Abstract
Rai is a recently identified member of the family of Shc-like proteins, which are cytoplasmic signal transducers characterized by the unique PTB-CH1-SH2 modular organization. Rai expression is restricted to neuronal cells and regulates in vivo the number of postmitotic sympathetic neurons. We report here that Rai is not a common substrate of receptor tyrosine kinases under physiological conditions and that among the analyzed receptors (Ret, epidermal growth factor receptor, and TrkA) it is activated specifically by Ret. Overexpression of Rai in neuronal cell lines promoted survival by reducing apoptosis both under conditions of limited availability of the Ret ligand glial cell line-derived neurotrophic factor (GDNF) and in the absence of Ret activation. Overexpressed Rai resulted in the potentiation of the Ret-dependent activation of phosphatidylinositol 3-kinase (PI3K) and Akt. Notably, increased Akt phosphorylation and PI3K activity were also found under basal conditions, e.g., in serum-starved neuronal cells. Phosphorylated and hypophosphorylated Rai proteins form a constitutive complex with the p85 subunit of PI3K: upon Ret triggering, the Rai-PI3K complex is recruited to the tyrosine-phosphorylated Ret receptor through the binding of the Rai PTB domain to tyrosine 1062 of Ret. In neurons treated with low concentrations of GDNF, the prosurvival effect of Rai depends on Rai phosphorylation and Ret activation. In the absence of Ret activation, the prosurvival effect of Rai is, instead, phosphorylation independent. Finally, we showed that overexpression of Rai, at variance with Shc, had no effects on the early peak of mitogen-activated protein kinase (MAPK) activation, whereas it increased its activation at later time points. Phosphorylated Rai, however, was not found in complexes with Grb2. We propose that Rai potentiates the MAPK and PI3K signaling pathways and regulates Ret-dependent and -independent survival signals.
Shc-like gene sequences are present in all multicellular organisms analyzed to date, from nematodes to Homo sapiens (18). Three mammalian Shc genes have been identified, termed Shc (ShcA), Sli (ShcB/SCK), and Rai (ShcC/N-Shc) (26-28). Due to alternative initiation codon usage and splicing patterns, these three genes encode at least six proteins (p66Shc, p52Shc, p46Shc, p64Rai, p52Rai, and p68Sli), all of which contain a unique PTB-CH1-SH2 modular organization. PTB and SH2 are phosphotyrosine-binding domains that are found in hundreds of different proteins. The concomitant presence of the PTB and SH2 domains (in the N-PTB-SH2-C order) is unique to the proteins of the Shc family (18). The CH1 region contains tyrosine phosphorylation sites and several putative SH3-binding sites that might be important for the signaling function(s) of the Shc-like proteins.
The modular organization of the proteins of the Shc family suggests that they function as adaptors within cytoplasmic signaling pathways. Indeed, several lines of evidence support a general role for the p52/46Shc proteins in the transduction of signals from activated tyrosine kinases (TKs) to Ras. p52/46Shc proteins are rapidly and efficiently tyrosine-phosphorylated by all TKs tested to date (2). Three major Shc phosphorylation sites have been identified within its CH1 region (31, 39). Once phosphorylated, the p52/46Shc proteins act to bridge receptor TKs (RTKs) and the Grb2 adaptor protein (2). Grb2, in turn, is constitutively complexed to SOS, a Ras guanine nucleotide exchange factor (4). Recruitment of the Grb2/SOS complex by p52/46Shc results in the membrane relocalization of SOS, an event sufficient to induce Ras activation. Consistent with this model, overexpression of p52/p46Shc enhances epidermal growth factor (EGF)- or granulocyte-macrophage colony-stimulating factor-induced Ras downstream signaling events (2), such as mitogen-activated protein kinase (MAPK) and fos promoter activation. Moreover, fibroblasts derived from Shc-null mice display a reduced MAPK activation in response to physiological concentrations of growth factors (16). In line with their role in the activation of Ras, the p52/p46Shc proteins are ubiquitously expressed in the adult mice.
Recent results have suggested that the Shc proteins could have further, apparently unrelated, functions in addition to Ras activation. Homozygous inactivation of p66Shc in mice extends their life spans and increases cellular resistance to oxidative stress-induced apoptosis (21). p66Shc is not involved in Ras activation (22). Instead, it is implicated in pathways activated by environmental stresses, as shown by its serine phosphorylation in cells exposed to oxidative stress (21).
Unlike Shc, which is variably expressed in many different tissues, expression of the other two Shc-related genes is either exclusively (Rai) or predominantly (Sli) restricted to the nervous system (25-27). Null mutations of Sli and Rai in mice resulted in no overt neuroanatomical abnormalities within the central nervous system. However, analysis of sympathetic and sensory neuronal populations revealed loss of sympathetic neurons in the mice lacking both Sli and Rai (but not in the single-null mutants) as well as loss of sensory neurons (associated with nociceptive function) in the Sli-deficient mice (30). These findings suggest that Rai and Sli regulate neural development and that they perform redundant functions in supporting sympathetic neuron development and survival while Sli plays a nonredundant role in the survival of sensory neurons. However, the biochemical mechanisms underlying the neural functions of Sli and Rai remain largely unknown.
Neurons of the developing sympathetic superior cervical ganglia (SCG) express high levels of both Rai and Sli transcripts and are significantly reduced in number in mice carrying double mutations of Sli and Rai (30). Loss of either the TrkA (9, 36) or the Ret (34) receptor, but not of the TrkB or TrkC receptors (9), results in virtual elimination of SCG neurons, thereby suggesting that the Rai/Sli mutant phenotype may be caused by the disruption of TrkA and/or Ret signaling. Indeed, preliminary evidences suggest that, like the Shc proteins, Sli and Rai may function as cytoplasmic signaling proteins downstream of activated TK receptors. Both Rai and Sli can bind activated TK receptors, including the EGF receptor (EGFR) and Trk receptor (10, 23, 24, 26). Whether Rai or Sli activates Ras or other intracellular pathways remains to be determined, as do the biological consequences of their activities.
Here, we report that Rai is a physiological substrate of the Ret receptor and that it exerts a prosurvival function in neuronal cells by activating the phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway.
MATERIALS AND METHODS
Proteins and plasmids.
The Shc, Grb2, Rai, p85, and glutathione S-transferase (GST) fusion proteins have been described elsewhere (27, 33, 37). GST-Ret(COOH) (residues 1010 to 1072) was expressed in the bacterial strain TKB1 harboring an inducible Elk kinase, and it was tyrosine phosphorylated and purified by following the manufacturer's instructions (Amersham-Pharmacia Biotech). The human p52Rai and p52Shc cDNAs were subcloned within the enhanced green fluorescent protein-selectable Pinco retroviral vector (12) or the pcDNA3.1 vector. All of the Ret constructs used in this work expressed the Ret9 protein form. The Ret plasmid encoding the MEN2A-associated mutation, C634R, and those encoding the Hirschsprung disease-associated mutation, ΔN1059, or the Y905F mutation were previously described (11, 20, 32). The chimeric EGFR/Ret (E/R) receptor was reported previously (33).
Tyrosine-to-phenylalanine mutations were introduced in the p52Rai and Ret-MEN2A cDNAs by in vitro site-directed mutagenesis. The bovine p85 (into pcDNA3.1) cDNA was a gift of R. Hooshmand-Raad. To generate the Shc(CH1Rai) and Rai(CH1Shc) constructs, the Shc and Rai CH1 regions were amplified by PCR, sequenced, and inserted in frame into the corresponding location of hemagglutinin (HA)-Rai and HA-Shc, respectively.
Cell culture, infection, and transfection.
SK-N-MC E/R and PC12coRα cells were obtained by stably transfecting SK-N-MC or PC12 cells with E/R and GFRα1 (gift from S. Jing) expression vectors, respectively. Transfection of the Phoenix packaging cell line and infection of target cells were performed as described previously (12). The efficiency of infection, measured as GFP positivity, was evaluated by fluorescence-activated cell sorter (FACS) analysis, and it was consistently stable over months and >95%. Transfections of SK-N-BE(2) cells were performed using Lipofectamine (Gibco BRL).
Differentiation of PC12coRα cells and apoptosis detection.
PC12coRα cells were plated onto 60-mm-diameter polylysine-coated dishes in 1% horse serum at a density of 10,000 cells/dish in the absence or presence of glial cell line-derived neurotrophic factor (GDNF) (1 to 100 ng/ml). Every second day, the cells were examined under light microscopy and fresh medium and growth factors were added.
Neurite outgrowth was quantitated by scoring the number of cells with neuritis longer than the size of two cell bodies. Results were expressed as increases (n-fold) in percentage with respect to unstimulated control cells. The statistical analysis was performed on the basis of the results of three experiments, each of them done in triplicate.
Apoptosis was measured by FACS analysis of propidium iodide-stained cells.
Immunoprecipitation, in vitro binding assay, and Far Western experiments.
Cell cultures were serum starved for 24 h and treated with EGF (100 ng/ml), GDNF (1 to 100 ng/ml), nerve growth factor (NGF) (50 ng/ml), or neurturin (NTN) (100 ng/ml). The cells were then lysed at 4°C in ice-cold lysis buffer (27, 33). For immunoprecipitations, clarified lysates were incubated with polyclonal antibodies against p85, Ret, the CH1 regions of Rai and Shc, or the monoclonal anti-EGFR antibody (27, 33). For in vitro binding assays, total protein lysates (1 mg) were incubated for 2 h at 4°C with 10 μg of the appropriate GST fusion protein. For Far Western experiments, blots were blocked in 2% bovine serum albumin (BSA) in TTBS (20 mM Tris-HCl [pH 7.5], 150 mM NaCl, 0.05% Tween 20) for 2 h at room temperature and then incubated with reduced glutathione (3 μM) in TTBS with 0.5% BSA (wt/vol) for 1 h at room temperature. Blots were then incubated with the GST p85/N-SH2 or GST p85/C-SH2 proteins (10 nM) in TTBS in the presence of reduced glutathione and BSA. After extensive washing in TTBS, blots were detected with the affinity-purified anti-GST antibody.
