Abstract
In spite of intensified efforts to understand cell signaling from endosomes, there is no direct evidence demonstrating that endosomal signaling is sufficient to activate signal transduction pathways and no evidence to demonstrate that endosomal signaling is able to produce a biological outcome. The lack of breakthrough is due in part to the lack of means to generate endosomal signals without plasma membrane signaling. In this paper, we report the establishment of a system to specifically activate epidermal growth factor (EGF) receptor (EGFR) when it endocytoses into endosomes. We treated cells with EGF in the presence of AG-1478, a specific EGFR tyrosine kinase inhibitor, and monensin, which blocks the recycling of EGFR. This treatment led to the internalization of nonactivated EGF-EGFR complexes into endosomes. The endosome-associated EGFR was then activated by removing AG-1478 and monensin. During this procedure we did not observe any surface EGFR phosphorylation. We also achieved specific activation of endosome-associated EGFR without using monensin. By using this system, we provided original evidence demonstrating that (i) the endosome can serve as a nucleation site for the formation of signaling complexes, (ii) endosomal EGFR signaling is sufficient to activate the major signaling pathways leading to cell proliferation and survival, and (iii) endosomal EGFR signaling is sufficient to suppress apoptosis induced by serum withdrawal.
The growth-stimulatory signal of epidermal growth factor (EGF) is mediated by the transmembrane EGF receptor (EGFR). The binding of EGF at the cell surface induces dimerization of EGFR, which results in the activation of EGFR tyrosine kinase activity and trans-autophosphorylation (5, 35). Sites of tyrosine autophosphorylation in an activated EGFR bind signaling proteins that contain phosphotyrosine (pTyr)-binding domains such as Src homology 2 and phosphotyrosine binding (32, 33). These signaling proteins include Grb2, SHC, phospholipase C-γ1 (PLC-γ1), the p85α subunit of phosphatidylinositol 3-kinase (PI3K), GAP, and Cbl (32, 33, 44). Formation of the receptor-signaling protein complexes then initiates the activation of various signaling pathways. For example, the interaction between EGFR and SHC/Grb2 results in the recruitment of Sos to the plasma membrane to activate Ras. Activated Ras mediates Raf activation, which then phosphorylates and activates mitogen-activated protein kinase kinase and extracellular-signal-regulated kinase (ERK), leading to the activation of transcriptional factors c-Fos and c-Jun (27, 28, 32). This “Ras pathway” has been elucidated and is necessary for EGF-stimulated DNA synthesis. In addition, activation of PI3K by EGFR stimulates Akt/protein kinase B activity, which protects against apoptosis (3, 15).
Concomitant with the activation of various signaling pathways by EGF, the binding of EGF also rapidly triggers the clustering and internalization of ligand-receptor complexes via coated pits and coated vesicles (5, 34). The fate of internalized EGF and EGFR is primarily proteolytic degradation in lysosomes (5). However, whether endocytosis leads exclusively to receptor inactivation or is important in maintaining, triggering, or amplifying cellular responses to growth factors remains an important question (7, 12, 13). It is well established that endocytosis of EGFR from the cell surface to lysosomes results in degradation of the receptor, which can attenuate receptor signaling and may even be conceived of as a tumor suppressor pathway (10, 13). On the other hand, accumulated evidence suggests that the internalized EGF-EGFR complex may maintain its ability to generate cell signaling from endosomes. Internalized EGFR is autophosphorylated and catalytically active (11, 21, 23). Various signaling molecules that regulate Ras activity, including Grb2, SHC, GAP, and Cbl, are cointernalized with EGFR into endosomes and remain associated with the receptor in endosomes (10, 14, 17, 25, 40). Recently, more results confirmed the interaction between EGFR and various signaling proteins in endosomes (4, 19, 22, 31, 37).
More evidence supporting endosomal signaling comes from endocytosis-blocking experiments. Inhibition of EGFR endocytosis by a dominant-negative mutant dynamin enhances cell proliferation but decreases ERK activation (39). In a recent study of EGFR transactivation by G protein-coupled receptors, it was found that inhibition of EGFR endocytosis by either mutant dynamin or β-arrestin abolished ERK activation (1, 29). Inhibition of EGFR endocytosis by phospholipase D also blocks EGF-stimulated ERK activation (36). However, in the few cases where biological end points were measured, inhibition of endocytosis did not result in attenuation of biological effects (6, 39). Very recently, it was reported that retrograde support of neuronal survival does not require the retrograde transport (endocytosis) of nerve growth factor (26). These results argue against the physiological relevance of endosome-originated signals (12). So far, the controversy over endosomal signaling and its physiological relevance remains unsolved. The lack of breakthrough is due to the limitation of current approaches. For example, while it has made a significant contribution and remains a powerful tool to study endosomal signaling, the endocytosis inhibition approach has its limitations. While inhibition of EGFR endocytosis eliminates endosomal signaling, the retention of EGFR at the cell surface also enhances signaling from the plasma membrane. Thus, it is difficult to determine whether the observed effects are due to the lack of endosomal signaling or due to prolonged plasma membrane signaling. Blocking EGFR endocytosis by mutant dynamin or β-arrestin affects all endocytic events mediated by these factors. Thus, it is difficult to determine whether the observed effects are due to the inhibition of EGFR endosomal signaling or due to a broad inhibition of endocytosis. Moreover, this approach is not suitable for studying the dynamics of endosomal signaling. Up to now, none of the current approaches offered a means to get activated receptors inside a cell without initial activation at the cell surface (43).
Here we report the establishment of a system that allows the specific activation of endosome-associated EGFR without the activation of the plasma membrane-associated EGFR and without disrupting the overall endocytosis pathway. By using this novel system, we provided original evidence demonstrating that (i) the endosome can serve as a nucleation site for the formation of signaling complexes, (ii) endosomal EGFR signaling is sufficient to activate the major signaling pathways leading to cell proliferation and survival, and (iii) endosomal EGFR signaling is sufficient to suppress apoptosis induced by serum withdrawal.
MATERIALS AND METHODS
Antibodies and chemicals.
Mouse anti-pTyr (py99), rabbit anti-EGFR, anti-ERK, anti-Grb2, anti-Raf, and anti-SHC antibodies were from Santa Cruz Biotechnology (Santa Cruz, Calif.). Rabbit anti-p85, mouse anti-Ras, and anti-phospho-EGFR (p-EGFR) antibodies were from Upstate Biotechnology Inc. (Lake Placid, N.Y.). Rabbit anti-phospho-Akt (Ser 473) antibodies were from Cell Signaling Technology (Beverly, Mass.). The mouse anti-EGFR antibody (Ab1) was from Oncogene (Boston, Mass.). The mouse anti-phospho-PLC-γ1 antibody was from Medicore (Montreal, Quebec, Canada). Mouse anti-Rab5 and anti-early endosome autoantigen 1 (EEA1) antibodies were from BD Signal Transduction. Glutathione cross-linked to 4% agarose and goat anti-mouse immunoglobulin G (IgG) conjugated with agarose were from Sigma (St. Louis, Mo.). Protein A-Sepharose 6MB was from Pharmacia BioProcess (Uppsala, Sweden). AG-1478 and monensin were from Calbiochem (La Jolla, Calif.). EGF was from Upstate Biotechnology. Unless otherwise specified, all the chemicals were purchased from Sigma.
Cell culture and treatment.
MDCK and BT20 cells were grown at 37°C in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum (FBS) and were maintained in a 5% CO2 atmosphere. To specifically activate EGFR after its endocytosis into endosomes, MDCK and BT20 cells were serum starved for 24 h. After the cells were pretreated with 0.5 μM AG-1478 for 15 min, monensin and EGF/Texas red-conjugated EGF (TR-EGF) were added to final concentrations of 100 μM and 100 ng/ml, respectively. After a 30-min treatment, the cells were washed with phosphate-buffered saline (PBS) three times and then maintained in the serum-free medium for various times indicated in figures. Alternatively, MDCK and BT20 cells were pretreated with 0.5 μM AG-1478 for 15 min, and then EGF/TR-EGF was added to a final concentration of 100 ng/ml. After a 30-min treatment, the cells were cooled down to 4°C and washed with acidic stripping buffer (100 mM acetic acid, 150 mM NaCl [pH 2.7]) (42) for 1 min. The cells were then washed with PBS three times and incubated in serum-free medium for various times.
