Abstract
A recessive mutation was identified that constitutively activated the ethylene response pathway in Arabidopsis and resulted in a rosette-lethal phenotype. Positional cloning of the gene corresponding to this mutation revealed that it was allelic to responsive to antagonist1 (ran1), a mutation that causes seedlings to respond in a positive manner to what is normally a competitive inhibitor of ethylene binding. In contrast to the previously identified ran1-1 and ran1-2 alleles that are morphologically indistinguishable from wild-type plants, this ran1-3 allele results in a rosette-lethal phenotype. The predicted protein encoded by the RAN1 gene is similar to the Wilson and Menkes disease proteins and yeast Ccc2 protein, which are integral membrane cation-transporting P-type ATPases involved in copper trafficking. Genetic epistasis analysis indicated that RAN1 acts upstream of mutations in the ethylene receptor gene family. However, the rosette-lethal phenotype of ran1-3 was not suppressed by ethylene-insensitive mutants, suggesting that this mutation also affects a non-ethylene-dependent pathway regulating cell expansion. The phenotype of ran1-3 mutants is similar to loss-of-function ethylene receptor mutants, suggesting that RAN1 may be required to form functional ethylene receptors. Furthermore, these results suggest that copper is required not only for ethylene binding but also for the signaling function of the ethylene receptors.
INTRODUCTION
The gaseous hormone ethylene influences many aspects of plant growth and development, including germination, leaf and flower senescence and abscission, fruit ripening, nodulation, and root hair development (Abeles et al., 1992). Many components of ethylene signaling have been identified, principally through the use of a simple genetic screen that uses the ethylene-mediated seedling morphology known as the triple response. The triple response of Arabidopsis is characterized by an inhibition of elongation of the seedling root and hypocotyl, radial expansion of the hypocotyl, and exaggeration of the curvature of the apical hook (Figure 1). Three classes of response mutants have been identified: ethylene-insensitive mutants, constitutive ethylene response mutants, and those mutants that affect only a subset of organs (reviewed in Kieber, 1997; Johnson and Ecker, 1998).
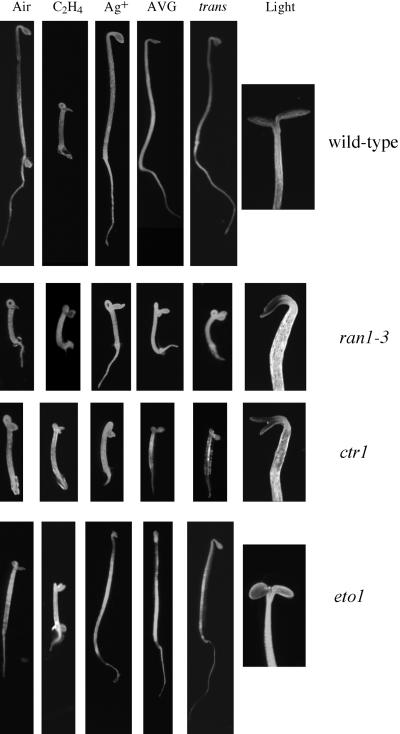
Figure 1.
The ran1-3 Mutation Constitutively Activates the Ethylene Response Pathway in Seedlings.
Phenotypes of 3-day-old etiolated wild-type, ran1-3, ctr1, and eto1 (as indicated at right) seedlings were grown on Murashige and Skoog (MS) medium in air, ethylene (C2H4), or trans-cyclooctene (trans) or were grown in air on MS medium containing AgNO3 (Ag+) or aminoethoxyvinylglycine (AVG) as indicated above each column of seedlings. At right are seedlings that were grown for 3 days in the dark on MS medium in air and then placed in a lighted growth chamber for 12 hr (Light).
The ETR1 gene was identified by a series of dominant, ethylene-insensitive mutations (Bleecker et al., 1988). ETR1 encodes a protein that is similar to bacterial two-component histidine kinases (Chang et al., 1993). Two-component systems are the major route by which bacteria sense and respond to external stimuli, such as phosphate availability and osmolarity (Stock et al., 1990). The C terminus of ETR1 is similar to both the histidine kinase domain of the sensor component and the receiver domain of the response regulators. Yeast cells expressing wild-type, but not mutant, ETR1 bound ethylene with an affinity consistent with the observed concentrations that cause physiological effects. This, coupled with other genetic and molecular data, indicates that ETR1 encodes an ethylene receptor (Schaller and Bleecker, 1995). ETR1 forms disulfide-linked dimers in vivo and has been shown to possess intrinsic histidine kinase activity in vitro (Schaller et al., 1995; Gamble et al., 1998).
ETR1 is part of a small gene family of ethylene receptors in Arabidopsis, and dominant, ethylene-insensitive mutations have been found in several of the other genes in this family (Hua et al., 1995, 1998; Sakai et al., 1998). Loss-of-function mutations in ETR1 result in little or no phenotype, but disruption of multiple homologs results in a constitutive ethylene response phenotype that becomes increasingly strong as additional homologs are disrupted (Hua and Meyerowitz, 1998). This suggests that ETR1 and its homologs function as negative regulators of ethylene signaling and that binding of ethylene serves to inactivate receptor function.
Recessive ethylene-insensitive3 (ein3) mutations result in a weak ethylene-insensitive phenotype (Roman et al., 1995). EIN3 is the founding member of the EIN3/EIL gene family, the members of which encode transcription factors that are involved in regulating ethylene-mediated gene expression (Chao et al., 1997). Overexpression of EIN3 or the other EIL homologs results in a constitutive ethylene response phenotype. Strong alleles of the recessive ein2 mutation completely abolish all ethylene responses that have been tested (Roman et al., 1995). EIN2 encodes a 12-pass transmembrane protein that is most similar to the Nramp family of metal transporters (Alonso et al., 1999). However, although EIN2 has been demonstrated to be an integral membrane protein, its biochemical function remains unknown.
CONSTITUTIVE TRIPLE RESPONSE1 (CTR1), a negative regulator of ethylene signaling, acts downstream of ETR1 and encodes a protein with similarity to the Raf family of protein kinases (Kieber et al., 1993). Recessive mutations in CTR1 have pleiotropic effects on plant development, including a reduction in the size of roots, leaves, and stems; delayed flowering; delayed opening of the apical hook; and ectopic formation of root hairs (Kieber et al., 1993). These phenotypes can be linked to a constitutive activation of the ethylene response pathway and are all suppressed by downstream ethylene-insensitive mutations, such as ein2. The smaller organs in ctr1 mutants have been linked to a reduction in cell expansion. In many plant tissues, this process is regulated by ethylene. Interestingly, when four of the five Arabidopsis ETR1 homologs are disrupted simultaneously, the resulting plants have a phenotype more severe than that of ctr1 null alleles (Hua and Meyerowitz, 1998).
responsive to antagonist1 (ran1) was identified in a screen for mutants that displayed a triple response in response to trans-cyclooctene, a compound that inhibits ethylene action in wild-type plants (Sisler et al., 1990; Hirayama et al., 1999). ran1 mutations are recessive and do not affect the response to exogenous ethylene. Importantly, neither the ran1-1 nor the ran1-2 mutants have any appreciable phenotype in the absence of trans-cyclooctene. Analysis of ethylene-regulated gene expression revealed that ran1 also confers on adult plants the ability to respond positively to trans-cyclooctene. RAN1 has significant similarity to the human Wilson and Menkes disease proteins and yeast Ccc2p, which are involved in copper trafficking (Chelly et al., 1993; Mercer et al., 1993; Vulpe et al., 1993; Fu et al., 1995). Consistent with a role in copper trafficking, the phenotype of the ran1 mutant was partially reverted by growth on copper-supplemented medium, and RAN1 was able to suppress the phenotype of a yeast ccc2 mutant. Biochemical analysis of the ethylene receptor ETR1 has demonstrated that it contains a copper cofactor (Rodriguez et al., 1999), which may be added by a RAN1-dependent mechanism.
Here, we describe ran1-3, a mutation that results in the constitutive activation of ethylene responses in seedlings and adults. Analysis of the interaction with other mutants affected in ethylene action indicates that dominant, ethylene-insensitive receptor mutations are epistatic to ran1-3, indicating that the RAN1 gene product acts very early in signaling. In addition to its role in ethylene signaling, our analysis indicates that RAN1 function is required for cell elongation in an ethylene-independent manner.
