Abstract
Amplicon transgenes from potato virus X (PVX) are based on a modified version of the viral genome and are efficient activators of post-transcriptional gene silencing (PTGS). To determine whether PVX amplicons activate PTGS in Arabidopsis, we used constructs based on the genome of PVX carrying a green fluorescent protein (GFP) reporter gene. Our analysis of the transgene phenotype exploited previous observations indicating that PTGS is associated with short 25-nucleotide RNA species, transgene methylation, and homology-dependent virus resistance. We also used the ability of turnip mosaic virus to suppress gene silencing as a means of dissecting stages of the mechanism. The results showed that a PVX:GFP amplicon induces weak PTGS and that this PTGS was enhanced in the presence of a GFP reporter gene. Our interpretation of these data is that the PTGS induced by the amplicon was genetically determined and equivalent to the initiation stage of the PTGS mechanism. The PTGS induced by the combined amplicon and reporter gene was equivalent to the maintenance stage and was associated with an epigenetic conversion of the transgene. The distinction between genetic and epigenetic PTGS explains the well-characterized effects of transgene dosage on PTGS that have been previously interpreted in terms of RNA expression thresholds.
INTRODUCTION
Amplicons from potato virus X (PVX) are transgenes based on a modified viral genome in which the key elements are cis- and trans-acting elements required for virus replication. These elements include the gene for the virus-encoded replication enzyme and the cis-acting elements required for replication of the viral RNA. Originally, the amplicon transgenes were introduced into plants as a possible means of achieving high-level expression of genes incorporated into the viral genome. However, from the analysis of transgenic plants expressing a PVX vector with a β-glucuronidase (GUS) reporter gene (PVX:GUS), we found that there was an unexpectedly low level of GUS expression (Angell and Baulcombe, 1997).
Subsequently, it was found that this low level of GUS expression was due to activation of a sequence-specific mechanism of RNA degradation by the PVX:GUS amplicon. The targets were RNA molecules that shared sequence identity with the amplicon transgene, and the underlying mechanism was the same as that of post-transcriptional gene silencing (PTGS) in plants exhibiting cosuppression and other related phenomena (Angell and Baulcombe, 1997). An important feature of amplicon-mediated PTGS is the lack of between-line variation that is normally associated with sense or antisense transgenes: in tobacco and tomato, each tested line exhibited a high level of amplicon-mediated PTGS. Therefore, amplicons can be exploited for consistent and efficient transgene silencing. Recently, we have shown that PTGS could be targeted against endogenous RNA by PVX amplicons with fragments of endogenous genes and that the silencing phenotype was reproducible in all of the tested tomato and tobacco lines (Angell and Baulcombe, 1999).
There are two features of the PVX amplicons that are important for the PTGS phenotypes. First, there is the production of replicating viral RNA that is known, from several systems, to be a potent activator of PTGS (Lindbo et al., 1993; Kumagai et al., 1995; Covey et al., 1997; Ratcliff et al., 1997, 1999; Jones et al., 1998a; Ruiz et al., 1998). Plants probably have an RNA surveillance system that can detect the double strands of these replicating RNAs (Waterhouse et al., 1998) and that is part of an RNA-mediated, anti-viral defense system (Ratcliff et al., 1999). The second feature of PVX influencing the amplicon phenotype is the absence of a virus-encoded suppressor of PTGS. Many viruses encode these suppressors as a strategy to counteract the antiviral defense, but PVX does not (Brigneti et al., 1998; Voinnet et al., 1999). Obviously, if an amplicon transgene encodes a suppressor of silencing, it would compromise the PTGS phenotype.
There are several reports of transgenic plants expressing replicating viral RNA or DNA associated with phenotypes that can be explained, at least in part, in terms of PTGS (Kaido et al., 1995; Angell and Baulcombe, 1997; Atkinson et al., 1998). Based on these reports, it seemed likely that amplicon-mediated PTGS could be exploited in host plant–virus combinations other than those with solanaceous species and PVX. To explore this idea, we investigated the phenotype of PVX amplicons in Arabidopsis. It was known that PVX is able to replicate in Arabidopsis (T. Dalmay, unpublished data), although it is not able to spread and accumulate systemically. The results presented here confirm that PVX amplicon transgenes activate PTGS in Arabidopsis. However, from the analysis of transgene methylation, virus resistance, and the effects of viral suppressors, we have been able to dissect different types of gene silencing, depending on whether the amplicon is present alone or in combination with another homologous transgene. These data indicate the importance of an epigenetic transgene conversion in PTGS and address the roles of RNA thresholds and transgene interactions in PTGS.
RESULTS
PTGS by a PVX:GFP Amplicon in Arabidopsis
PVX amplicons are transgene constructs that consistently activate gene silencing in independent lines of tobacco and tomato (Angell and Baulcombe, 1997, 1999). To determine whether PVX amplicons also activate PTGS in Arabidopsis, we used the constructs presented schematically in Figure 1. The main elements of these constructs are the cauliflower mosaic virus 35S promoter coupled with the cDNA of the PVX:green fluorescent protein (GFP) vector (35S–PVX:GFP). The transcriptional terminator was from the nopaline synthase gene, and the construct was incorporated into the T-DNA of an Agrobacterium transformation vector. Nine Arabidopsis transformants carrying this cDNA were generated. DNA gel blot analysis and segregation data (T. Dalmay, unpublished data) revealed that five of these transformants carried a single copy and two carried multiple copies of the transgene. The other two lines carried the bar selectable marker gene from the transformation vector, but due to rearrangement of the integrated T-DNA, the 35S–PVX:GFP amplicon was not present.
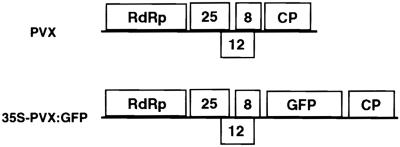
Figure 1.
PVX and the 35S–PVX:GFP Construct.
A schematic but not-to-scale representation of the viral component of the 35S–PVX:GFP transgene compared with the genome of unmodified PVX. The PVX genes are RdRp, for RNA-dependent RNA polymerase; 25, 12, and 8, the “triple gene block” (encoding proteins of 25, 12, and 8 kD, respectively); and CP, coat protein. In 35S–PVX:GFP, the viral cDNA was inserted between the 35S promoter of cauliflower mosaic virus and the terminator sequence of the nopaline synthase gene of the plasmid vector pSLJ755/5.
