Abstract
In the frq-wc-based circadian feedback loops of Neurospora, two PAS domain-containing transcription factors, WHITE COLLAR-1 (WC-1) and WC-2, form heterodimeric complexes that activate the transcription of frequency (frq). FRQ serves two roles in these feedback loops: repressing its own transcription by interacting with the WC complex and positively upregulating the levels of WC-1 and WC-2 proteins. We report here that the steady-state level of WC-1 protein is independently regulated by both FRQ and WC-2 through different posttranscriptional mechanisms. The WC-1 level is extremely low in wc-2 knockout strains, and this low level of expression is independent of wc-1 transcription and FRQ protein expression. In addition, our data show that the PAS domain of WC-2 mediates the interactions of this protein with both WC-1 and FRQ in vivo. Such interactions are essential for maintaining the steady-state level of WC-1 and the proper function of WC-1 and WC-2 in circadian clock and light responses.
Circadian clocks are responsible for controlling a wide variety of physiological, behavioral, cellular, and biochemical activities in most eukaryotic and certain prokaryotic organisms. The circadian oscillators are composed of positive and negative elements which form the core of the oscillators generating the basic circadian rhythmicity (16). In these networks the positive elements of the loop activate the transcription of the negative elements, while the negative elements feed back to block their own activation by the positive elements. To date, all of the identified positive elements in Neurospora, Drosophila, and mammals are PER-ARNT-SIM (PAS) domain-containing transcription factors (1, 10, 18, 23, 24, 40). These factors form heterodimeric complexes that activate the transcription of the negative elements in each system, generating protein products of the negative elements that feed back to inhibit their own expression via interactions with the positive elements (2, 7, 12, 15, 25, 27, 45, 48). In Drosophila, mammals, and Neurospora, it was also found that the negative elements of the oscillator activate the expression of one or two of the positive elements (8, 20, 26, 28, 34, 44). Therefore, the negative and the positive elements form positive feedback loops interlocked with the negative feedback loop. The similar arrangement in different clock systems suggests that it may be a common aspect in the eukaryotic circadian oscillators. Studies with Neurospora suggest that the interlocked positive feedback loops are important for maintaining the robustness and stability of the clock (8, 28, 34).
In the Neurospora circadian feedback loops, the heterodimeric complexes formed by WHITE COLLAR-1 (WC-1) and WC-2 (15, 46), two PAS domain-containing transcription factors (containing GATA-type zinc finger DNA binding domains) (46) are the positive components (8, 10), while two forms of the FREQUENCY (FRQ) protein function as the negative elements (2, 17, 30). In constant darkness, WC-1 and WC-2 are required for the activation of frq and the generation of circadian rhythms. In wc-1 and wc-2 mutants, frq mRNA and FRQ protein levels are very low and the clock is defective under normal conditions (10). By inducing the expression of wc-1 or wc-2 in these mutants, frq expression can be activated and thus the clock can be restarted (8). WC-1 and WC-2 proteins are also essential components for the light responses in Neurospora, including light induction of frq and light resetting of the clock (5, 10, 29). In the WC-1/WC-2 heterodimeric complex, WC-1 is the limiting factor for the formation of the complex (8, 15); thus, regulation of the level of WC-1 is important for the proper function of the Neurospora circadian clock.
The protein products of the frq gene are the negative elements in the Neurospora circadian feedback loop (2, 11, 16, 31). Both forms of FRQ protein (large and small FRQ [SFRQ] forms) are present in homodimeric complexes that feed back to repress their own transcription, probably by interacting with the WC-1/WC-2 complexes (2, 7, 15, 17, 30, 34). FRQ also positively regulates protein levels of both WC-1 and WC-2, forming positive feedback loops interlocked with the negative feedback loop (8, 28, 34). FRQ appears to regulate wc-1 and wc-2 through two different mechanisms: FRQ positively regulates WC-1 through a posttranscriptional mechanism, leading to its rhythmic expression, while FRQ increases the steady-state level of wc-2 mRNA (8, 28). These positive feedback loops appear to be important for maintaining the robustness and stability of the clock, since higher levels of WC-1 and WC-2 expression lead to more robust and stable oscillation of the clock (8, 28).
The fact that the positive elements in Neurospora, Drosophila, and mammals are all heterodimeric transcription activation complexes consisting of PAS domain-containing factors suggests that a key aspect of the clock mechanism is similar in different systems. Although these PAS domains are thought to function chiefly as protein-protein interaction modules (4, 22), their role in the assembly of these heterodimeric complexes remains unclear. For the PAS-containing transcription factors of the mammalian and Drosophila clocks, presently no in vivo evidence exists regarding the molecular basis of their interactions. Heterodimerization among other basic helix-loop-helix (bHLH) PAS proteins is thought to be mediated by their bHLH DNA binding domains, while their PAS domains might determine the specificity of the interactions (37, 39, 49). In Neurospora, WC-1 and WC-2 were shown to interact through their PAS domains by in vitro binding assay (4), but this has yet to be demonstrated in vivo. In this study, we show that the PAS domain of WC-2 is essential for the formation of the WC-1/WC-2 complex in vivo and that formation of this complex does not require most of the protein regions outside of the PAS domain. More importantly, our data show that the formation of WC-1/WC-2 complex is essential for maintaining the steady-state level of WC-1 protein and for the functions of both proteins in circadian clock generation and light responses in Neurospora. Together our data indicate that the level of WC-1 protein is regulated independently by FRQ and WC-2 through different posttranscriptional mechanisms, and both regulations are important in maintaining WC-1 levels in the wild type.
MATERIALS AND METHODS
Strains and culture conditions.
The bd, a (wild-type clock) strain was used as the wild-type strain in this study. wc-2 mutants ER33 (Fungal Genetics Stock Center [FGSC] #4408, a point mutation in the conserved Zn finger region) and 234w (FGSC #3817, a point mutation that generates a premature stop codon downstream of the coiled coil domain, creating a truncated protein of 356 amino acids [aa]) (29) were obtained from FGSC. 93-4 (his-3 bd frq10) (frq−), 87-12 (wild-type clock, his-3 bdA), 161-8 (his-3 bd wc-1ER53), 241-23 (his-3 bd wc-2ko), and 304-4 (his-3 bd wc-2ko frq9) were the host strains for various his-3 targeting constructs used (3, 9, 10). In the frq10 (frq−) strain, 5.3 kb of the frq locus has been deleted (3). The wc-1 strain used in this study was derived from FGSC #4397(ER53) (10, 14), and it makes an ∼80-kDa truncated WC-1 protein (data not shown). In the wc-2ko (wc-2−)strain the wild-type wc-2 locus is replaced by a hph gene (9); thus, no endogenous WC-2 protein is made.
