Abstract
Gonadotropin-releasing hormone (GnRH) is the central regulator of the reproductive axis. Normal sexual maturation depends on the migration of GnRH neurons from the olfactory placode to the hypothalamus during development. Previously, we showed restricted expression of the membrane receptor adhesion-related kinase (Ark) in immortalized cell lines derived from migratory but not postmigratory GnRH neurons. In addition, Ark and GnRH transcripts were detected along the GnRH neuron migratory route in the E13 mouse cribriform plate. In the present study, we examined the role of Ark and its ligand, Gas6 (encoded by growth arrest-specific gene 6), in GnRH neuron migration. Gas6 stimulated lamellipodial extension, membrane ruffling, and chemotaxis of immortalized NLT GnRH neuronal cells via the Ark receptor. Gas6/Ark signaling promoted activation of the Rho family GTPase Rac, and adenoviral-mediated expression of dominant negative N17Rac abolished Gas6/Ark-induced actin cytoskeletal reorganization and migration of GnRH neuronal cells. In addition, p38 MAPK was activated downstream of Ark and Rac, and inhibition of p38 MAPK with either SB203580 or adenoviral dominant negative p38α also blocked Gas6/Ark-mediated migration. Finally, downstream of Rac and p38 mitogen-activated protein kinase (MAPK), Gas6/Ark signaling promoted activation of MAPK-activated protein kinase 2 and induced phosphorylation of HSP25, a known regulator of cortical actin remodeling. The data are the first to demonstrate a migratory signaling pathway downstream of Ark/Axl family receptors and suggest a previously unidentified role for p38 MAPK in neuronal migration. Furthermore, these studies support a potential role for Ark in the regulation of GnRH neuronal migration.
The acquisition of reproductive competence relies on a hierarchy of events initiated by gonadotropin-releasing hormone (GnRH). Neurons producing GnRH are unique, originating in the olfactory placode and migrating into the central nervous system during development (59, 66). Traveling in association with vomeronasal nerve fibers, the GnRH neurons migrate through the nasal septum, cross the cribriform plate (the nasal-brain border), and continue along vomeronasal nerve fibers until they reach the preoptic area and hypothalamus (67, 70). After completing the journey, GnRH neurons project axons toward the median eminence, establishing contacts with pituitary portal vessels. GnRH release is tightly regulated and occurs in a pulsatile manner to stimulate pituitary gonadotropin production (60).
In humans, X-linked Kallmann’s syndrome, arising from mutations in the KAL-1 gene, results in aberrant olfactory and GnRH neuron migration (11, 24, 32, 58). KAL-1 encodes a cell adhesion molecule, anosmin-1, that is believed to be required for olfactory nerve migration (58, 62). Since the GnRH neurons travel in association with olfactory nerves, the defect in olfactory nerve migration is thought to disrupt the GnRH neuron pathway, resulting in loss of GnRH neuronal migration. Although the path of the GnRH neurons from the olfactory placode to the forebrain during development has been well characterized, the cellular signaling mechanisms that directly govern GnRH neuron motility are unknown.
Because primary GnRH neurons are extremely limited in number (∼800 neurons in the mouse, several thousand in the primate) (54, 66), studies of their development and differentiation have been difficult. We and others have utilized immortalized GnRH neuronal cell lines as a model system to identify factors potentially involved in GnRH neuronal physiology. Simian virus 40 T-antigen-targeted tumorigenesis was used to establish GnRH neuronal cell lines at two distinct developmental phases, migratory and postmigratory (39, 49). GN10, GN11, and NLT GnRH neuronal cells were isolated from an olfactory tumor of migration-arrested GnRH neurons (49), while GT-1 cells (and subclones GT1-1, GT1-3, and GT1-7) were derived from a postmigratory hypothalamic tumor (39). Characterization of these cell lines has provided evidence that many GnRH neuronal attributes were retained during the immortalization process. For example, GT-1 cells secrete GnRH in a pulsatile fashion similarly to GnRH neurons in vivo (39, 65) and can restore reproductive function when implanted into the brains of hypogonadal mice (61). Furthermore, the GT cells resemble GnRH neurons found in vivo displaying perikarya and neurites and express neuron-specific transcripts, including neuron-specific enolase and the 68-kDa neurofilament protein (39). Although the GN/NLT cell lines have not been as well characterized, they synthesize GnRH, albeit at a much lower level than the GT clones, and express neuron-specific proteins, including tau and microtubule-associated peptide 2 (49, 72). Furthermore, recent studies have indicated that the GN/NLT neuronal cells are intrinsically motile like GnRH neurons found in the olfactory placode in vivo (36; M. P. Allen et al., 30th Ann. Meet. Soc. Neurosci., abstr. 225.2, 2000). Thus, the immortalized cell lines provide excellent models that mimic both the migratory and postmigratory phenotypes of in vivo GnRH neurons.
To elucidate factors that may regulate GnRH neuron motility, differential display was used to compare the gene expression profiles of postmigratory GT1-7 and migratory GN10 neuronal cell lines (10). One candidate gene that was differentially expressed in the GN10 GnRH cells encoded adhesion-related kinase (Ark), a unique membrane receptor consisting of an extracellular domain akin to cell adhesion molecules and a cytoplasmic tyrosine kinase domain (10). Ark is the murine homolog of the human protein Axl (also known as UFO and Tyro7) (23, 28, 45, 52) and belongs to a novel receptor family that includes Tyro3 (also known as etk2, Dtk, brt, Rse, Sky, and tif) and Mer (also known as Tyro12, eyk, and Nyk) (reviewed in reference 8). Members of this family are expressed in the central nervous system as well as other tissues (28, 47, 52). Ark/Axl family receptors have been shown to promote cell growth and survival (2, 5, 12, 15, 16, 33, 35), adhesion (4, 38), and migration (13) via binding to a common ligand, Gas6 (encoded by growth arrest-specific gene 6) (42, 64). Gas6 is also expressed in the brain, suggesting that Gas6/Ark signaling may play a role in central nervous system physiology (48).
Previous studies from our laboratory demonstrated that Gas6 promotes survival of Ark-expressing migratory GnRH neuronal cells (2). More recent findings have shown that Ark regulates GnRH gene expression (1). Moreover, Ark and GnRH transcripts were detected along the GnRH neuron migratory route in mouse E13 cribriform plate RNA (1). Given the differential expression of Ark in cell lines derived from migratory but not postmigratory GnRH neurons and Ark expression in the cribriform plate during embryogenesis, we explored a role for Gas6/Ark signaling in GnRH neuron motility. In migratory GnRH neuronal cells, Gas6 stimulates Ark-dependent actin cytoskeletal reorganization and chemotaxis via activation of the Rho family GTPase, Rac. Although Rac has previously been implicated in neuronal migration, its downstream targets in neurons remain largely undefined (73). The results illustrate that p38 mitogen-activated protein kinase (MAPK), a protein previously implicated in neuronal apoptosis (68), is required downstream of Rac in Gas6/Ark-induced GnRH neuronal-cell motility. The data are the first to describe an Ark/Axl receptor-mediated migratory signaling pathway. In addition, the data support a novel function for p38 MAPK in regulating neuronal migration.
MATERIALS AND METHODS
Reagents.
Recombinant human Gas6 was generated as described previously (64). Ark318 immunoglobulin G (IgG) and preimmune (PI) IgG were generously provided by Claudio Basilico and Paola Bellosta (New York University) (5). PD98059, SB203580, and protein A/G agarose were purchased from Calbiochem (San Diego, Calif.). Rhodamine phalloidin was obtained from Molecular Probes (Eugene, Oreg.). Rabbit polyclonal antibodies that recognize the Tyr/Thr-dually phosphorylated (active) forms of p38 and ERK1/2 were purchased from New England Biolabs (Beverly, Mass.) and Promega (Madison, Wis.), respectively. Polyclonal heat shock protein 25 (HSP25) antibody was obtained from Stressgen (Victoria, Canada). Monoclonal (M2) Flag antibody was from Sigma (St. Louis, Mo.). Polyclonal antibodies to ERK2 and p38 were obtained from Santa Cruz (Santa Cruz, Calif.). Rac activation and MAPK-activated protein (MAPKAP) kinase 2 activation kits were purchased from Upstate Biotechnology (Lake Placid, N.Y.). Purified collagen was purchased from Worthington (Freehold, N.J.). Transwell migration chambers (6.5-mm diameter, 8-μm pore size) were obtained from Costar (Corning, N.Y.). The bicinchoninic acid protein assay kit was purchased from Pierce (Rockford, Ill.). The complete protease inhibitor cocktail was from Boehringer Mannheim (Indianapolis, Ind.). Hybond polyvinylidene difluoride (PVDF) blotting membranes, enhanced chemiluminescence reagents, and horseradish peroxidase-conjugated secondary antibodies were purchased from Amersham (Piscataway, N.J.). Dulbecco’s modified Eagle’s medium (DMEM) and antibiotics were from Life Technologies (Rockville, Md.), and fetal calf serum was from Gemini Bio-Products (Calabasas, Calif.). The DiffQuik staining kit was from Dade Behring (Newark, Del.). Glass coverslips (12-mm circles) were purchased from Fisher Scientific (Pittsburgh, Pa.).
Cell culture.
GN10, GN11, NLT (49), and GT1-7 (39) GnRH neuronal cell lines were grown in DMEM supplemented with 10% fetal calf serum, 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 0.25 μg of amphotericin B per ml at 37°C in humidified 5% CO2-95% air.
Boyden chamber migration assays.
Neuronal cells were rinsed once in PBS and incubated for 16 to 18 h in DMEM supplemented with 0.5% fetal calf serum, 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 0.25 μg of amphotericin B per ml at 37°C in humidified 5% CO2-95% air. The cells were removed from cell culture plates by trypsin digestion, pelleted by centrifugation at 2,000 × g for 5 min, and resuspended in migration medium (DMEM, 0.5% fetal bovine serum [FBS], 0.5% bovine serum albumin, 4 mM MgCl2, 4 mM CaCl2). A total of 30,000 cells were plated into the upper chamber of collagen (0.1 mg/ml)-coated Transwells and allowed to migrate for 24 h at 37°C in humidified 5% CO2-95% air. For determination of basal migration rates, migration medium was included in the lower Transwell chamber. For chemotaxis experiments, vehicle or Gas6 (400 ng/ml) was added to the lower chamber containing the migration medium. For blocking experiments, the Axl extracellular domain (ECD) was added to the lower chamber at a final concentration of 800 ng/ml. For experiments using PD98059 (30 μM), SB203580 (30 μM), PI IgG (10 μg/ml), and Ark318 IgG (10 μg/ml), the reagents were added to both the upper and lower compartments of the Transwell. For adenoviral experiments, NLT cells were infected for 24 to 48 h and then incubated for 16 to 18 h in low-serum medium (0.5% FBS) like uninfected cells prior to setting up the migration assay. Following the 24-h migration, the cells were fixed and stained by using a DiffQuik kit. The number of migrating cells for each condition was determined by counting cells in four different fields on each membrane.
