Abstract
Objective
Flavor is the primary dimension by which young children determine food acceptance. However, children are not merely miniature adults because sensory systems mature postnatally and their responses to certain tastes differ markedly from adults. Among these differences are heightened preferences for sweet-tasting and greater rejection of bitter-tasting foods. The present study tests the hypothesis that genetic variations in the newly discovered TAS2R38 taste gene as well as cultural differences are associated with differences in sensitivity to the bitter taste of propylthiouracil (PROP) and preferences for sucrose and sweet-tasting foods and beverages in children and adults.
Design
Genomic DNA was extracted from cheek cells of a racially and ethnically diverse sample of 143 children and their mothers. Alleles of the gene TAS2R38 were genotyped. Participants were grouped by the first variant site, denoted A49P, because the allele predicts a change from the amino acid alanine (A) to proline (P) at position 49. Henceforth, individuals who were homozygous for the bitter-insensitive allele are referred to as AA, those who were heterozygous for the bitter-insensitive allele are referred to as AP, and those who were homozygous for the bitter-sensitive allele are referred to as PP. Using identical procedures for children and mothers, PROP sensitivity and sucrose preferences were assessed by using forced-choice procedures that were embedded in the context of games that minimized the impact of language development and were sensitive to the cognitive limitations of pediatric populations. Participants were also asked about their preferences in cereals and beverages, and mothers completed a standardized questionnaire that measured various dimensions of their children’s temperament.
Results
Genetic variation of the A49P allele influenced bitter perception in children and adults. However, the phenotype-genotype relationship was modified by age such that 64% of heterozygous children, but only 43% of the heterozygous mothers, were sensitive to the lowest concentration (56 micromoles/liter) of PROP. Genotypes at the TAS2R38 locus were significantly related to preferences for sucrose and for sweet-tasting beverages and foods such as cereals in children. AP and PP children preferred significantly higher concentrations of sucrose solutions than did AA children. They were also significantly less likely to include milk or water as 1 of their 2 favorite beverages (18.6% vs 40%) and were more likely to include carbonated beverages as 1 of their most preferred beverages (46.4% vs 28.9%). PP children liked cereals and beverages with a significantly higher sugar content. There were also significant main effects of race/ ethnicity on preferences and food habits. As a group, black children liked cereals with a significantly higher sugar content than did white children, and they were also significantly more likely to report that they added sugar to their cereals.
Unlike children, there was no correspondence between TAS2R38 genotypes and sweet preference in adults. Here, the effects of race/ethnicity were the strongest determinants, thus suggesting that cultural forces and experience may override this genotype effect on sweet preferences. Differences in taste experiences also affected mother–child interaction, especially when the 2 resided in different sensory worlds. That is, children who had 1 or 2 bitter-sensitive alleles, but whose mothers had none, were perceived by their mothers as being more emotional than children who had no bitter-sensitive alleles.
Conclusion
Variations in a taste receptor gene accounted for a major portion of individual differences in PROP bitterness perception in both children and adults, as well as a portion of individual differences in preferences for sweet flavors in children but not in adults. These findings underscore the advantages of studying genotype effects on behavioral outcomes in children, especially as they relate to taste preferences because cultural forces may sometimes override the A49P genotypic effects in adults. New knowledge about the molecular basis of food likes and dislikes in children, a generation that will struggle with obesity and diabetes, may suggest strategies to overcome diet-induced diseases.
Keywords: children, genetic predisposition, racial differences, taste, temperament
ABBREVIATIONS: PTC, phenylthiocarbamide; PROP, propylthiouracil
Flavor is the primary dimension by which young children determine food acceptance. However, children are not merely miniature adults because sensory systems mature postnatally and their responses to certain tastes differ markedly from adults.1 Among these differences are heightened preferences for sweet-tasting and greater rejection of bitter-tasting foods.2–4 From an evolutionary perspective, these responses serve important biological functions. In nature, sweetness is associated with readily available calories from carbohydrates, whereas bitterness is often associated with toxins.5
One of the most widely studied individual differences is the genetically determined sensitivity to certain bitter tastes. From birth to old age, the ability to taste compounds that contain an N-C = S group, such as phenylthiocarbamide (PTC) and its chemical relative propylthiouracil (PROP), is evident in human populations.6–10 Although these chemicals taste bitter to some, others either cannot taste them or require high concentrations to recognize its presence. The degree of taste sensitivity has been shown in some studies11–14 to be associated with disliking/ liking of bitter and sweet tastes, which, in turn, has important long-term health implications.15,16
Because much of the phenotypic variation in PTC taste sensitivity can be explained by the DNA sequence diversity in the TAS2R38 gene on chromosome 7q,17,18 the present study assessed how alleles of this gene, which is a member of a family that functions as bitter taste receptors,19 influence taste-and food-related behaviors in children and adults. The study goals were 4-fold. First, we determined whether the genotype that confers sensitivity to PTC would generalize to the chemically similar compound PROP. Second, we determined whether there were age-related changes in bitter sensitivity and, if so, the relationship between genotype and phenotype. Third, we explored how variation in this gene influenced preferences for other stimuli, such as sucrose, and liking of sweet-tasting foods and beverages. We focused on preference rather than other measures such as detection threshold because PROP sensitivity has found to be associated with sweet preferences in children.14 Fourth, we determined how discordance in genotype affected mother–child interaction. Because mothers often offer their children the types of foods that they themselves like,20 we hypothesized that a mother would perceive her child differently if she was insensitive to some bitter tastes but her child was not.
