Abstract
v-Crk, an oncogene product of avian sarcoma virus CT10, efficiently transforms chicken embryo fibroblasts (CEF). We have recently reported that constitutive activation of the phosphoinositide 3-kinase (PI3K)/AKT pathway plays a critical role in the v-Crk-induced transformation of CEF. In the present study we investigated the molecular mechanism by which v-Crk activates the PI3K/AKT pathway. First, we found that v-Crk promotes the association of the p85 regulatory subunit of PI3K with focal adhesion kinase (FAK) by inducing the phosphorylation of the Y397 residue in FAK. This FAK phosphorylation needs activation of the Src family tyrosine kinase(s) for which the v-Crk SH2 domain is responsible. v-Crk was unable to activate the PI3K/AKT pathway in FAK-null cells, indicating the functional importance of FAK. In addition, we found that H-Ras is also required for the activation of the PI3K/AKT pathway. The v-Crk-induced activation of AKT was greatly enhanced by the overexpression of H-Ras or its guanine nucleotide exchange factor mSOS, which binds to the v-Crk SH3 domain, whereas a dominant-negative mutant of H-Ras almost completely suppressed this activation. Furthermore, we showed that v-Crk stimulates the interaction of H-Ras with the Ras binding domain in the PI3K p110 catalytic subunit. Our data indicated that the v-Crk-induced activation of PI3K/AKT pathway was cooperatively achieved by two distinct interactions. One is the interaction of p85 with tyrosine-phosphorylated FAK promoted by the v-Crk SH2 domain, and another is the interaction of p110 with H-Ras dictated by the v-Crk SH3 domain.
v-Crk is the oncogene product of CT10 avian sarcoma virus which efficiently transforms chicken embryo fibroblasts (CEF) in tissue culture and induces tumors in newborn chickens (24). v-Crk consists of a viral Gag sequence fused to cellular SH2 and SH3 domains. This protein is the first molecule identified as the adaptor protein comprised primarily of SH2 and SH3 domains mediating protein-protein interactions (4). What was both surprising and interesting was that v-Crk has no catalytic domain of enzyme, and yet these SH2 and SH3 domains alone cause cell transformation (24). Therefore, many attempts have been made to understand the roles of these domains in signal transduction. It seems likely that, to achieve oncogenic transformation, v-Crk must have many interactions with various signaling molecules. Although several molecules interacting with the v-Crk SH2 and SH3 domains have been identified, the overall scheme leading to transformation has remained unclear.
Recently, we reported that v-Crk can activate the phosphoinositide 3-kinase (PI3K)/AKT pathway, and this activation seems essential for v-Crk-induced transformation based on the following observations (2). (i) In v-Crk-transformed CEF, the PI3K/AKT pathway was constitutively activated, whereas the MAPK or the JNK pathway was not. (ii) Studies with several v-Crk mutants showed a close correlation between the activation of the PI3K/AKT pathway and cell transformation. (iii) A constitutively active mutant of PI3K was shown to transform CEF, indicating that activation of the PI3K/AKT pathway is sufficient for transformation. (iv) A specific inhibitor of PI3K significantly suppresses the v-Crk-induced transformation of CEF. Stam et al. have also reported that the PI3K/AKT pathway is activated in v-Crk-transformed NIH 3T3 cells (35).
PI3K was first described as a lipid kinase associated with viral oncoproteins, v-Src, v-Ros, and polyomavirus middle T antigen (7). PI3K consists of a p85 regulatory subunit containing two SH2 domains and one SH3 domain, as well as a p110 catalytic subunit that phosphorylates inositol lipids specifically at the D-3 position of the inositol ring (41). The lipid products of PI3K serve as a second messenger involved in many biological functions. AKT is a serine/threonine protein kinase, which was first identified as the oncogene product of the AKT8 murine leukemia virus (22). It is now well established that AKT is a downstream target of PI3K. AKT binds to the lipid products of PI3K by using its pleckstrin homology domain and is then activated through the phosphorylation at threonine 308 and serine 473 by the PI3K-dependent kinases PDK1 and PDK2 (10). Activated AKT exerts effects on many growth controlling processes, including suppression of apoptosis (9), translational control (13), glucose metabolism, and cell cycle progression (21). The PI3K/AKT pathway is activated in response to a wide variety of extracellular stimuli, which include growth factors and cytokines, as well as adhesion to extracellular matrices. Involvements of several adaptor proteins such as IRS-1/2 (42), Gab1/2 (17), and Cbl (39) in the activation of this pathway have been well analyzed. However, the relationship between Crk adaptor protein and the PI3K/AKT pathway remains to be elucidated.
In the present study, we tried to clarify the molecular mechanisms by which the adaptor type oncogene v-Crk activates the PI3K/AKT pathway. The results obtained can explain how two SH domains play independent roles in interacting with other cellular proteins and then synergistically contribute to cell transformation.
MATERIALS AND METHODS
Plasmids.
Mammalian expression vectors for hemagglutinin (HA) epitope-tagged wild-type (WT) focal adhesion kinase (FAK) and various mutant FAK (FAK Y397F, FAK Y407F, FAK Y576/7F, FAK Y861F, FAK Y925F, and FAK KD) in pRc/CMV plasmid were kindly provided by S. Hanks, Vanderbilt University (6). Myc epitope-tagged AKT1 construct in the pUSEamp mammalian expression vector was purchased from Upstate Biotechnology. Mammalian expression vectors for Flag-tagged H-Ras, Rap1, and R-Ras in pCXN2-Flag plasmid were kindly provided by M. Matsuda, Osaka University (25). The mammalian expression vector for mSOS, pSRαSOS, was kindly provided by M. Kasuga, Kobe University (32). WT H-Ras was subcloned into pcDNA3 and mutated by using the QuickChange site-directed mutagenesis kit (Stratagene) to make effector loop mutants H-Ras T35S and H-Ras Y40C. The Ras-binding domain of human PI3K p110α (amino acids 128 to 325) was amplified by PCR and cloned into a mammalian glutathione S-transferase (GST) fusion vector pEBG (36) in frame, and the resulting plasmid was designated pEBG-p110 RBD. Mammalian expression vector for H-Ras N17 (pcDNA3-Ras-N17) (37), WT v-Crk (pCXbsr/v-Crk), v-Crk SH2 mutant (pCXbsr/v-Crk R273N), v-Crk SH3 mutant (pCXbsr/v-Crk BSP) (2), Csk (pcDNA1 Csk) (30), kinase-deficient c-Abl (pcDNA3 c-Abl KD) (34), and C3G (pCAGGS-C3G) (38) were also used.
Cell culture and transfection.