Immunoblotting.
Neuronal cells were washed once in PBS at 4°C and lysed in 0.1 ml of cell lysis buffer containing 20 mM HEPES (pH 7.4), 1% Triton X-100, 50 mM NaCl, 1 mM EGTA, 20 mM sodium orthovanadate, and 50 mM sodium fluoride and supplemented with complete protease inhibitors. Cell debris was removed by centrifugation at 14,000 × g for 15 min at 4°C. The protein concentration of the supernatant was determined with a bicinchoninic acid protein assay kit. Protein (20 to 50 μg) was resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 7.5 to 12% gels and transferred to PVDF. The membranes were blocked in 5% nonfat milk-TBS-T buffer (137 mM NaCl, 0.1% Tween-20, 20 mM Tris-Cl; pH 7.6) for 1 h at room temperature (RT) or overnight at 4°C. Membranes were incubated for 1 h in primary antibody and washed three times in TBS-T for 15 min. The membranes were incubated in horseradish peroxidase-linked secondary antibody for 30 to 60 min, washed three times in TBS-T for 15 min, and incubated in enhanced chemiluminescence immunodetection reagents according to the manufacturer’s instructions. Antibodies to the following proteins were used at the indicated dilutions: phospho-ERK1/2, 1:5,000; ERK2, 1:1,000; phospho-p38, 1:1,000; p38, 1:2,000; Ark318, 1:1,000; HSP25, 1:2,000; and Flag, 1 μg/ml. The Rac activation assay and the MAPKAP kinase 2 activation assay were performed according to the manufacturer’s instructions. For adenoviral experiments, NLT cells were infected for 48 h, incubated in low-serum medium (0.5% FBS) for 16 h, and then stimulated with Gas6 (400 ng/ml) for different lengths of time. Following incubation, cell lysates were resolved by SDS-PAGE and immunoblotted for the appropriate proteins as described above. Densitometry analysis was performed with the Bio-Rad Fluor-S multi-imager and Quantity One software.
Actin localization.
NLT cells (15,000) were seeded onto sterile glass coverslips. On the following day, the cells were washed once in PBS and incubated for 16 to 18 h in DMEM supplemented with 0.5% fetal calf serum, 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 0.25 μg of amphotericin B per ml at 37°C in humidified 5% CO2-95% air. For blocking experiments including the Axl ECD (800 ng/ml), PI IgG (10 μg/ml), Ark318 IgG (10 μg/ml), PD98059 (30 μM), and SB203580 (30 μM), the cells were pretreated for 4 h and then stimulated with 400 ng of Gas6 per ml for 10 min. Following Gas6 treatment, the cells were rinsed once in PBS and fixed in 4% paraformaldehyde-PBS for 30 min at RT followed by two additional PBS rinses. The cells were permeabilized in 5% bovine serum albumin-0.2% Triton X-100-PBS for 30 min at RT. F-actin was stained with 25 μl of rhodamine phalloidin per ml in the permeabilization solution for 30 min at RT. The cells were rinsed three times with PBS, mounted on slides, and viewed with a Zeiss Axioskop II microscope. For adenoviral experiments, the cells were infected for 24 to 48 h, trypsinized, and replated at 15,000 cells/coverslip as described above for uninfected cells. To quantitate the percentage of cells that displayed membrane ruffles and/or lamellipodia, cells in three fields were counted on each coverslip. For statistical analysis, n is the number of fields counted.
Adenoviruses.
The wild-type p38α, dominant negative p38α (p38αDN), and constitutively active MKK6 (MKK6CA) plasmids were generously provided by Roger Davis (University of Massachusetts Medical School). p38αDN contains two mutations, Thr-180 to Ala and Tyr-182 to Phe (50). In the MKK6CA mutant, Ser-207 and Thr-211 were replaced by Glu (51). Adenoviruses containing p38α, p38αDN, and MKK6CA were generated by subcloning the various cDNAs into the shuttle plasmid pACCMV (14) followed by overlap recombination with the adenoviral vector Ad5dl327BSTβ-gal (57). Large-scale virus stocks were purified by cesium chloride gradient centrifugation, and titers were determined by plaque assays in 293 cells.
To construct Rac1 adenoviruses, Rac1 was cloned from a mouse cDNA library, and mutations (Rac1G12V and Rac1T17N) were generated by PCR-based site-directed mutagenesis. The adenoviral green fluorescent protein (GFP), GFP/N17Rac, and GFP/V12Rac were generated with the AdEasy system (19). The Rac adenoviruses coexpress GFP and the respective mutant Rac protein. For immunoblotting experiments, GFP-, GFP/N17Rac-, and GFP/V12Rac-expressing adenoviruses were used at a multiplicity of infection (MOI) of 40 PFU per cell, and the MKK6CA- and wild-type-p38α-bearing adenoviruses were used at an MOI of 100 PFU/cell. For migration experiments, GFP and GFP/N17Rac were used at 10 to 20 PFU/cell, and the p38αDN adenovirus was used at 50 PFU/cell. The empty adenovirus (Ad-CMV) was used at the same MOI as others in the respective experiment and served as a negative control. For actin localization, GFP, GFP/N17Rac, and GFP/V12Rac were used at 40 to 80 PFU/cell, and MKK6CA and p38α were used at 100 PFU/cell.
32P incorporation into HSP25.
NLT cells were incubated overnight in DMEM containing 0.5% FBS. The cells were then incubated for 4 h in phosphate-free DMEM-0.5% FBS and 2 mCi of 32Pi. Subsequently, the cells were washed twice in phosphate-free medium and stimulated with Gas6 (400 ng/ml) for 10 to 30 min. Cells were lysed (see “Immunoblotting” above), and the lysate was incubated with anti-HSP25 antibody for 2 h at 4°C with constant mixing. Protein A/G agarose was added to the lysate, and incubation was continued for another 2 h. The protein A/G agarose was washed three times in cold lysis buffer, and the samples were boiled and loaded onto a SDS-12.5% PAGE gel. The proteins were transferred to PVDF, and the membrane was exposed to film for 4 h at RT to detect phosphorylated HSP25. Subsequently, the membrane was probed with anti-HSP25 antibody to detect the total HSP25 protein present.
RESULTS
Gas6 promotes migration of Ark-expressing GnRH neuronal cell lines.
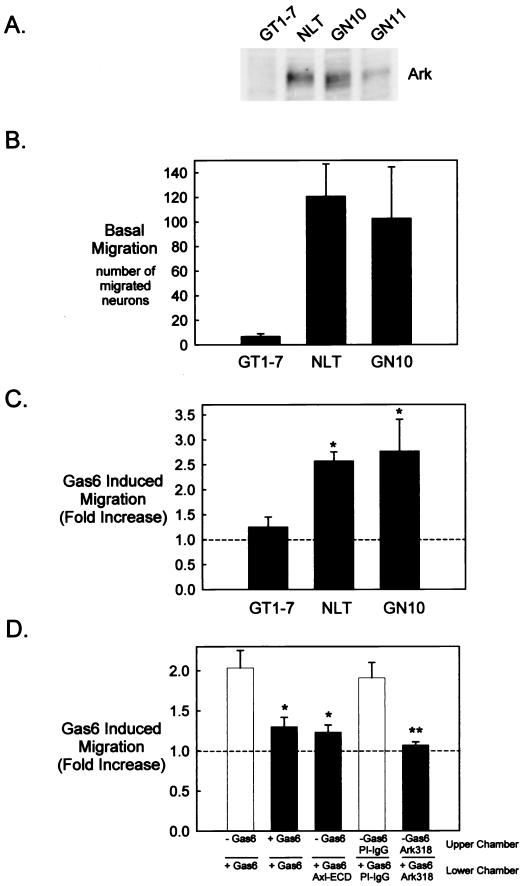
Previous differential display analysis demonstrated Ark expression in migratory (GN10) but not postmigratory (GT1-7) GnRH neuronal cells (10). In addition to the GN10 cells, two other cell lines were established from simian virus 40 T-antigen immortalized migratory GnRH neurons, the GN11 and NLT lines (49). Immunoblot analysis with a polyclonal Ark antibody (Ark318) revealed the restricted expression of Ark in all three cell lines derived from migratory GnRH neurons (GN10, GN11, and NLT) but not GT1-7 cells (Fig. 1A). However, Ark expression was substantially lower in the GN11 subclone than in the GN10 and NLT cells. Therefore, the GN11 cell line was not further investigated in this study.
FIG. 1.
Gas6 stimulates migration of Ark-expressing GnRH neuronal cell lines. (A) GT1-7, NLT, GN10, and GN11 cell lysates (20 μg) were analyzed by immunoblotting with Ark318 IgG. (B to D) The migratory behavior of the cells was examined with a Boyden chamber system as described in Materials and Methods. (B) Twenty-four-hour migration in the presence of 0.5% FBS in the lower chamber. Values are as follows: GT1-7, 7 ± 2 (n = 4); NLT, 121 ± 26 (n = 4); GN10, 103 ± 42 (n = 6). (C) Twenty-four-hour migration in the presence of Gas6 (400 ng/ml) in the lower chamber (n = 4 for GT1-7 and n = 62 for NLT and GN10). *, statistically significant by the t test: P < 0.01 for NLT and P < 0.05 for GN10. (D) Effects of Gas6, recombinant Axl ECD (800 ng/ml), Ark318 IgG (10 μg/ml), and PI IgG (10 μg/ml) on migration (24 h). *, statistically significantly different from the control (Gas6 in the lower chamber only) by the Tukey-Kramer multiple comparisons test, P < 0.05 (n = 3). **, statistically different from the control (PI IgG in the upper and lower chambers) by the t test, P < 0.05 (n = 3).
