Abstract
Nhlh1 is a basic helix-loop-helix transcription factor whose expression is restricted to the nervous system and which may play a role in neuronal differentiation. To directly study Nhlh1 function, we generated null mice. Homozygous mutant mice were predisposed to premature, adult-onset, unexpected death. Electrocardiograms revealed decreased total heart rate variability, stress-induced arrhythmia, and impaired baroreceptor sensitivity. This predisposition to arrhythmia is a likely cause of the observed death in the mutant mice. Heterozygosity for the closely related transcription factor Nhlh2 increased the severity of the Nhlh1-null phenotype. No signs of primary cardiac structural or conduction abnormalities could be detected upon necropsy of the null mice. The pattern of altered heart rhythm observed in basal and experimental conditions (stress and pharmacologically induced) suggests that a deficient parasympathetic tone may contribute to the arrhythmia in the Nhlh1-null mouse. The expression of Nhlh1 in the developing brain stem and in the vagal nuclei in the wild-type mouse further supports this hypothesis. The Nhlh1 mutant mouse may thus provide a model to investigate the contribution of the autonomic nervous system to arrhythmogenesis.
The basic helix-loop-helix (bHLH) family of transcription factors is involved in the regulation of multiple developmental processes, such as hematopoiesis (36, 40), neurogenesis (27), cardiac muscle development (39), mesodermal cell determination (8), dermogenesis (8, 28), and myogenesis (2, 34). bHLH proteins function as either homo- or heterodimers and bind to a consensus DNA sequence. The basic region mediates DNA binding while the HLH domain is involved in protein-protein interaction (45). Distinct subgroups that share particular amino acid homology within the bHLH domain can be defined within the family. Members of such subgroups, like Nhlh1 and Nhlh2, generally share overlapping patterns of expression and possess similar functional properties. Nhlh1 and Nhlh2 protein products exhibit very high homology, with 98% amino acid identity in the bHLH region and 66% identity over the entire protein sequence (15, 29).
Nhlh1 was originally discovered because of its homology within the bHLH motif to the hematopoietic transcription factor and oncogene SCL (5). Nhlh1 expression is confined to the nervous system, is observed first at e9.0, peaks at e12.5 to e13.5, and continues throughout embryonic development. Nhlh1 can be detected in the subependymal layer of the neuroepithelium throughout the central nervous system (CNS), in the dorsal root ganglia, and in the cranial ganglia. Expression can also be observed in the sensory nasal epithelium and developing optic cup (5). Postnatally, Nhlh1 is expressed in the developing cerebellum (13, 42) and other distinct brain areas in the adult (reference 16 and data not shown). Nhlh1 and Nhlh2 have a partially overlapping expression pattern in the CNS and peripheral nervous system during development (15, 29). The expression of Nhlh1 and Nhlh2 in postmitotic neurons suggests a role in neuronal differentiation. A previous study described how the loss of Nhlh2 function results in disruption of the hypothalamic-pituitary axis controlling fertility and body weight (16), implicating this gene in the development of specific populations of hypothalamic neurons and anterior pituitary cells.
To explore the function of Nhlh1, mice carrying a deletion of the Nhlh1 coding region were generated. Analysis of the phenotype of Nhlh1−/− mice suggests the presence of an autonomic nervous system dysfunction affecting the regulation of heart rhythm.
MATERIALS AND METHODS
Generation of Nhlh1 mutant mice.
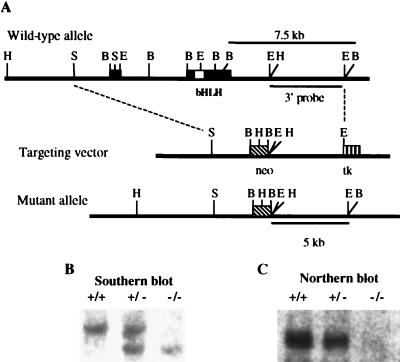
Nhlh1 genomic clones were isolated by screening a 129Sv/J mouse genomic library (Stratagene) with the full-length Nhlh1 cDNA (5). To construct the Nhlh1 targeting vector, a pBluescript plasmid (Stratagene) containing the Nhlh1 3.0-kb 5′ SacI-BamHI fragment was linearized at the EcoRI site in the multiple cloning site and ligated to a 5.5-kb EcoRI fragment from the 3′ region of the Nhlh1 gene (Fig. 1A). This 5.5-kb EcoRI fragment does not contain any Nhlh1 exons and is 3′ of the transcription termination site. The new plasmid, containing both 5′ and 3′ regions of the Nhlh1 gene, was linearized with SalI and ligated to a SalI fragment from pBSII-TK, which contains the herpes simplex virus thymidine kinase gene driven by the mutant polyoma virus enhancer from pIC19R/MC1-TK (31). A BamHI fragment from the pPNT vector (41) containing the neomycin resistance gene (PGK-neo) was then ligated into a unique BamHI site between the two genomic fragments of Nhlh1. Prior to electroporation, the targeting vector was linearized with PvuI, which also released a 1-kb fragment from the vector backbone (pBluescript). All embryonic stem (ES) cell clones tested negative for insertion of this vector fragment (data not shown). Cell culture, electroporation of ES cells (a gift of H. Westphal), and generation of Nhlh1−/− mice from ES cells were performed as described previously (16). Recombination generated a BamHI genomic mutant fragment of approximately 5.0 kb, which was smaller than the 7.5-kb wild-type fragment detected by Southern blot analysis (Fig. 1B). Animals were housed and all experiments were conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Experimental Animals.
FIG. 1.