CEF transduced with WT v-Crk, v-Crk SH2 mutant (R273N), v-Crk SH3 mutant (BSP), or a control vector were generated and maintained as described previously (2). Chicken wild-type Csk was expressed in CEF by the use of a mouse-avian chimeric retroviral vector system as described previously (2). For the soft agar colony assay, 5 × 104 of each infected CEF were plated per 6-cm culture dish in 3 ml of Dulbecco modified Eagle medium containing 10% calf serum and 0.4% agar on a layer of 5 ml of the same medium containing 0.7% agar. Plates were incubated at 37°C for 3 to 4 weeks until colonies developed. FAK-deficient fibroblast cell lines isolated from fak-knockout (FAK-KO) mouse embryos (a kind gift from T. Yamamoto, University of Tokyo, and M. Hamaguchi, Nagoya University) were cultured as described previously (18). Mouse WT FAK and FAK F397 (phosphorylation site mutant) were expressed in FAK-KO fibroblast cell lines by using the retroviral vector pCXpur according to the procedure described previously (2). 293T cells and COS-7 cells were grown in Dulbecco modified Eagle medium supplemented with 10% fetal calf serum. Transient transfections were performed by using FuGENE 6 transfection reagent (Roche) as indicated by the manufacturer.
Immunoblotting.
Immunoblotting was performed essentially as described previously (1). Briefly, cells were lysed in Laemmli sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer containing 1 mM Na3VO4. Each lysate corresponding to 10 μg of protein was fractionated by SDS-10% PAGE, and the proteins were electrophoretically transferred to Immobilon (Millipore). After a blocking step performed with 3% bovine serum albumin in TBS (10 mM Tris-HCl, pH 7.4; 140 mM NaCl) for 1 h at room temperature, the membranes were incubated with appropriate primary antibodies in blocking buffer overnight at 4°C. The membranes were then washed extensively with TBS containing 0.1% Tween 20 and incubated for 1 h at room temperature with horseradish peroxidase-conjugated secondary antibodies. After being washed, the membranes were developed with the ECL Plus chemiluminescence reagent (Amersham Pharmacia) as directed by the manufacturer.
Immunoprecipitation.
Cells were lysed in 1% NP-40 lysis buffer containing 10 mM Tris-HCl (pH 8.0), 150 mM NaCl, 10% glycerol, 2.5 mM EDTA, 2 mM Na3VO4, 50 mM NaF, 20 mM Na4P2O7, 1 mM phenylmethylsulfonyl fluoride, 10 μg of leupeptin/ml, and 1% aprotinin for 20 min on ice. Insoluble material was removed by centrifugation at 4°C for 20 min at 10,000 × g. After quantitation of the protein with BCA protein assay reagent (Pierce), lysates corresponding to 500 μg of protein were incubated with antibodies for 3 h at 4°C and then with protein G-Sepharose beads (Amersham Pharmacia) for 1 h at 4°C. Immunoprecipitates were washed three times with lysis buffer, suspended in Laemmli SDS-PAGE sample buffer containing 1 mM Na3VO4, and analyzed by SDS-PAGE.
Antibodies.
Anti-Gag mouse monoclonal antibody 3C2 was kindly provided by D. Boettiger, University of Pennsylvania (26). Anti-PI3-kinase p85 mouse monoclonal antibody AB6, anti-PI3-kinase p85 rabbit polyclonal antibody, anti-phosphotyrosine mouse monoclonal antibody 4G10, and anti-Fak mouse monoclonal antibody 4.47 were purchased from Upstate Biotechnology. Anti-Cas mouse monoclonal antibody and anti-Ras mouse monoclonal antibody were purchased from Transduction Laboratories. Anti-Cbl rabbit polyclonal antibody C-15, anti-c-Myc mouse monoclonal antibody 9E10, anti-C3G rabbit polyclonal antibody C-19, anti-SOS1 rabbit polyclonal antibody C-23, and anti-GST rabbit polyclonal antibody Z-5 were purchased from Santa Cruz Biotechnology. Anti-phospho-Fak(Y397) rabbit polyclonal antibody, anti-phospho-Src(Tyr527) rabbit polyclonal antibody, and anti-Src rabbit polyclonal antibody were purchased from Biosource International. Anti-Akt rabbit polyclonal antibody and anti-phospho-Akt(Thr308) rabbit polyclonal antibody were purchased from New England Biolabs. Anti-phospho-Src(Tyr416) rabbit polyclonal antibody was purchased from Cell Signaling Technology. Anti-Fyn mouse monoclonal antibody and anti-Yes mouse monoclonal antibody were purchased from Wako. Anti-FLAG mouse monoclonal antibody M2 was purchased from Sigma.
GST pull-down assay.
The GST-p85 SH2N construct contains amino acid residues 330 to 436 of human p85. The GST-p85 SH2C construct contains amino acid residues 586 to 720 of human p85. The GST-p85 SH3 construct contains amino acid residues 1 to 113 of human p85. These three GST fusion constructs were kindly provided by Y. Fukui, University of Tokyo. pGEX-p85, a GST fusion construct containing full-length bovine p85, was a kind gift from J. Backer, Albert Einstein College of Medicine (43). GST fusion proteins were expressed in bacteria and affinity purified with glutathione-Sepharose beads (Amersham Pharmacia) as described previously (31).
For the GST pull-down assay, 500 μg of cell lysates prepared in 1% NP-40 lysis buffer was incubated with 5 μg of GST fusion proteins coupled to glutathione-Sepharose beads for 2 h at 4°C. Precipitates were washed three times with lysis buffer, suspended in Laemmli SDS-PAGE sample buffer containing 1 mM Na3VO4, and analyzed by SDS-PAGE.
RESULTS
Tyrosine-phosphorylated proteins associated with PI3K p85 in v-Crk-transformed CEF.
It is well known that the activation of PI3K is triggered by the binding of its p85 regulatory subunit to phosphorylated tyrosine residues of activated growth factor receptors or their substrates (41). Therefore, to understand the mechanism of activatation of the PI3K/AKT pathway by v-Crk, it is important to know the nature of tyrosine-phsphorylated proteins interacting with PI3K p85. Immunoprecipitations of total cell lysates from v-Crk-transformed or control cells with anti-p85 antibody, followed by immunoblotting with an anti-phosphotyrosine (P-Tyr) antibody showed that several tyrosine-phosphorylated proteins were associated with endogenous p85, specifically with lysates from v-Crk-transformed CEF (Fig. 1A). Among them a band of ca. 120 kDa was most prominent. An 85-kDa band is supposed to be p85 itself. We also performed an in vitro precipitation assay by using GST fusion proteins with the individual SH2 and SH3 domains of p85 (Fig. 1B). A tyrosine-phosphorylated protein of ca. 120 kDa was again precipitated only from the lysate of v-Crk-transformed CEF, with the GST fusion protein of p85 N-terminal SH2 domain (SH2N).