Liquid culture conditions were the as same those previously described (2, 13, 31), except a lower glucose concentration was used in the media for strains used for quinic acids (QA) induction (1× Vogel’s, 0.1% glucose, 0.17% arginine, 10−2 M QA) (8). Race tube assay medium contains 1× Vogel’s, 0.1% glucose, 0.17% arginine, 50 ng of biotin/ml, and 1.5% agar. Calculations of period length were performed as described previously (30) with Chrono II, version 11.1 (Till Roenneberg, Ludwigs-Maximillian University, Munich, Germany).
Plasmids.
The qa-WC1 and qa-WC-2 his-3 targeting constructs were made previously (8). To create the qa-SFRQ::qa-WC-1 construct, the XbaI-NdeI fragment of pYL74 (containing qa-SFRQ) was blunt ended and inserted into the NdeI (blunt-ended) site of pqa-WC-1. The Myc-WC-2 and Myc-WC-1 constructs (pMyc-WC-2 and pMyc-WC-1) were made by inserting a PCR fragment containing the 5 c-Myc epitope tags (80 aa) (7) into the BstXI site of pWC2-1 or the NsiI site of pWC1-1. pWC2-1 contains a SmaI fragment of the wc-2 locus in pDE3dBH which can rescue the circadian and light phenotype of the wc-2 mutant (29). pUC19-Myc-WC-2 is the plasmid template for all in vitro site-specific mutagenesis, and it contains a SmaI-StuI fragment of wc-2 (includes the entire open reading frame [ORF]). All the deletions and point mutations of the WC-2 ORF were made by using the Transformer Site-Directed Mutagenesis kit (Clontech Laboratories, Inc., Palo Alto, Calif.), and the pSspI/EcoRV primer was used as the selection primer. The mutagenic primers used were WC2(33-127) (to delete WC-2 aa 33 to 127), WC2.PAS (to delete WC-2 aa 187 to 211), and WC2.309RR (to change both aa 309 isoleucine and 312 leucine to arginines). All resulting plasmid constructs were targeted by transformation to the his-3 locus of the host strains as previously described (6).
Protein and RNA analyses.
Protein extraction, quantification, Western blot analysis, and immunoprecipitation assays are as previously described (7, 17). The purification of Neurospora nuclei was performed as previously described (33). Equal amounts of total protein (40 to 100 μg) were loaded in each protein lane, and after the blots were developed by chemiluminescence (Amersham) they were stained by amido black to verify equal loading of protein (30).
RNA extraction and Northern blot analysis were performed as previously described (11). Equal amounts of total RNA (40 μg) were loaded onto agarose gels for electrophoresis, and the gels were blotted and probed with RNA probe specific for frq, wc-1, or wc-2 (5, 11, 29).
RESULTS
FRQ differentially regulates WC-1 and WC-2, and its effect on WC-1 is independent of WC-2.
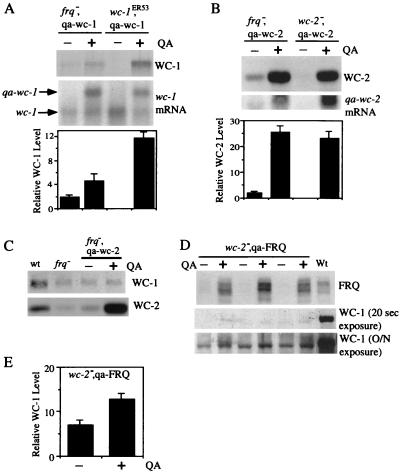
Although it is clear that FRQ positively regulates levels of wc-2 mRNA and both WC-1 and WC-2 proteins (8, 28, 34), the mode of regulation of FRQ on WC-1 is unclear due to conflicting data on how FRQ affects the abundance of wc-1 mRNA. The results of Lee et al. (28) and our results (8) show that comparable levels of wc-1 mRNA are produced in wild-type and frq− strains (frq10), indicating that a posttranscriptional mechanism is responsible for the regulation. This contrasts with data in a recent report by Merrow et al., who found that the abundance of wc-1 mRNA is much less in frq null strains than in the wild-type strain, suggesting that FRQ affects the steady-state level of wc-1 mRNA (34). To clarify whether FRQ posttranscriptionally regulates WC-1, we introduced a QA-inducible promoter (qa-2)-controlled WC-1 construct, qa-WC-1 (8), into frq10 and wc-1ER53 (making a truncated WC-1 protein of about 80 kDa) strains and examined the levels of WC-1 protein and wc-1 mRNA generated by induction with 0.01 M QA. In the presence of QA, genes under control of the qa-2 promoter are constitutively expressed (2, 19). If FRQ positively regulates WC-1 by increasing its mRNA level, we should expect that WC-1 should be similarly induced in these two strains. We found that this is not the case. As shown in Fig. 1A, the induced WC-1 protein level in the frq−, qa-WC-1 strain is significantly lower than that of the wc-1ER53, qa-WC-1 strain, even though the induction levels of qa-wc-1 mRNA were comparable in both strains. The larger size of the qa-wc-1 mRNA is due to the lack of the transcription terminator sequence in the part of the wc-1 3′ untranslated region in the qa-WC-1 construct, leading to the extension of the transcript (5, 8). These data indicate that FRQ posttranscriptionally regulates the steady-state level of WC-1 protein.
FIG. 1.
FRQ posttranscriptionally regulates WC-1 independent of WC-2. WC-1, WC-2, or FRQ was induced with 0.01 M QA in either frq10 or wc mutant strains in LL. The strains used are labeled at the top of each panel. (A and B) Western and Northern blot analyses were performed to show the expression levels of WC proteins or mRNA. Densitometric analysis of Western blots from three independent experiments is shown in the bottom graphs. Error bars represent standard deviation. The two arrows in panel A indicate the endogenous wc-1 mRNA and the induced qa-wc-1 mRNA. The larger size of the qa-wc-1 mRNA is due to a transcriptional extension at the 3′ untranslated region of qa-wc-1. (C) Western blot analysis showing that the induction of WC-2 in frq− strains fails to increase WC-1 levels. wt, wild type. (D) Western blot analysis showing that FRQ can increase WC-1 levels in the absence of WC-2. Triplicate samples are shown. Two different exposures of the WC-1 signal are shown, including an overnight (O/N) exposure to visualize the weak WC-1 signals in a wc-2−, qa-FRQ strain. (E) Densitometric analysis of the Western blot results shown in panel D. Error bars represent standard deviations.
In contrast to the differential induction of WC-1 that we observed, WC-2 was induced to similar levels in frq−, qa-WC-2 and wc-2−, qa-WC-2 strains (Fig. 1B). Combined with previous data (8), this result suggests that the positive role that FRQ has on WC-2 protein level is achieved by increasing the steady-state level of wc-2 mRNA.
