Abstract
Background
Members of the matrix metalloproteinase (MMP) family of proteases are required for the degradation of the basement membrane and extracellular matrix in both normal and pathological conditions. In vitro, MT1-MMP (MMP-14, membrane type-1-MMP) expression is higher in more invasive human breast cancer (HBC) cell lines, whilst in vivo its expression has been associated with the stroma surrounding breast tumours. MMP-1 (interstitial collagenase) has been associated with MDA-MB-231 invasion in vitro, while MMP-3 (stromelysin-1) has been localised around invasive cells of breast tumours in vivo. As MMPs are not stored intracellularly, the ability to localise their expression to their cells of origin is difficult.
Methods
We utilised the unique in situ-reverse transcription-polymerase chain reaction (IS-RT-PCR) methodology to localise the in vitro and in vivo gene expression of MT1-MMP, MMP-1 and MMP-3 in human breast cancer. In vitro, MMP induction was examined in the MDA-MB-231 and MCF-7 HBC cell lines following exposure to Concanavalin A (Con A). In vivo, we examined their expression in archival paraffin embedded xenografts derived from a range of HBC cell lines of varied invasive and metastatic potential. Mouse xenografts are heterogenous, containing neoplastic human parenchyma with mouse stroma and vasculature and provide a reproducible in vivo model system correlated to the human disease state.
Results
In vitro, exposure to Con A increased MT1-MMP gene expression in MDA-MB-231 cells and decreased MT1-MMP gene expression in MCF-7 cells. MMP-1 and MMP-3 gene expression remained unchanged in both cell lines. In vivo, stromal cells recruited into each xenograft demonstrated differences in localised levels of MMP gene expression. Specifically, MDA-MB-231, MDA-MB-435 and Hs578T HBC cell lines are able to influence MMP gene expression in the surrounding stroma.
Conclusion
We have demonstrated the applicability and sensitivity of IS-RT-PCR for the examination of MMP gene expression both in vitro and in vivo. Induction of MMP gene expression in both the epithelial tumour cells and surrounding stromal cells is associated with increased metastatic potential. Our data demonstrate the contribution of the stroma to epithelial MMP gene expression, and highlight the complexity of the role of MMPs in the stromal-epithelial interactions within breast carcinoma.
Background
Human breast carcinoma (HBC) is the most predominant cancer in females worldwide. Breast cancers can be classified histologically based upon the types and patterns of cells of which they are composed. Carcinomas can be invasive, extending into the surrounding stroma or non-invasive, confined to the epithelial cells of ducts or lobules [1]. In conjunction with other protease systems such as the serine, cysteine and aspartyl proteases, members of the matrix metalloprotease (MMP) family of proteolytic enzymes degrade constituents of the extracellular matrix surrounding invasive breast carcinomas [2]. Currently, 28 human MMPs have been identified and classified according to both their substrate specificities and structural similarities. There are four major subgroups: i) interstitial collagenases; ii) gelatinases; iii) stromelysins; and iv) the membrane-type (MT) -MMPs [3,4]. Collectively, MMPs degrade all extracellular matrix proteins as well as a growing number of key regulatory genes such as cytokines, growth factors, cell surface receptors and adhesion molecules [5,6]. Although MMPs are expressed by tissues at various stages of development, they are typically absent in normal cells of the adult organism [2], and the high frequency at which MMP transcripts or proteins are detected in invasive tumours has implicated these enzymes in the establishment, growth, invasion and/or metastasis of tumours [6]. The expression of MMPs is usually tightly regulated, and a number of studies provide evidence of carefully controlled MMP involvement in developmentally regulated processes such as ovulation, embryogenic growth and differentiation, and organ development [6-8]. In general terms, MMP activity is regulated at least at three levels: transcription/translation, proteolytic activation of the zymogen, and inhibition of the active enzyme [2] with upregulation of each of these associated with pathological events [6]. Importantly in all mammalian cells except polymorphonuclear (PMN) leucocytes, most MMPs are not stored intracellularly but are rapidly secreted after biosynthesis and post-translational processing [9]. As a result it is difficult to identify their cellular origin using standard immunohistochemical techniques [10].
MT1-MMP is one of the membrane bound MMPs. In vivo, MT1-MMP expression has been localised to the stroma surrounding breast tumours [11,12], whilst in vitro, our recent data confirms previous studies where basal levels of MT1-MMP have been shown to be higher in the more invasive MDA-MB-231 cells as compared to the less invasive MCF-7 cells [13,14]. MT1-MMP has been shown to activate pro-gelatinase-A (proMMP-2) in human breast carcinoma cells [15] and can also activate proMMP-13 [16]. MT1-MMP degrades native type I collagen, fibronectin, laminin, fibrin, gelatin and cartilage proteoglycan core protein [2] and has also been classified as an interstitial collagenase [3]. MMP-1 is the most ubiquitously expressed interstitial collagenase [3]. MMP-1 cleaves fibrillar collagens including collagens types I, II and III, resulting in cleavage products that are rapidly denatured at body temperature to gelatin, the substrate preferred by the gelatinases (MMP-2 and MMP-9) [2]. MMP-1 is produced by a wide variety of normal cells (eg stromal fibroblasts, macrophages and endothelial cells) [3], and is involved in tissue remodelling and repair [3,17]. It has been implicated in matrix invasion by the MDA-MB-231 HBC cells in vitro [18,19], but has a low incidence of expression in the tumour cells of breast carcinomas [2,11]. MMP-3 is a member of the stromelysin sub-family, which show broad substrate specificity, degrading type IV collagen, laminin, fibronectin and proteoglycans. MMP-3 is expressed in areas of tissue growth [20], focally expressed around invasive cells in the stromal component of breast tumours, and expressed in both benign and malignant breast phenotypes [19].
As MT1-MMP, MMP-1 and MMP-3 have all been implicated in the processes involved in human breast cancer, this study utilised HBC cell lines both in culture and grown as xenografts in nude mice, to investigate gene expression changes of MT1-MMP, MMP-1 and MMP-3 in vitro and in vivo using IS-RT-PCR. In order to demonstrate IS-RT-PCR detection to examine MMP expression level changes, we utilised Concanavalin A (Con A), an agent known to modulate MMP expression, and to upregulate at least one MMP [15,21,22], in our in vitro studies. Nude mouse xenografts are heterogenous, containing neoplastic human parenchyma with mouse stroma and vasculature with many histologic features of the original tumour maintained [23]. As xenograft growth varies with tumour type, the ability to mimic human tumours in vivo provides a reproducible model system that can be correlated to the human disease state. We have previously applied the IS-RT-PCR technique to examine in vitro gene expression of MT1-MMP in MDA-MB-231 HBC cells [21], while others have examined expression of MMP-2, MMP-9 and their inhibitors TIMP-1 and TIMP-2 in cervical cancer [24]. Thus the objective of this study was to utilise the unique IS-RT-PCR methodology to examine the localised gene expression of members of the MMP family of proteases implicated in breast cancer. Our findings here with IS-RT-PCR demonstrate the detection of in vitro and in vivo gene expression patterns of MMPs in HBC cell lines, and suggest a contribution from the stroma to MMP expression in breast carcinomas.
Methods
Cell culture
HBC cell lines MDA-MB-231, MCF-7, ZR-75-1 and MDA-MB-453, were originally obtained from the ATCC (Virginia, USA) and maintained by the Lombardi Cancer Center Shared Cell Culture Resource. They were grown as a monolayer culture in RPMI 1640 media (Invitrogen, USA) supplemented with 10% foetal calf serum (Biowhittaker, USA) in the presence of 5% CO2. Cells (approx. 1 × 105 cells/slide) were passaged onto two eight-chambered chamber slides (Lab-Tek, Nunc, USA) and allowed to attach for 8–12 hrs. Cells were then grown for a further 16–24 hrs in RPMI 1640 with 10% foetal calf serum in the presence or absence of Con A (25 μg/ml, Sigma, USA).
