Abstract
Endocytosis is required for efficient mitogen-activated protein kinase (MAPK) activation by activated growth factor receptors. We examined if H-Ras and K-Ras proteins, which are distributed across different plasma membrane microdomains, have equal access to the endocytic compartment and whether this access is necessary for downstream signaling. Inhibition of endocytosis by dominant interfering dynamin-K44A blocked H-Ras but not K-Ras-mediated PC12 cell differentiation and selectively inhibited H-Ras- but not K-Ras-mediated Raf-1 activation in BHK cells. H-Ras- but not K-Ras-mediated Raf-1 activation was also selectively dependent on phosphoinositide 3-kinase activity. Stimulation of endocytosis and endocytic recycling by wild-type Rab5 potentiated H-Ras-mediated Raf-1 activation. In contrast, Rab5-Q79L, which stimulates endocytosis but not endocytic recycling, redistributed activated H-Ras from the plasma membrane into enlarged endosomes and inhibited H-Ras-mediated Raf-1 activation. Rab5-Q79L expression did not cause the accumulation of wild-type H-Ras in enlarged endosomes. Expression of wild-type Rab5 or Rab5-Q79L increased the specific activity of K-Ras-activated Raf-1 but did not result in any redistribution of K-Ras from the plasma membrane to endosomes. These results show that H-Ras but not K-Ras signaling though the Raf/MEK/MAPK cascade requires endocytosis and endocytic recycling. The data also suggest a mechanism for returning Raf-1 to the cytosol after plasma membrane recruitment.
The localization of Ras to the inner surface of the plasma membrane is essential for biological activity because Ras effectors must be recruited to the plasma membrane for activation. Recent studies have shown that the different Ras isoforms H-, N-, and K-Ras are differentially distributed across plasma membrane microdomains. Inactive H-Ras associates with lipid rafts and caveolae, but activation results in a redistribution of H-Ras from rafts and caveolae to the disordered plasma membrane. In contrast, K-Ras is predominantly (>85%) localized to disordered plasma membrane irrespective of its activation state (43). The GTP-regulated dynamic equilibrium of H-Ras between lipid rafts and disordered plasma membrane is critical for H-Ras function, because confining H-Ras to lipid rafts by N-myristoylation or by mutations in the hypervariable linker domain severely inhibits H-Ras-mediated Raf-1 and phosphoinositide 3-kinase (PI3-K) activation and blocks H-Ras-stimulated PC12 cell differentiation (26, 43). Also, H-Ras- but not K-Ras-mediated signaling is functionally dependent on plasma membrane lipid rafts (53).
Numerous studies have now shown that H-, N-, and K-Ras generate quantitatively different signal outputs through effectors such as Raf, PI3-K, and Rac, which in turn results in qualitatively or quantitatively distinct biological responses (5, 20, 27, 29, 62, 64, 65, 69, 70). When taken together, these observations strongly suggest that the environment of the plasma membrane microdomain in which each Ras isoform operates dictates signal output (42).
The plasma membrane is also a highly dynamic structure because of the constant internalization and recycling of endocytic vesicles (22). Endocytosis occurs through clathrin-dependent and -independent mechanisms, although the precise nature of the clathrin-independent pathways remains unclear. Activation of many growth factor receptors also stimulates endocytosis. This is important for signal termination because internalization of growth factor receptor-ligand complexes into acidified endosomes disengages the ligand and allows recycling of inactive receptor back to the cell surface (63, 67). The role of the endosome, however, is more complex, because in certain experimental systems endocytosis of signaling complexes is required for signal propagation (10, 16). For example, dominant-interfering dynamin mutants that block the release of clathrin-coated vesicles from the plasma membrane inhibit epidermal growth factor (EGF)- and insulin-stimulated mitogen-activated protein kinase (MAPK) activation (9, 63). This inhibitory effect could be at the level of Ras activation, because activated EGF receptor continues to stimulate Ras GTP loading from the endosome (8, 23). However, blocking endocytosis has no effect on EGF- or lysophosphatidic acid-stimulated activation of Ras, Raf-1, or MEK in COS cells (30), leading to the interesting hypothesis that MEK, activated at the plasma membrane, may need endosomal transport through the cytosol to efficiently activate MAPK (10, 30).
In contrast, Romero and colleagues report that insulin stimulation of HIRcB cells results in endosomal accumulation of all components of the MAPK cascade, including insulin receptor, Ras, Raf, activated MEK, and MAPK (47, 49). Endosomal trafficking of Ras and Raf-1 appears to be critical for insulin-stimulated MAPK activation (47), a conclusion in part supported by the study of Pol et al., who identified Ras, Raf-1, MEK, and MAPK in endosomes purified from EGF-stimulated rat liver (41). In PC12 cells, pharmacological inhibitors of endocytosis or PI3-K block Ras-mediated B-Raf activation but have no effect on nerve growth factor (NGF)-stimulated Ras GTP loading. Interestingly, the same inhibitors also block Rap1-mediated B-Raf activation but in this case by an effect on Rap1 GTP loading (71). Thus, endocytosis is required at different levels in Ras- and Rap1-mediated activation of the Raf/MEK/MAPK cascade in PC12 cells (71). Consistent with these results, a recent study has shown that clathrin-coated vesicles prepared from NGF-stimulated PC12 cells contain all elements of the Ras/Raf/MEK/MAPK cascade (25).
Since activation of growth factor receptors can stimulate endocytosis, it is unclear from the studies carried out to date whether Ras and Raf-1 simply traffic together with the activated receptor, perhaps as part of a multicomponent signaling complex, or whether Ras and Raf can traffic through the endosomal compartment independently of growth factor receptors. In addition, no study has yet examined whether endocytosis of plasma membrane-localized Ras is influenced by the C-terminal membrane anchor. To address these questions, we investigated to what extent two Ras isoforms, H-Ras and K-Ras, enter the endocytic compartment and whether perturbing endosomal trafficking interferes with their ability to activate the Raf-1/MEK/MAPK cascade.
MATERIALS AND METHODS
Plasmids.
Expression vectors for hemagglutinin (HA)-tagged K44A mutant dynamin-1 (15) were kindly donated by Sandra Schmid. cDNAs for Myc-tagged wild-type Rab5 and Rab5-Q79L were kindly donated by Harald Stenmark (56) and were cloned downstream of the cytomegalovirus promoter in the pC1 expression vector. H-RasG12V, K-RasG12V, green fluorescent protein (GFP)-H-RasG12V, GFP-K-RasG12V, GFP-tH, and GFP-H-Ras expression vectors have been described previously (2, 21, 43). An expression vector for GFP-Raf was constructed by cloning the Raf-1 cDNA into pEGFP-N3 (Clontech). H-Ras/K-Ras chimeras were constructed by introducing silent point mutations at codons 162 and 163 in K-Ras to create a BstYI restriction site, followed by an exchange of cDNA sequences 3′ of the new BstYI site in K-Ras and a naturally occurring BstYI site in H-Ras. The resulting chimeric cDNAs were sequenced and then cloned into pEGFP-C2.
Antibodies.
Monoclonal antibodies against HA, Myc (9E10), and Ras (Y13-259) were made from hybridomas acquired from the American Type Culture Collection. Raf-1 antibodies were purchased from Life Technologies. Phospho-Akt (Ser-473) antibody was purchased from Cell Signaling. Polyclonal anti-H-, N-, and K-Ras antibodies were purchased from Santa Cruz.
Cell transfection and immunofluorescence.
Baby hamster kidney (BHK) cells were grown and maintained in HEPES-buffered Dulbecco's modified Eagle's medium containing 10% donor calf serum, as described previously (53). BHK cells were seeded onto either coverslips for immunofluorescence or 10-cm dishes for biochemical assays and transfected by using Lipofectamine (Life Technologies) according to the manufacturer's instructions. The efficiency of transfection was typically 65 to 80%. Cells on coverslips were fixed 24 h after lipofection. Cells on 10-cm dishes were switched to serum-free medium 18 to 24 h after lipofection and incubated for a further 4 h before being harvested. Where indicated, cells were then treated with 20 μM LY294002 in serum-free medium for 60 min. Cells were washed and scraped on ice into 0.5 ml of buffer A (10 mM Tris-HCl [pH 7.5], 25 mM NaF, 5 mM MgCl2, 1 mM EGTA, 1 mM dithiothreitol, 100 μM NaVO4). After 10 min on ice, cells were passed 25 times through a 23-gauge needle, and the nuclei were removed by low-speed centrifugation.
Postnuclear supernatants were spun at 100,000 × g. The supernatant (S100) was removed, and the sedimented fraction (P100) was rinsed and sonicated for 5 min in 100 μl of ice-cold buffer A. The S100 fraction and resuspended P100 fractions were snap-frozen and stored at −70°C in aliquots after the protein content was measured by the Bradford reaction. PC12 cells were cultured in Dulbecco's modified Eagle's medium supplemented with 5% horse serum, 10% calf serum, and 2 mM l-glutamine and transfected on coverslips by using Lipofectamine. At 16 h after lipofection, the cells were returned to standard PC12 maintenance medium and incubated a further 48 h prior to processing for confocal microscopy. Where indicated in the text, PC12 cultures were supplemented with 50 ng of NGF per ml.
Confocal microscopy.
Transfected PC12 or BHK cells were washed with phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde for 30 min at room temperature. Cells were washed for 10 min in PBS, permeabilized with 0.2% Triton X-100 in PBS, and blocked in 3% bovine serum albumin in PBS. The primary antibodies (anti-Myc and anti-HA) were diluted in blocking buffer at a 1:2 to 1:30 dilution, and the secondary antibodies, indocarbocyanin (CY3)-conjugated anti-mouse immunoglobulin and fluorescein isothiocyanate-conjugated anti-rat immunoglobulin, were used at a 1:300 dilution. Coverslips were mounted in Mowiol for confocal microscopy (2).
Ras GTP loading assays.