RESULTS
Isolation and Phenotypic Characterization of ran1-3
We screened small families of mutagenized Arabidopsis for lines that segregated seedlings displaying a constitutive triple response (Ctr−) phenotype. The use of a family screen allowed the recovery of lethal or infertile mutants. From a screen of 6300 individual ethyl methanesulfonate–mutagenized M2 seeds (in 2100 families), two new ctr1 alleles were identified (data not shown). In addition, a single line was identified that segregated for a Ctr− seedling phenotype that died after growing for 2 weeks in soil. We identified a sibling from this family that segregated for the Ctr− mutant phenotype. The mutation in this line was originally called constitutive triple response2 but has been renamed ran1-3 because it is allelic to the ran1 mutation. ran1-3 complemented ctr1 (42 of 42 F1 seedlings from a ctr1/ctr1 × RAN1/ran1-3 cross displayed a wild-type phenotype, and the F2 from all F1 plants segregated both wild-type and Ctr− mutant seedlings).
Inhibitors of ethylene biosynthesis (aminoethoxyvinylglycine) or binding (AgNO3 and trans-cyclooctene) did not revert the seedling phenotype of ran1-3, indicating that the mutation probably affects ethylene signaling rather than ethylene biosynthesis (Figure 1). Consistent with this, ran1-3 mutant and wild-type 3-day-old etiolated seedlings produced comparable amounts of ethylene (0.17 ± 0.07 pL of ethylene seedling−1 hr−1 for ran1-3 versus 0.19 ± 0.05 pL of ethylene seedling−1 hr−1 for wild-type seedlings).
When grown in air, the hypocotyls of 3-day-old etiolated ran1-3 seedlings were shorter than those of the ctr1-5 mutant, which is a strong ctr1 allele (2.4 ± 0.5 mm for ran1-3 versus 3.1 ± 0.7 mm for ctr1). In contrast, the roots of ran1-3 mutant seedlings were not significantly shorter (1.5 ± 0.3 mm) than those of ctr1-5 (1.4 ± 0.4 mm). Like ctr1 mutant and ethylene-treated wild-type seedlings, the apical hook and cotyledons of ran1-3 mutants take longer to open when etiolated seedlings are shifted to the light, and the cotyledons are darker green (Figure 1).
ran1-3 segregated as a single-gene, recessive mutation. However, the progeny of a self-fertilized ran1-3 heterozygote segregated a Ctr− phenotype at a frequency that deviated from the expected 25% (554 wild type: 115 ran1-3, χ2 = 22, P < 0.05). We examined reciprocal crosses between ran1-3 heterozygotes and wild-type plants to determine if the transmission of ran1-3 is reduced through the male, the female, or both gametes (Table 1). Transmission of ran1-3 through the female gamete did not differ from the expected 1:1 segregation ratio in the F1 in crosses to wild-type males. However, when ran1-3 heterozygotes were used as males in crosses to wild-type females, the F1 showed a marked departure from the expected 1:1 ratio; wild-type alleles were approximately twice as likely as ran1-3 to be transmitted to the F1 through the pollen. In contrast, ctr1 mutations display a defect in transmission through the female gametes (Kieber et al., 1993).
Table 1.
The ran1-3 Allele Is Transmitted Poorly through the Male Gamete
| F1 Genotypeb
|
|||
|---|---|---|---|
| Crossa | RAN1/RAN1 | RAN1/ran1-3 | χ2c |
|
ran1-3/RAN1 × RAN1/RAN1 |
49 | 44 | 0.54 (P > 0.5) |
|
RAN1/RAN1 × RAN1/ran1-3 |
31 | 16 | 4.8 (P < 0.5) |
The first plant listed in each cross is the female.
Determined by progeny testing each F1 plant.
Calculated for an expected 1:1 ratio.
When grown on soil, ran1-3 mutants die after forming only two to three sets of true leaves (Figures 2A and 2B). The cotyledons and leaves of ran1-3 senesce much earlier than those of wild-type plants and display high amounts of anthocyanins (Figure 2B). The rosette of ran1-3 is quite compact (<5.0 mm in diameter), and the true leaves are much smaller than those of the wild type or the ctr1-5 mutant. ran1-3 mutants do not bolt, and they rarely survive for >21 days on soil. Like ctr1, ran1-3 mutants have a greatly reduced root system and display an increase in the number of root hairs (data not shown). These adult phenotypes cosegregate with the seedling phenotype (i.e., within <1.9 map units of the seedling phenotype with a 95% degree of confidence), suggesting that they result from a single mutation. This was confirmed by complementation of both the seedling and the adult phenotypes with the same gene (see below).
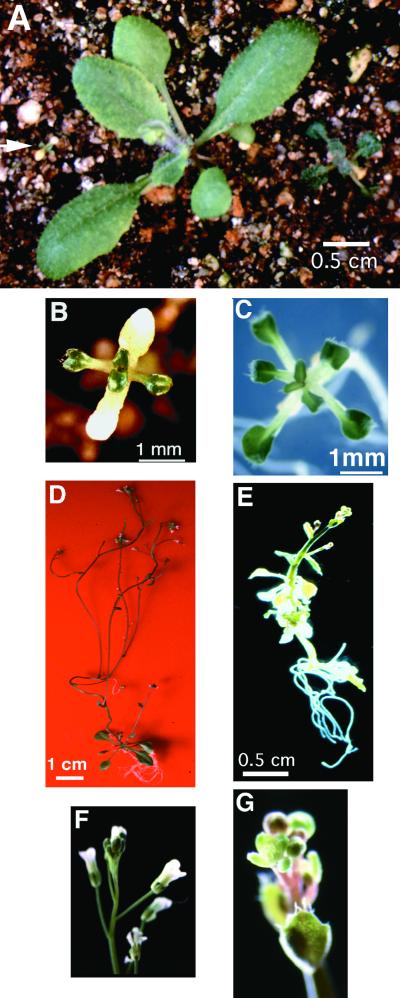
Figure 2.
Phenotypes of the Adult ran1-3 Mutants.
(A) Phenotype, from left to right, of ran1-3, wild-type, and ctr1 18-day-old adult plants grown in soil during long days. The ran1-3 plant is indicated by an arrow.
(B) Close-up of a ran1-3 mutant grown in soil as given in (A). Note the senescence of the cotyledons.
(C) Phenotype of a 21-day-old ran1-3 plant grown in vitro on Gamborg's medium.
(D) Phenotype of a 28-day-old wild-type plant grown in vitro on Gamborg's medium.
(E) Phenotype of a 28-day-old ran1-3 mutant grown in vitro on Gamborg's medium.
(F) Close-up of a flower from a 28-day-old wild-type plant grown in vitro on Gamborg's medium.
(G) Close-up of a flower from a 28-day-old ran1-3 mutant grown in vitro on Gamborg's medium. The ran1-3 mutant floral structures do not develop past this point. Note the accumulation of anthocyanin in the ran1-3 floral structures.
When grown in vitro on Gamborg's media, ran1-3 mutants were more robust than their soil-grown counterparts (Figure 2C). The rosette was slightly larger, and early senescence was not observed. In contrast to soil-grown ran1-3 plants, ran1-3 mutants grown in vitro produced an inflorescence, although flowering was delayed relative to the wild-type plants, and the inflorescence was very small (Figure 2E). ran1-3 mutants formed flowers in vitro, but these never fully developed or opened, and the plants were not fertile (Figure 2G). These rudimentary ran1-3 flowers displayed high concentrations of anthocyanins.