Xenograft tissue
Tumour xenografts of the MDA-MB-231, MDA-MB-435, MCF-7 and Hs578T HBC cell lines were xenografted into intact female mice as described previously [25]. Animal studies were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals with approval of the Georgetown University Medical Center Animal Ethics Committee. The tissue was excised, and material from the xenografted tumours fixed in 10% neutral buffered formalin (Fisher Scientific, USA) and embedded in paraffin for routine histological analysis. The Lombardi Cancer Center Tissue Resource performed sectioning of the paraffin blocks, and tissue sections of 5 μm were mounted onto Pro-Bond +/- slides (Fisher Scientific, USA).
Pre-treatment of sections
Following sectioning, slides were placed under vacuum overnight with desiccant. Our previously described RNA ISH procedure [26] was modified as follows. All slides were de-waxed in xylene (4 × 10 min), washed in ethanol (100%, 3 × 3 mins), and rehydrated through a graded ethanol series (85%, 70% and 50% in 0.9% NaCl × 2 min each). Sections were then washed in saline (0.9% NaCl, 1X × 2 min) and PBS (1X × 2 min) prior to fixation with 4% paraformaldehyde (PFA; 250 ml H2O, 1 g NaOH, 10 g PFA, 1.7 g sodium acetate pH 6.5) for 20 minutes. Following fixation, slides were rinsed in 2X PBS (2 mins), followed by Proteinase K digestion (10 mg/ml; Roche Diagnostics, USA; 25 ml 1 M Tris pH 8.0, 250 μl 10 mg/ml Proteinase K, 25 ml 0.5 M EDTA pH 8.0, 200 ml H2O). Slides were then rinsed in 2% glycine (2 mins) and re-fixed in 4% PFA (10 mins) to ensure complete deactivation of Proteinase K. Slides were rinsed in triethanolamine (TEA, Sigma, USA; 0.1 M pH 8.0 × 3 min) in preparation for further digests.
RNase digestion
Following pre-treatment all slides were rinsed in RNase buffer (10 mM HEPES, 20 mM NaCl, 1 mM EDTA) without enzyme (2 × 2 mins). Negative control sections undergoing RNase digestion were then overlayed with RNase Digest mixture containing RNase cocktail (8 mg/ml RNase A and 160 U/ml RNase T1 final volume, Bresatec, Australia) in RNase buffer, placed in a humid chamber and incubated at 37°C for 8 hrs. Untreated slides were overlayed in RNase buffer and also incubated at 37°C for 8 hrs.
DNase pre-treatment
Prior to DNase digest, all slides were rinsed in DNase buffer (0.1 M C2H3O2Na, 5 mM MgSO4 pH 5.0, 2 × 2 min). DNase buffer (200 μl) containing 1.5 U/ml final volume of RNase-free DNase (Roche Diagnostics, USA) was added to each slide and these were incubated overnight at 37°C in a humid chamber. After incubation the slides were rinsed thoroughly in DNase buffer without enzyme, followed by washes in sterile water and dehydration through a graded ethanol series (50%, 70%, and 90% in 0.9% saline, 100% × 3 mins each).
Primers
Primers encoding MT1-MMP, MMP-1 and MMP-3 (Bresatec, Australia) were designed using the Amplimer program, product design checked by the Amplify v1.2 program, and BLASTed (NCBI, USA) for homology to the mRNA of these genes, with significant sequence homology demonstrated between the human and mouse MMPs examined. Primers encoding human β-actin were used as a positive control (Research Genetics, USA). Primer sequences are listed in Table 1. All primers were tested using traditional RT-PCR on mRNA extracted from MDA-MB-231 and MCF-7 HBC cells using the High Pure RNA Isolation Kit (Roche Diagnostics, USA). Reverse transcription was performed using 20 ng of isolated mRNA, by AMV reverse transcriptase (Promega Corporation, USA) at 50°C for 30 minutes, followed by PCR amplification using Taq DNA Polymerase (Perkin-Elmer Corporation, Applied Biosystems Division, USA). The linear phase of amplification was determined to be between cycles 20–25 using a standard two-step PCR cycle.
Table 1.
Primer sequences encoding the human β-actin, MT1-MMP, MMP-1 and MMP-3 genes.
| Gene | Primer Sequence |
| β-actin sense | 5'- ACC CAC ACT GTG CCC ATC TA |
| β-actin antisense | 5'- CGG AAC CGC TCA TTG CC |
| MT1-MMP sense | 5'- AGT GGA TGG ACA CGG AGA AT |
| MT1-MMP antisense | 5'- TCC ATC CAT CAC TTG GTT AT |
| MMP-1 sense | 5'- AGC GTG TGA CAG TAA GCT AA |
| MMP-1 antisense | 5'- GTT TTC CTC AGA AAG AGC AGC AT |
| MMP-3 sense | 5'- GTC TCA AGA TGA TAT AAA TG |
| MMP-3 antisense | 5'- AAT TGA TTT CCT TTA AAA ATG A |
In situ-Reverse Transcription-Polymerase Chain Reaction (IS-RT-PCR)
Prior to the IS-RT-PCR procedure Gene-Frame gaskets (Advanced Biotechnologies, UK) were placed around the sections. IS-RT-PCR was performed on consecutive sections for each xenograft: section A) RNase/DNase negative control; B) β-actin positive control; C) MT1-MMP assay slide; D) MMP-1 assay slide; and E) MMP-3 assay slide. Reverse transcription was performed as previously reported [18] with the following modifications: dCTP concentration was 100 μM, biotin-dCTP 80 μM, MnCl2 10 mM and 7.5 U of rTth DNA Polymerase (Perkin-Elmer Corporation, Applied Biosystems Division, USA) was used in the 30 μl mix added to each slide. Linear amplification efficiency was determined using traditional RT-PCR as outlined above, to be between cycles 20 and 25. For the amplification step the initial denaturation was at 95°C for 4 minutes, the MgCl2 concentration was 15 mM, and 30 μl were added to each slide. Amplification was performed for 20 cycles.
Detection
Post IS-RT-PCR detection was performed as reported previously [21] with the following modifications: Incubation in the NBT/BCIP/ASB solution was for 15 minutes, slides were counterstained with eosin (Australian Biostain Pty Ltd, Australia) and mounted in Ultramount (Fronine, Australia). Consistency of observed gene expression in consecutive tumour sections demonstrated the reliability of the IS-RT-PCR technique and as expected, all cells were found to express the ubiquitous β-actin gene.
Signal quantitation
The level of signal intensity was assigned a value from zero to four (0+ to 4+). Slides showing no purple precipitation and only eosin counterstain were assigned a value of zero (0+), whilst cells stained with precipitate were assigned a value between one and four (1+ to 4+). The observed signal intensity was assessed based on the observed level of signal intensity and tissue morphology using light microscopy. Visual characteristics including the overall level of precipitation, direct comparison of the entire tissue area to compensate for background, and the number and intensity of cells exhibiting purple precipitation were also considered. In addition, a coded labelling system was used to ensure that slide examination was unbiased. In vitro signal intensity following exposure to Con A and cytokines was compared to the untreated or basal level allowing an estimate of up/down-regulation to be determined. The in vivo xenograft material was histologically assessed and scored from 0+ to 4+ (with 0 = no expression; 1+ = low expression; 2+ = moderate expression; and 3+ = strong expression). We compared the relative levels of gene expression of each MMP within the xenografts analysed to controls. Following independent examination of slides and sections by three individuals the signal level intensity was averaged. The photographs presented in Figures 1, 2 and 3 are of the same area within consecutive sections and are therefore a representation of the overall observed gene expression levels.