Subcellular fractionation and total-cell lysates.
Isolation of plasma membrane (PM), endosomal (EN), and cytosolic (CY) fractions was carried out by our previously described method (41). Briefly, following treatment cells were scraped into homogenization buffer (0.25 M sucrose, 20 mM Tris-HCl [pH 7], 1 mM MgCl2, 4 mM NaF, 0.5 mM Na3VO4, 0.02% NaN3, 0.1 mM 4-[2-aminoethyl]-benzenesulfonyl fluoride, 10 μg of aprotinin/ml, 1 μM pepstatin A) and homogenized. The homogenates were centrifuged at 200 × g for 5 min to remove cell debris and nuclei (p1). The postnuclear supernatant (S1) was then centrifuged at 1,500 × g for 10 min to yield a supernatant (S2) and a pellet (P2). Next, P2 was resuspended in homogenization buffer (0.25 M sucrose), overlaid upon an equal volume of 1.42 M sucrose buffer, and centrifuged at 82,000 × g for 1 h. The pellicule at the 0.25-to-1.42 M interface was collected as the PM fraction. The S2 fraction was centrifuged at 100,000 × g for 30 min to yield the soluble CY fraction and a microsomal pellet. This pellet was resuspended in 0.25 M sucrose buffer and overlaid on a discontinuous sucrose gradient containing equal volumes of homogenization buffer at 1.00 and 1.15 M sucrose. The resuspension was centrifuged at 200,000 × g for 1.5 h to obtain the purified EN fraction at the 0.25-to-1.00 M interface. For a typical experiment, the total yield is 30 μg for the plasma membrane, 30 μg for the EN fraction, and 1 mg for the CY fraction. The total yields of each fraction were very consistent for all of the treatments.
Indirect immunofluorescence.
Indirect immunofluorescence was performed as described previously (41). Briefly, cells were grown on glass coverslips and serum starved for 24 h. After treatment, the cells were fixed by methanol and permeabilized with 0.2% Triton X-100. Next, the cells were incubated with primary antibodies at room temperature for 1 h followed by fluorescence-labeled secondary antibodies for 1 h.
Immunoprecipitation and immunoblotting.
Immunoprecipitation experiments were carried out as described previously (41). Cells were lysed with immunoprecipitation buffer (20 mM Tris [pH 7.5], 150 mM NaCl, 1% NP-40, 0.1% sodium deoxycholate, 100 mM NaF, 0.5 mM Na3VO4, 0.02% NaN3, 0.1 mM 4-[2-aminoethyl]-benzenesulfonyl fluoride, 10 μg of aprotinin/ml, 1 μM pepstatin A) overnight at 4°C. Cell lysates were then centrifuged at 21,000 × g for 30 min to remove debris. The supernatants, containing 1 mg of total protein, were incubated with 1 μg of mouse anti-EGFR antibody to immunoprecipitate EGFR from BT20 and MDCK cells. For control experiments, primary antibodies were replaced with normal mouse or sheep IgG (Sigma), and no EGFR was precipitated by normal IgG.
Ras activation assay.
Ras activation was assayed by the method described by Herrmann et al. (20). Briefly, BT20 cells which had been treated as required were lysed and scraped into 0.5 ml of BOS buffer (50 mM Tris-HCl [pH 7.4], 200 mM NaCl, 1% NP-40, 10% glycerol, 10 mM NaF, 2.5 mM MgCl2, 1 mM EDTA) and then centrifuged at 21,000 × g and 4°C for 30 min. Glutathione S-transferase (GST) fused to the Raf Ras binding domain (GST-RBD), precoupled to glutathione-agarose beads in BOS buffer, was added, and the lysates were incubated at 4°C for 1 h. Beads were collected by centrifugation and washed three times with BOS buffer, and then loading buffer was added. Ras was detected with the monoclonal anti-Ras antibody, followed by a horseradish peroxidase (HRP)-coupled anti-mouse antibody.
Immunoblotting.
Immunoblotting was performed as described previously (40). For the detection of EGFR, pTyr, phospho-ERK, phospho-Akt, phospho-PLC-γ1, and Raf in total lysates of MDCK and BT20 cells, aliquots containing 20 μg of protein from each cell lysate were used. For the detection of EGFR, SHC, Grb2, PLC-γ1, and the p85α subunit of PI3K in the anti-EGFR immunoprecipitates, 1/10 of the immunoprecipitate from each lysate was used. To examine EGFR in each subcellular fraction of BT20 and MDCK cells, aliquots containing 5 μg of protein from each fraction were used. The 5 μg of protein represents one-sixth of the total yield for both the PM and EN fractions. Protein samples were separated by electrophoresis through sodium dodecyl sulfate-10% polyacrylamide-containing gels and electrophoretically transferred onto nitrocellulose filter paper. Filters were then probed with the respective primary antibody. The primary antibodies were detected with a polyclonal goat anti-rabbit IgG coupled to HRP or a polyclonal goat anti-mouse IgG coupled to HRP followed by enhanced chemiluminescence development (Pierce Chemical, Rockford, Ill.) and light detection with Fuji (Tokyo, Japan) Super RX film. Quantification of the results was achieved by using a FluorChem digital imaging system (Alpha Innotech Corporation).
TUNEL assay.
BT20 and MDCK cells (10,000 per coverslip) were serum starved for 24 (for BT20 cells) or 72 h (for MDCK cells) to initiate a significant level of apoptosis. Some of the serum-starved cells were treated with AG-1478 for 15 min and then with EGF (20 ng/ml) and monensin for 30 min followed by a washing with PBS and incubation with serum-free medium for 12 h. Alternatively, some cells were treated with AG-1478 and EGF (20 ng/ml) for 30 min followed by a 1-min washing with acidic stripping buffer. Cells were then incubated with serum-free medium for 12 h. For controls, some of the serum-starved cells were stimulated with EGF for 30 min, followed by incubation with serum-free medium for 12 h. Apoptosis was assayed by a terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay using an apoptosis detection system kit (Promega) according to the manufacturer's instructions. The percentage of apoptotic cells was calculated as the number of apoptotic nuclei/total nuclei analyzed × 100. For each experimental treatment, a minimum of 250 cells were counted.
RESULTS
Establishment of a novel system that specifically activates endosome-associated EGFR.
