Abstract
With increasing frequency during serial passage in culture, primary human keratinocytes express p16INK4A (p16) and undergo senescence arrest. Keratinocytes engineered to express hTERT maintain long telomeres but typically are not immortalized unless, by mutation or other heritable event, they avoid or greatly reduce p16 expression. We have confirmed that keratinocytes undergo p16-related senescence during growth in culture, whether in the fibroblast feeder cell system or in the specialized K-sfm medium formulation, and that this mechanism can act as a barrier to immortalization following hTERT expression. We have characterized the p16-related arrest mechanism more precisely by interfering specifically with several regulators of cell cycle control. Epidermal, oral mucosal, corneal limbal, and conjunctival keratinocytes were transduced to express a p16-insensitive mutant cdk4 (cdk4R24C), to abolish p16 control, and/or a dominant negative mutant p53 (p53DD), to abolish p53 function. Expression of either cdk4R24C or p53DD alone had little effect on life span, but expression of both permitted cells to divide 25 to 43 population doublings (PD) beyond their normal limit. Keratinocytes from a p16+/− individual transduced to express p53DD alone displayed a 31-PD life span extension associated with selective growth of variants that had lost the wild-type p16 allele. Cells in which both p53 and p16 were nonfunctional divided rapidly during their extended life span but experienced telomere erosion and ultimately ceased growth with very short telomeres. Expression of hTERT in these cells immortalized them. Keratinocytes engineered to express cdk4R24C and hTERT but not p53DD did not exhibit an extended life span. Rare immortal variants exhibiting p53 pathway defects arose from them, however, indicating that the p53-dependent component of keratinocyte senescence is telomere independent. Mutational loss of p16 and p53 has been found to be a frequent early event in the development of squamous cell carcinoma. Our results suggest that such mutations endow keratinocytes with extended replicative potential which may serve to increase the probability of neoplastic progression.
The replicative life span of normal human fibroblasts is limited by a senescence mechanism that responds to partial telomere shortening by triggering a p53- and p21cip1 (p21)-dependent growth arrest (5, 8, 12). Expression of hTERT, the telomerase catalytic subunit, in presenescent fibroblasts and several other cell types, including retinal pigmented epithelial cells, vascular endothelial cells, and mesothelial cells, is sufficient to permanently evade this senescence mechanism and immortalize these cell types (7, 17, 78). Recent studies have indicated, however, that telomere shortening alone cannot completely explain the replicative life span limit and immortalization barrier exhibited by a diverse set of epithelial cell types.
For keratinocytes (17, 31), mammary epithelial cells (11, 21, 31, 56), bladder urothelial cells (51), and prostate epithelial cells (28, 57), senescence has been found to be associated with expression and accumulation of high levels of p16INK4A, an inhibitor of cdk4 and cdk6 (48, 59). Keratinocytes and mammary epithelial cells engineered to express hTERT extend and stabilize their telomeres but typically are not immortalized. The emergence of rare, rapidly dividing variants from hTERT-transduced keratinocyte cultures has been found to correlate in many cases with loss or substantial reduction of p16 expression, loss of heterozygosity or homozygous deletion of the p16 locus, or hypermethylation of the p16 promoter (17, 20, 31). These studies have provided compelling yet circumstantial evidence that the replicative potential of keratinocytes and several other epithelial cell types in culture typically does not first become limited by telomere shortening but rather by a mechanism that detects a different, as yet uncharacterized form of cell aging or replicative history and triggers a growth arrest enforced by p16 induction.
Several previous reports (17, 31) indicated that hTERT-expressing keratinocytes are still subject to a p16-associated senescence arrest which acts as a barrier to immortalization and that this occurs whether the cells are cultured in the fibroblast feeder layer system (4, 55) or in a feeder cell-free, nutritionally optimized keratinocyte culture medium, K-sfm (50). A more recent report (52) comparing the behavior of one primary keratinocyte line cultured in the fibroblast feeder system and in a different keratinocyte medium formulation, MCDB154/KGM (9), concluded that p16 senescence is not an important factor in determining the replicative potential of keratinocytes and is not a barrier to immortalization when these cells are cultured in the fibroblast feeder system.
In order to investigate the relationships among the various mechanisms implicated in keratinocyte senescence, we have introduced dominantly acting, mutant forms of cell cycle-regulatory proteins that specifically abolish p16-dependent or p53-dependent growth arrest mechanisms in the presence or absence of constitutive telomerase activity. Our observations indicate that keratinocyte proliferation is limited by a p16-enforced growth arrest irrespective of culture conditions or telomerase expression. We also have identified a second, p53-dependent and telomere-independent arrest mechanism that contributes to the limited replicative potential of normal keratinocytes and which acts as a barrier to immortalization in keratinocytes lacking functional p16. These results define the essential elements of a telomere-independent senescence arrest mechanism in keratinocytes and provide a new perspective on the possible roles played by mutations affecting p16INK4A and p53 in the neoplastic progression of keratinocytes toward squamous cell carcinoma in vivo.
(Some of this research was conducted as part of a Harvard Biochemical Sciences honors thesis by K.M.O.)
MATERIALS AND METHODS
Cell lines.
Primary cell lines (also known as cell strains) were cultured from clinically normal tissues: R2F (dermal fibroblast from newborn foreskin) (69), HM3 (peritoneal mesothelial cell from a 29-year-old female) (41), strain N (epidermal keratinocyte from newborn foreskin) (54, 58), OKF4 (oral floor of mouth keratinocyte from a 28-year-old male) (34, 58), HuCL-22 (corneal limbal keratinocyte from a 30-year-old male), and ConjEp-1 (conjunctival keratinocyte from an 82-year-old male). Two primary cell lines studied were not genetically normal: K107 (epidermal keratinocytes cultured from phenotypically normal skin of a 52-year-old female previously determined to have inherited a heterozygous loss-of-function mutation [G101W] of p16INK4A) and POE9n (premalignant oral mucosal keratinocytes possessing a homozygous deletion at the p16INK4A/p14ARF locus, lacking p53 expression, and exhibiting an extended but finite replicative life span, cultured from a severely dysplastic oral epithelial lesion of a 65-year-old male) (17; J. G. Rheinwald, J. Benwood, A. Palanisamy, R. Feldman, and E. Sauter, unpublished data). N/E6E7 is an immortalized line we derived from strain N by transduction to express the E6 and E7 oncoproteins of human papillomavirus type 16 HPV16 (J. G. Rheinwald, J. Benwood, and A. Palanisamy, unpublished data).
Culture media and methods.
Primary keratinocyte lines were initiated either from explanted, minced tissue fragments or from single cells that had been released from such fragments by trypsinization. These cell populations were expanded through one to four serial passages by cocultivating with mitomycin-treated Swiss 3T3J2 cells (55) in flavin adenine dinucleotide (FAD) medium, consisting of Dulbecco's modified Eagle's medium (DMEM)/F12 (1:1, vol/vol) medium (Gibco, Invitrogen Corp.), 5% calf serum (HyClone), 10 ng of epidermal growth factor (EGF) per ml, 0.4 μg of hydrocortisone per ml, 5 μg of insulin per ml, 10 × 10−10 M cholera toxin (CT), 2 × 10−11 M triiodothyronine, and 1.8 × 10−4 M adenine (4, 76). The primary lines and derived transductants were subsequently cultured either in this feeder cell system or without feeder cells in a nutritionally optimized, keratinocyte serum-free medium (K-sfm) (50) (Gibco, Invitrogen Corp.), supplemented as described previously (58) with 25 μg of bovine pituitary extract (BPE) per ml, 0.2 ng of EGF per ml, and 0.4 mM CaCl2.
R2F fibroblasts and derived transductants were cultured in DMEM/F12 medium plus 15% iron-fortified newborn calf serum (HyClone, Inc.) plus 10 ng of EGF per ml. HM3 mesothelial cells and derived transductants were cultured in M199/MCDB105 (1:1, vol/vol) medium (Sigma) plus 15% calf serum, 10 ng of EGF per ml, and 0.4 μg of hydrocortisone per ml, as described previously (14, 41). 3T3J2NHP cells (resistant to G418, hygromycin, and puromycin [17]) and the retroviral vector producer cell lines PA317 and PT67 were cultured in pyruvate-free DMEM (Gibco, Invitrogen Corp.) plus 10% calf serum.
Organotypic cultures were prepared as described previously (47, 58). Briefly, keratinocytes were seeded at 2 × 105 cells/cm2 of surface area onto contracted, human foreskin fibroblast-containing collagen gels formed on a porous membrane filter unit. These were cultured submerged for 4 days and then at the air-liquid interface for 10 days. The cultures were fixed in 10% formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin.
Retroviral vectors and transductions.
Retroviral vectors constructed from pBABE-puro and pBABE-hygro (40) and pLXSN (39) were used: pL(p53DD)SN, which expresses a dominant-negative fragment of p53 (23, 63); pBABE(cdk4R)hygro, which expresses a p16-resistant point mutant (R24C) form of cdk4 (61, 74); and pBABE(hTERT)puro, which expresses the catalytic subunit of human telomerase (17, 24). The parent, “empty” pBABE and pLXSN vectors were used as controls.
Transductions and selection were performed essentially as described previously (17, 58). Nearly confluent cultures of PA317 or PT67 amphotropic packaging cells producing the above vectors were incubated for 7 h or overnight in K-sfm or in a 1:1 mixture of K-sfm and a DMEM/F12-based, serum-free keratinocyte medium formulation (29). The resulting retroviral supernatants were passed through a 0.45-μm filter and stored in 2-ml aliquots at −80°C until use. Human cells plated 1 or 2 days earlier at ≈105 cells per 9-cm2 well were transduced by refeeding them for 5 to 7 h with retroviral supernatant plus 2 μg of Polybrene (Sigma) per ml. The transduced cells were subcultured the next day into 75-cm2 flasks. Drug selection (0.2 mg of G418, 10 μg of hygromycin, or 1 μg of puromycin per ml for keratinocytes; 0.4 mg of G418, 50 μg of hygromycin, or 1 μg of puromycin per ml for fibroblasts and mesothelial cells) was started 2 days after transduction and continued for 6 to 10 days. Some cdk4R and some hTERT transductants were generated by coplating keratinocytes with mitomycin-treated retroviral producer cells in FAD medium. Three or four days later, the producer cells were selectively removed by brief incubation with EDTA and vigorous pipetting, mitomycin-treated 3T3J2NHP cells were added back to the cultures, and drug selection was started the next day.
Replicative life span determination.
Cells were plated at 0.2 × 105 to 2 × 105 cells per T75 flask or p100 dish in their appropriate growth medium, refed every 2 to 3 days, and subcultured 5 to 8 days after plating, before growth was slowed by high cell density. Population doublings (PD) per passage was calculated as log2 (number of cells at time of subculture/number of cells plated). No correction was made for cells that failed to reattach or reinitiate growth at subculture. Cumulative PD was plotted against total time in culture to determine replicative life span and the onset of senescence or crisis. Immortalization was judged to have occurred if cells grew for at least 50 PD beyond the life span of the parent cell line.
Telomere length measurement.
Telomere length was estimated from the average terminal restriction fragment length, detected by hybridizing a 32P-labeled telomeric (CCCTAA)3 probe to HinfI- and RsaI-digested genomic DNA separated on agarose gels (15).
Western blots.
