Abstract
Plants are sessile organisms, and their ability to adapt to stress is crucial for survival in natural environments. Many observations suggest a relationship between stress tolerance and heat shock proteins (HSPs) in plants, but the roles of individual HSPs are poorly characterized. We report that transgenic Arabidopsis plants expressing less than usual amounts of HSP101, a result of either antisense inhibition or cosuppression, grew at normal rates but had a severely diminished capacity to acquire heat tolerance after mild conditioning pretreatments. The naturally high tolerance of germinating seeds, which express HSP101 as a result of developmental regulation, was also profoundly decreased. Conversely, plants constitutively expressing HSP101 tolerated sudden shifts to extreme temperatures better than did vector controls. We conclude that HSP101 plays a pivotal role in heat tolerance in Arabidopsis. Given the high evolutionary conservation of this protein and the fact that altering HSP101 expression had no detrimental effects on normal growth or development, one should be able to manipulate the stress tolerance of other plants by altering the expression of this protein.
INTRODUCTION
Organisms have evolved a wide array of mechanisms for adapting to stressful environments. One of the most closely studied of these is the induction of heat shock proteins (HSPs), which comprise several evolutionarily conserved protein families. All of the major HSPs (that is, those expressed in very high amounts in response to heat and other stresses) have related functions: they ameliorate problems caused by protein misfolding and aggregation. However, each major HSP family has a unique mechanism of action. Some promote the degradation of misfolded proteins (Lon, ubiquitin, and various ubiquitin-conjugating enzymes); others bind to different types of folding intermediates and prevent them from aggregating (Hsp70 and Hsp60); and still another (Hsp100) promotes the reactivation of proteins that have already aggregated (Parsell and Lindquist, 1993, 1994).
Although all organisms synthesize HSPs in response to heat, the balance of proteins synthesized and the relative importance of individual HSP families in stress tolerance vary greatly among organisms. For example, in yeast, a member of the Hsp100 (ClpB/C) family, Hsp104, is strongly expressed in the nuclear-cytoplasmic compartment in response to stress and plays a particularly pivotal role in tolerance to extreme conditions (Sanchez et al., 1992; Parsell et al., 1994). Yeast cells expressing Hsp104 survive exposure to high temperatures or high concentrations of ethanol 1000- to 10,000-fold better than do cells not expressing Hsp104. Members of the Hsp100 family also play critical roles in the stress tolerance of bacterial cells (Schirmer et al., 1996), including photosynthetic cyanobacteria (Eriksson and Clarke, 1996). In contrast, the fruit fly Drosophila makes no protein of this type in response to stress; instead, the induction of Hsp70 plays the central role in stress tolerance in this organism (Solomon et al., 1991; Welte et al., 1993).
Determining which proteins play the most crucial roles in stress tolerance in different types of organisms requires genetic analysis. Among organisms amenable to such analysis, higher plants present a particularly interesting subject. First, their immobility limits the range of their behavioral responses to stress and places a strong emphasis on cellular and physiologic mechanisms of protection. Second, their natural environments subject them to wide variations in temperature, both seasonally and diurnally. Third, they are developmentally complex, and the nature of the stresses to which they are exposed as well as their responses to stress are likely to vary in different tissues. Even for a particular organ—among leaves, for example—temperatures can vary dramatically with position on the plant (sun exposure) and can change abruptly with a shift in shading. Finally, the ability to withstand heat stress, especially in combination with water stress, may be of great importance in agricultural productivity (Levitt, 1980; Frova, 1997).
Surprisingly, the critical factors conferring temperature tolerance in higher plants are still poorly understood. Much indirect evidence suggests that HSPs, as a general class, are likely to play some role. Several studies have correlated the induction of HSPs by mild heat stress with the induction of tolerance to much more severe stress (Ougham and Howarth, 1988; Vierling, 1991; Howarth and Skot, 1994). In addition, overexpression of certain transcriptional regulators of HSP expression, HSF1 and HSF3, causes plants to constitutively express at least some HSPs and produces somewhat higher basal thermotolerance (Lee et al., 1995; Prändl et al., 1998). The only direct evidence for the function of an individual HSP in stress tolerance in plants comes from transgenic carrot culture cells and plants. Changes in the expression of HSP17.7 cause modest changes in growth rates of tissue culture cells and electrolyte leakage of leaves after heat stress (Malik et al., 1999).
Hsp100 family members, which play such a major role in the stress tolerance of bacteria and fungi, have also been identified in higher plants (Lee et al., 1994; Boston et al., 1996; Schirmer et al., 1996; Wells et al., 1998). Similar to many other HSP families, the Hsp100 protein family comprises both heat-inducible and constitutive members. Among plants, bacteria, and yeast, these heat-inducible members are more closely related to each other than they are to their own constitutively expressed relatives (Schirmer et al., 1996). Their sequence homology and similar patterns of induction suggest a related function in stress tolerance. Moreover, the Arabidopsis, soybean, wheat, and tobacco Hsp100 homologs can at least partially restore thermotolerance to yeast cells carrying an hsp104 deletion (Lee et al., 1994; Schirmer et al., 1994; Wells et al., 1998). Here, we directly address the question of HSP101 function in the thermotolerance of whole plants by manipulating its expression levels in transgenic Arabidopsis plants.
RESULTS
Generation of Plants with Altered HSP101 Levels
To create transgenic Arabidopsis plants with altered levels of HSP101 expression, the full-length cDNA sequence derived from the Columbia ecotype (Col-0; Schirmer et al., 1994) was placed under the control of the constitutive cauliflower mosaic virus 35S promoter (Koncz et al., 1992) in the sense or antisense orientation. These constructs, or the corresponding vector without an insert, were introduced into plants by selection for the kanamycin resistance marker on the vector. Both root tissue culture transformants of the Nössen (No-0) ecotype and vacuum infiltration transformants of Col-0 were obtained (Koncz et al., 1992; Bechtold and Pelletier, 1998). Independent transgenic lines were screened to evaluate HSP101 levels by immunoblotting with an HSP101-specific antiserum.
Among the antisense lines tested, 12 of 27 No-0 transformants had considerably diminished HSP101 expression after a mild heat stress as compared with vector controls (data not shown). Surprisingly, none of the 11 Col-0 antisense plants tested exhibited a marked decrease in HSP101 expression.
Of plants transformed with the sense construct, only one No-0 line and two Col-0 lines expressed HSP101 constitutively. However, 17 of 25 Col-0 transformants showed markedly less HSP101 after heat stress, presumably as a result of cosuppression of the introduced and endogenous genes (Matzke and Matzke, 1995). For all vector-alone transformants, HSP101 expression patterns were the same as those for untransformed wild-type plants: HSP101 was undetectable in plants grown at 22°C, and the protein was strongly induced by heat treatments at 38°C for 90 min.
The five Nössen antisense lines (No-AS1 to No-AS5) and the five Columbia cosuppression lines (Col-SUP1 to Col-SUP5) with the greatest decreases in HSP101 expression as well as one Nössen antisense line (No-AS6) having an intermediate decrease of HSP101 expression were propagated for further analysis. All three constitutive expression lines (No-C1, Col-C1, and Col-C2) and several vector control lines were also propagated. Homozygous lines of each genotype were produced, and the No-0 plants were backcrossed twice to reduce the likelihood of propagating adventitious mutations introduced by the tissue culture transformation.
Why antisense inhibition was apparently more effective with No-0 plants and why cosuppression was more effective with Col-0 plants are questions of great interest. However, these will require a separate investigation. Here, we focus on the use of these plants in studying the role of HSP101 in stress tolerance.
Quantification of HSP101 Expression
To quantify HSP101 expression in these lines, 14-day-old seedlings were analyzed by protein blotting with an HSP101 antibody and 125I-labeled protein A (Figure 1 and Table 1). Constitutive expression was assessed in plants maintained at their normal growth temperature of 22°C. Inducible expression was assessed after exposure to a standard conditioning pretreatment of 38°C for 90 min. In wild-type plants of both ecotypes, this heat treatment strongly induced HSPs, including HSP101 (Figure 1 and Table 1) (Osteryoung et al., 1993; Schirmer et al., 1994; Wehmeyer et al., 1996); it also induced tolerance to more severe heat shocks (W. Zolotor and E. Vierling, unpublished results); and by itself it did not reduce viability (data not shown). To control for variations in protein loading, blots were also reacted with antibody 7.1O (Velazquez et al., 1983), which recognizes both constitutive and heat-inducible members of the Hsp70 family. (Because these proteins of ∼70 kD comigrated on our gels, all control samples should have had the same levels of expression, and all heat-shocked samples should have had two- to threefold higher levels.) Changes in HSP101 levels did not noticeably affect the expression of the small HSPs, as determined by protein gel blot analysis, or the expression of other proteins, at least as detected by Coomassie staining (data not shown).