The ran1-3 Mutation Affects Cell Size and Ethylene-Regulated Gene Expression
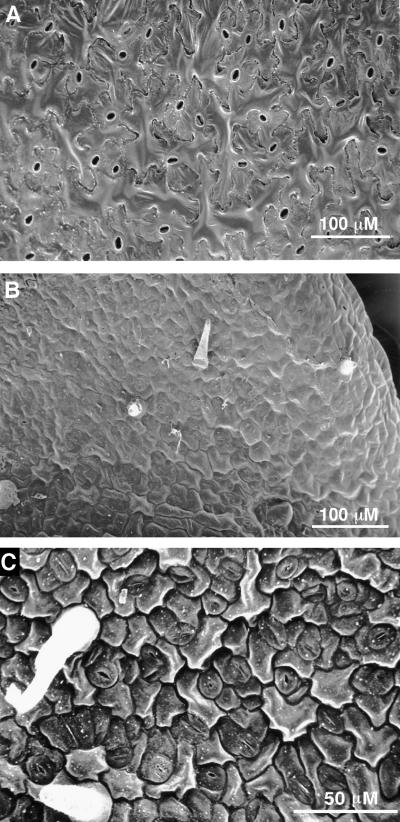
To determine the basis for the reduced size of ran1-3 mutants, we examined the size of leaf epidermal cells by using scanning electron microscopy. As shown in Figure 3, ran1-3 epidermal cells were much smaller than those of the wild type and were only slightly larger than stomatal cells. The ran1-3 epidermal cells were also considerably smaller than those from the ctr1 mutant or from wild-type plants grown in ethylene (data not shown), consistent with the smaller size of the ran1-3 leaves. ran1-3 epidermal cells were much rounder than wild-type cells, similar to unexpanded cells. In addition to the cell size difference, there were considerably fewer trichomes on ran1-3 mutant leaves, and the ones that did form were generally misshapen, often developing only a single point, in contrast to the three found in wild-type trichomes.
Figure 3.
The ran1-3 Mutant Displays a Reduction in the Size of Epidermal Cells.
(A) and (B) Wild-type (A) or ran1-3 (B) plants were grown on Gamborg's medium for 13 days, and leaf epidermal cells were observed by scanning electron microscopy, as described in Methods. Note that the ran1-3 epidermal cells are much smaller than their wild-type counterparts. The spikes protruding from the ran1-3 leaves are the trichomes, which in wild-type leaves have three points.
(C) Close-up of ran1-3 epidermal cells. The doughnut-shaped stomata are clearly visible, as are two trichomes (white spikes).
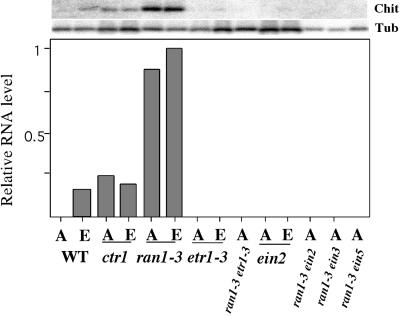
The expression of the β-chitinase gene is induced by ethylene in adult Arabidopsis plants (Samac et al., 1990). We examined the steady state amount of β-chitinase mRNA in air- and ethylene-grown wild-type and mutant plants by using RNA gel blot analysis (Figure 4). As previously observed (Kieber et al., 1993), the β-chitinase gene was expressed to a greater extent in ctr1 mutants in both the presence and the absence of ethylene. This is consistent with the constitutive activation of the ethylene response pathway in the ctr1 mutant. In both control and ethylene-treated ran1-3 mutants, the steady state expression of β-chitinase mRNA was very high, exceeding that observed in wild-type plants treated with high amounts of ethylene or in the ctr1-2 mutant. This indicates that like the ctr1 mutation, the ran1-3 mutation results in the constitutive activation of the ethylene response pathway in adult plants.
Figure 4.
The ran1-3 Mutation Results in Increased Expression of β-Chitinase.
RNA gel blot analysis of wild-type (WT), ctr1, ran1-3, etr1-3, ran1-3 etr1-3, ein2, ran1-3 ein2, ran1-3 ein3, and ran1-3 ein5 (as indicated) adult tissues grown on soil. Plants were placed in an enclosed chamber containing either air (A) or 10 μL L−1 ethylene (E) for 48 hr as indicated. Total RNA was extracted, separated by gel electrophoresis, blotted to a nylon membrane, and hybridized with either a β-chitinase probe (Chit) or a β-tubulin probe (Tub). The signals were quantified by using a PhosphorImager. The signal from the β-chitinase blot was normalized to the β-tubulin signal, and the highest ratio was then assigned a value of 1; the others were plotted relative to that. The original image of the gel blot is shown at top, and the lanes correspond to the labeling of the graph's x axis.
Genetic Interaction between ran1-3 and Other Ethylene Action Mutants
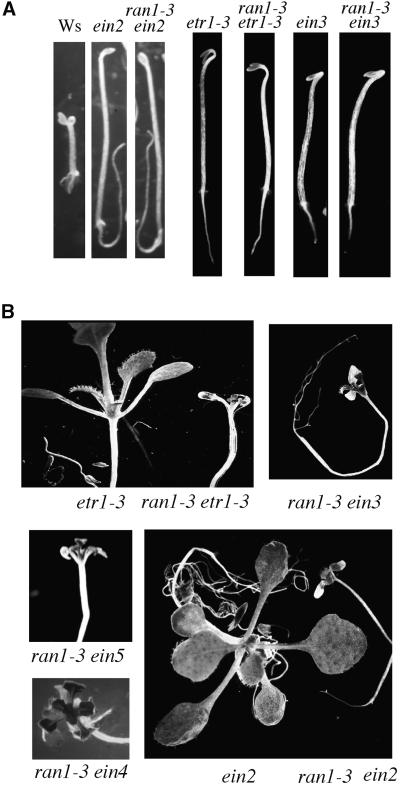
To determine the order of action of RAN1 relative to other ethylene-signaling components, we analyzed the epistasis between ran1-3 and various ein mutations (Figure 5). These ein mutations all affect ethylene perception or signal transduction in both seedlings and adult plants. Double mutants of ran1-3 with etr1-3, ein2, ein3, ein4, ein5, and ein6 were generated, and their seedling and adult phenotypes were examined. All the double mutants displayed ethylene insensitivity as 3-day-old etiolated seedlings (Figure 5; data not shown), indicating that the RAN1 gene product acts at the same place as or upstream of the products of these genes. Seedlings doubly mutant for both ran1-3 and each of these ethylene-insensitive mutations could not be visually distinguished from the ethylene-insensitive single mutants grown under the same conditions, indicating that the suppression of the ran1-3 phenotype was essentially complete. However, when all six of these ran1-3 ein double mutants were transferred to soil, the adult plants displayed the phenotype of the ran1-3 mutant (Figure 5; data not shown). The rosettes of double mutant plants were similar to ran1-3 single mutants in size and appearance. These results were confirmed with multiple F2 lines for each double mutant combination.
Figure 5.
Phenotype of Various Double Mutants.
(A) Seedling phenotype of wild-type (Ws) and single and double mutant seedlings (as indicated at the top) grown in ethylene in the dark for 3 days.
(B) Eighteen-day-old wild-type and single and double mutant plants (as indicated below each photograph). All the adult plants were grown under long days on soil, except for the ran1-3 ein4 double mutant, which was grown under long days on Gamborg's media.
To determine whether the phenotype of the double mutants reflected constitutive activation of the ethylene response pathway, we examined expression of the β-chitinase gene (Figure 4). As expected, the steady state amount of β-chitinase mRNA was not induced by ethylene in the etr1-3 or ein2 mutants grown in either air or ethylene. Despite their ran1-3 morphology, the adult plants of the ran1-3 etr1-3, ran1-3 ein2, ran1-3 ein3, and ran1-3 ein5 double mutants did not have elevated expression levels of β-chitinase. This suggests that the double mutant adult plants do not constitutively activate the ethylene response pathway, which is consistent with the phenotypes of the double mutant etiolated seedlings.
Cloning of Gene Corresponding to ran1-3
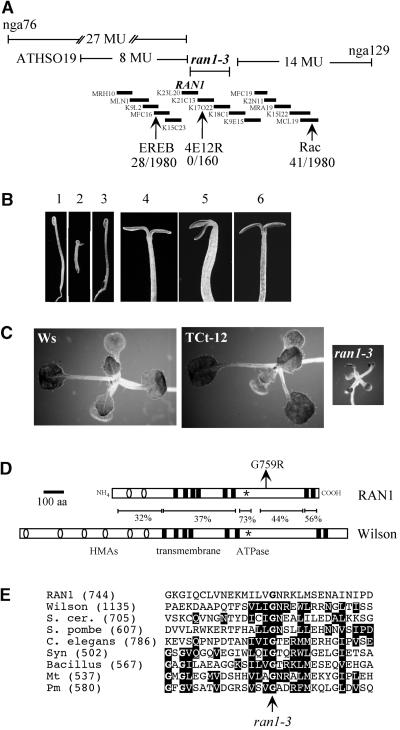
We mapped the ran1-3 mutation to chromosome 5 by using a series of simple sequence length polymorphic (SSLP) markers. This map position was refined by using cleaved amplified polymorphic sequences (CAPS) markers that we generated using sequence information obtained from the Arabidopsis genome sequencing project (see Methods). We found that ran1-3 mapped very close to the RAN1 gene, which also had been linked to ethylene signaling (Hirayama et al., 1999). We thus sequenced the RAN1 gene amplified from ran1-3 mutant as well as wild-type Wassilewskija (Ws) genomic DNA (because the original RAN1 sequence was from the Columbia ecotype). Comparison of the sequences revealed that the ran1-3 mutation was correlated with a single nucleotide change in the RAN1 coding region (Figure 6). This mutation is a G-to-A transition at nucleotide 3061 of the genomic sequence (in which the A of the predicted start codon is defined as residue 1), which results in the replacement of Gly-759 with an Arg residue. Gly-759 is in the loop of RAN1 that contains the predicted ATPase domain, even though it does not occur within the highly conserved ATPase signature sequence that surrounds the Asp residue involved in the aspartyl phosphate intermediate (Figure 6D). This Gly is invariant in all sequenced cation transport P-type ATPases, including genes from eubacteria and archaebacteria (Figure 6E).