To prepare cells for Ras GTP loading assays, PC12 cells were cultured in reduced serum (0.1% horse serum, 0.5% calf serum) for 16 h and then treated for 5 and 30 min with 50 ng of NGF/ml. Ras GTP loading was measured in Raf-RNA binding domain (RBD) pulldown assays by using a high-affinity Raf-RBD-A85K mutant as described previously (14, 19, 26). Separate pulldowns from three aliquots of the same cell lysate were immunoblotted with anti-H-, N-, and K-Ras antisera to identify the Ras isoform that was activated by NGF stimulation. Aliquots of the starting lysates were also blotted with pan-Ras (Y13-259) and isotype-specific antisera to verify Ras protein normalization.
Western blotting.
Expression and subcellular localization of ectopically expressed proteins were determined by immunoblotting. Cellular fractions were normalized for protein content and then resolved on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels. When S100 and P100 fractions were compared, 20 μg of each S100 fraction was loaded together with an equivalent fraction of the corresponding P100 fraction as described previously (21). Proteins were transferred to polyvinylidene difluoride membranes by semidry transfer. Blots were probed with anti-Raf-1, Y13-259 (Ras), 9E10 (anti-Myc), or anti-HA monoclonal antibodies or phospho-Akt(Ser-473), developed by enhanced chemiluminescence, and quantified by phosphorimaging (Bio-Rad) as described before (52).
Raf-1 kinase assays.
P100 aliquots of transfected BHK cells were normalized for protein content and assayed for Raf activity by using a two-stage coupled MEK/ERK assay with phosphorylation of myelin basic protein used as a readout, as described previously (52).
RESULTS
H-Ras- but not K-Ras-mediated PC12 cell differentiation is blocked by dominant interfering mutant dynamin.
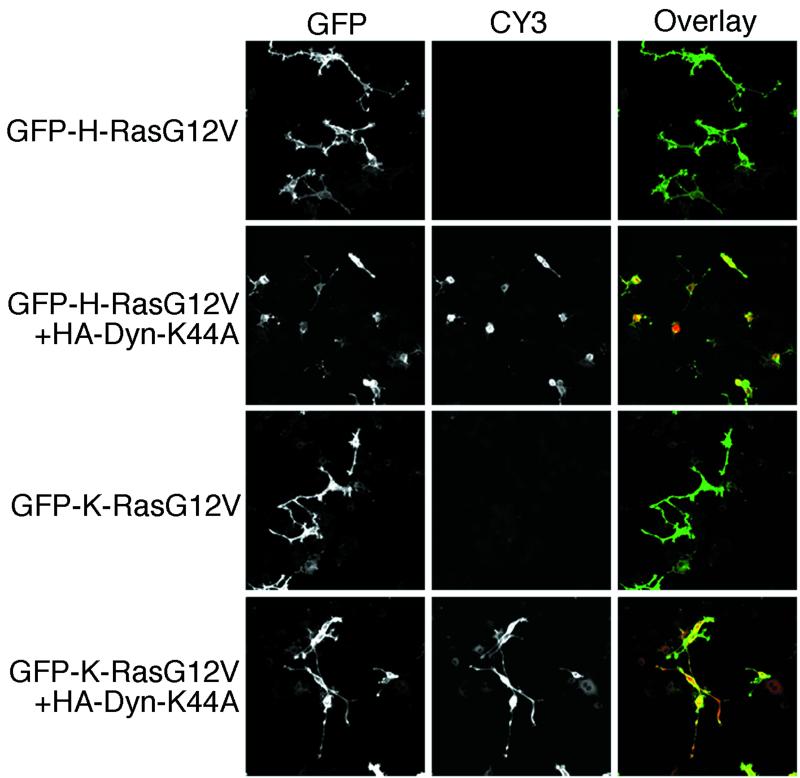
To assess whether clathrin-mediated endocytosis was required for Ras signaling, we first examined whether PC12 cell differentiation induced by activated H-RasG12V or K-RasG12V was inhibited by coexpression of dynamin-K44A. This well-characterized GTPase-defective, dominant interfering mutant of dynamin blocks the release of clathrin-coated vesicles from the plasma membrane (63). Figure 1 shows that GFP-tagged H-RasG12V and K-RasG12V both drive differentiation of PC12 cells, although the extent and complexity of neurite outgrowth are less marked with GFP-K-RasG12V than with GFP-H-RasG12V. Dynamin-K44A expression, detected by anti-HA indirect immunofluorescence, blocked neurite outgrowth in cells expressing GFP-H-RasG12V but had no detectable effect on neurite outgrowth in cells expressing GFP-K-RasG12V (Fig. 1). The inhibitory effect of dynamin-K44A was not due to an effect on H-Ras localization, because plasma membrane targeting of both GFP-H-RasG12V and GFP-K-RasG12V was unaffected (Fig. 1). PC12 cell differentiation has been shown previously to be mediated predominantly via the Raf/MEK/MAPK pathway (35, 46, 61). Our data therefore strongly suggest that endocytic function is important for H-Ras but not K-Ras activation of this kinase cascade.
FIG. 1.
PC12 cell differentiation induced by H-RasG12V but not K-RasG12V is inhibited by dynamin-K44A. PC12 cells were transfected with GFP-H-RasG12V or GFP-K-RasG12V in combination with an empty vector or HA-tagged dynamin-K44A, as indicated. Cells were incubated for 48 h prior to fixation. GFP-tagged proteins were visualized by direct immunofluorescence, and HA-tagged dynamin proteins were visualized by indirect immunofluorescence. Dynamin-K44A expression blocked neurite outgrowth in cells expressing GFP-H-RasG12V but had no detectable effect on neurite outgrowth in cells expressing GFP-K-RasG12V. The inhibitory effect of dynamin-K44A was not due to an affect on H-Ras localization because plasma membrane targeting of both GFP-H-RasG12V and GFP-K-RasG12V was unaffected.
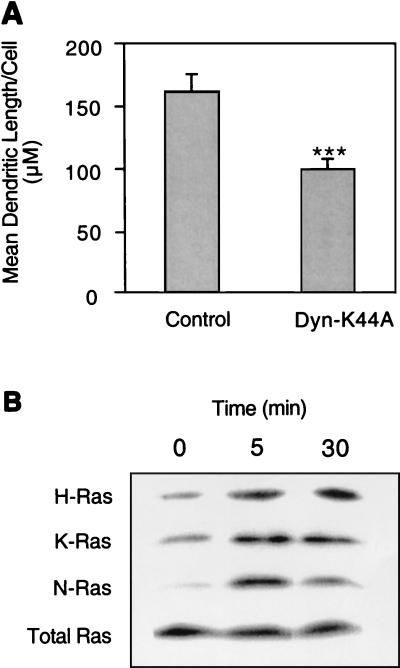
To establish the potential physiological relevance of these observations, we examined whether dynamin-K44A could also block NGF-induced PC12 cell differentiation. Figure 2A shows that expression of dynamin-K44A significantly reduced but did not completely abolish neurite outgrowth from PC12 cells cultured in NGF. To explore possible reasons for this incomplete effect of dominant interfering dynamin, we investigated which Ras isoforms are activated in PC12 cells in response to NGF treatment. Figure 2B shows that all three Ras isoforms are expressed in PC12 cells and that all three Ras isoforms are activated by NGF, albeit to different extents.
FIG. 2.
PC12 cell differentiation induced by NGF is partially inhibited by dynamin-K44A. (A) PC12 cells transfected with GFP-tK alone or in combination with dynamin-K44A were cultured with 50 ng of NGF/ml for 72 h and then fixed. Membrane-targeted GFP-tK (GFP with the membrane anchor of K-Ras) is transfected to allow clear visualization of neurites in the control and dynamin-expressing cells. The mean total neurite length per transfected cell (± standard error of the mean) was measured for 40 to 50 cells across multiple microscopic fields from six independent transfections. The difference between means, examined in a t test, shows that dynamin-K44A significantly (P < 0.001) inhibited NGF-stimulated neurite outgrowth. (B) Untransfected, serum-starved PC12 cells were treated for 0 (control), 5, or 30 min with 50 ng of NGF/ml and analyzed for endogenous H-, N-, and K-Ras activation in Raf-RBD pulldown assays. Starting lysates were probed with a pan-Ras antibody to check protein normalization.
H- and K-Ras have different requirements for endocytosis to activate Raf-1.
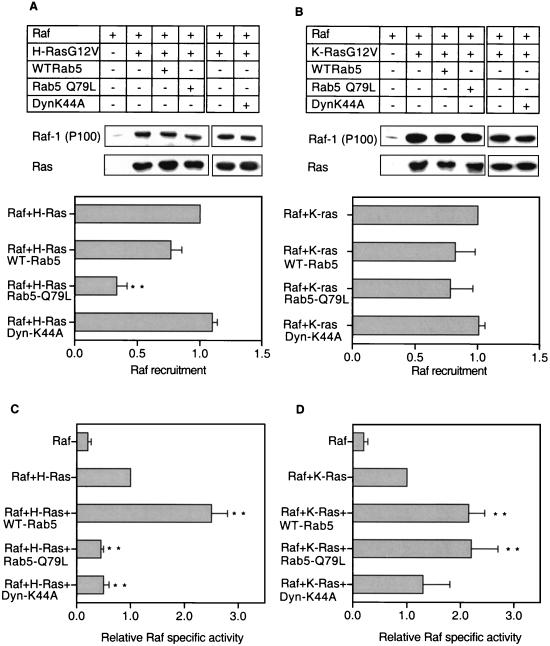
In light of these observations, we investigated whether preventing clathrin-coated vesicle release from the plasma membrane had any direct effect on the ability of Ras to recruit Raf-1 or initiate Raf-1 activation at the plasma membrane. BHK cells transiently transfected with Ras and Raf-1 with or without dynamin-K44A were fractionated into S100 (cytosolic) and P100 (membrane) fractions. Raf-1 recruitment was assayed by quantitative immunoblotting of S100 and P100 fractions, and Raf-1 activation was assayed in a coupled MEK/ERK kinase assay with P100 fractions normalized for Raf-1 content. Figure 3 shows that expression of dynamin-K44A significantly (P < 0.01) inhibited H-Ras-mediated Raf-1 activation but had no effect on K-Ras-mediated Raf-1 activation. Membrane recruitment of Raf-1 by H-RasG12V or K-RasG12V was unaffected by expression of dynamin-K44A (Fig. 3).
