Abstract
A wide variety of tumor cells exhibit overexpression of urokinase plasminogen activator (uPA) and its receptor (uPAR). In breast cancer, expression of uPA and uPAR is essential for tumor cell invasion and metastasis. It is also known that uPA binds to uPAR and activates the RAS extra-cellular signal regulated kinase (ERK) signaling pathway. In our study, we have introduced small interfering RNA (siRNA) to downregulate the expression of uPA and uPAR in two breast cancer cell lines (MDA MB 231 and ZR 75 1). uPA and uPAR were downregulated individually using single constructs, and in combination using a bicistronic construct driven by a CMV promoter in a pcDNA-3 mammalian expression vector. Reverse transcription PCR (RT-PCR) and Western blot analyses indicated downregulation at both the mRNA and protein levels. In vitro angiogenesis studies using conditioned medium in HMEC-1 cells indicated a decrease in the angiogenic potential of conditioned media from treated cells when compared to the controls. This decrease in angiogenic potential was remarkably higher with the bicistronic construct. Similarly, the invasive potential of these cells decreased dramatically when treated with the bicistronic construct, thereby suggesting a synergistic effect from the downregulation of both uPA and uPAR. Furthermore, when uPA and uPAR were downregulated simultaneously, the apoptotic cascade was triggered as indicated by the upregulation of both initiator and effector caspases as well as other pro-apoptotic molecules. A mitochondrial permeability assay and FACS analysis revealed an increase in apoptotic cells in the uPA/uPAR treatment as compared to the other treatments. This overexpression of pro-apoptotic caspases in relation to the RNAi-induced downregulation of uPA and uPAR clearly suggests the involvement of the uPA-uPAR system in cell survival and proliferation in addition to their role in tumor progression.
Keywords: breast cancer cells, invasion, angiogenesis, uPA, uPAR
Introduction
In multicellular organisms, invasion plays a pivotal role in several diverse physiological and pathological processes including tissue remodeling associated with embryonic development (1), inflammation (2,3), angiogenesis (4), wound healing (5), and tumor metastasis (6-9). Among the large number of proteases involved in invasion, urokinase plasminogen activator (uPA) and urokinase-specific cell surface receptor (uPAR) are of particular importance (10-12). uPA is a serine protease and when bound to its receptor, uPAR, initiates the activation of metalloproteases as well as the conversion of plasminogen to plasmin (13,14). In addition, it has been widely reported that the ability of uPAR to localize the proteolytic activity of uPA on the cell surface is extremely important for the invasive ability of tumor cells (15). In addition to its proteolytic function as a zymogen, the uPA/uPAR complex functions as a vitronectin receptor and has been shown to participate in normal and tumor cell motility processes including monocyte migration and tumor cell migration and invasion (16-18). The major change associated with cellular transformation is the regulation of mitogenic pathways, which become constitutively activated (19). Oncogenic cells show a dramatic enhancement of protease production (e.g. uPA), which may promote increased pericellular proteolysis leading to the release of angiogenic and growth factors.
As such, the uPA/uPAR system presents a viable therapeutic target for anti-cancer studies. Here, we have developed siRNA constructs for the inhibition of these proteins. RNA interference is the process of sequence specific, post-transcriptional gene silencing mediated by double stranded RNA molecules (21-23 bp in length) homologous to the sequence of the silenced gene. Elbashir et al (20) showed that synthetic 21-23 nucleotide siRNA could induce efficient RNA interference in mammalian cells. In order to circumvent the high cost of synthetic siRNA and establish gene knockdown cell lines by siRNA, several plasmid vector systems have been designed to produce siRNA inside cells. These siRNA sequences are driven by RNA polymerase III dependent promoters such U6 and H1-RNA. In addition, siRNA has been shown to be potent since only a few molecules of dsRNA per cell are necessary to trigger gene silencing throughout the treated animal (21). Earlier studies in our laboratory have successfully demonstrated the efficiency of CMV promoter-driven siRNA sequences in the downregulation of ECM-degrading proteases (22). In the current study, we selected two breast cancer cell lines (MDA MB 231, an aggressively invasive cell line with high levels of uPA & uPAR, and ZR 75 1, a moderately aggressive cell line with moderate levels of uPA and uPAR expression).
Earlier studies with MDA MB 231 cells have shown the use of uPAR antagonists and uPA inhibitors to downregulate the invasive ability of breast cancer cells (23). Here, we present evidence of RNAi-induced downregulation of uPA and uPAR at both the mRNA and protein level by reverse transcription PCR and western blot analysis respectively. Furthermore, we show that when uPA and uPAR are downregulated, the invasiveness and angiogenic potential of cancers are inhibited. The uPA/uPAR system not only plays a role in proteolytic cleavage of the ECM but also plays a significant role in the signaling involved in cell survival and proliferation. Here, we can demonstrate that inhibition of this signaling triggers the apoptotic cascade.
Materials and Methods
Cell lines and culture conditions
The two breast cancer cell lines (MDA MB 231 and ZR 75 1) were obtained from ATCC (Manassas, VA). As suggested by ATCC, MDA MB 231 cells were grown in L-15 medium supplemented with glutamine, 10% FBS, 100 μg/mL streptomycin, and 100 units/mL penicillin (Invitrogen, Carlsbad, CA) at 37°C. ZR 75 1 cells were maintained in RPMI 1640 medium supplemented with 10% FBS, 10 mM HEPES, 1 mM sodium pyruvate, 4.5 g/L glucose, 1.5 g/L sodium bicarbonate, 100 μg/mL streptomycin, and 100 units/mL penicillin. Human microvascular endothelial cells (HMEC) were cultured with advanced DMEM supplemented with 2% FBS, 100 μg/mL streptomycin, 100 units/mL penicillin, 1 μg/mL hydrocortisone and 10 mM EGF.
siRNA vectors
Small interfering RNA sequences targeting uPAR and uPA were introduced into a mammalian expression vector pcDNA-3 downstream of the cytomegalovirus promoter. An uPAR sequence (+348 to +369) was used as the target sequence, and for convenience, a self-complimentary oligo was used. An uPAR sequence that was 23 bases in length with a 9 base loop region with BamHI sites incorporated at the ends (gatcctacagcagtggagagcgattatatataataatcgctctccactgctgtag) was used. The self-annealed oligo was ligated into the BamHI site of pcDNA-3 vector. Similarly, an uPA sequence (+78 to +98) was used as the target sequence. The 21 base uPA sequence with a 9 base loop sequence incorporated between the repeats and HindIII site on either ends (agctgagagccctgctggcgcgccatatataatggcgcgccagcagggctctca) was used. The bicistronic construct carried both the uPAR and uPA sequences and the BamHI and HindIII sites respectively. BGH poly A terminator served as a stop signal for RNA synthesis for all three constructs. The vectors carrying uPAR and uPA were named as pU and pUPA respectively and the bicistronic construct was named as pU2. The plasmid vectors were cloned and maintained in JM109 cells. A scrambled sequence of nucleotides was also introduced into the expression cassette and used as a mock vector (pSV). Plasmid DNA was isolated using the Qiagen plasmid purification system and analyzed for quality and quantity (Qiagen, Valenica, CA).