Because the GN10 and NLT GnRH neuronal cells were isolated during migration, we suspected that they retained motile characteristics that had been lost by the postmigratory GT1-7 cells. To test this hypothesis, a Boyden chamber system was utilized to examine the migratory behavior of the cell lines. A Boyden chamber consists of an upper porous membrane on which the cells of interest are seeded and a lower chamber containing a chemoattractant. In a typical assay, cells migrate through the pores and attach to the lower side of the membrane, and subsequently the number of migrated cells is determined. As predicted, the GN10 and NLT GnRH neuronal cells were intrinsically migratory, unlike the GT1-7 cells (Fig. 1B).
To examine a role for Ark in GnRH neuronal-cell motility, Gas6, the Ark ligand, was included as the chemoattractant in the migration assay. As shown in Fig. 1C, Gas6 (400 ng/ml) potentiated NLT and GN10 migration 2.6-fold (n = 4, P < 0.01) and 2.7-fold (n = 6, P < 0.05), respectively, while having no significant effect on Ark-negative GT1-7 cells (1.2-fold increase; n = 4, P = 0.36). Inclusion of Gas6 in both the upper and lower compartments effectively attenuated NLT migration, indicating that the effect was chemotactic rather than chemokinetic (Fig. 1D). Moreover, the Axl ECD and the polyclonal anti-Ark318 IgG antagonized NLT migration, demonstrating that the effect was both ligand and receptor dependent, respectively (Fig. 1D). Since similar results were obtained with the GN10 neuronal cell line (data not shown), in the remainder of the studies we utilized the NLT cell line as representative of migratory GnRH neurons.
Gas6 stimulates actin cytoskeletal remodeling and migration of NLT GnRH neuronal cells via Ark and the small G protein Rac.
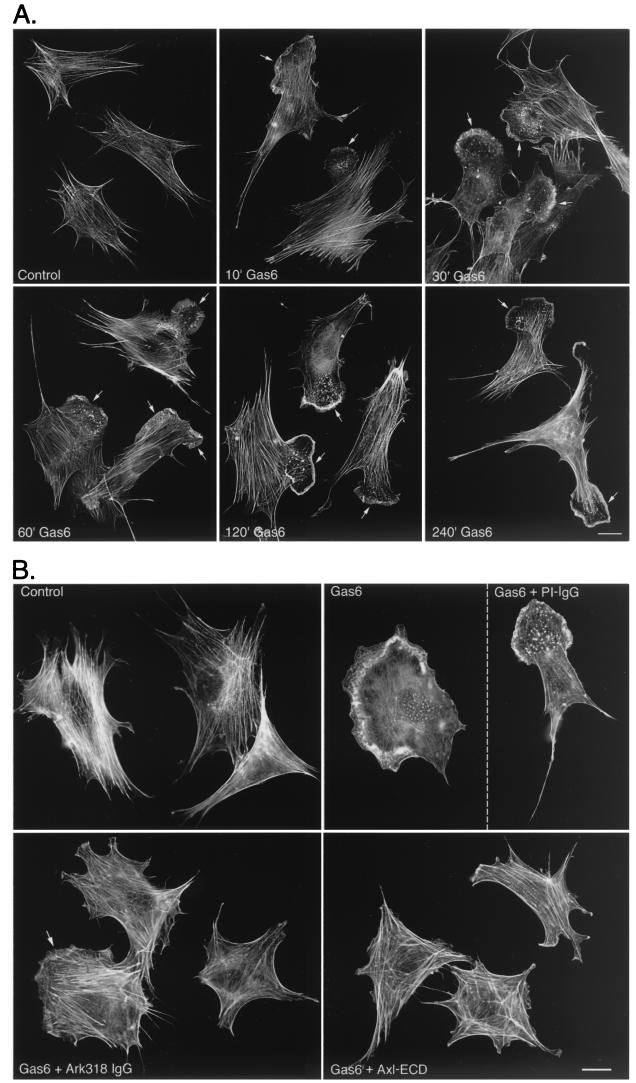
A morphological hallmark of cellular motility is the development of membrane ruffles and lamellipodia that are readily visualized by staining cellular filamentous actin (F- actin). Examination of unstimulated NLT cells revealed an intense actin stress fiber network (Fig. 2A). Gas6 treatment (400 ng/ml) resulted in dramatic reorganization of the NLT actin cytoskeleton to a motile phenotype that occurred within 10 min and persisted for at least 4 h (Fig. 2A). In this assay, 9% ± 1.5% of control NLT cells displayed membrane ruffles and/or lamellipodia. After 10 min of Gas6 treatment, 63% ± 3% of NLT cells displayed these cytoskeletal modifications (n = 9, P < 0.0001). Consistent with the results obtained for NLT migration (Fig. 1D), Gas6-mediated cytoskeletal remodeling was significantly abolished by the Axl ECD and the anti-Ark318 IgG, while PI IgG had no effect (Fig. 2B) (percentages of cells displaying membrane ruffles and/or lamellipodia: Gas6, 65% ± 5%; Gas6 plus PI IgG, 54% ± 10%; Gas6 plus Ark318 IgG, 23% ± 2%; Gas6 plus Axl ECD, 22% ± 3%; n = 6; for the last two values, P < 0.001 versus the Gas6 value). Thus, Gas6-induced actin cytoskeletal remodeling and migration of NLT GnRH neuronal cells are Ark dependent.
FIG. 2.
Gas6 stimulates Ark-dependent formation of lamellipodia and membrane ruffles in NLT GnRH neuronal cells. (A) NLT cells were incubated with Gas6 (400 ng/ml) for the indicated times. Following fixation, F-actin was visualized with rhodamine phalloidin. Arrows indicate lamellipodia and membrane ruffles. The data are representative of three independent experiments (see Materials and Methods for quantitation of actin cytoskeletal remodeling). Bar = 20 μm. (B) NLT cells were pretreated (4 h) with the Axl ECD (800 ng/ml), Ark318 IgG (10 μg/ml), or PI IgG (10 μg/ml) followed by a 10-min stimulation with Gas6 (400 ng/ml). F-actin was visualized as in panel A. The partial effectiveness of the Ark318 IgG at blocking the Gas6 effect is indicated by the arrow. The data are representative of two independent experiments. Bar = 20 μm.
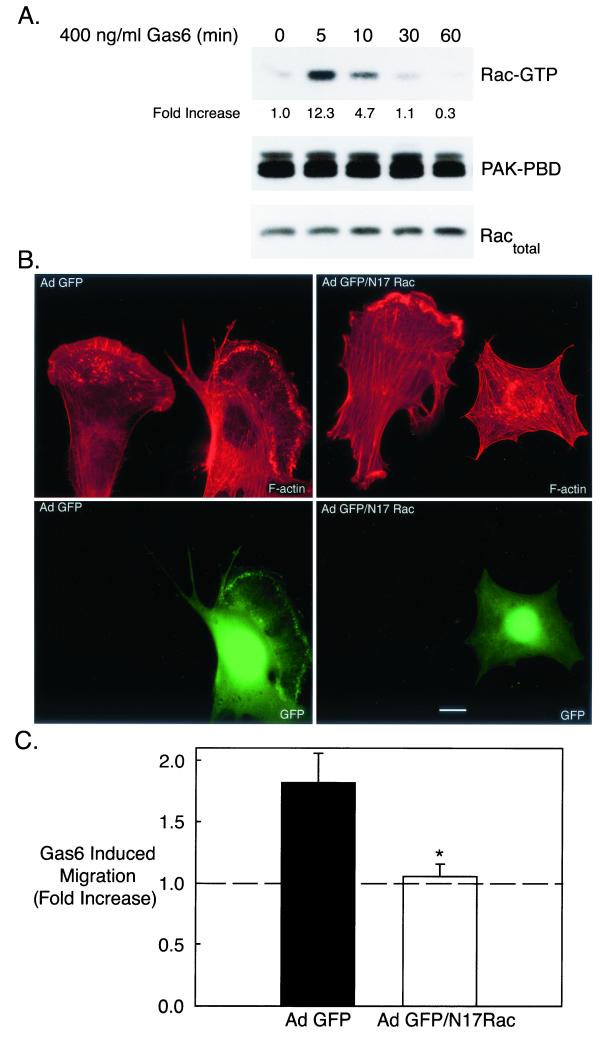
Actin cytoskeletal dynamics are regulated by the Rho family of monomeric GTPases (17). Of the Rho family GTPases, Rho, Rac, and Cdc42, Rac is the principal regulator of membrane ruffling and lamellipodial formation (53). To examine whether Gas6 stimulated Ark signaling to Rac, active (GTP-bound) Rac was precipitated from Gas6-treated cells with the Rac binding domain of the effector protein PAK (PAK-PBD). Subsequently, precipitated Rac was detected by immunoblot analysis. Since the PAK-PBD interacts exclusively with active (GTP-bound) Rac, the Rac immunoblot represents the amount of active Rac in each sample (6). Gas6 treatment of NLT cells resulted in significant Rac activation that peaked within 5 min (12.3-fold), remained elevated at 10 min, and returned to near baseline by 30 min (Fig. 3A, top). Gas6 addition had no effect on the amount of total Rac (GDP plus GTP bound) detected in NLT lysates (Fig. 3A, bottom).
FIG. 3.
Gas6/Ark-stimulated NLT migration and cytoskeletal reorganization requires the Rho family GTPase Rac. (A) NLT cells were treated with Gas6 (400 ng/ml) for the indicated times followed by a Rac activation assay. Briefly, GTP-bound Rac (Rac-GTP) was precipitated with PAK-PBD agarose, and GTP-bound Rac was visualized by Rac immunoblotting. Equal amounts of PAK-PBD were used to precipitate Rac-GTP (PAK-PBD blot), and each sample contained a similar amount of Rac protein (for the Ractotal blot, 1/20 of each sample was loaded). The data are representative of three independent experiments. The increase in Rac-GTP was calculated by dividing the Rac-GTP density by the Ractotal density at each time point and setting the value at the zero time point to 1. (B) NLT cells were infected with Ad GFP or Ad GFP/N17Rac (see Materials and Methods), stimulated for 10 min with Gas6 (400 ng/ml), and stained with rhodamine phalloidin. Actin is shown in red and GFP is in green. The data are representative of two independent experiments. Bar = 20 μm. (C) NLT cells infected with Ad GFP or GFP/N17Rac were tested in the Boyden chamber migration assay with or without Gas6. *, statistically different from the Ad GFP value by the t test, P < 0.05 (n = 5).
