Abstract
Fas and Fas ligand (FasL) expression has been detected in chronic vascular lesions, and Fas-mediated apoptosis of vascular smooth muscle cells (VSMC) may influence the integrity of the atherosclerotic plaque. Here we report that FasL is not expressed by normal VSMC, but its expression is upregulated by stresses that induce apoptosis, including serum deprivation, exposure to the phosphatidylinositol 3-kinase (PI 3-kinase) inhibitor wortmannin, and ablation of Akt signaling. Conversely, constitutive activation of Akt signaling diminished FasL expression in VSMC cultures exposed to low-mitogen media or wortmannin. Under conditions of suppressed PI 3-kinase/Akt signaling, VSMC apoptosis was partially inhibited by treatment with neutralizing antibody against FasL. Suppression of Akt signaling increased the activity of c-Jun N-terminal kinase, and transduction of dominant-negative c-Jun inhibited FasL induction under these conditions. Diminished Akt signaling promoted the cleavage of caspase 3, and both caspase 3 cleavage and FasL induction were inhibited by transduction of dominant-negative caspase 9 or the caspase 8 inhibitor CrmA. Similarly, induction of FasL by the Akt-regulated forkhead transcription factor FKHRL1 was dependent upon caspase and c-Jun activation. Taken together, these results indicate that the sequential activation of caspase 3 and c-Jun participates in the induction of FasL under conditions of suppressed Akt signaling or FKHRL1 activation and that FasL participates in a positive-feedback loop to promote cell death under conditions of cellular stress.
Vascular smooth muscle cell (VSMC) apoptosis is prevalent in the proliferative lesions of the vessel wall (72). VSMC apoptosis has been documented in specimens from atherosclerotic and restenotic lesions (23, 32, 37, 45), and VSMC cultured from atherosclerotic coronary atherectomy specimens proliferate more slowly and undergo higher frequencies of apoptosis than VSMC cultured from normal vessels (2). Thus, an imbalance between cell death and growth in VSMC may contribute to diminished cellularity and atherosclerotic plaque rupture (41). In support of this hypothesis, apoptosis is detected in recently ruptured plaques as well as the fibrous cap and adjacent media of nonulcerated lesions (3, 8). Despite the potential importance of VSMC apoptosis in vascular lesions, little is known about the biochemical mechanisms that regulate VSMC viability (72).
The serine/threonine protein kinase Akt/PKB regulates diverse cellular processes in various cell types including VSMC (16, 68). Activation of Akt occurs through the direct binding of the inositol lipid products of the phosphatidylinositol 3-kinase (PI 3-kinase) reaction to its pleckstrin homology domain (10). PI 3-kinase-dependent activation of Akt also occurs through PDK1-mediated phosphorylation of threonine 308, which leads to the autophosphorylation of serine 473 (67). Akt signaling is implicated in the physiological regulation of organ size (69), glucose homeostasis (9), vasomotor tone (43), and angiogenesis (40). Other studies have shown that Akt signaling inhibits apoptosis in a variety of cell types in vitro (10) and can protect cardiomyocytes from ischemia-reperfusion injury in vivo (20, 48). The antiapoptotic function of Akt is reported to be mediated by its ability to phosphorylate apoptosis regulatory molecules including BAD, caspase 9, IKK-α, and the forkhead transcriptional factor FKHRL1 (10). Although Akt is likely to have an antiapoptotic role in VSMC, this has not been formally documented.
Fas (also called APO-1 or CD95) is a type I membrane protein that transmits a suicide signal to the cell (50). The clustering of Fas following the binding of Fas ligand (FasL) or anti-Fas antibodies leads to caspase 8-dependent cell death (34, 65). Whereas many cell types express Fas, FasL expression is much more restricted. FasL is predominantly expressed in immune cells such as activated T cells, natural killer (NK) cells, and macrophages (15, 51, 52, 64). FasL can also be expressed by some tumors or host tissues that function as immune barriers (27, 28, 55, 59, 62, 63). In some cases, dysregulated expression of Fas or FasL may contribute to pathogenesis (7, 39, 58, 61). VSMC normally express Fas but not FasL (57), and they undergo apoptosis when exposed to FasL in vitro and in vivo (44, 46, 56). It is proposed that FKHRL1 activates the FasL gene promoter and that Akt-mediated phosphorylation of FKHRL1 favors cellular survival by promoting the retention of FKHRL1 in the cytoplasm (4).
Here, we examined the role of PI 3-kinase signaling in controlling FasL expression in VSMC. Serum deprivation and inhibition of either PI 3-kinase or Akt signaling were found to upregulate FasL expression. This upregulation was associated with caspase activation, mitogen-activated protein kinase-Erk kinase kinase-1 (MEKK1) cleavage, and the induction of stress-activated protein kinase-c-Jun kinase (SAPK/JNK). Under these conditions, caspase inhibition markedly inhibited MEKK1 cleavage, JNK activation, and FasL expression. FasL expression was also reduced by inhibition of c-Jun-dependent transcription. Likewise, FasL induction by FKHRL1 was suppressed by caspase or c-Jun inhibition. These data suggest that cellular stresses that suppress Akt signaling or activate FKHRL1 will induce FasL expression on the VSMC surface via a caspase- and c-Jun-dependent pathway. This, in turn, establishes a positive-feedback loop that enhances cell death and tissue destruction.
MATERIALS AND METHODS
Cell culture.
Human VSMC were isolated from an internal mammary artery obtained during coronary bypass surgery. These cells were maintained in Dulbecco’s modified Eagle medium containing 10% fetal bovine serum (complete medium) and identified as VSMC on the basis of typical morphology and positive immunostaining with α-actin antibody. VSMC were grown to confluence on 10-cm-diameter dishes or six-well plates. Complete medium was replaced with fresh medium, with or without serum, typically at the time of test reagent addition. Caspase inhibitors Z-VAD-FMK and Z-DEVD-FMK were from Calbiochem (San Diego, Calif.) and were added to the culture medium at 20 μM each.
Adenoviral constructs.
Replication-defective adenovirus vectors expressing dominant-negative and constitutively active forms of murine Akt tagged with the hemagglutinin epitope were constructed as described previously (19). The dominant-negative Akt mutant (Adeno-dnAkt) has alanine residues substituted for threonine at position 308 and serine at position 473. The constitutively active Akt (Adeno-myrAkt) has the c-Src myristoylation sequence fused in frame to the N terminus of the wild-type Akt coding sequence that targets the fusion protein to the membrane. Like the Akt vectors, Adeno-βGal expresses the lacZ gene from the cytomegalovirus (CMV) promoter (60). Adenovirus vector expressing a dominant-negative c-Jun mutant (Adeno-TAM67) and the C287A caspase 9 dominant-negative mutant (Adeno-dnCasp-9) from the CMV promoter were from Harris Perlman (Northwestern University Medical School, Chicago, Ill.). The adenovirus expressing CrmA (Adeno-CrmA) was generated by homologous recombination in human embryonic 293 kidney cells. In brief, the 1.4-kbp CrmA cDNA (generously provided by David Pickup, Duke University) was ligated into EcoRI sites of our adenovirus shuttle vector designated Adeno-CrmA. The resulting shuttle plasmid was rescued into recombinant adenovirus as described previously (36). The hemagglutinin-tagged human FKHRL1 triple mutant sequence cDNA (from Michael E. Greenberg, Harvard Medical School) was subcloned into shuttle vector pAdTrack-CMV, which contains green fluorescent protein (GFP) under the control of a separate CMV promoter (from Bert Vogelstein, Johns Hopkins Oncology Center). The FKHRL1-AAA triple mutant is not phosphorylatable because three phosphorylation sites, Thr32, Ser253, and Ser315, were replaced by alanine residues (4). Shuttle vector containing the FKHRL1 cDNA was linearized and cotransformed into Escherichia coli with the adenoviral backbone plasmid pAdEasy-1. The resultant recombinant adenoviral DNA with FKHRL1 cDNA was transfected into packaging cell line 293 cells to produce the recombinant adenoviral vectors. For experiments with these reagents, control cultures were infected with an adenoviral vector expressing only the GFP transgene (Adeno-GFP) prepared by the same system (29). All viral constructs were purified by CsCl ultracentrifugation. VSMC were infected for 24 h with adenoviral constructs at a multiplicity of infection (MOI) of 50 or 100, achieving greater than 90% transduction efficiency (data not shown).
Protein detection by flow cytometry.
After various treatments, cells were washed by rinsing with phosphate-buffered saline (PBS) and adherent cells were harvested with PBS containing 0.5% EDTA. VSMC were incubated with PBS containing 5% fetal calf serum and 10 μg of hamster antibody against FasL (4H9; MBL, Nagoya, Japan) per ml or 10 μg of hamster immunoglobulin G (IgG) per ml for 1 h at 4°C. After three washes in PBS, cells were incubated with 10 μg of fluorescein isothiocyanate (FITC)-conjugated goat anti-hamster IgG (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.) per ml for 30 min at 4°C. To analyze cell surface expression of Fas, detached cells were incubated with 10 μg of mouse antibody against Fas (UB2, MBL) per ml or 10 μg of mouse IgG per ml for 1 h at 4°C. After three washes in PBS, cells were incubated with 10 μg of FITC-conjugated rat anti-mouse IgG (Pharmingen, San Diego, Calif.) per ml for 30 min at 4°C. Immunofluorescence staining on the cell surface was analyzed by a method using a flow cytometer (FACS; Becton Dickinson) on the FL-1 channel.