Figure 6.
Cloning of RAN1-3.
(A) Physical mapping of ran1-3. The thick bars represent ordered bacterial artificial chromosome (BAC) clones from the physical map of the 16- to 18-Mb region of chromosome 5 as determined by the Kazusa sequencing group. The BAC containing the RAN1 gene is indicated. The identities of each BAC are indicated to the left of the bars. Shown above are the map distances from the initial mapping with the SSLP markers. The distances from the ran1-3 mutation to each marker are indicated. Note that the lines are not to scale. The results of fine-structure mapping of 990 ran1-3 seedlings plants (1980 chromosomes) from the F2 progeny of a cross between ran1-3 and the wild type (ecotype Columbia) are indicated below. The Rac and the EREB CAPS markers are described in Methods. MU, map units.
(B) The RAN1 gene complements the seedling phenotype of ran1-3. Phenotype of 3-day-old etiolated seedlings (seedlings 1 to 3) and etiolated seedlings shifted to the light for 14 hr (seedlings 4 to 6). Wild-type (seedlings 1 and 4), ran1-3 (seedlings 2 and 5), and TCt-12 seedlings (seedlings 3 and 6) were grown on MS media at 23°C, and representative seedlings were photographed. The TCt-12 seedlings are T2 progeny of a transformed line that harbors a wild-type RAN1 transgene and which is homozygous mutant at the endogenous ran1-3 locus, as determined by molecular analysis (see Methods). Note that the RAN1 gene complements both the Ctr− and the delayed hook opening phenotypes of ran1-3.
(C) The RAN1 gene complements the adult phenotype of mature ran1-3 plants. Phenotype of wild-type (Ws), TCt-12 (see [B]), and ran1-3 12-day-old plants grown on Gamborg's medium are shown.
(D) Diagram of RAN1 and the Wilson's disease proteins. The numbers represent the percentage identities between the various domains of the two protein sequences. The various domains are indicated below the Wilson's (Wilson) gene. The heavy metal binding domains (HMAs) are represented by open circles, the transmembrane domains are indicated by black boxes, and the P-type cation transport ATPase signature motif is indicated with asterisks. The position of the amino acid alteration predicted from the ran1-3 mutation is indicated with an arrow. The HMAs and the ATPase signature motif were identified by using a Prosite search. The transmembrane domains were predicted with the Tmpred program at EMBnet (www.ch.embnet.org/index.html). aa, amino acids.
(E) Lineup of the region surrounding the ran1-3 mutation. Various P-type cation transport ATPases were aligned by using the Clustal program; the region surrounding the ran1-3 mutation is shown. The numbers within parentheses indicate the residue number of the first amino shown in the lineup. The protein sequences were from the predicted amino acid sequences from genes from the following species (GenBank accession numbers are given in parentheses): Wilson, human (4502323); S. cer., Saccharomyces cerevisiae CCC2 gene (P38995); S. pombe, Schizosaccharomyces pombe (CAA18378); C. elegans, Caenorhabditis elegans (BAA20550); Syn, Synechoccus sp PCC7942 (P37279); Bacillus, Bacillus subtilis (CAB15355); Mt, Methanobacterium thermoautotrophicum (AAB86009); and Pm, Proteus mirabilis (AAB01764). The residues in reverse type indicate identity to the RAN1 amino acid sequence.
To confirm that this gelne corresponds to the ran1-3 mutations, we tested whether a genomic fragment spanning the RAN1 gene could complement the ran1-3 mutation. A population segregating for ran1-3 mutants was transformed with a RAN1 genomic fragment comprising the entire coding region plus 1 kb of the 5′ and 0.5 kb of the 3′ flanking DNA. Transformants were selected, and a line (TCt-12) was identified that was segregating ∼17% Ctr− seedlings. The kanamycin resistance marker, which was contained on the T-DNA, cosegregated 100% with wild-type progeny (40 of 40 wild-type seedlings were kanamycin resistant, and 120 of 120 Ctr− seedlings were kanamycin sensitive). We conclude that the RAN1 gene complements the ran1-3 mutation and that line TCt-12 was a homozygous mutant at the endogenous RAN1 locus and heterozygous for the transgene. We confirmed that the endogenous RAN1 locus in this line was homozygous mutant in the phenotypically wild-type seedlings by using a CAPS marker created by the ran1-3 mutation (see Methods). Importantly, the RAN1 gene complemented both the seedling and adult ran1-3 phenotypes, indicating that this mutation is responsible for all the phenotypes observed in this line (Figures 6B and 6C).
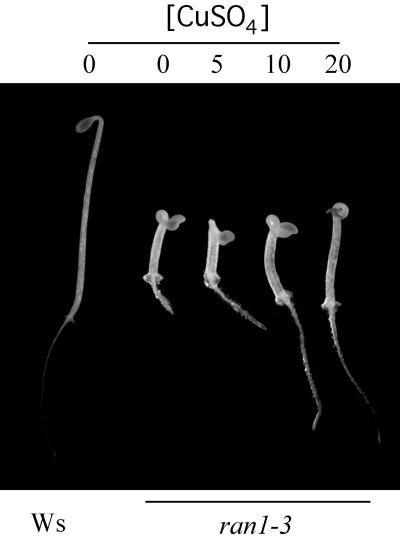
Copper Ion Partially Suppresses the ran1-3 Seedling Phenotype
Addition of copper ions to the media suppresses the phenotype of both the ran1 and the yeast ccc2 mutants (Fu et al., 1995; Yuan et al., 1995; Hirayama et al., 1999), consistent with their role in mediating copper trafficking. To test whether copper could suppress the phenotype of ran1-3, we examined seedlings grown on Murashige and Skoog (MS) media supplemented with various concentrations of CuSO4 (Figure 7). Addition of as much as 20 μM CuSO4 to the media had little or no effect on the germination or growth of 3-day-old etiolated wild-type or eto1 seedlings (data not shown). The roots of 3-day-old etiolated ran1-3 mutant seedlings were progressively longer when greater concentrations of CuSO4 were included in the media (up to 20 μM), which indicates that cupric ion is able to partially suppress this aspect of the triple response phenotype of the ran1-3 mutant. In contrast to the roots, there was little or no change in the length of the hypocotyl or the curvature of the apical hook of etiolated ran1-3 seedlings, even at 20 μM of CuSO4. Greater concentrations of copper had toxic effects on seedling growth (data not shown). We detected no amelioration of the delayed hook opening phenotype or the adult phenotypes of ran1-3 mutants by supplementing with various concentrations of CuSO4 up to 20 μM (data not shown).
Figure 7.
Effect of CuSO4 on the Phenotype of ran1-3 Seedlings.
Seeds were plated on MS media containing the indicated amounts (in μm) of CuSO4. After a 4-day cold treatment, the plates were incubated in the dark for 3 days, and representative seedlings were photographed.
