Abstract
The p38 mitogen-activated protein kinase (MAPK) pathway is an important mediator of cellular responses to environmental stress. Targets of p38 include transcription factors, components of the translational machinery, and downstream serine/threonine kinases, including MAPK-activated protein kinase 5 (MK5). Here we have used enhanced green fluorescent protein fusion proteins to analyze the subcellular localization of MK5. Although this protein is predominantly nuclear in unstimulated cells, MK5 shuttles between the nucleus and the cytoplasm. Furthermore, we have shown that the C-terminal domain of MK5 contains both a functional nuclear localization signal (NLS) and a leucine-rich nuclear export signal (NES), indicating that the subcellular distribution of this kinase reflects the relative activities of these two signals. In support of this, we have shown that stress-induced activation of the p38 MAPK stimulates the chromosomal region maintenance 1 protein-dependent nuclear export of MK5. This is regulated by both binding of p38 MAPK to MK5, which masks the functional NLS, and stress-induced phosphorylation of MK5 by p38 MAPK, which either activates or unmasks the NES. These properties may define the ability of MK5 to differentially phosphorylate both nuclear and cytoplasmic targets or alternatively reflect a mechanism whereby signals initiated by activation of MK5 in the nucleus may be transmitted to the cytoplasm.
The mammalian p38 mitogen-activated protein kinase (MAPK) pathway is activated by UV radiation, sodium arsenite, heat shock, bacterial lipopolysaccharide, and proinflammatory cytokines and is an important mediator of the cellular response to environmental stress (18, 26, 39). Among the cellular responses to p38 signaling are the production of inflammatory cytokines and phosphorylation of the small heat shock proteins. The physiological processes affected by p38 signaling include cell cycle progression, differentiation, apoptosis, and the inflammatory response. In addition, studies of Drosophila, Xenopus, and mouse have revealed important roles for p38 MAPK signaling during early development (32).
Four distinct p38 MAPKs have been identified in mammalian cells: p38α (stress-activated protein kinase 2a [SAPK2a]), p38β (SAPK2b), p38γ (SAPK3), and p38δ (SAPK4). These enzymes are all phosphorylated and activated by MAPK kinase 6 (MKK6) (15). A second MKK, MKK3, is able to activate p38α, p38γ, and p38δ, but not p38β (49). The four p38 MAPK isoforms are 60 to 70% identical at the amino acid sequence level and have overlapping substrate specificities in vitro. This has made the identification of physiological substrates and functions for p38 MAPKs a difficult task. However, the discovery that p38α and p38β, but not p38γ or 38δ, are inhibited by the pyridinyl imidazole SB203580 coupled with the more recent generation of mice which lack either individual p38 MAPKs or their upstream activators has proven extremely useful in delineating functional pathways for these enzymes (6, 25, 29, 32)
Targets of the p38 pathway include the transcription factors activating transcription factor 2, CHOP/GADD153, and myocyte enhancer factor 2 and components of the translational machinery (31, 38, 50, 53). A further complication in the identification of physiological roles and substrates for the p38 family of MAPKs came with the discovery that a number of p38 substrates are themselves serine/threonine protein kinases. These MAPK-activated protein kinases (MKs) include MK2 and MK3 (28, 30, 39), the MAP-kinase interacting kinases 1 and 2 (MNK1 and 2) (14, 51), and the p38-regulated and -activated protein kinases (PRAK) or MK5 (34, 35) and mitogen- and stress-activated kinases (MSKs) 1 and 2 (10). Targets for the MKs include the small heat shock proteins and transcription factors such as CREB (43), serum response factor (20), and the basic helix-loop-helix protein E47 (33). The physiological importance of p38-activated kinases as effectors of p38 MAPK signaling has recently been illustrated in knockout experiments in which mice lacking MK2 showed increased stress resistance and survived lipopolysaccharide-induced septic shock (23).
With the apparent complexity of MAPK signaling pathways and the identification of multiple targets and effectors which include both nuclear and cytoplasmic proteins, there is increasing interest in the spatio-temporal organization of these signaling cascades. Inactive MAPKs reside predominantly in the cytoplasm, and upon activation they translocate to the cell nucleus (5). This translocation seems to require both phosphorylation and subsequent dimerization of MAPK, but the molecular details of the transport process and its dynamics remain unclear. Sequestration of extracellular signal-regulated kinase 1/2 MAPK within the cytoplasm prevents the activation of transcription factors such as Elk-1 and blocks the ability of cells to initiate DNA synthesis in response to growth factor stimulation, underlining the importance of nuclear translocation in mediating physiological responses to MAPK signaling (4).
Despite the evidence for the regulated nucleocytoplasmic shuttling of MAPKs, these proteins do not appear to contain amino acid sequence motifs corresponding to known nuclear localization signals (NLS) or nuclear export signals (NES). In addition, no direct interactions have been detected between MAPKs and purified importins or exportins, which are critical components of these transport pathways (9). Thus, the translocation of MAPKs across the nuclear membrane most likely occurs through interactions with NES- and NLS-containing proteins, and increasing attention has been given to the role of MAPK activators, regulators, and substrates as mediators of these transport events. Most of our information about the subcellular distribution of mammalian MAPKs derives from studies of extracellular signal-regulated kinase 1/2 and Jun N-terminal protein kinase, while remarkably little is known about the subcellular distribution of the p38 MAPKs.
PRAK/MK5 was first identified as an expressed sequence tag by database searching for sequences homologous to MK2 (34, 35). MK5 is strongly activated in response to cellular stress and proinflammatory cytokines and its activation is mediated by p38 dependent phosphorylation of Threonine 182 within the activation loop of the kinase. However, other than the suggestion that the small heat shock protein Hsp27 and the regulatory light chain of myosin II may be substrates for MK5, information about its subcellular localization or its physiological targets is lacking (34, 35).
In the present study we have investigated the regulation of the subcellular distribution of MK5. We find that this enzyme is constitutively nuclear when expressed in resting mammalian cells and that this pattern of localization is dependent on a functional lysine- and arginine-rich NLS located within the carboxy terminus of the protein. Interestingly, this NLS overlaps with a p38-docking site within MK5, and overexpression of p38 was sufficient to cause the relocalization of MK5 to the cytoplasm in a process which requires p38 docking but not p38 activity. MK5 is also relocalized to the cytoplasm in response to stress treatments such as sodium arsenite and sorbitol. This relocalization is blocked by leptomycin B (LMB), demonstrating the involvement of chromosomal region maintenance 1 (CRM1)/exportin 1 in this process. Furthermore, a region of MK5 immediately upstream of the NLS spanning amino acid residues 332 to 342 contains a functional leucine-rich NES. Finally we show that stress-induced relocalization of MK5 is dependent on p38-mediated phosphorylation of threonine 182 but not on the resulting kinase activity of MK5. Our results suggest that nucleocytoplasmic transport of MK5 is regulated in response to stress via the balance in activities of opposing NLS and NES and that two different activities of p38 are involved. Firstly, binding of p38 to MK5 masks the NLS of the enzyme, and secondly phosphorylation of MK5 at threonine 182 by p38 MAPK increases the activity of its functional NES.
MATERIALS AND METHODS
Reagents.
LMB was generously provided by Minoru Yoshida (University of Tokyo, Tokyo, Japan). SB203580 was purchased from Alexis Corp. Sodium arsenite, sorbitol, bovine serum albumin and cycloheximide were purchased from Sigma Aldrich. Anti-MK5 (PRAK) antibodies, recombinant MK5(PRAK), PRAKtide and recombinant glutathione S-transferase (GST)-p38 have been described previously (19) and were kindly provided by Chris Armstrong (Department of Biochemistry, University of Dundee, Dundee, Scotland).
DNA constructs.
The murine MK5 cDNA was amplified by PCR using Pfu polymerase (Stratagene) and the following primers: 5′-GGGAATTCGTCGGAGGACAGCGACATGG-3′ and 5′-CCGCTCGAGCTACTGGGGCTCGTGGGGAAG-3′. The amplified product was ligated into pEGFP-C1 (Clontech) as an EcoRI-XhoI fragment. The hemagglutinin (HA) epitope tag was added to the N terminus of MK5 by PCR, and the resulting product was ligated into pcDNA 3.1 (Invitrogen) as an EcoRI-XhoI fragment. The C-terminal deletion mutants of MK5 were generated by PCR using the forward primer 5′-GGGAATTCGTCGGAGGACAGCGACATGG-3′ and the following reverse primers: 1-308, 5′-CGGAATTCTTACTCTGTCGAGTTGAGCCAGG-3′; 1-358, 5′-CGGAATTCTTAGTTGTTGACAGAGTGCAGGG-3′; and 1-368, 5′-CGGAATTCTTAGCCCAGCAGCTTCCTCTTCC-3′. All the deletion mutants were cloned as EcoRI fragments into pEGFP-C1. All point mutations were introduced into open reading frames using QuikChange (Stratagene). The various point mutants of MK5 were made by using the following complementary primers (only the forward primer is shown): K51E, 5′-CGGTTTGCACTGGAAATTCTTCTTGATCG-3′; T182A, 5′-GGTGATTTGATGGCGCCCCAGTTTACC-3′; T182D, 5′-GGTGATTTGATGGATCCCCAGTTTACC-3′; S212A, 5′-GGCATCATACCTACCGCGCCAACACCCTACACTTAC-3′; S212E, 5′-GGCATCATACCTACCGAGCCAACACCCTACCTTAC-3′; mutNLS, 5′-GTCAACAACCCCATTCTCGGGACGCTGCTGGGCACCAAG-3′; L337G, 5′-CAGGCGCATGCCGAGCAGGCGGCAAACATGAGGATC-3′; and mutNES, 5′-ATCCAGGACGCCAAGGTCAGCGCCAAACCCGCGCACTCTGTTAACAACCCC-3′. The p38β2 CDm was made using complementary primer (only the forward primer is shown) 5-CAGCCAGTACCACAACCCCAACAACGAGCCAGAGGCC-3. To construct pEGFP 3XNLS the two complementary oligonucleotides: 5′AGCTTTCAGGAAGAGGAAGCTGCTGAGGAAGAGGAAGCTGCTGAGGAAGAGGAAGCTGCTGCTGCA-3′ and 5′-AAGTCCCTTCTCCTTCGACGACTCCTTCTCCTTCGACGACTCCTTCTCCTTCGACGACG-3′ were annealed and ligated into pEGFP-C1 digested with HindIII and PstC1. All mutants were verified by sequencing, and the expression of all enhanced green fluorescent protein (EGFP) fusion proteins, p38 isoforms, and MKK6E/E was verified by Western blotting (data not shown). pSG5p38α expressing HA-tagged p38α MAPK has been described elsewhere (17). The constructs containing HA-tagged constitutively active MKK6 (MKK6E/E), FLAG-tagged p38α, and FLAG-tagged p38β2 were kindly provided by J. Han (Scripps Institute, La Jolla, Calif.) (22, 27). The expression vector encoding inactive p38α MAPK (p38αAGF) (38) was kindly provided by R. Davis (University of Massachusetts). The expression vector encoding the EGFP-MK2 fusion protein was kindly provided by M. Gaestel (University of Hanover, Hanover, Germany) (11).