DNA analysis by flow cytometry.
Apoptosis was monitored by measuring hypodiploid DNA content. After various treatments, attached and floating VSMC were harvested and fixed in cold 90% ethanol for 20 min and then resuspended in staining buffer consisting of 1 mg of RNase A per ml, 20 μg of propidium iodide per ml, and 0.01% NP-40. DNA content was analyzed by flow cytometry on the FL-2 channel and gating was set to exclude debris and cellular aggregates. Ten thousand events were counted for each analysis.
RT-PCR analysis.
Reverse transcriptase PCR (RT-PCR) assays were used to assess FasL expression in human umbilical vein endothelial cells, human VSMC, and activated and nonactivated Jurkat cells. Total RNA was isolated from human umbilical vein endothelial cells, human VSMC, and activated and nonactivated Jurkat cells and was reverse transcribed to cDNA and amplified by PCR according to the directions of the manufacturer (TaKaRa, Shiga, Japan), generating a 534-bp fragment of FasL (forward primer, 5′-GTTCTGGTTGCCTTGGTAGG-3′; reverse primer, 5′-GACCAGAGAGAGCTCAGATACG-3′) or a fragment of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) that is 983 bp (forward primer, 5′-TGAAGGTCGGAGTCAACGGATTTGGT-3′; reverse primer, 5′-CATGTGGGCCATGAGGTCCACCAC-3′; Clontech). The following conditions were used: 1 cycle at 94°C for 5 min and then 35 cycles at 95°C for 40 s, 58°C for 1 min, and 72°C for 1.5 min. The PCR products were electrophoresed in a 2% agarose gel. FasL plasmid was used as a positive control.
Coculture cytotoxicity assay.
Jurkat cells were labeled with 51Cr by incubation for 4 h in medium containing 20 μCi of 51Cr sodium (45). After washing in fresh medium twice, labeled Jurkat cells were cocultured with effector cells (VSMC) at various ratios for 24 h. 51Cr content of the supernatant fraction in counts per minute (cpm) was determined using a gamma counter. Percent specific release was calculated according to the formula 100 × [(experimental cpm)−(spontaneous cpm)]/[(maximum cpm)−(spontaneous cpm)], where spontaneous cpm is the cpm obtained in the absence of effector cells and maximum cpm is the cpm released when cells are incubated with 1 N HCl.
Immunoblot analysis of c-Jun N-terminal kinase (JNK).
Cells were washed by rinsing twice with PBS and harvested by scraping. Cell lysates were prepared in 50 mM Tris-HCl (pH 8.0)-20 mM EDTA-1% sodium dodecyl sulfate (SDS)-100 mM NaCl. Protein concentration was determined with a protein assay kit (Bio-Rad). Protein (20 μg) was separated in SDS-polyacrylamide electrophoresis gels and transferred to a polyvinylidene difluoride membrane (Millipore). The membrane was blocked with T-PBS (1× PBS, 0.3% Tween 20) containing 3% dry milk and incubated with primary antibody (anti-JNK1 antibody [Santa Cruz]) overnight at 4°C. After three washes with T-PBS, the membrane was reblocked and incubated with secondary antibody (anti-rabbit IgG horseradish peroxidase conjugate [Promega]) for 1 h. ECL (Amersham) was used for detection.
JNK activity assay.
JNK assays were performed using a SAPK/JNK assay kit (New England Biolabs). Cells were washed twice with PBS and lysed in a solution containing 20 mM Tris (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM β-glycerolphosphate, 1 mM Na3VO4, 1 μg of leupeptin per ml, and 1 mM phenylmethylsulfonyl fluoride. Lysates were incubated with beads bound to c-Jun fusion protein overnight at 4°C and then washed twice with lysis buffer and once with kinase reaction buffer (25 mM Tris [pH 7.5], 5 mM β-glycerolphosphate, 2 mM dithiothreitol, 2 mM Na3VO4, 10 mM MgCl2). Beads were resuspended with 50 μl of kinase reaction buffer and incubated with 100 μM ATP for 30 min at 37°C. The reaction was terminated by adding 25 μl of 6% SDS. JNK activity was assessed by Western immunoblotting using anti-phospho-c-Jun antibody.
Analysis of caspase 3 and MEKK1.
Cell lysates (300 μg) were prepared using an immunoprecipitation kit (Boehringer Mannheim) according to the directions of the manufacturer and were precleaned with protein G-agarose for 3 h at 4°C and immunoprecipitated overnight with anti-caspase 3 antibody (Pharmingen) or anti-MEKK1 antibody (Pharmingen) at 4°C. Immunoprecipitated protein was separated by electrophoresis on 15% (for caspase 3) or 7% (for MEKK1) polyacrylamide gels and analyzed by Western blotting using anti-caspase 3 or anti-MEKK1 antibody.
Caspase 3 activity.
Caspase 3 activity was measured using the caspase 3 colorimetric assay kit according to the directions of the manufacturer (R&D Systems). Cell lysates (from 106 cells) were incubated with 5 mM dithiothreitol and the caspase 3 colorimetric substrate that is conjugated to color reporter molecule p-nitroanilide (DEVD-pNA) in reaction buffer for 2 h at 37°C. Cleavage of substrate was quantified using a microplate reader to determine absorbance at 405 nm.
RESULTS
Disruption of Akt signaling induces FasL expression in VSMC.
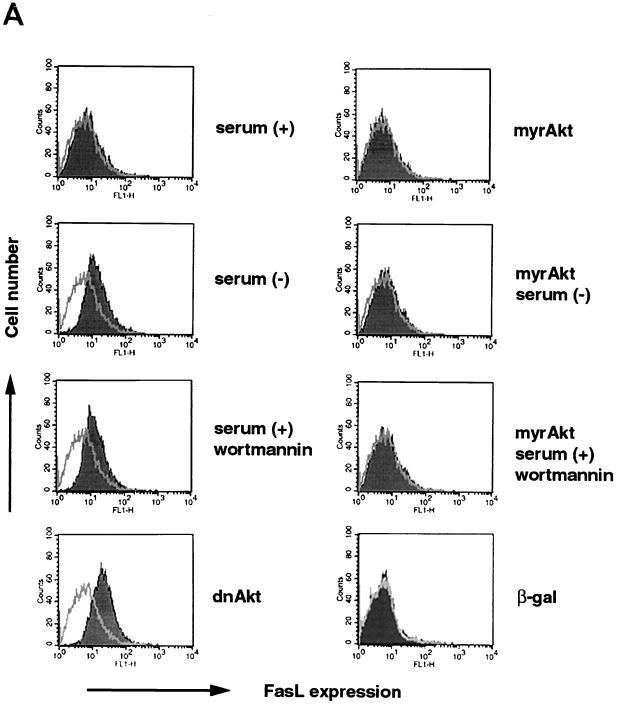
Cell surface FasL expression on human VSMC was analyzed by flow cytometry (Fig. 1A). Consistent with previously reported data (57), FasL was undetectable on the cell surface of control VSMC. However, cell surface FasL expression was readily detected when cells were incubated under conditions of serum deprivation for 48 h or when cells were treated with the PI 3-kinase inhibitor wortmannin (200 nM) for 48 h in the presence of medium containing 10% fetal bovine serum. Incubation with a replication-defective adenoviral construct expressing an inactive form of Akt (dnAkt), which functions in a dominant-negative manner in VSMC (68) and other cell types (19–22, 49), also upregulated cell surface FasL expression on human VSMC. Under these conditions, the intensity of the FasL signal was enhanced by coincubation with 10 nM KB8301 (data not shown), a metalloproteinase inhibitor which inhibits shedding of membrane-bound FasL (46, 66). Furthermore, cell surface FasL expression induced by serum deprivation or treatment with wortmannin was abolished when cells were preinfected with replication-defective adenoviral vector expressing constitutively active Akt (myrAkt) at an MOI of 100.
FIG. 1.
