Abstract
Grb14 is a member of the Grb7 family of adapters and acts as a negative regulator of insulin-mediated signaling. Here we found that the protein kinase Cζ (PKCζ) interacting protein, ZIP, interacted with Grb14. Coimmunoprecipitation experiments demonstrated that ZIP bound to both Grb14 and PKCζ, thereby acting as a link in the assembly of a PKCζ-ZIP-Grb14 heterotrimeric complex. Mapping studies indicated that ZIP interacted through its ZZ zinc finger domain with the phosphorylated insulin receptor interacting region (PIR) of Grb14. PKCζ phosphorylated Grb14 under in vitro conditions and in CHO-IR cells as demonstrated by in vivo labeling experiments. Furthermore, Grb14 phosphorylation was increased under insulin stimulation, suggesting that the PKCζ-ZIP-Grb14 complex is involved in insulin signaling. The PIR of Grb14, which also interacts with the catalytic domain of the insulin receptor (IR) and inhibits its activity, was preferentially phosphorylated by PKCζ. Interestingly, the phosphorylation of Grb14 by PKCζ increased its inhibitory effect on IR tyrosine kinase activity in vitro. The role of ZIP and Grb14 in insulin signaling was further investigated in vivo in Xenopus laevis oocytes. In this model, ZIP potentiated the inhibitory action of Grb14 on insulin-induced oocyte maturation. Importantly, this effect required the recruitment of PKCζ and the phosphorylation of Grb14, providing in vivo evidences for a regulation of Grb14-inhibitory action by ZIP and PKCζ. Together, these results suggest that Grb14, ZIP, and PKCζ participate in a new feedback pathway of insulin signaling.
Molecular adapters are proteins composed of the juxtaposition of various protein-protein or protein-lipid interacting domains and devoided of enzymatic activity. These proteins are essential components of signal transduction pathways. The Grb7 family of adapters, which comprises Grb7, Grb10, and Grb14, is implicated in receptor tyrosine kinase (RTK) signaling (7, 41). Growing evidence is emerging for an inhibitory role of Grb14 and Grb10 in insulin signaling. Grb14 is selectively expressed in insulin-sensitive tissues and upon insulin stimulation interacts in vivo with the insulin receptor (IR). Moreover, the overexpression of Grb14 in the CHO-IR cell line was shown to inhibit insulin-stimulated tyrosine phosphorylation of specific proteins, like IRS-1, and distal effects, like DNA and glycogen synthesis (23, 28). Despite controversial findings (46, 52, 71), overexpression studies of Grb10 isoforms are also consistent with a negative role in insulin signaling (34, 44, 48). The molecular analysis of the interaction between the Grb7 family of proteins and IR recently led to clues on their inhibitory action. These proteins interact in an insulin-dependent manner with the activated tyrosine kinase loop of the IR, and this interaction is mediated by their C-terminal region, containing the phosphorylated IR interacting region (PIR, also known as BPS, between the pleckstrin homology [PH] domain and the Src 2 homology [SH2] domain) and SH2 domains (17, 21, 22, 27, 28, 34). Using in vitro tyrosine kinase assays, it was recently shown that the binding of the PIR inhibits IR tyrosine kinase activity (2, 67).
In addition to the PIR and SH2 domains, members of the Grb7 family of proteins contain several conserved interacting regions, including a central PH domain, and an N-terminal proline-rich motif, which conforms to the consensus sequence of an SH3 binding site. These domains are binding sites for various proteins which are potentially involved in RTK signaling. Many reports have described interactions of the Grb proteins with various RTKs, but fewer studies have identified partners involved in post-receptor signaling steps, most of them concerning Grb10 partners (for a review, see reference 20). Kinases, like the serine/threonine kinases MEK1 and Raf1 or the tyrosine kinases Src and Tec, and the ubiquitin ligase Nedd4 have been shown to interact with the SH2 domain of Grb10 (33, 38, 45, 50). It has also been proposed that cAbl interacts with the proline-rich motif of Grb10 (17). To date, there is only one report of a nonreceptor Grb14 interacting protein, a novel human tankyrase that has been identified in association with the N-terminal domain of Grb14. This tankyrase is an ankyrin repeat-containing protein which is likely to be involved in the subcellular localization of Grb14 (36).
To identify new downstream partners of Grb14, we performed a two-hybrid screen of a rat liver cDNA library using the C-terminal domain of Grb14 as a bait. In this study we showed that the molecular adapter ZIP (protein kinase Cζ [PKCζ] interacting protein), also known as p62 (55, 60), interacted with Grb14. ZIP linked the atypical PKCζ to Grb14 into a heterotrimeric complex in vivo. In addition, we demonstrated that PKCζ phosphorylated Grb14 and increased its inhibitory action on IR catalytic activity in vitro. Of functional relevance, these data correlated with the regulation by Grb14 and ZIP of insulin-induced maturation of Xenopus laevis oocyte. Together, these findings suggest that Grb14, ZIP, and PKCζ are involved in a common down-regulation pathway of insulin signaling.
MATERIALS AND METHODS
Reagents and antibodies.
The following reagents were purchased from the indicated sources: oligonucleotides, culture media, and G418 (Geneticin) were from Life Technologies; human recombinant PKCζ was from Upstate Biotechnology Inc.; cell-permeable myristoylated PKCζ pseudosubstrate inhibitor (Myr-SIYRRGARRWKRL) was from Calbiochem; hygromycin and monoclonal antihemagglutinin (anti-HA) antibodies were from Roche Molecular Biochemicals; monoclonal anti-myc and anti-V5 antibodies were from Invitrogen; monoclonal anti-ZIP (p62) antibodies were from Transduction Laboratories, Inc.; polyclonal anti-PKCζ and anti-extracellular signal-regulated kinase 2 (anti-ERK2) antibodies were from Santa Cruz; and poly Glu-Tyr (4:1) and all chemicals were from Sigma France. Polyclonal anti-Grb14 antibodies were described previously (28). Polyclonal anti-ZIP antibodies were raised against a glutathione S-transferase (GST)-ZIPΔAID fusion protein and purified on protein A-agarose before use.
Yeast two-hybrid screen.
A yeast two-hybrid screen was performed in the Saccharomyces cerevisiae strain L40 using on, one hand, pFBL23-Grb14, encoding the C-terminal domain of Grb14 (amino acids 346 to 538) in N-terminal fusion with the LexA binding domain and, on the other hand, an oligo(dT)-primed cDNA library from a rat liver, cloned in fusion with the Gal4 activation domain in the pGAD3S2X plasmid, as previously described (28). The plasmids of the library producing yeast colonies of a His+ LacZ+ phenotype were isolated, and the specificity of association of their products with Grb14 was tested using pLex-lamin as a negative control. The cDNA inserts of these positive plasmids were sequenced.
5′ RACE and cDNA cloning.
pGAD-3′ZIP fragment, encompassing amino acids 104 to 440 of rat ZIP, was obtained by the two hybrid screen. To clone the 5′ end of the ZIP cDNA, the 5′ rapid amplification of cDNA ends (5′ RACE) technique was used on a rat liver Marathon Ready premade cDNA library (Clontech), with the Advantage cDNA PCR kit (Clontech). We used a primer corresponding to the 5′ sequence of ZIP, upstream from the ATG, according to the published sequence of rat ZIP (GenBank accession no.Y08355) and an antisense primer designed in the cloned insert. We obtained a 940-bp fragment corresponding to the 5′ end of the cDNA. This fragment was subcloned by TA cloning into the pTAdv vector (Clontech). An EcoRI/BamHI fragment (750 bp long) was then excised from the pTAdv-5′ZIP plasmid and ligated to pGAD-3′ZIP digested by EcoRI and BamHI, generating pGAD-ZIP (full length).
Plasmid constructions.
ZIPα, ZIPβ, ZIPΔAID+ZZ (Δ1-163), ZIPΔAID (Δ1-103) ZIPZZ (104-163), ZIPAID+ZZ (1-163) were amplified from the pGAD-ZIP plasmid using primers to introduce an EcoRI site in the 5′ end and an XhoI site in the 3′ end and then cloned in frame in a pGEX4T2 vector (Amersham Pharmacia Biotech) to produce GST-ZIP fusion proteins. ZIPα and ZIPβ were also cloned in pcDNA6/V5-HisC (Invitrogen) for expression of a V5 epitope-tagged version. All constructs were verified by DNA sequence analysis. pECE-myc-Grb14, pGEX-Grb7, pGEX-Grb10, and all pGEX-Grb14 constructs were described previously (2, 28).
Cell culture and preparation of lysates.
Chinese hamster ovary cells stably overexpressing IR (CHO-IR) or IR and Grb14 (CHO-IR-Grb14) were grown in Ham F-12 medium supplemented with 1 mM glutamine, penicillin (100 U/ml), streptomycin (100 μg/ml), and 10% fetal bovine serum, in the presence of G418 (75 μg/ml) (CHO-IR) and with the addition of hygromycin (100 μg/ml) (CHO-IR-Grb14) (28). COS cells were grown in Dulbecco's modified Eagle's medium, supplemented with 1 mM glutamine, penicillin (100 U/ml), streptomycin (100 μg/ml), and 10% fetal bovine serum. Confluent cells were transiently transfected using Lipofectamine (Invitrogen) as recommended by the manufacturer. Cells were serum starved for 24 h and stimulated or not with insulin (10−7 M) for 10 min at 37°C. They were then solubilized at 4°C in lysis buffer (20 mM Tris-HCl [pH 7.5], 150 mM NaCl, 5 mM EDTA, 30 mM sodium pyrophosphate, 50 mM NaF, 1% Triton X-100, pepstatin [1 μg/ml], leupeptin [2 μg/ml], aprotinin [5 μg/ml], 1 mM phenylmethylsulfonyl fluoride, 1 mM sodium orthovanadate) with gentle rotation at 4°C for 30 min. Cell lysates were then cleared by a 15,000 × g centrifugation at 4°C for 15 min.
Production of GST fusion proteins.
Purified GST fusion proteins were produced as described previously (29). Fusion proteins were quantified by Coomassie blue staining after sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis. For in vitro phosphorylation experiments and Xenopus oocyte microinjections, the fusion proteins were eluted from the glutathione-Sepharose beads with 50 mM Tris-HCl, pH 8, containing 10 mM reduced glutathione, and quantified as described above.