FIG. 3.
Dynamin-K44A and Rab5-Q79L expression reduces H-Ras- but not K-Ras-mediated Raf activation. BHK cells transfected with the plasmid combinations indicated were fractionated, and 20 μg of each membrane (P100) fraction was immunoblotted for H-Ras and Raf-1 (A) or K-Ras and Raf-1 (B). Under the conditions of this assay, approximately 50% of ectopically expressed Raf is recruited to the P100 fraction in the control cells expressing H- or K-Ras and Raf alone (49). A representative P100 immunoblot is shown together with a graph of relative Raf recruitment estimated from eight independent experiments. Differences from the respective control values (H- or K-Ras + Raf was set at 1) were examined in t tests, with significant differences indicated (∗∗, P < 0.01). P100 fractions from the H-Ras transfection series (C) and the K-Ras transfection series (D) were normalized for Raf-1 content and assayed for Raf activity by using a coupled MEK/ERK assay. The results show Raf specific activities ± standard errors of the means (n = 18) from three to four independent experiments for the H-Ras series (C), and Raf specific activities ± standard errors of the means (n = 21 to 22) from five to six independent experiments for the K-Ras series (D). Expression of ectopically expressed WT-Rab5, Rab5-Q79L, or dynamin-K44A was verified by Western blotting (not shown). Differences between each mean and the value for the H- or K-RasG12V control were examined in t tests, and significant differences are indicated (∗, P < 0.05; ∗∗, P < 0.01).
To extend these results, we examined whether stimulating endocytosis in BHK cells would potentiate H-Ras-mediated Raf activation. Expression of wild-type Rab5 (WT-Rab5) and expression of the GTPase-deficient mutant Rab5-Q79L increase the rate of clathrin-mediated endocytosis in BHK cells (56). WT-Rab5 expression also increases recycling through the sorting endosome back to the cell surface, resulting in only a minor increase in the size of the endosomal compartment (56). In contrast, Rab5-Q79L expression significantly reduces recycling through the sorting endosome and stimulates homotypic endosome fusion, resulting in an enlarged endosomal compartment (56).
To assess the effect of Rab5 expression on H-Ras-mediated Raf-1 activation, Myc-tagged WT-Rab5 or Rab5-Q79L was cotransfected into BHK cells with Raf-1 and H-RasG12V. Figure 3 shows that coexpression of WT-Rab5 had no measurable effect on Raf-1 membrane recruitment but resulted in a significant (P < 0.01), 2.5-fold increase in Raf-1 specific activity (Fig. 3C). In contrast, Rab5-Q79L expression significantly reduced (P < 0.01) the amount of Raf-1 recruited to the P100 fraction (Fig. 3A) and significantly decreased (P < 0.01) the specific activity of membrane-recruited Raf-1 (Fig. 3C). Expression of neither Rab5 protein altered the total amount of H-RasG12V associated with the P100 fraction (Fig. 3A). The simplest interpretation of these results, given the known effects of the two Rab5 proteins on vesicular trafficking, is that potentiation of H-Ras-stimulated Raf-1 activity requires increased endocytosis to be coupled with efficient endocytic recycling.
These experiments were then repeated by using K-RasG12V as the Raf-1 activator. Intriguingly, expression of both WT-Rab5 and Rab5-Q79L significantly (P < 0.01) increased Raf-1 specific activity (Fig. 3D). Neither Rab5 protein altered the amount of Raf-1 recruited to the P100 fraction or the amount of P100-associated K-RasG12V (Fig. 3B). We conclude that although endocytosis is not essential for K-Ras-mediated Raf-1 activation, it can be potentiated by increasing the rate of endocytosis. However, in contrast to H-Ras-mediated Raf-1 activation, increased endocytic recycling is not required for the effect.
H-Ras- but not K-Ras-mediated Raf-1 activation is dependent on PI3-K activity.
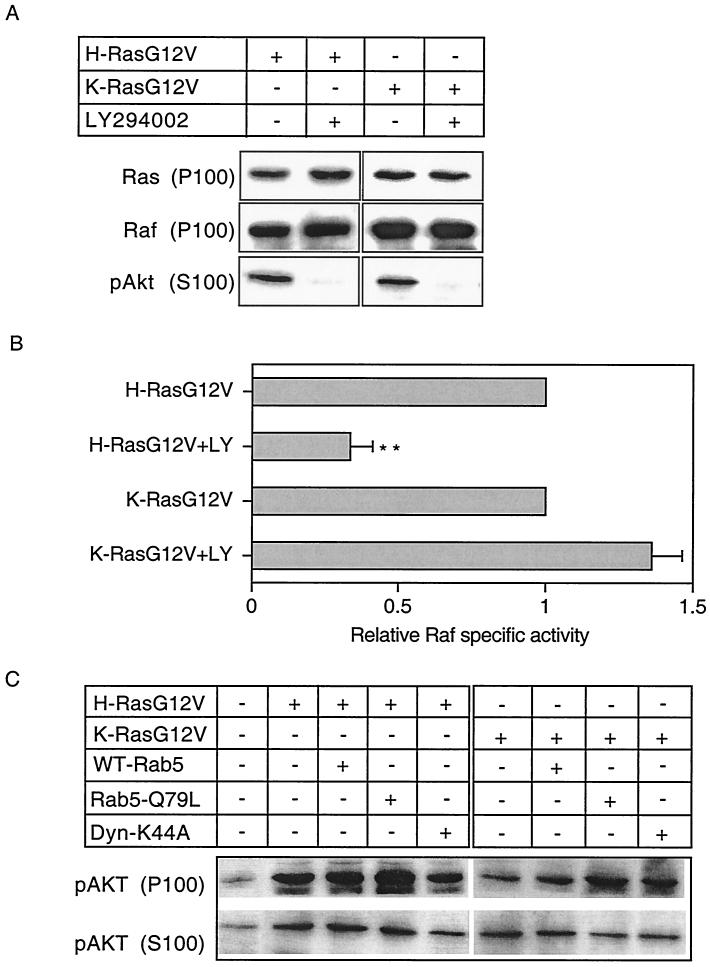
Previous data have demonstrated that inhibitors of PI3-K and endocytosis block Ras-mediated B-Raf and ERK activation in PC12 cells (55). In light of these results, we investigated whether inhibition of PI3-K activity would differentially affect H- and K-Ras-mediated Raf-1 activation in BHK cells. BHK cells transiently expressing H-RasG12V or K-RasG12V were treated for 60 min with the PI3-K inhibitor LY294002. This treatment completely blocked activation of Akt, assayed by immunoblotting with phosphorylation-specific antisera (Fig. 4A). LY294002 treatment did not affect the amount of H- or K-Ras associated with the membrane fraction and did not inhibit Raf-1 membrane recruitment (Fig. 4A). Confocal microscopy also revealed no effect of LY294002 on the plasma membrane localization of GFP-tagged H-RasG12V or K-RasG12V (data not shown). Nevertheless, assays of Raf-1 specific activity showed that LY294002 treatment significantly (P < 0.01) inhibited H-Ras-mediated Raf-1 activation but had no effect on K-Ras-mediated Raf-1 activation (Fig. 4B).
FIG. 4.
H-Ras- but not K-Ras-mediated Raf-1 activation is selectively dependent on PI3-K activity. BHK cells were transfected with either H-RasG12V or K-RasG12V. Cells were incubated in serum-free medium overnight and then either treated with 20 μM LY249002 for 60 min before harvesting or harvested without treatment (control). (A) Cells were fractionated, and 20 μg of the cytosolic (S100) fraction was immunoblotted for phospho-Akt and 20 μg of the membrane (P100) fraction was immunoblotted for Ras and Raf-1. (B) P100 fractions were normalized for Raf-1 content and assayed for Raf activity by using a coupled MEK/ERK assay. The results show relative Raf specific activities ± standard errors of the means (n = 6 to 7) from three independent experiments. Differences between each mean and the value for the H- or K-RasG12V control were examined in t tests, and significant differences are indicated (∗, P < 0.05; ∗∗, P < 0.01). In this set of experiments, the actual control H- and K-Ras Raf activities (estimated as described before [52]) were 2.9 ± 0.2 and 4.65 ± 0.4 pmol of phosphate transfer/20 min/10 μg of P100 fraction/unit of Ras, respectively. (C) S100 and P100 fractions prepared from BHK cells transfected with H-RasG12V or K-RasG12V with or without Rab5-Q79L, WT-Rab5, or dynamin-K44A or mock transfected (control) and normalized for protein content were immunoblotted for phospho-Akt. Duplicate Western blots were immunoblotted with Y13-259 to confirm equivalent levels of expression of H- and K-Ras across each transfection series and anti-HA and anti-Myc antisera to demonstrate ectopic expression of dynamin and Rab5 proteins (not shown).
These results prompted the additional question of whether H- and K-Ras have differential requirements for endocytosis to activate PI3-K. Figure 4C shows that this is not the case. Expression of dynamin-K44A, WT-Rab5, or Rab5-Q79L did not inhibit H- or K-Ras-stimulated Akt activation in cell membranes (P100 fraction). In fact, expression of Rab5-Q79L resulted in a small (1.5-fold) but reproducible increase in H- and K-Ras-stimulated P100-associated phospho-Akt levels (Fig. 4C). Dynamin-K44A coexpression with H-RasG12V, however, did result in reduced levels of cytosolic phospho-Akt, suggesting that release of activated Akt from the plasma membrane may in part be dependent on endocytosis.
H-RasG12V but not Raf-1 is sequestered in Rab5-Q79L enlarged endosomes.