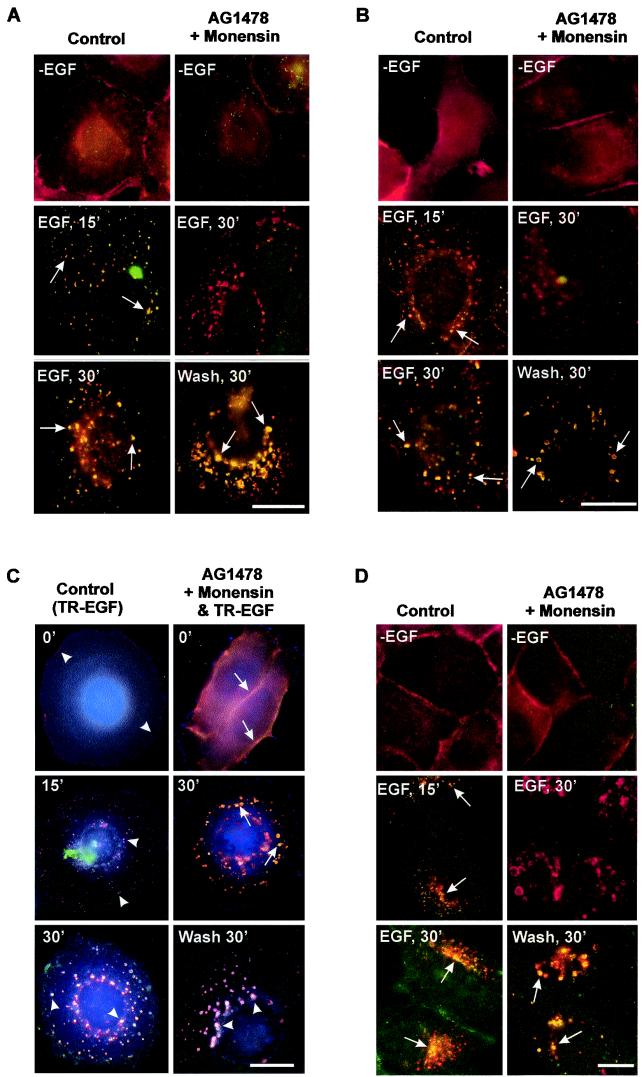
A mutation resulting in the loss of the intrinsic tyrosine kinase activity of EGFR abolishes ligand-induced down-regulation; however, whether this is due to impaired internalization or impaired lysosomal targeting remains unsolved (8, 16, 18). Some studies showed that the kinase-deficient mutant EGFR K721A is internalized into endosomes in response to EGF. Unlike wild-type EGFR, however, EGFR K721A recycles back to the plasma membrane rather than trafficking to lysosomes for degradation (16). Treatment of cells with monensin, which inhibits EGFR recycling, results in the retention of EGFR K721A in endosomes (16). Based on these observations, we examined whether treatment of cells with AG-1478 and monensin to specifically inhibit EGFR kinase activation and recycling would result in the internalization of inactive EGF-EGFR complexes into endosomes in response to EGF stimulation. BT20 cells were treated with AG-1478 for 15 min and then stimulated with EGF in the presence of monensin. Immunofluorescence showed that EGFR completely internalized into endosomes after addition of EGF and monensin for 30 min (Fig. 1A ). There is no overlap between EGFR and pTyr staining, indicating that the endosome-associated EGFR was not phosphorylated (inactive) (Fig. 1A). For control cells treated only with EGF (we term this treatment standard EGF stimulation), EGFR was phosphorylated and internalized into endosomes (Fig. 1A). To more specifically determine the phosphorylation status of endosome-associated EGFR, we double stained BT20 cells with anti-EGFR and anti-p-EGFR antibodies following the treatment described above. Our results confirmed that treatment of BT20 cells with AG-1478, EGF, and monensin induced internalization of nonphosphorylated EGFR into endosomes (Fig. 1B).
FIG.1.
Analysis by immunofluorescence of selective activation of EGFR after its endocytosis into endosomes. (A and B) BT20 cells were treated with AG-1478 for 15 min and then stimulated with EGF in the presence of monensin at 37°C for 30 min. Some of the cells were then washed three times with PBS and incubated with serum-free medium for 30 min. BT20 cells that were serum starved or treated only with EGF for 15 and 30 min at 37°C were used as controls; EGFR (red) and pTyr (green) (A) or p-EGFR (green) (B) localization was determined by indirect immunofluorescence. Arrows, colocalization (yellow) of EGFR and pTyr (A) or EGFR and p-EGFR (B). (C) BT20 cells were treated with AG-1478 for 15 min and then stimulated with TR-EGF in the presence of monensin at 4°C for 30 min followed by incubation at 37°C for the indicated times. Some of the cells were then washed with PBS three times and incubated with serum-free medium for the indicated times. BT20 cells that were treated only with TR-EGF at 4°C for 30 min and then at 37°C for indicated times were used as controls; TR-EGF (red), EGFR (green), and pTyr (blue) localization was determined by triple indirect immunofluorescence as described in Materials and Methods. Arrows, colocalization (yellow) of TR-EGF and EGFR; arrowheads, colocalization (light purple) of TR-EGF, EGFR, and pTyr. (D) MDCK cells were treated with AG-1478 for 15 min and then stimulated with EGF in the presence of monensin at 37°C for 30 min. Some of the cells were then washed with PBS three times and incubated with serum-free medium for 30 min. MDCK cells that were serum starved or treated only with EGF for 15 and 30 min at 37°C were used as controls; EGFR (red) and pTyr (green) localization was determined by indirect immunofluorescence. Arrows, colocalization (yellow) of EGFR and pTyr. Bars, 20 μm.
To determine whether endosome-associated nonphosphorylated EGFR binds to EGF, we treated BT20 cells with TR-EGF in the presence of AG-1478 and monensin. Triple-immunofluorescence staining indicated that both TR-EGF and the nonphosphorylated EGFR colocalized at endosomes (Fig. 1C). For control cells treated only with TR-EGF, EGFR was phosphorylated and cointernalized with TR-EGF into endosomes (Fig. 1C).
To determine whether endosome-associated nonphosphorylated EGFR could be activated, BT20 cells were washed with medium. The endosome-associated EGFR was significantly activated 30 min following the wash (Fig. 1A to C). The activated EGFR remained colocalized with TR-EGF (Fig. 1C). The fact that similar results were obtained with MDCK cells by immunofluorescence (Fig. 1D) suggests that our observation is not limited to BT20 cells.
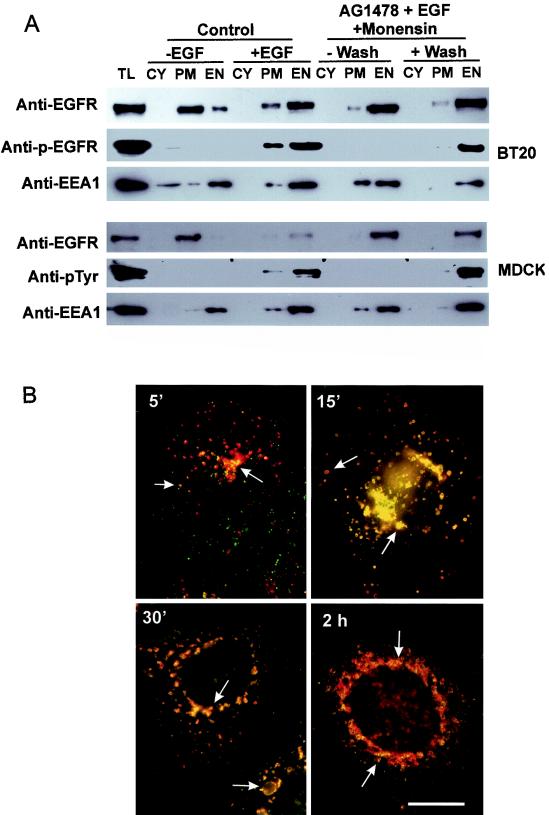
These observations were further confirmed by subcellular-fractionation experiments (Fig. 2). It is well established that EGFR is localized at the plasma membrane and not phosphorylated without EGF stimulation and is primarily phosphorylated and localized in endosomes following EGF stimulation for 30 min. Thus EGFR itself could be used as a marker for the plasma membrane and endosomes. Indeed, our results showed that for both BT20 and MDCK cells EGFR was primarily in the PM fraction and was not phosphorylated without EGF stimulation, while EGFR was primarily in the EN fraction and was phosphorylated following standard EGF stimulation (Fig. 2A and Table 1). Additionally, EEA1, a marker for endosomes, is highly enriched in our EN fractions. Treatment with AG-1478, monensin, and EGF resulted in the internalization of nonphosphorylated EGFR into endosomes, and the endosome-associated EGFR was phosphorylated following washing and incubation in medium (Fig. 2A and Table 1). Washing away of AG-1478 and monensin did not result in an increase of plasma membrane-associated EGFR (Fig. 2A and Table 1), which suggests that there is no detectable recycling of EGFR from the endosome to the plasma membrane following the removal of monensin. More importantly, no phosphorylated EGFR was detected at the plasma membrane (Fig. 2A and Table 1). A time course of immunofluorescence further showed that there was no detectable EGFR and EGFR phosphorylation at the plasma membrane following the removal of monensin, which further indicates that there is no detectable recycling of EGFR from the endosome to the plasma membrane following the removal of monensin (Fig. 2B). Together, these results suggest that we have established a system to specifically activate EGFR in endosomes without any detectable EGFR activation at the plasma membrane.