Figure 1.
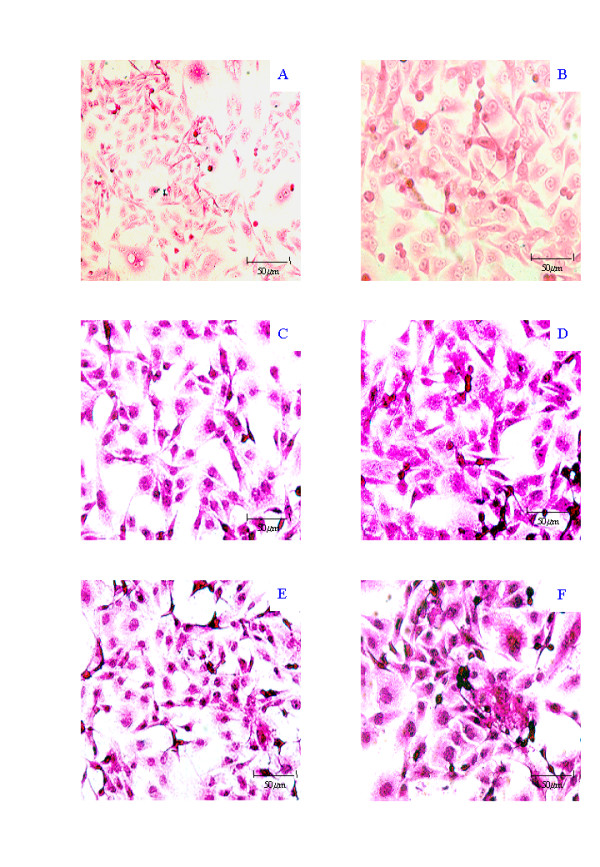
Representative photomicrographs of IS-RT-PCR analysis of Con A effects in MDA-MB-231 cells counterstained with Eosin. Negative controls include, RNase/DNase treated sections (A) and No RT (B). β-actin staining provided a positive Control without (C) or with (D) Con A treatment. MT1-MMP levels before (E) and after (F) Con A treatment are also shown. Photographs were on an Nikon Eclipse TE300 Microscope with a Nikon F-600 camera attachment and are × 40 (except for A, which is × 20).
Figure 2.
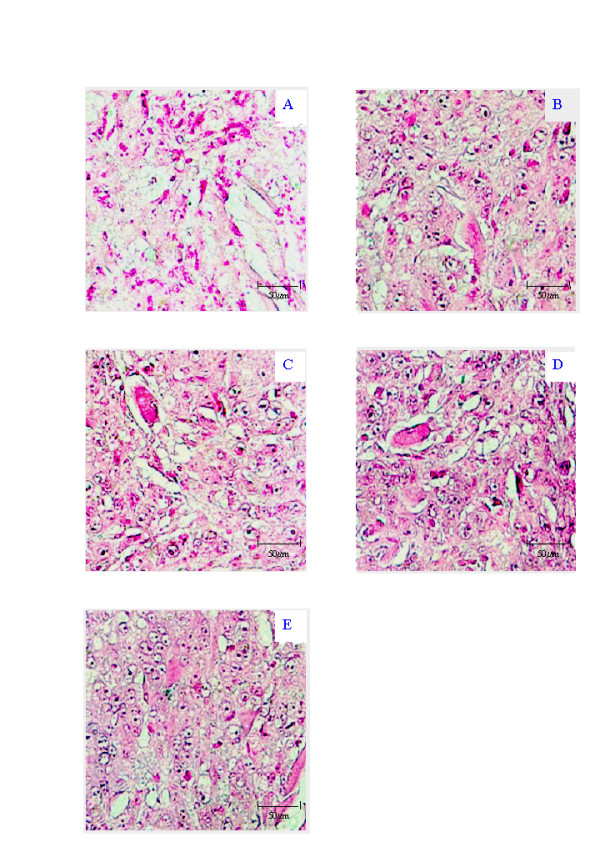
Representative photomicrographs of IS-RT-PCR results of MCF-7 xenograft showing the RNase/DNase negative control (A), positive control β-actin (B), MT1-MMP gene expression (C), MMP-1 gene expression (D) and MMP-3 gene expression (E). All photographs ×40 taken on an Olympus Microscope with a Nikon F-600 camera attachment.
Figure 3.
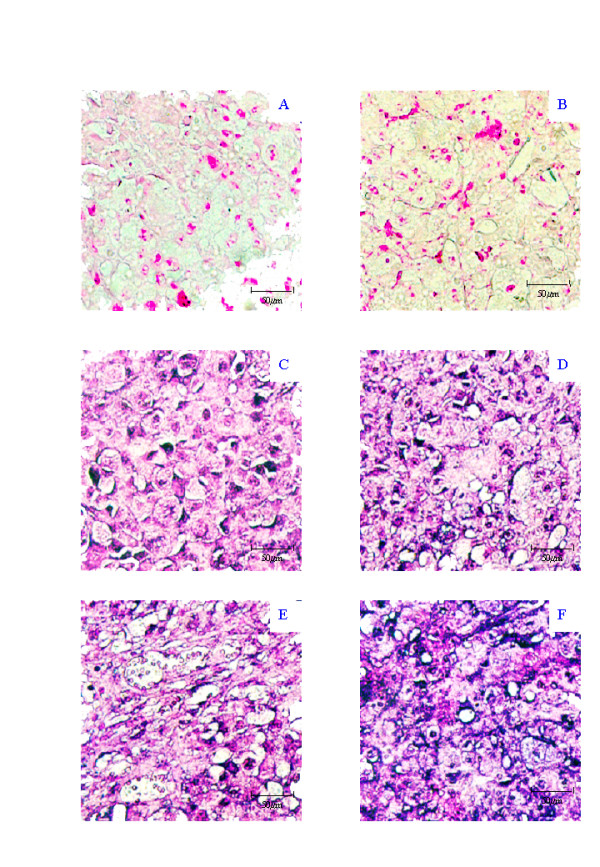
Representative photomicrographs of IS-RT-PCR results of MDA-MB-435 xenograft showing the No RT control (A), RNase/DNase negative control (B), positive control β-actin (C), MT1-MMP gene expression (D), MMP-1 gene expression (E) and MMP-3 gene expression (F). All photographs ×40 taken on an Olympus Microscope with a Nikon F-600 camera attachment.
Results
Primer specificity
Primers were tested using traditional RT-PCR on mRNA extracted from cultured MDA-MB-231 cells. Bands of the appropriate size for β-actin (289 bp), MT1-MMP (274 bp), MMP-1 (330 bp) and the MMP-3 (330 bp) transcripts were visualised by ethidium bromide agarose gel electrophoresis (not shown). For IS-RT-PCR two negative controls were used to ensure assay validity. The "No RT" control was used as a measure of the detection system specificity. Lack of precipitation in this control ensures that a positive signal is due to expression of the gene of interest. The RNase/DNase control was used for two reasons, to control for false positive results due to insufficient genomic DNA digestion, and to ensure that there was no signal due to any residual biotin-dCTP in the tissue section. Examples of these controls are given for the MDA-MB-231 cell line (Figure 1A and 1B), where the only colouration in the slides is due to the eosin counterstain, and purple precipitation which would be indicative of positive gene expression signal, is not observed. Primers amplifying β-actin mRNA were used as a positive control for demonstration of mRNA preservation in the tissue, and the ability to detect gene expression. We found a clear positive signal, the purple precipitate localised to all cells examined, demonstrating β-actin expression. An example of this is given in Figure 1C for the MDA-MB-231 cell line.