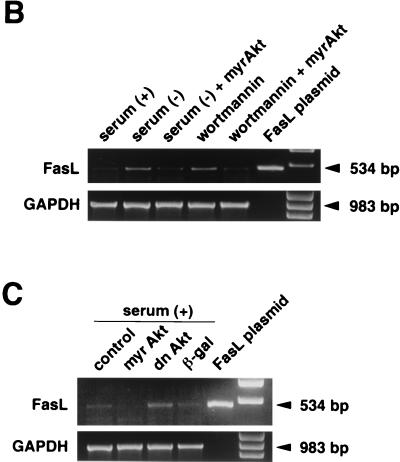
FasL expression is induced by serum deprivation or suppression of PI 3-kinase/Akt signaling. (A) Cell surface expression of FasL on human VSMC was determined by flow cytometry. VSMC were cultured with or without 10% serum, in the presence or absence of wortmannin (200 nM) for 48 h. Alternatively, cells were incubated with an adenoviral construct expressing dominant-negative Akt (dnAkt) (MOI = 100) for 48 h prior to analysis. Other cultures were preinfected with an adenoviral construct expressing constitutively active Akt (myrAkt) (MOI = 100) for 24 h prior to exposure to wortmannin or serum deprivation medium for 48 h. After treatments, cells were detached from the culture plate with 0.5% EDTA, incubated with anti-human FasL antibody (hamster IgG; 4H9), and stained with FITC-conjugated anti-hamster antibody (shaded curve). Hamster IgG was used as a negative control (open curve). Representative data from three replicate experiments are shown. PI 3-kinase (B) and Akt (C) signaling regulates FasL mRNA expression in human VSMC. Semiquantitative RT-PCR analysis was performed on FasL and GAPDH transcripts. RNA was isolated by a guanidine isothiocyanate-acid phenol method and reverse transcribed. A plasmid encoding human FasL cDNA served as a positive control for the FasL RT-PCR signal. The GAPDH signal shows uniformity of the RT-PCR analysis. Marker bands appear in the lanes on the far right. Representative data from two replicate experiments are shown in each panel.
To examine FasL regulation in greater detail, RT-PCR analysis was performed to assay for FasL transcripts in VSMC. Consistent with previously reported data (57), FasL mRNA was detected in untreated VSMC. The level of this transcript increased under conditions of serum deprivation or wortmannin treatment (Fig. 1B). Likewise, transduction of dnAkt upregulated FasL transcripts (Fig. 1C). Collectively, these data show that PI 3-kinase/Akt signaling is both essential and sufficient to suppress FasL expression by VSMC. In contrast, modulation of Akt signaling did not affect cell surface expression of the receptor molecule Fas on VSMC, nor did it affect the constitutive FasL expression on human endothelial cells (data not shown).
Cytotoxic activity of FasL on VSMC toward cocultured Jurkat cells.
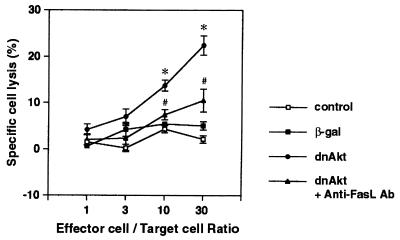
To further document the induction of FasL expression on the VSMC cell surface following inhibition of Akt signaling, treated cultures were cocultured with Jurkat cells that had been prelabeled with 51Cr. Jurkat cells express abundant Fas on their cell surface, and exposure to FasL-expressing cells will result in cell lysis and 51Cr release (47). After 24 h of coculture, VSMC preinfected with an adenoviral vector expressing dnAkt at an MOI of 100 for 24 h induced significant death of Jurkat cells in a manner dependent upon the effector cell/target cell ratio (Fig. 2). In contrast, mock-infected VSMC or VSMC infected with the control vector expressing β-galactosidase (β-Gal) (MOI = 100, 24 h) did not exhibit toxicity toward Jurkat cells. Similarly, VSMC preinfected with vector expressing myrAkt did not lead to Jurkat cell lysis (data not shown). To demonstrate that the cytotoxicity of dnAkt-transduced VSMC toward Jurkat cells is mediated by the Fas-FasL interaction, VSMC cultures treated in this manner were incubated with a neutralizing anti-FasL antibody for 30 min prior to the coculture assay. Treatment with this antibody significantly suppressed the cytotoxicity of dnAkt-transduced VSMC toward Jurkat cells (Fig. 2).
FIG. 2.
Cytotoxic activity of VSMC toward Jurkat cells following suppression of Akt signaling. Jurkat cells were prelabeled with 51Cr and cocultured with VSMC at the indicated ratio in the presence or absence of neutralizing anti-FasL antibody (500 ng/ml). VSMC were preinfected with adenovirus vector expressing dnAkt or β-Gal at an MOI of 100. Release of 51Cr in culture media was measured after 24 h of coculture. Percent specific release data are presented as means ± standard errors of the means (SEM) of four replicated experiments.
Suppression of Akt signaling promotes Fas-mediated fratricide in VSMC.
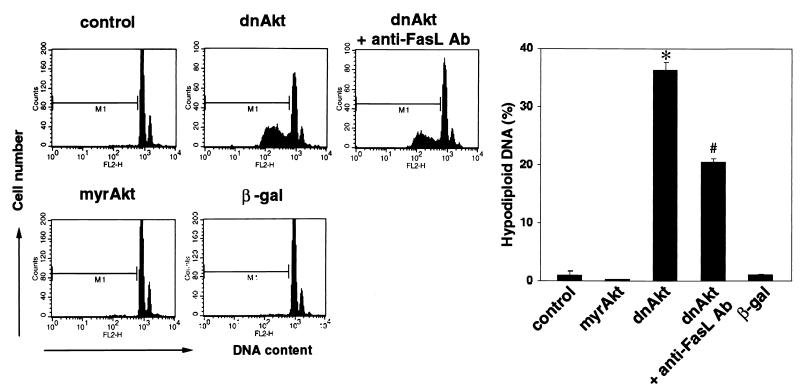
To examine the functional significance of FasL induction with regard to VSMC viability, cell death was quantified in the VSMC cultures by analyzing DNA fragmentation, an indicator of apoptosis. DNA fragmentation was assessed by quantifying hypodiploid DNA content by flow cytometry using propidium iodide staining. As shown in Fig. 3, infection of human VSMC with the adenoviral vector expressing dnAkt (MOI of 100, 96 h) led to marked cell death in complete medium, whereas little or no cell death was observed in mock-, β-Gal-, or myrAkt-transduced cultures. The inclusion of neutralizing anti-FasL antibody in the dnAkt-transduced cultures significantly inhibited DNA fragmentation.
FIG. 3.
Neutralizing antibodies against FasL partially reverse VSMC apoptosis in inhibition of Akt signaling. VSMC were cultured in complete medium with 10% fetal bovine serum, with or without neutralizing anti-FasL antibody (0.5 μg/ml), 72 h after transduction with the indicated adenoviral constructs (MOI = 100). Cells were fixed with 90% ethanol and stained with propidium iodide, and the DNA content was analyzed by flow cytometry. Representative data are shown on the left. The graph on the right shows values of means ± SEM from four experiments.
Treatment of human VSMC with the PI 3-kinase inhibitor wortmannin also led to significant production of hypodiploid DNA (Table 1). Under these conditions, coincubation with 0.5 μg of neutralizing anti-FasL antibody per ml significantly reduced the extent of hypodiploid DNA induced by 100 or 200 nM wortmannin at the 72-h time point. Collectively, these data show that Fas-mediated apoptosis contributes to VSMC death under conditions where PI 3-kinase or Akt signaling is inhibited.
TABLE 1.
Fas-mediated apoptosis contributes to VSMC death under conditions of PI 3-kinase inhibitiona
| Treatment (concn) | Hypodiploid DNA (%) |
|---|---|
| None | 0.3 ± 0.3 |
| Wortmannin (100 nM) | 2.8 ± 0.1 |
| Wortmannin plus anti-FasL antibody | 0.6 ± 0.1b |
| Wortmannin (200 nM) | 7.6 ± 0.7 |
| Wortmannin plus anti-FasL antibody (200 nM) | 2.7 ± 0.4b |
Human VSMC were incubated in complete medium in the presence or absence of the indicated concentration of wortmannin for 72 h. Some cultures were also incubated with neutralizing anti-FasL antibody over this time course. Hypodiploid DNA was assessed by flow cytometry. Shown are the values of the means ± SEM from four experiments.
P < 0.05 relative to parallel cultures containing the same level of wortmannin but no antibody.
Suppression of Akt signaling promotes c-Jun-dependent FasL induction.
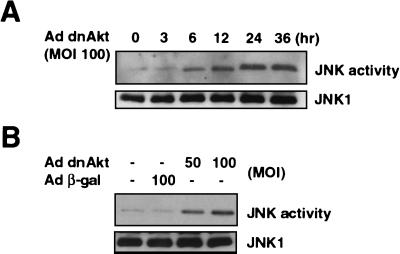
Previous studies have reported that JNK signaling can be an important regulator of Fas-mediated apoptosis (35, 38, 70). Thus, to examine the consequence of Akt signaling on the modulation of JNK activity in VSMC, an immunocomplex kinase assay was performed on JNK1 protein by Western blotting. As shown in Fig. 4A, infection with an adenoviral vector expressing dnAkt (MOI = 100) led to a time-dependent increase in JNK activation. Maximum activation of JNK occurred at approximately 24 h after infection, with little or no increase in the level of JNK protein. JNK1 activation was also dependent on the dose of the dnAkt-expressing viral vector, and infection with a control vector expressing β-Gal had no effect (Fig. 4B).
FIG. 4.
