Abstract
The cellular response to oncogenic Ras depends upon the presence or absence of cooperating mutations. In the absence of immortalizing oncogenes or genetic lesions, activation of the Ras/Raf pathway results in a p21Cip1-dependent cellular arrest. The human papillomavirus oncoprotein E7 transforms primary cells in cooperation with Ras and abolishes p21Cip1-mediated growth arrest in the presence of various antimitogenic signals. Here we have utilized a conditional Raf molecule to investigate the effects of E7 on p21Cip1 function in the context of Raf-induced cellular arrest. E7 bypassed Raf-induced arrest and alleviated inhibition of cyclin E-CDK2 without suppressing Raf-specific synthesis of p21Cip1 or derepressing p21Cip1-associated CDK2 complexes. Activation of Raf led to nuclear accumulation of p21Cip1, and we provide evidence that this effect is mediated by inhibition of Akt, a regulator of p21Cip1 localization. Loss of Akt activity appears to be an important event in the cellular arrest associated with Raf-induction, since maintenance of Akt activity was necessary and sufficient to bypass Raf-induced arrest. In agreement, expression of E7 sustained Akt activity and reduced nuclear accumulation of p21Cip1, resulting in decreased association between p21Cip1 and cyclin E-CDK2. Taken together, these data suggest that E7 inhibits p21Cip1 function in the context of Raf signaling by altering Raf-Akt antagonism and preventing the proper subcellular localization of p21Cip1. We propose that E7 elicits a proliferative response to Raf signaling by targeting p21Cip1 function via a novel mechanism.
Tumorigenesis occurs through the accumulation of genetic alterations that collectively transform normal cells into malignant derivatives (23). Increased cell division associated with transformation of primary cells usually requires the cooperation of at least two oncogenic mutations, while expression of individual oncogenes can promote arrest, senescence, and apoptosis (20, 39, 64), suggesting the presence of cellular safeguards to oncogenic stress. This is illustrated by the distinct cellular responses to hyperactivation of the Ras/Raf signaling cascade. Stimulation of Ras or Raf can induce cell cycle arrest in various primary cell types and at high signaling strength in immortalized fibroblasts (29, 37, 56, 65, 72). However, genetic lesions resulting in activation of cooperating cellular oncogenes (e.g., myc) or loss of tumor suppressors (e.g., p53) disables the growth-inhibitory effects of Ras/Raf and potentiates their mitogenic activity (30, 38). Similarly, viral oncoproteins such as adenovirus E1A, simian virus 40 (SV40) large T antigen, and human papillomavirus E7 cooperate with Ras (70), suggesting that such factors impinge upon critical effectors of Ras-induced arrest.
p21Cip1 is an important determinant in the cellular response to Ras/Raf activation. p21Cip1 was independently isolated as an inducer of cellular senescence, a transcriptional target of the p53 tumor suppressor, and a direct inhibitor of CDK2 (19, 24, 46). p21Cip1 is regulated at the transcriptional and posttranscriptional levels, with transcription of p21Cip1 activated by p53-dependent and p53-independent mechanisms (19, 40). p21Cip1 expression is also induced during senescence-derived arrest and in terminal differentiation of myoblast, epithelial, and hematopoietic cell lineages (40, 46, 48). Ras and Raf induce a p21Cip1-dependent cell cycle arrest in fibroblasts, epithelial keratinocytes, and Schwann cells (37, 58, 64, 65). Furthermore, genetic loss of p21Cip1 confers a proliferative advantage to Ras-transduced embryonic fibroblasts and promotes Ras-induced epithelial tumorigenesis (41, 65), implicating p21Cip1 as a logical target of transforming oncogenes. This is highlighted by observations that oncogenes or genetic lesions that cooperate with Ras in cellular transformation frequently abolish p21Cip1 function (19, 37, 49, 64, 70).
Human papillomaviruses (HPV) are small DNA viruses that require unscheduled S-phase entry in terminally differentiated epithelial keratinocytes in order for viral genome amplification to occur (34). Not surprisingly, HPV have evolved several strategies for uncoupling differentiation from cell cycle arrest. The E7 early gene product of HPV subtype 16 (HPV-16) stimulates cellular progression through the G1-to-S transition in the presence of various G1 arrest signals, suggesting that E7 has evolved to interact with key components of cellular growth-regulatory pathways. The best-described target of E7 is the retinoblastoma (RB) family of pocket proteins (RB, p107, and p130) (11, 16, 17, 26). E7 interacts with the pocket proteins through an LXCXE motif and can disrupt RB-mediated gene regulation (16). In addition, expression of E7 leads to a reduction in the steady-state level of RB by ubiquitin-dependent degradation (5, 13, 31). E7 has also been suggested to obviate the CDK-inhibitory function of p21Cip1 (21, 31). In accordance, E7 abolishes G1 arrest induced by DNA damage, epithelial differentiation, and transforming growth factor β (TGF-β), stimuli that negatively regulate proliferation via p21Cip1 (12). Importantly, E7 can transform primary rodent cells in cooperation with oncogenic Ras (52, 69), suggesting that E7 abolishes p21Cip1-mediated growth inhibition elicited by Ras/Raf signaling.
In the present study, we have examined the effects of E7 on p21Cip1 function in the context of Raf-induced arrest. Utilizing a conditional Raf kinase, we demonstrate that E7 abolishes p21Cip1 function induced by Raf. Expression of E7 prevents inhibition of cyclin E-CDK2 and rescues cell cycle progression. Activation of Raf induced p21Cip1 nuclear accumulation, and we provide evidence that this effect is mediated by inhibition of Akt activity, a regulator of p21Cip1 localization. Furthermore, maintenance of Akt activity bypassed Raf-induced arrest, suggesting that loss of Akt activity is necessary for growth arrest elicited by Raf. Consistent with this interpretation, expression of E7 prevented loss of Akt activity and reduced nuclear accumulation of p21Cip1, resulting in a decreased association between p21Cip1 and cyclin E-CDK2. Taken together, these results suggest that E7 targets p21Cip1 function by sustaining Akt-mediated regulation of p21Cip1 localization during Raf signaling.
MATERIALS AND METHODS
Cell culture.
NIH 3T3 cells stably expressing the RafAR fusion protein have been described previously (65) and were kindly provided by Hartmut Land, University of Rochester (Rochester, N.Y.). This cell line and all derivatives were cultured in Dulbecco's modified Eagle's medium (DMEM) without phenol red (GIBCO-BRL) supplemented with 10% charcoal-stripped newborn calf serum (NCS; HyClone). Cell lines stably expressing E7, E7.C24G, or human cyclin E were established via the recombinant amphotropic pBabe retroviral system described elsewhere (43). Upon infection, cells were selected in 2.5-μg/ml puromycin (Sigma) and were used for limited generations. Asynchronous cells were seeded at low density (5 × 105 cells per 15-cm-diameter dish) and were subsequently treated with RafAR-inducing 1.0 μM R1881 (methyltrienolone; Dupont) or vehicle (ethanol) for the indicated times. For TGF-β experiments, Mv1Lu cells with doxycycline-inducible E7 expression, tet-E7 Mv1Lu (a kind gift from M. O'Reilly), were treated with 3 ng of TGF-β/ml with or without 2 μg of doxycycline/ml for 24 h.
Cell cycle analysis.
Cells were pulsed with 10 μM bromodeoxyuridine (BrdU) for 30 min, trypsinized, and fixed in 70% ethanol for 12 h at 4°C. Subsequently, cells were labeled with fluorescein isothiocyanate-conjugated anti-BrdU (Boehringer Mannheim), treated with RNase A (1 mg/ml; Sigma), and stained with propidium iodide (20 μg/ml; Sigma) following standard protocols. Data were collected and analyzed by FACSCaliber (ELITE) and Multicycle software.
Immunoblotting and IP.
Cell pellets were lysed in HLB lysis buffer (50 mM Tris, pH 8.0, 150 mM NaCl, 1% Triton X-100, 1 mM dithiothreitol, and protease inhibitor cocktail [Sigma P8340]) for 30 min with vortexing. After centrifugation, protein content was quantitated via a standard Bio-Rad Bradford assay. For immunoblotting, 30 to 50 μg of cell lysate was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto nitrocellulose membrane (Schleicher & Schuell). After incubation with primary and secondary antibodies, antigen detection was performed by using the enhanced chemiluminescence kit from NEN. The following antibodies were obtained from Santa Cruz: anti-cyclin D1 (sc-450, Western blotting), anti-cyclin D2 (sc-593, Western), anti-cyclin E (sc-481, immunoprecipitation [IP] and Western), anti-human cyclin E (sc-198, IP and Western), anti-CDK2 (sc-163g, IP and Western), anti-CDK4 (sc-260, IP and Western), anti-p21Cip1 (sc-6246, Western), anti-p21Cip1 (sc-397, IP and immunofluorescence), anti-p27Kip1 (sc-528, Western and immunofluorescence), and antiphosphothreonine (sc-5267, Western). HPV-16 E7-specific and RB-specific antibodies were purchased from Zymed and Pharmingen, respectively. Antibodies recognizing Akt or Akt phosphorylated at serine-473 were obtained from Cell Signaling. For IPs, cell lysates were incubated with primary antibodies for 2 h at 4°C, and immune complexes were collected on protein A-agarose beads (Santa Cruz) for an additional 1 h. The complexes were washed four times with HLB buffer and resolved by SDS-12% PAGE for immunoblotting.
Kinase assays.
After IP, immune complexes were washed two additional times in kinase buffer (50 mM Tris, pH 7.5, 10 mM MgCl2, and 1 mM dithiothreitol). Kinase assays were performed in kinase buffer with 30 μM ATP, 3 μCi of [γ-32P]ATP, and 15 μg of histone H1 (for cyclin E-CDK2 complexes) or 2 μg of glutathione trasnferase (GST)-RB C terminus (for CDK4 complexes) per reaction for 15 min at 23°C. Radiolabeled substrate was resolved on SDS-12% PAGE and quantified with a PhosphorImager and ImageQuant (Molecular Dynamics) software. For in vitro p21Cip1 inhibition experiments, purified recombinant GST-p21Cip1 was incubated with 20 μg of target control or E7-expressing cell lysates for 30 min at 30°C before assaying for cyclin E-associated histone H1 kinase activity as described above.