One-quarter- to one-half-confluent cultures were trypsinized, rinsed in phosphate-buffered saline (PBS), pelleted, lysed in 20 mM Tris buffer (pH 7.3) plus 2% sodium dodecyl sulfate plus a protease inhibitor cocktail, and sonically disrupted. Then 40 μg of protein (assayed with the Bio-Rad protein reagent) from each extract was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis with precast, 4 to 20% gradient polyacrylamide gels (Bio-Rad). Gels were electrotransferred to nitrocellulose paper, and proteins were detected with antibodies specific for p16INK4A (JC1) and p21cip1 (CP36) (both provided by E. Harlow, Massachusetts General Hospital, Boston) and for p14ARF (Neomarkers, Inc.), p53 (Pab-421 [Ab-1; Oncogene Sciences] and DO-1 [Santa Cruz Biotechnology]), and cdk4 (DCS-156) (36), followed by peroxidase-labeled secondary antibody (Southern Biotechnologies) and chemiluminescence reagent (ECL; Amersham Corp.).
Immunocytochemical staining and DNA damage response assessment.
Antibodies specific for the following antigens were used: bromodeoxyuridine (BUdR) (Pharmingen), p16INK4A JC2 (from E. Harlow, Massachusetts General Hospital, Boston) (17, 43), p53 (BP53.12; Zymed), p14ARF (Neomarkers, Inc.), involucrin (antibody SY5, from F. Watt, I.C.R.F. Laboratories, London, United Kingdom) (26), and keratin K10 (antibody AE20, from C. A. Loomis, N.Y.U. School of Medicine, New York) (37).
Cells growing on p100 dishes (Falcon/Becton Dickinson) were fixed in 4% paraformaldehyde-PBS for 30 min and permeabilized with 0.1% Triton X-100-PBS for 5 min. Keratinocytes growing in the fibroblast feeder cell system were fed for the final 18 h before fixation with FAD medium containing the reduced CaCl2 concentration of 0.1 mM to cause detachment of the upper, stratified, differentiated cells, thereby making the underlying cells in the potentially proliferative compartment accessible to antibodies. Some cultures received 0.5 nM actinomycin D for the final 2 days before fixation to elicit a DNA damage response. Some cultures received 0.1 mM BUdR for the final 20 h before fixation to label cells that were in S phase during this time period. After fixation and permeabilization, cultures that had received BUdR were treated for 1 h with 0.2 M HCl to make DNA containing BUdR accessible to the antibody. HCl-treated cultures were then neutralized with 0.1 M borate buffer (pH 8.5) and rinsed with PBS before proceeding with immunostaining. Bound antibody was detected with ABC peroxidase and NovaRed color substrate (Vector Labs). For double-label experiments to colocalize BUdR and p16INK4A, cultures were incubated first with anti-BUdR, detected with peroxidase and NovaRed, then rinsed and incubated with anti-p16INK4A and detected with alkaline phosphatase and Vector Blue color substrate.
Analysis for p16INK4A loss of heterozygosity.
Single-strand conformational polymorphism analysis to distinguish wild-type from mutant alleles of p16INK4A exon 2 was performed as described previously (68). DNA from 3 × 105 to 1.5 × 106 cells was isolated with the Qiagen DNeasy tissue kit (Qiagen Inc., Valencia, Calif.). Normal human placental DNA was used as a control. The primer pair F (forward) 5′-AGCCCAACTGCGCCGAC-3′ and R (reverse) 5′-GGAAGCTCTCAGGGTACAAATTC-3′ was used to amplify the segment of exon 2 of p16INK4A that spans codon 101, which is the site of the missense mutation inherited in heterozygous form by the donor of the K107 primary keratinocyte line.
RESULTS
Senescence arrest is closely associated with expression of p16INK4A for human keratinocytes in culture.
We and others have previously reported that p16 expression becomes upregulated in keratinocytes with increasing frequency as they are serially passaged, ultimately yielding a growth-arrested, senescent cell population in which all cells contain a high level of p16 protein (17, 31, 35). We investigated whether this p16 increase closely precedes growth arrest and is therefore likely to be the cause of senescence rather than an event that follows senescence arrest, as has been described for fibroblasts (3, 66). Because a recent report (52) concluded that p16-related senescence arrest does not occur at a significant frequency in keratinocytes cultured in the presence of fibroblast feeder cells, we examined keratinocytes grown in that culture system, which we had developed previously (4, 55), as well as cells grown in K-sfm, the semidefined medium system that we and others have used in previous studies of keratinocyte immortalization (17, 31).
Mid- to late life span cultures of the primary oral keratinocyte line OKF4, serially passaged in K-sfm beginning at the third passage or serially passaged always in the fibroblast feeder system, were fed with medium containing BUdR for 24 h before fixation and double immunostaining for BUdR and p16. As shown in Fig. 1, in both culture systems >90% of cells that were p16 negative or had low levels of p16 incorporated BUdR, indicating that they had been in S phase at some time during the previous day, whereas >90% of cells that contained moderate to high levels of p16 had not entered S phase during that time. We obtained the same results for two other primary keratinocyte lines derived from different individuals and grown in these two culture systems (data not shown). We concluded that p16 expression very closely precedes senescence arrest for the great majority of keratinocytes growing in either of these culture systems and therefore is likely to play a major role in limiting the replicative potential of primary human keratinocyte cell lines.
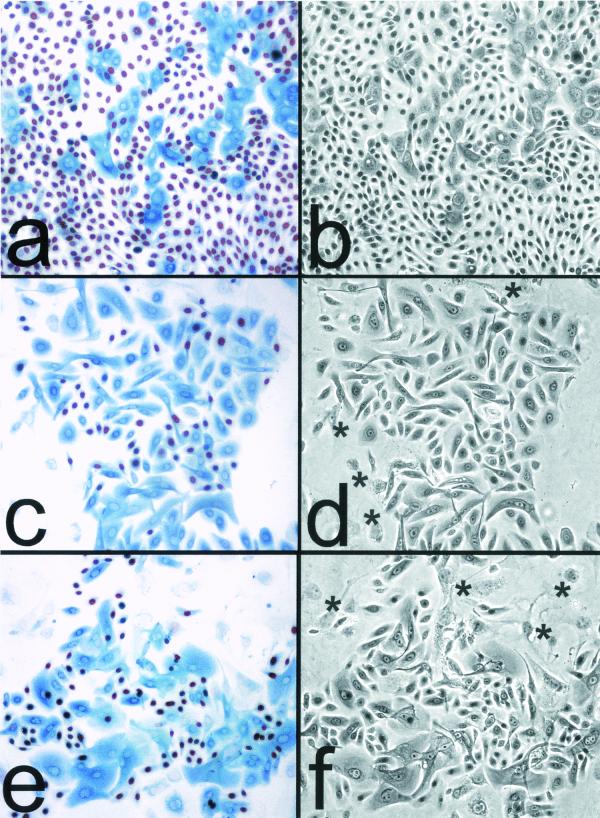
FIG. 1.
Cessation of division at the end of life span by primary human keratinocytes in culture is tightly linked to the expression and accumulation of p16INK4A. (a and b) Mid-life-span OKF4 cells serially passaged in K-sfm medium (at ≈25 PD of their ≈37-PD life span). (c and d) Late-life-span OKF4 cells serially passaged in the feeder fibroblast/FAD medium system (at ≈40 PD of their ≈45-PD life span). (e and f) Immortalized OKF4/TERT (early, feeders) cells serially passaged in the feeder fibroblast/FAD medium system (at 70 PD, which is 25 PD beyond the life span limit of the parent cell line). All three cell lines were plated at a low density (≈103 cells/cm2) and allowed to grow for 6 to 8 days before fixation. The fields shown are typical colonies that were the progeny of single cells. (a, c, and e) BUdR had been added to the medium 24 h before fixation, and cells were coimmunostained for p16 (blue) and for BUdR (red/brown). Asterisks in panels d and f indicate large, flat, p16- and BUdR-negative 3T3 fibroblast feeder cells at the periphery of keratinocyte colonies. Note that in the three cell lines shown, nearly all cells were cycling except for those containing moderate to high levels of p16. Small p16-positive cells are rare, indicating that cells become large, flat, and nondividing within several days of expressing p16. (b, d, and f) Phase-contrast images.
We had previously concluded from experiments examining strain N and OKF4 that serial passage of hTERT-transduced primary keratinocytes in the fibroblast feeder system versus K-sfm medium yields the same general result—namely, that immortalized lines that arise from hTERT-expressing keratinocytes typically represent a variant subpopulation that is partly or completely deficient in p16 expression (17). In light of recent work that concluded that growth in the feeder cell system abolishes induction of p16 and subsequent growth arrest in hTERT-expressing keratinocytes (52), we reexamined this question. We transduced OKF4 cells that had been serially passaged always in the fibroblast feeder system with pBABE(hTERT)puro, either relatively early (19 PD) or late (41 PD) in their ≈45-PD life span. The transductants were generated and drug selected in the feeder layer system, and this population was then divided, with half the cells passaged subsequently in the feeder cell system and half in K-sfm medium without feeder cells.
As shown in Fig. 2a and b, OKF4/TERT (early) cells yielded immortalized lines in both culture systems, although the K-sfm-grown cells exhibited a more prolonged “slow growth phase” (17) before more rapidly dividing cells took over the population. As shown in Fig. 2a, an inflection point is apparent in the growth curves of cells expressing hTERT whether they were grown in the presence or absence of fibroblast feeder cells. This inflection point occurred at the PD level at which untransduced control cells became senescent, consistent with the interpretation that the immortalized line arose from selective growth of a minor subpopulation or rare variant of the total hTERT-expressing cell population. As shown in Fig. 1e and f, the OKF4/TERT (early, feeders) immortalized line continued to generate nondividing, p16-positive cells during serial passage, as we had noted for several hTERT-immortalized lines arising in K-sfm as described in an earlier report (17). In contrast, OKF4 cells transduced to express hTERT late in their life span (Fig. 2b) did not yield immortalize lines when cultured in either the feeder cell or K-sfm system; the transduced population exhibited a modestly (≈10 PD) longer life span than untransduced control cells, but all the OKF4/TERT (late) cells senesced without emergence of an immortalized variant.
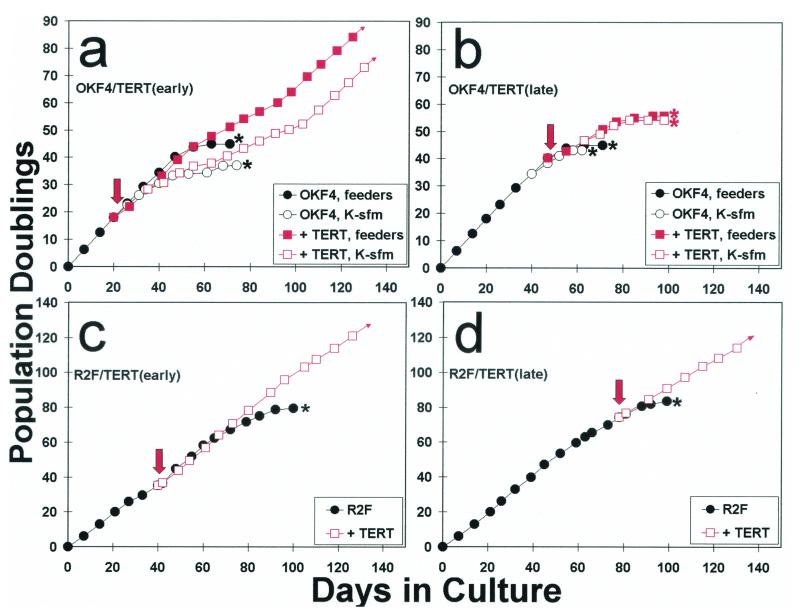
FIG. 2.