FIG. 2.
Further analysis of selective activation of endosome-associated EGFR by subcellular fractionation and by the time course of immunofluorescence. (A) Subcelllular fractionation. BT20 and MDCK cells were treated with AG-1478, EGF, and monensin as described in Materials and Methods. The cells were then subcellularly fractionated into PM, EN, and CY fractions. The subcellular fractions were subjected to immunoblotting with anti-EGFR, anti-pTyr, and anti-EEA1 antibodies. TL, total lysate. (B) Time course by immunofluorescence of the specific activation of endosome-associated EGFR following wash. BT20 cells were treated with AG-1478, EGF, and monensin for 30 min and then washed with PBS for the indicated times. EGFR (red) and pTyr (green) localization was determined by indirect immunofluorescence. Arrows, colocalization (yellow) of EGFR and pTyr. Bar, 20 μm.
TABLE 1.
Analysis by subcellular fractionation of the selective activation of endosome-associated EGFR
| Cell type and marker | Yield (%) of indicated fractiona for:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control cells
|
Cells treated with AG1478 + EGF + monensin
|
|||||||||||
| Without EGF
|
With EGF
|
Without wash
|
With wash
|
|||||||||
| CY | PM | EN | CY | PM | EN | CY | PM | EN | CY | PM | EN | |
| BT20 | ||||||||||||
| EGFR | 0 | 78 | 22 | 0 | 28 | 72 | 0 | 9 | 91 | 0 | 10 | 90 |
| p-EGFR | 0 | 0 | 0 | 0 | 27 | 73 | 0 | 0 | 0 | 0 | 2 | 98 |
| EEA1 | 29 | 8 | 63 | 3 | 17 | 80 | 2 | 35 | 63 | 4 | 2 | 94 |
| MDCK | ||||||||||||
| EGFR | 0 | 94 | 5 | 5 | 15 | 80 | 0 | 4 | 95 | 2 | 2 | 96 |
| p-EGFR | 0 | 0 | 0 | 0 | 9 | 91 | 0 | 0 | 0 | 0 | 3 | 97 |
| EEA1 | 12 | 7 | 81 | 5 | 14 | 81 | 3 | 19 | 78 | 3 | 6 | 91 |
Data are the quantification of the results from Fig. 2A normalized against the total yield of the three fractions. Each value is the percentage of the total of each set of three fractions following normalization.
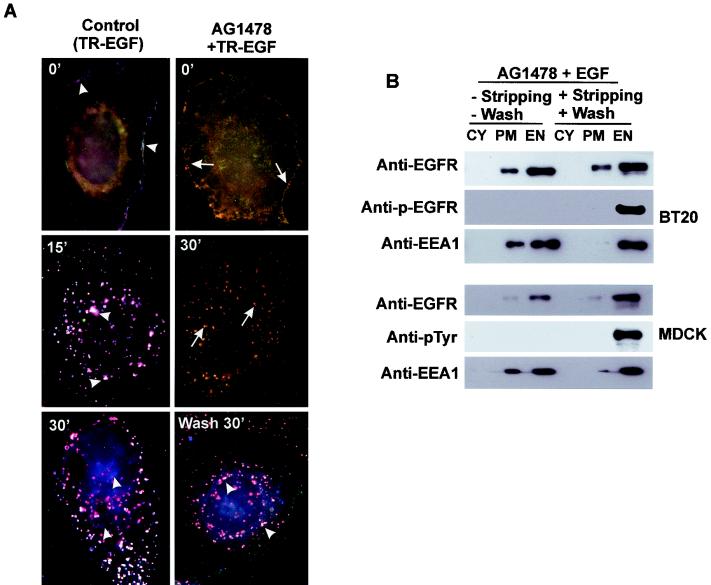
Although monensin was used in this system to block the recycling of inactive EGFR back to the plasma membrane, it was washed away prior to the activation of EGFR and thus its nonphysiological effects on the endosomal signaling of EGFR are minimum. Nevertheless, to completely eliminate any possible nonphysiological effects of monensin, we developed another technique to specifically activate endosome-associated EGFR without monensin. BT20 cells were treated with TR-EGF and AG-1478 in the absence of monensin. Interestingly, following incubation at 37°C for 30 min, TR-EGF and EGFR were mostly localized to endosomes, with a small amount of EGFR localized to the plasma membrane (Fig. 3A). There is no detectable p-EGFR staining, indicating that the endosome-associated EGFR was nonphosphorylated (Fig. 3A). To dissociate TR-EGF from EGFR at the plasma membrane, cells were cooled down to 4°C and washed with acidic stripping buffer for 1 min. No TR-EGF was detectable at the plasma membrane following stripping. We then activate endosome-associated EGFR by washing the cells with PBS and incubating the cells in serum-free medium at 37°C. At 30 min, the endosome-associated EGFR was significantly activated but no EGFR at the plasma membrane was phosphorylated (Fig. 3A). These observations were further confirmed by subcellular-fractionation experiments (Fig. 3B). Treatment with AG-1478, monensin, and EGF resulted in the internalization of nonphosphorylated EGFR into endosomes, and the endosome-associated EGFR was phosphorylated following washing and incubation in medium. No phosphorylated EGFR was detected at the plasma membrane (Fig. 3B). These results indicate that we are able to specifically activate endosome-associated EGFR without monensin. With these two techniques, we have established a system to specifically activate endosome-associated EGFR without any detectable activation of EGFR at the plasma membrane.
FIG. 3.
Selective activation of endosome-associated EGFR without monensin. (A) BT20 cells were treated with AG-1478 for 15 min and then stimulated with TR-EGF at 4°C for 30 min, followed by a wash with acidic stripping buffer at 4°C for 1 min. The cells were then washed with PBS three times and incubated with medium at 37°C for the indicated times. BT20 cells were triple fluorescence stained for TR-EGF (red), EGFR (green), and p-EGFR (blue) as described in Materials and Methods. Arrows, colocalization (yellow) of TR-EGF and EGFR; arrowheads, colocalization (light purple) of TR-EGF, EGFR, and p-EGFR. Bar, 20 μm. (B) Subcelllular fractionation. BT20 and MDCK cells were treated with AG-1478 for 15 min and then stimulated with EGF at 37°C for 30 min, followed by a wash with acidic stripping buffer at 4°C for 1 min. The cells were then washed with PBS three times and incubated with medium at 37°C for the indicated times. The cells were then subcellularly fractionated into the PM, EN, and CY fractions. The subcellular fractions were subjected to immunoblotting with anti-EGFR, anti-pTyr, and anti-EEA1 antibodies as described in Materials and Methods. TL, total lysate.
EGFR activity and trafficking in our established system.
