Abstract
Depletion of immune elements before adoptive cell transfer (ACT) can dramatically improve the antitumor efficacy of transferred CD8+ T cells, but the specific mechanisms that contribute to this enhanced immunity remain poorly defined. Elimination of CD4+CD25+ regulatory T (T reg) cells has been proposed as a key mechanism by which lymphodepletion augments ACT-based immunotherapy. We found that even in the genetic absence of T reg cells, a nonmyeloablative regimen substantially augmented CD8+ T cell reactivity to self-tissue and tumor. Surprisingly, enhanced antitumor efficacy and autoimmunity was caused by increased function rather than increased numbers of tumor-reactive T cells, as would be expected by homeostatic mechanisms. The γ C cytokines IL-7 and IL-15 were required for augmenting T cell functionality and antitumor activity. Removal of γ C cytokine–responsive endogenous cells using antibody or genetic means resulted in the enhanced antitumor responses similar to those seen after nonmyeloablative conditioning. These data indicate that lymphodepletion removes endogenous cellular elements that act as sinks for cytokines that are capable of augmenting the activity of self/tumor-reactive CD8+ T cells. Thus, the restricted availability of homeostatic cytokines can be a contributing factor to peripheral tolerance, as well as a limiting resource for the effectiveness of tumor-specific T cells.
The immune system precisely controls the levels and the activation state of each cellular compartment through homeostatic regulation, a process triggered during development and after the induction of a lymphopenic state (1–7). It has been long observed in mice that depletion of immune cells before adoptive cell transfer (ACT) can considerably enhance the antitumor efficacy of transferred CD8+ T cells (8–10). Recently, lymphodepletion followed by ACT has emerged as a promising treatment for patients with metastatic solid cancer (11, 12), but the molecular and cellular mechanisms that contribute to this antitumor effect have not been completely elucidated. Homeostatic expansion and T cell activation have been proposed to explain the enhanced antitumor responses seen after ACT into lymphodepleted hosts (13, 14). In addition, experiments indicate that lymphodepletion may enhance the antitumor efficacy of transferred CD8+ T cells by removal of competition at the surfaces of APCs (7, 15). T reg cells are critically involved in maintaining immunological tolerance to self/tumor antigens (16–20), and their removal is also considered a key mechanism underlying the effectiveness of lymphodepletion (21).
We describe the surprising finding that the augmentation of the antitumor efficacy of an ACT regimen after lymphodepletion is not a result of increased numbers of tumor-reactive T cells. Rather, lymphodepletion acts by enhancing the effector functions of transferred T cells. We demonstrate that the mere removal of T reg cell immune suppression is not the only mechanism accountable for the enhanced antitumor immunity observed after lymphodepletion: eradication of cellular sinks and the resultant increase in the availability of cytokines is also a central mechanism in activating self/tumor antigen–specific CD8+ T cells.
Results and discussion
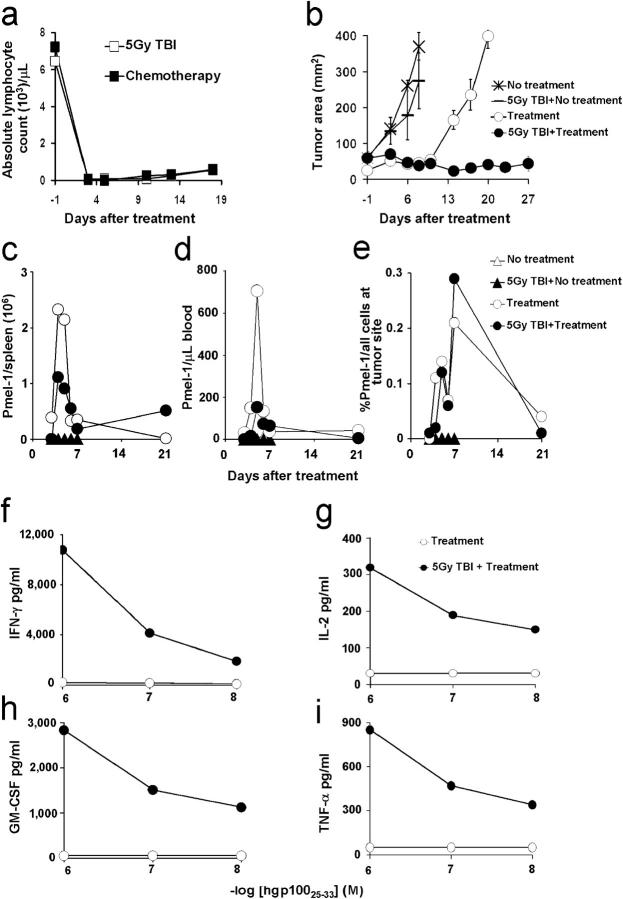
We have recently reported that an ACT regimen that combines the transfer of TCR transgenic (Tg) CD8+ T cells (pmel-1) reactive against the self/tumor antigen gp100, altered ligand vaccination, and the administration of exogenous IL-2 can cause substantial tumor regression of large, established s.c. B16 melanoma (22). To determine whether lymphodepletion could enhance antitumor immunity of transferred CD8+ T cells, we evaluated this ACT regimen (22) in tumor-bearing C57BL/6 WT hosts and hosts rendered lymphopenic by nonmyeloablative sublethal 5-Gy total body irradiation (TBI). In mice, TBI induced a severe lymphopenia similar to that induced by a nonmyeloablative chemotherapy regimen of 250 mg/kg Cytoxan + 50 mg/kg fludarabine (a combination currently used in the clinic; Fig. 1 a) (11). Transfer of 107 pmel-1 cells in combination with vaccination and IL-2 can eradicate established B16 tumors (22). We thus gave a log fewer (106 cells in all experiments) to generate a treatment window. Tumor treatment was significantly improved in TBI compared with nonirradiated hosts (P = 0.0014) when this tripartite regimen was used (Fig. 1 b).
