Abstract
The integral membrane protein linker for activation of T cells (LAT) is a central adapter protein in the T-cell receptor (TCR)-mediated signaling pathways. The cellular localization of LAT is extremely sensitive to intracellular redox balance alterations. Reduced intracellular levels of the antioxidant glutathione (GSH), a hallmark of chronic oxidative stress, resulted in the membrane displacement of LAT, abrogated TCR-mediated signaling and consequently hyporesponsiveness of T lymphocytes. The membrane displacement of LAT is accompanied by a considerable difference in the mobility of LAT upon native and nonreducing denaturing polyacrylamide gel electrophoresis analysis, a finding indicative of a conformational change. Targeted mutation of redox-sensitive cysteine residues within LAT created LAT mutants which remain membrane anchored under conditions of chronic oxidative stress. The expression of redox-insensitive LAT mutants allows for restoration of TCR-mediated signal transduction, whereas CD28-mediated signaling pathways remained impaired. These results are indicative that the membrane displacement of LAT as a result of redox balance alterations is a consequence of a conformational change interfering with the insertion of LAT into the plasma membrane. Conclusively, the data suggest a role for LAT as a crucial intermediate in the sensitivity of TCR signaling and hence T lymphocytes toward chronic oxidative stress.
A central and crucial role in T-cell signaling is attributed to the adapter protein LAT (for linker for activation of T cells) (72, 76, 78). LAT is an integral membrane protein that localizes predominantly to the glycolipid (GPI)-enriched microdomains in the plasma membrane. The presence of LAT in these microdomains is required for its function and is primarily dependent on the palmitoylation of cysteine residue C26, just outside of the α-helical transmembrane structure of LAT (7, 46, 76, 77, 79). Upon T-cell receptor (TCR) activation, the concerted action of Src and Syk protein tyrosine kinases results in the rapid phosphorylation of LAT. ZAP-70 (the ζ-associated protein kinase of 70 kDa) is directly responsible for the phosphorylation of LAT on several tyrosine residues. LAT does not contain any Src homology 2 (SH2), SH3, or phosphotyrosine-binding domains and interacts with other signaling proteins exclusively through these phosphorylated tyrosine residues (13, 66, 76). LAT mediates the assembly of multiprotein signaling complexes which serve to amplify and diverge the TCR-mediated signal and initiate the downstream signaling cascades. Signaling molecules that associate with LAT after phosphorylation, either directly or indirectly, include the adapter proteins Grb2 (growth factor receptor bound protein 2); SLP-76 (SH2 domain-containing leukocyte protein of 76 kDa); Gads (Grb2-related adapter downstream of Shc); Grap (Grb2-related adapter protein); Shb; SLAP (Src-like adapter protein); a phospholipase, PLC-γ1; the lipid kinase phosphatidylinositol 3-kinase, and the Tec kinase Itk (inducible T-cell kinase) (2, 11, 18, 43, 48, 49, 70, 71, 76). The functioning of proteins such as SLP-76 and PLC-γ1 is dependent on their association with LAT and subsequent phosphorylation (15, 79, 80). The association of PLC-γ1 with LAT couples the TCR-induced signal to the calcium-dependent activation of calcineurin, while the association of the Grb2/Sos complex with LAT results in the activation of the Ras/Raf-1/ERK (extracellular signal-regulated kinase) pathway, and the association of an SLP-76/Vav complex activates the mitogen-activated protein (MAP) kinase p38/Mpk2 through a Rac-1-dependent signaling route. The coordinated activation of these signaling pathways finally culminate in the activation of cytokine gene transcription, e.g., interleukin-2 (IL-2), necessary for T lymphocytes to fulfill their cellular function, through transcription factors such as NFAT (nuclear factor of activated T cells), AP-1 (activator protein-1), and NF-κB (nuclear factor-κB) (6, 15, 20, 30, 38, 39, 50, 66, 67, 76, 80).
Rheumatoid arthritis (RA) is a chronic inflammatory joint disease characterized by the presence of large numbers of hyporesponsive T lymphocytes in the synovium of the inflamed joints, together with activated neutrophils, macrophages, B lymphocytes, and dendritic cells. Antigen-independent cell-cell contact of the hyporesponsive T lymphocytes with macrophages has been reported to contribute to damage of the joint cartilage (55, 57, 61, 68, 69).
The hyporesponsiveness of the synovial T lymphocytes in RA is believed to be due to dysfunctional TCR-mediated signaling as a result of chronic oxidative stress. The excessive production of radical oxygen species depletes the antioxidant capacity of the T lymphocytes present in the synovium. Synovial T lymphocytes have severely decreased intracellular levels of the protective antioxidant glutathione (GSH). Also, a markedly increased extracellular concentration of the antioxidant thioredoxin has been reported in the synovial fluid (24, 52, 53, 54, 74).
Under conditions of chronic oxidative stress, such as in RA, LAT is displaced from the plasma membrane, remaining unphosphorylated upon stimulation of the T lymphocytes through the TCR/CD3 complex and thus blocking the progression of the TCR-mediated signaling pathways. We have previously established that the cellular localization of LAT is very sensitive to redox balance alterations by chemically inducing changes in the intracellular levels of GSH (24). In contrast to acute oxidative stress, which often results in activation of TCR signaling pathways and ultimately of several MAP kinases and transcription factors (1, 4, 21, 75, 76), chronic oxidative stress is disabling to a cell because it interferes with the oxidation status and therefore often the functioning of proteins containing sulfhydryl groups (26, 33, 42).
In the present study, we set out to elucidate how chronic oxidative stress and the decreased intracellular GSH levels in particular affects the cellular localization of LAT, and we also sought to gain further insight in how the TCR-mediated signaling pathways downstream from LAT and the CD28-mediated signaling pathways are affected by chronic oxidative stress. We present evidence establishing that chronic oxidative stress results in the membrane displacement of LAT by inducing a conformational change of the protein which we hypothesize interferes with the insertion of the α-helical structure into the plasma membrane. The expression of a redox-insensitive cysteine-to-serine mutant of LAT allows for a partial restoration of the TCR signaling pathways, leading to the transcriptional activation of the IL-2 gene expression. In addition to LAT, the costimulatory CD28 signaling pathways seem to be very sensitive to chronic oxidative stress.
MATERIALS AND METHODS
T-lymphocyte isolation.
Human peripheral blood T lymphocytes were obtained from healthy volunteer platelet donors, and mononuclear cell suspensions were prepared by Ficoll-Hypaque density gradient centrifugation. T lymphocytes were isolated by 2-aminoethylisothiouronium bromide (AET)-treated sheep red blood cell (SRBC) rosetting. The rosetted T lymphocytes were collected by centrifugation, and subsequently the SRBC were lysed with 155 mM NH4Cl, 10 mM KHCO3, and 0.1 mM EDTA according to standard procedures. The remaining cell preparations contained >92% T lymphocytes as assessed by fluorescence-activated cell-sorting (FACS) analysis after staining with a peridinin chlorophyl protein-conjugated anti-CD3 monoclonal antibody (MAb; Becton Dickinson, San Jose, Calif.). After isolation, lymphocytes were kept at 37°C in 5% CO2 in Iscove modified Dulbecco medium (Gibco-BRL/Life Technologies, Gaithersburg, Md.) containing 10% fetal calf serum (FCS; Gibco-BRL) supplemented with 100 U of penicillin/ml and 100 μg of streptomycin/ml (both from Roche, Mannheim, Germany).
The human leukemic T-cell line TAg Jurkat was cultured in RPMI 1640 medium (Gibco-BRL) containing 10% FCS supplemented with 2 mM ℓ-glutamine, 100 of U penicillin/ml, and 100 μg of streptomycin/ml.
Stimulation.
Jurkat cells (106/ml) or T lymphocytes (2.5 × 106/ml) were stimulated for various time periods with 1 μg of anti-CD3 MAb (1XE; Central Laboratory of The Netherlands Red Cross Blood Transfusion Service [CLB], Amsterdam, The Netherlands)/ml in combination with 1 μg of anti-CD28 MAb (15E8; CLB)/ml as indicated. Stimulation of cells with phorbol 12-myristate 13-acetate (PMA; Sigma, St. Louis, Mo.) occurred at a final concentration of 50 ng/ml, in combination with ionomycin (Sigma) at a final concentration of 1 μg/ml. Cells were preincubated for 68 to 72 h as indicated with BSO [𝒹ℓ-buthionine-(S,R)-sulfoximine; Sigma] at a final concentration of 200 μM.
Plasmids and mutagenesis.
The reporter plasmids used have been described before: pCAT3e-IL-2(−319/+47) contains the complete IL-2 promoter (23), pCAT3e-3xNFAT/IL-2 contains three copies of the distal NFAT binding site from the IL-2 promoter (22), pCAT-3xAP-1/IL-2 (also known as pX3CAT) contains three copies of the AP-1 binding site from the IL-2 promoter (44), and pCAT-2xNF-κB (also known as DR6) contains two NF-κB binding sites.
The expression plasmid pcDNA3/LAT(wt)-flag (a gift from Lawrence Samelson, Section of Lymphocyte Signaling, Cell Biology and Metabolism Branch, National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Md.) was used to generate LAT mutants containing cysteine-to-serine mutations (76). Point mutations were introduced by using a PCR-based mutagenesis strategy to substitute the cysteines at positions C9 (TGC→TCC), C26 (TGT→AGT), C29 (TGC→TCC), and C117 (TGT→AGT) for serine residues. All constructs were verified by sequencing. The expression plasmid pApuro/myc-BLNK(wt) was kindly provided by Andrew Chan, HHMI, Washington University School of Medicine, St. Louis, Mo. (17).
Transfection.
A total of 6 × 106 Jurkat cells were resuspended in 400 μl of RPMI 1640 medium containing 10% FCS, 2 mM ℓ-glutamine, antibiotics, and 10 μg of reporter plasmid DNA plus 25 μg of expression plasmid DNA was added. Also, 100 ng of pRL-SV40 (Promega Corporation, Madison, Wis.) was added as an internal control. After a 10-min incubation, cells were electroporated by using a Bio-Rad Gene Pulser (Bio-Rad Laboratories, Richmond, Calif.) at 250 V and 950 μF. Cells were then transferred to RPMI 1640 medium. At 1 h after electroporation, BSO was added as indicated at a final concentration of 200 μM. Then, 68 h later, cells were either left unstimulated or stimulated with anti-CD3, anti-CD3 plus anti-CD28, or PMA plus ionomycin. After 24 h, cells were harvested, and total cell extracts were prepared by lysis of the cells in 120 μl of passive lysis buffer (Promega) for 30 min at room temperature (RT). Insoluble debris was spun down in a microcentrifuge for 5 min.
CAT ELISA and Renilla luciferase assay.