Production of Nhlh1-null mice. (A) The Nhlh1 wild-type allele, the targeting vector, the region of Nhlh1 for targeted gene disruption (dashed lines), and the expected Nhlh1 mutant allele from appropriate homologous recombination events between the two are shown. H, HindIII; S, SacI; B, BamHI; E, EcoRI; solid boxes including open box (bHLH), Nhlh1 coding sequence; diagonally striped box, neo; vertically striped box, tk. (B) Southern blot analysis of tail DNA (5 μg of DNA per lane). The 7.5-kb germ line and 5.0-kb recombinant BamHI restriction fragments were detected by using the internal probe shown in panel A. (C) Northern blot analysis of newborn mouse head RNA (10 μg of total RNA per lane). The Nhlh1 full-length cDNA probe was used.
Telemetry.
Subcutaneous radio frequency transmitters (TA 10ETA-F20; Data Sciences) were implanted as described previously (32) in nine wild-type, two Nhlh1−/−, and eight Nhlh1−/− Nhlh2+/− (KH) mice. Four to 10 days after implantation, electrocardiograms (ECGs) were recorded for the unrestrained animals while at rest for 30 min. While still on monitor, the mice were placed in a pool of 30°C water in which they freely swam for 5 min. The mice were removed from the water and recorded for an additional 30 min during the recovery period after this swimming stress.
Baroreceptor sensitivity assay.
Baroreceptor sensitivity was tested for five wild-type and five KH animals. After anesthesia with intraperitoneal pentobarbital (25 mg/kg of body weight), ketamine (58 mg/kg), and xylazine (7 mg/kg), bilateral inguinal dissections were performed, allowing cannulation of the femoral artery and contralateral femoral vein. Arterial blood pressure was monitored with a modified Millar manometer, and the surface ECG was recorded as described below during bolus infusion of escalating doses of phenylephrine (10 to 40 μg/kg) alternating with increasing doses of nitroprusside (10 to 40 μg/kg). Core temperature was maintained at 37°C. Fourteen points per animal, representing the difference in blood pressure and RR interval, were selected randomly during rising or falling blood pressure and plotted. A linear regression was fit to the steepest part of the resulting sigmoidal curve.
Resting ECG.
The surface ECG was measured after anesthesia with inhaled methoxyfurane. Twenty-eight wild-type, 23 Nhlh1−/−, and 27 KH mice between 10 and 12 months of age underwent measurement of digitized ECG lead I for 3 min with subcutaneous needle electrodes (Biopac; 2,000-Hz sampling frequency). Signals were high-pass filtered (1 Hz), which may have prolonged the QT intervals slightly without affecting the overall comparison among different groups of animals. Data analysis was performed off-line on a workstation (Sun Microsystems, Inc.) with a customized, automated algorithm as described previously (3). The QT variability index (QTVI) was calculated from the ratio of normalized QT variability (QTV) to heart rate variability (HRV) power by using the following formula: QTVI = log [(QTV total power/mean QT2)/(HRV total power/mean HR2)], where HR is the heart rate. This analysis generates frequency spectra for heart rate and QT duration, allowing assessment of total heart rate power as well as low (0 to 1 Hz)- and high (1 to 5 Hz)-frequency components of heart rate variability, an indirect measure of the relative contributions of the sympathetic and parasympathetic nervous systems to fluctuations in the heart rate.
β-AR density measurement.
β-Adrenergic receptor (β-AR) binding on myocardial membranes was performed as previously described (20) by using the nonselective β-AR ligand 125I-cyanopindolol. Nonspecific binding was determined in the presence of 10 μM alprenolol. Reactions were conducted in 500 μl of binding buffer (50 mM HEPES [pH 7.3], 5 mM MgCl2, 0.1 mM ascorbic acid) at 37°C for 1 h and then terminated by vacuum filtration through glass-fiber filters. All assays were performed in triplicate, and receptor density (in femtomoles) was normalized to milligrams of membrane protein.
In situ hybridization.
Nhlh1 in situ hybridization on 16-μm frozen embryo sections was performed by using standard methods described on the following website: http://intramural.nimh.nih.gov/lcmr/snge/Protocols/ISHH/ISHH.html. Mouse 35S-UTP-labeled riboprobes were transcribed from cDNA fragments (Nhlh1 nucleotides 2067 to 2398) generated by PCR amplifications with primers containing either T3 (sense) or T7 (antisense) promoter sequences upstream of Nhlh1-specific nucleotide sequences: forward primer, 5′-CGCGCAATTAACCCTCACTAAAGGTGCTTTAGCTGAGGTCCTCTTGC-3′; reverse primer, 5′-GCGCGTAATACGACTCACTATAGGGTGCAACCAGACAAAGACACACAG-3′. Before hybridization, the frozen sections were fixed in 4% phosphate-buffered-saline-buffered formaldehyde for 10 min. After two rinses for 5 min in 1× phosphate-buffered saline, the sections were acetylated for 10 min with 0.25% acetic anhydride in 0.1 M triethanolamine-HCl (pH 8.0), rinsed in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), dehydrated in ethanol, and air dried. Sections were hybridized overnight at 55°C with 106 cpm of 35S-UTP-labeled antisense probes in 80 μl of hybridization buffer (50% formamide, 250 mM Tris-HCl [pH 7.4], 1 mM EDTA [pH 8], 300 mM NaCl, 10% dextran sulfate, 1× Denhardt's solution, 25 mg of yeast tRNA/ml, 100 μg of salmon sperm DNA/ml, 250 μg of total yeast RNA/ml, 100 mM dithiothreitol, 0.1% sodium thiosulfate, 0.1% sodium dodecyl sulfate). After hybridization, the nonspecifically bound probes were washed out as described previously (7). Sections were dehydrated in ethanol, air dried, and exposed to autoradiographic films (Kodak Biomax MR) for 3 days. Slides were then coated with autoradiographic emulsion (Kodak NTB3). After 2 weeks of incubation in dark, dry boxes at 4°C, the silver grains were developed with Kodak Dektol at 18°C and counterstained with 0.5% Giemsa solution. The sections were then air dried and mounted with Cytoseal 60 mounting medium (Stephens Scientific).
