Abstract
The proinflammatory cytokine tumor necrosis factor alpha (TNF-α) regulates immune responses, inflammation, and programmed cell death (apoptosis). TNF-α exerts its biological activities by activating multiple signaling pathways, including IκB kinase (IKK), c-Jun N-terminal protein kinase (JNK), and caspases. IKK activation inhibits apoptosis through the transcription factor NF-κB, whose target genes include those that encode inhibitors of both caspases and JNK. Despite activation of the antiapoptotic IKK/NF-κB pathway, TNF-α is able to induce apoptosis in cells sensitive to it, such as human breast carcinoma MCF-7 and mouse fibroblast LM cells. The molecular mechanism underlying TNF-α-induced apoptosis is incompletely understood. Here we report that in TNF-α-sensitive cells activation of the IKK/NF-κB pathway fails to block TNF-α-induced apoptosis, although its inactivation still promotes TNF-α-induced apoptosis. Interestingly, TNF-α-induced apoptosis is suppressed by inhibition of the JNK pathway but promoted by its activation. Furthermore, activation of JNK by TNF-α was transient in TNF-α-insensitive cells but prolonged in sensitive cells. Conversion of JNK activation from prolonged to transient suppressed TNF-α-induced apoptosis. Thus, absence of NF-κB-mediated inhibition of JNK activation contributes to TNF-α-induced apoptosis.
The proinflammatory cytokine tumor necrosis factor alpha (TNF-α) regulates immune responses, inflammation, and programmed cell death (apoptosis) (5). TNF-α exerts its biological activity by binding to type 1 and type 2 receptors (TNF-R1 and TNF-R2), thereby activating multiple signaling pathways (5, 31, 51, 53). The TNF-R1 signaling complex is composed of the trimerized receptor, the TNF-R1-associated death domain protein, the Fas-associated death domain protein, TNF receptor-associated factors 2 and 5, and the receptor-interacting protein (5, 51). The Fas-associated death domain protein recruits and activates procaspase 8 (39), initiating the apoptotic pathway, in which caspases 3 and 7 are two major effector caspases (9). Activated caspase 8 also cleaves Bid (BH3 interaction domain death agonist) (24, 32, 58), which triggers the release of cytochrome c from mitochondria to induce apoptosis (62). TNF receptor-associated factors 2 and 5 and the receptor-interacting protein are involved in activation of IκB kinase (IKK) and c-Jun N-terminal protein kinase (JNK) (1, 5, 30), leading to activation of NF-κB and c-Jun, respectively.
The IKK complex contains two catalytic subunits, IKKα and IKKβ (IKK-1 and IKK-2) (12, 23, 37, 42, 59, 61), and an essential regulatory subunit, NEMO/IKKγ/IKKAP1 (36, 43, 60), and can be activated by a variety of stimuli (20). Activated IKK phosphorylates IκBs, a group of cytoplasmic inhibitors of NF-κB, on specific serines (Ser-32 and -36 in IκBα and Ser-19 and -23 in IκBβ), triggering their ubiquitination and subsequent degradation by the 26S proteosome (20). Degradation of IκB unmasks NF-κB's nuclear translocation signals. This allows NF-κB to translocate into the nucleus, where it stimulates transcription of target genes that are involved in immune responses, inflammation, viral infection, and cell survival (2-4, 13, 46, 52, 55). Compelling evidence shows that the IKK/NF-κB pathway is required for cell survival (20). Genetic disruption experiments reveal that mice deficient in RelA, a major activating subunit of NF-κB, die from massive apoptosis of hepatocytes in the liver (6). A similar phenotype was observed in mice deficient in IKKβ (26, 27, 48) or IKKγ alleles (33, 44, 45). In contrast, disruption of IKKα alleles only slightly affects NF-κB activation by proinflammatory cytokines such as TNF-α and interleukin-1 (IL-1), although mice die of perinatal lethality with severe defects in keratinocyte proliferation and differentiation (17, 25, 47). Biochemical data also show that NF-κB controls expression of several inhibitors of apoptosis (IAPs), including c-IAP1, c-IAP2, and X chromosome-linked IAP (XIAP) (8, 18, 29, 56, 57). Indeed, overexpression of NF-κB suppresses apoptosis (6, 30, 54), while the “superrepressor” IκBα(A32/36) mutant, which can no longer be phosphorylated and is therefore resistant to ubiquitin-mediated degradation, suppresses NF-κB activation and sensitizes cells to apoptotic insults (57).