Figure 1.
Lymphodepletion enhances antitumor efficacy of adoptively transferred CD8+ T cells. (a) 5 Gy TBI induces severe lymphopenia. Mice were treated with 5 Gy TBI or a nonmyeloablative chemotherapy regimen with 250 mg/kg Cytoxan + 50 mg/kg fludarabine. Absolute lymphocyte count was determined at the indicated time points. (b) Lymphodepletion augments antitumor responses. TBI or nonirradiated WT mice bearing 12-d-old established s.c. B16 tumors were left untreated or received adoptive transfer of 106 cultured pmel-1 T cells in conjunction with rFPhgp100 vaccination and rhIL-2. Data shown are representative of multiple independent experiments. Values represent the mean ± SEM. (c–e) Lymphodepletion does not result in increased numbers of adoptively transferred T cells. Absolute numbers of adoptively transferred pmel-1 cells (CD8+Thy1.1+) in the spleens (c) and in the blood (d) of tumor-bearing, TBI, and nonirradiated mice. Percentages of adoptively transferred pmel-1 cells (CD8+Thy1.1+) in the tumor (e) under conditions specified. (f–i). Lymphodepletion enhances effector functions of adoptively transferred T cells. 6 d after adoptive transfer, pmel-1 thy1.1+ cells were isolated from the spleens of irradiated and nonirradiated mice and co-cultured with irradiated splenocytes pulsed with the indicated doses of hgp10025–33. Unpulsed splenocytes were used as controls. Data shown are representative of two independent experiments.
No significant differences (P > 0.05) in tumor growth were observed in untreated WT or TBI mice (Fig. 1 b), indicating that irradiation did not act by directly killing the tumor but rather acted as a result of its impact on host cells. Indeed, up to 20 Gy could be delivered directly to the tumor while shielding the host with little effect (Fig. S1 a, available at http://www.jem.org/cgi/content/full/jem.20050732/DC1). Conversely, shielding the tumor with irradiation delivered solely to the host resulted in profound antitumor effects (Fig. S1 b). Because chemotherapy had some direct antitumor effect (unpublished data), we chose to use TBI as a lymphodepleting regimen in the following experiments.
Transfer of T cells into lymphopenic recipients results in their proliferation and differentiation (3, 6, 7), but it was not known whether homeostatic proliferation was the mechanism responsible for the increased antitumor activity of pmel-1 cells after TBI. Surprisingly, when the tripartite regimen was given to tumor-bearing WT mice with TBI, we did not find an increased number of pmel-1 cells. Similar numbers or percentages of pmel-1 cells were found in the spleen (Fig. 1 c), blood (Fig. 1 d), tumor (Fig. 1 e), and tumor-draining lymph nodes (not depicted) of lymphodepleted and lymphoreplete animals. In addition, kinetic analyses using bromodeoxyuridine labeling indicated that there were no important differences in cell proliferation in nondepleted and lymphodepleted settings (unpublished data). Thus, the absolute number of tumor-reactive CD8+ T cells alone did not explain the enhanced antitumor responses into lymphodepleted recipients.
However, when pmel-1 CD8+ T cells were isolated from the same animals and tested for antigen-specific function, considerable differences were observed in lymphodepleted versus lymphoreplete recipients (Fig. 1, f–i). pmel-1 cells from lymphodepleted animals produced greater amounts of IFN-γ, IL-2, granulocyte-macrophage CSF, and TNF-α, as well as macrophage inflammatory protein–1α and regulated on activation, normal T cell expressed and secreted (not depicted) 6 d after transfer. Thus, the lymphopenic environment induces qualitative rather than quantitative improvements of adoptively transferred T cells.
