Abstract
Leaves originate from the shoot apical meristem, a small mound of undifferentiated tissue at the tip of the stem. Leaf formation begins with the selection of a group of founder cells in the so-called peripheral zone at the flank of the meristem, followed by the initiation of local growth and finally morphogenesis of the resulting bulge into a differentiated leaf. Whereas the mechanisms controlling the switch between meristem propagation and leaf initiation are being identified by genetic and molecular analyses, the radial positioning of leaves, known as phyllotaxis, remains poorly understood. Hormones, especially auxin and gibberellin, are known to influence phyllotaxis, but their specific role in the determination of organ position is not clear. We show that inhibition of polar auxin transport blocks leaf formation at the vegetative tomato meristem, resulting in pinlike naked stems with an intact meristem at the tip. Microapplication of the natural auxin indole-3-acetic acid (IAA) to the apex of such pins restores leaf formation. Similarly, exogenous IAA induces flower formation on Arabidopsis pin-formed1-1 inflorescence apices, which are blocked in flower formation because of a mutation in a putative auxin transport protein. Our results show that auxin is required for and sufficient to induce organogenesis both in the vegetative tomato meristem and in the Arabidopsis inflorescence meristem. In this study, organogenesis always strictly coincided with the site of IAA application in the radial dimension, whereas in the apical–basal dimension, organ formation always occurred at a fixed distance from the summit of the meristem. We propose that auxin determines the radial position and the size of lateral organs but not the apical–basal position or the identity of the induced structures.
INTRODUCTION
Leaf primordia develop in regular patterns from the shoot apical meristem. In the majority of flowering plants, the leaves are arranged in spirals, with the divergence angle between successive leaves approaching the Fibonacci angle of 137.5°, the so-called golden ratio (Steeves and Sussex, 1989; Lyndon, 1990, 1998; Jean, 1994). Molecular and genetic analyses have recently identified various genes that regulate meristem development and leaf formation. Some of these genes encode transcription factors of the homeobox class (Vollbrecht et al., 1991; Long et al., 1996; Kerstetter et al., 1997; Taylor, 1997; Mayer et al., 1998), whereas others, such as clavata1, clavata3, and zwille, appear to be involved in cell-to-cell signaling (Clark et al., 1997; Moussian et al., 1998; Fletcher et al., 1999; Trotochaud et al., 1999). Current models postulate that these genes control the balance between meristem self-propagation and the production of organogenic tissue.
Whereas genetic analyses provide us with an ever more detailed description of meristem maintenance and propagation, the molecular nature of the mechanisms that trigger leaf initiation and ensure that leaves form at the proper angles relative to each other remains to be established. In many mutants, disorganized phyllotaxis is part of a pleiotropic phenotype, and the effects on phyllotaxis may well be indirect. Only in the recently described maize abphyl1 mutant has a specific phyllotactic alteration, from distichous (alternate) to decussate (opposite), been observed (Jackson and Hake, 1999).
Most of the information on the control of phyllotaxis stems not from genetic analyses but from surgical and pharmacological experiments conducted between 20 and 60 years ago (reviewed in Steeves and Sussex, 1989; Lyndon, 1990, 1998; Callos and Medford, 1994). These experiments provided evidence that factors of either a chemical or a physical nature could affect leaf position and the angle between leaves. Not surprisingly, the classical plant hormones, particularly auxin and gibberellin, were implicated, although their specific role in organ initiation and positioning has not been determined (Snow and Snow, 1937; Gorter, 1949, 1951; Bedesem, 1958; Kiermayer, 1960; Schwabe, 1971; Maksymowych and Maksymowych, 1973; Maksymowych et al., 1976; Maksymowych and Erickson, 1977; Meicenheimer, 1981).
Here, we focus on the possible function of auxin. Auxin is thought to be synthesized in young apical tissues and to be transported downward to the maturing stem and to the roots by a polar transport system that can be blocked with specific inhibitors (Davies, 1995). Exactly which cells within the shoot tip synthesize auxin remains to be determined, and it is entirely open whether the meristem proper is a source of auxin. Similarly, the polar transport pathway has been thoroughly studied in stem tissues (Davies, 1995), but whether or not it operates within the meristem is not known.
In this study, we describe two complementary systems, namely, vegetative meristems of tomato and inflorescence meristems of the Arabidopsis pin-formed1-1 (pin1-1) mutant, which we used to study the role of auxin in the control of phyllotaxis. Tomato shoot apical meristems are easily accessible, can be cultured on defined media, and are amenable to micromanipulation (Fleming et al., 1997, 1999). We show that their cultivation on auxin transport inhibitors specifically inhibits leaf initiation, leading to meristems devoid of leaf primordia. Likewise, organ formation is blocked in the inflorescence meristem of the Arabidopsis auxin transport mutant pin1-1. To assess the role of auxin in organ formation and phyllotaxis more directly, we micromanipulated the auxin concentrations in both systems.
RESULTS
Inhibition of Polar Auxin Transport Blocks Leaf Initiation but Not Meristem Propagation
Vegetative tomato shoot apices were cultured on a synthetic medium containing the auxin transport inhibitor N-1-naphthylphthalamic acid (NPA). This treatment completely inhibited the formation of new leaf primordia, whereas the meristem continued to grow and form stem tissue, leading to a pinlike structure devoid of leaves (cf. Figures 1A and 1B with Figure 1C). Preexisting leaf primordia continued to develop, but leaflets were not initiated and the leaf blade was deformed (Figure 1A, P1 and P2). Note that at the beginning of the experiment, the preexisting primordia of the apex shown in Figure 1A (P1 and P2) were at the stages of P1 and P2 shown in Figure 1C. Axillary meristems grew out (Figure 1A), indicating that apical dominance was reduced. The structurally unrelated auxin transport inhibitors 2,3,5-triiodobenzoic acid (TIBA) and 9-hydroxyfluorene-9-carboxylic acid (HFCA) gave similar results, but the flavonoids quercetin and apigenin, which are putative endogenous regulators of auxin efflux (Jacobs and Rubery, 1988), had no effect. Although 10 μM NPA always blocked primordium formation, elongation of the pins was variable. Approximately 50% of the pins grew to only a few hundred micrometers long and then ceased to grow, whereas the remaining pins recovered and grew as much as several millimeters. For further studies, these vigorously growing pins were transferred weekly to new plates containing 10 μM NPA, at which time the base of the pins was trimmed to leave ∼200 μm of stem length.