Ability of keratinocytes and fibroblasts to be immortalized by hTERT expression early versus late in their life spans. The primary oral keratinocyte line OKF4 and the primary dermal fibroblast line R2F were transduced with the pBABE(hTERT)puro vector at either an early (a and c) or late (b and d) stage of their respective life spans. Downward arrows indicate the time of transduction. The OKF4 parent line and hTERT transductants were cultured in the fibroblast feeder cell system until drug selection was complete, after which half the cells were subsequently cultured in the feeder system and half in K-sfm medium. ∗ indicates the PD level at which cell populations were completely senescent. Note that R2F fibroblasts were immortalized by hTERT expression at any time during their life span, whereas OKF4 keratinocytes were not immortalized if hTERT was expressed late in the life span in either culture system. OKF4 cells yielded immortalized lines when hTERT was expressed earlier in the life span, but a long period of initially slow population doubling rates preceded the appearance of more rapidly dividing immortalized cells for OKF4/TERT (early) cells cultured in K-sfm, and a modest inflection of the growth curve and progressive selection of more rapidly dividing cells were also evident for OKF4/TERT (early) cells cultured with feeder cells. Small arrowheads at the ends of some lines in the graphs indicate immortalization.
This result was very different from that which we obtained in a similar experiment with the primary dermal fibroblast line R2F. Immortalized lines resulted from transduction of these cells with pBABE(hTERT)puro whether it was introduced relatively early or late in the life span of this line, and no slow growth phase or inflection in the life span curve was apparent. This result is consistent with previous studies that concluded that prevention of telomere shortening is sufficient to prevent normal senescence and yield an immortalized phenotype in human dermal fibroblasts (7, 17, 31).
Expression of mutant cell cycle-regulatory proteins in primary human cells can specifically abolish endogenous p16INK4A and p53-dependent growth arrest mechanisms.
We next sought to determine experimentally whether p16 is an essential enforcer of keratinocyte senescence, in contrast to the p53/p21-dependent mechanism found previously to be responsible for fibroblast senescence (8, 12). Accordingly, we compared the effects of stably expressing dominant-negative mutant forms of two cell cycle-regulatory proteins that specifically abolish either the activity of p16 or the activity of p53 in primary cell lines. cdk4R24C (cdk4R) is a naturally occurring point mutant of cdk4 which is unable to bind p16 yet retains cyclin D-dependent kinase activity (74). p53DD is a C-terminal fragment of p53 that retains the multimerization domain but lacks the transcriptional transactivation domain of p53. When overexpressed in cells, p53DD forms transcriptionally inactive multimers with endogenous wild-type p53 protein (63).
Cells transduced with the pBABE(cdk4R)hygro or the pL(p53DD)SN retroviral vector overexpressed the respective protein (Fig. 3a). As expected, levels of endogenous, wild-type p53 increased greatly in cells expressing p53DD because the latter prevents p53-mediated transcriptional activation of mdm2, which normally ubiquitinates p53 and targets it for proteasomal degradation (45, 63). The cdk4R and p53DD proteins expressed by transductants were biologically active: cdk4R-expressing cells approaching the end of their normal life span were able to enter S phase even after they had expressed and accumulated high levels of endogenous p16 (Fig. 3b), in contrast to the behavior of normal control keratinocytes (Fig. 1a), and p53DD-expressing cells failed to undergo p53-mediated cell cycle arrest in response to DNA damage (Fig. 3c).
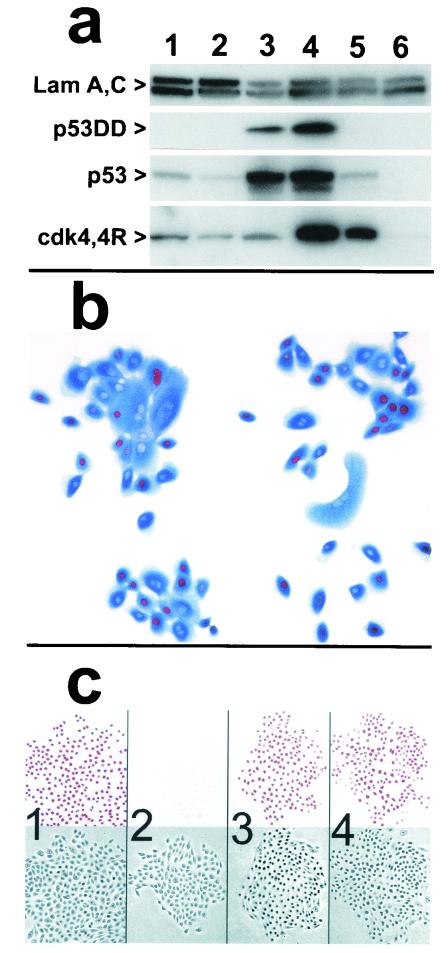
FIG. 3.
Expression and activity of cdk4R and p53DD in keratinocytes. (a) Western blots detecting expression of p53DD, p53, cdk4, the mutant cdk4R, and nuclear lamins A and C (as loading controls). Lane 1, strain N (34 PD); lane 2, strain N (44 PD); lane 3, N/p53DD (53 PD); lane 4, N/cdk4R/p53DD (69 PD); lane 5, N/cdk4R (46 PD); lane 6, POE9n (40 PD). Note elevated levels of endogenous p53 protein in p53DD-expressing cells and mutant cdk4R protein at severalfold higher levels than endogenous cdk4 protein in cdk4R-expressing cells. (b) cdk4R function in transductants. N/cdk4R cells at 54 PD (near senescence) treated with BUdR 24 h before fixation and stained immunocytochemically for BUdR (red) and p16 (blue). Note S-phase entry (BUdR incorporation) in cells containing high levels of p16. (c) Expression of p53DD in keratinocytes prevents growth arrest in response to DNA damage. Panels 1 and 2, strain N. Panels 3 and 4, N/p53DD. Cells shown in panels 2 and 4 were treated with 0.5 nM actinomycin D for the final 2 days before fixation. Cells shown in all panels were treated with 1 nM BUdR for the final 20 h before fixation. In each panel, the upper square shows BUdR immunocytochemical staining and the lower square is the phase contrast image of the same field. Note absence of normal growth arrest response in N/p53DD cells.
p53 but not p16INK4A plays an essential role in the mechanism responsible for enforcing replicative senescence in fibroblasts and mesothelial cells
We first assessed the effects of expressing cdk4R or p53DD on the replicative life spans of normal human dermal fibroblasts and peritoneal mesothelial cells, two cell types that were found previously to bypass senescence and become immortalized directly following expression of hTERT (7, 17). R2F fibroblasts transduced to express p53DD bypassed their normal senescence limit of 80 PD and continued to divide rapidly for an additional 43 PD (Fig. 4a; Table 1) before entering a state resembling crisis, in which some cells continued to cycle but there was a net decrease in cell number with each passage. This result confirms an earlier report (8) that expression of a dominant negative mutant p53 extends the life span of primary human fibroblasts.
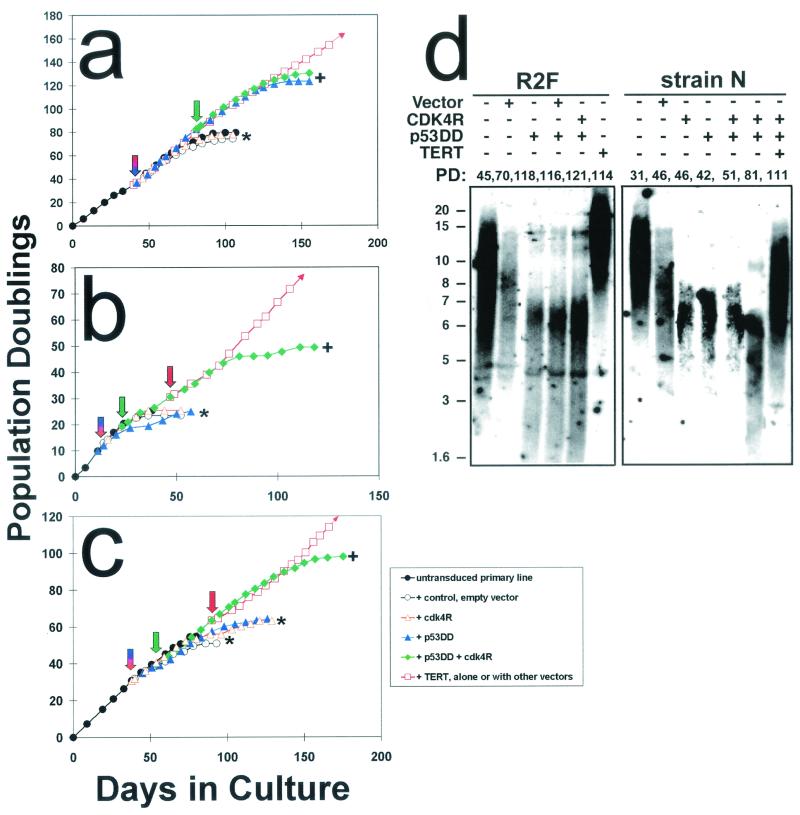
FIG. 4.
Effect on timing of senescence and susceptibility to hTERT immortalization of inhibiting p53- and p16INK4A-dependent growth arrest mechanisms. Graphs show life span progressions of human primary lines of the following different cell types and transductants thereof: (a) R2F fibroblasts, (b) HuCL-22 keratinocytes, and (c) strain N keratinocytes. Downward arrows indicate the points at which cells were stably transduced with the various vectors. Asterisk (∗) indicates the PD level at which senescence occurred for control cells and for transductants that did not exhibit significant life span extension. + indicates the PD level at which extended life span transductants ceased net population expansion. Small arrowheads at the ends of some lines in the graphs indicate immortalization. (d) Telomere status of normal fibroblasts and keratinocytes and derivatives engineered to bypass their respective senescence mechanisms. Terminal restriction fragment hybridization gels showing terminal restriction fragment lengths (in kilobases on the left) of fibroblast strain R2F and keratinocyte strain N control cells and designated transductants. The PD levels of the cultures analyzed are indicated above each lane.
TABLE 1.
Replicative life spans of primary human cell lines engineered to evade p16INK4A-, p53-, and telomere length-dependent growth arrest mechanisms
| Cell line (cell type) | PD (change from control)a for cells transfected in the presence of:
|
|||||
|---|---|---|---|---|---|---|
| No vector | Empty vector | p53DD | cdk4R | p53DD and cdk4R | p53DD, cdk4R, and hTERT | |
| R2F (dermal fibroblast) | 80 | 74 (−6) | 123 (+43*) | 78 (−2) | 130 (+50*) | n.d. (imm. by hTERT alone) |
| HM-3 (peritoneal mesothelial cell) | 43 | 40 (−3) | 65 (+22*) | 40 (−3) | n.d. | n.d. (imm. by hTERT alone) |
| HuCL-22 (corneal keratinocyte) | 24 | 23 (−1) | 22 (−2) | 25 (+1) | 49 (+25*) | >86 (imm.) |
| Strain N (epidermal keratinocyte) | 55 | 51 (−1) | 64 (+9) | 63 (+8) | 98 (+43*) | >114 (imm.) |
| OKF4 (oral mucosal keratinocyte) | 37 | 32 (−5) | 32 (−5) | 42 (+5) | 61 (+24*) | >76 (imm.) |
| ConjEp-1 (conjunctival keratinocyte) | 18 | n.d. | 17 (−1) | n.d. | 33 (+15*) | >52 (imm.) |
| K107 (p16INK4A+/− epidermal keratinocyte) | 23 | 22 (−1) | 54 (+31*) (p16−/− variant) | 27 (+4) | 54 (+31*) | >67 (imm.) |
*, extended life span but not immortal; imm, immortalized; n.d., not determined.