Induction of in vitro MMP gene expression
IS-RT-PCR results following Con A treatment
Expression levels of the three MMPs were examined with respect to β-actin in the MDA-MB-231, and MCF-7 cell lines, with or without exposure to Con A. In the MDA-MB-231 cell line, MT1-MMP expression was increased from 2+ to 4+ by Con A treatment, while MMP-1 and MMP-3 remained unchanged at 2+ following exposure to Con A. MT1-MMP induction by Con A in the MDA-MB-231 cell line is shown in Figure 1E and 1F. In the MCF-7 cell line, the MMP-1, MMP-3 and β-actin expression levels also remained unaffected by Con A at 2+, however, the MT1-MMP expression was decreased by Con A from 3+ in the absence of Con A to 1+ in the presence of Con A (not shown).
Adaptation of IS-RT-PCR to paraffin tissue
We adapted the IS-RT-PCR protocol for analyses of these MMPs in paraffin embedded xenografts of MCF-7, MDA-MB-231, MDA-MB-435, and Hs578T HBC cell lines grown subcutaneously in the mammary region of nude mice. Primary xenograft material from each cell line (except MDA-MB-231) were examined, as were metastatic deposits of MDA-MB-231 cells in the spleen and mesentery. The primary MDA-MB-231 tumour was unavailable for examination. A summary of the results obtained from the xenograft tissue is given in Table 2.
Table 2.
Summary of IS-RT-PCR results obtained for expression of β-actin, MT1-MMP, MMP-1 and MMP-3 in HBC cell line derived xenograft tissues. The intensity is a value from zero to four (0+ to 4+) based on the overall level of signal intensity observed throughout the entire section.
| Cell Line | β-actin | MT1-MMP | MMP-1 | MMP-3 |
| MCF-7 | Tumour 2+ | Tumour 1+ | Tumour 1+ | Tumour 1+ |
| Stroma 1+ | Stroma 1+ | Stroma 1+ | Stroma 1+ | |
| MDA-MB-231 | Tumour 2+ | Tumour 3+ | Tumour 0+ | Tumour 1+ |
| Spleen | Stroma 2+ | Stroma 0+ | Stroma 0+ | Stroma 0+ |
| MDA-MB-231 | Tumour 2+ | Tumour 2+ | Tumour 2+ | Tumour 1+ |
| Mesenteric | Stroma 2+ | Stroma 1+ | Stroma 2+ | Stroma 1+ |
| MDA-MB-435 | Tumour 2+ | Tumour 2+ | Tumour 2+ | Tumour 3+ |
| Stroma 2+ | Stroma 0+ | Stroma 0+ | Stroma 0+ | |
| Hs578T | Tumour 1+ | Tumour 1+ | Tumour 1+ | Tumour 1+ |
| Stroma 1+ | Stroma 1+ | Stroma 0+ | Stroma 2+ |
In the MCF-7 xenograft, low expression of MT1-MMP, MMP-1 and MMP-3 was observed in the tumour cells (1+) as indicated by the faint purple precipitation (Figure 2C, 2D, 2E). A low level of gene expression for MT1-MMP, MMP-1 and MMP-3 (1+) was also seen in the stromal compartment surrounding the MCF-7 cells (Figure 2C, 2D, 2E). However, β-actin expression was low in the stromal component (1+) and at a moderate level in the tumour component (2+) suggesting that the relative stromal levels are even higher (Figure 2B). A low level of expression of β-actin was consistent between the two compartments (1+). The MDA-MB-435 No RT control (Figure 3B) served as the No RT control section for the MCF-7 experiment, with the experiments run concurrently. In the MDA-MB-435 cell line xenograft, we observed moderate expression of MT1-MMP and MMP-1 (2+) (Figure 3C and 3D), and high levels of MMP-3 (3+) (Figure 3E) in the tumour cells. No expression of MT1-MMP (0+), MMP-1 (0+) or MMP-3 (0+) was observed in the stromal tissue surrounding the MDA-MB-435 cells (Figure 3D, 3E and 3F). β-actin expression was consistently moderate in both compartments (2+) (Figure 3C).
The Hs578T xenograft showed low expression of MT1-MMP (1+), MMP-1 (1+) and MMP-3 (1+) in the tumour cells. In the surrounding stromal cells, we observed low MT1-MMP expression (1+), moderate MMP-3 expression (2+) and no MMP-1 expression (0+). β-actin expression levels were consistently low in both compartments (1+) (Table 2). Although the primary MDA-MB-231 xenograft was unavailable for examination, mesenteric deposits in the spleen and mesentery of the same mouse were examined (Table 2). In the MDA-MB-231 spleen metastasis, the tumour cell showed high MT1-MMP (3+), and low MMP-3 expression (1+), but no detectable MMP-1 gene expression (0+). No detectable expression of MT1-MMP, MMP-1 and MMP-3 was observed in the host stromal cells (0+). β-actin gene expression was consistent between the two compartments at a moderate level (2+) (Table 2). In the mesenteric metastasis, the MDA-MB-231 tumour cells showed a moderate expression of both MT1-MMP and MMP-1 (2+), and low expression of MMP-3 (1+). In contrast to the splenic metastasis, the host stromal tissue surrounding the mesenteric metastasis showed low expression of MT1-MMP (1+), MMP-3 (1+) and moderate MMP-1 expression (2+). Again, β-actin was consistent in both compartments at a moderate level (2+) (Table 2).
Discussion
The central aim of investigations into molecular carcinogenesis is the identification of gene products involved in cancer progression during which tumour cells acquire the capacity for invasion with resulting metastasis [27]. Metastasis is an active process involving the altered attachment of the tumour cell to the basement membrane, localised degradation of connective tissue, and migration through stromal tissue [28,29]. Such matrix degradation is mediated by members of several proteinase families (the serine, cysteine, aspartate proteinases and MMPs), with substantial tissue destruction being carried out by members of the MMP family [3]. The expression of members of the MMP family and their inhibitors, the TIMPs, have been examined in both physiological and pathological conditions by many methods both in vitro and in vivo, including the use of total RNA from tissues and cell lines, Northern analysis, RT-PCR in situ and standard in situ hybridisation (ISH) [13,21,24,30-33]. Xenografts of cell lines representing increased progression of breast cancer were examined in the present study using the IS-RT-PCR technique. The samples ranged from the poorly invasive, estrogen-receptor positive cells (MCF-7), through to examples of more aggressive, estrogen-receptor negative human breast carcinoma (MDA-MB-231, MDA-MB-435, Hs578T) [25]. We analysed and compared the localised expression of MT1-MMP, MMP-1, MMP-3 and β-actin, in both the tumour parenchyma and the surrounding stroma. Both the MDA-MB-231 splenic and MDA-MB-435 xenografts demonstrate no detectable MMP gene expression in the stromal compartment. This highlights that although significant sequence homology exists between the human and mouse MMPs examined, minor differences may influence the detection of gene expression. Localised gene expression in both the epithelial tumour cells and the stroma compartments in the remaining xenografts however, demonstrate the detectable homology between human and mouse for the IS-RT-PCR primer sequences used.