SDS-PAGE and Western blotting.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting were performed according to established procedures. The following monoclonal antibodies were used: anti-EGFR (33), anti-pTyr (Upstate Biotechnology), anti-vinculin (Sigma), and anti-ErkP (Cell Signaling). The polyclonal antibodies anti-CH1Rai, anti-SH2Rai (27), anti-CH1Shc (27), anti-p85 (Upstate Biotechnology), anti-Ret (32), anti-AktP (Ser437; New England Biolabs), anti-Akt (New England Biolabs), anti-Erks (Santa Cruz Biotechnology), and anti-Grb2(Santa Cruz Biotechnology) were also used. Densitometry measurements of both total and phosphoprotein-specific Erks immunoblots were performed by PhosphorImager analysis (Typhoon 8600; Molecular Dynamics).
In vitro PI3K assay.
Lysate (300 μg) was immunoprecipitated with anti-p85 antibodies. The kinase reaction was performed for 30 min at room temperature in the PI3K buffer (20 mM Tris-HCl [pH 7.5], 100 mM NaCl, 0.5 mM EGTA [pH 8], 25 mM MgCl2, 10 μCi of [γ-32P]ATP, 1 μg of phosphatidylinositol) and terminated by the addition of HCl. Thin-layer chromatography was conducted on Whatman silica gel plates in a closed thin-layer chromatography tank with methanol-chloroform-water-ammonia (47:60:11.3:2 [vol/vol/vol/vol]) as a buffer. The plates were processed for autoradiography, and the level of incorporation of radioactive ATP was quantitated with a phosphorimager.
In vitro kinase assay.
Ret proteins were immunoprecipitated from Cos cells transiently transfected with Ret/MEN2A or RETY905F constructs. The immunoprecipitated Ret proteins were suspended in a kinase buffer (40 mM HEPES-KOH [pH 8], 40 mM potassium glutamate, 1 mM EGTA, 0.5 mM EDTA, 8 mM magnesium acetate, 2 mM dithiothreitol, 10 mM sodium fluoride) with radiolabeled [γ-32P]ATP and 3 μg of MBP (Sigma Chemical Co.) or immunoprecipitated Rai proteins derived from lysates of Cos cells transiently transfected with Rai or RaiFFF cDNAs. The kinase reaction was carried out for 25 min in a 30°C water bath and was terminated by adding SDS sample buffer with 2-mercaptoethanol. The proteins were resolved on 12% denaturing gels.
RESULTS
Rai is a physiological substrate of the Ret TK receptor.
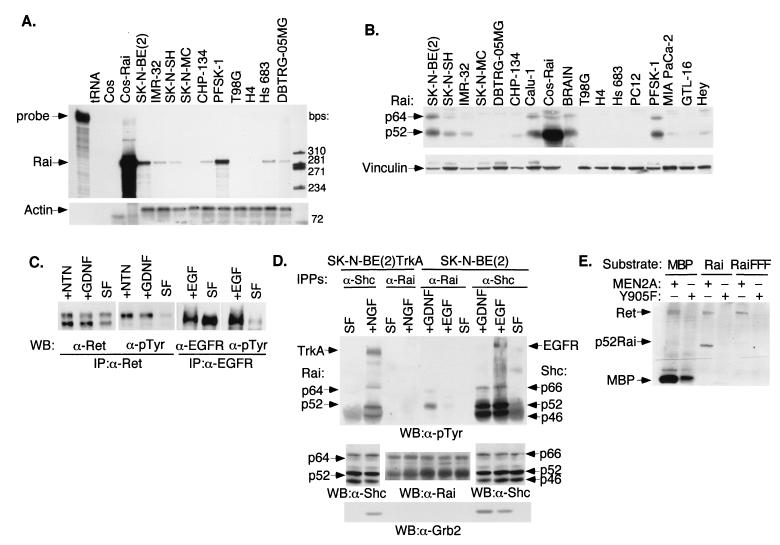
To investigate the role of Rai in receptor signal transduction pathways, we first screened cell lines of different neural origins for expression of endogenous Rai and RTKs. The SK-N-BE(2) neuroblastoma cell line was selected for further experiments because it expressed high levels of Rai transcripts (by RNase protection) (Fig. 1A) and protein (by Western blotting) (Fig. 1B) as well as functional Ret and EGFR (as demonstrated by ligand-induced receptor autophosphorylation) (Fig. 1C). In the case of Ret, as expected, only the fully glycosylated mature 170-kDa protein product was phosphorylated by GDNF or NTN treatments (Fig. 1C).
FIG. 1.
Expression pattern of Rai in neuronal cell lines and phosphorylation by activated Ret and EGFR. (A) RNase protection analysis of Rai expression in different tumor cell lines using 10 μg of total RNA and Rai (SH2 domain) (upper panel) or actin (lower panel) antisense probes. First lane, intact Rai probe; tRNA, tRNA used as a negative control; Cos-Rai, Cos cells transiently transfected with the p52Rai cDNA and used as a positive control. The specific Rai- and actin-protected fragments used are indicated above the remaining lanes. (B) Western blot analysis of Rai expression with 50 μg of whole-cell lysates from the indicated cell lines and polyclonal antibodies against the Rai CH1 region (upper panel) or vinculin for normalization (lower panel). The cell lines used in panels A and B were SK-N-BE(2), SK-N-SH, IMR-32, and CHP-134 (neuroblastomas); SK-N-MC (neuroepithelioma); PFSK-1 (primitive neuroectodermal tumor); DBTRG-05MG and T98G (glioblastomas); Hs 683 (glioma); H4 (neuroglioma); Calu-1 (lung carcinoma); Hey (ovarian carcinoma); MIA PaCa-2 (pancreatic carcinoma); GTL-16 (gastric carcinoma); and PC12 (phaeochromocytoma). Intact mouse brains (BRAIN) were also used. (C) Expression of functional Ret and EGFR in SK-N-BE(2) cells. Lysates from serum-starved (SF) cells (24 h) stimulated for 5 min with 100 ng of GDNF/ml (+GDNF), 100 ng of NTN/ml (+NTN), or 100 ng of EGF/ml (+EGF) were immunoprecipitated with anti-Ret (α-Ret) or anti-EGFR (α-EGFR) antibodies and immunoblotted with anti-Ret, anti-EGFR, and anti-pTyr (α-pTyr) antibodies, as indicated. (D) SK-N-BE(2) cells expressing endogenous Shc or Rai proteins and SK-N-BE(2)TrkA cells (engineered to express TrkA) were serum starved (24 h) (SF) and stimulated for 5 min with 100 ng of EGF/ml (+EGF), 100 ng of GDNF/ml (+GDNF), or 100 ng of NGF/ml (+NGF). Total lysates were immunoprecipitated with antibodies against the CH1 region of Shc (α-Shc) or Rai (α-Rai) and immunoblotted with antibodies against pTyr (α-pTyr) (upper panel), Shc or Rai (middle panel), or Grb2 (α-Grb2) (lower panel). Activated receptors and Grb2, Shc, and Rai polypeptides are indicated by arrows. (E) Cos cells were transiently transfected with the expression plasmids encoding Ret MEN2A, Ret Y905F, Rai, or Rai FFF. The in vitro kinase assay was performed with immunoprecipitated Ret proteins (MEN2A and Y905F) and immunoprecipitated Rai proteins (Rai and Rai FFF) as the substrates. As a positive control, we used the synthetic substrate MBP (myelin basic protein). IP, immunoprecipitate; WB, Western blot; +, present; −, absent.
To determine whether Rai is involved in EGFR and Ret signaling in SK-N-BE(2) cells, we analyzed anti-Rai immunoprecipitates by anti-phosphotyrosine blotting before and after 10 min of treatment with either GDNF (100 ng/ml) or EGF (100 ng/ml). Activation of Ret, but not EGFR, resulted in tyrosine phosphorylation of the p52 and p64 Rai proteins (Fig. 1D). No Rai phosphorylation was detected following EGF treatment even after longer exposures (up to 6 h) (data not shown). An in vitro kinase assay with immunoprecipitated Ret and Rai proteins confirmed that Rai is a specific substrate of Ret (Fig. 1E).
Similar experiments were performed in SK-N-BE(2) cells engineered to express the NGF receptor (TrkA). As in the case of EGF, NGF treatment failed to induce detectable tyrosine phosphorylation of the Rai proteins (Fig. 1D). Notably, EGF, GDNF, or NGF induced tyrosine phosphorylation of the cognate Shc proteins in the same cells (under identical experimental conditions) (Fig. 1D). It appears, therefore, that Rai, at variance with Shc, is not a common substrate of receptor TKs and that, of the analyzed receptors, it is activated specifically by Ret. We cannot exclude, however, that Rai functions as an adaptor protein for other activated receptors. Although we were unable to demonstrate phosphorylation of endogenous Rai proteins following EGF or NGF treatment of neural cells, we observed Rai phosphorylation in Cos cells transiently transfected with Rai and treated with pharmacological (100 ng/ml) doses of EGF (unpublished results) or cotransfected with Rai and TrkA and treated with NGF (unpublished results). Similarly, phosphorylation of overexpressed Rai has been recently reported in PC12 cells treated with NGF (23). Therefore, it is possible that, under specific physiological conditions, Rai may function as a substrate of activated growth factor receptors other than Ret.