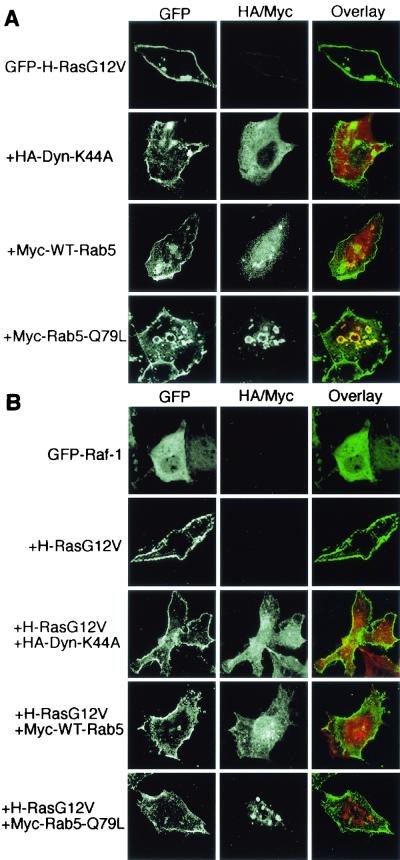
We next used confocal microscopy to address whether the subcellular distributions of GFP-tagged Ras and Raf proteins were altered by ectopic expression of dynamin or Rab5. Figures 5A and 6A show that plasma membrane localization of GFP-H-RasG12V and GFP-K-RasG12V was unaffected by the expression of either WT-Rab5 or dynamin-K44A. However, in the majority of cells expressing Rab5-Q79L, GFP-H-RasG12V was partially redistributed from the plasma membrane to enlarged endosomes that costained for the ectopically expressed Rab5-Q79L protein (Fig. 5A). In contrast, GFP-K-RasG12V remained localized exclusively at the plasma membrane in cells coexpressing Rab5-Q79L (Fig. 6A).
FIG. 5.
Rab5-Q79L expression causes endosomal accumulation of H-Ras but not H-Ras-recruited Raf-1. BHK cells were cotransfected with GFP-H-RasG12V (A) or untagged H-RasG12V with GFP-Raf-1 (B) in combination with WT-Rab5, Rab5-Q79L, or dynamin-K44A, as indicated. GFP-tagged proteins were visualized by direct immunofluorescence, and Myc-tagged Rab5 or HA-tagged dynamin proteins were visualized by indirect immunofluorescence. GFP-H-RasG12V is localized predominantly at the plasma membrane in cells coexpressing dynamin-K44A or WT-Rab5 but accumulates within enlarged endosomes when coexpressed with Rab5-Q79L (A). In contrast, GFP-Raf-1 does not accumulate within enlarged endosomes when coexpressed with H-RasG12V and Rab5-Q79L but is seen predominantly decorating the plasma membrane (B). Note that in all of the experiments shown in panel B and in Fig. 6B that are tritransfections, the expression of RasG12V can be inferred because GFP-Raf is recruited to the plasma membrane (compare with the control cells expressing GFP-Raf-1 alone).
FIG. 6.
Dynamin-K44A or Rab5 expression does not affect the plasma membrane localization of K-Ras or K-Ras-recruited Raf-1. BHK cells were transfected with GFP-K-RasG12V (A) or untagged K-RasG12V with GFP-Raf-1 (B) in combination with WT-Rab5, Rab5-Q79L, or dynamin-K44A, as indicated. Proteins were visualized by direct or indirect immunofluorescence. GFP-K-RasG12V coexpressed with WT-Rab5, Rab5-Q79L, or dynamin-K44A is found exclusively at the plasma membrane (A). GFP-Raf-1 coexpressed with K-RasG12V and either WT-Rab5, Rab5-Q79L, or dynamin-K44A is also found exclusively at the plasma membrane (B).
To determine the subcellular localization of Raf-1, GFP-Raf was coexpressed with H-RasG12V or K-RasG12V in the presence of dynamin-K44A or Rab5. The control panels in Fig. 5B and 6B show that both RasG12V proteins efficiently recruit GFP-Raf from the cytosol to the plasma membrane. Expression of dynamin-K44A or WT-Rab5 had no effect on the plasma membrane recruitment of GFP-Raf by either H-RasG12V (Fig. 5B) or K-RasG12V (Fig. 6B). Similarly, K-Ras-mediated GFP-Raf plasma membrane recruitment was unaffected by coexpression of Rab-Q79L (Fig. 6B). Most interestingly, there was no decoration of the enlarged Rab5-positive endosomes with GFP-Raf in the cells coexpressing Rab5-Q79L and H-RasG12V (Fig. 5B). Thus, activated H-Ras does not remain complexed with Raf-1 on early endosomal membranes. This result correlates well with the decrease in P100-associated Raf-1 detected by immunoblotting in cells expressing H-RasG12V and Rab5-Q79L (Fig. 3A).
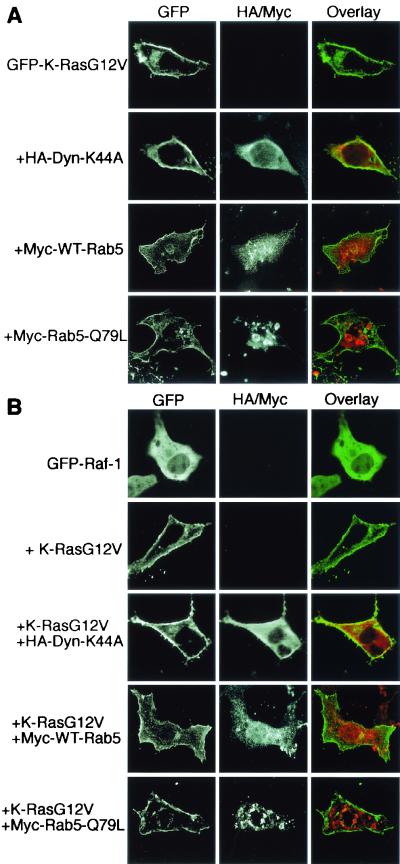
We next examined whether the Rab5-Q79L-induced endosomal accumulation of H-RasG12V was dependent on the activation state of H-Ras. To this end, wild-type GFP-H-Ras or GFP-tH (GFP targeted to the plasma membrane by the minimal C-terminal targeting sequences of H-Ras [43]) was coexpressed with Rab5-Q79L. Figure 7 shows that the plasma membrane localization of GFP-H-Ras and GFP-tH was unaffected by expression of Rab5-Q79L. Specifically, no GFP-H-Ras or GFP-tH accumulated in the large Rab5-Q79L-positive endosomes. We conclude from these results that activated H-Ras is sensitive to Rab5-Q79L-stimulated endocytosis, whereas inactive GDP-bound H-Ras is not.
FIG. 7.
Neither wild-type H-Ras nor GFP-tH localization is affected by WT-Rab5 or Rab5-Q79L expression. BHK cells were transfected with GFP-H-Ras or GFP-tH with or without Rab5-Q79L, as indicated, and proteins were visualized by direct or indirect immunofluorescence. There was no effect on the plasma membrane localization of GFP-H-Ras (A) or GFP-tH (B) when the proteins were coexpressed with Rab5-Q79L.
The HVR and not the effector activation profile renders H-Ras sensitive to endosomal sequestration.
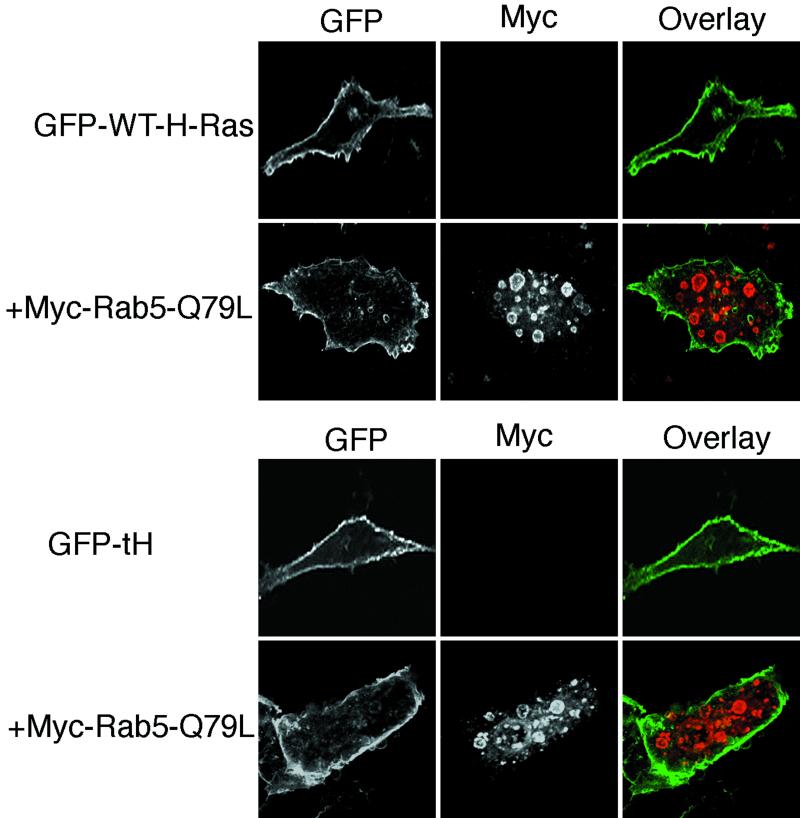
Taken together, the data presented in Fig. 7 strongly suggest that plasma membrane microlocalization, governed by the C-terminal hypervariable region (HVR), renders H-Ras differentially sensitive to endocytosis. To formally demonstrate this, we replaced the C-terminal HVR of K-RasG12V with the C-terminal HVR of H-Ras. Figure 8A shows that this GFP-K/CTH-G12V chimeric protein localized to the plasma membrane, but when coexpressed with Rab5-Q79L, it behaved exactly like H-RasG12V and accumulated in the enlarged Rab5-Q79L-generated endosomes. The reciprocal GFP-H/CTK-G12V chimera H/K (H-RasG12V with the C-terminal HVR of K-Ras) behaved like K-RasG12V and did not accumulate in Rab5-Q79L endosomes (data not shown).
FIG. 8.
