Abstract
Epithelial tumors of the pancreas exhibit a wide spectrum of histologies with varying propensities for metastasis and tissue invasion. The histogenic relationship among these tumor types is not well established; moreover, the specific role of genetic lesions in the progression of these malignancies is largely undefined. Transgenic mice with ectopic expression of transforming growth factor alpha (TGF-α) in the pancreatic acinar cells develop tubular metaplasia, a potential premalignant lesion of the pancreatic ductal epithelium. To evaluate the cooperative interactions between TGF-α and signature mutations in pancreatic tumor genesis and progression, TGFα transgenic mice were crossed onto Ink4a/Arf and/or p53 mutant backgrounds. These compound mutant mice developed a novel pancreatic neoplasm, serous cystadenoma (SCA), presenting as large epithelial tumors bearing conspicuous gross and histological resemblances to their human counterpart. TGFα animals heterozygous for both the Ink4a/Arf and the p53 mutation showed a dramatically increased incidence of SCA, indicating synergistic interaction of these alleles. Inactivation of p16Ink4a by loss of heterozygosity, intragenic mutation, or promoter hypermethylation was a common feature in these SCAs, and correspondingly, none of the tumors expressed wild-type p16Ink4a. All tumors sustained loss of p53 or Arf, generally in a mutually exclusive fashion. The tumor incidence data and molecular profiles establish a pathogenic role for the dual inactivation of p16Ink4a and p19Arf-p53 in the development of SCA in mice, demonstrating that p16Ink4a is a murine tumor suppressor. This genetically defined model provides insights into the molecular pathogenesis of SCA and serves as a platform for dissection of cell-specific programs of epithelial tumor suppression.
The human pancreatic ducts are susceptible to a complex array of neoplasms with distinct clinical behaviors and prognoses. These include highly malignant pancreatic ductal adenocarcinoma (56) and the less aggressive pancreatic cystic neoplasms, serous cystadenoma (SCA) (6) and mucinous cystadenoma (58). Despite differences in mutational profiles and histologic features, the pathogenic interrelation of pancreatic tumor types is not understood and processes of tumor progression in the pancreatic ducts remain to be identified.
Transgenic mouse strains in which human transforming growth factor alpha (TGF-α), a ligand for the epidermal growth factor receptor (EGFR), is expressed in the pancreatic acinar cells (20) have served as a model for premalignant lesions of the pancreas. The pancreas in these animals undergoes progressive histologic transformation, characterized by diffuse fibrosis and tubular metaplasia, displaying islands of proliferating cells within the tubules (5); however, pancreatic tumors do not occur. The pathogenic role of TGF-α, mediated by RAS, phosphoinositide 3′-kinase, and other signaling pathways, involves promotion of proliferation, survival, invasion, and angiogenesis (32). High levels of EGFR signaling may also result in growth-antagonizing effects due to RAS-mediated induction of p19Arf-p53 and p16Ink4a (47); hence, loss of these tumor suppressors in association with TGF-α overexpression could cooperate in inducing cellular transformation.
The p19Arf-p53 pathway is of central importance for tumor suppression in mice and humans (47). p53- or Arf-deficient mice develop spontaneous tumors with high penetrance and short latency (9, 18, 24). Mouse embryonic fibroblasts lacking either of these genes escape from passage-induced growth arrest and can be transformed by oncogenic H-RAS alone (9, 24). Furthermore, consistent with the role of this pathway in monitoring aberrant proliferation, retinoblastoma protein (RB) deficiency or potent oncogenic stimuli such as Myc, E1a, E2F-1, and Abl (8, 30, 35, 37, 61; S. Bates, A. C. Phillips, P. A. Clark, F. Stott, G. Peters, R. L. Ludwig, and K. H. Vousden, Letter, Nature 395:124–125, 1998) induce an apoptotic program that is strictly dependent on p19Arf and p53 function. The grossly similar tumor-prone phenotypes of p53 and Arf mutant mice (9, 24) and the mutually exclusive patterns of p53 and Arf (or Ink4a/Arf) mutations in tumor cells (35, 47, 51) provide genetic support for biochemical studies demonstrating that these genes comprise a common pathway (23, 35, 51).
The other product of the Ink4a/Arf locus, p16Ink4a, inhibits the phosphorylation of RB by CDK4 and -6, restraining cells in the G1 phase of the cell cycle. p16Ink4a is an important tumor suppressor in humans (39); however, its significance in the mouse has remained in question due to the comparable phenotypes of Arf-null mice (24) and of mice harboring a deletion that inactivates both p16Ink4a and p19Arf (42). Loss of the p16Ink4a-RB pathway is a universal feature in the establishment of human cell lines in vitro (22, 25); conversely, this pathway is typically left intact in the immortalization of mouse embryonic fibroblasts. On the other hand, a hypomorphic p16Ink4a allele in the BALB/c mouse strain is associated with increased susceptibility to plasmacytoma or lung tumors following treatment with the carcinogen pristane or urethane, respectively (16, 60). Taken together, these observations suggest that the relative importance of the p16Ink4a-RB and p53 pathways in regulating abnormal proliferation may represent variable programs with respect to cell type identities and growth contexts.
In this study, we have analyzed the genetic interactions between loss of the Ink4a/Arf and p53 tumor suppressor genes and overexpression of TGF-α in the neoplastic transformation of the pancreas. We report that local overexpression of TGF-α cooperates with Ink4a/Arf and p53 deficiency to produce a pancreatic ductal neoplasm that is a virtual histological and clinical phenocopy of SCA in humans. Molecular analysis reveals specific loss of the p16Ink4a product of the Ink4a/Arf locus and either p19Arf or p53 loss, demonstrating that both p16Ink4a and the p19Arf-p53 pathway are critical suppressors of this neoplasm in the mouse. This study yields insight into the mechanisms of tumorigenic progression in the pancreatic ducts and provides a model system for evaluation of the histogenic and genetic relationships among the various pancreatic neoplasms.
MATERIALS AND METHODS
Mouse strains and tumor surveillance and characterization.
Metallothionein-TGFα transgenic mice of the MT42 strain (20) were crossed onto Ink4a/Arf (42) and/or p53 mutant strains (18) on a mixed genetic background (∼50% CD1, 32.5% C57BL/6, 12.5% CBA, 5% 129/sv). Similar crosses were performed using the independently derived metallothionein-TGFα transgenic line, designated MT100 (20), on an inbred, FVB/N genetic background. Morbid animals or animals with appropriate tumor burdens were sacrificed and autopsied. Tumor specimens were prepared for histologic and molecular analysis as described previously (48). TGF-α and vascular endothelial growth factor (VEGF) levels were measured using enzyme-linked immunosorbent assay (ELISA) kits (Oncogene Research).
Histology and immunohistochemistry.
Specimens were prepared and processed for histopathology and immunohistochemistry as described previously (48). Polyclonal antisera to Pdx-1 (a gift of C. Wright) were used at a 1:1,000 dilution.
Molecular analyses of tumor samples.
For loss-of-heterozygosity (LOH) analysis, Southern blotting of genomic DNA was performed according to standard protocols. Blots were hybridized with probes to p53 exon 1 (18) or to sequences downstream of Ink4a/Arf exon 3 (42). PhosphorImager analysis (Fuji BAS) was used to quantitate the signal intensities of mutant and wild-type alleles. Reverse transcription-PCR (RT-PCR) analysis was performed as described previously (4) using primer pairs to amplify p16Ink4a exon 1 or the region spanning exons 1 and 2.