Rai expression enhances the effects of activated Ret on survival and differentiation of PC12 cells.
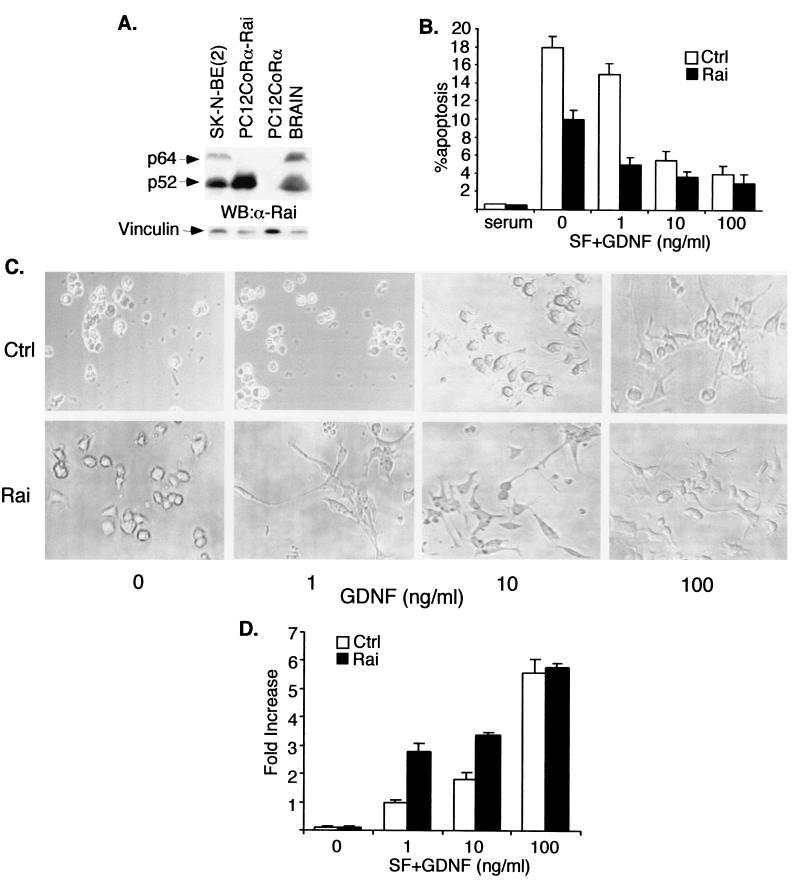
To investigate its function in the Ret signaling pathway, Rai was ectopically expressed in PC12coRα cells. PC12 is a well-established model system for studying differentiation and survival in a neuronal cell setting. Furthermore, PC12 cells do not express Rai (Fig. 1B), thereby allowing analysis of the effects of its ectopic expression. Although parental PC12 cells express Ret, they respond poorly to GDNF, due to the absence of GFRα1 (coRα), a glycosyl phosphatidylinositol-linked GDNF receptor that is necessary for the activation of Ret by GDNF (15). Stable expression of GFRα1 in PC12 cells (PC12coRα cells) rendered cells responsive to GDNF, as revealed by Ret autophosphorylation following GDNF (data not shown). We selected Rai-transduced PC12coRα clones with levels of overexpressed protein comparable to those of endogenous Rai of SK-N-BE(2) cells or adult mouse brain (Fig. 2A).
FIG. 2.
Ectopic Rai expression increases Ret-induced survival and differentiation of PC12coRα cells. (A) Western blot (WB) analysis of Rai expression with 50 μg of whole-cell lysates from the indicated cell lines and polyclonal antibodies against the Rai CH1 region (upper panel) or vinculin for normalization (lower panel). α-Rai, anti-Rai antibodies. (B) The percentage of apoptosis was measured in PC12coRα cells infected with either the Pinco retrovirus expressing Rai (Rai) or the empty vector (Ctrl) and maintained for 48 h in complete medium (serum), SF medium (0), or SF medium supplemented with GDNF at concentrations of 1, 10, and 100 ng/ml. The percentage of apoptotic cells was measured by FACS analysis of phosphatidylinositol-stained nuclei. Values shown are the means (± standard errors) of three independent experiments, each performed in triplicate. (C) Morphology of control (Ctrl) and Rai-expressing (Rai) PC12coRα cells grown in medium containing 1% serum and supplemented with different concentrations of GDNF (0, 1, 10, 100 ng/ml). Photographs were taken 7 days after the addition of GDNF. These results are representative of three experiments which gave comparable results. (D) Neurite outgrowth was quantitated by counting the cells with neurites longer than the size of two cell bodies. Results were expressed as the increase in percentage (n-fold) with respect to that of unstimulated control cells.
Serum starvation of PC12coRα cells led to a loss of cell viability after 48 h, due to induction of apoptosis (Fig. 2B). High doses (100 ng/ml) of GDNF partially reversed this response, leading to the survival of serum-starved cells (Fig. 2B) and allowing their terminal differentiation (Fig. 2C). In contrast, treatment with low doses (1 ng/ml) of GDNF had only a marginal effect on survival (Fig. 2B) and differentiation (Fig. 2C and D) of PC12coRα cells. Notably, the expression of Rai-sensitized PC12coRα cells had effects similar to those of low doses (1 ng/ml) of GDNF on both survival (Fig. 2B) and differentiation (Fig. 2C and D). Intermediate doses of GDNF (10 ng/ml) almost completely suppressed serum starvation-induced apoptosis of PC12coRα cells (Fig. 2B) but failed to induce terminal differentiation (Fig. 2C and D), in the absence of Rai. In contrast, neurite outgrowth was easily detected in the PC12coRα cells expressing Rai following intermediate doses of GDNF (Fig. 2C and D).
These results suggest that Rai is a functional substrate of Ret and that it enhances the survival and differentiable effects of Ret in neuronal cells. Interestingly, we noticed that survival of serum-starved PC12coRα cells expressing Rai was slightly, yet consistently, higher than that of control cells (Fig. 2B), suggesting that Rai exerts a slightly inhibitory effect on apoptosis of PC12 cells even in the absence of GDNF treatment and Ret activation. This putative function of Rai in established neuronal cells parallels those previously reported in central nervous system progenitor cells obtained from embryonic-day-14 rat striatum and grown in a minimal medium (5).
Rai does not bind Grb2 and increases late (GDNF-induced) MAPK activation.
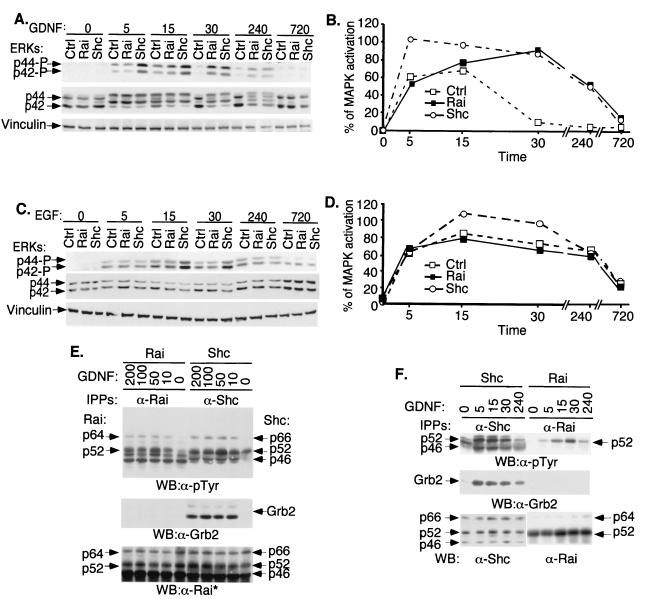
To investigate the molecular mechanisms underlying the effects of Rai on survival and differentiation, we first analyzed its ability to activate the Ras signaling pathway by measuring the extent and the kinetics of MAPK activation in Shc- or Rai-overexpressing cells following GDNF or EGF treatment. In the GDNF-treated control SK-N-BE(2) cells, phosphorylated p44 and p42 Erk2 were detected at 5 and 15 min following treatment while they were almost undetectable after 30 min (Fig. 3A and B). Under the same experimental conditions, Shc overexpression induced increased MAPK activation at both early (5 and 15 min) and later (30 min) time points. Rai overexpression, instead, had no effects on the early peak (5 and 15 min) of MAPK activation, whereas it increased activation at later time points (30 min) (Fig. 3A and B). No effect of Rai overexpression was, instead, observed on EGF-induced MAPK activation (Fig. 3C and D).
FIG. 3.
