Abstract
Purpose. Lymphatic vessel endothelial hyaluronic acid receptor (LYVE-1) is a newly discovered lymphatic-specific marker. To date, there is no report of its expression on conjunctival cells. The purpose of this study was to investigate the expression of LYVE-1 in normal conjunctiva, to phenotype LYVE-1+ cells, and to study changes in their expression levels during corneal inflammation.
Methods. Flatmounted conjunctivae or cross sections of eye-balls were harvested from BALB/c mice (6-8 weeks of age) for immunofluorescent confocal microscopic studies.
Results. The data demonstrate, for the first time, that in addition to its expression on lymphatic vessels, LYVE-1 was expressed on CD45+, CD11b+, and CD31- conjunctival cells, indicating a bone-marrow-derived monocytic lineage. Surprisingly, the number of cells that expressed LYVE-1 decreased during corneal inflammation, in conjunction with ingrowth of lymphatics into the cornea.
Conclusions. A new population of monocytic cells has been found to express LYVE-1 in normal conjunctiva. These cells that normally express LYVE-1 may act as a reservoir for lymphangiogenesis and cell recruitment when the immune system is challenged.
Though the lymphatic system was discovered at the same time as the blood circulation, study in this field has made little progress during the past several decades, until recently when several lymphatic endothelium-specific markers, including lymphatic vessel endothelial hyaluronic acid receptor (LYVE-1), were identified.1-5 The lymphatic system is crucial in tissue fluid regulation, fat absorption, cancer metastasis, and immune responses. To initiate an immune response, foreign materials are taken up by antigen-presenting cells (APCs) in the periphery and transported to regional lymph nodes by the lymphatic vessels for priming of naïve T cells.6 Previous data from our laboratory indicate that lymphatic function plays an important role in corneal immunity. For example, corneal transplantation using transgenic green fluorescence protein (GFP) donor tissue with wild-type mice as recipients, has shown that several hours after surgery graft-derived GFP+ cells appear in the ipsilateral host cervical lymph nodes, and that this traffic was significantly enhanced in vascularized and lymphatic-vessel-rich beds.7 Another related study showed that corneally grafted hosts that had their cervical lymph nodes excised before surgery demonstrate indefinite graft acceptance without any form of pharmaceutical immune modulation.8 A more recent study of vascular endothelial growth factor receptor (VEGFR)-3, another lymphatic marker, has shown its signaling to be critical for induction of corneal alloimmunity, and the blockade of this pathway significantly suppressed corneal APC trafficking to local draining lymph nodes and rejection of corneal transplants.9 Furthermore, direct inhibition of lymphangiogenesis after keratoplasty has been shown to reduce the risk of immune rejections after corneal transplantation.10 It is therefore possible to modulate adaptive immunity in the periphery by regulating lymphatic-specific candidates at the molecular level.
LYVE-1 is a newly identified lymphatic-specific marker. It was identified by searching expressed sequence tag (EST) databases for sequences homologous to the hyaluronan (HA) receptor CD44, which is widely expressed on leukocytes, dendritic cells, and tumor cells.1 LYVE-1 is a receptor for HA, an important component of the extracellular matrix and also a key mediator of cell migration in tissues during inflammation, wound healing, and neoplasia.11 HA degradation occurs in regional lymph nodes or in the liver and the major transport route to these sites is through the lymphatic system. LYVE-1 is expressed on both sides of lymphatic endothelial cells and may be involved in lymph node homing of leukocytes and tumor cells expressing CD44.2,12-15 It is suggested that LYVE-1 may be involved in HA metabolism in the lymphatic system and the LYVE-1-HA interactions may regulate the migration of CD44+ leukocytes, dendritic cells, and tumor cells. Of note, to date, there is no report of its expression on conjunctival cells. The purpose of this study was to identify and characterize LYVE-1 expression in the conjunctiva.