METHODS
Genotype and behavioral analyses were performed on 143 children and their mothers who were either of African-American, non-Hispanic or European, non-Hispanic descent, hereafter referred to as black and white, respectively. The sample included 21 sibling pairs and 4 sibling triads. All children were the biological offspring of their mothers, and all siblings within a family shared the same mother and father. These relationships were assessed through family history and verified by genotyping. Race/ethnicity was assigned by maternal report according to standard US Census categories; we use the term race/ethnicity in describing our groups because it best represents both the genetic and the cultural components of the study sample.21 Children and their parents were racially concordant.
The mothers were 35.3 ± 0.6 years of age, and children ranged in age from 5 to 10 years (mean: 8.0 ± 0.2 years). Although there were no significant effects of race/ethnicity on the parity or age of the mother, black women had lower household incomes (P < .001) and fewer years of education when compared with white women (P < .001). Testing procedures were approved by the Office of Regulatory Affairs at the University of Pennsylvania. Informed consent was obtained from each mother, and assent was obtained from each child who was 7 years of age or older.
Procedures
After a 1-hour fast, each participant was tested individually in a closed room designed for sensory testing. Because previous research demonstrated that children tend to answer questions in the affirmative, PROP sensitivity and sucrose preferences were assessed by using forced-choice procedures that were embedded in the context of games that were fun for children, minimized the impact of language development, and were sensitive to the cognitive limitations of pediatric populations. Procedures were identical for children and mothers. PROP sensitivity was determined at the end of the test session, after sucrose preferences were obtained.
Bitter Taste Sensitivity
Participants were presented with a cup that contained 5 mL of water and told to rinse the contents in their mouth and then expectorate. If the solution tasted like water, then they were told to give it to a stuffed toy of Big Bird (a likable, well-known television character puppet); but if it tasted “yucky” or bitter, then they should give it to Oscar the Grouch, “so that he can throw it in his trash can.” This procedure was repeated, and participants tasted, in ascending order, 3 solutions of PROP (56, 180, and 560 μmol/L), rinsing with water before and after each tasting. The concentrations chosen followed from the research of Anliker et al.22 During the tasting of each solution, the researcher recorded whether the participant displayed any facial expression during sampling and, if so, whether it was a facial grimace or negative facial expression.
Participants were classified into 4 groups on the basis of which was the first sample, if any, given to Oscar the Grouch. Those who gave all samples to Big Bird were categorized as “none tasted bitter.” Independent groupings were formed on the basis of the first solution, if any, that participants displayed negative facial expressions during sampling. Six children and 2 mothers were excluded because responses were inconsistent. PROP testing was conducted a second time on a random sample of 7 children, all of whom were sensitive to PROP; reliability was 85.7%.
Sucrose Preferences
A forced-choice, paired-comparison, tracking technique was used to determine sucrose preferences.23 Participants were presented with pairs of solutions that differed in sucrose concentration (3, 6, 12, 24, and 36 g/100 mL). They tasted, without swallowing, each solution and then pointed to which of the pair they liked better. The procedure continued until the participant chose either a given concentration of sucrose when it was paired with a higher and lower concentration or the highest or lowest solution 2 consecutive times. The entire task was repeated with stimulus pairs presented in reverse order. Nine children and 5 mothers were excluded because their responses were inconsistent.
Food Preferences and Mothers’ Perception of Child Temperament
Because maternal reports of children’s preferences are often inaccurate,24 children were asked directly about their favorite cereals and beverages by inquiring, “What are your favorite cereals (or beverages) in the whole world?” and, “Which cereals (or beverages) do you ask your mom to buy the most?” There were no pictures of cereals to prompt memory; rather, we relied on each participant’s straight recall. Also included were questions regarding whether the participant liked to add sugar to his or her cereal. The sugar (range: 4–56 g/100 g) and sodium (range: 8–966 mg/ 100 g) contents of their favorite cereals (N = 55 different brands) and the sugar (range: 0–18.3 g/100 mL) and sodium (range: 0–258 mg/100 mL) contents of their favorite beverages (N = 57 different types) then were determined from product labels. All but 20 mothers answered similar questions about their cereal preferences. We did not ask mothers to list their favorite beverages but rather asked them to identify their favorite soft drinks and whether they drank coffee and, if so, how many spoonfuls of sugars they added to each 8-ounce cup. Seven of the mothers reported using artificial sweeteners in their coffee. Because previous reports equated 1 packet of artificial sweeteners to 2 teaspoons of sugar,25 we corrected the data accordingly so that comparisons could be made on the level of sweetness preferred. They also completed a 25-item scale that has been shown to have satisfactory internal and test-retest reliabilities.26 This scale measures the child temperament dimensions of emotionality, shyness, activity, sociability, and negative reactivity to food. Scores for each of the dimensions could range from 1 to 5, with higher scores indicating “more” of that particular temperament characteristic.