Tyrosine phosphorylation, Grb2 binding, and effects on MAPK activation of Rai proteins. (A) SK-N-BE(2) cells infected with the empty retroviral vector (Ctrl) or with vectors expressing Shc (Shc) or Rai (Rai) were serum deprived (24 h) and then stimulated for different time periods (0, 5, 15, 30, 240, or 720 min) with 100 ng of GDNF/ml. Lysates were analyzed by Western blotting with antibodies against phosphorylated ERKs (upper panel), anti-ERK antibodies (middle panel), or vinculin (lower panel). (B) The quantitation of the MAPK activation results shown in panel A was determined as described in Materials and Methods. (C) The same cells described for panel A were serum deprived (24 h) and then stimulated for different time periods (0, 5, 15, 30, 240, or 720 min) with 100 ng of EGF/ml. Lysates were analyzed by Western blotting with antibodies against phosphorylated ERKs (upper panel), anti-ERK antibodies (middle panel), or vinculin (lower panel). (D) The quantitation of the MAPK activation results shown in panel C was determined as described in Materials and Methods. (E) SK-N-BE(2) cells overexpressing Rai (Rai) or Shc (Shc) were serum starved (0) and then stimulated for 5 min with different doses of GDNF as indicated (in nanograms/milliliter) and lysed. Specific anti-Rai (anti-CH1 antibodies) (α-Rai) or anti-Shc (anti-CH1 antibodies) (α-Shc) immunoprecipitates (IPPs) were analyzed by Western blotting (WB) with anti-pTyr (α-pTyr) (upper panel), anti-Grb2 (α-Grb2) (middle panel), or anti-Rai (anti-SH2) (lower panel) antibodies. This anti-Rai antibody (anti-SH2) also recognizes overexpressed Shc polypeptides. (F) Specific anti-Rai (anti-CH1 antibodies) or anti-Shc (anti-CH1 antibodies) immunoprecipitates derived from the lysates of the experiment described for panel A were analyzed by Western blotting with anti-pTyr (upper panel), anti-Grb2 (middle panel), or anti-Rai (anti-CH1) and anti-Shc (anti-SH2) antibodies (lower panel).
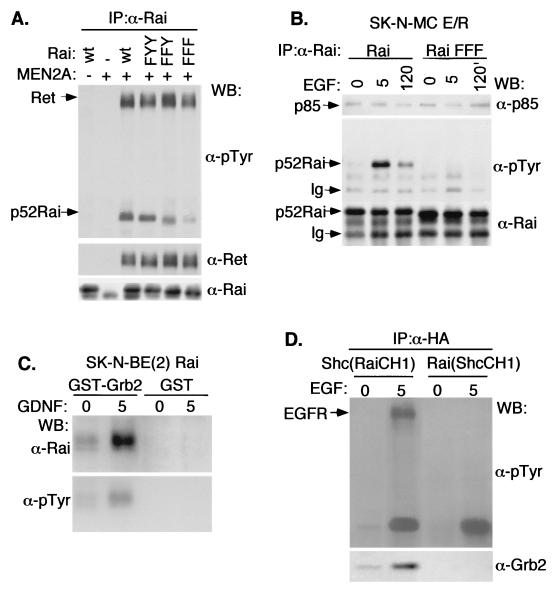
To investigate the mechanisms underlying the different effects of Shc and Rai on MAPK activation, we evaluated the ability of tyrosine-phosphorylated Rai proteins to form stable complexes with Grb2, measuring levels of Grb2-Rai association in vivo. Lysates from SK-N-BE(2) cells overexpressing either Rai or Shc and treated with different concentrations of GDNF were immunoprecipitated with an antibody specific for Rai or Shc, respectively, and analyzed by Western blotting with anti-Grb2 antibodies. While the Shc-Grb2 complexes were easily detected, we did not observe Rai-Grb2 complexes (Fig. 3E, middle panel). This is likely not due to low stoichiometry of Rai phosphorylation, since the amounts of immunoprecipitated tyrosine-phosphorylated Shc and Rai polypeptides were comparable (Fig. 3E, upper and lower panels). We then analyzed the kinetics of Shc/Rai tyrosine phosphorylation and Grb2-binding following GDNF treatment of SK-N-BE(2) cells (Fig. 3F). In the Shc-overexpressing cells, we observed similar kinetics of Shc phosphorylation and Shc-Grb2 complex formation (with a peak at 5 min and a slow subsequent decline). In the case of Rai-overexpressing cells, instead, Rai phosphorylation peaked at 30 min and no Rai-Grb2 complex was detected at any time point (Fig. 3F). Similarly, GDNF-induced Shc-Grb2, but not Rai-Grb2, complexes were detected in the SK-N-BE(2) cells expressing endogenous Shc and Rai (Fig. 1D). The inability of Rai to bind Grb2 is surprising in light of the high conservation in Rai of the Shc consensus sequences for Grb2 binding (corresponding to Y231, Y232, and Y304 in Rai) (27). Therefore, we investigated whether the Rai Y231, Y232, and Y304 residues are sites of Ret-dependent phosphorylation and Grb2 binding. Tyrosine-to-phenylalanine mutations were introduced at single (Y304; FYY mutant), double (Y231 and Y232; FFY mutant), and triple (Y304, Y231, and Y232; FFF mutant) sites of p52Rai. The resulting mutants were cotransfected in Cos cells together with activated Ret (Ret-MEN2A). The use of this mutant, rather than the wild-type receptor, circumvents the necessity to cotransfect the GFRα coreceptor and confines the analysis to Ret-mediated signaling. Indeed, the C634R MEN2A mutation induces Ret activation through disulfide-linked receptor dimerization (32). Protein lysates were immunoprecipitated with specific anti-Rai antibodies and analyzed by anti-phosphotyrosine blots. Rai tyrosine phosphorylation decreased in the FYY and FFY mutants and almost disappeared in the FFF mutant (Fig. 4A). Similar results were obtained in SK-N-MC cells expressing the chimeric E/R receptor and transfected with the parental or the phosphorylation-defective FFF Rai cDNAs (Fig. 4B). In these cells, the extracellular domains of the EGFR are fused to the cytoplasmic portion of Ret, thereby allowing the activation of the Ret signaling pathway by EGF (33). Furthermore, the use of this artificial construct allows analysis of Ret independent of autonomous Ret signals initiated by GFRα1, when cells are triggered by GDNF (38). EGF stimulation induced tyrosine phosphorylation of the wild-type, but not of the FFF, Rai protein (Fig. 4B), thereby suggesting that, as for the Shc proteins, Y231, Y232, and Y304 are the major Rai tyrosine phosphorylation sites. These results differ from those of a previous report, which showed that NGF treatment induces phosphorylation of overexpressed Rai only at the Y304 site, possibly due to differences in the experimental systems used (GDNF versus NGF and endogenous versus overexpressed Rai proteins) (23).
FIG. 4.
The Rai Y231, Y232, and Y304 residues are sites of Ret-dependent phosphorylation. (A) Cos cells were cotransfected with cDNAs encoding activated Ret (Ret-MEN2A), phosphorylation-defective Rai mutants, or the wild-type (wt) p52Rai protein. Lysates from serum-starved cells were immunoprecipitated with antibodies against the CH1 region of Rai and visualized by immunoblotting with anti-pTyr (α-pTyr) (upper panel), anti-Ret (α-Ret) (middle panel), or anti-Rai (α-Rai) (lower panel) antibodies. The differences in electrophoretic mobility of the various Rai mutants were consistently observed and remain unexplained. (B) SK-N-MC E/R cells stably expressing the parental (Rai) or the FFF (Rai FFF) Rai proteins were serum starved, stimulated with 100 ng of EGF/ml, and lysed at the indicated times (0, 5, or 120 min). Lysates were immunoprecipitated with anti-Rai (anti-CH1) antibodies and detected by immunoblotting with anti-p85 (α-p85) (upper panel), anti-pTyr (middle panel), or anti-Rai (lower panel) antibodies. Ig, cross-reactive immunoglobulins. (C) In vitro binding experiment with immobilized GST or GST-Grb2 (full-length Grb2) and lysates from SK-N-BE(2) cells overexpressing Rai that were serum starved (0 min) or stimulated (5 min) with 100 ng of GDNF/ml. Bound proteins were analyzed by Western blotting (WB) with anti-Rai (anti-CH1) (upper panel) and anti-pTyr (lower panel) antibodies. (D) Shc−/− mouse embryonic fibroblasts were transiently transfected with cDNAs encoding HA-tagged Shc (RaiCH1) and Rai (ShcCH1) chimeric proteins that were serum starved and stimulated with 100 ng of EGF/ml for 0 or 5 min. Anti-HA immunoprecipitates were analyzed by immunoblotting with anti-pTyr (upper panel) and anti-Grb2 (lower panel) antibodies.
We then measured the ability of a GST-Grb2 recombinant protein to interact with phosphorylated Rai. In vitro binding experiments revealed that the GST-Grb2 fusion protein bound phosphorylated Rai obtained from lysates of GDNF-treated SK-N-BE(2) cells overexpressing Rai (Fig. 4C). The discrepancy between in vivo and in vitro interaction data suggested that phosphorylated Rai has the potential to bind Grb2 but that, in vivo, binding is prevented by other factors, such as protein conformation or subcellular localization. To test this hypothesis, we swapped the CH1 regions of Rai and Shc and evaluated the potential of the resulting hybrid proteins to form stable complexes with Grb2. Notably, Shc hybrid proteins carrying the Rai CH1 region [Shc(RaiCH1)] maintained the ability to complex with Grb2, whereas the Rai hybrid carrying the Shc CH1 region [Rai(ShcCH1)] did not acquire the ability of Shc to bind Grb2 (Fig. 4D). It appears, therefore, that the tyrosine-phosphorylated Rai and Shc CH1 regions have equal potential to bind Grb2 but that the ability of Shc and Rai to form in vivo complexes with Grb2 depends on the specific molecular context (PTB and/or SH2 domains) of their CH1 regions. In conclusion, these data indicate that, although Rai becomes tyrosine-phosphorylated following Ret activation, it does not recruit the Grb2-SOS complex in vivo.
Rai expression increases PI3K activation and Akt phosphorylation by activated Ret.