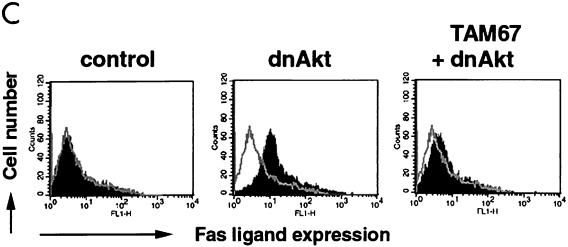
Suppression of Akt signaling activates JNK and induces FasL through a c-Jun-dependent mechanism. (A) Human VSMC cultures were infected with adenovirus vector expressing dnAkt at an MOI of 100, and cell lysates were prepared at the indicated times. JNK activity was measured by immunocomplex kinase assay using a c-Jun fusion protein bound to beads as a substrate. JNK1 was determined by immunoblotting with specific antibody against JNK1. (B) Human VSMC cultures were infected with adenovirus vector expressing dnAkt or β-Gal at the indicated MOI, and cell lysates were prepared after 24 h. JNK activity was measured by immunocomplex kinase assay. (C) Induction of FasL by Akt suppression is dependent on c-Jun. Human VSMC were mock infected or infected with adenoviral vectors expressing dnAkt and/or TAM67, a dominant-negative mutant of c-Jun. Control cells were infected with an adenoviral vector encoding β-Gal. All vectors were exposed to cells for 24 h and were used at an MOI of 100. At 48 h postinfection, cells were detached from the culture plate with 0.5% EDTA, incubated with anti-human FasL antibody (4H9), and stained with FITC-conjugated anti-hamster antibody (closed curve). FasL expression was analyzed by flow cytometry. Hamster IgG was used as negative control (open curve). Representative data from two replicate experiments are shown in each panel.
To examine the functional significance of JNK activation on FasL induction under these conditions, VSMC cultures were transduced with an adenoviral vector expressing a dominant-negative c-Jun mutant (Adeno-TAM67). Preinfection with Adeno-TAM67 largely reversed the induction of FasL expression on VSMC caused by infection with the viral construct expressing dnAkt (Fig. 4C). Collectively, these data are consistent with the hypothesis that JNK activation is an essential step in the induction of FasL following suppression of Akt signaling.
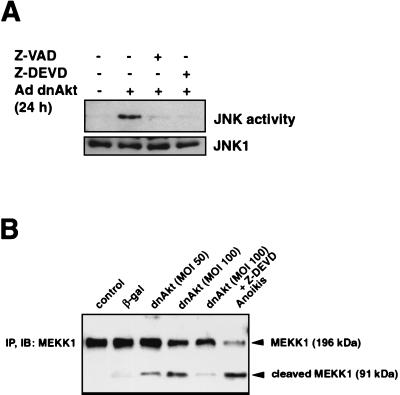
Caspase activation was examined as a potential mechanism involved in JNK induction because previous studies have shown that caspase-dependent cleavage of MEKK1 will activate the JNK pathway in MDCK and embryonic stem cells (6, 74). Therefore, VSMC were infected with the adenoviral vector expressing dnAkt (MOI of 100 for 48 h) in the presence or absence of Z-VAD-FMK (20 μM), a broad-spectrum caspase inhibitor, or Z-DEVD-FMK (20 μM), which has specificity for caspase 3. Treatment with either Z-VAD-FMK or Z-DEVD-FMK abolished the induction of JNK activity caused by the suppression of Akt signaling (Fig. 5A).
FIG. 5.
Effect of caspase inhibitors on JNK activation and MEKK1 cleavage under conditions of suppressed Akt signaling. (A) Effect of caspase inhibitors on JNK activation. VSMC were infected with an adenovirus vector expressing dnAkt (MOI = 100) in the presence or absence of the caspase inhibitors Z-VAD-FMK (20 μM) or Z-DEVD-FMK (20 μM). After 24 h, JNK activity was measured by an immunocomplex kinase assay using c-Jun fusion protein bound to beads as a substrate. JNK1 was determined by immunoblotting with specific antibody against JNK1. (B) Suppression of Akt signaling leads to MEKK1 cleavage. Human VSMC were infected with adenovirus vector expressing dnAkt or β-Gal at an MOI of 50 or 100 for 15 h with or without Z-DEVD-FMK (20 μM). Control cultures were incubated with no virus or V-DEVD-FMK. Anoikis was induced by placing cultures in suspension for 15 h. Cell lysates underwent immunoblot (IB) analysis with anti-MEKK1 antibody performed on anti-MEKK1-immunoprecipitated (IP) material. Representative data from two replicate experiments are shown in each panel.
MEKK1 is an endogenous substrate of DEVD-directed caspases that has been implicated in the regulation of JNK (6, 25, 73, 74). Therefore, we examined whether inhibition of Akt signaling would lead to cleavage of MEKK1. Transduction of VSMC with dnAkt led to a dose-dependent induction of the 91-kDa MEKK1 cleavage product (Fig. 5B). This process was blocked when VSMC cultures were incubated with Z-DEVD-FMK. Little or no MEKK1 cleavage was detected in the control cultures. VSMC undergoing anoikis contained this fragment, consistent with observations made in MDCK cells that have lost matrix attachment (6). Collectively, these data show that, under conditions of suppressed Akt signaling, caspase activation and cleavage of MEKK1 coincide with enhanced JNK signaling and FasL expression.
Suppression of Akt signaling promotes caspase-dependent FasL induction.
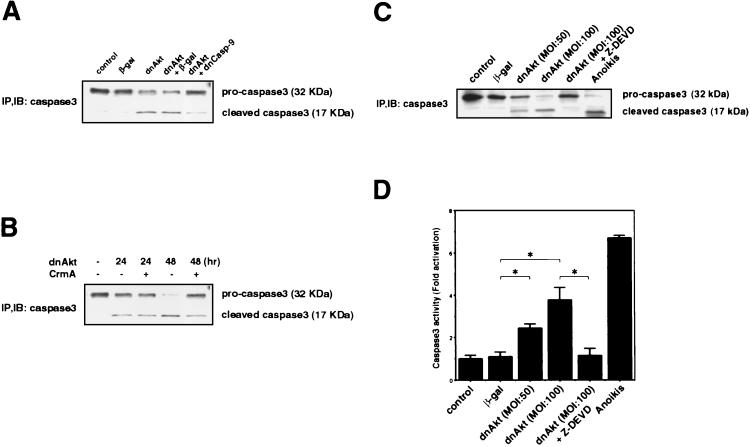
To further investigate the role of caspases in the Akt-JNK-FasL pathway, we tested whether suppression of Akt signaling will induce caspase 3 cleavage and activation. Immunoblot analysis of human VSMC transduced with dnAkt revealed cleavage of caspase 3 by 24 h while no caspase 3 cleavage was observed in mock- or β-Gal-transduced cultures (Fig. 6A). Caspase 9, which is activated upon mitochondrial dysfunction, can act to cleave caspase 3. To examine the role of caspase 9 in this process, VSMC were infected with combinations of adenoviral vectors expressing dominant-negative Akt and dominant-negative caspase 9 (Fig. 6B). Preinfection with Adeno-dnCasp-9 inhibited caspase 3 cleavage caused by infection with Adeno-dnAkt for 24 h. Adenoviral transduction of CrmA, a caspase 8 inhibitor, also inhibited caspase 3 cleavage. However, this protection was most notable 48 h after infection with Adeno-dnAkt (Fig. 6B). Finally, treatment with the general caspase inhibitor Z-DEVD-FMK inhibited caspase 3 cleavage in cultures transduced with dnAkt, and a similar cleavage pattern was observed when cells were placed in suspension culture to induce anoikis (Fig. 6C). These last results were corroborated by examining caspase 3 activity with a colorimetric assay. As shown in Fig. 6D, infection with adenovirus encoding dnAkt led to a dose-dependent increase of caspase 3 activity, and activation was blocked when cultures were incubated with Z-DEVD-FMK. Also consistent with the caspase 3 cleavage data (Fig. 6C), caspase 3 activity was low in mock- and β-Gal-transduced cultures and high in cultures undergoing anoikis (Fig. 6D).
FIG. 6.
Caspase 3 is cleaved and activated when Akt signaling is suppressed. (A) Human VSMC were infected with adenovirus vector expressing dominant-negative Akt (dnAkt) (MOI = 100) after preinfection with adenovirus vectors expressing β-Gal or dominant-negative caspase 9 (dnCasp-9) at an MOI of 100. Control (mock infected) and Adeno-β Gal-infected cultures were incubated in parallel with the Adeno-dnAkt-infected cultures. Cell lysates were prepared 24 h after the second infection. Immunoblot (IB) analysis with anti-caspase 3 antibody was performed on anti-caspase 3-immunoprecipitated (IP) material. (B) VSMC were preinfected with an adenoviral vector expressing CrmA 12 h prior to infection with Adeno-dnAkt. Cell lysates were prepared 24 or 48 h after infection with Adeno-dnAkt. (C) VSMC were infected with adenovirus vector expressing dnAkt or β-Gal (MOI of 50 or 100) in the presence or absence of Z-DEVD (20 μM). Control cultures were incubated in parallel but did not receive treatment with adenovirus or Z-DEVD. Anoikis was used as a positive control in which cells were placed in suspension cultures for 15 h. Cell lysates were extracted 15 h after infection, and immunoblot analysis with anti-caspase 3 antibody was performed on anti-caspase 3-immunoprecipitated material. (D) Caspase activity was determined using a caspase 3 colorimetric assay which involved the incubation of cell lysates with caspase 3-specific peptide and quantitation with a scanning multiwell spectrophotometer. Values are presented as means ± SEM (n = 4).