Chloramphenicol acetyltransferase (CAT) concentrations in total cell extracts were measured by using the CAT enzyme-linked immunosorbent assay (ELISA) kit (Roche) as recommended by the manufacturer. The Renilla luciferase activities in total cell extracts were quantified by counting the light units in a Tropix luminometer after mixing of 20 μl of cell extract with 50 μl of luciferase assay reagent II (LAR II; Promega) and 100 μl of Stop&Glo reagent (Promega), followed by vortexing for 5 s. The relative CAT expression was calculated by dividing the CAT expression measured in the CAT ELISA by the Renilla luciferase counts to correct for transfection efficiency.
Preparation of whole-cell lysates.
Whole-cell lysates, for detection of LAT or flag-tagged LAT mutants, were prepared from either 5 × 106 T lymphocytes or 2 × 107 transfected Jurkat cells, when indicated after incubation with BSO. Cells were harvested, washed twice with phosphate-buffered saline (PBS), and lysed in 0.5 or 1 ml, respectively, of TX lysis buffer (25 mM Tris-HCl [pH 7.6], 150 mM NaCl, 5 mM EDTA, 1 mM Na3VO4, 50 mM β-glycerophosphate, 1% Triton X-100) supplemented with protease inhibitors (10 μg of leupeptin [Sigma]/ml, 10 μg of pepstatin A [Sigma]/ml, 0.4 mM phenylmethylsulfonyl fluoride [PMSF; Sigma]) during 30 min on ice. Insoluble debris was spun down in a microcentrifuge for 10 min at 4°C.
For detection of tyrosine-phosphorylated flag-tagged LAT mutants, whole-cell lysates were prepared from 2 × 107 transfected Jurkat cells, unstimulated or stimulated with anti-CD3 for 3 min, when indicated after incubation with BSO. Cells were harvested, washed twice with PBS, and lysed in 1 ml of IPB lysis buffer (10 mM triethanolamine [pH 7.8], 150 mM NaCl, 5 mM EDTA, 10 mM Na3VO4, 10 mM NaF, 1% Nonidet P-40) supplemented with protease inhibitors (10 μg of leupeptin/ml, 10 μg of pepstatin A/ml, 0.4 mM PMSF) for 45 min on ice. Insoluble debris was spun down in a microcentrifuge for 10 min at 4°C.
Immunoprecipitations and detection of proteins by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and enhanced chemiluminescence (ECL) Western blotting were performed as described below.
Isolation of detergent-soluble and -insoluble cell fractions.
Whole-cell lysates were prepared from 5 × 107 transfected Jurkat cells, when indicated after incubation with BSO. Cells were harvested, washed twice with PBS, and lysed in 1 ml of TNEX buffer (25 mM Tris-HCl [pH 7.6], 150 mM NaCl, 5 mM EDTA, 1 mM Na3VO4, 10 mM NaF, 0.5% Triton X-100) supplemented with protease inhibitors (10 μg of leupeptin/ml, 10 μg of pepstatin A/ml, 0.4 mM PMSF) for 1 h on ice. The lysate was mixed with an equal volume of ice-cold TNES80 buffer (25 mM Tris-HCl [pH 7.6], 150 mM NaCl, 5 mM EDTA, 80% [wt/vol] sucrose plus protease inhibitors), transferred to an ultracentrifuge tube, layered with 6 ml of ice-cold TNES30 and 4 ml of ice-cold TNES5, and centrifuged at 125,000 × g for 16 h at 4°C. Then, 1-ml fractions were collected from the top; fractions 2 to 4 were pooled and constitute of the detergent-insoluble proteins, including the glycolipid-enriched membrane fraction; fractions 8 to 11 were pooled and contain the detergent-soluble proteins. The separation of both fractions was checked by detecting ZAP-70, which should only be present in the detergent-soluble fraction.
Immunoprecipitation, native and (non-)reducing denaturing PAGE, Western blotting, and immunodetection.
LAT was immunoprecipitated from the whole-cell lysates by incubation with 4 μg of rabbit anti-LAT polyclonal antibody (PAb; 06-807; Upstate Biotechnology, Lake Placid, N.Y.) for 16 h at 4°C while being rotated and for an additional 2 h after the addition of 25 μl of protein A/G Plus-agarose beads (50% slurry; Santa Cruz Biotechnology, Santa Cruz, Calif.). The flag-tagged LAT mutants were immunoprecipitated with 5 μg of rabbit anti-flag (sc-807; Santa Cruz Biotechnology). ZAP-70 was immunoprecipated with 4 μg of rabbit anti-ZAP-70 (sc-574; Santa Cruz Biotechnology). The immunoprecipitates were spun down in a microcentrifuge for 30 s at 4°C, washed twice with lysis buffer, and then resuspended in 1× sample buffer (nondenaturing: 110 mM Tris-HCl [pH 6.8], 40 mM EDTA, 8% glycerol, 0.01% bromophenol blue; nonreducing: nondenaturing plus 2.3% SDS; reducing: nonreducing plus 585 μM 2-mercaptoethanol). The proteins were separated by native PAGE on a 12% nondenaturing gel, by using electrophoresis buffer without SDS, or by SDS-PAGE on a 10% denaturing gel, and transferred onto a polyvinylidene difluoride membrane (Millipore, Bedford, Mass.). The membrane was blocked in PBS containing 5% skim milk and 0.1% Tween 20 for 1 h. Detection of LAT or the flag-tagged LAT mutants was performed by incubating the membranes with a rabbit PAb against LAT (1:1,000) for 16 h. Detection of tyrosine-phosphorylated LAT was performed by incubating with a mouse MAb against phospho-Tyr (PY99; 1:1,000; sc-7020; Santa Cruz Biotechnology), and ZAP-70 was detected by incubating with a rabbit PAb against ZAP-70 (1:1,000). The membranes were subsequently incubated with the appropriate secondary antibodies: swine anti-rabbit immunoglobulin-horseradish peroxidase (HRP; 1:5,000; Dako, Glostrup, Denmark) or goat anti-mouse immunoglobulin-HRP (1:5,000; Dako) for 4 h and then assayed by ECL (Amersham, Little Chalfont, United Kingdom). Membranes were stripped of bound antibodies by incubating the membranes for 30 min at 50°C in stripping buffer (100 mM 2-mercaptoethanol, 2% SDS, 62.5 mM Tris-HCl [pH 6.7]) to allow for a second round of detection.
Immunofluorescence staining and microscopy.
A total of 5 × 105 transfected Jurkat cells were mounted onto adhesive microscope slides, air dried, and kept frozen until staining. Prior to staining, the cells were fixed in 4% para-formaldehyde in PBS for 15 min at RT. After three washes in PBS containing 5% bovine serum albumin (BSA), cells were permeabilized by using 0.1% Triton X-100 in PBS for 3 min at RT, washed three times (PBS-BSA), and preblocked for 45 min at RT in PBS containing 5% BSA. Cells were then incubated with primary antibodies diluted in PBS-BSA (rabbit anti-LAT PAb at 1:250 [Upstate Biotechnology], rabbit anti-flag PAb at 1:150 [Zymed, San Francisco, Calif.], mouse anti-BLNK MAb at 1:50 [a kind gift of Andrew Chan]) for 45 min at RT, washed three times (PBS-BSA), and incubated as appropriate with secondary antibodies diluted in PBS-BSA (fluorescein isothiocyanate [FITC]-conjugated swine anti-rabbit immunoglobulin at 1:200 [Dako], tetramethylrhodamine isothiocyanate [TRITC]-conjugated rabbit anti-mouse immunoglobulin at 1:200 [Dako]) for 45 min at RT. A negative control was incubated with the secondary antibody only. After three final washes (PBS), cells were embedded in 1 mg of p-phenylenediamine (PPD; Sigma)/ml in 90% glycerol-10% PBS and covered with a coverslip. Cells were viewed by using a Leitz Aristoplan microscope (Leica, Wetzlar, Germany) equipped with a ×100 objective and optics for FITC and TRITC. Images were taken with a Sony 3CCD Color Video Camera, model DXC-9508.
Statistical analysis.
Statistical analyses were performed on the data by using Student’s t test for paired observations. The statistical significance of the data was set at P < 0.05.
RESULTS
LAT undergoes a conformational change as a result of chronic oxidative stress.
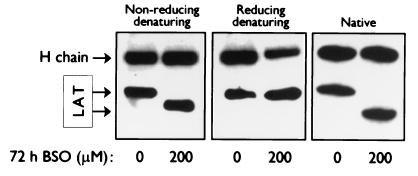
The cellular localization of the adapter protein LAT, a crucial module of the TCR-mediated signaling pathways, is very sensitive to changes in the intracellular levels of the antioxidant GSH. In T lymphocytes, which have been treated with BSO, an inhibitor of γ-glutamyl-cysteine synthetase, an essential enzyme for GSH synthesis, to deplete intracellular GSH, the normally membrane-anchored protein becomes displaced from the plasma membrane, rendering the T lymphocytes hyporesponsive and dysfunctional (24). To determine whether LAT undergoes a conformational change, which could possibly interfere with the positioning of the N-terminal α-helix of LAT in the bilipid layer of the plasma membrane, we immunoprecipitated LAT from whole-cell extracts of peripheral blood T lymphocytes which were cultured in either normal medium or medium containing 200 μM BSO for 72 h and assessed any changes of LAT in charge or conformational properties by native and both reducing and nonreducing denaturing PAGE, followed by Western blotting analysis. A noticeable difference in migratory pattern of LAT isolated from BSO-treated cells was observed by comparison of reducing and nonreducing denaturing conditions, which is consistent with the presence of an intramolecular disulfide bond in oxidated LAT (Fig. 1). Also, a considerable difference in the mobility of LAT from nontreated and BSO-treated cells was revealed on a nondenaturing gel: LAT that had been immunoprecipitated from cells treated with BSO displayed increased mobility compared to LAT in its normal conformation (Fig. 1). These analyses indicate that lowered intracellular GSH levels indeed affect the conformation of LAT significantly.
FIG. 1.
Conformational changes of LAT are induced in T lymphocytes upon depletion of the intracellular GSH levels. T lymphocytes from healthy donors were cultured for 72 h in normal medium (0 μM BSO) or medium containing 200 μM BSO. LAT was immunoprecipitated from whole-cell lysates of an equivalent number of cells and separated by nonreducing denaturing (left panel), reducing denaturing (middle panel), or native (right panel) PAGE. LAT was detected by ECL Western blotting with anti-LAT antibodies and HRP-conjugated swine anti-rabbit antibodies. The detected heavy (H) chain comes from the LAT antibodies used for the immunoprecipitation. The data are representative of three independent experiments.
Several cysteine-to-serine mutants of LAT are insensitive to redox balance alterations and remain membrane anchored.
