Abstract
Cytokinesis, the final stage of eukaryotic cell division, ensures the production of two daughter cells. It requires fine coordination between the plasma membrane and cytoskeletal networks, and it is known to be regulated by several intracellular proteins, including the small GTPase Rho and its effectors. In this study we provide evidence that the protein Nir2 is essential for cytokinesis. Microinjection of anti-Nir2 antibodies into interphase cells blocks cytokinesis, as it results in the production of multinucleate cells. Immunolocalization studies revealed that Nir2 is mainly localized in the Golgi apparatus in interphase cells, but it is recruited to the cleavage furrow and the midbody during cytokinesis. Nir2 colocalizes with the small GTPase RhoA in the cleavage furrow and the midbody, and it associates with RhoA in mitotic cells. Its N-terminal region, which contains a phosphatidylinositol transfer domain and a novel Rho-inhibitory domain (Rid), is required for normal cytokinesis, as overexpression of an N-terminal-truncated mutant blocks cytokinesis completion. Time-lapse videomicroscopy revealed that this mutant normally initiates cytokinesis but fails to complete it, due to cleavage furrow regression, while Rid markedly affects cytokinesis due to abnormal contractility. Rid-expressing cells exhibit aberrant ingression and ectopic cleavage sites; the cells fail to segregate into daughter cells and they form a long unseparated bridge-like cytoplasmic structure. These results provide new insight into the cellular functions of Nir2 and introduce it as a novel regulator of cytokinesis.
Cytokinesis ensures the separation of the cytoplasm between daughter cells at the final stage of eukaryotic cell division (12). It can be divided into four major steps: cleavage plane specification, contractile ring assembly, cleavage furrow constriction, and daughter cell separation (62). Specification of the cleavage plane is dictated by the mitotic spindle or microtubule asters (36), while assembly of the contractile ring involves local reorganization of actin and myosin filaments just beneath the plasma membrane. Sliding of the actin and myosin filaments pulls the membrane inward and provides the necessary force to constrict the cytoplasm of the dividing cells. This contraction results in cleavage furrow constriction (10, 32, 40). At the final stage of cytokinesis, the contractile ring at the cleavage furrow disassembles, followed by the fusion of opposing plasma membranes and cell separation (46). Defects in any of these steps prevent cytokinesis progression and subsequent cell division, a phenomenon which is usually associated with the production of multinucleate cells (38).
The Rho family of small GTPases controls a diverse array of cellular processes, including cell motility, morphogenesis, and cytokinesis (3, 15, 39, 49, 50). While it is not well understood how these proteins regulate cytokinesis, it is evident that their inactivation can induce multinucleate cell formation (38). Several Rho upstream regulators with GDP-GTP exchange factor or GAP (GTPase-activating protein) activities have been previously shown to play a critical role in cytokinesis (21, 37, 52). Drosophila melanogaster Pebble, a putative exchange factor for Rho, is required for the formation of the contractile ring and initiation of cytokinesis, whereas the nematode CYK-4, which encodes a GAP for Rho, is required for cytokinesis completion (20). Embryos from a cyk-4 mutant initiate but fail to complete cytokinesis. In addition to these upstream regulators, several Rho effectors, including citron kinase (20, 30), Rho-associated kinase (63), and the formin homology (FH) proteins (11, 60), have also been shown to regulate different steps of cytokinesis. Among the FH family members, the nematode CYK-1, the Drosophila DIA, and the yeast Bni1p, Bnr1p, cdc12 and SepA proteins have been shown to play a role in this process (60). Mutation in the Drosophila diaphanous gene causes cytokinesis defects and the production of highly polyploid cells (4), whereas microinjection of specific anti-mDia1 antibody into NIH 3T3 cells produces binucleate cells (55). CYK-1 is required for late cytokinesis events, as cytokinesis initiates normally in cyk-1 embryo mutants but cleavage furrows ingress extensively (48).
Recently, we have shown that the protein Nir2 binds the Rho small GTPase via a novel Rho-inhibitory domain (Rid) and regulates cell morphogenesis (54). Nir2 belongs to a highly conserved family of proteins that have been isolated from many species, including mammals, worms, flies, and fish (5, 8, 14, 26). The first family member, the Drosophila retinal degeneration B (rdgB) protein, was cloned in 1991 by Vihtelic et al. (56). Drosophila rdgB is implicated in the visual transduction cascade in flies, as rdgB mutant flies exhibit light-enhanced retinal degeneration and abnormal electroretinograms (16, 18, 45). More recently, four different mammalian genes similar to rdgB have been cloned by using different cloning strategies (1, 5, 14, 26, 28). The Nirs, Nir1, Nir2 (also known as H-RdgB and mRdgB1), and Nir3, were isolated as interacting proteins with the N-terminal region of the tyrosine kinase PYK2 by using a yeast two-hybrid screen (26). The Nir/rdgB family members share high sequence homology and several conserved structural domains, including an N-terminal phosphatidylinositol (PI) transfer domain, an acidic region that binds calcium, six hydrophobic stretches, and a conserved C-terminal domain (26). Although the mammalian Nirs/rdgBs are highly expressed in the retina (5, 26, 28), they are also abundantly expressed in other tissues and cell types, including hematopoietic and epithelial cells and different subtypes of neuronal cells (26).
Recently, we identified an additional functional domain in Nir2, Rid, which inhibits Rho-mediated stress fiber formation and lyophosphatidic acid (LPA)-induced Rho activation. This domain resides within the N-terminal region of Nir2, adjacent to its PI transfer domain (54). Since the Rho small GTPase is an important regulator of cytokinesis (11, 38, 60), we investigated the role of Nir2 in this process. We show here, for the first time, that a member of the Nir/rdgB family, Nir2, is essential for normal cytokinesis. Microinjection of anti-Nir2 antibodies into interphase cells blocks cytokinesis completion and results in the production of multinucleate cells. By using different Nir2-truncated mutants, we show that the N-terminal region is critical for cytokinesis, as overexpression of a Nir2 N-terminal-truncated mutant results in the production of multinucleate cells due to cleavage furrow regression. On the other hand, overexpression of Rid markedly affects cytokinesis and produces a phenotype which is characterized by poorly regulated ingression, an elongated cleavage furrow, the appearance of ectopic furrows, and severe impairment of contractility. Confocal microscopy analysis of Nir2 localization during the cell cycle revealed that it is mainly localized to the Golgi apparatus in interphase cells, but it is recruited to the cleavage furrow and the midbody during cytokinesis. In dividing cells, Nir2 and RhoA are colocalized in the cleavage furrow and the midbody and can be found in the same immunocomplex. Based on these results, we propose that Nir2 is required for late cytokinesis events and that its N-terminal region plays a critical role in this process.
MATERIALS AND METHODS
cDNA constructs and antibodies.