We sought to more completely understand the mechanism of increased CD8+ T cell function after transfer into an ablated recipient. T reg cells are involved in maintaining immunological tolerance to self/tumor antigens (16–20). Experiments using genetic knockouts of T reg cells as well as the add-back of these cells has incontrovertibly indicated their critical regulatory role in the antitumor activities of pmel-1 cells (21). We have extended these findings by exploring the role of TBI in mice devoid of T reg cells. The tripartite regimen was administered to irradiated or nonirradiated tumor-bearing Rag-1−/− mice and MHC class II−/− mice. We found that irradiation significantly augmented the antitumor efficacy of our treatment even in mice lacking endogenous T reg cells (P < 0.05; Fig. 2, a and b). Similar results were observed in other knockout mice devoid of T reg cells, such as CD4−/− (unpublished data). Enhanced autoimmune vitiligo was observed in irradiated versus nonirradiated mice genetically devoid of T reg cells (Rag-1−/−, MHC class II−/−, and CD4−/−; P < 0.0001) 1 mo after therapy (Fig. 2 c). These data suggested that the mere removal of T reg cell immune suppression is not the only mechanism accountable for the enhanced immune responses observed after irradiation.
Figure 2.
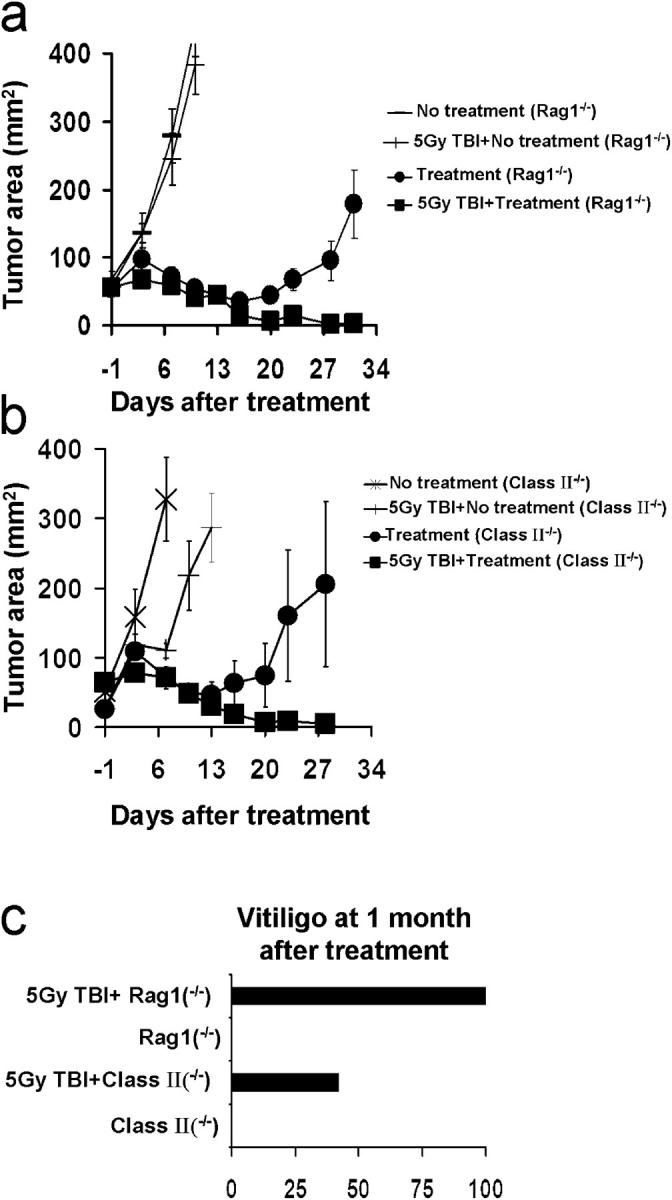
Even in the absence of T reg cells, ablation enhances antitumor efficacy of adoptively transferred T cells. (a) Ablation augments tumor responses in Rag1−/− mice. TBI or nonirradiated Rag1−/− mice bearing 14-d-old established s.c. B16 tumors were left untreated or received adoptive transfer of 106 cultured pmel-1 T cells in conjunction with rFPhgp100 vaccination and rhIL-2. Values represent the mean ± SEM. (b) Ablation augments tumor responses in MHC class II−/− mice. TBI or nonirradiated MHC class II−/− mice bearing 14-d-old established s.c. B16 tumors were left untreated or received adoptive transfer of 106 cultured pmel-1 T cells in conjunction with rFPhgp100 vaccination and rhIL-2. Values represent the mean ± SEM. (c) Ablation of mice genetically devoid of T reg cells augments autoimmunity. 30 d after treatment, mice were evaluated in a blinded fashion for the development of vitiligo. The percentages of mice with vitiligo are shown.