As we predicted, since human dermal fibroblasts do not exhibit a marked increase in p16-expressing cells as they approach senescence, expression of cdk4R in R2F had little or no effect on its replicative life span. Expression of cdk4R in R2F/p53DD cells also resulted in little change in its extended proliferative potential, also as expected. Expression of hTERT in R2F cells resulted in direct immortalization, confirming previous studies (7, 17). The primary mesothelial cell line HM3 responded similarly to R2F fibroblasts to the expression of cdk4R, p53DD, and hTERT (Table 1). Our results for fibroblasts and mesothelial cells were consistent with previous studies (5, 8, 12) and with a model in which replicative senescence of these cell types results from a p53-dependent cell cycle arrest instigated by telomere shortening.
Inhibition of the activities of both p16INK4A and p53 is necessary to bypass senescence and extend the replicative life span of keratinocytes.
Previous studies had suggested that keratinocyte life span is limited by a p16-dependent mechanism activated independently of telomere status (17, 20, 31). We therefore predicted that keratinocytes would respond differently from fibroblasts to expression of cdk4R and p53DD. As we expected, expression of p53DD in primary human keratinocyte lines cultured from four different stratified squamous epithelial tissues did not permit any of them to evade senescence (i.e., to divide significantly longer than the life span of the parent cell line) (Fig. 4b and c and Table 1). We were surprised to find that expression of cdk4R in these primary keratinocyte lines also resulted in little or no life span extension (Fig. 4b and c and Table 1) despite the demonstrable ability of cdk4R-expressing keratinocytes to enter S phase after having begun to express p16 (Fig. 3b).
When transduced to express both p53DD and cdk4R before they had reached the end of their normal life span, all four keratinocyte lines examined evaded senescence and continued to divide rapidly for an average of 27 PD (range, o 15 to 43 PD) beyond the normal limit of the respective primary line (Fig. 4b and c and Table 1). During their extended life span phase, cdk4R- and p53DD-transduced keratinocytes continued to experience telomere erosion (Fig. 4d) and ultimately ceased further population expansion in a crisis-like state, marked by slow proliferation and cell death without net population increase. At any time during their extended life span before crisis, p53DD and cdk4R double transductants could be “rescued” as immortalized cell lines by transduction to express hTERT (Fig. 4b and c and Table 1), which caused elongation and stabilization of telomere sequences (Fig. 4d). From these observations, we concluded that inhibition of both p16 and p53 function is required to evade keratinocyte senescence and that, additionally, expression of hTERT is required for immortalization.
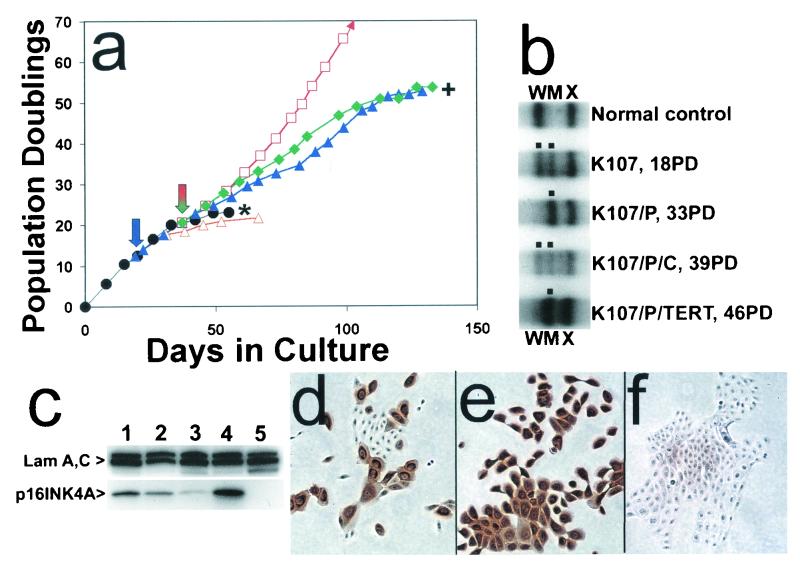
We wished to eliminate the possibility that the observed requirement for keratinocytes to lose p53 function in addition to p16 susceptibility in order to evade senescence was an artifact of employing cdk4R expression to permit keratinocytes that had accumulated high levels of endogenous p16 to continue dividing. We therefore examined the replicative life span, the integrity of the p16 locus, and the p16 protein expression status of K107, a primary keratinocyte line cultured from an individual who inherited a heterozygous loss-of-function point mutation (G101W) in exon 2 of p16INK4A. The wild-type and mutant alleles of K107 could be distinguished by single strand conformational polymorphism analysis, and the inactive protein product of the mutant allele has a very short half-life in cells (22), so that expression of that allele results in very low steady-state levels of immunochemically detectable p16 protein (70). As shown in Fig. 5a, K107 cells had a replicative life span of ≈23 PD. Immunocytochemical analysis disclosed that wild-type p16 protein accumulated in K107 cells as they became senescent, just as in genetically normal keratinocytes, and no p16-negative cells were observed in these cultures when the population had become completely senescent (data not shown).
FIG. 5.
Senescence bypass of p16INK4A+/− keratinocytes engineered to be p53 deficient, displayed by variants that spontaneously lose the wild-type p16INK4A allele. (a) Life span progression of the p16INKA+/− keratinocyte strain K107 and transductants. Symbols are as in Fig. 4. (b) PCR-based single-strand conformational polymorphism analysis detecting wild-type and mutant allele in K107 and transductants thereof at designated PD levels. W, wild-type allele; M, mutant allele; X, unrelated sequence. Note loss of wild-type p16 allele in extended life span K107/p53DD and K107/p53DD/TERT cells but retention of both alleles in K107/p53DD cells transduced to express cdk4R, which presumably acts as a phenocopy of p16 deficiency and removes selection pressure for loss of the wild-type allele. (c) Western blot analysis of p16 expression by K107 and transductants. Lane 1, strain N (40 PD); lane 2, K107 (18 PD); lane 3, K107/p53DD (44 PD); lane 4, K107/p53DD/cdk4R (46 PD); lane 5, POE9n (42 PD). Note the very faint p16 band, presumably of the unstable mutant p16 protein, in lane 3 and the high level of presumably wild-type p16 protein in lane 4. POE9n, which has a homozygous deletion at the p16INK4A locus, served as a negative control. (d to f) p16 immunocytochemical staining. (d) K107 (20 PD). (e) K107/p53DD/cdk4R (39 PD). (f) K107/p53DD (37 PD). Note that extended life span K107/p53DD cells in panel f, which retain only the mutant allele, stained very weakly, presumably because of the short half-life of this mutant p16.
When K107 cells were transduced to express both p53DD and cdk4R before the end of the line's normal life span, the resulting double transductants behaved in the same way as such transductants generated from genetically normal keratinocytes in that they bypassed their normal senescence limit and divided for an additional 31 PD (Fig. 5a and Table 1). Extended life span K107/p53DD/cdk4R cells retained both the mutant and wild-type p16INK4A alleles (Fig. 5b) and expressed high levels of p16 protein (Fig. 5c and e), just like normal keratinocytes transduced to express both p53DD and cdk4R. In contrast to genetically normal keratinocytes, however, K107 cells transduced to express p53DD alone continued to divide rapidly for an additional 31 PD past their normal senescence limit. K107/p53DD cells exhibiting this extended replicative life span expressed very little immunochemically detectable p16 protein (Fig. 5c and f) and were found to have lost the wild-type p16INK4A allele (Fig. 5b).
K107/p53DD cells transduced to express hTERT during their extended life span were immortalized. Thus, rare spontaneous p16INK4A−/− variants do arise within the K107 population during serial culture, but they have a selective advantage over p16INK4A+/− cells (in the form of a longer replicative life span) only if the separate, p53-dependent senescence arrest mechanism can be overcome. This result demonstrates the phenotypic equivalence in this system of cdk4R expression and loss of wild-type p16 expression. More importantly, we concluded from this and the previous experiments that keratinocyte replicative life span is limited by a mechanism that enforces senescence arrest by inducing or activating both p16 and p53.
p53-dependent component of the keratinocyte senescence mechanism is activated by a signal other than telomere shortening
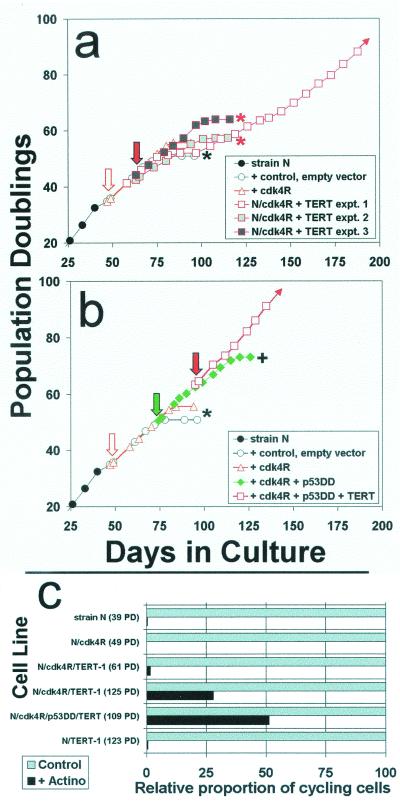
We initially interpreted the requirement for abolishing p53 in addition to p16 in order to evade keratinocyte senescence as indicating that telomere length-sensitive, p53-dependent arrest typically is activated in cells after the same number of population doublings as is the separate, p16-dependent arrest mechanism. If this hypothesis were true, then keratinocytes expressing cdk4R should escape senescence arrest if subsequently transduced to express either p53DD (thereby inhibiting the p53-dependent mechanism triggered by telomere shortening) or hTERT (thereby preventing telomere shortening such that p53 upregulation should never occur).
The results of an experiment to test this hypothesis are shown in Fig. 6. Strain N keratinocytes previously transduced at mid-life span to express cdk4R proved not to be immortalized as an immediate result of hTERT expression (Fig. 6a). In two of three such experiments, the N/cdk4R/TERT cell population senesced soon after the N/cdk4R cells did. In one of the three experiments, most of the N/cdk4R/TERT cells senesced and a rare, immortalized variant eventually emerged, similar to the result described following expression of hTERT in the parent strain N cell line (17). The precise nature of the heritable alteration acquired by the immortalized N/cdk4R/TERT variant remains to be characterized, but analysis of these cells disclosed a partially defective DNA damage response (Fig. 6c), consistent with a lesion in the p53-dependent growth arrest pathway.
FIG. 6.