Cell culture and transfection.
COS-1 cells, HEK 293, and HeLa cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum (Gibco BRL), 2 mM l-glutamine, penicillin (100 U/ml), and streptomycin (100 μg/ml) and transfected using a standard calcium phosphate method as previously described (40). NIH 3T3 cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% newborn calf serum (Bio-Whittaker), penicillin (100 U/ml), and streptomycin (100 μg/ml) and transfected with Lipofectamine plus reagent (Life Technologies Inc.) according to the manufacturer's instructions.
Western blotting.
For detection of epitope tagged p38 in transfected cells and in GST pull down experiments, samples were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (4 to 12% NU-PAGE; Invitrogen Inc.) transferred to 0.45-mm-pore-size polyvinylidene difluoride membrane (Millipore) and probed with either an anti-HA antibody (12CA5; Roche Molecular Biochemicals) to detect HA-tagged p38 or an anti-FLAG-M2 antibody to detect FLAG-tagged p38 (Stratagene) as previously described (40)
Expression of GST fusion proteins in Escherichia coli.
To produce wild-type and mutant GST-MK5 fusion proteins, the appropriate open reading frames were ligated into pGEK4-T3 (Amersham-Pharmacia) digested with EcoRI and XhoI. GST fusion proteins were expressed in E. coli (BL21DE3[pLysS]) by induction with 0.5 μM isopropyl-1-thio-β-d-galactopyranoside at 23°C for 3 h. Fusion proteins were then purified with glutathione-Sepharose (Amersham Pharmacia) using standard techniques. Both the expression and yield of these fusion proteins were analyzed by SDS-PAGE and Coomassie blue staining.
GST pull down assays.
COS-1 cells transfected with HA-tagged p38α or HEK cells transfected with FLAG-tagged p38β2 were lysed in buffer A (20 mM Tris-acetate, pH 7.0; 0.27 M sucrose; 1 mM EDTA; 1 mM EGTA; 1 mM orthovanadate; 10 mM β-glycerophosphate; 50 mM sodium fluoride; 5 mM sodium pyrophosphate; 1% [vol/vol] Triton X-100; 0.1% [vol/vol] 2-mercaptoethanol), with addition of complete protease inhibitor cocktail (Roche Molecular Biochemicals). Lysates were incubated with GST recombinant proteins for 1 h at 4°C; glutathione agarose was then added and the lysates were incubated for a further 30 min at 4°C. The precipitates were then washed three times with buffer A and twice with 50 mM Tris-HCl, pH 7.5. Finally, proteins were analyzed by SDS-PAGE and Western blotting.
Fluorescence microscopy.
To determine the subcellular localization of EGFP fusion proteins, cells were seeded in 24-well plates at a density of 3 × 104 cells per well the day before transfection. NIH 3T3 cells were transfected with expression vectors encoding the various EGFP fusions (0.4 μg per well). Twenty-four hours after transfection EGFP fusion proteins were visualized by fluorescence microscopy using a Leitz DMIRB inverted microscope equipped with a Leica DC100 digital camera. For DAPI (4′,6′-diamidino-2-phenylindole) staining, cells were simultaneously fixed and permeabilized using 4% paraformaldehyde containing 0.1% Triton X-100 and stained with DAPI (1 μg/ml; Roche Diagnostic, GmbH) for 10 min at room temperature. To detect endogenous MK5, HeLa cells were fixed and incubated with 3% bovine serum albumin in phosphate-buffered saline for 1 h at room temperature. Anti-MK5(PRAK) antibody was then added to a final concentration of 12 μg/ml, and cells were incubated for 1 h at room temperature. Finally, immunostaining was detected using a fluorescein isothiocyanate-conjugated anti-sheep immunoglobulin G (F7634; Sigma) at a dilution of 1:80.
Immune complex kinase assays.
Cells were washed twice in phosphate-buffered saline, lysed in 0.5 ml (per 10-cm-diameter dish) of ice-cold buffer A, and harvested using a cell scraper. Lysates were centrifuged for 10 min at 15,000 × g at 4°C, and supernatants were transferred to a clean Eppendorf tube. Protein concentration was then measured using a Bradford assay (Bio-Rad). Kinases were immunoprecipitated from these lysates (500 μg of protein) using 2 μl of anti-GFP antibody ab290 (Abcam, Cambridge, United Kingdom). Samples were then mixed for 1 h at 4°C, before addition of 30 μl of protein G-agarose (50% slurry preequilibrated in buffer A), and incubated for a further 30 min. Immune precipitates were washed three times with buffer A containing 0.5 M NaCl and twice with 50 mM Tris-HCl, pH 7.5. MK5 kinase activity was assayed by addition of 30 μM PRAKtide (kindly provided by C. Armstrong, University of Dundee) in 50 μl of 50 mM Tris HCl (pH 7.5), 0.1 mM EGTA, 10 mM magnesium acetate, and 1 μCi of [γ-32P]ATP (3,000 Ci/mmol; Amersham Pharmacia). Following incubation for 15 min with shaking at 30°C, 20-μl aliquots were spotted onto Whatman p81 paper and washed extensively with 75 mM phosphoric acid before measurement of radioactive incorporation by scintillation counting.
FLIP analysis.
Fluorescence loss in photobleaching (FLIP) analysis was performed using a Zeiss LSM510 confocal laser-scanning microscope as previously described (48). For photobleaching and image capture an argon laser (maximum output 25mW) was used at 25% power. Images of fluorescent cells were captured every 5 s using this laser at 4% transmission. An area of the cytoplasm of one cell was repeatedly bleached after the capture of every fourth image, with 15 iterations at 100% laser transmission. The relative fluorescence in the nucleus of the bleached cell and an adjacent (unbleached control) cell was then measured, as was fluorescence in the bleached area using LSM510 software (Zeiss).
RESULTS
MK5 contains a functional nuclear localization sequence.
Transfection of NIH 3T3 cells with an expression vector encoding a fusion protein between EGFP and MK5 (EGFP-MK5) yielded a predominantly nuclear pattern of fluorescence (Fig. 1). This contrasts with the expression pattern for EGFP alone, which was distributed diffusely in both the nucleus and cytoplasm. Similar results were obtained after transfection of HEK293, HeLa, and COS-1 cells (results not shown). In addition, cells transfected with an HA-tagged MK5 expression vector also showed a nuclear distribution for the protein (data not shown). Finally, we have examined the subcellular distribution of endogenous MK5 protein in resting HeLa cells using an anti-MK5 antiserum, and the results reveal a predominantly nuclear localization (see Fig. 4C). These results demonstrate that, at least in unstimulated (resting) cells, the MK5 protein is nuclear and that our EGFP-MK5 fusion protein accurately reflects the subcellular localization of the endogenous protein.
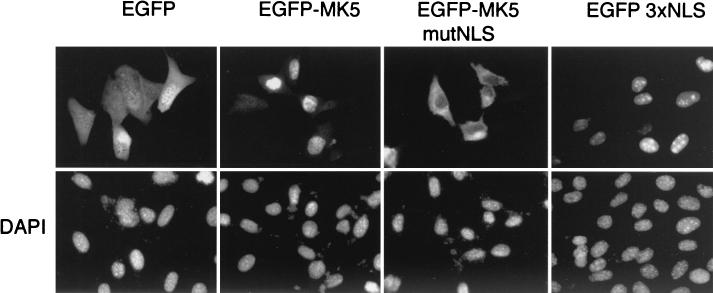
FIG. 1.
MK5 is nuclear in resting or unstimulated NIH 3T3 cells and contains a functional NLS. NIH 3T3 cells were transfected with plasmids encoding either EGFP, EGFP-MK5, pEGFP-MK5mutNLS (where the arginines and lysines [in boldface type in Fig. 2A] in the NLS signal have been changed to glycine and threonine, respectively), or pEGFP 3XNLS (containing three copies of the NLS fused to EGFP). These EGFP proteins were visualized by fluorescence microscopy (upper panel). The nuclei of the transfected NIH 3T3 cells were visualized by DAPI staining (lower panel). Several fields of cells were examined, and representative images are shown.
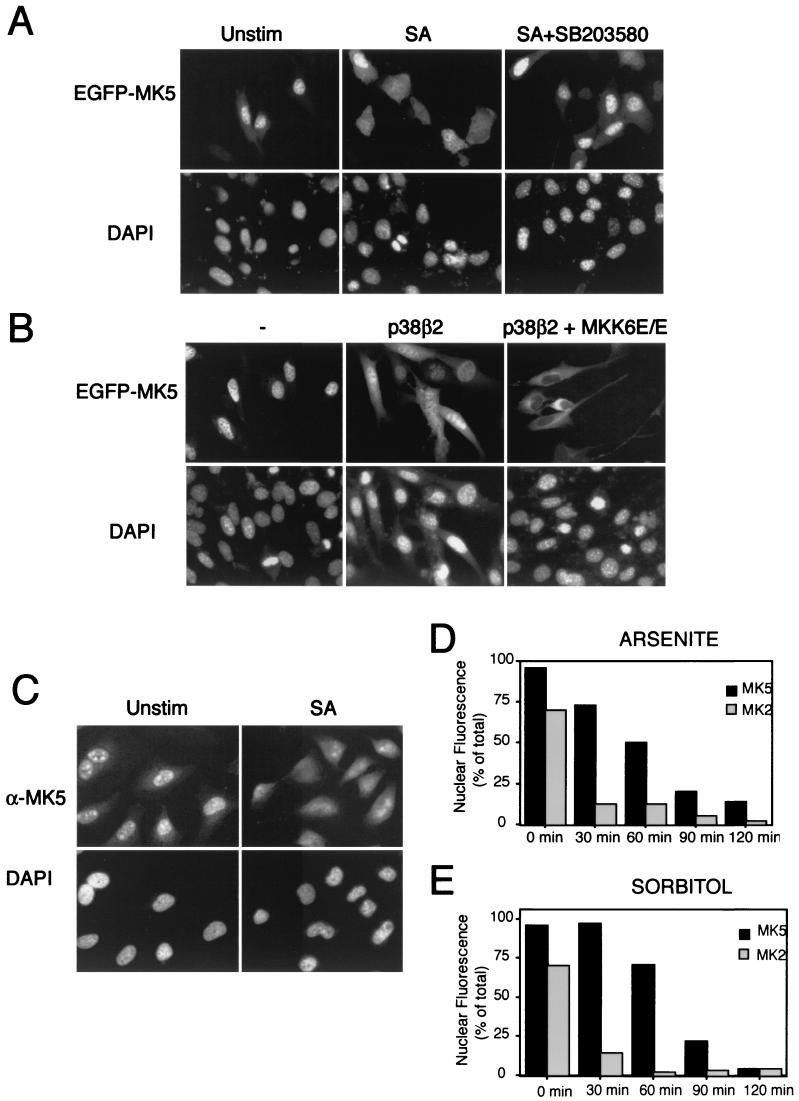
FIG.4.