FIG. 1.
Tyrosine-phosphorylated proteins associated with PI3K p85 in v-Crk-transformed CEF. (A) Coimmunoprecipitation (Co-IP) assay. Total cell lysates from control or v-Crk-transformed CEF were subjected to immunoprecipitation with anti-PI3K p85 rabbit polyclonal antibody, and the precipitates were then subjected to immunoblot analysis with anti-P-Tyr mouse monoclonal antibody (upper panel) or anti-PI3K p85 mouse monoclonal antibody (lower panel). (B) GST pull-down assay. Total cell lysates from control or v-Crk-transformed CEF were incubated with the GST fusion proteins indicated above, and proteins associated with each GST-fusion protein were subjected to immunoblot (IB) analysis with anti-phosphotyrosine (P-Tyr) mouse monoclonal antibody.
FAK is associated with PI3K p85 in v-Crk-transformed CEF.
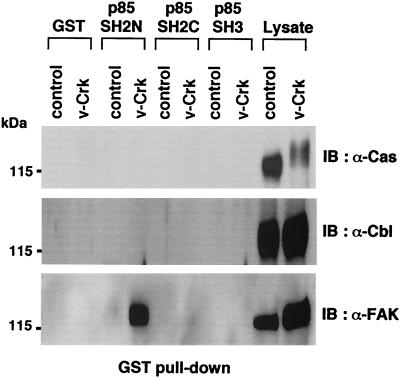
There are several candidate proteins with this molecular weight range that are tyrosine phosphorylated in v-Crk-transformed cells, such as Cas, c-Cbl, and FAK. Immunoblotting of the same GST pull-down samples as in Fig. 1B with each specific antibody against these candidate proteins revealed that only the anti-FAK antibody reacted with the protein bound to the N-terminal SH2 domain of p85 (Fig. 2).
FIG. 2.
FAK in v-Crk-transformed CEF binds to N-terminal SH2 domain of PI3K p85. The same GST-pull down samples as in Fig. 1B were subjected to immunoblot analysis with anti-Cas mouse monoclonal antibody (upper panel), anti-Cbl rabbit polyclonal antibody (middle panel), or anti-FAK mouse monoclonal antibody (lower panel). Total cell lysates were also included to confirm the expression of each protein.
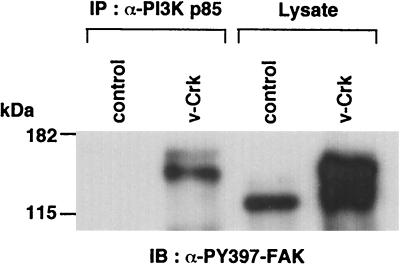
Chen et al. previously reported that Y397 in FAK is phosphorylated (PY397) upon integrin stimulation and provides a binding site for the SH2 domain of PI3K p85 (8). Therefore, we first tested whether v-Crk induces phosphorylation of this site. We transfected HA epitope-tagged FAK, with or without v-Crk, into 293T cells, and performed immunoblotting with an antibody against FAK phosphorylated at Y397 (Fig. 3). A series of Tyr-to-Phe mutants of FAK were also examined to confirm the specificity of this immunoblotting. Expression of v-Crk strongly induced phosphorylation of Y397 in FAK. We also performed an in vitro pull-down assay with a GST fusion protein of full-length p85 and found that WT FAK bound to GST-p85 only in the presence of v-Crk. Among the Tyr-to-Phe mutants of FAK, the Y397F mutant was unable to bind to GST-p85 even in the presence of v-Crk, whereas the binding of all other mutants was successful. These results indicated that v-Crk induces the phosphorylation of Y397 in FAK, and this site is used for binding with the p85 regulatory subunit of PI3K. Interestingly, this v-Crk-induced phosphorylation of FAK Y397 and the binding of p85 to this site were observed even in the cells cultured in a suspended condition, indicating that these events are independent of the integrin-mediated cell adhesion (Fig. 3, lane 9). More importantly, not only in the overexpression system with 293T cells but also in v-Crk-transformed CEF, phosphorylation of Y397 in endogenous FAK was greatly enhanced, and the association of this Y397 phosphorylated FAK with endogenous p85 was observed (Fig. 4). FAK in v-Crk-transformed CEF showed retarded electrophoretic mobility; this was most likely due to the tyrosine phosphorylation, as reported previously in Src-transformed NIH 3T3 cells (29).
FIG. 3.
FAK Y397 is phosphorylated by v-Crk and provides the binding site for PI3K p85. HA epitope-tagged WT or one of the indicated Tyr(Y) to Phe(F) mutants of FAK was transiently transfected with either v-Crk or a control vector into 293T cells. After 48 h, total cell lysates were prepared and subjected to immunoblot analysis with an antibody specific for phosphorylated Y397 (PY397) in FAK (A), anti-HA antibody (B), or anti-Gag antibody for the detection of v-Crk (C). (D) The same total cell lysates were also subjected to GST pull-down (PD) assay by using GST fusion protein with full-length p85 (GEX-p85), and the FAK proteins bound to GEX-p85 were detected by immunoblotting with anti-HA antibody. In lanes 8 and 9, cells were cultured in suspended condition for 24 h prior to harvesting by using culture dishs coated with an anti-adhesive polymer poly(2-hydroxyethyl methacrylate) as described previously (11).
FIG. 4.
Endogenous FAK is highly tyrosine phosphorylated at Y397 and associates with endogenous PI3K p85 in v-Crk-transformed CEF. The same immunoprecipitates with anti-PI3K p85 antibody were used as in Fig. 1B, and the total cell lysates from control or v-Crk-transformed CEF were subjected to immunoblot analysis with an antibody specific for phosphorylated Y397 (PY397) in FAK.
FAK is required for the activation of AKT by v-Crk.