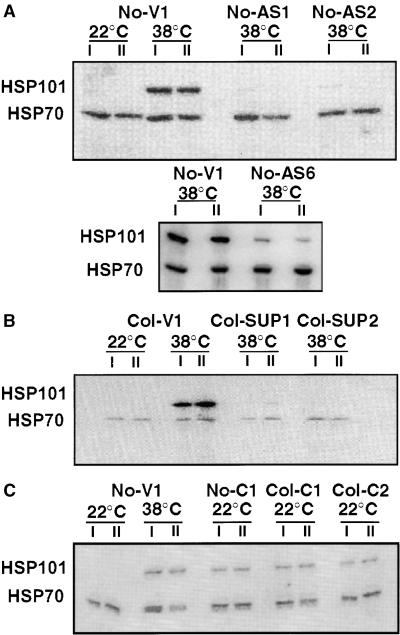
Figure 1.
Altered HSP101 Expression in Transgenic Plants.
Protein gel blot analysis of representative transgenic plants from the No-0 and Col-0 ecotypes.
(A) Vector control line No-V1 and antisense lines No-AS1, No-AS2, and No-AS6.
(B) Vector control line Col-V1 and cosuppression lines Col-SUP1 and Col-SUP2.
(C) Vector control line No-V1 and constitutive expression lines No-C1, Col-C1, and Col-C2.
Total cellular proteins from whole plants maintained at 22°C or heat shocked at 38°C for 90 min were electrophoretically separated on SDS–polyacrylamide gels and transferred to filters for reaction with an antiserum specific for HSP101 and a monoclonal antibody that recognized both constitutive and inducible members of the Hsp70 family. Immune complexes were detected with radiolabeled protein A and visualized by using a PhosphorImager. Samples prepared from different individual plants in the same experiment (I and II) illustrate the reproducibility of HSP101 alterations.
Table 1.
Quantification of HSP101 Expression in 14-Day-Old Transgenic Plantsa
| Transgenic Line | Expression at 22°C | Expression after 90 Min at 38°C |
|---|---|---|
| No-AS1 | Undetectable | Undetectable to 5% |
| No-AS2 | Undetectable | Undetectable to 10% |
| No-AS3 | Undetectable | Undetectable to 10% |
| No-AS4 | Undetectable | 5–10% |
| No-AS5 | Undetectable | 5–15% |
| No-AS6 | Undetectable | 50–60% |
| Col-SUP1 | Undetectable | Undetectable |
| Col-SUP2 | Undetectable | 5–10% |
| Col-SUP3 | Undetectable | 5–10% |
| Col-SUP4 | Undetectable | 10–20% |
| Col-SUP5 | Undetectable | 20–30% |
| No-C1 | 75–85% | 100–115% |
| Col-C1 | 60–65% | NDb |
| Col-C2 | 40–50% | ND |
Values of HSP101 expression in transgenic lines after heat shock or at 22°C were estimated using data from at least three independent experiments for each line, as described in Figure 1. Values are given relative to HSP101 expression in vector controls after exposure to a heat treatment of 38°C for 90 min.
ND, not done.
In 14-day-old seedlings, all vector control plants strongly expressed HSP101 after the 38°C treatment. In the five antisense lines, No-AS1 to No-AS5, HSP101 expression was severely diminished, being either undetectable or present at only 5 to 10% of the amounts observed in the vector control (Table 1). In antisense line No-AS6, the extent of reduction was intermediate, with Hsp101 levels at 50 to 60% of the vector control amounts (Table 1). In cosuppression lines, HSP101 was undetectable in Col-SUP1 and ranged from 5 to 30% of that of the vector control in the other lines (Col-SUP2 to Col-SUP5; Table 1).
As expected, in all wild-type plants, vector controls, antisense lines, and cosuppression lines, HSP101 was not detectable at normal growth temperatures (22°C). In the three constitutive lines, however, HSP101 was expressed in substantial amounts. In different lines, expression at 22°C ranged from 40 to 85% of that obtained in wild-type and vector controls after a full tolerance-inducing heat treatment (38°C for 90 min).
Altered HSP101 Expression Does Not Affect Growth in the Absence of Severe Heat Stress
The selected transgenic lines were first analyzed for general growth phenotypes at different life stages. Neither reduced nor constitutive HSP101 expression caused any obvious phenotype (Figure 2). Germination times and rates, growth rates, times to flowering, and seed yields were all comparable with plants transformed with the vector alone. Moreover, no differences were observed when antisense plants and control plants were grown to flowering under continuous mild heat stress (at 30°C). Thus, the amounts of HSP101 found in wild-type plants were not required for growth at normal or moderately increased temperatures, and constitutive expression of the protein resulted in no detectable harm.
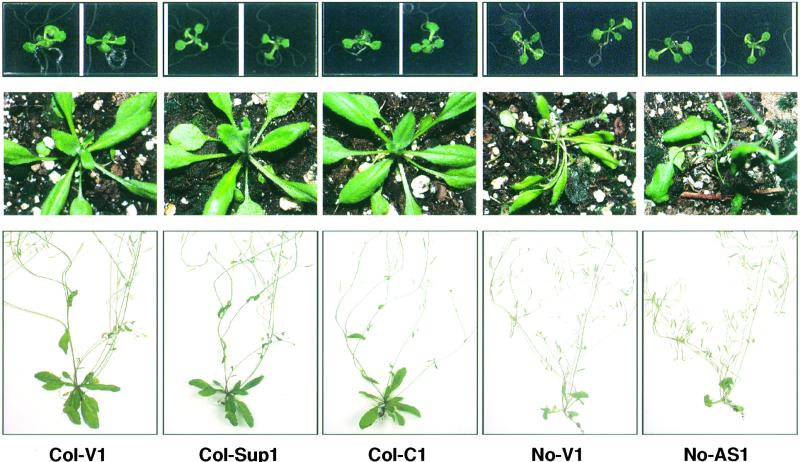
Figure 2.
Altered HSP101 Expression Has No Noticeable Effect on Growth and Development.
Representative plants from two vector control lines (No-V1 and Col-V1), an antisense line (No-AS1), a cosuppression line (Col-SUP1), and a constitutive expression line (Col-C1) are shown at different stages of development.
(Top) After 14 days.
(Center) After 3 weeks.
(Bottom) After 5 weeks.
Ecotype-specific morphological differences exist between Col-0 and No-0 lines, but no growth rate or morphological changes were associated with HSP101 transgenes.
HSP101 Is Essential for Induced Thermotolerance
Induced thermotolerance is defined as the ability of an organism to survive a normally lethal temperature if it is first conditioned by pretreatment at a milder temperature. To determine whether HSP101 plays a role in induced thermotolerance, we analyzed vector controls and plants with decreased HSP101 in assays involving pretreatment, severe heat stress, or combinations thereof. In these and all other experiments presented here, plants with altered HSP101 levels were grown and heat-treated on the same plates as the vector control plants to reduce other sources of variation.
Plants were grown on defined germination medium (GM plates) for 14 days and then were subjected to a 45°C heat shock for 2 hr, with or without a conditioning pretreatment at 38°C for 90 min (Figure 3). The plants were then returned to 22°C. Their viability was assessed daily and photographically recorded. Because of ecotype-specific variations in thermotolerance, phenotypes were clearest on day 5 after stress for No-0 plants and on day 6 for Col-0 plants. We tested two vector control lines from each ecotype, six No-0 antisense lines, and five Col-0 cosuppression lines.
Figure 3.
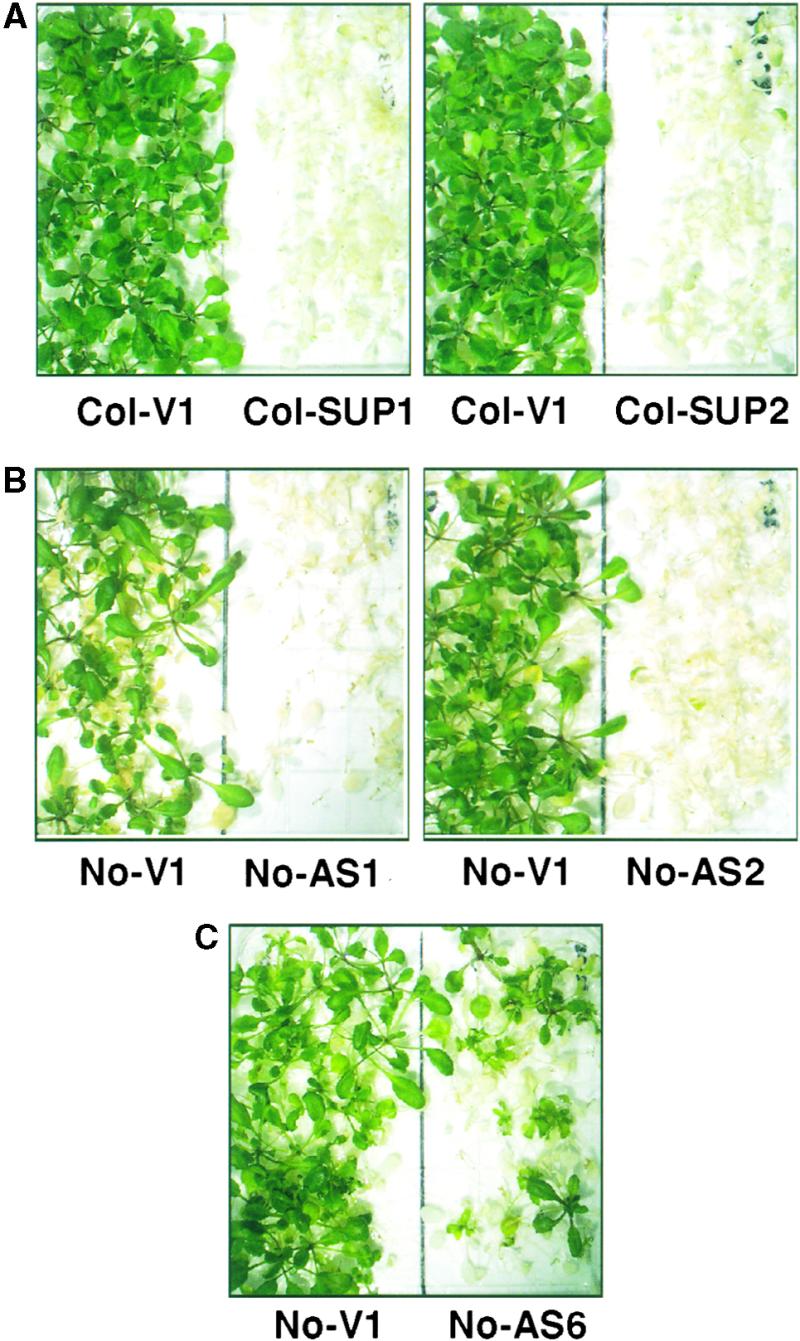
Reducing HSP101 Expression Impairs the Acquisition of Thermotolerance in a Dosage-Dependent Manner.