IL-7 and IL-15 have supportive roles in the survival and proliferation of adoptively transferred T cells (1, 2, 4, 23, 24). Other immune cells may serve as sinks for these cytokines. Because we found that radiation improved treatment in experiments using Rag1−/− mice, which lack both T cells and B cells but have NK cells, we hypothesized that NK cells may serve as sinks for cytokines. It is known that IL-15 is required for the survival of NK cells (25, 26). To determine whether NK cells were sinks in Rag-1−/− mice, we tested NK cell depletion using anti-NK1.1 antibody in mice that received our tripartite regimen with or without TBI. 3 d after ACT, high absolute numbers of NK cells were found in the blood of nonirradiated mice. Fewer NK cells were detected in either irradiated or NK-depleted Rag1−/− mice (isotype-treated vs. irradiated Rag-1−/− mice, P = 0.0013; isotype-treated vs. NK-depleted Rag-1−/− mice, P = 0.002; Fig. 3 a). No significant differences (P > 0.05) in NK cell numbers were found between irradiated and NK-depleted Rag-1−/− mice. As shown in Fig. 2 a, we again observed increased responses in irradiated compared with nonirradiated Rag-1−/− mice (P = 0.0172; Fig. 3 b). Astonishingly, merely removing NK cells with an mAb significantly improved ACT (isotype-treated vs. NK-depleted Rag-1−/− mice, P = 0.0264) and was as effective as irradiation (Fig. 3 b). These data indicated that in the absence of endogenous T reg cells, the removal of NK cells results in the enhancement antitumor efficacy of adoptively transferred CD8+ T cells.
Figure 3.
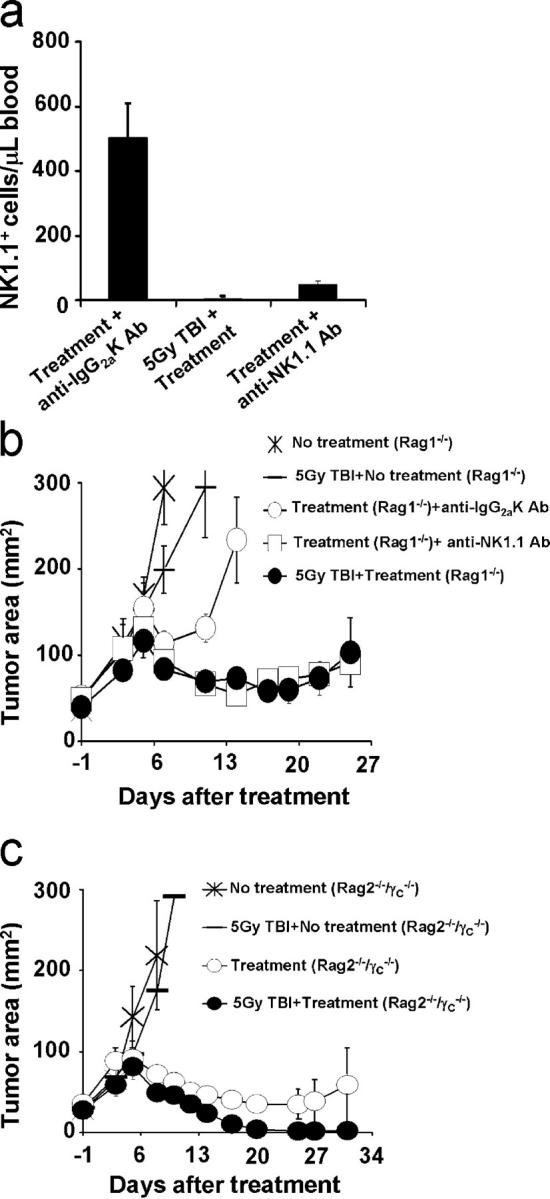
Removal of NK cells enhances antitumor efficacy of adoptively transferred T cells. (a) Irradiation or administration of NK1.1 antibody efficiently depletes NK cells. Absolute numbers of NK1.1+ cells in the blood of tumor-bearing TBI Rag-1−/− mice and nonirradiated Rag-1−/− mice treated with NK1.1 antibody or Ig2aK antibody. Results are shown 3 d after the administration of 106 cultured pmel-1 T cells in conjunction with rFPhgp100 vaccination and rhIL-2. Values represent the mean ± SEM. (b) Removal of NK cells by antibody or by irradiation enhances antitumor efficacy of adoptively transferred T cells. Rag-1−/− mice bearing 9-d-old established s.c. B16 tumors received TBI or antibodies (anti-NK1.1 or Ig2aK antibody). Mice then received adoptive transfer of 106 cultured pmel-1 T cells in conjunction with rFPhgp100 vaccination and rhIL-2 or were left untreated. Values represent the mean ± SEM. (c) The genetic depletion of T cells, B cells, and NK cells recapitulates the effect of TBI. Rag-2−/−/γC −/− mice bearing 8-d-old established s.c. B16 tumors were either left untreated or received TBI. Where designated, mice received adoptive transfer of 106 cultured pmel-1 T cells in conjunction with rFPhgp100 vaccination and rhIL-2. Irradiation improved antitumor responses in control WT mice (not depicted). Values represent the mean ± SEM.