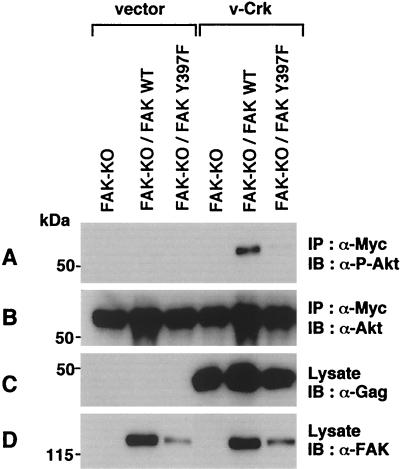
To examine the functional importance of FAK in v-Crk-induced activation of the PI3K/AKT pathway, we utilized a fibroblast cell line derived from the FAK knockout mouse (18). Myc-tagged AKT constructs were transiently transfected with or without v-Crk, and the activation of AKT was monitored by immunoblotting with an antibody specific for the phosphorylated form of AKT (Fig. 5). While v-Crk did not activate AKT in FAK knockout cells, the expression of WT FAK restored the activation. On the other hand, expression of FAK Y397F mutant could not restore it. These results clearly show that FAK is required for the v-Crk-induced activation of AKT and demonstrate the critical role of Y397 residue for this function.
FIG. 5.
FAK is required for the activation of AKT by v-Crk. Myc-tagged AKT was transiently cotransfected with v-Crk or a control vector into FAK knockout fibroblast cell line (FAK-KO) or its derivatives in which FAK WT or FAK Y397F mutant was expressed with a retroviral vector pCXpur (2). After 48 h, AKT was immunoprecipitated with anti-Myc antibody and subjected to immunoblot analysis with anti-phospho-Akt(Thr308) antibody (A) or anti-Akt antibody (B). Total cell lysates were also subjected to immunoblot analysis with anti-Gag antibody for the detection of v-Crk (C) or anti-FAK antibody (D).
Src family tyrosine kinase (SFK) is needed for the v-Crk-induced phosphorylation of Y397 in FAK.
Since v-Crk has no tyrosine kinase activity, the next important question is how v-Crk phosphorylates Y397 in FAK. Y397 is known to be a major autophosphorylation site of FAK (33); therefore, one possibility is that FAK itself phosphorylates this site. However, even the kinase-negative mutant of FAK (FAK KD) was phosphorylated at this site by v-Crk when expressed in FAK knockout cells, indicating that some other tyrosine kinase is involved in this process (Fig. 6A). To examine the possible involvement of c-Abl and Src family tyrosine kinases, we expressed the kinase-negative mutant of c-Abl (c-Abl KD) as a dominant-negative mutant and WT Csk (Csk WT), a negative regulator of SFKs. Although c-Abl KD had no effect, Csk WT almost completely suppressed the v-Crk-induced phosphorylation of Y397 in FAK (Fig. 6B). These results strongly suggest that SFKs mediate this phosphorylation and that v-Crk somehow activates SFK to phosphorylate this site.
FIG. 6.
v-Crk-induced phosphorylation of FAK Y397 is mediated by SFKs. (A) v-Crk induces Y397 phosphorylation in a kinase-negative mutant of FAK (FAK KD) expressed in FAK-KO cells. Either FAK WT, FAK Y397F mutant, or FAK KD mutant was transiently cotransfected with v-Crk or a control vector into FAK-KO fibroblast cell line. After 48 h, total cell lysates were prepared and subjected to immunoblot analysis with anti-PY397-FAK antibody (upper panel), anti-FAK antibody (middle panel), or anti-Gag antibody for the detection of v-Crk (lower panel). (B) Csk suppresses FAK Y397 phosphorylation induced by v-Crk. FAK KD mutant was transiently cotransfected with the expression vectors indicated above each lane. After 48 h, total cell lysates were prepared and subjected to immunoblot analysis with anti-PY397-FAK antibody (upper panel), anti-FAK antibody (middle panel), or anti-Gag antibody for the detection of v-Crk (lower panel).
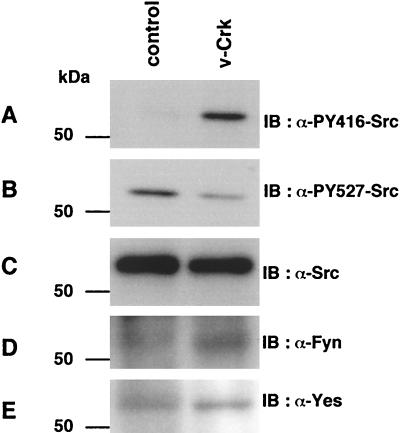
Activities of SFK are known to be regulated by phosphorylation at two tyrosine residues with opposing effects. In the case of c-Src, phosphorylation of Y416 in the activation loop of the kinase domain upregulates the enzyme, and phosphorylation of the c-terminal Y527 residue by Csk downregulates the activity (5). Here, we confirmed the activation of endogenous c-Src in v-Crk-transformed CEF by immunoblotting with phosphospecific antibodies against these tyrosine residues in c-Src. Compared to normal CEF, v-Crk-transformed CEF showed a clearly elevated level of phosphorylation at Y416 and a slightly reduced level of phosphorylation at Y527 (Fig. 7). This finding is consistent with our previous observation of the elevated specific activity of c-Src kinase in v-Crk-transformed cells as observed in an in vitro kinase assay (31). The sequences surrounding these regulatory Tyr residues are almost completely conserved in many other related SFK, and we confirmed that Fyn and Yes are expressed in CEF (Fig. 7). Therefore, the possibility remains that these kinases are also activated in v-Crk-transformed CEF.
FIG. 7.
SFK is activated in v-Crk-transformed CEF. Total cell lysates from control or v-Crk-transformed CEF were subjected to immunoblot analysis with anti-phospho-Src(Tyr416) antibody (A), anti-phospho-Src(Tyr527) antibody (B), anti-Src antibody (C), anti-Fyn antibody (D), or anti-Yes antibody (E).
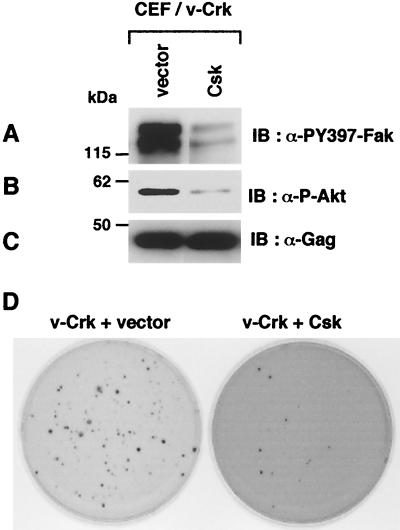
We further demonstrated that the decrease in FAK Y397 phosphorylation by the overexpression of Csk, a negative regulator of SFK, resulted in the significant reduction of the AKT phosphorylation and subsequently in the marked suppression of soft agar colony formation of v-Crk-transformed CEF (Fig. 8). These results strongly support the notion that FAK Y397 phosphorylation by SFK plays a critical role in the activation of PI3K/AKT pathway and thereby in the cellular transformation induced by v-Crk.