Since FRQ positively regulates the levels of both WC-1 and WC-2 proteins, it is possible that its effect on WC-1 is through WC-2, since these two proteins are present in heterodimeric complexes. To test this possibility we examined the effect of various WC-2 protein levels on WC-1 in two different strains where WC-2 or FRQ expression was under the control of a qa-2-inducible promoter, frq−, qa-WC-2 and wc-2−, qa-FRQ (2, 8). As shown in Fig. 1C, the dramatic induction of WC-2 protein in the frq−, qa-WC-2 strain failed to change the level of WC-1 (lanes 3 and 4), indicating that the positive role of FRQ on WC-1 is independent of WC-2. Consistent with this result, the induction of FRQ in the wc-2−, qa-FRQ strain increased WC-1 levels (Fig. 1D and E). In addition, we found that levels of WC-1 in the wc-2−, qa-FRQ strain are much lower than those of the wild type, suggesting that WC-2 is important for maintaining the normal level of WC-1 (see below).
WC-1 protein level is extremely low in wc-2 knockout strains, and the effect of wc-2 on wc-1 is posttranscriptional and independent of frq.
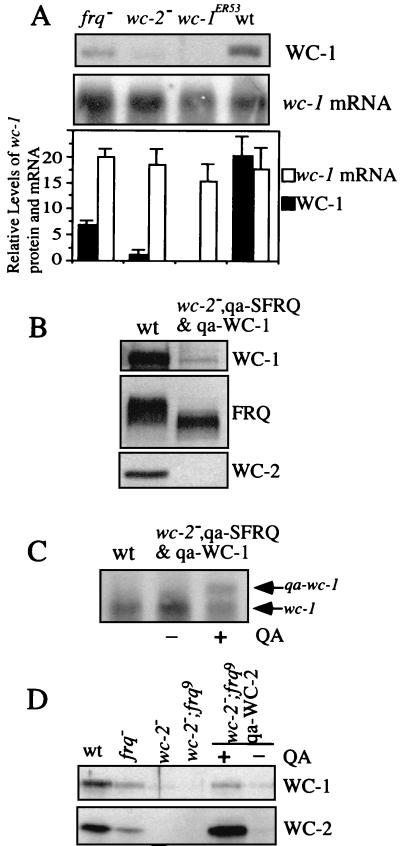
As shown in Fig. 1D, the level of WC-1 is very low in the wc-2−, qa-FRQ strain. To examine the effect of WC-2 on the level of WC-1 protein, we examined the WC-1 level in a wc-2 knockout (wc-2−) strain (9). We found that the WC-1 level was also extremely low in this strain, much lower than what was found in the frq− strain (Fig. 2A). Northern blot analysis revealed that the levels of wc-1 mRNA were comparable in different mutant strains, indicating that the effect of WC-2 on WC-1 is posttranscriptional. Similar results were obtained for cultures grown in either constant light (LL) or constant darkness (DD24) conditions (data not shown).
FIG. 2.
The WC-1 level is extremely low in wc-2 knockout strains, and the effect of WC-2 on WC-1 is independent of FRQ expression. (A) The levels of wc-1 protein (top) and mRNA (middle) were examined in various mutants and wild-type (wt) strains at DD24. Densitometric analysis of blots from three independent experiments is shown in the bottom graph. Error bars represent standard deviations. (B and C) The level of WC-1 is extremely low in a wc-2ko strain despite the induction of both SFRQ (B) and qa-wc-1 mRNA at the same time (C). In panel B, Western blot analysis shows that the induction of SFRQ failed to restore WC-1 levels back to wild-type amounts. In panel C, the Northern blot results corresponding to the samples depicted in panel B show that the qa-wc-1 mRNA was induced in the presence of QA. (D) The levels of WC-1 and WC-2 were examined by Western blot analysis in LL with the indicated mutant strains. To induce WC-2 in the wc-2, frq double mutant (wc-2−, frq9, qa-WC-2), 0.01 M QA was used.
The comparison of WC-1 levels in frq− and wc-2− strains suggests that the low level of WC-1 observed in the wc-2− strain is not due solely to the low abundance of FRQ in that strain, indicating that additional regulation is involved. To further rule out the possibility that the low FRQ level is responsible for the low WC-1 level, we made a construct where both WC-1 and the SFRQ (100 aa smaller than the large form of FRQ) were put under the control of the inducible qa-2 promoter (qa-WC-1 and qa-SFRQ) and introduced into a wc-2− strain. Our preliminary experiments showed that either FRQ form was able to positively regulate WC-1 (data not shown). As shown in Fig. 2B and C, the level of WC-1 protein is still very low in this strain despite the fact that both SFRQ and qa-wc-1 mRNA were induced. This result indicates that while FRQ can increase the WC-1 level in a wc-2− strain (Fig. 1D), the absence of the wc-2 gene leads to a very low level of WC-1.
To further confirm our conclusion, a wc-2−, frq− (frq9) double mutant was made and the qa-WC-2 construct was introduced into this strain (8). As shown in Fig. 2D, the level of WC-1 is also extremely low in the wc-2−, frq− double mutant, similar to that of the wc-2− strain. By inducing WC-2 expression in this strain, we could restore the level of WC-1 to that of the frq− strain. Consistent with the results shown in Fig. 1C, the inability for the overexpressed WC-2 to increase WC-1 protein to a level that is above that of a frq− strain indicates that FRQ and WC-2 both independently regulate WC-1 and that the combination of these effects is important for maintaining WC-1 protein level in the wild type.
Functional WC-2 is not required for maintaining WC-1 level.
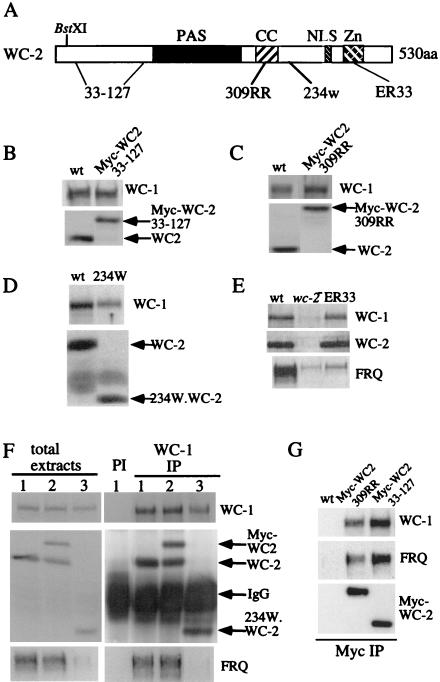
The extremely low level of WC-1 in wc-2 knockout strains contrasts with the moderate WC-1 levels previously reported in a wc-2 truncation strain (234w) (42, 46), raising the possibility that functional WC-2 protein is not required for maintaining the WC-1 protein level. To test this possibility, WC-1 protein levels were examined in two existing wc-2 mutants and several newly created wc-2 mutants. Figure 3A depicts the linear structure of the WC-2 ORF. Sequence analysis revealed that it contains a PAS domain, a coiled coil domain, a putative nuclear localization domain, and a GATA-type zinc finger DNA binding domain (29). Two existing mutants are affected in the C-terminal half of the WC-2 protein: 234w produces a truncated WC-2 protein consisting of 356 aa, while ER33 contains a point mutation in the conserved Zn finger region (10, 29). To study the functions of the rest of the protein, a 5 c-Myc epitope tag (7) was inserted into the amino terminus of the protein at the BstXI site to create the Myc-WC-2 construct. Our data showed that Myc-WC-2 is able to rescue all of the defects of the wc-2 knockout strain, including normal WC-1 level, light responses, and circadian rhythms (see Fig. 6 and data not shown). Starting with this construct, we generated additional constructs containing various mutations. The Myc-WC-2(33-127) construct contains an in-frame deletion (from aa 33 to 127) of most of the protein to the amino-terminal side of the PAS domain. In contrast, the Myc-WC-2(309RR) construct contains two point mutations (I309R and L312R) designed to disrupt the protein-protein interaction surface of the putative coiled-coil domain. Similar mutations have been shown to severely impair the function of the coiled coil domain of FRQ (7). These mutant constructs were introduced into a wc-2− strain.