Statistical analysis.
All results are presented as means ± standard errors of the mean. Statistical analyses were performed with GraphPad Prism (GraphPad Software). The survival fraction and multiple-sample comparison tests were calculated by using Statistica (StatSoft). P values of <0.05 were considered significant.
RESULTS
Targeted disruption of the Nhlh1 gene.
The Nhlh1 targeting construct was designed to replace the entire coding sequence of Nhlh1 contained in exons I and II (Fig. 1A). No Nhlh1 RNA expression was detected in Nhlh1−/− embryonic mouse heads, confirming targeted deletion of the gene (Fig. 1C). Nhlh1−/− mice were also crossed to Nhlh2−/− mice (16) to produce double-null mice (Nhlh1−/− Nhlh2−/−) and various other intermediate allelic combinations. In this paper, we focus primarily on the Nhlh1−/− and Nhlh1−/− Nhlh2+/− (Nhlh1-null Nhlh2 heterozygote [KH]) mice.
Mice lacking Nhlh1 are viable and develop normally to maturity but have a reduced life expectancy.
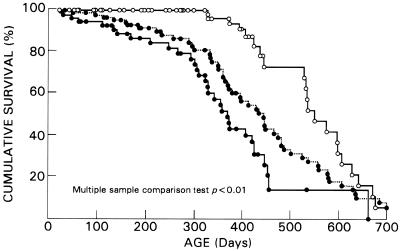
Mice heterozygous for the Nhlh1 deletion (Nhlh1+/−) appeared normal. Homozygous mutant offspring (Nhlh1−/−) were born at the expected Mendelian frequency. These offspring were viable to adulthood, fertile, and initially indistinguishable from wild-type littermates. Despite the lack of an overt anatomical or physiological phenotype, earlier death was observed in the Nhlh1−/− population (Fig. 2). By 1 year of age, about 25% of the Nhlh1−/− mice died, while there was 100% survival in the wild-type group (P < 0.01 by the log rank test). Survival curves showed a slightly higher incidence of deaths in the KH group (45% by 1 year of age; P < 0.0001 versus the wild-type group by the log rank test), suggesting a compounding effect of Nhlh2 heterozygosity on the reduction of the life expectancy in the KH group. The double-null Nhlh1−/− Nhlh2−/− mouse died perinatally in apparent cardiorespiratory distress (data not shown). Nhlh1−/− and KH deaths were unexpected. They were not preceded by any noticeable health problems, such as acute weight loss, dehydration, or obvious discomfort. Blood and urine analyses were normal. Necropsy and histopathology revealed no evidence of cerebrovascular accident or gross abnormalities in the CNS, skeletal muscles, or visceral organs. Similarly, no difference in heart weight (wild type, 192 ± 32.4 mg, n = 6; Nhlh1−/−, 194 ± 50.1 mg, n = 9), no evidence of myocardial infarction, no chamber enlargement, and no altered cardiac morphology were found. Frequent and prolonged monitoring of the mice in the colony did not reveal the occurrence of spontaneous epileptic seizures. Therefore, we hypothesized that cardiac arrhythmia might explain the deaths with no apparent antemortem or postmortem signs.
FIG. 2.
Percent cumulative survival analysis of Nhlh1 mutant mice based on a population of 274 animals. Open and closed circles represent wild-type (n = 123) and mutant animals, respectively. For the mutants, the dotted line represents Nhlh1−/− mice (n = 89) and the solid line represents Nhlh1−/− Nhlh2+/− (KH) mice (n = 62).
Nhlh1−/− mice show unstable cardiac repolarization and decreased heart rate variability.
We first recorded ECGs for methoxyfurane-anesthetized Nhlh1−/−, KH, and wild-type control mice. No difference in resting heart rates among the groups was observed (Table 1). In humans and other large mammals, arrhythmia in structurally normal hearts that results in sudden cardiac death has been well documented in conditions associated with prolongation of cardiac repolarization (long QT syndrome) (44), as indexed by the QT interval on the surface ECG. Therefore, we studied cardiac repolarization in Nhlh1 mutant mice. No difference in mean QT interval or heart rate-corrected QT interval [QTc = QT/√(RR/100)] was found between mutant and wild-type mice (Table 1). Thus, we excluded the possibility of the mutant mice having long QT syndrome.
TABLE 1.
ECG parameters in anesthetized micea
| Genotype | HR (bpm) | QT (ms) | QTc (ms) | QTVIb | HRP (ms2)c | LF/HF HRVc |
|---|---|---|---|---|---|---|
| Wild type (n = 28) | 456 ± 8.3 | 74 ± 1.3 | 64.2 ± 1.13 | −0.39 ± 0.176 | 31.7 ± 6.11 | 1.16 ± 0.166 |
| Nhlh1−/− (n = 23) | 447 ± 8.4 | 75 ± 1.0 | 64.6 ± 0.87 | 0.17 ± 0.142d | 14.2 ± 1.96d | 2.23 ± 0.653 |
| KH (n = 27) | 489 ± 9.2 | 76 ± 1.7 | 68.2 ± 1.55 | 0.42 ± 0.148e | 17.2 ± 3.26d | 3.77 ± 0.833d |
Figures are means ± standard errors of the mean. HR, heart rate; QT, QT interval; QTc, adjusted QT interval; HRP, heart rate power; LF/HR HRV, low-frequency-to-high-frequency heart rate variability ratio.
Statistical significance, by analysis of variance: P < 0.001.
Statistical significance, by analysis of variance: P < 0.05.