Cellular stress induces MK5 relocalization. (A) NIH 3T3 cells transfected with pEGFP-MK5 were either left untreated (Unstim), treated for 90 min with 250 μM sodium arsenite (SA), or pretreated for 60 min with a 10 μM concentration of the p38-specific inhibitor SB203580 before 90 min of treatment with 250 μM sodium arsenite (SA+SB203580). EGFP fluorescence is shown in the upper panels, while DAPI staining is shown in the lower panels. Several fields of cells were examined, and representative images are shown. (B) NIH 3T3 cells were cotransfected with pEGFP-MK5 and either wild-type p38β2 or wild-type p38β2 together with constitutively active MKK6 (p38β2 + MKK6E/E). EGFP fluorescence is shown in the upper panels, while DAPI staining is shown in the lower panels. Several fields of cells were examined, and representative images are shown. (C) HeLa cells were either left untreated or exposed to 250 μM sodium arsenite for 120 min. Cells were then fixed, and endogenous MK5 protein was detected using an antibody against MK5 (19). (D) NIH 3T3 cells transfected with either pEGFP-MK2 or pEGFP-MK5 either were left untreated (0 min) or were treated with 250 μM sodium arsenite. The cells were then fixed at the indicated time after induction. One hundred cells were counted at each time point, and the fraction of cells (% of total) exhibiting exclusively nuclear localization of EGFP fusion proteins are shown. (E) Same as panel D except that cells were treated with 0.3 M sorbitol. Experiments were performed three times with reproducible results.
The observation that our EGFP-MK5 fusion protein is nuclear, coupled with its molecular mass of approximately 84 kDa, indicates that it is most likely to enter the nucleus via an active transport process. Carrier proteins of the importin family mediate active nuclear import of substrate proteins, which contain specific amino acid sequences, referred to as NLS (16). A database search using the predictNLS server (http://maple.bioc.columbia.edu/predict NLS/), an automated tool for the analysis and determination of NLS (7), reveals a potential NLS within the carboxy terminus of MK5 at residues 360 to 365 (LRKRKL). This sequence motif is also found in the human nuclear proto-oncogene c-ski (36) and nuclear transcription factors in Drosophila melanogaster and Saccharomyces cerevisiae (3, 21). To determine whether this sequence was functional, we generated a mutant in which the putative NLS was disrupted by replacing four basic amino acids (amino acids 361 to 364 [RKRK]) with the sequence GTGT (pEGFP-MK5mutNLS).
In contrast to the wild-type protein, NIH 3T3 cells transfected with an expression plasmid encoding EGFP-tagged MK5 mutNLS fusion protein displayed significant accumulation of this mutant protein within the cytoplasm (Fig. 1). Nuclear exclusion of this mutant was also observed using an HA-tagged form of MK5 carrying this mutated NLS (data not shown), indicating that the cytosolic localization of MK5mutNLS is not influenced by its fusion partner. Finally, to conclusively prove that the identified sequence motif is a bona fide NLS and can drive nuclear localization of a heterologous protein; three copies of this NLS were fused to EGFP (EGFP3xNLS). In contrast to EGFP alone, which was distributed equally through the cell, the EGFP3xNLS fusion protein exhibited a clear nuclear localization when expressed in NIH 3T3 cells (Fig. 1).
The NLS in MK5 overlaps with a binding site for p38 MAPK.
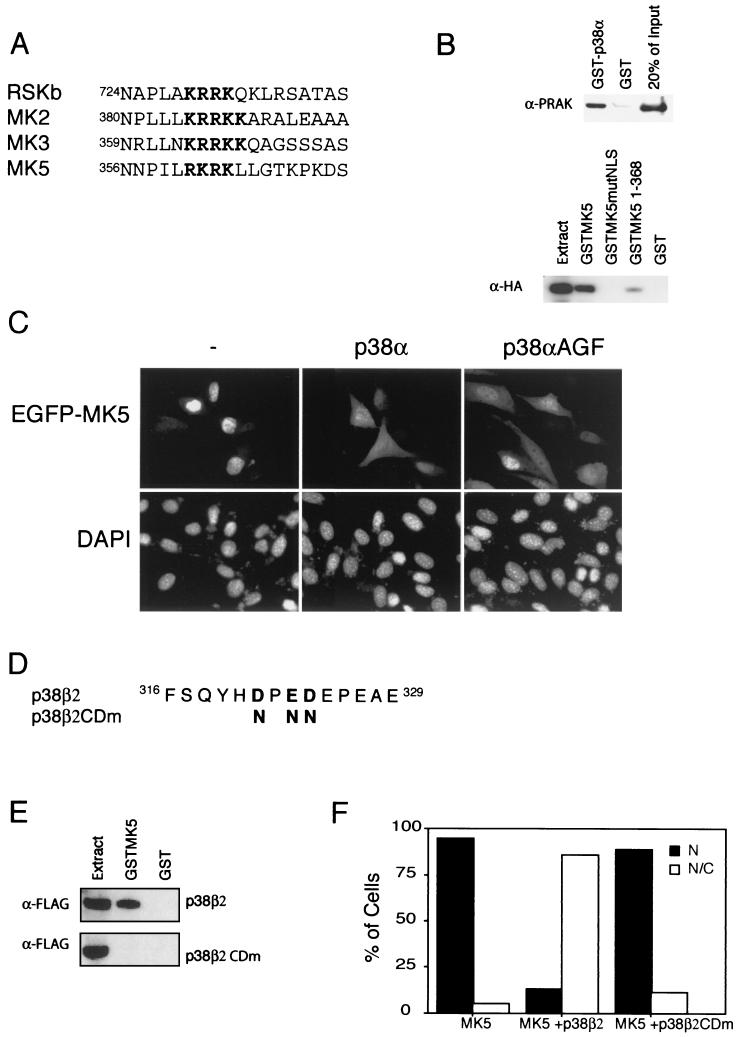
The NLS which we have identified in MK5 overlaps with a motif which has been proposed by several investigators to be an MAPK docking site (Fig. 2A) (42, 44). Tanoue and coworkers recently demonstrated that MK5 interacts physically with p38 MAPK (SAPK2), consistent with the known role of this MAPK in phosphorylating and activating MK5 (45). This interaction is direct, as we can readily demonstrate an interaction between purified recombinant p38α and MK5 in a GST pull down assay (Fig. 2B, upper panel). We have then gone on to investigate whether the MK5 NLS participates in this docking. GST fusion proteins of wild-type MK5 and MK5mutNLS were expressed and purified. These fusion proteins were then used in a GST pull down assay with extracts of Cos-1 cells transfected with an expression vector encoding HA-tagged p38α MAPK.
FIG. 2.
The NLS of MK5 overlaps with a p38 docking site. (A) Alignment of proposed p38 docking sites in various MKs (42, 44). (B) To detect direct interaction between MK5 and p38, in vitro-purified recombinant MK5 (1 μg) was mixed with either GST-p38 (1 μg) or GST alone (1 μg) and protein complexes were pulled down using glutathione-agarose. Coprecipitated MK5 was detected by Western blotting using ananti-MK5 antibody (upper panel). Lysates from COS-1 cells transfected with a plasmid encoding the HA-tagged version of p38α (pSG5p38α) were mixed with the indicated GST fusion protein. Coprecipitated HA-p38 was detected by Western blotting using an anti-HA antibody (lower panel). Similar results were obtained in three independent experiments. (C) NIH 3T3 cells transfected with either pEGFP-MK5 alone or cotransfected with pEGFP-MK5 together with either wild-type p38α or kinase dead p38α (p38αAGF). EGFP fluorescence is shown in the upper panels, while DAPI staining is shown in the lower panels. Several fields of cells were examined, and representative images are shown. (D) Amino acid sequence of the CD domains of human p38α and p38β2. Boldface type indicates the negatively charged amino acid known to be important for p38α's ability to interact with MK5 and the corresponding residues in p38β2, which we have changed to asparagines in p38β2CDm. (E) Lysates from HEK 293 cells transfected with a plasmid encoding a FLAG-tagged version of p38β2 (upper panel) and p38β2CDm (lower panel) were mixed with the indicated GST fusion protein. Coprecipitated FLAG-p38β2 was detected by Western blotting using an anti-FLAG antibody. Similar results were obtained in three separate experiments. (F) NIH 3T3 cells transfected with either pEGFP-MK5 alone or cotransfected with pEGFP-MK5 together with plasmids encoding either wild-type p38β2 (p38β2) or mutant p38β2 (p38β2CDm). One hundred cells were counted and scored as either displaying exclusively nuclear fluorescence (N) or both nuclear and cytosolic fluorescence (N/C). Similar results were obtained in three separate experiments.
Our results clearly show that wild-type MK5 but not MK5 mutNLS interacts with p38α MAPK (Fig. 2B, lower panel), indicating that there is functional overlap between the NLS and the MAPK docking site and that the NLS is necessary for p38 docking. A truncated MK5 protein (consisting of residues 1 to 368), which still includes the NLS, was also able to bind to p38α, though not as potently as the full-length protein. Although three copies of the MK5 NLS were sufficient to direct EGFP into the nucleus (Fig. 1), this triple motif produced as a GST fusion was unable to bind to p38α MAPK in a GST pull down assay (data not shown). This indicates that the NLS motif is necessary but not sufficient for p38 docking and that MAPK binding involves additional sequences which lie outside the minimal NLS motif. These additional sequences are probably C terminal to the NLS since the truncated mutant containing residues 1 to 368 had reduced affinity for p38α.
Binding of p38 MAPK to MK5 interferes with NLS function.