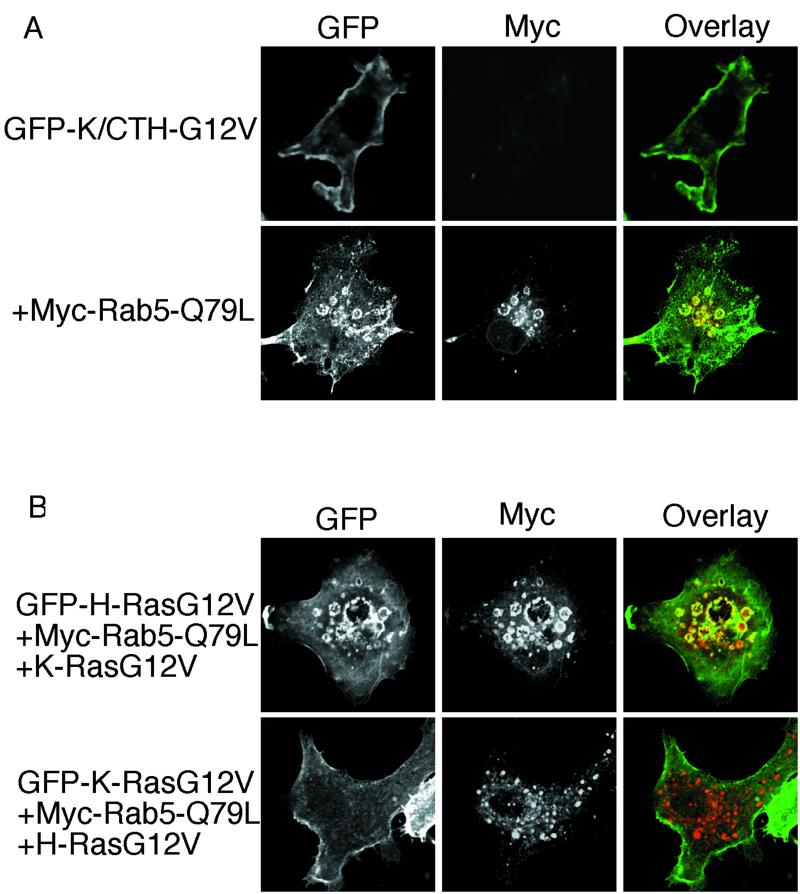
The HVR but not the effector activation profile renders H-Ras sensitive to endosomal sequestration. (A) BHK cells were transfected with chimeric GFP-K/CTH-G12V or GFP-H/CTK-G12V with or without Rab5-Q79L, as indicated, and proteins were visualized by direct or indirect immunofluorescence. There was significant accumulation of GFP-K/CTH-G12V within enlarged endosomes when coexpressed with Rab5-Q79L. No effect was seen on the plasma membrane localization of GFP-H/CTK-G12V (data not shown). (B) BHK cells were transfected with GFP-K-RasG12V together with H-RasG12V and Rab5-Q79L or with GFP-H-RasG12V together with K-RasG12V and Rab5-Q79L, as indicated, and proteins were visualized by direct or indirect immunofluorescence. There was no effect on GFP-K-RasG12V plasma membrane localization in cells coexpressing H-RasG12V and Rab5-Q79L. Similarly, expression of K-RasG12V did not inhibit the accumulation of GFP-H-RasG12V in Rab5-Q79L enlarged endosomes (compare with Fig. 5A).
Recent work from Walsh and Bar-Sagi (65) with chimeric Ras proteins has shown that the effector activation profiles of H- and K-Ras are also determined by the C-terminal HVR sequences. These data are entirely consistent with the general hypothesis that different microlocalizations govern Ras signal output, but given that the Ras HVR sequences govern both effector utilization and microlocalization, then by swapping HVR sequences between H- and K-Ras, the effector activation profile and microlocalization will both be changed. Therefore, we coexpressed GFP-K-RasG12V with H-RasG12V in cells expressing Rab5-Q79L to see if activation of the H-RasG12V effector complement is sufficient to trap GFP-K-RasG12V in the endosomal compartment. The results show that this is not the case, because in cells coexpressing H-RasG12V, GFP-K-RasG12V did not accumulate in Rab5-Q79L enlarged endosomes (Fig. 8B). Similarly, coexpression of K-RasG12V had no effect on the accumulation of GFP-H-RasG12V in Rab5-Q79L-positive endosomes (data not shown). We conclude that microlocalization, dictated by the C-terminal membrane anchor, and not differences in signaling output determine the differential sensitivity of the Ras isoforms to clathrin-mediated endocytosis.
DISCUSSION
Recent studies have demonstrated that the closely related Ras isoforms H-, K-, and N-Ras generate distinct signaling outputs despite having a common set of activators and effectors (5, 20, 27, 29, 62, 64, 65, 69, 70). We and others have proposed that these signaling differences are a result of compartmentalization of the Ras proteins to different microdomains of the plasma membrane (42, 43). K-Ras, anchored by a polybasic sequence and farnesylation, is localized predominantly to the disordered plasma membrane. H-Ras anchored by palmitoylation and farnesylation is in equilibrium between lipid rafts and disordered plasma membrane. We show in the present study that the different Ras membrane anchors, which mediate microcompartmentalization within the plasma membrane, also render H- and K-Ras differentially sensitive to regulation by membrane trafficking. Our focus was the initial event in the MAPK cascade, namely, Ras-dependent plasma membrane recruitment and activation of Raf. We used ectopic expression of Rab5 proteins to enhance endocytosis and dominant interfering mutant dynamin proteins to block endocytosis and found strikingly different effects on H- and K-Ras-mediated Raf activation.
In BHK cells, H-Ras- and not K-Ras-mediated Raf activation was selectively inhibited by dynamin-K44A. The simplest interpretation of these data are that H-Ras/Raf complexes must enter the endosomal compartment to complete the multistep process of Raf activation initiated at the plasma membrane (Fig. 9, loop A), whereas K-Ras completes Raf activation efficiently at the plasma membrane without a contribution from the endosome (Fig. 9, loop B). Interestingly, the inhibitory effect of dynamin-K44A on H-Ras function was more complete in the biological assay of PC12 cell differentiation than was evident in our biochemical Raf kinase assays in BHK cells. Possible explanations for this discrepancy are that endocytosis may also be required for MEK to MAPK communication, as suggested by Kranenberg et al. (30), and/or is needed to activate additional H-Ras signaling pathways that contribute to PC12 cell growth or viability.
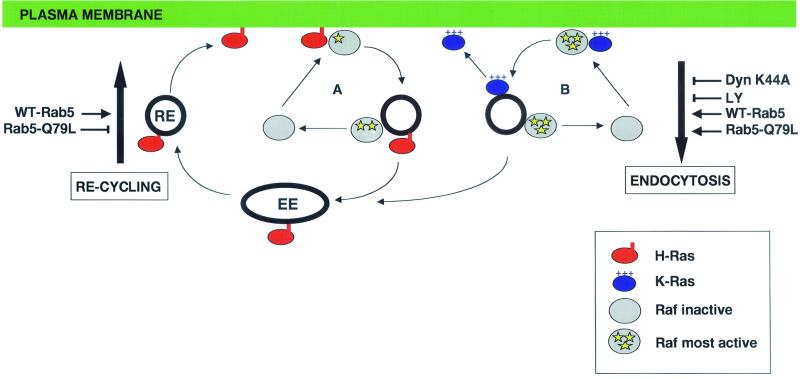
FIG. 9.
Model of the differential endosomal trafficking of H- and K-Ras. In loop A, activated H-Ras recruits Raf to the plasma membrane, where Raf activation is initiated. H-Ras and Raf are internalized together because they occupy the same microdomain in the disordered plasma membrane. Raf is released directly from the endosome to the cytosol, but H-Ras remains associated with the endosome because of its C-terminal membrane anchor and is reliant on endocytic recycling for return to the plasma membrane. Endocytosis of H-Ras/Raf complexes is required to complete H-Ras-mediated Raf-1 activation. In loop B, activated K-Ras recruits Raf to the plasma membrane. Raf activation in the K-Ras microdomain is both initiated and completed. K-Ras and activated Raf-1 are endocytosed together. K-Ras immediately diffuses back to the negatively charged plasma membrane down an electrostatic gradient, and Raf is released directly from the endosome to the cytosol. Recycling of K-Ras is therefore not dependent on endocytic recycling. Raf-1 is probably released after clathrin-coated vesicles have fused with early endosomes because activated Ras and Raf are found together in clathrin-coated vesicles in NGF-stimulated PC12 cells (25). Raf-1 is inactive in the cytosol (32, 52, 57), so release from the endosome must be accompanied by deactivation. Manipulations that increase or decrease endocytosis or recycling are marked on either side of the diagram with an arrow or T-bar, respectively. EE, early endosome; RE, recycling endosome.
In contrast to the nearly complete block of H-RasG12V-induced neurite outgrowth, dynamin-K44A only partially inhibited NGF-induced PC12 cell differentiation. A similar observation has also been made in PC12 cells expressing a temperature-sensitive dominant negative dynamin G273D (72). PC12 cells express H-, N-, and K-Ras, and we show here that NGF efficiently activates all three Ras isoforms. Therefore, one explanation of the incomplete inhibition of NGF-stimulated PC12 neurite outgrowth by dominant interfering dynamin mutants is that endogenous K-Ras-mediated Raf activation is sufficient to differentiate PC12 cells when clathrin-mediated endocytosis is blocked.
Interestingly, like dynamin-K44A, LY294002 selectively inhibited H-Ras- but not K-Ras-mediated Raf-1 activation. PI3-Ks are involved in multiple vesicular trafficking events, including cooperating with Rab5 in regulating early endosomal function (13). The precise role that PI3-Ks play in Raf-1 activation continues to be debated (50, 58, 68), but in the context of the dynamin-K44A result, our preferred interpretation is that the selective effect of LY294002 on H-Ras signaling is due to an inhibition of endocytosis. Similarly, York et al. (71) showed that activation of B-Raf by H-RasG12V in PC12 cells is abolished by LY294002 and concluded that this was likely due to an inhibition of Ras endocytosis. In contrast to the activation of Raf, the generation of membrane-associated phospho-Akt by H- and K-Ras was unaffected by dynamin-K44A, suggesting that neither Ras isoform requires clathrin-mediated endocytosis to activate PI3-K at the plasma membrane. This is also consistent with an earlier study showing that PI3-K activation in NGF-stimulated PC12 cells is actually terminated by endocytosis of activated Trk receptors (72).