DISCUSSION
We identified ran1-3, a constitutive ethylene response mutant with a rosette-lethal phenotype, by using a family screen for seedlings displaying the triple response in the absence of exogenous ethylene. Several lines of evidence indicate that ran1-3 is a constitutive ethylene-signaling mutant. First, ran1-3 mutant seedlings display a Ctr− phenotype. This phenotype is not reverted by inhibitors of ethylene biosynthesis or binding, and ran1-3 seedlings produce wild-type amounts of ethylene. The seedling phenotype of ran1-3 mutants is suppressed by ethylene-insensitive mutants, indicating that the Ctr− phenotype results from an activation of the ethylene response pathway. Finally, ran1-3 adult plants display a constitutive activation of an ethylene-inducible gene, and this phenotype also is suppressed by ethylene-insensitive mutations. However, the morphological changes that result from the ran1-3 mutation are not observed in plants grown continuously in the presence of saturating concentrations of ethylene (Kieber et al., 1993) and are not suppressed by ethylene-insensitive mutations, suggesting that ran1-3 also affects a non-ethylene-dependent signaling pathway that affects cell expansion.
Molecular analysis indicated that ran1-3 disrupts the RAN1 locus. This result was surprising because the previously identified ran1 mutants are phenotypically distinct from the ran1-3 mutant. ran1-1 and ran1-2 were identified as seedlings that displayed a triple response when exposed to the ethylene antagonist trans-cyclooctene (Hirayama et al., 1999). Apart from their altered response to trans-cyclooctene, ran1-1 and ran1-2 have no discernable effect on the morphology of seedlings or adult plants. RAN1 encodes a protein with substantial amino acid similarity to the human Menkes and Wilson disease proteins (Bull et al., 1993; Chelly and Monaco, 1993; Chelly et al., 1993; Mercer et al., 1993; Tanzi et al., 1993; Vulpe et al., 1993; Yamaguchi et al., 1993) and to yeast Ccc2p (Fu et al., 1995). These proteins are P-type cation transport ATPases that are involved in copper trafficking. Ccc2p is required in yeast to provide copper to the Fet3 protein (Yuan et al., 1995), which is a multicopper oxidase involved in high-affinity iron transport (Askwith et al., 1994). In the absence of Ccc2p, no functional Fet3 protein is made, and the yeast cells become iron deficient. Hirayama et al. (1999) have proposed that RAN1 functions to add copper ions to the ETR1 family of ethylene receptors.
Copper is required for the full ethylene binding activity of ETR1 expressed in yeast (Rodriguez et al., 1999), and the ran1-1 and ran1-2 mutations have been suggested to cause decreased loading of copper ions to the ethylene receptors, resulting in a change in receptor output in response to trans-cyclooctene (Hirayama et al., 1999). The ran1-1 and ran1-2 mutations are not amorphic, because cDNAs containing these mutations still can complement a yeast ccc2 mutant, albeit not as efficiently as the wild-type RAN1 gene (Hirayama et al., 1999). In contrast to ran1-3 mutants, the ran1-1 and ran1-2 mutants have no observable distinct adult phenotype, consistent with the notion that these two mutations represent very subtle changes in the function of RAN1. Although it is unclear whether ran1-3 is an amorphic mutation, it does disrupt a residue that is absolutely conserved in all described cation-transporting P-type ATPases across several kingdoms (Solioz et al., 1994; Lutsenko and Kaplan, 1995; Solioz and Vulpe, 1996). This, coupled with its striking phenotype and the lack of morphological phenotype of the ran1-1 and ran1-2 mutations, suggests that if not amorphic, ran1-3 represents at least a very strong loss-of-function allele. Phenotypically, ran1-3 mutants resemble cosuppressed RAN1 lines, although the latter have not been well characterized (Hirayama et al., 1999).
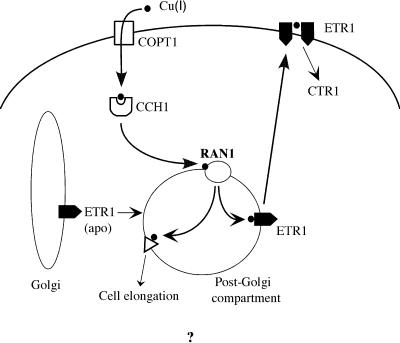
In yeast, Ccc2p acts downstream of a high-affinity copper transporter, CTR1 (not related to the Arabidopsis CTR1 gene), and Atx1p, a copper chaperone protein that binds copper en route to Ccc2p (Dancis et al., 1994; Lin et al., 1997). Yeast two-hybrid analysis demonstrated that Atx1p, which contains one copper binding motif, was able to interact directly with the putative copper binding domain of Ccc2p (Pufahl et al., 1997; Hamza et al., 1999). Because this interaction was dependent on copper ions, it suggested that Atx1p could donate copper to Ccc2p by direct interaction and copper exchange. Homologs of both the yeast CTR1 and Atx1 genes have been identified in Arabidopsis (Kampfenkel et al., 1995; Himelblau et al., 1998). Using the yeast system as a guide, the model for RAN1 function presented in Figure 8 postulates that copper enters the cell by way of COPT1, the Arabidopsis homolog of the yeast CTR1 gene. The CCH1 protein (the Atx1 homolog) then brings the copper to RAN1, which may reside in a post-Golgi compartment vesicle. Here, RAN1 delivers copper ion to the ethylene receptors, which then become functional and ultimately may be sent to the plasma membrane. RAN1 also may act to deliver copper to other cuproenzyme(s), which when defective may lead to the rosette-lethal phenotype observed in the ran1-3 mutant.
Figure 8.
Model of RAN1 Function.
Copper ion (filled circle) is hypothesized to be transported into the cell and brought to the RAN1 protein by the Arabidopsis homologs of the yeast CTR1 and ATX1 genes, called COPT1 and CCH1, respectively. The RAN1 protein (open circle) then delivers copper ion to the ETR1 apoprotein (apo), which then becomes functional and possibly is transported to the plasma membrane. In the absence of ethylene, ETR1 functions to activate CTR1, which is a negative regulator of the ethylene response pathway. Analysis of the ran1-3 mutant suggests that the protein also is required for the function of additional cuproenzymes, which are represented by the open triangle. Arrows indicate either direct or indirect interactions between elements. See the text for additional details.
The constitutive ethylene response phenotype of ran1-3 mutants is consistent with a disruption in the function of the ethylene receptors (Hua and Meyerowitz, 1998). This suggests that RAN1 is required to form functional ethylene receptors and, by extension, that copper is required for their function. Copper has been shown to be required for high-level ethylene binding of ETR1 purified from yeast; however, the results presented here indicate that copper also is required for the signaling function of the ethylene receptors. The suppression of the ran1-3 constitutive ethylene phenotype by the ein4 and etr1-3 mutants suggests that that the phenotype of ran1-3 mutants is not likely to be due to the receptors getting caught up in the secretory pathway through a lack of copper addition. Furthermore, this suppression indicates that the etr1-3 and ein4 mutations result in a catalytically hyperactive receptor that can function in the absence of copper. This is consistent with the results of Hall et al. (1999), who found that some etr1 alleles that resulted in dominant ethylene insensitivity did not impair ethylene binding. However, the etr1-3 mutation did markedly reduce ethylene binding, which suggests that this mutation both disrupts ligand binding and results in a catalytically hyperactive receptor. Interestingly, although gain-of-function receptor mutations suppressed ran1-3, silver ion did not. How silver ion blocks ethylene responses is unclear (Beyer, 1976), but it has been shown to enhance the binding of ethylene to purified ETR1 (Rodriguez et al., 1999). The lack of reversion of the ran1-3 phenotype by silver ion suggests that silver ion does not act by constitutively activating the signaling function of the receptors, or if it does, then this activation requires copper ion.
The ran1-3 mutation could cause rosette lethality in several ways. Copper, an essential micronutrient required for the activity of several cuproenzymes (Linder, 1991), is, however, very reactive in biological systems; disruption of copper homeostasis results in the rapid generation of reactive oxygen species. The Wilson and Menkes disease proteins are localized to the trans-Golgi network and relocalize to the plasma membrane when intracellular copper concentrations increase, presumably facilitating copper efflux (Vulpe et al., 1993; Petris et al., 1996; Hung et al., 1997). Thus, these proteins have been postulated to act both in the assembly of cuproenzymes and in removing excess copper from the cell. Perhaps the RAN1 protein plays a similar role in plant cells. If so, then the ran1-3 mutation could disrupt copper homeostasis, resulting in an increase of intracellular copper to toxic concentrations. However, this seems unlikely because ran1-3 mutants do not display increased sensitivity to copper (K.E. Woeste and J.J. Kieber, unpublished observations). Alternatively, the lethality of ran1-3 could result from the loss of catalytic activity of an essential cuproenzyme or enzymes, as we have postulated in the model presented in Figure 8. Also, disruption of the function of all the ethylene receptors could result in the rosette lethality. Although disruption of four of the five Arabidopsis ethylene receptors is not lethal, it results in a stronger phenotype than null ctr1 mutations, raising the possibility that these proteins may act in pathways other than just ethylene signaling.