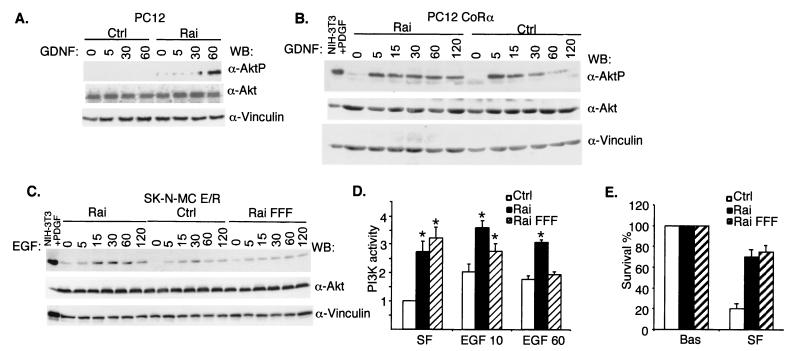
Activated RTKs promote membrane recruitment of PI3K, which then leads to the local formation of PI(3,4)P2 and PI(3,4,5)P3 (17). Akt is a kinase which, upon binding to PI(3,4)P2 and PI(3,4,5)P3, is phosphorylated by other serine/threonine kinases (PDK1 and PDK2) and, in turn, phosphorylates several substrates involved in the suppression of apoptosis (7). Thus, to investigate further the mechanisms of the anti-apoptotic function of Rai, we analyzed its effects on the Ret-mediated activation of the PI3K/Akt pathway by using three different cell systems: PC12 cells, PC12CoRα cells, and SK-N-MC neuronal precursors expressing an E/R chimera. We evaluated the effects of Rai on Akt activation by measuring the status of Akt phosphorylation with anti-phospho-Akt antibodies. Following Ret stimulation, ectopic expression of Rai protein induced Akt activation in PC12 cells (Fig. 5A). In PC12CoRα cells, Akt phosphorylation rapidly declined after 15 min of GDNF stimulation, and it remained elevated for 120 min in Rai-expressing cells (Fig. 5B). In SK-N-MC cells expressing the E/R chimera, Akt phosphorylation was potentiated by Rai expression (Fig. 5C). Interestingly, also in the absence of added growth factors, Rai expression slightly increased basal phospho-Akt levels in PC12, PC12CoRα, and SK-N-MC E/R cells (Fig. 5A to C).
FIG. 5.
Rai expression increases activation of PI3K/Akt by Ret. PC12 (A), PC12coRα (B), and SK-N-MC E/R (C) cells were infected with the empty vector (Ctrl) or with the vector containing Rai (Rai), serum starved, and treated with GDNF (100 ng/ml for results shown in panel A and 1 ng/ml for results shown in panel B) or EGF (100 ng/ml for results shown in panel C) at the indicated time points (0, 5, 15, 30, 60, or 120 min). Lysates were analyzed for activated Akt with antibodies specific for the phosphorylated Ser-473 residue of Akt (α-AktP) (top panels). Anti-Akt (α-Akt) and anti-vinculin (α-vinculin) antibodies were used to normalize the amount of proteins loaded. WB, Western blot; PDGF, platelet-derived growth factor. (D) In vitro kinase assay of anti-p85 immunoprecipitates from SK-N-MC E/R cells serum starved (SF) and stimulated with 100 ng of EGF/ml. Equal amounts of PI3K were confirmed by immunoblotting p85 in the cell lysates. PI3K activity is expressed in arbitrary units. The data in the graphs are presented as the ratios between the values obtained at each condition and those of the control cells (Ctrl) prior to EGF treatment (SF). The data represent the averages (± standard errors) of five independent experiments. Variations of PI3K activity that scored statistically significant (by Student's t test) with respect to the control cells are indicated with stars. In particular, for the control cells, the P values were 0.02 for 10 min of treatment and 0.007 for 60 min of treatment. For Rai-overexpressing cells, the P values were 0.09 for 10 min of treatment and 0.27 for 60 min of treatment. For Rai-overexpressing cells versus control cells, the P values were 0.009 for SF conditions, 0.019 for 10 min of treatment, and 0.0014 for 60 min of treatment. (E) Percent survival was measured for PC12coRα cells infected with either the Pinco retrovirus expressing Rai (Rai), the empty vector (Ctrl), or Rai FFF (Rai FFF), which had been maintained for 48 h in SF medium. Survival was determined by trypan blue exclusion. Bas, growing cells before starvation.
Since Akt can be activated through both PI3K-independent and -dependent pathways (7), we evaluated whether the effects of Rai on Akt activation were mediated by PI3K. We performed PI3K enzymatic assays on SK-N-MC E/R cells stably expressing Rai and control cells. Cells were serum starved for 24 h and then treated with EGF (to induce Ret activation) for 10 or 60 min. Anti-PI3K (anti-p85) immunoprecipitates were then analyzed for their enzymatic activity with phosphatidylinositol as the substrate. As previously reported (1, 35), 10 to 60 min of Ret stimulation in SK-N-MC E/R cells (control) induced an approximately twofold increase in PI3K activity (Fig. 5D). Notably, under the same experimental conditions, an increase of PI3K activity of three- to fourfold was observed when the cells expressed Rai (Fig. 5D) in comparison to the control cells in serum-free (SF) conditions. The ability of overexpressed Rai to potentiate Ret-mediated PI3K activation was dependent on the integrity of Y231, Y232, and Y304, since the Rai FFF mutant, which is not phosphorylated upon Ret stimulation (Fig. 4B), was unable to do so (Fig. 5D). Notably, Rai overexpression potentiated PI3K activation (about 2.5-fold) also in the absence of Ret stimulation, and this ability was retained by the FFF mutant (Fig. 5D). It appears, therefore, that Rai functions as an antiapoptotic protein which potentiates both basal and Ret-dependent activation of PI3K and Akt. This model is consistent with the observed effects of Rai on Ret-induced neuronal differentiation, since activated PI3K can also promote differentiation in different neuronal cell systems, including GDNF-stimulated dopaminergic neurons (29).
Binding of Rai to the p85 subunit of PI3K.
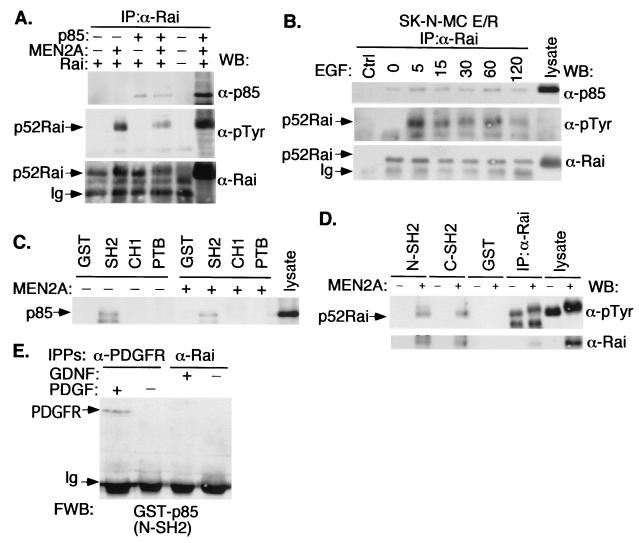
To study the mechanism(s) underlying the effects of Rai on PI3K, we first investigated whether Rai forms a stable complex with PI3K in vivo by using cells that had been transiently transfected with cDNAs encoding Rai, Ret-MEN2A, and the p85 subunit of PI3K. Anti-p85 Western blots of anti-Rai immunoprecipitates revealed the existence of a p85-Rai complex in both the presence and the absence of cotransfected Ret-MEN2A (Fig. 6A, upper panel). However, anti-phosphotyrosine blots of the same anti-Rai immunoprecipitates revealed the presence of phosphorylated Rai proteins only when Ret-MEN2A was cotransfected (Fig. 6A, middle panel). Similar experiments performed with SK-N-MC E/R cells revealed the existence of a stable Rai-p85 complex (Fig. 6B, upper panel) both in SF conditions (where Rai is hypophosphorylated) and following Ret activation by EGF (where Rai is hyperphosphorylated) (Fig. 6B, middle panel). Though these experiments were performed with overexpressed proteins, the results suggest that the phosphorylated and hypophosphorylated Rai proteins form a constitutive complex with p85 in vivo.
FIG. 6.
Rai forms a complex with the p85 regulatory subunit of PI3K. (A) Cos cells cotransfected with p85, p52Rai, and Ret-MEN2A cDNAs were serum deprived, immunoprecipitated with antibodies against the CH1 region of Rai (α-Rai), and immunoblotted with antibodies against p85 (α-p85) (upper panel), p-Tyr (α-pTyr) (middle panel), and Rai (lower panel). WB, Western blot; Ig, immunoglobulin. (B) SK-N-MC E/R cells infected with the empty vector (Ctrl) or with Rai cDNA were serum starved and stimulated with EGF (100 ng/ml) for the indicated times (0, 5, 15, 30, 60, or 120 min). Lysates were immunoprecipitated (IP) with anti-Rai antibodies and immunoblotted with anti-p85 (upper panel), anti-pTyr (middle panel), and anti-Rai (lower panel) antibodies. (C) Lysates from serum-starved untransfected (−) or Ret-MEN2A-transfected (+) Cos cells overexpressing p85 were incubated with immobilized GST or the indicated Rai GST fusion proteins. GST-bound proteins were then visualized by Western blotting with anti-p85 antibodies. (D) Lysates from serum-starved untransfected (−) or Ret-MEN2A-transfected (+) Cos cells expressing Rai were incubated with immobilized GST or the indicated p85 GST fusion proteins. GST-bound proteins were then visualized with anti-pTyr (upper panel) or anti-Rai (lower panel) antibodies. (E) Lysates from serum-starved (−) or stimulated (+) (for 5 min with 100 ng of GDNF/ml or 50 ng of platelet-derived growth factor [PDGF]/ml) SK-N-BE(2) cells were immunoprecipitated with the indicated antibodies and analyzed by Far Western blotting (FWB) with the p85-N-SH2 GST fusion protein (see Materials and Methods). The identity of the band indicated with an arrow was assessed by subsequent reprobing of the filter with anti-PDGF receptor (α-PDGFR) antibodies (data not shown). Similar results were obtained with the p85-C-SH2 GST fusion protein. IPPs, immunoprecipitates.