Plasmids and transfections.
pBabe retroviral constructs expressing E7 and E7.C24G were generated and are described elsewhere (45). The pBabe derivative expressing human cyclin E was kindly provided by B. Amati (67). The pcDNA3 constructs encoding Akt K179 M (6) and myristoylated Akt (32) were the generous gifts of Paul Coffer and Richard Roth, respectively. The green fluorescent protein (GFP) expression plasmid, pEGFPC1, is commercially available (Clontech). For transient transfections, control or E7-expressing cells were seeded on 60-mm-diameter dishes at a density of 1.5 × 105 cells/plate. Cells were transfected 24 h later with the indicated plasmids using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. After a 5-h incubation, the transfection medium was replaced with DMEM without phenol red (GIBCO-BRL) supplemented with 10% charcoal-stripped NCS (HyClone) for an additional 6 h. Subsequently, cells were treated with vehicle (ethanol) or R1881 for an additional 30 h, pulsed with 10 μM BrdU during the last 10 h of treatment, and analyzed for BrdU incorporation by immunofluorescence (see below).
Immunofluorescence.
For localization experiments, cells were plated in six-well dishes and treated accordingly. After treatment, cells were fixed in 3.7% paraformaldehyde, permeabilized in 0.2% Triton X-100 and 10% fetal bovine serum (FBS) in phosphate-buffered saline (PBS) for 10 min, and incubated with p21Cip1- or p27Kip1-specific antibodies (1:100 dilution) or normal immunoglobulin G (IgG) for 12 h at 4°C. Cells were washed three times in PBS with 10% FBS and stained with fluorophore-conjugated anti-rabbit (1:200 dilution; Molecular Probes) for 30 min. All antibody solutions were prepared in 10% FBS in PBS. For transfection experiments, cells were pulsed with BrdU for 10 h prior to fixation. Cells were fixed and permeabilized as described for cyclin kinase inhibitor (CKI) localization experiments, and cellular DNA was denatured by incubation with DNase I (100 U/ml; Gibco) for 1 h at 37°C. BrdU-positive cells were stained with fluorophore-conjugated anti-BrdU (Molecular Probes) for 12 h at 4°C. Antigen staining was visualized by inverted-fluorescence microscopy (Olympus CK40), and images were captured with a Quality Imaging camera and software. Exposure times were kept constant for each experiment.
RESULTS
E7 overcomes Raf-induced growth arrest.
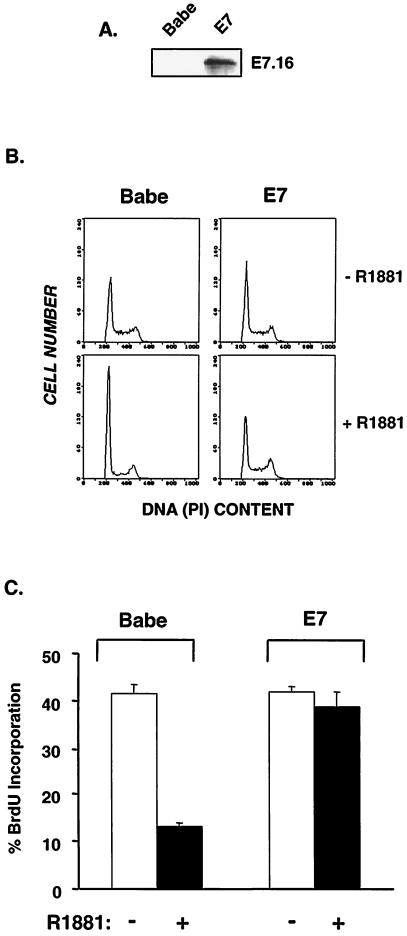
The E7 oncoprotein from high-risk HPV-16 can transform primary fibroblasts in cooperation with oncogenic Ras (52, 69). Activation of the Ras/Raf/mitogen-activated protein kinase (MAPK) pathway induces cell cycle arrest (29, 36, 37, 56, 64, 65, 72), indicating that E7 must impair Ras-induced arrest. To explore the molecular basis for E7-mediated bypass of Ras-induced arrest, we utilized a conditional RafAR molecule in which an activated Raf kinase was fused to the androgen receptor hormone-binding domain (65). Importantly, Raf acts directly downstream of Ras (42, 68), and Ras effector loop mutants that preferentially activate Raf (but not phosphatidylinositol 3-kinase [PI 3-K] or Ral.GDS) confer growth arrest similar to that conferred by oncogenic Ras (36). Additionally, Raf renders growth and morphological phenotypes similar to those of Ras in primary and immortal fibroblasts (36, 65). In order to assess the effects of E7 in this system, RafAR-expressing NIH 3T3 cells were infected with the retroviral vector pBabe (43) or its derivative encoding HPV-16 E7. Infected cells were pooled, examined for E7 expression (Fig. 1A), and used for subsequent experiments. Activation of RafAR with 1.0 μM R1881 led to morphological changes, including elongation and development of extended processes (data not shown). These RafAR-induced alterations in cell morphology, which are consistent with previous descriptions in NIH 3T3 and other cell types (36, 37, 65), were not affected in E7-expressing cells, indicating that at least some components of Raf signaling are not disrupted by the presence of E7. Upon examination of cell proliferation, RafAR activation led to G1 arrest in control cells (Babe), with >85% of cells accumulating in G1 (Fig. 1B). This was accompanied by inhibition of DNA synthesis as shown in Fig. 1C. However, cells expressing E7 continued cell cycle progression (Fig. 1B) and DNA synthesis (Fig. 1C) in the presence of activated RafAR. Similar observations have been made in separately generated clonal and pooled E7-expressing cell lines. These results indicate that E7 perturbs Raf-induced G1 arrest.
FIG. 1.
E7 abolishes RafAR-induced arrest. (A) NIH 3T3 cells stably expressing the RafAR fusion protein were infected with amphotrophic retroviruses expressing HPV-16 E7 (E7) or empty vector (Babe). Lysates were prepared, and 100 μg of protein was resolved by SDS-15% PAGE, transferred to nitrocellulose membrane, and probed with a monoclonal antibody directed against HPV-16 E7. (B and C) Pools of Babe- or E7-expressing cells growing in DMEM plus 10% NCS were treated with 0.02% ethanol (−) or 1.0 μM R1881 (+) for 30 h and pulsed with BrdU for 30 min. Cells were trypsinized, fixed in 70% ethanol, and stained with propidium iodide (PI) for detection of total DNA content (B) and with anti-BrdU-fluorescein isothiocyanate for detection of DNA synthesis (C).
E7 prevents inactivation of cyclin E-CDK2 by p21Cip1.
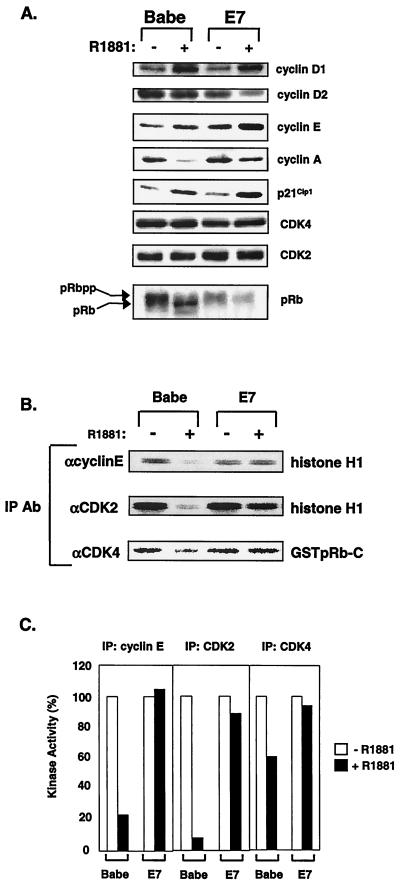
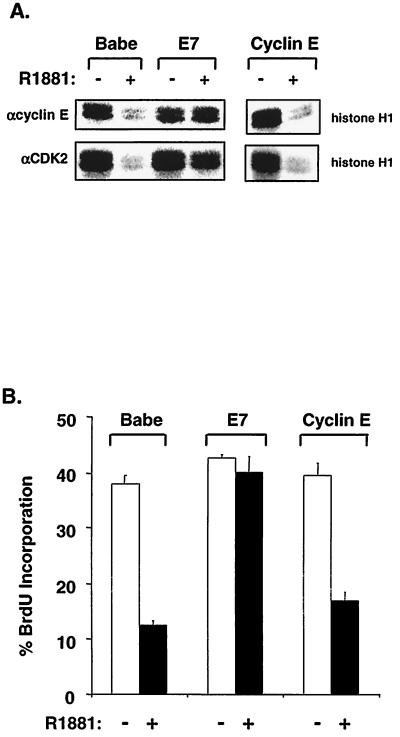
In NIH 3T3 fibroblasts, Raf activation leads to inhibition of DNA synthesis preceded by increased expression of p21Cip1 and loss of cyclin E-CDK2 kinase activity (65, 72). To determine the nature of resistance to RafAR-induced arrest in E7-expressing cells, we examined the expression levels and activities of G1-specific cyclins, CDKs, and p21Cip1. Activation of RafAR led to induction of cyclin D1 and cyclin E in control and E7-expressing cells (Fig. 2A). Importantly, p21Cip1 was also elevated in both cell lines upon RafAR stimulation (Fig. 2A). Consistent with observations in other p21Cip1-dependent arrest systems (21, 31, 61), this implies that E7 does not overcome Raf-induced arrest by preventing p21Cip1 expression. In RafAR-arrested control cells, stimulation of RafAR resulted in the loss of steady-state cyclin A and accumulation of hypophosphorylated RB (Fig. 2A). Cyclin E-CDK2 activity is required for hyperphosphorylation of RB and cyclin A expression (60, 71, 74). In accordance, control cells exhibited significant loss of cyclin E-CDK2 activity and a less dramatic decrease in CDK4-associated kinase activity (Fig. 2B and C), likely due to a greater sensitivity of CDK2 to inhibition by Kip/Cip CKIs (8, 33, 53). In contrast, cyclin E-CDK2 and CDK4 kinase activities were maintained upon RafAR stimulation of E7-expressing cells (Fig. 2B and C). In addition, cyclin A expression and RB hyperphosphorylation were similar in asynchronous and RafAR-activated cells in the presence of E7. Since biochemical and genetic approaches have demonstrated that p21Cip1 is an essential mediator of Raf-induced arrest (65, 72), these results suggest that E7 overcomes RafAR-induced arrest by abolishing the CDK2-inhibitory function of p21Cip1.