The ran1-3 mutation, like ctr1, demonstrates reduced genetic transmission. However, in contrast to ctr1, which shows reduced transmission only through the female gamete (Kieber and Ecker, 1994), ran1-3 appears to affect transmission through male gametes. This unusual effect on one gamete but not the other by different constitutive ethylene-signaling mutants seems to suggest that the defects in transmission seen in each case are not the result of constitutive ethylene signaling or of ethylene-mediated inhibition of cell expansion. Otherwise, one would expect that both mutations would affect gametes of the same gender. The role of ctr1 and ran1-3 in gamete development or fertilization (or both) may be related to pleiotropic effects of these genes that remain to be elucidated.
METHODS
Growth of Plants
The Columbia and Wassilewskija (Ws) ecotypes of Arabidopsis thaliana were used in this study. The ein6 and ein7 mutants were obtained from the Arabidopsis Biological Resource Center (Ohio State University, Columbus). Seeds were surface-sterilized and grown on sterile Murashige and Skoog (MS) media (GIBCO), or they were grown on Gamborg's B5 medium plus 0.8% agar as described in Vogel et al. (1998b), or they were sown directly onto Metro mix 200 (Grace, Boca Raton, FL) as indicated. CuSO4, aminoethoxyvinylglycine (10 μM) and AgNO3 (17 μg/mL) were added directly to MS agar before the seeds were plated. For trans-cyclooctene treatment, plates were placed in a sealed 3-L polystyrene chamber (Billups-Rothenberg, Del Mar, CA) containing 4 μL of liquid trans-cyclooctene. Adult plants were grown in soil under continuous illumination at 23°C.
Isolation of Mutants by Family Screen
Arabidopsis seeds (ecotype Ws) were mutagenized as described by Vogel et al. (1998b) and sown directly onto soil in 4-inch-diameter (∼10 cm) pots. Plants were thinned to three or four plants per pot and grown to maturity. Seeds from each pot were harvested separately, and a portion of the seeds from each family was screened for triple response mutants as described previously (Kieber et al., 1993). Lethal Ctr− mutants were rescued by identifying heterozygous siblings from the family by progeny testing.
Genetic Analysis
ran1-3 heterozygotes were crossed to etr1-3, ein2, ein3, ein4, ein5, and ein6 mutants to create double mutant stocks for analysis of epistasis. The ran1-3 mutations were isolated from the Ws ecotype, ein6 from the Landsberg erecta ecotype, and the other ethylene-insensitive mutants from the Columbia ecotype. Plants used in crosses were verified as ran1-3 heterozygotes by progeny testing. To identify ran1-3 ein double mutants, we identified homozygous Ein− F2 plants either by their phenotype (for recessive mutations) or by progeny testing (for dominant mutations). We examined progeny from multiple homozygous ein lines for adults that displayed the phenotype of the ran1-3 mutant. Plants that exhibited both the seedling Ein− phenotype and the adult ran1-3 phenotype were used as the double mutants for the RNA gel blot analysis.
Measurement of Ethylene Production
Wild-type and ran1-3 heterozygous seeds were surface-sterilized, plated on MS agar, and incubated at 4°C for 4 days. They then were incubated in the dark for 48 hr at 23°C. Wild-type and ran1-3 mutant plants were identified, and ∼15 were transferred to 22-mL gas chromatography vials containing 3 mL of MS medium. The vials were flushed with hydrocarbon-free air and sealed for 24 hr. The accumulated ethylene was measured with a gas chromatograph (Perkin-Elmer) as described previously (Vogel et al., 1998b). All observations were from at least three replicates, and each experiment was repeated at least once with comparable results.
RNA Gel Blot Analysis
Seeds were germinated on MS agar, and 3-day-old seedlings then were transferred to soil and grown for 1 to 2 weeks under constant light at 23°C. For the ran1-3 ein double mutants, Ein− seedlings were picked, and those that displayed a ran1-3 mutant adult phenotype were chosen for RNA isolation. For ran1-3 mutants, Ctr− seedlings were picked from the progeny of a selfed heterozygote. Plants treated with ethylene were grown in soil and placed in an illuminated incubator at 23°C with a continuous flow of ethylene (10 μL L−1) for 48 hr. Total RNA was isolated, separated by agarose gel electrophoresis, and blotted to a nylon membrane as described (Ausubel et al., 1994). The filters were hybridized with radiolabeled probes by using Rapid-hyb buffer as described by the manufacturer (Amersham). Signals were quantified by using a PhosphorImager (Molecular Dynamics, Sunnyvale, CA). The β-chitinase gene was obtained from plasmid pMON8817 (Samac et al., 1990), and the β-tubulin gene was obtained by polymerase chain reaction (PCR) amplification from genomic Arabidopsis DNA (Vogel et al., 1998a).
Organ and Cell Size
The roots and hypocotyls of 3-day-old etiolated seedlings grown on MS agar were measured to the nearest 0.5 mm with a dissecting microscope. At least 23 individuals of each genotype were measured.
For the evaluation of epidermal cell size, wild-type Ws and ran1-3 mutant plants were grown on Gamborg's agar under sterile conditions with continuous illumination for 13 days after germination at 23°C. Plants were prepared for scanning electron microscopy by immersion in 2.5% gluteraldehyde in 50 mM sodium cacodylate buffer for 2 hr at room temperature, followed by two 10-min washes in 50 mM sodium cacodylate. Tissues then were dehydrated in an ethanol concentration gradient of 25, 50, 75, 95, and 100% ethanol. After dehydration, the tissue was dried by using a critical-point drier and then coated with gold to a thickness of 300 Å with a sputter coater and mounted on Al stumps. Micrographs were taken with a DS 130 scanning electron microscope (Topcon Corp., Paramus, NJ).
Cloning the Gene Corresponding to the ran1-3 Mutation
ran1-3 heterozygotes (ecotype Ws) were backcrossed to wild-type plants of the Columbia ecotype. Three-day-old etiolated F2 seedlings were isolated that displayed the Ctr− phenotype and then were grown for 10 days on Gamborg's medium agar to confirm their phenotype. DNA was prepared as described (Vogel et al., 1998a) and used in PCRs to amplify simple sequence length polymorphisms (SSLPs; Bell and Ecker, 1994) and cleaved amplified polymorphic sequences (CAPS; Konieczny and Ausubel, 1993) markers. The SSLPs were used to map ran1-3 to chromosome 5. To further refine the map position of ran1-3, we generated a series of CAPS markers near the region of ran1-3 by sequencing genomic DNA isolated by PCR amplification with primers derived from the Arabidopsis genomic sequencing project. We identified sequence differences between the two ecotypes that resulted in restriction polymorphisms. Two sets of primers then were designed, one nested relative to the other, to amplify the DNA surrounding the polymorphic site. These primers were used in a single PCR or in two sequential PCRs (to increase the robustness of the markers) as indicated, with 1 μL of 1:100 dilution of the first reaction used as a substrate for the second. The two reactions consisted of 30 cycles of 94°C for 30 sec, 58°C for 1 min, and 72°C for 2 min. The reactions then were cleaved with the appropriate enzymes, and the products were analyzed by gel electrophoresis. The primers and the restriction enzymes used for the CAPS markers are as follows. For the EREB marker, the primers for the first round of PCR were 5′-CCCTCGTTGACCGTCTCTT-3′ and 5′-CCTCTTACTTAGCCAAAAACATAGGA-3′, and for the second round of PCR the primers were 5′-GTCGTCGGTGGTTATGCTTC-3′ and 5′-TAATTA-TTTTCCGGCAATGTAAAATAC-3′. The products were cleaved with TaqI to yield DNA fragments of 148, 75, and 35 bp for Ws and 129, 75, 35, and 19 bp for the Columbia ecotype. For the Rac CAPS marker, the primers for the first round of PCR were 5′-CAGAGA-CATCCTCCTTACCAAT-3′ and 5′-GCGTACCGGCAGTATCCCA-3′, and for the second round of PCR the primers were 5′-CCACCACTTCTCTTCTCTTCTG-3′ and 5′-GGTACTCCCGTCGACCAC-3′. The products were cleaved with DdeI to yield DNA fragments of 366 and 211 bp for Ws and 298, 211, and 68 bp for the Columbia ecotype.
