Abstract
The assembly of photoreceptor outer segments into stacked discs is a complicated process, the precise regulation of which remains a mystery. It is known that the integrity of the outer segment is heavily dependent upon surrounding cell types including the retinal pigment epithelium and Müller cells; however the role played by Müller cells within this photoreceptor-specific process has not been fully explored. Using an RPE-deprived but otherwise intact Xenopus laevis eye rudiment preparation, we reveal that Müller cell involvement in outer segment assembly is dependent upon the stimulus provided to the retina. Pigment epithelium-derived factor is able to support proper membrane folding after inhibition of Müller cell metabolism by alpha-aminoadipic acid, while isopropyl beta-D-thiogalactoside, a permissive glycan, requires intact Müller cell function. These results demonstrate that both intrinsic and extrinsic redundant mechanisms exist to support the ability of photoreceptors to properly assemble their outer segments. Our study further suggests that the receptor for pigment epithelium-derived factor resides in photoreceptors themselves while that for permissive glycans is likely localized to Müller cells, which in turn communicate with photoreceptors to promote proper membrane assembly.
Keywords: retina, membrane assembly, pigment epithelium-derived factor, sugar, glycan
INTRODUCTION
Photoreceptors are highly polarized and ultrastructurally unique retinal neurons. At one pole of the neuron is the chemical synapse; while at the other is the outer segment, the most highly specialized region of the photoreceptor cell. Functional and anatomical integrity of the photoreceptor outer segments is essential for proper detection of light and optimal vision. Furthermore, integrity of outer segments is heavily dependent on the contributions of other surrounding cells. To this end, the importance of an intact and fully functional retinal pigment epithelium (RPE) on photoreceptor development and survival has been known for many years. Each photo-receptor outer segment is continuously being renewed at its proximal end, while the distal tips are shed and phagocytized by the RPE (Young, 1967). Outer segment development is impaired in the absence of the RPE, indicating that interactions between these two cell types are of fundamental importance for the differentiation of photoreceptors (Hollyfield and Witkovsky, 1974). Moreover, photoreceptor outer segments rapidly degenerate after separation from the RPE and the degree of recovery is inversely proportional to the length of time of the detachment (Anderson et al., 1986; Erickson et al., 1983; Guérin et al., 1989; Guérin et al., 1993; Lewis et al., 1991).
Like the RPE, Müller cells are important role players in photoreceptor development and survival. Müller cells are coupled embryologically, physically, and metabolically to photoreceptors (Reichenbach et al., 1993). It has also been proposed that Müller cells provide trophic support to photo-receptors to promote their survival (Cao et al., 1997; Newman and Reichenbach, 1996; Reichenbach et al., 1993). Increasing evidence also documents that glial cells may regulate synapto-genesis (Ullian et al., 2001; Ullian et al., 2004) and neuronal processing (Newman, 2004) through bidirectional communication (Araque and Perea, 2004). During development, Müller cells, photoreceptors and a subset of inner retinal neurons descend from a single retinal progenitor cell and arrange themselves in a columnar fashion (Turner and Cepko, 1987; Reichenbach et al., 1993) in which Müller cells surround photoreceptors from the synaptic terminals to the inner segments (Robinson and Dreher, 1990) where the two cells are joined via the adherens junctions that comprise the outer limiting membrane (reviewed in Jablonski and Ervin, 2000 and Tepass, 2002). In addition, Müller cells express voltage-gated ion channels, neurotransmitter receptors and various uptake carrier systems, which enable them to modulate the activity of retinal neurons (Reichenbach et al., 1997; Lin and Bergles, 2004). The coupling between Müller cells and photo-receptors is further demonstrated by our studies which document that targeted disruption of Müller cell metabolism with α-aminoadipic acid (i.e. α-AAA) results in disorganization of nascent photoreceptor outer segments despite normal levels of opsin expression (Jablonski and Iannaccone, 2000). These data suggest that Müller cells interact with photoreceptors through mechanisms that may regulate, at least in part, the assembly of photoreceptor outer segment membranes.