In vitro interaction assays.
Cell lysates (250 μg) were incubated overnight at 4°C with 3 μg of immobilized GST fusion proteins. For in vitro interaction with PKCζ, 2.5 ng of human recombinant PKCζ was incubated during 1 h at 4°C in 500 μl of lysis buffer with 1.5 μg of immobilized GST fusion proteins. After extensive washing, bound proteins were eluted by heating in SDS sample buffer, separated by SDS-PAGE, and transferred to nitrocellulose. We verified by Ponceau red staining of the nitrocellulose membranes that equal amounts of GST fusions were used. Membranes were then subjected to immunodetection with the indicated antibodies. Immunoreactive bands were revealed using an ECL detection kit (Amersham Pharmacia Biotech).
Immunoprecipitation and Western blot analysis.
For coimmunoprecipitation experiments, cells were transiently transfected with the indicated expression plasmids. Beginning at 48 h after transfection, cells were serum starved for 24 h and stimulated or not with insulin (10−7 M) for 10 min at 37°C. Cell lysates were prepared as described above. After a preclearing step, 500 μg of cell lysates was incubated overnight at 4°C with the indicated antibodies in the presence of 100 μl of protein A-agarose. The immunoprecipitates were washed three times in lysis buffer, subjected to SDS-PAGE, and immunodetected with the indicated antibodies. Immunoreactive bands were revealed as explained above.
For in vivo studies in rats, liver proteins were extracted in lysis buffer, and then lysates were solubilized with gentle rotation for 90 min at 4°C and cleared by a 15,000 × g centrifugation at 4°C for 15 min. Protein concentrations were determined using a bicinchoninic acid protein assay (Pierce). Immunoprecipitations were performed as described above.
In vitro phosphorylation of Grb14 by PKCζ.
The PKCζ kinase assays were performed essentially as recommended by the manufacturer (Upstate Biotechnology Inc.). Human recombinant PKCζ (50 ng) was preincubated with the indicated amounts of eluted GST fusion proteins during 10 min at room temperature in assay dilution buffer II (20 mM MOPS [pH 7.2], 25 mM β-glycerophosphate, 1 mM sodium orthovanadate, 1 mM dithiothreitol, 1 mM calcium chloride). The kinase reaction (final reaction mixture volume, 60 μl) was initiated by the addition of 5 μg of phosphatidylserine, 7.5 mM MgCl2, 50 μM ATP, and 5μCi of [γ-32P]ATP. After a 30-min incubation at 30°C, the reaction was stopped by the addition of Laemmli sample buffer and the samples were boiled for 5 min. Samples were then subjected to SDS-10% PAGE, and the bands corresponding to phosphorylated proteins were detected by autoradiography. For in vitro IR tyrosine kinase assays with GST-PIR-SH2, and for microinjection of phosphorylated GST-PIR into Xenopus oocytes, 2 μg of GST fusion proteins was incubated with 50 ng of PKCζ as described above. Various amounts of GST fusion proteins were obtained by dilution with assay dilution buffer II.
Metabolic labeling.
Confluent CHO-IR cells were transiently transfected with myc-Grb14, HA-PKCζ, and pcDNA6 (empty vector) as indicated. Beginning at 24 h after transfection, cells were serum starved for 16 h. Cells were labeled for 3 h in the same medium containing 100 μCi/ml of [32P]orthophosphate (Amersham Pharmacia Biotech) and then stimulated or not with insulin (10−7M) for 10 min at 37°C. When indicated, 20 μM PKCζ pseudosubstrate inhibitor was added 30 min before insulin stimulation. Cells were washed twice in KRBH buffer, pH 7.4 (107 mM NaCl, 5 mM KCl, 3 mM CaCl2, 1 mM MgSO4, 20 mM HEPES, 10 mM glucose, 1% bovine serum albumin, 7 mM NaHCO3) and scraped into lysis buffer. Lysates were cleared by a 15,000 × g centrifugation at 4°C for 15 min. The supernatant fractions were immunoprecipitated with anti-Grb14 polyclonal antibodies. Immunocomplexes were washed three times with lysis buffer, boiled in Laemmli sample buffer, separated by SDS-10% PAGE, and transferred to nitrocellulose. Phosphorylated Grb14 was detected with a PhosphorImager (Molecular Dynamics) and quantified (ImageQuant). The nitrocellulose membranes were then immunodetected using anti-myc antibodies.
IR tyrosine kinase activity in vitro assays.
IRs were partially purified by affinity chromatography on wheat germ agglutinin (WGA), essentially as described previously (2). WGA-purified receptors were incubated for 1 h at room temperature in the presence or in the absence of 100 nM insulin, in 30 mM HEPES buffer (pH 7.6), supplemented with 30 mM NaCl, 0.1% Triton X-100, and 0.03% bovine serum albumin. The autophosphorylation of receptors was initiated by the addition of phosphorylation buffer (8 mM MgCl2, 4 mM MnCl2, 20 μM ATP, and 5 μCi of [γ-32P]ATP per sample) for 30 min at room temperature, in the presence of variable amounts of GST-PIR-SH2 of Grb14, previously phosphorylated by PKCζ or not. The tyrosine kinase assay was performed using 15 μg of the synthetic substrate poly Glu-Tyr (4:1). After 30 min the reaction was stopped by spotting the mixture onto Whatman ET31 filter paper squares (2 by 2 cm), which were treated as described previously (2). Results were expressed as a percentage of the maximal insulin-stimulated kinase activity measured in the absence of added fusion proteins.
Xenopus oocyte studies.
After anesthesia with MS 222 (1 g/liter; Sandoz), Xenopus laevis ovarian fragments were surgically removed and placed in modified OR2 medium lacking potassium (82.5 mM NaCl, 1 mM MgCl2, 1 mM CaCl2, 1 mM Na2HPO4, 5 mM HEPES, adjusted to pH 7.4), supplemented with streptomycin-penicillin (each at 50 μg/ml; Eurobio), tetracycline (50 μg/ml; Sigma), sodium pyruvate (225 μg/ml; Sigma), and soybean trypsin inhibitor (30 μg/ml; Sigma). Stage VI oocytes were harvested by using 1-h treatment with collagenase A (1 mg/ml; Boehringer Mannheim). Complete defolliculation of the oocytes was achieved by manual dissection. The oocytes were kept at 19°C in the modified OR2 medium. Microinjections of eluted GST fusion proteins were performed in the equatorial region of the oocyte. Germinal vesicle breakdown (GVBD) was determined by the appearance of a white spot at the center of the animal pole. For ERK2 phosphorylation analysis, oocytes were lysed in ice-cold homogenization buffer (60 mM β-glycerophosphate, 15 mM nitrophenylphosphate, 15 mM EGTA, 15 mM MgCl2, 2 mM dithiothreitol, 1 mM sodium orthovanadate, 1 mM NaF, leupeptin [10 μg/ml], aprotinin [10 μg/ml], soybean trypsin inhibitor [10 μg/ml], 1 mM phenylmethylsulfonyl fluoride, 100 μM benzamidine, 25 mM MOPS [pH 7.2]). Electrophoresis was performed on 15% modified polyacrylamide gels (30% acrylamide and 0.2% bisacrylamide). Western blot analysis was performed as described above.
RESULTS
Cloning of ZIP by interaction with Grb14 in a yeast two-hybrid screen.
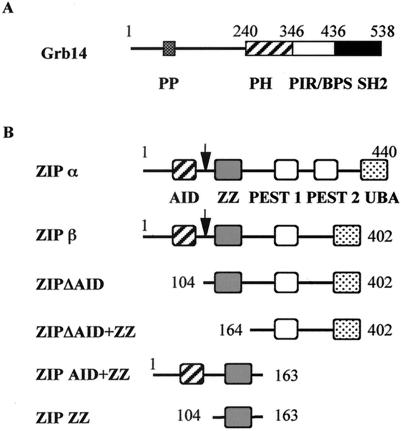
A rat liver cDNA library was screened using the C-terminal domain of Grb14, including the PIR and SH2 domains as a bait (amino acids 346 to 538) (Fig. 1A). The rat used for the library construction had been starved for 48 h and refed with a high-carbohydrate diet, to favor the identification of genes involved in insulin-stimulated metabolism. From 2 × 106 tested colonies, eight clones contained plasmids encoding proteins interacting with Grb14, and not with a nonspecific control like lamin. Sequence analysis of the cDNAs inserted in these plasmids revealed that all encoded the same protein, although they can be divided into two groups, differing by a deletion of 114 bp in the 3′ end. The longest insert was 1.6 kb and encoded the last 337 amino acids of the protein ZIP (amino acids 104 to 440). ZIP was previously isolated by its interaction with the atypical PKCζ (55, 60). ZIP also interacts with several proteins like p56lck (26), RIP (62), TRAF6 (61), COUP-TFII (40), and Kvβ2 (18). The full-length rat ZIP cDNAs were cloned using the 5′-RACE technique. The full-length 440-amino-acid ZIP protein was called ZIPα. The shorter form, encoded by the cDNA with 114 bp deleted, carried a deletion of amino acids 349 to 387 and was called ZIPβ. This variant is different from the previously reported isoform ZIP2 (deletion of amino acids 222 to 248) (18). ZIP is a molecular adapter, characterized by the succession of several conserved protein domains (Fig. 1B): an N-terminal atypical PKC-interacting domain (AID), a cysteine-rich sequence called ZZ zinc finger domain, two central PEST domains, which are likely to be implicated in protein stability, and a C-terminal ubiquitin-associated domain potentially involved in ubiquitination-mediated protein degradation (55, 61, 70). The ZIPβ isoform lacked the second PEST domain.
FIG. 1.
Schematic representation of Grb14 and ZIP proteins. (A) Grb14. Shown are the proline-rich motif (PP); the PH domain; the PIR (28), also called BPS, between the PH and SH2 domain (22); and the SH2 domain. (B) ZIP and various ZIP constructs. The arrow indicates the length of the ZIP protein isolated in the two-hybrid screen. Shown are the AID (47), ZZ, PEST sequences (PEST2 is lacking in the ZIPβ isoform), and ubiquitin-associated (UBA) domain (70).
ZIP forms a heteromultimeric complex with Grb14 and PKCζ.