P < 0.05 compared to the wild type, by Dunnett's multiple-comparison test.
P < 0.01 compared to the wild type, by Dunnett's multiple-comparison test.
Predisposition to arrhythmic events can be predicted also by measurements of beat-to-beat QT interval fluctuations (temporal QT interval variability) expressed in the QTVI (see Materials and Methods). The QTVI is a unitless negative number for the normal heart and increases in the presence of significant repolarization abnormalities (increased repolarization lability) (3). The QTVI was a positive number and significantly higher for Nhlh1−/− mice than for wild-type mice (Table 1), consistent with augmented lability despite a normal average duration of repolarization. In the KH group the QTVI was further increased compared with that of the Nhlh1−/− mice, indicating a more severe phenotype in the former.
Heart rate variability represents beat-to-beat fluctuations in heart rate that are generated by the opposing effects of sympathetic and parasympathetic activity (35). Total heart rate power, an index of heart rate variability, was significantly reduced in Nhlh1−/− mice compared to that of wild-type mice (Table 1). Since certain frequency components of heart rate variability reflect primarily parasympathetic activity (high frequency, 1.0 to 5.0 Hz) while others reflect primarily sympathetic activity (low frequency, 0 to 1.0 Hz), heart rate variability analysis can reveal abnormalities in heart rate regulation which are determined by the interaction between sympathetic and parasympathetic influences (21). The ratio of low-frequency to high-frequency heart rate variability tended to increase in the Nhlh1−/− group and reached statistical significance in the KH mice compared with that of the wild-type mice (Table 1). Heart rate variability analysis therefore suggested the hypothesis of reduced parasympathetic activation in the mutants (30, 46).
Nhlh1−/− mice show stress-induced arrhythmia.
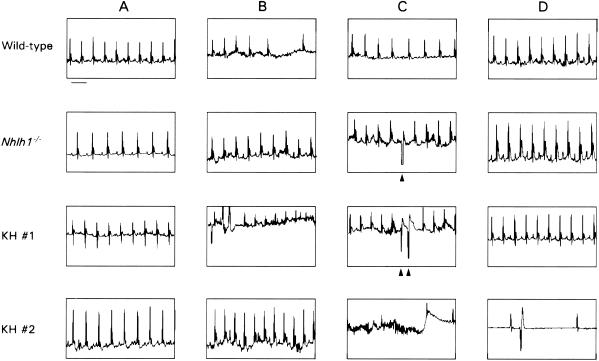
To test whether spontaneous arrhythmias could be observed in waking animals or could be provoked by exposure to stress, we recorded ECGs by telemetry in conscious mice at rest and during and after exposure to 5 min of exercise (swimming) stress (Fig. 3). ECG recordings of Nhlh1−/− and KH mice revealed the presence of premature ventricular complexes, couplets, and triplets during swimming (Fig. 3C). The only ectopic beats seen in wild-type mice were isolated ventricular escape beats during “diving”-induced atrioventricular block (data not shown). Notably, one KH mouse (KH no. 2) developed a rapid rhythm (800 to 850 beats per min [bpm]) following immersion without consistent atrial activity (Fig. 3B). The animal abruptly ceased swimming and, after a burst of chaotic electrical activity (Fig. 3C), developed an agonal bradycardia (Fig. 3D) despite removal from the water. Due to the unstable electrical baseline, the precise antemortem rhythm may not be described with absolute certainty. However, it appears to be most consistent with ventricular fibrillation, as suggested for mice under other circumstances (43).
FIG. 3.
Representative ECGs from wild-type, Nhlh1−/−, and KH mice at rest (A), during the first 5 s after diving (B), after 1 min in water (C), and during the recovery period after stress (D). The scale bar underneath the wild-type ECG in column A represents 100 ms. The arrowheads in column C indicate premature ventricular complexes and couplets during swimming.
Absence of diving-induced bradycardia and reduction in baroreceptor sensitivity in Nhlh1 mutant mice.
Wild-type and mutant animals could be distinguished by their initial responses to water immersion (Fig. 3B). In 7 of 9 wild-type animals, an immediate bradycardia occurred (at least one pause of >0.20 s; mean for wild-type mice, 0.30 ± 0.04 s) that was not seen in any of the mutant animals (0 of 2 Nhlh1−/− and 0 of 8 KH; P < 0.01 between KH and wild-type mice by chi-square Mann-Whitney U test). For the first 30 beats after immersion, the heart rate was higher in the Nhlh1−/− (644 ± 40 bpm, n = 2) and KH (717 ± 19 bpm, n = 8) mice than in wild-type animals (569 ± 34 bpm, n = 9; unpaired t test between KH and wild-type mice, P < 0.01). Bradycardia that results from an increased parasympathetic stimulus to the sinoatrial and atrioventricular nodes is a well-established response to sudden water immersion in humans and other mammals (9, 17). Lack of a vagal response to water immersion of Nhlh1 mutant mice suggested the presence of a depressed parasympathetic response, consistent with the heart rate variability analysis.