Engineering keratinocytes to resist p16INK4A inhibition and to express hTERT is not sufficient to immortalize them. (a) Strain N cells were first transduced to express cdk4R. Then, in three separate experiments, aliquots of the N/cdk4R population were transduced to express hTERT and serially passaged. Downward arrows indicate timing of the respective transductions. Asterisks indicate complete senescence of the cell population. + indicates evasion of senescence and growth for a substantially extended life span. Note that in all three experiments, the cdk4R/TERT double transductants senesced at the same time or only slightly later than strains N and N/cdk4R. Only experiment 1 yielded a rare immortalized variant. (b) In another experiment, the same N/cdk4R line shown in panel a was first transduced to express p53DD and then to express hTERT. Note that the N/cdk4R/p53DD/TERT cells immediately exhibited an immortalized phenotype, without any inflection in the life span plot that would have suggested outgrowth of a rare variant. (c) Loss of the DNA damage response by rare variants of N/cdk4R/TERT-1 cells that evaded senescence and became immortalized. Cells growing in K-sfm medium were untreated or pretreated with low concentrations of actinomycin D tointroduce DNA strand breaks, received BUdR for the final 20 h before fixation, and were immunostained for BUdR as described in the text. From 150 to 500 cells in at least four randomly selected fields were scored for labeled nuclei. The labeling index (percent cycling cells) of control cultures was 70 to 80% for all lines shown. These control values were normalized to 100% (gray bars), and the relative numbers of labeled nuclei in the actinomycin-treated culture of each line (black bars) are shown in the graph. Note that the parent primary line strain N cells, presenescent N/cdk4R cells, and early N/cdk4R/TERT-1 cells exhibited the normal DNA damage response of cell cycle arrest. In contrast, the later-passage, rapidly dividing, immortalized N/cdk4/TERT-1 cultures and N/cdk4R/p53DD/TERT cultures contained a substantial frequency of cells that were not arrested and continued to cycle following DNA damage. Note also that N/TERT-1, a line previously shown to be deficient in p16 expression but having normal p53 function (17), displayed a normal DNA damage response, demonstrating that p16 deficiency and telomere stabilization alone do not impair this response.
As we had expected from the experiments described in Fig. 4c and Table 1, transduction of the N/cdk4R line to express p53DD resulted in substantial but finite life span extension, and the N/cdk4R/p53DD cells were directly immortalized by hTERT expression (Fig. 6b). This experiment provided further evidence that both the p16-dependent and p53-dependent components of the keratinocyte senescence mechanism that we had identified function as barriers to hTERT immortalization. More importantly, it disclosed that the p53-dependent component is activated independently of telomere shortening and therefore that p53 is involved in keratinocyte senescence in a different way than it has been proposed to be involved in fibroblast senescence.
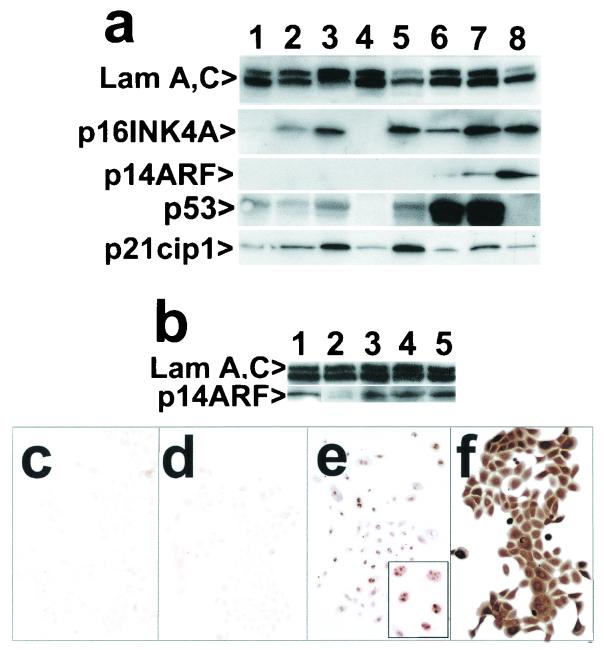
Increase in p14ARF expression occurs as engineered keratinocytes evade their normal life span limit.
Western blot analysis (Fig. 7a) disclosed progressively increasing levels of p16 and p21 in normal keratinocytes as they approached senescence. Some of the p21 increase presumably resulted from increased p53 activity in some cells in response to telomere shortening, but interestingly, even higher levels of p21 were found in late-life span, cdk4R-expressing cells. We investigated the possibility that p53 is activated in cdk4R-expressing keratinocytes as the result of an increase in expression of p14ARF, which increases p53 levels indirectly by inhibiting mdm2-mediated ubiquitination of p53 (65, 67, 71, 79).
FIG. 7.
Expression of p14INK4A, p21cip1, and p14ARF in normal keratinocytes and cells engineered to bypass senescence by evading p16- and p53-dependent cell cycle controls. (a) Western blot of strain N and derivatives. Lane 1, strain N (26 PD); lane 2, (44 PD); lane 3, strain N (55 PD); lane 4, POE9n (42 PD); lane 5, N/cdk4R (49 PD); lane 6, N/p53DD (55 PD); lane 7, N/cdk4R/p53DD (67 PD); lane 8, N/E6E7 (124 PD). Note that POE9n, which has a homozygous deletion at the p16/p14ARF locus, served as a negative control and N/E6E7 served as a positive control for expression of p16 and p14ARF proteins. (b) Western blot of K107 and derivatives. Lane 1, N/E6E7 (128 PD); lane 2, K107 (20 PD); lane 3, K107/p53DD (44 PD); lane 4, K107/p53DD/cdk4R (40 PD); lane 5, K107/p53DD/TERT (54 PD). (c to f) Immunocytochemical staining of strain N and derivatives for p14ARF (panels c to e) and for p16INK4A (panel f). (c) Strain N (51 PD); (d) N/p53DD/cdk4R (51 PD); (e and f) N/p53DD/cdk4R (58 PD). Inset in panel e is an enlargement showing the characteristic nucleolar location of p14ARF.
p14ARF was not expressed at detectable levels by normal keratinocytes approaching or at senescence or in cdk4R-expressing keratinocytes (Fig. 7a, b, and c), however. It was detectable immunocytochemically in only occasional p53DD transductants nearing senescence (data not shown). Western blot and immunocytochemical analysis revealed that p53DD transductants that also expressed cdk4R or that had become p16INK4A−/−, such that they could resist cell cycle arrest by both p14ARF and p16, had detectably elevated levels of p14ARF protein, identifiable in the nucleoli of most cells in these populations (Fig. 7). Importantly, this increase in p14ARF levels did not occur in p53DD/cdk4R-engineered cells as an immediate response to loss of p53- and p16-dependent cell cycle control. Instead, p14ARF protein was detected only after such engineered cells had proliferated beyond the PD level at which control cells had all expressed p16 and become senescent.
Our experimental method of blocking p53-dependent events by p53DD expression could not distinguish between p14ARF-instigated activation of endogenous p53 and other mechanisms that might accomplish such activation. However, the close correlation between the appearance of p14ARF and the timing of the p53-dependent component of senescence arrest raises the possibility that telomere-independent senescence in keratinocytes results from sequential induction of p16 and p14ARF, the latter occurring only if the former fails to arrest growth.
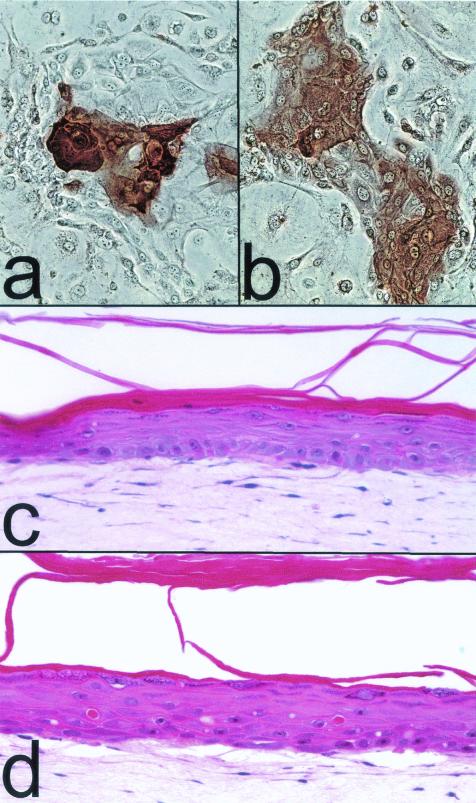
Keratinocytes engineered to evade p16INK4A- and p53-dependent arrest mechanisms divide rapidly and retain the ability to differentiate during their extended life span.
As shown in Fig. 4b and c, keratinocytes engineered to evade senescence arrest continued to divide rapidly until limited by a crisis presumably related to very short telomeres. These cells retained the ability to stratify and express suprabasal terminal differentiation proteins K10 and involucrin during their extended life span (Fig. 8a and b). Organotypic cultures of cdk4R- and p53DD-expressing epidermal keratinocytes that had been immortalized by hTERT expression and which had undergone a total of 116 PD stratified and underwent an epidermis-type suprabasal differentiation program, including formation of a granular cell layer and an enucleated stratum corneum (Fig. 8c and d). We did not analyze these cell lines for markers of hyperplasia during experimental tissue formation, as was reported recently to be exhibited by some hTERT-immortalized keratinocyte lines (20). We were able to conclude from these experiments, however, that keratinocytes engineered to evade arrest from the telomere-unrelated aging and senescence mechanism are not functionally impaired, so that, if they are able to maintain their telomeres, they can divide and differentiate for an indefinite period under the culture conditions used in these studies.
FIG. 8.
Differentiation potential of keratinocytes engineered to bypass senescence. (a and b) Epidermal keratinocytes transduced to express cdk4R and p53DD and cultured to within 10 PD of crisis, immunocytochemically stained for K10 (a) and involucrin (b). (c and d) Hematoxylin- and eosin-stained sections of organotypic cultures of the primary epidermal keratinocyte line strain N at 32 PD (c) and of N/cdk4R/p53DD/TERT at 116 PD (d).
DISCUSSION
Two-stage growth arrest mechanism, initiated by p16INK4A, restricts the replicative life span of keratinocytes.
We have demonstrated here that p16INK4A is an essential element of a growth arrest mechanism in keratinocytes that substantially limits their replicative potential in culture and can enforce a telomere status-independent barrier to immortalization. These results confirm and extend prior observations by several groups (17, 20, 31) indicating that simultaneous loss or relaxation of p16 and Rb control together with expression of active telomerase is necessary for immortalization of human keratinocytes whether such cells are cultured in an optimized keratinocyte medium formulation (K-sfm) or cocultured with fibroblast feeder cells. Surprisingly, we observed that blocking the interaction of p16 with cdk4 by expressing a dominantly acting mutant cdk4 (R24C) in the presence of active telomerase was not sufficient to immortalize human keratinocytes. Instead, we found that inhibition of p53-dependent control was additionally required for immortalization.
These results have defined the central elements of a model for keratinocyte aging and senescence that accommodates data reported previously by ourselves and others (17, 20, 31, 52) that are related to mechanisms that limit proliferative potential and restrict immortalization in this cell type. In this model, progressive telomere shortening and a telomere-independent cell aging process occur simultaneously in keratinocytes. Senescence arrest triggered by the latter, via p16 expression, occurs first in the majority of cells produced during growth of keratinocytes in culture and therefore is typically preeminent in determining the replicative life span, measured as expansion potential, of primary keratinocyte cell lines.
Keratinocyte senescence mechanism has a p53-dependent component which is telomere status independent.
Our study revealed an unexpected role for p53 as an element of the telomere-unrelated keratinocyte senescence mechanism. The p53-dependent component was sufficient to enforce senescence arrest in engineered, p16-resistant keratinocytes and in genetically p16-deficient keratinocytes, as demonstrated by the fact that such cells did not exhibit an extended life span unless they were also engineered to eliminate p53 function. Unlike the situation in fibroblasts, the p53-dependent component of this arrest mechanism that we identified proved not to be activated by telomere shortening. Keratinocytes engineered to express both cdk4R (to confer resistance to p16) and hTERT (to extend and maintain telomeres) senesced after approximately the same number of population doublings as did those of the control, parent cell line or cells expressing cdk4R alone. A rare, immortalized variant that arose from one N/cdk4R/TERT line was found to exhibit an impaired DNA damage response, consistent with acquisition of a lesion in the p53-dependent growth control pathway. It remains to be determined whether p14ARF, levels of which we observed to increase in extended-life-span keratinocytes, is an essential upstream mediator of the p53 component of senescence in this cell type. We can conclude that telomere stabilization (and consequent avoidance of a telomere length-sensitive, p53-dependent arrest) does not prevent activation of a p53-dependent immortalization barrier in keratinocytes.