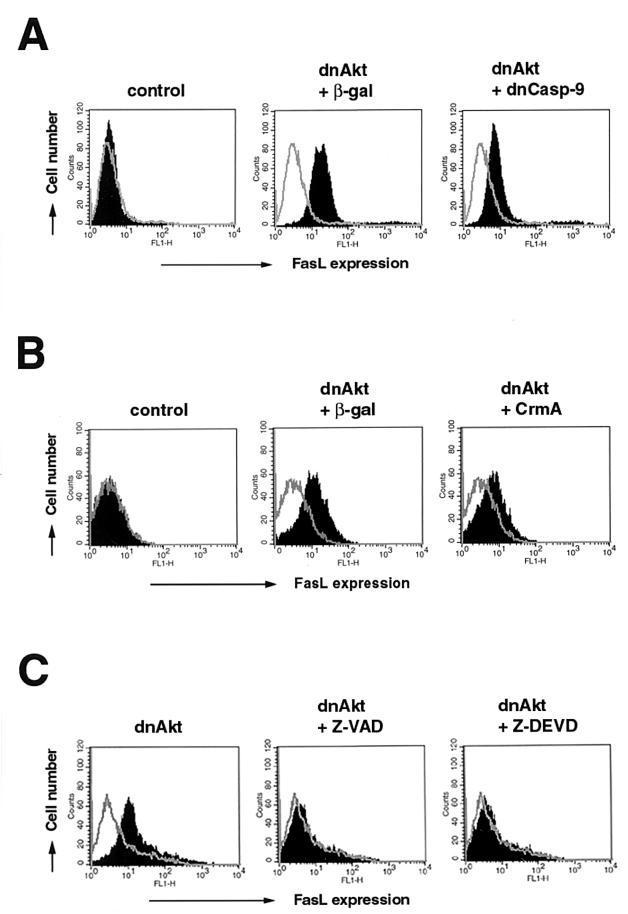
Fluorescence-activated cell sorter analysis was performed to examine the role of caspase activation in cell surface FasL expression (Fig. 7). Transduction of dominant-negative caspase 9 inhibited FasL expression under conditions of suppressed Akt signaling (Fig. 7A). Similarly, preinfection with Adeno-CrmA inhibited cell surface FasL expression (Fig. 7B). Like the time course of caspase 3 cleavage, the effect of CrmA on FasL expression was detectable at 48 but not 24 h after infection with Adeno-dnAkt (data not shown). Finally, the general caspase inhibitors Z-DEVD-FMK and Z-VAD-FMK effectively blocked FasL expression under conditions of suppressed Akt signaling (Fig. 7C). Taken together, there was a good correlation between the reduction in FasL expression and inhibition of caspase 3 activation under conditions of suppressed Akt signaling.
FIG. 7.
Caspase inhibition reduces FasL expression under conditions of suppressed Akt signaling. (A) Human VSMC were preinfected with Adeno-βGal or Adeno-dnCasp-9 (MOI = 100) 12 h prior to infection with Adeno-dnAkt (MOI = 100). Cells were harvested 24 h after infection with Adeno-dnAkt. (B) VSMC were preinfected with Adeno-CrmA (MOI = 100) 12 h prior to infection with Adeno-dnAkt (MOI = 100). Cells were harvested 48 h after infection with Adeno-dnAkt. (C) VSMC were infected with an adenovirus vector expressing dnAkt (MOI = 100) in the presence or absence of the caspase inhibitors Z-VAD-FMK (20 μM) or Z-DEVD-FMK (20 μM). Cells were harvested after 48 h. Representative data from two replicate experiments are shown. Cells were detached from the culture plate with 0.5% EDTA and assayed for cell surface FasL expression by flow cytometry. Cells were incubated with anti-human FasL antibody (4H9) and then stained with FITC-conjugated anti-hamster antibody (solid curve). Hamster IgG was used as a negative control (open curve).
FKHRL1 promotes caspase- and Jun-dependent FasL induction.
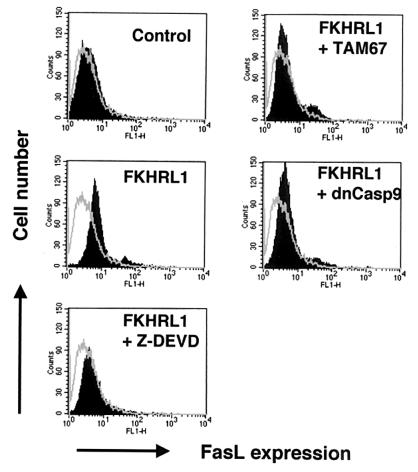
It has been shown that Akt phosphorylates and inhibits the nuclear translocation of the FKHRL1 transcription factor (4). Furthermore, it was reported that FKHRL1 transactivates the FasL promoter and promotes apoptosis via a FasL-dependent mechanism. Thus, to examine the role of FKHRL1 in VSMC, we constructed an adenoviral vector expressing a nonphosphorylatable form of FKHRL1 (Adeno-FKHRL1). Adeno-FKHRL1 induced FasL cell surface expression (Fig. 8) and promoted DNA fragmentation (Table 2). Coinfection of Adeno-FKHRL1 with Adeno-dnCasp-9 or Adeno-TAM67 or incubation of FKHRL1-transduced cells with Z-DEVD-FMK reduced cell surface FasL expression (Fig. 8), implicating caspase 9 activation and c-Jun-dependent transcription in this process. Similarly, coinfection with Adeno-dnCasp-9 or Adeno-TAM67 reduced FKHRL1-dependent apoptosis (Table 2), indicating the functional significance of caspase 9 and c-Jun in cell survival under these conditions.
FIG. 8.
Induction of FasL by FKHRL1 is dependent on caspase activity and c-Jun. Human VSMC were infected with adenoviral vectors expressing GFP (control) or FKHRL1 or a combination of vectors expressing FKHRL1 and TAM67 or dominant-negative caspase 9 at an MOI of 100 each. Adeno-dnCasp-9 and Adeno-TAM67 were added to cells 12 h before the addition of Adeno-FKHRL1. Other cultures were incubated with Adeno-FKHRL1 and Z-DEVD (20 μM) simultaneously. After 24 h of infection with Adeno-FKHRL1, the medium was changed to fresh medium in the presence or absence of Z-DEVD. Cells were detached from the culture plate with 0.5% EDTA 24 h following the medium change. Cell surface FasL expression was assessed by flow cytometry using anti-human FasL antibody (4H9) and FITC-conjugated anti-hamster antibody (solid curve). Hamster IgG was used as a negative control (open curve).
TABLE 2.
FKHRL1-induced VSMC apoptosis is dependent upon caspase 9 and c-Jun activationa
| Treatment | Hypodiploid DNA (%) |
|---|---|
| Adeno-GFP | 3.8 ± 0.4 |
| Adeno-FKHRL1 | 14.7 ± 0.6 |
| Adeno-FKHRL1 plus Adeno-dnCasp-9 | 7.7 ± 0.4b |
| Adeno-FKHRL1 plus Adeno-TAM67 | 8.6 ± 0.7b |
Human VSMC were infected with the indicated adenoviral constructs at an MOI of 100 for 24 h, and hypodiploid DNA was assessed by flow cytometry 48 h following transfection. Control cultures were treated with Adeno-GFP. Values are reported as means ± SEM from four experiments, and the level of hypodiploid DNA from the nontransduced cultures was excluded from each value.
P < 0.05 relative to parallel cultures transduced with Adeno-FKHRL1 alone.
DISCUSSION
This study examined the role of PI 3-kinase/Akt signaling in the regulation of FasL expression and cellular survival in VSMC. It is shown that serum deprivation, incubation with PI 3-kinase inhibitor wortmannin, or transduction of an inactivating Akt mutant will lead to increases in FasL mRNA and cell surface expression of the FasL protein. VSMC express Fas and are susceptible to Fas-mediated apoptosis both in vitro and in vivo (1, 24, 46, 56). Thus, the coexpression of Fas and FasL on the VSMC surface would be expected to contribute to toxicity under conditions of diminished Akt signaling. Indeed, it was found that incubation with neutralizing anti-FasL antibody partially blocked apoptosis induced by the PI 3-kinase inhibitor wortmannin or transduction of a dominant-negative Akt mutant, demonstrating the functional significance of FasL expression under these conditions. Consistent with these data, VSMC exhibited a cytotoxic activity toward cocultured Jurkat cells following suppression of Akt signaling, a process that could also be inhibited by neutralizing anti-FasL antibodies.