Fourteen-day-old seedlings grown at 22°C were pretreated at 38°C for 90 min, immediately subjected to a severe heat shock at 45°C for 2 hr, and then returned to 22°C for recovery.
(A) Representative Col-0 vector control plants (Col-V1) and cosuppression plants from the lines with the greatest reductions in HSP101 expression (Col-SUP1 or Col-SUP2) were photographed after 6 days of recovery at 22°C.
(B) Representative No-0 vector control plants (No-V1) and plants from the two antisense lines with the most severe reductions in HSP101 (No-AS1 or No-AS2) at 5 days after return to 22°C.
(C) Representative plants from the antisense line that had an intermediate decrease (No-AS6) after 5 days of recovery at 22°C.
Plants of all genotypes died within 3 days of direct exposure to 45°C (data not shown). As seen with wild-type plants (data not shown), conditioning allowed vector controls (Figure 3) of both the No-0 and Col-0 ecotypes to survive this otherwise lethal heat stress. These plants exhibited some delay in growth after heat shock, but after 5 days of recovery at 22°C, virtually all plants were green and healthy.
Immediately after heat shock, Col-0 cosuppression plants and No-0 antisense plants appeared to be identical to the vector controls. However, in the ensuing days of recovery at 22°C, most of the cosuppression and antisense plants stopped growing (Figure 3; data not shown). Because they survived >3 days, these plants exhibited some tolerance relative to unconditioned plants. However, their ability to survive extreme heat stress after pretreatment was greatly reduced compared with vector controls in a manner that varied with the extent of HSP101 inhibition.
In the lines with the most severe decreases in HSP101 (e.g., Col-SUP1, Col-SUP2, No-AS1, and No-AS2; Table 1 and Figure 3, top and center), no plants survived after 5 to 6 days of recovery. In lines with somewhat less severe decreases in HSP101 (Col-SUP4, Col-SUP5, and No-AS5), some plants survived. Survival rates in the different experiments varied from 0 to 10% (data not shown). Survival was reproducibly greatest in the line that retained the most HSP101, No-AS6. In this line, survival varied from 20 to 30% (Figure 3, bottom).
To extend our analysis beyond one particular growth stage and the simplicity of a life-and-death outcome, we tested five No-0 antisense lines for inducible thermotolerance in quantitative hypocotyl elongation assays. As will be described elsewhere, Arabidopsis HSP101 is developmentally regulated and induced during the course of seed formation (S.-W. Hong, N. Wehmeyer, and E. Vierling, manuscript in preparation), is present in mature seeds, and disappears during germination. To analyze the effects of HSP101 on hypocotyl elongation, we first had to determine when seedlings had lost most of this developmentally regulated protein. Quantitative protein gel blot analysis demonstrated that only a small quantity of HSP101 remained in vector controls after 2.5 days of growth at 22°C. Moreover, heat treatment of these seedlings at 38°C demonstrated that they were able to induce HSP101 strongly in response to stress. Antisense seedlings had no detectable HSP101 at 22°C, and induction by heat treatment was severely impaired (Figure 4A). Note that induction of the small HSPs was unaffected in the transgenic plants.
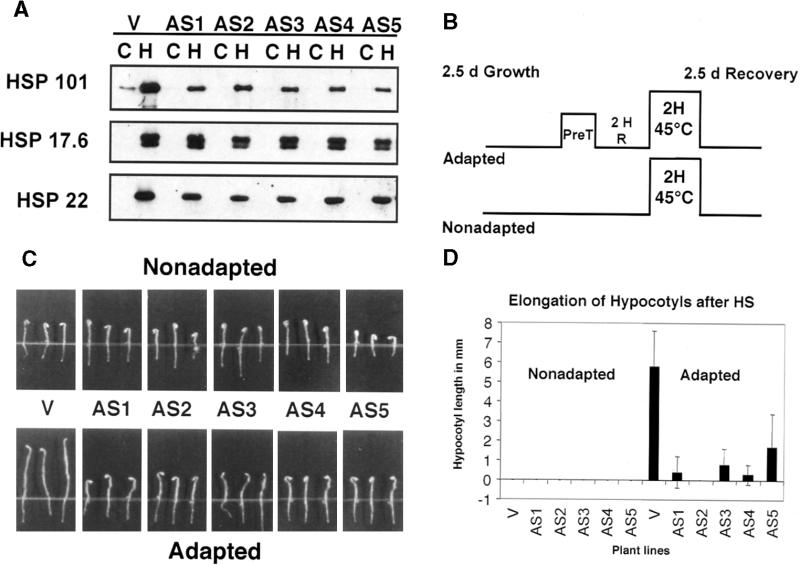
Figure 4.
Quantitative Hypocotyl Elongation Assay.
(A) HSP101, HSP17.6, and HSP22 expression in 2.5-day-old vector control (V) and antisense (AS) seedlings. Total proteins from 40 to 46 seedlings that had been maintained at 22°C (C) or heated to 38°C for 90 min (H) were electrophoretically separated on SDS–polyacrylamide gels, transferred to filters, and reacted with antibodies against HSP101, HSP17.6, and HSP22. The HSP101 blot was exposed longer than those in Figure 1 to visualize the low amount of developmentally induced HSP101 remaining in the vector control seedlings at 22°C (lane C).
(B) Schematic representation of a hypocotyl elongation assay with adapted and nonadapted seedlings. Seedlings grown at 22°C for 2.5 days (d) were transferred directly to 45°C (Nonadapted). Seedlings pretreated (PreT) at 38°C for 90 min were allowed to recover for 2 hr (2 H R) before the 45°C treatment (Adapted). After the 45°C heat shock, all seedlings were returned to 22°C for 2.5 days of recovery before the hypocotyl elongation was measured.
(C) Results of a representative hypocotyl elongation assay. One vector control (V; No-V1) and five antisense lines (AS1 to AS5 and No-AS1 to No-AS5) were subjected to the experimental conditions described in (B). Vector control seedlings showed marked hypocotyl elongation when heat shocked after a conditioning mild pretreatment (adapted seedlings). In contrast, hypocotyl elongation of antisense seedlings was greatly affected despite adaptation.
(D) Graphical presentation of combined results of two independent hypocotyl elongation assays for five antisense lines and one vector control. Error bars represent standard deviation and are based on data for at least 22 seedlings of each genotype. HS, heat shock.
Figure 4C shows a typical hypocotyl elongation assay for five antisense lines (No-AS1 to No-AS5) and one vector control. Seedlings were grown in the dark for 2.5 days and then heat-shocked at 45°C for 2 hr after a 38°C pretreatment (adapted) or without pretreatment (nonadapted). After heat shock, seedlings were allowed to recover for 2.5 days at 22°C, and the extent of hypocotyl elongation during recovery was measured (Figure 4B). Both vector control seedlings and antisense seedlings were unable to elongate their hypocotyls after direct heat shock at 45°C. In contrast, vector control seedlings that had received a mild pretreatment before the 45°C stress displayed substantial hypocotyl elongation. Antisense seedlings failed to elongate their hypocotyls under these conditions and resembled the nonadapted seedlings (Figure 4C). Results of two independent hypocotyl elongation assays for all five antisense lines and one vector control are presented in Figure 4D.
To provide a more immediate assessment of viability, we stained seedlings with 2,3,5-triphenyltetrazolium chloride (TTC) at 2 and 4 hr after the 45°C heat shock. TTC is normally colorless but was reduced to deep-red insoluble formazan in all seedlings (adapted and nonadapted), confirming their viability soon after heat shock (data not shown). Thus, reduced amounts of HSP101 did not cause immediate lethality but rather failure to recover from heat shock.