Differences between fibroblast and keratinocyte senescence.
The results of our experiments to characterize the replicative senescence mechanism of human fibroblasts and mesothelial cells are consistent with previous studies of these cell types and of several others, including retinal pigmented epithelial cells and vascular endothelial cells. For these four cell types, the reported data (5, 7, 8, 12, 17, 78) support a model in which partial telomere shortening is recognized by the DNA damage response mechanism, triggering an increase in p53 activity, consequent induction of p21, and permanent cell cycle arrest. In our study, expression of a dominant negative mutant p53 in fibroblasts was sufficient to confer a long extended life span limited eventually by an apparent crisis associated with very short telomeres. In contrast, fibroblasts that we engineered by cdk4R expression to be resistant to p16 did not exhibit an extended life span, providing direct evidence that induction of increased p16 levels is not an essential element of the mechanism that normally limits fibroblast replicative potential. An increase in p16 levels has been detected previously in senescent fibroblasts, but only after they had already undergone growth arrest in response to increased p21 levels (3). A recent analysis of p21 and p16 expression in senescing fibroblasts and the effects of these inhibitors on cyclinE/cdk2 and cyclin D1/cdk4 activities also concluded that p16 is not responsible for initiating senescence arrest in this cell type (66).
Although p14ARF has been found to be an essential component of premature senescence in human fetal lung fibroblasts caused by experimental overexpression of E2F (18), recent reports have concluded that p14ARF is not involved in the p53-dependent DNA damage response pathway (30) or in the p53-dependent mechanism responsible for normal senescence in human skin fibroblasts (72). Consistent with the latter report, we found no increase in p14ARF protein levels in senescing fibroblasts or in p53DD-expressing, extended-life-span fibroblasts (data not shown), leading to our conclusion that the pathway leading to p53-dependent senescence arrest in fibroblasts is different from that which activates the p53-dependent component of the p16-initiated keratinocyte senescence mechanism.
Some discrepancy among results from different laboratories about whether Rb regulation plays a critical role in fibroblast senescence is likely to be associated with recently identified differences between dermal fibroblasts (such as the R2F primary line that we studied here) and primary fetal lung fibroblast lines (such as IMR-90 and WI-38), which have been reported recently to exhibit an unusual sensitivity to oxygen-induced damage that limits life span, possibly independent of telomere shortening and involving p16 expression [6, 75]). There are also important species differences with respect to the mechanisms responsible for limiting fibroblast life span. Several studies, culminating in recent reports comparing the proliferation potential and spontaneous immortalization frequency of murine embryo fibroblasts cultured from normal, p16INK4A-null, and ARF-null mice (32, 62), have demonstrated clearly that upregulation of ARF, reinforced by coordinate upregulation of p16, is responsible for determining the limited replicative potential of murine embryo fibroblasts in culture, a senescence mechanism that is not triggered by telomere shortening (64).
p16-related arrest mechanism is active and restricts the proliferation capacity of keratinocytes cultured in the feeder cell system and in defined culture medium formulations.
Our present study has demonstrated clearly that p16 upregulation eventually occurs in the vast majority of keratinocytes immediately preceding their senescence arrest in all media that support their proliferation, including the fibroblast feeder cell system (4, 55) and another, semidefined medium formulation (K-sfm) that is permissive for a long (i.e., >40 PD) replicative life span of some normal primary keratinocyte lines. As found in previous studies (17, 52), we observed an inflection in the growth curves of hTERT-expressing keratinocytes at the approximate PD level at which untransduced control cells upregulate p16 and become senescent. This inflection, which we had termed the slow growth phase (17), preceded the emergence of rapidly dividing, immortalized cell lines in both the feeder cell and K-sfm systems. As discussed below, the mechanism by which hTERT-expressing keratinocytes become immortalized without undergoing a complete loss of p16 expression remains to be determined. It is important to note that such lines can arise in both culture systems and that there is not an apparent qualitative difference in the activation of the p16-dependent senescence arrest mechanism and immortalization barrier between cells cultured in the two systems.
We used K-sfm medium for most of the experiments presented here and in an earlier study (17) characterizing p16-related keratinocyte senescence. This medium promotes rapid, exponential growth of primary keratinocytes, yielding colony-forming efficiencies during serial passage and replicative life spans for most primary lines we have tested that are similar to those achieved in the fibroblast feeder cell system (M. Ramsey and J. Rheinwald, unpublished data). The five oral epithelial and epidermal primary lines that were examined in the present and earlier (17) studies exhibited life spans of 23, 35, 37, 42, and 55 PD in K-sfm medium, compared with the ≈36-PD life span of the primary epidermal line cultured in the feeder layer system in a recent study (52). The two ocular keratinocyte primary lines that we examined in the present study, ConjEp-1 and HuCL-22, had shorter life spans of 18 and 24 PD, respectively, consistent with our earlier study of the in vitro replicative potential of conjunctival and corneal limbal keratinocytes in the feeder cell culture system (33).
One can readily calculate whether the expansion potential exhibited by normal keratinocytes in culture is consistent with the expected replication capacity of keratinocyte stem cells in vivo. The renewal rate of the human epidermis, expressed as average turnover or transit time of cells from the proliferative basal layer up through the differentiated layers and ultimate desquamation, has been estimated as about 1 month (73). Even if the renewal rate were twice as frequent, this would require formation during the maximum human life span of (130 years) × (24 new cells per year) = 3,120 ≈ 212 total cells from each original basal cell. Thus, a 12-PD replicative life span would endow the average cell in the basal, proliferative compartment with sufficient expansion potential to maintain a stratified squamous epithelium. Allowing for additional reserve capacity to accommodate occasional demands of wound repair and the possibility that the longest-lived stem cells comprise only 1/10 to 1/100 of all the basal cells in an epithelium, the life spans that we have observed for oral and epidermal keratinocytes cultured in K-sfm medium still exceed what is likely to be required of these cells in vivo.
Seeking upstream events in the pathway leading to p16INK4A-enforced keratinocyte senescence.
It is important to characterize the elements of the keratinocyte aging and senescence pathway that precede p16 expression. p16 does not appear to be expressed during normal renewal and differentiation of stratified squamous epithelia in vivo (43, 53), so it is possible that the stem cells and their differentiating progeny in these tissues normally elude p16-related senescence. The behavior of one cell line described in a recent study (52) suggested to the authors that, provided that hTERT-expressing keratinocytes are maintained in the feeder layer system, p16-enforced senescence can be avoided indefinitely and therefore yield an immortalized line that retains the normal p16 expression mechanism.
The BUdR and p16 double immunocytochemical staining data that we presented here (Fig. 1) showed that p16-associated senescence occurs in the great majority of cells serially passaged either in the feeder layer system or in K-sfm and that it persists, albeit with a reduced frequency of induction, in many hTERT-immortalized keratinocyte lines passaged continuously in the feeder cell system. The experiment shown in Fig. 2 suggests that the ability of the p16-initiated senescence mechanism to function as an absolute barrier to formation of an hTERT-immortalized line can vary with the stage during the life span at which hTERT is introduced and with the conditions of culture. Close examination of the shape of the life span curves reported here (Fig. 2) and in a previous study (17), as well as one recently reported by others (52), reveals that hTERT-transduced keratinocyte lines are not directly immortalized but undergo a progressive evolution toward more rapid population doubling times during serial passage. It will be important to determine whether the ultimately immortalized, rapidly dividing lines have acquired a lesion in some step of the p16-related aging/senescence pathway that reduces the probability with which p16-expressing cells are generated during serial passage to a level permitting indefinite population expansion, i.e., the immortalized phenotype.
Several recent studies of keratinocytes and other cell types have identified potential molecular candidates for regulators of steps in the keratinocyte aging/senescence pathway upstream of p16 induction. A role for the transcription factors Ets1 and Ets2 in the induction of p16 expression in response to hyperactivation of the ras-raf-MEK pathway in human fibroblasts has been reported (44). A correlation between loss of Id1 expression and an increase in p16 expression has been identified in human keratinocytes (42). Reports that Id1 overexpression in keratinocytes results in life span extension (42) and occasional evolution of an immortalized line (1) are consistent with the possibility that Id1 directly or indirectly acts as a repressor of the p16 promoter, as has been reported recently for mouse embryo fibroblasts (2). Bmi-1 has been found to repress the p16/ARF locus in mouse embryo fibroblasts (27), so this protein is another candidate for a regulator that could block expression of p16 in “young” keratinocytes.
Two especially interesting candidates for essential components of the pathway upstream of p16 induction in keratinocytes have been identified recently. One is 14-3-3σ, which is expressed in the suprabasal, differentiating cell layers of the epidermis in vivo and has been found to be upregulated in cultured human keratinocytes. Increased levels of this protein correlate with reduced proliferative potential and increased p16 expression as keratinocytes approach senescence, and expression of antisense 14-3-3σ RNA in keratinocytes permanently prevented p16 upregulation during serial passage and led to formation of immortalized lines expressing endogenous telomerase (16). Expression of p63, the predominant isotype of which acts as a p53 inhibitor (77), has been found to correlate with a long remaining replicative potential for keratinocytes cultured in the fibroblast feeder cell system (49). It remains to be determined whether, and by what mechanisms, alterations in the expression of these and other regulatory proteins during the course of keratinocyte aging ultimately cause p16 induction and consequent senescence arrest.
Toward understanding the function of keratinocyte aging and the p16INK4A-enforced senescence arrest mechanism in vivo.
Keratinocytes lacking p16, p14ARF, or p53 expression or function differentiate normally, as shown for human cells in our studies and in studies of knockout mice (19, 60). Immunohistochemical staining for p16 has revealed either no detectable expression or scattered expression of this protein with no apparent pattern in normal stratified squamous epithelia (43, 53). Thus, these proteins appear to have no essential role in the normal development, renewal, differentiation, or homeostasis of these tissues. p16INK4A and p14ARF deletions and deletions and point mutations of the p53 gene are detected in many premalignant dysplastic lesions of oral mucosal epithelium (10, 13, 38, 46), consistent with the conclusion that ability to express these proteins becomes important as a tumor suppressor mechanism at an early stage of neoplastic progression in stratified squamous epithelial tissues.
Integration and expression of the E6 and E7 oncoproteins of certain types of papillomaviruses, such as HPV16 and HPV18, are frequently found in premalignant dysplasias of the cervical epithelium. Among the functions of the E6 and E7 oncoproteins are inactivation of p53 and Rb (25). Extended replicative potential conferred by escape from p16 and p53 control, whether by mutation of cellular genes or acquired by HPV infection, would be predicted to greatly assist epithelial neoplastic progression in vivo.
Our results suggest that primary keratinocytes engineered to express cdk4R and p53DD should serve as valuable, well-defined models of premalignant human epithelial cells for investigating subsequent genetic and epigenetic events, including induction of endogenous hTERT expression, necessary to confer a malignant phenotype.
Acknowledgments
We thank K. Münger for the p53DD retroviral vector, I. Gipson for providing ocular tissue specimens, D. Galloway for the HPV16 E6E7 retroviral vector, and J. Koh, C. Ngwu, E. Harlow, J. Bartek, F. Watt, and C. Loomis for providing antibodies. We thank James Rocco for helpful discussions.