We have previously demonstrated that photoreceptor outer segment assembly in RPE-deprived isolated Xenopus laevis tadpole retinas can be modulated by the addition of specific molecules. We have shown that exogenously added purified pigment epithelium-derived factor (PEDF) supports normal levels of opsin expression and is a potent promoter of proper assembly of nascent photoreceptor outer segment membranes in RPE-deprived, but otherwise intact retinas from Xenopus laevis tadpoles (Jablonski et al., 2000). Similarly, lactose, galactose, isopropyl beta-D-thiogalactoside (i.e. IPTG, a non-metabolizable form of galactose), and structurally-related glycans also support photoreceptor outer segment assembly in RPE-deprived Xenopus retinas (Stiemke and Hollyfield, 1994; Stiemke and Hollyfield, 1995; Jablonski et al., 1999; Jablonski and Ervin, 2000) through a mechanism that appears to be receptor-mediated (Wang et al., 2003).
While several of the events that lead to formation and organization of outer segments have been elucidated, the precise mechanisms that regulate photoreceptor outer segment assembly and the contributions of neighboring cell types remains incompletely characterized. Likewise, the mechanism(s) via which PEDF and the aforementioned permissive glycans support photoreceptor outer segment assembly remain to be elucidated. In the present paper, we demonstrate that photo-receptor outer segment assembly is differentially dependent upon Müller cell involvement and suggest that PEDF exerts its effect directly on photoreceptors, while the putative receptor for permissive glycans is localized to Müller cells.
OBJECTIVE
The purpose of the present study was to evaluate the response of photoreceptors to external stimuli that have been shown to support proper outer segment folding (i.e. PEDF and IPTG) in intact RPE-deprived Xenopus laevis tadpole eyes when Müller cell function has been compromised and test the hypothesis that Müller cells contribute to the ability of photo-receptors to properly fold and assemble their outer segment membranes.
METHODS
The experimental culture preparation used in these studies has been previously described (Stiemke and Hollyfield, 1994). In brief, human chorionic gonadotropin (Sigma Chemical Co., St Louis, MO) was used to induce adult Xenopus laevis to breed. The external staging system of Nieuwkoop and Faber (Nieuwkoop and Faber, 1956) was used to determine retinal maturity. Xenopus laevis embryos and isolated eye rudiments were maintained under cyclic lighting conditions (12h light: 12h dark).
In all experiments, eye rudiments were removed from embryos at stage 33/34, when the eyes consist of a structure that is still devoid of the sclero-choroidal complex and the RPE represents the outermost layer. At this stage, photoreceptor outer segments are just beginning to be elaborated (Stiemke et al., 1994). The RPE was left intact in RPE-supported control eye rudiments, while it was gently peeled away from the neuro-retina in RPE-deprived eye rudiments. Purified bovine PEDF (generously provided by Joyce Tombran-Tink, University of Missouri, Kansas City), IPTG (Sigma Chemical Co., St Louis, MO), and alpha-aminoadipic acid (α-AAA) (Sigma Chemical Co., St Louis, MO) were prepared as previously described (Jablonski et al., 2000; Jablonski and Iannaccone, 2000; Jablonski et al., 2001b; Wang et al., 2003).
To begin teasing out the contribution of Müller cells toward the support of photoreceptor outer segment organization, we cultured RPE-deprived eye rudiments in the presence of either PEDF or IPTG, and compared the level of organization attained by photoreceptor outer segments under these conditions with that reached when co-culturing RPE-deprived eye rudiments also in the presence of α-AAA, an inhibitor of Müller cell metabolism. Concentrations of supplements were 50 ng/ml purified bovine PEDF or 5 × 10-5 M IPTG. In previous studies, these concentrations were shown to be those yielding the most effective Rport to photoreceptor outer segment assembly (Jablonski et al., 2000; Jablonski et al., 2001b; Wang et al., 2003). In the parallel Müller cell inhibition experiments, 1 × 10-5 M α-AAA was added to culture, i.e. at the concentration that we previously determined that photoreceptor outer segment assembly was most effectively disrupted, yet opsin expression by photoreceptors remained unaffected (Jablonski and Iannaccone, 2000). Control experiments included intact Xenopus laevis eye rudiments cultured with (i.e. positive control) and without (i.e. negative control) an adherent RPE in otherwise non-Rplemented standard Niu Twitty medium (Stiemke et al., 1994).