HSP101 Is Required for Basal Thermotolerance during Germination
The observation that HSP101 is developmentally regulated and expressed in seeds prompted us to examine basal thermotolerance in early development and the possible role of HSP101. First, we examined basal thermotolerance in germinating seeds from vector control lines. Seeds were plated on medium and allowed to germinate for various periods at 22°C before they were heat shocked at 47°C for 2 hr. Germinating seeds exhibited a remarkable ability to recover from the detrimental effects of this severe heat shock. When plants were scored after the heat shock, development was delayed by 5 to 7 days in comparison with the unstressed controls; however, virtually all seeds heat shocked either 30 min or 30 hr after plating eventually produced healthy plants (Figure 5; data not shown). In the next 18 hr, as the HSP101 decreased (S.-W. Hong, N. Wehmeyer, and E. Vierling, manuscript in preparation), this high basal thermotolerance was lost. Most germinating seeds that were heat shocked after 36 hr of development recovered, but survival rates were slightly lower than those for seeds heat shocked after 30 hr. None of the seedlings heat shocked after 48 hr of imbibition was able to survive the 47°C heat shock (Figure 5; data not shown).
Figure 5.
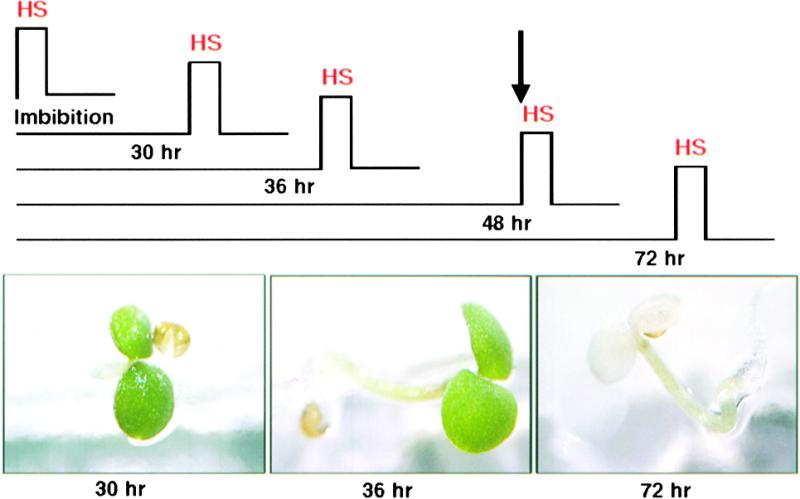
Germinating Seedlings Have High Thermotolerance, Which Is Lost after 2 Days of Growth.
Seeds germinated on plates at 22°C for 30 min, 30 hr, 36 hr, 48 hr, or 72 hr were exposed to 47°C for 2 hr (HS). Representative plants were photographed 5 days after heat stress. The arrow marks the time (48 hr of germination) after which all heat-shocked seedlings died.
To test directly the role of HSP101 in the high basal thermotolerance of germinating seeds, we examined antisense and cosuppression lines together with vector controls. First, HSP101 expression levels were determined. All of the antisense lines showed much less expression of HSP101 in mature (dry) seeds (Figure 6A). Decreased HSP101 expression did not, however, appear to affect expression of class 1 small HSPs, which are also present in seeds (Wehmeyer et al., 1996). Surprisingly, in mature seeds of the cosuppression lines, HSP101 was expressed in nearly the same amounts as in wild-type seeds (data not shown).
Figure 6.

Reduced HSP101 Expression Impairs Basal Thermotolerance in Seeds from Antisense Plants.
(A) Levels of developmentally regulated HSP101 and HSP17.6 expression in mature seeds of a vector control line (V; No-V1) and three antisense lines (AS1 to AS3 and No-AS1 to No-AS3). Seeds (10 mg) of each genotype were used to prepare protein samples, and the proteins were electrophoretically separated on SDS–polyacrylamide gels. For HSP101 and HSP17.6, 0.5 mg of total protein was loaded per lane; for 5 × HSP101, 2.5 mg of total protein per lane was loaded.
(B) Seeds of the three antisense lines and two control lines analyzed in (A) were germinated for 30 hr and then exposed directly to 47°C for 2 hr. Representative plates were photographed 10 days after heat shock.
Next, seeds from antisense lines, cosuppression lines, and vector control lines were exposed to 47°C for 2 hr immediately after seed plating or after 30, 36, 48, or 72 hr of germination. The majority of germinating vector control and cosuppression seeds continued to develop after the heat shock at the first three time points and eventually produced healthy plants (Figure 6B; data not shown). Germinating antisense seeds, however, failed to develop in all cases (Figure 6B; data not shown). A close examination of antisense seeds that were heat stressed after 36 hr of imbibition showed that the radicle emerged in some cases, indicating that elongation continued for some time (data not shown). However, the seedlings then stopped growing and died. Thus, as with the other assays, decreased levels of HSP101 did not cause immediate lethality but rather failure to recover from heat shock.
Constitutive HSP101 Expression Provides an Advantage to Plants Heat Shocked without Conditioning
The previously described experiments demonstrate that HSP101 is required both for induced thermotolerance and for the naturally high basal thermotolerance observed in germinating seedlings. However, many factors are likely to be involved in stress tolerance, and it does not necessarily follow that overexpression of HSP101 alone would provide tolerance to otherwise sensitive plants. To investigate this possibility, we examined plants that constitutively express HSP101 at normal temperatures in the absence of a conditioning pretreatment. As demonstrated earlier, 14-day-old plants were extremely sensitive to high temperatures if they were not given a conditioning pretreatment. When plants of this age from all three constitutive expression lines (No-C1, Col-C1, and Col-C2) were exposed to our standard killing heat shock (45°C for 2 hr), they also died. Thus, constitutive expression of HSP101 alone at a moderate level (see Table 1; 40 to 85% of that normally attained with heat treatment) did not provide the remarkable degree of thermotolerance that is conferred by a fully conditioning heat pretreatment.
To determine whether this extent of HSP101 expression might provide a survival advantage under less severe conditions, 14-day-old seedlings were given shorter heat shocks at 45°C, after which their viability was assessed daily for the next 10 days. In this case, all three constitutive expression lines, No-C1, Col-C1, and Col-C2, showed a marked advantage in comparison with the vector controls (Table 2; representative examples are shown in Figure 7).
Table 2.
Growth Advantage of Heat-Shocked Plants Constitutively Expressing HSP101
| Survival after a Period at 45°Ca
|
||||
|---|---|---|---|---|
| 15 Min | 30 Min | 45 Min | 60 Min | |
| Vector | ++++ | ++ | ± | − |
| No-C1 | ++++ | +++ | +++ | ++ |
| Col-C1 | ++++ | +++ | +++ | ++ |
| Col-C2 | ++++ | +++ | +++ | ± |
Plants from several experiments, such those shown in Figure 7, were scored on day 6. (++++), plants appeared as healthy as unheated controls; (+++), plants appeared almost as healthy as unheated controls, with some yellow tissue evident; (++), most plants had some bleached and withered leaves, all exhibited developmental delay, and some individual plants died; (±), most plants died, and green tissue was still evident on many plants after 6 days; (−), all plants died within 6 days, and patches of green tissue were visible on only a few.
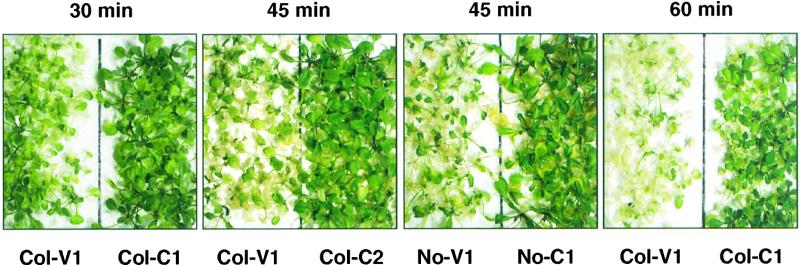
Figure 7.
Constitutive Expression of HSP101 Provides a Growth Advantage to Unconditioned 14-Day-Old Plants.
Fourteen-day-old plants grown at 22°C were shifted directly to 45°C for 30, 45, or 60 min and then returned to 22°C. Representative plates containing vector controls (No-V1 and Col-V1) and constitutive expression plants (No-C1, Col-C1, and Col-C2) were photographed 5 days after return to 22°C (6 days for Col-V1 and Col-C2 after 60 min of heat shock).
With a short (15-min) heat shock, all plants looked as healthy as unstressed plants, and there were no distinctions between lines even after 5 days of recovery (Table 2). No differences were apparent between the constitutive lines and the vector controls immediately after heat shock with exposures of 30 min. Subsequently, however, the vector controls bore obvious signs of stress: most plants had some bleached and withered leaves, and some individual plants died. In contrast, plants from all three constitutive lines appeared as healthy as unstressed plants of the same age (Table 2 and Figure 7).