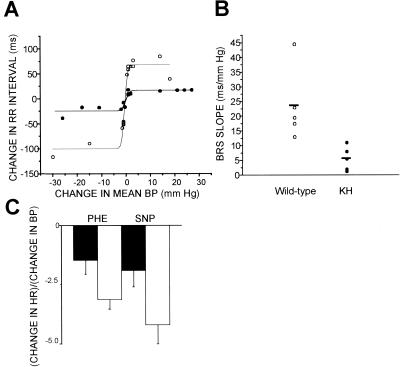
To test this hypothesis, we measured baroreceptor-mediated changes in heart rate in anesthetized wild-type and KH mice in response to phenylephrine (increased blood pressure) and nitroprusside (decreased blood pressure). We focused on the KH group due to the exacerbated phenotype observed in the diving reflex. Despite a similar resting systolic blood pressure (wild type, 63 ± 5 mm of Hg, n = 5; KH, 79 ± 13 mm of Hg, n = 5) and pulse (wild type, 315 ± 11 bpm; KH, 316 ± 12 bpm) in both wild-type and KH mice, only KH mice demonstrated a marked reduction in baroreceptor sensitivity to changes in mean blood pressure (Fig. 4A). The slope of the linear segments of the sigmoid relationship between changes in blood pressure and heart rate interval was significantly reduced in the KH group compared to that of the wild-type group (Fig. 4B). The ratio of the change in heart rate to the maximum mean blood pressure change in response to 20 μg of phenylephrine/kg was also reduced (Fig. 4C). This indicates the presence of reduced baroreceptor sensitivity and impairment of inducible vagal tone in the KH group (4).
FIG. 4.
Baroreceptor sensitivity assay. (A) Sigmoidal relationship between changes in mean blood pressure (BP) and beat-to-beat interval (RR) in two representative littermates. Changes in mean blood pressure were plotted against changes in the RR interval occurring spontaneously or with drug infusion. (B) Comparison of regression coefficients calculated for each animal from the sigmoidal curve (P < 0.02 by Fisher's R to z test). Open and closed circles in panels A and B represent wild-type and KH animals, respectively. (C) Ratio of changes in heart rate to the maximum change in blood pressure in response to 20 μg of either phenylephrine (PHE) or nitroprusside (SNP)/kg in KH (black bars) and wild-type (white bars) mice. Significance, as determined by unpaired Student's t test, was as follows: P = 0.05 during infusion of phenylephrine and P = 0.06 during infusion of nitroprusside.
Heart rate variability, diving reflex, and baroreceptor sensitivity seemed to indicate a functional parasympathetic deficit in Nhlh1−/− and KH mice. However, we could not exclude the possibility of an imbalance due to excessive sympathetic activity. Chronic sympathetic overactivity has been associated with the reduction of cardiac β-AR density (20). When we measured the total β-AR density in myocardial membranes, no changes were observed in the Nhlh1−/− group (19.2 ± 5.42 fmol/mg of protein, n = 16) or the KH group (15.2 ± 4.2 fmol/mg of protein, n = 16) compared to the wild-type group (18.2 ± 5.02 fmol/mg of protein, n = 22). These results exclude a direct involvement of the sympathetic nervous system.
Nhlh1 expression is restricted to the developing nervous system and detected in the brain stem.
Evidence of stress-induced arrhythmia raised the question of possible pathological effects of the Nhlh1 mutation on the heart muscle. No expression of Nhlh1 or Nhlh2 in the heart, the conduction system, or their primordia could be detected by in situ hybridization at any stage of embryonic development or in the adult. Moreover, no signs of cardiac abnormalities (cardiac morphology, infarction, or chamber enlargement) were detected at necropsy.
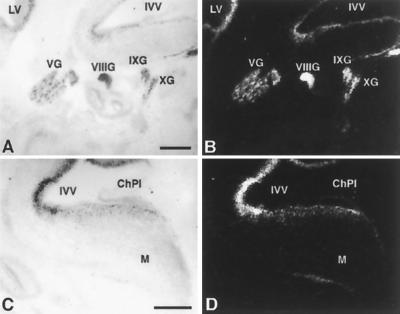
Expression of Nhlh1 can be detected at e11.5 in cells that correspond to vagus nerve cell bodies which are migrating to the developing heart (33). We detected Nhlh1 by in situ hybridization throughout embryonic development from e12.5 to e16.5 in the brain stem, where cardiovascular regulatory centers are located (Fig. 5 and data not shown). Moreover, Nhlh2 is expressed in these same areas, possibly explaining the more severe phenotype of the KH mice. These data suggest that the observed lack of diving reflex and reduced baroreceptor sensitivity might be caused by a developmental defect in the autonomic nervous system independent of any heart structural abnormality.
FIG. 5.
Expression of Nhlh1 in the mouse head at e13.5. Bright field (A and C) and dark field (B and D) in situ hybridizations on parasagittal sections are shown. LV, lateral ventricle; IVV, fourth ventricle; VG, trigeminal ganglion; VIIIG, vestibulocochlear ganglion; IXG, glossopharyngeal ganglion; XG, vagal ganglion; ChPl, choroid plexus; M, medulla oblongata. Scale bars represent 500 μm. No signal was observed when sections were hybridized with a sense probe (data not shown).
DISCUSSION
Besides being controlled by intrinsic electrical impulses generated by the conduction system, the mechanical activity of the heart is also modulated by neuronal activity through the autonomic nervous system (22). Autonomic nervous system imbalance is recognized to have a significant impact on arrhythmia susceptibility. In the present study, we demonstrate that deletion of the neuronal bHLH transcription factor Nhlh1 results in depression of parasympathetic tone, stress-induced arrhythmia, and increased mortality in mice. Therefore, autonomic nervous system imbalance per se, in situations of stress and/or exercise, may contribute to the onset of serious life-threatening ventricular arrhythmias and death.
Conclusive evidence of depressed resting and reflex vagal tone in the Nhlh1-null (Nhlh1−/− and KH) mice is derived from three different sets of observations. (i) Nhlh1-null mice had reduced heart rate variability, consistent with decreased resting vagal tone. (ii) Nhlh1-null mice manifested significantly less baroreceptor sensitivity (an indicator of reflex vagal tone) than did wild-type mice. (iii) Nhlh1-null mice failed to show transient bradycardia upon water immersion, implying a loss of the diving reflex, a phenomenon that depends on reflex parasympathetic activity. Heart rate variability (marker of tonic vagal activity) and baroreceptor sensitivity (marker of reflex vagal activity) have been widely used to reflect the autonomic activity in the heart (6, 11, 12, 18). Both experimental data from animals (19, 23, 38) and human clinical studies (14, 26) suggest a strong correlation between depressed heart rate variability and/or reduced baroreceptor sensitivity and a greater incidence of sudden cardiac death.