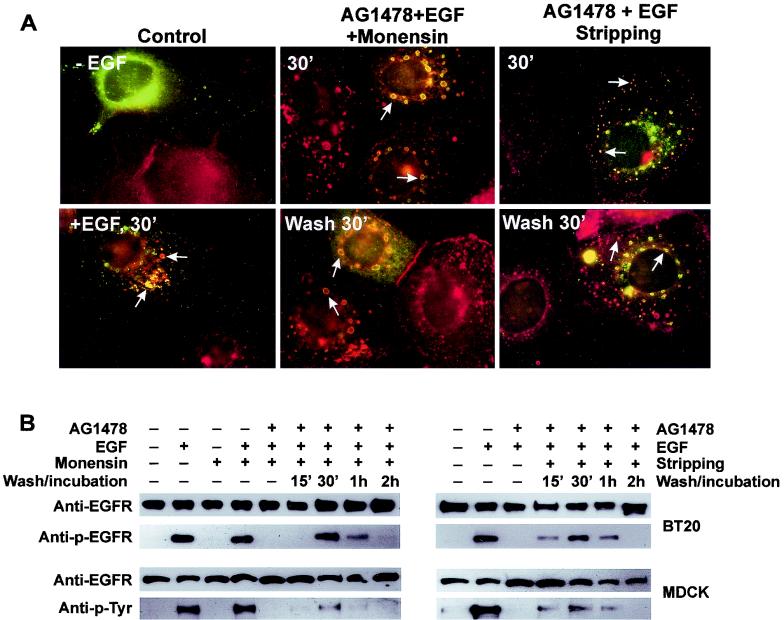
We next compared EGFR activity and trafficking in our system with those following standard EGF stimulation. We first determined whether treatment with AG-1478 and EGF with or without monensin resulted in the internalization of EGFR into the same pool of endosomes as that following standard EGF stimulation. It is well established that, following EGF stimulation, phosphorylated EGFR is internalized into Rab5-positive endosomes prior to its trafficking to late endosomes and lysosomes (2, 9, 24). BT20 cells were transiently transfected with Rab5 (9). We showed that treatment with AG-1478 and EGF with or without monensin also results in the internalization of nonphosphorylated EGFR into Rab5-positive endosomes (Fig. 4A). Following washing and incubation with medium for 30 min to activate the endosome-associated EGFR, EGFR was still mostly localized to Rab5-positive endosomes (Fig. 4A). In control cells treated with EGF for 30 min, EGFR was localized to Rab5-positive endosomes as expected (Fig. 4A). These results indicate that treatment with AG-1478 and EGF with or without monensin results in the internalization of EGFR into the same pool of endosomes as that following standard EGF stimulation. We then determined the phosphorylation and degradation of EGFR. BT20 and MDCK cells were treated with AG-1478 and EGF with or without monensin as described above. Immunoblotting with anti-EGFR, anti-pTyr, and anti-p-EGFR antibodies showed that before washing no EGFR was phosphorylated (Fig. 4B). Washing and incubation gradually induced the phosphorylation of EGFR. After 30 min of washing, phosphorylation of EGFR reached a maximum, approximately 50% of the level reached with standard EGF stimulation for 30 min. EGFR phosphorylation was reduced with incubation for 1 and 2 h (Fig. 4B). The pattern of dephosphorylation was very similar to that following standard EGF stimulation (data not shown). These results indicate that EGFR activation, trafficking, and deactivation in our system are very similar to those following standard EGF stimulation.
FIG. 4.
Trafficking and activity of EGFR following its selective activation in endosomes. (A) Internalization of inactive EGFR into Rab5-positive endosomes and its subsequent activation. BT20 cells were transfected with wild-type Rab5. Cells were either not treated or treated with EGF for 30 min as controls (left). Some cells were treated with AG-1478 for 15 min, followed by addition of EGF and monensin for 30 min without or with washing and incubation (middle). Some cells were treated with AG-1478 and EGF for 30 min without washing or followed by washing with acidic stripping buffer for 1 min and incubation with medium for 30 min. EGFR (red) and Rab5 (green) localization was determined by indirect immunofluorescence. Arrows, colocalization (yellow) of EGFR and Rab5. (B) Immunoblot analysis of the trafficking and inactivation of EGFR following its selective activation in the endosome. BT20 and MDCK cells were treated with AG-1478 and EGF with or without monensin as described for panel A. The cells were washed and then incubated with serum-free medium for the indicated times. Cell lysates were subjected to immunoblot analysis with mouse anti-pTyr, anti-p-EGFR, and rabbit anti-EGFR antibodies.
Recruitment of signaling proteins into endosomes by activation of endosome-associated EGFR.
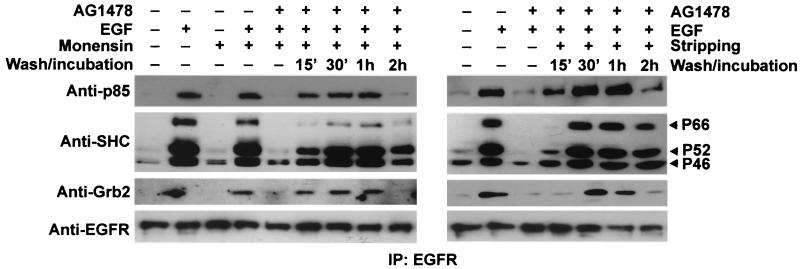
We next used this system to specifically study EGFR-mediated signal transduction from endosomes. Following EGF stimulation, various signaling proteins, including Grb2, SHC, the p85α subunit of PI3K, and PLC-γ1, were recruited to the plasma membrane by the interaction between EGFR pTyr sites and the signaling proteins (32, 44). Many of these proteins, including Grb2, SHC, and GAP, were then cointernalized with activated EGFR and remained associated with the EGFR in endosomes (10, 14, 17, 25, 40). However, it is unclear whether the direct activation of endosome-associated EGFR is able to directly recruit these signaling proteins into endosomes or whether the interaction between EGFR and these signaling proteins has to be initiated at the plasma membrane. To answer this question, BT20 and MDCK cells were treated with AG-1478 and EGF with or without monensin as described above. The cells were then lysed and immunoprecipitated with an anti-EGFR antibody. Immunoblotting the immunoprecipitates revealed the association of EGFR with various signaling proteins following the activation of endosome-associated EGFR (Fig. 5). The signaling proteins that are coimmunoprecipitated with EGFR include SHC, Grb2, and the p85α subunit of PI3K. Our results indicate that direct activation of endosome-associated EGFR is sufficient to recruit some signaling proteins into endosomes.
FIG. 5.
Interactions between signaling proteins and endosome-associated EGFR in BT20 cells. (A) BT20 cells were treated with AG-1478 and EGF with or without monensin as described in Materials and Methods. The cells were washed and then incubated with serum-free medium for the indicated times. The cells were lysed and immunoprecipitated (IP) with a rabbit anti-EGFR antibody as described in Materials and Methods. Immunoprecipitates were subjected to immunoblot analysis with mouse anti-EGFR, anti-Grb2, anti-SHC, and anti-p85α antibodies. BT20 cells that were serum starved or treated with EGF, monensin, or EGF plus monensin were used as controls.
Stimulation of various signaling pathways by endosomal signaling of EGFR.
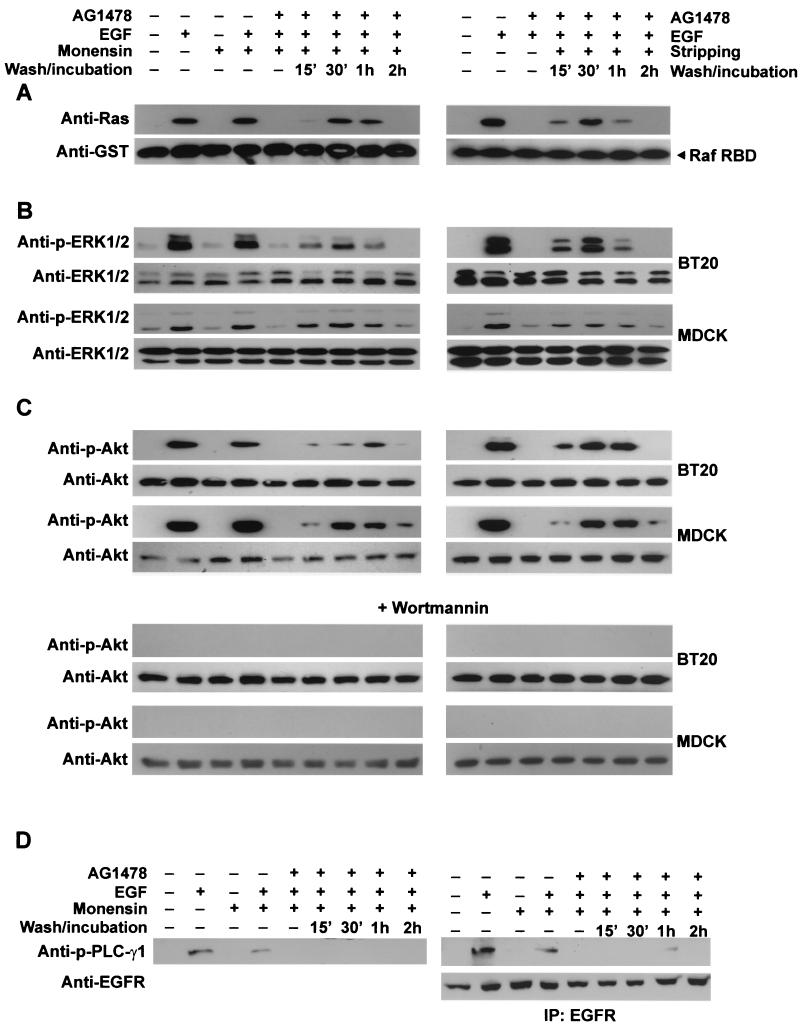
We further determined whether endosomal signaling of EGFR is sufficient to stimulate various signaling pathways. It is well established that, following standard EGF stimulation, several signal transduction pathways including the Ras-ERK pathway, which is essential for cell mitogenesis (28), and the PI3K-Akt pathway, which protects against apoptosis, were activated (3, 15). We showed that activation of endosome-associated EGFR also significantly activated Ras (Fig. 6A), ERK1/2 (Fig. 6B), and Akt (Fig. 6C). Interestingly, while the activation level of endosome-associated EGFR is approximately 50% of that following standard EGF stimulation (Fig. 3), the activation levels of Ras, ERK1/2, and Akt were also approximately 50% of those following standard EGF stimulation (Fig. 6). These results suggest that activation of endosome-associated EGFR and the standard activation of EGFR may have similar effects on the activation of the Ras-ERK and Akt pathways.