The assembly of the nascent photoreceptor outer segments under these four experimental conditions was then graded in a masked fashion using our previously described grading algorithm and six-step scale (Jablonski et al., 2001a). The key criterion of this grading scale is the amount of stacked and organized photoreceptor outer segment membrane that was associated with each evaluated photoreceptor cell. In brief, each step in grade represented a linear progression by an approximate 25% interval, ranging from 100% organization (grade 4) as seen in retinas with an adherent RPE to complete absence of organization (grade 0) despite the presence of whorls of membranous outer segment material attributable to the underlying photoreceptor cell. The grading also included a sixth level (grade—1) to account for the complete absence of the photoreceptor outer segment. Using this grading system, eight contiguous photoreceptors from six individual retinas, for a total of 48 individual photoreceptors per experimental condition, were evaluated. Grading data were then statistically analyzed by one-way analysis of variance (ANOVA) using SAS statistical software (SAS Institute Inc., Cary, NC). Post-hoc analyses were performed using least squares means. Because six comparisons were made, a Bonferroni's adjustment was applied and a p value of 0.0083 (i.e. 0.05/6) was considered statistically significant. Data are presented as mean ± standard error (S.E.).
RESULTS
Grading of the structure and organization of photoreceptor outer segments revealed significant differences in the organization of outer segments under the various culture protocols utilized in these studies. By one-way ANOVA, the overall F-test for differences among the six groups was highly significant (F = 26.79; p value < 0.0001). In retinas that completed morphogenesis with an adherent RPE, the vast majority of photoreceptor outer segments were highly structured, properly folded and contained discs of equal diameter (Fig. 1A). Based on our grading scale, this corresponded to a grade of 3.60 ± 0.08 (Fig. 1G), with a grade of 4 representing the highest level (100%) of organization. In the absence of the RPE, the average grade of photoreceptor outer segment organization decreased to 0.98 ± 0.27 (Fig. 1B,G), which indicated that on average, less than 25% of the outer segment material was organized into stacked flattened membranous saccules.
Fig. 1.
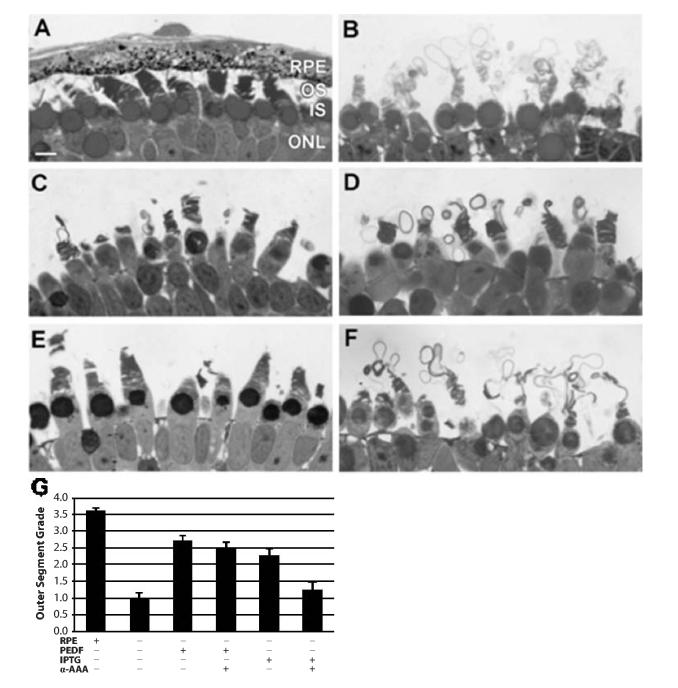
Illustrative examples of photoreceptor outer segment organization under the experimental conditions utilized in this study. (A) Control outer retina in which photoreceptors elaborated outer segments with a juxtaposed retinal pigment epithelium (RPE) layer. (B) Negative control RPE-deprived retina in which outer segments were synthesized in non-Rplemented Niu-Twitty medium. (C) RPE-deprived retina in which outer segment membranes were elaborated in medium Rplemented with 50 ng/ml purified bovine pigment epithelial-derived factor (PEDF). (D) RPE-deprived retina that was maintained in medium Rplemented with both 50 ng/ml purified bovine PEDF and 10-5 M alpha-amino adipic acid (α-AAA). (E) RPE-deprived retina that was exposed to 5 × 10-5 M isopropyl beta-D-thiogalactoside (IPTG), a permissive glycan. (F) RPE-deprived retina that was exposed to both 5 × 10-5 M IPTG and α-AAA. (G) Graphic illustration of photoreceptor outer segment grading from the various experimental conditions. RPE=retinal pigment epithelium; OS=photoreceptor outer segments; IS=photoreceptor inner segments; ONL=outer nuclear layer. Magnification bar=10 μm.