The underlying physiologic mechanism of decreased heart rate variability is likely to be an alteration in the cardiac sympathetic-parasympathetic balance, which is characterized by a relative sympathetic dominance that is probably secondary to reduced parasympathetic activity (19). Indeed, heart rate variability is low in congestive heart failure, which is characterized by a reduced vagal and an increased sympathetic outflow to the heart (37). Many data show the close association of depressed heart rate variability with increased cardiac mortality after myocardial infarction in humans (23) and in animals (19). Baroreceptor sensitivity is indicative of reflex responses to increases in arterial blood pressure. For humans, the predictive value for lethal arrhythmia or decreased baroreceptor sensitivity has been shown only after myocardial infarction due to the lack of prospective studies (14, 25). For the dog, decreased baroreceptor sensitivity shows a correlation with risk of ventricular fibrillation both before and after myocardial infarction (19). In our mice, we observed reductions of both heart rate variability and baroreceptor sensitivity in the absence of heart damage. In view of current knowledge, it is conceivable that reduced heart rate variability and especially baroreceptor sensitivity in the Nhlh1-null mice are predictive of susceptibility to lethal arrhythmia even in the intact heart. Furthermore, the failure of Nhlh1-null mice to respond to water immersion with transient reflex bradycardia and the occurrence of ectopies during swim exercise confirm the prediction of defective reflex vagal activity and the hypothesis of increased susceptibility to arrhythmia under these circumstances.
An additional finding that might predispose Nhlh1-null mice to lethal arrhythmia is unstable cardiac repolarization. We found no evidence of prolongation of the QT interval. However, Nhlh1-null mice manifested an increase in the beat-to-beat QTVI, consistent with a depression in the control of repolarization. Increased QTVI has been shown to be a promising predictor of future arrhythmic events in humans after myocardial infarction (3). Although the mechanism by which unstable repolarization leads to lethal arrhythmia is still unclear, it is assumed that alterations in the duration of action potential in the myocardium facilitate the induction of functional reentry, a condition necessary for the most common lethal tachyarrhythmias (1). At present we are unaware of a direct physiological connection between depression of vagal activity (both tonic and reflex) and unstable repolarization. Therefore, each of these abnormalities acting independently or both in concert might provoke lethal arrhythmic events in Nhlh1−/− mice.
A recent report (24) describes a different strain of Nhlh1−/− mice that are viable and fertile and do not show any gross sign of neurological malfunction. These authors did not observe a tendency of Nhlh1−/− mice to die prematurely and suffer from arrhythmia. The difference from our results may be a function of the relatively small number of animals used (n = 20) and the fact that these authors monitored them for only 1 year. Strain variability may also explain this difference, although it does not seem to affect the phenotype of the double-null Nhlh1−/− Nhlh2−/− mice. In fact, like us, these authors report that the double-null Nhlh1−/− Nhlh2−/− mice die perinatally.
Recent studies have provided evidence that bHLH proteins like Nhlh1 expressed in postmitotic neurons have a role in the specification of neuronal subtypes (10). Attempts by us and others (24) to identify abnormalities in the CNS of Nhlh1−/− mice have revealed so far only normal gross morphology. In spite of this, we cannot rule out the abnormal development of a specific lineage in the brain stem as the origin of the physiological defect that we described.
In summary, we now believe that our initial observation of increased mortality in Nhlh1-null mice is likely explained by the occurrence of lethal arrhythmia, to which Nhlh1-null mice are more susceptible. It is compelling that in the course of this work we were able to observe reproducible arrhythmias and even document an episode of death in a mutant mouse during swim exercise. The Nhlh1-deficient mouse thus provides a reagent for studying aspects of arrhythmogenesis and lethal arrhythmic events.
Acknowledgments
We thank F. D. Porter, S.-P. Huang, and H. Westphal for embryonic stem cells and help with generating the Nhlh1−/− mouse; E. J. Lee for blastocyst injections; J. Czaja for excellent skills in performing surgical procedures; E. Mezey and M. Rusnák for assistance with the in situ hybridization; K. F. Shotwell for β-AR measurements; R. Molina for technical support in the mouse room; J. Quigley for statistical analysis; N. Montano and A. Rapacciuolo for fruitful discussions; and R. D. Berger, W. M. Kuehl, K. S. Kuehl, J. J. O'Shea, S. Wray, M. Nau, and C. Coyle for valuable comments on the manuscript. Special thanks go to S. Izraeli and G. Tonon for continuous support, ideas, and critical review of the manuscript.