A DNA fragment encoding Rid (amino acids [aa] 205 to 424) was amplified by PCR using the sense and antisense oligonucleotide primers 5′-GCGGATTCAAGATCGAGCAGTTCATC-3′ and 5′-CCGCTCGAGTTAGCATGCCTCAGCCCCCAG-3′, respectively. The amplified DNA fragment was subcloned in frame into the pCMV-Neo expression vector containing either an N-terminal Myc or hemagglutinin (HA) epitope tag. The DNA constructs were verified by restriction enzyme analysis and sequencing. Other constructs used in this study have been previously described (54). Complete cloning details are available upon request. The mammalian expression vector pEGFP-C1 was purchased from Clontech. pCNG2, encoding the green fluorescent protein (GFP)-β-1,2-N-acetylglucosaminyltransferase I (NAGT-I) fusion protein under control of the cytomegalovirus promoter, was kindly provided by D. Shima (Imperial Cancer Research Fund, London, United Kingdom). Two different polyclonal anti-Nir2 antibodies were used in this study: antipeptide antibody against Nir2 was raised in rabbits immunized with a keyhole limpet hemocyanin-conjugated peptide (TPDGPEAPPGPDAS) corresponding to aa 287 to 300 of Nir2 (anti-Nir2-N), and antibody against a glutathione S-transferase fusion protein containing aa 524 to 678 (anti-Nir2-M). The antibodies were raised in rabbits and affinity purified using standard procedures. Monoclonal anti-β-tubulin antibody was purchased from Sigma. Monoclonal anti-Myc and anti-RhoA antibodies and polyclonal anti-HA antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.), and anti-HA monoclonal antibody was purchased from Boehringer Mannheim Biotechnology. Alexa-488- donkey anti-mouse immunoglobulin G (IgG), and Oregon green-donkey anti-rabbit IgG were purchased from Molecular Probes. Cy3-conjugated goat anti-rabbit or goat anti-mouse IgG was from Jackson ImmunoResearch Laboratories (West Grove, Pa.).
Indirect immunofluorescence.
HeLa cells grown on glass coverslips were transfected as indicated and fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) for 20 min at room temperature. When indicated, the cells were pretreated with nocodazole (10 μM) or with brefeldin A (BFA; 5 μM) for 3 h and 0.5 h, respectively, at 37°C. For recovery studies, cells were washed three times with BFA- or nocodazole-free medium and incubated for 1 h at 37°C in a regular growth medium. The fixed cells were permeabilized with 0.1% (vol/vol) Triton X-100 in PBS for 5 min and then incubated for 30 min in blocking buffer (10 mM Tris [pH 7.5], 150 mM NaCl, 2% bovine serum albumin, 1% glycine, 10% goat serum, and 0.1% Triton X-100). For endogenous Nir2 immunostaining using anti-Nir2-N antibody, cells were fixed in methanol for 10 min at −20°C, washed with PBS, and blocked as described above. For costaining with anti-RhoA and anti-Nir2 (anti-Nir2-N) antibodies, the cells were fixed in 10% trichloroacetic acid for 15 min and blocked as described above. After 1 h of incubation with various primary antibodies, the cells were washed with PBS and incubated with fluorescent-labeled secondary antibodies as indicated. Tetramethyl rhodamine isocyanate (TRITC)-phalloidin (Sigma) was used to visualize filamentous actin, while Hoechst 33258 (2 μg/ml; Molecular Probes) was used to stain DNA. Coverslips were mounted and analyzed by fluorescence microscopy as previously described (27). When indicated, the specimens were analyzed with a confocal laser microscope (Zeiss 510) equipped with filters for fluorescein and Cy3 epifluorescence. Both 488- and 543-nm laser lines were used for excitation, and 505- to-530-nm band-pass and 560-nm long-pass filters were used for emission. Image analysis was performed using the standard system operating software provided with the Zeiss 510 microscope (version 2.01).
Time-lapse imaging.
HeLa cells were plated onto a 6-cm culture dish containing glass coverslips 24 h before transfection. Cells were cotransfected with expression vectors encoding the indicated Nir2 mutant and GFP at a 10:1 molar ratio. Approximately 18 h after transfection, GFP-expressing cells were monitored by time-lapse phase-contrast videomicroscopy. Time-lapse imaging was performed on a Zeiss Axiovert 135 microscope equipped with a heated imaging chamber (Warner Instruments Corp.), a Sensicam charge-coupled device camera (PCO), and Axon Imaging Workbench version 2.1 software. The movies were transferred to a Macintosh computer by using NIH Image software and QuickTime format. The movies can be viewed at http://www.weizmann.ac.il/neurobiology/labs/lev/movies.html.
Immunoprecipitation from interphase and mitotic cells.
HeLa cells were split into normal growth medium containing Dulbecco's modified Eagle's medium, 10% fetal bovine serum, penicillin (100 U/ml), and streptomycin (100 mg/ml). After 24 h the cells were incubated for 18 h in the absence or presence of 100 ng of nocodazole/ml as previously described (47). The mitotic index after treatment with nocodazole was typically 95 to 98%. Interphase or mitotic HeLa cells were lysed in lysis buffer (50 mM Tris-HCl [pH 7.5], 100 mM NaCl, 1 mM dithiothreitol, 5 mM MgCl2, 50 mM NaF, 1 mM EDTA, 1 mM Na3VO4, 10% glycerol, 0.5% NP-40, 1 mM phenylmethylsulfonyl fluoride, 10 μg of leupeptin/ml, and 10 μg of aprotinin/ml) and immunoprecipitated as previously described (26).
Microinjection of anti-Nir2 antibodies.
Microinjection of mononucleate HeLa cells grown on coverslips was performed on a Zeiss Axiovert 135 microscope equipped with an Eppendorf microinjection system. Antibodies were dialyzed against PBS and concentrated to 1 mg/ml. Immediately after microinjection, coverslips were washed with PBS and incubated in growth medium for 24 or 36 h. The cells were fixed and stained as indicated and subsequently analyzed by fluorescence microscopy.
RESULTS
Nir2 localizes to the cleavage furrow during cytokinesis.
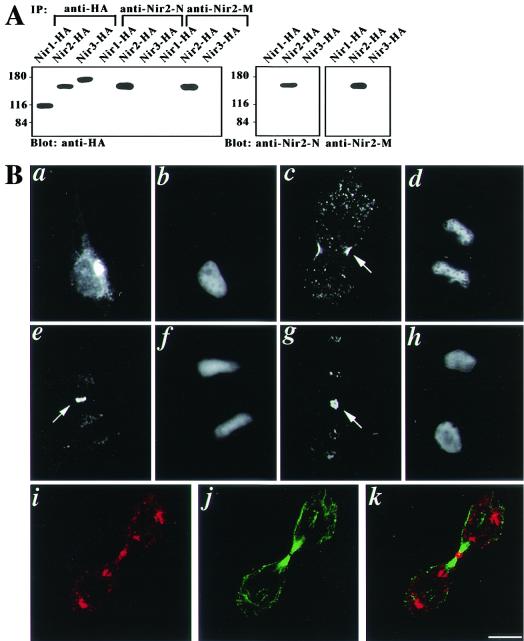
To determine the localization of endogenous Nir2 in dividing cells, an antipeptide antibody against Nir2 was raised in rabbits as described in Materials and Methods. Briefly, a 14-aa peptide derived from the Nir2 N-terminal region was used for polyclonal antibody production. The peptide sequence has no significant homology to any other known proteins, including the Nir/rdgB family members, as determined by a BLASTP sequence homology search of the GenBank database. The specificity of this antibody was determined by immunoprecipitation, immunoblotting (Fig. 1A), and immunofluorescence (data not shown) analyses. As shown in Fig. 1A, this antibody (anti-Nir2-N) specifically recognizes Nir2 and does not cross-react with the other family members (Nir1 and Nir3). Dividing HeLa cells were immunostained with this antibody and analyzed by fluorescence microscopy. As shown in Fig. 1B, Nir2 immunostaining appeared in the cleavage furrow in anaphase and in the midbody during telophase. Double-immunostaining analysis of Nir2 and β-tubulin revealed Nir2 in the middle of the bridge, between the microtubule bundles. In contrast, in interphase cells Nir2 immunostaining appeared in a perinuclear reticular structure resembling the Golgi apparatus, with a more diffuse and punctate staining throughout the rest of the cytoplasm (Fig. 1B, panel a).