This research was supported by grant R01-DE13178 from the NIDCR to J.G.R.; by grants from CIBAvision, Inc., and Inspire Pharmaceuticals, Inc., to J.G.R.; by Oral Cancer Program Project grant PO1-DE12467 from the NIDCR and by a Doris Duke Charitable Foundation Clinical Scientist Award to W.C.H.; and by a Howard Temin Award (CA94223) from the U.S. National Cancer Institute to W.C.H.
The research described in this report resulted from an ongoing collaboration between the laboratories of J.G.R. and W.C.H. and included key contributions from each of the authors. The order of the authors reflects their relative contributions to the project, without reference to senior or junior investigator status.
Footnotes
This paper is dedicated to Howard Green and I. C. (Gunny) Gunsalus, who encouraged, inspired, and supported J.G.R. as a student to embark upon a career as research scientist 30 years ago.
REFERENCES
- 1.Alani, R. M., J. Hasskarl, M. Grace, M. C. Hernandez, M. A. Israel, and K. Munger. 1999. Immortalization of primary human keratinocytes by the helix-loop-helix protein, Id-1. Proc. Natl. Acad. Sci. USA 96:9637-9641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alani, R. M., A. Z. Young, and C. B. Shifflett. 2001. Id1 regulation of cellular senescence through transcriptional repression of p16/Ink4a. Proc. Natl. Acad. Sci. USA 98:7812-7816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alcorta, D. A., Y. Xiong, D. Phelps, G. Hannon, D. Beach, and J. C. Barrett. 1996. Involvement of the cyclin-dependent kinase inhibitor p16 (INK4a) in replicative senescence of normal human fibroblasts. Proc. Natl. Acad. Sci. USA 93:13742-13747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allen-Hoffmann, B. L., and J. G. Rheinwald. 1984. Polycyclic aromatic hydrocarbon mutagenesis of human epidermal keratinocytes in culture. Proc. Natl. Acad. Sci. USA 81:7802-7806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allsopp, R. C., H. Vaziri, C. Patterson, S. Goldstein, E. V. Younglai, A. B. Futcher, C. W. Greider, and C. B. Harley. 1992. Telomere length predicts replicative capacity of human fibroblasts. Proc. Natl. Acad. Sci. USA 89:10114-10118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atamna, H., A. Paler-Martinez, and B. N. Ames. 2000. N-t-Butyl hydroxylamine, a hydrolysis product of α-phenyl-N-t-butyl nitrone, is more potent in delaying senescence in human lung fibroblasts. J. Biol. Chem. 275:6741-6748. [DOI] [PubMed] [Google Scholar]
- 7.Bodnar, A. G., M. Ouellette, M. Frolkis, S. E. Holt, C. P. Chiu, G. B. Morin, C. B. Harley, J. W. Shay, S. Lichtsteiner, and W. E. Wright. 1998. Extension of life-span by introduction of telomerase into normal human cells. Science 279:349-352. [DOI] [PubMed] [Google Scholar]
- 8.Bond, J. A., F. S. Wyllie, and D. Wynford-Thomas. 1994. Escape from senescence in human diploid fibroblasts induced directly by mutant p53. Oncogene 9:1885-1889. [PubMed] [Google Scholar]
- 9.Boyce, S. T., and R. G. Ham. 1983. Calcium-regulated differentiation of normal human epidermal keratinocytes in chemically defined clonal culture and serum-free serial culture. J. Investig. Dermatol. 81:33s-40s. [DOI] [PubMed] [Google Scholar]
- 10.Boyle, J. O., J. Hakim, W. Koch, P. van der Riet, R. H. Hruban, R. A. Roa, R. Correo, Y. J. Eby, J. M. Ruppert, and D. Sidransky. 1993. The incidence of p53 mutations increases with progression of head and neck cancer. Cancer Res. 53:4477-4480. [PubMed] [Google Scholar]
- 11.Brenner, A. J., M. R. Stampfer, and C. M. Aldaz. 1998. Increased p16 expression with first senescence arrest in human mammary epithelial cells and extended growth capacity with p16 inactivation. Oncogene 17:199-205. [DOI] [PubMed] [Google Scholar]
- 12.Brown, J. P., W. Wei, and J. M. Sedivy. 1997. Bypass of senescence after disruption of p21CIP1/WAF1 gene in normal diploid human fibroblasts. Science 277:831-834. [DOI] [PubMed] [Google Scholar]
- 13.Califano, J., P. van der Riet, W. Westra, H. Nawroz, G. Clayman, S. Piantadosi, R. Corio, D. Lee, B. Greenberg, W. Koch, and D. Sidransky. 1996. Genetic progression model for head and neck cancer: implications for field cancerization. Cancer Res. 56:2488-2492. [PubMed] [Google Scholar]
- 14.Connell, N. D., and J. G. Rheinwald. 1983. Regulation of the cytoskeleton in mesothelial cells: reversible loss of keratin and increase in vimentin during rapid growth in culture. Cell 34:245-253. [DOI] [PubMed] [Google Scholar]
- 15.Counter, C. M., F. M. Botelho, P. Wang, C. B. Harley, and S. Bacchetti. 1994. Stabilization of short telomeres and telomerase activity accompany immortalization of Epstein-Barr virus-transformed human B lymphocytes. J. Virol. 68:3410-3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dellambra, E., O. Golisano, S. Bondanza, E. Siviero, P. Lacal, M. Molinari, S. D'Atri, and M. De Luca. 2000. Downregulation of 14-3-3sigma prevents clonal evolution and leads to immortalization of primary human keratinocytes. J. Cell Biol. 149:1117-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dickson, M. A., W. C. Hahn, Y. Ino, V. Ronfard, J. Y. Wu, R. A. Weinberg, D. N. Louis, F. P. Li, and J. G. Rheinwald. 2000. Human keratinocytes that express hTERT and also bypass a p16INK4a-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol. Cell. Biol. 20:1436-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dimri, G. P., K. Itahana, M. Acosta, and J. Campisi. 2000. Regulation of a senescence checkpoint response by the E2F1 transcription factor and p14ARF tumor suppressor. Mol. Cell. Biol. 20:273-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donehower, L. A., M. Harvey, B. L. Slagle, M. J. McArthur, C. A. Montgomery, Jr., J. S. Butel, and A. Bradley. 1992. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature 356:215-221. [DOI] [PubMed] [Google Scholar]
- 20.Farwell, D. G., K. A. Shera, J. I. Koop, G. A. Bonnet, C. P. Matthews, G. W. Reuther, M. D. Coltrera, J. K. McDougall, and A. J. Klingelhutz. 2000. Genetic and epigenetic changes in human epithelial cells immortalized by telomerase. Am. J. Pathol. 156:1537-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foster, S. A., D. J. Wong, M. T. Barrett, and D. A. Galloway. 1998. Inactivation of p16 in human mammary epithelial cells by CpG island methylation. Mol. Cell. Biol. 18:1793-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gombart, A. F., R. Yang, M. J. Campbell, J. D. Berman, and H. P. Koeffler. 1997. Inhibition of growth of human leukemia cell lines by retrovirally expressed wild-type p16INK4A. Leukemia 11:1673-1680. [DOI] [PubMed] [Google Scholar]
- 23.Gottlieb, E., R. Haffner, T. von Ruden, E. F. Wagner, and M. Oren. 1994. Down-regulation of wild-type p53 activity interferes with apoptosis of IL-3-dependent hematopoietic cells following IL-3 withdrawal. EMBO J. 13:1368-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hahn, W. C., C. M. Counter, A. S. Lundberg, R. L. Beijersbergen, M. W. Brooks, and R. A. Weinberg. 1999. Creation of human tumour cells with defined genetic elements. Nature 400:464-468. [DOI] [PubMed] [Google Scholar]
- 25.Howley, P. M., and D. R. Lowy. 2001. Papillomaviruses and their replication, p. 2198-2229. In D. M. Knipe and P. M. Howley (ed.), Virology. Lippincott, Philadelphia, Pa.
- 26.Hudson, D. L., K. L. Weiland, T. P. Dooley, M. Simon, and F. M. Watt. 1992. Characterisation of eight monoclonal antibodies to involucrin. Hybridoma 11:367-379. [DOI] [PubMed] [Google Scholar]
- 27.Jacobs, J. J., K. Kieboom, S. Marino, R. A. DePinho, and M. van Lohuizen. 1999. The oncogene and Polycomb-group gene bmi-1 regulates cell proliferation and senescence through the ink4a locus. Nature 397:164-168. [DOI] [PubMed] [Google Scholar]
- 28.Jarrard, D. F., S. Sarkar, Y. Shi, T. R. Yeager, G. Magrane, H. Kinoshita, N. Nassif, L. Meisner, M. A. Newton, F. M. Waldman, and C. A. Reznikoff. 1999. p16/pRb pathway alterations are required for bypassing senescence in human prostate epithelial cells. Cancer Res. 59:2957-2964. [PubMed] [Google Scholar]
- 29.Johnson, E. W., S. F. Meunier, C. J. Roy, and N. L. Parenteau. 1992. Serial cultivation of normal human keratinocytes: a defined system for studying the regulation of growth and differentiation. In Vitro Cell Dev. Biol. 28A:429-435. [DOI] [PubMed] [Google Scholar]
- 30.Kamijo, T., E. van de Kamp, M. J. Chong, F. Zindy, J. A. Diehl, C. J. Sherr, and P. J. McKinnon. 1999. Loss of the ARF tumor suppressor reverses premature replicative arrest but not radiation hypersensitivity arising from disabled atm function. Cancer Res. 59:2464-2469. [PubMed] [Google Scholar]
- 31.Kiyono, T., S. A. Foster, J. I. Koop, J. K. McDougall, D. A. Galloway, and A. J. Klingelhutz. 1998. Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature 396:84-88. [DOI] [PubMed] [Google Scholar]
- 32.Krimpenfort, P., K. C. Quon, W. J. Mooi, A. Loonstra, and A. Berns. 2001. Loss of p16Ink4a confers susceptibility to metastatic melanoma in mice. Nature 413:83-86. [DOI] [PubMed] [Google Scholar]
- 33.Lindberg, K., M. E. Brown, H. V. Chaves, K. R. Kenyon, and J. G. Rheinwald. 1993. In vitro propagation of human ocular surface epithelial cells for transplantation. Investig. Ophthalmol. Vis. Sci. 34:2672-2679. [PubMed] [Google Scholar]
- 34.Lindberg, K., and J. G. Rheinwald. 1990. Three distinct keratinocyte subtypes identified in human oral epithelium by their patterns of keratin expression in culture and in xenografts. Differentiation 45:230-241. [DOI] [PubMed] [Google Scholar]
- 35.Loughran, O., A. Malliri, D. Owens, P. H. Gallimore, M. A. Stanley, B. Ozanne, M. C. Frame, and E. K. Parkinson. 1996. Association of CDKN2A/p16INK4A with human head and neck keratinocyte replicative senescence: relationship of dysfunction to immortality and neoplasia. Oncogene 13:561-568. [PubMed] [Google Scholar]
- 36.Lukas, C., S. K. Jensen, J. Bartkova, J. Lukas, and J. Bartek. 1999. Immunohistochemical analysis of the D-type cyclin-dependent kinases Cdk4 and Cdk6, with a series of monoclonal antibodies. Hybridoma 18:225-234. [DOI] [PubMed] [Google Scholar]