Methods
Animals
Seven to 10-week-old male BALB/c mice (Taconic Farms, Germantown, NY, or from our own breeding facility) were used for these experiments. All protocols were approved by the Schepens Eye Research Institute Animal Care and Use Committee, and all animals were treated according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Mice were anesthetized with using a mixture of ketamine and xylazine (120 and 20 mg/kg body weight, respectively) for each surgical procedure.
Suture Placement in the Corneal Surface
Three interrupted sutures (1-0 nylon, Sharpoint; Vanguard) were placed in the central cornea of one eye of each mouse to induce inflammatory corneal neovascularization, which is also associated with significant lymphangiogenesis, as described previously.7,16,17 One and 2 weeks later, the eyeballs were collected and fixed in acetone for immunohistochemical studies.
Antibodies
The following antibodies were used: FITC-conjugated rat-anti-mouse CD45 (panleukocyte marker); FITC-conjugated rat-anti-mouse CD11b (M1/70); FITC-conjugated rat-anti-mouse CD31 (PECAM-1, Santa Cruz Biotechnology, Santa Cruz, CA); rabbit anti-mouse LYVE-1; and rat anti-mouse CD16/32 (2.4G2, FcγIII/II receptor). The secondary antibodies were R-conjugated donkey-anti-rabbit IgG. Isotype controls included rat IgG2b-FITC, rat IgG2a-FITC, and rabbit serum. All primary mAbs (except where noted) and isotype-matched controls were purchased from BD PharMingen (San Diego, CA), and secondary IgG Abs were purchased from Santa Cruz Biotechnology. The anti-LYVE-1 Ab was a kind gift of David Jackson (Weatherall Institute of Molecular Medicine, Oxford, UK).
Immunohistochemical Studies
The experiments were performed as described previously.7,17 Full-thickness corneal and conjunctival tissues or 8-μm frozen sections of eyeballs were fixed in acetone for immunofluorescence staining. To block nonspecific staining, sections were incubated with 2% bovine serum albumin (BSA) and anti-FcR mAb (CD16/CD32) for 30 minutes before they were immunostained with primary antibodies or control antibodies for 2 hours. Thereafter, the sections were incubated with secondary antibodies for 1 hour. All staining procedures were performed at room temperature, and each step was followed by three thorough washings in PBS for 5 minutes each. Finally, sections were covered with mounting medium (Vector, Burlingame, CA) and examined by confocal microscope (TCS 4D; Leica, Heidelberg, Germany) or epifluorescence microscope (Eclipse E800; Nikon, Tokyo, Japan). For each antibody staining study, tissues samples from three to five mice were examined. For cross-sectional studies, at least five sections were analyzed for each tissue sample derived from each animal. Correlation analysis between the number of LYVE-1+ cells/section in the normal and inflamed conditions was performed with Student’s t-test (two-sided). P < 0.05 was considered significant. The degree of change was calculated as follows: percentage of change = (mean number of the normal group - mean number of the inflamed group)/mean number of the normal group.
Results
LYVE-1 Expression on Vessels and Cells in the Normal Conjunctiva
Because lymphatic vessels are normally present in the conjunctiva and limbus, but not the cornea, we first stained the whole-mounted tissues with LYVE-1. Immunofluorescence studies demonstrated LYVE-1+ endothelialized structures only in the conjunctival and limbal areas (Fig. 1A). In that it an avascular and lymphatic-free tissue under normal conditions, the cornea, as expected, did not show any vascular staining (Fig. 1A). However, unexpectedly, in addition to its presence on vessels, LYVE-1 expression was detected on a large population of cells located in the conjunctival stroma.
Figure 1.