JNK is a member of the mitogen-activated protein (MAP) kinase family and is activated by a variety of extracellular stimuli through a MAP kinase cascade consisting of JNK kinases (JNKK1/MKK4/SEK1 and JNKK2/MKK7) and multiple MAP kinase kinase kinases (7, 10, 11, 16, 21, 22, 28, 38). Activated JNK, in turn, phosphorylates and activates c-Jun, a major component of the transcription factor AP-1, as well as other targets (7, 10). The contribution of JNK activation to apoptosis has been shown to be cell type and stimulus dependent (7, 10). Moreover, the precise role of JNK activation in TNF-α-induced apoptosis is less clear (30, 40). Recently, we have shown that negative regulation of JNK activation by NF-κB contributes to inhibition of TNF-α-induced apoptosis (49). Thus, regulation of JNK activation by NF-κB may play a critical role in cell survival in response to TNF-α.
We report here that activation of the IKK/NF-κB pathway is necessary, but not sufficient, for suppression of TNF-α-induced apoptosis in cells sensitive to it, such as MCF-7 cells. This is most likely due to absence of NF-κB-mediated inhibition of prolonged JNK activation.
MATERIALS AND METHODS
Cell culture, transfection, and transcription assays.
TNF-α-sensitive human breast carcinoma MCF-7 (50) and mouse fibroblast LM (American Type Culture Collection) cells, as well as TNF-α-insensitive MCF-7 (MCF-7-R), COS-1, and human fibrosarcoma HT-1080 cells, were grown in RPMI 1640 or MEDM (41) medium supplemented with 10% fetal bovine serum, 2 mM glutamine, 100 U of penicillin per ml, and 100 μg of streptomycin per ml. Transfections were performed by the Ca2+ phosphate method, and 2× NF-κB-luciferase reporter gene activity was determined as previously described (50).
Reagents.
Antibodies against JNK and hemagglutinin (HA) were purchased from PharMingen. Antibody against ΙKKα, IKKβ, ΙKKγ, and ΙκBα was purchased from Santa Cruz. Anti-actin antibody was purchased from Sigma. Anti-phospho antibodies of p38 and ERK were from New England BioLabs. IL-1 and human TNF-α were purchased from R & D Systems. The proteosome inhibitor N-acetyl-Leu-Leu-Nle-CHO (ALLN), 12-O-tetradecanoylphorbol-13-acetate (TPA), and sodium vanadate (OV) were purchased from Sigma. The specific JNK inhibitor SP600125 was synthesized and purified as previously published (15), and the specific IKK inhibitor NEMO-binding domain (NBD) peptide (34) was synthesized by the Peptide Synthesis Core Facility, University of Chicago. The caspase 7 substrate DEVD-AFC was purchased from Clontech. [γ-32P]ATP (3,000 mCi/nmol) was from Dupont NEN.
Plasmids and adenovirus.
Mammalian HA-IKKβ, HA-JNKK2-JNK1, HA-JNKK2(K149M), HA-IKKβ(EE), HA-RelA, HA-XIAP, and M2-JNK1 expression vectors, adenovirus IκBα(A32/36) and green fluorescent protein vectors, glutathione S-transferase (GST)-IκBα, and GST-c-Jun(1-79) have been described previously (41, 49, 50, 63).
Protein kinase assays and immunoblotting.
Immune complex kinase assays were performed as previously described (28). Kinase activity was quantitated with a PhosphorImager. Immunoblot analysis was performed as previously described (28). The antibody-antigen complexes were visualized by the enhanced chemiluminescence detection system (Amersham).
Apoptosis assays.
Cells were cotransfected with various constructs in the presence of a GFP plasmid at a ratio of 4:1. Under these conditions, cells expressing GFP also expressed the cotransfected plasmid (50). Cells were subsequently infected with Ad/GFP or Ad/IκBα(A32/A36) (multiplicity of infection [MOI], 500) or left uninfected. At the time points indicated, cells were treated with stimuli and stained with Hoechst. Cell nucleus condensation was detected by fluorescence microscopy. Caspase 7 activity was measured with the synthetic fluorogenic substrate AC-Asp-Glu-Val-Asp-7-amino-4-trifluoromethylcoumarin (AC-DEVD-AFC) in accordance with the manufacturer's (Clontech) manual. The liberation of 7-amino-4-trifluoromethylcoumarin from AC-DEVD-AFC was read by a cytofluorometer at a 400-nm excitation wavelength and a 505-nm emission wavelength.