FIG. 2.
E7 prevents p21Cip1-mediated inhibition of cyclin E-CDK2 activity. (A) Expression levels of proteins involved in regulating the G1-to-S transition were examined during RafAR activation in the presence or absence of E7. Lysates were prepared from Babe- or E7-expressing cells treated with 0.02% ethanol (−) or 1.0 μM R1881 (+) for 30 h, and 30 μg of protein was resolved by SDS-12% PAGE, transferred to nitrocellulose membrane, and probed with antibodies to the indicated proteins. (B and C) Kinase activities of cyclin E-, CDK2-, and CDK4-associated complexes were assessed during RafAR activation. Lysates (50 μg) prepared as described for panel A were immunoprecipitated with the indicated antibodies, and immune complexes were collected on protein A-Sepharose beads and assayed for kinase activity using histone H1 (for cyclin E-CDK2) and GST-RB C terminus (for CDK4) as substrates (B). Quantitation of relative kinase activities in panel B is represented as a percentage of kinase activity from vehicle only (C).
E7 does not derepress p21Cip1-associated CDK2 activity.
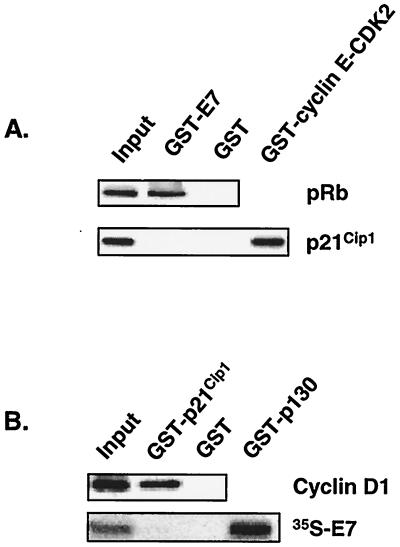
E7 has been shown to bypass several p21Cip1-mediated G1 arrest signals, and a model has been proposed stipulating that p21Cip1-associated CDK2 complexes are derepressed via a p21Cip1-E7 interaction (21). However, others have reported that E7 does not associate with p21Cip1 (28, 61). We explored the possibility that E7 derepresses p21Cip1-cyclin E-CDK2 complexes within the context of Raf signaling by testing two key predictions: (i) E7 should interact with p21Cip1 in cell lysates, and (ii) p21Cip1 should be associated with active cyclin E-CDK2 complexes. To test the first corollary of this model, we utilized standard co-IPs with E7- and p21Cip1-specific antibodies. No interaction between E7 and p21Cip1 was detected (data not shown). However, E7 expression is low in this system, raising the possibility that the putative E7-p21Cip1 interaction was not detected due to technical limitations. To confirm the co-IP results, purified recombinant GST-E7 was mixed with control cell lysates and precipitated complexes were examined for the presence of p21Cip1 by Western blot analysis. p21Cip1 was not found in GST-E7 precipitates (Fig. 3A). As controls, p21Cip1 was detected in GST-cyclin E-CDK2 precipitates, and RB was associated with GST-E7. In the reciprocal experiment, GST-p21Cip1 was mixed with cell lysate and an excess of radiolabeled E7. No p21Cip1-E7 interaction was observed, while GST-p130-35S-E7 and GST-p21Cip1-cyclin D1 complexes were detected (Fig. 3B). These results suggest that E7 does not associate with p21Cip1 in this system.
FIG. 3.
p21Cip1 does not associate with E7 in C4 cells. Lysate was prepared from Babe-transduced cells treated with 1.0 μM R1881 for 30 h. The indicated purified GST fusion proteins were incubated with lysate (A) or lysate supplemented with radiolabeled 35S-E7 (B) for 12 h at 4°C. Coprecipitated proteins were separated by SDS-12% PAGE and transferred to a nitrocellulose membrane, and the indicated proteins were detected with the appropriate antibodies (both gels in panel A and top gel in panel B) or by phosphorimager analysis (B, bottom gel). Input lanes represent 10% of extract or 35S-E7 used per reaction.
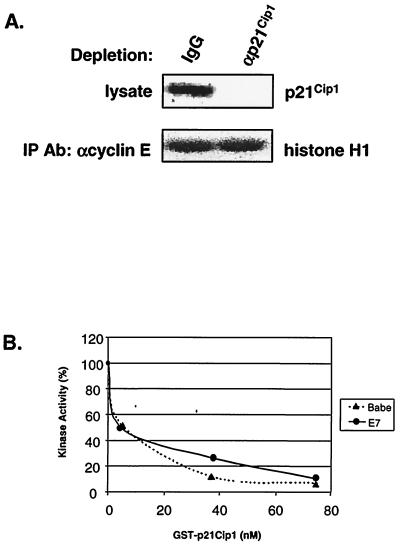
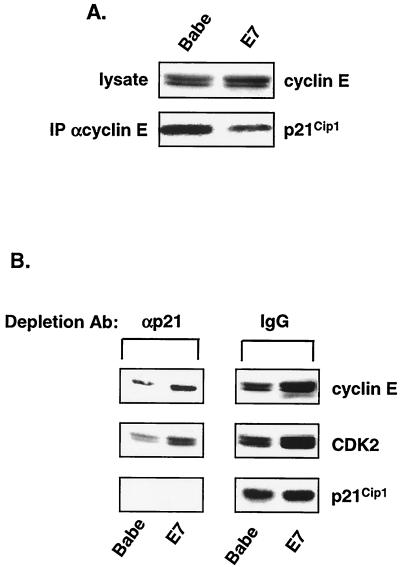
In order to address the second corollary that p21Cip1 is associated with active cyclin E-CDK2 in E7-expressing cells, an immunodepletion approach was used. We asked if depletion of p21Cip1-containing complexes from cell lysates would reduce the level of remaining cyclin E-CDK2 activity. To this end, RafAR-induced E7-expressing cell lysates were subjected to three rounds of immunodepletion with control or p21Cip1-specific antibodies shown previously to precipitate all known cyclin-CDK-p21Cip1 complexes (7). p21Cip1 was efficiently depleted as monitored by Western blot analysis (Fig. 4A). Cyclin E-CDK2 complexes were then immunoprecipitated from depleted lysates and assessed for kinase activity. p21Cip1-specific immunodepletion reduced p21Cip1 levels by >95% but did not alter the residual cyclin E-CDK2 kinase activity (Fig. 4A), indicating that any depleted p21Cip1-cyclin E-CDK2 complexes were inactive and that E7 does not derepress p21Cip1-associated CDK2 in RafAR-activated cells. In addition, cyclin E and CDK2 are not rendered intrinsically resistant to p21Cip1 by E7, as cyclin E-associated kinase activity in lysates of control and E7-expressing cells was equally sensitive to inhibition by purified recombinant GST-p21Cip1 (Fig. 4B).
FIG. 4.
E7 does not derepress p21Cip1-associated cyclin E-CDK2. (A) p21Cip1 is not associated with active cyclin E-CDK2 in the presence of E7. Whole-cell lysates prepared from RafAR-induced E7-expressing cells were subjected to three rounds of immunodepletion with normal rabbit IgG or p21Cip1-specific antibodies (Ab). Depleted lysates were analyzed by Western blotting with an antibody specific for p21Cip1 (top gel). IPs were performed on depleted lysates with a cyclin E-specific antibody and assayed for histone H1 kinase activity (bottom gel) as described in Fig. 2 legend. (B) E7 does not render cyclin E-CDK2 resistant to p21Cip1. Lysates (20 μg) from asynchronous Babe (▴) or E7 (♦) cells were mixed with increasing concentrations of purified recombinant GST-p21Cip1 and assayed for cyclin E-associated histone H1 kinase activity. Initial activity is represented as 100%.
Enhanced expression of cyclin E does not overcome RafAR-induced arrest.
Expression of E7 increased cyclin E protein levels approximately 2.5- to 3-fold (Fig. 2A), which is consistent with the ability of E7 to dysregulate RB-E2F transcriptional regulation of the cyclin E gene (21, 73). This effect is independent from and additive to the RafAR-induced elevation of cyclin E (Fig. 2A). Since E7 did not alter the intrinsic sensitivity of cyclin E-CDK2 to p21Cip1, we considered that dysregulation of cyclin E expression by E7 may be sufficient to overcome RafAR-induced, p21Cip1-mediated arrest. This scenario was examined by stable expression of human cyclin E in RafAR-NIH 3T3. Exogenous human cyclin E associates with and activates endogenous murine CDK2 (1, 67), as human cyclin E-specific antisera precipitated robust levels of kinase activity (Fig. 5A, top row). However, retroviral expression of cyclin E did not abolish RafAR-induced arrest (Fig. 5B). In addition, IP of human cyclin E complexes or total endogenous CDK2 revealed that exogenous cyclin E expression did not prevent p21Cip1-mediated inhibition of cyclin E-CDK2 (Fig. 5A), suggesting that the activity of E7 in this system is not defined solely by induction of cyclin E levels. These observations are consistent with other reports that arrest imposed by Kip/Cip CKIs cannot be overcome by elevated physiological accumulation of cyclin E (1, 50).