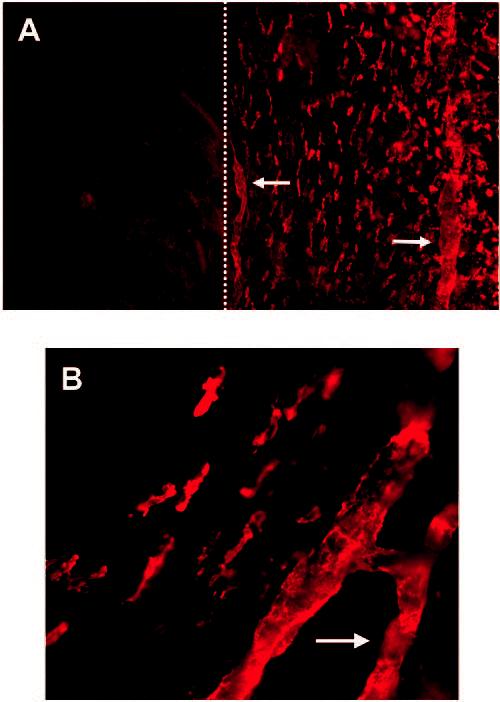
LYVE-1 expression on both vascular structures and cells in the normal conjunctiva. Representative micrographs of immunostained wholemount tissues showing LYVE-1 expression on limbal and conjunctival (right of the dotted line) cells and vessels (arrows) under low (A) and high (B) magnification. No LYVE-1+ vessels were present in the cornea (left of the dotted line). Original magnification: (A) ×100, (B) ×400.
LYVE-1 Expression on Nonvascular Cells in the Normal Conjunctiva
To exclude the endothelial origin of the LYVE-1+ cells in the normal conjunctiva, we used anti-CD31, a panendothelial marker. The double staining studies demonstrated that the LYVE-1+ cells distributed throughout the normal conjunctiva were CD-31- (Fig. 2). Serial laser confocal scanning also confirmed that these cells were not connected to the vascular structures.
Figure 2.
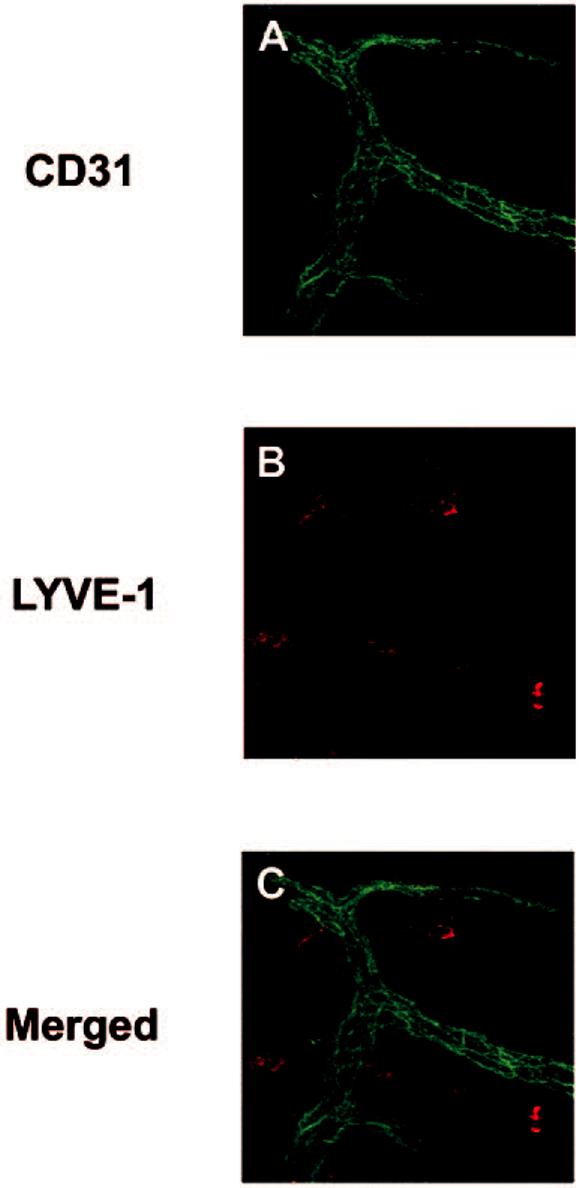
LYVE-1 expression on nonvascular cells. Micrographs demonstrating the distribution of LYVE-1+ cells (B, red) in the vascular-free areas of the conjunctiva. Vascular structures were CD31+ (A, green). (C) Merger of (A) and (B). Original magnification, ×400.