In plants given a 45-min heat shock, most of the vector controls died during the subsequent recovery at 22°C, whereas most of the constitutive expression plants survived (Table 2 and Figure 7; shown are representative plates at a time when this difference in survival was first clearly visible). After exposure to 45°C for 60 min, the constitutive expression plants No-C1 and Col-C1 had withered leaves and were developmentally delayed, but they were noticeably more healthy than were the vector controls. By day 10, most of the constitutive expression plants had clearly returned to normal growth; all of the vector control plants, however, had died (Table 2). The line with the lowest constitutive expression of HSP101, Col-C2, did not recover from the 60-min heat shock as well as those lines with higher levels, No-C1 and Col-C1, did. By day 6, the fraction of bleached plant tissue was greater in Col-C2 plants than in No-C1 and Col-C1 plants, and by day 10, some Col-C2 plants had died. However, even plants of this line were much less affected than were vector controls (Table 2).
We also examined the effects of constitutive HSP101 expression on newly germinated seedlings. In contrast to wild-type and vector control lines, 3-day-old seedlings of all constitutive lines contained substantial amounts of HSP101 protein (Figure 8A; data not shown). When 3-day-old seedlings of all genotypes were exposed to 47°C for 2 hr, none survived. However, with less severe heat shocks (47°C for 30 or 45 min), survival rates were strikingly different between the constitutive and the vector control lines. Similar results were obtained for all three constitutive lines.
Figure 8.
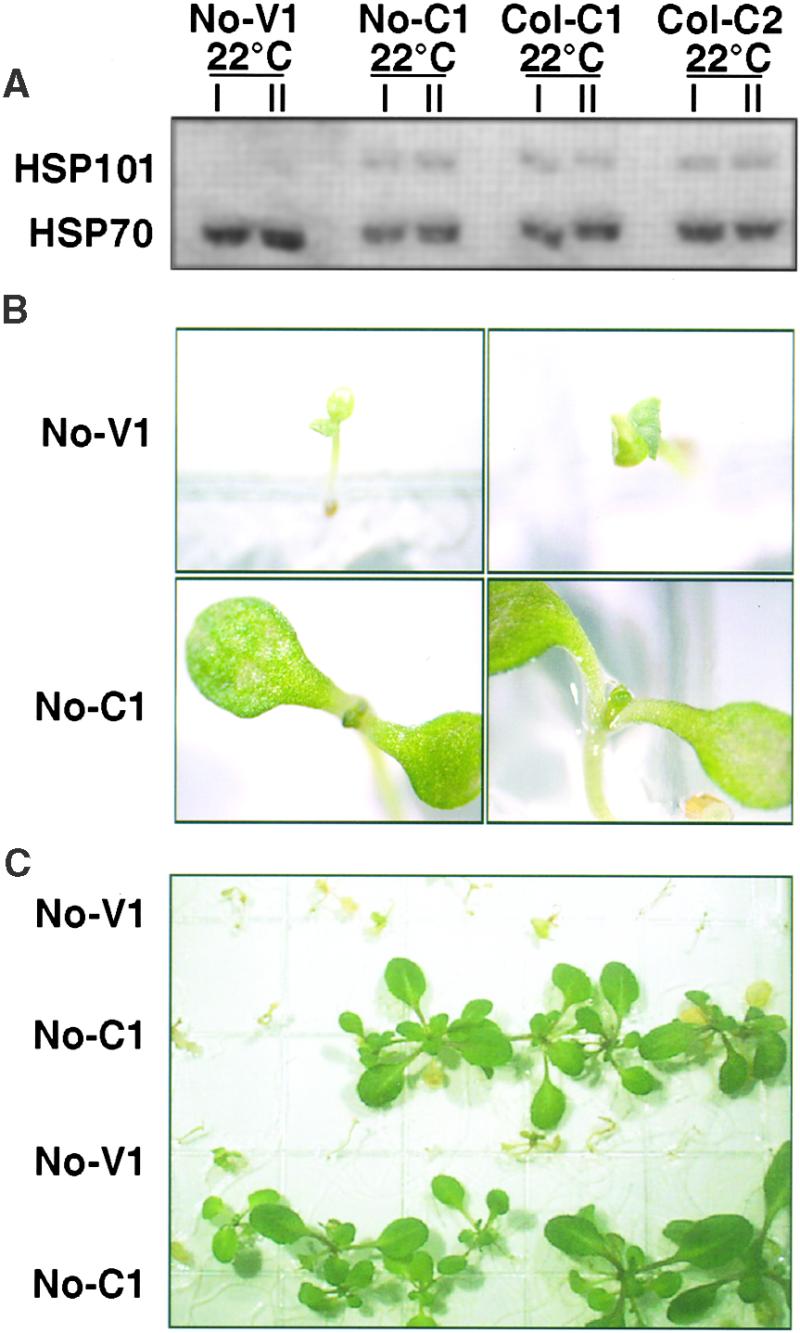
Constitutive Expression of HSP101 Provides a Growth Advantage to 3-Day-Old Seedlings.
(A) Analysis of HSP101 expression in 3-day-old seedlings of one vector control line (No-V1) and three constitutive expression lines (No-C1, Col-C1, and Col-C2). Total proteins from pooled seedlings of each genotype grown at 22°C were analyzed as in Figure 1. In constitutive lines, expression of HSP101 at day 3 was not as high relative to Hsp70 as in 14-day-old plants of the same genotype (see Figure 1). Vector controls did not contain HSP101 at this developmental stage. Samples prepared from different individual plants in the same experiment (I and II) illustrate the reproducibility of HSP101 alterations.
(B) Seeds of vector controls and constitutive lines were plated together and germinated for 3 days. Seedlings were heat shocked at 47°C for 30 min and then returned to 22°C. Representative seedlings of vector control (No-V1) and constitutive line No-C1 are shown 2 days after exposure to heat shock. All four photographs were taken at the same magnification.
(C) A lower magnification photograph of plants from the same experiment shown in (B) at 10 days after heat shock.
Representative data for the No-C1 line and one vector control are shown in Figures 8B and 8C. Two days after a 30-min heat shock at 47°C, stress-related damage was seen in both vector control and constitutive expression seedlings. However, most seedlings from constitutive lines were much further developed, displaying their first pair of adult leaves and expanded cotyledons; in contrast, vector control seedlings had no adult leaves and only small cotyledons with bleached patches. Two weeks after the heat shock, these early signs of recovery had translated into vigorous growth for most constitutive expression plants, but the vector controls had grown little, if at all (Figure 8C). Thus, the loss of basal thermotolerance that occurs in early development, as seedlings lose their store of HSP101, can be partially reversed by constitutive expression of HSP101.
DISCUSSION
We have demonstrated that the expression of a specific HSP plays a crucial role in the thermotolerance of a plant. Numerous studies from other laboratories have previously documented a correlation between HSP induction and adaptation to stress in plants (Nover, 1990; Vierling, 1991; Howarth and Skot, 1994; Yeh et al., 1994; Lee et al., 1995; Lee and Schöffl, 1996; Prändl et al., 1998), but these experiments did not address the question of which HSPs might play a crucial role. Indeed, because the same plants generally underwent other physiological changes, it could not be determined whether HSP induction served vital or peripheral functions. The role of HSP101 is here established by several mutually supportive arguments.
First, alterations in thermotolerance were linked to alterations in heat tolerance by three different types of genetic manipulation: inhibiting HSP101 expression through the production of antisense RNAs or by cosuppression of impaired thermotolerance, whereas overexpressing HSP101 enhanced it. Second, in each case, multiple independent transformants that affected HSP101 in the same way displayed the same change in thermotolerance, and no transformants that substantially affected HSP101 expression failed to affect thermotolerance. Third, in experiments in which conditions were sensitive enough to detect them, dosage relationships were apparent. Constitutive lines with the highest levels of HSP101 expression were the best able to withstand heat stress, and antisense lines with the strongest inhibition of HSP101 expression were the most severely affected by heat stress. Fourth, changes in HSP101 expression altered both acquired and basal thermotolerance.
Finally, when the effects of antisense and cosuppression on HSP101 expression diverged, their effects on tolerance also diverged: both forms impaired HSP101 expression, and both impaired thermotolerance in 14-day-old seedlings; only antisense expression diminished the developmentally regulated induction of HSP101 in seeds, and only antisense decreased thermotolerance during seed germination. The importance of HSP101 in thermotolerance recently has been confirmed using the hypocotyl elongation assay to screen for mutants defective in thermotolerance. A point mutation in HSP101 was one of the mutants isolated (Hong and Vierling, 2000).
Our experiments were prompted by the identification of Arabidopsis HSP101 as a protein that is strongly induced by heat, homologous to the well-studied yeast protein Hsp104, which is able to partially compensate for the loss of thermotolerance caused by hsp104 deletions in yeast (Schirmer et al., 1994). Even so, the remarkably similar effects of Hsp104 in a simple microbe and HSP101 in a complex vascular plant are surprising. Yeast cells have multiple strategies for surviving stress (Eleutherio et al., 1993; Ruis and Schuller, 1995; Zahringer et al., 1997; Moskvina et al., 1998; Singer and Lindquist, 1998; Simon et al., 1999), and one would expect plants to have at least as many (Bohnert et al., 1995; Smirnoff, 1998). Moreover, plants typically have numerous redundant and closely related genes. Indeed, several other members of the Hsp100 family, including other stress-inducible members, are present in Arabidopsis (Shanklin et al., 1995; Nakashima et al., 1997; Nielsen et al., 1997; Weaver et al., 1999; E. Vierling, unpublished results).