To more definitively assess the role of cellular sinks in our model, we administered pmel-1 cells in conjunction with vaccine and IL-2 into TBI or nonirradiated Rag-2−/−/γC −/− mice. The mice lack T cells, B cells, and, most importantly, NK cells. We did not observe an improved treatment outcome after irradiation in Rag-2−/−/γC −/− mice, which lack all cellular immune components dependent on γC cytokines. Collectively, these finding suggest that endogenous cellular components, including NK cells, act as cellular sinks for cytokines capable of activating antitumor responses.
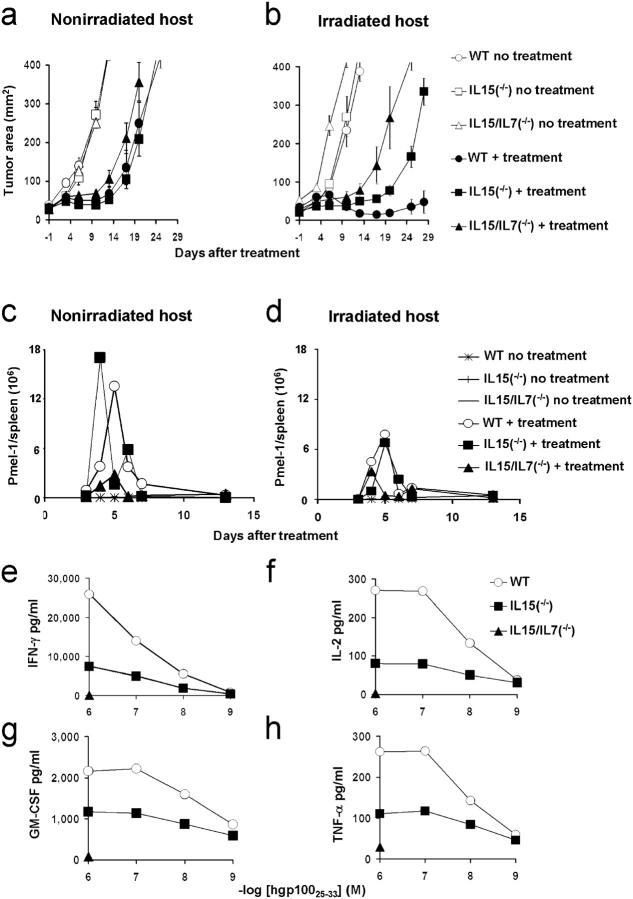
To more precisely determine the role of γC cytokines, we evaluated our regimen in WT, IL-15−/−, IL-7−/−, and IL-7−/−IL-15−/− tumor-bearing mice with or without TBI. The enhancement of the treatment derived from TBI was partially abrogated on ACT into IL-15−/− (P = 0.0009; Fig. 4 b). A slight impairment, not reaching statistical significance (P > 0.05), was seen in mice deficient of IL-7 (not depicted). Most importantly, treatment was dramatically impaired into mice deficient of both IL-7 and IL-15 (WT vs. IL-7−/−IL-15−/−, P < 0.0001; IL-15−/− vs. IL-7−/−IL-15−/−, P = 0.0003; Fig. 4 b). The lack of both these cytokines completely abrogated the benefit of the ablation; there were no significant differences (P > 0.05) in tumor treatment in nonirradiated WT mice versus irradiated IL-7−/−IL-15−/− mice. In contrast, the absence of IL-7 and IL-15 did not influence the treatment in mice that did not receive TBI. These homeostatic cytokines may not be limiting in the nonirradiated setting (Fig. 4 a). Thus, endogenous leukocytes appear to compete with the transferred T cells for supportive cytokines in nonirradiated hosts.
Figure 4.