- 37.Manabe, M., H. W. Lim, M. Winzer, and C. A. Loomis. 1999. Architectural organization of filiform papillae in normal and black hairy tongue epithelium: dissection of differentiation pathways in a complex human epithelium according to their patterns of keratin expression. Arch. Dermatol. 135:177-181. [DOI] [PubMed] [Google Scholar]
- 38.Mao, L., J. S. Lee, Y. H. Fan, J. Y. Ro, J. G. Batsakis, S. Lippman, W. Hittelman, and W. K. Hong. 1996. Frequent microsatellite alterations at chromosomes 9p21 and 3p14 in oral premalignant lesions and their value in cancer risk assessment. Nat. Med. 2:682-685. [DOI] [PubMed] [Google Scholar]
- 39.Miller, A. D., and G. J. Rosman. 1989. Improved retroviral vectors for gene transfer and expression. BioTechniques 7:980-982, 984-986, 989-990. [PMC free article] [PubMed] [Google Scholar]
- 40.Morgenstern, J. P., and H. Land. 1990. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 18:3587-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murphy, J. E., and J. G. Rheinwald. 1997. Intraperitoneal injection of genetically modified, human mesothelial cells for systemic gene therapy. Hum. Gene Ther. 8:1867-1879. [DOI] [PubMed] [Google Scholar]
- 42.Nickoloff, B. J., V. Chaturvedi, P. Bacon, J. Z. Qin, M. F. Denning, and M. O. Diaz. 2000. Id-1 delays senescence but does not immortalize keratinocytes. J. Biol. Chem. 275:27501-27504. [DOI] [PubMed] [Google Scholar]
- 43.Nielsen, G. P., A. O. Stemmer-Rachamimov, J. Shaw, J. E. Roy, J. Koh, and D. N. Louis. 1999. Immunohistochemical survey of p16INK4A expression in normal human adult and infant tissues. Lab. Investig. 79:1137-1143. [PubMed] [Google Scholar]
- 44.Ohtani, N., Z. Zebedee, T. J. Huot, J. A. Stinson, M. Sugimoto, Y. Ohashi, A. D. Sharrocks, G. Peters, and E. Hara. 2001. Opposing effects of Ets and Id proteins on p16INK4a expression during cellular senescence. Nature 409:1067-1070. [DOI] [PubMed] [Google Scholar]
- 45.Oren, M. 1999. Regulation of the p53 tumor suppressor protein. J. Biol. Chem. 274:36031-36034. [DOI] [PubMed] [Google Scholar]
- 46.Papadimitrakopoulou, V., J. Izzo, S. M. Lippman, J. S. Lee, Y. H. Fan, G. Clayman, J. Y. Ro, W. N. Hittelman, R. Lotan, W. K. Hong, and L. Mao. 1997. Frequent inactivation of p16INK4a in oral premalignant lesions. Oncogene. 14:1799-1803. [DOI] [PubMed] [Google Scholar]
- 47.Parenteau, N. L., C. M. Nolte, P. Bilbo, M. Rosenberg, L. M. Wilkins, E. W. Johnson, S. Watson, V. S. Mason, and E. Bell. 1991. Epidermis generated in vitro: practical considerations and applications. J. Cell Biochem. 45:245-251. [DOI] [PubMed] [Google Scholar]
- 48.Parry, D., S. Bates, D. J. Mann, and G. Peters. 1995. Lack of cyclin D-Cdk complexes in Rb-negative cells correlates with high levels of p16INK4/MTS1 tumour suppressor gene product. EMBO J. 14:503-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pellegrini, G., E. Dellambra, O. Golisano, E. Martinelli, I. Fantozzi, S. Bondanza, D. Ponzin, F. McKeon, and M. De Luca. 2001. p63 identifies keratinocyte stem cells. Proc. Natl. Acad. Sci. USA 98:3156-3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pirisi, L., S. Yasumoto, M. Feller, J. Doniger, and J. A. DiPaolo. 1987. Transformation of human fibroblasts and keratinocytes with human papillomavirus type 16 DNA. J. Virol. 61:1061-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Puthenveettil, J. A., M. S. Burger, and C. A. Reznikoff. 1999. Replicative senescence in human uroepithelial cells. Adv. Exp. Med. Biol. 462:83-91. [DOI] [PubMed] [Google Scholar]
- 52.Ramirez, R. D., C. P. Morales, B. S. Herbert, J. M. Rohde, C. Passons, J. W. Shay, and W. E. Wright. 2001. Putative telomere-independent mechanisms of replicative aging reflect inadequate growth conditions. Genes Dev. 15:398-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reed, A. L., J. Califano, P. Cairns, W. H. Westra, R. M. Jones, W. Koch, S. Ahrendt, Y. Eby, D. Sewell, H. Nawroz, J. Bartek, and D. Sidransky. 1996. High frequency of p16 (CDKN2/MTS-1/INK4A) inactivation in head and neck squamous cell carcinoma. Cancer Res. 56:3630-3633. [PubMed] [Google Scholar]
- 54.Rheinwald, J. G., and M. A. Beckett. 1980. Defective terminal differentiation in culture as a consistent and selectable character of malignant human keratinocytes. Cell 22:629-632. [DOI] [PubMed] [Google Scholar]
- 55.Rheinwald, J. G., and H. Green. 1975. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell 6:331-343. [DOI] [PubMed] [Google Scholar]
- 56.Romanov, S. R., B. K. Kozakiewicz, C. R. Holst, M. R. Stampfer, L. M. Haupt, and T. D. Tlsty. 2001. Normal human mammary epithelial cells spontaneously escape senescence and acquire genomic changes. Nature 409:633-637. [DOI] [PubMed] [Google Scholar]
- 57.Sandhu, C., D. M. Peehl, and J. Slingerland. 2000. p16INK4A mediates cyclin dependent kinase 4 and 6 inhibition in senescent prostatic epithelial cells. Cancer Res. 60:2616-2622. [PubMed] [Google Scholar]
- 58.Schon, M., and J. G. Rheinwald. 1996. A limited role for retinoic acid and retinoic acid receptors RAR alpha and RAR beta in regulating keratin 19 expression and keratinization in oral and epidermal keratinocytes. J. Investig. Dermatol. 107:428-438. [DOI] [PubMed] [Google Scholar]
- 59.Serrano, M., G. J. Hannon, and D. Beach. 1993. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature 366:704-707. [DOI] [PubMed] [Google Scholar]
- 60.Serrano, M., H. Lee, L. Chin, C. Cordon-Cardo, D. Beach, and R. A. DePinho. 1996. Role of the INK4a locus in tumor suppression and cell mortality. Cell 85:27-37. [DOI] [PubMed] [Google Scholar]
- 61.Shapiro, G. I., C. D. Edwards, M. E. Ewen, and B. J. Rollins. 1998. p16INK4A participates in a G1 arrest checkpoint in response to DNA damage. Mol. Cell. Biol. 18:378-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sharpless, N. E., N. Bardeesy, K. H. Lee, D. Carrasco, D. H. Castrillon, A. J. Aguirre, E. A. Wu, J. W. Horner, and R. A. DePinho. 2001. Loss of p16Ink4a with retention of p19Arf predisposes mice to tumorigenesis. Nature 413:86-91. [DOI] [PubMed] [Google Scholar]
- 63.Shaulian, E., A. Zauberman, D. Ginsberg, and M. Oren. 1992. Identification of a minimal transforming domain of p53: negative dominance through abrogation of sequence-specific DNA binding. Mol. Cell. Biol. 12:5581-5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sherr, C. J., and R. A. DePinho. 2000. Cellular senescence: mitotic clock or culture shock? Cell 102:407-410. [DOI] [PubMed] [Google Scholar]
- 65.Sherr, C. J., and J. D. Weber. 2000. The ARF/p53 pathway. Curr. Opin. Genet. Dev. 10:94-99. [DOI] [PubMed] [Google Scholar]
- 66.Stein, G. H., L. F. Drullinger, A. Soulard, and V. Dulic. 1999. Differential roles for cyclin-dependent kinase inhibitors p21 and p16 in the mechanisms of senescence and differentiation in human fibroblasts. Mol. Cell. Biol. 19:2109-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tao, W., and A. J. Levine. 1999. p19(ARF) stabilizes p53 by blocking nucleo-cytoplasmic shuttling of Mdm2. Proc. Natl. Acad. Sci. USA 96:6937-6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tsao, H., X. Zhang, K. Kwitkiwski, D. M. Finkelstein, A. J. Sober, and F. G. Haluska. 2000. Low prevalence of germline CDKN2A and CDK4 mutations in patients with early-onset melanoma. Arch. Dermatol. 136:1118-1122. [DOI] [PubMed] [Google Scholar]
- 69.Tubo, R. A., and J. G. Rheinwald. 1987. Normal human mesothelial cells and fibroblasts transfected with the EJras oncogene become EGF-independent, but are not malignantly transformed. Oncogene Res. 1:407-421. [PubMed] [Google Scholar]
- 70.Walker, G. J., B. G. Gabrielli, M. Castellano, and N. K. Hayward. 1999. Functional reassessment of P16 variants with a transfection-based assay. Int. J. Cancer 82:305-312. [DOI] [PubMed] [Google Scholar]
- 71.Weber, J. D., L. J. Taylor, M. F. Roussel, C. J. Sherr, and D. Bar-Sagi. 1999. Nucleolar Arf sequesters Mdm2 and activates p53. Nat. Cell Biol. 1:20-26. [DOI] [PubMed] [Google Scholar]
- 72.Wei, W., R. M. Hemmer, and J. M. Sedivy. 2001. Role of p14ARF in replicative and induced senescence of human fibroblasts. Mol. Cell. Biol. 21:6748-6757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weinstein, G. D., and E. J. Van Scott. 1965. Autoradiographic analysis of turnover times of normal and psoriatic epidermis. J. Investig. Dermatol. 45:257-262. [DOI] [PubMed] [Google Scholar]
- 74.Wolfel, T., M. Hauer, J. Schneider, M. Serrano, C. Wolfel, E. Klehmann-Hieb, E. De Plaen, T. Hankeln, K. H. Meyer zum Buschenfelde, and D. Beach. 1995. A p16INK4a-insensitive CDK4 mutant targeted by cytolytic T lymphocytes in a human melanoma. Science 269:1281-1284. [DOI] [PubMed] [Google Scholar]
- 75.Wright, W. E., and J. W. Shay. 2001. Cellular senescence as a tumor-protection mechanism: the essential role of counting. Curr. Opin. Genet. Dev. 11:98-103. [DOI] [PubMed] [Google Scholar]
- 76.Wu, Y. J., L. M. Parker, N. E. Binder, M. A. Beckett, J. H. Sinard, C. T. Griffiths, and J. G. Rheinwald. 1982. The mesothelial keratins: a new family of cytoskeletal proteins identified in cultured mesothelial cells and nonkeratinizing epithelia. Cell 31:693-703. [DOI] [PubMed] [Google Scholar]
- 77.Yang, A., M. Kaghad, Y. Wang, E. Gillett, M. D. Fleming, V. Dotsch, N. C. Andrews, D. Caput, and F. McKeon. 1998. p63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol. Cell 2:305-316. [DOI] [PubMed] [Google Scholar]
- 78.Yang, J., E. Chang, A. M. Cherry, C. D. Bangs, Y. Oei, A. Bodnar, A. Bronstein, C. P. Chiu, and G. S. Herron. 1999. Human endothelial cell life extension by telomerase expression. J. Biol. Chem. 274:26141-26148. [DOI] [PubMed] [Google Scholar]
- 79.Zhang, Y., Y. Xiong, and W. G. Yarbrough. 1998. ARF promotes MDM2 degradation and stabilizes p53: ARF-INK4a locus deletion impairs both the Rb and p53 tumor suppression pathways. Cell 92:725-734. [DOI] [PubMed] [Google Scholar]