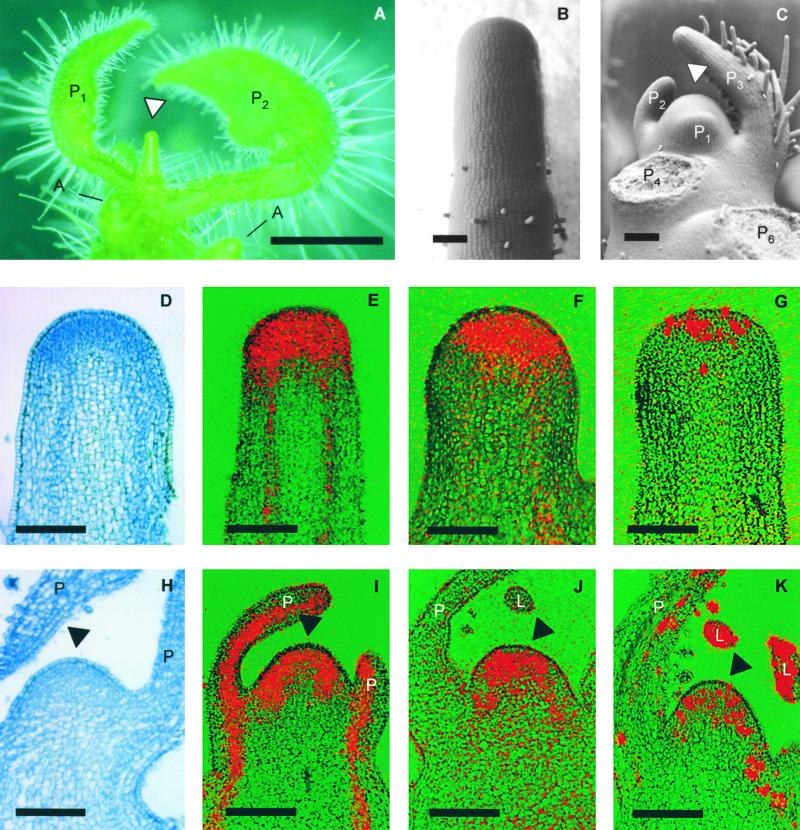
Figure 1.
Inhibition of Leaf Primordium Initiation by the Auxin Transport Inhibitor NPA and Expression of Diagnostic Genes in NPA-Treated Apices.
(A) Culture of tomato apices on NPA-containing medium results in a naked pin (arrowhead), outgrowth of axillary meristems (A), and altered development of preexisting primordia (P1 and P2, with P1 being the youngest primordium at the beginning of the experiment).
(B) Close-up of an NPA pin visualized by low-vacuum scanning electron microscopy.
(C) Control tomato apex with the meristem (arrowhead) and the three youngest leaf primordia (P1 to P3, with P1 being the youngest), as well as the leaf bases of older primordia (P4 and P6).
(D) to (G) Longitudinal sections of NPA pins.
(H) to (K) Longitudinal sections of control apices.
(D) and (H) show staining with toluidine blue. (E) to (G) and (I) to (K) are the result of in situ hybridizations. (E) and (I) show hybridization with a probe against Rpl2, which encodes a ribosomal protein. (F) and (J) show hybridization with a probe against the homeobox gene LeT6. (G) and (K) are the result of hybridization with a probe against histone H4. Arrowheads indicate the meristem; L, leaflet primordium; P, primordium.  ;
;  .
.
The pinlike structures, further referred to as NPA pins, exhibited small, densely staining cells at the tip that were arranged in layers as in control meristems (cf. Figures 1D and 1H; Steeves and Sussex, 1989). The tip of the NPA pins expressed the ribosomal protein Rpl2, a general marker for biosynthetic activity (Figure 1E; Fleming et al., 1993). Similarly, LeT6, a gene encoding a homeobox protein that is expressed in meristems (Chen et al., 1997), was highly expressed in the same tissue (Figure 1F). Histone H4, which is a marker for cells during S phase (Fleming et al., 1993; Brandstätter et al., 1994), was expressed only in the most apical cells of the pins, whereas in control apices, histone H4 was also detected in the meristem flanks (Figures 1G and 1K). Because Rpl2, LeT6, and histone H4 are expressed in meristems (Figures 1I to 1K), their presence indicates that NPA pins contain meristematic tissues at the tip. Cortical stripes that stained with toluidine blue (Figures 1D and 1H) and express Rpl2 (Figures 1E and 1I) presumably represent provascular strands and therefore indicate that the basic stem anatomy in NPA pins was not affected. Thus, auxin transport inhibitors specifically inhibit primordium initiation but not meristem self-perpetuation or formation of stem tissues.
Local Treatment with Indole-3-Acetic Acid Induces Leaf Primordia on NPA Pins
Although auxin is thought to be produced in the shoot apex, whether it is produced in the meristem itself is not known (Davies, 1995). If auxin is produced in the meristem, then NPA would be expected to cause accumulation of auxin within the meristem, and any resulting inhibition of leaf initiation could be attributed to inhibitory concentrations of auxin. If auxin is not produced in the meristem but is transported there from subtending tissues by an NPA-sensitive transporter, then NPA treatment would deplete auxin in the meristem, and inhibition of organ formation would result from the decrease in auxin concentrations in the meristem. In the latter case, application of auxin to the meristem would be expected to rescue organogenesis.
We applied small amounts (estimated as <5% of the volume of the meristem) of lanolin paste containing 0.1 to 30 mM indole-3-acetic acid (IAA) to various positions on the NPA pins, particularly on the flank and tip of the meristem. By taking into account retention in lanolin and photooxidation and diffusion in the tissue, we can reasonably expect these applied concentrations to cover the physiologic concentration range of IAA (Uggla et al., 1996). Treatment with auxin at the flank of the meristems induced the formation of bulges, whereas lanolin alone had no effect (Figures 2A and 2B, and Table 1). These bulges, which always coincided with the radial position of IAA application, differentiated into leaf primordia (Figures 2C and 2D). In the longitudinal (apical–basal) dimension, the primordia always formed at a fixed position from the summit, that is, within a ring-shaped zone roughly equivalent to the peripheral zone in untreated meristems (Steeves and Sussex, 1989; Lyndon, 1990, 1998).
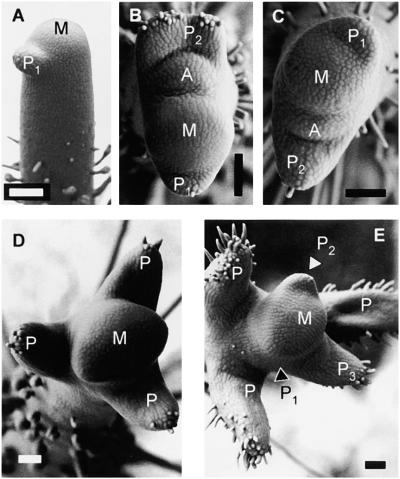
Figure 2.

Induction of Leaves on NPA Pins by the Natural Auxin IAA.
(A) NPA pin 4 days after treatment with control paste (white arrowhead) at the flank of the meristem (black arrowhead).
(B) to (D) Induction of a leaf primordium (arrows in [B] to [D]; P in [D]) on an NPA pin treated with red lanolin paste containing 10 mM IAA (white arrowheads in [B] to [D]). Black arrowheads point to the meristem. The same pin was photographed after 1 day (B), 2 days (C), and 4 days (D).
(E) Leaf induced by IAA on an NPA pin.
(F) to (K) Leaf primordia induced on NPA pins 1 week after local IAA treatment (red paste) at concentrations of 0.1, 0.3, 1, 3, 10, and 30 mM, respectively.
 ;
;  .
.
Table 1.
IAA Concentration Determines the Frequency of Leaf Induction on Tomato NPA Pins
| Number of Effects (%)
|
|||
|---|---|---|---|
| Treatmenta | No. of Treatments | Bulgingb | Primordiumc |
| Lanolin only | 18 | 0 | 0 |
| 0.1 mM IAA | 18 | 5 (27) | 3 (17) |
| 0.3 mM IAA | 18 | 10 (55) | 6 (33) |
| 1.0 mM IAA | 17 | 15 (88) | 9 (53) |
| 3.0 mM IAA | 18 | 17 (94) | 12 (67) |
| 10 mM IAA | 18 | 18 (100) | 14 (78) |
| 30 mM IAA | 18 | 16 (88) | 11 (61) |
Apices were cultured on 10 μM NPA for 17 days. The resulting pins were locally treated with IAA at the flank. Apices were examined 8 days after treatment with IAA.