FIG. 1.
Endogenous Nir2 localizes to the cleavage furrow and the midbody during cytokinesis. (A) Specificity of anti-Nir2 antibodies. Two different polyclonal antibodies against Nir2, anti-Nir2-N (against aa 287 to 300) and anti-Nir2-M (against aa 524 to 678), were raised in rabbits as described in Materials and Methods. These antibodies specifically recognize Nir2. Western analysis of HEK 293 cells expressing either HA-tagged Nir1, -Nir2 or -Nir3 revealed their specificity to Nir2. Likewise, the two antibodies exclusively immunoprecipitated Nir2. (B) HeLa cells were immunostained with affinity-purified rabbit polyclonal antibody against Nir2 (a, c, e, g, and i) (anti-Nir2-N). DNA was stained with Hoechst 33258 (b, d, f, and h). In interphase cells, Nir2 is mainly localized to the Golgi apparatus (a). The localization of Nir2 at the cleavage furrow in anaphase (c and e) and to the midbody during telophase (g) is indicated by an arrow. Confocal images of double immunostaining for Nir2 (i) and β-tubulin (j) and the merged image (k) localize Nir2 to the middle of the microtubule bundles. Bar, 10 μm.
The localization of Nir2 in the Golgi apparatus in interphase cells was confirmed by immunostaining of HeLa cells, which express the Golgi marker GFP-NAGT-I (43), with anti-Nir2 antibodies. Laser confocal microscopy analysis revealed that Nir2 is extensively colocalized with NAGT-I (Fig. 2A). Furthermore, treatment with nocodazole, which induces microtubule depolymerization and subsequent scattering of the Golgi complex (53), led to complete dispersal of Nir2 and NAGT-I. The two proteins were distributed throughout the cytoplasm and were colocalized in many scattered structures of various sizes. This effect was reversible, and both Nir2 and GFP-NAGT-I returned to their original subcellular localization following nocodazole removal (data not shown). Likewise, colocalization of Nir2 and NAGT-I was obtained upon treatment with BFA, which leads to a dramatic redistribution of Golgi proteins in the endoplasmic reticulum (24). After treatment with BFA, Nir2 was observed in a reticular pattern characteristic of endoplasmic reticulum labeling and returned to its original localization following BFA removal (Fig. 2A). These results indicate that in interphase cells Nir2 is mainly, but not exclusively, located in the Golgi apparatus.
FIG. 2.
Nir2 is mainly localized to the Golgi apparatus in interphase cells. Shown are confocal images of HeLa cells expressing GFP-NAGT-I (green) immunostained with anti-Nir2 antibodies (red) before and after treatment with nocodazole or BFA, after recovery from BFA treatment (A) or during cytokinesis (B). Colocalization appears in yellow. The distribution of Nir2 and NAGT-I at late anaphase and telophase is shown. Bars, 10 μm.
To determine whether Nir2 and NAGT-I are also colocalized during cytokinesis, dividing HeLa cells that express GFP-NAGT-I were immunostained with anti-Nir2 antibodies. The confocal images shown in Fig. 2B demonstrate that in late anaphase NAGT-I is localized in punctuated structures dispersed throughout the poles of the two daughter cells—a typical distribution for Golgi markers (33). In contrast, Nir2 immunoreactivity appeared in the cleavage furrow, distinct from NAGT-I. During telophase, when the Golgi apparatus starts to reform (44), GFP-NAGT-I was mainly visualized near the centrosomes. A minor pool was also observed adjacent to the bridge. Although weak colocalization of NAGT-I and Nir2 was observed at this stage, strong and exclusive immunoreactivity of Nir2 was detected in the midbody. These results clearly demonstrate the discrete localization of Nir2 during cytokinesis. It is worth noting that similar localization of Nir2 was also found in other cell types, including TE671 and MCF-7, using at least two different antibodies against Nir2 (data not shown).
Microinjection of anti-Nir2 antibodies inhibits cytokinesis.
The specific localization of Nir2 in the cleavage furrow and midbody during cytokinesis suggests that Nir2 plays a role in this process. Its essential role in cytokinesis was demonstrated by microinjection of anti-Nir2 antibodies into interphase HeLa cells. Two different affinity-purified polyclonal antibodies against Nir2 were used: one antibody was raised against a peptide derived from its N-terminal region (anti-Nir2-N), while the second one was raised against aa 524 to 678 in the middle region of the protein (anti-Nir2-M). These two antibodies are specific to Nir2; they do not cross-react with the other family members (Fig. 1A), and they specifically recognize endogenous Nir2 in HeLa cells (Fig. 3A). The injected cells were grown for 24 or 36 h, fixed, immunostained with Cy3-conjugated goat anti-rabbit IgG, and analyzed by fluorescence microscopy. Nuclei were visualized by Hoechst staining. The results shown in Fig. 3B and C clearly demonstrate the inhibitory effect of the two anti-Nir2 antibodies on cytokinesis. Twenty-four hours after microinjection, approximately 40% of the cells injected with anti-Nir2-N antibody had become binucleated, as had ≈27% of the cells injected with the second antibody, anti-Nir2-M. Control rabbit IgG had no apparent effect on multinucleate cell formation. After 36 h, 58% of the cells injected with anti-Nir2-N antibody and 55% of those injected with anti-Nir2-M antibody contained two or more nuclei (Fig. 3C). These results strongly suggest that endogenous Nir2 is essential for normal cytokinesis.
FIG. 3.
Microinjection of anti-Nir2 antibodies inhibits cytokinesis. (A) Anti-Nir2 antibodies recognize endogenous Nir2 in HeLa cells. Shown is a Western analysis of Nir2 immunoprecipitated from HeLa cells using either anti-Nir2-N or anti-Nir2-M antibodies or their corresponding preimmune serum (P.I.) as a control. (B) HeLa cells grown on coverslips were microinjected with either antibodies against Nir2 or control rabbit IgG. Two different affinity-purified polyclonal antibodies against Nir2 were injected: anti-Nir2-N and anti-Nir2-M. Cells were grown for 24 or 36 h after injection, fixed, and immunostained with Cy3-conjugated goat anti-rabbit IgG. DNA was stained with Hoechst 33258. Shown are representative images of microinjected cells 36 h postinjection. Bar, 50 μm. (C) Number of microinjected cells bearing a single nucleus, two nuclei, or more than two nuclei, as obtained in three independent experiments.
Deletion of the Nir2 N-terminal region results in the production of multinucleate cells.