GSH can affect the conformation of proteins by altering the oxidation status of their sulfhydryl groups. Human LAT contains four cysteine residues, which contain such sulfhydryl groups, at positions C9 and C26, in the transmembrane α-helix, at position C29, intracellular and just proximal of the α-helix, and at position C117, approximately halfway from the cytoplasmic tail (72, 76). To explore how chronic oxidative stress through the depletion of the intracellular GSH levels can affect the conformation of LAT, we constructed a series of LAT mutants in which the cysteine residues are replaced by serine residues and used them in transient-transfection experiments. The mutants were tagged with a flag tag to distinguish them from endogenous LAT. After transfection of Jurkat cells with the different expression plasmids, the cells were cultured for 72 h either in normal medium or medium containing 200 μM BSO, and the subcellular localization of both endogenous LAT and the flag-tagged LAT mutants was analyzed by immunofluorescence staining on cytospins. The expression levels of all flag-tagged LAT mutants after transfection in either untreated- or BSO-treated Jurkat cells was verified by immunostaining and FACS analysis and appeared to be similar and stable (data not shown).
In Jurkat cells cultured in normal medium, LAT was always clearly detected in the plasma membrane, while endogenous LAT in Jurkat cells treated with BSO was always displaced from the membrane and localized in the cytoplasm (data not shown). The same pattern of cellular localization depending on the status of the intracellular GSH levels was observed for flag-tagged wild-type LAT in transfected Jurkat cells and also for the LAT mutants in which either cysteine residue C9 [LAT(C9S)], C26 [LAT(C26S)], or C29 [LAT(C29S)] was replaced by a serine residue (Fig. 2).
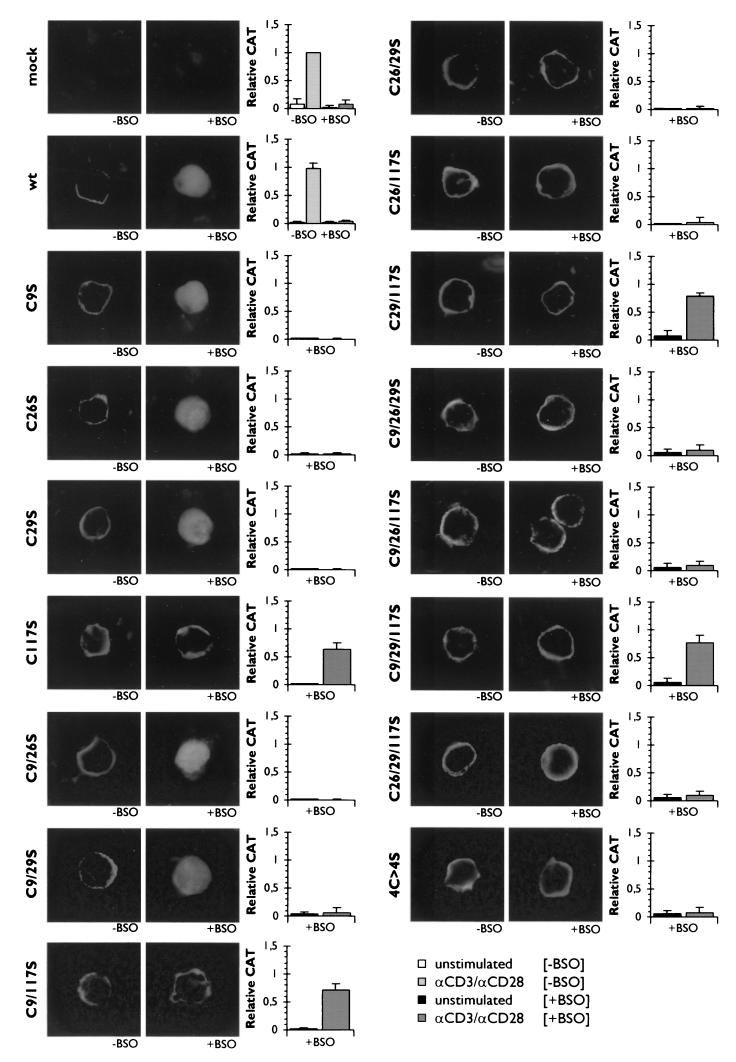
FIG. 2.
The cysteine-to-serine mutations at cysteine residues C117 and C26/C29 confer redox insensitivity to LAT, which thus remains localized in the plasma membrane upon treatment with BSO, and allows for the partial restoration of the signaling pathways leading to IL-2 promoter-regulated CAT expression in transfected Jurkat T cells. Jurkat T cells were cotransfected with pCAT3e-IL-2(−319/+47) and either an empty expression plasmid (mock), an expression plasmid encoding flag-tagged wild-type LAT (wt), or an expressing plasmid for a series of flag-tagged LAT mutants containing cysteine-to-serine substitutions at C9, C26, C29, and/or C117. Transfected cells were cultured for 68 h in normal medium (−BSO) or medium containing 200 μM BSO (+BSO) and afterward either fixed, permeabilized, labeled with specific antibodies against the flag-tag, and then stained with FITC-conjugated swine anti-rabbit antibodies (left panels) or left unstimulated or stimulated with anti-CD3 plus anti-CD28 (αCD3/αCD28) for another 24 h (right panels). The CAT expression was measured and corrected for as described in Materials and Methods. The relative CAT expression induced by endogenous LAT as determined in anti-CD3/anti-CD28-stimulated mock-transfected Jurkat T cells which were cultured in BSO-free medium was set at 1. The mean ± the SD found for the relative CAT expression in six to eight independent experiments are shown.
In contrast, the LAT mutant in which the cysteine residue at C117 is replaced by a serine residue [LAT(C117S)] was targeted to the plasma membrane both under normal conditions and under conditions of chronic oxidative stress (Fig. 2). In addition, all double and triple mutants containing the C117-to-S117 mutation [LAT(C9/117S), LAT(C26/117S), LAT(C29/117S), LAT(C9/26/117S), LAT(C9/29/117S), and LAT(C26/29/117S)], as well as the mutant in which all four cysteine residues have been mutated to serines [LAT(4C>4S)], were still detected in the plasma membrane after GSH depletion (Fig. 2). Moreover, the expression of the double mutant LAT(C26/29S) in transfected Jurkat cells also showed membrane localization independent of the intracellular levels of GSH. In line with this expression pattern, the triple mutant LAT(C9/26/29S) was also detected in the plasma membrane after expression in transfected Jurkat cells, cultured in either normal medium or medium supplemented with BSO, indicating that they are insensitive to chronic oxidative stress (Fig. 2).
In contrast also, the double mutants LAT(C9/26S) and LAT(C9/29S) were localized in the plasma membrane in untreated Jurkat cells, whereas both of these LAT mutants were only detected in the cytoplasm after depletion of the intracellular GSH levels (Fig. 2).
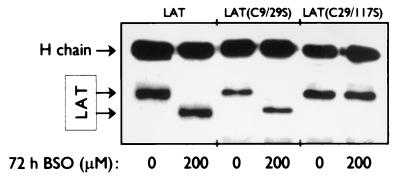
Native PAGE analysis of the redox-sensitive mutant LAT(C9/29S) and redox-insensitive mutant LAT(C29/117S), immunoprecipitated from whole-cell extracts of BSO- or untreated transfected Jurkat cells, showed that the conformational change as observed for endogenous LAT is also apparent for the redox-sensitive LAT mutant but absent in the redox-insensitive mutant (Fig. 3).
FIG. 3.
Conformational changes of wild-type LAT and the redox-sensitive mutant LAT(C9/29S) but not the redox-insensitive mutant LAT(C29/117S) upon depletion of the intracellular GSH levels. T lymphocytes from healthy donors and Jurkat T cells, transfected with either flag-tagged LAT(C9/29S) or flag-tagged LAT(C29/117S) were cultured for 72 h in normal medium (0 μM BSO) or medium containing 200 μM BSO. LAT was immunoprecipitated from whole-cell lysates of T lymphocytes with anti-LAT antibodies, while LAT mutants were immunoprecipitated from whole-cell lysates of transfected Jurkat T cells with anti-flag antibodies. The immunoprecipitated proteins were separated by native PAGE. LAT and LAT mutants were detected by ECL Western blotting with anti-LAT antibodies and HRP-conjugated swine anti-rabbit antibodies. The detected heavy (H) chain comes from the antibodies used for the immunoprecipitation. The data are representative of three independent experiments.
These results signify that the modification of cysteine residues in LAT in cells under conditions of chronic oxidative stress contributes to the displacement of LAT from the cellular membrane by conformational changes.
T-cell function is partially restored in transfected cells expressing redox-insensitive LAT mutants which localize to the GPI-enriched membrane microdomains.
Since the membrane localization of LAT is crucial for its function, the hyporesponsiveness of T lymphocytes due to chronic oxidative stress is believed to be primarily the result of the membrane displacement of LAT. We have previously shown that the proximal TCR-mediated signaling cascade leading to LAT phosphorylation, i.e., the successive activation of the Src tyrosine kinase p56lck, the recruitment of ZAP-70 to the phosphorylated immunoreceptor tyrosine-based activation motifs of the ζ chain of the TCR/CD3 complex, and the activation through phosphorylation of ZAP-70, are intact in cells with lowered intracellular levels of GSH (24). The membrane localization of the redox-insensitive LAT mutants allowed us to examine the effect of chronic oxidative stress on the TCR-mediated signaling pathways downstream from LAT phosphorylation. First, cotransfection studies of Jurkat cells were performed with the expression plasmids encoding the whole series of LAT mutants in combination with an IL-2 promoter-driven CAT reporter construct to establish which LAT mutants are capable of TCR signaling transmission in the presence of BSO, by measuring their ability to induce IL-2 gene transcription after stimulation.
To quantitate the CAT reporter gene expression, the IL-2 promoter-driven CAT expression induced by endogenous LAT in Jurkat cells, which were transfected with an empty expression plasmid (mock) and stimulation with anti-CD3 plus anti-CD28 (anti-CD3/anti-CD28) for 24 h after culture for 72 h in normal medium, was set at 1 (100%; n = 13) (Fig. 2). As anticipated, the expression of CAT induced by endogenous LAT in transfected Jurkat cells was completely blocked in the presence of BSO due to the displacement of LAT from the plasma membrane (Fig. 2). The level of CAT expression in Jurkat cells transfected with an expression plasmid encoding wild-type LAT after culture in normal medium and stimulation with anti-CD3/anti-CD28 was similar to that induced by endogenous LAT: 98% ± 11% (mean ± the standard deviation [SD]; n = 3; Fig. 2). Again, after the addition of BSO to the medium to deplete the intracellular GSH levels, the CAT expression after stimulation with anti-CD3/anti-CD28 was completely abolished (Fig. 2). The anti-CD3/anti-CD28 stimulation of Jurkat cells which were transfected with the expression plasmids encoding the cysteine-to-serine LAT mutants and cultured in normal medium in the absence of BSO always resulted in a level of CAT expression similar to that observed with the empty expression plasmid, ensuring that none of the mutated LAT proteins nor wild-type LAT functions as a dominant-negative inhibitor of endogenous LAT (data not shown).