Apices formed a local bulge at the site of treatment.
Apices in which the local bulge developed into a leaf primordium.
When auxin was applied to the very tip of the meristems, bulges never initiated at the tip but rather emerged at the flank at unpredictable radial positions (data not shown). The primordia induced by IAA exhibited dorsoventrality, turned green, and formed the trichomes typical for leaves (Figures 2C to 2E). At later stages, lateral leaflets were formed in ∼50% of the cases; after 2 months, the leaves had reached a length of several centimeters and exhibited all of the structures found in normal leaves (Figure 2E). However, the proportions of the different parts deviated from those of normal leaves; in particular, the petiole was thicker than normal, and only one pair of leaflets was formed (Figure 2E). The size of induced leaf primordia depended on the concentration of auxin (Figures 2F to 2K). Whereas lower concentrations (0.1 and 0.3 mM) induced primordia of approximately normal size (Figures 2F and 2G), higher concentrations (1 to 30 mM) induced primordia that were wider than normal (Figures 2H to 2J) and, in some cases, encompassed the entire meristem (Figure 2K). The frequency of primordium initiation was also dependent on auxin concentration (Table 1).
These experiments show that the NPA pins contain functional meristems capable of leaf formation when supplied with auxin. The site of auxin treatment determined the site of primordium formation in the radial position, but in the longitudinal position, primordia always originated from a ring-shaped region at the flanks of the meristem, as in natural leaf formation. The concentration of auxin determined the number of cells recruited into primordia.
Various growth regulators that have been shown to control cell division and cell expansion in other systems are therefore candidates for a role in primordium formation. We applied gibberellin A3, the kinetin 6-benzylaminopurine, and brassinolide to the flanks of the meristems of NPA pins. In addition, the effects of fusicoccin, a compound that mimics some aspects of IAA-induced growth by activating H+ extrusion into the cell wall (Marrè, 1979), were tested. In contrast to IAA, none of these substances was able to induce primordium formation (Table 2). Thus, induction of primordium formation by IAA on NPA pins is a specific effect.
Table 2.
Induction of Primordium Formation on Tomato NPA Pins by Various Hormones
| Experiment 1
|
Experiment 2
|
|||
|---|---|---|---|---|
| Treatmenta | No. of Treatments | No. of Effectsb (%) | No. of Treatments | No. of Effectsb (%) |
| Lanolin only | 9 | 0 | 17 | 0 |
| 10 mM IAA | 17 | 9 (53) | 18 | 11 (61) |
| 10 mM gibberellin | 19 | 0 | 18 | 0 |
| 10 mM brassinolide | 17 | 0 | 17 | 0 |
| 10 mM kinetin | n.d.c | n.d. | 18 | 0 |
| 10 mM fusicoccin | n.d. | n.d. | 19 | 0 |
All treatments were performed by applying lanolin paste containing the indicated hormones to the flanks of tomato NPA pins. Apices were examined 7 days after treatment.
Local induction of a leaf primordium.
These treatments were not performed in experiment 1. n.d., not determined.
NPA and IAA Interfere with Primordium Positioning at the Meristem
If local differences in auxin concentration determine leaf position, then it should be possible to disrupt phyllotaxis in a normal meristem by manipulating the auxin concentrations present with exogenous auxin as well as with auxin transport inhibitors. With this in mind, we applied auxin or auxin transport inhibitors to the I1 and I2 positions of normal meristems, that is, at the positions where the next two primordia would form (Figure 3A), or to the entire flank of the meristem. Application of IAA to I1, the site of incipient primordium formation, increased the size of the primordium to be formed at this position (cf. Figures 3B and 3C; see Table 3). Specifically, the base of the primordium was enlarged, often to an extent that it was thicker than the next older primordium, suggesting that more cells were engaged in primordium initiation than normal. Treatment at I2 (between P1 and P2) caused the initiation of ectopic primordia (Figure 3D and Table 3), which were fused to various degrees with P1. When the entire flank of the meristem was treated with IAA, oversized fused primordia were induced (Figure 3E and Table 3). Note that in Figures 3D and 3E, tissues on different sides of P1 were induced to form primordia. In Figure 3D, these tissues were in the space between P1 and P2, whereas in Figure 3E, they were in the space between P1 and I1. This indicates that on both sides of P1, the flank is able to form primordia if supplied with auxin. However, in the proximity of P2, the flank tissue did not respond to IAA; consequently, whereas ectopic primordia were always fused to some extent with P1, they were never fused with P2 (Figures 3D and 3E). Perhaps at stage P2, primordia are surrounded by an inhibitory field (Lyndon, 1990).
Figure 3.
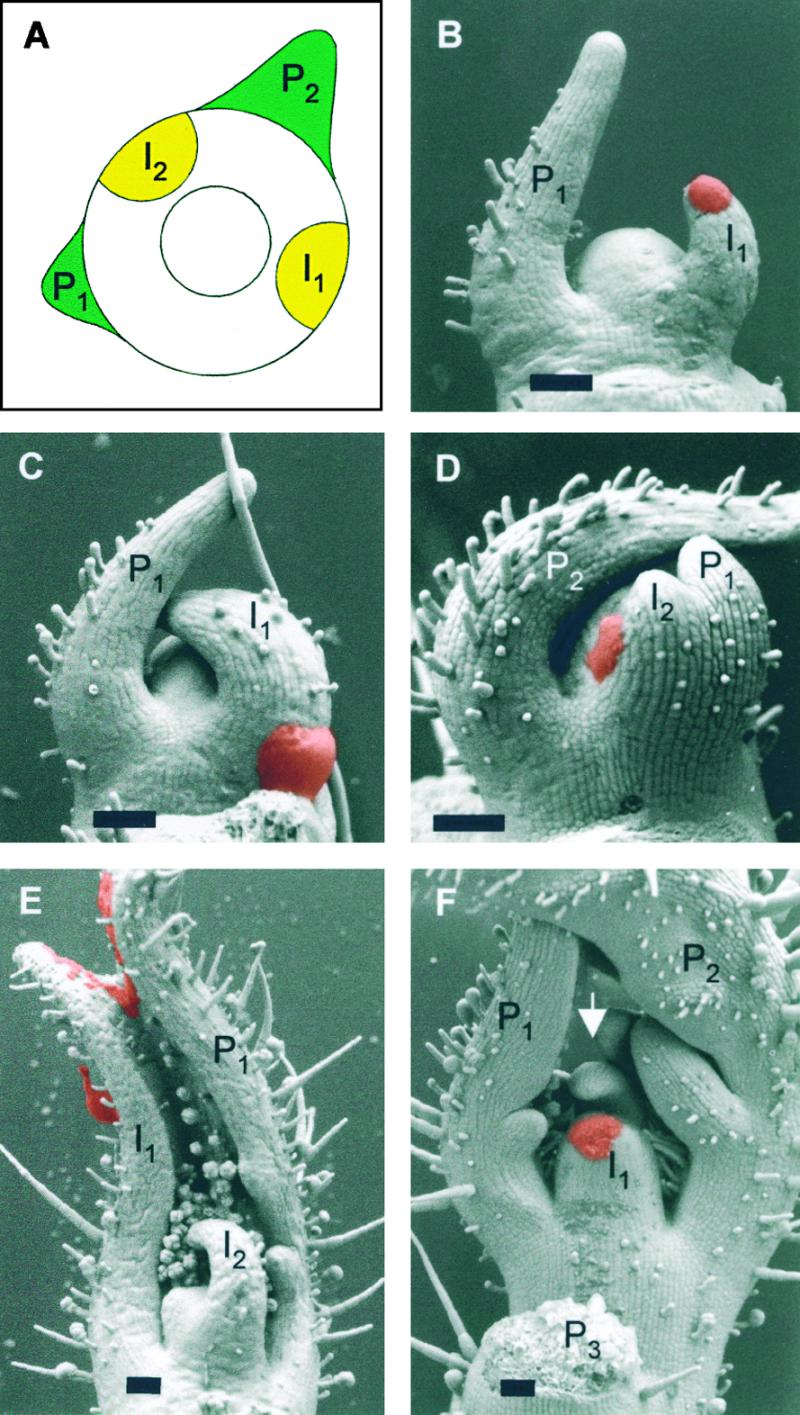
Effects of Treatments of Meristems with IAA and NPA.