To better understand the role of Nir2 in cytokinesis, we overexpressed it in mammalian cells and assessed its effect on cytokinesis progression. This experimental approach has been previously used to determine the role of critical proteins in cytokinesis, including ECT2 (52), ROCK (63), and citron kinase (30, 31), among others. HeLa cells were transiently transfected with expression vectors encoding either the full-length Nir2 or Nir2-truncated mutants in which different Nir2 structural domains had been deleted (Fig. 4B). Two days after transfection, the cells were fixed, immunostained with anti-HA antibodies, and analyzed by fluorescence microscopy. The most pronounced effect on cytokinesis was obtained in cells that overexpressed the ΔN2 truncated mutant, in which the entire N-terminal region of Nir2 had been deleted. As seen in Fig. 4A, nearly all of the cells that strongly expressed the ΔN2 mutant became multinucleated. Of the total ΔN2 transfectants, more than 60% became multinucleated, whereas approximately 35% of the ΔN1 transfectants had two or more nuclei. The wild type as well as the ΔPI and ΔPB mutants, in which the PI transfer domain or the C-terminal PYK2-binding (PB) domain had been deleted, had only minor effects on multinucleus formation compared to the control GFP-transfected cells (Fig. 4B). These results were also confirmed by fluorescence-activated cell sorter analysis (data not shown). Thus, overexpression of the ΔN2 mutant specifically blocked cytokinesis without markedly affecting cell cycle progression or nuclear division.
FIG. 4.
Overexpression of ΔN2 truncated mutant results in the production of multinucleated cells. (A) HeLa cells were transiently transfected with DNA encoding HA-tagged ΔN2 truncated mutant. The cells were fixed 2 days later and immunostained with anti-HA antibodies. Nuclei were stained with Hoechst 33258 and visualized by fluorescence microscopy. Bar, 50 μm. (B) HeLa cells were transiently transfected with expression vectors encoding either GFP or the indicated Nir2-truncated mutants. Schematic illustration of the different mutants is shown in the left panel. Two days after transfection, the cells were fixed and stained as described for panel A. The percentage of transfectants bearing multinuclei was scored. Data are means ± standard deviations of at least five independent experiments. At least 600 cells per sample were counted.
To better understand the effect of the ΔN2 truncated mutant on cytokinesis, we used time-lapse videomicroscopy analysis. As shown in Fig. 5B (and in the movie that can be viewed at http://www.weizmann.ac.il/neurobiology/labs/lev/movies.html), cytokinesis initiated normally and the cleavage furrow formed, but the cells failed to separate. Instead, furrow regression was evident approximately 30 min later. In many cases, the cells were “frozen” for a very long time after cleavage furrow formation (up to 4 h), and then cytokinesis reversed. In contrast, the control, nontransfected HeLa cells completed cytokinesis within approximately 15 min under the same experimental conditions (Fig. 5A). These results suggest that the ΔN2 truncated mutant normally initiates cytokinesis but fails to complete it due to late-cytokinesis defects.
FIG. 5.
Time-lapse imaging of cells expressing ΔN2 truncated mutant. (A) Time-lapse imaging of HeLa cells expressing GFP represents normal cytokinesis. Bar, 10 μm. (B) HeLa cells were cotransfected with expression vectors encoding ΔN2 mutant and GFP at a 10:1 molar ratio; 18 h after transfection, GFP-expressing cells were monitored by time-lapse phase-contrast videomicroscopy. Images are shown at selected time points. Bar, 10 μm. The full movie is available at http://www.weizmann.ac.il/neurobiology/labs/lev/movies.html.
Rid affects cytokinesis progression.
The profound effect of the ΔN2 mutant on cytokinesis completion suggests that its N-terminal region is required for normal cytokinesis. Recently, we showed that the N terminal of Nir2 contains a Rid (Fig. 4B). Since Rho inactivation is required for late cytokinesis events (23, 29, 34, 35), we assessed the effect of Rid overexpression on cytokinesis progression. As shown in Fig. 6, Rid-expressing cells failed to form the contractile ring (Fig. 6A, panel a) and exhibited severe irregularities. During telophase, the cells failed to segregate into daughter cells, and they formed a long unseparated bridge-like structure between them (Fig. 6A, panel c). The furrow elongates and other regions of the cells develop additional sites of ingression or ectopic furrowing that result in the formation of anucleate fragments (Fig. 6A, panel b). The unseparated bridge was coincidentally stained with antibodies against Rid and TRITC-phalloidin, and in many cases it appeared as a thin, long, bead-like structure (Fig. 6A, panel c). Furthermore, double-immunofluorescence analysis using antibodies against Rid and β-tubulin revealed that during telophase the microtubule bundles can be visualized in some of the Rid transfectants, but the two daughter cells are still connected by an unseparated cytoplasmic region (Fig. 6B, panels c and d). The cytoplasmic bridge of Rid transfectants was elongated, forming a long structure that was barely detected by anti-β-tubulin staining (Fig. 6B, panels i and j). The microtubule bundles disassemble and are not detected near the ectopic furrows (Fig. 6B, panels f and g). The Rid phenotype shares some properties with the phenotype obtained by overexpression of C3 exoenzyme (35), which causes inactivation of the small GTPase Rho (42). It was previously shown that microinjection of C3 exoenzyme into NIH 3T3 or NRK cells causes abnormal cytokinesis, generating irregular ingressions and ectopic cleavage sites (35). These results are consistent with our previous findings demonstrating that Rid negatively regulates Rho-mediated downstream signals (54).
FIG. 6.
Rid affects cytokinesis progression. (A) HeLa cells grown on coverslips were transfected with Rid-encoding vector. The cells were fixed 15 h later and costained with anti-Rid antibodies (Rid), TRITC-phalloidin (Actin), and Hoechst 33258 (DNA). No detectable contractile ring was visualized in the Rid-expressing cells (a, arrowhead). The cleavage furrow elongates (c) and the cells develop additional sites of ingression or ectopic furrows that result in the formation of anucleate fragments (b, arrowheads). Bar, 10 μm. (B) Representative images of Rid-expressing cells during cytokinesis. HeLa cells were grown on coverslips and transfected as described for panel A and then costained with antibodies against Rid (c, f, and i) or β-tubulin (a, d, g, and j) and with Hoechst 33258. In the control (a and b) nontransfected HeLa cells, microtubule bundles are visualized in the midbody. Microtubule bundles were also obtained in Rid-transfected cells (a), but the cells were still connected by a cytoplasmic bridge (arrowhead). This cytoplasmic bridge elongated (f and i), and ectopic sites developed (f and g, arrowheads). The ectopic sites were hardly detected by β-tubulin immunostaining (g). The microtubule bundles disassembled and were not detected in the long cytoplasmic bridge (j). Instead, a large gap of β-tubulin immunostaining was observed. Bar, 10 μm. (C) Videomicroscopy of cells expressing Rid. HeLa cells were cotransfected with expression vectors encoding Rid and GFP at a 10:1 molar ratio; 12 to 15 h later, GFP-expressing cells were monitored by time-lapse phase-contrast videomicroscopy. Images are shown at selected time points (in minutes). Bar, 10 μm. The full movie is available at http://www.weizmann.ac.il/neurobiology/labs/lev/movies.html.
Rid-expressing cells exhibit abnormal contractility during cytokinesis.