MT1-MMP analysis of the HBC xenografts demonstrated mRNA in the tumour cells of all of the samples examined. In vitro, we have previously demonstrated MT1-MMP expression in MCF-7 cells by IS-RT-PCR [21], however Northern analysis of MCF-7 cells failed to find MT1-MMP in MCF-7 cells, whereas more invasive HBC cell lines showed MT1-MMP expression in concordance with their ability to be induced by Con A to activate MMP-2 [12,34,35]. MT1-MMP has also been shown to correlate with increased invasion and to enhance migration of MCF-10A epithelial cells [6,12,36], and transfection of MT1-MMP into MCF-7 cells stimulates higher migration and invasiveness [37,38], and also stimulates VEGF production, angiogenic stimulation, and xenograft take rate in nude mice [39,40]. Presumably the IS-RT-PCR method is more sensitive than Northern analysis, as may be expected. Indeed, we detected lower levels of MT1-MMP in the MCF-7 xenografts than in the majority of other xenografts derived from more invasive HBC cells. More recently, using isolated RNA for quantitative analyses, we were unable to detect MT1-MMP in MCF-7 derived xenografts, but consistent with our observations here, increasing levels of MT1-MMP was detected in MDA-MB-231 derived xenografts as compared to the parental cells [13]. The reasons for these differences are not clear and require further experimentation, but it is important to note that the cultured MCF-7 cells would have received estrogenic stimuli from both the phenol red and foetal calf serum in the medium [41].
MT1-MMP overexpression in the mammary gland results in abnormalities including hyperplasia, fibrosis, lymphocytic infiltration and adenocarcinoma [42], suggesting a pivotal role for MT1-MMP in carcinogenesis. However, ISH analyses of human breast carcinomas have primarily localised MT1-MMP mRNA expression to the stromal cells [11,29,43-45], although one study found localised MT1-MMP in the tumour [46]. An immunocytochemical analysis showed expression of MT1-MMP in both tumour and stromal cells [47]. More recently, one study by Bisson et al, combining ISH and IHC data has co-localised MT1-MMP to the α-smooth muscle positive myofibroblast cells in close contact with tumour cells [48]. Our studies certainly show the potential for the cell lines examined to make MT1-MMP, even the relatively well-conserved cell lines such as MCF-7. Although it is acknowledged that in vitro propagation of cell lines may alter some genetic pathways, it is also apparent that cell lines and tumour samples have distinctive gene expression patterns in common [49]. Being ER-positive, and predominantly epithelial, MCF-7 cells far better represent what one may find clinically in breast cancers than the other cell lines examined here [50]. The invasive HBC cell lines show a mesenchymal-like phenotype that may have resulted from epithelial-mesenchymal transition (EMT) in breast carcinoma. EMT is thought to have occurred in the MDA-MB-231, MDA-MB-435 and Hs578T cell lines [51]. The demonstration of MT1-MMP expression observed in the stromal component is not totally unexpected. MT1-MMPs ability to stimulate invasion and metastasis in in vitro systems along with the estrogen receptor status of the cells is well documented as described above. Also, the basal levels observed between the MCF-7 and more invasive HBC cell lines may also be of impact in respect to the higher invasive potential of the cells.
MMP-1 mRNA was detected in the tumour cells of five of the six xenografts examined, the exception, surprisingly, being the MDA-MB-231 splenic metastasis. This is unexpected since MDA-MB-231 cell overexpress MMP-1 in culture [52], and these cells have a mutation/polymorphism in the promotor region which drives strong MMP-1 expression [18]. This transcriptional repression has not been found to occur extensively in breast cancer [18]. The reasons for suppression of the MMP-1 expression in splenic metastasis are not clear, but could include a strong repression, possibly due to altered signalling from the stroma. The splenic metastasis stroma was notably also lacking all three MMPs examined, but showed a strong β-actin signal in both compartments. One cannot rule out the possibility of selective aberrant loss of MMP mRNA rather than the β-actin, however, this is unprecedented. Further analysis of splenic metastases of the MDA-MB-231 cells, and primary human tumours, would be warranted. MMP-1 has been previously demonstrated to be equally expressed in vitro in both MCF-7 and MDA-MB-231 cells [53], although in a similar study using Northern analysis we found selective expression in MDA-MB-231 cells but not MCF-7 [51]. Increased production and secretion of MMP-1 has been correlated with increased metastatic potential [52,54]. More recently, Bachmeir et al have demonstrated a cell density-dependent regulation of gene expression of several MMPs in HBC cell lines [31]. Increased cell density resulted in decreasing levels of both MMP-1 and MMP-3 expression levels, with a resultant decreased invasion concurrent with invasive potential [31-33]. Increased expression of MMP-1 has been reported on contact with Matrigel [55], and also in response to fibronectin fragments [56], attesting to the potential regulation of this MMP by the microenvironment. In vivo, MMP-1 expression has been demonstrated in the stroma of nine of thirty-four breast carcinomas [11] when examined by ISH, localised to stromal constituents during tumour formation [57], and to be upregulated in the stromal component of ductal carcinomas when examined by ISH and IHC [57-59]. Thus, again, our observation that MMP-1 expression was absent in the stroma of three out of six HBC xenografts appears to contrast the clinical situation. This may reflect the increased sensitivity of IS-RT-PCR and may also reflect the stage in tumour formation of the MCF-7 and MDA-MB-231 mesenteric xenografts that we examined.
MMP-3 mRNA was localised to the tumour component in all six xenograft samples examined. In vitro, MMP-3 mRNA has been demonstrated in the MDA-MB-231 HBC cell line [55], where it is stimulated by fibroblasts via the extracellular matrix metalloproteinase inducer (EMMPRIN) [60], and can enhance tumourigenicity and migration [54]. In vivo, MMP-3 is centrally involved in mammary gland development [61], and has been demonstrated to promote tumour initiation and formation in the tetracycline-regulated mouse mammary model [62,63]. IHC and ISH in vivo analysis has demonstrated MMP-3 in both the tumour and stroma cellular compartments of both invasive and non-invasive tumours, with the level of stromal expression increasing with tumourigenicity [59,64,65], and in the extracellular matrix adjacent to breast tumours [66]. Our observation of MMP-3 gene expression in the stroma of four out of six of the xenografts examined, and in particular in the Hs578T xenograft, further support a role for this MMP in breast cancer.
Although HBC cell lines have been demonstrated to express certain MMPs, this is less evident in vivo where, as detailed above, the surrounding stromal cells contribute to much of the MMP activity. Important differences in MMP expression between the stromal cells recruited by each cell line and the tumour cells themselves, were detected in the current study. The MCF-7 xenograft stroma demonstrated low stromal MT1-MMP, MMP-1 and MMP-3 expression. MT1-MMP expression was observed at a lower level in the stroma associated with the MDA-MB-231 mesenteric metastasis. In the Hs578T xenograft stroma, MMP-1 was absent while MMP-3 was observed at a higher level in the stroma as compared to the tumour cells. No stromal expression of the three MMPs examined was observed in either the MDA-MB-231 spleen metastasis or the MDA-MB-435 xenografts. The level of stromal gene expression will depend on signals from the different HBC lines in the primary site, and also on the different responsivities in the different host sites for the splenic and mesenteric metastases. In regard to the primary site, overall recruitment of the stroma will differ among the HBC lines; however, β-actin mRNA detection was used to normalise the data. We did detect lower levels of β-actin in the Hs578T xenograft, and indeed higher levels of β-actin in the tumour cell component of the MCF-7 xenograft. However, this does not appear to have influenced the detection of the MMPs as indicated by the moderate levels of all MMPs examined in both compartments in the MCF-7 xenograft, and the moderate level of MMP-3 in the Hs578T tumour compartment.
Conclusion
These data combined indicate communication, and in particular HBC cell-line specific communication, between the epithelial and stromal cellular compartments for MMP production and utilisation. Considerable work has been performed in vitro to examine the effects of different HBC cell lines on MMP expression by co-culture with fibroblasts and other factors known to be produced by the tumour cells which mediate induction of MMPs (eg Con A, TPA, EMMPRIN) [12,15,21,31-33,53,60]. These and other studies, implicate increasing MMP levels associated with both epithelial tumour cells and surrounding stromal cells, in association with increasing tumourigenicity and metastatic potential [12,15,21,24,53,54,60,67]. Our in vivo data indicate that, the MDA-MB-231, MDA-MB-435 and Hs578T HBC cell lines are able to influence MMP gene expression in the surrounding stroma. This is reinforced by a recent study demonstrating the influence of tumour associated fibroblasts on MCF-7 tumour cells to activate MMP-2 in vitro and increase tumour size in vivo [68]. These data combined indicate IS-RT-PCR may serve as a more sensitive, one-step procedure for the examination of MMP gene expression in human breast cancers.