RESULTS
Activation of the IKK/NF-κB pathway is insufficient to suppress TNF-α-induced apoptosis in sensitive MCF-7 cells.
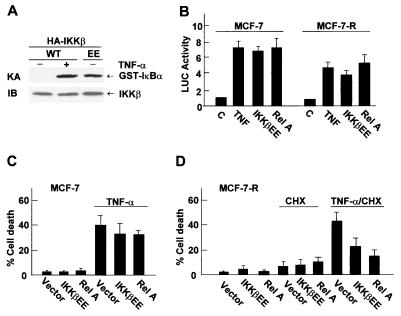
Despite activation of the antiapoptotic IKK/NF-κB pathway, TNF-α induces apoptosis in a subline of human breast carcinoma MCF-7 cells by activation of caspases (30, 50). This indicates that activation of NF-κB alone may be insufficient to suppress TNF-α killing of MCF-7 cells. To test this possibility, TNF-α-insensitive MCF-7 (MCF-7-R) cells and TNF-α-sensitive MCF-7 cells were transfected with an expression vector encoding GFP and either RelA, which is active when overexpressed (20, 49), or the constitutively active IKKβ(EE) mutant (37, 41) or an empty vector. In the absence of stimuli, IKKβ(EE) was fully active (20), as measured by immune complex kinase assays with GST-IκBα as the substrate (Fig. 1A). Expression of IKKβ(EE) or RelA also significantly stimulated NF-κB transcriptional activity, as measured by NF-κB-luciferase reporter gene assays (Fig. 1B). Treatment with TNF-α induced apoptosis in MCF-7 cells (30, 50), including nucleus condensation as measured by Hoechst staining (Fig. 1C) or caspase activation (F. Tang and A. Lin, unpublished data; Fig. 2D). However, expression of IKKβ(EE) or RelA did not significantly inhibit TNF-α-induced apoptosis in MCF-7 cells (Fig. 1C). In the presence of the protein synthesis inhibitor cycloheximide (CHX), which inhibits the synthesis of antiapoptotic proteins induced by NF-κB (30, 50), TNF-α also induced apoptosis in TNF-α-insensitive MCF-7-R cells (Fig. 1D). Expression of RelA or IKKβ(EE), which results in activation of NF-κB and accumulation of antiapoptotic proteins prior to TNF-α-CHX treatment, suppressed TNF-α-CHX-induced apoptosis in MCF-7-R cells (Fig. 1D, 50 and 64% less death than with TNF-α-CHX alone, respectively). Thus, activation of the IKK/NF-κB pathway alone is insufficient to block TNF-α killing of MCF-7 cells.
FIG. 1.
Activation of NF-κB is insufficient to suppress TNF-α-induced apoptosis in MCF-7 cells. (A) MCF-7 cells were transfected with an expression vector encoding wild-type HA-IKKβ (WT) or HA-IKKβ(EE) (1.5 μg of each). After 48 h, cells were treated with or without TNF-α (20 ng/ml) for 15 min. The activity of wild-type HA-IKKβ or HA-IKKβ(EE) was measured by immune complex kinase assays (KA) with GST-IκBα as the substrate, and their expression was analyzed by immunoblotting with anti-HA antibody (IB). (B) MCF-7 or MCF-7-R cells were transfected with the 2× NF-κB-Luc reporter plasmid (0.2 μg each), along with expression vectors encoding either HA-IKKβ(EE), HA-RelA, or an empty vector (0.4 μg of each). After 30 h, cells were treated with or without TNF-α (20 ng/ml) for 8 h. Relative luciferase (LUC) activity was determined as previously described (28). The results are presented as means ± standard errors and represent three separate experiments done in duplicate. (C and D) MCF-7 (C) or MCF-7-R (D) cells were transfected with expression vectors encoding HA-IKKβ(EE), HA-RelA, or an empty vector (2.0 μg of each), along with the transfection marker GFP (0.5 μg). After 48 h, cells were treated with or without TNF-α (20 ng/ml) for 11 h (C) or treated with or without TNF-α (20 ng/ml) plus CHX (10 μg/ml) or CHX alone for 6 h (D). Cells were stained with Hoechst, and nuclear condensation was visualized by fluorescence microscopy. The apoptotic death of transfected (GFP-positive) cells was calculated by counting eight randomly selected areas (total, 40 to 50 cells per area). The results are presented as means ± standard errors and represent three separate experiments.