FIG. 3.
Functional WC-2 is not required for maintaining WC-1 level or forming complexes with WC-1. (A) Schematic depiction of the domain architecture of the WC-2 protein, along with the location of mutations in various mutants used in this work. CC, coiled coil domain; NLS, nuclear localization signal; Zn, GATA-type Zn finger DNA binding domain; BstXI, restriction site used to introduce the 5 c-Myc epitope tag. (B to E) Western blot analyses showing WC-1 levels in various wc-2 mutants in LL. (F) Immunoprecipitation assay (IP) using WC-1 antiserum shows that the mutant WC-2 proteins can still form complexes with WC-1 in vivo. The Neurospora protein extracts were either immunoprecipitated by WC-1 antiserum (right panels) or analyzed directly by Western blot analyses. Lane 1, wild-type (wt) strain; lane 2, wc-2+ Myc-WC-2(33-127) (note the endogenous WC-2 band); lane 3, 234w. PI, wild-type extracts were immunoprecipitated with the preimmune antiserum. (G) Immunoprecipitation assay using c-Myc monoclonal antibody showing that Myc-WC2.309RR and Myc-WC2(33-127) can form complexes with WC-1 and FRQ. The wild-type extract was used as the control, since it does not contain c-Myc-tagged WC-2.
FIG. 6.
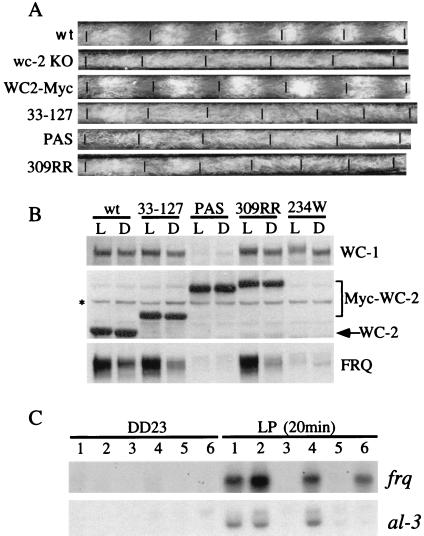
The WC-2 PAS domain is essential for WC-2 functions in circadian clock and light responses. (A) Race tube assays showing the circadian conidiation rhythms of various wc-2 mutants. The short black lines mark the growth fronts of Neurospora every 24 h in DD24. KO, knockout. (B) Western blot analyses showing the expression of WC-1, WC-2, and FRQ in various wc-2 mutants in LL (L) and DD24 (D). The asterisk indicates a nonspecific band recognized by our WC-2 antiserum. (C) Northern blot analyses showing the light induction of frq and al-3 mRNA in various wc-2 mutants. LP, cultures were given a 20-min light pulse at DD23 before harvest. Lane 1, wild type (wt); lane 2, wc-2−, Myc-WC2(33-127); lane 3, wc-2−, Myc-WC2.PAS; lane 4, wc-2−, Myc-WC2.309RR; lane 5, wc-2234w; lane 6, wc-2ER33.
As shown in Fig. 3B to E, WC-1 protein levels in these wc-2 mutants are comparable to those of the wild type, except in 234w. In addition, the mutant WC-2 proteins were found to be expressed at about the wild-type level in these strains. Although the predicted size of Myc-WC-2(33-127) is about the same as that of endogenous WC-2, the 27 acidic aa in the 5 c-Myc epitope tag is likely responsible for the slower mobility of the Myc-WC-2(33-127) protein (Fig. 3B). Similar to previously reported results (42), the WC-1 level in 234w is only slightly lower than that of the wild type. These data indicate that a fully functional WC-2 protein is not required for maintaining the WC-1 level. The low but detectable FRQ level and the level in the ER33 mutant comparable to that of wild-type WC-1 suggest that even a low level of FRQ is able to support a near-normal level of WC-1 (Fig. 3E).
Since the PAS domain of WC-2 is predicted to mediate the interactions between WC-1 and WC-2, it is likely that these mutated WC-2 proteins can still form complexes with WC-1. To directly examine this possibility, we performed immunoprecipitation assays with WC-1 antiserum. As expected, we found that the mutant WC-2 forms can still form complexes with WC-1 (Fig. 3F and data not shown). As shown in Fig. 3F, the truncated WC-2 proteins in Myc-WC-2(33-127) (in a wild-type background) and 234w strains were coprecipitated with WC-1. By performing additional immunoprecipitation assays with a c-Myc monoclonal antibody, we showed that Myc-WC-2(33-127) and Myc-WC-2(309RR) can still form complexes with WC-1 and FRQ (Fig. 3G). These results clearly suggest that the N terminus and the coiled coil regions of WC-2 are not essential for its interactions with WC-1 and FRQ. Due to low levels of FRQ in 234W and ER33 strains, we do not know whether WC-2 in these two strains can still form complexes with FRQ.
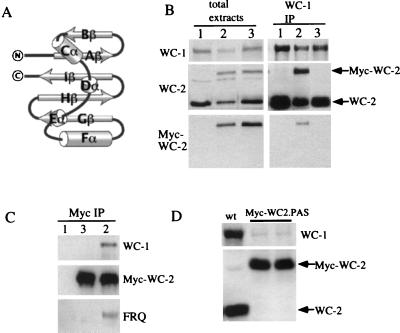
Alteration of WC-2 PAS domain results in loss of WC-1/WC-2 and WC-2/FRQ interactions and an extremely low level of WC-1.