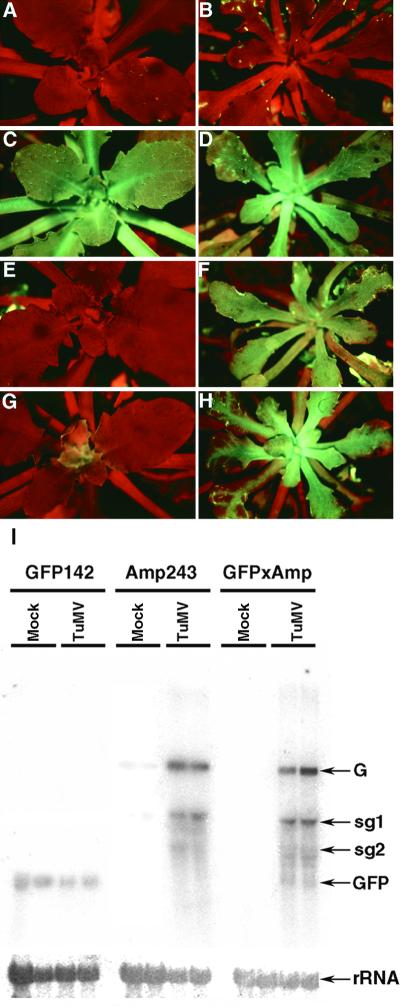
None of the transformed plants carrying the 35S–PVX:GFP amplicon exhibited GFP fluorescence, as shown for the Amp243 line in Figures 2A, 2D, 2G, and 2J. In principle, this observation could indicate either that there was PTGS or that the PVX vector replicated at a very low level, if at all, in Arabidopsis. To investigate these possibilities, we crossed the amplicon lines with a line (GFP142) carrying a single copy of a 35S–GFP transgene. This line exhibited bright green fluorescence under UV illumination due to GFP expression (Figures 2B, 2E, 2H, and 2K). We reasoned that if the phenotype of the plants carrying the 35S–PVX:GFP amplicon was due to PTGS, then the 35S–GFP transgene would be silenced in the progeny of this cross and there would be little or no GFP fluorescence in these plants. Alternatively, if PVX were not able to replicate in Arabidopsis, the PVX:GFP transgene would not cause PTGS, and the progeny would be as fluorescent green as the 35S–GFP parent. As a control in these analyses, we also generated plants carrying the 35S–GFP transgene combined with a replication defective amplicon (35S–PVXΔrep:GFP). The PVX cDNA in this construct has a deletion between nucleotides 965 and 2694 in the gene encoding the replication enzyme.
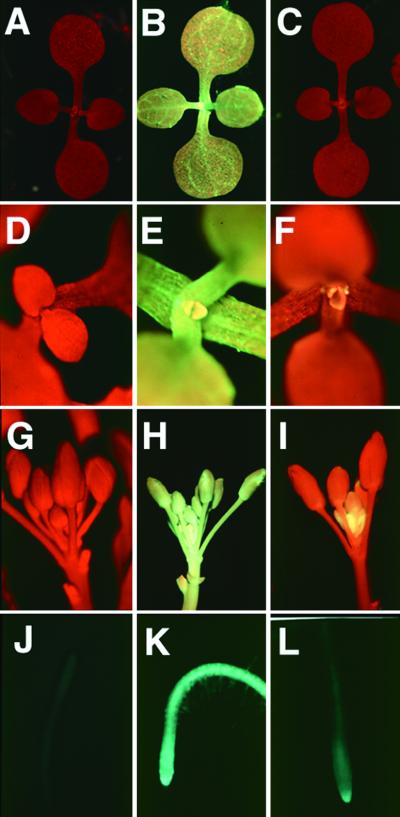
Figure 2.
GFP Fluorescence in Plants Carrying 35S–GFP and 35S–PVX:GFP Transgenes.
GFP fluorescence in plants carrying 35S–GFP ([B], [E], [H], and [K]), 35S–PVX:GFP ([A], [D], [G], and [J]), or a combination of both transgenes ([C], [F], [I], and [L]). The images were produced under UV light in a dissecting microscope, and the red fluorescence is due to chlorophyll. GFP fluorescence appears green, yellow, or blue-green in different tissues and also depends on the photographic exposure time. Nontransformed plants look the same as the Amp243 plants ([A], [D], [G], and [J]).
(A) to (C) Young seedlings.
(D) to (F) Close-up of the growing point of young seedlings.
(G) to (I) Immature flowers.
(J) to (L) Roots.
There was no detectable fluorescence from the roots of the plants shown in (A), (D), (G), and (J); in the plants shown in (C), (F), (I), and (L), there was GFP fluorescence only in the growing point.
For each of the seven 35S–PVX:GFP lines, we made 10 replicate crosses with the 35S–GFP plants, and from each cross, 10 progeny were examined for GFP fluorescence. These progeny all exhibited GFP fluorescence that was restricted to the growing points of the shoot (Figures 2C and 2F), flower (Figure 2I), and root (Figure 2L). The other parts of the plant lacked any detectable green fluorescence. We could rule out that the absence of green fluorescence was due to dilution of the 35S–GFP transgene, because the progeny of a cross between 35S–GFP and nontransgenic plants were fully green fluorescent (data not shown). Moreover, the suppression of the 35S–GFP phenotype was dependent on virus replication, because the lines carrying 35S–GFP together with the 35S–PVXΔrep:GFP transgene were fully green fluorescent (data not shown). Therefore, the PVX:GFP amplicon was able to cause silencing of a homologous GFP transgene in trans, and as occurred with PVX amplicons in tobacco, the silencing was dependent on replication of viral RNA. Also, as occurred with the PVX amplicons in tobacco, the silencing phenotype was consistent in independent lines. It is likely that this silencing is dependent on virus replication, because there was no silencing in any of the four lines carrying the 35S–PVXΔrep:GFP construct in the GFP background.
To investigate further the silencing phenotype, we analyzed RNA and transcription in the lines GFP142 (35S–GFP), Amp243 (35S–PVX:GFP), and the progeny of a cross between these lines. These progeny are referred to hereafter as GFP142 × Amp243. Figure 3 shows the result of RNA gel blot analysis of the respective RNA samples and indicates, as expected, that GFP mRNA levels were substantially higher in line GFP142 (Figure 3, lanes 5 to 8) than in GFP142 × Amp243 (Figure 3, lanes 9 to 12). We conclude from these results that the PVX:GFP amplicon from Amp243 was able to silence the GFP transgene from GFP142.
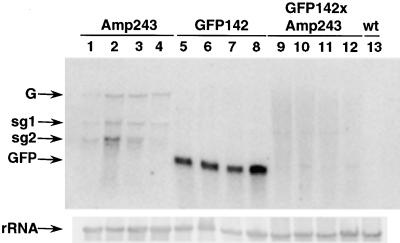
Figure 3.
GFP and PVX:GFP RNA Accumulation.
RNA was isolated from leaves of wild-type plants (wt; lane 13) and lines carrying 35S–PVX:GFP (Amp243; lanes 1 to 4), 35S–GFP (GFP142; lanes 5 to 8), or a combination of both transgenes (GFP142 × Amp243; lanes 9 to 12). RNA gel blot analysis was conducted using 32P-labeled probes corresponding to the full-length GFP sequence (top) and rRNA (bottom). The RNA species detected were the genomic (G) and subgenomic (sg) RNAs of PVX:GFP, GFP mRNA (GFP), and rRNA.
Figure 3 also shows that in Amp243, the PVX:GFP RNA was present as both genomic and subgenomic species (Figure 3, lanes 1 to 4). The subgenomic RNAs would have been produced by the PVX replicase, using negative strand RNA as a template. Thus, the presence of subgenomic RNAs confirms that PVX is able to replicate in Arabidopsis. We obtained further confirmation of this replication by demonstrating that infectious PVX could be isolated from Amp243 and other lines carrying the 35S–PVX:GFP construct (data not shown).