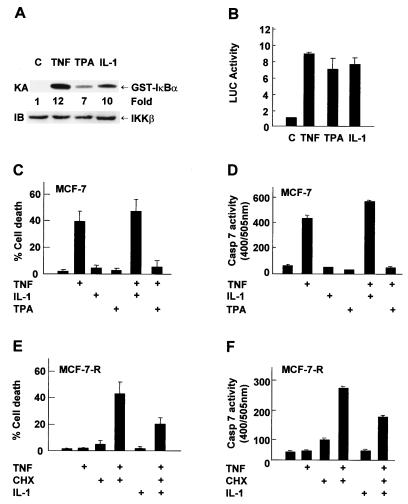
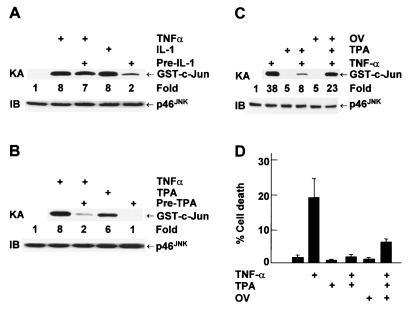
FIG. 2.
TPA, but not IL-1, inhibits TNF-α-induced apoptosis in MCF-7 cells. (A) MCF-7 cells were treated with or without TNF-α (20 ng/ml) for 15 min, TPA (100 ng/ml) for 30 min, or IL-1 (5 ng/ml) for 15 min. IKK activity and expression were examined as described in the legend to Fig. 1. (B) MCF-7 cells were transfected with the 2× NF-κB-Luc reporter plasmid (0.2 μg). After 30 h, cells were treated with or without TNF-α (20 ng/ml), TPA (1.0 ng/ml), or IL-1 (5 ng/ml) for 8 h. Luciferase (LUC) activity was determined as previously described (28). The results are presented as means ± standard errors and represent three separate experiments done in duplicate. (C) MCF-7 cells were pretreated with TPA (100 ng/ml) or IL-1 (5 ng/ml) for 3 h and then stimulated with TNF-α (20 ng/ml) for 11 h. Apoptotic cell death was calculated by counting six to eight randomly selected areas (total, 150 to 200 cells per area) as described in the legend to Fig. 1. The results shown represent three separate experiments. (D) MCF-7 cells were pretreated with or without TPA (1.0 ng/ml) or IL-1 (5 ng/ml) for 3 h and then stimulated with TNF-α (20 ng/ml) for 11 h. Caspase (Casp) activity was measured with DEVD-AFC as the substrate in accordance with the manufacturer's (Clontech) manual. Caspase 7 activity is presented as means ± standard errors and represents three separate experiments. (E) MCF-7-R cells were pretreated with or without IL-1 (5 ng/ml) for 3 h and then stimulated with TNF-α (20 ng/ml)-CHX (10 μg/ml) for 10 h. Apoptotic cell death was calculated as described for panel C, and the results shown represent three separate experiments. (F) MCF-7-R cells were pretreated with or without IL-1 (5 ng/ml) for 3 h and then stimulated with TNF-α (20 ng/ml) and CHX (10 μg/ml) for 6 h. Caspase activity was measured as described for panel D.
Not all NF-κB inducers are able to inhibit TNF-α killing of MCF-7 cells.
Pretreatment with NF-κB inducers can inhibit TNF-α killing (30, 34, 50). We tested whether pretreatment with IL-1 or TPA can suppress TNF-α killing of MCF-7 cells. Both IL-1 and TPA activated IKK and NF-κB transcriptional activity, as measured by immune complex kinase assays (Fig. 2A) and NF-κB-luciferase reporter gene assays (Fig. 2B), respectively. Unlike TNF-α, neither IL-1 nor TPA induced apoptosis in MCF-7 cells (Fig. 2C). Pretreatment with IL-1 had little effect on TNF-α killing or caspase 7 activity (Fig. 2C and D). The failure of IL-1 to prevent TNF-α killing was not generic, since it was able to suppress TNF-α-CHX-mediated apoptosis of MCF-7-R cells (Fig. 2E, 50% less death than with TNF-α-CHX alone). Since IL-1 induced similar levels of NF-κB activation in both MCF-7 and MCF-7-R cells (Tang and Lin, unpublished), it is unlikely that the inability of IL-1 to protect MCF-7 cells from TNF-α-induced apoptosis was caused by weaker activation of NF-κB. In contrast to IL-1, pretreatment with TPA significantly suppressed TNF-α-induced apoptosis in MCF-7 cells (Fig. 2C, 89% less death than with TNF-α alone), likely through a classical protein kinase C pathway (Tang and Lin, unpublished) (14, 35). Taken together, preactivation of NF-κB alone cannot inhibit TNF-α-induced apoptosis in MCF-7 cells.