Since the regulation of WC-2 on WC-1 is posttranscriptional, it is possible that the interaction between WC-1 and WC-2 is important for maintaining the steady-state level of WC-1. Although the PAS regions of WC proteins interact with each other in vitro (4), no in vivo experimental evidence has yet been generated to support this hypothesis. Unlike WC-1 and most other eukaryotic PAS domain-containing proteins, WC-2 has only a single PAS repeat. Although the primary amino acid sequences of PAS domains are poorly conserved, these domains all appear to adopt a similar mixed α/β-type fold (Fig. 4A) (21, 35, 36). To mutate the WC-2 PAS structure, two strands and its linker region (Gβ and Hβ) of the central β sheet were deleted (Myc-WC-2.PAS), which should destroy the formation of the PAS structure. To examine whether PAS-mutated WC-2 can still interact with WC-1, this construct was introduced into a wild-type strain so that endogenous WC-1, WC-2, and FRQ could be used as the internal controls for immunoprecipitation assays. As expected, the PAS-deleted Myc-WC-2 protein failed to form a complex with WC-1, although both the endogenous WC-2 and the Myc-tagged wild-type WC-2 proteins can be coprecipitated by WC-1 (Fig. 4B). With c-Myc monoclonal antibody to perform immunoprecipitation assay, our data also showed that Myc-WC-2.PAS could not form complexes with either WC-1 or FRQ (Fig. 4C). Together these data indicate that the PAS domain of WC-2 is essential for the interactions among WC-1, WC-2, and FRQ in vivo.
FIG. 4.
Deletion of part of the WC-2 PAS domain leads to the loss of its interactions with WC-1 and FRQ and results in an extremely low level of WC-1. (A) Schematic diagram of conserved secondary structure elements among all PAS domains, labeled in accordance with the nomenclature of Gong et al. (21). (B and C) Immunoprecipitation assays (IP) showing that the WC-2 PAS domain is essential for WC-2 interaction with WC-1 (B) and FRQ (C). The constructs were introduced into a wild-type (wt) strain, and Neurospora protein extracts were either immunoprecipitated by WC-1 (B) or c-Myc (C) antiserum or were analyzed directly by Western blot analysis. WC-1, WC-2, c-Myc, and FRQ antisera were used for Western blot analysis. Lane 1, wild type; lane 2, wild type, Myc-WC-2; lane 3, wild type, Myc-WC-2.PAS. (D) Western blot analyses show that the WC-1 level is extremely low in two independent wc-2−, Myc-WC-2.PAS transformants. The Myc-WC-2.PAS construct was introduced into the wc-2 null strain.
If the interaction between WC-1 and WC-2 is important for the stability of WC-1, we should expect that the PAS-mutated WC-2 protein cannot maintain a normal WC-1 level in a wc-2− background. This is exactly what we found: although the Myc-WC-2.PAS proteins were normally expressed in wc-2− strains, the level of WC-1 protein was low and similar to what was found for wc-2− strains (Fig. 4D). Together with the results depicted in Fig. 3, these data strongly suggest that the WC-2 PAS domain-mediated WC-1/WC-2 interaction is important for maintaining the steady-state level of WC-1 protein. In the absence of WC-2, we suggest that WC-1 protein is unstable and is quickly degraded. Despite the strong influence of WC-2 on WC-1 levels, our data also indicate that WC-2 protein is stable in the absence of WC-1 or WC-1/WC-2 interaction (Fig. 4 and 6B).
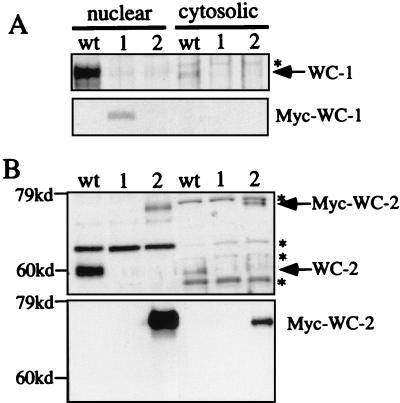
In Drosophila, the PAS domain-mediated PERIOD-TIMELESS (PER-TIM) interaction affects both nuclear localization and the stability of PER protein (38, 41, 43, 47). Thus, it is possible that the low WC-1 level is due to its failure to enter into the nucleus in the absence of WC-2. A previous report has shown that the nuclear entries of WC-1 and WC-2 are independent from each other (42), although it should be noted that the wc-2 mutant (234w) used in that study can still form complexes with WC-1 (Fig. 3F). To clarify this issue, we examined the nuclear localization of WC-1 in wc-2− strains. Because of the low level of WC-1 in wc-2− strains and the limited specificity of our WC-1 antiserum, we generated a strain in which a 5 c-Myc epitope tag was inserted into the N-terminal end of WC-1 (Myc-WC-1). The Myc-WC-1 construct was able to rescue the circadian and light responses of the wc-1 mutant strain (data not shown). Thus, it could function as the endogenous WC-1 protein. Nuclear localization of Myc-WC2.PAS in the wc-2−, Myc-WC2.PAS strain was also examined to see whether the WC-1/WC-2 interaction is needed for the nuclear entry of WC-2. As shown in Fig. 5, Myc-WC-1 (lane 1 of panel A) and Myc-WC2.PAS (lane 2 of panel B) proteins were enriched in the nuclear fractions. These data indicate that the interaction of WC-1/WC-2 is not required for the nuclear localization of either WC-1 or WC-2.
FIG. 5.
Nuclear localization of WC-1 and WC-2 are independent from each other. Western blot analyses of the nuclear and cytosolic preparations from wild-type (wt), wc-2−, Myc-WC-1 (lane 1) and wc-2−, Myc-WC2.PAS (lane 2) strains were performed. The strains were grown in LL. (A) Western blot analysis was performed with WC-1 (top) or c-Myc (bottom) monoclonal antibodies to show the levels of WC-1 and Myc-WC-1. (B) The blots were probed with WC-2 (top) or c-Myc (bottom) monoclonal antibodies to show the levels of WC-2 and Myc-WC-2. An asterisk indicates nonspecific bands recognized by our WC-1 and WC-2 antisera.
Circadian phenotypes and light responses in the wc-2 mutants reveal that the PAS domain of WC-2 is essential for WC-2 function in these two processes.
To study the functional role played by various regions of WC-2 in regulating the circadian clock, we used race tube assays to examine the circadian conidiation rhythms in the wc-2 mutant strains (in the wc-2− background) (Fig. 6A). The levels of FRQ protein in these mutants were also examined under LL and DD24 conditions (Fig. 6B). As previously reported, the wc-2 knockout strain exhibits no circadian conidiation rhythms under normal conditions (9). While the wild-type Myc-WC-2 construct can fully rescue the robust conidiation rhythm of the wc-2− strain, none of the wc-2 mutant constructs did (Fig. 6A). The PAS mutation of WC-2 (Myc-WC-2.PAS) led to complete loss of conidiation rhythm (Fig. 6A) and very low levels of FRQ (Fig. 6B), indicating that the WC-2 PAS domain is essential for its circadian clock function. Less severe phenotypes were observed with Myc-WC-2(33-127) and Myc-WC-2(309RR) strains, both of which can still interact with WC-1 and FRQ (Fig. 3G) and support FRQ expression in LL and in DD24. In contrast to the complete loss of conidiation rhythm in the Myc-WC-2.PAS strain, both mutants exhibited conidiation rhythm for 1 to 2 days in DD24 before becoming arrhythmic. These data suggest that both mutations result in partially functional WC-2 protein and that both regions are important for robust oscillation of the circadian clock.