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
L.M.H. performed all in vitro and in vivo studies and drafted the manuscript. E.W.T. contributed toward the design of the study and manuscript finalisation. A.E.O.T. contributed toward the design of the in situ sample preparation. R.E.I. contributed toward data management and manuscript finalisation. M.G.I. participated in the conception and design of the study. L.R.G. participated in the conception and design of the study and its coordination. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgements
L.M.H. was supported by the Queensland Cancer Fund, Kathleen Cunningham Foundation. We also thank the Tissue Culture Shared Resources of Lombardi Cancer Center, which are partially supported by PHS grant NIH 1P30-CA-51008 (Cancer Center Support Grant) to Lombardi Cancer Center.
Contributor Information
Larisa M Haupt, Email: lhaupt@imcb.a-star.edu.sg.
Erik W Thompson, Email: rik@foo.medstv.unimelb.edu.au.
Ann EO Trezise, Email: ann.Trezise@uq.edu.au.
Rachel E Irving, Email: rairving@staff.bond.edu.au.
Michael G Irving, Email: michael_irving@bond.edu.au.
Lyn R Griffiths, Email: l.griffiths@griffith.edu.au.
References
- Elston CW, Ellis IO, Pinder SE. Pathological prognostic factors in breast cancer. Crit Rev Oncol Hematol. 1999;31:209–23. doi: 10.1016/s1040-8428(99)00034-7. [DOI] [PubMed] [Google Scholar]
- Stamenkovic I. Matrix metalloproteinases in tumor invasion and metastasis. Semin Cancer Biol. 2000;10:415–33. doi: 10.1006/scbi.2000.0379. [DOI] [PubMed] [Google Scholar]
- Brinckerhoff CE, Rutter JL, Benbow U. Interstitial collagenases as markers of tumor progression. Clin Cancer Res. 2000;6:4823–30. [PubMed] [Google Scholar]
- Reno F, Sabbatini M, Stella M, Magliacani G, Cannas M. Effect of in vitro mechanical compression on Epilysin (matrix metalloproteinase-28) expression in hypertrophic scars. Wound Repair Regen. 2005;13:255–61. doi: 10.1111/j.1067-1927.2005.130307.x. [DOI] [PubMed] [Google Scholar]
- Chung KF. Cytokines in chronic obstructive pulmonary disease. Eur Respir J Suppl. 2001;34:50s–59s. doi: 10.1183/09031936.01.00229701. [DOI] [PubMed] [Google Scholar]
- Sato H, Seiki M. Membrane-type matrix metalloproteinases (MT-MMPs) in tumor metastasis. J Biochem (Tokyo) 1996;119:209–15. doi: 10.1093/oxfordjournals.jbchem.a021223. [DOI] [PubMed] [Google Scholar]
- Ries C, Petrides PE. Cytokine regulation of matrix metalloproteinase activity and its regulatory dysfunction in disease. Biol Chem Hoppe Seyler. 1995;376:345–55. [PubMed] [Google Scholar]
- Lochter A, Sternlicht MD, Werb Z, Bissell MJ. The significance of matrix metalloproteinases during early stages of tumor progression. Ann N Y Acad Sci. 1998;857:180–93. doi: 10.1111/j.1749-6632.1998.tb10116.x. [DOI] [PubMed] [Google Scholar]
- Woessner JF., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. Faseb J. 1991;5:2145–54. [PubMed] [Google Scholar]
- Osteen KG, Rodgers WH, Gaire M, Hargrove JT, Gorstein F, Matrisian LM. Stromal-epithelial interaction mediates steroidal regulation of metalloproteinase expression in human endometrium. Proc Natl Acad Sci USA. 1994;91:10129–33. doi: 10.1073/pnas.91.21.10129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada A, Bellocq JP, Rouyer N, Chenard MP, Rio MC, Chambon P, Basset P. Membrane-type matrix metalloproteinase (MT-MMP) gene is expressed in stromal cells of human colon, breast, and head and neck carcinomas. Proc Natl Acad Sci USA. 1995;92:2730–4. doi: 10.1073/pnas.92.7.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulyaeva H, Bueno J, Polette M, Birembaut P, Sato H, Seiki M, Thompson EW. MT1-MMP correlates with MMP-2 activation potential seen after epithelial to mesenchymal transition in human breast carcinoma cells. Clin Exp Metastasis. 1997;15:111–20. doi: 10.1023/A:1018444609098. [DOI] [PubMed] [Google Scholar]
- Lafleur MA, Drew AF, de Sousa EL, Blick T, Bills M, Walker EC, Williams ED, Waltham M, Thompson EW. Upregulation of matrix metalloproteinases (MMPs) in breast cancer xenografts: a major induction of stromal MMP-13. Int J Cancer. 2005;114:544–54. doi: 10.1002/ijc.20763. [DOI] [PubMed] [Google Scholar]
- Yu M, Sato H, Seiki M, Spiegel S, Thompson EW. Elevated cyclic AMP suppresses ConA-induced MT1-MMP expression in MDA-MB-231 human breast cancer cells. Clin Exp Metastasis. 1998;16:185–91. doi: 10.1023/A:1006580406314. [DOI] [PubMed] [Google Scholar]
- Yu M, Sato H, Seiki M, Thompson EW. Complex regulation of membrane-type matrix metalloproteinase expression and matrix metalloproteinase-2 activation by concanavalin A in MDA-MB- 231 human breast cancer cells. Cancer Res. 1995;55:3272–7. [PubMed] [Google Scholar]
- Murphy G, Stanton H, Cowell S, Butler G, Knauper V, Atkinson S, Gavrilovic J. Mechanisms for pro matrix metalloproteinase activation. Apmis. 1999;107:38–44. doi: 10.1111/j.1699-0463.1999.tb01524.x. [DOI] [PubMed] [Google Scholar]
- McDonnell S, Matrisian LM. Stromelysin in tumor progression and metastasis. Cancer Metastasis Rev. 1990;9:305–19. doi: 10.1007/BF00049521. [DOI] [PubMed] [Google Scholar]
- Benbow U, Rutter JL, Lowrey CH, Brinckerhoff CE. Transcriptional repression of the human collagenase-1 (MMP-1) gene in MDA231 breast cancer cells by all-trans-retinoic acid requires distal regions of the promoter. Br J Cancer. 1999;79:221–8. doi: 10.1038/sj.bjc.6690037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basset P, Bellocq JP, Wolf C, Stoll I, Hutin P, Limacher JM, Podhajcer OL, Chenard MP, Rio MC, Chambon P. A novel metalloproteinase gene specifically expressed in stromal cells of breast carcinomas. Nature. 1990;348:699–704. doi: 10.1038/348699a0. [DOI] [PubMed] [Google Scholar]
- Bruner KL, Rodgers WH, Gold LI, Korc M, Hargrove JT, Matrisian LM, Osteen KG. Transforming growth factor beta mediates the progesterone suppression of an epithelial metalloproteinase by adjacent stroma in the human endometrium. PNAS. 1995;92:7362–7366. doi: 10.1073/pnas.92.16.7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haupt LM, Thompson EW, Griffiths LR, Irving MG. IS-RT-PCR assay detection of MT-MMP in a human breast cancer cell line. Biochem Mol Biol Int. 1996;39:553–61. doi: 10.1080/15216549600201611. [DOI] [PubMed] [Google Scholar]
- Azzam HS, Arand G, Lippman ME, Thompson EW. Association of MMP-2 activation potential with metastatic progression in human breast cancer cell lines independent of MMP-2 production. J Natl Cancer Inst. 1993;85:1758–64. doi: 10.1093/jnci/85.21.1758. [DOI] [PubMed] [Google Scholar]