Inhibition of the IKK/NF-κB pathway promotes TNF-α killing.
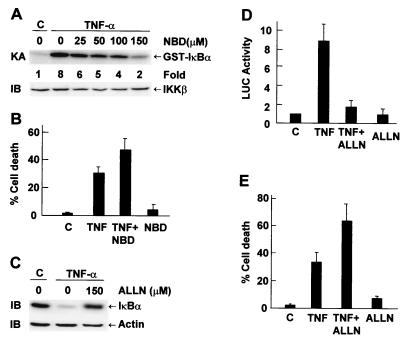
The inability of NF-κB activation to suppress TNF-α killing raised the question of whether the IKK-NF-κB pathway is needed for survival of MCF-7 cells. To address this, we used a synthetic peptide that resembles the NBD of IKKα and IKKβ to inhibit IKK activation by TNF-α. This synthetic peptide inhibits IKK by competing with IKKα and IKKβ for binding to IKKγ/NEMO (34). Immune complex kinase assays showed that pretreatment with NBD inhibited TNF-α-induced IKK activation in a dose-dependent manner (Fig. 3A). This inhibition was not a result of decreased expression of IKKβ (Fig. 3A), IKKα, or IKKγ (Tang and Lin, unpublished). Pretreatment with NBD promoted TNF-α killing of MCF-7 cells (Fig. 3B). Consistently, pretreatment with ALLN, a proteosome inhibitor that inhibits IκBα degradation (Fig. 3C) and NF-κB transcriptional activity (Fig. 3D), promoted TNF-α killing of MCF-7 cells (Fig. 3E). These data suggest that activation of the IKK/NF-κB pathway is still necessary for survival of MCF-7 cells.
FIG. 3.
Inhibition of the IKK/NF-κB pathway promotes TNF-α-induced apoptosis in MCF-7 cells. (A) MCF-7 cells were pretreated with or without NBD at various doses for 3 h and stimulated with TNF-α (20 ng/ml) for 15 min. IKK activity and expression were measured as described in the legend to Fig. 2. (B) MCF-7 cells were pretreated with NBD (150 μM) for 3 h and stimulated with TNF-α (20 ng/ml) for 11 h. Cells were Hoechst stained, and apoptotic cells were calculated as described in the legend to Fig. 2C. The results are presented as means ± standard errors and represent three separate experiments. (C) MCF-7 cells were pretreated with ALLN (150 μM) for 2 h and stimulated with TNF-α (20 ng/ml) for 15 min. Degradation of IκBα proteins was analyzed by immunoblotting with anti-IκBα antibody. (D) MCF-7 cells were transfected with the 2× NF-κB-Luc reporter plasmid (0.2 μg). After 30 h, cells were pretreated with or without ALLN (150 μM) for 2 h and stimulated with TNF-α (20 ng/ml) for 8 h or left untreated. Cells were harvested, and relative luciferase (LUC) activity was determined. The results are presented as means ± standard errors and represent three separate experiments done in duplicate. (E) MCF-7 cells were pretreated with ALLN (150 μM) for 2 h and stimulated with TNF-α (20 ng/ml) for 11 h. Apoptotic cell death was calculated as described for panel B.
JNK activation is required for TNF-α killing.
The results reported above suggest that other TNF-α effectors may play a critical role in TNF-α-induced apoptosis. To test this possibility, we examined whether activation of JNK is involved in TNF-α killing.