Genotype Analyses
Cells from the cheek were obtained, and genomic DNA was extracted following the directions of the manufacturer (Epicenter, Madison, WI). Alleles of the gene TAS2R38 (accession no. AF494231) were genotyped for a variant site using allele-specific probes and primers purchased from Applied Biosystems (ABI no. hCV8876467). Although there are 3 variant sites in the gene associated with bitter sensitivity, they are in strong linkage disequilibrium.17 Therefore, participants were grouped by the first variant site, denoted A49P, because the allele predicts a change from the amino acid alanine (A) to proline (P) at position 49. Henceforth, individuals who are homozygous for the bitter-insensitive allele are referred to as AA, those who are heterozygous for the bitter-insensitive allele are referred to as AP, and those who are homozygous for the bitter-sensitive allele are referred to as PP.
Statistical Analyses
The associations between the ability to taste PROP and TAS2R38 genotypes were analyzed with χ2 analyses for k independent samples.27 To determine the effects on children’s sweet preference and temperament measures, we conducted separate 2-way analyses of variance with A49P genotype and race/ethnicity as the between-subjects factors; children’s ages were covaried in each analysis. When significant, post hoc analyses (eg, Fisher least significant difference tests) were conducted. Similar analyses were conducted on the mothers’ data, but because the sample size of PP white mothers was <5, the A49P alleles grouping was reduced to 2 groups: AA versus PP/AP.
The trait heritability of PROP threshold and 2 indices of sugar preference (sucrose and cereal preference) were estimated using a variance component method provided by the Sequential Oligo-genic Linkage Analysis Routines28 using the computing resources of the Medical Research Council Rosalind Franklin Centre for Genomics Research (Cambridge, United Kingdom). The type of family relationship, eg, mother–child, and their degree of genetic similarity was contrasted with the trait similarity between family members to provide estimates of heritability, and the analysis was conducted with and without age, gender, and race/ethnicity as covariates. Significance of the estimated heritability was determined by likelihood ratio tests, in which the obtained likelihood of the model with the additive genetic variance component and covariates was compared with the obtained likelihood of the model with the additive genetic variance component constrained to be 0.
RESULTS
Heritability
PROP thresholds displayed marked heritability that was uninfluenced by age, gender, or race/ethnicity (Table 1). Approximately 55% of the trait variance was accounted for by genetic relatedness. Heritability for sweet preference measures had a different pattern from that of PROP and was overall less heritable (range: 4%–20%) and significantly influenced by age and race/ethnicity. Children preferred more concentrated sucrose solutions and liked cereals with higher sugar, but not salt, contents than their mothers. Race/ethnicity had a significant influence on sugar content of favorite cereals but did not affect sucrose preference as measured in the laboratory.
TABLE 1.
Heritability of PROP Sensitivity, Sucrose Preference, and Composition of Preferred Cereal
| Trait | Covariates | N | H2 | SE | P Value |
|---|---|---|---|---|---|
| PROP sensitivity | None | 250 | 0.55 | 0.15 | <.001* |
| Age, gender, race/ethnicity | 250 | 0.56 | 0.15 | <.001* | |
| Sucrose preference | None | 247 | 0.04 | 0.15 | .39 |
| Age,* gender, race/ethnicity | 247 | 0.14 | 0.16 | .19 | |
| Cereal (sugars) | None | 234 | 0.16 | 0.18 | .19 |
| Age,* gender, race/ethnicity* | 234 | 0.37 | 0.19 | .03† | |
| Cereal sodium | None | 233 | 0.16 | 0.18 | .19 |
| Age, gender, race/ethnicity | 233 | 0.15 | 0.19 | .21 | |
| Cereal kcal | None | 233 | 0.00 | NA | .50 |
| Age, gender, race/ethnicity | 233 | 0.00 | NA | .50 |
H2 indicates proportion of genetic variance; NA, not applicable.
P < .001.
P < .05.
Allelic Distribution
There were no age-, race/ethnicity-, or gender-related differences in the distribution of the TAS2R38 genotypes (Table 2).
TABLE 2.
Distribution of A49P Genotypes of the TAS2R38 Gene in Girls and Boys and Their Mothers by Race/Ethnicity
| AA | AP | PP | Total | |
|---|---|---|---|---|
| Children | ||||
| Black girls | 15 | 21 | 10 | 46 |
| White girls | 10 | 14 | 6 | 30 |
| Black boys | 11 | 22 | 11 | 44 |
| White boys | 9 | 11 | 3 | 23 |
| Total | 45 | 68 | 30 | 143 |
| % of total | 31.5 | 47.6 | 20.9 | |
| Mothers | ||||
| Black | 23 | 40 | 11 | 74 |
| White | 11 | 25 | 4 | 40 |
| Total | 34 | 65 | 15 | 114 |
| % of total | 29.8 | 57.0 | 13.2 | |
Bitter Taste Sensitivity and Facial Expressions
Genotypes at TAS2R38 predicted PROP sensitivity, as determined by either the forced-choice procedure (Fig 1) or display of facial expressions. Although there was no difference in the 2 methods in identifying a PROP-sensitive adult, the grouping that was based on the forced-choice procedures was significantly more likely to identify bitter taste sensitivity among AP and PP children with the lowest concentration of PROP (56 μmol/L) offered than that based on facial expression. Whereas 70% of the AP and PP children indicated that this PROP solution tasted bitter, only 49% displayed a negative facial expression during the tasting (P < .001). The bitter-sensitive form of the receptor gene displayed a heterozygote effect such that 2 copies conferred greater PROP sensitivity than did a single copy in both children (P = .04) and adults (P = .007). However, children who were heterozygous at TAS2R38 A49P were significantly more likely to perceive the lowest concentration of PROP offered when compared with adults with the same genotype (64% vs 43%; P = .02).
