32.Minden, A., A. Lin, T. Smeal, B. Derijard, M. Cobb, R. Davis, and M. Karin. 1994. c-Jun N-terminal phosphorylation correlates with activation of the JNK subgroup but not the ERK subgroup of mitogen-activated protein kinases. Mol. Cell. Biol. 14:6683–6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mueller, B. K. 1999. Growth cone guidance: first steps towards a deeper understanding. Annu. Rev. Neurosci. 22:351–388. [DOI] [PubMed] [Google Scholar]
- 34.Newsome, T. P., S. Schmidt, G. Dietzl, K. Keleman, B. Asling, A. Debant, and B. J. Dickson. 2000. Trio combines with dock to regulate Pak activity during photoreceptor axon pathfinding in Drosophila. Cell 101:283–294. [DOI] [PubMed] [Google Scholar]
- 35.Nobes, C. D., and A. Hall. 1995. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 81:53–62. [DOI] [PubMed] [Google Scholar]
- 36.Qu, J., M. S. Cammarano, Q. Shi, K. C. Ha, P. de Lanerolle, and A. Minden. 2001. Activated PAK4 regulates cell adhesion and anchorage-independent growth. Mol. Cell. Biol. 21:3523–3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reid, T., T. Furuyashiki, T. Ishizaki, G. Watanabe, N. Watanabe, K. Fujisawa, N. Morii, P. Madaule, and S. Narumiya. 1996. Rhotekin, a new putative target for Rho bearing homology to a serine/threonine kinase, PKN, and rhophilin in the rho-binding domain. J. Biol. Chem. 271:13556–13560. [DOI] [PubMed] [Google Scholar]
- 38.Ridley, A. J., and A. Hall. 1992. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 70:389–399. [DOI] [PubMed] [Google Scholar]
- 39.Ridley, A. J., H. F. Paterson, C. L. Johnston, D. Diekmann, and A. Hall. 1992. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell 70:401–410. [DOI] [PubMed] [Google Scholar]
- 40.Sander, E. E., J. P. ten Klooster, S. van Delft, R. A. van der Kammen, and J. G. Collard. 1999. Rac downregulates Rho activity: reciprocal balance between both GTPases determines Cellular morphology and migratory behavior. J. Cell Biol. 147:1009–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sarner, S., R. Kozma, S. Ahmed, and L. Lim. 2000. Phosphatidylinositol 3-kinase, Cdc42, and Rac1 Act downstream of Ras in integrin-dependent neurite outgrowth in N1E-115 neuroblastoma Cells. Mol. Cell. Biol. 20:158–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sastry, P. S., and K. S. Rao. 2000. Apoptosis and the nervous system. J. Neurochem. 74:1–20. [DOI] [PubMed] [Google Scholar]
- 43.Self, A. J., and A. Hall. 1995. Purification of recombinant Rho/Rac/G25K from Escherichia coli. Methods Enzymol. 256:3–10. [DOI] [PubMed] [Google Scholar]
- 44.Sells, M. A., and J. Chernoff. 1997. Emerging from the Pak: the p21-activated protein kinase family. Trends Cell Biol. 7:162–167. [DOI] [PubMed] [Google Scholar]
- 45.Sells, M. A., U. G. Knaus, S. Bagrodia, D. M. Ambrose, G. M. Bokoch, and J. Chernoff. 1997. Human p21-activated kinase (Pak1) regulates actin organization in mammalian Cells. Curr. Biol. 7:202–210. [DOI] [PubMed] [Google Scholar]
- 46.Serafini, T., S. A. Colamarino, E. D. Leonardo, H. Wang, R. Beddington, W. C. Skarnes, and M. Tessier-Lavigne. 1996. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell 87:1001–1014. [DOI] [PubMed] [Google Scholar]
- 47.Stanyon, C. A., and O. Bernard. 1999. LIM-kinase 1. Int. J. Biochem. Cell Biol. 31:389–394. [DOI] [PubMed] [Google Scholar]
- 48.Steven, R., T. J. Kubiseski, H. Zheng, S. Kulkarni, J. Mancillas, A. Ruiz Morales, C. W. Hogue, T. Pawson, and J. Culotti. 1998. UNC-73 activates the Rac GTPase and is required for Cell and growth cone migrations in C. elegans. Cell 92:785–795. [DOI] [PubMed] [Google Scholar]
- 49.Taylor, S. S., D. R. Knighton, J. Zheng, L. F. Ten Eyck, and J. M. Sowadski. 1992. Structural framework for the protein kinase family. Annu. Rev. Cell Biol. 8:429–462. [DOI] [PubMed] [Google Scholar]
- 50.Tigyi, G., D. J. Fischer, A. Sebok, F. Marshall, D. L. Dyer, and R. Miledi. 1996. Lysophosphatidic acid-induced neurite retraction in PC12 Cells: neurite-protective effects of cyclic AMP signaling. J. Neurochem. 66:549–558. [DOI] [PubMed] [Google Scholar]
- 51.Tigyi, G., D. J. Fischer, A. Sebok, C. Yang, D. L. Dyer, and R. Miledi. 1996. Lysophosphatidic acid-induced neurite retraction in PC12 Cells: control by phosphoinositide-Ca2+ signaling and Rho. J. Neurochem. 66:537–548. [DOI] [PubMed] [Google Scholar]
- 52.van Leeuwen, F. N., H. E. Kain, R. A. van der Kammen, F. Michiels, O. W. Kranenburg, and J. G. Collard. 1997. The guanine nucleotide exchange factor Tiam1 affects neuronal morphology: opposing roles for the small GTPases Rac and Rho. J. Cell Biol. 139:797–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang, F., X. Li, M. Sharma, M. Zarnegar, B. Lim, and Z. Sun. 2001. Androgen receptor specifically interacts with a novel p21-activated kinase, PAK6. J. Biol. Chem. 276:15345–15353. [DOI] [PubMed] [Google Scholar]
- 54.Zhang, S., J. Han, M. A. Sells, J. Chernoff, U. G. Knaus, R. J. Ulevitch, and G. M. Bokoch. 1995. Rho family GTPases regulate p38 mitogen-activated protein kinase through the downstream mediator Pak1. J. Biol. Chem. 270:23934–23936. [DOI] [PubMed] [Google Scholar]
- 55.Zipkin, I. D., R. M. Kindt, and C. J. Kenyon. 1997. Role of a new Rho family member in Cell migration and axon guidance in C. elegans. Cell 90:883–894. [DOI] [PubMed] [Google Scholar]