(A) Schematic representation of a tomato apex with the youngest and the second youngest primordia (P1 and P2, respectively). The large circle delimits the apical meristem, and the small circle marks the central zone, which is not involved in organogenesis. The sites of incipient primordium formation (I1) and the site of the following primordium (I2) are indicated as yellow areas.
(B) Control treatment with lanolin (red paste) at the site of incipient primordium formation (I1). The primordium that formed at the I1 position has a normal size relative to P1 (cf. with Figure 1C).
(C) Increased primordium size caused by IAA treatment (red paste) at I1. P1 denotes the preexisting primordium. Note that the base of I1 is extended toward P1.
(D) Induction of an ectopic primordium by IAA (red paste) at the I2 position (between preexisting primordia P1 and P2). The ectopic primordium is fused to P1.
(E) Induction of a large fused leaf primordium caused by IAA treatment (red paste) on the entire flank of the meristem. The fused leaf includes P1 (right side) and I1 (left side). P2 (which would have been toward the viewer) was removed before scanning electron microscopic analysis.
(F) Inhibition of primordium initiation by NPA treatment at I1 (red paste) and formation of an ectopic primordium at I2 (arrow, between preexisting primordia P1 and P2).
Apices shown in (B) to (D) were examined 3 days after treatment; apices shown in (E) and (F) were analyzed 6 days after treatment.  .
.
Table 3.
Inhibition of Leaf Primordium Formation and Induction of Ectopic Leaf Primordia by Local Application to Tomato Apices of Lanolin Paste Containing Various Auxin Transport Inhibitors or Auxin
| Treatmenta | No. of Treatments | Site of Application | No. of Effects (%) |
|---|---|---|---|
| Lanolin only | 22 | I1 | 0 |
| Lanolin only | 15 | I2 | 0 |
| 10 mM NPA | 22 | I1 | 17b (77) |
| 10 mM HFCA | 12 | I1 | 8b (66) |
| 10 mM TIBA | 12 | I1 | 5b (42) |
| 10 mM apigenin | 12 | I1 | 0 |
| 10 mM quercetin | 12 | I1 | 0 |
| 10 mM IAA | 14 | I1 | 10c (71) |
| 10 mM IAA | 64 | I2 | 22d (34) |
| 10 mM IAA | 14 | Entire | 7e (50) |
All treatments were performed by applying lanolin paste containing the indicated substances to freshly dissected tomato apices at the flank of the meristems either at the position of incipient leaf formation (I1) or at the site where the following primordium was expected (I2). Alternatively, the entire flank was treated. Apices were examined 7 days after treatment.
Leaf formation inhibited.
Primordia thicker than normal.
Ectopic primordia fused to P1.
Large ectopic primordia fused to P1.
To determine the effects of local inhibition of auxin transport within the meristem, apices were treated with NPA at I1. Primordium formation was inhibited at the site of treatment (Figure 3F), whereas on the opposite side of the meristem, that is, at I2, a primordium was initiated, resulting in a reversal of phyllotaxis. The auxin transport inhibitors 2,3,5-triiodobenzoic acid and 9-hydroxyfluorene-9-carboxylic acid had similar effects, whereas apigenin and quercetin had no effects (Table 3). Taken together, auxin and NPA both affected organ positioning but had opposite effects.
Phyllotaxis in Recovering NPA Pins Is Variable
Leaf positioning is known to be affected by preexisting leaf primordia (Snow and Snow, 1931, 1933; Callos and Medford, 1994). The NPA pins are devoid of leaves and therefore provide a simple system with which to test the role of preexisting leaves. After 5 weeks of culture on NPA and repeated removal of the basal stem portion, the pins lacked any trace of leaves. Three days after transfer to synthetic medium without NPA, pins spontaneously initiated new primordia (Figure 4A). These always originated from the flank of the meristem. Primordium formation continued and resulted in distichous (Figure 4B; 30%), spiral (Figure 4C; 18%), or whorled phyllotaxis (Figure 4D; 27%); the remaining pins either were fasciated or did not recover at all ( ). Interestingly, recovering apices returned to spiral phyllotaxis after production of a few whorled leaves (Figure 4E). In general, the apices initiated axillary meristems soon after primordium formation (Figures 4B and 4C). These results show that preexisting leaves are not required for primordium formation per se but for correct phyllotactic patterning. Both the occurrence of distichous phyllotaxis and the symmetry observed in whorled phyllotaxis (Figures 4B and 4D) suggest the involvement of lateral inhibition in the patterning of the plant apex (Lyndon, 1990; Meinhardt, 1994).
). Interestingly, recovering apices returned to spiral phyllotaxis after production of a few whorled leaves (Figure 4E). In general, the apices initiated axillary meristems soon after primordium formation (Figures 4B and 4C). These results show that preexisting leaves are not required for primordium formation per se but for correct phyllotactic patterning. Both the occurrence of distichous phyllotaxis and the symmetry observed in whorled phyllotaxis (Figures 4B and 4D) suggest the involvement of lateral inhibition in the patterning of the plant apex (Lyndon, 1990; Meinhardt, 1994).
Figure 4.
Phyllotaxis in Pins Recovering from NPA.
(A) Primordium initiation on an NPA pin 3 days after transfer to NPA-free medium.
(B) to (E) Phyllotaxis on apices 7 days after transfer to NPA-free medium (n = 60). Shown are distichous phyllotaxis (B), spiral phyllotaxis (C), whorled phyllotaxis (D), and reversion from whorled to spiral phyllotaxis (E).
A, axillary meristem; M, apical meristem; P, primordia initiated concomitantly; P1, P2, P3, successive primordia, with P1 being the youngest.  .
.
Local Treatment with IAA Induces Flower Primordia on Inflorescence Apices of the Arabidopsis Mutant pin1-1
The effects of NPA on tomato apices resemble the phenotype of the Arabidopsis pin1-1 mutant (Okada et al., 1991). This mutant is affected in auxin transport in the inflorescence stem, the genetic defect for which was recently traced to a putative auxin efflux carrier (Gälweiler et al., 1998). The pin1-1 mutation affects leaf formation and development of the inflorescence. Two aspects of the mutant phenotype are particularly striking in the context of our experiments with tomato apices. In pin1-1 plants, leaves are often fused or cup shaped and are oversized from the initial stages of development (cf. Figure 5A with Figures 5B and 5C), suggesting that the number of cells recruited is larger than normal. This leaf phenotype resembles the leaves triggered on NPA pins by IAA at higher concentrations (Figures 2I to 2K). Second, mutant inflorescences are greatly reduced to a pinlike shoot that resembles the pins produced by tomato meristems in the presence of NPA (Okada et al., 1991; cf. Figures 1B and 5D).