Fig 1.
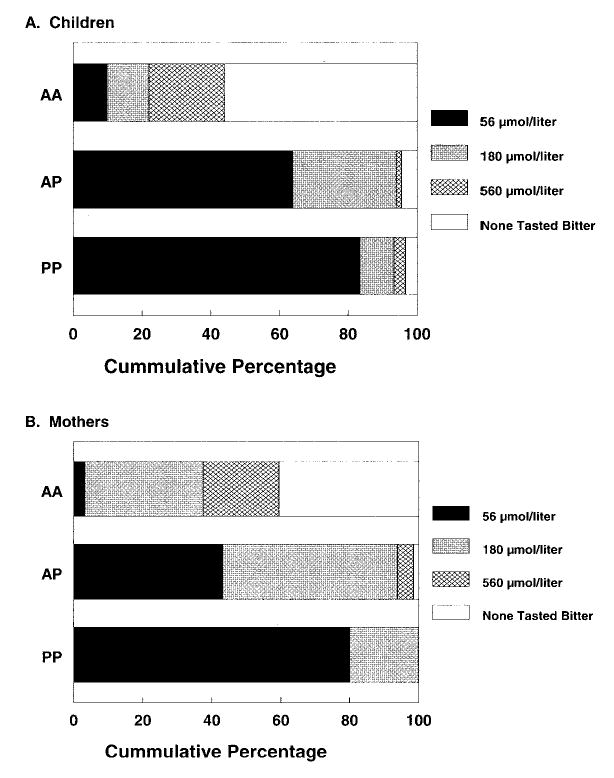
Effect of A49P genotype on sensitivity to the bitter taste of PROP. The cumulative percentage of children (A) and mothers (B) in each of the 3 allele groups (AA, AP, PP) who first detected a bitter or “yucky” taste when sampling 560, 180, and 56 μmol/L of PROP or who never detected a bitter taste when sampling each of these PROP solutions (none tasted bitter).
Sucrose Preferences
There was a significant effect of genotype on sucrose preference in children (P = .01). As shown in Table 3, AP and PP children preferred significantly higher concentrations of sucrose solutions than AA children. Although there was a significant genotype and race/ethnicity interaction on mothers’ preference for sucrose (P = .03), post hoc analysis revealed only a race/ethnicity difference among AP/PP mothers. That is, AP/PP black mothers preferred higher levels of sucrose than AP/PP white mothers.
TABLE 3.
Effects of A49P Genotype and Race/Ethnicity on Preferred Level of Sucrose, the Sugar and Sodium Content of Favorite Cereals, and the Sugar and Sodium Content of Favorite Beverages in Children
| Children | A49P | Sucrose Preference (g/100 mL) | Sugar Content of Cereal (g/100 g) | Sodium Content of Cereal (mg/100 g) | Sugar Content of Beverage (g/100 mL) | Sodium Content of Beverage (mg/100 mL) |
|---|---|---|---|---|---|---|
| Black | AA | 14.8 ± 1.9 | 36.7 ± 1.5 | 57.1 ± 2.8 | 9.8 ± 0.7 | 14.4 ± 1.9 |
| AP | 21.2 ± 1.8 | 35.1 ± 1.8 | 60.5 ± 2.3 | 10.2 ± 0.5 | 14.9 ± 1.2 | |
| PP | 24.5 ± 2.0 | 41.6 ± 1.0 | 55.8 ± 2.6 | 11.0 ± 0.4 | 22.9 ± 3.6 | |
| Total | 20.2 ± 1.2 | 37.9 ± 1.1a | 58.4 ± 1.5 | 10.3 ± 0.3 | 16.6 ± 1.2 | |
| White | AA | 16.4 ± 2.7 | 29.5 ± 3.1 | 65.8 ± 3.7 | 8.6 ± 0.8 | 20.4 ± 2.6 |
| AP | 22.8 ± 2.3 | 32.3 ± 2.4 | 65.3 ± 3.9 | 10.6 ± 0.7 | 21.1 ± 5.3 | |
| PP | 16.4 ± 4.1 | 37.8 ± 4.5 | 58.5 ± 5.3 | 11.0 ± 1.3 | 24.7 ± 8.6 | |
| Total | 19.3 ± 1.6 | 32.2 ± 1.8b | 64.3 ± 2.4 | 10.0 ± 0.5 | 21.4 ± 3.0 | |
| All children | AA | 15.5 ± 1.6c | 35.3 ± 1.7c | 60.8 ± 2.3 | 9.3 ± 0.5c | 16.9 ± 1.6 |
| AP | 21.8 ± 1.4d | 34.1 ± 1.5c | 62.3 ± 2.1 | 10.3 ± 0.4 | 17.2 ± 2.1 | |
| PP | 22.0 ± 1.9d | 40.4 ± 1.5d | 56.6 ± 2.4 | 11.0 ± 0.5d | 23.4 ± 3.6 |
Values are means ± SEM. To convert values for sucrose to moles per liter, multiply by 0.0292. Within each measure, a is significantly different from b; c is significantly different from d.