Since wc-2 is also an essential component of light responses in Neurospora (29), Northern blot analyses were performed to examine the light induction of frq and albino-3 (al-3) mRNA in these wc-2 mutant strains (Fig. 6C). Neither frq nor al-3 demonstrated any light induction at the mRNA level in the Myc-WC-2.PAS and 234w strains, indicating that both the PAS domain and the C-terminal region of WC-2 are essential for the light induction of gene expression. Consistent with WC-2 proteins being partially functional in the Myc-WC-2(33-127) and Myc-WC-2(309RR) mutants, light induction of the frq and al-3 mRNAs were observed and the induction level was comparable to that of the wild type (Fig. 6C). As previously reported, we found that frq but not al-3 mRNA is induced in ER33 (10, 29). This final result is an intriguing one, suggesting that the light induction of frq and al-3 have different requirements for the functional activity of WC-2.
DISCUSSION
In the Neurospora frq-wc-based circadian feedback loops, the two PAS domain-containing transcription factors, WC-1 and WC-2, form heterodimeric complexes that activate the transcription of frq, while FRQ protein products have two roles: to repress FRQ’s own transcription by interacting with the WC complex and to positively regulate the levels of WC-1 and WC-2 proteins, forming interlocked circadian feedback loops (Fig. 7) (32). In this study, we report that the positive regulation of FRQ on WC-1 is a posttranscriptional one (8, 28) and is independent of WC-2. A central observation of this paper was our finding of extremely low levels of WC-1 in wc-2− strains. Further, the level of WC-1 in these strains is independent of wc-1 transcription and FRQ protein expression. Thus, the steady-state level of WC-1 is posttranscriptionally regulated independently by FRQ and WC-2. Both regulations are important in maintaining the steady-state level of WC-1 protein in wild-type strains that are required for normal light responses and normal circadian rhythmicity. Our data further showed that the PAS domain of WC-2 is essential for its in vivo interactions with WC-1 and FRQ and its function in circadian clock and light responses.
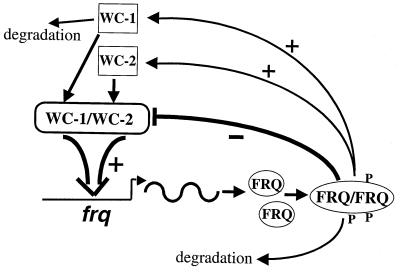
FIG. 7.
The Neurospora circadian feedback loops. WC-1 and WC-2 form a heterodimeric complex to activate the transcription of frq. If WC-1 cannot form this complex with WC-2, it is quickly degraded. FRQ proteins form homodimers that interact with the WC-1/WC-2 complex to inhibit their transcriptional activation, forming the negative feedback loop. FRQ also positively regulates the levels of both WC-1 and WC-2, forming the positive feedback loops. Progressive phosphorylation of FRQ leads to its degradation.
By examining the WC-1 level and WC-1/WC-2 protein-protein interaction in various wc-2 mutants, we found that a fully functional WC-2 is not required for maintaining WC-1 levels, provided that protein-protein interactions between WC-1 and WC-2 can still be maintained. The PAS domain of WC-2 is central to these interactions, as shown by the failure of the Myc-WC2.PAS construct to maintain WC-1 levels in a wc-2− strain. Significantly, the deletion of the WC-2 PAS also impairs its ability to interact with FRQ, suggesting that the WC-2 PAS domain also mediates the WC interaction with FRQ to close the circadian negative feedback loop. However, we could not exclude the possibility that the deletion of part of the WC-2 PAS region changed the overall structure of WC-2, and such change might affect its ability to interact with WC-1.
The very low level of WC-1 in the absence of WC-1/WC-2 interaction indicates that such interaction is important for maintaining the steady-state level of WC-1. Although it is possible that WC-2 might regulate WC-1 at the level of translation, we think the most likely scenario is that WC-1 protein becomes very unstable in the absence of WC-2 or when it cannot form a complex with WC-2. Consistent with our notion, a very low level of WC-1 was found in a wc-1 PAS mutant (42). Although it is not known whether mutant WC-1 can still interact with WC-2 in that mutant, it is reasonable to speculate that the PAS domain of WC-1 mediates its interaction with WC-2. This contrasts with the regulation of FRQ on WC-1, where the absence of FRQ does not seem to affect the stability of WC-1 (28). To directly assay the effect that WC-2 has on WC-1 protein stability in wild-type and wc-2− strains, we carried out 35S-pulse-chase labeling experiments. However, we were unable to detect WC-1 protein in [35S]methionine-labeled Neurospora protein extracts for the wc-2− strains, probably due to the low level of WC-1 protein and specificity of our WC-1 antiserum, although we were able to detect WC-1 in a wild-type strain (data not shown).
Similar PAS-mediated protein-protein interactions have also been found for PER and TIM proteins of Drosophila, the negative clock elements in the Drosophila circadian feedback loop. It was shown that two domains of PER, including the PAS domain of this protein, interact with two other non-PAS domains of TIM, and such interactions are important for the nuclear localization for both proteins and for the stability of PER (38, 41, 43, 47). In tim01 and per01 flies, PER and TIM proteins were only found in the cytoplasm and the level of PER is low in tim01 flies. The inability of these two proteins to migrate into the nucleus results in the loss of circadian behavioral rhythms and molecular oscillations. In Neurospora, although the stability of WC-1 may dependent on the WC-1/WC-2 interaction, the nuclear entries of both proteins are independent from each other (Fig. 5) (42).
Previously it was found that WC-2 may mediate the interaction between FRQ and WC-1 (15). In addition, we found that FRQ interacts with itself through its coiled coil domain (7) and that the formation of the FRQ coiled coil structure is essential for its self association and its interaction with the WC complex. The presence of a putative coiled coil motif in WC-2 raises the possibility that FRQ and WC-2 may associate via these motifs. However, when we directly tested this hypothesis with Myc-WC2(309RR), a mutant specifically designed to disrupt the hydrophobic interface of the coiled coil region of WC-2, we found that WC-2 can still interact with FRQ and WC-1 and can activate FRQ in light and dark conditions (Fig. 6). This result suggests that WC-2’s coiled coil structure is not essential for the protein interactions. Despite these near-normal phenotypes at the molecular level, this mutant has a severe defect in the generation of a robust circadian conidiation rhythm (Fig. 6A). Specifically, this mutant exhibits low amplitude conidiation rhythms for about 2 days before becoming arrhythmic. These data suggest that the coiled coil domain of WC-2 plays a role in maintaining robust rhythmicity in DD24. Although it is possible that the coiled coil of WC-2 may still be involved in mediating its interaction with WC-1 and FRQ, it is also possible that it serves as an interaction surface with another unidentified clock protein. In this study we found that WC-2 protein with the PAS deletion lost its ability to interact with FRQ, suggesting that the WC-2 PAS domain mediates the FRQ/WC-2 interaction. However, this simple interpretation of the results is complicated by the fact that the WC-1 level is low and cannot form a complex with WC-2 in the mutant, since the formation of the WC-1/WC-2 complex may be important for the FRQ-WC interaction.