We further investigated the mechanisms underlying the interaction of p85 with Rai by in vitro binding experiments. Rai GST-SH2 (but not Rai GST-PTB or GST-CH1) bound p85 from lysates of Cos cells transiently transfected with p85 cDNA in both the presence and the absence of coexpressed Ret-MEN2A (Fig. 6C). The N- and C-terminal SH2 domains of p85, instead, only interacted with hyperphosphorylated Rai following Ret stimulation (Fig. 6D). These results suggest that p85 can form a stable complex in vitro with both hypo- and hyperphosphorylated Rai polypeptides and that the molecular determinants of these interactions under the two conditions are different. To investigate whether Rai binds to p85 directly, anti-Rai immunoprecipitates from SK-N-BE(2) cells unstimulated and stimulated with GDNF were resolved by SDS-PAGE and blotted with GST-p85 protein. As shown in Fig. 6E, GST-p85 was unable to interact with immunoprecipitated Rai, suggesting that Rai binds p85 in vivo indirectly.
To investigate the biological role of Rai-tyrosine phosphorylation, we analyzed the capacity of the phosphorylation-defective Rai FFF mutant to stimulate PI3K activity, Akt phosphorylation, and survival of neuronal cells. In the absence of Ret activation (that is, under SF conditions), the Rai FFF mutant retained the capacity of the wild-type protein to activate PI3K (Fig. 5D) and Akt (Fig. 5C), to promote survival (Fig. 5E), and to interact with p85 (Fig. 4B). In contrast, following Ret stimulation, the levels of activation of PI3K (Fig. 5D) and Akt (Fig. 5C) were comparable in the cells not expressing Rai (controls) and in those expressing the Rai FFF mutant. It appears, therefore, that the effects of Rai expression on the PI3K/Akt pathway and on survival are based on two different mechanisms, one which is dependent on Ret activation and Rai phosphorylation and another which does not require Ret or Rai phosphorylation. In both cases, however, a stable Rai-p85 complex is formed.
Phosphorylation of the Ret-MEN2A tyrosine Y1062 residue is indispensable for recruitment and phosphorylation of Rai and for PI3K/Akt activation.
Mutation of the Ret-MEN2A Tyr 1062 residue (Y1062F) eliminates binding of Ret to p85 as well as Ret-mediated activation of PI3K and Akt (8, 35). Similarly, deletion of an asparagine residue close to Y1062 (ΔN1059) impairs Shc binding to Ret and the subsequent activation of downstream signaling pathways (11). Notably, asparagine 1059 has been found to be missing in patients affected by congenital megacolon or Hirschsprung's disease, a defect of development of the enteric nervous system which is caused by loss of function of the Ret receptor (11).
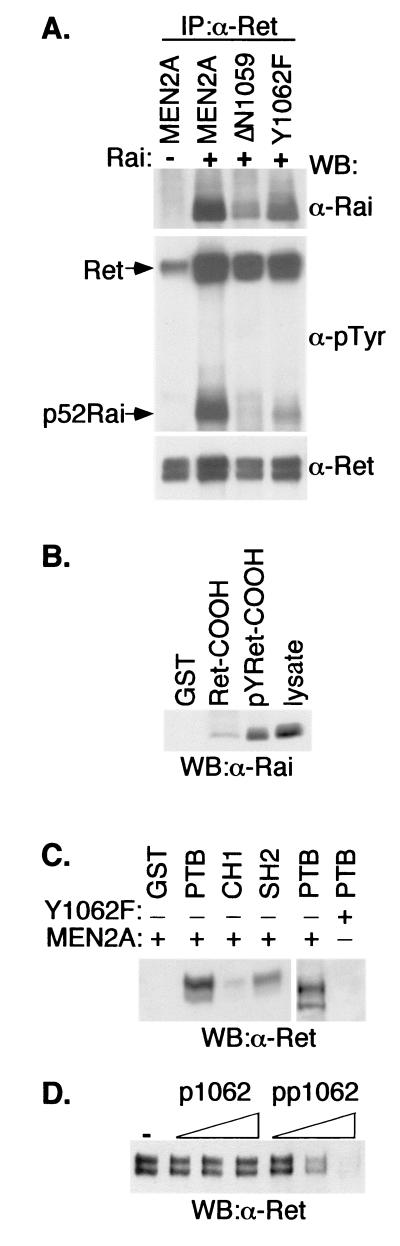
To explore the mechanisms through which Rai potentiates the effects of Ret on PI3K, we investigated whether Y1062 is involved in Rai phosphorylation and recruitment. The cDNAs for p52Rai and Ret-MEN2A (or its mutants) were transiently cotransfected into Cos cells, and the extent of Rai phosphorylation and Ret-Rai interaction was analyzed. Probing of anti-Ret immunoprecipitates with an anti-Rai antibody revealed the existence of a stable complex between the wild-type Ret-MEN2A protein and Rai (Fig. 7A, upper panel). Anti-phosphotyrosine staining of the same immunoprecipitates showed that the Ret-bound Rai polypeptides are phosphorylated (Fig. 7A, middle panel). Both the Y1062F and ΔN1059 mutations markedly reduced the degree of Rai phosphorylation and association with Ret-MEN2A, indicating that the Ret-MEN2A tyrosine 1062 residue is a docking site for Rai. This was confirmed by in vitro binding experiments, which showed the following. (i) A GST fusion protein of the cytoplasmic tail of Ret containing Y1062 (Ret-COOH) binds Rai in a pull-down experiment, and such binding is dependent on GST-Ret-COOH tyrosine phosphorylation (Fig. 7B). (ii) Rai binds Ret through its PTB and (to a lesser extent) SH2 domains, and the Y1062F Ret mutation prevents this binding (Fig. 7C). (iii) Progressively increasing amounts of a Ret peptide encompassing the phosphorylated tyrosine 1062 (pp1062) (but not the corresponding dephosphorylated peptide, p1062) compete with binding of the Rai-PTB domain to Ret (Fig. 7D). Together, these results suggest a model in which phosphorylated Rai functions as an adaptor molecule for the recruitment of PI3K to phosphorylated Ret tyrosine 1062.
FIG. 7.
Tyrosine 1062 of Ret-MEN2A is a docking site for Rai protein. (A) Lysates from serum-starved Cos cells transiently cotransfected with cDNAs encoding p52Rai and different Ret mutants were immunoprecipitated (IP) with anti-Ret antibodies and immunoblotted with either anti-Rai (α-Rai) (upper panel), anti-pTyr (α-pTyr) (middle panel), or anti-Ret (α-Ret) (lower panel) antibodies. WB, Western blot. (B) Serum-starved Cos cells transiently transfected with p52Rai were lysed and incubated with immobilized GST or the GST fusion proteins representative of unphosphorylated (Ret-COOH) or tyrosine-phosphorylated (pYRet-COOH) Ret. GST-bound proteins were revealed by using anti-Rai antibodies. (C) Cos cells were transfected with Ret-MEN2A or Ret-MEN2A Y1062F cDNAs and serum starved. Lysates were incubated with immobilized GST or the indicated Rai GST fusion proteins. Immunoblotting with anti-Ret antibodies revealed GST-bound proteins. +, present; −, absent. (D) Lysates from Cos cells transiently transfected with Ret-MEN2A and serum starved were incubated with Rai GST-PTB in the presence of increasing amounts of an unphosphorylated (p1062) or a tyrosine-phosphorylated (pp1062) peptide spanning Ret tyrosine 1062. GST-bound proteins were visualized with anti-Ret antibodies.
DISCUSSION
Apoptosis plays a major role in the nervous system, both in physiological and pathological conditions. During development, a large fraction of immature neurons undergoes apoptosis, which is triggered by deprivation of neurotrophic factors. In the adult brain, toxic insults from biochemical or genetic accidents might lead to neuronal apoptosis and neurodegenerative diseases (41). Neurons share the same basic survival programs with all other cell types. The survival of developing neurons depends on the availability of neurotrophic factors which specifically activate a number of neurotrophin receptors, including Trk and Ret. Activated Trk or Ret receptors stimulate survival of neural cells through the PI3K/Akt and the Ras-MAPK pathways. We report here that Rai proteins stimulate survival of neural cells both constitutively and upon Ret-mediated phosphorylation.
Mice lacking both Rai and Sli, but neither mutant alone, exhibit a significant loss of neurons within the SCG. Notably, the number of sympathetic SCG neurons in the double-mutant mice was reduced at birth and by 8 weeks postnatal, but it had been normal at embryonic day 13.5, which suggests a loss of postmitotic neurons during the later phase of embryonic development rather than a defect in neurogenesis (30). Since mutations of either TrkA or Ret result in a virtual elimination of SCG neurons, it has been suggested that Rai and Sli possess functionally redundant roles in the cytoplasmic propagation of survival signals from activated TrkA and/or Ret in SCG neurons (30). Here, we have demonstrated that in cultured neural cells, Rai is a physiological substrate of Ret and that its expression potentiates the survival effects of activated Ret. Therefore, one of the physiological functions of Rai appears to be that of transducing Ret-dependent survival signals in a subpopulation of sympathetic neurons. We cannot exclude, however, that Rai functions as an adaptor protein for other signaling pathways. Although we were unable to demonstrate phosphorylation of endogenous Rai proteins following EGF or NGF treatment of neural cells, Rai can become phosphorylated upon overexpression and activation of other receptors (this report and reference 23).