Food and Beverage Preferences
There were significant main effects of genotype (P = .05) and race/ethnicity (P = .01) on the sugar content of the children’s favorite cereals (Table 3). Although there were no differences in the salt content of the cereals, PP children liked cereals with significantly higher sugar contents when compared with the remaining children. As a group, black children liked cereals with significantly higher sugar contents than white children.
Genotype (P = .048) but not race/ethnicity was related to the sugar content of the children’s favorite beverages. PP children liked beverages with significantly higher sugar contents when compared with AA children. The level of sucrose preferred in the laboratory was significantly correlated with the sugar content of children’s favorite beverages (P = .009). AP and PP children were significantly less likely to include milk or water as 1 of their top 2 beverages (18.6% vs 40%; P = .006) and were more likely to include carbonated beverages as 1 of their most preferred beverages (46.4% vs 28.9%; P = .05). There was no significant difference between the groups in the ranking of noncarbonated beverages and juices. Black children (76.4%) were significantly more likely to report that they liked to add sugar to their cereals when compared with white children (43.4%; P = .00007). There was also a tendency for AP and PP (69.1%) children to report that they liked to add more sugar to their cereals (53.3%; P = .07) and to their foods in general (80.6% vs 66.7%; P = .07) when compared with AA children. No such tendencies were evident for adding salt, however.
Although no genotype effects were observed for the sugar content of the mothers’ favorite cereals, there were significant effects of race/ethnicity (P = .001; Table 4). Like their children, black mothers liked cereals with higher sugar contents when compared with white mothers. They were also more likely to consume regular rather than diet sodas when compared with white mothers (black vs white: 95.2% vs 68.6%; P = .0003). Although there were no race/ethnicity differences in the proportion of mothers who drank coffee, black mothers added more spoonfuls of sugar (3.5 ± 0.4) to their coffee than did white mothers (1.7 ± 0.5; P = .005). The level of sucrose that the mother preferred in the laboratory was correlated with the number of spoonfuls of sugar that mothers reported adding to their coffee (r[64df] = 0.28; P = .002) but not the sugar content of their favorite cereals.
TABLE 4.
Effects of A49P Genotype and Race/Ethnicity on Preferred Level of Sucrose and the Sugar and Sodium Content of Favorite Cereals in Mothers
| Mothers | A49P | Sucrose Preference (g/100 mL) | Sugar Content of Cereal (g/100 g) | Sodium Content of Cereal (mg/100 g) |
|---|---|---|---|---|
| Black | AA | 12.3 ± 1.9 | 30.0 ± 2.8 | 69.9 ± 3.3 |
| AP/PP | 15.5 ± 1.5a | 31.8 ± 2.1 | 59.1 ± 3.5 | |
| Total | 14.5 ± 1.2 | 31.2 ± 1.7a | 62.7 ± 3.5 | |
| White | AA | 16.2 ± 3.3 | 21.4 ± 4.1 | 51.0 ± 1.0 |
| AP/PP | 9.7 ± 1.8b | 18.5 ± 3.0 | 71.1 ± 5.0 | |
| Total | 11.5 ± 1.6 | 19.4 ± 3.4b | 65.1 ± 4.9 | |
| All mothers | AA | 13.5 ± 1.7 | 27.4 ± 2.4 | 64.3 ± 4.0 |
| AP/PP | 13.4 ± 1.2 | 27.5 ± 1.9 | 63.1 ± 2.9 |
Values are means ± SEM. To convert values for sucrose to moles per liter, multiply by 0.0292. Within each measure, a is significantly different from b.
Child Temperament and Food Neophobia
Children who had 1 or 2 bitter-sensitive alleles but whose mothers had none were perceived by their mothers as being more emotional when compared with remaining children (Fig 2). There was also a significant effect of genotype (P = .05) and race/ ethnicity (P = .009) on mothers’ perceptions of children’s activity. PP children were perceived as being more active (3.11 ± 0.10) when compared with AP children (2.80 ± 0.07: P = .006), whereas black children were perceived by their mothers as being less active (black vs white: 2.82 ± 0.06 vs 3.09 ± 0.08; P = .009) as well as reacting more negatively to food (black vs white: 3.28 ± 0.09 vs 2.97 ± 0.12; P = .02) when compared with white children.
Fig 2.
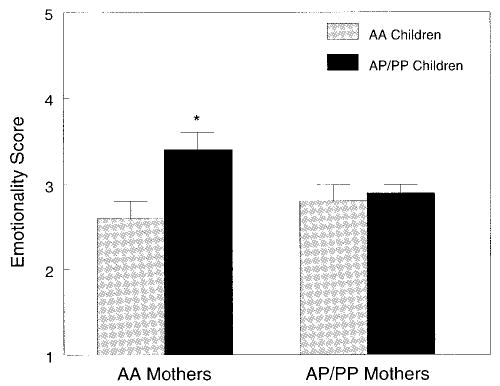
Effect of A49P genotype on maternal perception of children’s emotionality (scores could range from 1 to 5). ▪, Children who are heterozygous or homozygous for the bitter-sensitive allele; □, children who are homozygous for the insensitive nontaster allele. AP/PP children of AA mothers were significantly more likely to be perceived as emotional when compared with the remaining groups (*P = .03).