Transfection studies
1×106 cells were plated in 100 mm Petri dishes for each transfection experiment. The cells were transfected in serum-free L-15 media using 10 μg of lipofectamine reagent (Invitrogen, Grand Island, NY) as per manufacturer's instructions. The following constructs were used for transfection: pU, pUPA, pU2 and pSV. No plasmid was introduced in the control plates. After allowing 12 h for transfection, the serum-free media was replaced with serum-containing media and left in the incubator at 37°C for 24 h. The media was then replaced with serum-free media and conditioned media was collected after 12 h. Cells were harvested for isolation of total RNA and/or total cell lysate. Conditioned media was used for fibrin zymography and in vitro angiogenic assay.
RT-PCR
Total RNA was isolated from control and transfected cells using the PARIS™ protein and RNA isolation system (Ambion, Austin, TX). Total RNA was treated with DNase I (Invitrogen). First strand cDNA was prepared using Superscript III reverse transcriptase (Invitrogen) using oligo dT. 100ng of first strand cDNA was used for PCR amplification. We used the following primers for uPAR: uPAR-S- CATGCAGTGTAAGACCCAACGGGGA & uPAR-AS- AATAGGTGACAGCCCGGCCAGAGT. The following primers were used for uPA: uPA-S-TGCGTCCTGGTCGTGAGCGA & uPA-AS- CAAGCGTGTCAGCGCTGTAG. GAPDH was used as an internal control. PCR analysis was carried out using puReTaq™ Ready-To-Go PCR beads (Amersham Biosciences, UK).
Western blot analysis
MDA MB 231 and ZR 75 1 cells were transfected with pSV, pU, pUPA and pU2 as described above. At the end of the designated incubation period, cells were harvested, washed twice with cold PBS, and lysed in a buffer (150 mM NaCl, 50 mM Tris-Hcl, 2 mM EDTA, 1% NP-40, pH 7.4) containing protease inhibitors. Equal amounts of protein (20 μg/lane) from cell lysate were electrophoresed under non-reducing conditions on 10% acrylamide gels. After SDS–PAGE, proteins were transferred to a polyvinylidene difluoride membrane (BioRad). The membrane was incubated for 2 h in PBS (0.1% Tween-20 [T-PBS], 5% nonfat skim milk) to block non-specific binding. Subsequently, the membrane was incubated for 2 h with antibody against uPAR (R&D Systems), ERK (Santa Cruz Biotech Inc), pERK (Santa Cruz Biotech Inc), p38 (Santa Cruz Biotech Inc), p-p38 (Santa Cruz Biotech Inc), caspases 9, 8, 6 & 3 (Cell Signal) and APAF-1 (MBL International) respectively in T-PBS + 5% non-fat milk. After washing in T-PBS, proteins were visualized using the ECL™ detection kit with appropriate HRP-conjugated secondary antibody (Amersham Pharmacia Biotech, UK) as per manufacturer's instructions. The membranes were stripped and probed with monoclonal antibodies for GAPDH for loading control as per standard protocols.
In vitro angiogenesis
Conditioned media collected from transfected MDA MB 231 and ZR 75 1 cells was tested for induction of angiogenesis in human microvascular endothelial cells (HMEC) plated onto 8-well chamber slides (4×104/well). Once the cells had attached to the surface of the slide, the media was removed and replaced with conditioned media collected from RNAi-transfected cells. After 72 h in conditioned media, cells were washed gently with PBS and stained with hematoxylin and eosin (H&E). Angiogenic network formation was visualized under the microscope and Image Pro software was used for quantification of angiogenesis. The degree of angiogenesis was measured by the following method: number of branch points and the total number of branches per point were counted at random (per 10 fields), with the product indicating the degree of angiogenesis as compared to the controls.
Boyden chamber invasion assay
The in vitro invasiveness of MDA MB 231 and ZR 75 1 cells in the presence of vectors expressing siRNA for uPAR and uPA individually and in combination was assessed using a modified Boyden chamber assay. Breast cancer cells were transfected with pSV, pU, pUPA and pU2 vectors as described above. After allowing 24 h for transfection, 5×105 cells were suspended in 600 μL of serum-free medium and placed in the upper compartment of the transwell chambers [12 μm pore size, 12 mm diameter (Corning Costar Fischer Scientific, Pittsburg, PA] coated with Matrigel (100 μg/cm2). The lower compartment of the chambers was filled with 200 μL of serum-containing medium and the cells were allowed to migrate for 24 h. After incubation, the cells were fixed and stained with Hema-3 and photographed. Quantification of the invasion assay was performed as described previously (24-26).
MITO-PT 100 and FACS analysis
Induction of apoptosis in response to RNAi-induced downregulation of uPAR and uPA individually and in combination was tested using the MITO-PT 100 assay (Serotec). MDA MB 231 and ZR 75 1 cells were transfected with pSV, pU, pUPA and pU2 as described above. After 72 h, the cells were trypsinized and pelleted. The cells were then resuspended in MitoPT dye diluted in serum-free media as per manufacturer's instructions. After an incubation period, 100 μL of the cell suspension was visualized under a fluorescent microscope. MitoPT kit allows distinction between non-apoptotic (red fluorescence) and apoptotic (green fluorescence) cells. The remaining cells were used for FACS analysis. FACS analysis was performed according to manufacturer's instructions and 10,000 cells were recorded for each transfection. When cells stained with MitoPT are run through a flow cytometer, the instrument measures apoptosis by monitoring the amount of red fluorescence in each region. As the mitochondrial membrane collapses, a reduction in the amount of red fluorescence is observed as MitoPT dye is converted from a dimer form to a monomer form. These experiments were performed in triplicate for each cell line.