Based on the results obtained for the measured CAT expression by the transfected Jurkat stimulated with anti-CD3/anti-CD28, cultured for 72 h in medium containing BSO, the LAT mutants could be divided in three groups. The first group consisted of the LAT mutants that were expressed in the cytoplasm of the transfected Jurkat cells, after treatment of the cells with BSO to deplete the intracellular GSH levels [LAT(C9S), LAT(C26S), LAT(C29S), LAT(C9/26S), and LAT(C9/29S)]. In this group, no expression of CAT could be detected after stimulation of the cells with anti-CD3/anti-CD28 (Fig. 2).
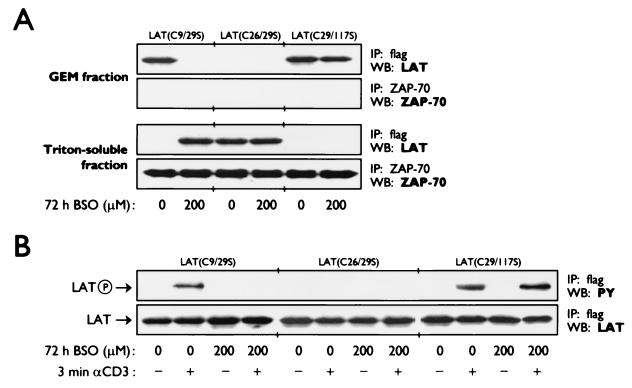
A second group was made up of the LAT mutants that, although membrane anchored after treatment of transfected Jurkat cells with BSO, were unable to relay the TCR-induced signal to downstream signaling cascades resulting in the absence of CAT expression after anti-CD3/anti-CD28 stimulation [LAT(C26/29S), LAT(C26/117S), LAT(C9/26/29S), LAT(C9/26/117S), LAT(C26/29/117S), and LAT(4C>4S)] (Fig. 2). These mutants have in common the replacement of cysteine residue C26 with a serine and hence are lacking the palmitoylation group at C26, which is necessary for the positioning of LAT in the GPI-enriched microdomains in the plasma membrane. Further analysis of the subcellular localization by fractionation of lysates into detergent-insoluble and -soluble cell fractions showed that LAT(C26/29S), as a representative of this group, was present within the plasma membrane but outside the lipid rafts (Fig. 4A). The localization of LAT in these microdomains has previously been shown to be necessary for LAT to participate in the TCR-mediated signaling pathways (46, 78, 80). In agreement with this, we observed that the tyrosine phosphorylation of LAT(C26/29S) was also deficient, both in the absence and in the presence of BSO (Fig. 4B), thus confirming that the expression of LAT mutants containing the C26-to-S26 mutation renders the cells unresponsive to stimulation due to placement of LAT outside the GPI-enriched microdomains.
FIG. 4.
Only the redox-insensitive mutant LAT(C29/117S), and not the redox-sensitive mutant LAT(C9/29S) or the redox-insensitive mutant LAT(C26/29S), is present within the glycolipid-enriched microdomains (GEM) of the plasma membrane upon depletion of the intracellular GSH levels and consequently becomes tyrosine-phosphorylated upon T-cell activation. Jurkat T cells, transfected with either the flag-tagged LAT mutant LAT(C9/29S), LAT(C26/29S), or LAT(C29/117S) were cultured for 72 h in normal medium (0 μM BSO) or medium containing 200 μM BSO. (A) Whole-cell lysates were prepared and subjected to sucrose gradient ultracentrifugation to separate the detergent (Triton X-100)-insoluble and -soluble proteins. The detergent-insoluble fraction contains the proteins present within the glycolipid-enriched microdomains of the plasma membrane. LAT mutants were immunoprecipated from both fractions with anti-flag antibodies, separated by SDS-PAGE, and detected by ECL Western blotting with anti-LAT antibodies and HRP-conjugated swine anti-rabbit antibodies. The presence of ZAP-70 (which should only be detected in the Triton-soluble fraction) in both fractions was determined to ascertain complete fractionation of the whole-cell lysates. (B) After stimulation with anti-CD3 (αCD3) for 3 min, whole-cell lysates were prepared, from which the LAT mutants were immunoprecipitated with anti-flag antibodies. After SDS-PAGE, the phosphorylation status of the LAT mutants was determined by ECL Western blotting with anti-phosphotyrosine (PY) antibodies and HRP-conjugated goat anti-mouse antibodies. The equal expression of the LAT mutants was verified by ECL Western blotting with anti-LAT antibodies and HRP-conjugated swine anti-rabbit antibodies.
The third group contained four LAT mutants [LAT(C117S), LAT(C9/117S), LAT(C29/117S), and LAT(C9/29/117S)] that were not displaced from the cellular membrane after expression in transfected Jurkat cells after treatment with BSO and were all capable of signal transduction leading to IL-2 promoter-driven CAT expression after anti-CD3/anti-CD28 stimulation. The CAT expression after BSO treatment in stimulated Jurkat cells transfected with the expression plasmid for LAT(C117S) was only 63% ± 11% (mean ± SD; n = 4; P = 0.006) compared with the level of CAT expression measured in the control group (Fig. 2). The expression of the redox-insensitive LAT mutant LAT(C9/117S) in BSO-treated transfected Jurkat cells resulted in 71% ± 11% (mean ± SD; n = 4; P = 0.012) of the IL-2 promoter-driven CAT expression after anti-CD3/anti-CD28 stimulation (Fig. 2). Similarly, the CAT expression in stimulated Jurkat cells transfected with the expression plasmids encoding either LAT(C29/117S) or LAT(C9/29/117S) reached, respectively, 68% ± 7% (mean ± SD; n = 7; P < 0.005) and 76% ± 7% (mean ± SD; n = 4; P = 0.04) after BSO treatment (Fig. 2). As expected, we found that LAT(C29/117S), as a representative of this group, was detected in the GPI-enriched microdomains in the plasma membrane and became tyrosine phosporylated upon anti-CD3 stimulation, independent of the intracellular GSH levels (Fig. 4).
TCR-mediated signaling pathways are differentially sensitive to chronic oxidative stress, while the CD28-induced signaling pathways are almost completely inhibited.
Downstream from LAT, the TCR-induced signal diverges into several parallel signaling pathways which have different but also overlapping targets in the IL-2 promoter, such as the AP-1, NFAT, and NF-κB binding elements (31). To examine the effect of chronic oxidative stress on these different TCR-mediated signaling pathways and also the CD28-mediated pathway, we used different CAT reporter constructs driven by several copies of either the AP-1, NFAT, or NF-κB elements from the IL-2 promoter in cotransfection studies. For each of the different reporter constructs, the CAT expression induced by endogenous LAT was determined by stimulation of Jurkat cells, transfected with an empty expression plasmid (mock), with anti-CD3 for 24 h after culture for 72 h in normal medium, and set at 1 (100%; n = 5). Again, the treatment of mock-transfected Jurkat cells with BSO completely inhibited either the AP-1-, NFAT-, or NF-κB-mediated CAT expression (data not shown). Two LAT mutants that were representative for the first and second group, respectively, were used for the cotransfection studies: (i) LAT(C9/29S) from the redox-sensitive group of LAT mutants that are displaced from the cellular membrane upon BSO treatment and (ii) LAT(C29/117S), used to represent the second group of LAT mutants that remain membrane localized after BSO treatment and allow for IL-2 promoter-driven CAT expression. A representative of the third group of LAT mutants, which remain membrane bound after BSO treatment but are unable to transduce the TCR signal to downstream effectors since they are not localized within the lipid rafts due to the C26S mutation, was omitted since no CAT expression could be detected, similar to the group of redox-sensitive mutants.
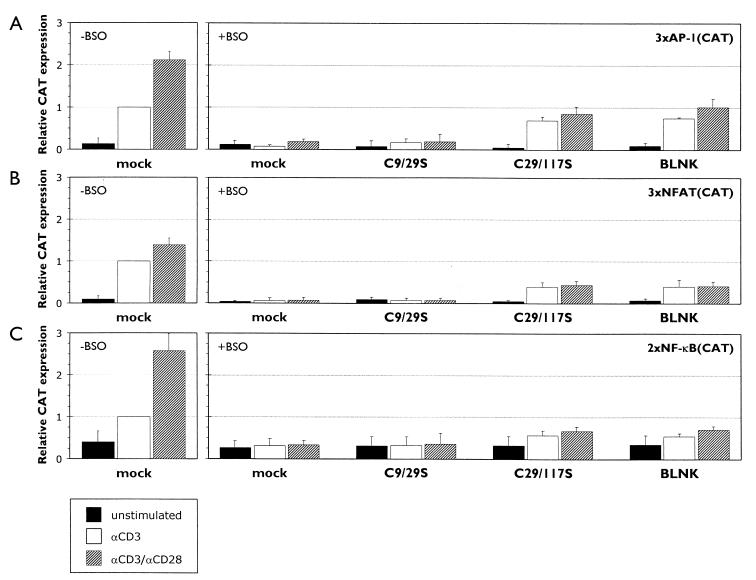
The AP-1 element of the IL-2 promoter is bound by a homo- or heterodimeric complex existing of c-jun and/or c-fos, whose expression and transactivation activity is regulated through different MAP kinases (9, 36). Both p38/Mpk2 and ERK are activated as a result of different TCR-initiated signaling cascades, while JNK (Jun N-terminal kinase) is activated as a result of the combined activation of both the TCR- and the CD28-mediated signaling pathways (16, 27, 30, 59, 65). The AP-1-driven CAT expression measured in Jurkat cells transfected with the redox-insensitive LAT mutant LAT(C29/117S), cultured in BSO-containing medium, and then stimulated with anti-CD3 was only 70% ± 13% (mean ± SD; n = 5; P = 0.005) of the CAT expression measured in the control group (Fig. 5A). This result indicates that either or both of the TCR-mediated signaling pathways leading to p38/Mpk2 and ERK activation are affected by chronic oxidative stress. Costimulation of mock-transfected Jurkat cells, which have been cultured in normal medium, with anti-CD3 and anti-CD28 enhanced the AP-1-driven CAT expression more than 2-fold compared to the anti-CD3-induced CAT expression: (2.1 ± 0.19)-fold (mean ± SD; n = 5; P < 0.005) (Fig. 5A). In BSO-treated Jurkat cells transfected with LAT(C29/117S), the AP-1 driven CAT expression increased only with a factor 1.2 ± 0.15 (mean ± SD) after costimulation with anti-CD3/anti-CD28 to 85% ± 17% (mean ± SD; n = 5; P = 0.07) (Fig. 5A), suggesting that the CD28-mediated signaling pathways are significantly inhibited by changes induced by chronic oxidative stress.
FIG. 5.