DISCUSSION
Genotypes at the TAS2R38 locus predicted sensitivity to the bitter compound PROP in children and adults. The gene effect was incompletely dominant, with heterozygotes having an intermediate phenotype, confirming earlier suggestions about the mode of inheritance.29,30 Although the data presented herein suggest that TAS2R38 genotype is predictive of a large proportion of the individual differences in the concentration at which people can first detect PROP, there was not a one-to-one correspondence, perhaps because of the sensitivity or types of the methods used (eg, TAS2R38 genotype does not predict the perceived intensity of PROP at suprathreshold concentrations31) or the existence of other genetic and environmental modifiers.18,30–34
Two important age-related differences were observed. First, the phenotype–genotype relationship in children was best captured by forced-choice methods; thus, classifications that were based on facial expressions alone underestimated the proportion of children who were bitter sensitive. Second, the phenotype–genotype relationship was modified by age, with heterozygous children being more sensitive to the bitterness of lower concentrations of PROP when compared with adults with the same genotype, a finding that is consistent with previous reports of a smaller number of “nontaster” children when compared with adults.9,35 Longitudinal studies are warranted, but we suggest that because human sensory systems develop postnatally, the ability to taste some bitter substances in bitter-sensitive children might alter the developmental course of the taste system. Moreover, some individuals who are born sensitive to PROP may become less sensitive with age because of experience, aging, and/or disease.30,36
The genotype at this locus partially explained individual differences in sweet preferences for children but not for adults. PP and AP children preferred significantly higher concentrations of sugars in liquids and solid foods when compared with AA children, a finding that is in disagreement with a previous study on children.14 The discrepancy may be attributable to the different methods used in assessing sucrose liking (eg, the pattern of hedonic ratings to 3 sucrose concentrations14 vs forced-choice procedures to assess preference for sucrose and liking of sweet-tasting foods and beverages). Sweet preferences were also strongly influenced by age and race/ ethnicity, confirming previous reports that children prefer higher levels of sweets than adults and that individuals of African descent prefer higher levels of sweet than those of European descent.37–39
Why should genotypes of a bitter-taste gene predict sweet preference in children? Three hypotheses, not mutually exclusive, might account for this relationship. First, alleles of the TAS2R38 receptor could either bind sweet compounds directly or indirectly influence the intracellular processes of taste receptor cells, resulting in a change in the perception of sweetness. Second, the TAS2R38 genes and its alleles could be in linkage disequilibrium with nearby genes that influence sweet taste perception. Third, TAS2R38 allele frequency may be an especially sensitive genetic marker of racial ancestry, a variable with reliable effects on sweet preference, as demonstrated herein.
Unlike children, there was no correspondence between TAS2R38 genotypes and sweet preference in adults. Here, the effects of race/ethnicity were the strongest determinants, thus suggesting that cultural forces and experience may override this genotype effect on sweet preferences. Demographic factors, such as income, can also play a role on food availability because sugars, as well as fats, constitute one of the most palatable and low-priced nutrients.40
These findings underscore advantages of studying genotype effects on behavioral outcome in children, especially as it relates to taste preferences. First, unlike adults, young children’s preferences are less constrained by experiential and cognitive factors,41 and their taste preferences determine intake. Second, although preferences are influenced by early experiences,42,43 the impact of experience is less than that observed in the adult. Whereas children respond to the sensory qualities of foods, mothers have food-related beliefs and experiences that influence their behaviors. These beliefs and experiences also affected mother–child interaction, especially when the 2 resided in different sensory worlds. That is, children who were sensitive to bitter tastes but whose mothers were not were more likely to be perceived as being emotional. Why such differences were not observed for the temperament dimension of negative reaction to foods remains unknown, however. Direct assessments of mothers’ perceptions of their children during feeding situations should be the focus of future research.
CONCLUSION
The current study described a specific relationship between inborn individual differences in bitter-taste perception and sweet preferences and the genetic variation in 1 bitter-taste receptor gene. Thus, parents and their children live in different sensory worlds not only because of age but, in some cases, because of genetics. These findings help to explain some obstacles that parents face when negotiating with children about food choices and pave the way for other research. New knowledge about the molecular basis of food likes and dislikes in children, a generation that will struggle with obesity and diabetes, may suggest strategies to overcome diet-induced diseases. The influence of genetics and culture on the ontogeny of complex behaviors related to food preferences remains an important research area that needs to be explored fully.
Acknowledgments
This work was supported by grants HD37119 and DC004698 from the National Institutes of Health.
We acknowledge Janice Kennedy and Kirsten J. Mascioli for expert technical assistance; Dr R. Arlen Price for statistical analysis; and Drs Gary Beauchamp, Joseph Brand, and Paul Breslin for comments on an earlier version of the manuscript.
Footnotes
No conflict of interest declared.