Increased access to supportive endogenous cytokines into irradiated hosts enhances antitumor efficacy of transferred T cells. (a and b) Treatment is impaired in irradiated mice genetically deficient in IL-15 or IL-7 and IL-15 versus WT mice. WT, IL-15−/−, or IL-7−/−IL-15−/− mice bearing 9-d-old established s.c. B16 tumors were left untreated or received adoptive transfer of 106 cultured pmel-1 T cells in conjunction with rFPhgp100 vaccination and rhIL-2 with or without TBI. Values represent the mean ± SEM. (c and d) Absence of both IL-7 and IL-15 impaired proliferative responses of adoptively transferred T cells. Absolute numbers of adoptively transferred pmel-1 cells (CD8+Thy1.1+) in the spleens of tumor-bearing, nonirradiated WT, IL-15−/−, or IL-7−/−IL-15−/− mice (c). Absolute numbers of adoptively transferred pmel-1 cells (CD8+Thy1.1+) in the spleens of tumor-bearing, irradiated WT, IL-15−/−, or IL-7−/−IL-15−/− mice (d). (e–h) Increased access to IL-7 and IL-15 enhances effector functions of adoptively transferred T cells. 6 d after adoptive transfer pmel-1 thy1.1+ cells were isolated from the spleens of TBI WT, IL-15−/−, or IL-7−/−IL-15−/− mice and co-cultured with irradiated splenocytes pulsed with the indicated doses of hgp10025–33. Unpulsed splenocytes were used as controls. Because of the limited numbers of cells isolated from IL-7−/−IL-15−/− mice, pmel-1 cells were tested only against 1 μg/ml of peptide and unpulsed control.
To determine whether adoptively transferred CD8+ T cells underwent quantitative or qualitative changes in the presence of IL-7 and IL-15, pmel-1 thy.1.1 T cells were administered in conjunction with vaccine and IL-2 into IL-15−/− and IL-7−/−IL-15−/− with or without TBI. Again, we did not observe higher numbers of pmel-1 cells in irradiated mice compared with nonirradiated controls (Fig. 4, c and d). IL-15 was required to maintain the complete functionality of CD8+ T cells but not the numbers of pmel-1 T cells (Fig. 4, c–h). These findings are consistent with the concept that IL-7 can maintain the proliferation and survival, but not the function, of adoptively transferred cells in IL-15−/− mice. Most importantly, pmel-1 cells were profoundly impaired in both quantity and quality when IL-7 and IL-15 were absent (Fig. 4, c–h). Conversely, the addition of exogenous IL-7 and IL-15 alone or in combination with our tripartite regimen increased antitumor responses (Fig. S2, a–d, available at http://www.jem.org/cgi/content/full/jem.20050732/DC1). These data demonstrate that IL-15 and IL-7 are critical for sustaining the proliferation and function of transferred T cells.
Collectively, these findings demonstrate that a key mechanism underlying the enhanced function of ACT therapies after lymphodepletion is the transient eradication of cellular sinks and the resultant increase in the availability of homeostatic cytokines, which in turn results in the augmentation in the function of adoptively transferred T cells. The ability of T cells to receive these signals maybe critically dependent on their state of differentiation; less-differentiated cells express higher levels of receptors for key homeostatic cytokines (27). These findings may furthermore indicate that limiting access to activating cytokines may be a mechanism whereby otherwise self-reactive T cells are maintained in a quiescent state (22). A deeper understanding of the mechanisms underlying the enhanced effectiveness of immunotherapy after lymphodepletion may allow us to more precisely achieve enhanced immunity without the use of nonspecific modalities such as chemotherapy or TBI, with all their life-threatening side effects.
MATERIALS AND METHODS
Mice and tumor lines.
All mice used in these experiments were bred and housed at NIH facilities. Female pmel-1 TCR Tg mice (22) were crossed with C57BL/6-Thy1.1 Tg mice (The Jackson Laboratory) to derive pmel-Thy1.1 double Tg mice (C57BL/6-pmel-1-Thy1.1 mice; The Jackson Laboratory). Female C57BL/6, Rag-1−/−, MHC class II−/− (The Jackson Laboratory), IL-15−/−, Rag-2−/−/γC −/− (Taconic), IL-7−/−, and IL-7−/−IL-15−/−mice were used as recipients in ACT experiments. Experiments were conducted with the approval of the NCI Animal Use and Care Committee. B16-F10 (H-2b), a spontaneous, transplantable gp100+ murine melanoma, was maintained in culture media as previously described (22).
In vitro activation of pmel-1 T cells.
pmel-1 splenocytes were isolated as described previously (22) and cultured in the presence of 1 μM hgp10025–33 and culture media containing 30 IU/ml of recombinant human IL-2 (rhIL-2; Chiron Corp.). Cells were used for ACT 6–7 d after the start of the culture.
ACT, vaccination, and cytokine administration.
6–12-wk-old mice (n = 5 for all groups) were injected s.c. with 2–5 × 105 B16-F10 melanoma cells and treated 10–14 d later with i.v. adoptive transfer of pmel-1 CD8+ T cell in vitro activated–splenocytes. Lymphopenia was induced by sublethal TBI (5 Gy) of tumor-bearing mice on the day of treatment. NK cells were depleted by i.p. administration of 100 μg/mouse of NA/LE anti-NK1.1 antibody (BD Biosciences) every 3 d for a total of five doses. 100 μg/mouse of NA/LE anti-Ig2aK was used as a control antibody (BD Biosciences). Mice were vaccinated with 2 × 107 PFU of a previously described (22) recombinant fowlpox virus expressing human gp100 (rFPhgp100; Therion Biologics). 0–108 μg/dose of rhIL-2 (Chiron Corp.), rhIL-7, or rhIL-15 (PeproTech) were administered by i.p. injection twice daily for a total of six doses. Tumors were measured using calipers, and the products of the perpendicular diameters were recorded. All experiments were performed in a blind, randomized fashion (the measuring investigator had no knowledge of the experimental group) and performed independently at least twice with similar results.