To further characterize the effect of Rid on cytokinesis, we used a time-lapse videomicroscopy analysis. As shown in Fig. 6C (and in the movie that can be viewed at http://www.weizmann.ac.il/neurobiology/labs/lev/movies.html), although cytokinesis was initiated the transfected cells consistently failed to separate and exhibited abnormal contractile movements both inside and outside the equator. In the first 16 min, contraction-expansion cycles were observed which repeated almost every 30 s. Such abnormal contractile movements were not observed in the control GFP-transfected HeLa cells (Fig. 5A). Generally (≈50% of cells recorded), the cleavage furrow pinched in from one side of the equator and progressed asymmetrically. This abnormality resulted in unequal segregation of the cytoplasm between the two connected daughter cells. Most of the transfected cells also formed protrusions at their cell surface, including the furrow region (Fig. 6C; 19 to 36 min) and could not complete cytokinesis. In contrast, the control cells usually completed cytokinesis within 15 to 30 min and produced two separated daughter cells. Abnormal contractile movements were also observed upon overexpression of a citron kinase-truncated mutant (30). However, in the latter case, furrow regression was observed and a multinucleate phenotype was obtained.
Endogenous Nir2 colocalizes and coimmunoprecipitates with RhoA in dividing cells.
Since Nir2 binds Rho via Rid (54) and Rid markedly affects cytokinesis (Fig. 5 and 6), it could be that Nir2 and RhoA interact with each other during cytokinesis. It was previously shown that the small GTPase Rho is localized to the cleavage furrow and midbody in dividing cells (30, 51). Since Nir2 is also found in the cleavage furrow during cytokinesis (Fig. 1B), we assessed whether Nir2 and RhoA are colocalized in dividing HeLa cells. Double immunostaining of endogenous Nir2 and RhoA was carried out using anti-Nir2 polyclonal antibody and anti-RhoA monoclonal antibody as described in Materials and Methods. Confocal microscopy analysis revealed colocalization of endogenous Nir2 and RhoA in the cleavage furrow during anaphase and in the midbody during telophase (Fig. 7A and B). In anaphase cells, RhoA immunoreactivity accumulated in the equatorial region, where it overlapped with Nir2 immunoreactivity, as seen in the Z-series optical sections obtained by confocal microscopy (Fig. 7A, Anaphase I). The three-dimensional image, which was reconstructed by these Z-series images, clearly shows that both Nir2 and RhoA accumulated and colocalized in a ring-like structure at the equatorial region, the contractile ring. The colocalization of Nir2 and Rho was maintained until late telophase, when both of them appeared in the middle of the intercellular bridge (Fig. 7B). The apparent colocalization of endogenous Nir2 and RhoA in the cleavage furrow and midbody during cytokinesis suggests that Nir2 interacts with RhoA in vivo. This interaction is probably mediated via Rid, as demonstrated by in vitro binding assays using recombinant Rid and RhoA (54). To further confirm the association between endogenous Nir2 and RhoA during cytokinesis, coimmunoprecipitation experiments were carried out. Nir2 and RhoA were immunoprecipitated from either interphase or mitotic HeLa cells and their association was determined by Western analysis by using the appropriate antibodies, as described in the legend for Fig. 7C. As shown, Nir2 specifically interacts with RhoA in mitotic cells. Although a weak association of Nir2 and RhoA was detected in interphase cells, the association between these two proteins was significantly enhanced during mitosis. These results are consistent with the localization studies shown in Fig. 7A and B and indicate that the interaction between Nir2 and Rho occurs in dividing cells.
FIG. 7.
Association between endogenous Nir2 and RhoA. Endogenous Nir2 colocalizes with RhoA during cytokinesis. Shown are confocal images of HeLa cells double immunostained with anti-Nir2 antibodies (red) and anti-RhoA antibodies (green) during anaphase I (A), anaphase II and telophase (B). Colocalization appears in yellow. Images shown in anaphase I are individual optical sections from a Z-series of 1-μm intervals obtained by confocal microscopy. The three-dimensional image was constructed from nine optical sections, and it is shown in the right panel. Bar, 10 μm. (C) Coimmunoprecipitation of Nir2 and RhoA in mitotic cells. Nir2 or RhoA were immunoprecipitated from either interphase or mitotic cells and their interaction was determined by Western analysis using anti-RhoA or anti-Nir2 antibodies, respectively. Preimmune serum (P.I.) was used as a control.
DISCUSSION
The final result of cytokinesis is the creation of two new membrane compartments surrounding the cytoplasm and nuclei of the newly formed daughter cells, a process that requires coordinated movements of the plasma membrane and the cytoskeletal networks (46). This coordination is achieved by dynamic reorganization of the cytoskeleton at the cleavage furrow and of the plasma membrane. Many proteins that play a crucial role in contractile ring organization, such as the ERM (ezrin-radixin-moesin) proteins (41), the septin family of proteins (9, 22), and the Rho small GTPase and its effector proteins (13, 30, 61), are recruited to the cleavage furrow during cytokinesis. In the present study, we showed that the Nir2 protein is located mainly in the Golgi apparatus in interphase cells (Fig. 2). This localization was observed in different cell types, and it is consistent with a previous report demonstrating the localization of mouse Nir2, PITPnm, mainly in the Golgi apparatus (2). However, during cytokinesis, Nir2 is recruited to the cleavage furrow and midbody (Fig. 1B and 2B), where it colocalizes with the small GTPase RhoA (Fig. 7A and B). Microinjection of specific antibodies against Nir2 inhibited cytokinesis completion and resulted in the production of multinucleate cells (Fig. 3). These results clearly indicate that Nir2 is essential for cytokinesis. To better understand the role of Nir2 in cytokinesis, we have used Nir2-truncated mutants and time-lapse videomicroscopy analysis. Our results indicate that the N-terminal region of Nir2 is essential for normal cytokinesis completion, as overexpression of an N-terminal-truncated mutant results in multinucleated cell formation (Fig. 4). By using time-lapse videomicroscopy, we could show that this mutant fails to complete cytokinesis due to late-cytokinesis defects (Fig. 5B).
The N-terminal region of Nir2 contains a PI transfer domain and a novel Rid (aa 205 to 424) (54). We have previously shown that Rid shares sequence homology with the Rho-binding domain of FH proteins and inhibits Rho-mediated stress fiber formation and LPA-induced RhoA activation (54). Here we show that Rid markedly affects cytokinesis progression. Overexpression of Rid produces a phenotype which is characterized by poorly regulated ingressions, an elongated cleavage furrow, the appearance of ectopic furrows (Fig. 6A and B), and severe impairment of contractility, as demonstrated by time-lapse videomicroscopy (Fig. 6C). Although some Rid-expressing cells eventually do contract, Rid appears to cause abortive cytokinesis after repeated attempts to continue cytokinesis (Fig. 6C), thereby causing elongation of normal stimulus time for cytokinesis. The Rid phenotype shares some properties with the phenotypes obtained by overexpression of negative regulators of Rho or several mutants of its downstream effectors. For example, ectopic furrows are common in C3-injected cells (35), whereas abnormal contractile movements were obtained by overexpression of a citron kinase-truncated mutant (30, 31). Elongated furrows have been previously observed upon overexpression of a glial fibrillary acidic protein mutant in which Rho kinase phosphorylation sites had been mutated (13, 63). Thus, disruption of Rho activation-inactivation or its downstream signal transduction during cytokinesis causes different effects on cytokinesis progression. The effect of Rid on cytokinesis (Fig. 6) might be related to its ability to inhibit Rho downstream signals. Rid-expressing cells showed cleavage ingression without obvious concentration of actin filaments along the cleavage furrow (Fig. 6A), consistent with our previous results demonstrating the apparent loss of F-actin staining in Rid-overexpressing cells (54). Similarly, microinjection of C3 exoenzyme into dividing NRK cells caused an apparent loss of actin filaments along the furrow (35). Thus, both Rid and C3 exoenzyme affect the organization of the actin filaments in the cleavage furrow. Nevertheless, the phenotypes of Rid and C3 exoenzyme are not identical, as C3 has no effect on contractility (unpublished results), whereas Rid-expressing cells undergo repeated contraction-expansion cycles over an extended period of time. These differences are probably related to the irreversible inactivation of Rho by C3 due to ADP ribosylation (42). In contrast, Rid binds Rho and causes its inactivation in a reversible manner like other Rho-binding proteins.