For methylation-specific PCR, genomic DNA was modified by bisulfite treatment as described previously (14). Methylated alleles of the p16Ink4a promoter-5′ untranslated region (5′ UTR) were amplified with primers p16-M1 (5′-CGATTGGGCGGGTATTGAATTTTCGC-3′) and p16-M2 (5′-CACGTCATACACACGACCCTAAACCG-3′). Unmethylated alleles were amplified with primers p16-U1 (5′-GTGATTGGGTGGGTATTGAATTTTTGTG-3′) and p16-U2 (5′-CACACATCATACACACAACCCTAAACCA-3′). Primers to unmodified DNA were used as a control for complete bisulfite modification of the treated DNA.
For immunoblot analysis, cell lysates were prepared and analyzed as described previously (4). The antibodies used in this study were as follows: for p16Ink4a, M156 (Santa Cruz); for p19Arf, NB 200–106 (Novus); and for p53, Pab 240 (Santa Cruz).
RESULTS
Tumor spectrum and survival.
The development of premalignant pancreatic lesions in transgenic mice that ectopically express TGF-α in the acinar cells, coupled with the frequent mutation of the INK4a/ARF locus in human pancreatic adenocarcinoma, prompted us to assess the potential cooperative effects of TGFα overexpression and Ink4a/Arf deficiency in the pancreas. Compound mice transgenic for TGFα under the control of the metallothionein-1 promoter (the MT42 strain) and mutant for Ink4a/Arf were generated on a mixed genetic background. As reported previously, MT-TGFα animals show both pancreatic and extrapancreatic expression of TGFα and develop progressive interstitial fibrosis of the pancreas that culminates in tubular metaplasia with 100% penetrance (20). Some MT-TGFα animals also develop hepatocellular carcinoma with a late onset. None of the MT-TGFα Ink4a/Arf+/+ mice (n = 25) or nontransgenic Ink4a/Arf+/− or Ink4a/Arf−/− mice (n = 65) exhibited pancreatic neoplasia. Consistent with previous reports, Ink4a/Arf−/− mice succumbed to soft tissue sarcomas or lymphomas at a median age of 35 weeks, while heterozygous mice developed a similar tumor spectrum at a median age of 69 weeks (42).
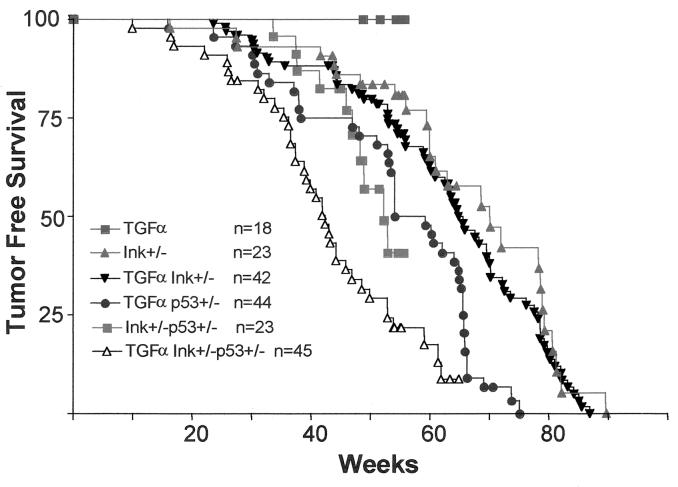
In contrast, a novel neoplastic phenotype of the pancreas was observed in 6 of 65 (9.5%) MT-TGFα Ink4a/Arf−/− animals (Table 1). This neoplasm appears to phenocopy SCA (microcystic adenoma) of the pancreas in humans (see Fig. 2A and discussion below). These animals became ill and were sacrificed at a mean age of 35.8 weeks (range, 25 to 44 weeks). MT-TGFα Ink4a/Arf+/− mice also developed SCA, but with a lower incidence (3%) and a longer latency (mean, 70 weeks). To determine whether loss of p53 modifies the ductal epithelial neoplastic phenotype elicited in MT-TGFα Ink4a/Arf mutant mice, a p53-null allele was introduced into the cohort. Transgenic TGFα expression in conjunction with p53 heterozygosity was associated with a low incidence of SCA (1 of 41 animals), a rate similar to that observed with Ink4a/Arf heterozygosity. In contrast, MT-TGFα Ink4a/Arf+/− p53+/− mice exhibited a marked increase in incidence, with 13 of 37 (35%) displaying large SCAs at the time of death or sacrifice (Table 1; P < 0.001 for comparison with MT-TGFα p53+/− mice). Tumor-free survival analysis showed statistically significant differences for MT-TGFα Ink4a/Arf+/− p53+/− mice versus MT-TGFα p53+/− mice (P < 0.001), and versus Ink4a/Arf+/− p53+/− animals (Fig. 1; P = 0.01); notably, in each comparison the excess mortality was attributable to SCA. MT-TGFα p53−/− mice also developed SCA with increased penetrance (4 of 15 [26%]). The SCAs arising in a p53+/− or p53−/− background were grossly and histologically indistinguishable from those arising in the p53+/+ background, with no evidence for progression to a higher-grade histology. In summary, MT-TGFα Ink4a/Arf+/− p53+/− mice experience rates of SCA genesis that are significantly higher than those observed with heterozygosity at either locus alone, demonstrating that in a setting of inappropriate growth factor stimulation, deficiency at the Ink4a/Arf locus can act in synergy with p53 mutations to promote the development of this malignancy.
TABLE 1.
Results of TGFα crosses: synergy of Ink4a/Arf and p53 loss in SCA
| Cohort | Genetic background | Cystadenoma incidencea | Latency (no. of wks) |
|---|---|---|---|
| MT42 | CD1/C57BL/6 | 0/25 (0) | |
| MT42 Ink/Arf−/− | CD1/C57BL/6 | 8/67 (11.9) | 35.8 |
| MT42 Ink/Arf+/− | CD1/C57BL/6 | 3/100 (3.0) | 70.0 |
| MT42 p53+/− | CD1/C57BL/6 | 1/41 (2.5) | 50.0 |
| MT42 Ink/Arf+/−p53+/− | CD1/C57BL/6 | 13/37 (35.1) | 39.3 |
| MT42 p53−/− | CD1/C57BL/6 | 4/15 (26.7) | 26.4 |
| MT42 Ink/Arf+/−p53−/− | CD1/C57BL/6 | 3/10 (30.0) | 19.3 |
| MT42 Ink/Arf−/−p53−/− | CD1/C57BL/6 | 1/4 (25.0) | 16.0 |
| MT100 | FVB/n | 0/12 (0) | |
| MT100 Ink/Arf+/− | FVB/n | 5/12 (42.0) | 40.0 |
| MT100 p53+/− | FVB/n | 2/4 (50) | 26.4 |
| Ink/Arf−/− | CD1/C57BL/6 | 0/42 (0) | |
| p53−/− | CD1/C57BL/6 | 0/31 (0) | |
| Ink/Arf+/−p53+/− | CD1/C57BL/6 | 0/18 (0) |
Expressed as the number of animals with SCA/total number of animals (percent animals with SCA).