LYVE-1 Expression on Bone-Marrow-Derived Monocytes in the Normal Cornea
To characterize the phenotype of LYVE-1+ nonvascular cells, we stained the wholemounted tissues with anti-CD45 (panleukocyte marker) and anti-CD11b (monocyte/macrophage marker). Nearly all nonvascular LYVE-1+ cells in the conjunctiva were CD45+, indicating their bone marrow origin (Fig. 3). Furthermore, these LYVE-1+ cells uniformly expressed CD11b, confirming that they were of a monocyte/macrophage lineage (Fig. 4).
Figure 3.
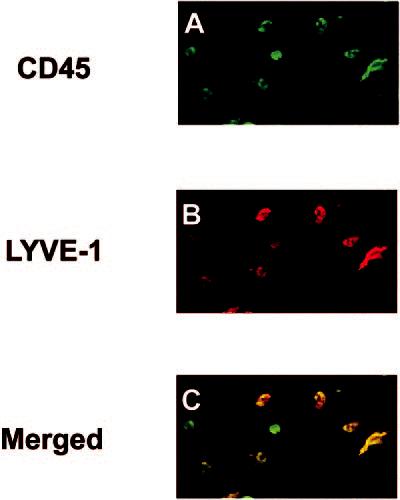
LYVE-1+ cells in the conjunctiva were bone-marrowderived. Wholemounted conjunctivae were double-stained with CD45 (A, green) and LYVE-1 (B, red). Confocal micrograph showed that LYVE-1+ cells coexpressed CD45 (C, yellow). Original magnification, ×200.
Figure 4.
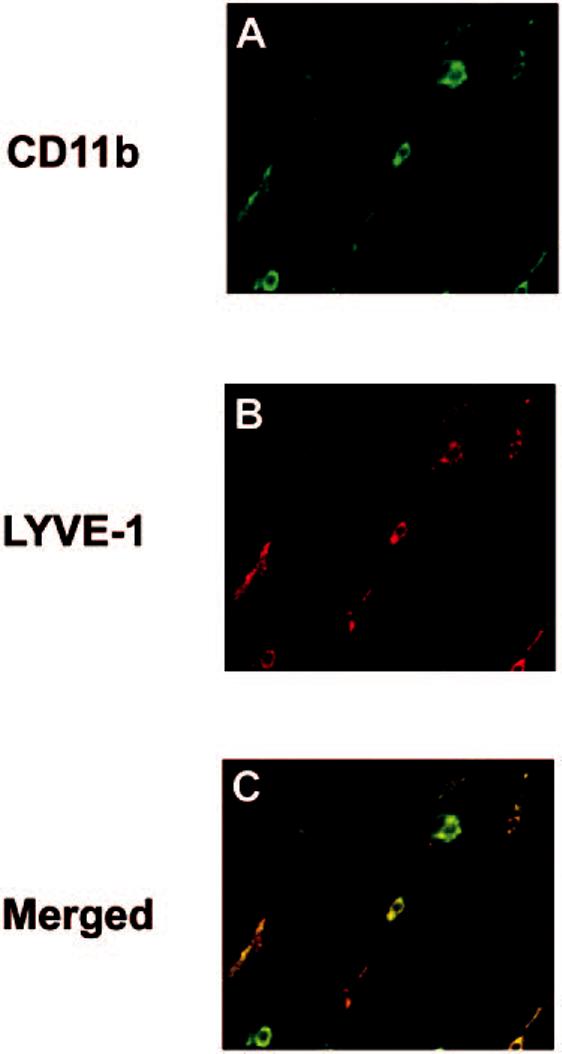
LYVE-1+ cells in the conjunctiva were monocytes. CD11b (A, green) expression of the LYVE-1+ (B, red) cells indicates that they are of the monocytic lineage. (C) Merged image showing coexpression (yellow). Original magnification, ×400.