FIG. 5.
Enhanced cyclin E expression cannot overcome RafAR-induced arrest. (A) Cyclin E-CDK2 activity was not restored by exogenous cyclin E expression. NIH 3T3 cells stably expressing the RafAR fusion protein were transduced with constructs expressing empty vector (Babe), HPV-16 E7 (E7), or human cyclin E (Cyclin E). Pools of infected cells were treated with 0.02% ethanol (−) or 1.0 μM R1881 (+) for 30 h and assessed either for cyclin E-associated (upper gels) or CDK2-associated (bottom gels) histone H1 kinase activity. An antibody specifically recognizing human cyclin E was utilized for immunoprecipitating cyclin E-associated kinase activity (top right gel) from cells expressing human cyclin E. (B) Exogenous cyclin E expression cannot abolish RafAR-induced arrest. Cell lines were treated as described for panel A and assessed for DNA synthesis via BrdU incorporation.
E7 alters the stoichiometry between p21Cip1 and cyclin E-CDK2.
Upon RafAR activation, cells expressing E7 maintained cyclin E-CDK2 activity (Fig. 2A) in complexes free of p21Cip1 (Fig. 4A), suggesting that E7 may prevent the association of p21Cip1 with a pool of cyclin E-CDK2 complexes. Equal amounts of p21Cip1 were associated with cyclin E-immunoprecipitates in RafAR-induced control and E7-expressing cells (data not shown). However, since there is a significant increase of cyclin E steady-state levels in E7-expressing cells (Fig. 2A), this observation would imply that the stoichiometry of p21Cip1 and cyclin E-CDK2 is altered in the presence of E7. To illustrate this contention more clearly, cyclin E-containing complexes were immunoprecipitated from RafAR-induced control or E7 cell lysate, standardizing on the level of cyclin E expression (Fig. 6A, top row). As seen in Fig. 6A (bottom row), cyclin E-associated p21Cip1 was significantly lower in E7-expressing cells. This suggests that E7 expression may lead to accumulation of p21Cip1-free cyclin E-CDK2 complexes. In order to examine this hypothesis more directly, RafAR-induced control or E7 cell lysates were depleted with p21Cip1-specific antisera as described for Fig. 4A. Mock or p21Cip1-depleted lysates were subsequently analyzed for remaining cyclin E, CDK2, and p21Cip1. The levels of cyclin E and CDK2 were significantly reduced by the p21Cip1-specific antisera in both cell types (Fig. 6B, left column). This indicates that a substantial quantity of CDK2 complexes is associated with p21Cip1, consistent with previous reports (7, 75, 76). However, E7-expressing cell lysates retained approximately threefold more cyclin E and CDK2 than the control counterparts in p21Cip1-depleted lysates. Since data from Fig. 4A demonstrate that cyclin E-associated kinase activity is not associated with p21Cip1, the increased pool of p21Cip1-free cyclin E-CDK2 in E7 cells (Fig. 6B) is likely responsible for E7-specific maintenance of cyclin E-CDK2 activity during RafAR activation (Fig. 2B and C). Altogether, these observations indicate that the presence of E7 hinders p21Cip1 association with and inhibition of cyclin E-CDK2 complexes in RafAR-activated cells.
FIG. 6.
E7 alters p21Cip1/cyclin E-CDK2 stoichiometry. (A) Lysates were prepared from Babe- or E7-expressing cells treated with 1.0 μM R1881 for 30 h. The amount of lysate used was standardized to cyclin E steady-state levels as determined by Western blotting (top gel). Cyclin E was immunoprecipitated and immune complexes were analyzed for cyclin E-associated p21Cip1 by Western blotting with a p21Cip1-specific antibody (bottom gel). (B) Lysates prepared from RafAR-induced Babe- or E7-expressing cells were subjected to three rounds of immunodepletion with normal rabbit IgG or p21Cip1-specific antibodies (Ab). Depleted lysates were analyzed by Western blotting with antibodies specific for cyclin E (top gels), CDK2 (middle gels), or p21Cip1 (bottom gels).
E7 prevents CKI nuclear accumulation.
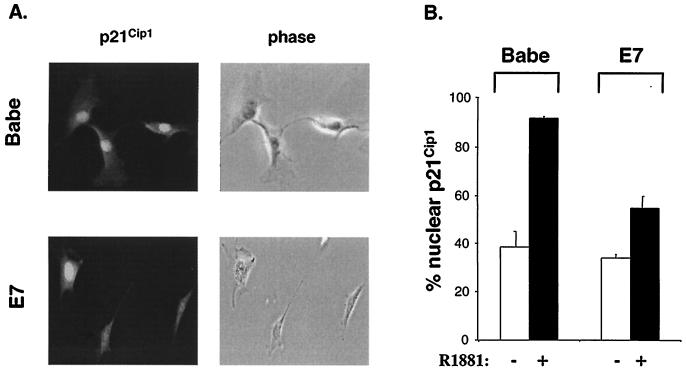
Induction of p21Cip1 expression by p53 or other antimitogenic stimuli is accompanied by its nuclear accumulation. Since the localization of p21Cip1 is considered to be important in its function as an inhibitor of proliferation (22, 66), the effects of RafAR on p21Cip1 cellular localization were examined. Upon staining with p21Cip1-specific antibodies, a similar fraction (∼40%) of control and E7-expressing cells exhibited strong nuclear fluorescence (Fig. 7). This observation is consistent with reports that p21Cip1 localizes to the nucleus during mid-G1 (14), as 50 to 55% of asynchronous cells were in G1 (Fig. 1B). Interestingly, induction of RafAR resulted in a dramatic increase in p21Cip1 nuclear accumulation, with >90% of control cells showing nuclear p21Cip1-staining (Fig. 7). This observation suggests that Raf signaling activates p21Cip1 function by regulating its cellular localization as well as increasing its synthesis. Notably, E7 expression markedly reduced the RafAR-specific nuclear localization of p21Cip1 (Fig. 7), without altering induction of p21Cip1 expression (Fig. 2A). As cyclin E is primarily a nuclear protein (47), these results are consistent with the idea that E7 prevents p21Cip1 association with cyclin E-CDK2 by inhibiting RafAR-induced nuclear compartmentalization of p21Cip1.
FIG. 7.
E7 impairs RafAR-induced p21Cip1 nuclear accumulation. Babe- or E7-expressing cells were treated with 0.02% ethanol (−) or 1.0 μM R1881 for 30 h, fixed with 3.7% paraformaldehyde, and stained with p21Cip1 specific antibody as described in Materials and Methods. Cells were scored for nuclear or whole-cell localization of p21Cip1. (A) Representative fluorescent (left) and phase-contrast (right) micrographs of RafAR-induced Babe- (top row) or E7-expressing cells (lower row) are shown. (B) Quantitation of cells exhibiting nuclear localization of p21Cip1. The number of cells with nuclear p21Cip1 accumulation is represented as a percentage of total cells counted. The average and deviation values shown are from two independent experiments with at least 200 cells counted per experiment.
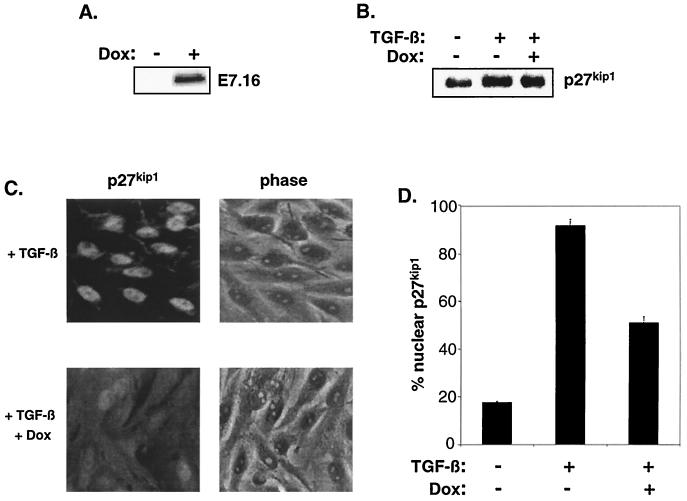
Since E7 has also been shown to abolish the CKI function of the related p27Kip1 during cellular arrest (63) (data not shown), we examined if mislocalization was a more general strategy by which E7 impinges on CKI function. TGF-β signaling leads to p27Kip1-mediated inhibition of cyclin E-CDK2 and cell cycle arrest in the epithelial cell line Mv1Lu (55). We investigated the effects of E7 on p27Kip1 localization in the context of TGF-β utilizing an Mv1Lu derivative that expresses E7 in response to doxycycline (Fig. 8A). Consistent with previous observations (12), E7 prevented the arrest imposed by TGF-β (data not shown). Interestingly, TGF-β induced a robust nuclear accumulation of p27Kip1 that was alleviated in the presence of E7 (Fig. 8C and D). E7 altered p27Kip1 localization without affecting expression levels of the CKI (Fig. 8B). We have also observed this mislocalization of p27Kip1 by E7 during growth factor deprivation in fibroblasts (data not shown). These results suggest that E7 hinders Kip/Cip CKI function through a conserved mechanism (mislocalization) and in response to multiple antimitogenic signals.
FIG. 8.