From the RNA gel blot, we found, surprisingly, that the PVX RNAs were present at a lower level in the GFP142 × Amp243 progeny than in the Amp243 lines (Figure 3, lanes 9 to 12). One interpretation of this result is that the activation of gene silencing required both types of transgene. In this scenario, the absence of GFP fluorescence in Amp243 would be due to poor replication of the PVX:GFP RNA rather than gene silencing. A second possibility is that a low level of PTGS in Amp243 was enhanced by the 35S–GFP transgene in the GFP142 × Amp243 line. To resolve these alternatives, it was first necessary to determine whether the silencing mechanism in the GFP142 × Amp243 line was transcriptional or post-transcriptional.
To distinguish between transcriptional or PTGS in the GFP142 × Amp243 plants, we used transcription run-off analysis. The data, shown in Figure 4, reveal that the high-level transcription of GFP in GFP142 × Amp243 and GFP142 plants does not reflect the marked differential accumulation of GFP mRNA in these lines. Therefore, these data indicate that there is silencing of GFP at the post-transcriptional level. PTGS was also implicated by the similar levels of PVX run-off transcripts in the Amp243 and GFP142 × Amp243 lines, despite the different levels of PVX:GFP RNA (Figure 3).
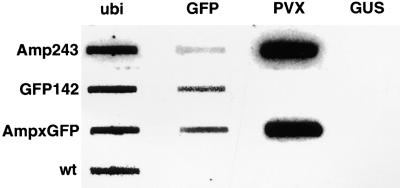
Figure 4.
Nuclear Run-Off Analysis of Transgene Transcription.
Ubiquitin (ubi), GFP, PVX, and GUS DNA was fixed onto nitrocellulose membranes that were hybridized with the 32P-labeled in vitro–labeled transcripts of isolated nuclei from lines carrying 35S–GFP (GFP142), 35S–PVX:GFP (Amp243), or a combination of both transgenes (AmpxGFP). wt, wild type.
The transcription analyses included three controls. First, GUS DNA was used as negative control. Second, to validate the comparisons of transgene transcription, we showed that ubiquitin transcription was similar in extracts from the different lines. Third, we showed that transcription was almost completely eliminated by the inclusion of actinomycin D as an inhibitor of DNA-dependent RNA polymerase in the transcription run-off analysis (data not shown). In the absence of this third control, it remained possible that incorporation of labeled nucleotide in the run-off assay was due to the RNA-dependent RNA polymerase encoded in the PVX:GFP of the amplicon constructs.
We noticed that PVX gave a stronger signal than did GFP in these run-off assays with the Amp243 lines (Figure 3). In part, the differential representation may be because the transcripts were hybridized with PVX DNA that was longer than the GFP DNA. It is likely also that there was premature termination of transcription so that the GFP transcripts from the 3′ end of the transgene would have been underrepresented in the final run-off product.
Virus Infection of Arabidopsis Plants Exhibiting PTGS
In previous analyses, we have used viruses as a tool to dissect the mechanism of PTGS. For example, we have shown that viruses encode suppressors of PTGS and that these suppressors can be used to dissect the underlying mechanism (Brigneti et al., 1998; Voinnet et al., 1999). We have also shown that viruses can be used to report PTGS, provided that there is sequence similarity in the virus and the PTGS transgene (English et al., 1996).
Here, we exploited a viral suppressor of PTGS to determine whether the low level of GFP in Amp243 plants was due to PTGS or to inefficient replication of PVX. If there had been inefficient replication, the introduction of a suppressor of PTGS into these plants would not have affected GFP expression from the PVX:GFP amplicon. However, if there had been PTGS, PVX:GFP accumulation and consequent GFP expression would have increased in the presence of the viral suppressor.
The source of the suppressor of PTGS was turnip mosaic virus (TuMV; potyvirus group), which is highly infectious on Arabidopsis. The TuMV-encoded helper component protease (HC-PRO) is functionally similar to homologous proteins encoded in the genomes of potato virus Y and tobacco etch virus that have been previously characterized as suppressors of PTGS (Anandalakshmi et al., 1998; Brigneti et al., 1998; Kasschau and Carrington, 1998).
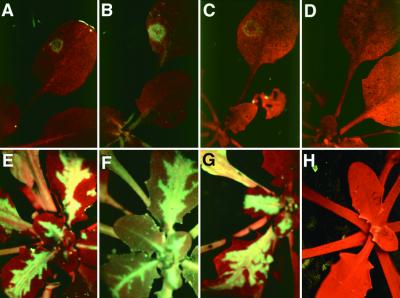
Two weeks after TuMV inoculation of Amp243 plants, the systemically infected leaves exhibited uniform green fluorescence, as shown in Figure 5F. The increase in GFP fluorescence was similar but more pronounced on GFP142 × Amp243 plants (Figure 5H), whereas on the mock inoculated or infected wild-type (Figures 5A and 5B) and the mock-inoculated Amp243 or GFP142 × Amp243 plants (Figures 5E and 5G), there was no green fluorescence. TuMV had no effect on the fluorescence of the 35S–GFP plants (Figures 5C and 5D).
Figure 5.
Effect of TuMV Infection on GFP Fluorescence.
(A) to (H) Nontransformed ([A] and [B]), GFP142 ([C] and [D]), Amp243 ([E] and [F]), and GFP142 × Amp243 ([G] and [H]) plants were inoculated with water ([A], [C], [E], and [G]) or TuMV ([B], [D], [F], and [H]). GFP fluorescence was assessed in the plants under UV light in a dissecting microscope, and the photographs were taken at 14 days after inoculation.
(I) RNA was isolated from the top three or four leaves of mock- or TuMV-inoculated GFP142, Amp243, and GFP142 × Amp243 plants. RNA gel blot analysis was performed using 32P-labeled probes corresponding to the full-length GFP sequence (top) and rRNA (bottom). The RNA species detected were the genomic (G) and subgenomic (sg) RNAs of PVX:GFP, GFP mRNA (GFP), and rRNA.
To rule out the possibility that TuMV might increase transcription from the 35S promoter, we analyzed the RNA from infected and noninfected plants. Figure 5I shows the result of RNA gel blot analysis of the respective RNA samples and indicates that TuMV did not increase the level of GFP RNA in the nonsilenced GFP142 plants. However, consistent with the fluorescence data, there was a substantial increase in the PVX:GFP RNA in the Amp243 plants and of PVX:GFP and GFP RNA in the GFP142 × Amp243 plants. From these data, we conclude that the TuMV-mediated increase of GFP in GFP142 × Amp243 plants and Amp243 plants is due to suppression of PTGS.
Tobacco and tomato exhibiting PTGS are resistant to viruses that share sequence similarity with the transgene (English et al., 1996). To determine whether PTGS in GFP142 × Amp243 and Amp243 plants can also target viral RNA, we inoculated these plants with tobacco rattle virus (TRV) carrying the GFP sequence (TRV:GFP) (Ratcliff et al., 1999). Figure 6 illustrates that the nontransformed plants and GFP142 plants showed green fluorescent spots on the inoculated leaves a few days after inoculation (Figures 6A and 6B) and very strong green fluorescence on the systemically infected leaves a week later (Figures 6E and 6F). In contrast, there was no green fluorescence on GFP142 × Amp243 plants (Figures 6D and 6H). These data show that TRV:GFP is a potential target of PTGS. However, on the Amp243 plants, despite the PTGS, the appearance of GFP fluorescence indicated that TRV:GFP had accumulated and spread as rapidly as it did on the nontransgenic plants (Figures 6C and 6G). Thus, the data from TuMV infection (Figure 5), PVX:GFP RNA analyses (Figure 3), and TRV:GFP susceptibility (Figure 6) collectively indicate that the Amp243 plants exhibit PTGS but that it is weaker than in GFP142 × Amp243 plants.