Results
RT-PCR
In order to study the effects of downregulation of uPA and uPAR, individually and in combination, on tumor cell induced angiogenesis, cancer cell invasion & survival, we transfected breast cancer cell lines MDA MB 231 and ZR 75 1 with pcDNA mammalian expression vectors harboring small interfering RNA sequences driven by a CMV promoter. mRNA levels were determined using semi-quantitative RT-PCR. Briefly, 48 h after transfection, total RNA was isolated from the cells and first strand cDNA was prepared using Superscript III reverse transcriptase and oligo-dT. PCR analysis was carried out using the appropriate primers for uPAR and uPA. Clear amplification was observed in the control and scrambled vector at approximately 280 bp with the primers for uPAR, and at 780 bp with the primers for uPA. While no change was observed in the levels of amplified product in cells treated with the scrambled vector as compared to the control, significant decreases in the levels of uPAR was observed in treatments with pU and pU2 (Fig. 1). Similarly, uPA levels were significantly decreased in pUPA and pU2 when the primers for uPA were used. The levels of GAPDH remain unchanged in all the treatments, indicating uniform levels of first strand cDNA taken in all the cases
Figure 1.
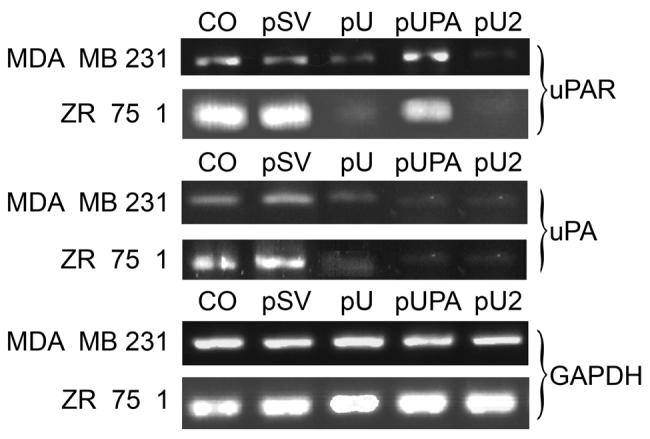
Reverse transcription PCR analysis of RNAI transfected breast cancer cells. 1×106 cells were plated on 100 mm petri plate for each transfection experiment. Cells were transfected with CO (control with no plasmid). Scrambled vector (pSV), uPAR (pU), pUPA and pU2. After 48 hrs., total RNA was isolated from the cells to prepare first strand cDNA followed by PCR analysis using the primers for uPA and uPAR. GAPDH was used as an internal control.
uPAR and uPA protein expression
The levels of uPAR protein in the total cell lysate and uPA in the conditioned media was assessed by western blot and fibrin zymography respectively. Western blot analyses using the anti-uPAR antibody revealed significant decreases in uPAR expression after transfection with pU in MDA MB 231 and ZR 75 1 cells. (Fig. 2A). However, no change in uPAR expression was observed after transfection with pUPA levels when compared to the control (Fig. 2A). Quantitative analysis of uPAR protein by densitometry revealed a decrease in protein expression with pU by 40% and 60% and in pU2 by 75% and 80%, respectively for MDA MB 231 and ZR 75 1 compared to controls and pSV and pUPA transfected cells (Fig. 2B).
Figure 2.
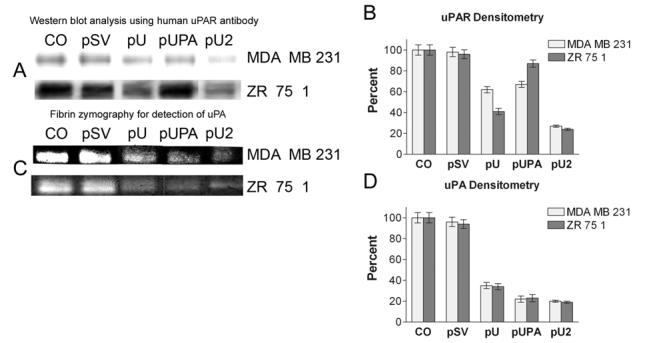
Western blot analysis and fibrin zymography. Transfection was carried out as described earlier. 1×106 cells were plated on 100 mm petri plate for each transfection experiment. Cells were transfected with CO (control with no plasmid). Scrambled vector (pSV), uPAR (pU), pUPA and pU2. After 36 hours, serum containing media from the plate was replaced with serum-free media. After 48 hrs., cells were harvested for protein extraction and conditioned media was collected 20μg of total protein from each of the samples was loaded on SDS-PAGE and blotted on nitrocellulose membranes. The membrane was probed with anti-uPAR antibody (A). 0.2μg of conditioned media was loaded for fibrin zymography (C). Quantification of uPAR protein (B) and uPA enzymatic activity (D) was obtained by scanning the auto-radiograms with a densitometer. Data are shown mean values ± S.D. of three different experiments in each group.
Fibrin zymography using conditioned medium from treated cells revealed a decrease in the levels of uPA activity in all the treatments as compared to the control and SV-treated group (Fig. 2C). Treatment with pUPA and pU2 showed uPA activity was decreased, ranging from 77% to 81% in MDA MB 231 and ZR 75 1 cells, respectively. pU treatment also showed reduced uPA activity of 65% and 66% (Fig. 2D) in MDA MB 231 and ZR 75 1 cells, respectively. No significant change was observed in the case of scrambled vector when compared to the control.
Inhibition of angiogenesis in HMEC-1 cells
In order to maintain the flow of nutrients for the growing number of cells, tumor cells induce angiogenesis. Since uPAR has been implicated in the regulation of angiogenesis, we assessed the effects of pU, pUPA and pU2 on tumor cell-induced angiogenesis. HMEC, grown in conditioned media from MDA MB 231 cells and ZR 75 1 cells transfected with pSV, pU, pUPA, pU2 or untransfected control, were stained with H&E. The results clearly demonstrated that endothelial cells form capillary-like structures in the presence of control and pSV-transfected cells within 72 h, in the case of conditioned media from MDA MB 231 cells (Fig. 3A), and within 96 h in the case of ZR 75 1 cells (data not shown). However, pU2 significantly inhibited tumor cell-induced angiogenesis. The quantification of the branch points and number of branches was almost undetectable in conditioned media from pU2-transfected cells, which decreased 94% with conditioned media from MDA MB 231 cells and 92% with conditioned media from ZR 75 1 cells as compared to the control (Fig. 3B). Conditioned media from pU- and pUPA-treated cells also demonstrated decreases in tumor cell-induced angiogenesis ranging from 60% for pU in ZR 75 1 to 70% in pUPA-transfected MDA MB 231 (Fig. 3B).
Figure 3.