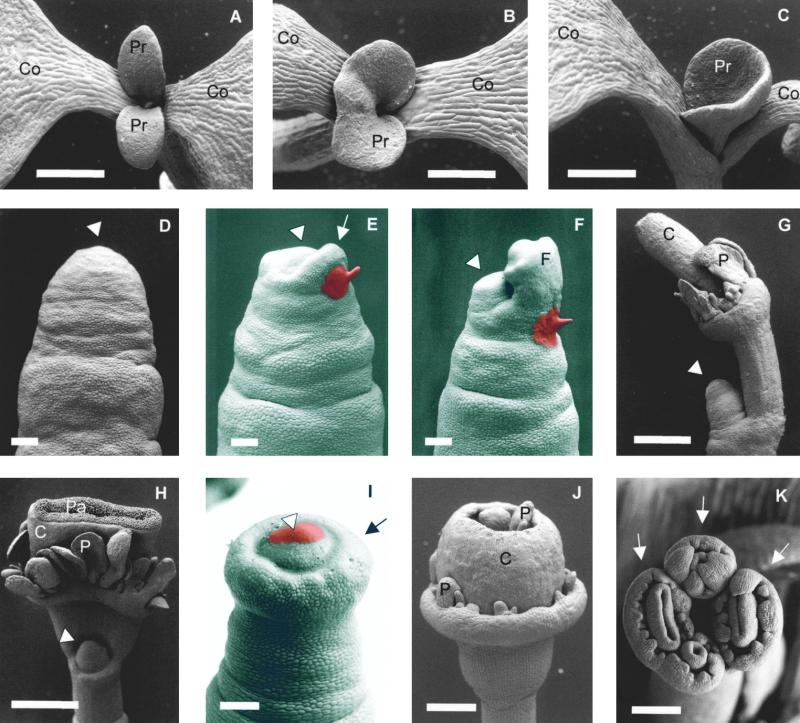
Figure 5.
Leaf and Inflorescence Phenotype of the Arabidopsis pin1-1 Mutant and Induction of Flowers by IAA.
(A) Wild-type Arabidopsis seedling with two leaf primordia.
(B) pin1-1 seedling with one fused leaf primordium.
(C) pin1-1 seedling with a cup-shaped leaf primordium.
(D) Inflorescence apex of a pin1-1 mutant plant devoid of flowers. The arrowhead indicates the meristem.
(E) to (H) Induction of flowers by treatment with 1 mM IAA (red paste) at the flank. Apices were analyzed 38 hr (arrow points to local bulge) (E), 4 days (F), 7 days (G), and 11 days (H) after treatment. Arrowheads in (E) to (H) denote the meristem.
(I) Induction of a circular bulge (arrow) around the flank by treatment for 2 days with 1 mM IAA at the top of the meristem (arrowhead).
(J) Circular flower induced after 6 days by treatment as given in (I). Note that the circular flower does not correspond to a single normal flower but rather is a ring of several flowers that are laterally fused; consequently, petaloid organs are found on the adaxial side of the circular carpel. In the center, the indeterminate inflorescence meristem is maintained (not visible).
(K) Flowers (arrows) induced after 4 days by treatment as given in (I); in this case, the initial ring bulge broke into several individual flowers. Two of these are partially fused (on the lower left side).
C, carpel; Co, cotyledon; F, flower primordium; P, petal; Pa, papilla; Pr, leaf primordium.  ;
; 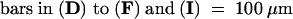 ;
; 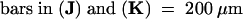 .
.
In light of our results, the phenotype of the pin1-1 mutant might be caused by abnormally high amounts or mislocalization of auxin in vegetative meristems, leading to induction of broad leaf primordia, and by the lack of auxin in the apical inflorescence meristem, which prevents flower formation. To test this hypothesis, we applied auxin in lanolin paste to the flanks of inflorescence meristems of pin1-1 mutants. This treatment induced the formation of flower primordia at the site of treatment (85%;  ). In control treatments with lanolin, primordium formation was observed in only two cases (
). In control treatments with lanolin, primordium formation was observed in only two cases ( ). A low frequency of spontaneous organ formation may be expected because pin1-1 mutant plants occasionally form aberrant flowers after the pins have reached a length of several centimeters (Okada et al., 1991). Therefore, for all further analyses of auxin-induced flower formation, we used young pins <1 cm long just after their emergence from the rosette. In such young pins, spontaneous organ formation was never observed.
). A low frequency of spontaneous organ formation may be expected because pin1-1 mutant plants occasionally form aberrant flowers after the pins have reached a length of several centimeters (Okada et al., 1991). Therefore, for all further analyses of auxin-induced flower formation, we used young pins <1 cm long just after their emergence from the rosette. In such young pins, spontaneous organ formation was never observed.
Treatment with 1 mM IAA at the flank of pin1-1 apices induced bulges at the site of treatment after 38 hr (Figure 5E and Table 4), and the bulges later developed into flower primordia (Figure 5F). These flowers initiated two to four whorls of organs; after a week, they had reached a length of ∼0.5 mm (Figure 5G). Although the flowers reached normal sizes, their architecture was aberrant. Each had a large carpel with papillae at the tip surrounded by a variable number of petaloid organs (Figure 5H). Similar defects have been described for the flowers of Arabidopsis wild-type plants that had been treated with auxin transport inhibitors and in the escape flowers in pin1-1 mutants (Okada et al., 1991).
Table 4.
Induction of Floral Primordia by Local Treatment of Arabidopsis pin1-1 Inflorescence Apices with Lanolin Paste Containing IAA
| No. of Effects (%)
|
||||||
|---|---|---|---|---|---|---|
| Treatment | Site of Application | No. of Treatments | No Effect | Local Primordiuma | Incomplete Ringb | Complete Ringc |
| Lanolin only | At the flank | 18 | 18 (100) | 0 | 0 | 0 |
| 1 mM IAA | At the flank | 20 | 1 (5) | 19 (95) | 0 | 0 |
| 0.1 mM IAA | At the flank | 16 | 2 (13) | 14 (87) | 0 | 0 |
| 0.01 mM IAA | At the flank | 14 | 14 (100) | 0 | 0 | 0 |
| 1 mM IAA | 100 μm below the flank | 22 | 16 (73) | 6 (27)d | 0 | 0 |
| 1 mM IAA | 200 μm below the flank | 14 | 14 (100) | 0 | 0 | 0 |
| Lanolin only | On the summit | 18 | 18 (100) | 0 | 0 | 0 |
| 1 mM IAA | On the summit | 18 | 1 (6) | 0 | 7 (39) | 10 (55) |
| 0.1 mM IAA | On the summit | 18 | 6 (33) | 0 | 11 (61) | 1 (6) |
| 0.01 mM IAA | On the summit | 15 | 15 (100) | 0 | 0 | 0 |
Formation of a local primordium at the radial position of treatment.
Formation of an incomplete ring-shaped primordium or several individual bulges.
Formation of a complete ring-shaped primordium.
Primordia were always formed at the flank above the site of treatment at the same radial position as IAA application.