The addition of 50 ng/ml purified PEDF to the medium Rported proper outer segment organization compared to RPE-deprived retinas (p < 0.0001, Fig. 1C,G). The average organizational grade attained by PEDF-Rported RPE-deprived retinas was 2.69 ± 0.17, which indicates that between 50-75% of the outer segment membranes were highly structured. The addition of 1 × 10-5 M α-AAA did not significantly diminish the ability of PEDF to Rport photoreceptor outer segment assembly. Under these conditions, the average organizational grade attained by photoreceptor outer segment despite RPE deprivation was 2.44 ± 0.23, which was still significantly greater than that of RPE-deprived negative control conditions (p < 0.0001, Fig. 1D,G), yet it was not different from retinas maintained in PEDF alone (p = 0.354).
The response of IPTG-Rported retinas to the addition of 1 × 10-5 M α-AAA was very different from that observed in PEDF-exposed retinas. In retinas Rported with 5 × 10-5 M IPTG, the average organizational grade of outer segments was 2.25 ± 0.23, consistent with approximately 50 to 60% level of outer segment organization. This was significantly greater than RPE-deprived retinas (p < 0.0001, Fig. 1E,G). When α-AAA was added to IPTG-Rported retinas, though, the average organizational grade dropped to 1.23 ± 0.24 (Fig. 1F,G). Outer segments elaborated under this experimental condition were significantly less organized than those exposed to IPTG only (p < 0.0001), and they were no better organized than in the RPE-deprived negative control conditions (p = 0.247).
CONCLUSIONS
In RPE-deprived Xenopus laevis eye rudiments, photo-receptor outer segment membranes are synthesized, yet they are not properly assembled.
Both PEDF and IPTG Rport the proper folding of nascent photoreceptor outer segments in lieu of the RPE.
α-AAA does not significantly diminish the ability of PEDF to promote photoreceptor outer segment assembly.
In contrast, α-AAA prevents the ability of IPTG to promote proper photoreceptor outer segment folding.
DISCUSSION
We have previously demonstrated that both the neurotrophic agent, PEDF (Jablonski et al., 2000), and permissive glycans including lactose, galactose and IPTG (Jablonski and Ervin, 2000; Stiemke and Hollyfield, 1994; Stiemke and Hollyfield, 1995; Wang et al., 2003) Rport photoreceptor outer segment assembly in isolated RPE-deprived Xenopus laevis eye rudiments. We have also previously demonstrated that Müller cells are likely directly involved in the process of photoreceptor outer segment assembly (Jablonski and Iannaccone, 2000). The results of the present study lend further evidence in favor of Müller cells playing an important role in this process, but show also that such a role differs substantially depending on the neurotrophic factor that is used to promote photoreceptor outer segment assembly.