The TCR-mediated signaling pathways leading to activation of AP-1, NFAT, and NF-κB are partially and differentially functional in transfected Jurkat T cells upon depletion of the intracellular GSH levels, while the CD28-mediated signaling pathways are almost completely inhibited. Jurkat T cells were cotransfected with pCAT-3xAP-1/IL-2 (A), pCAT3e-3xNFAT/IL-2 (B), or pCAT-2xNF-κB (C) in combination with either an empty expression plasmid (mock), an expression plasmid encoding the redox-sensitive flag-tagged LAT mutant C9/29S, an expressing plasmid encoding the redox-insensitive flag-tagged LAT mutant C29/117S, or an expression plasmid encoding myc-tagged BLNK. Transfected cells were cultured for 68 h in normal medium (−BSO) or medium containing 200 μM BSO (+BSO) and afterward left unstimulated or stimulated with anti-CD3 (αCD3) or anti-CD3 plus anti-CD28 (αCD3/αCD28) for another 24 h. The CAT expression was measured and corrected for as described in Materials and Methods. The relative CAT expression induced by endogenous LAT was determined separately for all three CAT constructs in anti-CD3-stimulated mock-transfected Jurkat T cells which were cultured in BSO-free medium and set at 1. The mean ± the SD found for the relative CAT expression in four to five independent experiments are shown.
The NFAT elements in the IL-2 promoter are recognized by a multiprotein complex composed of AP-1 with a T-cell-specific subunit NFAT1, which is dephosphorylated and translocated to the nucleus after activation of calcineurin through a TCR-mediated calcium-dependent signaling pathway (12, 50, 62, 63). The expression of the redox-insensitive mutant LAT(C29/117S) in transfected Jurkat cells which have been treated with BSO and stimulated with anti-CD3 resulted in an NFAT-driven CAT expression that was significantly less than the CAT expression in the control group: 39% ± 5% (mean ± SD; n = 5; P < 0.005) (Fig. 5B), suggesting that, in addition to the TCR-mediated signaling pathways resulting in AP-1 activity, the calcium/calcineurin signaling pathway leading to NFAT1 activity is severely affected by lowered intracellular GSH levels. While we detected a consistent, significant (1.4 ± 0.16)-fold (mean ± SD; n = 5; P = 0.005) upregulation of the NFAT-driven CAT expression in the control group after costimulation with anti-CD3 and anti-CD28 (Fig. 5B), costimulation with anti-CD3/anti-CD28 of BSO-treated Jurkat cells that were transfected with the expression plasmid for LAT(C29/117S) did not enhance the CAT expression by these cells due to stimulation with anti-CD3 alone: 44% ± 10% (mean ± SD; n = 5; P = 0.15) (Fig. 5B), again implying that the CD28-mediated signaling pathways seem to be very sensitive to chronic oxidative stress.
The activation of NF-κB requires the phosphorylation and subsequent ubiquitination and degradation of its inhibitor IκB, which sequesters the transcription factor inactive in the cytoplasm. A distinct subset of MEK kinases (MAPK/ERK kinase kinases, MEKKs) is involved in the activation of the IκB kinases (IKKs), which are responsible for the phosphorylation of IκB: while the TCR-mediated signaling pathways lead to activation of Raf-1, a member of the MEKK family, the CD28-mediated signaling pathways lead to strong NF-κB activity due to the activation of two other MEKK family members, NIK (NF-κB-inducing kinase) and MEKK1 (3, 34, 35, 37, 47, 56, 58). In unstimulated transfected Jurkat cells, independent of the status of the intracellular GSH levels, a moderate amount of NF-κB-dependent CAT protein was detected due to the basal level of NF-κB activity in resting cells, which measured ca. 35% ± 14% (mean ± SD; n = 4) of the CAT expression by mock-transfected anti-CD3-stimulated Jurkat cells in the control group (Fig. 5C). The NF-κB-driven CAT expression measured in anti-CD3-stimulated Jurkat cells transfected with the redox-insensitive LAT mutant LAT(C29/117S) after culture in BSO-containing medium was only 56% ± 12% (mean ± SD; n = 4; P = 0.005) (Fig. 5C), suggesting that the TCR-mediated signaling pathway resulting in the activation of Raf-1 is sensitive to chronic oxidative stress. The CAT expression induced in mock-transfected Jurkat cells, which have been cultured in normal medium, by costimulation with anti-CD3 and anti-CD28 was increased by a factor of 2.6 ± 0.5 (mean ± SD; n = 4; P = 0.008) compared to the CAT expression measured after stimulation with anti-CD3 alone (Fig. 5C). However, the treatment of transfected Jurkat cells expressing the LAT(C29/117S) mutant with BSO largely inhibited this upregulation of the NF-κB-dependent CAT expression due to costimulation, since these cells produced only slightly larger amounts of CAT protein after stimulation with both anti-CD3 and anti-CD28 (67% ± 10% [mean ± SD; n = 4; P = 0.26]) compared to stimulation with anti-CD3 (56% ± 12% [mean ± SD; n = 4]) (Fig. 5C), confirming the previous observations that the anti-CD28-mediated signaling pathways are almost completely blocked under conditions of chronic oxidative stress.
The B-cell adapter protein BLNK can substitute for redox-insensitive LAT mutants and partially restore T-cell function.
The central adapter protein in the B cell receptor (BCR)-mediated signaling pathways is BLNK (B-cell linker protein), also known as SLP-65 (SH2 domain-containing leukocyte protein of 65 kDa) or BASH (B-cell adapter containing SH2 domain), an adapter protein which has taken on both functions that LAT and SLP-76 fulfill in the TCR-mediated signaling pathways. Unlike LAT, BLNK is not an integral membrane protein and is only recruited to the plasma membrane upon activation of the BCR complex (17, 29, 30, 41, 73). To investigate whether BLNK could substitute for LAT in the TCR-mediated signaling pathways under conditions of chronic oxidative stress and restore T-cell activation, we again performed cotransfection experiments with an expression plasmid encoding wild-type BLNK in combination with the above-mentioned different CAT reporter constructs. In all experiments, BLNK behaved similarly to the redox-insensitive LAT mutant LAT(C29/117S), partially restoring the signaling pathways modulating and regulating the transcription of the IL-2 gene in transfected Jurkat cells which have been cultured in BSO-containing medium (Fig. 5).
DISCUSSION
The integral membrane adapter protein LAT plays an essential role both in T-cell activation and T-cell development (76, 77, 78, 79, 80). The formation of multimolecular signaling complexes at the cellular membrane is necessary to initiate several parallel and cross-talking signaling cascades and provides T lymphocytes with a mechanism to amplify the antigen-induced signal, ultimately leading to T-cell activation (15, 32, 46, 79, 80). Due to its central role in the TCR-mediated signaling pathways, the disablement of LAT debilitates the functioning of T lymphocytes, rendering them hyporesponsive upon TCR engagement. Chronic oxidative stress and the accompanying depletion of the intracellular GSH levels severely affects the activation of T lymphocytes because it causes the membrane displacement of LAT, thus inhibiting the phosphorylation of LAT, the subsequent association with other signaling proteins, and the downstream signaling events (22). The present study shows that chronic oxidative stress affects the conformation of LAT, possibly inducing the formation of disulfide bonds that interfere with the integration of the α-helical structure of LAT into the plasma membrane. Furthermore, we show that other signaling proteins working downstream from LAT are affected by chronic oxidative stress.
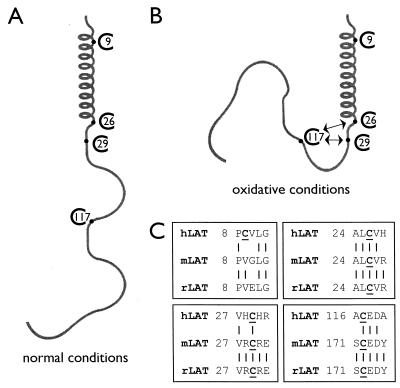
Analysis of the effect of chronic oxidative stress by native PAGE shows that the conformation of LAT changes significantly in T lymphocytes with depleted levels of GSH. The mutation of either cysteine residue C117 or the combination of C26 with C29 to serine residues is sufficient to render LAT insensitive to chronic oxidative stress, since the mutant LAT proteins remain membrane anchored in GSH-depleted transfected Jurkat T cells, as shown by intracellular immunofluorescence staining. Analysis under reducing and nonreducing denaturing conditions shows that oxidated LAT contains one or more disulfide bonds. We hypothesize that, in oxidative conditions, the sulfhydryl group of C117 forms a disulfide bond with the sulfhydryl group on either C26 or C29. It remains to be determined whether this is a direct intramolecular disulfide bond or whether a second small sulfhydryl-containing molecule is involved in the formation of an intermolecular disulfide bond. Cysteine residue C26 is part of the α-helix, and C29 is present just proximal of the α-helix that is inserted into the plasma membrane under normal conditions, and hence we speculate that any interactions, either with the cytoplasmic tail of LAT through C117 or with an unknown second molecule, will result in sterical hindrance, preventing the α-helix of LAT from becoming integrated into the plasma membrane under these conditions (Fig. 6). The cysteine residue at position C9, which is present within the transmembrane α-helix, does not seem to play any role in the disturbed conformation of LAT due to chronic oxidative stress. This cysteine residue C9 is only present in human LAT and is not conserved in either mouse or rat LAT. However, the cysteine residues C26 and C29 are completely conserved between species, while cysteine residue C117 is present in the cytoplasmic tail of mouse and rat LAT at a different position but in exactly the same amino acid sequence (Fig. 6C) (72, 76). It will be interesting to see whether mouse and rat LATs are more or less sensitive to chronic oxidative stress due to the different location of this cysteine residue within the cytoplasmic tail.
FIG. 6.
Hypothetical model for the membrane displacement of LAT under conditions of chronic oxidative stress. (A) Under normal conditions, the N-terminal α-helix of LAT is inserted into the lipid bilayer of the cellular membrane. (B) Under oxidative conditions, the sulfhydryl group at cysteine residue C117 in the cytoplasmic tail of LAT forms an intramolecular (or an intermolecular) disulfide bond with the sulfhydryl group of either cysteine C26 or C29, which is present at the end of the α-helix or just proximal of the α-helix, respectively. The induced conformational change causes sterical hindrance, thus preventing the α-helix from becoming integrated into the plasma membrane and localizing LAT to the cytoplasm, consequently causing the hyporesponsiveness of T lymphocytes upon antigenic stimulation. (C) The cysteine residues C26 and C29 of LAT are conserved between human, mouse, and rat LATs, while cysteine C9 is only present in human LAT. The cysteine residue in the cytoplasmic tail is in human LAT located at position 117, while in both mouse and rat this cysteine residue in a similar amino acid sequence is present at position 172.
All LAT mutants containing either the C117S or the double C26/29S mutation display redox insensitivity and are targeted to the cellular membrane under conditions of chronic oxidative stress. Cotransfection experiments demonstrate that some of these mutants can also restore the signaling pathways, resulting in the transcriptional activation of the IL-2 gene promoter, upon costimulation of the transfected Jurkat T cells through both the TCR/CD3 complex and the CD28 receptor. However, a consistent and significant decrease in the level of transcriptional activation is observed, indicating that either the TCR-mediated signaling pathways downstream of LAT and/or the CD28-mediated signaling pathways are also sensitive to the cellular changes induced by chronic oxidative stress.