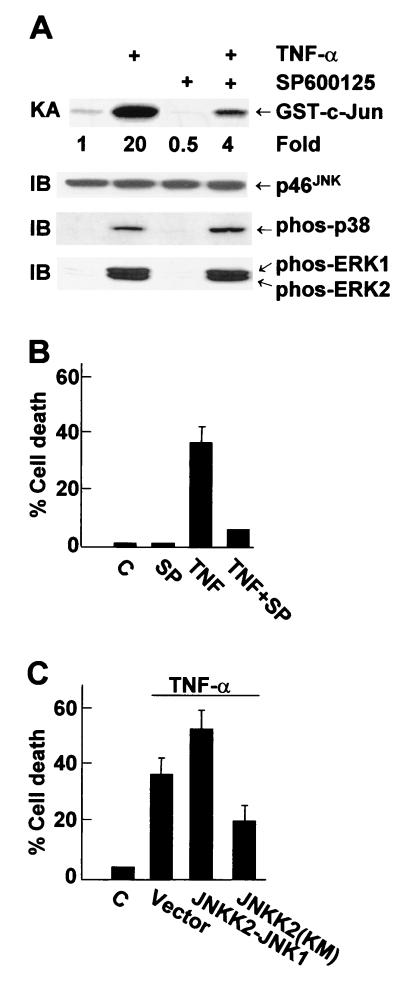
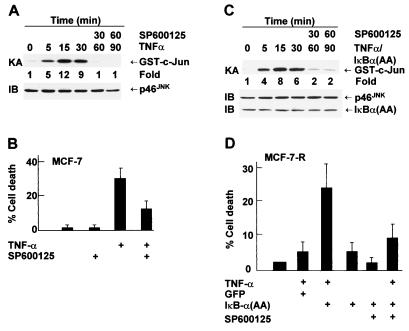
First, we tested whether the specific JNK inhibitor SP600125 (15) can suppress TNF-α killing of MCF-7 cells. SP600125 inhibited TNF-α-induced activation of JNK, as measured by immune complex kinase assays (Fig. 4A). SP600125 had no detectable effect on activation and expression of p38 or ERK, as measured by immunoblotting with anti-phospho or pan antibodies, respectively (Fig. 4A) or by immune complex kinase assays, respectively (Tang and Lin, unpublished). Pretreatment of MCF-7 cells with SP600125 significantly suppressed TNF-α-induced apoptosis (Fig. 4B). Furthermore, expression of the dominant negative JNKK2(K149M) mutant, which blocks TNF-α-induced JNK activation (Tang and Lin, unpublished) (48), suppressed TNF-α-induced apoptosis (Fig. 4C). Conversely, expression of the JNKK2-JNK1 fusion protein, which has constitutive Jun kinase activity (49, 63), promoted TNF-α killing (Fig. 4C). Expression of the JNKK2-JNK1 fusion protein alone had no detectable effect on apoptosis of MCF-7 cells (Tang and Lin, unpublished). These results suggest that JNK activation may contribute to TNF-α killing of MCF-7 cells.
FIG. 4.
JNK activation is involved in TNF-α killing of MCF-7 cells. (A) MCF-7 cells were pretreated with SP600125 (20 μM) for 30 min and stimulated with TNF-α (20 ng/ml) for 15 min. JNK was immunoprecipitated with anti-JNK1 antibody, and its activity was measured by immune complex kinase assay with GST-c-Jun(1-79) as the substrate. JNK content was analyzed by immunoblotting with anti-JNK antibody. The same cell lysates were also analyzed for activation of p38 or ERK by immunoblotting with corresponding anti-phospho antibodies. (B) MCF-7 cells were pretreated with SP600125 (SP; 20 μM) for 30 min and stimulated with TNF-α (20 ng/ml) for 11 h. Apoptotic cell death was calculated as described in the legend to Fig. 2C. (C) MCF-7 cells were cotransfected with expression vectors encoding GFP (0.5 μg) and either the HA-JNKK2-JNK1 fusion protein, the dominant negative mutant HA-JNKK2(K149M), or an empty vector (2.0 μg of each). Cells were treated with or without TNF-α (20 ng/ml) for 11 h and stained with Hoechst. The death of transfected (GFP-positive) cells was calculated as described in the legend to Fig. 1.
Since pretreatment with TPA, but not IL-1, suppresses TNF-α killing of MCF-7 cells (Fig. 2C), we were curious if this was due to their differential ability to inhibit TNF-α-mediated JNK activation. Indeed, pretreatment with IL-1 had little inhibitory effect on TNF-α-induced JNK activation, as measured by immune complex kinase assays (Fig. 5A). In contrast, pretreatment with TPA significantly inhibited TNF-α-induced JNK activation (Fig. 5B). The inhibition was not the result of decreased expression of p46JNK (Fig. 5B) or p54 JNK (Tang and Lin, unpublished). The inhibitory effect of TPA on TNF-α-induced JNK activation and apoptosis was significantly abrogated by the tyrosine phosphatase inhibitor OV (Fig. 5C and D), suggesting that an OV-sensitive JNK phosphatase(s) may, in part, mediate the inhibitory effect of TPA on JNK activation and apoptosis in MCF-7 cells.