E7 diminishes TGF-β-induced p27Kip1 nuclear localization. (A) tet-E7 Mv1Lu cells were treated for 24 h with 2 μg of doxycycline (Dox) or vehicle per ml. Cell lysates were analyzed by Western blotting with antibody specific for HPV-16 E7. (B) tet-E7 Mv1Lu cells were treated for 24 h with 3 ng of TGF-β/ml in the presence or absence of 2 μg of doxycycline/ml and subsequently analyzed for p27Kip1 expression via Western blot. (C and D) tet-E7 Mv1Lu cells were treated as described for panel B, fixed with 3.7% paraformaldehyde, and stained with p27Kip1 specific antibody as described in Materials and Methods. (C) Representative fluorescent (left) and phase-contrast (right) micrographs of TGF-β-treated cells in the absence (top) or presence (bottom) of doxycycline. (D) Quantitation of cells exhibiting nuclear localization of p27Kip1. The number of cells with nuclear p27Kip1 accumulation is represented as a percentage of total cells counted. The average and deviation values shown are from two independent experiments with at least 200 cells counted per experiment.
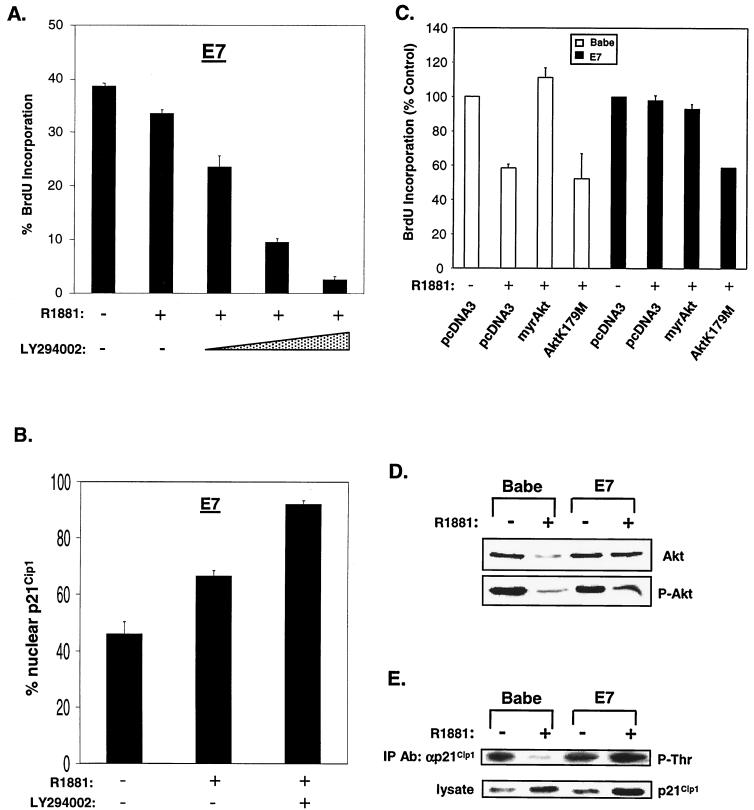
The PI 3-K/Akt pathway is required for E7-mediated abolition of RafAR-induced arrest.
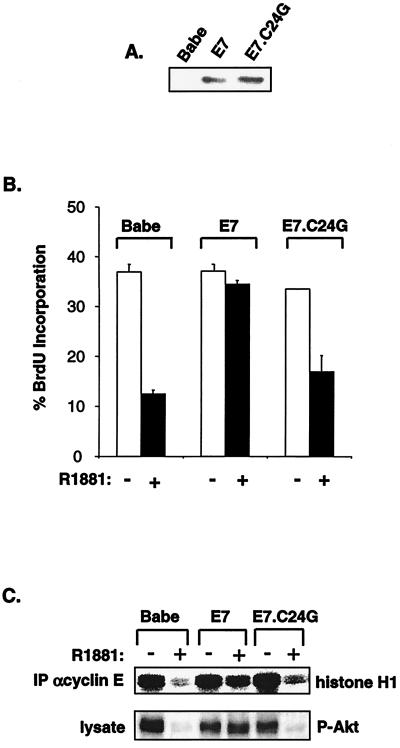
p21Cip1 contains a bipartite nuclear localization sequence (NLS) in its C terminus (22). Mutation of the NLS reduces the capacity of p21Cip1 to inhibit CDK activity and cellular proliferation (59, 66, 77). Recently, Akt has been shown to phosphorylate threonine-145 within the p21Cip1 NLS, leading to cytoplasmic localization of p21Cip1 (77). In accordance, inhibition of Akt or its activator, PI 3-K, results in reduced p21Cip1 phosphorylation, promoting nuclear accumulation of p21Cip1 and growth arrest (77). In order to assess the role of Akt in E7-mediated abolition of RafAR-induced arrest, RafAR was activated in E7-expressing cells in the presence or absence of LY294002, an inhibitor of PI 3-K. As previously demonstrated, E7-expressing cells continue through the G1-to-S transition following RafAR activation. However, cell cycle progression of RafAR-induced, E7-expressing cells was diminished in the presence of LY294002 (Fig. 9A). Incubation with LY294002 also restored Raf-induced nuclear accumulation of p21Cip1 (Fig. 9B), suggesting that PI 3-K/Akt activity is required for E7 to impair p21Cip1 localization and overcome RafAR-induced G1 arrest. Because inhibition of PI 3-K could have pleiotropic effects beyond the specific activity of Akt, we further explored the role of Akt in this system by utilizing a dominant-negative mutant of Akt, Akt K179 M. RafAR-activated control or E7-expressing cells were transiently transfected with vector or Akt K179 M expression plasmids in conjunction with a plasmid encoding GFP. DNA synthesis of the transfected, GFP-positive cells was measured by BrdU incorporation. As shown in Fig. 9C, introduction of Akt K179 M reduced BrdU incorporation of RafAR-induced E7-expressing cells to a level similar to that for RafAR-induced control cells, indicating that Akt activity is required for E7-mediated bypass of RafAR arrest. In addition, Akt activity appears sufficient to rescue G1-to-S progression during RafAR signaling, since transfection of RafAR-activated control cells with a myristoylated, constitutively active form of Akt restored BrdU incorporation to asynchronous levels (Fig. 9C). These results suggest that Akt antagonizes Raf-induced arrest. Consequently, we examined the effects of RafAR signaling on the status of Akt using antibodies that detect total or serine-473 phosphorylated (active) Akt. In control cells, the steady-state levels of total and active Akt were decreased upon RafAR activation by 66 and 79%, respectively (Fig. 9D). This RafAR-induced reduction in Akt activity correlated with a significant decrease in threonine-phosphorylated p21Cip1 despite elevated levels of total p21Cip1 (Fig. 9E). In contrast, E7-expressing cells maintained total and activated Akt at or near asynchronous levels and exhibited a modest increase in threonine-phosphorylated p21Cip1 upon RafAR activation (Fig. 9D and E). Taken together, these observations suggest that RafAR signaling may converge on p21Cip1 in two ways: inducing transcription of p21Cip1 and stimulating p21Cip1 nuclear accumulation via negative regulation of Akt. While E7 does not interfere with RafAR-specific expression of p21Cip1, the ability of RafAR to induce Akt down-regulation and p21Cip1 nuclear localization is prevented by E7, consistent with the idea that maintenance of Akt is important in E7-mediated cell cycle progression in the presence of RafAR activation. This hypothesis is further supported by analysis of the E7.C24G point mutation within the context of the RafAR system. Residue 24 resides in the LXCXE motif of E7 and is essential for the E7-RB interaction (3, 44). Importantly, it has been demonstrated that mutation of the LXCXE motif disrupts the ability of E7 to cooperate with an activated Ras pathway in cellular transformation (2, 18, 51). As represented in Fig. 10B, RafAR-NIH 3T3 cells stably expressing E7.C24G were as sensitive to RafAR-induced inhibition of DNA synthesis as cells transduced with empty retrovirus, although the E7- and E7.C24G-expressing cell lines expressed comparable levels of E7 as determined by Western blot analysis (Fig. 10A). This suggests that the LXCXE motif is essential in E7-mediated abolition of RafAR-induced arrest. In contrast to cells expressing wild-type E7, E7.C24G-expressing cells exhibited RafAR-induced nuclear accumulation of p21Cip1 (data not shown) and were incapable of maintaining active Akt and cyclin E-associated kinase activity upon RafAR induction (Fig. 10C). Altogether, these observations support the hypothesis that persistence of Akt activity may play a role in the ability of E7 to abolish p21Cip1 function and RafAR-induced arrest.
FIG. 9.
The PI 3-K/Akt pathway is involved in E7-mediated abolition of RafAR-induced arrest. (A) E7-expressing cells were treated for 30 h with 1.0 μM R1881 in the absence or presence of increasing concentrations of LY294002 (50, 100, and 200 μM), an inhibitor of PI 3-K activity. DNA synthesis was measured via BrdU incorporation. (B) E7-expressing cells were treated with 1.0 uM R1881 in the presence or absence of 100 μM LY294002, stained with p21Cip1-specific antibody, and quantitated for nuclear p21Cip1 localization as for Fig. 7. (C) Babe- or E7-expressing cells were cotransfected with a GFP expression plasmid (400 ng) and the indicated plasmids (3.2 μg). After transfection, cells were treated with 0.02% ethanol or 1.0 mM R1881 for 30 h, with BrdU added for the last 10 h of treatment. Cells were stained with anti-BrdU, and GFP-positive cells were scored for BrdU incorporation via indirect immunofluorescence. Percentages of BrdU incorporation were calculated by defining the number obtained from vehicle-treated cells as 100%. The average and deviation values shown are from three independent experiments with at least 150 GFP-positive cells counted per experiment. Low deviation values for some samples could not be resolved for this figure. (D) Lysates were prepared from Babe- or E7-expressing cells treated with 0.02% ethanol (−) or 1.0 μM R1881 (+) for 30 h and analyzed by Western blotting with antibodies directed against total Akt (top gel) or Akt phosphorylated on serine 473 (P-Akt), representing the active form of Akt. (E) Lysates prepared from Babe- or E7-expressing cells treated as described for panel D were subjected to IP with an antibody (Ab) specific for p21Cip1. Precipitated p21Cip1 was analyzed for threonine phosphorylation by Western blotting with a phosphothreonine-specific antibody (top panel). p21Cip1 expression was analyzed in lysates used for above IPs by Western blotting with a p21Cip1-specific antibody (bottom panel).