FIG. 6.
Stimulation of various signal transduction pathways by activation of endosome-associated EGFR. BT20 and MDCK cells were treated with AG-1478 and EGF with or without monensin as described in Materials and Methods. The cells were washed and then incubated with serum-free medium for the indicated times. (A) Activation of Ras. BT20 cell lysates were incubated with GST-RBD conjugated with glutathione beads. The glutathione beads were then subjected to immunoblot analysis with a mouse anti-Ras antibody. GST-Raf RBD was stained by a mouse anti-GST antibody to show equal loading. (B) Activation of ERK. BT20 and MDCK cell lysates were subjected to immunoblot analysis with a rabbit anti-phospho-ERK1/2 (p-ERK1/2) antibody. Total ERK1/2 was used as the control. (C) Activation of Akt and its inhibition by wortmannin. Activation of Akt was determined by an anti-phospho-Akt (p-Akt) antibody. To determine the effects of wortmannin on the activation of Akt, BT20 and MDCK cells were treated with wortmannin (100 nM) for 15 min before the treatment with AG-1478, EGF, and monensin. Cell lysates were subjected to immunoblot analysis with an anti-p-Akt antibody. (D) Activation of PLC-γ1. Activation of PLC-γ1 was determined by immunoblotting of BT20 cell lysates or EGFR immunoprecipitates (IP) with anti-phospho-PLC-γ1 (p-PLC-γ1) antibody.
Moreover, Akt phosphorylation was blocked by PI3K inhibitor wortmannin, indicating that Akt activation is mediated by PI3K (Fig. 6C). Together, these results indicate that endosomal signaling of EGFR is sufficient to stimulate the signal transduction pathways implicated in cell mitogenesis and survival.
However, activation of endosome-associated EGFR only slightly activated PLC-γ1. While we did not detect phosphorylation of PLC-γ1 in the total lysates following the activation of endosome-associated EGFR (Fig. 6D), we detected phosphorylation of PLC-γ1 from EGFR immunoprecipitates (Fig. 6D).
Support of cell survival by endosomal signaling of EGFR.
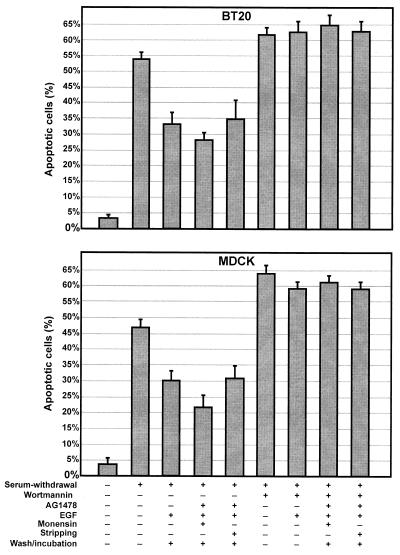
We finally determined whether pure endosomal signaling of EGFR is sufficient to produce a biological outcome. We examined the effects of pure endosomal signaling of EGFR on serum withdrawal-induced apoptosis by a TUNEL assay (Fig. 7). For BT20 cells cultured in 10% FBS, only 5% of cells are apoptotic; however, serum withdrawal for 24 h induced 54% apoptosis. For MDCK cells serum withdrawal for 72 h induced 47% apoptosis, compared with 3% apoptosis in 10% FBS (Fig. 7). To determine the effects of activation of endosome-associated EGFR on apoptosis induced by serum withdrawal, BT20 and MDCK cells were stimulated with EGF (20 ng/ml) for 30 min in the presence of AG-1478 with or without monensin to allow the internalization of inactive EGFR into endosomes. After the subsequent activation of endosome-associated EGFR by stripping and/or washing and incubating with serum-free medium for 12 h, the percentage of apoptotic cells was significantly reduced to a level comparable to that following standard EGF (20 ng/ml) stimulation for 30 min (Fig. 7). These results demonstrate that pure endosomal signaling of EGFR is sufficient to produce a biological effect. Moreover, when the cells were pretreated with wortmannin (100 nM) to block PI3K activity, the activation of endosome-associated EGFR was unable to suppress apoptosis induced by serum withdrawal (Fig. 7). This indicates that, similar to standard activation of EGFR, activation of endosome-associated EGFR supports cell survival by stimulating the PI3K-Akt pathway.
FIG. 7.
Activation of endosome-associated EGFR and the effects on cell survival. BT20 and MDCK cells were incubated in serum-free medium to induce apoptosis. The cells were then treated AG-1478 and EGF (20 ng/ml) with or without monensin followed by washing and incubation as described above. To determine the effects of wortmannin, the cells were pretreated with wortmannin (100 nM). The apoptosis was determined by TUNEL assay.
DISCUSSION
In spite of intensified efforts to understand cell signaling from endosomes (4, 19, 22, 31, 37), many questions remain due to the limitations of current approaches. So far, the most comprehensive approach is to block receptor endocytosis and examine the effects on cell signaling. The most commonly used tools are dominant-negative constructs of the GTPase dynamin and arrestin (1, 29, 39). While it has made a significant contribution and remains a powerful tool to study endosomal signaling, this endocytosis inhibition approach has serious limitations in demonstrating and analyzing receptor-mediated signaling from endosomes. The questions that are essential for understanding endosomal signaling but that remain unanswered include (i) whether specific activation of the endosome-associated receptor is able to recruit signaling proteins directly into the endosome, (ii) whether endosomal signaling is sufficient to stimulate various signaling pathways leading to cell mitogenesis and survival, and (iii) whether endosomal signaling is sufficient to produce a biological outcome. To answer these questions, a novel system to activate endosome-associated receptors without activation of the receptors at the cell surface needs to be established (4).
As demonstrated in this paper, we have established such a system to specifically activate EGFR in endosomes without initial activation at the plasma membrane (Fig. 1 to 4). Following treatment with AG-1478 to inhibit EGFR kinase activation, addition of EGF resulted in the internalization of inactivated EGF-EGFR complex into endosomes with or without monensin (Fig. 1 to 3). In the presence of monensin more than 90% of EGFR was internalized and remained in endosomes following EGF treatment for 30 min (Fig. 2), while only 70 to 80% of EGFR was in the endosome fractions without monensin, possibly due to the recycling of the inactivate EGF-EGFR complex (Fig. 3). We did not observe any increase of the plasma membrane-associated EGFR following the removal of monensin and AG-1478 by washing, which suggests that washing does not lead to the recycling of EGFR. This is not surprising because washing results in the activation of EGFR and activated EGFR is targeted to the lysosome for degradation instead of being recycled back to the plasma membrane. However, in both cases we did not detect any activation of EGFR at the plasma membrane following the activation of endosome-associated EGFR by stripping surface EGF and/or washing away AG-1478 (Fig. 1 to 3). The total phosphorylation level of EGFR following its specific activation in endosomes is approximately 50% of that following the standard activation of EGFR at the plasma membrane (Fig. 4). Two possible processes may explain the lower phosphorylation level: some EGF may be dissociated from EGFR in endosomes, and AG-1478 may not be completely washed away. Nevertheless, our results demonstrate that we have established a system to specifically activate endosome-associated EGFR without detectable activation of surface EGFR.