Our present study confirms our previous finding (Jablonski et al., 2000) that PEDF Rports photoreceptor outer segment assembly in lieu of an adherent RPE layer and are, more in general, consistent with the evidence that PEDF is not only a very promising antiangiogenic factor (Mori et al., 2002) but also a potent neurotrophic agent (Cayouette et al., 1999; Cao et al., 1999; Imai et al., 2004). Our data further demonstrate that the Rportive effect remains virtually unaltered by the presence of Müller cell specific inhibitor, α-AAA, in the culture media. These results suggest that the organizational effect of PEDF on outer segment membranes is direct upon photo-receptors themselves and is not mediated by Müller cells. This finding is consistent with previous studies conducted by Aymerich et al. who demonstrated that the distribution of PEDF binding sites were most abundant in the inner segments of photoreceptor cells (Aymerich et al., 2001). They suggested that the PEDF binding sites likely represented cell-surface receptors that were available to interact with the extracellular ligand (Aymerich et al., 2001). Our results agree with the protective and morphogenetic effect of PEDF on photoreceptors and indicate that PEDF likely acts directly on photoreceptor cells, possibly through the aforementioned cell-specific putative receptor, and not through a Müller cell-mediated mechanism.
Previously we demonstrated that in addition to its neuro-Rportive properties, PEDF also had a powerful morpho-genetic effect upon Müller cells and that it was able to prevent many of the dysmorphic and biosynthetic alterations in the glial cell population that were induced by removal of the RPE (Jablonski et al., 2001b). These data lead us to hypothesize that the glioprotective effects of PEDF may further Rport the proper folding of photoreceptor outer segments (Jablonski et al., 2001b), given the positive influence that Müller cells have upon outer segment assembly (Jablonski and Iannaccone, 2000). To our surprise, PEDF was able to exert its neuro-Rportive effects in the absence of a Müller cell contribution. This data could be interpreted in several ways including that PEDF does interact with Müller cells yet that this interaction may not be essential toward photoreceptor outer segment organization. Another interpretation may be that in the presence of PEDF photoreceptors also provide Rport for Müller cells in the RPE-deprived state via bidirectional communication as proposed by Araque and Perea (Araque and Perea, 2004) and therefore in our previous study, PEDF may have Rported Müller cells indirectly through its interactions with photoreceptors. The precise mechanism underlying our previous findings, however, is yet to be revealed.
Our present results also confirm that IPTG Rports photo-receptor outer segment assembly. However, in contrast to our results with PEDF, the addition of the Müller cell inhibitor, α-AAA, markedly prevented the ability of the permissive glycan to promote proper folding of nascent outer segment membranes. These findings are strongly suggestive of the possibility that the organizational stimulus of permissive glycans is not received directly by photoreceptors, but rather indirectly through Müller cells. Previously, we demonstrated that targeted disruption of Müller cell metabolism induces photoreceptor dysmorphogenesis, which provided direct evidence that Müller cells influence significantly the assembly of photoreceptor outer segment membranes (Jablonski and Iannaccone, 2000), and that the organizational effect of permissive glycans upon photoreceptor outer segments displays the characteristics of a receptor-mediated phenomenon (Wang et al., 2003).
Together with these earlier findings, the results of the present study strongly suggest that the putative receptor for the permissive glycans localizes in all likelihood to Müller cells. Moreover, while a significant body of evidence indicates that factors Rplied by the RPE are critical for the development and survival of photoreceptors, data in the scientific literature demonstrated that photoreceptors themselves do not always directly respond to the stimulus. For example, when retinas are presented in vivo with a stimulus of brain-derived neurotrophic factor, ciliary neurotrophic factor, fibroblast growth factor or α2-adrenergic agonists, it is the Müller cells that respond by upregulating c-fos, c-jun and/or the mitogen-activated protein (MAP) kinase pathways, even though these neuroprotective agents Rport photoreceptor survival (Peng et al., 1998; Wahlin et al., 2000; Cao et al., 1998). Although the precise mechanisms governing these phenomena have not yet been fully elucidated, these observations indicate that Müller cells are able to Rport photoreceptor health and integrity, and are likely to play an important role in this endeavor under both physiologic and pathologic conditions.