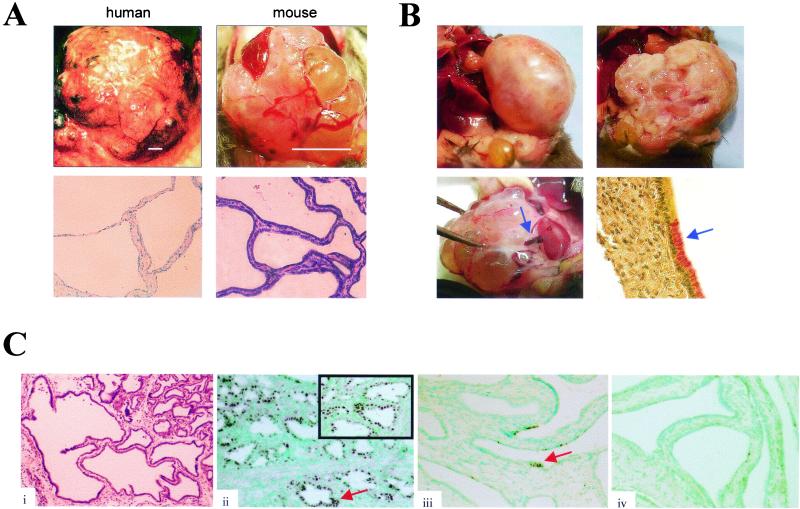
FIG. 2.
(A) Resemblance of human and murine SCAs. (Upper panels) Gross anatomy of human (left) and mouse (right) SCAs, showing intercystic vascular arcades. Bars, 4 and 1 cm in the human and mouse images, respectively. (Lower panels) Light micrographs showing hematoxylin and eosin staining of human (left) and mouse (right) SCAs, demonstrating characteristic low cuboidal epithelium with thin fibrous septa. (B) Structural features of murine SCA. (Upper left) SCA presenting as an encapsulated mass. (Upper right) Cross-sectional anatomy shows multilocular cystic architecture and pseudocapsule. (Lower left) Arrow depicts vascular pedicle. (Lower right) Immunohistochemical detection of limited focal mucicarmine staining in the tumors identifies these lesions as SCAs rather than mucinous cystadenomas. (C) Histological progression in the development of SCA. (i) Low-power photomicrograph depicting transition from tubular metaplasia (upper right) into SCA (left and bottom regions). (ii through iv) Immunohistochemistry shows a decreasing gradient of Pdx-1 expression in SCA progression, going from regions of tubular metaplasia (shown in a low-power view [ii] and a high-power view [ii, inset]) to the SCA tumor base (iii) and SCA growing edge (iv).
FIG. 1.
Tumor-free survival in the metallothionein-TGFα cohort (MT42 transgenic strain).
The cooperative interactions between TGFα and Ink4a/Arf and/or p53 were also assessed in an independently derived MT-TGFα transgenic strain (MT100) maintained on an inbred FVB/n genetic background. These animals exhibit pronounced tubular metaplasia and also sustain biliary ductal hyperplasia, gastric foveolar hyperplasia, and dilation of the esophagus (20, 52). As in the MT42 transgenic strain discussed above, Ink4a/Arf or p53 deficiency greatly enhanced the pancreatic phenotype conferred by TGFα expression in the MT100 strain (Table 1). Specifically, on an Ink4a/Arf+/− background, 5 of 12 animals developed symptomatic SCA (mean latency, 40 weeks); 2 of 4 p53+/− mice developed SCA (mean latency, 26.4 weeks). Survival analysis of the mice heterozygous for Ink4a/Arf or p53 mutation demonstrated a statistically significant dependence on transgene status (P < 0.001).
Tumor characteristics.
The SCAs are strikingly similar in gross morphological appearance to the human lesion (55) (Fig. 2A, upper panels). Approximately half of these tumors presented as encapsulated masses (Fig. 2B, upper left). The tumors on cross-section showed cystic architecture (Fig. 2B, upper right), in some cases with areas of focal hemorrhage, as seen in the human disease. A vascular pedicle was frequently evident (Fig. 2B, lower left). On the light-microscopic level, the appearance is again remarkably similar to that of the human lesion (6) (Fig. 2A, lower panels). The cysts consist of a single layer of uniform low cuboidal cells, separated by a region of stromal cells. Mucicarmine staining revealed only rare focal positivity (Fig. 2B, lower right), further supporting the diagnosis of SCA rather than mucinous cystadenoma. On all backgrounds, SCA invariably arose in contiguity with regions of tubular metaplasia. Often, a transition zone from metaplasia to neoplasia was histologically evident (Fig. 2C, panel i). The pancreatic stem and β-cell marker, Pdx-1, confined to the islets of the normal adult mouse (21), has been shown to be induced in association with tubular metaplasia, a phenomenon that may reflect the fact that these lesions arise from a rare stem cell population in the pancreatic ducts (50). In our cohort, we detected frequent nuclear positivity for Pdx-1 in the metaplastic tubules by immunohistochemistry. A gradient of Pdx-1 was seen in the SCAs (Fig. 2C, panels ii through iv), with nearly uniform nuclear staining of smaller cysts at the tumor base and diminishing, punctate staining of scattered nuclei in the larger, more uniform cysts toward the growing edge. This suggests that these tumors may have arisen from Pdx-1-expressing cells, derived either from the tubular metaplastic epithelium or directly from a stem cell population, that subsequently differentiate to lose Pdx-1 expression during tumorigenic progression.
Molecular profile of SCAs.
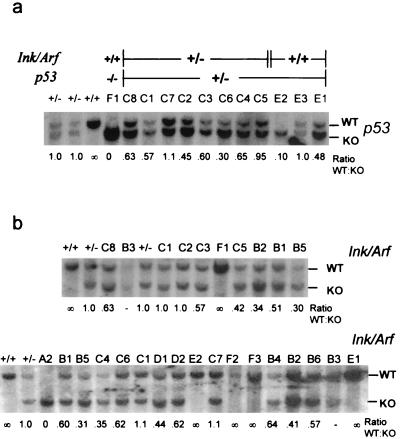
Since deficiency of Ink4a/Arf and p53 cooperated with TGFα to induce SCA, we assessed LOH at these loci by Southern blot analysis of tumor DNA. LOH was measured as a relative decrease in the ratio of the wild-type to the mutant allele due to the intermixture of stromal cells within the tumor specimens (often >50% as assessed by histological review). All SCAs analyzed arising in MT-TGFα Ink4a/Arf+/− animals lost the wild-type Ink4a/Arf allele (n = 6) (Fig. 3b and Table 2). Two of three tumors from MT-TGFα p53+/- animals lost the wild-type p53 allele (Fig. 3a and Table 2). Among SCAs arising in double heterozygotes, five of eight showed LOH at Ink4a/Arf and six of eight showed LOH at p53. One of these tumors, C5, lost the wild-type Ink4a/Arf allele while retaining wild-type p53, whereas two tumors, C1 and C2, showed LOH at p53 while retaining wild-type Ink4a/Arf; only tumor C7 remained heterozygous for both p53 and Ink4a/Arf (but see below). Significantly, LOH of Ink4a/Arf was detected in two of two SCAs assayed from MT-TGFα p53−/− Ink4a/Arf+/− mice.
FIG. 3.