In this system EGFR follows the same endocytic pathway as the control: the EGFR first is internalized into Rab5-positive endosomes and eventually traffics to lysosomes for degradation. The only difference is that EGFR is not activated during its internalization from the plasma membrane to endosomes and stops at endosomes until being activated. Thus, this system not only allows the generation of pure or nearly pure endosomal signaling of EGFR but also allows it under a condition very similar to the endosomal signaling of EGFR following its activation at the plasma membrane. The establishment of this system allows us to specifically determine EGFR-mediated signaling from endosomes and to address related questions that cannot be elucidated by previous approaches.
The first question we addressed is whether the activated-endosome-associated EGFR is able to recruit various signaling proteins to endosomes. It has been argued that clathrin-coated pits function as nucleation sites for the organization of signaling complexes on the plasma membrane (30). However, we showed by coimmunoprecipitation that activation of endosome-associated EGFR resulted in the interaction with various signaling proteins including SHC, Grb2, and the p85α subunit of PI3K (Fig. 5). Our results indicate that endosomes could also serve as nucleation sites for the organization of signaling complexes.
The second question we addressed is whether endosomal signaling of EGFR itself is sufficient to stimulate various signaling pathways. Previous studies using an endocytosis inhibition approach suggest that endosomal signaling of EGFR is necessary for EGF-induced ERK activation (1, 29, 36, 39). Here we showed that the endosomal signaling of EGFR itself is sufficient to activate various signaling proteins including Ras, ERK, and Akt (Fig. 6). Interestingly, we also showed that PLC-γ1 is only weakly phosphorylated by the endosomal signaling of EGFR, which suggests that the endosomal signaling of EGFR is not identical to the standard EGFR signaling.
Finally, we determined the physiological relevance of EGFR-mediated signaling from endosomes. Inhibition of EGFR endocytosis blocks EGF-stimulated ERK activation (36). However, in the few cases where biological end points were measured, inhibition of endocytosis did not result in attenuation of biological effects (6, 39). Very recently, it was reported that retrograde support of neuronal survival does not require the retrograde transport (endocytosis) of nerve growth factor (26). These results argue against the physiological relevance of endosome-originated signals (12). Here we showed that endosomal signaling of EGFR itself stimulated the PI3K-Akt pathway, which suggests a role for endosomal EGFR signaling in antiapoptosis. Indeed, we showed by TUNEL assay that activation of endosome-associated EGFR suppressed apoptosis induced by serum withdrawal (Fig. 7). Moreover, this suppression was blocked by treating the cell with wortmannin. We have also shown that endosomal signaling of EGFR is sufficient to activate Akt and that this activation is abolished by wortmannin. Together, our results indicate that the molecular mechanism by which endosomal signaling of EGFR supports cell survival is through the activation of the PI3K-Akt pathway. This provides the original evidence to show that endosomal signaling is sufficient to produce biological outcome.
If we divide the whole-EGFR signaling into two components, signaling from the plasma membrane and signaling from endosomes, we clearly demonstrate that eliminating the initial signaling from the plasma membrane does not affect the ability of EGFR to activate major signaling pathways and to provide a physiological response. Moreover, following EGF stimulation, activated EGFR only remains at the plasma membrane briefly (5 to 10 min) but remains at the endosome much longer (1 h) (5, 38). This argues for a more important role for endosome signaling.
In summary, we have successfully established a system to allow the specific activation of endosome-associated EGFR without the initial activation at the plasma membrane. By using this system, we determined the roles of endosomal EGFR signaling in cell mitogenesis and antiapoptosis. We provided original evidence demonstrating that the endosome can serve as a nucleation site for the formation of signaling complexes and that endosomal EGFR signaling is sufficient to activate the major signaling pathways leading to cell proliferation and antiapoptosis. We also demonstrate that pure endosomal EGFR signaling is sufficient to suppress apoptosis induced by serum withdrawal. The same approach could be applied to study endosomal signaling of other receptor tyrosine kinases including platelet-derived growth factor receptor and nerve growth factor receptor.
Acknowledgments
We thank Jim Stone for GST-Raf RBD and R. Rachubinski, B. Posner, and D. Brindley for comments on the manuscript.
This work was supported in part by grants from the Canadian Institutes of Health Research (CIHR), the Alberta Heritage Foundation for Medical Research (AHFMR), and the Natural Science and Engineering Council of Canada. Z.W. is a CIHR Scholar and an AHFMR Scholar.
REFERENCES
- 1.Ahn, S., S. Maudsley, L. M. Luttrell, R. J. Lefkowitz, and Y. Daaka. 1999. Src-mediated tyrosine phosphorylation of dynamin is required for beta2-adrenergic receptor internalization and mitogen-activated protein kinase signaling. J. Biol. Chem. 274:1185-1188. [DOI] [PubMed] [Google Scholar]
- 2.Barbieri, M. A., R. L. Roberts, A. Gumusboga, H. Highfield, C. Alvarez-Dominguez, A. Wells, and P. D. Stahl. 2000. Epidermal growth factor and membrane trafficking. EGF receptor activation of endocytosis requires Rab5a. J. Cell Biol. 151:539-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burgering, B. M., and P. J. Coffer. 1995. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature 376:599-602. [DOI] [PubMed] [Google Scholar]
- 4.Burke, P., K. Schooler, and H. S. Wiley. 2001. Regulation of epidermal growth factor receptor signaling by endocytosis and intracellular trafficking. Mol. Biol. Cell 12:1897-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carpenter, G. 1987. Receptors for epidermal growth factor and other polypeptide mitogens. Annu. Rev. Biochem. 56:881-914. [DOI] [PubMed] [Google Scholar]
- 6.Ceresa, B. P., A. W. Kao, S. R. Santeler, and J. E. Pessin. 1998. Inhibition of clathrin-mediated endocytosis selectively attenuates specific insulin receptor signal transduction pathways. Mol. Cell. Biol. 18:3862-3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ceresa, B. P., and S. L. Schmid. 2000. Regulation of signal transduction by endocytosis. Curr. Opin. Cell Biol. 12:204-210. [DOI] [PubMed] [Google Scholar]
- 8.Chen, W. S., C. S. Lazar, K. A. Lund, J. B. Welsh, C. P. Chang, G. M. Walton, C. J. Der, H. S. Wiley, G. N. Gill, and M. G. Rosenfeld. 1989. Functional independence of the epidermal growth factor receptor from a domain required for ligand-induced internalization and calcium regulation. Cell 59:33-43. [DOI] [PubMed] [Google Scholar]