Based on our findings we reasoned that the binding of p38 MAPK to MK5 might mask the NLS and interfere with its ability to mediate nuclear import of the protein. In order to test this, the effects of expressing p38α MAPK on the localization of EGFP-MK5 were determined in NIH 3T3 cells. Coexpression of p38α MAPK caused the localization of MK5 to redistribute dramatically from a predominantly nuclear pattern to a diffuse staining throughout the cell (Fig. 2C). This effect is mediated by p38 binding, as a p38β2 mutant form (45) unable to bind MK5 (p38β2CDm), did not cause this redistribution (Fig. 2D to F). Finally, we can demonstrate that this ability of p38 to cause redistribution of MK5 is dependent only on binding and not on the activity of the MAPK, as expression of a “kinase dead” mutant (p38αAGF) of p38α (38) also causes relocalization of MK5 when coexpressed in NIH 3T3 cells (Fig. 2C). Overall, our data strongly suggest that the binding of p38 MAPK and NLS function are mutually exclusive.
MK5 shuttles between the nucleus and the cytosol.
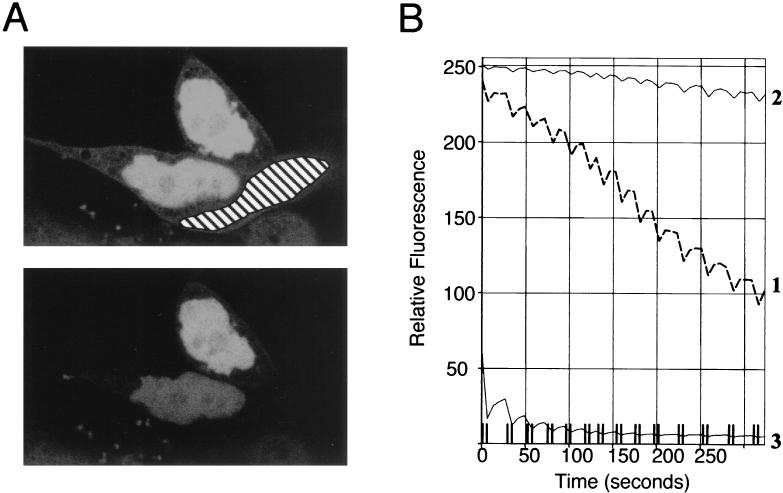
The above data demonstrate that MK5 is predominantly a nuclear protein when expressed in NIH 3T3 cells. However, our results using the NLS mutant and the fact that binding to p38 MAPK causes a redistribution of MK5 such that it becomes cytoplasmic may be indicative of a nuclear export pathway for MK5. Therefore, we set out to determine if MK5 is able to shuttle between the nucleus and the cytosol when expressed in NIH 3T3 cells. To study this we have used FLIP analysis (48). In these experiments we repeatedly photobleached an area of the cytosol within a cell expressing the MK5 protein fused to EGFP and then monitored the level of EGFP fluorescence in the nucleus of that cell and an adjacent cell expressing EGFP-MK5 which was not photobleached. We observed a rapid decrease in the levels of nuclear fluorescence in the photobleached cell (Fig. 3). In contrast, nuclear fluorescence in the adjacent (unbleached) cell was unaffected. Thus, despite the apparently constitutive nuclear localization of MK5 this experiment shows that this protein in fact rapidly shuttles between the nucleus and the cytosol.
FIG. 3.
MK5 shuttles between the nucleus and the cytosol. (A) A fraction of the cytosol (as marked) of an NIH 3T3 cell transfected with EGFP-MK5 was repeatedly photobleached. Images as scanned before photobleaching (upper panel) and after repeated photobleaching (lower panel) are shown. (B) Fluorescence in the nucleus of the bleached cell (line 1), in the nucleus of an adjacent control cell (line 2), and in the bleached cytosol (line 3) were measured at the indicated times. Cells were pretreated with cycloheximide (10 μg/ml) for 30 min before commencing the FLIP experiment.
Cellular stress causes nuclear export of MK5.
It has recently been demonstrated that cellular stress can cause the rapid translocation of MK2 from the nucleus to the cytoplasm (1, 11). As MK5 is highly related to MK2 (34), we explored the possibility that the localization of MK5 was regulated in a similar fashion. NIH 3T3 cells expressing EGFP-MK5 were treated with 250 μM sodium arsenite, a potent activator of the p38 MAPK pathway. Relocalization of EGFP-MK5 from the nucleus to the cytoplasm was observed 90 to 120 min after treatment (Fig. 4A). Sorbitol, another known p38 activator, was also able to induce redistribution of the EGFP-MK5 fusion protein, while stimuli such as platelet-derived growth factor, epidermal growth factor, and 12-O-tetradecanoyl-phorbol acetate, which preferentially activate the classical ERK1/2 MAPK pathway, did not affect the nuclear localization of EGFP-MK5 (data not shown). No redistribution was observed in treated cells expressing EGFP alone (data not shown). To ensure that the stress-induced export was not the result of the EGFP domain, HA-tagged MK5 was also analyzed. This protein was also exported to the cytoplasm in response to treatment with either sodium arsenite or sorbitol (data not shown). These results show that EGFP-MK5, like EGFP-MK2, is exported from the nucleus in response to stress. Kinetic studies revealed that EGFP-MK2 relocalizes more rapidly from the nucleus to the cytoplasm when compared with EGFP-MK5 in response to both arsenite and sorbitol (Fig. 4D and E). Clear redistribution of EGFP-MK5 was observed only after at least 90 min of stimulation, while almost all of the EGFP-MK2 was relocalized to the cytoplasm within 40 to 60 min. In previous studies of MK2 localization the protein was either epitope tagged or fused to EGFP, and the subcellular distribution of the endogenous protein was not studied (1, 11). We wished to confirm that our EGFP-MK5 fusion protein behaved in a similar way to endogenous MK5. HeLa cells were either left untreated or exposed to 250 μM sodium arsenite for 120 min before fixation and detection of endogenous protein using an antibody against MK5 (19) We can clearly see that the endogenous MK5 is a predominantly nuclear protein and that like EGFP-MK5 a significant fraction of the protein relocalizes to the cytoplasm in response to sodium arsenite (Fig. 4C). We conclude that the behavior of the EGFP-MK5 protein accurately reflects that of endogenous MK5.
Activation of p38 MAPK is required for stress-induced nuclear export of MK5.
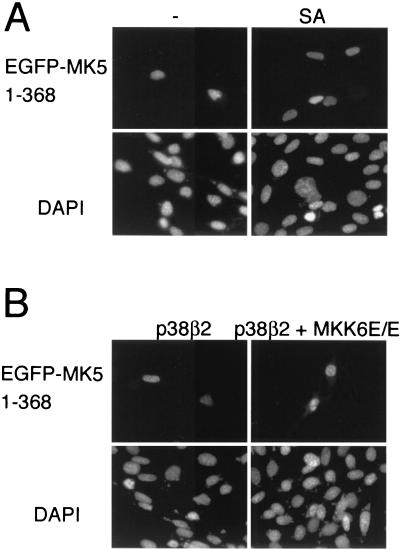
The enzymatic activity of MK5 is regulated by p38 MAPK (34), and the p38 activators sorbitol and arsenite both induced nuclear export of MK5. To further explore the role of p38 activation in stress-induced MK5 nuclear export, NIH 3T3 cells expressing EGFP-MK5 were pretreated for 30 min with the p38α/β-specific inhibitor SB203580 (8) before exposure to sodium arsenite. Pretreatment with SB203580 completely blocked stress-induced translocation of MK5 (Fig. 4A). These results suggest that activation of p38 (-α and/or -β) by sodium arsenite is able to cause the export of MK5 from the nucleus. We have already demonstrated that overexpression of p38α MAPK alone caused redistribution of the MK5 protein and that this was mediated by the ability of p38 to bind to MK5 with no requirement for the p38α to be active (Fig. 2C). In order to dissect out the roles played by either p38 binding or p38 activation we compared the effects of expressing p38β2 MAPK with or without coexpression of a constitutively activated form of MKK6 (MKK6E/E), the upstream activator of p38β2 MAPK (Fig. 4B). As seen before for p38α, expression of p38β2 alone induced redistribution of the MK5 such that protein was found in both the cytoplasm and in the nucleus. In contrast, the coexpression of p38β2 and activated MKK6 reproducibly resulted in complete export of the MK5 protein from the nucleus to the cytosol, leading to nuclear exclusion of MK5. Our results strongly suggest that both p38 binding to MK5 and activation of p38 MAPK may play a role in stimulating the translocation of MK5 from the nucleus to the cytoplasm in response to stress. Because coexpression of active MKK6 and p38β2 gave rise to complete nuclear exclusion and was less cytotoxic than extended treatment with either sodium arsenite or sorbitol we have used this as a tool in our subsequent experiments to investigate the mechanism of stress-induced nuclear export of MK5.
p38-mediated phosphorylation but not activation of MK5 is required for stress-induced nuclear export.