Next, Rac participation in Gas6/Ark-mediated cytoskeletal remodeling and migration was evaluated. NLT cells expressing adenoviral GFP (Ad GFP) developed prominent lamellipodia and membrane ruffles upon exposure to Gas6 in a manner similar to that of uninfected NLT cells in the same culture (Fig. 3B, left panels) (71% ± 5% of GFP-expressing cells underwent actin cytoskeletal reorganization following Gas6 treatment). In contrast, NLT cells expressing GFP and dominant negative Rac (Ad GFP/N17Rac) did not form lamellipodia in the presence of agonist, whereas uninfected neighboring neuronal cells responded to Gas6 with profound actin reorganization (Fig. 3B, right panels) (7% ± 2% of GFP/N17Rac-expressing cells responded to Gas6; n = 9, P < 0.0001). Similarly, NLT cells expressing Ad GFP migrated in response to Gas6 (1.82-fold increase), whereas cells expressing Ad GFP/N17Rac were unresponsive to agonist treatment (1.05-fold increase relative to control) (Fig. 3C) (n = 4, P < 0.05). In addition, the basal migration of GFP/N17Rac-expressing neuronal cells was only 29% ± 8% of that of GFP-expressing cells. Together, these data demonstrate that Rac activity is obligatory for both basal and Gas6/Ark-mediated cytoskeletal reorganization and chemotaxis of NLT GnRH neuronal cells.
The ERK pathway is not essential for Gas6/Ark-induced migration of GnRH neuronal cells.
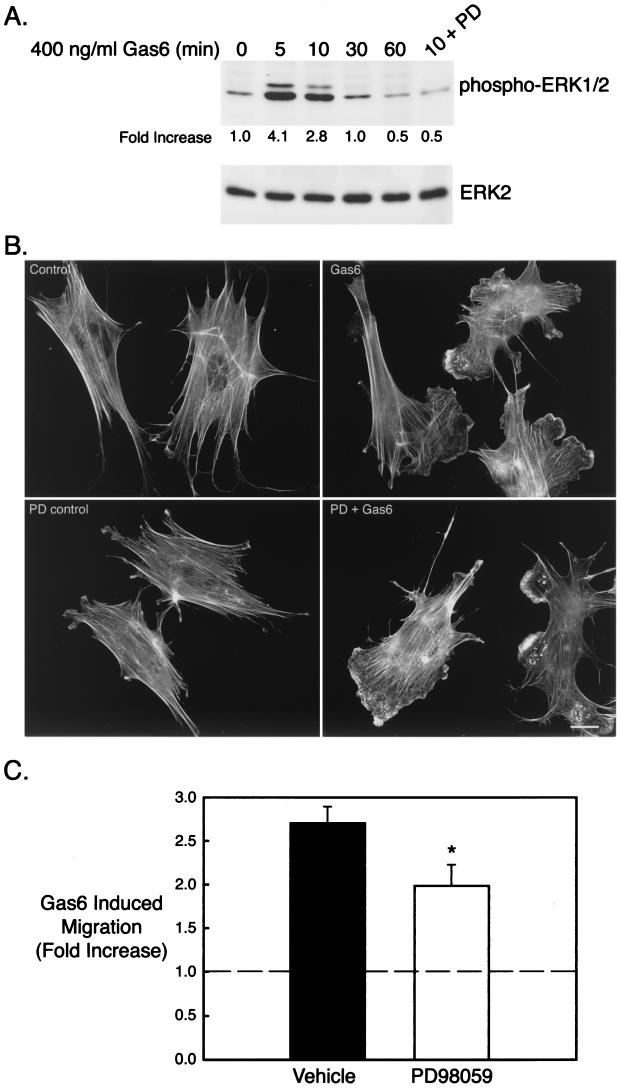
Previous studies from our laboratory implicated the ERK pathway in Gas6/Ark mediated GnRH neuronal survival and regulation of GnRH gene expression (2; M. P. Allen et al., 82nd Ann. Meet. Endocr. Soc. abstr. 519, 2000). Furthermore, ERK is required for migration of other cell types (69). Therefore, we investigated whether the ERK pathway played a role in Gas6/Ark-mediated GnRH neuronal migration. In NLT GnRH neuronal cells, Gas6 stimulated a rapid and transient activation of the ERK pathway that peaked at 5 min (4.1-fold) and returned to baseline by 30 min (Fig. 4A). Furthermore, Gas6-stimulated ERK activation was effectively abolished by the MEK1/2 inhibitor PD98059 (Fig. 4A). To determine if the ERK pathway was required for Gas6/Ark-induced ruffling and lamellipodial formation, NLT cells were incubated with PD98059 (30 μM, 4 h), stimulated with agonist for 10 min, and stained with rhodamine phalloidin (Fig. 4B). Although PD98059 clearly blocked ERK activation (Fig. 4A), it had no discernible effect on Gas6-mediated actin remodeling of NLT cells (Fig. 4B) (Gas6 plus dimethyl sulfoxide [DMSO], 71% ± 5% ruffling and/or lamellipodia, versus Gas6 plus PD98059, 61% ± 3%; n = 9, P = 0.13). Likewise, the MEK1/2 inhibitor only partially attenuated Gas6-stimulated NLT migration (vehicle, 2.7-fold increase; PD98059, 2.0-fold increase; n = 5, P = 0.05) (Fig. 4C) (similar results were obtained with the structurally distinct MEK inhibitor, UO126; data not shown). Thus, although the ERK pathway acts downstream of Gas6 and Ark to influence GnRH neuronal survival and gene expression, it is not essential in the migratory signaling pathway.
FIG. 4.
The ERK pathway is not essential for development of the motile phenotype induced by Gas6/Ark signaling. (A) NLT cells were treated for the indicated times with Gas6 (400 ng/ml) and immunoblotted for phospho-ERK1/2 and total ERK2. In the last lane, cells were pretreated for 4 h with PD98059 (PD) (30 μM) and then stimulated with Gas6 for 10 min. The data are representative of two independent experiments. The increase in phospho-ERK1/2 was calculated by dividing the phospho-ERK1/2 density by the total ERK2 density at each time point and then setting the zero time point value to 1. (B) NLT cells were pretreated for 4 h with PD (30 μM), stimulated with Gas6 (10 min), and then stained with rhodamine phalloidin. The data are representative of four independent experiments. Bar = 20 μm. (C) NLT cells were subjected to the migration assay in the presence of vehicle (DMSO) or PD (30 μM) as described in Materials and Methods. The data are the fold increase in migration in the presence of Gas6 (400 ng/ml). *, P = 0.05 (n = 5, t test).
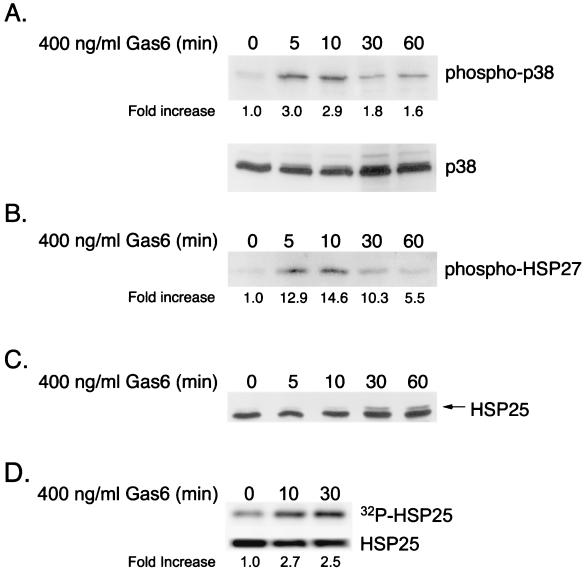
Gas6/Ark signaling induces migration of GnRH neuronal cells via a Rac → p38 MAPK → MAPKAP kinase 2 → HSP25 pathway.
Recent studies have suggested a role for p38 MAPK in smooth muscle cell motility (20). Therefore, Gas6/Ark-induced p38 activation in NLT neuronal cells was examined by immunoblotting with a phospho-specific p38 antiserum (Fig. 5A. Agonist treatment resulted in marked p38 activation within 5 min (3.0-fold) that remained elevated for at least 1 h. In smooth muscle cells, growth factor-induced migration involves activation of a p38 MAPK → MAPKAP kinase 2 → heat shock protein 27 (HSP27) pathway, where HSP27 functions as an actin-capping protein and is thought to modulate actin polymerization and cell shape (29). Gas6/Ark-induced MAPKAP kinase 2 activity was evaluated in NLT cells by using an immunoprecipitation-kinase assay (Fig. 5B). MAPKAP kinase 2 was immunoprecipitated from Gas6-treated cells and incubated with recombinant human HSP27 in a kinase assay, and phosphorylated HSP27 was detected by using a phospho-specific HSP27 antiserum. As shown in Fig. 5B, Gas6 treatment resulted in a 12.9-fold increase in MAPKAP kinase 2 activity within 5 min that remained elevated for at least 1 h. To examine if endogenous NLT HSP25 (the murine isoform of HSP27) was activated (phosphorylated) following agonist treatment, an HSP25 immunoblot was performed (Fig. 5C). Exposure to Gas6 resulted in the increased formation of a slower-migrating form of HSP25 within 30 min that was sustained to 60 min, suggesting that Gas6 had indeed stimulated phosphorylation of HSP25. To confirm the phosphorylation of HSP25, NLT cells were pretreated with 32Pi to radiolabel the cellular ATP, and HSP25 was immunoprecipitated from lysates that had been treated with Gas6 for 10 to 30 min. Gas6 treatment resulted in a 2.7-fold increase in the incorporation of 32Pi into HSP25 within 10 min (Fig. 5D, top) while having no effect on the total amount of HSP25 present in the cells (Fig. 5D, bottom). Thus, Gas6/Ark signaling promotes the sequential activation of p38 MAPK, MAPKAP kinase 2, and HSP25 in NLT GnRH neuronal cells.
FIG. 5.