Treatments with 0.1 mM IAA at the flank had similar effects, but the frequency was less and the induced flowers were smaller than those developing after treatment with 1 mM IAA (Table 4). Application of IAA at 10 μM had no effect (Table 4). Treatment with 1 mM IAA at a position ∼100 μm below the flank induced only small bulges at a reduced frequency, and treatments 200 μm below the flank had no effect (Table 4), indicating that the maximal range of auxin diffusion in the apical tissues is between 100 and 200 μm. When Arabidopsis inflorescence pins were treated with 1 mM IAA on the summit of the meristem, ring-shaped bulges were induced around the flank of the entire meristem (Figure 5I and Table 4); however, the center of the meristem never responded to IAA treatment. At later times, the ring-shaped bulges developed into circular flowers that consisted of a large, ring-shaped carpel with petaloid organs both inside and outside the carpel (Figure 5J), or the ring broke into discrete flowers arranged in a circle (Figure 5K). Treatments with 0.1 mM IAA on the top resulted in the induction of incomplete ring-shaped bulges (Table 4); application on the top of IAA at 10 μM had no effect (Table 4).
DISCUSSION
Auxin Transport Is Required for Lateral Organ Formation
Tomato apices can be dissected and cultured in vitro on defined media. Under such conditions, the apices grow normally and initiate leaves in the regular spiral phyllotactic pattern (Fleming et al., 1997, 1999). Apices cultured on medium containing NPA or other specific inhibitors of polar auxin transport, however, were unable to initiate new leaf primordia. Stem growth in such pinlike structures was not affected, and according to histology and gene expression analysis, normal-structured meristems were maintained at the summit of the pins (Figure 1). Thus, inhibition of auxin transport specifically blocked primordium initiation but not meristem self-propagation or the formation of stem tissues. The tomato NPA pins are reminiscent of the pin-shaped inflorescences of the Arabidopsis pin1-1 mutant, which has a defect in auxin transport caused by the inactivation of a putative membrane efflux carrier for auxin (Okada et al., 1991; Gälweiler et al., 1998).
Exogenous Auxin Restores Organ Formation on Tomato NPA Pins and on Arabidopsis pin1-1 Inflorescence Apices
Leaf initiation in NPA-treated tomato meristems could be restored by applying microdroplets of auxin to defined positions on the meristem. The effect was specific for auxin, because no other hormone was able to trigger primordium formation. Fusicoccin, an inducer of plasma membrane proton pumps (Marrè, 1979), had no effect either, indicating that acidification of the extracellular space was not sufficient to induce primordium formation.
Whereas auxin induced leaf primordia on vegetative tomato pins, it induced flower primordia on Arabidopsis pin1-1 inflorescence apices. Therefore, auxin appears to trigger organogenesis without determining organ identity, which suggests that other factors in the meristem determine organ identity. In this context, it would be interesting to analyze organ formation on floral NPA pins of tomato. Unfortunately, we were not able to perform such experiments because the floral apices could not be cultured in vitro.
Whether the meristem itself produces auxin is not known; therefore, inhibition of auxin transport could lead to either an accumulation or a depletion of auxin in the meristem, depending on whether or not the meristem is a source of auxin. The fact that application of exogenous auxin can relieve the block of primordium formation in tomato and Arabidopsis pins suggests that meristems do not produce sufficient amounts of auxin to permit organ formation and that, under natural conditions, auxin is transported acropetally into the meristem by a specific NPA-inhibitable transport system. However, as long as the actual auxin concentrations within the meristem are not known, alternative explanations cannot be excluded. In particular, under natural conditions, auxin transport and reallocation within the meristem are conceivably the basis of primordium initiation rather than acropetal transport.
Auxin Plays a Role in the Radial Positioning of Leaf Primordia
A striking aspect of our work is the correlation between the site at which auxin is applied and the site of organ initiation. In the radial dimension, the correlation was perfect in both tomato and Arabidopsis pins: primordia formed exactly at the position where auxin was applied. In contrast, in the apical–basal dimension, organ initiation invariantly occurred at a fixed distance of ∼50 to 100 μm from the tip of the meristem, just as in natural meristems (Figure 1C). This was true even when auxin was applied slightly above or below this area or at the very tip of the meristem (Figures 2 and 5). This zonation is reminiscent of the classical zonation model in which, according to histology and gene expression patterns, the meristem is subdivided into central and peripheral zones (Steeves and Sussex, 1989; Lincoln et al., 1994; Laufs et al., 1998a; Lyndon, 1998; Fletcher et al., 1999; Nishimura et al., 1999). The central zone has been proposed to serve as a pool of stem cells, whereas the peripheral zone presumably is the site of organogenesis (Medford, 1992; Clark, 1997; Meyerowitz, 1997). Although this zonation model convincingly explains the apical–basal patterning of the meristem, it does not shed light on the radial patterning.
We propose the existence of two independent patterning systems in the meristem. The first is the apical–basal pattern, which may be specified by genes such as shoot meristemless, wuschel, clavata, and mgoun (Long et al., 1996; Clark et al., 1997; Laufs et al., 1998b; Mayer et al., 1998; Fletcher et al., 1999) and the maintenance of which does not depend on auxin. The second, the radial patterning system, is conditioned by auxin and determines where within the peripheral zone organs are initiated. The results of the experiments presented in Figure 3 show that the effect of auxin is not limited to pins but is probably critical in normal meristems as well. In the presence of preexisting leaves, auxin can induce ectopic and fused primordia at the flank of the meristem (Figures 3B and 3C). Conversely, NPA inhibits leaf formation at the site of application and leads to primordium initiation at the opposite side. In keeping with the results of previous experiments, the simplest explanation for these findings is that under natural conditions, localized accumulation of auxin in the meristem, mediated by an NPA-inhibitable auxin transporter, determines the radial position of organ initiation.
Interestingly, interference with auxin transport also affects the radial patterning of embryos. When developing embryos were incubated in media containing auxin transport inhibitors, they exhibited defects in bilateral symmetry, ranging from the lack of cotyledons to collarlike cotyledons (Liu et al., 1993; Hadfi et al., 1998). pin1-1 mutant embryos exhibit similar defects (Okada and Shimura, 1994), indicating that auxin transport is important for the establishment of bilateral symmetry in embryogenesis and possibly for the initiation of cotyledons.
A Model for Patterning of the Meristem
If auxin determines radial leaf position, then how could auxin transport itself be directed? Preexisting primordia are known to influence future primordium position (Snow and Snow, 1931, 1933; Callos and Medford, 1994). Therefore, we analyzed phyllotaxis in meristems that were grown for prolonged periods on NPA and in which all radial patterning information that derived from preexisting primordia was erased. When such apices were no longer treated with NPA, they resumed primordium formation, but phyllotaxis was virtually random (Figure 4). These results show that preexisting leaves, although dispensable for leaf initiation per se, are required for correct leaf positioning. We propose that the influence of preexisting leaves on phyllotaxis is mediated by their vasculature, possibly by defining the routes of auxin transport to the meristem. Such a relationship between vasculature and phyllotaxis has been suggested on the basis of histologic analysis (Larson, 1975; Kirchoff, 1984). Auxin transport is associated with the vasculature; conversely, auxin induces vascular differentiation (Sachs, 1991; Davies, 1995). Such a positive feedback mechanism (Sachs, 1991), in which the vascular prepattern in the apex is used as a template to determine further leaf positioning, could explain the remarkable regularity and stability of phyllotactic systems (Steeves and Sussex, 1989; Lyndon, 1990, 1998; Jean, 1994).