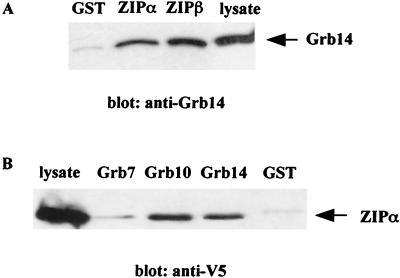
The Grb14-ZIP interaction observed in yeast was first confirmed in vitro using GST pull down assays. Immobilized GST-ZIPα and GST-ZIPβ were incubated with CHO-IR-Grb14 cell lysates, and retained proteins were analyzed on anti-Grb14 immunoblots. As shown in Fig. 2A, Grb14 was similarly retained by GST-ZIPα and GST-ZIPβ. GST alone did not bind to Grb14. Based on the observation that PIR and SH2 domains are conserved between the members of Grb7 family (8, 28), we compared their respective binding abilities to that of ZIPα. A series of constructs of each Grb7 family protein fused to GST were immobilized on glutathione-Sepharose and used to pull down ZIPα from lysates of transiently transfected COS cells. As shown in Fig. 2B, Grb10 and Grb14 bound to ZIPα, whereas no interaction was detected with Grb7.
FIG. 2.
Interaction of Grb14 with ZIP in vitro. (A) ZIP isoforms expressed as GST-fusion proteins or GST control were immobilized on glutathione-Sepharose beads and incubated with lysates of CHO-IR-Grb14 cells. Bound proteins were immunodetected with anti-Grb14 polyclonal antibodies. (B) Grb7 family members expressed as immobilized GST-fusion proteins were incubated with lysates of COS cells transiently transfected with V5 tagged ZIPα. Bound proteins were detected by immunodetection with anti-V5 monoclonal antibodies. These blots are representative of 3 different experiments.
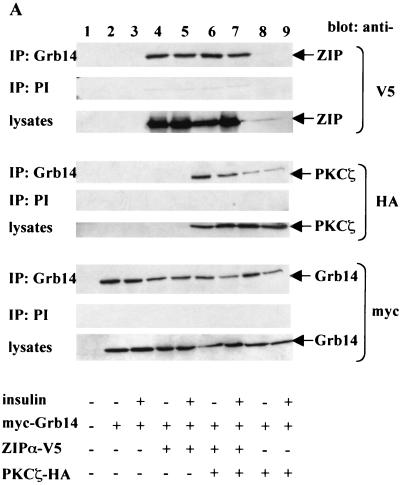
To verify that the interaction between Grb14 and ZIP also occurs in intact mammalian cells, CHO-IR cells were transiently transfected with myc-Grb14 and V5-ZIPα. At 48 h after transfection, cells were stimulated or not with insulin, and cell lysates were immunoprecipitated with anti-Grb14 polyclonal antibodies. The anti-Grb14 immunoprecipitates were analyzed with anti-V5 antibodies. As shown in Fig. 3A (upper blots, lanes 4 and 5), V5-ZIPα coprecipitated with myc-Grb14 under basal conditions as well as after insulin stimulation. Similar results were obtained when using the V5-ZIPβ expression plasmid (data not shown).
FIG. 3.
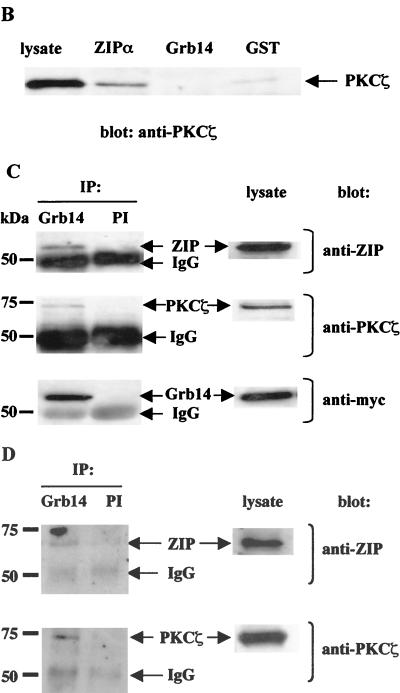
ZIP forms a ternary complex with Grb14 and PKCζ in vivo. (A) CHO-IR cells were transiently transfected as indicated with 1 μg of either pECE myc-Grb14, pcDNA6 V5-ZIPα, or pcDNA3 HA-PKCζ. pcDNA6 empty plasmid was used to normalize total DNA amount to 3 μg. At 48 h after transfection, cells were stimulated or not with 10−7 M insulin. Cell extracts were immunoprecipitated with polyclonal anti-Grb14 antibodies. Control immunoprecipitations using rabbit preimmune serum were simultaneously performed (PI). The immunoprecipitates were separated by SDS-PAGE and immunoblotted with monoclonal anti-HA, anti-V5, or anti-myc antibodies. Lysates of the same cells were separated by SDS-PAGE and immunoblotted with the same antibodies to confirm equal expressions levels of proteins. (B) Grb14 does not interact with PKCζ in vitro. ZIPα and Grb14 expressed as GST fusion proteins were incubated with human recombinant PKCζ. Bound PKCζ was detected by Western blotting using anti-PKCζ antibodies. (C) Lysates from COS cells transiently transfected with myc-Grb14 were immunoprecipitated with polyclonal anti-Grb14 antibodies. Control immunoprecipitation using rabbit preimmune serum was simultaneously performed. The immunoprecipitates were separated by SDS-PAGE and immunoblotted as indicated with monoclonal anti-ZIP antibodies, anti-myc antibodies, or polyclonal anti-PKCζ antibodies. Lysates of the same cells were analyzed in parallel. (D) Rat liver extracts were immunoprecipitated with polyclonal anti-Grb14 antibodies cross-linked to protein A-agarose. Control immunoprecipitation using rabbit preimmune serum was simultaneously performed. Immunoprecipitates were analyzed by sequential immunodetection using polyclonal anti-ZIP and anti-PKCζ antibodies. In this figure the blots are representative of two or three independent experiments, and lysates and preimmune controls were analyzed on the same gels as experimental samples.
Previous studies have shown that ZIP interacts with PKCζ and behaves as a link between PKCζ and other proteins, such as RIP (62) or Kvβ2 (18), in ternary complexes. Therefore, we next investigated whether ZIP could simultaneously interact with Grb14 and PKCζ. CHO-IR cells were transfected with myc-Grb14, V5-ZIPα, and HA-PKCζ, and cell extracts were immunoprecipitated with anti-Grb14 antibodies. The immunoprecipitates were then analyzed with either anti-V5 or anti-HA antibodies. The results of Fig. 3A (lanes 6 and 7) indicate that V5-ZIPα and HA-PKCζ coprecipitated with myc-Grb14, in the basal state as well as after insulin stimulation. To further demonstrate that ZIP is the link that connects PKCζ to Grb14, CHO-IR cells were transfected with only myc-Grb14 and HA-PKCζ. Interestingly, HA-PKCζ was immunoprecipitated with the myc-Grb14 antibodies. However, the amount of PKCζ immunoprecipitated was lower than in the presence of coexpressed V5-ZIPα (middle blots [compare lanes 8 and 9 to lanes 6 and 7]). As ZIP is expressed in CHO cells (data not shown), endogenous ZIP was likely to mediate the interaction between Grb14 and PKCζ. To verify this hypothesis, we tested direct in vitro interaction between PKCζ and Grb14. Immobilized GST-ZIPα and GST-Grb14 were incubated with recombinant PKCζ, and retained proteins were analyzed on anti-PKCζ immunoblots. As shown in Fig. 3B, PKCζ was retained by GST-ZIPα as expected, whereas it did not interact with GST-Grb14 or GST alone. All together, these results indicate that ZIP forms a bridge between Grb14 and PKCζ.
Next, we attempted to confirm that these interactions also occur under more-physiological conditions. Since Grb14 was not expressed in various cell lines, like CHO or COS cells (data not shown), we investigated whether endogenous ZIP and PKCζ can form a complex with ectopically expressed Grb14. COS cells were transiently transfected with myc-Grb14. Under these conditions, the level of expression of Grb14 protein was about fourfold higher than that in rat liver (data not shown). Cell extracts were immunoprecipitated with anti-Grb14 antibodies, and the immunoprecipitates were analyzed with either monoclonal anti-ZIP or polyclonal anti-PKCζ antibodies. As reported in Fig. 3C, endogenous ZIP and PKCζ coprecipitated with myc-Grb14, whereas no interaction was detected with nonspecific rabbit immunoglobulin G.
Grb14 is specifically expressed in insulin-sensitive tissues (28). Then, we used rat liver extracts to further demonstrate the heterotrimeric complex formation in vivo, in a physiological target of insulin. In agreement with the data obtained with transfected cell lines, we were able to detect a ternary complex between endogenous Grb14, ZIP, and PKCζ in a coimmunoprecipitation experiment using anti-Grb14 antibodies (Fig. 3D). This result suggests that the constitution of the heterotrimeric complex may be physiologically relevant.
Mapping of regions involved in the Grb14-ZIP association.
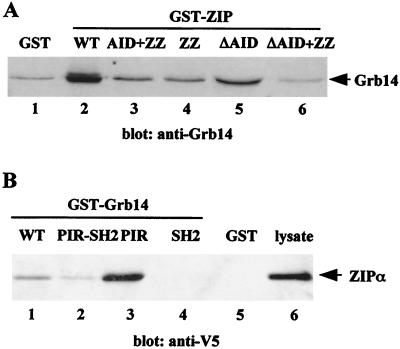
In order to determine which region of ZIP is required for the interaction with Grb14, a series of ZIP constructs fused to GST (Fig. 1B) was used to pull down Grb14 from lysates of CHO-IR-Grb14 cells. As shown in Fig. 4A, deletion of the N terminus of ZIP, containing the AID and ZZ domains, suppressed Grb14 interaction (lanes 1, 2, and 6). The complementary construct, amino acids 1 to 163 of ZIP, retained Grb14, but to a lower extent than wild-type (WT) ZIP (compare lanes 2 and 3). This suggests that the presence of the following domains in C terminus of the ZZ domain is required for a correct folding of the protein, and thus for a full binding activity. The ΔAID construct, containing the ZZ domain and the C terminus of the protein, bound Grb14, but also with a lower affinity than WT ZIP (compare lanes 2 and 5). This decreased binding could be explained by a role of the AID domain, ZIP amino acids 1 to 103, in Grb14 binding. However, this is unlikely since (i) a similar amount of Grb14 was retained by the AID-ZZ and the ZZ domains (lanes 3 and 4); (ii) the clones isolated in the two-hybrid screen did not contain the AID domain (Fig. 1B), suggesting that it is dispensable for the ZIP-Grb14 interaction in yeast; and (iii) similar amounts of WT ZIP and ZIPΔAID were retained in anti-Grb14 immunoprecipitation experiments performed in mammalian cells (data not shown). Thus, more probably, the presence of the GST in N terminus of the ZIPΔAID construct had altered the protein folding and partially masked the ZZ interaction domain. Although we cannot exclude from these experiments the possibility that other domains of ZIP participate in the interaction with Grb14, these results are in favor of a role of the ZZ domain (amino acids 104 to 163) in Grb14 binding.