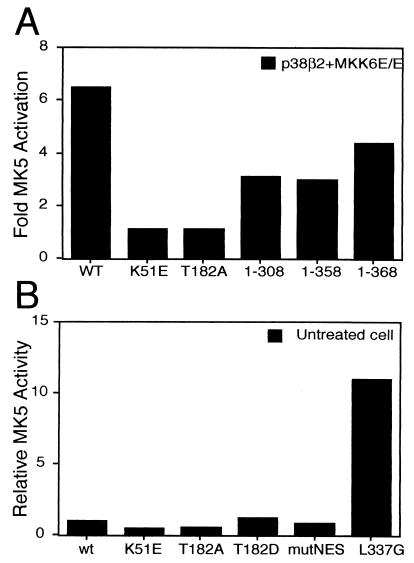
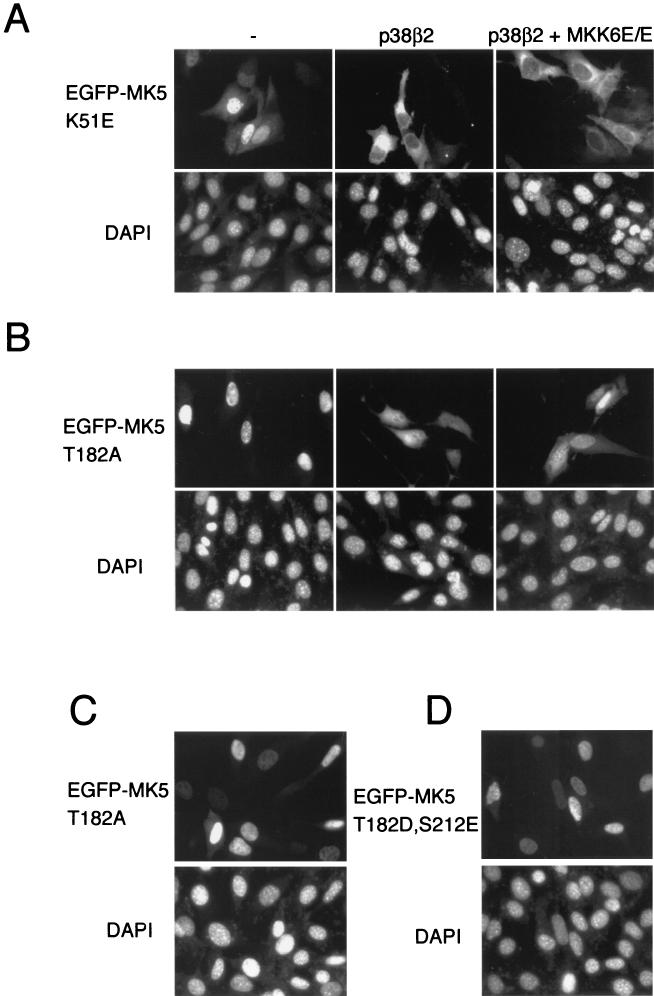
To explore whether activation of MK5 itself is necessary for its translocation, we generated a kinase dead mutant by replacing an essential lysine residue (Lys-51) within the ATP-binding site of MK5 with glutamic acid (K51E). This mutation has previously been shown to abolish all kinase activity (34), and unlike the wild-type protein, this mutant cannot be activated by coexpression of MKK6 E/E and p38 (see Fig. 6A). The K51E substitution did not alter the nuclear localization of MK5 in unstimulated cells (Fig. 5A). Furthermore, coexpression of activated MKK6 together with p38 still provoked cytoplasmic translocation of MK5 (K51E) (Fig. 5A). Interestingly, coexpression of p38 alone induced efficient nuclear export of this mutant (Fig. 5A). Thus, it would appear that there is no requirement for MK5 activity itself in order for the protein to be exported from the nucleus in response to stress.
FIG. 6.
p38-mediated activation of MK5. (A) HEK 293 cells were cotransfected with the indicated plasmids, together with p38β2 and MKK6E/E. Twenty-four hours following transfection cells were lysed and the kinase activities of the various EGFP-MK5 fusion proteins were assayed in an immune complex kinase assay using PRAKtide as substrate. The results are presented as activation relative to the kinase activity in a lysate from cells transfected with the indicated EGFP-MK5 vector alone (no p38β2 and MKK6E/E). (B) Comparison of the relative basal kinase activities measured in lysates from cells transfected with the indicated EGFP-MK5 plasmids. The kinase activity of pEGFP-MK5 (WT) was set as 1. That equal amounts of protein were assayed in these experiments was verified by Western blotting of immune precipitates using an antibody against EGFP. Experiments were performed at least three times, and the results of a single representative experiment are shown.
FIG. 5.
MK5 relocalization is dependent on p38-mediated phosphorylation. (A) NIH 3T3 cells were transfected with either a kinase dead MK5 (pEGFP-MK5 K51E), or cotransfected with either pEGFP-MK5 K51E and wild-type p38β2 or pEGFP-MK5 K51E together with both wild-type p38β2 and constitutive active MKK6 (p38β2 + MKK6E/E). (B) NIH 3T3 cells were transfected as in panel A except that a pEGFP-MK5 T182A plasmid in which the phosphoacceptor site Thr182 was changed into an Ala was used. (C) NIH 3T3 cells transfected with a mutant of MK5 in which the regulatory Thr182 is converted to an Asp (pEGFP-MK5 T182D). (D) NIH 3T3 cells were transfected with a double mutant of MK5 in which both p38 phosphorylation sites were changed to acidic residues (pEGFP-MK5 T182D,S212E). EGFP fluorescence is shown in the upper panels, while DAPI staining is shown in the lower panels. Several fields of cells were examined, and representative images are shown.
Activated p38 MAPK phosphorylates two residues within MK5, threonine 182, which is part of a conserved LMTP site in the T-loop, and serine 212. Of these, only phosphorylation of Thr182 is essential for activation of MK5 (34). We have mutated Thr182 in MK5 by replacing it with a nonphosphorylable alanine residue (MK5T182A). This protein, like the kinase dead MK5 mutant, cannot be activated by coexpression of MKK6E/E and p38 (Fig. 6A) and, like the wild-type MK5, resided in the nucleus in unstimulated cells (Fig. 5B). Coexpression of p38β2 caused a significant redistribution of this mutant protein just as was seen with wild-type MK5 (compare Fig. 4B and 5B). However, in contrast to either the wild-type or the kinase dead MK5 proteins, MK5T182A was not excluded from the nucleus when coexpressed with MKK6E/E and p38β2 MAPK (Fig. 5B). Changing Thr182 into Asp, which mimics phosphorylation, did not affect the subcellular distribution in resting cells, nor did this mutation lead to nuclear export in response to activation of the p38 pathway (Fig. 5C and data not shown). However, this mutant was not significantly more active than wild-type MK5 (Fig. 6B), indicating that this amino acid substitution is unable to effectively mimic phosphorylation at this site. A Ser212Ala substitution MK5 mutant was exported from the nucleus as effectively as the wild-type MK5 (data not shown), confirming that phosphorylation of this residue by p38 MAPK plays no role in the export process. A double mutant (MK5 T182D/S212E), in which both phosphorylation sites are replaced by acidic amino acids to mimic phosphorylation, was still localized in the nucleus of untreated NIH 3T3 cells (Fig. 5D).
We conclude that p38-mediated phosphorylation of Thr182 but not the resulting increase in the kinase activity of MK5 is necessary for the stress-induced relocalization of the protein.
LMB inhibits stress-induced MK5 relocalization.
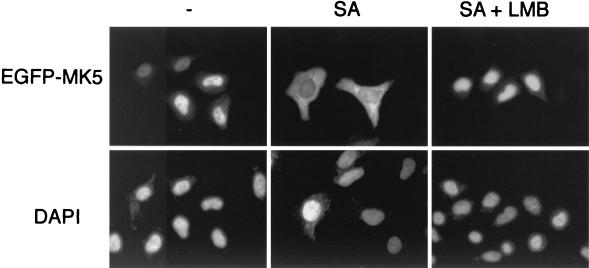
Active nuclear export of proteins is accomplished by transport receptors, called exportins, which bind to specific sequences in cargo proteins. One such exportin, CRM1, was originally shown to directly interact with a leucine-rich motif in the human immunodeficiency virus type 1 Rev protein (12). This motif was defined as an NES, and functional Leu-rich NESs have been identified in several other proteins (reviewed in reference 16). CRM1-dependent nuclear export is blocked by the Streptomyces metabolite LMB (12, 13, 24, 52). To determine whether the observed stress-induced relocalization of MK5 is CRM1 dependent, NIH 3T3 cells transfected with wild-type EGFP-MK5 were pretreated with LMB (5 ng/ml) for 1 h, and the cells were then exposed to 250 μM sodium arsenite. LMB blocked MK5 export induced both by sodium arsenite and by cotransfection with constitutively activated MKK6E/E plus p38 (Fig. 7 and data not shown). These findings indicate that the stress-induced relocalization of MK5 from the nucleus to the cytoplasm is CRM1 dependent and suggests the presence of a leucine-rich NES in MK5.
FIG. 7.
LMB blocks the translocation of MK5. HeLa cells transfected with pEGFP-MK5 were either left untreated (-) or were treated with 250 μM sodium arsenite (SA) either in the absence or presence of LMB (5 ng/ml) (SA + LMB). EGFP fluorescence is shown in the upper panels, while DAPI staining is shown in the lower panels. Several fields of cells were examined, and representative images are shown.
MK5 contains a leucine-rich NES.
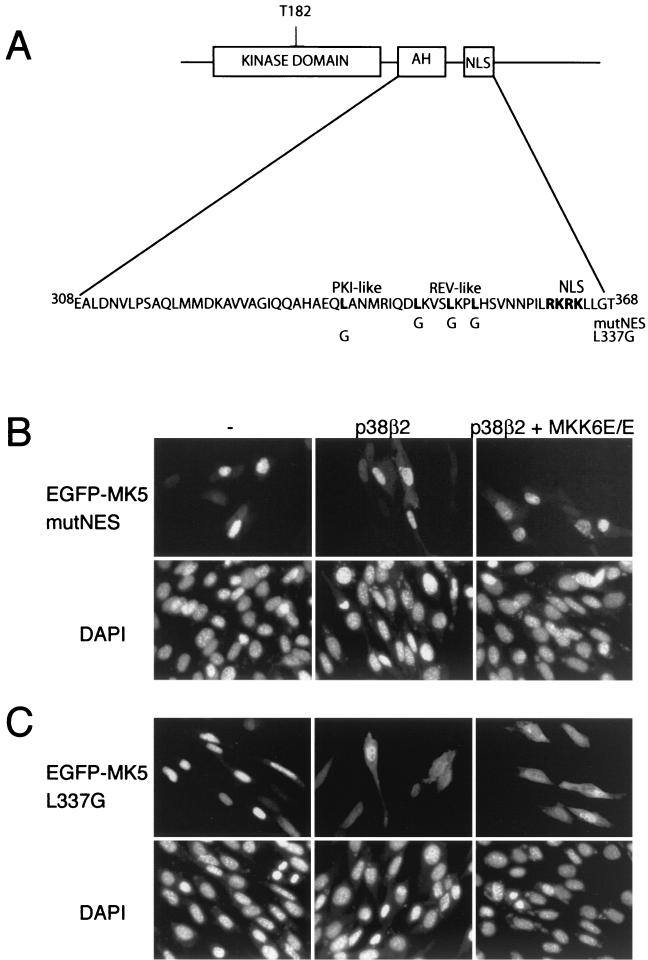
An EGFP-MK5 mutant truncated at residue 358, which is just amino terminal to the NLS, showed a distribution in the cell that was indistinguishable from EGFP and consistent with loss of the functional NLS. However, coexpression of p38 and MKK6E/E caused redistribution of this fusion protein such that it was excluded from the nucleus (Table 1). This was surprising, as we have previously shown that MK5 which lacks a functional NLS (MK5 mutNLS) is unable to bind to p38 MAPK (Fig. 2B). However, both MK5 mutNLS and MK5 1-358 can still be activated by coexpression of p38β2 and constitutively activated MKK6, indicating that they are still able to be phosphorylated at threonine 182 by p38 MAPK (Fig. 6A). Interestingly, a mutant form of MK2 lacking a functional NLS is also activated in response to sodium arsenite, albeit with reduced efficiency (1). An EGFP-MK5 fusion protein truncated at residue 308 showed the same distribution in the cell as EGFP alone, and its localization was not modified by exposure to stress (Table 1). Careful examination of the primary sequence of MK5 in the region of residues 308 to 358 identified a putative NES immediately N-terminal to the NLS (LKVSLKPLHS, residues 345 to 354) (hydrophobic residues are shown in boldface type throughout this paragraph), resembling the Rev-like NES consensus L(X)2-3L(X)2-3LXL (Fig. 8A). In order to investigate its role, a mutant was generated in which the essential leucines were replaced by glycines. This NES mutant form of MK5 resided in the nucleus of nonstressed cells, and its subcellular localization was not altered after arsenite treatment (data not shown) or following overexpression of activated MKK6 and p38 (compare Fig. 4B and 8B). A region of four spaced hydrophobic residues in the sequence MTSALATMRV (Fig. 8A) resembling an inhibitor of protein kinase A (PKI)-like NES has previously been identified as a putative NES in MK2, and mutation of the central leucine in this sequence was enough to disrupt nuclear export of MK2 (11). This sequence is partly conserved in MK5 (HAEQLANMRI, residues 333 to 342), and mutation of the corresponding leucine (Leu337) in MK5 impaired the nuclear export of MK5 following coexpression of p38 in combination with activated MKK6 (Fig 8C). Interestingly, this single point mutation also caused constitutive activation of MK5, indicating that this region of the protein may also be involved in auto-regulation of MK5 activity (Fig. 6B).