In conclusion, our findings demonstrate that the regulation of photoreceptor outer segment assembly can be modulated by various external stimuli. Moreover, we provide additional evidence that Müller cells can play an important role in promoting the proper folding of nascent outer segment membranes. The critical factor that determines whether photo-receptors or other retinal cells such as the Müller cell are directly or indirectly involved is very likely the cellular location of the receptor for the stimulus. Given this, it appears that there are redundant mechanisms, both intrinsic and extrinsic, that collectively Rport photoreceptors and promote the ability to assemble outer segment membranes into the thermodynamically unfavorable conformation that is required to stabilize the structure and maintain viability of the cell. In the case of PEDF the receptor is likely localized to photoreceptors, while for permissive glycans the receptor is very likely to be found on Müller cells that in turn stimulate photoreceptors via pathways that are yet to be revealed. In future studies, it will be also of interest to determine if these seemingly independent mechanisms could be simultaneously stimulated to result in a synergistic additional beneficial effect towards outer segment membrane assembly.
ACKNOWLEDGEMENTS
supported by NEI grant EY10853 (M.M.J.), Fight for Sight Grant-in Aid #GA02046 (M.M.J.), a Knights Templar Eye Foundation grant (X.F.W.), NEI core grant EY013080 to the University of Tennessee Health Science Center at Memphis, and an unrestricted grant to the Department of Ophthalmology from Research to Prevent Blindness, Inc., New York, NY. M.M.J. is the recipient of a Research to Prevent Blindness William and Mary Greve Special Scholar Award. A.I. is the recipient of a Research to Prevent Blindness Career Development Award. The authors gratefully acknowledge the technical assistance of Sharon Frase of The Integrated Microscopy Center at the University of Memphis. The authors also are grateful to Patricia Becerra at the National Eye Institute for stimulating discussions during the planning phases of this study, Joyce Tombran-Tink, Ph.D. at the University of Missouri, Kansas City for providing purified PEDF and Elissa Chesler, Ph.D., at the University of Tennessee Health Science Center for statistical assistance.
REFERENCES
- Anderson DH, Guérin CJ, Erickson PA, Stern WH, Fisher SK. Morphological recovery in the reattached retina. Investigative Ophthalmology and Visual Science. 1986;27:168–183. [PubMed] [Google Scholar]
- Araque A, Perea G. Glial Modulation of Synaptic Transmission in Culture. Glia. 2004;47:241–248. doi: 10.1002/glia.20026. [DOI] [PubMed] [Google Scholar]
- Aymerich MS, Alberdi EM, Martinez A, Becerra SP. Evidence for Pigment Epithelium-Derived Factor Receptors in the Neural Retina. Investigative Ophthalmology and Visual Science. 2001;42:3287–3293. [PubMed] [Google Scholar]
- Cao W, Li F, Steinberg RH, La Vail MM. Induction of c-fos and c-jun mRNA expression by basic fibroblast growth factor in cultured rat Müller cells. Investigative Ophthalmology and Visual Science. 1998;39:565–573. [PubMed] [Google Scholar]
- Cao W, Tombran-Tink J, Chen W, Mrazek D, McGinnis JF. Pigment epithelium-derived factor protects cultured retinal neurons against hydrogen peroxide-induced cell death. Journal of Neuroscience Research. 1999;57:789–800. [PubMed] [Google Scholar]
- Cao W, Wen R, Li F, Cheng T, Steinberg RH. Induction of basic fibroblast growth factor mRNA by basic fibroblast growth factor in Muller cells. Investigative Ophthalmology and Visual Science. 1997;38:1358–1366. [PubMed] [Google Scholar]