FIG. 4.
Mapping of domains involved in the Grb14-ZIP interaction. (A) ZIPα and various ZIP constructs expressed as GST fusion proteins were incubated with lysates of CHO-IR-Grb14 cells. Bound proteins were immunodetected with anti-Grb14 antibodies. (B) Various Grb14 constructs expressed as GST fusion proteins were incubated with lysates of COS cells transiently transfected with V5-ZIPα. Bound proteins were immunodetected using anti-V5 antibodies. Ponceau red staining of the proteins after transfer confirmed that equivalent amounts of GST fusions were loaded. These blots are representative of three different experiments.
By the same approach, using a series of Grb14 deletion constructs fused to GST, we investigated the role of the different domains of Grb14 in its binding to ZIP. Figure 4B clearly shows that the strongest binding was detected with the PIR domain alone. By contrast, the SH2 domain was unable to bind to ZIPα. The PIR-SH2 domain and the full-length Grb14 bound to ZIP to a lower extent than did the PIR alone. This decreased binding could indicate that the presence of the other domains modifies the conformation of the PIR and reduces its binding affinity to ZIP. Collectively, these results suggest that the Grb14-ZIP binding is mediated by the PIR-ZZ interaction.
PKCζ phosphorylates Grb14 in vitro and in intact cells.
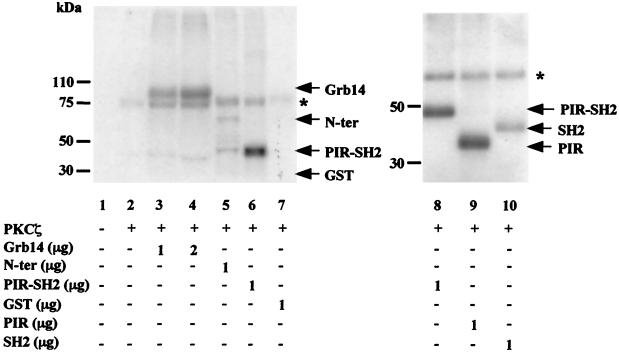
Previous studies reported that ZIP allows PKCζ to be associated with specific effectors in signaling complexes and to phosphorylate them (18). Thus, to determine whether PKCζ was able to phosphorylate Grb14, we performed in vitro phosphorylation assays using human recombinant PKCζ and GST-Grb14 fusion protein. Incubation of PKCζ with increasing amounts of GST-Grb14 resulted in an increased phosphorylation of GST-Grb14, whereas control GST was not phosphorylated (Fig. 5, lanes 3, 4, and 7). The addition of GST-ZIPα or GST-ZIPβ to the reaction did not modify the level of Grb14 phosphorylation (data not shown), suggesting that under in vitro conditions, ZIP was dispensable for Grb14 phosphorylation by PKCζ. To determine if a particular domain of Grb14 was preferentially phosphorylated by PKCζ, similar experiments were performed using various GST-Grb14 constructs. The C-terminal domain of Grb14, including the PIR and SH2 domains (amino acids 346 to 538), was more intensively phosphorylated than the N-terminal domain (amino acids 1 to 346) (Fig. 5, lanes 5 and 6). Interestingly, the PIR was a much better substrate for PKCζ than the SH2 domain (Fig. 5, lanes 8 to 10).
FIG. 5.
PKCζ phosphorylates Grb14 in vitro. Phosphorylation assays were performed in vitro using human recombinant PKCζ as described in Material and Methods. GST fusion proteins were used as substrate as indicated. Phosphorylated proteins were subjected to SDS-PAGE and analyzed by autoradiography. The asterisk indicates autophosphorylated PKCζ. These blots are representative of four different experiments.
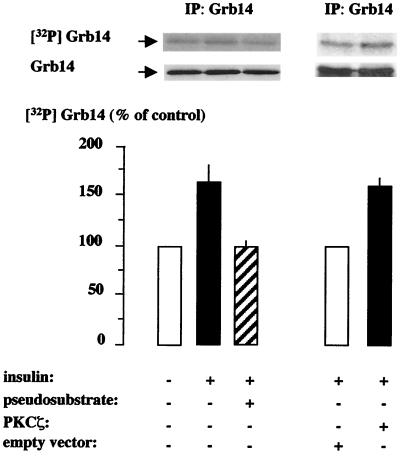
In order to assess the phosphorylation state of Grb14 in intact cells, we performed in vivo metabolic labeling experiments. First, CHO-IR cells were transiently transfected with myc-Grb14, labeled with [32P]orthophosphate, and stimulated or not with insulin for 10 min. Consistent with previous results (8, 58), Grb14 was phosphorylated under basal conditions. Treatment of the cells with insulin induced a 60% increase in Grb14 level of phosphorylation (Fig. 6). The addition of cell-permeable myristoylated PKCζ pseudosubstrate (Myr-SIYRRGARRWRKL), which specifically inhibits PKCζ enzyme (65), decreased insulin-stimulated Grb14 phosphorylation, restoring phosphorylation to the level observed in the absence of insulin. Cotransfection of CHO-IR cells with HA-PKCζ resulted in a 60% increase in insulin-stimulated Grb14 phosphorylation. By contrast, in the absence of insulin stimulation, the overexpression of PKCζ did not increase Grb14 phosphorylation (data not shown). Together, these results support the hypothesis that PKCζ may account for the in vivo insulin-induced Grb14 phosphorylation.
FIG. 6.
Grb14 phosphorylation in intact cells. CHO-IR cells transiently transfected with myc-Grb14 and labeled with [32P]orthophosphate were stimulated (+) or not (−) with insulin as indicated. Cells were either cotransfected with HA-PKCζ or empty vector (right part) or treated 30 min with 20 μM of myristoylated PKCζ (Myr-SIYRRGARRWRKL) pseudosubstrate prior to insulin stimulation (left part). Cell lysates were immunoprecipitated with anti-Grb14 antibodies, separated by SDS-PAGE, transferred to nitrocellulose membrane, and quantified with a PhosphorImager (upper blot). Anti-myc immunoblots were then performed to confirm that equal amounts of Grb14 were immunoprecipitated (lower blot). Results are expressed as a percentage of their respective control, in the absence of insulin (left part) or in the absence of HA-PKCζ (right part), and represent the means + standard errors of the means (error bars) of three (right part) or four (left part) independent experiments.
The phosphorylation of Grb14 by PKCζ increases its inhibitory effect on in vitro IR tyrosine kinase activity.
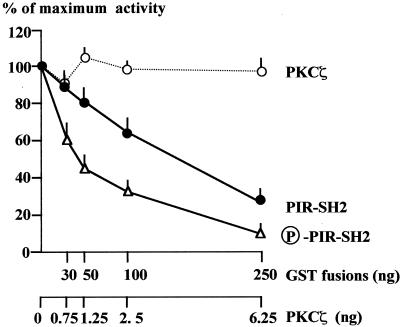
The PIR of Grb14 mediates both the binding to the IR and the inhibitory action of the protein on the catalytic activity of the receptor (2, 28). As the PIR was the main domain of Grb14 phosphorylated by PKCζ, we hypothesized that this phosphorylation could modify the inhibitory action of Grb14 on IR catalytic activity. IR tyrosine kinase activity was then investigated in vitro in the presence of increasing amounts of GST-PIR-SH2, which was or was not previously phosphorylated by PKCζ. As shown in Fig. 7, the dose-response curve of IR kinase inhibition by phosphorylated GST-PIR-SH2 was shifted to the left compared to that of inhibition by the nonphosphorylated protein, reflecting an increased sensitivity of the IR to its inhibitory effect. Fifty percent inhibition of IR tyrosine kinase activity was observed for 40 ng of phosphorylated GST-PIR-SH2 versus 160 ng for the nonphosphorylated form. When performing the IR kinase assay, PKCζ was added together with phosphorylated GST-PIR-SH2. As serine-threonine phosphorylation of IR has been described to inhibit IR tyrosine kinase activity (4, 68, 69), we tested the ability of PKCζ to modify the IR catalytic activity under the same conditions. However, when added alone, the same amounts of PKCζ did not modify IR tyrosine kinase activity. The displacement of the dose-response curve was then attributable to the phosphorylation of the PIR-SH2 domain. Thus, these results clearly show that phosphorylation of Grb14 by PKCζ increases its inhibitory action on IR catalytic activity.
FIG. 7.
The phosphorylation of PIR-SH2 of Grb14 increases its inhibitory effect on IR catalytic activity. Tyrosine kinase activity of WGA-purified IR was measured using a synthetic substrate (poly Glu-Tyr 4:1), in the presence of increasing amounts of GST-PIR-SH2 previously phosphorylated or not by PKCζ, or in the presence of PKCζ alone. Results are expressed as a percentage of the maximal insulin effect measured in the absence of GST fusion proteins, and are the means + standard errors of the means of three to five different experiments.
ZIP potentiates the Grb14 inhibitory action on insulin-induced reinitiation of meiosis in X. laevis oocytes.