FIG. 8.
Csk suppresses v-Crk-induced transformation of CEF. Total cell lysates from v-Crk-transformed CEF infected with control retroviral vector or with Csk expressing retroviral vector were subjected to immunoblot analysis with anti-PY397-FAK antibody (A), anti-phospho-Akt(Ser473) antibody (B), or anti-Gag antibody (C). (D) Both CEF were subjected to soft agar colony formation assay. At 3 weeks after plating, colonies were stained with 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl tetrazolium bromide, and photographs of the stained colonies were taken as described previously (2).
H-Ras is involved in the v-Crk-induced activation of PI3K/AKT pathway.
Although the important role of the phosphorylation of FAK Y397 for the activation of PI3K/AKT pathway by v-Crk was well established by studies described above, analysis with v-Crk mutants suggested that this is not sufficient. A v-Crk SH3 mutant could induce phosphorylation of Y416 in c-Src and Y397 in FAK but could not activate AKT, indicating that some pathway involving the SH3 domain is also necessary for the activation of AKT by v-Crk (Fig. 9).
FIG. 9.
v-Crk SH3 mutant can induce phosphorylation of Y416 in c-Src and Y397 in FAK but cannot activate AKT. Total cell lysates from control CEF or CEF expressing the indicated version of v-Crk were subjected to immunoblot analysis with anti-PY397-FAK antibody (A), anti-phospho-Src(Tyr416) antibody (B), anti-phospho-Akt(Ser473) antibody (C), or anti-Gag antibody (D).
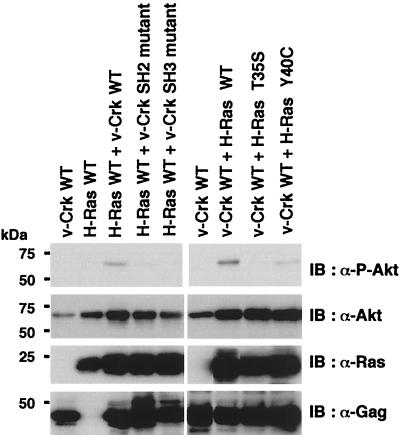
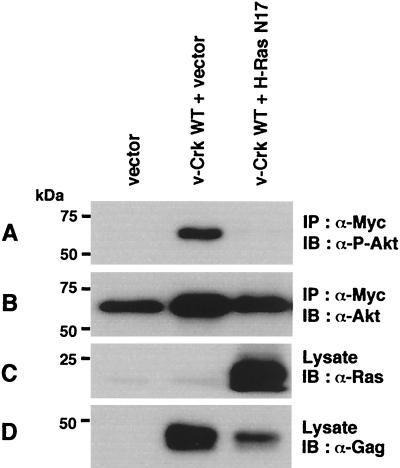
In this context, it is noteworthy that v-Crk SH3 is known to bind to guanine nucleotide exchange factors (GEFs) for Ras family small G proteins such as C3G and mSOS (23), and active GTP-loaded forms of Ras are reported to activate PI3K by binding to the p110 catalytic subunit (28). To examine the possible involvement of Ras family small G proteins in the activation of the PI3K/AKT pathway, we transiently expressed each wild-type of H-Ras, Rap1, and R-Ras, along with v-Crk and AKT, in COS-7 cells, and the activation of AKT was analyzed. Among the Ras family small G proteins examined, only H-Ras was able to significantly enhance the v-Crk-induced activation of AKT (Fig. 10A). Consistent with this, coexpression of mSOS, a GEF for H-Ras, but not C3G, a GEF for Rap1 and R-Ras (14), enhanced the AKT activation by v-Crk (Fig. 10B). Mutational analysis revealed that only the WT v-Crk and neither the SH2 mutant nor the SH3 mutant synergized with H-Ras to activate AKT (Fig. 11). Moreover, as expected, H-Ras 40C, a mutant which can bind to PI3K p110 but not Raf (27) synergized with v-Crk, whereas H-Ras 35S, a mutant which can bind to Raf but not PI3K p110 (27), did not (Fig. 11). Furthermore, we confirmed that binding of H-Ras to the p110 Ras-binding domain is significantly enhanced by the expression of v-Crk (Fig. 12). Finally, we found that the expression of H-Ras N17, a dominant-negative mutant of H-Ras (15), almost completely suppressed the AKT activation induced by v-Crk (Fig. 13). These results clearly indicated the functional importance of H-Ras in the v-Crk-induced activation of the PI3K/AKT pathway.
FIG. 10.
H-Ras and mSOS can enhance the activation of AKT induced by v-Crk. (A) Effect of Ras family small G proteins on v-Crk-induced AKT activation. Flag-tagged Ras family small G proteins indicated on the top were transiently coexpressed with AKT in the absence (−) or presence (+) of v-Crk in COS-7 cells. After 48 h, total cell lysates were prepared and subjected to immunoblot analysis with anti-phospho-Thr308-Akt(P-Akt) antibody, anti-Akt antibody, anti-FLAG antibody, or anti-Gag antibody as indicated on the right. (B) Effect of guanine nucleotide exchange factors on v-Crk-induced AKT activation. mSOS or C3G was transiently coexpressed with AKT in the presence (+) or absence (−) of v-Crk in COS-7 cells. After 48 h, total cell lysates were prepared and subjected to immunoblot analysis with anti-phospho-Thr308-Akt(P-Akt) antibody, anti-Akt antibody, anti-mSOS, anti-C3G antibody, or anti-Gag antibody as indicated on the right.
FIG. 11.
Mutational analysis of synergistic activation of AKT by v-Crk and H-Ras. Proteins indicated on the top were transiently coexpressed with AKT in COS-7 cells. After 48 h, total cell lysates were prepared and subjected to immunoblot analysis with anti-phospho-Thr308-Akt(P-Akt) antibody, anti-Akt antibody, anti-Ras antibody, or anti-Gag antibody as indicated on the right. At this exposure, activation of AKT by v-Crk alone was not evident.
FIG. 12.
v-Crk enhances the interaction of H-Ras with PI3K p110 Ras-binding domain. pEBG-p110 RBD, a mammalian GST fusion expression vector for p110 Ras-binding domain, and H-Ras expression vector were transiently transfected with v-Crk expression vector or control vector into COS-7 cells. After 48 h, GST-p110 RBD proteins were affinity precipitated with glutathione-Sepharose beads (PD) and subjected to immunoblot analysis with anti-Ras antibody (A) or anti-GST antibody (B). Total cell lysates were also subjected to immunoblot analysis with anti-Ras antibody (C) or anti-Gag antibody for the detection of v-Crk (D).