(a) LOH analysis of p53 in murine SCAs. Numbers below the gel indicate the normalized ratio of wild-type(WT) to mutant (knockout [KO]) alleles in SCAs relative to heterozygous DNA specimens isolated from normal tissue. Tumor names and mouse genotypes are shown above the gel. (b) LOH analysis of Ink4a/Arf in murine SCAs.
TABLE 2.
Molecular analysis of SCAs in MT-TGFα mice
| Genotype | LOHa
|
Methylation of p16Ink4a | Expression
|
Other lesion | Gene inactivationb
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| p53 | Ink/Arf | p16 | p19 | p53 | p19Arf | p16Ink4a | p53 | |||
| Ink/Arf−/− | ||||||||||
| A1 | − | Null | − | − | − | − | Y | Y | N | |
| A2 | − | Null | − | − | − | − | Y | Y | N | |
| A3 | − | Null | − | − | − | − | Y | Y | N | |
| A4 | − | Null | − | − | − | − | Y | Y | N | |
| Ink/Arf+/− | ||||||||||
| B1 | NDc | ND | − | − | − | − | Y | Y | N | |
| B2 | − | + | − | − | − | − | Y | Y | N | |
| B3 | − | + | − | − | − | − | Y | Y | N | |
| B4 | − | + | − | − | − | − | Y | Y | N | |
| B5 | − | + | − | − | − | − | Y | Y | N | |
| B6 | − | + | − | ND | ND | ND | Y | Y | N | |
| B7 | − | + | − | ND | ND | ND | Y | Y | N | |
| Ink/Arf+/−p53+/− | ||||||||||
| C1 | + | − | − | − | − | − | 1 | 1 | Y | |
| C2 | + | − | − | − | − | − | 1 | 1 | Y | |
| C3 | + | + | − | − | − | − | Y | Y | Y | |
| C4 | + | + | − | − | − | − | Y | Y | Y | |
| C5 | − | + | − | − | − | − | Y | Y | N | |
| C6 | + | + | − | − | − | − | Y | Y | Y | |
| C7 | − | − | − | −d | − | − | Splice defecte | Y | Y | N |
| C8 | + | + | − | ND | ND | ND | Y | Y | Y | |
| Ink/Arf+/−p53−/− | ||||||||||
| D1 | Null | + | − | − | − | − | Y | Y | Y | |
| D2 | Null | + | − | − | − | − | Y | Y | Y | |
| p53+/− | ||||||||||
| E1 | + | − | − | − | − | − | 1 | 1 | Y | |
| E2 | + | − | + | − | + | − | N | Y | Y | |
| E3 | − | − | − | − | − | − | Splice defecte | Y | Y | N |
| p53−/− | ||||||||||
| F1 | Null | − | + | − | + | − | N | Y | Y | |
| F2 | Null | − | − | − | − | − | 1 | 1 | Y | |
| F3 | Null | − | + | ND | ND | ND | N | Y | Y | |
In the LOH column, − indicates lack of LOH in heterozygotes or absence of deletion in +/+ animals.
Y, yes; N, no; 1, no lesions of Ink4a/Arf locus detected, but lack of p19Arf overexpression despite p53 nullizygosity is consistent with a defective Ink4a/Arf locus.
ND, not done.
p16Ink4a protein is detected but migrates aberrantly at 9 to 11 kDa.
RT-PCR analysis detects transcripts from exon 1α and 1β but no products containing exons 2 and 3, suggesting defects in splicing or truncation of message.
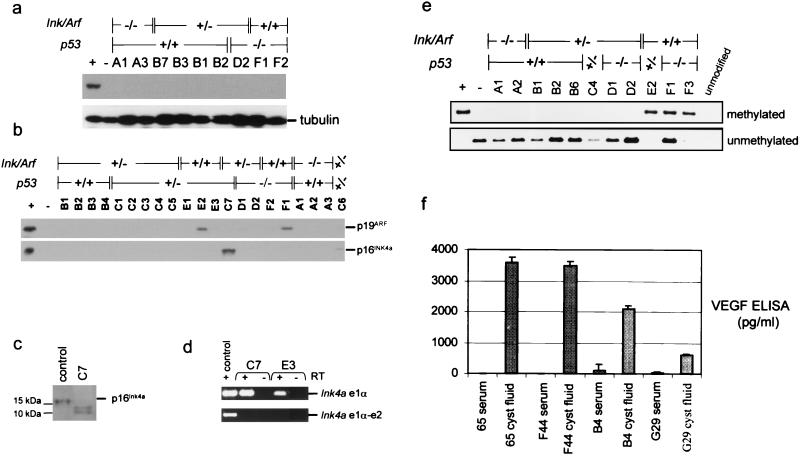
To extend our studies of the murine SCAs, we analyzed the expression of p53, p16Ink4a, and p19Arf by immunoblotting of cell lysates from these tumors. None of the tumors showed overexpression of p53, suggesting that stabilizing p53 point mutations are absent in these samples (Fig. 4a and Table 2). In tumors arising on an Ink4a/Arf-deficient, p53 wild-type background, it appears that p53 function is retained, since there is no aberrant p53 overexpression and no deletion or rearrangement of the p53 locus (Fig. 4a and data not shown). Hence, the predominant means of p53 inactivation in the SCAs is loss of the wild-type allele in mice heterozygous for mutant p53. p19Arf expression was detectable only in tumors E2 and F1 (Fig. 4b, upper panel, and Table 2). The high level of p19Arf in these tumors correlates with the absence of p53, consistent with the well-established negative feedback loop between p53 and p19Arf (36, 51). p16Ink4a expression was detected only in tumor C7, arising in a double heterozygous animal; however, the anti-p16Ink4a immunoreactive band showed aberrantly rapid migration (Fig. 4b, lower panel, and 4c). Tumors C7 and E3 were the only SCAs that did not have LOH at either Ink4a/Arf or p53 (Table 2). RT-PCR analysis of RNAs derived from these tumors was consistent with an aberrant Ink4a/Arf transcript, since amplification products were detected with primers used to amplify exon 1α or 1β but not with primers that amplify transcripts spanning either of these exons and exon 2 (Fig. 4d and data not shown). Thus, it appears that there is disruption of the 3′ portions of transcripts encoding both p16Ink4a and p19Arf. Southern blot analysis of C7 and E3 failed to detect deletion or rearrangements of the Ink4a/Arf locus, and sequence analysis of the exon 2 splice acceptor region failed to detect mutations (data not shown); thus, the precise molecular lesions at this locus remain undefined.
FIG. 4.