References
- 1.Mennella JA. Taste and smell. In: Swaiman KF, Ashwall S, eds. Pediatric Neurology: Principles and Practice 3rd ed. Philadelphia, PA: CV Mosby Company; 1999:104–113
- 2.Desor JA, Maller O, Andrews K. Ingestive responses of human newborns to salty, sour, and bitter stimuli. J Comp Physiol Psychol. 1975;89:966–970. doi: 10.1037/h0077171. [DOI] [PubMed] [Google Scholar]
- 3.Mennella JA, Pepino MY, Beauchamp GK. Modification of bitter taste by a sodium salt in children. Dev Psychobiol. 2003;43:120–127. doi: 10.1002/dev.10127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desor JA, Maller O, Turner RE. Preference for sweet in humans: infants, children and adults. In: Weiffenbach JM, ed. Taste and Development: The Genesis of Sweet Preference. Washington, DC: US Government Printing Office; 1977
- 5.Glendinning JI. Is the bitter rejection response always adaptive? Physiol Behav. 1994;56:1217–1227. doi: 10.1016/0031-9384(94)90369-7. [DOI] [PubMed] [Google Scholar]
- 6.Kalmus H. PTC testing of infants. Ann Hum Genet. 1976;40:139–140. doi: 10.1111/j.1469-1809.1976.tb00172.x. [DOI] [PubMed] [Google Scholar]
- 7.Six in ten ‘tasteblind’ to bitter chemical. Sci News Lett. 1931;9:249. [Google Scholar]
- 8.Bartoshuk LM. Bitter taste of saccharin related to the genetic ability to taste the bitter substance 6-n-propylthiouracil. Science. 1979;205:934–935. doi: 10.1126/science.472717. [DOI] [PubMed] [Google Scholar]
- 9.Blakeslee AF. Genetics of sensory thresholds: taste for phenyl thio carbamide. Proc Natl Acad Sci U S A. 1932;18:120–130. doi: 10.1073/pnas.18.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Snyder LH. Inherited taste deficiency. Science. 1931;74:151–152. doi: 10.1126/science.74.1910.151. [DOI] [PubMed] [Google Scholar]
- 11.Duffy VB, Bartoshuk LM. Food acceptance and genetic variation in taste. J Am Diet Assoc. 2000;100:647–655. doi: 10.1016/S0002-8223(00)00191-7. [DOI] [PubMed] [Google Scholar]
- 12.Keller KL, Steinmann L, Nurse RJ, Tepper BJ. Genetic taste sensitivity to 6-n-propylthiouracil influences food preference and reported intake in preschool children. Appetite. 2002;38:3–12. doi: 10.1006/appe.2001.0441. [DOI] [PubMed] [Google Scholar]
- 13.Gent JF, Bartoshuk LM. Sweetness of sucrose, neohesperidin, dihydro-chalcone, and saccharin is related to genetic ability to taste the bitter substance 6-n-propylthiouracil. Chem Senses. 1983;7:265–272. [Google Scholar]
- 14.Looy H, Weingarten HP. Facial expressions and genetic sensitivity to 6-n-propylthiouracil predict hedonic response to sweet. Physiol Behav. 1992;52:75–82. doi: 10.1016/0031-9384(92)90435-5. [DOI] [PubMed] [Google Scholar]
- 15.Duffy VB, Peterson JM, Dinehart ME, Bartoshuk LM. Genetic and environmental variation in taste: associations with sweet intensity, preference and intake. Top Clin Nutr. 2003;18:209–220. [Google Scholar]
- 16.Tepper BJ. 6-n-Propylthiouracil: a genetic marker for taste, with implications for food preference and dietary habits. Am J Hum Genet. 1998;63:1271–1276. doi: 10.1086/302124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim U, Jorgenson E, Coon H, Leppert M, Risch N, Drayna D. Positional cloning of the human quantitative trait locus underlying taste sensitivity to phenylthiocarbamide. Science. 2003;299:1221–1225. doi: 10.1126/science.1080190. [DOI] [PubMed] [Google Scholar]
- 18.Drayna D, Coon H, Kim UK, et al. Genetic analysis of a complex trait in the Utah Genetic Reference Project: a major locus for PTC taste ability on chromosome 7q and a secondary locus on chromosome 16p. Hum Genet. 2003;112:567–572. doi: 10.1007/s00439-003-0911-y. [DOI] [PubMed] [Google Scholar]
- 19.Wooding S, Kim UK, Bamshad MJ, Larsen J, Jorde LB, Drayna D. Natural selection and molecular evolution in PTC, a bitter taste receptor gene. Am J Hum Genet. 2004;74:637–646. doi: 10.1086/383092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skinner JD, Carruth BR, Wendy B, Ziegler PJ. Children’s food preference: a longitudinal analysis. J Am Diet Assoc. 2002;102:1638–1647. doi: 10.1016/s0002-8223(02)90349-4. [DOI] [PubMed] [Google Scholar]
- 21.Sankar P, Cho MK. Toward a new vocabulary of human genetic variation. Science. 2002;298:1337–1338. doi: 10.1126/science.1074447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anliker JA, Bartoshuk L, Ferris AM, Hooks LD. Children’s food preferences and genetic sensitivity to the bitter taste of 6-n-propylthiouracil (PROP) Am J Clin Nutr. 1991;54:316–320. doi: 10.1093/ajcn/54.2.316. [DOI] [PubMed] [Google Scholar]