Jun is reported to positively regulate transcription of the FasL gene (17). Therefore, experiments were conducted to examine the role of JNK signaling in the Akt-mediated suppression of FasL expression. Inhibition of Akt signaling led to the induction of JNK activity in VSMC. The functional significance of this increase in JNK activity was demonstrated by the ability of a dominant-negative c-Jun construct to eliminate FasL expression under conditions of suppressed Akt signaling. Caspase activation was also found to be essential for JNK activation and FasL expression. Furthermore, caspase-dependent cleavage of MEKK1 occurred when Akt signaling was suppressed. It is well established that caspase-dependent MEKK1 cleavage results in JNK activation and apoptosis (6, 25, 73, 74). Therefore, we propose that Akt signaling serves to inhibit JNK activation and FasL expression by suppressing caspase activation and MEKK1 cleavage (Fig. 9). Reports that Akt phosphorylates and inactivates Bad (11) and procaspase 9 (5) are consistent with this hypothesis. Akt also phosphorylates and inactivates the forkhead transcription factor FKHRL1, and this factor transactivates the FasL promoter (4). Therefore, modulation of FKHRL1 activity represents an alternative mechanism of Akt-mediated FasL regulation. To explore the role of FKHRL1 in controlling VSMC viability, an adenoviral vector expressing a nonphosphorylatable form of FKHRL1 was constructed. This vector induced cell surface FasL expression and apoptosis in VSMC. Moreover, transduction of dominant-negative caspase 9 or dominant-negative c-Jun appreciably suppressed FKHRL1-induced FasL expression and apoptosis. Although these data do not rule out a direct link between FKHRL1 and FasL gene transcription (4), they suggest that activation of caspases and c-Jun can be a significant modulator of FasL expression downstream of FKHRL1 (Fig. 9) and that Akt- and FKHRL1-mediated control of Fas-mediated apoptosis is more complex than was previously appreciated. In this regard, induction of the pro-apoptotic Bcl-2 family member Bim by FKHRL1 (14) may be significant in the induction of FasL through a caspase-dependent mechanism.
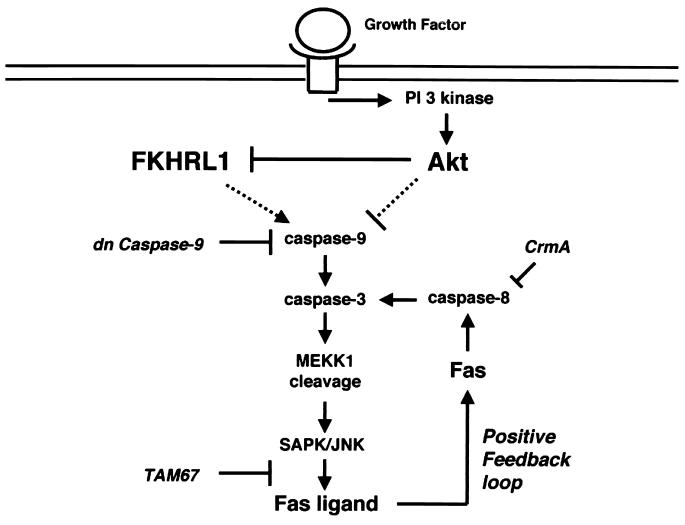
FIG. 9.
Model of FasL regulation by Akt signaling. Suppression of Akt signaling leads caspase-dependent cleavage of MEKK1, which promotes JNK activation and FasL expression. Like JNK activation, Fas-mediated apoptosis will serve to further escalate caspase activity, establishing a positive-feedback loop that facilitates destruction of the cell. In this study, caspase 9, caspase 8, and c-Jun transcription were inhibited by adenoviral transduction of dominant-negative caspase 9, CrmA, or TAM67, respectively. The mechanisms of caspase activation following the suppression of Akt signaling (or activation of FKHRL1) were not addressed in this study and these relationships are indicated by the dashed lines. An alternative mechanism not shown in this model is the direct transcriptional activation of FasL by FKHRL1 (4).
It is well established that anoikis, genotoxic agents, and other stresses promote a cytotoxic feedback loop involving caspase-mediated cleavage of MEKK1 and JNK signaling (6, 25, 73, 74). It is also reported that Fas activation can promote MEKK1 cleavage through caspase 8-mediated activation of the caspase cascade (12). The data from the present study suggest that Akt signaling can control MEKK1 cleavage and JNK-mediated activation of FasL expression (Fig. 9). Therefore, it follows that FasL may also participate in a positive-feedback loop that increases caspase activation following mitogen deprivation or other stresses that promote apoptosis via the inhibition of Akt signaling. The finding that cell surface FasL expression is involved in this process suggests that the induction of apoptosis via the caspase-MEKK1-JNK mechanism can lead to Fas-mediated destruction of neighboring cells, potentially facilitating tissue destruction. For example, this mechanism may be responsible for FasL induction and Fas-mediated cell death in an isolated heart following ischemia-reperfusion injury (33). Furthermore, focal tissue destruction through this type of mechanism may be an important component of the host response to pathogens (26).
The Akt-caspase-JNK regulatory axis reported here may also provide a mechanistic rationale to explain stress-induced FasL expression that has been reported in diverse cell types. For example, FasL is reported to be induced by serum deprivation in PC12 cells (42), c-Myc transformation in fibroblasts (30), oxidized lipids in endothelial cells (58), and chemotherapeutic drugs in hepatoma and leukemia cells (18, 31). Since all of these stresses promote apoptosis, it is conceivable that FasL expression is triggered downstream of caspase and JNK activation. Furthermore, this regulatory pathway may partly explain why inactivating mutations in the PTEN tumor suppressor gene, a negative regulator of Akt signaling, leads to impaired Fas-mediated apoptosis (13).
Apoptosis of VSMC is commonly found in the inflammatory fibroproliferative disorders of the vessel wall (71). Previous studies have identified some essential regulators of VSMC viability, including Bcl-XL (54) and Bcl-2 (53), but the signaling cascades involved in this process have not been examined in detail. The data presented here suggest that caspase-mediated cross talk between Akt and JNK is an important determinant of FasL expression and Fas-mediated cell death under conditions of cellular stress, providing a molecular framework for further analyses on the role of this signaling pathway in the control of VSMC apoptosis.
Acknowledgments
This work was supported by NIH grants AG17241, AG15052, HL50692, HD23681, and AR40197 to K.W. L.A.K. is a Canada Research Chair in Molecular Cardiology and supported by CHIR grants MT-13176 and MOP42402.
REFERENCES
- 1.Bennett, M., K. Macdonald, S. W. Chan, J. P. Luzio, R. Simari, and P. Weissberg. 1998. Cell surface trafficking of Fas: a rapid mechanism of p53-mediated apoptosis. Science 282:290–293. [DOI] [PubMed] [Google Scholar]
- 2.Bennett, M. R., G. I. Evan, and S. M. Schwartz. 1995. Apoptosis of human vascular smooth muscle cells derived from normal vessels and coronary atherosclerotic plaques. J. Clin. Investig. 95:2266–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bjorkerud, S., and B. Bjorkerud. 1996. Apoptosis is abundant in human atherosclerotic lesions, especially in inflammatory cells (macrophages and T cells), and may contribute to the accumulation of gruel and plaque instability. Am. J. Pathol. 149:367–380. [PMC free article] [PubMed] [Google Scholar]
- 4.Brunet, A., A. Bonni, M. J. Zigmond, M. Z. Lin, P. Juo, L. S. Hu, M. J. Anderson, K. C. Arden, J. Blenis, and M. E. Greenberg. 1999. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96:857–868. [DOI] [PubMed] [Google Scholar]
- 5.Cardone, M. H., N. Roy, H. R. Stennicke, G. S. Salvesen, T. F. Franke, E. Stanbridge, S. Frisch, and J. C. Reed. 1998. Regulation of cell death protease caspase-9 by phosphorylation. Science 282:1318–1321. [DOI] [PubMed] [Google Scholar]
- 6.Cardone, M. H., G. S. Salvesen, C. Widmann, G. Johnson, and S. M. Frisch. 1997. The regulation of anoikis: MEKK-1 activation requires cleavage by caspases. Cell 90:315–323. [DOI] [PubMed] [Google Scholar]
- 7.Chervonsky, A. V., Y. Wang, F. S. Wong, I. Visintin, R. A. Flavell, C. A. Janeway, Jr., and L. A. Matis. 1997. The role of Fas in autoimmune diabetes. Cell 89:17–24. [DOI] [PubMed] [Google Scholar]
- 8.Crisby, M., B. Kallin, J. Thyberg, B. Zhivotovsky, S. Orrenius, V. Kostulas, and J. Nilsson. 1997. Cell death in human atherosclerotic plaques involves both oncosis and apoptosis. Atherosclerosis 130:17–27. [DOI] [PubMed] [Google Scholar]
- 9.Czech, M. P., and S. Corvera. 1999. Signaling mechanisms that regulate glucose transport. J. Biol. Chem. 274:1865–1868. [DOI] [PubMed] [Google Scholar]