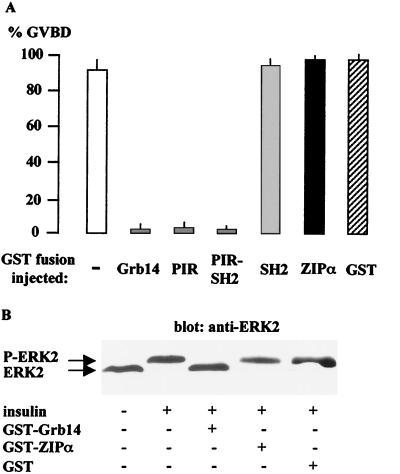
Xenopus oocyte, physiologically arrested at the G2 stage of the first meiotic prophase, is a suitable system for investigating the functional roles of signaling molecules initiated by several extracellular inducers like progesterone or insulin (15, 37, 42). The binding of these hormones to their receptors stimulates the entry of oocytes into the M phase, leading to a GVBD, an event that can be easily monitored and used as an indicator of reinitiation of meiosis. The effect of Grb14 and its domains on insulin-induced oocyte maturation was studied by microinjection of GST-fusion proteins 1 h prior to stimulation with insulin (10−6 M). As shown in Fig. 8A, microinjection of 100 ng of GST-Grb14 totally blocked the maturation induced by insulin. Injection of the same amount of GST had no effect on insulin-induced GVBD. In addition, this inhibitory action of Grb14 was specific for the insulin transduction pathway, since it was not observed when the oocytes were stimulated with progesterone (data not shown). Interestingly, the PIR-SH2 and the PIR-GST fusions were sufficient to inhibit the reinitiation of meiosis, whereas the GST-SH2 fusion had no effect. The microinjection of 100 ng of GST-ZIPα, (or GST-ZIPβ [data not shown]) did not alter the insulin-induced reinitiation of oocyte meiosis.
FIG. 8.
Grb14 inhibits insulin-induced X. laevis oocyte maturation. Oocytes were microinjected with 100 ng of the indicated GST fusion proteins 1 h before insulin stimulation and analyzed 20 h later for the appearance of GVBD. (A) Effect of the injection of various GST fusions on the insulin induction of oocyte maturation. Results are expressed as a percentage of GVBD. Data represent the means + standard errors of the means (error bars) of two to three animals, each concerning 20 oocytes. (B) Western blot analysis of oocyte lysates using anti-ERK2 antibodies. The blot is representative of three different experiments.
Numerous studies have shown the importance of p21ras-mitogen-activated protein (MAP) kinase cascade in insulin-induced GVBD (19, 54), and MAP kinase activation appears to be necessary and sufficient to induce oocyte maturation (19). Thus, to further investigate the molecular mechanism of Grb14 inhibitory action in X. laevis oocytes, we studied its effect on MAP kinase activation. Insulin-induced ERK2 phosphorylation was detected by immunoblot analysis using anti-ERK2 antibodies (Fig. 8B). A shift to a higher electrophoretic mobility of the 42-kDa protein, indicative of the presence of ERK2 phosphorylated form, was observed in oocytes stimulated with insulin but did not occur in oocytes previously microinjected with GST-Grb14. As expected, GST-ZIPα and GST did not alter ERK phosphorylation. These data support the notion that Grb14 inhibits the IR catalytic activity and subsequent activation of MAP kinase during insulin stimulation (2).
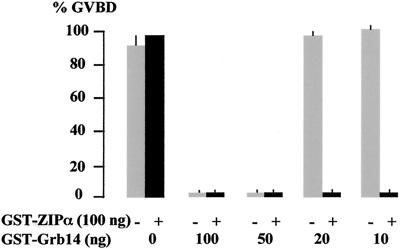
The study of the effect of decreasing amounts of Grb14 indicated that at least 50 ng of GST-Grb14 was required to inhibit insulin-induced GVBD (Fig. 9). Coinjection of 100 ng of GST-ZIPα with low amounts of GST-Grb14, which were unable to inhibit the action of insulin, restored the inhibition of insulin-induced GVBD (Fig. 9). Similar results were obtained when using ZIPβ (data not shown). Thus, these results suggest that ZIP potentiates the inhibitory action of Grb14 on oocyte maturation.
FIG. 9.
ZIP potentiates Grb14 inhibition of insulin-induced oocyte maturation. Oocytes were microinjected with decreasing amounts of GST-Grb14 as indicated, together with (filled bars) or without (grey bars) 100 ng of GST-ZIPα. Oocyte maturation in response to insulin was monitored as in Fig. 8. Data are the means + standard errors of the means (error bars) of three animals, each concerning 20 oocytes.
ZIP must interact with PKCζ to potentiate the action of Grb14.
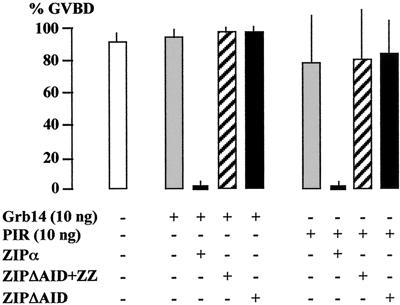
To further explore the mechanism by which ZIP regulates the action of Grb14, we used ZIP deletion constructs expressed as GST-fusion proteins: ZIPΔAID+ZZ, from which both Grb14 and PKCζ interacting domains were deleted, and ZIPΔAID, which could not bind to PKCζ but retained its Grb14 binding ability. As shown previously, coinjection of ZIPα with 10 ng of GST-Grb14 inhibited insulin-induced GVBD. By contrast, the coinjection of ZIPΔAID+ZZ did not allow the blocking of insulin-induced reinitiation of meiosis (Fig. 10). Interestingly, ZIPΔAID was also unable to potentiate the inhibitory effect of Grb14. These data suggest that the ZIP-Grb14 interaction was not sufficient to mediate the functional action of ZIP, and that ZIP must associate with both Grb14 and PKCζ to potentiate Grb14 inhibitory action on oocyte Xenopus maturation. Similar results were obtained when using the PIR of Grb14 instead of full-length protein (Fig. 10), whereas the SH2 domain had no effect (data not shown). Ten nanograms of GST-PIR was not sufficient to block the insulin-induced reinitiation of oocyte meiosis. Coinjected ZIP potentiated the inhibitory action of PIR, whereas ZIPΔAID+ZZ and ZIPΔAID were ineffective. These results reinforce the central role played by the PIR in Grb14 function.
FIG. 10.
Importance of Grb14-ZIP-PKCζ interaction in ZIP modulation of Grb14 action. Oocytes were coinjected with GST-Grb14 or GST-PIR in the presence of 100 ng of various GST-ZIP constructs, as indicated. Oocyte maturation in response to insulin was monitored as in Fig. 8. Data are the means + standard errors of the means (error bars) of three animals, each concerning 20 oocytes.
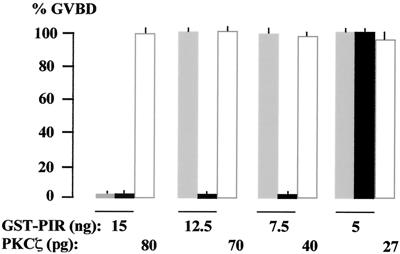
We have shown that phosphorylation of Grb14 increased its inhibitory effect on in vitro IR tyrosine kinase activity (Fig. 7). We reasoned that if the role of ZIP is to allow Grb14 to be phosphorylated by PKCζ, previous phosphorylation of the GST-PIR fusion would increase its inhibitory effect on the action of insulin in the Xenopus oocyte model. We thus tested the effect of decreasing amounts of GST-PIR, prephosphorylated or not, on insulin-induced oocyte maturation. As shown in Fig. 11, the injection of 12.5 ng of GST-PIR did not inhibit the effect of insulin. However, if the protein was prephosphorylated by recombinant PKCζ, the injection of 12.5 ng, as well as 7.5 ng, totally inhibited the effect of insulin. As a control, microinjection of the same amounts of activated PKCζ in the absence of GST-PIR did not modify insulin-induced oocyte maturation. These results confirm in vivo that the inhibitory action of Grb14 is increased when the protein is phosphorylated.
FIG. 11.
PIR phosphorylation increases its inhibition of insulin-induced oocyte maturation. Oocytes were either microinjected with the indicated amounts of GST-PIR, which was (black bars) or was not (grey bars) previously phosphorylated by recombinant PKCζ as described in Materials and Methods, or microinjected with PKCζ alone (white bars). Oocyte maturation in response to insulin was monitored as in Fig. 8. Data are the means + standard errors of the means (error bars) of three animals, each concerning 20 oocytes.
All together, these data are consistent with the notion that ZIP acts as an adapter, linking PKCζ to Grb14 into a new signaling pathway.
DISCUSSION
The atypical protein kinase C isoforms (aPKCs), which include PKCζ and PKCλ, are implicated in multiple intracellular pathways. They regulate critical cellular functions such as cell proliferation and apoptosis (3, 12, 30, 49), and they are also important mediators of physiological actions of insulin such as translocation of the insulin-responsive glucose transporter GLUT4 and enhancement of glucose transport in adipocytes and skeletal muscle (1, 6, 16, 65). Accumulating studies support the existence of adapter proteins coordinating physical association between PKC and substrates and thus acting as catalysts for specific reactions (47, 63). These proteins are key components for the determination of the selectivity of signaling events. Some of these effectors can modulate aPKC catalytic activity, like lambda interacting protein and Par-6 (partitioning-defective 6), acting as activators (10, 56), or Par-4 (prostate androgen response-4), acting as an inhibitor (9). Atypical PKC isotype-specific interacting protein (ASIP) binds the aPKC catalytic domain (24). Interestingly, overexpression of ASIP in 3T3-L1 adipocytes inhibits insulin-induced glucose uptake (31). However, the mechanism by which ASIP inhibits PKCλ effect in GLUT4 translocation remains unclear. Another aPKC-interacting protein, ZIP, binds selectively and constitutively to the V1 portion of their regulatory domain and does not modulate their catalytic activity (55, 60). ZIP contains a number of conserved motifs, suggesting a role as a scaffold protein linking aPKCs to other proteins implicated in various signaling pathways. ZIP has been shown to recruit the proteins RIP and TRAF6, connecting PKCζ to the TNF-α and IL-1 receptors, respectively (61, 62). These interactions appear to be of functional relevance since they stimulate NF-κB activity. In addition, it was recently reported that through its association with the NGF receptor TrkA, the ZIP-TRAF6 complex is implicated in the NGF-induced NF-κB activation (72). ZIP acts also as a link between PKCζ and the neuronal potassium channel Kvβ2 (18).