- Cayouette M, Smith SB, Becerra SP, Gravel C. Pigment epithelium-derived factor delays the death of photoreceptors in mouse models of inherited retinal degenerations. Neurobiology of Disease. 1999;6:523–532. doi: 10.1006/nbdi.1999.0263. [DOI] [PubMed] [Google Scholar]
- Erickson PA, Fisher SK, Anderson DH, Stern WH, Borgula GA. Retinal detachment in the cat:the outer nuclear and outer plexiform layers. Investigative Ophthalmology and Visual Science. 1983;24:927–942. [PubMed] [Google Scholar]
- Guérin CJ, Anderson DH, Fariss RN, Fisher SK. Retinal reattachment of the primate macula. Investigative Ophthalmology and Visual Science. 1989;30:1708–1725. [PubMed] [Google Scholar]
- Guérin CJ, Lewis GP, Fisher SK, Anderson DH. Recovery of photoreceptor outer segment length and analysis of membrane assembly rates in regenerating primate photoreceptor outer segments. Investigative Ophthalmology and Visual Science. 1993;34:175–183. [PubMed] [Google Scholar]
- Hollyfield JG, Witkovsky P. Pigmented retinal epithelium involvement in photoreceptor development and function. Journal of Experimental Zoology. 1974;189:357–378. doi: 10.1002/jez.1401890309. [DOI] [PubMed] [Google Scholar]
- Imai D, Yoneya S, Gehlbach PL, Wei LL, Mori K. Intraocular gene transfer of pigment epithelium-derived factor rescues photoreceptors from light-induced cell death. Journal of Cellular Physiology. 2004;202:570–578. doi: 10.1002/jcp.20155. [DOI] [PubMed] [Google Scholar]
- Jablonski MM, Ervin CS. A closer look at lactose-mediated Rport of retinal morphogenesis. Anatomical Record. 2000;259:205–214. doi: 10.1002/(SICI)1097-0185(20000601)259:2<205::AID-AR10>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Jablonski MM, Iannaccone A. Targeted disruption of Müller cell metabolism induces photoreceptor dysmorphogenesis. Glia. 2000;32:192–204. doi: 10.1002/1098-1136(200011)32:2<192::aid-glia80>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Jablonski MM, Tombran-Tink J, Mrazek DA, Iannaccone A. Pigment epithelium-derived factor Rports normal development of photoreceptor neurons and opsin expression after retinal pigment epithelium removal. Journal of Neuroscience. 2000;20:7149–7157. doi: 10.1523/JNEUROSCI.20-19-07149.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski MM, Graney MJ, Kritchevsky SB, Iannaccone A. Reliability assessment of a rod photoreceptor outer segment grading system. Experimental Eye Research. 2001a;72:573–579. doi: 10.1006/exer.2001.0987. [DOI] [PubMed] [Google Scholar]
- Jablonski MM, Tombran-Tink J, Mrazek DA, Iannaccone A. Pigment epithelium-derived factor Rports normal Müller cell development and glutamine synthetase expression after removal of the RPE. Glia. 2001b;35:14–25. doi: 10.1002/glia.1066. [DOI] [PubMed] [Google Scholar]
- Jablonski MM, Wohabrebbi A, Ervin CS. Molecular Vision 5. 1999. Lactose promotes organized photoreceptor outer segment assembly and preserves expression of photoreceptor proteins in retinal degeneration; p. 16. [PubMed] [Google Scholar]
- Lewis GP, Erickson PA, Anderson DH, Fisher SK. Opsin distribution and protein incorporation in photoreceptors after experimental retinal detachment. Experimental Eye Research. 1991;53:629–640. doi: 10.1016/0014-4835(91)90223-2. [DOI] [PubMed] [Google Scholar]
- Lin SC, Bergles DE. Synaptic Signaling Between Neurons and Glia. Glia. 2004;47:290–298. doi: 10.1002/glia.20060. [DOI] [PubMed] [Google Scholar]
- Mori K, Gehlbach P, Yamamoto S, Duh E, Zack DJ, Li Q, Berns KI, Raisler BJ, Hauswirth WW, Campochiaro PA. AAV-mediated gene transfer of pigment epithelium-derived factor inhibits choroidal neovascularization. Investigative Ophthalmology and Visual Science. 2002;43:1994–2000. [PubMed] [Google Scholar]