Although it has a critical role in regulating clathrin-mediated endocytosis, dynamin also interacts with numerous signaling proteins as well as the actin cytoskeleton (28, 45). Thus, we cannot exclude the possibility that interference with some other dynamin-regulated process could underlie the effects on Raf activation that we observed. Given these uncertainties, we therefore also used Rab5 proteins to stimulate clathrin-mediated endocytosis. The results of these experiments can most readily be explained if the normal pathway of H-Ras-mediated Raf activation and the return of Raf to the cytosol involve trafficking through the endosome (Fig. 9, loop A). We speculate that Raf is released directly from the endosome to the cytosol, whereas H-Ras, because of its C-terminal membrane anchor, is more avidly membrane associated and is reliant on endocytic recycling for return to the plasma membrane. Thus, when Rab5-Q79L expression stimulates endocytosis and blocks endocytic recycling, the model predicts that H-Ras but not Raf-1 will accumulate within enlarged endosomes, exactly as seen in Fig. 5.
The sequestration of H-RasG12V in the Rab-Q79L enlarged endosomes reduces the amount of H-RasG12V available at the plasma membrane to recruit and activate Raf, explaining the kinase assay and fractionation results shown in Fig. 3. Activated H-RasG12V but not H-Ras or GFP-tH was sequestered in the Rab-Q79L-positive endosomes. Our previous studies have shown that although these three proteins are tethered to the plasma membrane by the same membrane anchor, activated H-RasG12V is localized almost completely to disordered plasma membrane, whereas H-Ras and GFP-tH are approximately 50% and 100% localized to lipid rafts, respectively (42). Together, these results suggest that H-Ras must be released from lipid rafts in order to be captured by clathrin-coated pits.
A different situation exists in cells expressing WT-Rab5, where increased endocytosis is matched by increased endocytic recycling (56). Endocytosed H-Ras will be efficiently returned to the plasma membrane, and endocytosed Raf-1 will be released to the cytosol, where it is available for reactivation by H-Ras (Fig. 9, loop A). The net effect of WT-Rab5 expression then is to stimulate Raf-1 and H-RasG12V recycling without altering the steady-state plasma membrane localization of H-Ras. In a previous study, we showed that under conditions in which H-Ras plasma membrane localization is maintained, increased recycling of Raf-1 between the cytosol and plasma membrane is accompanied by an increase in Raf specific activity (54), consistent with the effect of WT-Rab5 on Raf activation seen in Fig. 3.
A key assumption in our reasoning is that H-Ras and Raf are separated from each other after endocytosis in clathrin-coated vesicles or early endosomes (Fig. 9). How might this be achieved? A direct interaction between GTP-loaded Ras and the RBD of Raf-1 is essential for the recruitment of Raf-1 from the cytosol to the plasma membrane (18, 60). However, several lines of evidence suggest that Ras does not continue to operate as a membrane anchor for Raf after recruitment. First, H- and K-Ras are readily solubilized from membranes by 1% NP-40, whereas membrane-recruited Raf-1 is largely insoluble (52, 57). Second, recent studies have shown that Raf-1 can anchor to cell membranes, including endosomes, via an interaction with phosphatidic acid that is independent of the RBD (48, 49). Taking these data together, it is reasonable to conclude that after recruitment by Ras, Raf remains anchored to plasma and endosomal membranes independently of an interaction with Ras. Indeed, if the main anchor for Raf is phosphatidic acid, then turnover of this lipid in the endosome could be the mechanism that releases Raf back to the cytosol.
Unlike H-Ras-mediated Raf activation, K-Ras-mediated Raf activation was potentiated by WT-Rab5 and Rab5-Q79L expression. Importantly, Rab5-Q79L did not trap K-Ras in enlarged early endosomes. The simplest interpretation of these data are that K-Ras and Raf are endocytosed together, but K-Ras is returned to the cell surface from the endosome by a mechanism that it is independent of endosomal recycling (Fig. 9, loop B). The maintenance of K-Ras plasma membrane localization is thus independent of endosomal recycling, and so, both Rab5 proteins, by increasing the rate of endocytosis, will increase Raf-1 recycling and hence Raf-1 specific activity. There is some indirect evidence to support this hypothesis.
Much attention recently has been focused on how newly synthesized Ras proteins traffic to the plasma membrane after posttranslational processing has been completed on the cytosolic surface of the endoplasmic reticulum. Palmitoylated H-Ras traffics to the cell surface through the Golgi via the classical exocytic pathway (2, 12). The K-Ras transport pathway is as yet poorly defined, but it does not involve the Golgi, is unaffected by temperature blocks, and is independent of Sar1 and Arf1 function (2, 12; S. Roy and J. F. Hancock, unpublished data). Thus, by all established criteria, transport of K-Ras from the endoplasmic reticulum to the plasma membrane is independent of known vesicular transport mechanisms. These data have led to speculation that the charged polybasic domain of K-Ras drives diffusion through the cytosol to the negatively charged plasma membrane down an electrostatic gradient (3, 33, 37, 51). If this is correct, then K-Ras removed from the negatively charged plasma membrane by endocytosis could similarly diffuse back to the plasma membrane down a charge gradient, whereas the recruited Raf-1 remains anchored to the endosome by an interaction with phosphatidic acid.
Thus, our proposed recycling mechanisms for endocytosed H-Ras and K-Ras are analogous to their known mechanisms for transport from the endoplasmic reticulum to the plasma membrane, i.e., H-Ras is dependent on vesicular transport, whereas K-Ras is not. An alternative scenario, which we cannot formally exclude, is that Raf activated by K-Ras undergoes endocytosis, whereas K-Ras does not and simply remains at the plasma membrane. Further work is required to discriminate between these possibilities.
Although the proposed model is our favored interpretation of the data, it is substantially based on ectopic Rab5 and dynamin-K44A expression experiments, and several caveats are required. First, others have shown that clathrin-coated vesicles immunopurified from NGF-stimulated PC12 cells contain both Ras and Raf (25), whereas the Rab5-Q79L endosomes that we studied here contain H-Ras but not Raf. It is therefore possible that the enlarged Rab5-Q79L endosomes may not exactly replicate the function of normal early endosomes, i.e., may inappropriately retain or exclude specific proteins. An alternative conclusion, if the two data sets are taken together, is that Raf may be released to the cytosol when clathrin-coated vesicles fuse with early endosomes. Second, our focus has been on clathrin-mediated endocytosis, whereas there are also clathrin-independent endocytic pathways. Of particular relevance to Ras signaling are endocytic pathways that selectively internalize or sort lipid raft-associated proteins or endocytose caveolae (24, 38-40, 44). The nature of these pathways remains somewhat controversial, with different studies reporting somewhat conflicting results (38, 44, 55). Nevertheless, it is clear that some nonclathrin endocytic pathways are dynamin dependent or are regulated by Rho family GTPases (17, 31), although none are regulated by Rab5.
We did not examine the role of nonclathrin endocytosis in Ras signaling. Our rationale was that since constitutively activated H-RasG12V and K-RasG12V are predominantly (>85%) localized outside of lipid rafts, they may not be accessible to lipid raft-selective endocytic pathways. In the context of agonist-driven endocytosis, however, this may not be the case. Rizzo et al. (47) have shown that GFP-H-Ras and a lipid raft marker (GFP-with the C-terminal HVR of H-Ras, very similar to GFP-tH) are both internalized into endosomal structures when HIRcB cells are stimulated with insulin. Immunoisolation of insulin receptor-positive endosomes from these stimulated cells shows that they contain clathrin and EEA1 and, like the clathrin-coated vesicles from NGF-stimulated PC12 cells, retain Raf (47, 49). Thus, activation of insulin receptors drives the endocytosis of both H-Ras and the GFP-tH lipid raft marker, possibly into the same set of endosomal vesicles (although this has not yet been formally demonstrated). In contrast, our data show that activated H-Ras in the absence of an insulin stimulus is internalized away from the GFP-tH lipid raft marker, which remains at the plasma membrane.
These observations can be reconciled by proposing that either (i) the endocytic pathway utilized by H-Ras is controlled by signals from the insulin receptor or (ii) activation of the insulin receptor triggers the recruitment of lipid rafts into endocytic vesicles, whereas activated H-Ras signaling does not. Substantial support for the latter proposal comes from multiple studies showing that insulin receptor activation drives the assembly of multiprotein signaling complexes on lipid rafts (1, 7, 11, 36) and that activation of lipid raft-associated TC10 is required for GLUT4 translocation to the plasma membrane (11, 66). The C-terminal anchor of TC10 is nearly identical to H-Ras, and in fact a TC10 chimera with H-Ras C-terminal sequences functions like wild-type TC10 (66). The presence of ectopically expressed H-Ras and GFP-tH in insulin-stimulated vesicles (47) is therefore perhaps not unexpected if proteins recruited to the insulin receptor-positive lipid rafts at the plasma membrane are internalized together. Moreover, the presence of activated insulin receptor in the endocytic vesicles could maintain the levels of phosphatidic acid at a level sufficient to retain Raf. Nevertheless, further work is clearly required to unravel the sorting mechanisms involved in directing Ras proteins and other plasma membrane proteins into different endocytic pathways and the possible signaling consequences of such compartmentalization.
Activated Ras stimulates fluid-phase endocytosis via a Rab5-dependent mechanism (4, 6, 34). Recently Tall et al. (59) identified Rin1, a Rab5-guanine nucleotide exchange factor, as a novel Ras effector and showed that Rin1 stimulates endosomal fusion in a Ras-GTP-dependent manner. Rin1 expression drives the formation of enlarged early endosomes in NR6 cells, very similar to Rab5-Q79L endosomes, and attenuates EGF-stimulated MAPK activation. This result recapitulates the effect of Rab5-Q79L on H-Ras-mediated Raf activation and further emphasizes the interplay between Ras signaling and endocytosis.
In conclusion, although the final model remains speculative, our study clearly demonstrates differences in the dependence of H- and K-Ras on endosomal function that reflect their different mechanisms of plasma membrane attachment. Earlier studies have shown that the correct microlocalization of Ras proteins is intricately linked to their signaling function. The new observations presented here are fully consistent with this general hypothesis and highlight the potential role of endocytosis in isoform-specific regulation of Ras signaling.