In this study, we identified ZIP as a new downstream partner of Grb14. Although we isolated two isoforms, ZIPα and ZIPβ, we did not find any functional difference between them. They bound similarly to Grb14, and both of them potentiated the inhibitory role of Grb14 on insulin induced Xenopus oocyte maturation. Results of mapping studies indicated that the ZZ zinc finger domain of ZIP, which includes a novel atypical zinc finger motif (55), is required for the interaction with Grb14. This domain was also recently shown to interact with the death domain kinase RIP (62). All together, these results suggest that the ZZ zinc finger domain of ZIP is a new protein-protein interacting region. The PKCζ binding domain of ZIP, termed AID, is located N terminal of the ZZ zinc finger domain, indicating that ZIP can bind simultaneously Grb14 and PKCζ through two distinct but proximal modules. Using coimmunoprecipitation experiments in transfected CHO-IR and COS cells, we confirmed that these interactions are not antagonistic, resulting in a ternary complex including Grb14, ZIP, and PKCζ. Importantly, Grb14, ZIP, and PKCζ form a heterotrimer also in vivo in rat liver, demonstrating that endogenous proteins interact under physiological conditions. By contrast with previous studies reporting a ligand induction of ZIP multimeric complexes (61, 62, 72), we were unable to detect any effect of insulin on the formation of this heterotrimer (Fig. 3A and data not shown). Nevertheless, the functional regulation by insulin of the Grb14-ZIP-PKCζ complex may involve other modifications, like phosphorylations or conformational changes in response to the hormone. In this regard, we reported that Grb14 undergoes insulin-stimulated phosphorylation during labeling experiments in intact cells. Since we previously reported that Grb14 was not phosphorylated on tyrosine residues under insulin stimulation in CHO-IR-Grb14 cells (28), Grb14 is likely to be phosphorylated on serine/threonine residues. This is in agreement with previous studies reporting that Grb14 was phosphorylated in vivo mainly on serine residues (8, 58). In these studies, stimulation with PDGF (8) or fibroblast growth factor 2 (58) increased Grb14 phosphorylation to an extent similar to that after insulin stimulation. Other members of the Grb7 family of proteins are also phosphorylated on serine/threonine residues (20). More particularly, it was shown that insulin increased Grb10 serine phosphorylation, and it can be underlined that the effect of insulin on Grb10 and on Grb14 phosphorylation is of similar amplitude (13). The serine/threonine kinases involved in Grb7 protein phosphorylation are still unidentified (20), but they do not appear to be either cyclic AMP- or calcium-stimulated kinases (53) or classical or novel PKC isozymes (13, 53). Insulin-induced Grb10 phosphorylation was blocked by wortmannin, suggesting that insulin stimulates a kinase acting in a phosphatidyl inositol 3-kinase dependent manner (13). Since insulin is known to stimulate PKCζ catalytic activity (5, 64, 65), and since ZIP anchors PKCζ and Grb14 into a heterotrimeric complex, we hypothesized that Grb14 could be a substrate for the kinase. We thus tested whether PKCζ could be implicated in insulin-stimulated Grb14 phosphorylation. In intact cells, insulin-stimulated Grb14 phosphorylation is decreased by a PKCζ pseudosubstrate, which inhibits PKCζ enzymatic activity. Furthermore, the overexpression of PKCζ increased insulin-induced Grb14 phosphorylation. Together, these data suggest that the serine/threonine kinase PKCζ is a potential candidate for in vivo insulin-induced Grb14 phosphorylation. However, a role for other kinases cannot be excluded, and further experiments are needed to clarify this point. More particularly, the identity of kinases responsible for the high basal phosphorylation state of these proteins is still unclear.
Of functional relevance, we provided evidence that phosphorylation of Grb14 increased its inhibitory effect on IR tyrosine kinase activity. Interestingly, among the various domains of Grb14, the PIR, which is responsible for both the specific interaction with the activated IR tyrosine kinase loop and the inhibition of the IR catalytic activity (2, 28), was preferentially phosphorylated by PKCζ in vitro. Although this domain does not contain a conventional phosphorylation motif for PKC, it exhibits 4 serine residues followed in +1 by a hydrophobic amino acid, which has been shown to be present in all bona fide PKC phosphorylation sites (51). In addition, PKCζ was already described to phosphorylate IKKβ in a nonconsensus motif (32). The identification of the Grb14-phosphorylated sites and the mechanism whereby Grb14 phosphorylation increases its inhibitory effect deserve further investigation. However, a simple hypothesis could be that this increased activity reflects an increased binding affinity of the phosphorylated PIR for the IR kinase loop.
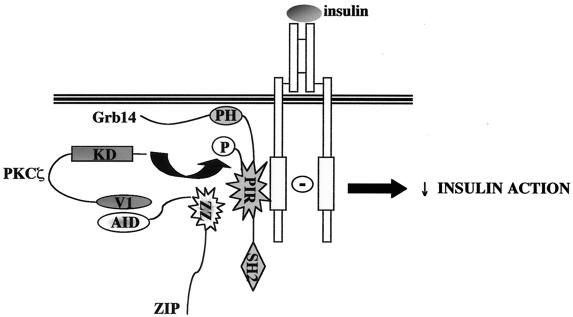
Xenopus oocyte, which endogenously expresses insulin-signaling molecules, provides a widely used experimental model to study in vivo insulin action (15, 25, 59, 66). We show here that injection of Grb14 into Xenopus oocyte specifically blocks insulin-induced reinitiation of meiosis. These data correlate with Grb14 inhibition of distal insulin effects, such as DNA or glycogen synthesis (28). By contrast, injection of ZIP alone did not alter oocyte maturation. Since PKCζ is a critical step for the mitogenic signal in oocytes (3, 11), this result seems to confirm that ZIP does not regulate PKCζ activity in vivo. On the other hand, ZIP was able to potentiate Grb14 inhibitory action. This effect required the recruitment of PKCζ and could be mimicked by previous Grb14 phosphorylation. These observations provide in vivo evidence that ZIP acts as a functional scaffold protein connecting PKCζ to Grb14, allowing its phosphorylation. Interestingly, the PIR expressed alone was sufficient to mediate both the inhibitory effect of Grb14 on insulin-induced reinitiation of meiosis and the potentiation by ZIP of this inhibitory effect. This clearly demonstrates that the interaction of the PIR with ZIP does not antagonize the regulation of its phosphorylation by PKCζ in vivo. These data also correlate with the observation that ZIP binding to Grb14 did not alter the insulin-induced Grb14-IR interaction (data not shown). The PIR of Grb14 appears thus to be the functional core of the protein in the regulation of insulin signaling. Considering our in vitro and in vivo findings, a model emerges whereby ZIP acts as a physical link in the assembly of a PKCζ-ZIP-Grb14 complex, targeting the PKCζ activity to Grb14 (Fig. 12). The constitution of this complex has major functional consequences, since it potentiates the inhibitory effect of Grb14 on insulin signaling.
FIG. 12.
A proposed model for the role of ZIP as an adapter connecting PKCζ to Grb14. ZIP interacts with the V1 domain of PKCζ through its AID domain, and with the PIR of Grb14 through its ZZ zinc finger domain, allowing the formation of a heterotrimeric complex. Under insulin stimulation, the PIR binds to the activated IR regulatory tyrosine kinase loop. PIR phosphorylation by PKCζ leads to an increased Grb14 inhibitory action on IR catalytic activity and insulin action.
PKCζ is known as a positive effector of the insulin signal transduction pathways, implicated in insulin-induced glucose transport, GLUT4 translocation, and protein synthesis (1, 16, 43, 64, 65). However, recent studies reported that PKCζ can also play an inhibitory role in insulin signal transduction, by the inhibition of Akt (14, 39) or by the serine phosphorylation of IRS-1 (35, 57). Serine phosphorylation of IRS-1 by PKCζ participates in a negative feedback control mechanism induced by insulin, which promotes the dissociation of the IR-IRS-1 complex and leads to the termination of insulin signal (35, 57). Interestingly, the present work suggests that by enhancing the inhibitory activity of Grb14, PKCζ can use an additional way to down-regulate insulin signaling.
Acknowledgments
We thank J. Camonis and R. Farese, respectively, for the kind gifts of the pFBL23 plasmid and the pcDNA3-PKCζ plasmid. We also gratefully acknowledge V. Le Marcis for GST fusions production and E. Clauser, T. Issad, and B. Desbuquois for critically reading the manuscript.
This work was supported by Servier and by the Association pour la Recherche sur le Cancer (grant 5237 to A.-F. Burnol). B.C. is supported by a Servier fellowship, and V.B. is the recipient of a fellowship from the Ministère de la Recherche.