- Malkinson AM. Primary lung tumors in mice as an aid for understanding, preventing, and treating human adenocarcinoma of the lung. Lung Cancer. 2001;32:265–79. doi: 10.1016/S0169-5002(00)00232-4. [DOI] [PubMed] [Google Scholar]
- Nuovo GJ. The foundations of successful RT in situ PCR. Front Biosci. 1996;1:c4–c15. doi: 10.2741/a110. [DOI] [PubMed] [Google Scholar]
- Thompson EW, Paik S, Brunner N, Sommers CL, Zugmaier G, Clarke R, Shima TB, Torri J, Donahue S, Lippman ME, et al. Association of increased basement membrane invasiveness with absence of estrogen receptor and expression of vimentin in human breast cancer cell lines. J Cell Physiol. 1992;150:534–44. doi: 10.1002/jcp.1041500314. [DOI] [PubMed] [Google Scholar]
- Trezise AE, Linder CC, Grieger D, Thompson EW, Meunier H, Griswold MD, Buchwald M. CFTR expression is regulated during both the cycle of the seminiferous epithelium and the oestrous cycle of rodents. Nat Genet. 1993;3:157–64. doi: 10.1038/ng0293-157. [DOI] [PubMed] [Google Scholar]
- Liotta LA, Stetler-Stevenson W. Metalloproteinases and malignant conversion: does correlation imply causality? J Natl Cancer Inst. 1989;81:556–7. doi: 10.1093/jnci/81.8.556. [DOI] [PubMed] [Google Scholar]
- Curran S, Murray GI. Matrix metalloproteinases: molecular aspects of their roles in tumour invasion and metastasis. Eur J Cancer. 2000;36:1621–30. doi: 10.1016/S0959-8049(00)00156-8. [DOI] [PubMed] [Google Scholar]
- Chambers AF, Matrisian LM. Changing views of the role of matrix metalloproteinases in metastasis. J Natl Cancer Inst. 1997;89:1260–70. doi: 10.1093/jnci/89.17.1260. [DOI] [PubMed] [Google Scholar]
- Tryggvason K, Hoyhtya M, Pyke C. Type IV collagenases in invasive tumors. Breast Cancer Res Treat. 1993;24:209–18. doi: 10.1007/BF01833261. [DOI] [PubMed] [Google Scholar]
- Bachmeier BE, Vene R, Iancu CM, Pfeffer U, Mayer B, Noonan D, Albini A, Jochum M, Nerlich AG. Transcriptional control of cell density dependent regulation of matrix metalloproteinase and TIMP expression in breast cancer cell lines. Thromb Haemost. 2005;93:761–9. doi: 10.1160/TH04-09-0601. [DOI] [PubMed] [Google Scholar]
- Kousidou OC, Roussidis AE, Theocharis AD, Karamanos NK. Expression of MMPs and TIMPs genes in human breast cancer epithelial cells depends on cell culture conditions and is associated with their invasive potential. Anticancer Res. 2004;24:4025–30. [PubMed] [Google Scholar]
- Bachmeier BE, Albini A, Vene R, Benelli R, Noonan D, Weigert C, Weiler C, Lichtinghagen R, Jochum M, Nerlich AG. Cell density-dependent regulation of matrix metalloproteinase and TIMP expression in differently tumorigenic breast cancer cell lines. Exp Cell Res. 2005;30:83–98. doi: 10.1016/j.yexcr.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Gilles C, Polette M, Seiki M, Birembaut P, Thompson EW. Implication of collagen type I-induced membrane-type 1-matrix metalloproteinase expression and matrix metalloproteinase-2 activation in the metastatic progression of breast carcinoma. Lab Invest. 1997;76:651–60. [PubMed] [Google Scholar]
- Deryugina EI, Ratnikov B, Monosov E, Postnova TI, DiScipio R, Smith JW, Strongin AY. MT1-MMP initiates activation of pro-MMP-2 and integrin alphavbeta3 promotes maturation of MMP-2 in breast carcinoma cells. Exp Cell Res. 2001;263:209–23. doi: 10.1006/excr.2000.5118. [DOI] [PubMed] [Google Scholar]
- Gilles C, Polette M, Coraux C, Tournier JM, Meneguzzi G, Munaut C, Volders L, Rousselle P, Birembaut P, Foidart JM. Contribution of MT1-MMP and of human laminin-5 gamma2 chain degradation to mammary epithelial cell migration. J Cell Sci. 2001;114:2967–76. doi: 10.1242/jcs.114.16.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deryugina EI, Bourdon MA, Reisfeld RA, Strongin A. Remodeling of collagen matrix by human tumor cells requires activation and cell surface association of matrix metalloproteinase-2. Cancer Res. 1998;58:3743–50. [PubMed] [Google Scholar]
- Deryugina EI, Bourdon MA, Jungwirth K, Smith JW, Strongin AY. Functional activation of integrin alpha V beta 3 in tumor cells expressing membrane-type 1 matrix metalloproteinase. Int J Cancer. 2000;86:15–23. doi: 10.1002/(SICI)1097-0215(20000401)86:1<15::AID-IJC3>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Sounni NE, Devy L, Hajitou A, Frankenne F, Munaut C, Gilles C, Deroanne C, Thompson EW, Foidart JM, Noel A. MT1-MMP expression promotes tumor growth and angiogenesis through an up-regulation of vascular endothelial growth factor expression. Faseb J. 2002;16:555–64. doi: 10.1096/fj.01-0790com. [DOI] [PubMed] [Google Scholar]
- Deryugina EI, Soroceanu L, Strongin AY. Up-regulation of vascular endothelial growth factor by membrane-type 1 matrix metalloproteinase stimulates human glioma xenograft growth and angiogenesis. Cancer Res. 2002;62:580–8. [PubMed] [Google Scholar]
- Katzenellenbogen BS, Kendra KL, Norman MJ, Berthois Y. Proliferation, hormonal responsiveness, and estrogen receptor content of MCF-7 human breast cancer cells grown in the short-term and long-term absence of estrogens. Cancer Res. 1987;47:4355–60. [PubMed] [Google Scholar]
- Ha HY, Moon HB, Nam MS, Lee JW, Ryoo ZY, Lee TH, Lee KK, So BJ, Sato H, Seiki M, Yu DY. Overexpression of membrane-type matrix metalloproteinase-1 gene induces mammary gland abnormalities and adenocarcinoma in transgenic mice. Cancer Res. 2001;61:984–90. [PubMed] [Google Scholar]
- Shimada T, Nakamura H, Yamashita K, Kawata R, Murakami Y, Fujimoto N, Sato H, Seiki M, Okada Y. Enhanced production and activation of progelatinase A mediated by membrane-type 1 matrix metalloproteinase in human oral squamous cell carcinomas: implications for lymph node metastasis. Clin Exp Metastasis. 2000;18:179–88. doi: 10.1023/A:1006749501682. [DOI] [PubMed] [Google Scholar]
- Polette M, Clavel C, Birembaut P, De Clerck YA. Localization by in situ hybridization of mRNAs encoding stromelysin 3 and tissue inhibitors of metallo-proteinases TIMP-1 and TIMP-2 in human head and neck carcinomas. Pathol Res Pract. 1993;189:1052–7. doi: 10.1016/S0344-0338(11)80679-5. [DOI] [PubMed] [Google Scholar]
- Heppner KJ, Matrisian LM, Jensen RA, Rodgers WH. Expression of most matrix metalloproteinase family members in breast cancer represents a tumor-induced host response. Am J Pathol. 1996;149:273–82. [PMC free article] [PubMed] [Google Scholar]