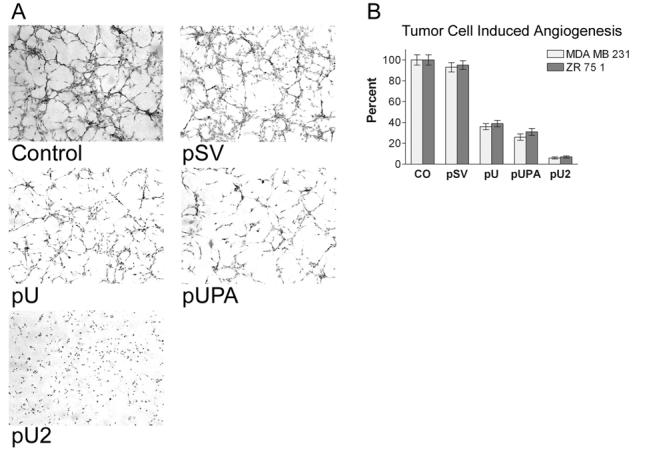
In vitro angiogenesis. Network formation by human microvascular endothelial cells in conditioned media from MDA MB 231/ZR 751 cells. Breast cancer cells were transfected with control (CO), scrambled vector (pSV) or RNAi constructs for uPAR (pU), uPA (pUPA), uPAR-uPA (pU2) and conditioned media was collected after 48 hrs. as described earlier. HMEC-1 cells grown in conditioned media were stained with H&E after 72 hours and examined under a confocal scanning laser microscope (A). Quantification of angiogenesis in co-cultures transfected with pSV, pU, pUPA and pU2 as described in Methods. Values are mean ± S.D. from three different experiments.
Inhibition of breast cancer cell invasion by pU, pUPA and pU2
To study the effect of siRNA-mediated downregulation of uPA and uPAR on their invasive potential, breast cancer cell lines MDA MB 231 and ZR 75 1 were transfected with pU, pUPA, pU2, or pSV and transferred to Matrigel-coated transwell chambers. Extensive invasion was observed within 48 h in the untransfected controls and cells transfected with pSV (Fig.4A). Figure 4A illustrates that the staining of pU, pUPA and pU2 transfected MDA MB 231 and ZR 251 cells was significantly less than that of the controls (Fig. 4A). In MDA MB 231 cells, pU2 treatment resulted in the least number of invading cells with 12% of the control while pUPA and pU treatment resulted in 19% of the control (Fig. 4B). In ZR 75 1 cells, inhibition of invasion was observed to a lesser extent with pU and pUPA treatments showing a less than 50% invasion as compared to the control. Once again, maximum inhibition was observed with pU2 treatment with only 21% invasion when compared to the control (Fig. 4B). Invasion of cells transfected with pSV in both MDA MB 231 and ZR 75 1 cells did not differ significantly from the controls.
Figure 4:
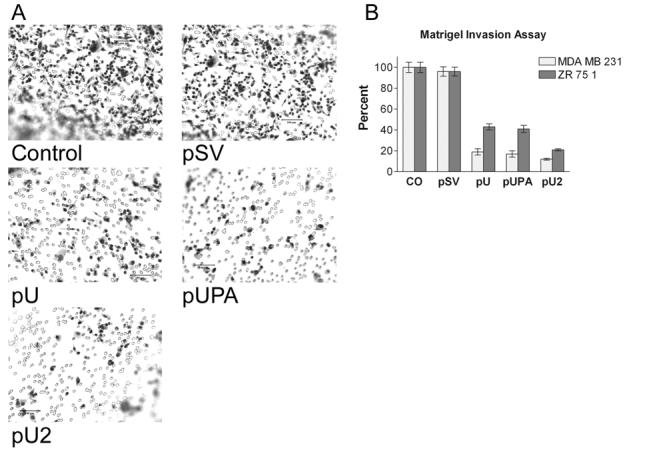
Matrigel invasion assay. Breast cancer cells transfected with control (CO), scrambled vector (pSV), or RNAi constructs for uPAR (pU), uPA (pUPA), uPAR-uPA (pU2) were transferred to 12μm transwell chambers coated with matrigel. After 48 hours, the cells were scraped from the chamber and stained to check for invasion of cells to the lower surface (A). The invasion was quantified as described in Methods (B). Values are mean ± S.D. of four experiments.
It has been reported previously that antibody-mediated inhibition of uPA induces the expression of caspase 3. To study the induction of apoptotic cell death due to downregulation of uPA and uPAR, FACS analysis was performed using the MitoPT™ mitochondrial permeability transition detection kit. 72 h after transfection, the cells were run through a flow cytometer. In the cells transfected with pSV, pU and pUPA, the majority of the cells were seen to fall in the red fluorescent region, indicating the presence of intact mitochondria. However, in the case of cells transfected with pU2, a significant drop in the number cells showing red fluorescence was observed, and also, a large number of events in the smaller size region was observed indicating the presence of extensive cellular debris. When MitoPT-stained cells were observed under the microscope, the induction of apoptosis in pU2-transfected cells could be clearly seen with more number of cells showing green fluorescence (Fig. 5B). As noted earlier, a large number of smaller particulate matter was observed in pU2-transfected cells as compared to the control indicating the presence of extensive cellular degradation.
Figure 5:
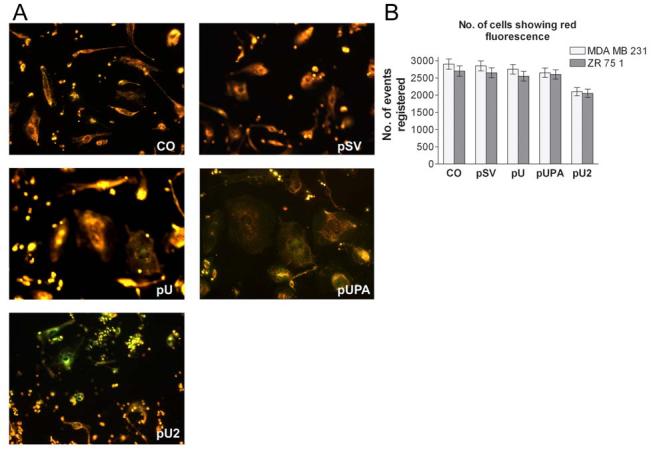
MITO-PT 100 and FACS analysis. MDA MB 231 cells were transfected with control (CO), scrambled vector (pSV), or RNAi constructs for uPAR (pU), uPA (pUPA), uPAR-uPA (pU2). After 48 hrs, the cells were resuspended in MitoPT dye diluted in serum-free media. After incubating at 37° C for 15 min, 100 μl of the suspension was visualized under a fluorescent microscope (A). MitoPT kit allows the distinction between non-apoptotic red fluorescent cells and apoptotic green fluorescent cells. FACS analysis was carried out according to manufacturer's instructions and 10,000 cells were recorded for each transfection. When cells stained with MitoPT are run through a flow cytometer, the instrument will measure apoptosis by monitoring the amount of red fluorescence in each region (B).