REFERENCES
- 1.Bandyopadhyay, G., M. L. Standaert, U. Kikkawa, Y. Ono, J. Moscat, and R. V. Farese. 1999. Effect of transiently expressed atypical (zeta, lambda), conventional (alpha, beta) and novel (delta, epsilon) protein kinase C isoforms on insulin-stimulated translocation of epitope-tagged GLUT4 glucose transporters in rat adipocytes: specific interchangeable effects of protein kinase C-zeta and C-lambda. Biochem. J. 337:461-470. [PMC free article] [PubMed] [Google Scholar]
- 2.Bereziat, V., A. Kasus-Jacobi, D. Perdereau, B. Cariou, J. Girard, and A. F. Burnol. 2002. Inhibition of insulin receptor catalytic activity by the molecular adapter Grb14. J. Biol. Chem. 28:4845-4852. [DOI] [PubMed] [Google Scholar]
- 3.Berra, E., M. T. Diaz-Meco, I. Dominguez, M. M. Municio, L. Sanz, J. Lozano, R. S. Chapkin, and J. Moscat. 1993. Protein kinase C zeta isoform is critical for mitogenic signal transduction. Cell 74:555-563. [DOI] [PubMed] [Google Scholar]
- 4.Bossenmaier, B., L. Mosthaf, H. Mischak, A. Ullrich, and H. U. Haring. 1997. Protein kinase C isoforms beta 1 and beta 2 inhibit the tyrosine kinase activity of the insulin receptor. Diabetologia 40:863-866. [DOI] [PubMed] [Google Scholar]
- 5.Braiman, L., A. Alt, T. Kuroki, M. Ohba, A. Bak, T. Tennenbaum, and S. R. Sampson. 2001. Activation of protein kinase C zeta induces serine phosphorylation of VAMP2 in the GLUT4 compartment and increases glucose transport in skeletal muscle. Mol. Cell. Biol. 21:7852-7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braiman, L., L. Sheffi-Friedman, A. Bak, T. Tennenbaum, and S. R. Sampson. 1999. Tyrosine phosphorylation of specific protein kinase C isoenzymes participates in insulin stimulation of glucose transport in primary cultures of rat skeletal muscle. Diabetes 48:1922-1929. [DOI] [PubMed] [Google Scholar]
- 7.Daly, R. J. 1998. The Grb7 family of signalling proteins. Cell. Signal. 10:613-618. [DOI] [PubMed] [Google Scholar]
- 8.Daly, R. J., G. M. Sanderson, P. W. Janes, and R. L. Sutherland. 1996. Cloning and characterization of Grb14, a novel member of the Grb7 family. J. Biol. Chem. 271:12502-12510. [DOI] [PubMed] [Google Scholar]
- 9.Diaz-Meco, M. T., M. M. Municio, S. Frutos, P. Sanchez, J. Lozano, L. Sanz, and J. Moscat. 1996. The product of par-4, a gene induced during apoptosis, interacts selectively with the atypical isoforms of protein kinase C. Cell 86:777-786. [DOI] [PubMed] [Google Scholar]
- 10.Diaz-Meco, M. T., M. M. Municio, P. Sanchez, J. Lozano, and J. Moscat. 1996. Lambda-interacting protein, a novel protein that specifically interacts with the zinc finger domain of the atypical protein kinase C isotype lambda/iota and stimulates its kinase activity in vitro and in vivo. Mol. Cell. Biol. 16:105-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dominguez, I., M. T. Diaz-Meco, M. M. Municio, E. Berra, A. Garcia de Herreros, M. E. Cornet, L. Sanz, and J. Moscat. 1992. Evidence for a role of protein kinase C zeta subspecies in maturation of Xenopus laevis oocytes. Mol. Cell. Biol. 12:3776-3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dominguez, I., L. Sanz, F. Arenzana-Seisdedos, M. T. Diaz-Meco, J. L. Virelizier, and J. Moscat. 1993. Inhibition of protein kinase C zeta subspecies blocks the activation of an NF-κB-like activity in Xenopus laevis oocytes. Mol. Cell. Biol. 13:1290-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong, L. Q., H. Du, S. G. Porter, L. F. Kolakowski, A. V. Lee, J. Mandarino, J. Fan, D. Yee, and F. Liu. 1997. Cloning, chromosome localization, expression, and characterization of an Src homology 2 and pleckstrin homology domain-containing insulin receptor binding protein hGrb10g. J. Biol. Chem. 272:29104-29112. [DOI] [PubMed] [Google Scholar]
- 14.Doornbos, R. P., M. Theelen, P. C. van der Hoeven, W. J. van Blitterswijk, A. J. Verkleij, and P. M. van Bergen en Henegouwen. 1999. Protein kinase C zeta is a negative regulator of protein kinase B activity. J. Biol. Chem. 274:8589-8596. [DOI] [PubMed] [Google Scholar]
- 15.El-Etr, M., S. Schorderet-Slatkine, and E. E. Baulieu. 1979. Meiotic maturation in Xenopus laevis oocytes initiated by insulin. Science 205:1397-1399. [DOI] [PubMed] [Google Scholar]
- 16.Etgen, G. J., K. M. Valasek, C. L. Broderick, and A. R. Miller. 1999. In vivo adenoviral delivery of recombinant human protein kinase C-zeta stimulates glucose transport activity in rat skeletal muscle. J. Biol. Chem. 274:22139-22142. [DOI] [PubMed] [Google Scholar]
- 17.Frantz, J. D., S. Giogetti-Peraldi, E. A. Ottinger, and S. E. Shoelson. 1997. Human Grb-IRβ/Grb10. Splice variants of an insulin and growth factor receptor-binding protein with PH and SH2 domains. J. Biol. Chem. 272:2659-2667. [DOI] [PubMed] [Google Scholar]
- 18.Gong, J., J. Xu, M. Bezanilla, K. Van Huizen, R. Derin, and M. Li. 1999. Differential stimulation of PKC phosphorylation of potassium channels by ZIP1 and ZIP2. Science 285:1565-1569. [DOI] [PubMed] [Google Scholar]
- 19.Haccard, O., A. Lewellyn, R. S. Hartley, E. Erikson, and J. L. Maller. 1995. Induction of Xenopus oocyte meiotic maturation by MAP kinase. Dev. Biol. 168:677-682. [DOI] [PubMed] [Google Scholar]
- 20.Han, D. C., T.-L. Shen, and J.-L. Guan. 2001. The Grb7 family proteins: structure, interactions with other signaling molecules and potential cellular functions. Oncogene 20:6315-6321. [DOI] [PubMed] [Google Scholar]
- 21.Hansen, H., U. Svensson, J. Zhu, L. Laviola, F. Giorgino, G. Wolf, R. J. Smith, and H. Riedel. 1996. Interaction between the Grb10 SH2 domain and the insulin receptor carboxyl terminus. J. Biol. Chem. 271:8882-8886. [DOI] [PubMed] [Google Scholar]
- 22.He, W., D. W. Rose, J. M. Olefsky, and T. A. Gustafson. 1998. Grb10 interacts differentially with the insulin receptor, insulin-like growth factor I receptor, and epidermal growth factor receptor via the Grb10 Src homology 2 (SH2) domain and a second novel domain located between the pleckstrin homology and SH2 domains. J. Biol. Chem. 273:6860-6867. [DOI] [PubMed] [Google Scholar]
- 23.Hemming, R., R. Agatep, K. Badiani, K. Wyant, G. Arthur, R. D. Gietz, and B. Triggs-Raine. 2001. Human growth factor receptor bound 14 binds the activated insulin receptor and alters the insulin-stimulated tyrosine phosphorylation levels of multiple proteins. Biochem. Cell Biol. 79:21-32. [PubMed] [Google Scholar]
- 24.Izumi, Y., T. Hirose, Y. Tamai, S. Hirai, Y. Nagashima, T. Fujimoto, Y. Tabuse, K. J. Kemphues, and S. Ohno. 1998. An atypical PKC directly associates and colocalizes at the epithelial tight junction with ASIP, a mammalian homologue of Caenorhabditis elegans polarity protein PAR-3. J. Cell Biol. 143:95-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janicot, M., and M. D. Lane. 1989. Activation of glucose uptake by insulin and insulin-like growth factor I in Xenopus oocytes. Proc. Natl. Acad. Sci. USA 86:2642-2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joung, I., J. L. Strominger, and J. Shin. 1996. Molecular cloning of a phosphotyrosine-independent ligand of the p56lck SH2 domain. Proc. Natl. Acad. Sci. USA 93:5991-5995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kasus-Jacobi, A., V. Bereziat, D. Perdereau, J. Girard, and A.-F. Burnol. 2000. Evidence for an interaction between the insulin receptor and Grb7. A role for two of its binding domains, PIR and SH2. Oncogene 19:2052-2059. [DOI] [PubMed] [Google Scholar]
- 28.Kasus-Jacobi, A., D. Perdereau, C. Auzan, E. Clauser, E. Van Obberghen, F. Mauvais-Jarvis, J. Girard, and A.-F. Burnol. 1998. Identification of the rat adapter Grb14 as an inhibitor of insulin actions. J. Biol. Chem. 273:26026-26035. [DOI] [PubMed] [Google Scholar]
- 29.Kasus-Jacobi, A., D. Perdereau, S. Tartare-Deckert, E. Van Obberghen, J. Girard, and A.-F. Burnol. 1997. Evidence for a direct interaction between insulin receptor substrate-1 and Shc. J. Biol. Chem. 272:17166-17170. [DOI] [PubMed] [Google Scholar]
- 30.Kieser, A., T. Seitz, H. S. Adler, P. Coffer, E. Kremmer, P. Crespo, J. S. Gutkind, D. W. Henderson, J. F. Mushinski, W. Kolch, and H. Mischak. 1996. Protein kinase C-zeta reverts v-raf transformation of NIH-3T3 cells. Genes Dev. 10:1455-1466. [DOI] [PubMed] [Google Scholar]
- 31.Kotani, K., W. Ogawa, M. Hashiramoto, T. Onishi, S. Ohno, and M. Kasuga. 2000. Inhibition of insulin-induced glucose uptake by atypical protein kinase C isotype-specific interacting protein in 3T3-L1 adipocytes. J. Biol. Chem. 275:26390-26395. [DOI] [PubMed] [Google Scholar]
- 32.Lallena, M. J., M. T. Diaz-Meco, G. Bren, C. V. Paya, and J. Moscat. 1999. Activation of IκB kinase beta by protein kinase C isoforms. Mol. Cell. Biol. 19:2180-2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Langlais, P., L. Q. Dong, D. Hu, and F. Liu. 2000. Identification of Grb10 as a direct substrate for members of the Src tyrosine kinase family. Oncogene 19:2895-2903. [DOI] [PubMed] [Google Scholar]