Nevertheless, this single protein plays such a pivotal role in both organisms that (1) inhibiting its expression during conditioning pretreatments has disastrous effects on the induction of thermotolerance; (2) inhibiting its developmentally regulated induction (in yeast, stationary phase cells and spores [Sanchez et al., 1992]; in plants, seeds) severely reduces the high basal thermotolerance that characterizes these stages of development; (3) the protein appears to be less crucial in preventing stress damage than in allowing recovery from it; (4) changing the expression levels of HSP101 (unlike many other HSPs and tolerance factors) had little effect on normal growth and development; and (5) expressing the protein at times when it would normally not be expressed was sufficient to confer higher basal levels of thermotolerance.
Now that it has been established that HSP101 plays a major role in thermotolerance, it is of interest to understand the mechanism by which the protein functions and to define the targets that are protected. Evidence from yeast suggests that Hsp104 acts in vivo to reactivate proteins aggregated by high temperatures (Parsell et al., 1994). In addition, the ability to reactivate denatured proteins has been demonstrated in vitro with purified Hsp104 and chemically denatured substrates. To reactivate proteins in vitro, Hsp104 requires the assistance of Hsp40 and Hsp70 (Glover and Lindquist, 1998). These data support a model in which Hsp104 performs the first step in dissociating protein aggregates so that Hsp70 and Hsp40 can recognize the denatured substrate and complete the refolding process. Consistent with these data, bacterial homologs of Hsp104, which are also required for stress tolerance, have recently been shown to have the same capacity to disaggregate proteins in vitro in cooperation with bacterial Hsp70 and Hsp40 homologs (Mogk et al., 1999; Motohashi et al., 1999; Zolkiewski, 1999). The function of Hsp104 in protein disaggregation also parallels the defined activities of other proteins belonging to a larger related family of ATPases, the AAA+ ATPases, many of which act to alter the oligomeric state of other protein complexes (Neuwald et al., 1999). Given the high sequence similarity of HSP101 with yeast HSP104 and their conserved functions in thermotolerance, one can reasonably propose that HSP101 in Arabidopsis is also acting to facilitate reactivation of proteins denatured by heat.
Another, although not necessarily mutually exclusive, activity has been suggested for HSP101 by Gallie and colleagues (Wells et al., 1998), who reported that HSP101 from tobacco and wheat positively regulates the translation of tobacco mosaic RNA through direct interaction with the sequence in the viral 5′ leader. Because HSP101 is strongly expressed in seedlings and mature plants after heat stress, this might represent a specific mechanism for plant viruses to regulate their replication and mobility in response to the health of their host and/or a mechanism for taking advantage of the host stress response upon infection. Alternatively, or in addition, HSP101 could affect the translation of some cellular mRNAs and thereby contribute to thermotolerance. However, the 5′ leader sequences to which HSP101 binds have not been identified in cellular mRNAs. Also, the significant decrease of HSP101 in our antisense and cosuppression lines did not lead to any noticeable changes in the expression of other proteins, including other HSPs, which might be the logical targets for translational enhancement during heat stress.
The finding that HSP101 plays a crucial role in thermotolerance in plants, together with the conserved function of HSP101, suggests that engineering plants to express increased HSP101 may improve survival during periods of acute environmental stress. In this regard, the fact that the constitutive HSP101 expression we achieved increases heat tolerance without compromising growth at normal temperatures is important—and is in contrast to other efforts to engineer stress tolerance in plants. Many of those attempts, such as constitutive expression of the multiple stress-response transcription factor DREB1A or of a subunit of trehalose synthase (TPS1) (Holmstrom et al., 1996; Kasuga et al., 1999; Smirnoff and Bryant, 1999), produce disadvantageous growth phenotypes. With inducible promoters that might produce even higher levels of HSP101 accumulation, much higher heat tolerance might be attainable, as has already been achieved with yeast (Lindquist and Kim, 1996). Manipulation of HSP101 expression, therefore, holds considerable promise in protecting plants at many life stages from irreversible stress-induced damage.
METHODS
Vector Construction and Plant Transformation
The EcoRI insert of pBSKHSP100 containing the full-length cDNA of Arabidopsis thaliana ecotype Columbia (Col) HSP101 (Schirmer et al., 1994) was cloned into the EcoRI site of pBICaMV, a plant transformation vector (a kind gift of J. Celenza, Boston University, MA). Sense and antisense constructs were identified by restriction analysis and subsequently were sequenced.
Plasmid DNAs for sense, antisense vector, or vector without insert were transformed into Agrobacterium tumefaciens strain LB4400 for tissue culture transformation and into A. tumefaciens strain GV3101 for vacuum infiltration (Koncz et al., 1992). Both strains of agrobacteria were a generous gift of B. Keith (University of Chicago, IL). The DNA of three independent transformants was isolated and then transformed in Escherichia coli (DH5α), and the plasmid DNA was prepared for restriction analysis to confirm the presence of the respective construct in the agrobacteria.
Root tissue culture transformation with Nössen (No-0) plants was performed as described (Koncz et al., 1992). For vacuum infiltration with Columbia (Col-0) plants, we followed a modified version of the protocol by Bechtold and Pelletier (1998), which we obtained from J. Mundy (University of Copenhagen, Denmark).
Quantification of HSP101 in Kanamycin-Resistant Plants
Transformed kanamycin-resistant plants (T1 generation) were grown on germination medium (GM) plates containing 50 mg/L kanamycin (GM per liter: Murashige and Skoog medium [Sigma], 1.0 mL of Murashige and Skoog vitamins [Sigma], 10 mg of sucrose, adjusted to pH 5.7 with KOH, and 2 mg of Phytagel [Sigma]) at 22°C in incubators (models I-35LVL and E-30B; Percival Scientific, Booner, IA) under continuous light (150 to 300 μmol m−2 sec−1). Fourteen-day-old plants were exposed to 38°C for 90 min in the light. Before heat treatment, two plants of each genotype were frozen in liquid nitrogen for analysis of HSP101 expression at 22°C. After heat treatment, two plants of each genotype were taken to assess HSP101 expression after heat stress. Total proteins were extracted by grinding individual frozen plants in plant lysis buffer (100 mM Tris-HCl, pH 8.0, 25 mM KCl, 4 mM CaCl2, 0.05 mg/mL bovine serum albumin, and protease inhibitor cocktail Complete, EDTA-free [Boehringer Mannheim], 1 tablet for each 50 mL of buffer). Insoluble debris was removed by centrifugation at 10,000g for 5 to 10 min. Protein concentrations were estimated using the Bio-Rad protein assay. Twelve micrograms of protein from each sample was suspended in 6 × sample buffer (300 mM Tris-HCl, pH 6.8, 12% [w/v] SDS, 60% [v/v] glycerol, 6% [v/v] 2-mercaptoethanol, and 0.12% [w/v] bromophenol blue), separated electrophoretically on 10% SDS–polyacrylamide gels, and after separation transferred to Immobilon-P membranes (Millipore Corp., Bedford, MA) for immunological analysis. Equal loading was confirmed by Coomassie Brilliant Blue R 250 staining of the membrane. Membranes were reacted with polyclonal antibodies against HSP101 (generated against an N-terminal fragment of HSP101) and a monoclonal antibody that recognizes constitutive and heat-inducible species of Hsp70 (7.1O; Velazquez et al., 1983). Immunocomplexes were visualized and quantified with 125I-labeled protein A, using a PhosphorImager and ImageQuant software (Molecular Dynamics, Sunnyvale, CA).
Vector controls, plants with decreased amounts of HSP101 (No-AS1 to No-AS6 and Col-SUP1 to Col-SUP5), and plants with constitutive expression of HSP101 at 22°C (No-C1, Col-C1, and Col-C2) were propagated to homozygosity and grown on GM media without kanamycin. HSP101 in these plants (T2 and T3 generations) was quantified as described earlier. Transgenic plants generated by tissue culture transformation were backcrossed twice to wild-type No-0 plants before analysis.
Phenotypic Analysis
To observe general plant growth phenotypes, we grew transgenic plants on soil or PNS medium (2.5 mM potassium phosphate, pH 5.5, 5 mM KNO3, 2 mM MgSO4, 2 mM Ca[NO3]2, 49 μM ethylenediamine-tetraacetic acid micronutrients, and 5 g/L sucrose) on a 16-hr-light/ 8-hr-dark cycle at 22°C/18°C in a growth chamber illuminated at ∼250 μmol m−2 sec−1. Plants were photographed after 14 days (grown in PNS), 3 weeks (grown in soil), or 5 weeks (grown in soil). Similar results were obtained when plants were grown under continuous light at 22°C. To assess growth under stressful conditions, we also grew antisense and vector control plants on a 16-hr-light/8-hr-dark cycle at 30°C/24°C with ∼250 μmol m−2 sec−1. Germination rates and frequencies for each genotype were monitored by plating ∼150 vector control seeds and ∼50 seeds of each antisense line (No-AS1 to No-AS5), cosuppression line (Col-SUP1 to Col-SUP5), and constitutive line (NO-C1, Col-C1, and Col-C2) together on GM plates. Plates were incubated at 22°C under continuous light. Germination was scored daily for 3 days. Similar results were obtained when the plates were incubated for 3 days at 4°C after plating before incubating at normal growth conditions at 22°C in continuous light (150 to 300 μmol m−2 sec−1) or when sterilized seeds were kept at 4°C for 3 days before plating.