Figure 6.
GFP Fluorescence Due to TRV:GFP.
(A) and (E) Nontransformed plants.
(B) and (F) GFP142.
(C) and (G) Amp243.
(D) and (H) GFP142 × Amp243.
Plants were inoculated with TRV:GFP. GFP fluorescence was assessed in the plants under UV light in a dissecting microscope, and photographs of the inoculated ([A] to [D]) and systemically infected ([E] to [H]) leaves were taken at 4 and 11 days after inoculation, respectively.
The 25-Nucleotide RNA
Recently, we reported the presence of a 25-nucleotide-long RNA species in tobacco and tomato plants exhibiting PTGS (Hamilton and Baulcombe, 1999). These short RNAs hybridized with both the plus and minus strands of the PTGS target sequences and were not found in tissues from nonsilenced tomato and tobacco. To further characterize PTGS in the Amp243 and GFP142 × Amp243 plants, we assayed for the short RNAs by RNA gel blotting of samples of low molecular weight RNAs. Figure 7, lane 3, shows that there was no 25-nucleotide GFP RNA in the GFP142 plants. However, there was a faint signal in samples from the Amp243 plants (Figure 7, lane 2) and a very strong signal with the sample from GFP142 × Amp243 plants (Figure 7, lane 4). The 25-nucleotide RNAs were detected at a low level in three independent experiments with Amp243. These data confirm that 25-nucleotide RNAs are associated with PTGS in Arabidopsis. Moreover, from the level of these RNAs, there is further evidence that weak PTGS in the Amp243 plants was increased by the addition of the 35S–GFP transgene from GFP142.
Figure 7.
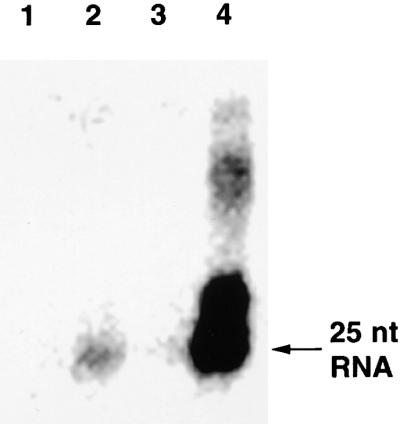
RNA Gel Blot Analysis of Low Molecular Weight RNAs.
Low molecular weight RNA was isolated from leaves of wild-type C24 plants (lane 1), lines carrying 35S–GFP (GFP142; lane 3), 35S–PVX:GFP (Amp243; lane 2), or a combination of both transgenes (GFP142 × Amp243; lane 4). RNA gel blot analysis was conducted using 32P-labeled probes corresponding to the full-length GFP sequence. The ∼27-nucleotide (nt) band visible in lane 4 was not detected when the experiment was repeated.
Although these analyses of the 25-nucleotide RNAs were conducted with GFP probes, it also would be expected that there would be short RNA species corresponding to the PVX component of the PVX:GFP amplicon. To test this prediction, RNA gel blot analysis of the short RNA was also performed with a PVX probe. Surprisingly, and in contrast to the results with the GFP probe, the 25-nucleotide RNAs from the Amp243 and GFP142 × Amp243 plants gave equal signals (data not shown). These signals were substantially weaker than those detected with the GFP probe. From these data, we conclude that the 25-nucleotide GFP RNAs from GFP142 × Amp243 plants were derived mainly from the 35S–GFP transgene rather than from the PVX:GFP amplicon.
Transgene Methylation
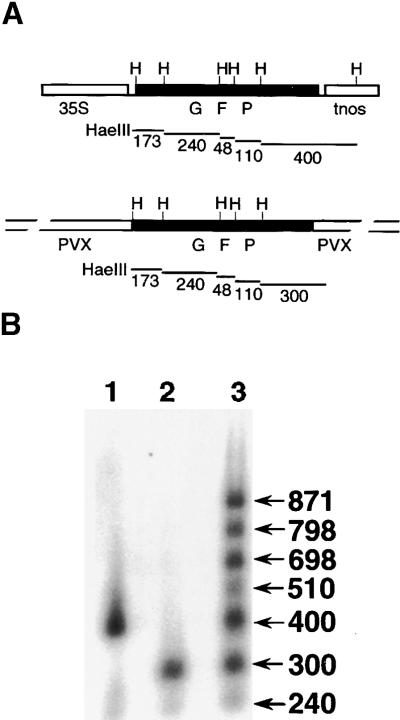
We previously presented a model of PTGS in which homology-dependent RNA–DNA interactions led to sequence-specific methylation of the participating DNA (Jones et al., 1999). To determine whether these processes could be implicated in the amplicon-mediated PTGS of GFP in Arabidopsis, we conducted DNA gel blot analysis with GFP transgenes using methylation-sensitive restriction endonucleases and with a GFP-specific probe. Figure 8A shows the predicted sizes of the GFP-specific HaeIII restriction fragments from 35S–GFP reporter and the 35S–PVX:GFP amplicon. The data shown in Figure 8B for HaeIII-digested DNA demonstrate that the 35S–GFP and 35S–PVX:GFP transgenes were not methylated in the GFP142 (Figure 8B, lane 1) and Amp243 plants (Figure 8B, lane 2) but were highly methylated in GFP142 × Amp243 plants (Figure 8B, lane 3). Similar data were obtained with AluI-digested DNA (data not shown). In contrast, when the GFP probe was removed and the filter was reprobed for the phytoene desaturase gene (data not shown), the three samples were all digested to the same extent, allowing us to rule out the possibility that there was incomplete digestion of the DNA.
Figure 8.
Differences in Transgene Methylation Associated with PTGS.
(A) Structure of the GFP transgene in 35S–GFP and 35S–PVX:GFP. Features shown are the 35S promoter (35S), GFP coding sequence (black box; GFP), nopaline synthase terminator (tnos), and the PVX sequence flanking GFP in 35S–PVX:GFP. The restriction sites for HaeIII (H) and the lengths (in base pairs) of the expected digestion products are shown.
(B) Gel blot analysis with DNA from leaves of GFP142 (35S–PVX:GFP; lane 1), Amp243 (35S–PVX:GFP; lane 2), and GFP142 × Amp243 (lane 3) plants using a GFP-coding sequence probe. The numbers at right indicate the estimated length (in base pairs) of the hybridizing DNA fragments.
With GFP probes, it was not possible to determine whether the methylated DNA in the GFP142 × Amp243 plants was the amplicon or the 35S–GFP transgene. However, by using PVX probes from the regions flanking GFP in the amplicon, this distinction could be made. It was apparent that the GFP sequence in the amplicon was methylated in GFP142 × Amp243 plants (data not shown). In contrast, by using a PVX probe from the 5′ end of the viral sequence, it was shown that the PVX sequence was not methylated at the 5′ end region in either the Amp243 or GFP142 × Amp243 plants (data not shown). From these results, we conclude that amplicons mediate the homology-dependent transgene methylation that is characteristic of other examples of PTGS. However, the amplicon mediated methylation only in the presence of a 35S–GFP transgene, and methylation was restricted to GFP sequences. These results indicate that the PVX:GFP amplicon could not mediate methylation autonomously.