We have previously shown in in vitro binding assays that Nir2 preferentially binds the inactive GDP-bound form of Rho via Rid (54). Here we show that endogenous Nir2 colocalizes with RhoA during cytokinesis (Fig. 7A and B) and associates with RhoA in mitotic cells (Fig. 7C). These results suggest that the interaction between these two proteins is physiologically relevant and further support the role of Nir2 in cytokinesis, as Rho is an important regulator of this process. Since Rho inactivation is required both in early mitosis for disassembly of stress fibers and focal contacts and late in cytokinesis for disassembly of the contractile ring (23, 29, 34, 35), it could be that Nir2 is involved in the disassembly of the contractile ring at the final step of cytokinesis. The pronounced effect of the ΔN2 mutant on cytokinesis may support this hypothesis. The ΔN2 truncated mutant lacks the entire N-terminal region of Nir2, which contains the PI transfer domain and Rid. In cells that overexpress ΔN2, the final step of cytokinesis is blocked due to cleavage furrow regression, and multinucleate cells are produced (Fig. 4).
While Rid probably mediates the interaction of Nir2 with Rho, it is not yet clear whether the PI transfer domain has any role in cytokinesis. Nevertheless, we found that Nir2 translocates from the Golgi to the cleavage furrow during cytokinesis, distinctly from other Golgi proteins, such as NAGT-I (Fig. 2B). Similar translocation was recently shown for the Rab6-binding kinesin, Rab6-KIFL (17). It was proposed that one of its functions is to deliver vesicles from the Golgi to the site of membrane fusion during cytokinesis. Likewise, it could be that Nir2 is required for vesicle trafficking and/or membrane fusion at the final stage of cytokinesis. It was previously shown that Nir/rdgB family members contain a functional PI transfer domain, which can transfer PI between membrane bilayers in vitro (26, 57). The role of PI transfer proteins in vesicle trafficking and membrane fusion has been previously demonstrated in different experimental settings (6), and the importance of membrane fusion in cytokinesis has been demonstrated in genetic studies carried out in plants (25, 58), flies (59), and worms (19). Thus, it could be that Nir2 via its PI transfer domain is involved in membrane trafficking at the late stage of cytokinesis. Although overexpression of a ΔPI mutant had no effect on this process (Fig. 4B), we cannot exclude the possibility that this mutant does not exert a dominant negative effect on endogenous Nir2, thereby allowing cytokinesis to continue normally. Similarly to the ΔPI mutant, the ΔPB mutant also had no effect on cytokinesis, suggesting that the PYK2-binding domain is not required for normal cytokinesis. Indeed, immunolocalization studies revealed that PYK2 is not recruited to the cleavage furrow or the midbody during cytokinesis, and biochemical studies indicate that Nir2 is not tyrosine phosphorylated in mitotic cells (data not shown). These results suggest that the Nir2-PYK2 interaction does not play a role in cytokinesis. On the other hand, the profound effect of the ΔN2 mutant on cytokinesis, and to a lesser extent that of ΔN1, suggests that Rid is crucial for normal cytokinesis and the Nir2-Rho interaction plays a role in this process. Since Nir2 preferentially binds the inactive form of Rho, we propose that it is required late in cytokinesis for contractile ring disassembly.
While our results suggest that Nir2 is necessary for cytokinesis, it was previously shown that it is expressed in a variety of cell types and tissues, including neurons (26). Its expression in postmitotic cells suggests that it has additional cellular roles, which remain to be elucidated. We have recently shown that Nir2 regulates cell morphology (54); therefore, it could be that Nir2 is required for neuronal morphogenesis and/or development. Other proteins that are essential for cytokinesis, such as RhoA and citron kinase, are also expressed in neurons. While Rho regulates neurite development and the cytoskeleton, citron kinase is essential for the development of a specific subset of neurons (7).
In summary, by using a variety of experimental approaches, including precise localization studies, antibody microinjection, truncated mutant overexpression, and time-lapse videomicroscopy, we have provided evidence that Nir2 is essential for cytokinesis. Our results indicate that the N-terminal region of Nir2 is critical for cytokinesis completion and, therefore, we propose that Nir2 is required late in cytokinesis for disassembly of the contractile ring and down-regulation of Rho functions.
Acknowledgments
We thank B. Geiger and S. Bershadsky for critical reading of the manuscript.
Sima Lev is an incumbent of the Helena Rubinstein Career Development Chair. We thank F. Attinger for his generous support of our studies. This work was supported by the Israel Science Foundation (grant no. 649/00-1), Minna-James-Heineman Foundation, Minerva Foundation, and the Israel Cancer Research Foundation.