- 10.Datta, S. R., A. Brunet, and M. E. Greenberg. 1999. Cellular survival: a play in three Akts. Genes Dev. 13:2905–2927. [DOI] [PubMed] [Google Scholar]
- 11.Datta, S. R., H. Dudek, X. Tao, S. Masters, H. Fu, Y. Gotoh, and M. E. Greenberg. 1997. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91:231–241. [DOI] [PubMed] [Google Scholar]
- 12.Deak, J. C., J. V. Cross, M. Lewis, Y. Qian, L. A. Parrott, C. W. Distelhorst, and D. J. Templeton. 1998. Fas-induced proteolytic activation and intracellular redistribution of the stress-signaling kinase MEKK1. Proc. Natl. Acad. Sci. USA 95:5595–5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Cristofano, A., P. Kotsi, Y. F. Peng, C. Cordon-Cardo, K. B. Elkon, and P. P. Pandolfi. 1999. Impaired Fas response and autoimmunity in Pten+/− mice. Science 285:2122–2125. [DOI] [PubMed] [Google Scholar]
- 14.Dijkers, P. F., R. H. Medemadagger, J. J. Lammers, L. Koenderman, and P. J. Coffer. 2000. Expression of the pro-apoptotic bcl-2 family member bim is regulated by the forkhead transcription factor FKHR-L1. Curr. Biol. 10:1201–1204. [DOI] [PubMed] [Google Scholar]
- 15.Dockrell, D. H., A. D. Badley, J. S. Villacian, C. J. Heppelmann, A. Algeciras, S. Ziesmer, H. Yagita, D. H. Lynch, P. C. Roche, P. J. Leibson, and C. V. Paya. 1998. The expression of Fas ligand by macrophages and its upregulation by human immunodeficiency virus infection. J. Clin. Investig. 101:2394–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duan, C., J. R. Bauchat, and T. Hsieh. 2000. Phosphatidylinositol 3-kinase is required for insulin-like growth factor-I-induced vascular smooth muscle cell proliferation and migration. Circ. Res. 86:15–23. [DOI] [PubMed] [Google Scholar]
- 17.Faris, M., K. M. Latinis, S. J. Kempiak, G. A. Koretzky, and A. Nel. 1998. Stress-induced Fas ligand expression in T cells is mediated through a MEK kinase 1-regulated response element in the Fas ligand promoter. Mol. Cell. Biol. 18:5414–5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friesen, C., I. Herr, H. Krammer, and K.-M. Debatin. 1996. Involvement of the CD95 (APO-1/Fas) receptor/ligand system in drug-induced apoptosis in leukemia cells. Nat. Med. 2:574–577. [DOI] [PubMed] [Google Scholar]
- 19.Fujio, Y., K. Guo, T. Mano, Y. Mitsuuchi, J. R. Testa, and K. Walsh. 1999. Cell cycle withdrawal promotes myogenic induction of Akt, a positive modulator of myocyte survival. Mol. Cell. Biol. 19:5073–5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujio, Y., T. Nguyen, D. Wencker, R. N. Kitsis, and K. Walsh. 2000. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation 101:660–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujio, Y., and K. Walsh. 1999. Akt mediates cytoprotection of endothelial cells by vascular endothelial growth factor in an anchorage-dependent manner. J. Biol. Chem. 274:16349–16354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fulton, D., J.-P. Gratton, T. J. McCabe, J. Fontana, Y. Fujio, K. Walsh, T. F. Franke, A. Papapetropoulos, and W. C. Sessa. 1999. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature 399:597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geng, Y. J., and P. Libby. 1995. Evidence for apoptosis in advanced human atheroma: colocalization with interleukin-1β converting enzyme. Am. J. Pathol. 147:251–266. [PMC free article] [PubMed] [Google Scholar]
- 24.Geng, Y.-J., L. E. Henderson, E. B. Levesque, M. Muszynzki, and P. Libby. 1997. Fas is expressed in human atherosclerotic intima and promotes apoptosis of cytokine-primed human vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 17:2200–2208. [DOI] [PubMed] [Google Scholar]
- 25.Gerwins, P., J. L. Blank, and G. L. Johnson. 1997. Cloning of a novel mitogen-activated protein kinase kinase kinase, MEKK4, that selectively regulates the c-Jun amino terminal kinase pathway. J. Biol. Chem. 272:8288–8295. [DOI] [PubMed] [Google Scholar]
- 26.Grassmé, H., S. Kirschnek, J. Riethmueller, A. Riehle, G. von Kürthy, F. Lang, M. Weller, and E. Gulbins. 2000. CD95/CD95 ligand interactions on epithelial cells in host defense to Pseudomonas aeruginosa. Science 290:527–530. [DOI] [PubMed] [Google Scholar]
- 27.Griffith, T. S., T. Brunner, S. M. Fletcher, D. R. Green, and T. A. Ferguson. 1995. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science 270:1189–1192. [DOI] [PubMed] [Google Scholar]
- 28.Hahne, M., D. Rimoldi, M. Schröter, P. Romero, M. Schreier, L. E. French, P. Schneider, T. Bornand, A. Fontana, D. Lienard, J.-C. Cerottini, and J. Tschopp. 1996. Melanoma cell expression of Fas(Apo-1/CD95) ligand: implications for tumor immune escape. Science 274:1363–1366. [DOI] [PubMed] [Google Scholar]
- 29.He, T. C., S. Zhou, L. T. Da Costa, J. Yu, K. W. Kinzler, and B. Vogelstein. 1998. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA 95:2509–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hueber, A.-O., M. Zörnig, D. Lyon, T. Suda, S. Nagata, and G. I. Evan. 1997. Requirement for the CD95 receptor-ligand pathway in c-Myc-induced apoptosis. Science 278:1305–1309. [DOI] [PubMed] [Google Scholar]
- 31.Hug, H., S. Strand, A. Grambihler, J. Galle, V. Hack, W. Stremmel, P. H. Krammer, and P. R. Galle. 1997. Reactive oxygen intermediates are involved in the induction of CD95 ligand mRNA expression by cytostatic drugs in hepatoma cells. J. Biol. Chem. 272:28191–28193. [DOI] [PubMed] [Google Scholar]
- 32.Isner, J. M., M. Kearney, S. Bortman, and J. Passeri. 1995. Apoptosis in human atherosclerosis and restenosis. Circulation 91:2703–2711. [DOI] [PubMed] [Google Scholar]
- 33.Jeremias, I., C. Kupatt, A. Martin-Villalba, H. Habazettl, J. Schenkel, P. Boekstegers, and K. M. Debatin. 2000. Involvement of CD95/Apo1/Fas in cell death after myocardial ischemia. Circulation 102:915–920. [DOI] [PubMed] [Google Scholar]
- 34.Juo, P., C. J. Kuo, J. Yuan, and J. Blenis. 1998. Essential requirement for caspase-8/FLICE in the initiation of the Fas-induced apoptotic cascade. Curr. Biol. 8:1001–1008. [DOI] [PubMed] [Google Scholar]
- 35.Kasibhatla, S., T. Brunner, L. Genestier, F. Echeverri, A. Mahboubi, and D. R. Green. 1998. DNA damaging agents induce expression of Fas ligand and subsequent apoptosis in T lymphocytes via the activation of NF-κB and AP-1. Mol. Cell 1:543–551. [DOI] [PubMed] [Google Scholar]
- 36.Kirshenbaum, L. A., and D. de Moissac. 1997. The bcl-2 gene product prevents programmed cell death of ventricular myocytes. Circulation 96:1580–1585. [DOI] [PubMed] [Google Scholar]
- 37.Kockx, M. M., G. R. De Meyer, J. Muhring, W. Jacob, H. Bult, and A. G. Herman. 1998. Apoptosis and related proteins in different stages of human atherosclerotic plaques. Circulation 97:2307–2315. [DOI] [PubMed] [Google Scholar]
- 38.Kolbus, A., I. Herr, M. Schreiber, K.-M. Debatin, E. F. Wagner, and P. Angel. 2000. c-Jun-dependent CD95-L expression is a rate-limiting step in the induction of apoptosis by alkylating agents. Mol. Cell. Biol. 20:575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kondo, T., T. Suda, H. Fukuyama, M. Adachi, and S. Nagata. 1997. Essential roles of the Fas ligand in the development of hepatitis. Nat. Med. 3:409–413. [DOI] [PubMed] [Google Scholar]
- 40.Kureishi, Y., Z. Luo, I. Shiojima, A. Bialik, D. Fulton, D. J. Lefer, W. C. Sessa, and K. Walsh. 2000. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat. Med. 6:1004–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee, R. T., and P. Libby. 1997. The unstable atheroma. Arterioscler. Thromb. Vasc. Biol. 17:1859–1867. [DOI] [PubMed] [Google Scholar]