Gas6/Ark signaling activates a p38 MAPK → MAPKAP kinase 2 → HSP25 signaling cascade in NLT GnRH neuronal cells. (A) NLT cells were treated for the indicated times with Gas6 (400 ng/ml) and immunoblotted for phospho-p38 and total p38. The data are representative of three independent experiments. The increase in phospho-p38 was calculated by dividing the phospho-p38 density by the total p38 density at each time point and setting the zero time point value to 1. (B) MAPKAP kinase 2 was immunoprecipitated from Gas6-treated neuronal cells and exposed to recombinant human HSP27 in a kinase assay, and phosphorylated HSP27 was detected by phospho-HSP27 immunoblotting. The data are representative of two independent experiments. The increase in phospho-HSP27 was calculated by setting the zero time point value at 1. (C) NLT cells were treated for the indicated times with Gas6 (400 ng/ml) and immunoblotted for HSP25. The arrow indicates a slower-migrating form of HSP25 consistent with phosphorylation. The data are representative of two independent experiments. (D) NLT cells were labeled with 32Pi and stimulated with Gas6. HSP25 was immunoprecipitated from the cell lysates, transferred to PVDF, and analyzed by autoradiography (top) or HSP25 immunoblotting (bottom). The increase in 32P-labeled HSP25 was calculated by dividing the 32P-labeled-HSP25 density by the total HSP25 density at each time point and setting the zero time point value to 1. The data are representative of two experiments.
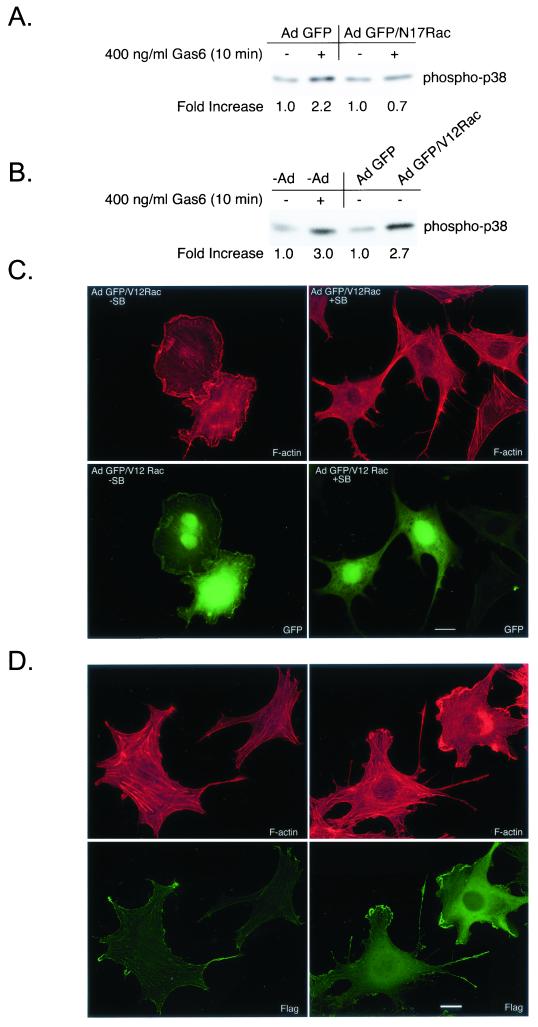
Rac and Cdc42 GTPases have been shown to function upstream of p38 MAPK (71). To resolve whether Gas6/Ark-stimulated p38 MAPK activation was downstream of Rac, NLT cells were infected with Ad GFP or with Ad GFP/N17Rac prior to Gas6 addition (Fig. 6A). Gas6 treatment of GFP-expressing NLT neuronal cells resulted in p38 activation (2.2-fold), while cells expressing GFP/N17Rac were unresponsive to the agonist (0.7-fold), demonstrating that p38 is downstream of Rac in this receptor system (Fig. 6A). In addition, expression of adenoviral constitutively active Rac (Ad GFP/V12Rac) in NLT cells resulted in ligand-independent p38 activation (2.7-fold) (Fig. 6B) and induced dramatic membrane ruffling (Fig. 6C, left panels). Furthermore, V12Rac-stimulated ruffling (GFP/V12Rac without SB203580, 82% ± 5%) was markedly inhibited upon exposure to the selective p38 inhibitor, SB203580 (34% ± 7%), demonstrating that p38 activation was required for V12Rac-induced cytoskeletal remodeling (Fig. 6C, right panels) (n = 12, P < 0.0001). Finally, NLT cells coinfected with adenoviral MKK6CA and Flag-tagged wild-type p38α were examined by immunoblot analysis (Fig. 7B) and in the actin assay (Fig. 6D). Expression of adenoviral MKK6CA and Flag-p38α in NLT neuronal cells resulted in robust p38 activation (Fig. 7B, phospho-p38 blot). Consistent with p38 activation promoting cytoskeletal reorganization, NLT cells positive for the Flag epitope (p38α) exhibited ligand-independent lamellipodial advance (Fig. 6D, right panels), whereas uninfected cells did not extend lamellipodia (Fig. 6D, left panels) (9% ± 2% for uninfected cells versus 76% ± 4% for MKK6CA/p38α-infected cells; n = 18, P < 0.0001). Collectively, these data demonstrate that p38 lies downstream of Rac and is the major Rac target engaged in NLT GnRH neuronal cells to elicit actin cytoskeletal remodeling.
FIG. 6.
p38 activation promotes actin reorganization downstream of Rac in NLT GnRH neuronal cells. (A) NLT cells expressing Ad GFP or GFP/N17Rac were treated for 10 min with Gas6 (400 ng/ml) and then immunoblotted for phospho-p38. The data are representative of two independent experiments. The increase in phospho-p38 was calculated by setting the density of the no-Gas6 samples to 1. (B) uninfected NLT cells or those expressing Ad GFP or GFP/V12Rac were treated for 10 min with Gas6 (400 ng/ml) or vehicle and then immunoblotted for phospho-p38. The data are representative of two independent experiments. (C) NLT cells were infected with adenoviruses expressing GFP/V12Rac (see Materials and Methods), treated for 4 h with vehicle (DMSO) or SB203580 (30 μM) and then stained with rhodamine phalloidin. The data are representative of two independent experiments. Actin is shown in red and GFP is in green. Bar = 20 μm. (D) NLT cells infected with adenoviral MKK6CA and Flag-p38α were visualized with rhodamine phalloidin and anti-Flag immunocytochemistry (left panels, uninfected cells; right panels, cells infected with MKK6CA and Flag-p38α). Bar = 20 μm.
FIG. 7.
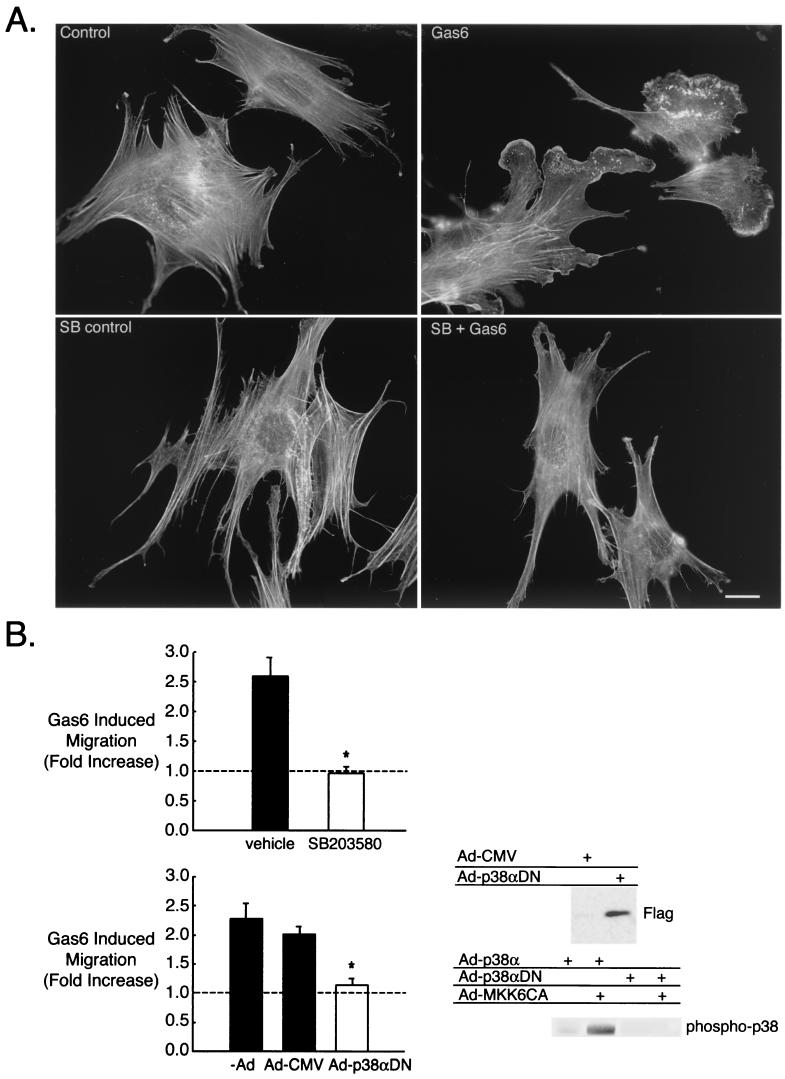
Gas6/Ark-mediated GnRH neuronal cytoskeletal remodeling and migration requires p38 MAPK. (A) NLT cells were pretreated for 4 h with SB203580 (SB) (30 μM), stimulated with Gas6 (10 min), and then stained with rhodamine phalloidin. Bar = 20 μm. The data are representative of four independent experiments. (B) (Left) The effect of Gas6 on NLT migration was examined in the presence of vehicle (DMSO) or SB (30 μM) and in neurons infected with either Ad-CMV or Ad-p38αDN as described in Materials and Methods. *, P < 0.05 for SB versus vehicle (n = 3, t test); P < 0.01 for p38αDN versus −Ad and P < 0.05 for p38αDN versus Ad-CMV (n = 4, Tukey-Kramer multiple comparisons test). (Right) Anti-Flag immunoblotting demonstrated that adenoviral Flag-p38αDN was efficiently expressed in the NLT neurons. The phospho-p38 blot demonstrated that wild-type p38α (Ad-p38α) was activated in the presence of Ad-MKK6CA, but Ad-p38αDN was not.
Next we investigated the role of p38 in Gas6/Ark-stimulated lamellipodial extension and migration. Pretreatment of NLT cells with the p38 inhibitor SB203580 (30 μM, 4 h), resulted in a striking inhibition of Gas6/Ark-mediated cytoskeletal remodeling (Fig. 7A) (71% ± 5% membrane ruffles and/or lamellipodia for Gas6 plus DMSO versus 20% ± 3% for Gas6 plus SB303580; n = 9, P < 0.0001). SB203580 also markedly blocked Gas6-induced NLT migration, decreasing the Gas6 effect from 2.6-fold to 0.96-fold (Fig. 7B) (n = 3, P < 0.05).