(a) Immunoblot for p53 expression in murine SCAs. There was no aberrant overexpression of p53 in the SCAs. Results for positive-control extracts from irradiated NIH 3T3 cells (+) and negative-control p53-null fibroblasts (−) are shown. (b) Immunoblots for p19Arf (upper panel) and p16Ink4a (lower panel) expression. The positive control (+) was a mouse melanoma cell lysate, and the negative control (−) was a lysate from Ink4a/Arf−/− fibroblasts. (c) Immunoblot shows aberrant migration of p16Ink4a expressed in tumor C7. Lysates were resolved on an 8-to-16% gradient gel. (d) RT-PCR analysis of the Ink4a mRNA. PCR of cDNAs from tumors C7 and E3 yielded products with primers amplifying Ink4a exon 1α (e1α) (upper panel) but not with primers spanning exons 1 and 2 (lower panel). RNA from a wild-type mouse embryonic fibroblast was used as a positive control. As a negative control for residual DNA contamination, RNA specimens were subjected to the same analysis without the addition of reverse transcriptase (−RT). (e) Methylation-specific PCR. DNA specimens were treated with bisulfite and analyzed by PCR using primers that recognize methylated (upper panel) or unmethylated (lower panel) sequences in the p16Ink4a regulatory region. The positive control (+) was the Sp6c murine lung cancer cell line (34), and the negative control (−) was normal pancreas DNA. The specificity of the primers was confirmed by the lack of amplification of DNA that was not subjected to bisulfite treatment (unmodified). (f) VEGF ELISA showing elevated VEGF levels in cyst fluid from SCAs.
Epigenetic silencing in association with promoter hypermethylation is a frequent mechanism of p16Ink4a inactivation in human tumors (15). We sought to determine whether such a mechanism is operative in murine SCAs that do not display structural alterations of the Ink4a/Arf locus. We evaluated the methylation status of the CpG island in the p16Ink4a promoter-5′ UTR region using the methylation-specific PCR assay (14). Methylated p16Ink4a alleles were detected in three tumors (Fig. 4e and Table 2); each of these arose on Ink4a/Arf+/+ backgrounds and harbored homozygous null alleles of p53. The promoter hypermethylation was confirmed by an independent primer set designed to interrogate other CpG dinucleotides in the p16Ink4a 5′ UTR and by Southern blot analysis using methylation-sensitive restriction enzymes (data not shown). Protein lysates were available for two of these tumors, and for both specimens, immunoblot analysis demonstrated the absence of p16Ink4a expression and high levels of p19Arf (Fig. 4b), suggesting that p16Ink4a is selectively inactivated and (together with p53 loss) induces SCA in TGFα transgenic mice. Notably, Rb loss was not detected in any of the SCAs, as determined by immunoblot analysis (data not shown). In 4 of 27 tumors analyzed (C1, C2, E3, and F2), we failed to detect lesions at the Ink4a/Arf locus. However, these tumors all failed to express p19Arf (and p16Ink4a) despite being nullizygous for p53. This lack of p19Arf upregulation despite loss of p53 may indicate that these tumors incurred disruption of the Ink4a/Arf locus that eluded detection or, conversely, that the locus may be subject to a trans regulatory repression mechanism (19).
Lack of VHL mutations and high VEGF levels in murine SCA.
In humans, hereditary predisposition to SCA is associated with the familial cancer syndrome von Hippel-Lindau disease (28). The gene mutated in this syndrome, VHL, is also frequently mutated, and shows LOH, in sporadic cases of SCA (29). To address a possible role of Vhl alterations in murine SCAs, we performed molecular analysis of this locus in the tumors. Sequence analysis of the Vhl exons failed to identify mutations, and methylation-specific PCR of the Vhl regulatory region failed to show aberrant hypermethylation (n = 12); hence, it is likely that Vhl remains intact in these tumors. Subsequently, we sought to determine whether these tumors display a correlate of Vhl loss, specifically, high VEGF levels. VEGF levels in the tumor cyst fluid were greatly increased relative to those in serum (Fig. 4f). These results suggest that the genetic lesions in the murine SCAs may impinge upon pathways common to VHL and that this mechanism may contribute to the pathogenic process.
DISCUSSION
In this study, we have demonstrated cooperative interactions between TGF-α, p16Ink4a, and p19Arf-p53 in the genesis of pancreatic neoplasia. SCA incidence was markedly increased in TGFα Ink4a/Arf+/− p53+/− mice over that in TGFα mice heterozygous for Ink4a/Arf or p53 alone (35% compared to 3 and 2.5%, respectively, in the MT42 strain of MT-TGFα mice), indicating strong synergy between these alleles. Molecular analysis revealed selective inactivation of p16Ink4a in pancreatic tumorigenesis, even in p53-null tumors in which p19Arf remained intact. Taken together, the tumor incidence data and molecular profiles provide strong evidence that p16Ink4a contributes to a potent tumor suppression activity which synergizes with the p19Arf-p53 pathway in protecting against neoplastic transformation of the murine pancreatic ducts. These conclusions are supported further by an increased incidence of spontaneous and carcinogen-induced cancers in mice harboring germ line mutations that specifically inactivate p16Ink4a (46). Thus, p16Ink4a is a bone fide murine tumor suppressor gene.
The prominence of p16Ink4a observed in this study may seem at odds with previous studies indicating that the tumor suppressor activity of the Ink4a/Arf locus in the mouse relates primarily to p19Arf function alone. However, this discrepancy may be readily explained by differences in the experimental designs of the studies. Notably, in previous studies employing transgenic Myc overexpression, it has been shown that Myc strongly selects for loss of Arf or p53, since these genes directly mediate an apoptotic program in response to aberrant Myc activity (10, 41, 61). Furthermore, since Myc can bypass p16Ink4a by increasing cyclin E/CDK2 activity, the need to eliminate p16Ink4a might be obviated in these animals (2, 4, 31). In addition, while the p19Arf-p53 axis appears to be the dominant tumor suppression pathway in murine lymphoid cells and fibroblasts, it is prudent to consider the possibility that there may exist cell type-specific differences in the prominence of various tumor suppressors. That p16Ink4a may exert a distinct tumor suppressor activity is also implied by its involvement in the response to diverse cellular stresses in vivo such as growth at high cell density (57), DNA damage (43), and extracellular-matrix-dependent proliferative stimuli (11).
Our phenotypic outcome contrasts with that of a recent report exploring the impact of TGFα overexpression and loss of p53 function in the pancreas (54). This previous report demonstrated that ectopic expression of TGFα in the murine pancreatic acinar cells, using the elastase promoter, cooperates with p53 deficiency to induce pancreatic adenocarcinoma rather than SCA. In the absence of tumor suppressor gene mutations, the elastase-TGFα mice exhibit a pancreatic phenotype similar to that of the metallothionein-TGFα strains (20, 40); however, on a p53 mutant background, pancreatic adenocarcinomas develop with high penetrance (54). These experiments were performed on the BALB/c mouse strain, which harbors a hypomorphic p16Ink4a allele (59); hence, these animals were effectively compromised in both p53 and p16Ink4a function. An important distinction in the elastase-TGFα mice is the continued expression of TGFα in their tubular metaplastic structures, correlating with elevated activity of the downstream Ras-mitogen-activated protein (MAP) kinase signaling pathway. In contrast, the MT-TGFα strains employed in our studies lose expression of TGFα in the pancreas as tubular complexes develop (5); furthermore, we failed to detect TGFα expression in the cyst fluid in association with the SCAs in these animals (data not shown). To confirm further that the Ras-MAP kinase pathway was not activated by another means in the SCAs, the Ras family GTPases were sequenced and found to be devoid of activating point mutations in N-Ras, K-Ras, or H-Ras (data not shown).
It is noteworthy that, in the SCA model, TGF-α does not behave strictly as an oncogene, since expression is extinguished both in the tubular metaplastic lesions and in the tumors; hence, it is not a direct ligand for the cells undergoing transformation. Instead, it is likely that expression of TGF-α in the acini alters the differentiation state within the pancreas (45). The consequent loss of acinar cells due to metaplastic change and/or cell death appears to stimulate aberrant ductal proliferation, and this may amplify a cell population susceptible to neoplastic transformation. Disruptions of tissue architecture within the metaplastic pancreas may also create a microenvironment more supportive of tumorigenesis (53).