FIG. 10.
The LXCXE motif of E7 is necessary to prevent p21Cip1-mediated inhibition of cyclin E-CDK2. (A) NIH 3T3 cells stably expressing the RafAR fusion protein were transduced with constructs expressing empty vector (Babe), E7, or E7.C24G. Lysates were prepared and examined for E7 expression with a monoclonal antibody directed against HPV-16 E7. (B) Pools of infected cells were treated with 0.02% ethanol (−) or 1.0 μM R1881 (+) for 30 h and assessed for DNA synthesis via BrdU incorporation. Low deviation values for some samples could not be resolved for this figure. (C) Babe-, E7-, or E7.C24G-expressing cells were treated as described for panel A and assessed for cyclin E-associated histone H1 kinase activity (top panel) or cellular levels of active Akt by Western blotting with an antibody specifically recognizing Akt phosphorylated on serine 473 (P-Akt, bottom gel).
DISCUSSION
E7 alters p21Cip1 localization.
Oncogenic activation of the Ras/Raf/MAPK pathway can lead to a p21Cip1-dependent cell cycle arrest (37, 65, 72). HPV-16 E7 transforms primary cells in cooperation with Ras and abolishes growth arrest elicited by various antimitogenic signals that induce p21Cip1 expression (12, 52, 69). In the present study, we demonstrate that HPV-16 E7 abolishes the CDK-inhibitory function of p21Cip1 in response to Raf activation by inhibiting its nuclear accumulation. Furthermore we show that Akt, a regulator of p21Cip1 localization, is required for the ability of E7 to impair p21Cip1 nuclear accumulation and Raf-induced arrest. The ability to impinge on p21Cip1 function is conserved in several Ras-cooperating oncogenes. However, these oncogenes target p21Cip1 by differing mechanisms. For instance, SV40 large T antigen interacts with and inactivates the p53 tumor suppressor, a transcriptional activator of p21Cip1 (15, 19). In rat Schwann cells, expression of large T antigen prevents Raf-induced, p53-mediated expression of p21Cip1, resulting in a mitogenic cellular response to Raf (37). Alternatively, other Ras-cooperating oncogenes do not affect the expression or accumulation of p21Cip1. Myc activates expression of factors that sequester p21Cip1 and the closely related p27Kip1 (49, 67). In the context of Raf signaling, Myc restores cyclin E-CDK2 activity by inducing expression of cyclin E and cyclin D2, with cyclin D-CDK complexes sequestering p21Cip1 and cyclin E feeding a p21Cip1-free pool of activable cyclin E-CDK2 (4, 49). Similar to Myc, E7 induces synthesis of cyclin E (Fig. 2A) (21, 73). Increased levels of cyclin E may elevate the pool of total cyclin E-CDK2 in E7-expressing cells but, similar to previous observations (50), is insufficient to bypass Raf-induced arrest or restore cyclin E-CDK2 activity (Fig. 5A and B).
Our experiments support a novel mechanism by which E7 prevents Raf-induced, p21Cip1-mediated inhibition of cyclin E-CDK2. We show that Raf activation enhances the nuclear localization of p21Cip1 (Fig. 7) and that E7 expression reduces this Raf-specific p21Cip1 nuclear accumulation. Since p21Cip1 is thought to establish a regulatory threshold that must be overcome for CDK2 activation (7, 25, 27), we predict that a reduction in nuclear p21Cip1 would effectively lower the “local threshold” of p21Cip1 in the nuclear compartment. This is consistent with the observation that E7-expressing cells exhibited an increased pool of p21Cip1-free cyclin E-CDK2 despite Raf-induced elevation in p21Cip1 levels (Fig. 6A and B). Previously, a model was proposed in which E7 abolishes p21Cip1 function by directly interacting with and derepressing p21Cip1-associated CDK2 complexes (21, 31). However, our experiments do not support such a model. Interaction between E7 and p21Cip1 was not observed in the context of Raf signaling (Fig. 3) and could not be detected in other cell types (data not shown) (28, 61). In addition, immunodepletion experiments indicate that p21Cip1-associated cyclin E-CDK2 complexes are not active in the presence of E7 (Fig. 4A), suggesting that E7 does not physically derepress p21Cip1-cyclin E-CDK2 complexes. Furthermore, we have shown that the intrinsic sensitivity of cyclin E-CDK2 to p21Cip1-mediated inhibition is not altered in E7-expressing cells (Fig. 4B). Taken together, these data imply that E7 impinges on p21Cip1 function via an alternative mechanism.
The ability of E7 to reduce nuclear accumulation of p21Cip1 and prevent inhibition of cyclin E-CDK2 suggests that p21Cip1 localization affects its regulation of CDK activity. Indeed, mutation of the nuclear localization sequence of p21Cip1 reduces its growth-inhibitory function (22, 77). Interestingly, subcellular location is an important aspect of other CKI function. For instance, the coordinated inhibition of CDK4 and CDK2 by p15Ink4b and p27Kip1 requires proper compartmentalization of both CKIs within the cell (54). We have also shown that E7 affects the nuclear localization of p27Kip1 upon serum withdrawal (data not shown) or TGF-β (Fig. 8), suggesting that the ability of E7 to mislocalize Kip/Cip CKIs is conserved in the context of other biological signals.
Akt has recently been shown to regulate p21Cip1 and p27Kip1 localization via phosphorylation of their respective NLSs (77) (J. M. Slingerland, personal communication). Interestingly, E7 prevents Raf-specific reduction in Akt levels and p21Cip1 phosphorylation (Fig. 9D), suggesting that E7 alters localization of p21Cip1 by targeting Akt. In accordance, inhibition of the PI 3-K/Akt signaling pathway abolished the effects of E7 on p21Cip1 localization (Fig. 9B). Further experiments are required to determine the role of Akt in E7-mediated bypass of other p21Cip1- and p27Kip1-associated arrest signals. However, Akt has been implicated in diverse contexts, including cell survival, proliferation, and transformation, making Akt an intriguing putative target of E7 (9, 10).
Antagonism between Ras effectors.
Ras transduces extracellular information through a multitude of signaling cascades. Raf and Akt, components of two “parallel” Ras signaling pathways, have been shown to interact, with Akt phosphorylating and negatively regulating Raf activity (57, 78). In this study, we provide evidence that Raf can functionally antagonize Akt. We demonstrate that Raf can down-regulate steady-state levels of total and active Akt (Fig. 9D). Importantly, transfection with a constitutively active Akt restored cell cycle progression during Raf signaling (Fig. 9C), indicating that loss of Akt activity may be essential in Raf-induced arrest. Consistent with this idea, expression of E7 maintained total and active Akt levels (Fig. 9D), and disruption of Akt activity prevented E7-mediated bypass of Raf arrest (Fig. 9A and C). Interestingly, an E7 mutant deficient in the ability to bind and disrupt RB does not maintain Akt activity (Fig. 10C), suggesting that RB may be involved in regulating Akt or factors that control Akt activity. However, the mechanism(s) by which Raf and E7 target Akt is presently unclear. Nevertheless, the observation that Raf down-regulates Akt function establishes precedent for bidirectional cross talk between these Ras effector pathways. The antagonism between Raf and Akt suggests that the cellular response elicited by simultaneous stimulation of Raf and Akt can be affected by the intensity and duration of the stimulation. For instance, anchorage detachment in primary fibroblasts leads to Raf-dependent anoikis (79). Activation of Raf in this context requires loss of Akt activity, and exogenously restored Akt activity can disrupt Raf activation and restore cell survival. Likewise, sustained Akt activity can prevent serum- and insulin-like growth factor 1-induced activation of the Raf/ERK signaling cascade, promoting myotube differentiation and hypertrophy (57, 78). Here we demonstrate that Raf activation leads to loss of Akt activity and that this may be an essential event in the cellular arrest imposed by Raf.
Cooperation between E7 and Ras signaling.
Activation of the Ras/Raf/MAPK pathway can stimulate transformation or cellular arrest, depending on the signaling intensity and presence or absence of cooperating oncogenic mutations (29, 35, 62, 65, 70, 72). In the present study, we have shown that the HPV-16 E7 oncoprotein abolishes growth arrest imposed by Raf activity, a signal that elicits growth and morphological phenotypes similar to those elicited by Ras in primary and immortal cells (36, 65). Consistent with these observations, E7 inhibits Ras-induced arrest and senescence and subsequently transforms primary cells in cooperation with activated Ras (52, 69). Raf-induced arrest is dependent on the CKI p21Cip1 (65, 72), and we demonstrate that E7 impairs p21Cip1 CDK-inhibitory function during Raf signaling. As previously mentioned, other oncogenes (e.g., Myc and SV40 large T antigen) that cooperate with Ras in cellular transformation also impair p21Cip1 function (15, 49). This convergence of function implies that inactivation of p21Cip1 may be an important mechanism by which oncogenic mutations alter the cellular response to the activated Ras/Raf pathway from growth arrest to proliferation. Indeed, genetic abolition of p21Cip1 confers a proliferative advantage in Ras-transduced fibroblasts and promotes aggressive Ras-induced epithelial tumorigenesis (41, 65). These observations suggest that p21Cip1 is critical in suppressing Ras-induced transformation. In light of our observations that E7 can abolish p21Cip1 function in the context of Raf signaling, this has important implications for HPV pathogenesis, since high-risk HPV infect and contribute to tumorigenesis in the cervical epithelium (34).
Acknowledgments
We thank E. Harlow, P. Coffer, R. Roth, R. Freeman, and B. Amati for plasmids and antibodies and M. O'Reilly for tet-E7 Mv1Lu cells. For feedback and support during the course of this work and on the manuscript, we acknowledge J. Zhao, S. Butler, S. Dewhurst, S. Huang, D. Patel, H. McMurray, L. Baglia, L. DeLeu, J. Mendler, and C. Westbrook. We are particularly grateful to H. Land for cell lines, reagents, and helpful discussions. We thank P. Keng for assistance with flow cytometry.