FIG. 5.
JNK phosphatase(s) mediates the inhibitory effect of TPA on JNK activation in MCF-7 cells. (A and B) MCF-7 cells were pretreated with IL-1 (5 ng/ml) or TPA (100 ng/ml) for 1 h and stimulated with TNF-α (20 ng/ml) for 15 min. JNK activity and expression was measured as described in the legend to Fig. 4. (C and D) MCF-7 cells were pretreated with OV (100 μM) for 30 min prior to treatment with TPA (100 ng/ml) for 1 h. Cells were stimulated with TNF-α (20 ng/ml) for either 15 min (C) or 11 h (D). JNK activity and expression were measured as described in the legend to Fig. 4, while apoptotic cell death was detected and calculated as described in the legend to Fig. 1. The results shown represent three separate experiments.
Absence of NF-κB-mediated inhibition of JNK activation contributes to TNF-α-induced apoptosis.
In the absence of NF-κB-mediated JNK inhibition, TNF-α induces prolonged JNK activation, which contributes to the apoptotic process (49). The fact that TNF-α killing of MCF-7 cells was blocked by inhibition of JNK, but not by activation of NF-κB, provoked us to test whether there is a defect in NF-κB-mediated inhibition of JNK in these cells and whether this contributes to killing by TNF-α.
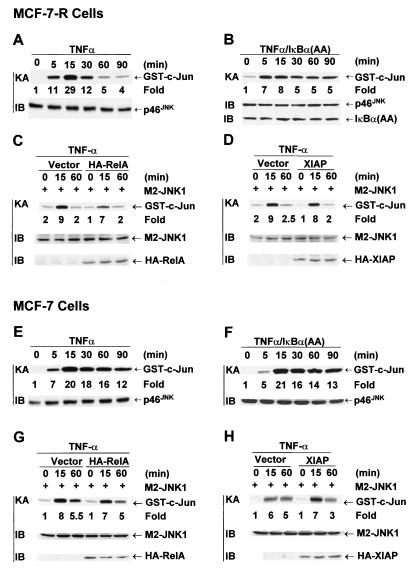
Immune complex kinase assays showed that JNK activation by TNF-α was transient in MCF-7-R cells (Fig. 6A). This transient activation of JNK is due to NF-κB-mediated inhibition since infection of MCF-7-R cells with adenovirus encoding the superrepressor IκBα(A32/36) mutant converted JNK activation from transient to prolonged (Fig. 6B). Suppression of protein synthesis by CHX also converted JNK activation by TNF-α from transient to prolonged (Tang and Lin, unpublished). Since JNK activation was already transient, expression of RelA or the JNK inhibitor XIAP only slightly inhibited TNF-α-induced activation of cotransfected M2-JNK1 (Fig. 6C and D). The activation of M2-JNK1 by TNF-α was weaker than that of endogenous JNK because of the higher basal activity of M2-JNK1 when it is ectopically expressed. In contrast, JNK activation by TNF-α was prolonged in MCF-7 cells (Fig. 6E). This prolonged activation did not result from increased expression of either p46JNK (Fig. 6E) or p54JNK (Tang and Lin, unpublished). Infection of MCF-7 cells with adenovirus encoding IκBα(A32/36) showed little effect on prolonged JNK activation by TNF-α (Fig. 6F). Conversely, expression of RelA in MCF-7 cells failed to convert JNK activation by TNF-α from prolonged to transient (Fig. 6G). However, expression of XIAP, an NF-κB-induced inhibitor of JNK activation (49), converted JNK activation by TNF-α from prolonged to transient (Fig. 6H). These data suggest that NF-κB activation may be decoupled from its inhibition of JNK activation in MCF-7 cells.
FIG. 6.
Prolonged JNK activation by TNF-α in MCF-7 cells is due to absence of NF-κB-mediated inhibition. (A and E) MCF-7-R or MCF-7 cells were treated with TNF-α (20 ng/ml) for various times as indicated. JNK activity and expression were measured as described in the legend to Fig. 4. (B and F) MCF-7-R or MCF-7 cells were infected with Ad/IκBα(A32/36) (MOI, 500). After 24 h, cells were treated with TNF-α (20 ng/ml) for various times as indicated. JNK activity and expression were measured as described in the legend to Fig. 4. (C, D, G, and H) MCF-7-R or MCF-7 cells were transfected with an expression vector encoding HA-XIAP or HA-RelA or with an empty vector (1 μg of each), along with M2-JNK (0.5 μg). After 48 h, cells were treated with TNF-α (20 ng/ml) for various times as indicated. JNK activity was measured as described in the legend to Fig. 4. (H) M2-JNK1 was immunoprecipitated from extracts from vector- and HA-XIAP-transfected cells that had been normalized to contain the same amount of M2-JNK1 protein. Expression of M2-JNK, HA-RelA, and HA-XIAP was analyzed by immunoblotting with anti-M2 or anti-HA antibody.