To study the effect of simultaneous downregulation of uPAR and uPA on apoptotic cascade signaling, western blot analysis was performed. Specifically, we tested for the expression of initiator and effector caspases as well as other genes involved in the apoptotic cascade. pU2 induced the expression of several genes involved in apoptosis, namely initiator caspases 9 & 8, and effector caspases 3 & 6 (Fig. 6). All the caspases mentioned above showed a 1.5-2 fold increase in pU2-transfected cells as compared to the control. We also observed a significant increase in the expression level of APAF-1, which is involved in apoptosome formation, in both cells lines after pU2 transfection. However, pUPA- and pU-transfected cells did not exhibit any changes in caspase expression level with the exception of caspase 9, which was seen to increase in pUPA-transfected MDA MB 231 cells. In addition to the increase in the expression levels of the genes involved in programmed cell death, changes were observed in the expression of proteins involved in cell survival. Significant decreases in the levels of phospho-ERK and phospho-p38 were observed in cells treated with pUPA and pU2, while the inactive non-phosphorylated forms remained unchanged when compared to control and pSV- and pU-transfected cells.
Figure 6.
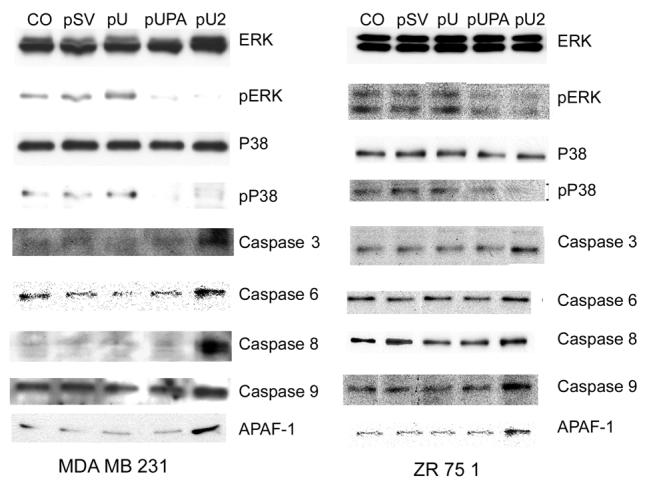
: Simultaneous inhibition of uPAR-uPA induces apoptotic cascade. Breast cancer cells were transfected with control (CO), scrambled vector (pSV), or RNAi constructs for uPAR (pU), uPA (pUPA), uPAR-uPA (pU2). After 48 hrs, cell lysates were prepared as per standard protocol and 15 μg of total protein was loaded on SDS-PAGE and blotted on nitrocellulose membrane. The membrane was probed with antibodies specific for ERK, pERK, p38, pP38, Caspases, 3,6,8 and 9 and APAF-1.
Discussion
Tumor cell invasion is associated with the destruction of the basement membrane and extracellular matrix. This process is associated with proteases, such as uPA and MMPs, secreted by malignant cells and stromal cells (6-9) Overexpression of uPA and uPAR has been well documented in wide variety of tumor cells. The urokinase plasminogen activator system has pleiotropic roles in tumor growth, angiogenesis and metastasis (27). In breast cancer, expression of uPA/uPAR is essential for tumor cell invasion and metastasis (28). Downregulation of matrix-degrading proteases and their receptors has potential therapeutic application in breast cancer. Antisense DNA and oligonucleotides are a convenient approach for the selective downregulation of protein expression (22). In a recent study, siRNA was quantitatively more efficient than antisense oligonucleotides at suppressing co-transfected GFP expression both in vitro and in vivo (29). Limitations to the use of RNAi in mammalian cells derive from unwarranted secondary responses to long dsRNA, such as induction of interferon synthesis (30). The recent development of 21-nucleotide siRNA duplexes has circumvented these problems and allowed successful RNAi in cultures of several types of mammalian cells (20). An important application of RNAi in mammals is the simultaneous inhibition of multiple genes in somatic cells. Previous observations demonstrated that there is no significant competition between co-transfected hairpin siRNA vectors.
In the present study, we have introduced siRNA sequences driven by a CMV promoter into breast cancer cell lines MDA MB 231 and ZR 75 1 to downregulate the expression of uPA and its receptor, individually and in combination, to assess the effects on cancer cell invasion, angiogenesis, survival and proliferation. Our results demonstrate that siRNA sequences can specifically reduce mRNA levels of uPAR and uPA without affecting the levels of housekeeping genes. Semi-quantitative RT-PCR analysis also revealed that when uPAR was downregulated, a decrease in the mRNA levels of uPA was also noticed. However, the reverse was not observed, suggesting transcriptional regulation of uPA expression by uPAR. Fibrin zymography also revealed a decrease in levels of uPA when compared to the control.
When conditioned media from transfected cells was assessed for angiogenic potential in HMEC, a significant decrease was seen in the number of branch points with conditioned media from pU- and pUPA-treated cells. The most remarkable decrease was observed in cells treated with pU2 where angiogenesis was reduced to less than 10% of the control in both cell lines. This decrease clearly indicates a synergistic rather than cumulative effect of the co-suppression of uPAR and uPA.
Li et al (31) have reported the inhibition of neo-vascularization in MDA MB 231 xenografts introduced into athymic mice, when uPA/uPAR antagonists were used. In another study, Min et al (32) have reported the inhibition of angiogenesis in an in vitro model when high-affinity murine urokinase receptor antagonists were used. Li et al (31) have suggested that the inhibition of angiogenesis observed in mice treated with uPAR antagonist might have occurred because of the hampering of bioavailability of angiogenic factors. Interestingly, the pattern of angiogenesis was similar with conditioned media from both breast cancer cell lines tested. The dramatic decrease in in vitro angiogenesis observed in our study when both uPAR and uPA were downregulated simultaneously suggests a signaling-induced downregulation in the release of angiogenic factors.