Our analysis indicated that ran1-3 mapped very close to the RAN1 gene (Hirayama et al., 1999). We thus amplified the RAN1 gene from ran1-3 homozygotes and from Ws (because RAN1 was sequenced from the Columbia ecotype) and identified a sequence change in the ran1-3 mutants by directly sequencing the PCR products, by using a series of gene-specific primers. We then isolated a genomic fragment corresponding to the full-length, wild-type RAN1 gene by PCR amplification of the entire coding region with 1 kb of 5′ and 250 bp of 3′ flanking DNA. The oligonucleotide primers used for this contained added SalI sites (lowercase letters) at the ends to simplify subsequent cloning. The primers used were 5′-aaagtcgacCTCGGGATCAGACTAGGCTACATCC-3′ and 5′-aaagtcgacATTAGAGGAATTCAC-TACTTTCACCATTTTAC-3′. The PCR fragment was cloned into the SalI site of a binary plant transformation vector (a pBI101 derivative that had the β-glucuronidase gene deleted). This construct, called pJK495, was transformed into a ran1-3 segregating population by the floral dip method (Clough and Bent, 1998). We identified kanamycin-resistant seedlings from the progeny of the transformed plants and allowed these to self. We identified a line (TCt-12) that was segregating 5:1 wild type to Ctr−, with 100% of linkage of the kanamycin resistance gene marker to the wild-type phenotype. This line then was checked to be homozygous for the ran1-3 mutation at the endogenous RAN1 locus by analysis of products derived from nested PCR amplification of the endogenous genes with use of primers flanking the site of the ran1-3 mutation. The primers were designed so that the 3′ antisense primer was complementary to sequences outside of the DNA present in the transgene. The ran1-3 mutation introduces an HphI restriction polymorphism, which simplified this analysis.
We amplified a full-length cDNA by using primers directed to the predicted 5′ and 3′ untranslated regions of the predicted RAN1 open reading frame. Sequence analysis (not shown) confirmed that the intron/exon structure previously described by Hirayama et al. (1999), which is distinct from that predicted by the Arabidopsis genome sequencing project, was correct.
The heavy metal binding domains (HMAs) and the ATPase signature motif in the predicted RAN1 protein were identified by using a ScanProsite program search at the Expert Protein Analysis System proteomics server of the Swiss Institute of Bioinformatics (www.expasy.ch/tools/scnpsit1.html). The transmembrane domains of RAN1 were predicted by using the Tmpred program at EMBnet (www.ch.embnet.org/index.html) and the default values. The lineup of the various P-type cation transport ATPases was generated by using the ClustalW 1.8 program at the Baylor College of Medicine Search Launcher (http://dot.imgen.bcm.tmc.edu:9331/multi-align/multi-align.html) also with the default parameters.
Acknowledgments
The authors thank Jack Gibbons for technical assistance with the scanning electron microscopy work. This work was supported in part by a National Aeronautics and Space Administration/National Science Foundation (NSF) grant (IBN-9416017) and an NSF grant (MCB-9816914) to J.J.K.
References
- Abeles, F.B., Morgan, P.W., and Saltveit, M.E., Jr. (1992). Ethylene in Plant Biology, 2nd ed. (San Diego, CA: Academic Press).
- Alonso, J.M., Hirayama, T., Roman, G., Nourizadeh, S., and Ecker, J.R. (1999). EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science 284 2148–2152. [DOI] [PubMed] [Google Scholar]
- Askwith, C., Eide, D., Van Ho, A., Bernard, P.S., Li, L., Davis-Kaplan, S., Sipe, D.M., and Kaplan, J. (1994). The FET3 gene of S. cerevisiae encodes a multicopper oxidase required for ferrous iron uptake. Cell 76 403–410. [DOI] [PubMed] [Google Scholar]
- Ausubel, F.M., Brent, R., Kingston, R.E., Moore, D.D., Seidman, J.G., Smith, J.A., and Struhl, K., eds (1994). Current Protocols in Molecular Biology. (New York: John Wiley and Sons).
- Bell, C., and Ecker, J. (1994). Assignment of thirty microsatellite loci to the linkage map of Arabidopsis. Genomics 19 137–144. [DOI] [PubMed] [Google Scholar]
- Beyer, E.M., Jr. (1976). A potent inhibitor of ethylene action in plants. Plant Physiol. 58 268–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleecker, A.B., Estelle, M.A., Somerville, C., and Kende, H. (1988). Insensitivity to ethylene conferred by a dominant mutation in Arabidopsis thaliana. Science 241 1086–1089. [DOI] [PubMed] [Google Scholar]
- Bull, P.C., Thomas, G.R., Rommens, J.M., Forbes, J.R., and Cox, D.W. (1993). The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene. Nat. Genet. 5 327–337. [DOI] [PubMed] [Google Scholar]
- Chang, C., Kwok, S.F., Bleecker, A., and Meyerowitz, E. (1993). Arabidopsis ethylene-response gene ETR1: Similarity of product to two-component regulators. Science 262 539–544. [DOI] [PubMed] [Google Scholar]
- Chao, Q., Rothenberg, M., Solano, R., Roman, G., Terzaghi, W., and Ecker, J.R. (1997). Activation of the ethylene gas response pathway in Arabidopsis by the nuclear protein ETHYLENE-INSENSITIVE3 and related proteins. Cell 89 1133–1144. [DOI] [PubMed] [Google Scholar]
- Chelly, J., and Monaco, A.P. (1993). Cloning the Wilson disease gene. Nat. Genet. 3 317–318. [DOI] [PubMed] [Google Scholar]
- Chelly, J., Tumer, Z., Tonnesen, T., Petterson, A., Ishikawa Brush, Y., Tommerup, N., Horn, N., and Monaco, A.P. (1993). Isolation of a candidate gene for Menkes disease that encodes a potential heavy metal binding protein. Nat. Genet. 3 14–19. [DOI] [PubMed] [Google Scholar]
- Clough, S.J., and Bent, A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16 735–743. [DOI] [PubMed] [Google Scholar]
- Dancis, A., Haile, D., Yuan, D.S., and Klausner, R.D. (1994). The Saccharomyces cerevisiae copper transport protein (Ctr1p). Biochemical characterization, regulation by copper, and physiologic role in copper uptake. J. Biol. Chem. 269 25660–25667. [PubMed] [Google Scholar]
- Fu, D., Beeler, T.J., and Dunn, T.M. (1995). Sequence, mapping and disruption of CCC2, a gene that cross-complements the Ca2+-sensitive phenotype of csg1 mutants and encodes a P-type ATPase belonging to the Cu2+-ATPase subfamily. Yeast 11 283–292. [DOI] [PubMed] [Google Scholar]
- Gamble, R.L., Coonfield, M.L., and Schaller, G.E. (1998). Histidine kinase activity of the ETR1 ethylene receptor from Arabidopsis. Proc. Natl. Acad. Sci. USA 95 7825–7829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, A.E., Chen, Q.G., Findell, J.L., Schaller, G.E., and Bleecker, A.B. (1999). The relationship between ethylene binding and dominant insensitivity conferred by mutant forms of the ETR1 ethylene receptor. Plant Physiol. 121 291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamza, I., Schaefer, M., Klomp, L.W.J., and Gitlin, J.D. (1999). Interaction of the copper chaperone HAH1 with the Wilson disease protein is essential for copper homeostasis. Proc. Natl. Acad. Sci. USA 96 13363–13368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himelblau, E., Mira, H., Lin, S.-J., Culotta, V.C., Peñarrubia, L., and Amasino, R.M. (1998). Identification of a functional homolog of the yeast copper homeostasis gene ATX1 from Arabidopsis. Plant Physiol. 117 1227–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayama, T., Kieber, J.J., Hirayama, N., Kogan, M., Guzman, P., Nourizadeh, S., Alonso, J.M., Dailey, W.P., Dancis, A., and Ecker, J.R. (1999). RESPONSIVE-TO-ANTAGONIST1, a Menkes/Wilson disease-related copper transporter, is required for ethylene signaling in Arabidopsis. Cell 97 383–393. [DOI] [PubMed] [Google Scholar]
- Hua, J., and Meyerowitz, E.M. (1998). Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell 94 261–271. [DOI] [PubMed] [Google Scholar]
- Hua, J., Chang, C., Sun, Q., and Meyerowitz, E. (1995). Ethylene-insensitivity conferred by Arabidopsis ERS gene. Science 269 1712–1714. [DOI] [PubMed] [Google Scholar]
- Hua, J., Sakai, H., Nourizadeh, S., Chen, Q.G., Bleecker, A.B., Ecker, J.R., and Meyerowitz, E.M. (1998). EIN4 and ERS2 are members of the putative ethylene receptor gene family in Arabidopsis. Plant Cell 10 1321–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung, I.H., Suzuki, M., Yamaguchi, Y., Yuan, D.S., Klausner, R.D., and Gitlin, J.D. (1997). Biochemical characterization of the Wilson disease protein and functional expression in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 272 21461–21466. [DOI] [PubMed] [Google Scholar]
- Johnson, P.R., and Ecker, J.R. (1998). The ethylene gas signal transduction pathway: A molecular perspective. Annu. Rev. Genet. 32 227–254. [DOI] [PubMed] [Google Scholar]
- Kampfenkel, K., Kushnir, S., Babiychuk, E., Inze, D., and Van Montagu, M. (1995). Molecular characterization of a putative Arabidopsis thaliana copper transporter and its yeast homologue. J. Biol. Chem. 270 28479–28486. [DOI] [PubMed] [Google Scholar]
- Kieber, J.J. (1997). The ethylene response pathway in Arabidopsis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48 277–296. [DOI] [PubMed] [Google Scholar]
- Kieber, J., and Ecker, J. (1994). Molecular and genetic analysis of the constitutive ethylene response mutant ctr1. In Plant Molecular Biology: Molecular Genetic Analysis of Plant Development and Metabolism, P. Puigdomenech and G. Coruzzi, eds (Heidelberg, Germany: Springer-Verlag), pp. 193–201.