To further verify that p38 MAPK was required for NLT migration via Gas6/Ark signaling, NLT cells infected with adenoviral Flag-tagged p38αDN were tested in the migration assay. As shown in Fig. 7B, the Flag-p38αDN was efficiently expressed in the NLT cells (Flag immunoblot). However, in the presence of adenovirally expressed MKK6CA, p38 activation was blocked in p38αDN-expressing neuronal cells, while those expressing wild-type p38α exhibited significant increases in active p38 (phospho-p38 blot). These data confirmed that p38αDN functioned in a dominant negative fashion. In addition, cells infected with empty adenovirus (Ad-CMV) responded to Gas6 similarly to uninfected cells (2.0- versus 2.3-fold increase relative to control). NLT cells infected with Ad-p38αDN, however, were unresponsive to Gas6 (1.14-fold stimulation) (n = 4, P < 0.01 for uninfected cells and P < 0.05 for cells infected with Ad-CMV). Together, the data illustrate that Gas6/Ark-mediated GnRH neuronal migration requires p38 MAPK.
DISCUSSION
The intrinsic signaling mechanisms that regulate GnRH neuron migration are unknown. In the present study, we used immortalized GnRH neuronal cell lines that retain properties found in vivo in either migratory or postmigratory GnRH neurons to investigate the mechanism underlying GnRH neuron migration. Because Ark is expressed in immortalized migratory GnRH neuronal cell lines (10) and along the GnRH neuron pathway during development (1), we examined a role for Ark in GnRH neuronal motility. Our results identified the Gas6/Ark interaction as a novel signaling complex that promotes NLT GnRH neuronal-cell migration. Characterization of the signaling pathway downstream of Gas6/Ark revealed that the small GTPase, Rac, signals to p38 MAPK to induce NLT GnRH neuronal motility. These data are the first to describe a receptor-initiated signal transduction cascade that directly regulates the migration of GnRH neuronal cells.
The Ark receptor ECD contains fibronectin type III repeats and immunoglobulin domains related to cell adhesion molecules. Not surprisingly, Ark has recently been implicated in cell adhesion and aggregation (4, 38). Moreover, Fridell et al. (13) recently provided evidence for Axl (the human homolog of Ark)-mediated chemotaxis of vascular smooth muscle cells. However, the signaling pathways downstream of Axl were not identified. While examining a role for Ark in GnRH neuron migration, we found that Gas6, the Ark ligand, potentiated the migration of Ark-expressing GN10 and NLT GnRH neuronal cell lines while having no discernible effect on GT1-7 cells. In addition, Gas6-induced motility was chemotactic and was blocked by an antibody to the Ark ECD and by sequestration of Gas6 with recombinant Axl ECD. Thus, Gas6 occupancy of the Ark receptor stimulates GnRH neuronal-cell migration.
To examine signaling pathways involved in motility downstream of Gas6/Ark, we first looked at changes in the actin cytoskeleton. In NLT cells, Gas6/Ark signaling stimulated dramatic lamellipodial extension and membrane ruffling. Members of the Rho family of small GTPases, Rho, Rac, and Cdc42, regulate morphological changes of the actin cytoskeleton and, as a result, elicit profound effects on cellular adhesion and motility (17). Rac is known to promote formation of lamellipodia and membrane ruffles (53). Thus, the actin remodeling observed upon Gas6 treatment suggested that Rac was activated downstream of the receptor-ligand interaction in GnRH neuronal cells. Indeed, direct measurement of active (GTP-bound) Rac revealed that Gas6 induced robust Rac activation in NLT cells. Moreover, dominant negative N17Rac significantly abolished both Gas6-induced actin cytoskeletal remodeling and migration of NLT cells. Together, these results demonstrated that the Ark receptor couples to the Rho family GTPase Rac to orchestrate actin cytoskeletal changes and chemotaxis of GnRH neuronal cells. These findings are consistent with a recently recognized role for Rac GTPase in the regulation of neuronal migration (73). Although several neuron-specific guanine nucleotide exchange factors, including Tiam-1 (9, 31), ASEF (25), and Trio (3, 34, 43), upstream of Rac have been described, downstream targets of Rac in neurons are largely unknown.
Both ERK and p38 MAPK pathways have previously been described to play a role in nonneuronal-cell migration (20, 69), and both pathways have been shown to be activated via Rac GTPase (7, 71). Therefore, we focused on the potential role of these two MAPK signaling pathways downstream of Rac in Gas6/Ark-stimulated GnRH neuronal-cell motility. Although the MEK1/2 inhibitor PD98059 clearly blocked Gas6/Ark-stimulated ERK activation, it had only modest effects on cytoskeletal changes and motility. The effect of PD98059 on Gas6-induced migration reached statistical significance (P = 0.05), however, suggesting that the ERK pathway may regulate a Rac-independent pathway involved in migration. For instance, recent studies have shown that Rho activity is regulated by the ERK-MAPK pathway (56). Since Rho is required for focal adhesion turnover at both the leading and trailing edges of the cell, disruption of Rho signaling results in decreased motility. Thus, in NLT cells, the ERK pathway blockade may inhibit motility via partial disruption of Rho signaling. Alternatively, PD98059 may affect NLT survival, consistent with our previous studies showing that Ark promotes GnRH neuronal survival via an ERK-dependent mechanism (2).
In contrast, pharmacological p38 MAPK inhibition dramatically attenuated Gas6/Ark-induced cytoskeletal reorganization and migration. Similarly, p38αDN completely blocked Gas6-induced GnRH neuronal chemotaxis. In addition, p38 MAPK was demonstrated to function as a downstream Rac target, since Gas6 activation of p38 was abolished in dominant negative-N17Rac-expressing cells. Moreover, constitutively active V12Rac stimulated p38 activation and induced dramatic membrane ruffling of NLT cells. The latter effect was significantly inhibited by blockade of p38 activation with SB203580, suggesting that p38 is the major Rac target engaged in GnRH neuronal cells to elicit cytoskeletal reorganization. Finally, overexpression of constitutively active p38 was sufficient to induce lamellipodial advance and membrane ruffling in NLT cells. Thus, p38 MAPK acts downstream of Rac GTPase to promote GnRH neuronal-cell motility.
The above findings were somewhat unexpected, since p38 activation in neurons was initially associated with increased apoptosis (27, 40, 68). Recently, p38 has also been shown to modulate PC12 pheochromocytoma cell neurite outgrowth during differentiation (18, 22, 41, 63). Coupled with our observation that p38 lies downstream of Rac in Gas6/Ark-stimulated GnRH neuron motility, these results are consistent with a much broader role for p38 MAPK in neurons, perhaps in the regulation of migration and neurite outgrowth during development and differentiation.
p38 MAPK has been shown to modulate nonneuronal-cell motility (20, 21, 26, 37, 44, 55). Recent studies suggest that p38 MAPK activates a MAPKAP kinase 2/HSP27 (HSP25 in mouse) signaling cascade that is important in smooth muscle cell actin cytoskeletal remodeling and motility (20, 30, 46, 55). HSP27 is an actin-capping protein whose activity is regulated via serine phosphorylation by upstream kinases such as MAPKAP kinase 2. Although the mechanism is not completely understood, HSP27 regulation of actin polymerization is involved in cortical actin cytoskeletal rearrangements such as membrane ruffling (29). Similar to growth factor stimulation of smooth muscle cells (20), we found that NLT GnRH neuronal cells treated with Gas6 exhibited increased MAPKAP kinase 2 activity and HSP25 phosphorylation. These data are consistent with a mechanism by which Gas6/Ark signaling stimulates GnRH neuronal migration via a Rac → p38 MAPK → MAPKAP kinase 2 → HSP25 pathway. The role of additional signaling pathways downstream of p38 that may also influence motility remains to be clarified.
In summary, we have utilized GnRH neuronal cell lines as a model system to identify and characterize factors potentially involved in GnRH neuron migration. Our studies point to several new candidate proteins, including Gas6, Ark, and p38 MAPK, as potential regulators of GnRH neuron migration in vivo. These factors will be targeted in future in vivo studies to determine if they are required for the proper migration of GnRH neurons during development.
Acknowledgments
We thank Carrie Swartz and Peter Watson for their assistance in developing the migration assays. We are also grateful to Tracey Laessig for preparing adenoviruses and Ron Bouchard for his expertise in cellular imaging and figure production. Finally, we thank Roger Davis for the p38 MAPK and MKK6CA plasmids.
This work was supported by National Institutes of Health grants HD08667-02 to M.P.A. and HD31191-03 to M.E.W.