Both in vitro and in vivo studies indicate that uPA and uPAR levels are closely associated with the degree of tumor cell invasion and that uPA and uPAR play key roles in tumor progression and metastasis. Expression of uPA and uPAR can be upregulated by mitogens, growth factors, oncogenes, and binding of integrin to extracellular matrix proteins. Generally, factors that upregulate uPA also upregulate uPAR, suggesting that uPA and uPAR may be co-regulated in cancer cells. In our study, we have observed that downregulation of uPAR exhibits a concomitant downregulation of uPA. We tested the effect of RNAi-induced downregulation on the invasive abilities of the two cancer cell lines, MDA MB 231 and ZR 75 1 cancer cell lines. A similar pattern was observed in the invasive abilities of transfected cells when compared to control and scrambled vector, with only pU2-treated cells showing 12% and 21% invasion when compared to control in MDA MB 231 and ZR 75 1 cells, respectively.
uPA has been known to induce chemotaxis and chemokinesis in a range of different cell types (33-39) Binding to uPAR seems to be required for all chemotaxic effects of uPA. The phenomena can be divided into two groups depending on whether uPA catalytic activity is also required. The binding of uPA to uPAR in some cell types causes the transmission of signaling over the cell membrane leading to increased motility (40). This ligand engagement of uPAR has been reported to cause the transient activation of a variety of signaling proteins including src-family of members (37,41,42), ERK-1 and ERK-2 (36,43), FAK (44,45), fos, jun and myc (46), and rac (47). In fact, inhibitors of these pathways block the effects of uPA (40). In our study, we observed that the levels of phospho-ERK and phospho-p38 in pU2-transfected cells in both breast cancer cell lines showed a significant decrease when compared to the control. The decrease in the expression levels of these pro-survival kinases can also be correlated with a significant increase in the levels of the pro-apoptotic caspases. A number of studies have demonstrated that activated ERK inhibits apoptosis (48-50). Western blot analysis revealed significant increases in levels of initiator and effector caspases in both breast cancer cell lines. While caspase 9 levels were seen to increase in pUPA, all the caspases tested were seen to increase with significantly pU2. We also observed an increase in APAF-1, the cytosolic protein which is the human homolog of the C. elegans CED-4. APAF-1 functions downstream to Bcl-2 (or its close family member BcLXL), which regulates the release of cytochrome c from mitochondria (51). Cecconi et al (52) have suggested an in vivo apoptotic pathway whereby apoptotic stimuli trigger apoptosis through a multimolecular complex critically involving APAF-1 and caspase 9 and ultimately leading to caspase 3 activation and apoptosis. In our study, the pU2-transfected cells showed significant increases in caspase activity.
We also used the MitoPT assay to further confirm apoptotic effects of pU2. The MitoPT assay follows the principle that the staining dye enters the mitochondria and aggregates, which show red fluorescence. In contrast, in apoptotic cells where the mitochondrial membrane is compromised, the dye diffuses into the cytoplasm and is visualized as diffuse green fluorescence. In flow cytometry analysis, apoptosis leads to a drop in the number of cells showing red fluorescence when compared to healthy cells. Here, we observed a drop in red fluorescent cells as well as a concomitant increase in small particulate matter, suggesting an increase in cellular debris in pU2-treated cells. The ratio of apoptotic and non-apoptotic cells clearly shows a 9-fold increase in the pU2-transfected cells from both cell lines indicating the efficacy of targeting the uPA/uPAR system in cancer cells.
The results clearly indicate that the uPAR/uPA system not only plays a significant role in the invasion of cancer cells, but also has a regulatory role in cell survival and proliferation. RNAi-induced, simultaneous downregulation of these proteins has been shown to inhibit the activation of the pro-survival kinases phospho-ERK and phospho-p38, and possibly inhibit the release of angiogenic factors, leading to reduced angiogenesis. Significantly, the apoptotic cascade is triggered leading to cell death. The relative ease of handling and delivery of siRNA constructs in plasmid vectors when compared to adenoviral vectors and the specific downregulation of genes of interest presents an effective therapeutic tool against breast cancer.
Acknowledgements
We would like to thank Shellee Abraham for preparing the manuscript and Sushma Jasti and Diana Meister for reviewing the manuscript.
Footnotes
- RNAi
- (RNA interference)
- siRNA
- (short-interfering RNA)
- uPA
- (urokinase-type plasminogen activator)
- uPAR
- (uPA receptor)
- ECM
- (extracellular matrix)
- SV
- (scrambled vector)
- GAPDH
- (glyceral dehyde-3-phospho dehydrogenase)
- PBS
- (phosphate-buffered saline)
- CMV
- (cytomegaloviruse)
This work was supported by N.I.H. grants CA75557, CA92393, CA95058, CA116708 and N.I.N.D.S. NS47699 and by Caterpillar, Inc. and OSF St. Francis, Inc., Peoria, IL (to J.S.R.)
Reference List
- 1.Symes K, Mercola M. Embryonic mesoderm cells spread in response to platelet-derived growth factor and signaling by phosphatidylinositol 3-kinase. Proc Natl Acad Sci U. S. A. 1996;93:9641–9644. doi: 10.1073/pnas.93.18.9641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blasi F. uPA, uPAR, PAI-1: key intersection of proteolytic, adhesive and chemotactic highways? Immunol. Today. 1997;18:415–417. doi: 10.1016/s0167-5699(97)01121-3. [DOI] [PubMed] [Google Scholar]
- 3.Leirisalo-Repo M.The present knowledge of the inflammatory process and the inflammatory mediators Pharmacol Toxicol 199475Suppl 21–3.1-3 [DOI] [PubMed] [Google Scholar]
- 4.Camussi G, Montrucchio G, Lupia E, Soldi R, Comoglio PM, Bussolino F. Angiogenesis induced in vivo by hepatocyte growth factor is mediated by platelet-activating factor synthesis from macrophages. J Immunol. 1997;158:1302–1309. [PubMed] [Google Scholar]