TABLE 1.
Percentages of transfected cells which exhibit either nuclear, cytoplasmic, or mixed nuclear/cytoplasmic localization of EGFP fusion proteinsa
| Protein(s) encoded | % of cells (mean ± SD) demonstrating localization:
|
||
|---|---|---|---|
| Nuclear | Cytoplasmic | Mixed | |
| MK5 1-308 | 0 | 0 | 100 |
| MK5 1-308 + MKK6E/E+p38 | 0 | 0 | 100 |
| MK5 1-358 | 0 | 12.5 ± 0.7 | 87.5 ± 0.7 |
| MK5 1-358 + MKK6E/E+p38 | 0 | 86 ± 4.2 | 14 ± 4.2 |
EGFP fusion proteins encoding either MK5 1-308 or MK5 1-358 alone or cotransfected with both constitutively active MKK6 and p38 MAPK.
FIG. 8.
A Rev-like NES motif in MK5 is necessary for stress induced export. (A) The amino acid sequence of MK5 showing the proposed autoinhibitory α-helix (AH). The AH region overlaps with the PKI-like and the Rev-like NES sequence and also lies close to the NLS. The critical residues in the two potential NESs (Rev-like and PKI-like) and in the NLS are marked in boldface type. The leucines in the two putative NESs were mutated to glycine and designated L337G and mutNES. (B) NIH 3T3 cells were transfected with either pEGFP-MK5mutNES alone or cotransfected with pEGFP-MK5mutNES together with plasmids encoding either wild-type p38β2 or both p38β2 and constitutively active MKK6 (p38β2 +MKK6E/E). EGFP fluorescence is shown in the upper panels, while DAPI staining is shown in the lower panels. Several fields of cells were examined, and representative images are shown. (C) NIH 3T3 cells were transfected with either pEGFP-MK5 L337G alone or cotransfected with pEGFP-MK5 L337G together with plasmids encoding either wild-type p38β2 or both p38β2 and constitutively active MKK6 (MKK6E/E). EGFP fluorescence is shown in the upper panels, while DAPI staining is shown in the lower panels. Several fields of cells were examined and representative images are shown.
Efficient p38 docking is essential for stress-mediated relocalization of MK5.
The data above showed that MK5 contains both a functional NLS and a functional NES(s) and is able to shuttle between the nucleus and the cytoplasm. The localization of the kinase at a given time will depend on the balance of the activity of the NES and the NLS. Phosphorylation of threonine 182 by p38 MAPK increases the activity of the NES, both in full-length MK5 (Fig. 4B and 5B) and in the truncated MK5, which lacks the NLS (MK5 1-358, Table 1). The NLS on the other hand overlaps with a p38 docking site, and coexpression of p38 is sufficient to mask this and cause nuclear export of the protein. Thus, p38 can increase the nuclear export of MK5 by two different mechanisms: by exposing the NES, which requires phosphorylation of threonine 182, and by masking the NLS by binding to MK5 and interfering with function of the NLS. We wanted to address the question of whether the latter mechanism plays any role in the stress-induced relocalization of MK5. To answer this we made use of the truncated form of MK5 (consisting of residues 1 to 368). This mutant retains the functional NLS and is strongly impaired in its ability to bind to p38 (Fig. 2B) but is still efficiently activated on coexpression of p38 and activated MKK6 (Fig. 6A). This mutant is nuclear in unstimulated cells, but in contrast to wild-type MK5 (Fig. 4B) it failed to relocalize to the cytoplasm in response to expression of either p38β2 alone or in combination with activated MKK6 or in response to sodium arsenite (Fig. 9). We conclude that the NLS is dominant over even a fully active NES within MK5 and that, in addition to phosphorylation of threonine182, p38 docking and subsequent masking of this NLS are essential for stress-mediated relocalization of MK5.
FIG. 9.
The mutant EGFPMK5 1-368 is not relocalized in response to stress. (A) NIH 3T3 cells were transfected with a plasmid encoding an EGFP-fusion protein comprising amino acid residues 1 to 368 of MK5 (pEGFP-MK5 1-368) and either left untreated (-) or exposed to 250 μM sodium arsenite (SA) for 90 min. (B) NIH 3T3 cells were cotransfected with pEGFP-MK5 1-368 together with either wild-type p38β2 (p38) or both wild-type p38β2 and constitutively active MKK6 (p38 + MKK6E/E). EGFP fluorescence is shown in the upper panels, while DAPI staining is shown in the lower panels. Several fields of cells were examined for each experiment and representative images are shown.
DISCUSSION
We have used EGFP fusion proteins to analyze the subcellular localization of the stress regulated enzyme MK5 and provide evidence that the C-terminal domain of the protein contains both a functional NLS and a leucine-rich NES. Furthermore, although the MK5 protein is predominantly nuclear in unstimulated cells, we have shown that this protein shuttles between the nucleus and the cytoplasm. This strongly suggests that the subcellular distribution of MK5 will be determined by the relative activities of these opposing localization signals and that these may be regulated in order to deliver the kinase into the appropriate cellular compartment. Consistent with this idea, we find that exposure to either sodium arsenite or sorbitol, both of which activate the p38 MAPK pathway, leads to relocalization of MK5 from the nucleus to the cytoplasm. This process is dependent on the activity of the CRM1-dependent nuclear export pathway as it is blocked by both LMB and by mutations within the leucine-rich NES.
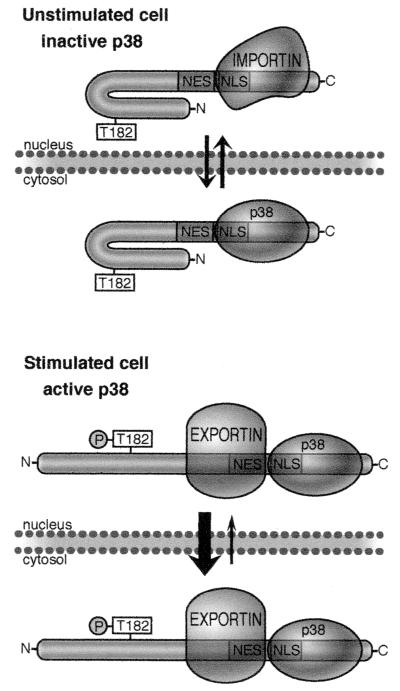
Surprisingly, we have found that the stress-induced NES-dependent relocalization of MK5 can be facilitated in two ways. Firstly, the NLS within MK5 overlaps with a putative docking site for p38 MAPK. Furthermore, coexpression of p38 MAPK in unstimulated cells is sufficient to trigger the translocation of a substantial fraction of MK5 to the cytoplasm. This translocation is critically dependent on the ability of p38 to bind to MK5, as p38β2 mutants that can no longer bind to MK5 do not trigger export. However, it is not dependent on the kinase activity of p38, as coexpression of a kinase dead mutant of p38α is equally effective in mediating export. These observations coupled with the fact that mutations which affect NLS function also interfere with the ability of p38α MAPK to physically interact with MK5 strongly suggest that p38 binding and NLS function are mutually exclusive and that binding of p38 down regulates NLS function, probably by restricting access to importin. A similar overlap between a functional NLS and a p38 docking site has also been identified in the p38-activated MKs MK3 and ribosomal S6 kinase B (45, 46). In both cases coexpression of p38 causes translocation of these proteins from the nucleus to the cytoplasm indicating that this mechanism may be of general significance.
The second mechanism by which MK5 translocation can be achieved is dependent on phosphorylation of MK5 by p38 MAPK. The specific p38 inhibitor SB203580 is able to block the arsenite-induced translocation of MK5 and mutation of threonine 182 within MK5 to a nonphosphorylable residue has the same effect. Threonine 182 is located within the activation loop of MK5 between subdomains VII and VIII, and phosphorylation of this residue alone is required for activation of MK5 (34). However, the kinase activity of MK5 itself is not required for stress-induced relocalization, as translocation of a kinase dead but phosphorylable mutant of MK5 is unimpaired. Some insight as to how this phosphorylation-dependent increase in translocation might occur has come from studies of the related enzyme MK2 (11). Unphosphorylated MK2 is proposed to adopt a closed conformation in which a C-terminal autoinhibitory α-helical motif makes contacts with the catalytic domain and masks an adjacent NES. This leaves the NLS exposed and results in nuclear accumulation of MK2. In MK2 there are at least two regulatory phosphorylation sites: threonine 205 (which is equivalent to threonine 182 in MK5) and threonine 317. Phosphorylation of the latter residue is proposed to open the conformation of MK2 and expose the NES, thus facilitating nuclear export of the protein. Threonine 317 lies in a putative hinge region between the catalytic domain of MK2 and the C-terminal region containing the autoinhibitory α-helix, NES and NLS. MK5 also contains a putative autoinhibitory α-helix between residues 308 and 350, but a functional equivalent to threonine 317 is not present in MK5 (34, 37). However, it could be envisaged that phosphorylation of threonine 182 alone in MK5 is accompanied by an equivalent conformational change to that caused by phosphorylation of threonine 317 in MK2. This is supported by the fact that while full activation of MK5 requires only phosphorylation of this single residue, MK2 is only fully active when multiple threonine residues (including threonine 317) are modified (2).