In conclusion, our data show that the steady-state level of WC-1 protein is regulated independently by FRQ and WC-2 posttranscriptionally. The WC-2 PAS domain mediates its interaction with WC-1 and FRQ, and the formation of the WC-1/WC-2 complex is essential for maintaining the steady-state level of WC-1 and the function of both proteins in circadian clock and light responses of Neurospora.
Acknowledgments
We thank Michael Collett, Jay Dunlap, and Jennifer Loros for providing the wc-2 knockout strain prior to its publication. We thank Duojia Pan for critical reading of the manuscript.
This study was supported by grants from the National Institutes of Health (GM 62591) to Y. Liu and from the Robert A. Welch Foundation and the Searle Scholars Program/The Chicago Community Trust to K. H. Gardner. Y. Liu (Louise W. Kahn Scholar) and K. H. Gardner (W. W. Caruth, Jr., Scholar) are both endowed scholars in Biomedical Research at the University of Texas Southwestern Medical Center.
REFERENCES
- 1.Allada, R., N. E. White, W. V. So, J. C. Hall, and M. Rosbash. 1998. A mutant Drosophila homolog of mammalian Clock disrupts circadian rhythms and transcription of period and timeless. Cell 93:791–804. [DOI] [PubMed] [Google Scholar]
- 2.Aronson, B., K. Johnson, J. J. Loros, and J. C. Dunlap. 1994. Negative feedback defining a circadian clock: autoregulation in the clock gene frequency. Science 263:1578–1584. [DOI] [PubMed] [Google Scholar]
- 3.Aronson, B. D., K. A. Johnson, and J. C. Dunlap. 1994. The circadian clock locus frequency: a single ORF defines period length and temperature compensation. Proc. Natl. Acad. Sci. USA 91:7683–7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballario, P., C. Talora, D. Galli, H. Linden, and G. Macino. 1998. Roles in dimerization and blue light photoresponses of PAS and LOV domains of Neurospora crassa white collar proteins. Mol. Microbiol. 29:719–729. [DOI] [PubMed] [Google Scholar]
- 5.Ballario, P., P. Vittorioso, A. Magrelli, C. Talora, A. Cabibbo, and G. Macino. 1996. White collar-1, a central regulator of blue-light responses in Neurospora crassa, is a zinc-finger protein. EMBO J. 15:1650–1657. [PMC free article] [PubMed] [Google Scholar]
- 6.Bell-Pedersen, D., J. C. Dunlap, and J. J. Loros. 1996. Distinct cis-acting elements mediate clock, light, and developmental regulation of the Neurospora crassa eas (ccg-2) gene. Mol. Cell. Biol. 16:513–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng, P., Y. Yang, C. Heintzen, and Y. Liu. 2001. Coiled-coil domain mediated FRQ-FRQ interaction is essential for its circadian clock function in Neurospora. EMBO J. 20:101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng, P., Y. Yang, and Y. Liu. 2001. Interlocked feedback loops contribute to the robustness of the Neurospora circadian clock. Proc. Natl. Acad. Sci. USA 98:7408–7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collett, M. A., J. C. Dunlap, and J. J. Loros. 2001. Circadian clock-specific roles for the light response protein WHITE COLLAR-2. Mol. Cell. Biol. 21:2619–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crosthwaite, S. K., J. C. Dunlap, and J. J. Loros. 1997. Neurospora wc-1 and wc-2: transcription, photoresponses, and the origins of circadian rhythmicity. Science 276:763–769. [DOI] [PubMed] [Google Scholar]
- 11.Crosthwaite, S. K., J. J. Loros, and J. C. Dunlap. 1995. Light-Induced resetting of a circadian clock is mediated by a rapid increase in frequency transcript. Cell 81:1003–1012. [DOI] [PubMed] [Google Scholar]
- 12.Darlington, T. K., K. Wager-Smith, M. F. Ceriani, D. Stankis, N. Gekakis, T. Steeves, C. J. Weitz, J. Takahashi, and S. A. Kay. 1998. Closing the circadian loop: CLOCK induced transcription of its own inhibitors, per and tim. Science 280:1599–1603. [DOI] [PubMed] [Google Scholar]
- 13.Davis, R. L., and D. deSerres. 1970. Genetic and microbial research techniques for Neurospora crassa. Methods Enzymol. 27A:79–143. [Google Scholar]
- 14.Degli Innocenti, F., and V. E. Russo. 1984. Isolation of new white collar mutants of Neurospora crassa and studies on their behavior in the blue light-induced formation of protoperithecia. J. Bacteriol. 159:757–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denault, D. L., J. J. Loros, and J. C. Dunlap. 2001. WC-2 mediates WC-1-FRQ interaction within the PAS protein-linked circadian feedback loop of Neurospora. EMBO J. 20:109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunlap, J. C. 1999. Molecular bases for circadian clocks. Cell 96:271–290. [DOI] [PubMed] [Google Scholar]
- 17.Garceau, N., Y. Liu, J. J. Loros, and J. C. Dunlap. 1997. Alternative initiation of translation and time-specific phosphorylation yield multiple forms of the essential clock protein FREQUENCY. Cell 89:469–476. [DOI] [PubMed] [Google Scholar]
- 18.Gekakis, N., D. Stankis, H. B. Nguyen, F. C. Davis, L. D. Wilsbacher, D. P. King, J. S. Takahashi, and C. J. Weitz. 1998. Role of the CLOCK protein in the mammalian circadian mechanism. Science 280:1564–1569. [DOI] [PubMed] [Google Scholar]
- 19.Giles, N. H., M. E. Case, J. Baum, R. Geever, L. Huiet, V. Patel, and B. Tyler. 1985. Gene organization and regulation in the qa (quinic acid) gene cluster of Neurospora crassa. Microbiol. Rev. 49:338–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glossop, N. R., L. C. Lyons, and P. E. Hardin. 1999. Interlocked feedback loops within the Drosophila circadian oscillator. Science 286:766–768. [DOI] [PubMed] [Google Scholar]
- 21.Gong, W., B. Hao, S. S. Mansy, G. Gonzalez, M. A. Gilles-Gonzalez, and M. K. Chan. 1998. Structure of a biological oxygen sensor: a new mechanism for heme-driven signal transduction. Proc. Natl. Acad. Sci. USA 95:15177–15182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang, Z. J., I. Edery, and M. Rosbash. 1993. PAS is a dimerization domain common to Drosophila period and several transcription factors. Nature 364:259–262. [DOI] [PubMed] [Google Scholar]