FIG. 13.
H-Ras N17, a dominant-negative mutant of H-Ras, suppresses the AKT activation induced by v-Crk. Myc-tagged AKT was transiently coexpressed with proteins indicated at the top in COS-7 cells. After 48 h, AKT was immunoprecipitated with anti-Myc antibody and subjected to immunoblot analysis with anti-phospho-Akt(Thr308) antibody (A) or anti-Akt antibody (B). Total cell lysates were also subjected to immunoblot analysis with anti-Ras antibody (C) or anti-Gag antibody for the detection of v-Crk (D).
DISCUSSION
In this study, we investigated how v-Crk activates the PI3K/AKT pathway that is critical in the transformation of CEF by v-Crk. First, we found that the p85 regulatory subunit of PI3K is associated with FAK in v-Crk-transformed CEF. This interaction is mediated by the N-terminal SH2 domain of p85 and the phosphorylated Y397 residue in FAK. v-Crk strongly induces phosphorylation of Y397 residue in FAK and thereby creates a binding site for PI3K p85. The sequence around FAK Y397 (TDDpYAEI) is not a good YXXM consensus motif for p85 SH2 binding but conforms well with the optimal p85 SH2 binding motif (EDDpYVEM), which was reported when a degenerated peptide library with varied residues both in N-and C-terminal to phosphotyrosine was used (44). In such a situation, the preference for Met at the +3 position might be less strict.
We previously reported that PI3K activity could be coimmunoprecipitated with v-Crk (12), and we actually detected the association of PI3K p85 subunit with v-Crk (T. Akagi and H. Hanafusa, unpublished data). This interaction might be mediated by FAK, since we found that v-Crk could bind to FAK (Akagi and Hanafusa, unpublished).
Since the phosphorylation of FAK Y397 by v-Crk is almost completely suppressed by the overexpression of Csk, SFKs most likely mediate this phosphorylation. Previously, similar induction of FAK Y397 phosphorylation by SFKs has been reported upon integrin stimulation in normal cells (20, 29). Interestingly, induction of FAK Y397 phosphorylation by v-Crk can be observed even in cells kept in suspension. These results seem to suggest that v-Crk can mimic integrin signaling even in the absence of cell adhesion to the extracellular matrix.
The precise molecular mechanism as to how v-Crk initially activates SFKs to induce FAK Y397 phosphorylation remains to be answered. As previously reported, no significant physical complex formation was observed either between v-Crk and c-Src or between v-Crk and Csk (30, 31). Previously, we proposed one model in which competitive binding of v-Crk and Csk to paxillin results in a failure in suppression of c-Src activity by Csk (31). Whatever the mechanism of initial activation is, once SFKs are activated by v-Crk to phosphorylate FAK Y397, FAK-SFKs bipartite kinase complex is formed, and the mutual activation of these kinases will take place as described previously (16, 40). Activation of these kinases will lead to the increased tyrosine phosphorylation of their substrates, such as Cas and paxillin. Tyrosine-phosphorylated Cas will then activate SFKs further through the direct interaction with both SH2 and SH3 domains of SFKs, as demonstrated in the case of Sin, a Cas-related protein (3). Again, these activated SFKs will enhance the phosphorylation of FAK Y397. Such a positive circuit might amplify the relatively weak initial activation of SFKs in v-Crk-transformed cells.
Chen et al. previously reported that PI3K p85 binds to phosphorylated Y397 in FAK in response to integrin-mediated cell adhesion or platelet-derived growth factor (PDGF) stimulation (8). This interaction will recruit PI3K to focal adhesion and will also cause an allosteric activation of the p110 catalytic subunit, since Chen et al. demonstrated in an in vitro assay that incubation of PI3K with FAK phosphopeptide containing Y397 increased its activity (8). Indeed, phosphorylation of FAK Y397 and subsequent association of p85 with this site is essential for v-Crk-induced PI3K/AKT activation since it could not be observed in FAK-null cells or in cells expressing the FAK Y397F mutant.
However, this FAK Y397 phosphorylation alone is not sufficient for the activation of the PI3K/AKT pathway since a v-Crk SH3 mutant is fully competent to induce phosphorylation of Y397 in FAK but fails to activate AKT. We found that H-Ras is also required in the activation of the PI3K/AKT pathway by v-Crk. Either H-Ras or mSOS, a GEF for H-Ras that binds to the SH3 domain in v-Crk, could enhance the v-Crk-induced AKT activation when overexpressed, and a dominant-negative mutant of H-Ras almost completely suppressed this AKT activation. Moreover, we obtained evidence showing that v-Crk promotes the interaction of H-Ras with the Ras-binding domain in the p110 catalytic subunit of PI3K. From these results, we speculate that v-Crk activates H-Ras through its SH3-binding partner mSOS, and the active H-Ras binds to the p110 catalytic subunit of PI3K. Matsuda et al. also demonstrated the potential of Crk to activate H-Ras (23). This interaction alone will increase its enzymatic activity only modestly, but when the binding of tyrosine phosphopeptide to the p85 regulatory subunit also takes place these two kinds of interaction will allow production of the optimal activation of PI3K in a synergistic manner, as proposed by Rodriguez-Viciana et al. (28). Experiments with PDGF receptor mutants have also shown that efficient activation of PI3K requires Ras function in addition to the binding of the p85 regulatory subunit to phosphorylated tyrosine residues generated on the PDGF receptor (19).
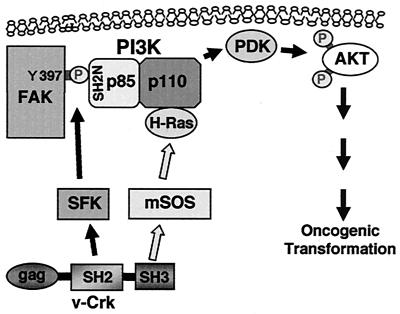
Taking all these findings into consideration, we propose that v-Crk activates PI3K by promoting both the interaction of the p85 regulatory subunit with tyrosine phosphorylated FAK and the interaction of the p110 catalytic subunit with active form of H-Ras. The resulting activation of AKT is supposed to lead to oncogenic transformation (Fig. 14). This model will illustrate the requirement of both the SH2 and SH3 domains of v-Crk in the activation of the PI3K/AKT pathway and is consistent with our previous results, indicating that v-Crk requires H-Ras function for transformation through a pathway other than the mitogen-activated protein kinase pathway (15).
FIG. 14.