- 42.Le-Niculescu, H., E. Bonfoco, Y. Kasuya, F.-X. Claret, D. R. Green, and M. Karin. 1999. Withdrawal of survival factors results in activation of the JNK pathway in neuronal cells leading to Fas ligand induction and cell death. Mol. Cell. Biol. 19:751–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luo, Z., Y. Fujio, Y. Kureishi, R. D. Rudic, G. Daumerie, D. Fulton, W. C. Sessa, and K. Walsh. 2000. Acute modulation of endothelial Akt/PKB activity alters nitric oxide-dependent vasomotor activity in vivo. J. Clin. Investig. 106:493–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luo, Z., M. Sata, T. Nguyen, J. M. Kaplan, G. Y. Akita, and K. Walsh. 1999. Adenovirus-mediated delivery of Fas ligand inhibits intimal hyperplasia after balloon injury in immunologically primed animals. Circulation 99:1776–1779. [DOI] [PubMed] [Google Scholar]
- 45.Mallat, Z., J. Ohan, G. Leseche, and A. Tedugi. 1997. Colocalization of CPP-32 with apoptotic cells in human atherosclerotic plaques. Circulation 96:424–428. [DOI] [PubMed] [Google Scholar]
- 46.Mano, T., Z. Luo, T. Suhara, R. C. Smith, S. Esser, and K. Walsh. 2000. Expression of wild-type and noncleavable Fas ligand by tetracycline-regulated adenoviral vectors to limit intimal hyperplasia in vascular lesions. Hum. Gene Ther. 11:1625–1635. [DOI] [PubMed] [Google Scholar]
- 47.McGahon, A. J., S. J. Martin, R. P. Bissonnette, A. Mahboubi, Y. Shi, R. J. Mogil, W. K. Nishioka, and D. R. Green. 1995. The end of the cell line: methods for the study of apoptosis in vitro, p.153–185. In L. M. Schwartz and B. A. Osborne (ed.), Cell death, vol. 46. Academic Press, Inc., San Diego, Calif. [DOI] [PubMed]
- 48.Miao, W., Z. Luo, R. N. Kitsis, and K. Walsh. 2000. Intracoronary, adenovirus-mediated Akt gene transfer in heart limits infarct size following ischemia-reperfusion injury in vivo. J. Mol. Cell. Cardiol. 32:2397–2402. [DOI] [PubMed] [Google Scholar]
- 49.Morales-Ruiz, M., G. Fulton, G. Sowa, L. R. Languino, Y. Fujio, K. Walsh, and W. C. Sessa. 2000. Vascular endothelial growth factor-stimulated actin reorganization and migration of endothelial cells is regulated via the serine/threonine kinase Akt. Circ. Res. 86:892–896. [DOI] [PubMed] [Google Scholar]
- 50.Nagata, S. 1997. Apoptosis by death factor. Cell 88:355–365. [DOI] [PubMed] [Google Scholar]
- 51.Oshimi, Y., S. Oda, Y. Honda, S. Nagata, and S. Miyazaki. 1996. Involvement of Fas ligand and Fas-mediated pathway in the cytotoxicity of human natural killer cells. J. Immunol. 157:2909–2915. [PubMed] [Google Scholar]
- 52.Perlman, H., L. J. Pagliari, C. Georganas, T. Mano, K. Walsh, and R. M. Pope. 1999. FLICE-inhibitory protein expression during macrophage differentiation confers resistance to Fas-mediated apoptosis. J. Exp. Med. 190:1679–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perlman, H., M. Sata, K. Krasinski, T. Dorai, R. Buttyan, and K. Walsh. 2000. Adenovirus-encoded hammerhead ribozyme to Bcl-2 inhibits neointimal hyperplasia and induces smooth muscle cell apoptosis. Cardiovasc. Res. 45:570–578. [DOI] [PubMed] [Google Scholar]
- 54.Pollman, M. J., Z. L. Hall, M. J. Mann, L. Zhang, and G. H. Gibbons. 1998. Inhibition of neointimal cell bcl-x expression induces apoptosis and regression of vascular disease. Nat. Med. 4:222–227. [DOI] [PubMed] [Google Scholar]
- 55.Saas, P., P. R. Walker, M. Hahne, A.-L. Quiquerez, V. Schnuriger, G. Perrin, L. French, E. G. Van Meir, N. deTribolet, J. Tschopp, and P.-Y. Dietrich. 1997. Fas ligand expression by astrocytoma in vivo: maintaining immune privilege in the brain? J. Clin. Investig. 99:1173–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sata, M., H. Perlman, D. A. Muruve, M. Silver, M. Ikebe, T. A. Libermann, P. Oettgen, and K. Walsh. 1998. Fas ligand gene transfer to the vessel wall inhibits neointima formation and overrides the adenovirus-mediated T cell response. Proc. Natl. Acad. Sci. USA 95:1213–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sata, M., T. Suhara, and K. Walsh. 2000. Vascular endothelial cells and smooth muscle cells differ in their expression of Fas and Fas ligand and in their sensitivity to Fas ligand-induced cell death: implications for vascular disease and therapy. Arterioscler. Thromb. Vasc. Biol. 20:309–316. [DOI] [PubMed] [Google Scholar]
- 58.Sata, M., and K. Walsh. 1998. Oxidized LDL activates Fas-mediated endothelial cell apoptosis. J. Clin. Investig. 102:1682–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sata, M., and K. Walsh. 1998. TNFα regulation of Fas ligand expression on the vascular endothelium modulates leukocyte extravasation. Nat. Med. 4:415–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith, R. C., D. Branellec, D. H. Gorski, K. Guo, H. Perlman, J.-F. Dedieu, C. Pastore, A. Mahfoudi, P. Denèfle, J. M. Isner, and K. Walsh. 1997. p21CIP1-mediated inhibition of cell proliferation by overexpression of the gax homeodomain gene. Genes Dev. 11:1674–1689. [DOI] [PubMed] [Google Scholar]
- 61.Strand, S., W. J. Hofmann, A. Grambihler, H. Hug, M. Volkmann, G. Otto, H. Wesch, S. M. Mariani, V. Hack, W. Stremmel, P. H. Krammer, and P. R. Galle. 1998. Hepatic failure and liver cell damage in acute Wilson’s disease involve CD95 (APO-1/Fas) mediated apoptosis. Nat. Med. 4:588–593. [DOI] [PubMed] [Google Scholar]
- 62.Strand, S., W. J. Hofmann, H. Hug, M. Müller, G. Otto, D. Strand, S. M. Mariani, W. Stremmel, P. H. Krammer, and P. R. Galle. 1996. Lymphocyte apoptosis induced by CD95 (APO-1/Fas) ligand expressing tumor cells—a mechanism of immune evasion? Nat. Med. 2:1361–1366. [DOI] [PubMed] [Google Scholar]
- 63.Stuart, P. M., T. S. Griffith, N. Usui, J. Pepose, X. Yu, and T. A. Ferguson. 1997. CD95 ligand (FasL)-induced apoptosis is necessary for corneal allograft survival. J. Clin. Investig. 99:396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Suda, T., T. Okazaki, Y. Naito, T. Yokota, N. Arai, S. Ozaki, K. Nakao, and S. Nagata. 1995. Expression of the Fas ligand in T cell lineage. J. Immunol. 154:3806–3813. [PubMed] [Google Scholar]
- 65.Suda, T., T. Takahashi, P. Golstein, and S. Nagata. 1993. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell 75:1169–1178. [DOI] [PubMed] [Google Scholar]
- 66.Tanaka, M., T. Itai, M. Adachi, and S. Nagata. 1998. Downregulation of Fas ligand by shedding. Nat. Med. 4:31–36. [DOI] [PubMed] [Google Scholar]
- 67.Toker, A., and A. C. Newton. 2000. Akt/protein kinase B is regulated by autophosphorylation at the hypothetical PDK-2 site. J. Biol. Chem. 275:8271–8274. [DOI] [PubMed] [Google Scholar]
- 68.Ushio-Fukai, M., R. W. Alexander, M. Akers, Q. Q. Yin, Y. Fujio, K. Walsh, and K. K. Griendling. 1999. Reactive oxygen species mediate the activation of Akt/protein kinase B by angiotensin II in vascular smooth muscle cells. J. Biol. Chem. 274:22699–22704. [DOI] [PubMed] [Google Scholar]
- 69.Verdu, J., M. A. Buratovich, E. L. Wilder, and M. J. Birnbaum. 1999. Cell-autonomous regulation of cell and organ growth in Drosophila by Akt/PKB. Nat. Cell Biol. 1:500–506. [DOI] [PubMed] [Google Scholar]
- 70.Villunger, A., D. C. Huang, N. Holler, J. Tschopp, and A. Strasser. 2000. Fas ligand-induced c-Jun kinase activation in lymphoid cells requires extensive receptor aggregation but is independent of DAXX, and Fas-mediated cell death does not involved DAXX, RIP, or RAIDD. J. Immunol. 165:1337–1343. [DOI] [PubMed] [Google Scholar]
- 71.Walsh, K., and J. M. Isner. 2000. Apoptosis in inflammatory-fibroproliferative disorders of the vessel wall. Cardiovasc. Res. 45:756–765. [DOI] [PubMed] [Google Scholar]
- 72.Walsh, K., R. C. Smith, and H. S. Kim. 2000. Vascular cell apoptosis in remodeling, restenosis and plaque rupture. Circ. Res. 87:184–188. [DOI] [PubMed] [Google Scholar]
- 73.Widmann, C., P. Gerwins, N. L. Johnson, M. B. Jarpe, and G. L. Johnson. 1998. MEK kinase 1, a substrate for DEVD-directed caspases, is involved in genotoxin-induced apoptosis. Mol. Cell. Biol. 18:2416–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yujiri, T., S. Sather, G. R. Fanger, and G. L. Johnson. 1998. Role of MEKK1 in cell survival and activation of JNK and ERK pathways defined by targeted gene disruption. Science 282:1911–1914. [DOI] [PubMed] [Google Scholar]