Changes in LYVE-1 Expression Levels during Ocular Surface Inflammation
We next studied the changes in expression levels of LYVE-1 in the conjunctiva after induction of corneal inflammation by suture placement, a standard experimental procedure to induce lymphangiogenesis in the cornea.7,17 It was found that the number of LYVE-1+ was significantly decreased (71%; P < 0.0001) in the inflamed conjunctiva compared with its normal condition (Figs. 5A, 5B). At the same time, new lymphatics were detected in the previously lymphatic-free cornea (Figs. 5C, 5D). Figure 5E summarizes the results of changes in the number of conjunctival LYVE-1+ cells.
Figure 5.
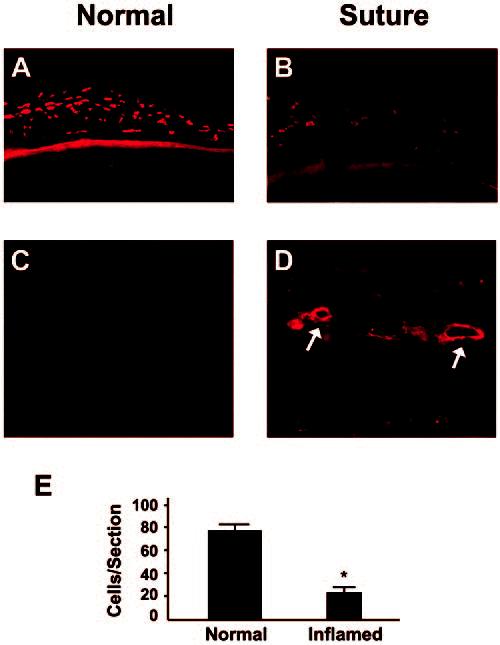
The number of LYVE-1+ cells in the conjunctiva decreased significantly during corneal inflammation. Cross-section staining shows that the number of LYVE-1+ cells diminished in the conjunctiva (A, B), whereas new lymphatic vessels (arrows) were formed in the cornea (C, D). Data on changes in the number of LYVE-1+ conjunctival cells are summarized in (E). *P < 0.0001. Original magnification: (A, B) ×100; (C, D) ×200.
Discussion
LYVE-1 has been extensively used as a lymphatic marker since its first identification in 1999 by Banerji et al.1-5,12,18 In addition to lymphatic vessels, its expression was also reported on sinusoidal endothelial cells within the liver and placental syncytiotrophoblasts; Kupfer cells; and, to a lesser extent, on cerebral cortex neurons, renal tubular cuboidal epithelium, and pancreatic exocrine cells/islets of Langerhans.1,19 However, the cellular origin of this expression has not been fullycharacterized, and there are no systematic studies to date regarding its expression by monocytes, macrophages, or other histiocytes besides Kupfer cells.1,15,19 Our studies are the first to demonstrate the presence of LYVE-1 on normal conjunctival cells. In addition, we showed that these cells are CD45+, CD11b+, and CD31-, indicating a monocytic lineage. Using the corneal inflammation model, we also demonstrated that LYVE-1 expression on the conjunctival cells was downregulated during corneal inflammation while lymphangiogenesis occurred.
Being the forefront medium in the passage of light to the retina, the cornea maintains its transparency under physiologic conditions, largely due to its lack of vascular structures. The conjunctiva, however, is normally endowed with both blood and lymphatic vessels that support the essential metabolic functions of the tissues and provide cellular immune effectors to the anterior compartment of the eye. Little is known about the mechanisms by which this vascular distinction between these two neighboring tissues is achieved and what resources are used to transform the cornea into a lymphatic-rich tissue when the system is challenged under pathoinflammatory conditions.