DISCUSSION
We have shown here that the PVX amplicon can mediate gene silencing in Arabidopsis. We have also confirmed that, as in other examples of PTGS, 25-nucleotide RNAs and sequence-specific methylation of DNA are associated with the presence of the amplicon. Our results indicate the potential utility of viral amplicons as a general strategy for gene silencing in transgenic plants, because in Arabidopsis, as in tobacco and tomato, PTGS was observed in all lines tested. However, in Arabidopsis, a nonhost of PVX, amplicon-mediated silencing was weaker than it was in host plants of PVX (Angell and Baulcombe, 1997, 1999). This nonhost effect most likely explains why PVX amplicons provoke weak silencing of endogenous genes in Arabidopsis (T. Dalmay, S. Angell, and D.C. Baulcombe, unpublished data). To produce strong gene silencing in Arabidopsis, it will be necessary to generate amplicons based on viruses for which Arabidopsis is a host.
Transgene Interactions Mediated by RNA
Although the PTGS induced by the PVX:GFP amplicon was weak in Arabidopsis, it has characteristics that are informative about the mechanism of gene silencing. One of these characteristics is the enhanced PTGS in lines carrying PVX:GFP together with a 35S–GFP transgene. In principle, this effect could be a consequence of transgene dosage. There are many examples in which PTGS is influenced by the number of homologous transgenes in a genome (Mueller et al., 1995; Goodwin et al., 1996). However, it is unlikely that this explanation applies to the Arabidopsis lines carrying 35S–GFP and 35S–PVX:GFP transgenes because other lines with two GFP transgenes, for example, GFP142 and Amp243 with homozygous transgenes, did not exhibit strong PTGS. A more plausible explanation is based on a homology-dependent interaction between the 35S–GFP and 35S–PVX:GFP transgenes in GFP142 and Amp243.
It is unlikely that this interaction would operate at the DNA level because there was no gene silencing of the 35S–GFP transgene in plants carrying a 35S–PVXΔrep:GFP transgene (data not shown). An alternative and more likely scenario is indicated by the methylation of the GFP transgenes in GFP142 × Amp243 plants. In several other experimental systems (Wassenegger et al., 1994; Jones et al., 1998b, 1999; Guo et al., 1999), transgene methylation is an indicator of an RNA–DNA interaction. Thus, the replicating RNA of the 35S–PVX:GFP transgenes, either directly or indirectly, could have participated in an interaction with the 35S–GFP transgene in GFP142 × Amp243.
Initiation and Maintenance of PTGS
When PVX:GFP was inoculated to Nicotiana benthamiana expressing 35S–GFP, there was virus-induced silencing of the transgene (Ruiz et al., 1998). Analysis of this manifestation of PTGS revealed that there are separate initiation and maintenance stages. Initiation PTGS is dependent on the presence of the replicating PVX:GFP. The maintenance stage follows from RNA–DNA interactions involving the GFP sequences and is associated with methylation of the GFP DNA. Maintenance PTGS is also associated with elimination of the viral RNA from the inoculated plant. To account for the persistence of PTGS in the absence of the viral RNA, we have suggested that there is an epigenetic conversion of the GFP transgene. In the maintenance stage, the epigenetically converted transgene rather than the PVX:GFP would be the mediator of PTGS (Jones et al., 1999).
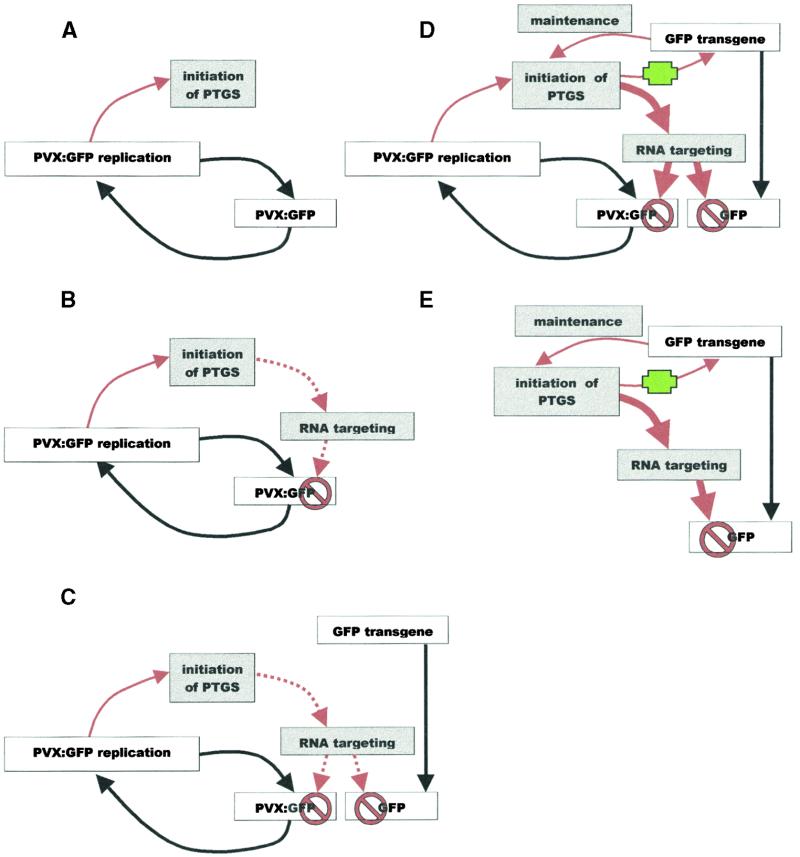
In these terms, the phenotype of Amp243 represents initiation, as shown schematically in Figures 9A and 9B. The PVX:GFP transgene is not methylated in Amp243 (Figure 8B, lane 2), indicating that it has not participated in the RNA–DNA interaction or undergone the epigenetic conversion that is characteristic of the maintenance stage (Jones et al., 1999). In Amp243, PTGS is considered weak because of the low abundance of the 25-nucleotide RNA (Figure 7, lane 2) and the accumulation of PVX:GFP RNA (Figure 3, lanes 1 to 4). The absence of resistance against TRV:GFP (Figures 6C and 6G) is also an indicator of weak PTGS. We speculate that PTGS in Amp243 is weak because PVX replicates inefficiently in the nonhost Arabidopsis.
Figure 9.
Different Stages of PTGS.
(A) The early initiation stage of PTGS in Arabidopsis cells carrying a PVX:GFP amplicon transgene. PVX:GFP replication leads to the accumulation of PVX:GFP RNA and initiates PTGS.
(B) The late initiation stage of PTGS in Arabidopsis cells carrying a PVX:GFP amplicon transgene. The initiation of PTGS induces an RNA targeting mechanism that constrains accumulation of PVX:GFP RNA. The PTGS depends on the continued presence of viral RNA so that there is feedback regulation that prevents the RNA targeting mechanism from eliminating the viral RNA from the cell. This stage represents the PTGS in mature cells of Amp243.
(C) The late initiation stage of PTGS in Arabidopsis cells carrying a PVX:GFP amplicon transgene and a GFP reporter gene. The situation is as shown in (B) except that the RNA targeting mechanism affects the GFP mRNA as well as the PVX:GFP RNA. There is also an RNA–DNA interaction affecting the GFP reporter gene. At this stage, there has not been an epigenetic conversion of the GFP reporter gene.