- 23.Kay, S. A. 1997. As time PASses: the first mammalian clock gene. Science 276:1093. [DOI] [PubMed] [Google Scholar]
- 24.King, D., Y. Zhao, A. Sangoram, L. Wilsbacher, M. Tanaka, M. Antoch, T. Steeves, M. Vitaterna, J. Kornhauser, P. Lowrey, F. Turek, and J. Takahashi. 1997. Positional cloning of the mouse circadian CLOCK gene. Cell 89:641–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kume, K., M. J. Zylka, S. Sriram, L. P. Shearman, D. R. Weaver, X. Jin, E. S. Maywood, M. H. Hastings, and S. M. Reppert. 1999. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell 98:193–205. [DOI] [PubMed] [Google Scholar]
- 26.Lee, C., K. Bae, and I. Edery. 1998. The Drosophila CLOCK protein undergoes daily rhythms in abundance, phosphorylation, and interactions with the PER-TIM complex. Neuron 21:857–867. [DOI] [PubMed] [Google Scholar]
- 27.Lee, C., K. Bae, and I. Edery. 1999. PER and TIM inhibit the DNA binding activity of a Drosophila CLOCK-CYC/dBMAL1 heterodimer without disrupting formation of the heterodimer: a basis for circadian transcription. Mol. Cell. Biol. 19:5316–5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee, K., J. J. Loros, and J. C. Dunlap. 2000. Interconnected feedback loops in the Neurospora circadian system. Science 289:107–110. [DOI] [PubMed] [Google Scholar]
- 29.Linden, H., and G. Macino. 1997. White collar-2, a partner in blue-light signal transduction, controlling expression of light-regulated genes in Neurospora crassa. EMBO J. 16:98–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu, Y., N. Garceau, J. J. Loros, and J. C. Dunlap. 1997. Thermally regulated translational control mediates an aspect of temperature compensation in the Neurospora circadian clock. Cell 89:477–486. [DOI] [PubMed] [Google Scholar]
- 31.Liu, Y., M. M. Merrow, J. J. Loros, and J. C. Dunlap. 1998. How temperature changes reset a circadian oscillator. Science 281:825–829. [DOI] [PubMed] [Google Scholar]
- 32.Loros, J. J., and J. C. Dunlap. 2001. Genetic and molecular analysis of circadian rhythms in NEUROSPORA. Annu. Rev. Physiol. 63:757–794. [DOI] [PubMed] [Google Scholar]
- 33.Luo, C., J. J. Loros, and J. C. Dunlap. 1998. Nuclear localization is required for function of the essential clock protein FREQUENCY. EMBO J. 17:1228–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Merrow, M., L. Franchi, Z. Dragovic, M. Gorl, J. Johnson, M. Brunner, G. Macino, and T. Roenneberg. 2001. Circadian regulation of the light input pathway in Neurospora crassa. EMBO J. 20:307–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morais Cabral, J. H., A. Lee, S. L. Cohen, B. T. Chait, M. Li, and R. Mackinnon. 1998. Crystal structure and functional analysis of the HERG potassium channel N terminus: a eukaryotic PAS domain. Cell 95:649–655. [DOI] [PubMed] [Google Scholar]
- 36.Pellequer, J. L., K. A. Wager-Smith, S. A. Kay, and E. D. Getzoff. 1998. Photoactive yellow protein: a structural prototype for the three-dimensional fold of the PAS domain superfamily. Proc. Natl. Acad. Sci. USA 95:5884–5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pongratz, I., C. Antonsson, M. L. Whitelaw, and L. Poellinger. 1998. Role of the PAS domain in regulation of dimerization and DNA binding specificity of the dioxin receptor. Mol. Cell. Biol. 18:4079–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Price, J. L., M. E. Dembinska, M. W. Young, and M. Rosbash. 1995. Suppression of PERIOD protein abundance and circadian cycling by the Drosophila clock mutation timeless. EMBO J. 14:4044–4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reisz-Porszasz, S., M. R. Probst, B. N. Fukunaga, and O. Hankinson. 1994. Identification of functional domains of the aryl hydrocarbon receptor nuclear translocator protein (ARNT). Mol. Cell. Biol. 14:6075–6086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rutila, J. E., V. Suri, M. Le, W. V. So, M. Rosbash, and J. C. Hall. 1998. CYCLE is a second bHLH-PAS clock protein essential for circadian rhythmicity and transcription of Drosophila period and timeless. Cell 93:805–813. [DOI] [PubMed] [Google Scholar]
- 41.Saez, L., and M. W. Young. 1996. Regulation of nuclear entry of the Drosophila clock proteins period and timeless. Neuron 17:911–920. [DOI] [PubMed] [Google Scholar]
- 42.Schwerdtfeger, C., and H. Linden. 2000. Localization and light-dependent phosphorylation of white collar 1 and 2, the two central components of blue light signaling in Neurospora crassa. Eur. J. Biochem. 267:414–422. [DOI] [PubMed] [Google Scholar]
- 43.Sehgal, A., J. Price, B. Man, and M. Young. 1994. Loss of circadian behavioral rhythms and per oscillations in the Drosophila mutant timeless. Science 263:1603–1606. [DOI] [PubMed] [Google Scholar]
- 44.Shearman, L. P., S. Sriram, D. R. Weaver, E. S. Maywood, I. Chaves, B. Zheng, K. Kume, C. C. Lee, G. T. van der Horst, M. H. Hastings, and S. M. Reppert. 2000. Interacting molecular loops in the mammalian circadian clock. Science 288:1013–1019. [DOI] [PubMed] [Google Scholar]
- 45.Shearman, L. P., M. J. Zylka, D. R. Weaver, L. F. Kolakowski, Jr., and S. M. Reppert. 1997. Two period homologs: circadian expression and photic regulation in the suprachiasmatic nuclei. Neuron 19:1261–1269. [DOI] [PubMed] [Google Scholar]
- 46.Talora, C., L. Franchi, H. Linden, P. Ballario, and G. Macino. 1999. Role of a white collar-1-white collar-2 complex in blue-light signal transduction. EMBO J. 18:4961–4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vosshall, L., J. Price, A. Sehgal, L. Saez, and M. Young. 1994. Specific block in nuclear localization of period protein by a second clock mutation. timeless. Science 263:1606–1609. [DOI] [PubMed] [Google Scholar]
- 48.Young, M. W. 1999. Molecular control of circadian behavioral rhythms. Recent Prog. Horm. Res. 54:87–94. [PubMed] [Google Scholar]
- 49.Zelzer, E., P. Wappner, and B. Z. Shilo. 1997. The PAS domain confers target gene specificity of Drosophila bHLH/PAS proteins. Genes Dev. 11:2079–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]