Acknowledgments
We thank Rob Parton for many insightful discussions, helpful advice, and numerous reagents.
This work was supported by grants from the National Health and Medical Research Council of Australia. J.F.H. is also supported by the Royal Children's Hospital Foundation, Queensland.
REFERENCES
- 1.Ahmed, Z., B. J. Smith, and T. S. Pillay. 2000. The APS adapter protein couples the insulin receptor to the phosphorylation of c-Cbl and facilitates ligand-stimulated ubiquitination of the insulin receptor. FEBS Lett. 475:31-34. [DOI] [PubMed] [Google Scholar]
- 2.Apolloni, A., I. A. Prior, M. Lindsay, R. G. Parton, and J. F. Hancock. 2000. H-Ras but not K-Ras traffics to the cell surface via the exocytic pathway. Mol. Cell. Biol. 20:2475-2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arbuzova, A., K. Martushova, G. Hangyas-Mihalyne, A. J. Morris, S. Ozaki, G. D. Prestwich, and S. McLaughlin. 2000. Fluorescently labeled neomycin as a probe of phosphatidylinositol-4,5-bisphosphate in membranes. Biochim. Biophys. Acta 1464:35-48. [DOI] [PubMed] [Google Scholar]
- 4.Barbieri, M. A., A. D. Kohn, R. A. Roth, and P. D. Stahl. 1998. Protein kinase B/Akt and Rab5 mediate Ras activation of endocytosis. J. Biol. Chem. 273:19367-19370. [DOI] [PubMed] [Google Scholar]
- 5.Bar-Sagi, D. 2001. A Ras by any other name. Mol. Cell. Biol. 21:1441-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bar-Sagi, D., and J. R. Feramisco. 1986. Induction of membrane ruffling and fluid-phase pinocytosis in quiescent fibroblasts by Ras proteins. Science 233:1061-1068. [DOI] [PubMed] [Google Scholar]
- 7.Baumann, C. A., V. Ribon, M. Kanzaki, D. C. Thurmond, S. Mora, S. Shigematsu, P. E. Bickel, J. E. Pessin, and A. R. Saltiel. 2000. CAP defines a second signalling pathway required for insulin-stimulated glucose transport. Nature 407:202-207. [DOI] [PubMed] [Google Scholar]
- 8.Burke, P., K. Schooler, and H. S. Wiley. 2001. Regulation of epidermal growth factor receptor signaling by endocytosis and intracellular trafficking. Mol. Biol. Cell 12:1897-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ceresa, B. P., A. W. Kao, S. R. Santeler, and J. E. Pessin. 1998. Inhibition of clathrin-mediated endocytosis selectively attenuates specific insulin receptor signal transduction pathways. Mol. Cell. Biol. 18:3862-3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ceresa, B. P., and S. L. Schmid. 2000. Regulation of signal transduction by endocytosis. Curr. Opin. Cell Biol. 12:204-210. [DOI] [PubMed] [Google Scholar]
- 11.Chiang, S. H., C. A. Baumann, M. Kanzaki, D. C. Thurmond, R. T. Watson, C. L. Neudauer, I. G. Macara, J. E. Pessin, and A. R. Saltiel. 2001. Insulin-stimulated GLUT4 translocation requires the CAP-dependent activation of TC10. Nature 410:944-948. [DOI] [PubMed] [Google Scholar]
- 12.Choy, E., V. K. Chiu, J. Silletti, M. Feoktisitov, T. Morimoto, D. Michaelson, I. E. Ivanov, and M. R. Philips. 1999. Endomembrane trafficking of Ras: the CAAX motif targets proteins to the ER and Golgi. Cell 98:69-80. [DOI] [PubMed] [Google Scholar]
- 13.Christoforidis, S., M. Miaczynska, K. Ashman, M. Wilm, L. Zhao, S. C. Yip, M. D. Waterfield, J. M. Backer, and M. Zerial. 1999. Phosphatidylinositol-3-OH kinases are Rab5 effectors. Nat. Cell Biol. 1:249-252. [DOI] [PubMed] [Google Scholar]
- 14.Clyde-Smith, J., G. Silins, M. Gartside, S. Grimmond, M. Etheridge, A. Apolloni, N. Hayward, and J. F. Hancock. 2000. Characterization of RasGRP2, a plasma membrane-targeted, dual specificity Ras/Rap exchange factor. J. Biol. Chem. 275:32260-32267. [DOI] [PubMed] [Google Scholar]
- 15.Damke, H., T. Baba, D. E. Warnock, and S. L. Schmid. 1994. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J. Cell Biol. 127:915-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Fiore, P. P., and P. De Camilli. 2001. Endocytosis and signaling: an inseparable partnership. Cell 106:1-4. [DOI] [PubMed] [Google Scholar]
- 17.Ellis, S., and H. Mellor. 2000. Regulation of endocytic traffic by Rho family GTPases. Trends Cell Biol. 10:85-88. [DOI] [PubMed] [Google Scholar]
- 18.Fabian, J. R., A. B. Vojtek, J. A. Cooper, and D. K. Morrison. 1994. A single amino acid change in Raf-1 inhibits Ras binding and alters Raf-1 function. Proc. Natl. Acad. Sci. USA 91:5982-5986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fridman, M., H. Maruta, J. Gonez, F. Walker, H. Treutlein, J. Zeng, and A. Burgess. 2000. Point mutants of c-Raf-1 RBD with elevated binding to v-Ha-Ras. J. Biol. Chem. 275:30363-30371. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton, M., and A. Wolfman. 1998. Ha-Ras and N-Ras regulate MAPK activity by distinct mechanisms in vivo. Oncogene 16:1417-1428. [DOI] [PubMed] [Google Scholar]
- 21.Hancock, J. F., A. I. Magee, J. E. Childs, and C. J. Marshall. 1989. All Ras proteins are polyisoprenylated but only some are palmitoylated. Cell 57:1167-1177. [DOI] [PubMed] [Google Scholar]
- 22.Hao, M., and F. R. Maxfield. 2000. Characterization of rapid membrane internalization and recycling. J. Biol. Chem. 275:15279-15286. [DOI] [PubMed] [Google Scholar]
- 23.Haugh, J. M., A. C. Huang, H. S. Wiley, A. Wells, and D. A. Lauffenburger. 1999. Internalized epidermal growth factor receptors participate in the activation of p21(Ras) in fibroblasts. J. Biol. Chem. 274:34350-34360. [DOI] [PubMed] [Google Scholar]
- 24.Henley, J. R., E. W. Krueger, B. J. Oswald, and M. A. McNiven. 1998. Dynamin-mediated internalization of caveolae. J. Cell Biol. 141:85-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howe, C. L., J. S. Valletta, A. S. Rusnak, and W. C. Mobley. 2001. NGF signaling from clathrin-coated vesicles: evidence that signaling endosomes serve as a platform for the Ras-MAPK pathway. Neuron 32:801-814. [DOI] [PubMed] [Google Scholar]
- 26.Jaumot, M., J. Yan, J. Clyde-Smith, J. Sluimer, and J. F. Hancock. 2002. The linker domain of the Ha-Ras hypervariable region regulates interactions with exchange factors, Raf-1 and phosphoinositide 3-kinase. J. Biol. Chem. 277:272-278. [DOI] [PubMed] [Google Scholar]
- 27.Johnson, L., D. Greenbaum, K. Cichowski, K. Mercer, E. Murphy, E. Schmitt, R. T. Bronson, H. Umanoff, W. Edelman, R. Kucherlapati, and T. Jacks. 1997. K-Ras is an essential gene in the mouse with partial functional overlap with N-Ras. Genes Dev. 11:2468-2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kessels, M. M., A. E. Engqvist-Goldstein, D. G. Drubin, and B. Qualmann. 2001. Mammalian Abp1, a signal-responsive F-actin-binding protein, links the actin cytoskeleton to endocytosis via the GTPase dynamin. J. Cell Biol. 153:351-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koera, K., K. Nakamura, K. Nakao, J. Miyoshi, K. Toyoshima, T. Hatta, H. Otani, A. Aiba, and M. Katsuki. 1997. K-Ras is essential for the development of the mouse embryo. Oncogene 15:1151-1159. [DOI] [PubMed] [Google Scholar]
- 30.Kranenburg, O., I. Verlaan, and W. H. Moolenaar. 1999. Dynamin is required for the activation of mitogen-activated protein (MAP) kinase by MAP kinase kinase. J. Biol. Chem. 274:35301-35304. [DOI] [PubMed] [Google Scholar]
- 31.Lamaze, C., T. H. Chuang, L. J. Terlecky, G. M. Bokoch, and S. L. Schmid. 1996. Regulation of receptor-mediated endocytosis by Rho and Rac. Nature 382:177-179. [DOI] [PubMed] [Google Scholar]
- 32.Leevers, S. J., H. F. Paterson, and C. J. Marshall. 1994. Requirement for Ras in Raf activation is overcome by targeting Raf to the plasma membrane. Nature 369:411-414. [DOI] [PubMed] [Google Scholar]
- 33.Leventis, R., and J. R. Silvius. 1998. Lipid-binding characteristics of the polybasic carboxy-terminal sequence of K-Ras4B. Biochemistry 37:7640-7648. [DOI] [PubMed] [Google Scholar]
- 34.Li, G., C. D'Souza Schorey, M. A. Barbieri, J. A. Cooper, and P. D. Stahl. 1997. Uncoupling of membrane ruffling and pinocytosis during Ras signal transduction. J. Biol. Chem. 272:10337-10340. [PubMed] [Google Scholar]
- 35.Marshall, C. J. 1995. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell 80:179-185. [DOI] [PubMed] [Google Scholar]
- 36.Moodie, S. A., J. Alleman-Sposeto, and T. A. Gustafson. 1999. Identification of the APS protein as a novel insulin receptor substrate. J. Biol. Chem. 274:11186-11193. [DOI] [PubMed] [Google Scholar]