The immunohistochemical detection of uniform Pdx-1 expression in the metaplastic ducts and the diminishing gradient of Pdx-1 expression during the progression of the SCAs (Fig. 2c) may provide insight into the cellular basis for SCA in our model. Based on this expression pattern and on the detection of transition zones between regions of tubular metaplasia and SCA, it appears that the SCAs arise from the transformation of precursor cells in the metaplastic ducts. Pdx-1 encodes a homeodomain transcription factor that is critical for pancreatic growth and morphogenesis in the embryo and for the maintenance of β-islet cell identity in the adult (1, 21). The normally quiescent adult pancreas displays a regenerative response to experimentally induced pancreatic injury, involving proliferation in the ducts (49). Accompanying this response is the reemergence of a Pdx-1 expressing ductal cell population that displays a multipotent differentiation capacity; hence, regenerating pancreatic ducts appear to harbor an expanded pool of pancreatic stem cells (27, 44, 50). Ultrastructural analysis of human SCAs suggests that these tumors arise from the centroacinar cells that constitute the junction between the duct system and the acini (6). Interestingly, the centroacinar cells have been proposed to have a pancreatic stem cell potential (13). Considering this hypothesis as well as the ultrastructural resemblance between human and murine SCAs and between human tubular metaplastic cells and centroacinar cells (5, 33), it is plausible to propose that the murine SCAs in our model may arise from centroacinar precursors.
SCAs, while very rare in the general population, occur at high frequency in association with von Hippel-Lindau disease (28). The product of the VHL gene negatively regulates hypoxia-inducible factor (Hif-1) activity under conditions of normoxia through the direction of ubiquitin-mediated proteolysis of the Hif-1α subunit (17). Consequently, when Vhl is inactivated, Hif-1α shows constitutive activity leading to aberrant transactivation of angiogenic target genes such as VEGF, thereby promoting tumorigenesis. SCAs tend to be hypervascular (55), consistent with deregulated angiogenic signaling conferred by VHL loss. Given the cooperative induction of murine SCA by Arf/p53 and p16Ink4a loss and TGFα expression, it is tempting to speculate about a possible mechanistic connection between these genes and the VHL pathway. Although TGF-α is proangiogenic and appears to be negatively regulated by VHL (7, 26), the lack of sustained TGF-α expression during progressive pancreatic neoplasia makes it unlikely that TGF-α is responsible for the high cystic levels of VEGF. Rather, our genetic observations point to a link between VHL and p19Arf-p53 or p16Ink4a. Along these lines, recent studies have demonstrated a biochemical role for both p53 and p19Arf in regulating Hif-1α function (12, 38). Additionally, there is evidence that VHL is important for mediating growth inhibition at high cell densities (3), a process in which p16Ink4a also plays a critical role (57); N. E. Sharpless and R. A. DePinho, unpublished data). The demonstration that, in the context of TGF-α-mediated disruption of pancreatic epithelial differentiation, the losses of p16Ink4a and p19Arf-p53 generate a lesion that is a phenocopy of a VHL-associated tumor provides a measure of genetic support of the proposed biochemical intersection of these pathways.
Acknowledgments
We thank N. Sharpless and M. Ivan for comments on the manuscript, G. David for helpful discussions during the course of this work, and S. Chan for expert technical assistance. We also thank T. Devereux and C. Wright for reagents.
N.B. is supported by a John Peter Hoffman Award from the American Cancer Society (ACS). J.M. is a recipient of NCI grant 5K08CA72744-05. M.L. is the recipient of NCI grant 5R01-CA81755. R.A.D. is an American Cancer Society Professor and recipient of the Steven and Michele Kirsch Foundation Investigator Award. This work is supported by grants to R.A.D. from the NIH and ACS.
N. Bardeesy and J. Morgan contributed equally to this work.
REFERENCES
- 1.Ahlgren, U., J. Jonsson, L. Jonsson, K. Simu, and H. Edlund. 1998. Beta-cell-specific inactivation of the mouse Ipf1/Pdx1 gene results in loss of the beta-cell phenotype and maturity onset diabetes. Genes Dev. 12:1763–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alevizopoulos, K., J. Vlach, S. Hennecke, and B. Amati. 1997. Cyclin E and c-Myc promote cell proliferation in the presence of p16INK4a and of hypophosphorylated retinoblastoma family proteins. EMBO J. 16:5322–5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baba, M., S. Hirai, S. Kawakami, T. Kishida, N. Sakai, S. Kaneko, M. Yao, T. Shuin, Y. Kubota, M. Hosaka, and S. Ohno. 2001. Tumor suppressor protein VHL is induced at high cell density and mediates contact inhibition of cell growth. Oncogene 20:2727–2736. [DOI] [PubMed] [Google Scholar]
- 4.Bardeesy, N., B. C. Bastian, A. Hezel, D. Pinkel, R. A. DePinho, and L. Chin. 2001. Dual inactivation of RB and p53 pathways in RAS-induced melanomas. Mol. Cell. Biol. 21:2144–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bockman, D. E., and G. Merlino. 1992. Cytological changes in the pancreas of transgenic mice overexpressing transforming growth factor alpha. Gastroenterology 103:1883–1892. [DOI] [PubMed] [Google Scholar]
- 6.Compton, C. C. 2000. Serous cystic tumors of the pancreas. Semin. Diagn. Pathol. 17:43–55. [PubMed] [Google Scholar]
- 7.de Paulsen, N., A. Brychzy, M. C. Fournier, R. D. Klausner, J. R. Gnarra, A. Pause, and S. Lee. 2001. Role of transforming growth factor-alpha in von Hippel-Lindau (VHL)−/− clear cell renal carcinoma cell proliferation: a possible mechanism coupling VHL tumor suppressor inactivation and tumorigenesis. Proc. Natl. Acad. Sci. USA 98:1387–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Stanchina, E., M. E. McCurrach, F. Zindy, S. Y. Shieh, G. Ferbeyre, A. V. Samuelson, C. Prives, M. F. Roussel, C. J. Sherr, and S. W. Lowe. 1998. E1A signaling to p53 involves the p19ARF tumor suppressor. Genes Dev. 12:2434–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donehower, L. A., M. Harvey, B. L. Slagle, M. J. McArthur, C. A. Montgomery, Jr., J. S. Butel, and A. Bradley. 1992. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature 356:215–221. [DOI] [PubMed] [Google Scholar]