- 9.Chen, X., and Z. Wang. 2001. Regulation of intracellular trafficking of the EGF receptor by Rab5 in the absence of phosphatidylinositol 3-kinase activity. EMBO Rep. 2:68-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clague, M. J., and S. Urbe. 2001. The interface of receptor trafficking and signalling. J. Cell Sci. 114:3075-3081. [DOI] [PubMed] [Google Scholar]
- 11.Cohen, S., and R. A. Fava. 1985. Internalization of functional epidermal growth factor: receptor/kinase complexes in A-431 cells. J. Biol. Chem. 260:12351-12358. [PubMed] [Google Scholar]
- 12.Di Fiore, P. P., and P. De Camilli. 2001. Endocytosis and signaling: an inseparable partnership. Cell 106:1-4. [DOI] [PubMed] [Google Scholar]
- 13.Di Fiore, P. P., and G. N. Gill. 1999. Endocytosis and mitogenic signaling. Curr. Opin. Cell Biol. 11:483-488. [DOI] [PubMed] [Google Scholar]
- 14.Di Guglielmo, G. M., P. C. Baass, W. J. Ou, B. I. Posner, and J. J. Bergeron. 1994. Compartmentalization of SHC, GRB2 and mSOS, and hyperphosphorylation of Raf-1 by EGF but not insulin in liver parenchyma. EMBO J. 13:4269-4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Downward, J. 1998. Mechanisms and consequences of activation of protein kinase B/Akt. Curr. Opin. Cell Biol. 10:262-267. [DOI] [PubMed] [Google Scholar]
- 16.Felder, S., K. Miller, G. Moehren, A. Ullrich, J. Schlessinger, and C. R. Hopkins. 1990. Kinase activity controls the sorting of the epidermal growth factor receptor within the multivesicular body. Cell 61:623-634. [DOI] [PubMed] [Google Scholar]
- 17.Fukazawa, T., S. Miyake, V. Band, and H. Band. 1996. Tyrosine phosphorylation of Cbl upon epidermal growth factor (EGF) stimulation and its association with EGF receptor and downstream signaling proteins. J. Biol. Chem. 271:14554-14559. [DOI] [PubMed] [Google Scholar]
- 18.Glenney, J. R., Jr., W. S. Chen, C. S. Lazar, G. M. Walton, L. M. Zokas, M. G. Rosenfeld, and G. N. Gill. 1988. Ligand-induced endocytosis of the EGF receptor is blocked by mutational inactivation and by microinjection of anti-phosphotyrosine antibodies. Cell 52:675-684. [DOI] [PubMed] [Google Scholar]
- 19.Haugh, J. M., A. C. Huang, H. S. Wiley, A. Wells, and D. A. Lauffenburger. 1999. Internalized epidermal growth factor receptors participate in the activation of p21(ras) in fibroblasts. J. Biol. Chem. 274:34350-34360. [DOI] [PubMed] [Google Scholar]
- 20.Herrmann, C., G. A. Martin, and A. Wittinghofer. 1995. Quantitative analysis of the complex between p21ras and the Ras-binding domain of the human Raf-1 protein kinase. J. Biol. Chem. 270:2901-2905. [DOI] [PubMed] [Google Scholar]
- 21.Kay, D. G., W. H. Lai, M. Uchihashi, M. N. Khan, B. I. Posner, and J. J. Bergeron. 1986. Epidermal growth factor receptor kinase translocation and activation in vivo. J. Biol. Chem. 261:8473-8480. [PubMed] [Google Scholar]
- 22.Kuruvilla, R., H. Ye, and D. D. Ginty. 2000. Spatially and functionally distinct roles of the PI3-K effector pathway during NGF signaling in sympathetic neurons. Neuron 27:499-512. [DOI] [PubMed] [Google Scholar]
- 23.Lai, W. H., P. H. Cameron, J. J. Doherty II, B. I. Posner, and J. J. Bergeron. 1989. Ligand-mediated autophosphorylation activity of the epidermal growth factor receptor during internalization. J. Cell Biol. 109:2751-2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lanzetti, L., V. Rybin, M. G. Malabarba, S. Christoforidis, G. Scita, M. Zerial, and P. P. Di Fiore. 2000. The Eps8 protein coordinates EGF receptor signalling through Rac and trafficking through Rab5. Nature 408:374-377. [DOI] [PubMed] [Google Scholar]
- 25.Levkowitz, G., H. Waterman, E. Zamir, Z. Kam, S. Oved, W. Y. Langdon, L. Beguinot, B. Geiger, and Y. Yarden. 1998. c-Cbl/Sli-1 regulates endocytic sorting and ubiquitination of the epidermal growth factor receptor. Genes Dev. 12:3663-3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacInnis, B. L., and R. B. Campenot. 2002. Retrograde support of neuronal survival without retrograde transport of nerve growth factor. Science 295:1536-1539. [DOI] [PubMed] [Google Scholar]
- 27.Marshall, C. J. 1994. MAP kinase kinase kinase, MAP kinase kinase and MAP kinase. Curr. Opin. Genet. Dev. 4:82-89. [DOI] [PubMed] [Google Scholar]
- 28.Marshall, C. J. 1996. Cell signalling. Raf gets it together. Nature 383:127-128. [DOI] [PubMed] [Google Scholar]
- 29.Maudsley, S., K. L. Pierce, A. M. Zamah, W. E. Miller, S. Ahn, Y. Daaka, R. J. Lefkowitz, and L. M. Luttrell. 2000. The beta(2)-adrenergic receptor mediates extracellular signal-regulated kinase activation via assembly of a multi-receptor complex with the epidermal growth factor receptor. J. Biol. Chem. 275:9572-9580. [DOI] [PubMed] [Google Scholar]
- 30.McPherson, P. S., B. K. Kay, and N. K. Hussain. 2001. Signaling on the endocytic pathway. Traffic 2:375-384. [DOI] [PubMed] [Google Scholar]
- 31.Miller, W. E., and R. J. Lefkowitz. 2001. Expanding roles for beta-arrestins as scaffolds and adapters in GPCR signaling and trafficking. Curr. Opin. Cell Biol. 13:139-145. [DOI] [PubMed] [Google Scholar]
- 32.Pawson, T. 1995. Protein modules and signalling networks. Nature 373:573-580. [DOI] [PubMed] [Google Scholar]
- 33.Pawson, T. 1997. New impressions of Src and Hck. Nature 385:582-585. [DOI] [PubMed] [Google Scholar]
- 34.Schlessinger, J. 1986. Allosteric regulation of the epidermal growth factor receptor kinase. J. Cell Biol. 103:2067-2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schlessinger, J., and A. Ullrich. 1992. Growth factor signaling by receptor tyrosine kinases. Neuron 9:383-391. [DOI] [PubMed] [Google Scholar]
- 36.Shen, Y., L. Xu, and D. A. Foster. 2001. Role for phospholipase D in receptor-mediated endocytosis. Mol. Cell. Biol. 21:595-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Slepnev, V. I., and P. De Camilli. 2000. Accessory factors in clathrin-dependent synaptic vesicle endocytosis. Nat. Rev. Neurosci. 1:161-172. [DOI] [PubMed] [Google Scholar]
- 38.Sorkin, A., and G. Carpenter. 1993. Interaction of activated EGF receptors with coated pit adaptins. Science 261:612-615. [DOI] [PubMed] [Google Scholar]
- 39.Vieira, A. V., C. Lamaze, and S. L. Schmid. 1996. Control of EGF receptor signaling by clathrin-mediated endocytosis. Science 274:2086-2089. [DOI] [PubMed] [Google Scholar]
- 40.Wang, Z., P. S. Tung, and M. F. Moran. 1996. Association of p120 ras GAP with endocytic components and colocalization with epidermal growth factor (EGF) receptor in response to EGF stimulation. Cell Growth Differ. 7:123-133. [PubMed] [Google Scholar]
- 41.Wang, Z., L. Zhang, T. K. Yeung, and X. Chen. 1999. Endocytosis deficiency of epidermal growth factor (EGF) receptor-ErbB2 heterodimers in response to EGF stimulation. Mol. Biol. Cell 10:1621-1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Waterman, H., I. Alroy, S. Strano, R. Seger, and Y. Yarden. 1999. The C-terminus of the kinase-defective neuregulin receptor ErbB-3 confers mitogenic superiority and dictates endocytic routing. EMBO J. 18:3348-3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wiley, H. S., and P. M. Burke. 2001. Regulation of receptor tyrosine kinase signaling by endocytic trafficking. Traffic 2:12-18. [DOI] [PubMed] [Google Scholar]
- 44.Yarden, Y., and M. X. Sliwkowski. 2001. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2:127-137. [DOI] [PubMed] [Google Scholar]