Our results provide the first association between the opposite directions of changes that occur in these two tissues during corneal inflammation. Although the number of conjunctival cells expressing LYVE-1 significantly diminished during ocular surface inflammation, LYVE-1+ lymphatics developed in the cornea. Because it is known that CD11b+ macrophages play an essential role in corneal lymphangiogenesis,17 it is likely that the conjunctiva, which we now demonstrate is normally endowed with a large constitutive population of LYVE-1+ cells, acts as a reservoir for the cells that induce corneal lymphangiogenesis. This concept is also supported by our previous findings that (1) during corneal inflammation, there is a significant influx of macrophages/CD11b+ cells into the cornea from the surrounding perilimbal tissue,20-22 and (2) depletion of conjunctival macrophages inhibits corneal lymphangiogenesis in inflammation.17 However, the source of these macrophages was never elucidated. Our present study not only makes the novel observation that conjunctival macrophages express the lymphatic endothelial marker LYVE-1, but also offers an explanation linking macrophages in the conjunctiva with development of corneal lymphatics, providing further evidence for the intimate link between these two cell subsets.
Acknowledgments
The authors thank Donald Pottle (Schepens Eye Research Institute) for technical assistance with the confocal microscopic studies.
Footnotes
Supported by National Eye Institute Grants EY-12963 (MRD) and EY-014786 (LC); an Award from Research to Prevent Blindness (MRD); and Deutsche Forschungsgemeinschaft Grant Cu 47/1-2 (CC) and IZKF Erlangen Grant A9 (CC).
Disclosure: L. Chen, None; C. Cursiefen, None; S. Barabino, None; Q. Zhang, None; M.R. Dana, None
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be marked “advertisement” in accordance with 18 U.S.C. §1734 solely to indicate this fact.
References
- Banerji S, Ni J, Wang SX, et al. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 1999;144:789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson DG. Biology of the lymphatic marker LYVE-1 and applications in research into lymphatic trafficking and lymphangiogenesis. APMIS. 2004;112:526–538. doi: 10.1111/j.1600-0463.2004.apm11207-0811.x. [DOI] [PubMed] [Google Scholar]
- Karkkainen MJ, Alitalo K. Lymphatic endothelial regulation, lymphoedema, and lymph node metastasis. Semin Cell Dev Biol. 2002;13:9–18. doi: 10.1006/scdb.2001.0286. [DOI] [PubMed] [Google Scholar]
- Oliver G, Detmar M. The rediscovery of the lymphatic system: old and new insights into the development and biological function of the lymphatic vasculature. Genes Dev. 2002;16:773–783. doi: 10.1101/gad.975002. [DOI] [PubMed] [Google Scholar]
- Chang L, Kaipainen A, Folkman J. Lymphangiogenesis new mechanisms. Ann N Y Acad Sci. 2002;979:111–119. doi: 10.1111/j.1749-6632.2002.tb04872.x. [DOI] [PubMed] [Google Scholar]
- Dana MR, Qian Y, Hamrah P. Twenty-five-year panorama of corneal immunology: emerging concepts in the immunopathogenesis of microbial keratitis, peripheral ulcerative keratitis, and corneal transplant rejection. Cornea. 2000;19:625–643. doi: 10.1097/00003226-200009000-00008. [DOI] [PubMed] [Google Scholar]