The model shown in Fig. 10 is proposed to explain the role of both MK5 activation and p38 docking in the subcellular distribution of MK5. Further evidence for the integration of the two mechanisms we have described in mediating stress-induced nuclear export of MK5 has come from studies using a deletion mutant of MK5 comprising residues 1 to 368. This mutant is truncated just C terminal to the NLS signal and contains both this and the NES. This mutant was still able to bind to p38 MAPK in vitro, albeit with reduced affinity. Furthermore, the NLS signal in the truncated protein was still functional, as MK5 1-368 was predominantly nuclear. However, this truncated form of MK5 was not translocated from the nucleus even when a constitutively active form of MKK6 and p38β2 were coexpressed. This failure to relocalize is not due to lack of MK5 phosphorylation, since MKK6 and p38β2 efficiently activated MK5 1-368 in vivo. Thus, the efficient translocation of MK5 from the nucleus to the cytoplasm in response to stress requires both an increase in the activity of the MK5 NES, which is dependent on p38 activity, and efficient docking of p38 to MK5. In presenting this model we must acknowledge that it is based on studies of MK5 expressed as an EGFP fusion protein in mammalian cells and not on the endogenous protein. However, we were able to demonstrate that endogenous MK5, like the EGFP-MK5 fusion protein, is predominantly nuclear in resting HeLa cells and that treatment with sodium arsenite caused a similar relocalization of the protein from the nucleus to the cytoplasm. This gives greatly increased confidence that the results we have obtained with both wild-type and mutant forms of MK5 fused to EGFP will accurately model the behavior of the endogenous protein.
FIG. 10.
Model describing the regulation of the subcellular distribution of MK5. In unstimulated cells (top panel) MK5 adopts a closed conformation in which the NES is less accessible to exportin. However, the NLS, which overlaps with the p38 docking site, is exposed, and both p38 and importin may compete for access to this site. The localization of MK5 will thus depend on the effective concentration of these two binding partners relative to MK5. In stimulated cells (lower panel) where p38 MAPK is activated, MK5 is exported to the cytoplasm, and two mechanisms appear to be important in this process. Firstly (as above) the binding of p38 to MK5 masks the functional NLS, thus impairing nuclear import. Secondly, MK5 becomes phosphorylated on threonine 182, which may induce a conformational change, leading to either activation or unmasking of the NES.
One of the major functions of MAPK docking motifs is to promote the specificity, efficiency, and accuracy of substrate recognition and phosphorylation (41). The overlapping NLS and MAPK docking domains identified in MK3 and MK5 may represent a novel function for such motifs in determining the subcellular distribution of both the MAPK and the substrate MK. Possible scaffold functions for members of the MAPK family are well documented, and both upstream activators and regulators of MAPK activity, including protein phosphatases, may play important roles in determining the subcellular distribution of these enzymes. The subcellular localization of mammalian p38 has not been well defined, but one study has shown that p38 resides in the nucleus of resting cells (1). Mammalian p38 translocates from the nucleus following stimulation by stress, and both MK2 and MK3 have been suggested to be involved in mediating this process. The results presented here suggest a similar role for MK5. Since p38 itself lacks an obvious NLS sequence, MKs have been suggested to be nuclear anchors for p38. However, the fact that a p38 docking site and a functional NLS overlap in MK2, -3, and -5 might argue against this.
The localization of MK5 is clearly affected both by physical association with, and phosphorylation by, p38 MAPK. As shown in Fig. 10, the relative levels of p38 and MK5 will have an impact on the subcellular distribution of MK5 in unstimulated cells. A high level of MK5 relative to p38 will favor nuclear localization of MK5, while a shift in this balance towards p38 will favor relocalization of MK5 to the cytoplasm.
In conclusion, we have shown that MK5 shuttles between the nucleus and the cytosol and that nuclear import and export are mediated by a functional NLS and NES, respectively. The localization of the MK5 protein at a given time is thus a reflection of the relative activities of these two localization signals, and we have demonstrated that these are reciprocally regulated by both binding of p38 MAPK, which masks the functional NLS, and stress induced phosphorylation of MK5 mediated by p38 MAPK, which appears to activate or unmask the NES. These properties may define the ability of MK5 to differentially phosphorylate both nuclear and cytoplasmic targets or alternatively reflect a mechanism whereby signals initiated by activation of MK5 in the nucleus may be transmitted to the cytoplasm. Finally, the regulated nuclear export of proteins following phosphorylation by p38 MAPK may extend beyond the family of MKs such as MK2 and MK5, as it has recently been demonstrated that the stress-induced cytoplasmic accumulation of hnRNP A1, a protein which regulates the alternative splicing of mRNAs, is dependent on activation of p38 MAPK (47).
Acknowledgments
We thank R. Davis, J. Han, and M. Gaestel for providing plasmids; Chris Armstrong (MRC Phosphorylation Unit, Dundee, Scotland) for PRAKtide, recombinant MK5(PRAK), anti-MK5(PRAK) antibodies, and purified GST-p38α; and Espen Michaelsen (University of Tromsø) for technical assistance.
This work was supported by grants from the Norwegian Cancer Society (DNK, project number A01037) and the Norwegian Research Council (project number 123686/310 and 135823/310), by the Erna and Olav Aakre Foundation for Fighting Cancer, and by Cancer Research UK. B.J. is supported by the Norwegian Cancer Society (DNK).
REFERENCES
- 1.Ben-Levy, R., S. Hooper, R. Wilson, H. F. Paterson, and C. J. Marshall. 1998. Nuclear export of the stress-activated protein kinase p38 mediated by its substrate MAPKAP kinase-2. Curr. Biol. 8:1049-1057. [DOI] [PubMed] [Google Scholar]
- 2.Ben Levy, R., I. A. Leighton, Y. N. Doza, P. Attwood, N. Morrice, C. J. Marshall, and P. Cohen. 1995. Identification of novel phosphorylation sites required for activation of MAPKAP kinase-2. EMBO J. 14:5920-5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benveniste, R. J., S. Thor, J. B. Thomas, and P. H. Taghert. 1989. Cell type-specific regulation of the FMRF-NH2 neuropeptide gene by Apterous, a LIM homeodomain transcription factor. Development 125:4757-4765. [DOI] [PubMed] [Google Scholar]
- 4.Brunet, A., D. Roux, P. Lenormand, S. Dowd, S. Keyse, and J. Pouyssegur. 1999. Nuclear translocation of p42/p44 mitogen-activated protein kinase is required for growth factor-induced gene expression and cell cycle entry. EMBO J. 18:664-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, R. H., C. Sarnecki, and J. Blenis. 1992. Nuclear localization and regulation of erk- and rsk-encoded protein kinases. Mol. Cell. Biol. 12:915-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen, P. 1997. The search for physiological substrates of MAP and SAP kinases in mammalian cells. Trends Cell Biol. 7:353-361. [DOI] [PubMed] [Google Scholar]
- 7.Cokol, M., R. Nair, and B. Rost. 2000. Finding nuclear localization signals. EMBO Rep. 1:411-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuenda, A., J. Rouse, Y. N. Doza, R. Meier, P. Cohen, T. F. Gallagher, P. R. Young, and J. C. Lee. 1995. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 364:229-233. [DOI] [PubMed] [Google Scholar]
- 9.Cyert, M. S. 2001. Regulation of nuclear localization during signaling. J. Biol. Chem. 276:20805-20808. [DOI] [PubMed] [Google Scholar]
- 10.Deak, M., A. D. Clifton, L. M. Lucocq, and D. R. Alessi. 1998. Mitogen- and stress-activated protein kinase-1 (MSK1) is directly activated by MAPK and SAPK2/p38, and may mediate activation of CREB. EMBO J. 17:4426-4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engel, K., A. Kotlyarov, and M. Gaestel. 1998. Leptomycin B-sensitive nuclear export of MAPKAP kinase 2 is regulated by phosphorylation. EMBO J. 17:3363-3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fornerod, M., M. Ohno, M. Yoshida, and I. W. Mattaj. 1997. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell 90:1051-1060. [DOI] [PubMed] [Google Scholar]
- 13.Fukuda, M., S. Asano, T. Nakamura, M. Adachi, M. Yoshida, M. Yanagida, and E. Nishida. 1997. CRM1 is responsible for intracellular transport mediated by the nuclear export signal. Nature 390:308-311. [DOI] [PubMed] [Google Scholar]
- 14.Fukunaga, R., and T. Hunter. 1997. MNK1, a new MAP kinase-activated protein kinase, isolated by a novel expression screening method for identifying protein kinase substrates. EMBO J. 16:1921-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goedert, M., A. Cuenda, M. Craxton, R. Jakes, and P. Cohen. 1997. Activation of the novel stress-activated protein kinase SAPK4 by cytokines and cellular stresses is mediated by SKK3 (MKK6); comparison of its substrate specificity with that of other SAP kinases. EMBO J. 16:3563-3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorlich, D., and U. Kutay. 1999. Transport between the cell nucleus and the cytoplasm. Annu. Rev. Cell. Dev. Biol. 15:607-660. [DOI] [PubMed] [Google Scholar]