It has been previously reported that the presence of LAT in the GPI-enriched microdomains in the plasma membrane is a prerequisite for its participation in TCR signaling and is dependent on the palmitoylation of cysteine residue C26 (77, 79). Our results confirm that membrane localization alone is not sufficient for functional TCR signaling but that the integrity of C26 is indispensable, since all LAT mutants with the C26S mutation and thus lacking the palmitoylation modification of the C26 residue, despite being localized in the membrane, are impaired in their ability to relay the TCR-induced signal from the membrane to the nucleus to initiate cytokine expression. The palmitoylation of C26 is not inhibited under conditions of chronic oxidative stress since all of the redox-insensitive LAT mutants containing an intact cysteine residue on position 26 can induce cytokine expression. In addition, LAT(C29/117S) can be detected within the lipid rafts within the plasma membrane in GSH-depleted cells.
The BCR-mediated signaling pathways show many similarities with those mediated through the TCR/CD3 complex; however, B lymphocytes do not express LAT. The central role in the BCR-mediated signaling pathways is played by the adapter protein BLNK. BLNK shows homology with SLP-76 and has integrated the functions of both SLP-76 and LAT in T lymphocytes. In contrast to LAT, BLNK is not a transmembrane protein but rather is recruited to the plasma membrane upon BCR engagement after phosphorylation and activation of upstream signaling proteins. Remarkably, BLNK can replace the redox-insensitive LAT mutants in the TCR-mediated signaling pathways resulting in activation of the transcriptional regulation of the IL-2 gene under conditions of chronic oxidative stress. BLNK contains only three cysteine residues, of which only two are conserved in mouse BLNK, and the oxidation of the sulfhydryl groups obviously does not interfere with the association of BLNK with other proteins (28). These results mark how crucial the membrane localization of LAT is for TCR signaling since LAT does not contain any domains through which it can interact with other proteins prior to becoming tyrosine phosphorylated, whereas the BCR-mediated signaling cascades might be far less sensitive to changes induced by an environment of chronic oxidative stress.
The TCR-induced signal diverges from LAT to three major pathways which are all involved in the regulation of cytokine gene expression. The current data indicate that the TCR-mediated signaling pathway leading to NF-κB activation is very sensitive to cellular changes due to exposure to chronic oxidative stress, since the anti-CD3-induced NF-κB-dependent CAT expression is almost completely abrogated in Jurkat T cells transfected with a redox-insensitive LAT mutant after treatment with BSO. Two signaling proteins in the TCR-mediated route modulating the activity of NF-κB likely to be affected by oxidative modifications are the serine-threonine kinase Raf-1 and the IKK complex. IKK activity has recently been shown to become inhibited by oxidation of cysteine residues (40). More proximal in this route, Raf-1 has been implicated in the activation of NF-κB through the activation of the serine-threonine kinase MEKK1 (3, 34, 45). A prerequisite for the activation of the kinase activity of Raf-1 is its recruitment to the plasma membrane through association with GTP-bound Ras via two distinct domains, the Ras-binding site and the zinc-containing cysteine-rich domain (CRD). The interactions between the Raf-1 CRD and Ras are essential for the Ras-mediated activation of Raf-1. Since an absolute requirement for correct Raf-1 CRD function has been established (51, 60), any alterations in the Raf-1 CRD structure due to oxidation of the sulfhydryl groups as a result of chronic oxidative stress seem likely to impair the association of Raf-1 with Ras and thus block any signaling pathways downstream from Raf-1, not only affecting the activation of NF-κB but also that of AP-1 through ERK. This conclusion is strengthened by another observation in synovial T lymphocytes from RA patients: Ras is constitutively active in these cells, despite the membrane displacement of LAT; however, the signaling pathway leading from Ras to ERK activation is defective (unpublished data). These results again suggest that the activation of Raf-1 might be severely affected as a result of oxidative changes due to chronic oxidative stress. Also consistent with this conclusion is our observation that the AP-1-dependent CAT expression upon anti-CD3 stimulation is significantly reduced (by ca. 30%) in BSO-treated Jurkat T cells transfected with a redox-insensitive LAT mutant. The remaining CAT expression is induced through the TCR-mediated signaling pathway leading to Vav/Rac1-dependent activation of the MAP kinase p38/Mpk2 and subsequently AP-1 activity (8, 14, 49). In accordance with this observation, the signaling cascade resulting in p38/Mpk2 activation has previously been shown not to become inhibited through BSO treatment in pulmonary vascular endothelial cells (25). The NFAT-dependent CAT expression is even further inhibited (by ca. 60%) than the AP-1-dependent CAT expression upon anti-CD3 stimulation of BSO-treated Jurkat T cells transfected with a redox-insensitive LAT mutant. Since the activity of NFAT is dependent on both the activation of AP-1 and the dephosphorylation and translocation of NFAT1 by the serine-threonine phosphatase calcineurin (12, 62), these data demonstrate that, in addition to the partially blocked activation of AP-1, the activation of calcineurin is severely impaired in an environment of chronic oxidative stress. Like any other phosphatase, calcineurin carries a critical cysteine residue in its catalytic domain. Oxidation of this cysteine residue will render calcineurin catalytically inactive, thus abrogating the dephosphorylation of NFAT1 and consequently the NFAT-dependent transcriptional regulation. Similarly, a suppression of the calcineurin activity has recently been described in natural killer cells which contain decreased intracellular levels of GSH, confirming our conclusions (19). Not only chronic oxidative stress but also acute oxidative stress has been shown to inactivate calcineurin through the oxidative formation of a disulfide bridge between two cysteine residues in the catalytic subunit of calcineurin (5, 64). Although GSH is the major component of the cellular defense mechanism against radical oxygen species and chronic oxidative stress, cells contain other antioxidants which can protect proteins against oxidation. The residual activity of calcineurin detected in the BSO-treated transfected Jurkat T cells might be explained by the presence of these other antioxidants, allowing part of the redox-sensitive proteins such as Raf-1, IKK, and calcineurin to remain active.
The activation of both NF-κB and AP-1 is strongly enhanced through the CD28-mediated signaling pathways under normal circumstances. Our results show that the CD28-mediated signaling pathways leading to NF-κB and AP-1 activation are severely perturbed under conditions of chronic oxidative stress. Further research will have to reveal the identity of the signaling proteins which transduce the CD28 signal that are severely inhibited in their function by oxidative modifications, although it is conceivable that these proteins play a role in the proximal CD28 signaling pathways since both the routes to AP-1 and NF-κB activation are affected.
Since chronic oxidative stress is involved in the pathogenesis of various degenerative diseases, including RA, cancer, and Alzheimer’s disease, it is of considerable importance to understand how it disables the functioning of cells so that therapies supplementing the antioxidant defense system to antagonize oxidative damage might be developed. In summary, our present findings indicate that chronic oxidative stress results in the displacement of LAT from the plasma membrane in T lymphocytes due to oxidative modicifications that induce a conformational change, possibly interfering with the integration of the α-helix of LAT in the membrane. Furthermore, the decreased intracellular GSH levels affect the TCR-mediated Raf-1-dependent signaling pathways leading to AP-1 and NF-κB activation, the TCR-mediated calcineurin-dependent signaling pathway leading to NFAT activation, and the CD28-mediated signaling pathways involved in the transcriptional regulation of cytokine gene expression.
Acknowledgments
We thank A. C. Chan for his generous gift of pApuro/myc-BLNK(wt) and anti-BLNK MAb and L. E. Samelson for providing the expression plasmid pcDNA3/LAT(wt)-flag. We are grateful to the Department of Cell Biology and Genetics at Erasmus University, Rotterdam, The Netherlands, for the use of their immunofluorescence microscope and video equipment.