- Dalberg K, Eriksson E, Enberg U, Kjellman M, Backdahl M. Gelatinase A, membrane type 1 matrix metalloproteinase, and extracellular matrix metalloproteinase inducer mRNA expression: correlation with invasive growth of breast cancer. World J Surg. 2000;24:334–40. doi: 10.1007/s002689910053. [DOI] [PubMed] [Google Scholar]
- Ishigaki S, Toi M, Ueno T, Matsumoto H, Muta M, Koike M, Seiki M. Significance of membrane type 1 matrix metalloproteinase expression in breast cancer. Jpn J Cancer Res. 1999;90:516–22. doi: 10.1111/j.1349-7006.1999.tb00778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisson C, Blacher S, Polette M, Blanc JF, Kebers F, Desreux J, Tetu B, Rosenbaum J, Foidart JM, Birembaut P, Noel A. Restricted expression of membrane type 1-matrix metalloproteinase by myofibroblasts adjacent to human breast cancer cells. Int J Cancer. 2003;105:7–13. doi: 10.1002/ijc.11012. [DOI] [PubMed] [Google Scholar]
- Ross DT, Perou CM. A comparison of gene expression signatures from breast tumors and breast tissue derived cell lines. Dis Markers. 2001;17:99–109. doi: 10.1155/2001/850531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D. Molecular portraits of human breast tumours. Nature. 2000;406:747–52. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- Gilles C, Polette M, Birembaut P, Brunner N, Thompson EW. Expression of c-ets-1 mRNA is associated with an invasive, EMT-derived phenotype in breast carcinoma cell lines. Clin Exp Metastasis. 1997;15:519–26. doi: 10.1023/A:1018427027270. [DOI] [PubMed] [Google Scholar]
- Benbow U, Schoenermark MP, Orndorff KA, Givan AL, Brinckerhoff CE. Human breast cancer cells activate procollagenase-1 and invade type I collagen: invasion is inhibited by all-trans retinoic acid. Clin Exp Metastasis. 1999;17:231–8. doi: 10.1023/A:1006639214618. [DOI] [PubMed] [Google Scholar]
- Barrett JM, Puglia MA, Singh G, Tozer RG. Expression of Ets-related transcription factors and matrix metalloproteinase genes in human breast cancer cells. Breast Cancer Res Treat. 2002;72:227–32. doi: 10.1023/A:1014993006190. [DOI] [PubMed] [Google Scholar]
- Bachmeier BE, Nerlich AG, Lichtinghagen R, Sommerhoff CP. Matrix metalloproteinases (MMPs) in breast cancer cell lines of different tumorigenicity. Anticancer Res. 2001;21:3821–8. [PubMed] [Google Scholar]
- Balduyck M, Zerimech F, Gouyer V, Lemaire R, Hemon B, Grard G, Thiebaut C, Lemaire V, Dacquembronne E, Duhem T, Lebrun A, Dejonghe MJ, Huet G. Specific expression of matrix metalloproteinases 1, 3, 9 and 13 associated with invasiveness of breast cancer cells in vitro. Clin Exp Metastasis. 2000;18:171–8. doi: 10.1023/A:1006762425323. [DOI] [PubMed] [Google Scholar]
- Huttenlocher A, Werb Z, Tremble P, Huhtala P, Rosenberg L, Damsky CH. Decorin regulates collagenase gene expression in fibroblasts adhering to vitronectin. Matrix Biol. 1996;15:239–50. doi: 10.1016/S0945-053X(96)90115-8. [DOI] [PubMed] [Google Scholar]
- Behrens P, Rothe M, Wellmann A, Krischler J, Wernert N. The Ets-1 transcription factor is up-regulated together with MMP 1 and MMP 9 in the stroma of pre-invasive breast cancer. J Pathol. 2001;194:43–50. doi: 10.1002/path.844. [DOI] [PubMed] [Google Scholar]
- Brummer O, Athar S, Riethdorf L, Loning T, Herbst H. Matrix-metalloproteinases 1, 2, and 3 and their tissue inhibitors 1 and 2 in benign and malignant breast lesions: an in situ hybridization study. Virchows Arch. 1999;435:566–73. doi: 10.1007/s004280050442. [DOI] [PubMed] [Google Scholar]
- Nakopoulou L, Giannopoulou I, Gakiopoulou H, Liapis H, Tzonou A, Davaris PS. Matrix metalloproteinase-1 and -3 in breast cancer: correlation with progesterone receptors and other clinicopathologic features. Hum Pathol. 1999;30:436–42. doi: 10.1016/S0046-8177(99)90120-X. [DOI] [PubMed] [Google Scholar]
- Caudroy S, Polette M, Tournier JM, Burlet H, Toole B, Zucker S, Birembaut P. Expression of the extracellular matrix metalloproteinase inducer (EMMPRIN) and the matrix metalloproteinase-2 in bronchopulmonary and breast lesions. J Histochem Cytochem. 1999;47:1575–80. doi: 10.1177/002215549904701209. [DOI] [PubMed] [Google Scholar]
- Werb Z, Ashkenas J, MacAuley A, Wiesen JF. Extracellular matrix remodeling as a regulator of stromal-epithelial interactions during mammary gland development, involution and carcinogenesis. Braz J Med Biol Res. 1996;29:1087–97. [PubMed] [Google Scholar]
- Lochter A, Werb Z, Bissell MJ. Transcriptional regulation of stromelysin-1 gene expression is altered during progression of mouse mammary epithelial cells from functionally normal to malignant. Matrix Biol. 1999;18:455–67. doi: 10.1016/S0945-053X(99)00036-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternlicht MD, Lochter A, Sympson CJ, Huey B, Rougier JP, Gray JW, Pinkel D, Bissell MJ, Werb Z. The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell. 1999;98:137–46. doi: 10.1016/S0092-8674(00)81009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebeau A, Nerlich AG, Sauer U, Lichtinghagen R, Lohrs U. Tissue distribution of major matrix metalloproteinases and their transcripts in human breast carcinomas. Anticancer Res. 1999;19:4257–64. [PubMed] [Google Scholar]
- Ioachim EE, Athanassiadou SE, Kamina S, Carassavoglou K, Agnantis NJ. Matrix metalloproteinase expression in human breast cancer: an immunohistochemical study including correlation with cathepsin D, type IV collagen, laminin, fibronectin, EGFR, c-erbB-2 oncoprotein, p53, steroid receptors status and proliferative indices. Anticancer Res. 1998;18:1665–70. [PubMed] [Google Scholar]
- Bodey B, Bodey B, Jr, Siegel SE, Kaiser HE. Matrix metalloproteinases in neoplasm-induced extracellular matrix remodeling in breast carcinomas. Anticancer Res. 2001;21:2021–8. [PubMed] [Google Scholar]
- Cao J, Sato H, Takino T, Seiki M. The C-terminal region of membrane type matrix metalloproteinase is a functional transmembrane domain required for pro-gelatinase A activation. J Biol Chem. 1995;270:801–5. doi: 10.1074/jbc.270.2.801. [DOI] [PubMed] [Google Scholar]
- Petersen OW, Nielsen HL, Gudjonsson T, Villadsen R, Rank F, Niebuhr E, Bissell MJ, Ronnov-Jessen L. Epithelial to mesenchymal transition in human breast cancer can provide a nonmalignant stroma. Am J Pathol. 2003;162:391–402. doi: 10.1016/S0002-9440(10)63834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