Induced ThermotoIerance Assays with 14-Day-Old Plants
Homozygous vector controls (No-V1 and No-V2, and Col-V1 and Col-V2) and homozygous plants with altered expression of HSP101 were plated together on GM plates (without kanamycin; 25 mL of medium per plate) and grown as described earlier for 14 days. Plates were exposed to one of the following heat treatments: 38°C for 90 min (pretreatment); 38°C for 90 min, followed by 45°C for 2 hr (conditioned); or 45°C for 15 min, 30 min, 45 min, 60 min, or 2 hr (unconditioned). After heat treatments, the plates were returned to 22°C, and viability was assessed daily for as long as 10 days. Results were documented photographically 5 or 6 days after heat stress. HSP101 protein concentrations were monitored in the same experiment immediately before and after pretreatment as described earlier.
Induced Thermotolerance Shown in Hypocotyl Elongation Assays
Vector control seeds and seeds of antisense lines No-AS1 to No-AS5 were plated in rows on PNS medium, and plates were covered with foil. After 3 days of cold treatment (4°C), the foil-wrapped plates were placed in a vertical position at room temperature for 2.5 days. Wrapped plates were then either kept at 22°C or subjected to one of the following treatments: 38°C for 90 min (pretreatment); 38°C for 90 min, followed by 2 hr of recovery at 22°C and then 2 hr at 45°C (conditioned); or 2 hr at 45°C (unconditioned). After heat treatments, the plates were briefly unwrapped, and the top and the bottom of the hypocotyl were marked; plates were then returned to growth in a vertical position at 22°C. After an additional 2.5 days, the hypocotyl elongation of all seedlings was measured and the results were photographed. Experiments included at least 11 seedlings from each genotype and were repeated at least two times.
Proteins were extracted from 2.5-day-old seedlings in the same experiment 2 hr after the 38°C pretreatment. Total proteins were prepared from 40 to 46 seedlings by grinding them in 50 μL of SDS sample buffer (60 mM Tris-HCL, pH 8.0, 60 mM DTT, 2% SDS, 15% sucrose, 5 mM ε-amino-N-caproic acid, and 1 mM benzamidine). Protein concentration was measured with a Coomassie blue binding assay. For analysis of HSP101 concentrations, proteins were electrophoretically separated on 10% SDS–polyacrylamide gels. For analysis of small HSPs—HSP21 (Osteryoung et al., 1993) and HSP17.6 (Wehmeyer et al., 1996)—the same samples were electrophoretically separated on 15% SDS–polyacrylamide gels. Proteins were visualized with an enhanced chemiluminescence detection kit and Hyperfilm-MP (both from Amersham).
To test the viability of stressed seedlings, seedlings were stained with 2% 2,3,5-triphenyltetrazolium chloride (TTC) 4 hr after heat treatment. Ten milliliters of 2% TTC in 50 mM potassium phosphate buffer, pH 7.0, was added to the seedlings on plates and vacuum infiltrated (∼10 in mm Hg) overnight. Stained seedlings were examined under the dissecting microscope.
Basal Thermotolerance Germination Assays
Vector control seeds and seeds of antisense lines No-AS1 to No-AS5 were plated together in rows on GM plates and exposed to 47°C for 2 hr immediately after sterilization and plating (30 min) or for 30, 36, 48, or 72 hr after sterilization and plating. Plates were then returned to normal growth conditions (22°C with continuous light at 150 to 300 μmol m−2 sec−1). Seed development was scored 2, 5, and 10 days after heat treatment and was photographed after 10 days. Similar results were obtained with sterilized seeds that had been cold treated (4°C for 3 days) before plating.
For analysis of HSP101 concentrations in seeds, 10 mg of seeds for each genotype was ground in 200 μL of sample buffer (60 mM Tris-HCl, pH 8.0, 60 mM DTT, 2% [w/v] SDS, 15% [w/v] sucrose, 5 mM ε-amino-N-caproic acid, and 1 mM benzamidine). Protein concentration was estimated with a Coomassie blue binding assay. Proteins (0.5 or 2.5 μg) were electrophoretically separated on 10% SDS–polyacrylamide gels. For analysis of small HSPs—HSP21 (Osteryoung et al., 1993) and HSP17.6 (Wehmeyer et al., 1996)—the same samples were electrophoretically separated on 15% SDS–polyacrylamide gels. Enhanced chemiluminescence (Amersham) was used for visualization.
Basal Thermotolerance Assays of 3-Day-Old Seedlings
Vector control seeds and seeds of constitutive expression lines No-C1, Col-C1, and Col-C2 were plated on GM plates and grown for 72 hr as described earlier. Plates were then directly exposed to 47°C for 30 min, 45 min, or 2 hr before being returned to normal growth conditions (22°C in continuous light at 150 to 300 μmol m−2 sec−1). Viability was assessed daily for as long as 10 days after heat treatment, and plants were photographed after 2 days (×10 magnification) and after 10 days (Figure 8C shows one-quarter of a plate). Similar results were obtained when seeds were cold treated (4°C for 3 days) before plating.
Acknowledgments
This work was supported by the Howard Hughes Medical Institute and a grant from the Department of Energy to S.L. (No. DE-FG02-95ER20207) and E.V. (No. DE-FG02-95ER26208). E.V. was also supported by a U.S. Department of Agriculture National Research Initiative Grant Program No. 96-351003232. C.Q. was supported by a doctoral stipend (No. D/95/09187) granted by Deutscher Akademischer Austauschdienst (DAAD). We thank Danielle M. Ware and Casey Ng for technical assistance; Drs. Brian Keith and John Celenza for supplying vectors and strains of agrobacteria; and Dr. Dora Raventos (laboratory of Dr. John Mundy) for the vacuum infiltration protocol. We also thank Sue Yamins, Kenneth Yliniemi, Sandra Suwanski, and John Zdenek of the University of Chicago Greenhouse for assistance. For advice and helpful discussions, we thank Drs. Brian Keith and Daphne Preuss (University of Chicago) and members of their laboratories.
References
- Bechtold, N., and Pelletier, G. (1998). In planta Agrobacterium-mediated transformation of adult Arabidopsis thaliana plants by vacuum infiltration. Methods Mol. Biol. 82, 259–266. [DOI] [PubMed] [Google Scholar]
- Bohnert, H.J., Nelson, D.E., and Jensen, R.G.. (1995). Adaptations to environmental stresses. Plant Cell 7, 1099–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boston, R.S., Viitanen, P.V., and Vierling, E. (1996). Molecular chaperones and protein folding in plants. Plant Mol. Biol. 32, 191–222. [DOI] [PubMed] [Google Scholar]
- Eleutherio, E.C., Araujo, P.S., and Panek, A.D. (1993). Protective role of trehalose during heat stress in Saccharomyces cerevisiae. Cryobiology 30, 591–596. [DOI] [PubMed] [Google Scholar]
- Eriksson, M.J., and Clarke, A.K. (1996). The heat shock protein ClpB mediates the development of thermotolerance in the cyanobacterium Synechococcus sp. strain PCC 7942. J. Bacteriol. 178, 4839–4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frova, C. (1997). Genetic dissection of thermotolerance in maize. In Physical Stress in Plants, S. Grillo and A. Leone, eds (New York: Springer-Verlag), pp. 31–38.
- Glover, J.R., and Lindquist, S. (1998). Hsp104, Hsp70, and Hsp40: A novel chaperone system that rescues previously aggregated proteins. Cell 94, 73–82. [DOI] [PubMed] [Google Scholar]
- Holmstrom, K.-O., Welin, E.M.B., Palva, E.M.B.W., Tunnela, O.E., and Londesborough, J. (1996). Drought tolerance in tobacco. Nature 379, 683–684. [Google Scholar]
- Hong, S.-W., and Vierling, E. (2000). Mutants of Arabidopsis thaliana defective in the acquisition of tolerance to high temperature stress. Proc. Natl. Acad. Sci. USA, in press. [DOI] [PMC free article] [PubMed]
- Howarth, C.J., and Skot, K.P. (1994). Detailed characterization of heat shock protein synthesis and induced thermotolerance in seedlings of Sorghum bicolor L. J. Exp. Bot. 45, 1353–1363. [Google Scholar]
- Kasuga, M., Liu, Q., Miura, S., Yamaguchi-Shinozaki, K., and Shinozaki, K. (1999). Improving plant drought, salt, and freezing tolerance by gene transfer of a single stress-inducible transcription factor. Nat. Biotechnol. 17, 287–291. [DOI] [PubMed] [Google Scholar]
- Koncz, C., Schell, J., and Redei, G.P. (1992). T-DNA transformation and insertion mutagenesis. In Methods in Arabidopsis Research, C. Koncz, N.-H. Chua, and J. Schell, eds (Singapore: World Scientific Publishing), pp. 224–273.