- Kieber, J.J., Rothenburg, M., Roman, G., Feldmann, K.A., and Ecker, J.R. (1993). CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the Raf family of protein kinases. Cell 72 427–441. [DOI] [PubMed] [Google Scholar]
- Konieczny, A., and Ausubel, F.M.. (1993). A procedure for mapping Arabidopsis mutations using co-dominant ecotype-specific PCR-based markers. Plant J. 4 403–410. [DOI] [PubMed] [Google Scholar]
- Lin, S.-J., Pufahl, R.A., Dancis, A., O'Halloran, T.V., and Culotta, V.C. (1997). A role for the Saccharomyces cerevisiae ATX1 gene in copper trafficking and iron transport. J. Biol. Chem. 272 9215–9220. [PubMed] [Google Scholar]
- Linder, M.C. (1991). Biochemistry of Copper. (New York: Plenum Publishing).
- Lutsenko, S., and Kaplan, J.H. (1995). Organization of P-type ATPases: Significance of structural diversity. Biochemistry 34 15607–15613. [DOI] [PubMed] [Google Scholar]
- Mercer, J.F., Livingston, J., Hall, B., Paynter, J.A., Begy, C., Chandrasekharappa, S., Lockhart, P., Grimes, A., Bhave, M., Siemieniak, D., and Glover, T.W. (1993). Isolation of a partial candidate gene for Menkes disease by positional cloning. Nat. Genet. 3 20–25. [DOI] [PubMed] [Google Scholar]
- Petris, M.J., Mercer, J.F.B., Culvenor, J.G., Lockhart, P., Gleeson, P.A., and Camakaris, J. (1996). Ligand-regulated transport of the Menkes copper P-type ATPase efflux pump from the Golgi apparatus to the plasma membrane: A novel mechanism of regulated trafficking. EMBO J. 15 6084–6095. [PMC free article] [PubMed] [Google Scholar]
- Pufahl, R.A., Singer, C.P., Peariso, K.L., Lin, S.J., Schmidt, P.J., Fahrni, C.J., Culotta, V.C., Penner-Hahn, J.E., and O'Halloran, T.V. (1997). Metal ion chaperone function of the soluble Cu(I) receptor Atx1. Science 278 853–856. [DOI] [PubMed] [Google Scholar]
- Rodriguez, F.I., Esch, J.J., Hall, A.E., Binder, B.M., Schaller, E.G., and Bleecker, A.B. (1999). A copper cofactor for the ethylene receptor ETR1 from Arabidopsis. Science 283 396–398. [DOI] [PubMed] [Google Scholar]
- Roman, G., Lubarsky, B., Kieber, J.J., Rothenberg, M., and Ecker, J.R. (1995). Genetic analysis of ethylene signal transduction in Arabidopsis thaliana: Five novel mutant loci integrated into a stress response pathway. Genetics 139 1393–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai, H., Hua, J., Chen, Q.G., Chang, C., Medrano, L.J., Bleecker, A.B., and Meyerowitz, E.M. (1998). ETR2 is an ETR1-like gene involved in ethylene signaling in Arabidopsis. Proc. Natl. Acad. Sci. USA 95 5812–5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samac, D., Hironaka, C., Yallaly, P., and Shah, D. (1990). Isolation and characterization of the genes encoding basic and acidic chitinase in Arabdopsis thaliana. Plant Physiol. 93 907–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller, G., and Bleecker, A. (1995). Ethylene-binding sites generated in yeast expressing the Arabidopsis ETR1 gene. Science 270 1809–1811. [DOI] [PubMed] [Google Scholar]
- Schaller, G., Ladd, A., Lanahan, M., Spanbauer, J., and Bleecker, A. (1995). The ethylene response mediator ETR1 from Arabidopsis forms a disulfide linked dimer. J. Biol. Chem. 270 12526–12530. [DOI] [PubMed] [Google Scholar]
- Sisler, E.C., Blankenship, S.M., and Guest, M. (1990). Competition of cyclooctenes and cyclooctadienes for ethylene binding and activity in plants. Plant Growth Reg. 9 157–164. [Google Scholar]
- Solioz, M., and Vulpe, C. (1996). CPx-type ATPases: A class of P-type ATPases that pump heavy metals. Trends Biochem. Sci. 21 237–241. [PubMed] [Google Scholar]
- Solioz, M., Odermatt, A., and Krapf, R. (1994). Copper pumping ATPases: Common concepts in bacteria and man. FEBS Lett. 346 44–47. [DOI] [PubMed] [Google Scholar]
- Stock, J.F., Stock, A.N., and Mottonen, J.M. (1990). Signal transduction in bacteria. Nature 344 395–400. [DOI] [PubMed] [Google Scholar]
- Tanzi, R.E., et al. (1993). The Wilson disease gene is a copper transporting ATPase with homology to the Menkes disease gene. Nat. Genet. 5 344–350. [DOI] [PubMed] [Google Scholar]
- Vogel, J.P., Schuerman, P., Woeste, K.W., Brandstatter, I., and Kieber, J.J. (1998. a). Isolation and characterization of Arabidopsis mutants defective in induction of ethylene biosynthesis by cytokinin. Genetics 149 417–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel, J.P., Woeste, K.W., Theologis, A., and Kieber, J.J. (1998. b). Recessive and dominant mutations in the ethylene biosynthetic gene ACS5 of Arabidopsis confer cytokinin insensitivity and ethylene overproduction, respectively. Proc. Natl. Acad. Sci. USA 95 4766–4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulpe, C., Levinson, B., Whitney, S., Packman, S., and Gitschier, J. (1993). Isolation of a candidate gene for Menkes disease and evidence that it encodes a copper-transporting ATPase. Nat. Genet. 3 7–13. [DOI] [PubMed] [Google Scholar]
- Yamaguchi, Y., Heiny, M.E., and Gitlin, J.D. (1993). Isolation and characterization of a human liver cDNA as a candidate gene for Wilson disease. Biochem. Biophys. Res. Commun. 197 271–277. [DOI] [PubMed] [Google Scholar]
- Yuan, D.S., Stearman, R., Dancis, A., Dunn, T., Beeler, T., and Klausner, R.D. (1995). The Menkes/Wilson disease gene homologue in yeast provides copper to a ceruloplasmin-like oxidase required for iron uptake. Proc. Natl. Acad. Sci. USA 92 2632–2636. [DOI] [PMC free article] [PubMed] [Google Scholar]