- 5.Kim HJ, Ingbar DH, Henke CA. Integrin mediation of type II cell adherence to provisional matrix proteins. Am J Physiol. 1996;271:L277–L286. doi: 10.1152/ajplung.1996.271.2.L277. [DOI] [PubMed] [Google Scholar]
- 6.Aznavoorian S, Murphy AN, Stetler-Stevenson WG, Liotta LA. Molecular aspects of tumor cell invasion and metastasis. Cancer. 1993;71:1368–1383. doi: 10.1002/1097-0142(19930215)71:4<1368::aid-cncr2820710432>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 7.Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E, Seiki M. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature. 1994;370:61–65. doi: 10.1038/370061a0. [DOI] [PubMed] [Google Scholar]
- 8.Stetler-Stevenson WG, Aznavoorian S, Liotta LA. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol. 1993;9:541–573. doi: 10.1146/annurev.cb.09.110193.002545. [DOI] [PubMed] [Google Scholar]
- 9.Vassalli JD, Pepper MS. Tumour biology. Membrane proteases in focus. Nature. 1994;370:14–15. doi: 10.1038/370014a0. [DOI] [PubMed] [Google Scholar]
- 10.Mazar AP. The urokinase plasminogen activator receptor (uPAR) as a target for the diagnosis and therapy of cancer. Anticancer Drugs. 2001;12:387–400. doi: 10.1097/00001813-200106000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Ossowski L, Aguirre-Ghiso JA. Urokinase receptor and integrin partnership: coordination of signaling for cell adhesion, migration and growth. Curr Opin Cell Biol. 2000;12:613–620. doi: 10.1016/s0955-0674(00)00140-x. [DOI] [PubMed] [Google Scholar]
- 12.Preissner KT, Nawroth PP, Kanse SM. Vascular protease receptors: integrating haemostasis and endothelial cell functions. J Pathol. 2000;190:360–372. doi: 10.1002/(SICI)1096-9896(200002)190:3<360::AID-PATH574>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 13.Dano K, Romer J, Nielsen BS, Bjorn S, Pyke C, Rygaard J, Lund LR. Cancer invasion and tissue remodeling--cooperation of protease systems and cell types. APMIS. 1999;107:120–127. doi: 10.1111/j.1699-0463.1999.tb01534.x. [DOI] [PubMed] [Google Scholar]
- 14.Mustjoki S, Alitalo R, Stephens RW, Vaheri A. Plasminogen activation in human leukemia and in normal hematopoietic cells. APMIS. 1999;107:144–149. doi: 10.1111/j.1699-0463.1999.tb01537.x. [DOI] [PubMed] [Google Scholar]
- 15.Yu W, Kim J, Ossowski L. Reduction in surface urokinase receptor forces malignant cells into a protracted state of dormancy. J Cell Biol. 1997;137:767–777. doi: 10.1083/jcb.137.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moller LB. Structure and function of the urokinase receptor. Blood Coagul. Fibrinolysis. 1993;4:293–303. [PubMed] [Google Scholar]
- 17.Stahl A, Mueller BM. Melanoma cell migration on vitronectin: regulation by components of the plasminogen activation system. Int J Cancer. 1997;71:116–122. doi: 10.1002/(sici)1097-0215(19970328)71:1<116::aid-ijc19>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 18.Wei Y, Lukashev M, Simon DI, Bodary SC, Rosenberg S, Doyle MV, Chapman HA. Regulation of integrin function by the urokinase receptor. Science. 1996;273:1551–1555. doi: 10.1126/science.273.5281.1551. [DOI] [PubMed] [Google Scholar]
- 19.Burgering BM, Bos JL. Regulation of Ras-mediated signalling: more than one way to skin a cat. Trends Biochem Sci. 1995;20:18–22. doi: 10.1016/s0968-0004(00)88944-6. [DOI] [PubMed] [Google Scholar]
- 20.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 21.Zamore PD. RNA interference: listening to the sound of silence. Nat Struct Biol. 2001;8:746–750. doi: 10.1038/nsb0901-746. [DOI] [PubMed] [Google Scholar]
- 22.Lakka SS, Gondi CS, Yanamandra N, Olivero WC, Dinh DH, Gujrati M, Rao JS. Inhibition of cathepsin B and MMP-9 gene expression in glioblastoma cell line via RNA interference reduces tumor cell invasion, tumor growth and angiogenesis. Oncogene. 2004;23:4681–4689. doi: 10.1038/sj.onc.1207616. [DOI] [PubMed] [Google Scholar]
- 23.Ma Z, Webb DJ, Jo M, Gonias SL. Endogenously produced urokinase-type plasminogen activator is a major determinant of the basal level of activated ERK/MAP kinase and prevents apoptosis in MDA-MB-231 breast cancer cells. J Cell Sci. 2001;114:3387–3396. doi: 10.1242/jcs.114.18.3387. [DOI] [PubMed] [Google Scholar]
- 24.Mohan PM, Lakka SS, Mohanam S, Kin Y, Sawaya R, Kyritsis AP, Nicolson GL, Rao JS. Downregulation of the urokinase-type plasminogen activator receptor through inhibition of translation by antisense oligonucleotide suppresses invasion of human glioblastoma cells. Clin Exp Metastasis. 1999;17:617–621. doi: 10.1023/a:1006779902978. [DOI] [PubMed] [Google Scholar]
- 25.Mohanam S, Sawaya R, McCutcheon I, Ali-Osman F, Boyd D, Rao JS. Modulation of in vitro invasion of human glioblastoma cells by urokinase-type plasminogen activator receptor antibody. Cancer Res. 1993;53:4143–4147. [PubMed] [Google Scholar]
- 26.Mohan PM, Chintala SK, Mohanam S, Gladson CL, Kim ES, Gokaslan ZL, Lakka SS, Roth JA, Fang B, Sawaya R, Kyritsis AP, Rao JS. Adenovirus-mediated delivery of antisense gene to urokinase-type plasminogen activator receptor suppresses glioma invasion and tumor growth. Cancer Res. 1999;59:3369–3373. [PubMed] [Google Scholar]
- 27.Guo Y, Higazi AA, Arakelian A, Sachais BS, Cines D, Goldfarb RH, Jones TR, Kwaan H, Mazar AP, Rabbani SA. A peptide derived from the nonreceptor binding region of urokinase plasminogen activator (uPA) inhibits tumor progression and angiogenesis and induces tumor cell death in vivo. FASEB J. 2000;14:1400–1410. doi: 10.1096/fj.14.10.1400. [DOI] [PubMed] [Google Scholar]
- 28.Huang S, New L, Pan Z, Han J, Nemerow GR. Urokinase plasminogen activator/urokinase-specific surface receptor expression and matrix invasion by breast cancer cells requires constitutive p38alpha mitogen-activated protein kinase activity. J Biol Chem. 2000;275:12266–12272. doi: 10.1074/jbc.275.16.12266. [DOI] [PubMed] [Google Scholar]
- 29.Bertrand JR, Pottier M, Vekris A, Opolon P, Maksimenko A, Malvy C. Comparison of antisense oligonucleotides and siRNAs in cell culture and in vivo. Biochem Biophys Res Commun. 2002;296:1000–1004. doi: 10.1016/s0006-291x(02)02013-2. [DOI] [PubMed] [Google Scholar]