Model for the activation of PI3K/AKT pathway by v-Crk. v-Crk induces phosphorylation of Y397 in FAK through the activation of SFK and creates a binding site for the N-terminal SH2 domain of PI3K p85. v-Crk also induces the interaction of PI3K p110 with H-Ras through its SH3 domain binding protein mSOS. These events will synergistically activate PI3K, and then its downstream kinases PI3K-dependent kinases (PDK) and AKT will be activated subsequently. This activation of the PI3K/AKT pathway is supposed to play an important role in oncogenic transformation by v-Crk.
In the present study, we examined the molecular mechanisms by which v-Crk activates the PI3K/AKT pathway and demonstrated that FAK and H-Ras play important roles in this process. Taking into consideration with the recent report by Zvara et al. that c-Crk is also capable of inducing FAK Y397 phosphorylation (45), our results raise the intriguing possibility that c-Crk may play some physiological roles in the activation of the PI3K/AKT pathway following integrin-mediated cell adhesion. Further studies to test this hypothesis are of great interest.
Acknowledgments
We are grateful to Kana Shibutani for technical assistance. We thank Steven Hanks, Michiyuki Matsuda, Masato Kasuga, Tadashi Yamamoto, Michinari Hamaguchi, David Boettiger, Yasuhisa Fukui, and Jonathan Backer for providing the reagents.
This work was supported by a Grant-in-Aid for Specially Promoted Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, as well as by a grant from the Kato Memorial Bioscience Foundation.
REFERENCES
- 1.Akagi, T., H. Ono, and K. Shimotohno. 1996. Expression of cell-cycle regulatory genes in HTLV-I infected T-cell lines: possible involvement of Tax1 in the altered expression of cyclin D2, p18Ink4, and p21Waf1/Cip1/Sdi1. Oncogene 12:1645-1652. [PubMed] [Google Scholar]
- 2.Akagi, T., T. Shishido, K. Murata, and H. Hanafusa. 2000. v-Crk activates the phosphoinositide 3-kinase/AKT pathway in transformation. Proc. Natl. Acad. Sci. USA 97:7290-7295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexandropoulos, K., and D. Baltimore. 1996. Coordinate activation of c-Src by SH3- and SH2-binding sites on a novel p130Cas-related protein, Sin. Genes Dev. 10:1341-1355. [DOI] [PubMed] [Google Scholar]
- 4.Birge, R. B., B. S. Knudsen, D. Besser, and H. Hanafusa. 1996. SH2 and SH3-containing adaptor proteins: redundant or independent mediators of intracellular signal transduction. Genes Cells 1:595-613. [DOI] [PubMed] [Google Scholar]
- 5.Bjorge, J. D., A. Jakymiw, and D. J. Fujita. 2000. Selected glimpses into the activation and function of Src kinase. Oncogene 19:5620-5635. [DOI] [PubMed] [Google Scholar]
- 6.Calalb, M. B., T. R. Polte, and S. K. Hanks. 1995. Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: a role for Src family kinases. Mol. Cell. Biol. 15:954-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cantley, L. C., K. R. Auger, C. Carpenter, B. Duckworth, A. Graziani, R. Kapeller, and S. Soltoff. 1991. Oncogenes and signal transduction. Cell 64:281-302. [DOI] [PubMed] [Google Scholar]
- 8.Chen, H. C., P. A. Appeddu, H. Isoda, and J. L. Guan. 1996. Phosphorylation of tyrosine 397 in focal adhesion kinase is required for binding phosphatidylinositol 3-kinase. J. Biol. Chem. 271:26329-26334. [DOI] [PubMed] [Google Scholar]
- 9.Datta, S. R., A. Brunet, and M. E. Greenberg. 1999. Cellular survival: a play in three Akts. Genes Dev. 13:2905-2927. [DOI] [PubMed] [Google Scholar]
- 10.Downward, J. 1998. Mechanisms and consequences of activation of protein kinase B/Akt. Curr. Opin. Cell Biol. 10:262-267. [DOI] [PubMed] [Google Scholar]
- 11.Fukazawa, H., S. Mizuno, and Y. Uehara. 1995. A microplate assay for quantitation of anchorage-independent growth of transformed cells. Anal. Biochem. 228:83-90. [DOI] [PubMed] [Google Scholar]
- 12.Fukui, Y., S. Kornbluth, S. M. Jong, L. H. Wang, and H. Hanafusa. 1989. Phosphatidylinositol kinase type I activity associates with various oncogene products. Oncogene Res. 4:283-292. [PubMed] [Google Scholar]
- 13.Gingras, A. C., B. Raught, and N. Sonenberg. 2001. Regulation of translation initiation by FRAP/mTOR. Genes Dev. 15:807-826. [DOI] [PubMed] [Google Scholar]
- 14.Gotoh, T., S. Hattori, S. Nakamura, H. Kitayama, M. Noda, Y. Takai, K. Kaibuchi, H. Matsui, O. Hatase, H. Takahashi, et al. 1995. Identification of Rap1 as a target for the Crk SH3 domain-binding guanine nucleotide-releasing factor C3G. Mol. Cell. Biol. 15:6746-6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greulich, H., and H. Hanafusa. 1996. A role for Ras in v-Crk transformation. Cell Growth Differ. 7:1443-1451. [PubMed] [Google Scholar]
- 16.Hanks, S. K., and T. R. Polte. 1997. Signaling through focal adhesion kinase. Bioessays 19:137-145. [DOI] [PubMed] [Google Scholar]
- 17.Hibi, M., and T. Hirano. 2000. Gab-family adapter molecules in signal transduction of cytokine and growth factor receptors, and T and B cell antigen receptors. Leuk. Lymphoma 37:299-307. [DOI] [PubMed] [Google Scholar]
- 18.Ilic, D., Y. Furuta, S. Kanazawa, N. Takeda, K. Sobue, N. Nakatsuji, S. Nomura, J. Fujimoto, M. Okada, and T. Yamamoto. 1995. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature 377:539-544. [DOI] [PubMed] [Google Scholar]
- 19.Klinghoffer, R. A., B. Duckworth, M. Valius, L. Cantley, and A. Kazlauskas. 1996. Platelet-derived growth factor-dependent activation of phosphatidylinositol 3-kinase is regulated by receptor binding of SH2-domain-containing proteins which influence Ras activity. Mol. Cell. Biol. 16:5905-5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klinghoffer, R. A., C. Sachsenmaier, J. A. Cooper, and P. Soriano. 1999. Src family kinases are required for integrin but not PDGFR signal transduction. EMBO J. 18:2459-2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawlor, M. A., and D. R. Alessi. 2001. PKB/Akt: a key mediator of cell proliferation, survival and insulin responses? J. Cell Sci. 114:2903-2910. [DOI] [PubMed] [Google Scholar]