T.F.W. was the recipient of a fellowship on NAIAID Training Grant T32AIO7362. This work was supported by NIAID grant RO1AI30798.
REFERENCES
- 1.Alevizopoulos, K., J. Vlach, S. Hennecke, and B. Amati. 1997. Cyclin E and c-Myc promote cell proliferation in the presence of p16INK4a and of hypophosphorylated retinoblastoma family proteins. EMBO J. 16:5322-5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banks, L., C. Edmonds, and K. Vousden. 1990. Ability of the HPV16 E7 protein to bind RB and induce DNA synthesis is not sufficient for efficient transforming activity in NIH3T3 cells. Oncogene 5:1383-1389. [PubMed] [Google Scholar]
- 3.Barbosa, M. S., C. Edmonds, C. Fisher, J. T. Schiller, D. R. Lowy, and K. H. Vousden. 1990. The region of the HPV E7 oncoprotein homologous to adenovirus E1a and Sv40 large T antigen contains separate domains for Rb binding and casein kinase II phosphorylation. EMBO J. 9:153-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouchard, C., K. Thieke, A. Maier, R. Saffrich, J. Hanley-Hyde, W. Ansorge, S. Reed, P. Sicinski, J. Bartek, and M. Eilers. 1999. Direct induction of cyclin D2 by Myc contributes to cell cycle progression and sequestration of p27. EMBO J. 18:5321-5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyer, S. N., D. E. Wazer, and V. Band. 1996. E7 protein of human papilloma virus-16 induces degradation of retinoblastoma protein through the ubiquitin-proteasome pathway. Cancer Res. 56:4620-4624. [PubMed] [Google Scholar]
- 6.Burgering, B. M., and P. J. Coffer. 1995. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature 376:599-602. [DOI] [PubMed] [Google Scholar]
- 7.Cai, K., and B. D. Dynlacht. 1998. Activity and nature of p21(WAF1) complexes during the cell cycle. Proc. Natl. Acad. Sci. USA 95:12254-12259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng, M., P. Olivier, J. A. Diehl, M. Fero, M. F. Roussel, J. M. Roberts, and C. J. Sherr. 1999. The p21(Cip1) and p27(Kip1) CDK ′inhibitors' are essential activators of cyclin D-dependent kinases in murine fibroblasts. EMBO J. 18:1571-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coffer, P. J., J. Jin, and J. R. Woodgett. 1998. Protein kinase B (c-Akt): a multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem. J. 335:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Datta, S. R., A. Brunet, and M. E. Greenberg. 1999. Cellular survival: a play in three Akts. Genes Dev. 13:2905-2927. [DOI] [PubMed] [Google Scholar]
- 11.Davies, R., R. Hicks, T. Crook, J. Morris, and K. Vousden. 1993. Human papillomavirus type 16 E7 associates with a histone H1 kinase and with p107 through sequences necessary for transformation. J. Virol. 67:2521-2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demers, G. W., E. Espling, J. B. Harry, B. G. Etscheid, and D. A. Galloway. 1996. Abrogation of growth arrest signals by human papillomavirus type 16 E7 is mediated by sequences required for transformation. J. Virol. 70:6862-6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Demers, G. W., S. A. Foster, C. L. Halbert, and D. A. Galloway. 1994. Growth arrest by induction of p53 in DNA damaged keratinocytes is bypassed by human papillomavirus 16 E7. Proc. Natl. Acad. Sci. USA 91:4382-4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duliæ, V., G. H. Stein, D. F. Far, and S. I. Reed. 1998. Nuclear accumulation of p21Cip1 at the onset of mitosis: a role at the G2/M-phase transition. Mol. Cell. Biol. 18:546-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dyson, N., K. Buchkovich, P. Whyte, and E. Harlow. 1989. Cellular proteins that are targetted by DNA tumor viruses for transformation. Princess Takamatsu Symp. 20:191-198. [PubMed] [Google Scholar]
- 16.Dyson, N., P. Guida, K. Munger, and E. Harlow. 1992. Homologous sequences in adenovirus E1A and human papillomavirus E7 proteins mediate interaction with the same set of cellular proteins. J. Virol. 66:6893-6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dyson, N., P. M. Howley, K. Munger, and E. Harlow. 1989. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science 243:934-937. [DOI] [PubMed] [Google Scholar]
- 18.Edmonds, C., and K. H. Vousden. 1989. A point mutational analysis of human papillomavirus type 16 E7 protein. J. Virol. 63:2650-2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.el-Deiry, W. S., T. Tokino, V. E. Velculescu, D. B. Levy, R. Parsons, J. M. Trent, D. Lin, W. E. Mercer, K. W. Kinzler, and B. Vogelstein. 1993. WAF1, a potential mediator of p53 tumor suppression. Cell 75:817-825. [DOI] [PubMed] [Google Scholar]
- 20.Evan, G. I., A. H. Wyllie, C. S. Gilbert, T. D. Littlewood, H. Land, M. Brooks, C. M. Waters, L. Z. Penn, and D. C. Hancock. 1992. Induction of apoptosis in fibroblasts by c-myc protein. Cell 69:119-128. [DOI] [PubMed] [Google Scholar]
- 21.Funk, J. O., S. Waga, J. B. Harry, E. Espling, B. Stillman, and D. A. Galloway. 1997. Inhibition of CDK activity and PCNA-dependent DNA replication by p21 is blocked by interaction with the HPV-16 E7 oncoprotein. Genes Dev. 11:2090-2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goubin, F., and B. Ducommun. 1995. Identification of binding domains on the p21Cip1 cyclin-dependent kinase inhibitor. Oncogene 10:2281-2287. [PubMed] [Google Scholar]
- 23.Hanahan, D., and R. A. Weinberg. 2000. The hallmarks of cancer. Cell 100:57-70. [DOI] [PubMed] [Google Scholar]
- 24.Harper, J. W., G. R. Adami, N. Wei, K. Keyomarsi, and S. J. Elledge. 1993. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 75:805-816. [DOI] [PubMed] [Google Scholar]
- 25.Harper, J. W., S. J. Elledge, K. Keyomarsi, B. Dynlacht, L. H. Tsai, P. Zhang, S. Dobrowolski, C. Bai, L. Connell-Crowley, and E. Swindell. 1995. Inhibition of cyclin-dependent kinases by p21. Mol. Biol. Cell 6:387-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heck, D. V., C. L. Yee, P. M. Howley, and K. Munger. 1992. Efficiency of binding the retinoblastoma protein correlates with the transforming capacity of the E7 oncoproteins of the human papillomaviruses. Proc. Natl. Acad. Sci. USA 89:4442-4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hengst, L., U. Gopfert, H. A. Lashuel, and S. I. Reed. 1998. Complete inhibition of Cdk/cyclin by one molecule of p21(Cip1). Genes Dev. 12:3882-3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hickman, E. S., S. Bates, and K. H. Vousden. 1997. Pertubation of the p53 response by human papillomavirus type 16 E7. J. Virol. 71:3710-3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirakawa, T., and H. E. Ruley. 1988. Rescue of cells from ras oncogene-induced growth arrest by a second, complementing, oncogene. Proc. Natl. Acad. Sci. USA 85:1519-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hunter, T. 1991. Cooperation between oncogenes. Cell 64:249-270. [DOI] [PubMed] [Google Scholar]
- 31.Jones, D. L., R. M. Alani, and K. Munger. 1997. The human papillomavirus E7 oncoprotein can uncouple cellular differentiation and proliferation in human keratinocytes by abrogating p21Cip1-mediated inhibition of cdk2. Genes Dev. 11:2101-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kohn, A. D., F. Takeuchi, and R. A. Roth. 1996. Akt, a pleckstrin homology domain containing kinase, is activated primarily by phosphorylation. J. Biol. Chem. 271:21920-21926. [DOI] [PubMed] [Google Scholar]
- 33.LaBaer, J., M. D. Garrett, L. F. Stevenson, J. M. Slingerland, C. Sandhu, H. S. Chou, A. Fattaey, and E. Harlow. 1997. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 11:847-862. [DOI] [PubMed] [Google Scholar]
- 34.Laimins, L. A. 1993. The biology of human papillomaviruses: from warts to cancer. Infect. Agents Dis. 2:74-86. [PubMed] [Google Scholar]
- 35.Land, H., A. C. Chen, J. P. Morgenstern, L. F. Parada, and R. A. Weinberg. 1986. Behavior of myc and ras oncogenes in transformation of rat embryo fibroblasts. Mol. Cell. Biol. 6:1917-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin, A. W., M. Barradas, J. C. Stone, L. van Aelst, M. Serrano, and S. W. Lowe. 1998. Premature senescence involving p53 and p16 is activated in response to constitutive MEK/MAPK mitogenic signaling. Genes Dev. 12:3008-3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lloyd, A. C., F. Obermuller, S. Staddon, C. F. Barth, M. McMahon, and H. Land. 1997. Cooperating oncogenes converge to regulate cyclin/cdk complexes. Genes Dev. 11:663-677. [DOI] [PubMed] [Google Scholar]
- 38.Lowe, S. W., T. Jacks, D. E. Housman, and H. E. Ruley. 1994. Abrogation of oncogene-associated apoptosis allows transformation of p53-deficient cells. Proc. Natl. Acad. Sci. USA 91:2026-2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lowe, S. W., and H. E. Ruley. 1993. Stabilization of the p53 tumor suppressor is induced by adenovirus 5 E1A and accompanies apoptosis. Genes Dev. 7:535-545. [DOI] [PubMed] [Google Scholar]