(D) The early maintenance stage of PTGS in Arabidopsis cells carrying a PVX:GFP amplicon transgene and a GFP reporter gene. The RNA–DNA interaction leads to an epigenetic conversion so that the PTGS is mediated by the GFP reporter gene as well as by the PVX:GFP RNA of the amplicon. The thick red arrows replacing the dotted arrows reflect the presence of two sources of PTGS resulting in stronger silencing than in (C).
(E) The late maintenance stage of PTGS in Arabidopsis cells carrying a PVX:GFP amplicon transgene and a GFP reporter gene. The strong PTGS shown in (C) results eventually in the complete elimination of PVX:GFP RNA from the cell. However, the PTGS persists due to the epigenetic conversion of the GFP transgene.
The red lines indicate processes involved in PTGS, the black lines indicate RNA synthesis, and the green cross indicates the RNA–DNA interaction that is proposed to initiate the epigenetic conversion of the transgene. The red “stop” sign indicates RNA degradation.
In the GFP142 × Amp243 plants, by comparison with the analysis of virus-induced gene silencing, it seems likely that the PTGS is at the maintenance stage. The transgenes are methylated (Figure 8B, lane 3), and the replicating amplicon RNA has been eliminated (Figure 3). Accordingly, the transgenes in GFP142 × Amp243 plants would have participated in an RNA–DNA interaction (Figure 9C) and undergone the epigenetic conversion (Figure 9D) typical of the maintenance stage of PTGS (Jones et al., 1999). Although initiation of PTGS in GFP142 × Amp243 plants would have involved replication of the amplicon RNA (Figure 9C), in the mature plant the amplicon RNA was eliminated, and PTGS would have been mediated by the 35S–GFP transgene (Figure 9E).
The epigenetic conversion in GFP142 × Amp243 plants would explain why the PTGS was stronger than in Amp243 plants: the PTGS would have been determined by the 35S–GFP transgene rather than the inefficiently replicating PVX:GFP RNA. This stronger gene silencing would then account for the elimination of both PVX:GFP and GFP RNA (Figure 3, lanes 9 to 12), the resistance against TRV:GFP (Figures 6D and 6H), and the high level of the 25-nucleotide GFP RNA (Figure 7, lane 4) in the GFP142 × Amp243 plants. At the stage at which PVX:GFP RNA had been eliminated (Figure 9E), the PTGS would be maintained by the epigenetically converted 35S–GFP transgene.
We had previously suggested that the HC-PRO suppressors of PTGS in potyviruses act at the maintenance rather than the initiation stage of PTGS (Brigneti et al., 1998). However, the symptoms of TuMV in Amp243 are not consistent with this interpretation because there was suppression of PTGS in Amp243 plants (Figure 5) in which, based on the absence of transgene methylation, we suggest that the maintenance stage is not active (Figures 9B and 9D). To reconcile the interpretations of these data, we now propose that HC-PRO interferes with an RNA targeting mechanism that is part of both initiation and maintenance stages of PTGS (Figure 9). Our previous data, showing that HC-PRO does not prevent DNA methylation associated with PTGS, are consistent with this interpretation (Jones et al., 1999).
Genetic and Epigenetic PTGS
As discussed above, PTGS in GFP142 × Amp243 plants is epigenetic, whereas in Amp243, the ability of the 35S–PVX:GFP locus to mediate PTGS is associated with virus replication and is genetically determined. We propose here that this distinction between genetic and epigenetic silencing allows many of the experimental observations about PTGS to be reconciled in variations of the conceptual model presented in Figure 9.
The most obvious examples of epigenetic PTGS are those in which the process is initiated by viruses and in which the virus is eliminated although the silencing persists (Lindbo et al., 1993; Ruiz et al., 1998; Jones et al., 1999). However, there are other examples in which viruses are not involved that may also be epigenetic. For example, there are reports of PTGS that was previously explained in terms of ectopic interactions between homologous transgene loci (Baulcombe and English, 1996). These examples include those in which PTGS follows from increasing the transgene dosage (Mueller et al., 1995; Goodwin et al., 1996). To account for these examples, it was proposed that PTGS requires two transgene functions referred to as silencer and receptor. PTGS would only ensue if silencer and receptor loci were present in the same background or if a single locus could perform both functions. In the present work, the 35S–PVX:GFP transgene has the characteristics of the silencer function (Baulcombe and English, 1996), and the 35S–GFP transgene, with its propensity to undergo epigenetic conversion, has the predicted attributes of the receptor locus.
It was originally suggested that the ectopic interaction would involve homologous DNAs (Baulcombe and English, 1996). However, in the example described here, the interaction between the PVX:GFP and GFP transgenes is most likely mediated by RNA because replication-defective amplicons did not cause the epigenetic conversion of 35S–GFP or activate PTGS.
Meiotically reversible PTGS (Elmayan and Vaucheret, 1995) and examples in which there is a correlation of transgene methylation with PTGS (Ingelbrecht et al., 1994; English et al., 1996) are likely to have an epigenetic component. It is also possible that delayed or developmentally regulated PTGS involves an epigenetic transgene conversion (Hart et al., 1992; Palauqui and Vaucheret, 1995). In these examples, the epigenetic conversion may be associated with systemic spread of a silencing signal (Palauqui et al., 1997; Voinnet and Baulcombe, 1997; Palauqui and Vaucheret, 1998; Voinnet et al., 1998; Jones et al., 1999).
In contrast, there are likely to be other examples, such as Amp243, in which the PTGS is genetically determined and in which there is no epigenetic component to the mechanism. Candidates for genetic PTGS include the examples with inverted repeat transgenes (Stam et al., 1998). Antisense transgenes may also initiate PTGS without an epigenetic component (Delange et al., 1995). A striking feature of these proposed examples of genetic PTGS is the potential to form double-stranded RNA: with amplicons, such structures are formed as part of the viral replication intermediate; and with inverted repeat transgenes, they could result from intramolecular interactions of complementary RNA. The double-stranded RNA could be formed with antisense transgenes as a result of intermolecular interactions between the sense and corresponding antisense RNAs. Perhaps, then, the initiator of genetic PTGS is double-stranded RNA, as it is in RNA interference in nematodes and other organisms (Sharp, 1999). In epigenetic silencing, the potential for double-stranded RNA formation is less obvious, and it seems likely that initiation may involve forms of aberrant RNA that are not necessarily double stranded.
METHODS
Transgenic Plants
The green fluorescent protein (GFP) insert in pPVX204 (Baulcombe et al., 1995) was replaced with the mGFP5 insert from pBin-35S-mGFP5 (Haseloff et al., 1997). This new potato virus X (PVX)–GFP5 sequence was inserted between the cauliflower mosaic virus 35S promoter and the transcriptional terminator of the nopaline synthase gene and transferred into the binary vector pSLJ755/5. The PVXΔrep:GFP construct was obtained by deleting a BglII fragment (positions 965 to 2694) from the full-length PVX:GFP. pBin-35S-mGFP5 (Haseloff et al., 1997) was used without any change. Transformation of Arabidopsis thaliana ecotype C24 with Agrobacterium tumefaciens GV3101 carrying 35S–PVX:GFP or 35S–GFP constructs was performed as described by Bechtold et al. (1993). The transformants were selected in vitro on medium supplemented with 10 mg/L l-phosphinotricin (Melford, Ipswich, UK) or 50 mg/L kanamycin, respectively.