REFERENCES
- 1.Aikawa, Y., H. Hara, and T. Watanabe. 1997. Molecular cloning and characterization of mammalian homologues of the Drosophila retinal degeneration B gene. Biochem. Biophys. Res. Commun. 236:559-564. [DOI] [PubMed] [Google Scholar]
- 2.Aikawa, Y., A. Kuraoka, H. Kondo, M. Kawabuchi, and T. Watanabe. 1999. Involvement of PITPnm, a mammalian homologue of Drosophila rdgB, in phosphoinositide synthesis on Golgi membranes. J. Biol. Chem. 274:20569-20577. [DOI] [PubMed] [Google Scholar]
- 3.Bar-Sagi, D., and A. Hall. 2000. Ras and Rho GTPases: a family reunion. Cell 103:227-238. [DOI] [PubMed] [Google Scholar]
- 4.Castrillon, D. H., and S. A. Wasserman. 1994. Diaphanous is required for cytokinesis in Drosophila and shares domains of similarity with the products of the limb deformity gene. Development 120:3367-3377. [DOI] [PubMed] [Google Scholar]
- 5.Chang, J. T., S. Milligan, Y. Li, C. E. Chew, J. Wiggs, N. G. Copeland, N. A. Jenkins, P. A. Campochiaro, D. R. Hyde, and D. J. Zack. 1997. Mammalian homolog of Drosophila retinal degeneration B rescues the mutant fly phenotype. J. Neurosci. 17:5881-5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cockcroft, S. 1998. Phosphatidylinositol transfer proteins: a requirement in signal transduction and vesicle traffic. Bioessays 20:423-432. [DOI] [PubMed] [Google Scholar]
- 7.Di Cunto, F., S. Imarisio, E. Hirsch, V. Broccoli, A. Bulfone, A. Migheli, C. Atzori, E. Turco, R. Triolo, G. P. Dotto, L. Silengo, and F. Altruda. 2000. Defective neurogenesis in citron kinase knockout mice by altered cytokinesis and massive apoptosis. Neuron 28:115-127. [DOI] [PubMed] [Google Scholar]
- 8.Elagin, V. A., R. B. Elagina, C. J. Doro, T. S. Vihtelic, and D. R. Hyde. 2000. Cloning and tissue localization of a novel zebrafish RdgB homolog that lacks a phospholipid transfer domain. Vis. Neurosci. 17:303-311. [DOI] [PubMed] [Google Scholar]
- 9.Field, C. M., and D. Kellogg. 1999. Septins: cytoskeletal polymers or signalling GTPases? Trends Cell Biol. 9:387-394. [DOI] [PubMed] [Google Scholar]
- 10.Fishkind, D. J., and Y. L. Wang. 1995. New horizons for cytokinesis. Curr. Opin. Cell Biol. 7:23-31. [DOI] [PubMed] [Google Scholar]
- 11.Frazier, J. A., and C. M. Field. 1997. Actin cytoskeleton: are FH proteins local organizers? Curr. Biol. 7:R414-R417. [DOI] [PubMed] [Google Scholar]
- 12.Glotzer, M. 1997. The mechanism and control of cytokinesis. Curr. Opin. Cell Biol. 9:815-823. [DOI] [PubMed] [Google Scholar]
- 13.Goto, H., H. Kosako, and M. Inagaki. 2000. Regulation of intermediate filament organization during cytokinesis: possible roles of Rho-associated kinase. Microsc. Res. Tech. 49:173-182. [DOI] [PubMed] [Google Scholar]
- 14.Guo, J., and F. X. Yu. 1997. Cloning and characterization of human homologue of Drosophila retinal degeneration B: a candidate gene for degenerative retinal diseases. Dev. Genet. 20:235-245. [DOI] [PubMed] [Google Scholar]
- 15.Hall, A. 1998. Rho GTPases and the actin cytoskeleton. Science 279:509-514. [DOI] [PubMed] [Google Scholar]
- 16.Harris, W. A., and W. S. Stark. 1977. Hereditary retinal degeneration in Drosophila melanogaster. A mutant defect associated with the phototransduction process. J. Gen. Physiol. 69:261-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill, E., M. Clarke, and F. A. Barr. 2000. The Rab6-binding kinesin, Rab6-KIFL, is required for cytokinesis. EMBO J. 19:5711-5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hotta, Y., and S. Benzer. 1970. Genetic dissection of the Drosophila nervous system by means of mosaics. Proc. Natl. Acad. Sci. USA 67:1156-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jantsch-Plunger, V., and M. Glotzer. 1999. Depletion of syntaxins in the early Caenorhabditis elegans embryo reveals a role for membrane fusion events in cytokinesis. Curr. Biol. 9:738-745. [DOI] [PubMed] [Google Scholar]
- 20.Jantsch-Plunger, V., P. Gonczy, A. Romano, H. Schnabel, D. Hamill, R. Schnabel, A. A. Hyman, and M. Glotzer. 2000. CYK-4:Rho family GTPase activating protein (GAP) required for central spindle formation and cytokinesis. J. Cell Biol. 149:1391-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kimura, K., T. Tsuji, Y. Takada, T. Miki, and S. Narumiya. 2000. Accumulation of GTP-bound RhoA during cytokinesis and a critical role of ECT2 in this accumulation. J. Biol. Chem. 275:17233-17236. [DOI] [PubMed] [Google Scholar]
- 22.Kinoshita, M., S. Kumar, A. Mizoguchi, C. Ide, A. Kinoshita, T. Haraguchi, Y. Hiraoka, and M. Noda. 1997. Nedd5, a mammalian septin, is a novel cytoskeletal component interacting with actin-based structures. Genes Dev. 11:1535-1547. [DOI] [PubMed] [Google Scholar]
- 23.Kishi, K., T. Sasaki, S. Kuroda, T. Itoh, and Y. Takai. 1993. Regulation of cytoplasmic division of Xenopus embryo by rho p21 and its inhibitory GDP/GTP exchange protein (rho GDI). J. Cell Biol. 120:1187-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klausner, R. D., J. G. Donaldson, and J. Lippincott-Schwartz. 1992. Brefeldin A: insights into the control of membrane traffic and organelle structure. J. Cell Biol. 116:1071-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lauber, M. H., I. Waizenegger, T. Steinmann, H. Schwarz, U. Mayer, I. Hwang, W. Lukowitz, and G. Jurgens. 1997. The Arabidopsis KNOLLE protein is a cytokinesis-specific syntaxin. J. Cell Biol. 139:1485-1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lev, S., J. Hernandez, R. Martinez, A. Chen, G. Plowman, and J. Schlessinger. 1999. Identification of a novel family of targets of PYK2 related to Drosophila retinal degeneration B (rdgB) protein. Mol. Cell. Biol. 19:2278-2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Litvak, V., D. Tian, Y. D. Shaul, and S. Lev. 2000. Targeting of PYK2 to focal adhesions as a cellular mechanism for convergence between integrins and G protein-coupled receptor signaling cascades. J. Biol. Chem. 275:32736-32746. [DOI] [PubMed] [Google Scholar]
- 28.Lu, C., T. S. Vihtelic, D. R. Hyde, and T. Li. 1999. A neuronal-specific mammalian homolog of the Drosophila retinal degeneration B gene with expression restricted to the retina and dentate gyrus. J. Neurosci. 19:7317-7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mabuchi, I., Y. Hamaguchi, H. Fujimoto, N. Morii, M. Mishima, and S. Narumiya. 1993. A rho-like protein is involved in the organisation of the contractile ring in dividing sand dollar eggs. Zygote 4:325-331. [DOI] [PubMed] [Google Scholar]
- 30.Madaule, P., M. Eda, N. Watanabe, K. Fujisawa, T. Masuoka, H. Bito, T. Ishizaki, and S. Narumiya. 1998. Role of citron kinase as a target of the small GTPase Rho in cytokinesis. Nature 394:491-494. [DOI] [PubMed] [Google Scholar]
- 31.Madaule, P., T. Furuyashiki, M. Eda, H. Bito, T. Ishizaki, and S. Narumiya. 2000. Citron, a Rho target that affects contractility during cytokinesis. Microsc. Res. Tech. 49:123-126. [DOI] [PubMed] [Google Scholar]