- 22.Marte, B. M., and J. Downward. 1997. PKB/Akt: connecting phosphoinositide 3-kinase to cell survival and beyond. Trends Biochem. Sci. 22:355-358. [DOI] [PubMed] [Google Scholar]
- 23.Matsuda, M., Y. Hashimoto, K. Muroya, H. Hasegawa, T. Kurata, S. Tanaka, S. Nakamura, and S. Hattori. 1994. CRK protein binds to two guanine nucleotide-releasing proteins for the Ras family and modulates nerve growth factor-induced activation of Ras in PC12 cells. Mol. Cell. Biol. 14:5495-5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mayer, B. J., M. Hamaguchi, and H. Hanafusa. 1988. A novel viral oncogene with structural similarity to phospholipase C. Nature 332:272-275. [DOI] [PubMed] [Google Scholar]
- 25.Mochizuki, N., Y. Ohba, S. Kobayashi, N. Otsuka, A. M. Graybiel, S. Tanaka, and M. Matsuda. 2000. Crk activation of JNK via C3G and R-Ras. J. Biol. Chem. 275:12667-12671. [DOI] [PubMed] [Google Scholar]
- 26.Potts, W. M., M. Olsen, D. Boettiger, and V. M. Vogt. 1987. Epitope mapping of monoclonal antibodies to gag protein p19 of avian sarcoma and leukaemia viruses. J. Gen. Virol. 68:3177-3182. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez-Viciana, P., P. H. Warne, A. Khwaja, B. M. Marte, D. Pappin, P. Das, M. D. Waterfield, A. Ridley, and J. Downward. 1997. Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by Ras. Cell 89:457-467. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez-Viciana, P., P. H. Warne, B. Vanhaesebroeck, M. D. Waterfield, and J. Downward. 1996. Activation of phosphoinositide 3-kinase by interaction with Ras and by point mutation. EMBO J. 15:2442-2451. [PMC free article] [PubMed] [Google Scholar]
- 29.Ruest, P. J., S. Roy, E. Shi, R. L. Mernaugh, and S. K. Hanks. 2000. Phosphospecific antibodies reveal focal adhesion kinase activation loop phosphorylation in nascent and mature focal adhesions and requirement for the autophosphorylation site. Cell Growth Differ. 11:41-48. [PubMed] [Google Scholar]
- 30.Sabe, H., M. Okada, H. Nakagawa, and H. Hanafusa. 1992. Activation of c-Src in cells bearing v-Crk and its suppression by Csk. Mol. Cell. Biol. 12:4706-4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sabe, H., S. E. Shoelson, and H. Hanafusa. 1995. Possible v-Crk-induced transformation through activation of Src kinases. J. Biol. Chem. 270:31219-31224. [DOI] [PubMed] [Google Scholar]
- 32.Sakaue, M., D. Bowtell, and M. Kasuga. 1995. A dominant-negative mutant of mSOS1 inhibits insulin-induced Ras activation and reveals Ras-dependent and -independent insulin signaling pathways. Mol. Cell. Biol. 15:379-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schaller, M. D., J. D. Hildebrand, J. D. Shannon, J. W. Fox, R. R. Vines, and J. T. Parsons. 1994. Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol. Cell. Biol. 14:1680-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shishido, T., T. Akagi, A. Chalmers, M. Maeda, T. Terada, M. M. Georgescu, and H. Hanafusa. 2001. Crk family adaptor proteins trans-activate c-Abl kinase. Genes Cells 6:431-440. [DOI] [PubMed] [Google Scholar]
- 35.Stam, J. C., W. J. Geerts, H. H. Versteeg, A. J. Verkleij, and P. M. van Bergen en Henegouwen. 2001. The v-Crk oncogene enhances cell survival and induces activation of protein kinase B/Akt. J. Biol. Chem. 276:25176-25183. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka, M., R. Gupta, and B. J. Mayer. 1995. Differential inhibition of signaling pathways by dominant-negative SH2/SH3 adapter proteins. Mol. Cell. Biol. 15:6829-6837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanaka, S., and H. Hanafusa. 1998. Guanine-nucleotide exchange protein C3G activates JNK1 by a ras-independent mechanism. JNK1 activation inhibited by kinase negative forms of MLK3 and DLK mixed lineage kinases. J. Biol. Chem. 273:1281-1284. [DOI] [PubMed] [Google Scholar]
- 38.Tanaka, S., T. Ouchi, and H. Hanafusa. 1997. Downstream of Crk adaptor signaling pathway: activation of Jun kinase by v-Crk through the guanine nucleotide exchange protein C3G. Proc. Natl. Acad. Sci. USA 94:2356-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thien, C. B., and W. Y. Langdon. 2001. Cbl: many adaptations to regulate protein tyrosine kinases. Nat. Rev. Mol. Cell. Biol. 2:294-307. [DOI] [PubMed] [Google Scholar]
- 40.Thomas, J. W., B. Ellis, R. J. Boerner, W. B. Knight, G. C. White II, and M. D. Schaller. 1998. SH2- and SH3-mediated interactions between focal adhesion kinase and Src. J. Biol. Chem. 273:577-583. [DOI] [PubMed] [Google Scholar]
- 41.Vanhaesebroeck, B., S. J. Leevers, G. Panayotou, and M. D. Waterfield. 1997. Phosphoinositide 3-kinases: a conserved family of signal transducers. Trends Biochem. Sci. 22:267-272. [DOI] [PubMed] [Google Scholar]
- 42.White, M. F. 1998. The IRS-signalling system: a network of docking proteins that mediate insulin action. Mol. Cell Biochem. 182:3-11. [PubMed] [Google Scholar]
- 43.Yu, J., C. Wjasow, and J. M. Backer. 1998. Regulation of the p85/p110α phosphatidylinositol 3′-kinase: distinct roles for the N-terminal and C-terminal SH2 domains. J. Biol. Chem. 273:30199-30203. [DOI] [PubMed] [Google Scholar]
- 44.Zhou, S., B. Margolis, M. Chaudhuri, S. E. Shoelson, and L. C. Cantley. 1995. The phosphotyrosine interaction domain of SHC recognizes tyrosine-phosphorylated NPXY motif. J. Biol. Chem. 270:14863-14866. [DOI] [PubMed] [Google Scholar]
- 45.Zvara, A., J. E. Fajardo, M. Escalante, G. Cotton, T. Muir, K. H. Kirsch, and R. B. Birge. 2001. Activation of the focal adhesion kinase signaling pathway by structural alterations in the carboxyl-terminal region of c-Crk II. Oncogene 20:951-961. [DOI] [PubMed] [Google Scholar]