REFERENCES
- 1.Allen, M. P., M. Xu, C. Zeng, S. A. Tobet, and M. E. Wierman. 2000. Myocyte enhancer factors-2B and -2C are required for adhesion related kinase repression of neuronal gonadotropin releasing hormone gene expression. J. Biol. Chem. 275:39662–39670. [DOI] [PubMed] [Google Scholar]
- 2.Allen, M. P., C. Zeng, K. Schneider, X. Xiong, M. K. Meintzer, P. Bellosta, C. Basilico, B. Varnum, K. A. Heidenreich, and M. E. Wierman. 1999. Growth arrest-specific gene 6 (Gas6)/adhesion related kinase (Ark) signaling promotes gonadotropin-releasing hormone neuronal survival via extracellular signal-regulated kinase (ERK) and Akt. Mol. Endocrinol. 13:191–201. [DOI] [PubMed] [Google Scholar]
- 3.Bateman, J., H. Shu, and D. Van Vactor. 2000. The guanine nucleotide exchange factor trio mediates axonal development in the Drosophila embryo. Neuron 26:93–106. [DOI] [PubMed] [Google Scholar]
- 4.Bellosta, P., M. Costa, D. A. Lin, and C. Basilico. 1995. The receptor tyrosine kinase ARK mediates cell aggregation by homophilic binding. Mol. Cell. Biol. 15:614–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellosta, P., Q. Zhang, S. P. Goff, and C. Basilico. 1997. Signaling through the ARK tyrosine kinase receptor protects from apoptosis in the absence of growth stimulation. Oncogene 15:2387–2397. [DOI] [PubMed] [Google Scholar]
- 6.Benard, V., B. P. Bohl, and G. M. Bokoch. 1999. Characterization of rac and cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J. Biol. Chem. 274:13198–13204. [DOI] [PubMed] [Google Scholar]
- 7.Clerk, A., F. H. Pham, S. J. Fuller, E. Sahai, K. Aktories, R. Marais, C. Marshall, and P. H. Sugden. 2001. Regulation of mitogen-activated protein kinases in cardiac myocytes through the small G protein Rac1. Mol. Cell. Biol. 21:1173–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crosier, K. E., and P. S. Crosier. 1997. New insights into the control of cell growth; the role of the AxI family. Pathology 29:131–135. [DOI] [PubMed] [Google Scholar]
- 9.Ehler, E., F. van Leeuwen, J. G. Collard, and P. C. Salinas. 1997. Expression of Tiam-1 in the developing brain suggests a role for the Tiam-1-Rac signaling pathway in cell migration and neurite outgrowth. Mol. Cell. Neurosci. 9:1–12. [DOI] [PubMed] [Google Scholar]
- 10.Fang, Z., X. Xiong, A. James, D. F. Gordon, and M. E. Wierman. 1998. Identification of novel factors that regulate GnRH gene expression and neuronal migration. Endocrinology 139:3654–3657. [DOI] [PubMed] [Google Scholar]
- 11.Franco, B., S. Guioli, A. Pragliola, B. Incerti, B. Bardoni, R. Tonlorenzi, R. Carrozzo, E. Maestrini, M. Pieretti, and P. Taillon-Miller. 1991. A gene deleted in Kallmann’s syndrome shares homology with neural cell adhesion and axonal path-finding molecules. Nature 353:529–536. [DOI] [PubMed] [Google Scholar]
- 12.Fridell, Y. W., Y. Jin, L. A. Quilliam, A. Burchert, P. McCloskey, G. Spizz, B. Varnum, C. Der, and E. T. Liu. 1996. Differential activation of the Ras/extracellular-signal-regulated protein kinase pathway is responsible for the biological consequences induced by the Axl receptor tyrosine kinase. Mol. Cell. Biol. 16:135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fridell, Y. W., J. Villa, Jr., E. C. Attar, and E. T. Liu. 1998. GAS6 induces Axl-mediated chemotaxis of vascular smooth muscle cells. J. Biol. Chem. 273:7123–7126. [DOI] [PubMed] [Google Scholar]
- 14.Gomez-Foix, A. M., W. S. Coats, S. Baque, T. Alam, R. D. Gerard, and C. B. Newgard. 1992. Adenovirus-mediated transfer of the muscle glycogen phosphorylase gene into hepatocytes confers altered regulation of glycogen metabolism. J. Biol. Chem. 267:25129–25134. [PubMed] [Google Scholar]
- 15.Goruppi, S., E. Ruaro, and C. Schneider. 1996. Gas6, the ligand of Axl tyrosine kinase receptor, has mitogenic and survival activities for serum starved NIH3T3 fibroblasts. Oncogene 12:471–480. [PubMed] [Google Scholar]
- 16.Goruppi, S., E. Ruaro, B. Varnum, and C. Schneider. 1999. Gas6-mediated survival in NIH3T3 cells activates stress signalling cascade and is independent of Ras. Oncogene 18:4224–4236. [DOI] [PubMed] [Google Scholar]
- 17.Hall, A. 1998. Rho GTPases and the actin cytoskeleton. Science 279:509–514. [DOI] [PubMed] [Google Scholar]
- 18.Hansen, T. O., J. F. Rehfeld, and F. C. Nielsen. 2000. Cyclic AMP-induced neuronal differentiation via activation of p38 mitogen-activated protein kinase. J. Neurochem. 75:1870–1877. [DOI] [PubMed] [Google Scholar]
- 19.He, T. C., S. Zhou, L. T. da Costa, J. Yu, K. W. Kinzler, and B. Vogelstein. 1998. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA 95:2509–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hedges, J. C., M. A. Dechert, I. A. Yamboliev, J. L. Martin, E. Hickey, L. A. Weber, and W. T. Gerthoffer. 1999. A role for p38(MAPK)/HSP27 pathway in smooth muscle cell migration. J. Biol. Chem. 274:24211–24219. [DOI] [PubMed] [Google Scholar]
- 21.Heuertz, R. M., S. M. Tricomi, U. R. Ezekiel, and R. O. Webster. 1999. C-reactive protein inhibits chemotactic peptide-induced p38 mitogen-activated protein kinase activity and human neutrophil movement. J. Biol. Chem. 274:17968–17974. [DOI] [PubMed] [Google Scholar]
- 22.Iwasaki, S., M. Iguchi, K. Watanabe, R. Hoshino, M. Tsujimoto, and M. Kohno. 1999. Specific activation of the p38 mitogen-activated protein kinase signaling pathway and induction of neurite outgrowth in PC12 cells by bone morphogenetic protein-2. J. Biol. Chem. 274:26503–26510. [DOI] [PubMed] [Google Scholar]
- 23.Janssen, J. W., A. S. Schulz, A. C. Steenvoorden, M. Schmidberger, S. Strehl, P. F. Ambros, and C. R. Bartram. 1991. A novel putative tyrosine kinase receptor with oncogenic potential. Oncogene 6:2113–2120. [PubMed] [Google Scholar]
- 24.Kallmann, F. J., W. A. Schoenfeld, and S. E. Barrera. 1944. The genetic aspects of primary eunuchoidism. Am. J. Ment. Defic. 48:203–236. [Google Scholar]
- 25.Kawasaki, Y., T. Senda, T. Ishidate, R. Koyama, T. Morishita, Y. Iwayama, O. Higuchi, and T. Akiyama. 2000. Asef, a link between the tumor suppressor APC and G-protein signaling. Science 289:1194–1197. [DOI] [PubMed] [Google Scholar]
- 26.Kimura, T., T. Watanabe, K. Sato, J. Kon, H. Tomura, K. Tamama, A. Kuwabara, T. Kanda, I. Kobayashi, H. Ohta, M. Ui, and F. Okajima. 2000. Sphingosine 1-phosphate stimulates proliferation and migration of human endothelial cells possibly through the lipid receptors, Edg-1 and Edg-3. Biochem. J. 348(Pt. 1):71–76. [PMC free article] [PubMed] [Google Scholar]
- 27.Kummer, J. L., P. K. Rao, and K. A. Heidenreich. 1997. Apoptosis induced by withdrawal of trophic factors is mediated by p38 mitogen-activated protein kinase. J.Biol. Chem. 272:20490–20494. [DOI] [PubMed] [Google Scholar]
- 28.Lai, C., and G. Lemke. 1991. An extended family of protein-tyrosine kinase genes differentially expressed in the vertebrate nervous system. Neuron 6:691–704. [DOI] [PubMed] [Google Scholar]
- 29.Landry, J., and J. Huot. 1995. Modulation of actin dynamics during stress and physiological stimulation by a signaling pathway involving p38 MAP kinase and heat-shock protein 27. Biochem. Cell Biol. 73:703–707. [DOI] [PubMed] [Google Scholar]
- 30.Landry, J., H. Lambert, M. Zhou, J. N. Lavoie, E. Hickey, L. A. Weber, and C. W. Anderson. 1992. Human HSP27 is phosphorylated at serines 78 and 82 by heat shock and mitogen-activated kinases that recognize the same amino acid motif as S6 kinase II. J. Biol. Chem. 267:794–803. [PubMed] [Google Scholar]
- 31.Leeuwen, F. N., H. E. Kain, R. A. Kammen, F. Michiels, O. W. Kranenburg, and J. G. Collard. 1997. The guanine nucleotide exchange factor Tiam1 affects neuronal morphology: opposing roles for the small GTPases Rac and Rho. J. Cell Biol. 139:797–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Legouis, R., J. P. Hardelin, J. Levilliers, J. M. Claverie, S. Compain, V. Wunderle, P. Millasseau, D. Le Paslier, D. Cohen, D. Caterina, et al. 1991. The candidate gene for the X-linked Kallmann syndrome encodes a protein related to adhesion molecules. Cell 67:423–435. [DOI] [PubMed] [Google Scholar]
- 33.Li, R., J. Chen, G. Hammonds, H. Phillips, M. Armanini, P. Wood, R. Bunge, P. J. Godowski, M. X. Sliwkowski, and J. P. Mather. 1996. Identification of Gas6 as a growth factor for human Schwann cells. J. Neurosci. 16:2012–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liebl, E. C., D. J. Forsthoefel, L. S. Franco, S. H. Sample, J. E. Hess, J. A. Cowger, M. P. Chandler, A. M. Shupert, and M. A. Seeger. 2000. Dosage-sensitive, reciprocal genetic interactions between the Abl tyrosine kinase and the putative GEF Trio reveal Trio’s role in axon pathfinding. Neuron 26:107–118. [DOI] [PubMed] [Google Scholar]
- 35.Lu, Q., M. Gore, Q. Zhang, T. Camenisch, S. Boast, F. Casagranda, C. Lai, M. K. Skinner, R. Klein, G. K. Matsushima, H. S. Earp, S. P. Goff, and G. Lemke. 1999. Tyro-3 family receptors are essential regulators of mammalian spermatogenesis. Nature 398:723–728. [DOI] [PubMed] [Google Scholar]
- 36.Maggi, R., F. Pimpinelli, L. Molteni, M. Milani, L. Martini, and F. Piva. 2000. Immortalized luteinizing hormone-releasing hormone neurons show a different migratory activity in vitro. Endocrinology 141:2105–2112. [DOI] [PubMed] [Google Scholar]