- 40.Missero, C., E. Calautti, R. Eckner, J. Chin, L. H. Tsai, D. M. Livingston, and G. P. Dotto. 1995. Involvement of the cell-cycle inhibitor Cip1/WAF1 and the E1A-associated p300 protein in terminal differentiation. Proc. Natl. Acad. Sci. USA 92:5451-5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Missero, C., F. Di Cunto, H. Kiyokawa, A. Koff, and G. P. Dotto. 1996. The absence of p21Cip1/WAF1 alters keratinocyte growth and differentiation and promotes ras-tumor progression. Genes Dev. 10:3065-3075. [DOI] [PubMed] [Google Scholar]
- 42.Moodie, S. A., B. M. Willumsen, M. J. Weber, and A. Wolfman. 1993. Complexes of Ras.GTP with Raf-1 and mitogen-activated protein kinase kinase. Science 260:1658-1661. [DOI] [PubMed] [Google Scholar]
- 43.Morgenstern, J. P., and H. Land. 1990. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 18:3587-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Munger, K., B. A. Werness, N. Dyson, W. C. Phelps, E. Harlow, and P. M. Howley. 1989. Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. EMBO J. 8:4099-4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nead, M. A., L. A. Baglia, M. J. Antinore, J. W. Ludlow, and D. J. McCance. 1998. Rb binds c-Jun and activates transcription. EMBO J. 17:2342-2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Noda, A., Y. Ning, S. F. Venable, O. M. Pereira-Smith, and J. R. Smith. 1994. Cloning of senescent cell-derived inhibitors of DNA synthesis using an expression screen. Exp. Cell Res. 211:90-98. [DOI] [PubMed] [Google Scholar]
- 47.Ohtsubo, M., A. M. Theodoras, J. Schumacher, J. M. Roberts, and M. Pagano. 1995. Human cyclin E, a nuclear protein essential for the G1-to-S phase transition. Mol. Cell. Biol. 15:2612-2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parker, S. B., G. Eichele, P. Zhang, A. Rawls, A. T. Sands, A. Bradley, E. N. Olson, J. W. Harper, and S. J. Elledge. 1995. p53-independent expression of p21Cip1 in muscle and other terminally differentiating cells. Science 267:1024-1027. [DOI] [PubMed] [Google Scholar]
- 49.Perez-Roger, I., S. H. Kim, B. Griffiths, A. Sewing, and H. Land. 1999. Cyclins D1 and D2 mediate Myc-induced proliferation via sequestration of p27Kip1 and p21Cip1. EMBO J. 18:5310-5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perez-Roger, I., D. L. C. Solomon, A. Sewing, and H. Land. 1997. Myc activation of cyclin E/Cdk2 kinase involves induction of cyclin E gene transcription and inhibition of p27Kip1 binding to newly formed complexes. Oncogene 14:2373-2381. [DOI] [PubMed] [Google Scholar]
- 51.Phelps, W. C., K. Munger, C. L. Yee, J. A. Barnes, and P. M. Howley. 1992. Structure-function analysis of the human papillomavirus type 16 E7 oncoprotein. J. Virol. 66:2418-2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Phelps, W. C., C. L. Yee, K. Munger, and P. M. Howley. 1988. The human papillomavirus type 16 E7 gene encodes transactivation and transformation functions similar to those of adenovirus E1A. Cell 53:539-547. [DOI] [PubMed] [Google Scholar]
- 53.Polyak, K., M. H. Lee, H. Erdjument-Bromage, A. Koff, J. M. Roberts, P. Tempst, and J. Massague. 1994. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell 78:59-66. [DOI] [PubMed] [Google Scholar]
- 54.Reynisdottir, I., and J. Massague. 1997. The subcellular locations of p15(Ink4b) and p27(Kip1) coordinate their inhibitory interactions with cdk4 and cdk2. Genes Dev. 11:492-503. [DOI] [PubMed] [Google Scholar]
- 55.Reynisdottir, I., K. Polyak, A. Iavarone, and J. Massague. 1995. Kip/Cip and Ink4 Cdk inhibitors cooperate to induce cell cycle arrest in response to TGF-beta. Genes Dev. 9:1831-1845. [DOI] [PubMed] [Google Scholar]
- 56.Ridley, A. J., H. F. Paterson, M. Noble, and H. Land. 1988. Ras-mediated cell cycle arrest is altered by nuclear oncogenes to induce Schwann cell transformation. EMBO J. 7:1635-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rommel, C., B. A. Clarke, S. Zimmermann, L. Nunez, R. Rossman, K. Reid, K. Moelling, G. D. Yancopoulos, and D. J. Glass. 1999. Differentiation stage-specific inhibition of the Raf-MEK-ERK pathway by Akt. Science 286:1738-1741. [DOI] [PubMed] [Google Scholar]
- 58.Roper, E., W. Weinberg, F. M. Watt, and H. Land. 2001. p19ARF-independent induction of p53 and cell cycle arrest by Raf in murine keratinocytes. EMBO Rep. 2:145-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rössig, L., A. S. Jadidi, C. Urbich, C. Badorff, A. M. Zeiher, and S. Dimmeler. 2001. Akt-dependent phosphorylation of p21Cip1 regulates PCNA binding and proliferation of endothelial cells. Mol. Cell. Biol. 21:5644-5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rudolph, B., R. Saffrich, J. Zwicker, B. Henglein, R. Muller, W. Ansorge, and M. Eilers. 1996. Activation of cyclin-dependent kinases by Myc mediates induction of cyclin A, but not apoptosis. EMBO J. 15:3065-3076. [PMC free article] [PubMed] [Google Scholar]
- 61.Ruesch, M., and L. A. Laimins. 1997. Initiation of DNA synthesis by human papillomavirus E7 oncoproteins is resistant to p21-mediated inhibition of cyclin E-cdk2 activity. J. Virol. 71:5570-5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ruley, H. E. 1990. Transforming collaborations between ras and nuclear oncogenes. Cancer Cells 2:258-268. [PubMed] [Google Scholar]
- 63.Schulze, A., B. Mannhardt, K. Zerfass-Thome, W. Zwerschke, and P. Jansen-Dürr. 1998. Anchorage-independent transcription of the cyclin A gene induced by the E7 oncoprotein of human papillomavirus type 16. J. Virol. 72:2323-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Serrano, M., A. W. Lin, M. E. McCurrach, D. Beach, and S. W. Lowe. 1997. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88:593-602. [DOI] [PubMed] [Google Scholar]
- 65.Sewing, A., B. Wiseman, A. C. Lloyd, and H. Land. 1997. High-intensity Raf signal causes cell cycle arrest mediated by p21Cip1. Mol. Cell. Biol. 17:5588-5597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sherr, C. J., and J. M. Roberts. 1995. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 9:1149-1163. [DOI] [PubMed] [Google Scholar]
- 67.Vlach, J., S. Hennecke, K. Alevizopoulos, D. Conti, and B. Amati. 1996. Growth arrest by the cyclin-dependent kinase inhibitor p27Kip1 is abrogated by c-Myc. EMBO J. 15:6595-6604. [PMC free article] [PubMed] [Google Scholar]
- 68.Vojtek, A. B., S. M. Hollenberg, and J. A. Cooper. 1993. Mammalian Ras interacts directly with the serine/threonine kinase Raf. Cell 74:205-214. [DOI] [PubMed] [Google Scholar]
- 69.Vousden, K. H., J. Doniger, J. A. DiPaolo, and D. R. Lowy. 1988. The E7 open reading frame of human papillomavirus type 16 encodes a transforming gene. Oncog. Res. 3:167-175. [PubMed] [Google Scholar]
- 70.Weinberg, R. A. 1989. Oncogenes, antioncogenes, and the molecular bases of multistep carcinogenesis. Cancer Res. 49:3713-3721. [PubMed] [Google Scholar]
- 71.Weinberg, R. A. 1995. The retinoblastoma protein and cell cycle control. Cell 81:323-330. [DOI] [PubMed] [Google Scholar]
- 72.Woods, D., D. Parry, H. Cherwinski, E. Bosch, E. Lees, and M. McMahon. 1997. Raf-induced proliferation or cell cycle arrest is determined by the level of Raf activity with arrest mediated by p21Cip1. Mol. Cell. Biol. 17:5598-5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zerfass, K., A. Schulze, D. Spitkovsky, V. Friedman, B. Henglein, and P. Jansen-Dürr. 1995. Sequential activation of cyclin E and cyclin A gene expression by human papillomavirus type 16 E7 through sequences necessary for transformation. J. Virol. 69:6389-6399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zerfass-Thome, K., A. Schulze, W. Zwerschke, B. Vogt, K. Helin, J. Bartek, B. Henglein, and P. Jansen-Dürr. 1997. p27KIP1 blocks cyclin E-dependent transactivation of cyclin A gene expression. Mol. Cell. Biol. 17:407-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang, H., G. J. Hannon, and D. Beach. 1994. p21-containing cyclin kinases exist in both active and inactive states. Genes Dev. 8:1750-1758. [DOI] [PubMed] [Google Scholar]
- 76.Zhang, H., G. J. Hannon, D. Casso, and D. Beach. 1994. p21 is a component of active cell cycle kinases. Cold Spring Harbor Symp. Quant. Biol. 59:21-29. [DOI] [PubMed] [Google Scholar]
- 77.Zhou, B. P., Y. Liao, W. Xia, B. Spohn, M. H. Lee, and M. C. Hung. 2001. Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat. Cell Biol. 3:245-252. [DOI] [PubMed] [Google Scholar]
- 78.Zimmermann, S., and K. Moelling. 1999. Phosphorylation and regulation of Raf by Akt (protein kinase B). Science 286:1741-1744. [DOI] [PubMed] [Google Scholar]
- 79.Zugasti, O., W. Rul, P. Roux, C. Peyssonnaux, A. Eychene, T. F. Franke, P. Fort, and U. Hibner. 2001. Raf-MEK-Erk cascade in anoikis is controlled by Rac1 and Cdc42 via Akt. Mol. Cell. Biol. 21:6706-6717. [DOI] [PMC free article] [PubMed] [Google Scholar]