- 17.Groom, L. A., A. A. Sneddon, D. R. Alessi, S. Dowd, and S. M. Keyse. 1996. Differential regulation of the MAP, SAP and RK/p38 kinases by Pyst1, a novel cytosolic dual-specificity phosphatase. EMBO J. 15:3621-3632. [PMC free article] [PubMed] [Google Scholar]
- 18.Han, J., J. D. Lee, L. Bibbs, and R. J. Ulevitch. 1994. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science 265:808-811. [DOI] [PubMed] [Google Scholar]
- 19.Hefner, Y., A. G. Borsch-Haubold, M. Murakami, J. I. Wilde, S. Pasquet, D. Schieltz, F. Ghomashchi, J. R. Yates III, C. G. Armstrong, A. Paterson, P. Cohen, R. Fukunaga, T. Hunter, I. Kudo, S. P. Watson, and M. H. Gelb. 2000. Serine 727 phosphorylation and activation of cytosolic phospholipase A2 by MNK1-related protein kinases. J. Biol. Chem. 275:37542-37551. [DOI] [PubMed] [Google Scholar]
- 20.Heidenreich, O., A. Neininger, G. Schratt, R. Zinck, M. A. Cahill, K. Engel, A. Kotlyarov, R. Kraft, S. Kostka, M. Gaestel, and A. Nordheim. 1999. MAPKAP kinase 2 phosphorylates serum response factor in vitro and in vivo. J. Biol. Chem. 274:14434-14443. [DOI] [PubMed] [Google Scholar]
- 21.Holmberg, S., and P. Schjerling. 1996. Cha4p of Saccharomyces cerevisiae activates transcription via serine/threonine response elements. Genetics 144:467-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang, Y., C. Chen, Z. Li, W. Guo, J. A. Gegner, S. Lin, and J. Han. 1996. Characterization of the structure and function of a new mitogen-activated protein kinase (p38β). J. Biol. Chem. 271:17920-17926. [DOI] [PubMed] [Google Scholar]
- 23.Kotlyarov, A., A. Neininger, C. Schubert, R. Eckert, C. Birchmeier, H. D. Volk, and M. Gaestel. 1999. MAPKAP kinase 2 is essential for LPS-induced TNF-alpha biosynthesis. Nat. Cell Biol. 1:94-97. [DOI] [PubMed] [Google Scholar]
- 24.Kudo, N., B. Wolff, T. Sekimoto, E. P. Schreiner, Y. Yoneda, M. Yanagida, S. Horinouchi, and M. Yoshida. 1998. Leptomycin B inhibition of signal-mediated nuclear export by direct binding to CRM1. Exp. Cell Res. 242:540-547. [DOI] [PubMed] [Google Scholar]
- 25.Kumar, S., P. C. McDonnell, R. J. Gum, A. T. Hand, J. C. Lee, and P. R. Young. 1997. Novel homologues of CSBP/p38 MAP kinase: activation, substrate specificity and sensitivity to inhibition by pyridinyl imidazoles. Biochem. Biophys. Res. Commun. 235:533-538. [DOI] [PubMed] [Google Scholar]
- 26.Lee, J. C., J. T. Laydon, P. C. McDonnell, T. F. Gallagher, S. Kumar, D. Green, D. McNulty, M. J. Blumenthal, J. R. Heys, S. W. Landvatter, et al. 1994. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature 372:739-746. [DOI] [PubMed] [Google Scholar]
- 27.Li, Z., Y. Jiang, R. J. Ulevitch, and J. Han. 1996. The primary structure of p38 gamma: a new member of p38 group of MAP kinases. Biochem. Biophys. Res. Commun. 228:334-340. [DOI] [PubMed] [Google Scholar]
- 28.Ludwig, S., K. Engel, A. Hoffmeyer, G. Sithanandam, B. Neufeld, D. Palm, M. Gaestel, and U. R. Rapp. 1996. 3pK, a novel mitogen-activated protein (MAP) kinase-activated protein kinase, is targeted by three MAP kinase pathways. Mol. Cell. Biol. 16:6687-6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin-Blanco, E. 2000. p38 MAPK signalling cascades: ancient roles and new functions. Bioessays 22:637-645. [DOI] [PubMed] [Google Scholar]
- 30.McLaughlin, M. M., S. Kumar, P. C. McDonnell, S. Van Horn, J. C. Lee, G. P. Livi, and P. R. Young. 1996. Identification of mitogen-activated protein (MAP) kinase-activated protein kinase-3, a novel substrate of CSBP p38 MAP kinase. J. Biol. Chem. 271:8488-8492. [DOI] [PubMed] [Google Scholar]
- 31.Morley, S. J., and L. McKendrick. 1997. Involvement of stress-activated protein kinase and p38/RK mitogen-activated protein kinase signaling pathway.s in the enhanced phosphorylation of initiation factor 4E in NIH 3T3 cells. J. Biol. Chem. 272:17887-17893. [DOI] [PubMed] [Google Scholar]
- 32.Nebreda, A. R., and A. Porras. 2000. p38 MAP kinases: beyond the stress response. Trends Biochem. Sci. 25:257-260. [DOI] [PubMed] [Google Scholar]
- 33.Neufeld, B., A. Grosse-Wilde, A. Hoffmeyer, B. W. Jordan, P. Chen, D. Dinev, S. Ludwig, and U. R. Rapp. 2000. Serine/threonine kinases 3pK and MAPK-activated protein kinase 2 interact with the basic helix-loop-helix transcription factor E47 and repress its transcriptional activity. J. Biol. Chem. 275:20239-20242. [DOI] [PubMed] [Google Scholar]
- 34.New, L., Y. Jiang, M. Zhao, K. Liu, W. Zhu, L. J. Flood, Y. Kato, G. C. Parry, and J. Han. 1998. PRAK, a novel protein kinase regulated by the p38 MAP kinase. EMBO J. 17:3372-3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ni, H., X. S. Wang, K. Diener, and Z. Yao. 1998. MAPKAPK5, a novel mitogen-activated protein kinase (MAPK)-activated protein kinase, is a substrate of the extracellular-regulated kinase (ERK) and p38 kinase. Biochem. Biophys. Res. Commun. 243:492-496. [DOI] [PubMed] [Google Scholar]
- 36.Nomura, N., S. Sasamoto, S. Ishii, M. Matsui, and R. Ishizaki. 1989. Isolation of human cDNA clones of ski and the ski-related gene, sno. Nucleic Acids Res. 17:5489-5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poteet-Smith, C. E., J. A. Smith, D. A. Lannigan, T. A. Freed, and T. W. Sturgill. 1999. Generation of constitutively active p90 ribosomal S6 kinase in vivo. Implications for the mitogen-activated protein kinase-activated protein kinase family. J. Biol. Chem. 274:22135-22138. [DOI] [PubMed] [Google Scholar]
- 38.Raingeaud, J., S. Gupta, J. S. Rogers, M. Dickens, J. Han, R. J. Ulevitch, and R. J. Davis. 1995. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J. Biol. Chem. 270:7420-7426. [DOI] [PubMed] [Google Scholar]
- 39.Rouse, J., P. Cohen, S. Trigon, M. Morange, A. Alonso-Llamazares, D. Zamanillo, T. Hunt, and A. R. Nebreda. 1994. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell 78:1027-1037. [DOI] [PubMed] [Google Scholar]
- 40.Seternes, O. M., B. Johansen, and U. Moens. 1999. A dominant role for the Raf-MEK pathway in forskolin, 12-O-tetradecanoyl-phorbol acetate, and platelet-derived growth factor-induced CREB (cAMP-responsive element-binding protein) activation, uncoupled from serine 133 phosphorylation in NIH 3T3 cells. Mol. Endocrinol. 13:1071-1083. [DOI] [PubMed] [Google Scholar]
- 41.Sharrocks, A. D., S. H. Yang, and A. Galanis. 2000. Docking domains and substrate-specificity determination for MAP kinases. Trends Biochem. Sci. 25:448-453. [DOI] [PubMed] [Google Scholar]
- 42.Smith, J. A., C. E. Poteet-Smith, K. Malarkey, and T. W. Sturgill. 1999. Identification of an extracellular signal-regulated kinase (ERK) docking site in ribosomal S6 kinase, a sequence critical for activation by ERK in vivo. J. Biol. Chem. 274:2893-2898. [DOI] [PubMed] [Google Scholar]
- 43.Tan, Y., J. Rouse, A. Zhang, S. Cariati, P. Cohen, and M. J. Comb. 1996. FGF and stress regulate CREB and ATF-1 via a pathway involving p38 MAP kinase and MAPKAP kinase-2. EMBO J. 15:4629-4642. [PMC free article] [PubMed] [Google Scholar]
- 44.Tanoue, T., M. Adachi, T. Moriguchi, and E. Nishida. 2000. A conserved docking motif in MAP kinases common to substrates, activators and regulators. Nat. Cell Biol. 2:110-116. [DOI] [PubMed] [Google Scholar]
- 45.Tanoue, T., R. Maeda, M. Adachi, and E. Nishida. 2001. Identification of a docking groove on ERK and p38 MAP kinases that regulates the specificity of docking interactions. EMBO J. 20:466-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tomas-Zuber, M., J. L. Mary, F. Lamour, D. Bur, and W. Lesslauer. 2001. C-terminal elements control location, activation threshold, and p38 docking of ribosomal S6 kinase B (RSKB). J. Biol. Chem. 276:5892-5899. [DOI] [PubMed] [Google Scholar]
- 47.van der Houven van Oordt, W., M. T. Diaz-Meco, J. Lozano, A. R. Krainer, J. Moscat, and J. F. Caceres. 2000. The MKK(3/6)-p38-signaling cascade alters the subcellular distribution of hnRNP A1 and modulates alternative splicing regulation. J. Cell Biol. 149:307-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Drogen, F., V. M. Stucke, G. Jorritsma, and M. Peter. 2001. MAP kinase dynamics in response to pheromones in budding yeast. Nat. Cell Biol. 3:1051-1059. [DOI] [PubMed] [Google Scholar]
- 49.Wang, X. S., K. Diener, C. L. Manthey, S. Wang, B. Rosenzweig, J. Bray, J. Delaney, C. N. Cole, P. Y. Chan-Hui, N. Mantlo, H. S. Lichenstein, M. Zukowski, and Z. Yao. 1997. Molecular cloning and characterization of a novel p38 mitogen-activated protein kinase. J. Biol. Chem. 272:23668-23674. [DOI] [PubMed] [Google Scholar]
- 50.Wang, X. Z., and D. Ron. 1996. Stress-induced phosphorylation and activation of the transcription factor CHOP (GADD153) by p38 MAP kinase. Science 272:1347-1349. [DOI] [PubMed] [Google Scholar]
- 51.Waskiewicz, A. J., A. Flynn, C. G. Proud, and J. A. Cooper. 1997. Mitogen-activated protein kinases activate the serine/threonine kinases Mnk1 and Mnk2. EMBO J. 16:1909-1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wolff, B., J. J. Sanglier, and Y. Wang. 1997. Leptomycin B is an inhibitor of nuclear export: inhibition of nucleo-cytoplasmic translocation of the human immunodeficiency virus type 1 (HIV-1) Rev protein and Rev-dependent mRNA. Chem. Biol. 4:139-147. [DOI] [PubMed] [Google Scholar]
- 53.Zhao, M., L. New, V. V. Kravchenko, Y. Kato, H. Gram, F. di Padova, E. N. Olson, R. J. Ulevitch, and J. Han. 1999. Regulation of the MEF2 family of transcription factors by p38. Mol. Cell. Biol. 19:21-30. [DOI] [PMC free article] [PubMed] [Google Scholar]