How could the action of auxin lead to the initiation of primordia? Previous experiments have shown that expansin, a protein with cell wall–loosening activity, can induce leaflike structures on the meristem (Fleming et al., 1997). In addition, a gene encoding an expansin is upregulated at the site of incipient leaf initiation under natural conditions (Reinhardt et al., 1998). Conceivably, expansin is a downstream effector of auxin action, and expansin expression possibly is directly regulated by auxin. However, the leaves induced by auxin differ from the structures induced by the exogenous expansin protein. The bulges in the latter case were devoid of vascular bundles, displayed only limited dorsoventrality, and rarely developed beyond the initial stages (Fleming et al., 1997). Thus, auxin is able to trigger the entire developmental program for leaf formation, whereas expansin protein triggers only a subset of the steps in leaf development.
Taken together, our results demonstrate that auxin plays a central role in organ initiation and positioning at the meristem in both tomato and Arabidopsis plants. We propose that when meristem cells are displaced from the center to the periphery, they become competent to respond to auxin with leaf formation. If auxin is absent during their passage through the peripheral region, then the cells lose the competence for organ initiation and acquire the default identity of stem tissue. If they are induced by auxin, then they acquire a new identity and become committed to organogenesis. Thus, auxin-mediated radial patterning is superimposed on the basic apical–basal pattern in the meristem (central, peripheral, and submeristematic zone), which is maintained independent of auxin. The combined positional information from these two patterning systems determines cell fate and thus phyllotaxis.
METHODS
Plant Growth and in Vitro Culture
Tomato plants (Lycopersicon esculentum cv Moneymaker) were grown as described previously (Reinhardt et al., 1998). Shoot apices were dissected and cultured according to Fleming et al. (1997). The auxin transport inhibitors N-1-naphthylphthalamic acid (NPA; Interchim, Montluçon, France), 9-hydroxyfluorene-9-carboxylic acid (HFCA; Sigma), and 2,3,5-triiodobenzoic acid (TIBA; Sigma) were added to the medium from 10 mM stock solutions in DMSO to a final concentration of 10 μM. The flavonoids quercetin and apigenin were added to the medium at concentrations of 10 and 100 μM each. For in situ hybridization analysis and treatments with indole-3-acetic acid (IAA), tomato apices were cultured on 10 μM NPA for 3 weeks before fixation or application of IAA. Pin-shaped apices used for the analysis of initial phyllotactic patterning were generated by culturing tomato apices for 5 weeks on 10 μM NPA. The bases of the apices were trimmed, and the apices were transferred to new NPA plates once a week.
Seeds (Arabidopsis thaliana; accession number CS8065 from the Arabidopsis Information Management System; http://aims.cps.msu edu/aims/) were surface-sterilized and grown on Murashige and Skoog plates (Serva, Heidelberg, Germany) for 1 week before potting. Plants were grown under an 8-hr-light/16-hr-dark regime at 22°C.
Treatments of Plant Apices
For local treatments of apices, IAA (Fluka, Buchs, Switzerland), 6-benzylaminopurine (Sigma), gibberellin A3 (Fluka), NPA, quercetin (Sigma), or apigenin (Sigma) 1 M stock solutions in DMSO were dissolved in a prewarmed (50°C) paste consisting of lanolin with 2.5% paraffin (Merck). Brassinolide (CIDtech Research, Cambridge, Ontario, Canada) and fusicoccin (Sigma) were dried under vacuum from 10 mM stock solutions in ethanol and dissolved directly in prewarmed lanolin paste. The paste was manually administered to cultured tomato apices with yellow, drawn plastic pipette tips. Tomato NPA pins treated with IAA were further cultured on plates containing 10 μM NPA. Arabidopsis pin-formed1-1 (pin1-1) inflorescence apices were treated similarly as tomato apices, but the apices were left on the intact plant.
In Situ Hybridizations and Microscopy
In situ hybridizations were performed as described by Reinhardt et al. (1998). Apices and seedlings were viewed with a variable-pressure scanning electron microscope (model S-3500N; Hitachi, Tokyo, Japan). Digital images were pseudocolored for clarity.
Acknowledgments
We thank Catherine Perrot-Rechenmann, Heinz Richner, Andrew J. Fleming, and Pia Stieger for critical reading of the manuscript and Jeroen Stuurman for discussion. This work was supported by a Swiss National Science Foundation grant to C.K. and D.R.
References
- Bedesem, P.P. (1958). Histogenetic effects of 2,3,5-triiodobenzoic acid on the shoot apices and leaf primordia of tomato. Bull. Torrey Bot. Club 85, 434–472. [Google Scholar]
- Brandstätter, J., Rossbach, C., and Theres, K. (1994). The pattern of histone H4 expression in the tomato shoot apex changes during development. Planta 192, 69–74. [DOI] [PubMed] [Google Scholar]
- Callos, J.D., and Medford, J.I. (1994). Organ positions and pattern formation in the shoot apex. Plant J. 6, 1–7. [Google Scholar]
- Chen, J.-J., Janssen, B.-J., Williams, A., and Sinha, N. (1997). A gene fusion at a homeobox locus: Alterations in leaf shape and implications for morpholocical evolution. Plant Cell 9, 1289–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, S.E. (1997). Organ formation at the vegetative shoot meristem. Plant Cell 9, 1067–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, S.E., Williams, R.W., and Meyerowitz, E.M. (1997). The CLAVATA1 gene encodes a putative receptor kinase that controls shoot and floral meristem size in Arabidopsis. Cell 89, 575–585. [DOI] [PubMed] [Google Scholar]
- Davies, P.J. (1995). Plant Hormones—Physiology, Biochemistry and Molecular Biology. (Dordrecht: The Netherlands: Kluwer Academic Publishers).
- Fleming, A.J., Mandel, T., Roth, I., and Kuhlemeier, C. (1993). The patterns of gene expression in the tomato shoot apical meristem. Plant Cell 5, 297–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming, A.J., McQueen-Mason, S., Mandel, T., and Kuhlemeier, C. (1997). Induction of leaf primordia by the cell wall protein expansin. Science 276, 1415–1418. [Google Scholar]
- Fleming, A.J., Caderas, D., Wehrli, E., McQueen-Mason, S., and Kuhlemeier, C. (1999). Analysis of expansin-induced morphogenesis on the apical meristem of tomato. Planta 208, 166–174. [Google Scholar]
- Fletcher, J.C., Brand, U., Running, M.P., Simon, R., and Meyerowitz, E.M. (1999). Signaling of cell fate decisions by CLAVATA3 in Arabidopsis shoot meristems. Science 283, 1911–1914. [DOI] [PubMed] [Google Scholar]
- Gälweiler, L., Guan, C., Müller, A., Wisman, E., Mendgen, K., Yephremov, A., and Palme, K. (1998). Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 282, 2226–2230. [DOI] [PubMed] [Google Scholar]
- Gorter, C.J. (1949). The influence of 2,3,5-triiodobenzoic acid on the growing points of tomatoes. Proc. Kon. Ned. Akad. Wet. 52, 1185–1193. [Google Scholar]
- Gorter, C.J. (1951). The influence of 2,3,5-triiodobenzoic acid on the growing points of tomatoes. II. The initiation of ring fasciations. Proc. Kon. Ned. Akad. Wet. Ser. C Biol. Med. Sci. 54, 181–190. [Google Scholar]
- Hadfi, K., Speth, V., and Neuhaus, G. (1998). Auxin-induced developmental patterns in Brassica juncea embryos. Development 125, 879–887. [DOI] [PubMed] [Google Scholar]
- Jackson, D., and Hake, S. (1999). Control of phyllotaxis in maize by the abphyl1 gene. Development 126, 315–323. [DOI] [PubMed] [Google Scholar]
- Jacobs, M., and Rubery, P.H. (1988). Naturally occurring auxin transport regulators. Science 241, 346–349. [DOI] [PubMed] [Google Scholar]
- Jean, R.V. (1994). Phyllotaxis: A Systematic Study in Plant Morphogenesis. (New York: Cambridge University Press).