- 10.Eischen, C. M., J. D. Weber, M. F. Roussel, C. J. Sherr, and J. L. Cleveland. 1999. Disruption of the ARF-Mdm2–p53 tumor suppressor pathway in Myc-induced lymphomagenesis. Genes Dev. 13:2658–2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fahraeus, R., and D. P. Lane. 1999. The p16INK4a tumour suppressor protein inhibits αvβ3 integrin-mediated cell spreading on vitronectin by blocking PKC-dependent localization of αvβ3 to focal contacts. EMBO J. 18:2106–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fatyol, K., and A. A. Szalay. 2001. The p14ARF tumor suppressor protein facilitates nucleolar sequestration of hypoxia-inducible factor-1α (HIF-1α) and inhibits HIF-1-mediated transcription. J. Biol. Chem. 276:28421–28429. [DOI] [PubMed] [Google Scholar]
- 13.Gasslander, T., I. Ihse, and S. Smeds. 1992. The importance of the centroacinar region in cerulein-induced mouse pancreatic growth. Scand. J. Gastroenterol. 27:564–570. [DOI] [PubMed] [Google Scholar]
- 14.Herman, J. G., J. R. Graff, S. Myohanen, B. D. Nelkin, and S. B. Baylin. 1996. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc. Natl. Acad. Sci. USA 93:9821–9826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herman, J. G., A. Merlo, L. Mao, R. G. Lapidus, J. P. Issa, N. E. Davidson, D. Sidransky, and S. B. Baylin. 1995. Inactivation of the CDKN2/p16/MTS1 gene is frequently associated with aberrant DNA methylation in all common human cancers. Cancer Res. 55:4525–4530. [PubMed] [Google Scholar]
- 16.Herzog, C. R., S. Noh, L. E. Lantry, K. L. Guan, and M. You. 1999. Cdkn2a encodes functional variation of p16INK4a but not p19ARF, which confers selection in mouse lung tumorigenesis. Mol. Carcinog. 25:92–98. [DOI] [PubMed] [Google Scholar]
- 17.Ivan, M., and W. G. Kaelin, Jr. 2001. The von Hippel-Lindau tumor suppressor protein. Curr. Opin. Genet. Dev. 11:27–34. [DOI] [PubMed] [Google Scholar]
- 18.Jacks, T., L. Remington, B. O. Williams, E. M. Schmitt, S. Halachmi, R. T. Bronson, and R. A. Weinberg. 1994. Tumor spectrum analysis in p53-mutant mice. Curr. Biol. 4:1–7. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs, J. J., K. Kieboom, S. Marino, R. A. DePinho, and M. van Lohuizen. 1999. The oncogene and Polycomb-group gene bmi-1 regulates cell proliferation and senescence through the ink4a locus. Nature 397:164–168. [DOI] [PubMed] [Google Scholar]
- 20.Jhappan, C., C. Stahle, R. N. Harkins, N. Fausto, G. H. Smith, and G. T. Merlino. 1990. TGF-α overexpression in transgenic mice induces liver neoplasia and abnormal development of the mammary gland and pancreas. Cell 61:1137–1146. [DOI] [PubMed] [Google Scholar]
- 21.Jonsson, J., L. Carlsson, T. Edlund, and H. Edlund. 1994. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature 371:606–609. [DOI] [PubMed] [Google Scholar]
- 22.Kamb, A., N. A. Gruis, J. Weaver-Feldhaus, Q. Liu, K. Harshman, S. V. Tavtigian, E. Stockert, R. S. Day III, B. E. Johnson, and M. H. Skolnick. 1994. A cell cycle regulator potentially involved in genesis of many tumor types. Science 264:436–440. [DOI] [PubMed] [Google Scholar]
- 23.Kamijo, T., J. D. Weber, G. Zambetti, F. Zindy, M. F. Roussel, and C. J. Sherr. 1998. Functional and physical interactions of the ARF tumor suppressor with p53 and Mdm2. Proc. Natl. Acad. Sci. USA 95:8292–8297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamijo, T., F. Zindy, M. F. Roussel, D. E. Quelle, J. R. Downing, R. A. Ashmun, G. Grosveld, and C. J. Sherr. 1997. Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell 91:649–659. [DOI] [PubMed] [Google Scholar]
- 25.Kiyono, T., S. A. Foster, J. I. Koop, J. K. McDougall, D. A. Galloway, and A. J. Klingelhutz. 1998. Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature 396:84–88. [DOI] [PubMed] [Google Scholar]
- 26.Knebelmann, B., S. Ananth, H. T. Cohen, and V. P. Sukhatme. 1998. Transforming growth factor alpha is a target for the von Hippel-Lindau tumor suppressor. Cancer Res. 58:226–231. [PubMed] [Google Scholar]
- 27.Kritzik, M. R., E. Jones, Z. Chen, M. Krakowski, T. Krahl, A. Good, C. Wright, H. Fox, and N. Sarvetnick. 1999. PDX-1 and Msx-2 expression in the regenerating and developing pancreas. J. Endocrinol. 163:523–530. [DOI] [PubMed] [Google Scholar]
- 28.Mohr, V. H., A. O. Vortmeyer, Z. Zhuang, S. K. Libutti, M. M. Walther, P. L. Choyke, B. Zbar, W. M. Linehan, and I. A. Lubensky. 2000. Histopathology and molecular genetics of multiple cysts and microcystic (serous) adenomas of the pancreas in von Hippel-Lindau patients. Am. J. Pathol. 157:1615–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore, P. S., G. Zamboni, A. Brighenti, D. Lissandrini, D. Antonello, P. Capelli, G. Rigaud, M. Falconi, and A. Scarpa. 2001. Molecular characterization of pancreatic serous microcystic adenomas: evidence for a tumor suppressor gene on chromosome 10q. Am. J. Pathol. 158:317–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morgenbesser, S. D., B. O. Williams, T. Jacks, and R. A. DePinho. 1994. p53-dependent apoptosis produced by Rb-deficiency in the developing mouse lens. Nature 371:72–74. [DOI] [PubMed] [Google Scholar]
- 31.O’Hagan, R. C., M. Ohh, G. David, I. M. de Alboran, F. W. Alt, W. G. Kaelin, Jr., and R. A. DePinho. 2000. Myc-enhanced expression of Cul1 promotes ubiquitin-dependent proteolysis and cell cycle progression. Genes Dev. 14:2185–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olayioye, M. A., R. M. Neve, H. A. Lane, and N. E. Hynes. 2000. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 19:3159–3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parsa, I., D. S. Longnecker, D. G. Scarpelli, P. Pour, J. K. Reddy, and M. Lefkowitz. 1985. Ductal metaplasia of human exocrine pancreas and its association with carcinoma. Cancer Res. 45:1285–1290. [PubMed] [Google Scholar]
- 34.Patel, A. C., C. H. Anna, J. F. Foley, P. S. Stockton, F. L. Tyson, J. C. Barrett, and T. R. Devereux. 2000. Hypermethylation of the p16 (Ink4a) promoter in B6C3F1 mouse primary lung adenocarcinomas and mouse lung cell lines. Carcinogenesis 21:1691–1700. [DOI] [PubMed] [Google Scholar]
- 35.Pomerantz, J., N. Schreiber-Agus, N. J. Liegeois, A. Silverman, L. Alland, L. Chin, J. Potes, K. Chen, I. Orlow, H. W. Lee, C. Cordon-Cardo, and R. A. DePinho. 1998. The Ink4a tumor suppressor gene product, p19Arf, interacts with MDM2 and neutralizes MDM2’s inhibition of p53. Cell 92:713–723. [DOI] [PubMed] [Google Scholar]