The PI3K/Akt pathway is an important regulator of neuronal survival in both central and peripheral populations. In the case of sympathetic neurons of the SCG, survival induced by NGF is critically dependent upon an intact PI3K pathway (6). The PI3K/Akt pathway is also essential for the transmission of Ret-dependent survival signals in neuronal cell cultures (8, 35). Here we show that Rai overexpression per se promotes PI3K/Akt activation and neuronal cell survival. These Rai activities are further potentiated by Ret triggering. Accordingly, we have also found that treatment of cells with a PI3K inhibitor blocks Rai prosurvival effects (data not shown).
The SH2 domain of Rai mediates a growth factor-independent physical interaction with p85. In turn, upon Ret triggering, the SH2 domain of p85 establishes a further contact with tyrosine-phosphorylated Rai proteins. Our data suggest that the p85-Rai interaction is not direct but is mediated by other adaptors.
It has been shown that Shc recruits PI3K to activated Ret receptors through the Grb2-Gab2 complex. In rat sympathetic neurons, PI3K can be recruited to Ret Tyr-1062 by Shc, through the Grb2-Gab2 complex. Gab2 is an adaptor protein that binds p85 (1, 14). Several results, however, argue against the possibility that the Rai-p85 interaction is also mediated by Shc proteins: (i) expression of Shc proteins is low or undetectable in postmitotic neurons (where Rai is highly expressed) (3) and (ii) a stable Ret-Rai complex is also formed in the absence of Shc protein expression (our unpublished results with fibroblasts derived from Shc-null embryos). Alternatively, Rai might recruit p85 through Gab2. Notably, we have preliminarily demonstrated that Rai forms a complex with Gab2 following Ret activation (unpublished results).
Experimental evidence points to phosphorylation of the Tyr-1062 residue of Ret as the key event for propagating PI3K/Akt activating signals (8, 35). This autophosphorylation site serves as a docking site for the recruitment of multiple proteins to activated Ret. Binding of both FRS2 and Shc to Tyr-1062 has been implicated in the assembly of two distinct complexes on Ret, respectively, one leading to Ras activation through recruitment of Grb2/SOS and another leading to the PI3K/Akt pathway through recruitment of Grb2/Gab-1 followed by p85 (1, 14, 20). In addition, the binding of IRS1 (19) and p62dok family members (13) to Ret Tyr-1062 leads primarily to PI3K or Ras activation, respectively. Here we show that Rai, mainly through its PTB domain, is another ligand of Tyr-1062 of Ret. In this frame, it is intriguing that a natural Ret mutant (ΔN1059) causing Hirschsprung's disease exerts a defective association with Rai. This suggests that a defect in the Ret-Rai pathway may contribute to the impaired development of enteric sympathetic neurons. Likewise, constitutive triggering of the Rai-PI3K pathway by oncogenic Ret mutants (Ret-MEN2A) may contribute to the neoplastic expansion of neural crest cells which are targeted by the MEN2 disease.
Our data propose a model whereby Rai proteins function as adaptors to facilitate recruitment of p85 by phosphorylated Tyr-1062 on activated Ret. According to this model, in response to GDNF treatment, a preexisting Rai/p85 complex is recruited to the phosphorylated Ret Tyr-1062 mainly through the Rai PTB domain. Upon receptor triggering, the stability of the p85-Rai interaction, which is mediated by the Rai SH2 domain in unstimulated cells, might be further increased via a direct contact of phosphorylated Rai with the p85 SH2 domains. This additional contact may also serve as a mechanism to modify the structure of the p85-p110 complex to potentiate p110 activation. Likely, another critical step in Ret-mediated potentiation of PI3K is the translocation of Rai-PI3K complexes from the cytosol to the plasma membrane where PI3K substrate lipids are located. The mechanisms through which the constitutive Rai-PI3K complex activates the PI3K/Akt pathway in the absence of receptor activation remain unexplained. Preliminary experiments from our laboratory indicate that treatment of neuronal cells with various toxic stimuli induces a redistribution of Rai proteins from the cytosol to other cellular compartments. Thus, it is possible that receptor-independent activation of p85 in Rai-expressing neuronal cells occurs through relocalization of the preexisting Rai-p85 complex.
Finally, we showed that Shc overexpression prolongs and potentiates the early peak (5 to 15 min) of Ras activation while Rai overexpression increases MAPK activation only at later time points (30 min). The two events (Shc- or Rai-induced MAPK activation) might be mechanistically unrelated, as only Shc proteins could be detected in stable complexes with Grb2. Since a number of published experiments suggest that formation of Shc-Grb2 complexes contribute to Ras activation, other mechanisms might be responsible for the ability of overexpressed Rai to increase late MAPK activation following GDNF treatment. Notably, recent findings suggest that PI3K activates the Ras-MAPK pathway, by acting both upstream of Ras and between Ras and ERK2 (40). In this report, inhibitors of PI3K are shown to suppress activation of both endogenous ERK2 and Ras in COS cells treated with physiological (1 to 2 ng/ml) doses of EGF. Therefore, the effect of Rai overexpression on the late peak of MAPK activation might be a consequence of its ability to increase PI3K activation following GDNF.
Thus, two different members of the Shc protein family, Shc and Rai, are recruited to the activated Ret receptor. Their association with Ret, mediated by the same site (e.g., Tyr-1062), is likely mutually exclusive. Therefore, Shc and Rai may regulate Ret signaling during different stages of neuronal development and elicit different functional consequences.
Acknowledgments
We thank B. Verducci-Galletti for excellent work in generating antibodies and plasmids, F. Dalla Valle for cell culture assistance, and S. Ronzoni for FACS analysis. We gratefully acknowledge M. Billaud for the Δ1059 Ret mutant, and V. Raker, A. Ventura, and K. Helin for helpful comments and critical readings of the manuscript.
This work was supported by grants from AIRC and EC.
REFERENCES
- 1.Besset, V., R. P. Scott, and C. F. Ibanez. 2000. Signaling complexes and protein-protein interactions involved in the activation of the Ras and phosphatidylinositol 3-kinase pathways by the c-Ret receptor tyrosine kinase. J. Biol. Chem. 275:39159-39166. [DOI] [PubMed] [Google Scholar]
- 2.Bonfini, L., E. Migliaccio, G. Pelicci, L. Lanfrancone, and P. G. Pelicci. 1996. Not all Shc's roads lead to Ras. Trends Biochem. Sci. 21:257-261. [PubMed] [Google Scholar]
- 3.Cattaneo, E., and P. G. Pelicci. 1998. Emerging roles for SH2/PTB-containing Shc adaptor proteins in the developing mammalian brain. Trends Neurosci. 21:476-481. [DOI] [PubMed] [Google Scholar]
- 4.Chardin, P., J. H. Camonis, N. W. Gale, L. van Aelst, J. Schlessinger, M. H. Wigler, and D. Bar-Sagi. 1993. Human Sos1: a guanine nucleotide exchange factor for Ras that binds to GRB2. Science 260:1338-1343. [DOI] [PubMed] [Google Scholar]
- 5.Conti, L., S. Sipione, L. Magrassi, L. Bonfanti, D. Rigamonti, V. Pettirossi, M. Peschanski, B. Haddad, P. Pelicci, G. Milanesi, G. Pelicci, and E. Cattaneo. 2001. Shc signaling in differentiating neural progenitor cells. Nat. Neurosci. 4:579-586. [DOI] [PubMed] [Google Scholar]
- 6.Crowder, R. J., and R. S. Freeman. 1998. Phosphatidylinositol 3-kinase and Akt protein kinase are necessary and sufficient for the survival of nerve growth factor-dependent sympathetic neurons. J. Neurosci. 18:2933-2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Datta, S. R., A. Brunet, and M. E. Greenberg. 1999. Cellular survival: a play in three Akts. Genes Dev. 13:2905-2927. [DOI] [PubMed] [Google Scholar]
- 8.De Vita, G., R. M. Melillo, F. Carlomagno, R. Visconti, M. D. Castellone, A. Bellacosa, M. Billaud, A. Fusco, P. N. Tsichlis, and M. Santoro. 2000. Tyrosine 1062 of RET-MEN2A mediates activation of Akt (protein kinase B) and mitogen-activated protein kinase pathways leading to PC12 cell survival. Cancer Res. 60:3727-3731. [PubMed] [Google Scholar]
- 9.Fagan, A. M., H. Zhang, S. Landis, R. J. Smeyne, I. Silos-Santiago, and M. Barbacid. 1996. TrkA, but not TrkC, receptors are essential for survival of sympathetic neurons in vivo. J. Neurosci. 16:6208-6218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ganju, P., J. P. O'Bryan, C. Der, J. Winter, and I. F. James. 1998. Differential regulation of SHC proteins by nerve growth factor in sensory neurons and PC12 cells. Eur. J. Neurosci. 10:1995-2008. [DOI] [PubMed] [Google Scholar]