- 37.Murray, D., A. Arbuzova, G. Hangyas-Mihalyne, A. Gambhir, N. Ben-Tal, B. Honig, and S. McLaughlin. 1999. Electrostatic properties of membranes containing acidic lipids and adsorbed basic peptides: theory and experiment. Biophys. J. 77:3176-3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nichols, B. J., A. K. Kenworthy, R. S. Polishchuk, R. Lodge, T. H. Roberts, K. Hirschberg, R. D. Phair, and J. Lippincott-Schwartz. 2001. Rapid cycling of lipid raft markers between the cell surface and Golgi complex. J. Cell Biol. 153:529-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oh, P., D. P. McIntosh, and J. E. Schnitzer. 1998. Dynamin at the neck of caveolae mediates their budding to form transport vesicles by GTP-driven fission from the plasma membrane of endothelium. J. Cell Biol. 141:101-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pelkmans, L., J. Kartenbeck, and A. Helenius. 2001. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat. Cell Biol. 3:473-483. [DOI] [PubMed] [Google Scholar]
- 41.Pol, A., M. Calvo, and C. Enrich. 1998. Isolated endosomes from quiescent rat liver contain the signal transduction machinery. Differential distribution of activated Raf-1 and Mek in the endocytic compartment. FEBS Lett. 441:34-38. [DOI] [PubMed] [Google Scholar]
- 42.Prior, I. A., and J. F. Hancock. 2001. Compartmentalization of Ras proteins. J. Cell Sci. 114:1603-1608. [DOI] [PubMed] [Google Scholar]
- 43.Prior, I. A., A. Harding, J. Yan, J. Sluimer, R. G. Parton, and J. F. Hancock. 2001. GTP-dependent segregation of H-Ras from lipid rafts is required for biological activity. Nat. Cell Biol. 3:368-375. [DOI] [PubMed] [Google Scholar]
- 44.Puri, V., R. Watanabe, R. D. Singh, M. Dominguez, J. C. Brown, C. L. Wheatley, D. L. Marks, and R. E. Pagano. 2001. Clathrin-dependent and -independent internalization of plasma membrane sphingolipids initiates two Golgi targeting pathways. J. Cell Biol. 154:535-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qualmann, B., M. M. Kessels, and R. B. Kelly. 2000. Molecular links between endocytosis and the actin cytoskeleton. J. Cell Biol. 150:F111-F116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qui, M. S., and S. H. Green. 1992. PC12 cell neuronal differentiation is associated with prolonged p21Ras activity and consequent prolonged ERK activity. Neuron 9:705-717. [DOI] [PubMed] [Google Scholar]
- 47.Rizzo, M. A., C. A. Kraft, S. C. Watkins, E. S. Levitan, and G. Romero. 2001. Agonist-dependent traffic of raft-associated Ras and Raf-1 is required for activation of the mitogen-activated protein kinase cascade. J. Biol. Chem. 276:34928-34933. [DOI] [PubMed] [Google Scholar]
- 48.Rizzo, M. A., K. Shome, C. Vasudevan, D. B. Stolz, T. C. Sung, M. A. Frohman, S. C. Watkins, and G. Romero. 1999. Phospholipase D and its product, phosphatidic acid, mediate agonist-dependent Raf-1 translocation to the plasma membrane and the activation of the mitogen-activated protein kinase pathway. J. Biol. Chem. 274:1131-1139. [DOI] [PubMed] [Google Scholar]
- 49.Rizzo, M. A., K. Shome, S. C. Watkins, and G. Romero. 2000. The recruitment of Raf-1 to membranes is mediated by direct interaction with phosphatidic acid and is independent of association with Ras. J. Biol. Chem. 275:23911-23918. [DOI] [PubMed] [Google Scholar]
- 50.Rodriguez-Viciana, P., P. H. Warne, A. Khwaja, B. M. Marte, D. Pappin, P. Das, M. D. Waterfield, A. Ridley, and J. Downward. 1997. Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by Ras. Cell 89:457-467. [DOI] [PubMed] [Google Scholar]
- 51.Roy, M. O., R. Leventis, and J. R. Silvius. 2000. Mutational and biochemical analysis of plasma membrane targeting mediated by the farnesylated, polybasic carboxy terminus of K-Ras4B. Biochemistry 39:8298-8307. [DOI] [PubMed] [Google Scholar]
- 52.Roy, S., A. Lane, J. Yan, R. McPherson, and J. F. Hancock. 1997. Activity of plasma membrane-recruited Raf-1 is regulated by Ras via the Raf zinc finger. J. Biol. Chem. 272:20139-20145. [DOI] [PubMed] [Google Scholar]
- 53.Roy, S., R. Luetterforst, A. Harding, A. Apolloni, M. Etheridge, E. Stang, B. Rolls, J. F. Hancock, and R. G. Parton. 1999. Dominant-negative caveolin inhibits H-Ras function by disrupting cholesterol-rich plasma membrane domains. Nat. Cell Biol. 1:98-105. [DOI] [PubMed] [Google Scholar]
- 54.Roy, S., R. A. McPherson, A. Apolloni, J. Yan, A. Lane, J. Clyde-Smith, and J. F. Hancock. 1998. 14-3-3 facilitates Ras-dependent Raf-1 activation in vitro and in vivo. Mol. Cell. Biol. 18:3947-3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sabharanjak, S., P. Sharma, R. G. Parton, and S. Mayor. 2002. Multiple GPI-anchored proteins are delivered to recycling endosomes via a distinct Cdc42-regulated, clathrin-independent pinocytic pathway. Dev. Cell 4:411-423. [DOI] [PubMed] [Google Scholar]
- 56.Stenmark, H., R. G. Parton, O. Steele-Mortimer, A. Lutcke, J. Gruenberg, and M. Zerial. 1994. Inhibition of Rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO J. 13:1287-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stokoe, D., S. G. Macdonald, K. Cadwallader, M. Symons, and J. F. Hancock. 1994. Activation of Raf as a result of recruitment to the plasma membrane. Science 264:1463-1467. [DOI] [PubMed] [Google Scholar]
- 58.Sun, H., A. J. King, H. B. Diaz, and M. S. Marshall. 2000. Regulation of the protein kinase Raf-1 by oncogenic Ras through phosphatidylinositol 3-kinase, Cdc42/Rac and Pak. Curr. Biol. 10:281-284. [DOI] [PubMed] [Google Scholar]
- 59.Tall, G. G., A. M. Barbieri, P. D. Stahl, and B. F. Horazdovsky. 2001. Ras-activated endocytosis is mediated by the Rab5 guanine nucleotide exchange activity of Rin1. Dev. Cell 1:73-82. [DOI] [PubMed] [Google Scholar]
- 60.Traverse, S., P. Cohen, H. Paterson, C. Marshall, U. Rapp, and R. J. Grand. 1993. Specific association of activated MAP kinase kinase kinase (Raf) with the plasma membranes of Ras-transformed retinal cells. Oncogene 8:3175-3181. [PubMed] [Google Scholar]
- 61.Traverse, S., N. Gomez, H. Paterson, C. Marshall, and P. Cohen. 1992. Sustained activation of the mitogen-activated protein (MAP) kinase cascade may be required for differentiation of PC12 cells. Comparison of the effects of nerve growth factor and epidermal growth factor. Biochem. J. 288:351-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Umanoff, H., W. Edelmann, A. Pellicer, and R. Kucherlapati. 1995. The murine N-Ras gene is not essential for growth and development. Proc. Natl. Acad. Sci. USA 92:1709-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vieira, A. V., C. Lamaze, and S. L. Schmid. 1996. Control of EGF receptor signaling by clathrin-mediated endocytosis. Science 274:2086-2089. [DOI] [PubMed] [Google Scholar]
- 64.Voice, J., R. Klemke, A. Le, and J. Jackson. 1999. Four human Ras homologs differ in their ability to activate Raf-1, induce transformation and stimulate cell motility. J. Biol. Chem. 274:17164-17170. [DOI] [PubMed] [Google Scholar]
- 65.Walsh, A. B., and D. Bar-Sagi. 2001. Differential activation of the Rac pathway by Ha-Ras and K-Ras. J. Biol. Chem. 276:15609-15615. [DOI] [PubMed] [Google Scholar]
- 66.Watson, R. T., S. Shigematsu, S. H. Chiang, S. Mora, M. Kanzaki, I. G. Macara, A. R. Saltiel, and J. E. Pessin. 2001. Lipid raft microdomain compartmentalization of TC10 is required for insulin signaling and GLUT4 translocation. J. Cell Biol. 154:829-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wells, A., J. B. Welsh, C. S. Lazar, H. S. Wiley, G. N. Gill, and M. G. Rosenfeld. 1990. Ligand-induced transformation by a noninternalizing epidermal growth factor receptor. Science 247:962-964. [DOI] [PubMed] [Google Scholar]
- 68.Wennstrom, S., and J. Downward. 1999. Role of phosphoinositide 3-kinase in activation of Ras and mitogen-activated protein kinase by epidermal growth factor. Mol. Cell. Biol. 19:4279-4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wolfman, J. C., and A. Wolfman. 2000. Endogenous c-N-Ras provides a steady-state anti-apoptotic signal. J. Biol. Chem. 275:19315-19323. [DOI] [PubMed] [Google Scholar]
- 70.Yan, J., S. Roy, A. Apolloni, A. Lane, and J. F. Hancock. 1998. Ras isoforms vary in their ability to activate Raf-1 and phosphoinositide 3-kinase. J. Biol. Chem. 273:24052-24056. [DOI] [PubMed] [Google Scholar]
- 71.York, R. D., D. C. Molliver, S. S. Grewal, P. E. Stenberg, E. W. McCleskey, and P. J. Stork. 2000. Role of phosphoinositide 3-kinase and endocytosis in nerve growth factor-induced extracellular signal-regulated kinase activation via Ras and Rap1. Mol. Cell. Biol. 20:8069-8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang, Y., D. B. Moheban, B. R. Conway, A. Bhattacharyya, and R. A. Segal. 2000. Cell surface Trk receptors mediate NGF-induced survival while internalized receptors regulate NGF-induced differentiation. J. Neurosci. 20:5671-5678. [DOI] [PMC free article] [PubMed] [Google Scholar]