- 37.Matsumoto, T., K. Yokote, K. Tamura, M. Takemoto, H. Ueno, Y. Saito, and S. Mori. 1999. Platelet-derived growth factor activates p38 mitogen-activated protein kinase through a Ras-dependent pathway that is important for actin reorganization and cell migration. J. Biol. Chem. 274:13954–13960. [DOI] [PubMed] [Google Scholar]
- 38.McCloskey, P., Y. W. Fridell, E. Attar, J. Villa, Y. Jin, B. Varnum, and E. T. Liu. 1997. GAS6 mediates adhesion of cells expressing the receptor tyrosine kinase Axl. J. Biol. Chem. 272:23285–23291. [DOI] [PubMed] [Google Scholar]
- 39.Mellon, P. L., J. J. Windle, P. C. Goldsmith, C. A. Padula, J. L. Roberts, and R. I. Weiner. 1990. Immortalization of hypothalamic GnRH neurons by genetically targeted tumorigenesis. Neuron 5:1–10. [DOI] [PubMed] [Google Scholar]
- 40.Michael, Z. W., M. K. Meintzer, P. Rao, J. Marotti, X. Wang, J. E. Esplen, E. D. Clarkson, C. R. Freed, and K. A. Heidenreich. 2001. Inhibitors of p38 MAP kinase increase the survival of transplanted dopamine neurons. Brain Res. 891:185–196. [DOI] [PubMed] [Google Scholar]
- 41.Morooka, T., and E. Nishida. 1998. Requirement of p38 mitogen-activated protein kinase for neuronal differentiation in PC12 cells. J. Biol. Chem. 273:24285–24288. [DOI] [PubMed] [Google Scholar]
- 42.Nagata, K., K. Ohashi, T. Nakano, H. Arita, C. Zong, H. Hanafusa, and K. Mizuno. 1996. Identification of the product of growth arrest-specific gene 6 as a common ligand for Axl, Sky, and Mer receptor tyrosine kinases. J. Biol. Chem. 271:30022–30027. [DOI] [PubMed] [Google Scholar]
- 43.Newsome, T. P., S. Schmidt, G. Dietzl, K. Keleman, B. Asling, A. Debant, and B. J. Dickson. 2000. Trio combines with Dock to regulate Pak activity during photoreceptor axon pathfinding in Drosophila. Cell 101:283–294. [DOI] [PubMed] [Google Scholar]
- 44.Nick, J. A., S. K. Young, K. K. Brown, N. J. Avdi, P. G. Arndt, B. T. Suratt, M. S. Janes, P. M. Henson, and G. S. Worthen. 2000. Role of p38 mitogen-activated protein kinase in a murine model of pulmonary inflammation. J. Immunol. 164:2151–2159. [DOI] [PubMed] [Google Scholar]
- 45.O’Bryan, J. P., R. A. Frye, P. C. Cogswell, A. Neubauer, B. Kitch, C. Prokop, R. Espinosa III, M. M. Le Beau, H. S. Earp, and E. T. Liu. 1991. axl, a transforming gene isolated from primary human myeloid leukemia cells, encodes a novel receptor tyrosine kinase. Mol. Cell. Biol. 11:5016–5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Piotrowicz, R. S., J. L. Martin, W. H. Dillman, and E. G. Levin. 1997. The 27-kDa heat shock protein facilitates basic fibroblast growth factor release from endothelial cells. J. Biol. Chem. 272:7042–7047. [DOI] [PubMed] [Google Scholar]
- 47.Prieto, A. L., J. L. Weber, and C. Lai. 2000. Expression of the receptor protein-tyrosine kinases Tyro-3, Axl, and mer in the developing rat central nervous system. J. Comp. Neurol. 425:295–314. [PubMed] [Google Scholar]
- 48.Prieto, A. L., J. L. Weber, S. Tracy, M. J. Heeb, and C. Lai. 1999. Gas6, a ligand for the receptor protein-tyrosine kinase Tyro-3, is widely expressed in the central nervous system. Brain Res. 816:646–661. [DOI] [PubMed] [Google Scholar]
- 49.Radovick, S., S. Wray, E. Lee, D. K. Nicols, Y. Nakayama, B. D. Weintraub, H. Westphal, G. B. Cutler, Jr., and F. E. Wondisford. 1991. Migratory arrest of gonadotropin-releasing hormone neurons in transgenic mice. Proc. Natl. Acad. Sci. USA 88:3402–3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raingeaud, J., S. Gupta, J. S. Rogers, M. Dickens, J. Han, R. J. Ulevitch, and R. J. Davis. 1995. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J. Biol. Chem. 270:7420–7426. [DOI] [PubMed] [Google Scholar]
- 51.Raingeaud, J., A. J. Whitmarsh, T. Barrett, B. Derijard, and R. J. Davis. 1996. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol. Cell. Biol. 16:1247–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rescigno, J., A. Mansukhani, and C. Basilico. 1991. A putative receptor tyrosine kinase with unique structural topology. Oncogene 6:1909–1913. [PubMed] [Google Scholar]
- 53.Ridley, A. J., H. F. Paterson, C. L. Johnston, D. Diekmann, and A. Hall. 1992. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell 70:401–410. [DOI] [PubMed] [Google Scholar]
- 54.Ronnekleiv, O. K., and J. A. Resko. 1990. Ontogeny of gonadotropin-releasing hormone-containing neurons in early fetal development of rhesus macaques. Endocrinology 126:498–511. [DOI] [PubMed] [Google Scholar]
- 55.Rousseau, S., F. Houle, J. Landry, and J. Huot. 1997. p38 MAP kinase activation by vascular endothelial growth factor mediates actin reorganization and cell migration in human endothelial cells. Oncogene 15:2169–2177. [DOI] [PubMed] [Google Scholar]
- 56.Sahai, E., M. F. Olson, and C. J. Marshall. 2001. Cross-talk between Ras and Rho signalling pathways in transformation favours proliferation and increased motility. EMBO J. 20:755–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schaack, J., S. Langer, and X. Guo. 1995. Efficient selection of recombinant adenoviruses by vectors that express beta-galactosidase. J. Virol. 69:3920–3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schwanzel-Fukuda, M., D. Bick, and D. W. Pfaff. 1989. Luteinizing hormone-releasing hormone (LHRH)-expressing cells do not migrate normally in an inherited hypogonadal (Kallmann) syndrome. Brain Res. Mol. Brain Res. 6:311–326. [DOI] [PubMed] [Google Scholar]
- 59.Schwanzel-Fukuda, M., and D. W. Pfaff. 1989. Origin of luteinizing hormone-releasing hormone neurons. Nature 338:161–164. [DOI] [PubMed] [Google Scholar]
- 60.Seminara, S. B., F. J. Hayes, and W. F. Crowley, Jr. 1998. Gonadotropin-releasing hormone deficiency in the human (idiopathic hypogonadotropic hypogonadism and Kallmann’s syndrome): pathophysiological and genetic considerations. Endocr. Rev. 19:521–539. [DOI] [PubMed] [Google Scholar]
- 61.Silverman, A. J., J. L. Roberts, K. W. Dong, G. M. Miller, and M. J. Gibson. 1992. Intrahypothalamic injection of a cell line secreting gonadotropin-releasing hormone results in cellular differentiation and reversal of hypogonadism in mutant mice. Proc. Natl. Acad. Sci. USA 89:10668–10672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Soussi-Yanicostas, N., J. P. Hardelin, M. M. Arroyo-Jimenez, O. Ardouin, R. Legouis, J. Levilliers, F. Traincard, J. M. Betton, L. Cabanie, and C. Petit. 1996. Initial characterization of anosmin-1, a putative extracellular matrix protein synthesized by definite neuronal-cell populations in the central nervous system. J. Cell Sci. 109(Pt. 7):1749–1757. [DOI] [PubMed] [Google Scholar]
- 63.Takeda, K., T. Hatai, T. S. Hamazaki, H. Nishitoh, M. Saitoh, and H. Ichijo. 2000. Apoptosis signal-regulating kinase 1 (ASK1) induces neuronal differentiation and survival of PC12 cells. J. Biol. Chem. 275:9805–9813. [DOI] [PubMed] [Google Scholar]
- 64.Varnum, B. C., C. Young, G. Elliott, A. Garcia, T. D. Bartley, Y. W. Fridell, R. W. Hunt, G. Trail, C. Clogston, and R. J. Toso. 1995. Axl receptor tyrosine kinase stimulated by the vitamin K-dependent protein encoded by growth-arrest-specific gene 6. Nature 373:623–626. [DOI] [PubMed] [Google Scholar]
- 65.Wetsel, W. C., M. M. Valenca, I. Merchenthaler, Z. Liposits, F. J. Lopez, R. I. Weiner, P. L. Mellon, and A. Negro-Vilar. 1992. Intrinsic pulsatile secretory activity of immortalized luteinizing hormone-releasing hormone-secreting neurons. Proc. Natl. Acad. Sci. USA 89:4149–4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wray, S., P. Grant, and H. Gainer. 1989. Evidence that cells expressing luteinizing hormone-releasing hormone mRNA in the mouse are derived from progenitor cells in the olfactory placode. Proc. Natl. Acad. Sci. USA 86:8132–8136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wray, S., S. Key, R. Qualls, and S. M. Fueshko. 1994. A subset of peripherin positive olfactory axons delineates the luteinizing hormone releasing hormone neuronal migratory pathway in developing mouse. Dev. Biol. 166:349–354. [DOI] [PubMed] [Google Scholar]
- 68.Xia, Z., M. Dickens, J. Raingeaud, R. J. Davis, and M. E. Greenberg. 1995. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270:1326–1331. [DOI] [PubMed] [Google Scholar]
- 69.Yamboliev, I. A., K. M. Wiesmann, C. A. Singer, J. C. Hedges, and W. T. Gerthoffer. 2000. Phosphatidylinositol 3-kinases regulate ERK and p38 MAP kinases in canine colonic smooth muscle. Am. J. Physiol. Cell Physiol. 279:C352–C360. [DOI] [PubMed] [Google Scholar]
- 70.Yoshida, K., S. A. Tobet, J. E. Crandall, T. P. Jimenez, and G. A. Schwarting. 1995. The migration of luteinizing hormone-releasing hormone neurons in the developing rat is associated with a transient, caudal projection of the vomeronasal nerve. J. Neurosci. 15:7769–7777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang, S., J. Han, M. A. Sells, J. Chernoff, U. G. Knaus, R. J. Ulevitch, and G. M. Bokoch. 1995. Rho family GTPases regulate p38 mitogen-activated protein kinase through the downstream mediator Pak1. J. Biol. Chem. 270:23934–23936. [DOI] [PubMed] [Google Scholar]
- 72.Zhen, S., I. C. Dunn, S. Wray, Y. Liu, P. E. Chappell, J. E. Levine, and S. Radovick. 1997. An alternative gonadotropin-releasing hormone (GnRH) RNA splicing product found in cultured GnRH neurons and mouse hypothalamus. J. Biol. Chem. 272:12620–12625. [DOI] [PubMed] [Google Scholar]
- 73.Zipkin, I. D., R. M. Kindt, and C. J. Kenyon. 1997. Role of a new Rho family member in cell migration and axon guidance in C. elegans. Cell 90:883–894. [DOI] [PubMed] [Google Scholar]