T. Cogliati and D. J. Good contributed equally to this work.
REFERENCES
- 1.Akar, F. G., K. R. Laurita, and D. S. Rosenbaum. 2000. Cellular basis for dispersion of repolarization underlying reentrant arrhythmias. J. Electrocardiol. 33:23-31. [DOI] [PubMed] [Google Scholar]
- 2.Arnold, H. H., and B. Winter. 1998. Muscle differentiation: more complexity to the network of myogenic regulators. Curr. Opin. Genet. Dev. 8:539-544. [DOI] [PubMed] [Google Scholar]
- 3.Atiga, W. L., H. Calkins, J. H. Lawrence, G. F. Tomaselli, J. M. Smith, and R. D. Berger. 1998. Beat-to-beat repolarization lability identifies patients at risk for sudden cardiac death. J. Cardiovasc. Electrophysiol. 9:899-908. [DOI] [PubMed] [Google Scholar]
- 4.Barron, H. V., and M. D. Lesh. 1996. Autonomic nervous system and sudden cardiac death. J. Am. Coll. Cardiol. 27:1053-1060. [DOI] [PubMed] [Google Scholar]
- 5.Begley, C. G., S. Lipkowitz, V. Gobel, K. A. Mahon, V. Bertness, A. R. Green, N. M. Gough, and I. R. Kirsch. 1992. Molecular characterization of NSCL, a gene encoding a helix-loop-helix protein expressed in the developing nervous system. Proc. Natl. Acad. Sci. USA 89:38-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Billman, G. E., P. J. Schwartz, and H. L. Stone. 1982. Baroreceptor reflex control of heart rate: a predictor of sudden cardiac death. Circulation 66:874-880. [DOI] [PubMed] [Google Scholar]
- 7.Bradley, D. J., H. C. Towle, and W. S. Young III. 1992. Spatial and temporal expression of alpha- and beta-thyroid hormone receptor mRNAs, including the beta 2-subtype, in the developing mammalian nervous system. J. Neurosci. 12:2288-2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burgess, R., P. Cserjesi, K. L. Ligon, and E. N. Olson. 1995. Paraxis: a basic helix-loop-helix protein expressed in paraxial mesoderm and developing somites. Dev. Biol. 168:296-306. [DOI] [PubMed] [Google Scholar]
- 9.Butler, P. J., and D. R. Jones. 1997. Physiology of diving of birds and mammals. Physiol. Rev. 77:837-899. [DOI] [PubMed] [Google Scholar]
- 10.Chien, C. T., C. D. Hsiao, L. Y. Jan, and Y. N. Jan. 1996. Neuronal type information encoded in the basic-helix-loop-helix domain of proneural genes. Proc. Natl. Acad. Sci. USA 93:13239-13244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiou, C. W., and D. P. Zipes. 1998. Selective vagal denervation of the atria eliminates heart rate variability and baroreflex sensitivity while preserving ventricular innervation. Circulation 98:360-368. [DOI] [PubMed] [Google Scholar]
- 12.Corr, P. B., K. A. Yamada, and F. X. Witkowski. 1986. Mechanisms controlling cardiac autonomic function and their relationship to arrhythmogenesis, p. 1343-1403. In H. A. Fozzard, E. Haber, R. B. Jennings, A. N. Katz, and H. E. Morgan (ed.), The heart and cardiovascular system. Raven Press, New York, N.Y.
- 13.Duncan, M. K., L. Bordas, E. Dicicco-Bloom, and K. K. Chada. 1997. Expression of the helix-loop-helix genes Id-1 and NSCL-1 during cerebellar development. Dev. Dyn. 208:107-114. [DOI] [PubMed] [Google Scholar]
- 14.Farrell, T. G., O. Odemuyiwa, Y. Bashir, T. R. Cripps, M. Malik, D. E. Ward, and A. J. Camm. 1992. Prognostic value of baroreflex sensitivity testing after acute myocardial infarction. Br. Heart J. 67:129-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gobel, V., S. Lipkowitz, C. A. Kozak, and I. R. Kirsch. 1992. NSCL-2: a basic domain helix-loop-helix gene expressed in early neurogenesis. Cell Growth Differ. 3:143-148. [PubMed] [Google Scholar]
- 16.Good, D. J., F. D. Porter, K. A. Mahon, A. F. Parlow, H. Westphal, and I. R. Kirsch. 1997. Hypogonadism and obesity in mice with a targeted deletion of the Nhlh2 gene. Nat. Genet. 15:397-401. [DOI] [PubMed] [Google Scholar]
- 17.Gooden, B. A. 1994. Mechanism of the human diving response. Integr. Physiol. Behav. Sci. 29:6-16. [DOI] [PubMed] [Google Scholar]
- 18.Hohnloser, S. H., T. Klingenheben, A. van de Loo, E. Hablawetz, H. Just, and P. J. Schwartz. 1994. Reflex versus tonic vagal activity as a prognostic parameter in patients with sustained ventricular tachycardia or ventricular fibrillation. Circulation 89:1068-1073. [DOI] [PubMed] [Google Scholar]
- 19.Hull, S. S., Jr., A. R. Evans, E. Vanoli, P. B. Adamson, M. Stramba-Badiale, D. E. Albert, R. D. Foreman, and P. J. Schwartz. 1990. Heart rate variability before and after myocardial infarction in conscious dogs at high and low risk of sudden death. J. Am. Coll. Cardiol. 16:978-985. [DOI] [PubMed] [Google Scholar]
- 20.Iaccarino, G., E. D. Tomhave, R. J. Lefkowitz, and W. J. Koch. 1998. Reciprocal in vivo regulation of myocardial G protein-coupled receptor kinase expression by beta-adrenergic receptor stimulation and blockade. Circulation 98:1783-1789. [DOI] [PubMed] [Google Scholar]
- 21.Ishii, K., M. Kuwahara, H. Tsubone, and S. Sugano. 1996. Autonomic nervous function in mice and voles (Microtus arvalis): investigation by power spectral analysis of heart rate variability. Lab. Anim. 30:359-364. [DOI] [PubMed] [Google Scholar]
- 22.Keating, M. T., and M. C. Sanguinetti. 2001. Molecular and cellular mechanisms of cardiac arrhythmias. Cell 104:569-580. [DOI] [PubMed] [Google Scholar]