GFP Imaging
GFP expression was monitored by using an MZ12 dissecting microscope (Leica, Heidelberg, Germany) coupled to an epifluorescence module. Photographs were taken using Kodak Ektachrome Panther (400 ASA) film.
RNA Gel Blot and Nuclear Run-Off Transcription Analyses
RNA gel blot analysis was performed as described previously (Mueller et al., 1995). Polymerase chain reaction–amplified full-length GFP DNA fragments were labeled by random-primed incorporation of 32P-dCTP. After hybridization, the signal present in the membranes was analyzed and quantified using Fujix Bio-Imaging Analyzer Bas 1000 (Fuji Photo Film Co., Fuji, Japan) equipment.
Nuclei were isolated as described by Covey et al. (1997), and incorporation of uridine 5′α-32P-triphosphate (Amersham) was determined by probing 1 μg of the appropriate DNA samples immobilized as slots on Hybond-N+ membranes (Amersham). Incorporation was assessed by using a Fujix Bio-Imaging Analyzer BAS 1000 (Fuji Photo Film Co.), as described by Mueller et al. (1995).
The 25-nucleotide RNAs were extracted and probed as described previously (Hamilton and Baulcombe, 1999). Total RNA was extracted by using an SDS-based extraction solution (Hamilton and Baulcombe, 1999) and precipitated with ethanol. The short RNAs do not bind to silica-based columns such as the RNAeasy columns (Qiagen). The pellet was dissolved in water, heated to 65°C for 5 min, and then placed on ice. Polyethylene glycol (molecular weight 8000; Sigma) was added to a final concentration of 5% and NaCl to a final concentration of 0.5 M. After a 30-min incubation on ice, the RNA was centrifuged at 10,000g for 10 min. Three volumes of ethanol were added to the supernatant, and the RNA was precipitated at −20°C for at least 2 hr. The low molecular weight RNAs were pelleted by centrifugation for 10 min at 10,000g. The pellet was dissolved in formamide and heated to 65°C for 5 min, and a one-third volume of 4 × loading solution was added (2 × TBE [1 × TBE is 0.09 M Tris-borate, pH 8.0, and 0.002 M EDTA], 40% sucrose, and 0.1% bromophenol blue) before loading on 15% polyacrylamide (19:1) gels containing 7 M urea in 0.5 × TBE. The samples were run until the bromophenol blue reached the bottom of the gel, then blotted to HybondNX (Amersham) membrane, and UV cross-linked. The membrane was prehybridized in 50% formamide, 7% SDS, 50 mM NaHPO4/NaH2PO4, pH 7.0, 0.3 M NaCl, 5 × Denhardt's solution (1 × Denhardt's solution is 0.02% Ficoll, 0.02% PVP, and 0.02% BSA), and 100 μg/mL sheared, denatured salmon sperm DNA at 40°C for at least 1 hr. Single-stranded 32P-labeled RNA probes were transcribed in vitro from plasmid templates as described by Mueller et al. (1995). The labeled RNA was hydrolyzed by adding 300 μL of 200 mM carbonate buffer (80 mM NaHCO3 and 120 mM Na2CO3) to a 20-μL probe and incubating at 60°C for 2 to 3 hr. Twenty microliters of 3 M NaOAc, pH 5.0, was added to the 320-μL hydrolyzed probe. The probe was then added to fresh hybridization solution. The hybridization was performed at 40°C overnight, and the membrane was subsequently washed at 50°C in 2 × SSC (1 × SSC is 0.15 M NaCl and 0.015 M sodium citrate) and 0.2% SDS.
DNA Gel Blot Analysis
Genomic DNA was extracted from flowers or leaves, and gel blot analysis was performed as described previously (Jones et al., 1999). 32P-labeled DNA probes corresponded to the entire 812 nucleotides of GFP5, a 5′ end sequence of PVX (positions 543 to 1528), a downstream viral flanking sequence to the GFP insert in PVX-GFP (positions 6469 to 7207), or a 5′ 500-bp cDNA fragment of the Arabidopsis gene encoding phytoene desaturase.
Wild-Type and Recombinant Viruses
A full-length cDNA clone of turnip mosaic virus (TuMV) (Sanchez et al., 1998) was obtained from Dr. F. Ponz (Instituto Nacional de Investigaciones Agrarias, Madrid, Spain). Sap of Nicotiana clevelandii plants inoculated with the cDNA was used to infect Arabidopsis plants. The tobacco rattle virus (TRV)-GFP was described previously (Ratcliff et al., 1999).
Acknowledgments
We are grateful to the Gatsby Charitable Foundation and the European Community framework IV COMREP project for support. The work with modified and imported viruses was licensed by the United Kingdom Ministry of Agriculture Fisheries and Food (PHL 24A/2921).
References
- Anandalakshmi, R., Pruss, G.J., Ge, X., Marathe, R., Smith, T.H., and Vance, V.B. (1998). A viral suppressor of gene silencing in plants. Proc. Natl. Acad. Sci. USA 95 13079–13084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angell, S.M., and Baulcombe, D.C. (1997). Consistent gene silencing in transgenic plants expressing a replicating potato virus X RNA. EMBO J. 16 3675–3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angell, S.M., and Baulcombe, D.C. (1999). Potato virus X amplicon–mediated silencing of nuclear genes. Plant J. 20 357–362. [DOI] [PubMed] [Google Scholar]
- Atkinson, R.G., Bieleski, L.R.F., Gleave, A.P., Jannsen, B.J., and Morris, B.A.M. (1998). Post-transcriptional silencing of chalcone synthase in petunia using a geminivirus-based episomal vector. Plant J. 15 593–604. [DOI] [PubMed] [Google Scholar]
- Baulcombe, D.C., and English, J.J. (1996). Ectopic pairing of homologous DNA and post-transcriptional gene silencing in transgenic plants. Curr. Opin. Biotechnol. 7 173–180. [Google Scholar]
- Baulcombe, D.C., Chapman, S.N., and Santa Cruz, S. (1995). Jellyfish green fluorescent protein as a reporter for virus infections. Plant J. 7 1045–1053. [DOI] [PubMed] [Google Scholar]
- Bechtold, N., Ellis, J., and Pelletier, G. (1993). In planta Agrobacterium-mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. C. R. Acad. Sci. Ser. III Sci. Vie 316 1194–1199. [Google Scholar]
- Brigneti, G., Voinnet, O., Li, W.X., Ji, L.H., Ding, S.W., and Baulcombe, D.C. (1998). Viral pathogenicity determinants are suppressors of transgene silencing in Nicotiana benthamiana. EMBO J. 17 6739–6746. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Covey, S.N., Al-Kaff, N.S., Langara, A., and Turner, D.S. (1997). Plants combat infection by gene silencing. Nature 385 781–782. [Google Scholar]
- Delange, P., Vanblokland, R., Kooter, J.M., and Mol, J.N.M. (1995). Suppression of flavonoid flower pigmentation genes in Petunia hybrida by the introduction of antisense and sense genes. Curr. Top. Microbiol. Immunol. 197 57–75. [DOI] [PubMed] [Google Scholar]
- Elmayan, T., and Vaucheret, H. (1995). Meiotically reversible silencing can affect single copies of a foreign transgene expressed at a very high level. Plant Cell 10 1747–1757. [Google Scholar]