Enumeration of adoptively transferred cells, NK cells, and ex vivo cytokine release assay.
At the time points indicated in the figures, adoptively transferred pmel-1 thy1.1 cells were enumerated as previously described (28). 3 d after treatment, the NIH clinical laboratory determined the white blood cell count on blood samples. Samples were analyzed by flow cytometry for Nk1.1 expression by cells. NK cell number was calculated by multiplying the white blood cell count by the percentage of NK1.1-positive cells. 6 d after adoptive transfer, pmel-1 thy1.1 cells were also used for cytokine release assay. pmel-1 thy1.1 cells were isolated from splenocytes as previously described (21) and were co-cultured at a 1:2 ratio with 105 irradiated splenocytes pulsed with titrated doses of hgp10025–33 peptide. Unpulsed splenocytes were used as negative controls. Supernatants were collected after 18 h and assayed by murine Lincoplex cytokine assay (Linco Research) or mouse IFN-γ (Endogen) ELISA kits.
Statistical analysis.
Tumor graphs were examined using the analysis of variance between groups test.
Online supplemental material
Fig. S1 shows how lymphodepletion enhances the antitumor activity of adoptively transferred CD8+ T cells by an indirect mechanism rather than directly killing the tumor. Fig. S2 shows the enhancement of ACT immunotherapy by the exogenous administration of combinations of IL-2, IL-7, and IL-15. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20050732/DC1.
Acknowledgments
This research was supported by the Intramural Research Program of the Center for Cancer Research, NCI, NIH.
The authors have no conflicting financial interests.
L. Gattinoni, S.E. Finkelstein, and C.A. Klebanoff contributed equally to this work.
References
- 1.Judge, A.D., X. Zhang, H. Fujii, C.D. Surh, and J. Sprent. 2002. Interleukin-15 controls both proliferation and survival of a subset of memory-phenotype CD8+ T cells. J. Exp. Med. 196:935–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tan, J.T., B. Ernst, W.C. Kieper, E. LeRoy, J. Sprent, and C.D. Surh. 2002. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J. Exp. Med. 195:1523–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldrath, A.W., L.Y. Bogatzki, and M.J. Bevan. 2000. Naive T cells transiently acquire a memory-like phenotype during homeostasis-driven proliferation. J. Exp. Med. 192:557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldrath, A.W., P.V. Sivakumar, M. Glaccum, M.K. Kennedy, M.J. Bevan, C. Benoist, D. Mathis, and E.A. Butz. 2002. Cytokine requirements for acute and Basal homeostatic proliferation of naive and memory CD8+ T cells. J. Exp. Med. 195:1515–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosen, S.D. 2004. Ligands for L-selectin: homing, inflammation, and beyond. Annu. Rev. Immunol. 22:129–156. [DOI] [PubMed] [Google Scholar]
- 6.Murali-Krishna, K., and R. Ahmed. 2000. Cutting edge: naive T cells masquerading as memory cells. J. Immunol. 165:1733–1737. [DOI] [PubMed] [Google Scholar]
- 7.Cho, B.K., V.P. Rao, Q. Ge, H.N. Eisen, and J. Chen. 2000. Homeostasis-stimulated proliferation drives naive T cells to differentiate directly into memory T cells. J. Exp. Med. 192:549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berenson, J.R., A.B. Einstein Jr., and A. Fefer. 1975. Syngeneic adoptive immunotherapy and chemoimmunotherapy of a Friend leukemia: requirement for T cells. J. Immunol. 115:234–238. [PubMed] [Google Scholar]
- 9.Cheever, M.A., P.D. Greenberg, and A. Fefer. 1980. Specificity of adoptive chemoimmunotherapy of established syngeneic tumors. J. Immunol. 125:711–714. [PubMed] [Google Scholar]
- 10.North, R.J. 1982. Cyclophosphamide-facilitated adoptive immunotherapy of an established tumor depends on elimination of tumor-induced suppressor T cells. J. Exp. Med. 155:1063–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dudley, M.E., J.R. Wunderlich, P.F. Robbins, J.C. Yang, P. Hwu, D.J. Schwartzentruber, S.L. Topalian, R. Sherry, N.P. Restifo, A.M. Hubicki, et al. 2002. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 298:850–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Childs, R.W., and J. Barrett. 2004. Nonmyeloablative allogeneic immunotherapy for solid tumors. Annu. Rev. Med. 55:459–475. [DOI] [PubMed] [Google Scholar]