REFERENCES
- 1.Abe, J., M. Okuda, Q. Huang, M. Yoshizumi, and B. C. Berk. 2000. Reactive oxygen species activate p90 ribosomal S6 kinase via Fyn and Ras. J. Biol. Chem. 275:1739–1748. [DOI] [PubMed] [Google Scholar]
- 2.Asada, H., N. Ishii, Y. Sasaki, K. Endo, H. Kasai, N. Tanaka, T. Takeshita, S. Tsuchiya, T. Konno, and K. Sugamura. 1999. Grf40, a novel Grb2 family member, is involved in T-cell signaling through interaction with SLP-76 and LAT. J. Exp. Med. 189:1383–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumann, B., C. K. Weber, J. Troppmair, S. Whiteside, A. Israel, U. R. Rapp, and T. Wirth. 2000. Raf induces NF-κB by membrane shuttle kinase MEKK1, a signaling pathway critical for transformation. Proc. Natl. Acad. Sci. USA 97:4615–4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beiqing, L., M. Chen, and R. L. Whisler. 1996. Sublethal levels of oxidative stress stimulate transcriptional activation of c-jun and suppress IL-2 promoter activation in Jurkat T cells. J. Immunol. 157:160–169. [PubMed] [Google Scholar]
- 5.Bogumil, R., D. Namgaladze, D. Schaarschmidt, T. Schmachtel, S. Hellstern, R. Mutzel, and V. Ullrich. 2000. Inactivation of calcineurin by hydrogen peroxide and phenylarsine oxide. Evidence for a dithiol-disulfide equilibrium and implications for redox regulation. Eur. J. Biochem. 267:1407–1415. [DOI] [PubMed] [Google Scholar]
- 6.Boriack-Sjodin, P. A., S. M. Margarit, D. Bar-Sagi, and J. Kuriyan. 1998. The structural basis of the activation of Ras by Sos. Nature 394:337–343. [DOI] [PubMed] [Google Scholar]
- 7.Brdicka, T., J. Cerny, and V. Horejsi. 1998. T cell receptor signalling results in rapid tyrosine phosphorylation of the linker protein LAT present in detergent-resistant membrane microdomains. Biochem. Biophys. Res. Commun. 248:356–360. [DOI] [PubMed] [Google Scholar]
- 8.Bubeck Wardenburg, J., R. Pappu, J. Y. Bu, B. Mayer, J. Chernoff, D. Straus, and A. C. Chan. 1998. Regulation of PAK activation and the T cell skeleton by the linker protein SLP-76. Immunity 9:607–616. [DOI] [PubMed] [Google Scholar]
- 9.Cahill, M. A., R. Janknecht, and A. Nordheim. 1996. Signaling pathways: jack of all cascades. Curr. Biol. 6:16–19. [DOI] [PubMed] [Google Scholar]
- 10.Canagarajah, B. J., A. Khoklatchev, M. H. Cobb, and E. J. Goldsmith. 1997. Activation mechanism of the MAP kinase ERK2 by dual phosphorylation. Cell 90:859–869. [DOI] [PubMed] [Google Scholar]
- 11.Ching, K. A., J. A. Grasis, P. Tailor, Y. Kawakami, T. Kawakami, and C. D. Tsoukas. 2000. TCR/CD3-induced activation and binding of Emt/Itk to linker of activated T cell complexes: requirement for the Src homology 2 domain. J. Immunol. 165:256–262. [DOI] [PubMed] [Google Scholar]
- 12.Crabtree, G. R. 1999. Generic signals and specific outcomes: signaling through Ca2+, calcineurin, and NF-AT. Cell 96:611–614. [DOI] [PubMed] [Google Scholar]
- 13.Di Bartolo, V., D. Mege, V. Germain, M. Pelosi, E. Dufour, F. Michel, G. Magistrelli, A. Isacchi, and O. Acuto. 1999. Tyrosine 319, a newly identified phosphorylation site of ZAP-70, plays a critical role in T cell antigen receptor signaling. J. Biol. Chem. 274:6285–6294. [DOI] [PubMed] [Google Scholar]
- 14.Enslen, H., J. Raingeaud, and R. J. Davis. 1998. Selective activation of p38 mitogen-activated protein (MAP) kinase isoforms by the MAP kinase kinases MKK3 and MKK6. J. Biol. Chem. 273:1741–1748. [DOI] [PubMed] [Google Scholar]
- 15.Finco, T. S., T. Kadlecek, W. Zhang, L. E. Samelson, and A. Weiss. 1998. LAT is required for TCR-mediated activation of PLCγ1 and the Ras pathway. Immunity 9:617–626. [DOI] [PubMed] [Google Scholar]
- 16.Franklin, R. A., A. Tordai, H. Patel, A. M. Gardner, G. L. Johnson, and E. W. Gelfand. 1994. Ligation of the T cell receptor complex results in activation of the Ras/Raf-1/MEK/MAPK cascade in human T lymphocytes. J. Clin. Investig. 93:2134–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu, C., C. W. Turck, T. Kurosaki, and A. C. Chan. 1998. BLNK: a central linker protein in B cell activation. Immunity 9:93–103. [DOI] [PubMed] [Google Scholar]
- 18.Fukazawa, T., K. A. Reedquist, G. Panchamoorthy, S. Soltoff, T. Trub, B. Druker, L. Cantley, S. E. Shoelson, and H. Band. 1995. T cell activation-dependent association between the p85 subunit of the phosphatidylinositol 3-kinase and Grb2/phopsholipase C-γ1-binding phosphotyrosyl protein pp36/38. J. Biol. Chem. 270:20177–20182. [DOI] [PubMed] [Google Scholar]
- 19.Furuke, K., M. Shiraishi, H. S. Mostowski, and E. T. Bloom. 1999. Fas ligand induction of human NK cells is regulated by redox through a calcineurin-nuclear factors of activated T cell-dependent pathway. J. Immunol. 162:1988–1993. [PubMed] [Google Scholar]
- 20.Genot, E., S. Cleverley, S. Henning, and D. Cantrell. 1996. Multiple p21ras effector pathways regulate nuclear factor of activated T cells. EMBO J. 15:3923–3933. [PMC free article] [PubMed] [Google Scholar]
- 21.Griffith, C. E., W. Zhang, and R. L. Wange. 1998. ZAP-70-dependent and -independent activation of ERK in Jurkat cells. Differences in signaling induced by H2O2 and CD3 cross-linking. J. Biol. Chem. 273:10771–10776. [DOI] [PubMed] [Google Scholar]
- 22.Gringhuis, S. I., L. F. M. H. de Leij, E. W. Verschuren, P. Borger, and E. Vellenga. 1997. Interleukin-7 upregulates the interleukin-2 gene expression in activated human T lymphocytes at the transcriptional level by enhancing the DNA binding activities of both NFAT and AP-1. Blood 90:2690–2700. [PubMed] [Google Scholar]
- 23.Gringhuis, S. I., L. F. M. H. de Leij, P. J. Coffer, and E. A. Velleng. 1998. Signaling through CD5 activates a pathway involving phosphatidylinositol 3-kinase, Vav, and Rac1 in human mature T lymphocytes. Mol. Cell. Biol. 18:1725–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gringhuis, S. I., A. Leow, E. A. M. Papendrecht-Van der Voort, P. H. J. Remans, F. C. Breedveld, and C. L. Verweij. 2000. Displacement of linker for activation of T cells from the plasma membrane due to redox balance alterations results in hyporesponsiveness of synovial fluid T lymphocytes in rheumatoid arthritis. J. Immunol. 164:2170–2179. [DOI] [PubMed] [Google Scholar]
- 25.Hashimoto, S., Y. Gon, K. Matsumoto, I. Takeshita, Y. Asai, Y. Asai, T. Machino, and T. Horie. 2000. Regulation of intracellular glutathione of TNF-α-induced p38 MAP kinase activation and RANTES production by human pulmonary vascular endothelial cells. Allergy 55:463–469. [DOI] [PubMed] [Google Scholar]
- 26.Hayes, J. D., and L. I. McLellan. 1999. Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defence against oxidative stress. Free Radical Res. 31:273–300. [DOI] [PubMed] [Google Scholar]
- 27.Hehner, S. P., T. G. Hofmann, O. Dienz, W. Droge, and M. L. Schmitz. 2000. Tyrosine-phosphorylated Vav1 as a point of integration for T-cell receptor- and CD28-mediated activation of JNK, p38, and interleukin-2 transcription. J. Biol. Chem. 275:18160–18171. [DOI] [PubMed] [Google Scholar]
- 28.Ishiai, M., M Kurosaki, R. Pappu, K. Okawa, I. Ronko, C. Fu, M. Shibata, A. Iwamatsu, A. C. Chan, and T. Kurosaki. 1999. BLNK required for coupling Syk to PLCγ2 and Rac1-JNK in B cells. Immunity 10:117–125. [DOI] [PubMed] [Google Scholar]
- 29.Ishiai, M., H. Sugawara, M. Kurosaki, and T. Kurosaki. 1999. Association of phospholipase C-γ2 Src homology domains with BLNK is critical for B cell antigen receptor signaling. J. Immunol. 163:1746–1749. [PubMed] [Google Scholar]
- 30.Jacinto, E., G. Werlen, and M. Karin. 1998. Cooperation between Syk and Rac1 leads to synergistic JNK activation in T lymphocytes. Immunity 8:31–41. [DOI] [PubMed] [Google Scholar]
- 31.Jain, J., C. Loh, and A. Rao. 1995. Transcriptional regulation of the IL-2 gene. Curr. Opin. Immunol. 7:333–342. [DOI] [PubMed] [Google Scholar]
- 32.Kane, L. P., J. Lin, and A. Weiss. 2000. Signal transduction by the TCR for antigen. Curr. Opin. Immunol. 12:242–249. [DOI] [PubMed] [Google Scholar]
- 33.Kanner, S. B., T. J. Kavanagh, A. Grossmann, S. L. Hu, J. B. Bolen, P. S. Rabinovitch, and J. A. Ledbetter. 1992. Sulfhydryl oxidation downregulates T-cell signaling and inhibits tyrosine phosphorylation of phospholipase Cγ1. Proc. Natl. Acad. Sci. USA 89:300–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kanno, T., and U. Siebenlist. 1996. Activation of nuclear factor-κB via T cell receptor requires a Raf kinase and Ca2+ influx. Functional synergy between Raf and calcineurin. J. Immunol. 157:5277–5283. [PubMed] [Google Scholar]
- 35.Karin, M., and Y. Ben-Neriah. 2000. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu. Rev. Immunol. 18:621–663. [DOI] [PubMed] [Google Scholar]
- 36.Karin, M., Z.-G. Liu, and E. Zandi. 1997. AP-1 function and regulation. Curr. Opin. Cell Biol. 9:240–246. [DOI] [PubMed] [Google Scholar]
- 37.Kempiak, S. J., T. S. Hiura, and A. E. Nel. 1999. The Jun kinase cascade is responsible for activating the CD28 response element of the IL-2 promoter: proof of cross-talk with the IkB kinase cascade. J. Immunol. 162:3176–3187. [PubMed] [Google Scholar]
- 38.Khokhlatchev, A. V., B. Canagarajah, J. Wilsbacher, M. Robinson, M. Atkinson, E. Goldsmith, and M. H. Cobb. 1998. Phosphorylation of the MAP kinase ERK2 promotes its homodimerization and nuclear translocation. Cell 93:605–615. [DOI] [PubMed] [Google Scholar]
- 39.King, A. J., H. Sun, B. Diaz, D. Barnard, W. Miao, S. Bagrodia, and M. S. Marshall. 1998. The protein kinase Pak3 positively regulates Raf-1 activity through phosphorylation of serine 338. Nature 396:180–183. [DOI] [PubMed] [Google Scholar]
- 40.Korn, S. H., E. F. M. Wouters, N. Vos, and Y. M. W. Janssen-Heiniger. 2001. Cytokine-induced activation of nuclear factor-κB is inhibited by hydrogen peroxide through oxidative inactivation of IκB kinase. J. Biol. Chem. 276:35693–35700. [DOI] [PubMed] [Google Scholar]
- 41.Kurosaki, T., and S. Tsukada. 2000. BLNK: connecting Syk and Btk to calcium signals. Immunity 12:1–5. [DOI] [PubMed] [Google Scholar]
- 42.Lahdenpohja, N., K. Savinainen, and M. Hurme. 1998. Pre-exposure to oxidative stress decreases the nuclear factor-κB-dependent transcription in T lymphocytes. J. Immunol. 160:1354–1358. [PubMed] [Google Scholar]