- 30.Stark JJ, Dillman RO, Schulof R, Wiemann MC, Barth NM, Honeycutt PJ, Soori G. Interferon-alpha and chemohormonal therapy for patients with advanced melanoma: final results of a phase I-II study of the Cancer Biotherapy Research Group and the Mid-Atlantic Oncology Program. Cancer. 1998;82:1677–1681. doi: 10.1002/(sici)1097-0142(19980501)82:9<1677::aid-cncr13>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 31.Li H, Lu H, Griscelli F, Opolon P, Sun LQ, Ragot T, Legrand Y, Belin D, Soria J, Soria C, Perricaudet M, Yeh P. Adenovirus-mediated delivery of a uPA/uPAR antagonist suppresses angiogenesis-dependent tumor growth and dissemination in mice. Gene Ther. 1998;5:1105–1113. doi: 10.1038/sj.gt.3300742. [DOI] [PubMed] [Google Scholar]
- 32.Min HY, Doyle LV, Vitt CR, Zandonella CL, Stratton-Thomas JR, Shuman MA, Rosenberg S. Urokinase receptor antagonists inhibit angiogenesis and primary tumor growth in syngeneic mice. Cancer Res. 1996;56:2428–2433. [PubMed] [Google Scholar]
- 33.Busso N, Masur SK, Lazega D, Waxman S, Ossowski L. Induction of cell migration by pro-urokinase binding to its receptor: possible mechanism for signal transduction in human epithelial cells. J Cell Biol. 1994;126:259–270. doi: 10.1083/jcb.126.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fazioli F, Resnati M, Sidenius N, Higashimoto Y, Appella E, Blasi F. A urokinase-sensitive region of the human urokinase receptor is responsible for its chemotactic activity. EMBO J. 1997;16:7279–7286. doi: 10.1093/emboj/16.24.7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fibbi G, Ziche M, Morbidelli L, Magnelli L, Del Rosso M. Interaction of urokinase with specific receptors stimulates mobilization of bovine adrenal capillary endothelial cells. Exp Cell Res. 1988;179:385–395. doi: 10.1016/0014-4827(88)90277-7. [DOI] [PubMed] [Google Scholar]
- 36.Nguyen DH, Hussaini IM, Gonias SL. Binding of urokinase-type plasminogen activator to its receptor in MCF-7 cells activates extracellular signal-regulated kinase 1 and 2 which is required for increased cellular motility. J Biol Chem. 1998;273:8502–8507. doi: 10.1074/jbc.273.14.8502. [DOI] [PubMed] [Google Scholar]
- 37.Resnati M, Guttinger M, Valcamonica S, Sidenius N, Blasi F, Fazioli F. Proteolytic cleavage of the urokinase receptor substitutes for the agonist-induced chemotactic effect. EMBO J. 1996;15:1572–1582. [PMC free article] [PubMed] [Google Scholar]
- 38.Sato Y, Rifkin DB. Autocrine activities of basic fibroblast growth factor: regulation of endothelial cell movement, plasminogen activator synthesis, and DNA synthesis. J Cell Biol. 1988;107:1199–1205. doi: 10.1083/jcb.107.3.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sato Y, Tsuboi R, Lyons R, Moses H, Rifkin DB. Characterization of the activation of latent TGF-beta by co-cultures of endothelial cells and pericytes or smooth muscle cells: a self-regulating system. J Cell Biol. 1990;111:757–763. doi: 10.1083/jcb.111.2.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sidenius N, Blasi F. The urokinase plasminogen activator system in cancer: recent advances and implication for prognosis and therapy. Cancer Metastasis Rev. 2003;22:205–222. doi: 10.1023/a:1023099415940. [DOI] [PubMed] [Google Scholar]
- 41.Chiaradonna F, Fontana L, Iavarone C, Carriero MV, Scholz G, Barone MV, Stoppelli MP. Urokinase receptor-dependent and -independent p56/59(hck) activation state is a molecular switch between myelomonocytic cell motility and adherence. EMBO J. 1999;18:3013–3023. doi: 10.1093/emboj/18.11.3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fazioli F, Blasi F. Urokinase-type plasminogen activator and its receptor: new targets for anti-metastatic therapy? Trends Pharmacol Sci. 1994;15:25–29. doi: 10.1016/0165-6147(94)90130-9. [DOI] [PubMed] [Google Scholar]
- 43.Aguirre Ghiso JA, Kovalski K, Ossowski L. Tumor dormancy induced by downregulation of urokinase receptor in human carcinoma involves integrin and MAPK signaling. J Cell Biol. 1999;147:89–104. doi: 10.1083/jcb.147.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu D, Aguirre Ghiso JA, Estrada Y, Ossowski L. EGFR is a transducer of the urokinase receptor initiated signal that is required for in vivo growth of a human carcinoma. Cancer Cell. 2002;1:445–457. doi: 10.1016/s1535-6108(02)00072-7. [DOI] [PubMed] [Google Scholar]
- 45.Tang H, Kerins DM, Hao Q, Inagami T, Vaughan DE. The urokinase-type plasminogen activator receptor mediates tyrosine phosphorylation of focal adhesion proteins and activation of mitogen-activated protein kinase in cultured endothelial cells. J Biol Chem. 1998;273:18268–18272. doi: 10.1074/jbc.273.29.18268. [DOI] [PubMed] [Google Scholar]
- 46.Rabbani SA, Gladu J, Mazar AP, Henkin J, Goltzman D. Induction in human osteoblastic cells (SaOS2) of the early response genes fos, jun, and myc by the amino terminal fragment (ATF) of urokinase. J Cell Physiol. 1997;172:137–145. doi: 10.1002/(SICI)1097-4652(199708)172:2<137::AID-JCP1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 47.Kjoller L, Hall A. Rac mediates cytoskeletal rearrangements and increased cell motility induced by urokinase-type plasminogen activator receptor binding to vitronectin. J Cell Biol %19. 2001;152:1145–1157. doi: 10.1083/jcb.152.6.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and - independent mechanisms. Science. 1999;286:1358–1362. doi: 10.1126/science.286.5443.1358. [DOI] [PubMed] [Google Scholar]
- 49.Guillonneau X, Bryckaert M, Launay-Longo C, Courtois Y, Mascarelli F. Endogenous FGF1-induced activation and synthesis of extracellular signal-regulated kinase 2 reduce cell apoptosis in retinal-pigmented epithelial cells. J Biol Chem. 1998;273:22367–22373. doi: 10.1074/jbc.273.35.22367. [DOI] [PubMed] [Google Scholar]
- 50.Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 51.Zou H, Henzel WJ, Liu X, Lutschg A, Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]
- 52.Cecconi F, Alvarez-Bolado G, Meyer BI, Roth KA, Gruss P. Apaf1 (CED-4 homolog) regulates programmed cell death in mammalian development. Cell. 1998;94:727–737. doi: 10.1016/s0092-8674(00)81732-8. [DOI] [PubMed] [Google Scholar]