- 23.Cowart BJ, Beauchamp GK. Early development of taste perception. In: McBride R, MacFie H, eds. Psychological Basis of Sensory Evaluation London, England: Elsevier; 1990:1–7
- 24.Liem DG, Mennella JA. Sweet and sour preferences during childhood: role of early experiences. Dev Psychobiol. 2002;41:388–395. doi: 10.1002/dev.10067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bielamowicz MK. Altering recipes for good health. North Central Regional Extension Publication 473;2000:13–15. Available at: fcs.tamu.edu/food_and_nutrition/PDF/alteringrecipes.pdf Accessed October 12, 2004
- 26.Pliner P, Loewen ER. Temperament and food neophobia in children and their mothers. Appetite. 1997;28:239–254. doi: 10.1006/appe.1996.0078. [DOI] [PubMed] [Google Scholar]
- 27.Siegel S, Castellan N Jr. The case of k related samples. In: Anker D, ed. Nonparametric Statistics for Behavioral Sciences 2nd ed. New York, NY: McGraw-Hill; 1988:168–189
- 28.Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62:1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Das SR. Inheritance of the P.T.C. taste character in man: an analysis of 126 Rarhi Brahmin families of West Bengal. Ann Hum Genet. 1958;22:200–212. doi: 10.1111/j.1469-1809.1958.tb01414.x. [DOI] [PubMed] [Google Scholar]
- 30.Bartoshuk LM, Duffy VB, Reed D, Williams A. Supertasting, earaches and head injury: genetics and pathology alter our taste worlds. Neurosci Biobehav Rev. 1996;20:79–87. doi: 10.1016/0149-7634(95)00042-d. [DOI] [PubMed] [Google Scholar]
- 31.Duffy VB, Davidson AC, Kidd JR, et al. Associations between PTC gene, 6-n-propylthiouracil (PROP) bitterness and alcohol intake. Alcohol Clin Exp Res. 2004;28:1629–1637. doi: 10.1097/01.ALC.0000145789.55183.D4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reed D, Nanthakumar E, North M, Bell C, Bartoshuk LM, Price RA. Localization of a gene for bitter taste perception to human chromosome 5p15. Am J Hum Genet. 1999;64:1478–1480. doi: 10.1086/302367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller IJ, Jr, Reedy FE., Jr Variations in human taste bud density and taste intensity perception. Physiol Behav. 1990;47:1213–1219. doi: 10.1016/0031-9384(90)90374-d. [DOI] [PubMed] [Google Scholar]
- 34.Olson JM, Boehnke M, Neiswanger K, Roche AF, Siervogel RM. Alternative genetic models for the inheritance of the phenylthiocarbamide taste deficiency. Genet Epidemiol. 1989;6:423–434. doi: 10.1002/gepi.1370060305. [DOI] [PubMed] [Google Scholar]
- 35.Karam E, Jr, Freire-Maia N. Phenylthiocarbamide and mental immaturity. Lancet. 1967;1:622–633. doi: 10.1016/s0140-6736(67)90474-6. [DOI] [PubMed] [Google Scholar]
- 36.Cowart BJ, Yokomukai Y, Beauchamp GK. Bitter taste in aging: compound-specific decline in sensitivity. Physiol Behav. 1994;56:1237–1241. doi: 10.1016/0031-9384(94)90371-9. [DOI] [PubMed] [Google Scholar]
- 37.Greene LS, Desor JA, Maller O. Heredity and experience: their relative importance in the development of taste preference in man. J Comp Physiol Psychol. 1975;89:279–284. doi: 10.1037/h0076802. [DOI] [PubMed] [Google Scholar]
- 38.Schiffman SS, Graham BG, Sanly-Miller EA, Peterson-Dancy M. Elevated and sustained desire for sweet taste in African-Americans: a potential factor in the development of obesity. Nutrition. 2000;16:886–893. doi: 10.1016/s0899-9007(00)00403-2. [DOI] [PubMed] [Google Scholar]
- 39.Pepino MY, Mennella JA. Factors contributing to individual differences in sucrose preference. Chem Senses. 2004, in press [DOI] [PMC free article] [PubMed]
- 40.Drewnowski A. Fat and sugar: an economic analysis. J Nutr. 2003;133:838S–840S. doi: 10.1093/jn/133.3.838S. [DOI] [PubMed] [Google Scholar]
- 41.Birch LL. Psychological influences on the childhood diet. J Nutr. 1998;128:407s–410s. doi: 10.1093/jn/128.2.407S. [DOI] [PubMed] [Google Scholar]
- 42.Mennella JA, Jagnow CJ, Beauchamp GK. Pre- and post-natal flavor learning by human infants. Pediatrics. 2001;107(6) doi: 10.1542/peds.107.6.e88. Available at: www.pediatrics.org/cgi/content/full/107/6/e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mennella JA, Griffin C, Beauchamp GK. Flavor programming during infancy. Pediatrics. 2004;113:840–845. doi: 10.1542/peds.113.4.840. [DOI] [PMC free article] [PubMed] [Google Scholar]