- Newman E. Glial Modulation of Synaptic Transmission in the Retina. Glia. 2004;47:268–274. doi: 10.1002/glia.20030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman E, Reichenbach A. The Müller cell: A functional element of the retina. Trends in Neuroscience. 1996;19:307–312. doi: 10.1016/0166-2236(96)10040-0. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal Table of Xenopus laevis (Daudin) North Holland Publishing Co.; Amsterdam: 1956. [Google Scholar]
- Peng M, Li Y, Liu C, Laties AM, Wen R. a2-adrenergic agonists selectively activate extracellular signal-regulated kinases in Müller cells in vivo. Investigative Ophthalmology and Visual Science. 1998;39:1721–1726. [PubMed] [Google Scholar]
- Reichenbach A, Faude F, Enzmann V, Bringmann A, Pannicke T, Francke M, Biedermann B, Kuhrt H, Stolzenburg JU, Skatchkov SN, Heinemann U, Wiedemann P, Reichelt W. The Müller (glial) cell in normal and diseased retina: a case for single-cell electrophysiology. Ophthalmic Research. 1997;29:326–340. doi: 10.1159/000268031. [DOI] [PubMed] [Google Scholar]
- Reichenbach A, Stolzenburg J-U, Eberhardt W, Chao TI, Dettmer D, Hertz L. What do retinal Müller (glial) cells do for their neuronal ‘small siblings’? Journal of Chemical Neuroanatomy. 1993;6:201–213. doi: 10.1016/0891-0618(93)90042-3. [DOI] [PubMed] [Google Scholar]
- Robinson SR, Dreher Z. Müller cells in adult rabbit retinae: Morphology, distribution and implications for function and development. Journal of Comparative Neurology. 1990;292:178–192. doi: 10.1002/cne.902920203. [DOI] [PubMed] [Google Scholar]
- Stiemke MM, Hollyfield JG. Outer segment disc membrane assembly in the absence of the pigment epithelium: The effect of exogenous sugars. Developmental Brain Research. 1994;80:285–289. doi: 10.1016/0165-3806(94)90115-5. [DOI] [PubMed] [Google Scholar]
- Stiemke MM, Hollyfield JG. In: In Degenerative Diseases of the Retina. Anderson RE, Hollyfield JG, LaVail MM, editors. Plenum Publishing Corp.; New York: 1995. pp. 129–137. [Google Scholar]
- Stiemke MM, Landers RA, Al-Ubaidi MR, Hollyfield JG. Photoreceptor outer segment development in Xenopus laevis: Influence of the pigment epithelium. Developmental Biology. 1994;162:169–180. doi: 10.1006/dbio.1994.1076. [DOI] [PubMed] [Google Scholar]
- Tepass U. Adherens junctions: new insight into assembly, modulation and function. Bioessays. 2002;24:690–695. doi: 10.1002/bies.10129. [DOI] [PubMed] [Google Scholar]
- Turner DL, Cepko CL. A common progenitor for neurons and glia persists in rat retina late in development. Nature. 1987;328:131–136. doi: 10.1038/328131a0. [DOI] [PubMed] [Google Scholar]
- Ullian EM, Christopherson KS, Barres BA. Role for Glia in Synaptogenesis. Glia. 2004;47:209–216. doi: 10.1002/glia.20082. [DOI] [PubMed] [Google Scholar]
- Ullian EM, Sapperstein SK, Christopherson KS, Barres BA. Control of synapse number by glia. Science. 2001;291:657–661. doi: 10.1126/science.291.5504.657. [DOI] [PubMed] [Google Scholar]
- Wahlin KJ, Campochiaro PA, Zack DJ, Adler R. Neurotrophic factors cause activation of intracellular signaling pathways in Muller cells and other cells of the inner retina, but not photoreceptors. Investigative Ophthalmology and Visual Science. 2000;41:927–936. [PubMed] [Google Scholar]
- Wang XF, Iannaccone A, Jablonski MM. Permissive glycan Rport of photoreceptor outer segment assembly occurs via a non-metabolic mechanism. Molecular Vision. 2003;9:701–709. [PubMed] [Google Scholar]
- Young RW. The renewal of photoreceptor cell outer segments. Journal of Cell Biology. 1967;33:61–72. doi: 10.1083/jcb.33.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]