- 36.Quelle, D. E., F. Zindy, R. A. Ashmun, and C. J. Sherr. 1995. Alternative reading frames of the INK4a tumor suppressor gene encode two unrelated proteins capable of inducing cell cycle arrest. Cell 83:993–1000. [DOI] [PubMed] [Google Scholar]
- 37.Radfar, A., I. Unnikrishnan, H. W. Lee, R. A. DePinho, and N. Rosenberg. 1998. p19Arf induces p53-dependent apoptosis during Abelson virus-mediated pre-B cell transformation. Proc. Natl. Acad. Sci. USA 95:13194–13199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ravi, R., B. Mookerjee, Z. M. Bhujwalla, C. H. Sutter, D. Artemov, Q. Zeng, L. E. Dillehay, A. Madan, G. L. Semenza, and A. Bedi. 2000. Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1α. Genes Dev. 14:34–44. [PMC free article] [PubMed] [Google Scholar]
- 39.Ruas, M., and G. Peters. 1998. The p16INK4a/CDKN2A tumor suppressor and its relatives. Biochim. Biophys. Acta 1378:F115–F177. [DOI] [PubMed] [Google Scholar]
- 40.Sandgren, E. P., N. C. Luetteke, R. D. Palmiter, R. L. Brinster, and D. C. Lee. 1990. Overexpression of TGF-α in transgenic mice: induction of epithelial hyperplasia, pancreatic metaplasia, and carcinoma of the breast. Cell 61:1121–1135. [DOI] [PubMed] [Google Scholar]
- 41.Schmitt, C. A., M. E. McCurrach, E. de Stanchina, R. R. Wallace-Brodeur, and S. W. Lowe. 1999. INK4a/ARF mutations accelerate lymphomagenesis and promote chemoresistance by disabling p53. Genes Dev. 13:2670–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Serrano, M., H. Lee, L. Chin, C. Cordon-Cardo, D. Beach, and R. A. DePinho. 1996. Role of the INK4a locus in tumor suppression and cell mortality. Cell 85:27–37. [DOI] [PubMed] [Google Scholar]
- 43.Shapiro, G. I., C. D. Edwards, M. E. Ewen, and B. J. Rollins. 1998. p16INK4A participates in a G1 arrest checkpoint in response to DNA damage. Mol. Cell. Biol. 18:378–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharma, A., D. H. Zangen, P. Reitz, M. Taneja, M. E. Lissauer, C. P. Miller, G. C. Weir, J. F. Habener, and S. Bonner-Weir. 1999. The homeodomain protein IDX-1 increases after an early burst of proliferation during pancreatic regeneration. Diabetes 48:507–513. [DOI] [PubMed] [Google Scholar]
- 45.Sharp, R., M. W. Babyatsky, H. Takagi, S. Tagerud, T. C. Wang, D. E. Bockman, S. J. Brand, and G. Merlino. 1995. Transforming growth factor alpha disrupts the normal program of cellular differentiation in the gastric mucosa of transgenic mice. Development 121:149–161. [DOI] [PubMed] [Google Scholar]
- 46.Sharpless, N. E., N. Bardeesy, K.-H. Lee, R. Carrasco, D. H. Castrillon, A. J. Aguirre, E. Wu, J. W. Horner, and R. A. DePinho. 2001. Loss of p16Ink4a with retention of p19Arf predisposes mice to tumorigenesis. Nature 413:86–91. [DOI] [PubMed] [Google Scholar]
- 47.Sharpless, N. E., and R. A. DePinho. 1999. The INK4A/ARF locus and its two gene products. Curr. Opin. Genet. Dev. 9:22–30. [DOI] [PubMed] [Google Scholar]
- 48.Signoretti, S., D. Waltregny, J. Dilks, B. Isaac, D. Lin, L. Garraway, A. Yang, R. Montironi, F. McKeon, and M. Loda. 2000. p63 is a prostate basal cell marker and is required for prostate development. Am. J. Pathol. 157:1769–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Slack, J. M. 1995. Developmental biology of the pancreas. Development 121:1569–1580. [DOI] [PubMed] [Google Scholar]
- 50.Song, S. Y., M. Gannon, M. K. Washington, C. R. Scoggins, I. M. Meszoely, J. R. Goldenring, C. R. Marino, E. P. Sandgren, R. J. Coffey, Jr., C. V. Wright, and S. D. Leach. 1999. Expansion of Pdx1-expressing pancreatic epithelium and islet neogenesis in transgenic mice overexpressing transforming growth factor alpha. Gastroenterology 117:1416–1426. [DOI] [PubMed] [Google Scholar]
- 51.Stott, F. J., S. Bates, M. C. James, B. B. McConnell, M. Starborg, S. Brookes, I. Palmero, K. Ryan, E. Hara, K. H. Vousden, and G. Peters. 1998. The alternative product from the human CDKN2A locus, p14ARF, participates in a regulatory feedback loop with p53 and MDM2. EMBO J. 17:5001–5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takagi, H., C. Jhappan, R. Sharp, and G. Merlino. 1992. Hypertrophic gastropathy resembling Menetrier’s disease in transgenic mice overexpressing transforming growth factor alpha in the stomach. J. Clin. Investig. 90:1161–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tlsty, T. D., and P. W. Hein. 2001. Know thy neighbor: stromal cells can contribute oncogenic signals. Curr. Opin. Genet. Dev. 11:54–59. [DOI] [PubMed] [Google Scholar]
- 54.Wagner, M., F. R. Greten, C. K. Weber, S. Koschnick, T. Mattfeldt, W. Deppert, H. Kern, G. Adler, and R. M. Schmid. 2001. A murine tumor progression model for pancreatic cancer recapitulating the genetic alterations of the human disease. Genes Dev. 15:286–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Warshaw, A. L., C. C. Compton, K. Lewandrowski, G. Cardenosa, and P. R. Mueller. 1990. Cystic tumors of the pancreas. New clinical, radiologic, and pathologic observations in 67 patients. Ann. Surg. 212:432–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Warshaw, A. L., and C. Fernandez-del Castillo. 1992. Pancreatic carcinoma. N. Engl. J. Med. 326:455–465. [DOI] [PubMed] [Google Scholar]
- 57.Wieser, R. J., D. Faust, C. Dietrich, and F. Oesch. 1999. p16INK4 mediates contact-inhibition of growth. Oncogene 18:277–281. [DOI] [PubMed] [Google Scholar]
- 58.Wilentz, R. E., J. Albores-Saavedra, and R. H. Hruban. 2000. Mucinous cystic neoplasms of the pancreas. Semin. Diagn. Pathol. 17:31–42. [PubMed] [Google Scholar]
- 59.Zhang, S., E. S. Ramsay, and B. A. Mock. 1998. Cdkn2a, the cyclin-dependent kinase inhibitor encoding p16INK4a and p19ARF, is a candidate for the plasmacytoma susceptibility locus, Pctr1. Proc. Natl. Acad. Sci. USA 95:2429–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang, S. L., W. DuBois, E. S. Ramsay, V. Bliskovski, H. C. Morse III, L. Taddesse-Heath, W. C. Vass, R. A. DePinho, and B. A. Mock. 2001. Efficiency alleles of the Pctr1 modifier locus for plasmacytoma susceptibility. Mol. Cell. Biol. 21:310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zindy, F., C. M. Eischen, D. H. Randle, T. Kamijo, J. L. Cleveland, C. J. Sherr, and M. F. Roussel. 1998. Myc signaling via the ARF tumor suppressor regulates p53-dependent apoptosis and immortalization. Genes Dev. 12:2424–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]