- Liu Y, Hamrah P, Zhang Q, et al. Draining lymph nodes of corneal transplant hosts exhibit evidence for donor major histocompatibility complex (MHC) class II-positive dendritic cells derived from MHC class II-negative grafts. J Exp Med. 2002;195:1–11. doi: 10.1084/jem.20010838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagami S, Dana MR. The critical role of lymph nodes in corneal alloimmunization and graft rejection. Invest Ophthalmol Vis Sci. 2001;42:1293–1298. [PubMed] [Google Scholar]
- Chen L, Hamrah P, Cursiefen C, et al. Vascular endothelial growth factor receptor-3 mediates induction of corneal alloimmunity. Nat Med. 2004;10:813–815. doi: 10.1038/nm1078. [DOI] [PubMed] [Google Scholar]
- Cursiefen C, Maruyama K, Liu Y, et al. Inhibition of hemangiogenesis and lymphangiogenesis after normal-risk corneal transplantation by neutralizing VEGF promotes graft survival. Invest Ophthalmol Vis Sci. 2004;45:2666–2673. doi: 10.1167/iovs.03-1380. [DOI] [PubMed] [Google Scholar]
- Lee JY, Spicer AP. Hyaluronan: a multifunctional, megaDalton, stealth molecule. Curr Opin Cell Biol. 2000;12:581–586. doi: 10.1016/s0955-0674(00)00135-6. [DOI] [PubMed] [Google Scholar]
- Jackson DG, Prevo R, Clasper S, et al. LYVE-1, the lymphatic system and tumor lymphangiogenesis. Trends Immunol. 2001;22:317–321. doi: 10.1016/s1471-4906(01)01936-6. [DOI] [PubMed] [Google Scholar]
- Sleeman JP, Krishnan J, Kirkin V, et al. Markers for the lymphatic endothelium: in search of the holy grail? Microsc Res Tech. 2001;55:61–69. doi: 10.1002/jemt.1157. [DOI] [PubMed] [Google Scholar]
- Reis-Filho JS, Schmitt FC. Lymphangiogenesis in tumors: what do we know. Microsc Res Tech. 2003;60:171–180. doi: 10.1002/jemt.10255. [DOI] [PubMed] [Google Scholar]
- Prevo R, Banerji S, Ferguson DJ, et al. Mouse LYVE-1 is an endocytic receptor for hyaluronan in lymphatic endothelium. J Biol Chem. 2001;276:19420–19430. doi: 10.1074/jbc.M011004200. [DOI] [PubMed] [Google Scholar]
- Dana MR, Streilein JW. Loss and restoration of immune privilege in eyes with corneal neovascularization. Invest Ophthalmol Vis Sci. 1996;37:2485–2494. [PubMed] [Google Scholar]
- Cursiefen C, Chen L, Borges LP, et al. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest. 2004;113:1040–1050. doi: 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cursiefen C, Schlötzer-Schrehardt U, Küchle M, et al. Lymphatic vessels in vascularized human corneas: immunohistochemical investigation using LYVE-1 and podoplanin. Invest Ophthalmol Vis Sci. 2002;43:2127–2135. [PubMed] [Google Scholar]
- Mouta Carreira C, Nasser SM, di Tomaso E, et al. LYVE-1 is not restricted to the lymph vessels: expression in normal liver blood sinusoids and down-regulation in human liver cancer and cirrhosis. Cancer Res. 2001;61:8079–8084. [PubMed] [Google Scholar]
- Zhu S, Dana MR. Expression of cell adhesion molecules on limbal and neovascular endothelium in corneal inflammatory neovascularization. Invest Ophthalmol Vis Sci. 1999;40:1427–1434. [PubMed] [Google Scholar]
- Zhu SN, Yamada J, Streilein JW, Dana MR. ICAM-1 deficiency suppresses host allosensitization and rejection of MHC-disparate corneal transplants. Transplantation. 2000;69:1008–1013. doi: 10.1097/00007890-200003150-00061. [DOI] [PubMed] [Google Scholar]
- Dana MR. Corneal antigen-presenting cells: diversity, plasticity, and disguise. Invest Ophthalmol Vis Sci. 2004;45:722–727. doi: 10.1167/iovs.03-0803. [DOI] [PubMed] [Google Scholar]