- English, J.J., Mueller, E., and Baulcombe, D.C. (1996). Suppression of virus accumulation in transgenic plants exhibiting silencing of nuclear genes. Plant Cell 8 179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin, J., Chapman, K., Swaney, S., Parks, T.D., Wernsman, E.A., and Dougherty, W.G. (1996). Genetic and biochemical dissection of transgenic RNA–mediated virus resistance. Plant Cell 8 95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, H.S., Lopez-Moya, J.J., and Garcia, J.A. (1999). Mitotic stability of infection-induced resistance to plum pox potyvirus associated with transgene silencing and DNA methylation. Mol. Plant-Microbe Interact 12 103–111. [DOI] [PubMed] [Google Scholar]
- Hamilton, A.J., and Baulcombe, D.C. (1999). A novel species of small antisense RNA in post-transcriptional gene silencing. Science 286 950–952. [DOI] [PubMed] [Google Scholar]
- Hart, C.M., Fischer, B., Neuhaus, J.M., and Meins, F., Jr. (1992). Regulated inactivation of homologous gene expression in transgenic Nicotiana sylvestris plants containing a defense-related tobacco chitinase gene. Mol. Gen. Genet. 235 179–188. [DOI] [PubMed] [Google Scholar]
- Haseloff, J., Siemering, K.R., Prasher, D.C., and Hodge, S. (1997). Removal of a cryptic intron and subcellular localization of green fluorescent protein are required to mark transgenic Arabidopsis plants brightly. Proc. Natl. Acad. Sci. USA 94 2122–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingelbrecht, I., Van Houdt, H., Van Montagu, M., and Depicker, A. (1994). Post-transcriptional silencing of reporter transgenes in tobacco correlates with DNA methylation. Proc. Natl. Acad. Sci. USA 91 10502–10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, A.L., Johanssen, I.E., Bean, S.J., Bach, I., and Maule, A.J. (1998. a). Specificity of resistance to pea seed–borne mosaic potyvirus in transgenic peas expressing the viral replicase (NIb) gene. J. Gen. Virol. 79 3129–3137. [DOI] [PubMed] [Google Scholar]
- Jones, A.L., Thomas, C.L., and Maule, A.J. (1998. b). De novo methylation and co-suppression induced by a cytoplasmically replicating plant RNA virus. EMBO J. 17 6385–6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, A.L., Hamilton, A.J., Voinnet, O., Thomas, C.L., Maule, A.J., and Baulcombe, D.C. (1999). RNA–DNA interactions and DNA methylation in post-transcriptional gene silencing. Plant Cell 11 2291–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaido, M., Mori, M., Mise, K., Okuno, T., and Furusawa, I. (1995). Inhibition of brome mosaic virus (BMV) amplification in protoplasts from transgenic tobacco plants expressing replicable BMV RNAs. J. Gen.Virol. 76 2827–2833. [DOI] [PubMed] [Google Scholar]
- Kasschau, K.D., and Carrington, J.C. (1998). A counterdefensive strategy of plant viruses: Suppression of post-transcriptional gene silencing. Cell 95 461–470. [DOI] [PubMed] [Google Scholar]
- Kumagai, M.H., Donson, J., Della-Cioppa, G., Harvey, D., Hanley, K., and Grill, L.K. (1995). Cytoplasmic inhibition of carotenoid biosynthesis with virus-derived RNA. Proc. Natl. Acad. Sci. USA 92 1679–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindbo, J.A., Silva-Rosales, L., Proebsting, W.M., and Dougherty, W.G. (1993). Induction of a highly specific antiviral state in transgenic plants: Implications for regulation of gene expression and virus resistance. Plant Cell 5 1749–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller, E., Gilbert, J.E., Davenport, G., Brigneti, G., and Baulcombe, D.C. (1995). Homology-dependent resistance: Transgenic virus resistance in plants related to homology-dependent gene silencing. Plant J. 7 1001–1013. [Google Scholar]
- Palauqui, J.-C., and Vaucheret, H. (1995). Field trial analysis of nitrate reductase co-suppression—A comparative-study of 38 combinations of transgene loci. Plant Mol. Biol. 29 149–159. [DOI] [PubMed] [Google Scholar]
- Palauqui, J.-C., and Vaucheret, H. (1998). Transgenes are dispensable for the RNA degradation step of cosuppression. Proc. Natl. Acad. Sci. USA 95 9675–9680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palauqui, J.-C., Elmayan, T., Pollien, J.-M., and Vaucheret, H. (1997). Systemic acquired silencing: Transgene-specific post-transcriptional silencing is transmitted by grafting from silenced stocks to non-silenced scions. EMBO J. 16 4738–4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff, F., Harrison, B.D., and Baulcombe, D.C. (1997). A similarity between viral defense and gene silencing in plants. Science 276 1558–1560. [DOI] [PubMed] [Google Scholar]
- Ratcliff, F., MacFarlane, S., and Baulcombe, D.C. (1999). Gene silencing without DNA: RNA-mediated cross protection between viruses. Plant Cell 11 1207–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz, M.T., Voinnet, O., and Baulcombe, D.C. (1998). Initiation and maintenance of virus-induced gene silencing. Plant Cell 10 937–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez, F., Martinez-Herrera, D., Aguilar, I., and Ponz, F. (1998). Infectivity of turnip mosaic potyvirus cDNA clones and transcripts on the systemic host Arabidopsis thaliana and local lesion hosts. Virus Res. 55 207–219. [DOI] [PubMed] [Google Scholar]
- Sharp, P. (1999). RNAi and double-strand RNA. Genes Dev. 13 139–141. [PubMed] [Google Scholar]
- Stam, M., Viterbo, A., Mol, J.N.M., and Kooter, J.M. (1998). Position-dependent methylation and transcriptional silencing of transgenes in inverted T-DNA repeats: Implications for post-transcriptional silencing of homologous host genes in plants. Mol. Cell. Biol. 18 6165–6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voinnet, O., and Baulcombe, D.C. (1997). Systemic signaling in gene silencing. Nature 389 553. [DOI] [PubMed] [Google Scholar]
- Voinnet, O., Vain, P., Angell, S., and Baulcombe, D.C. (1998). Systemic spread of sequence-specific transgene RNA degradation is initiated by localized introduction of ectopic promoterless DNA. Cell 95 177–187. [DOI] [PubMed] [Google Scholar]
- Voinnet, O., Pinto, Y.M., and Baulcombe, D.C. (1999). Suppression of gene silencing: A general strategy used by diverse DNA and RNA viruses. Proc. Natl. Acad. Sci. USA 96 14147–14152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassenegger, M., Heimes, S., Riedel, L., and Sänger, H.L. (1994). RNA-directed de novo methylation of genomic sequences in plants. Cell 76 567–576. [DOI] [PubMed] [Google Scholar]
- Waterhouse, P.M., Graham, H.W., and Wang, M.B. (1998). Virus resistance and gene silencing in plants can be induced by simultaneous expression of sense and antisense RNA. Proc. Natl. Acad. Sci. USA 95 13959–13964. [DOI] [PMC free article] [PubMed] [Google Scholar]