- 13.Dummer, W., B. Ernst, E. LeRoy, D. Lee, and C. Surh. 2001. Autologous regulation of naive T cell homeostasis within the T cell compartment. J. Immunol. 166:2460–2468. [DOI] [PubMed] [Google Scholar]
- 14.Dummer, W., A.G. Niethammer, R. Baccala, B.R. Lawson, N. Wagner, R.A. Reisfeld, and A.N. Theofilopoulos. 2002. T cell homeostatic proliferation elicits effective antitumor autoimmunity. J. Clin. Invest. 110:185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kedl, R.M., W.A. Rees, D.A. Hildeman, B. Schaefer, T. Mitchell, J. Kappler, and P. Marrack. 2000. T cells compete for access to antigen-bearing antigen-presenting cells. J. Exp. Med. 192:1105–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shimizu, J., S. Yamazaki, and S. Sakaguchi. 1999. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J. Immunol. 163:5211–5218. [PubMed] [Google Scholar]
- 17.Turk, M.J., J.A. Guevara-Patino, G.A. Rizzuto, M.E. Engelhorn, and A.N. Houghton. 2004. Concomitant tumor immunity to a poorly immunogenic melanoma is prevented by regulatory T cells. J. Exp. Med. 200:771–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang, H.Y., D.A. Lee, G. Peng, Z. Guo, Y. Li, Y. Kiniwa, E.M. Shevach, and R.F. Wang. 2004. Tumor-specific human CD4+ regulatory T cells and their ligands: implications for immunotherapy. Immunity. 20:107–118. [DOI] [PubMed] [Google Scholar]
- 19.Curiel, T.J., G. Coukos, L. Zou, X. Alvarez, P. Cheng, P. Mottram, M. Evdemon-Hogan, J.R. Conejo-Garcia, L. Zhang, M. Burow, et al. 2004. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 10:942–949. [DOI] [PubMed] [Google Scholar]
- 20.Woo, E.Y., H. Yeh, C.S. Chu, K. Schlienger, R.G. Carroll, J.L. Riley, L.R. Kaiser, and C.H. June. 2002. Cutting edge: regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J. Immunol. 168:4272–4276. [DOI] [PubMed] [Google Scholar]
- 21.Antony, P.A., C.A. Piccirillo, A. Akpinarli, S.E. Finkelstein, P.J. Speiss, D.R. Surman, D.C. Palmer, C.C. Chan, C.A. Klebanoff, W.W. Overwijk, et al. 2005. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J. Immunol. 174:2591–2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Overwijk, W.W., M.R. Theoret, S.E. Finkelstein, D.R. Surman, L.A. de Jong, F.A. Vyth-Dreese, T.A. Dellemijn, P.A. Antony, P.J. Spiess, D.C. Palmer, et al. 2003. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J. Exp. Med. 198:569–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schluns, K.S., W.C. Kieper, S.C. Jameson, and L. Lefrancois. 2000. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat. Immunol. 1:426–432. [DOI] [PubMed] [Google Scholar]
- 24.Becker, T.C., E.J. Wherry, D. Boone, K. Murali-Krishna, R. Antia, A. Ma, and R. Ahmed. 2002. Interleukin-15 is required for proliferative renewal of virus-specific memory CD8 T cells. J. Exp. Med. 195:1541–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prlic, M., B.R. Blazar, M.A. Farrar, and S.C. Jameson. 2003. In vivo survival and homeostatic proliferation of natural killer cells. J. Exp. Med. 197:967–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koka, R., P.R. Burkett, M. Chien, S. Chai, F. Chan, J.P. Lodolce, D.L. Boone, and A. Ma. 2003. Interleukin (IL)-15Rα–deficient natural killer cells survive in normal but not IL-15Rα–deficient mice. J. Exp. Med. 197:977–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gattinoni, L., C.A. Klebanoff, D.C. Palmer, C. Wrzesinski, K. Kerstann, Z. Yu, S.E. Finkelstein, M.R. Theoret, S.A. Rosenberg, and N.P. Restifo. 2005. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8(+) T cells. J. Clin. Invest. 115:1616–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palmer, D.C., S. Balasubramaniam, K. Hanada, C. Wrzesinski, Z. Yu, S. Farid, M.R. Theoret, L.N. Hwang, C.A. Klebanoff, L. Gattinoni, et al. 2004. Vaccine-stimulated, adoptively transferred CD8+ T cells traffic indiscriminately and ubiquitously while mediating specific tumor destruction. J. Immunol. 173:7209–7216. [DOI] [PMC free article] [PubMed] [Google Scholar]