- 43.Law, C.-L., M. K. Ewings, P. M. Chaudhary, S. A. Solow, T. J. Yun, A. J. Marshall, L. Hood, and E. A. Clark. 1999. GrpL, a Grb2-related adaptor protein, interacts with SLP-76 to regulate nuclear factor of activated T cell activation. J. Exp. Med. 189:1243–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee, W., P. Mitchell, and R. Tjian. 1987. Purified transcription factor AP-1 interacts with TPA-inducible enhancer elements. Cell 49:741–752. [DOI] [PubMed] [Google Scholar]
- 45.Li, S., and J. Sedivy. 1993. Raf-1 protein kinase activates the NF-κB transcription factor by dissociating the cytoplasmic NF-κB–IκB complex. Proc. Natl. Acad. Sci. USA 90:9247–9251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin, J., A. Weiss, and T. S. Finco. 1999. Localization of LAT in glycolipid-enriched microdomains is required for T cell activation. J. Biol. Chem. 274:28861–28864. [DOI] [PubMed] [Google Scholar]
- 47.Lin, X., E. T. Cunningham, Jr., Y. Mu, R. Geleziunas, and W. C. Greene. 1999. The proto-oncogene Cot kinase participates in CD3/CD28 induction of NF-κB acting through the NF-κB-inducing kinase and IκB kinases. Immunity 10:271–280. [DOI] [PubMed] [Google Scholar]
- 48.Lindholm, C. K., E. Gylfe, W. Zhang, L. E. Samelson, and M. Welsh. 1999. Requirement of the Src homology 2 domain protein Shb for T cell receptor-dependent activation of the interleukin-2 gene nuclear factor for activation of T cells nuclear element in Jurkat T cells. J. Biol. Chem. 274:28050–28057. [DOI] [PubMed] [Google Scholar]
- 49.Liu, S. K., N. Fang, G. A. Koretzky, and C. J. McGlade. 1999. The hematopoietic-specific adaptor protein Gads functions in T-cell signaling via interactions with the SLP-76 and LAT adaptors. Curr. Biol. 9:67–75. [DOI] [PubMed] [Google Scholar]
- 50.Loh, C., J. A. Carew, J. Kim, P. G. Hogan, and A. Rao. 1996. T-cell receptor stimulation elicits an early phase of activation and a later phase of deactivation of the transcription factor NFAT1. Mol. Cell. Biol. 16:3945–3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luo, Z., B. Diaz, M. S. Marshall, and J. Avruch. 1997. An intact Raf zinc finger is required for optimal binding to processed Ras and for ras-dependent Raf activation in situ. Mol. Cell. Biol. 17:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maurice, M. M., H. Nakamura, E. A. van der Voort, A. I. van Vliet, F. J. Staal, P. P. Tak, F. C. Breedveld, and C. L. Verweij. 1997. Evidence for a role of an altered redox state in hyporesponsiveness of synovial T cells in rheumatoid arthritis. J. Immunol. 158:1458–1465. [PubMed] [Google Scholar]
- 53.Maurice, M. M., A. J. Lankester, A. Z. Bezemer, M. F. Geertsma, P.-P. Tak, F. C. Breedveld, and C. L. Verweij. 1997. Defective TCR-mediated signaling in synovial T cells in rheumatoid arthritis. J. Immunol. 159:2973–2978. [PubMed] [Google Scholar]
- 54.Maurice, M. M., H. Nakamura, S. I. Gringhuis, T. Okamoto, S. Yoshida, F. Kullmann, S. Lechner, E. A. van der Voort, A. Leow, J. Versendaal, U. Muller-Ladner, J. Yodoi, P. P. Tak, F. C. Breedveld, and C. L. Verweij. 1999. Expression of the thioredoxin-thioredoxin reductase system in the inflamed joints of patients with rheumatoid arthritis. Arthritis Rheum. 42:2430–2439. [DOI] [PubMed] [Google Scholar]
- 55.McInnes, I. B., B. P. Leung, R. D. Sturrock, M. Field, and F. Y. Liew. 1997. Interleukin-15 mediates T cell-dependent regulation of tumor necrosis factor-α production in rheumatoid arthritis. Nat. Med. 3:189–195. [DOI] [PubMed] [Google Scholar]
- 56.Mercurio, F., B. W. Murray, A. Shevchenko, B. L. Bennett, D. B. Young, J. Wu Li, G. Pascual, A. Motiwala, H. Zhu, M. Mann, and A. M. Manning. 1999. IκB kinase (IKK)-associated protein 1, a common component of the heterogenous IKK complex. Mol. Cell. Biol. 19:1526–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Migita, K., K. Eguchi, Y. Kawabe, Y. Ichinose, T. Tsukada, T. Aoyagi, H. Nakamura, and S. Nagataki. 1996. TNF-α-mediated expression of membrane-type matrix metalloproteinase in rheumatoid synovial fibroblasts. Immunology 89:553–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nakano, H., M. Shindo, S. Sakon, S. Nishinaka, M. Mihara, H. Yagita, and K. Okumura. 1998. Differential regulation of IκB kinase α and β by two upstream kinases, NF-κB-inducing kinase and mitogen-activated protein kinase/ERK kinase kinase-1. Proc. Natl. Acad. Sci. USA 95:3537–3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nishina, H., M. Bachmann, A. J. Oliveira-dos-Santos, I. Kozieradzki, K. D. Fischer, B. Odermatt, A. Wakeham, A. Shahinian, H. Takimoto, A. Bernstein, T. W. Mak, J. R. Woodgett, P. S. Ohashi, and J. M. Penninger. 1997. Impaired CD28-mediated interleukin 2 production and proliferation in stress kinase SAPK/ERK1 kinase (SEK1)/mitogen-activated protein kinase 4 (MKK4)-deficient T lymphocytes. J. Exp. Med. 186:941–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Okada, T., C. D. Hu, T. G. Jin, K. Kariya, Y. Yamawaki-Kataoka, and T. Kataoka. 1999. The strength of interaction at the Raf cysteine-rich domain is a critical determinant of response of Raf to Ras family small GTPases. Mol. Cell. Biol. 19:6057–6064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Panayi, G. S. 1997. T-cell-dependent pathways in rheumatoid arthritis. Curr. Opin. Rheumatol. 9:236–240. [DOI] [PubMed] [Google Scholar]
- 62.Park, S., M. Uesugi, and G. L. Verdine. 2000. A second calcineurin binding site on the NFAT regulatory domain. Proc. Natl. Acad. Sci. USA 97:7130–7135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rao, A., C. Luo, and H. G. Hogan. 1997. Transcription factors of the NFAT family: regulation and function. Annu. Rev. Immunol. 15:707–747. [DOI] [PubMed] [Google Scholar]
- 64.Reiter, T. A., R. T. Abraham, M. Choi, and F. Rusnak. 1999. Redox regulation of calcineurin in T-lymphocytes. J. Biol. Inorg. Chem. 4:632–644. [DOI] [PubMed] [Google Scholar]
- 65.Salmon, R. A., I. N. Foltz, P. R. Young, and J. W. Schrader. 1997. The p38 mitogen-activated protein kinase is activated by ligation of the T or B lymphocyte antigen receptors, Fas or CD40, but suppression of kinase activity does not inhibit apoptosis induced by antigen receptors. J. Immunol. 159:5309–5317. [PubMed] [Google Scholar]
- 66.Salojin, K. V., J. Zhang, C. Meagher, and T. L. Delovitch. 2000. ZAP-70 is essential for the T cell antigen-receptor-induced plasma membrane targeting of SOS and Vav in T cells. J. Biol. Chem. 275:5966–5975. [DOI] [PubMed] [Google Scholar]
- 67.Salojin, K. V., J. Zhang, and T. L. Delovitch. 1999. TCR and CD28 are coupled via ZAP-70 to the activation of the Vav/Rac-1/PAK-1/p38 MAPK signaling pathway. J. Immunol. 163:844–853. [PubMed] [Google Scholar]
- 68.Salojin, K. V., J. Zhang, J. Madrenas, and T. L. Delovitch. 1998. T-cell anergy and altered T-cell receptor signaling: effects on autoimmune disease. Immunol. Today 19:468–473. [DOI] [PubMed] [Google Scholar]
- 69.Sebbag, M., S. L. Parry, F. M. Brennan, and M. Feldmann. 1997. Cytokine stimulation of T lymphocytes regulates their capacity to induce monocyte production of tumor necrosis factor-α, but not interleukin-10: possible relevance to pathophysiology of rheumatoid arthritis. Eur. J. Immunol. 27:624–632. [DOI] [PubMed] [Google Scholar]
- 70.Shan, X., and R. L. Wange. 1999. Itk/Emt/Tsk activation in response to CD3 cross-linking in Jurkat T cells requires ZAP-70 and Lat and is independent of membrane recruitment. J. Biol. Chem. 274:29323–29330. [DOI] [PubMed] [Google Scholar]
- 71.Tang, J., S. Sawasdikosol, J. H. Chang, and S. J. Burakoff. 1999. SLAP, a dimeric adapter protein, plays a functional role in T cell receptor signaling. Proc. Natl. Acad. Sci. USA 96:9775–9780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weber, J. R., S. Orstavik, K. M. Torgersen, N. C. Danbolt, S. F. Berg, J. C. Ryan, K. Taskén, J. B. Imboden, and J. T. Vaage. 1998. Molecular cloning of the cDNA encoding pp36, a tyrosine-phosphorylated adaptor protein selectively expressed by T cells and natural killer cells. J. Exp. Med. 187:1157–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wong, J., M. Ishiai, T. Kurosaki, and A. C. Chan. 2000. Functional complementation of BLNK by SLP-76 and LAT linker proteins. J. Biol. Chem. 275:33116–33122. [DOI] [PubMed] [Google Scholar]
- 74.Yoshida, S., T. Katoh, T. Tetsuka, K. Uno, N. Matsui, and T. Okamoto. 1999. Involvement of thioredoxin in rheumatoid arthritis: its costimulatory roles in the TNF-α-induced production of IL-6 and IL-8 from cultured synovial fibroblasts. J. Immunol. 163:351–358. [PubMed] [Google Scholar]
- 75.Yoshizumi, M., J. Abe, J. Haendeler, Q. Huang, and B. C. Berk. 2000. Src and Cas mediate JNK activation but not ERK1/2 and p38 kinases by reactive oxygen species. J. Biol. Chem. 275:11706–11712. [DOI] [PubMed] [Google Scholar]
- 76.Zhang, W., J. Sloan-Lancaster, J. Kitchen, R. P. Trible, and L. E. Samelson. 1998. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell 92:83–92. [DOI] [PubMed] [Google Scholar]
- 77.Zhang, W., R. P. Trible, and L. E. Samelson. 1998. LAT palmitoylation: its essential role in membrane microdomain targeting and tyrosine phosphorylation during T cell activation. Immunity 9:239–246. [DOI] [PubMed] [Google Scholar]
- 78.Zhang, W., C. L. Sommers, D. N. Burshtyn, C. C. Stebbins, J. B. DeJarnette, R. P. Trible, A. Grinberg, H. C. Tsay, H. M. Jacobs, C. M. Kessler, E. O. Long, P. E. Love, and L. E. Samelson. 1999. Essential role of LAT in T cell development. Immunity 10:323–332. [DOI] [PubMed] [Google Scholar]
- 79.Zhang, W., B. J. Irvin, R. P. Trible, R. T. Abraham, and L. E. Samelson. 1999. Functional analysis of LAT in TCR-mediated signaling pathways using a LAT-deficient Jurkat cell line. Int. Immunol. 11:943–950. [DOI] [PubMed] [Google Scholar]
- 80.Zhang, W., R. P. Trible, M. Zhu, S. K. Liu, C. J. McGlade, and L. E. Samelson. 2000. Association of Grb2, Gads, and phospholipase C-γ1 with phosphorylated LAT tyrosine residues. Effect of LAT tyrosine mutations on T cell antigen receptor-mediated signaling. J. Biol. Chem. 275:23355–23361. [DOI] [PubMed] [Google Scholar]