- 11.Geneste, O., C. Bidaud, G. De Vita, R. M. Hofstra, S. Tartare-Deckert, C. H. Buys, G. M. Lenoir, M. Santoro, and M. Billaud. 1999. Two distinct mutations of the RET receptor causing Hirschsprung's disease impair the binding of signalling effectors to a multifunctional docking site. Hum. Mol. Genet. 8:1989-1999. [DOI] [PubMed] [Google Scholar]
- 12.Grignani, F., T. Kinsella, A. Mencarelli, M. Valtieri, D. Riganelli, L. Lanfrancone, C. Peschle, G. P. Nolan, and P. G. Pelicci. 1998. High-efficiency gene transfer and selection of human hematopoietic progenitor cells with a hybrid EBV/retroviral vector expressing the green fluorescence protein. Cancer Res. 58:14-19. [PubMed] [Google Scholar]
- 13.Grimm, J., M. Sachs, S. Britsch, S. Di Cesare, T. Schwarz-Romond, K. Alitalo, and W. Birchmeier. 2001. Novel p62dok family members, dok-4 and dok-5, are substrates of the c-Ret receptor tyrosine kinase and mediate neuronal differentiation. J. Cell Biol. 154:345-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayashi, H., M. Ichihara, T. Iwashita, H. Murakami, Y. Shimono, K. Kawai, K. Kurokawa, Y. Murakumo, T. Imai, H. Funahashi, A. Nakao, and M. Takahashi. 2000. Characterization of intracellular signals via tyrosine 1062 in RET activated by glial cell line-derived neurotrophic factor. Oncogene 19:4469-4475. [DOI] [PubMed] [Google Scholar]
- 15.Jing, S., D. Wen, Y. Yu, P. L. Holst, Y. Luo, M. Fang, R. Tamir, L. Antonio, Z. Hu, R. Cupples, J. C. Louis, S. Hu, B. W. Altrock, and G. M. Fox. 1996. GDNF-induced activation of the ret protein tyrosine kinase is mediated by GDNFR-alpha, a novel receptor for GDNF. Cell 85:1113-1124. [DOI] [PubMed] [Google Scholar]
- 16.Lai, K. M., and T. Pawson. 2000. The ShcA phosphotyrosine docking protein sensitizes cardiovascular signaling in the mouse embryo. Genes Dev. 14:1132-1145. [PMC free article] [PubMed] [Google Scholar]
- 17.Leevers, S. J., B. Vanhaesebroeck, and M. D. Waterfield. 1999. Signalling through phosphoinositide 3-kinases: the lipids take centre stage. Curr. Opin. Cell Biol. 11:219-225. [DOI] [PubMed] [Google Scholar]
- 18.Luzi, L., S. Confalonieri, P. P. Di Fiore, and P. G. Pelicci. 2000. Evolution of shc functions from nematode to human. Curr. Opin. Genet. Dev. 10:668-674. [DOI] [PubMed] [Google Scholar]
- 19.Melillo, R. M., F. Carlomagno, G. De Vita, P. Formisano, G. Vecchio, A. Fusco, M. Billaud, and M. Santoro. 2001. The insulin receptor substrate (IRS)-1 recruits phosphatidylinositol 3-kinase to Ret: evidence for a competition between Shc and IRS-1 for the binding to Ret. Oncogene 20:209-218. [DOI] [PubMed] [Google Scholar]
- 20.Melillo, R. M., M. Santoro, S. H. Ong, M. Billaud, A. Fusco, Y. R. Hadari, J. Schlessinger, and I. Lax. 2001. Docking protein FRS2 links the protein tyrosine kinase RET and its oncogenic forms with the mitogen-activated protein kinase signaling cascade. Mol. Cell. Biol. 21:4177-4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Migliaccio, E., M. Giorgio, S. Mele, G. Pelicci, P. Reboldi, P. P. Pandolfi, L. Lanfrancone, and P. G. Pelicci. 1999. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature 402:309-313. [DOI] [PubMed] [Google Scholar]
- 22.Migliaccio, E., S. Mele, A. E. Salcini, G. Pelicci, K. M. Lai, G. Superti-Furga, T. Pawson, P. P. Di Fiore, L. Lanfrancone, and P. G. Pelicci. 1997. Opposite effects of the p52shc/p46shc and p66shc splicing isoforms on the EGF receptor-MAP kinase-fos signalling pathway. EMBO J. 16:706-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakamura, T., M. Komiya, N. Gotoh, S. Koizumi, M. Shibuya, and N. Mori. 2002. Discrimination between phosphotyrosine-mediated signaling properties of conventional and neuronal Shc adapter molecules. Oncogene 21:22-31. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura, T., S. Muraoka, R. Sanokawa, and N. Mori. 1998. N-Shc and Sck, two neuronally expressed Shc adapter homologs. Their differential regional expression in the brain and roles in neurotrophin and Src signaling. J. Biol. Chem. 273:6960-6967. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura, T., R. Sanokawa, Y. Sasaki, D. Ayusawa, M. Oishi, and N. Mori. 1996. N-Shc: a neural-specific adapter molecule that mediates signaling from neurotrophin/Trk to Ras/MAPK pathway. Oncogene 13:1111-1121. [PubMed] [Google Scholar]
- 26.O'Bryan, J. P., Z. Songyang, L. Cantley, C. J. Der, and T. Pawson. 1996. A mammalian adaptor protein with conserved Src homology 2 and phosphotyrosine-binding domains is related to Shc and is specifically expressed in the brain. Proc. Natl. Acad. Sci. USA 93:2729-2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pelicci, G., L. Dente, A. De Giuseppe, B. Verducci-Galletti, S. Giuli, S. Mele, C. Vetriani, M. Giorgio, P. P. Pandolfi, G. Cesareni, and P. G. Pelicci. 1996. A family of Shc related proteins with conserved PTB, CH1 and SH2 regions. Oncogene 13:633-641. [PubMed] [Google Scholar]
- 28.Pelicci, G., L. Lanfrancone, F. Grignani, J. McGlade, F. Cavallo, G. Forni, I. Nicoletti, T. Pawson, and P. G. Pelicci. 1992. A novel transforming protein (SHC) with an SH2 domain is implicated in mitogenic signal transduction. Cell 70:93-104. [DOI] [PubMed] [Google Scholar]
- 29.Pong, K., R. Y. Xu, W. F. Baron, J. C. Louis, and K. D. Beck. 1998. Inhibition of phosphatidylinositol 3-kinase activity blocks cellular differentiation mediated by glial cell line-derived neurotrophic factor in dopaminergic neurons. J. Neurochem. 71:1912-1919. [DOI] [PubMed] [Google Scholar]
- 30.Sakai, R., J. T. Henderson, J. P. O'Bryan, A. J. Elia, T. M. Saxton, and T. Pawson. 2000. The mammalian ShcB and ShcC phosphotyrosine docking proteins function in the maturation of sensory and sympathetic neurons. Neuron 28:819-833. [DOI] [PubMed] [Google Scholar]
- 31.Salcini, A. E., J. McGlade, G. Pelicci, I. Nicoletti, T. Pawson, and P. G. Pelicci. 1994. Formation of Shc-Grb2 complexes is necessary to induce neoplastic transformation by overexpression of Shc proteins. Oncogene 9:2827-2836. [PubMed] [Google Scholar]
- 32.Santoro, M., F. Carlomagno, A. Romano, D. P. Bottaro, N. A. Dathan, M. Grieco, A. Fusco, G. Vecchio, B. Matoskova, M. H. Kraus, et al. 1995. Activation of RET as a dominant transforming gene by germline mutations of MEN2A and MEN2B. Science 267:381-383. [DOI] [PubMed] [Google Scholar]
- 33.Santoro, M., W. T. Wong, P. Aroca, E. Santos, B. Matoskova, M. Grieco, A. Fusco, and P. P. di Fiore. 1994. An epidermal growth factor receptor/ret chimera generates mitogenic and transforming signals: evidence for a ret-specific signaling pathway. Mol. Cell. Biol. 14:663-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schuchardt, A., V. D'Agati, L. Larsson-Blomberg, F. Costantini, and V. Pachnis. 1994. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature 367:380-383. [DOI] [PubMed] [Google Scholar]
- 35.Segouffin-Cariou, C., and M. Billaud. 2000. Transforming ability of MEN2A-RET requires activation of the phosphatidylinositol 3-kinase/AKT signaling pathway. J. Biol. Chem. 275:3568-3576. [DOI] [PubMed] [Google Scholar]
- 36.Smeyne, R. J., R. Klein, A. Schnapp, L. K. Long, S. Bryant, A. Lewin, S. A. Lira, and M. Barbacid. 1994. Severe sensory and sympathetic neuropathies in mice carrying a disrupted Trk/NGF receptor gene. Nature 368:246-249. [DOI] [PubMed] [Google Scholar]
- 37.Taylor, S. J., and D. Shalloway. 1996. Cell cycle-dependent activation of Ras. Curr. Biol. 6:1621-1627. [DOI] [PubMed] [Google Scholar]
- 38.Trupp, M., R. Scott, S. R. Whittemore, and C. F. Ibanez. 1999. Ret-dependent and -independent mechanisms of glial cell line-derived neurotrophic factor signaling in neuronal cells. J. Biol. Chem. 274:20885-20894. [DOI] [PubMed] [Google Scholar]
- 39.van der Geer, P., S. Wiley, G. D. Gish, and T. Pawson. 1996. The Shc adaptor protein is highly phosphorylated at conserved, twin tyrosine residues (Y239/240) that mediate protein-protein interactions. Curr. Biol. 6:1435-1444. [DOI] [PubMed] [Google Scholar]
- 40.Wennström, S., and J. Downward. 1999. Role of phosphoinositide 3-kinase in activation of Ras and mitogen-activated protein kinase by epidermal growth factor. Mol. Cell. Biol. 19:4279-4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yuan, J., and B. A. Yankner. 2000. Apoptosis in the nervous system. Nature 407:802-809. [DOI] [PubMed] [Google Scholar]