- 23.Kleiger, R. E., J. P. Miller, J. T. Bigger, Jr., and A. J. Moss. 1987. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am. J. Cardiol. 59:256-262. [DOI] [PubMed] [Google Scholar]
- 24.Krüger, M., and T. Braun. 2001. The neuronal basic helix-loop-helix transcription factor NSCL-1 is dispensable for normal neuronal development. Mol. Cell. Biol. 22:792-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.La Rovere, M. T., J. T. J. Bigger, F. I. Marcus, A. Mortara, and P. J. Schwartz. 1998. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet 351:478-484. [DOI] [PubMed] [Google Scholar]
- 26.La Rovere, M. T., G. Specchia, A. Mortara, and P. J. Schwartz. 1988. Baroreflex sensitivity, clinical correlates, and cardiovascular mortality among patients with a first myocardial infarction. A prospective study. Circulation 78:816-824. [DOI] [PubMed] [Google Scholar]
- 27.Lee, J. E. 1997. Basic helix-loop-helix genes in neural development. Curr. Opin. Neurobiol. 7:13-20. [DOI] [PubMed] [Google Scholar]
- 28.Li, L., P. Cserjesi, and E. N. Olson. 1995. Dermo-1: a novel twist-related bHLH protein expressed in the developing dermis. Dev. Biol. 172:280-292. [DOI] [PubMed] [Google Scholar]
- 29.Lipkowitz, S., V. Gobel, M. L. Varterasian, K. Nakahara, K. Tchorz, and I. R. Kirsch. 1992. A comparative structural characterization of the human NSCL-1 and NSCL- 2 genes. Two basic helix-loop-helix genes expressed in the developing nervous system. J. Biol. Chem. 267:21065-21071. [PubMed] [Google Scholar]
- 30.Malliani, A., M. Pagani, F. Lombardi, and S. Cerutti. 1991. Cardiovascular neural regulation explored in the frequency domain. Circulation 84:482-492. [DOI] [PubMed] [Google Scholar]
- 31.Mansour, S. L., K. R. Thomas, and M. R. Capecchi. 1988. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature 336:348-352. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell, G. F., A. Jeron, and G. Koren. 1998. Measurement of heart rate and Q-T interval in the conscious mouse. Am. J. Physiol. 274:H747-H751. [DOI] [PubMed] [Google Scholar]
- 33.Murdoch, J. N., J. Eddleston, N. Leblond-Bourget, P. Stanier, and A. J. Copp. 1999. Sequence and expression analysis of Nhlh1: a basic helix-loop-helix gene implicated in neurogenesis. Dev. Genet. 24:165-177. [DOI] [PubMed] [Google Scholar]
- 34.Olson, E. N., and W. H. Klein. 1994. bHLH factors in muscle development: dead lines and commitments, what to leave in and what to leave out. Genes Dev. 8:1-8. [DOI] [PubMed] [Google Scholar]
- 35.Pagani, M., F. Lombardi, S. Guzzetti, O. Rimoldi, R. Furlan, P. Pizzinelli, G. Sandrone, G. Malfatto, S. Dell'Orto, E. Piccaluga, et al. 1986. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ. Res. 59:178-193. [DOI] [PubMed] [Google Scholar]
- 36.Robb, L., and C. G. Begley. 1997. The SCL/TAL1 gene: roles in normal and malignant haematopoiesis. Bioessays 19:607-613. [DOI] [PubMed] [Google Scholar]
- 37.Saul, J. P., Y. Arai, R. D. Berger, L. S. Lilly, W. S. Colucci, and R. J. Cohen. 1988. Assessment of autonomic regulation in chronic congestive heart failure by heart rate spectral analysis. Am. J. Cardiol. 61:1292-1299. [DOI] [PubMed] [Google Scholar]
- 38.Schwartz, P. J., E. Vanoli, M. Stramba-Badiale, G. M. De Ferrari, G. E. Billman, and R. D. Foreman. 1988. Autonomic mechanisms and sudden death. New insights from analysis of baroreceptor reflexes in conscious dogs with and without a myocardial infarction. Circulation 78:969-979. [DOI] [PubMed] [Google Scholar]
- 39.Srivastava, D., P. Cserjesi, and E. N. Olson. 1995. A subclass of bHLH proteins required for cardiac morphogenesis. Science 270:1995-1999. [DOI] [PubMed] [Google Scholar]
- 40.Staal, F. J., F. Weerkamp, A. W. Langerak, R. W. Hendriks, and H. C. Clevers. 2001. Transcriptional control of t lymphocyte differentiation. Stem Cells 19:165-179. [DOI] [PubMed] [Google Scholar]
- 41.Tybulewicz, V. L., C. E. Crawford, P. K. Jackson, R. T. Bronson, and R. C. Mulligan. 1991. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell 65:1153-1163. [DOI] [PubMed] [Google Scholar]
- 42.Uittenbogaard, M., D. R. Peavy, and A. Chiaramello. 1999. Expression of the bHLH gene NSCL-1 suggests a role in regulating cerebellar granule cell growth and differentiation. J. Neurosci. Res. 57:770-781. [PubMed] [Google Scholar]
- 43.Vaidya, D., G. E. Morley, F. H. Samie, and J. Jalife. 1999. Reentry and fibrillation in the mouse heart. A challenge to the critical mass hypothesis. Circ. Res. 85:174-181. [DOI] [PubMed] [Google Scholar]
- 44.Vatta, M., H. Li, and J. A. Towbin. 2000. Molecular biology of arrhythmic syndromes. Curr. Opin. Cardiol. 15:12-22. [DOI] [PubMed] [Google Scholar]
- 45.Voronova, A., and D. Baltimore. 1990. Mutations that disrupt DNA binding and dimer formation in the E47 helix-loop-helix protein map to distinct domains. Proc. Natl. Acad. Sci. USA 87:4722-4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wickman, K., J. Nemec, S. J. Gendler, and D. E. Clapham. 1998. Abnormal heart rate regulation in GIRK4 knockout mice. Neuron 20:103-114. [DOI] [PubMed] [Google Scholar]