- 34.Liu, F., and R. A. Roth. 1995. Grb-IR: a SH2-domain-containing protein that binds to the insulin receptor and inhibits its function. Proc. Natl. Acad. Sci. USA 92:10287-10291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu, Y. F., K. Paz, A. Herschkovitz, A. Alt, T. Tennenbaum, S. R. Sampson, M. Ohba, T. Kuroki, D. LeRoith, and Y. Zick. 2001. Insulin stimulates PKCzeta-mediated phosphorylation of insulin receptor substrate-1 (IRS-1). A self-attenuated mechanism to negatively regulate the function of IRS proteins. J. Biol. Chem. 276:14459-14465. [DOI] [PubMed] [Google Scholar]
- 36.Lyons, R. J., R. Deane, D. K. Lynch, Z. S. J. Ye, G. M. Sanderson, H. J. Eyre, G. R. Sutherland, and R. J. Daly. 2001. Identification of a novel human tankyrase through its interaction with the adaptor protein Grb14. J. Biol. Chem. 276:17172-17180. [DOI] [PubMed] [Google Scholar]
- 37.Maller, J. L., and J. W. Koontz. 1981. A study of the induction of cell division in amphibian oocytes by insulin. Dev. Biol. 85:309-316. [DOI] [PubMed] [Google Scholar]
- 38.Mano, H., K.-I. Ohya, A. Miyazato, Y. Yamashita, W. Ogawa, J. Inazawa, U. Ikeda, K. Shimada, K. Hatake, M. Kasuga, K. Ozawa, and S. Kajigaya. 1998. Grb10/GrbIR as an in vivo substrate of Tec tyrosine kinase. Genes Cells 3:431-441. [DOI] [PubMed] [Google Scholar]
- 39.Mao, M., X. Fang, Y. Lu, R. Lapushin, R. C. Bast, Jr., and G. B. Mills. 2000. Inhibition of growth-factor-induced phosphorylation and activation of protein kinase B/Akt by atypical protein kinase C in breast cancer cells. Biochem. J. 352 Pt 2:475-482. [PMC free article] [PubMed] [Google Scholar]
- 40.Marcus, S. L., C. J. Winrow, J. P. Capone, and R. A. Rachubinski. 1996. A p56(lck) ligand serves as a coactivator of an orphan nuclear hormone receptor. J. Biol. Chem. 271:27197-27200. [DOI] [PubMed] [Google Scholar]
- 41.Margolis, B. 1994. The Grb family of SH2 domain proteins. Prog. Biophys. Mol. Biol. 62:223-244. [DOI] [PubMed] [Google Scholar]
- 42.Masui, Y., and H. J. Clarke. 1979. Oocyte maturation. Int. Rev. Cytol. 57:185-282. [DOI] [PubMed] [Google Scholar]
- 43.Mendez, R., G. Kollmorgen, M. F. White, and R. E. Rhoads. 1997. Requirement of protein kinase C zeta for stimulation of protein synthesis by insulin. Mol. Cell. Biol. 17:5184-5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morrione, A. 2000. Grb10 proteins in insulin-like growth factor and insulin receptor signaling. Int. J. Mol. Med. 5:151-154. [DOI] [PubMed] [Google Scholar]
- 45.Morrione, A., P. Plant, B. Valentinis, O. Staub, S. Kumar, D. Rotin, and R. Baserga. 1999. mGrb10 interacts with Nedd4. J. Biol. Chem. 274:24094-24099. [DOI] [PubMed] [Google Scholar]
- 46.Morrione, A., B. Valentinis, M. Resnicoff, S.-Q. Xu, and R. Baserga. 1997. The role of mGrb10α in insulin-like growth factor I-mediated growth. J. Biol. Chem. 272:26282-26287. [DOI] [PubMed] [Google Scholar]
- 47.Moscat, J., and M. T. Diaz-Meco. 2000. The atypical protein kinase Cs. Functional specificity mediated by specific protein adapters. EMBO Rep. 1:399-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mounier, C., L. Lavoie, V. Dumas, K. Mohammad-Ali, J. Wu, A. Nantel, J. J. Bergeron, D. Y. Thomas, and B. I. Posner. 2001. Specific inhibition by hGrb10zeta of insulin-induced glycogen synthase activation: evidence for a novel signaling pathway. Mol. Cell. Endocrinol. 173:15-27. [DOI] [PubMed] [Google Scholar]
- 49.Murray, N. R., and A. P. Fields. 1997. Atypical protein kinase C iota protects human leukemia cells against drug-induced apoptosis. J. Biol. Chem. 272:27521-27524. [DOI] [PubMed] [Google Scholar]
- 50.Nantel, A., K. Mohammad-Ali, J. Sherk, B. I. Posner, and D. Y. Thomas. 1998. Interaction of the adapter Grb10 adapter protein with the Raf1 and MEK1 kinases. J. Biol. Chem. 273:10475-10484. [DOI] [PubMed] [Google Scholar]
- 51.Nishikawa, K., A. Toker, F. J. Johannes, Z. Songyang, and L. C. Cantley. 1997. Determination of the specific substrate sequence motifs of protein kinase C isozymes. J. Biol. Chem. 272:952-960. [DOI] [PubMed] [Google Scholar]
- 52.O'Neill, T. J., D. W. Rose, T. S. Pillay, K. Hotta, J. M. Olefsky, and T. A. Gustafson. 1996. Interaction of a Grb-IR splice variant (a human Grb10 homolog) with the insulin and insulin-like growth factor I receptors. J. Biol. Chem. 271:22506-22513. [DOI] [PubMed] [Google Scholar]
- 53.Ooi, J., V. Yajnik, D. Immanuel, M. Gordon, J. J. Moskow, A. M. Buchberg, and B. Margolis. 1995. The cloning of Grb10 reveals a new family of SH2 domain proteins. Oncogene 10:1621-1630. [PubMed] [Google Scholar]
- 54.Posada, J., and J. A. Cooper. 1992. Requirements for phosphorylation of MAP kinase during meiosis in Xenopus oocytes. Science 255:212-215. [DOI] [PubMed] [Google Scholar]
- 55.Puls, A., S. Schmidt, F. Grawe, and S. Stabel. 1997. Interaction of protein kinase C ζ with ZIP, a novel protein kinase C-binding protein. Proc. Natl. Acad. Sci. USA 94:6191-6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qiu, R. G., A. Abo, and G. S. Martin. 2000. A human homolog of the C. elegans polarity determinant Par-6 links Rac and Cdc42 to PKCζ signaling and cell transformation. Curr. Biol. 10:697-707. [DOI] [PubMed] [Google Scholar]
- 57.Ravichandran, L. V., D. L. Esposito, J. Chen, and M. J. Quon. 2001. Protein kinase C-zeta phosphorylates insulin receptor substrate-1 and impairs its ability to activate phosphatidylinositol 3-kinase in response to insulin. J. Biol. Chem. 276:3543-3549. [DOI] [PubMed] [Google Scholar]
- 58.Reilly, J. F., G. Mickey, and P. A. Maher. 2000. Association of fibroblast growth factor receptor 1 with the adaptor protein Grb14. Characterization of a new receptor binding partner. J. Biol. Chem. 275:7771-7778. [DOI] [PubMed] [Google Scholar]
- 59.Sadler, S. E., and J. L. Maller. 1987. In vivo regulation of cyclic AMP phosphodiesterase in Xenopus oocytes. Stimulation by insulin and insulin-like growth factor 1. J. Biol. Chem. 262:10644-10650. [PubMed] [Google Scholar]
- 60.Sanchez, P., G. De Carcer, I. V. Sandoval, J. Moscat, and M. T. Diaz-Meco. 1998. Localization of atypical protein kinase C isoforms into lysosome-targeted endosomes through interaction with p62. Mol. Cell. Biol. 18:3069-3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sanz, L., M. T. Diaz-Meco, H. Nakano, and J. Moscat. 2000. The atypical PKC-interacting protein p62 channels NF-75 B activation by the IL-1-TRAF6 pathway. EMBO J. 19:1576-1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sanz, L., P. Sanchez, M.-J. Lallena, M. T. Diaz-Meco, and J. Moscat. 1999. The interaction of p62 with RIP links the atypical PKCs to NF-75 B activation. EMBO J. 18:3044-3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schechtman, D., and D. Mochly-Rosen. 2001. Adaptor proteins in protein kinase C-mediated signal transduction. Oncogene 20:6339-6347. [DOI] [PubMed] [Google Scholar]
- 64.Standaert, M. L., G. Bandyopadhyay, L. Perez, D. Price, L. Galloway, A. Poklepovic, M. P. Sajan, V. Cenni, A. Sirri, J. Moscat, A. Toker, and R. V. Farese. 1999. Insulin activates protein kinases C-zeta and C-lambda by an autophosphorylation-dependent mechanism and stimulates their translocation to GLUT4 vesicles and other membrane fractions in rat adipocytes. J. Biol. Chem. 274:25308-25316. [DOI] [PubMed] [Google Scholar]
- 65.Standaert, M. L., L. Galloway, P. Karnam, G. Bandyopadhyay, J. Moscat, and R. V. Farese. 1997. Protein kinase C-zeta as a downstream effector of phosphatidylinositol 3-kinase during insulin stimulation in rat adipocytes. J. Biol. Chem. 272:30075-30082. [DOI] [PubMed] [Google Scholar]
- 66.Stefanovic, D., E. Erikson, L. J. Pike, and J. L. Maller. 1986. Activation of a ribosomal protein S6 protein kinase in Xenopus oocytes by insulin and insulin-receptor kinase. EMBO J. 5:157-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stein, E. G., T. A. Gustafson, and S. R. Hubbard. 2001. The BPS domain of Grb10 inhibits the catalytic activity of the insulin and IGF-1 receptors. FEBS Lett. 493:106-111. [DOI] [PubMed] [Google Scholar]
- 68.Strack, V., B. Stoyanov, B. Bossenmaier, L. Mosthaf, M. Kellerer, and H. U. Haring. 1997. Impact of mutations at different serine residues on the tyrosine kinase activity of the insulin receptor. Biochem. Biophys. Res. Commun. 239:235-239. [DOI] [PubMed] [Google Scholar]
- 69.Takayama, S., M. F. White, and C. R. Kahn. 1988. Phorbol ester-induced serine phosphorylation of the insulin receptor decreases its tyrosine kinase activity. J. Biol. Chem. 263:3440-3447. [PubMed] [Google Scholar]
- 70.Vadlamudi, R. K., I. Joung, J. L. Strominger, and J. Shin. 1996. p62, a phosphotyrosine-independent ligand of the SH2 domain of p56lck belongs to a new class of ubiquitin-binding proteins. J. Biol. Chem. 271:20235-20237. [DOI] [PubMed] [Google Scholar]
- 71.Wang, J., H. Dai, N. Yousaf, M. Moussaif, Y. Deng, A. Boufelliga, O. R. Swamy, M. E. Leone, and H. Riedel. 1999. Grb10, a positive, stimulatory signaling adapter in platelet-derived growth factor BB-, insulin-like growth factor I-, and insulin-mediated mitogenesis. Mol. Cell. Biol. 19:6217-6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wooten, M. W., M. L. Seibenhener, V. Mamidipudi, M. T. Diaz-Meco, P. A. Barker, and J. Moscat. 2001. The atypical protein kinase C-interacting protein p62 is a scaffold for NF-κB activation by nerve growth factor. J. Biol. Chem. 276:7709-7712. [DOI] [PubMed] [Google Scholar]