- Kerstetter, R.A., Laudencia-Chingcuanco, D., Smith, L.G., and Hake, S. (1997). Loss-of-function mutations in the maize homeobox gene, knotted1, are defective in shoot meristem maintenance. Development 124, 3045–3054. [DOI] [PubMed] [Google Scholar]
- Kiermayer, O. (1960). Die Formative Wirksamkeit der 2,3,5-Trijodobenzoesäure (TIBA) in Gegenwart von Gibberellinsäure (GA). Planta 55, 153–168. [Google Scholar]
- Kirchoff, B.K. (1984). On the relationship between phyllotaxy and vasculature: A synthesis. Bot. J. Linn. Soc. 89, 37–51. [Google Scholar]
- Larson, P.R. (1975). Development and organization of the primary vascular system in Populus deltoides according to phyllotaxy. Am. J. Bot. 62, 1084–1099. [Google Scholar]
- Laufs, P., Grandjean, O., Jonak, C., Kiêu, K., and Traas, J. (1998. a). Cellular parameters of the shoot apical meristem in Arabidopsis. Plant Cell 10, 1375–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufs, P., Dockx, J., Kronenberger, J., and Traas, J. (1998. b). MGOUN1 and MGOUN2: Two genes required for primordium initiation at the shoot apical and floral meristems in Arabidopsis thaliana. Development 125, 1253–1260. [DOI] [PubMed] [Google Scholar]
- Lincoln, C., Long, J., Yamaguchi, J., Serikawa, K., and Hake, S. (1994). A knotted1-like homeobox gene in Arabidopsis is expressed in the vegetative meristem and dramatically alters leaf morphology when overexpressed in transgenic plants. Plant Cell 6, 1859–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, C.-M., Xu, Z.-H., and Chua, N.-H. (1993). Auxin polar transport is essential for the establishment of bilateral symmetry during early plant embryogenesis. Plant Cell 5, 621–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long, J.A., Moan, E.I., Medford, J.I., and Barton, M.K. (1996). A member of the KNOTTED class of homeodomain proteins encoded by the STM gene of Arabidopsis. Nature 379, 66–69. [DOI] [PubMed] [Google Scholar]
- Lyndon, R.F. (1990). Plant Development—The Cellular Basis. (London: Unwin Hyman).
- Lyndon, R.F. (1998). The Shoot Apical Meristem—Its Growth and Development. (Cambridge, UK: Cambridge University Press).
- Maksymowych, R., and Erickson, R.O. (1977). Phyllotactic change induced by giberellic acid in Xanthium shoot apices. Am. J. Bot. 64, 33–44. [Google Scholar]
- Maksymowych, R., and Maksymowych, A.B. (1973). Induction of morphogenetic changes and acceleration of leaf initiation by giberellic acid in Xanthium pennsilvanicum. Am. J. Bot. 60, 901–906. [Google Scholar]
- Maksymowych, R., Cordero, R.E., and Erickson, R.O. (1976). Long-term developmental changes in Xanthium induced by giberellic acid. Am. J. Bot. 63, 1047–1053. [Google Scholar]
- Marrè, E. (1979). Fusicoccin: A tool in plant physiology. Annu. Rev. Plant Physiol. 30, 273–288. [Google Scholar]
- Mayer, K.F.X., Schoof, H., Haecker, A., Lenhard, M., Jürgens, G., and Laux, T., (1998). Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell 95, 805–815. [DOI] [PubMed] [Google Scholar]
- Medford, J.I. (1992). Vegetative apical meristems. Plant Cell 4, 1029–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meicenheimer, R.D. (1981). Changes in Epilobium phyllotaxy induced by N-1-naphthylphthalamic acid and α-4-chlorophenoxyisobutyric acid. Am. J. Bot. 68, 1139–1154. [Google Scholar]
- Meinhardt, H. (1994). Models of pattern formation and their application to plant development. In Positional Controls in Plant Development, P.W. Barlow and D.J. Carr, eds (Cambridge, UK: Cambridge University Press), pp. 1–32.
- Meyerowitz, E.M. (1997). Genetic control of cell division patterns in developing plants. Cell 88, 299–308. [DOI] [PubMed] [Google Scholar]
- Moussian, B., Schoof, H., Haecker, A., Jürgens, G., and Laux, T. (1998). Role of the zwille gene in the regulation of central shoot meristem fate during Arabidopsis embryogenesis. EMBO J. 17, 1799–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura, A., Tamaoki, M., Sato, Y., and Matsuoka, M. (1999). The expression of tobacco knotted1-type class 1 homeobox genes corresponds to regions predicted by the cytohistological zonation model. Plant J. 18, 337–347. [DOI] [PubMed] [Google Scholar]
- Okada, K., and Shimura, Y. (1994).The pin-formed gene. In Arabidopsis, An Atlas of Morphology and Development, J.J. Bowman, ed (New York: Springer-Verlag), pp. 180–183.
- Okada, K., Ueda, J., Komaki, M.K., Bell, C.J., and Shimura, Y. (1991). Requirement of the auxin polar transport system in early stages of Arabidopsis floral bud formation. Plant Cell 3, 677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt, D., Wittwer, F., Mandel, T., and Kuhlemeier, C. (1998). Localized upregulation of a new expansin gene predicts the site of leaf formation in the tomato meristem. Plant Cell 10, 1427–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs, T. (1991). Pattern Formation in Plant Tissues. (New York: Cambridge University Press).
- Schwabe, W.W. (1971). Chemical modification of phyllotaxis and its implications. Symp. Soc. Exp. Biol. 25, 301–322. [PubMed] [Google Scholar]
- Snow, M., and Snow, R. (1931). Experiments on phyllotaxis. I. The effect of isolating a primordium. Philos. Trans. R. Soc. Lond. Ser. B 221, 1–43. [Google Scholar]
- Snow, M., and Snow, R. (1933). Experiments on phyllotaxis. II. The effect of displacing a primordium. Philos. Trans. R. Soc. Lond. Ser. B 222, 354–400. [Google Scholar]
- Snow, M., and Snow, R. (1937). Auxin and leaf formation. New Phytol. 36, 1–18. [Google Scholar]
- Steeves, T.A., and Sussex, I.M. (1989). Patterns in Plant Development, 2nd ed. (New York: Cambridge University Press).
- Taylor, C.B. (1997). knox-on effects on leaf development. Plant Cell 9, 2102–2105. [Google Scholar]
- Trotochaud, A.E., Hao, T., Wu, G., Yang, Z., and Clark, S.E. (1999). The CLAVATA1 receptor-like kinase requires CLAVATA 3 for its assembly into a signaling complex that includes KAPP and a Rho-related protein. Plant Cell 11, 393–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uggla, C., Moritz, T., Sandberg, G., and Sundberg, B. (1996). Auxin as a positional signal in pattern formation in plants. Proc. Natl. Acad. Sci. USA 93, 9282–9286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollbrecht, E., Veit, B., Sinha, N., and Hake, S. (1991). The developmental gene Knotted-1 is a member of a maize homeobox gene family. Nature 350, 241–243. [DOI] [PubMed] [Google Scholar]