- Lee, J.H., and Schöffl, F. (1996). An HSP70 antisense gene affects the expression of HSP70/HSC70, the regulation of HSF, and the acquisition of thermotolerance in transgenic Arabidopsis thaliana. Mol. Gen. Genet. 252, 11–19. [DOI] [PubMed] [Google Scholar]
- Lee, J.H., Hubel, A., and Schöffl, F. (1995). Derepression of the activity of genetically engineered heat shock factor causes constitutive synthesis of heat shock proteins and increased thermotolerance in transgenic Arabidopsis. Plant J. 8, 603–612. [DOI] [PubMed] [Google Scholar]
- Lee, Y.R., Nagao, R.T., and Key, J.L. (1994). A soybean 101-kD heat shock protein complements a yeast HSP104 deletion mutant in acquiring thermotolerance. Plant Cell 6, 1889–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt, J. (1980). Responses of plants to environmental stresses. In Physiological Ecology, T.T. Kozlowski, ed (New York: Academic Press), pp. 347–448.
- Lindquist, S., and Kim, G. (1996). Heat-shock protein 104 expression is sufficient for thermotolerance in yeast. Proc. Natl. Acad. Sci. USA 93, 5301–5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik, M.K., Slovin, J.P., Hwang, C.H., and Zimmerman, J.L. (1999). Modified expression of a carrot small heat shock protein gene, HSP17.7, results in increased or decreased thermotolerance. Plant J. 20, 1–11. [DOI] [PubMed] [Google Scholar]
- Matzke, M.A., and Matzke, A.J.M. (1995). How and why do plants inactivate homologous (trans)genes? Plant Physiol. 107, 679–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogk, A., Tomoyasu, T., Goloubinoff, P., Rüdiger, S., Röder, D., Langen, H., and Bukau, B. (1999). Identification of thermolabile E. coli proteins: Prevention and reversion of aggregation by DnaK and ClpB. EMBO J. 18, 6934–6949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskvina, E., Schuller, C., Maurer, C.T., Mager, W.H., and Ruis, H. (1998). A search in the genome of Saccharomyces cerevisiae for genes regulated via stress. Yeast 14, 1041–1050. [DOI] [PubMed] [Google Scholar]
- Motohashi, K., Watanabe, Y., Yohda, M., and Yoshida, M. (1999). Heat-inactivated proteins are rescued by the DnaK/J-GrpE set and ClpB chaperones. Proc. Natl. Acad. Sci. USA 96, 7184–7189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima, K., Kiyosue, T., Yamaguchi-Shinozaki, K., and Shinozaki, K. (1997). A nuclear gene, erd1, encoding a chloroplast-targeted Clp protease regulatory subunit homolog is not only induced by water stress but also developmentally up-regulated during senescence in Arabidopsis thaliana. Plant J. 12, 851–861. [DOI] [PubMed] [Google Scholar]
- Neuwald, A.F., Aravind, L., Spouge, J.L., and Koonin, E.V. (1999). AAA+: A class of chaperone-like ATPases associated with the assembly, operation, and disassembly of protein complexes. Genome Res. 9, 27–43. [PubMed] [Google Scholar]
- Nielsen, E., Akita, M., Davila-Aponte, J., and Keegstra, K. (1997). Stable association of chloroplastic precursors with protein translocation complexes that contain proteins from both envelope membranes and a stromal Hsp100 molecular chaperone. EMBO J. 16, 935–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nover, L. (1990). Molecular cell biology of the heat stress response. I. Naturwissenschaften 77, 310–316. [DOI] [PubMed] [Google Scholar]
- Osteryoung, K.W., Sundberg, H., and Vierling, E. (1993). Poly(A) tail length of a heat shock protein RNA is increased by severe heat stress, but intron splicing is unaffected. Mol. Gen. Genet. 239, 323–333. [DOI] [PubMed] [Google Scholar]
- Ougham, H.J., and Howarth, C.J. (1988). Temperature shock proteins in plants. Symp. Soc. Exp. Biol. 42, 259–280. [PubMed] [Google Scholar]
- Parsell, D.A., and Lindquist, S. (1993). The function of heat-shock proteins in stress tolerance: Degradation and reactivation of damaged proteins. Annu. Rev. Genet. 27, 437–496. [DOI] [PubMed] [Google Scholar]
- Parsell, D.A., and Lindquist, S. (1994). Heat shock proteins and stress tolerance. In The Biology of Heat Shock Proteins and Molecular Chaperones, R.I. Morimoto, A. Tissieres, and C. Georgopoulos, eds (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press), pp. 457–494.
- Parsell, D.A., Kowal, A.S., Singer, M.A., and Lindquist, S. (1994). Protein disaggregation mediated by heat-shock protein Hsp104. Nature 372, 475–478. [DOI] [PubMed] [Google Scholar]
- Prändl, R., Hinderhofer, K., Eggers-Schumacher, G., and Schöffl, F. (1998). HSF3, a new heat shock factor from Arabidopsis thaliana, derepresses the heat shock response and confers thermotolerance when overexpressed in transgenic plants. Mol. Gen. Genet. 258, 269–278. [DOI] [PubMed] [Google Scholar]
- Ruis, H., and Schuller, C. (1995). Stress signaling in yeast. Bioessays 17, 959–965. [DOI] [PubMed] [Google Scholar]
- Sanchez, Y., Taulien, J., Borkovich, K.A., and Lindquist, S. (1992). Hsp104 is required for tolerance to many forms of stress. EMBO J. 11, 2357–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmer, E.C., Lindquist, S., and Vierling, E. (1994). An Arabidopsis heat shock protein complements a thermotolerance defect in yeast. Plant Cell 6, 1899–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmer, E.C., Glover, J.R., Singer, M.A., and Lindquist, S. (1996). HSP100/Clp proteins: A common mechanism explains diverse functions. Trends Biochem. Sci. 21, 289–296. [PubMed] [Google Scholar]
- Shanklin, J., DeWitt, N.D., and Flanagan, J.M. (1995). The stroma of higher plant plastids contain ClpP and ClpC, functional homologs of Escherichia coli ClpP and ClpA: An archetypal two-component ATP-dependent protease. Plant Cell 7, 1713–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon, J.R., Treger, J.M., and McEntee, K. (1999). Multiple independent regulatory pathways control UB14 expression after heat shock in Saccharomyces cerevisiae. Mol. Microbiol. 31, 823–832. [DOI] [PubMed] [Google Scholar]
- Singer, M.A., and Lindquist, S. (1998). Multiple effects of trehalose on protein folding in vitro and in vivo. Mol. Cell 1, 639–648. [DOI] [PubMed] [Google Scholar]
- Smirnoff, N. (1998). Plant resistance to environmental stress. Curr. Opin. Biotechnol. 9, 214–219. [DOI] [PubMed] [Google Scholar]
- Smirnoff, N., and Bryant, J.A. (1999). DREB takes the stress out of growing up. Nat. Biotechnol. 17, 229–230. [DOI] [PubMed] [Google Scholar]
- Solomon, J.M., Rossi, J.M., Golic, K., McGarry, T., and Lindquist, S. (1991). Changes in hsp70 alter thermotolerance and heat-shock regulation in Drosophila. New Biol. 3, 1106–1120. [PubMed] [Google Scholar]
- Velazquez, J.M., Sonoda, S., Bugaisky, G., and Lindquist, S. (1983). Is the major Drosophila heat shock protein present in cells that have not been heat shocked? J. Cell Biol. 96, 286–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierling, E. (1991). The roles of heat shock proteins in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 42, 579–620. [Google Scholar]
- Weaver, L.M., Froehlich, J.E., and Amasino, R.M. (1999). Chloroplast-targeted ERD1 protein declines but its mRNA increases during senescence in Arabidopsis. Plant Physiol. 119, 1209–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehmeyer, N., Hernandez, L.D., Finkelstein, R.R., and Vierling, E. (1996). Synthesis of small heat-shock proteins is part of the developmental program of late seed maturation. Plant Physiol. 112, 747–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells, D.R., Tanguay, R.L., Le, H., and Gallie, D.R. (1998). HSP101 functions as a specific translational regulatory protein whose activity is regulated by nutrient status. Genes Dev. 12, 3236–3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welte, M.A., Tetrault, J.M., Dellavalle, R.P., and Lindquist, S. (1993). A new method for manipulating transgenes: Engineering heat tolerance in a complex, multicellular organism. Curr. Biol. 3, 842–853. [DOI] [PubMed] [Google Scholar]
- Yeh, K.W., Jinn, T.L., Yeh, C.H., Chen, Y.M., and Lin, C.Y. (1994). Plant low-molecular-mass heat-shock proteins: Their relationship to the acquisition of thermotolerance in plants. Biotechnol. Appl. Biochem. 19, 41–49. [PubMed] [Google Scholar]
- Zahringer, H., Burgert, M., Holzer, H., and Nwaka, S. (1997). Neutral trehalase Nth1p of Saccharomyces cerevisiae encoded by the NTH1 gene is a multiple stress responsive protein. FEBS Lett. 412, 615–620. [DOI] [PubMed] [Google Scholar]
- Zolkiewski, M. (1999). ClpB cooperates with DnaK, DnaJ, and GrpE in suppressing protein aggregation. A novel multi-chaperone system from Escherichia coli. J. Biol. Chem. 274, 28083–28086. [DOI] [PubMed] [Google Scholar]