- 32.Mandato, C. A., H. A. Benink, and W. M. Bement. 2000. Microtubule-actomyosin interactions in cortical flow and cytokinesis. Cell Motil. Cytoskeleton 45:87-92. [DOI] [PubMed] [Google Scholar]
- 33.Misteli, T., and G. Warren. 1995. Mitotic disassembly of the Golgi apparatus in vivo. J. Cell Sci. 108:2715-2727. [DOI] [PubMed] [Google Scholar]
- 34.Moorman, J. P., D. A. Bobak, and C. S. Hahn. 1996. Inactivation of the small GTP binding protein Rho induces multinucleate cell formation and apoptosis in murine T lymphoma EL4. J. Immunol. 156:4146-4153. [PubMed] [Google Scholar]
- 35.O'Connell, C. B., S. P. Wheatley, S. Ahmed, and Y.-L. Wang. 1999. The small GTP-binding protein Rho regulates cortical activities in cultured cells during division. J. Cell Biol. 144:305-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oegema, K., and T. J. Mitchison. 1997. Rappaport rules: cleavage furrow induction in animal cells. Proc. Natl. Acad. Sci. USA 94:4817-4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prokopenko, S. N., A. Brumby, L. O'Keefe, L. Prior, Y. He, R. Saint, and H. J. Bellen. 1999. A putative exchange factor for Rho1 GTPase is required for initiation of cytokinesis in Drosophila. Genes Dev. 13:2301-2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prokopenko, S. N., R. Saint, and H. J. Bellen. 2000. Untying the Gordian knot of cytokinesis: role of small G proteins and their regulators. J. Cell Biol. 148:843-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ridley, A. J. 1995. Rho-related proteins: actin cytoskeleton and cell cycle. Curr. Opin. Genet. Dev. 5:24-30. [DOI] [PubMed] [Google Scholar]
- 40.Robinson, D. N., and J. A. Spudich. 2000. Towards a molecular understanding of cytokinesis. Trends Cell Biol. 10:228.. [DOI] [PubMed] [Google Scholar]
- 41.Sato, N., S. Yonemura, T. Obinata, S. Tsukita, and S. Tsukita. 1991. Radixin, a barbed end-capping actin-modulating protein, is concentrated at the cleavage furrow during cytokinesis. J. Cell Biol. 113:321-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sekine, A., M. Fujiwara, and S. Narumiya. 1989. Asparagine residue in the rho gene product is the modification site for botulinum ADP-ribosyltransferase. J. Biol. Chem. 264:8602-8605. [PubMed] [Google Scholar]
- 43.Shima, D. T., K. Haldar, R. Pepperkok, R. Watson, and G. Warren. 1997. Partitioning of the Golgi apparatus during mitosis in living HeLa cells. J. Cell Biol. 137:1211-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Souter, E., M. Pypaert, and G. Warren. 1993. The Golgi stack reassembles during telophase before arrival of proteins transported from the endoplasmic reticulum. J. Cell Biol. 122:533-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stark, W. S., and R. Sapp. 1987. Ultrastructure of the retina of Drosophila melanogaster: the mutant ora (outer rhabdomeres absent) and its inhibition of degeneration in rdgB (retinal degeneration-B). J. Neurogenet. 4:227-240. [PubMed] [Google Scholar]
- 46.Straight, A. F., and C. M. Field. 2000. Microtubules, membranes and cytokinesis. Curr. Biol. 10:R760-R770. [DOI] [PubMed] [Google Scholar]
- 47.Stuart, R. A., D. Mackay, J. Adamczewski, and G. Warren. 1993. Inhibition of intra-Golgi transport in vitro by mitotic kinase. J. Biol. Chem. 268:4050-4054. [PubMed] [Google Scholar]
- 48.Swan, K. A., A. F. Severson, C. J. Clayton, P. R. Martin, H. Schnabel, R. Schnabel, and B. Bowerman. 1998. cyk-1: a C. elegans FH gene required for a late step in embryonic cytokinesis. J. Cell Sci. 111:2017-2027. [DOI] [PubMed] [Google Scholar]
- 49.Symons, M., and J. Settleman. 2000. Rho family GTPases: more than simple switches. Trends Cell Biol. 10:415-419. [DOI] [PubMed] [Google Scholar]
- 50.Takai, Y., T. Sasaki, K. Tanaka, and H. Nakanishi. 1995. Rho as a regulator of the cytoskeleton. Trends Biochem. Sci. 20:227-231. [DOI] [PubMed] [Google Scholar]
- 51.Takaishi, K., T. Sasaki, T. Kameyama, S. Tsukita, S. Tsukita, and Y. Takai. 1995. Translocation of activated Rho from the cytoplasm to membrane ruffling area, cell-cell adhesion sites and cleavage furrows. Oncogene 11:39-48. [PubMed] [Google Scholar]
- 52.Tatsumoto, T., X. Xie, R. Blumenthal, I. Okamoto, and T. Miki. 1999. Human ECT2 is an exchange factor for Rho GTPases, phosphorylated in G2/M phases, and involved in cytokinesis. J. Cell Biol. 147:921-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thyberg, J., and S. Moskalewski. 1999. Role of microtubules in the organization of the Golgi complex. Exp. Cell Res. 246:263-279. [DOI] [PubMed] [Google Scholar]
- 54.Tian, D., V. Litvak, M. Toledo-Rodriguez, S. Carmon, and S. Lev. 2002. Nir2, a novel regulator of cell morphogenesis. Mol. Cell. Biol. 22:2650-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tominaga, T., E. Sahai, P. Chardin, F. McCormick, S. A. Courtneidge, and A. S. Alberts. 2000. Diaphanous-related formins bridge Rho GTPase and Src tyrosine kinase signaling. Mol. Cell 5:13-25. [DOI] [PubMed] [Google Scholar]
- 56.Vihtelic, T. S., D. R. Hyde, and J. E. O'Tousa. 1991. Isolation and characterization of the Drosophila retinal degeneration B (rdgB) gene. Genetics 127:761-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vihtelic, T. S., M. Goebl, S. Milligan, J. E. O'Tousa, and D. R. Hyde. 1993. Localization of Drosophila retinal degeneration B, a membrane-associated phosphatidylinositol transfer protein. J. Cell Biol. 122:1013-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Waizenegger, I., W. Lukowitz, F. Assaad, H. Schwarz, G. Jurgens, and U. Mayer. 2000. The Arabidopsis KNOLLE and KEULE genes interact to promote vesicle fusion during cytokinesis. Curr. Biol. 10:1371-1374. [DOI] [PubMed] [Google Scholar]
- 59.Warn, R. M., A. Warn, V. Planques, and M. Robert-Nicoud. 1990. Cytokinesis in the early Drosophila embryo. Ann. N. Y. Acad. Sci. 582:222-232. [DOI] [PubMed] [Google Scholar]
- 60.Wasserman, S. 1998. FH proteins as cytoskeletal organizers. Trends Cell Biol. 8:111-115. [DOI] [PubMed] [Google Scholar]
- 61.Watanabe, N., P. Madaule, T. Reid, T. Ishizaki, G. Watanabe, A. Kakizuka, Y. Saito, K. Nakao, B. M. Jockusch, and S. Narumiya. 1997. p140mDia, a mammalian homolog of Drosophila diaphanous, is a target protein for Rho small GTPase and is a ligand for profilin. EMBO J. 16:3044-3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wolf, W. A., T.-L. Chew, and R. L. Chisholm. 1999. Regulation of cytokinesis. Cell. Mol. Life Sci. 55:108-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yasui, Y., M. Amano, K. Nagata, N. Inagaki, H. Nakamura, H. Saya, K. Kaibuchi, and M. Inagaki. 1998. Roles of Rho-associated kinase in cytokinesis; mutations in Rho-associated kinase phosphorylation sites impair cytokinetic segregation of glial filaments. J. Cell Biol. 143:1249-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]