To determine whether prolonged JNK activation contributes to TNF-α-induced apoptosis in MCF-7 cells, cells were treated with the JNK inhibitor SP600125 30 min after TNF-α stimulation to convert JNK activation from prolonged to transient (Fig. 7A). Under this condition, TNF-α-induced apoptosis was significantly suppressed (Fig. 7B). In MCF-7-R cells expressing IκBα(A32/36), JNK activation was prolonged (Fig. 6B) and cells were sensitized to TNF-α-induced apoptosis (Fig. 7D). Treatment with SP600125 30 min after TNF-α stimulation converted JNK activation from prolonged back to transient (Fig. 7C) and suppressed TNF-α killing (Fig. 7D). These data suggest that the absence of NF-κB-mediated inhibition of JNK activation by TNF-α in MCF-7 cells contributes to TNF-α-induced apoptosis.
FIG. 7.
Prolonged JNK activation contributes to TNF-α-induced apoptosis in MCF-7 cells. (A) MCF-7 cells were stimulated with TNF-α (20 ng/ml). After 30 min, cells were treated with SP600125 (20 μM) and harvested at the time points indicated. JNK activity and expression were measured as described in the legend to Fig. 4. (B) MCF-7 cells were treated with TNF-α (20 ng/ml) and 30 min later were treated with or without SP600125 (20 μM). After 11 h, cells were stained with Hoechst and visualized by fluorescence microscopy. Cell death was calculated as described in the legend to Fig. 2C. (C) MCF-7-R cells infected with Ad/IκBα(A32/36) (MOI, 500) were treated with TNF-α (20 ng/ml). After 30 min, cells were treated with SP600125 (20 μM). JNK activity and expression were measured as described in the legend to Fig. 4. (D) MCF-7-R cells were infected with Ad/IκBα(A32/36) or Ad/GFP (MOI, 500) and treated with TNF-α (20 ng/ml). After 30 min, cells were treated with SP600125 (20 μM) and cell death was calculated 7 h later as described in Fig. 2C. The results shown represent three separate experiments.
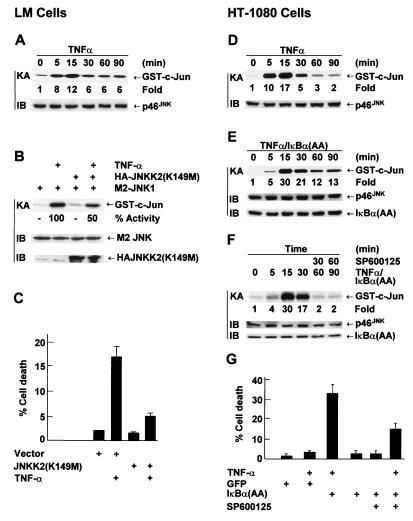
In the absence of NF-κB-mediated inhibition, prolonged JNK activation also contributes to TNF-α-induced apoptosis in other cell types. In TNF-α-sensitive LM fibroblasts, activation of JNK by TNF-α was prolonged (Fig. 8A) and TNF-α alone was able to induce apoptosis (Fig. 8C). TNF-α-induced apoptosis was suppressed by the dominant negative JNKK2(K149M) mutant, which inhibits JNK activation by TNF-α (Fig. 8B and C). In TNF-α-insensitive HT-1080 cells, JNK activation by TNF-α was transient (Fig. 8D). Infection of HT-1080 cells with adenovirus encoding IκBα(A32/36) converted JNK activation from transient to prolonged (Fig. 8E) and sensitized cells to TNF-α killing (Fig. 8G). Treatment of cells with SP600125 30 min after TNF-α stimulation converted JNK activation from prolonged back to transient (Fig. 8F) and suppressed TNF-α-induced apoptosis (Fig. 8G). Similar results were obtained with COS-1 cells (Tang and Lin, unpublished). Thus, the duration of JNK activation affects the susceptibility of cells to TNF-α-induced apoptosis.
FIG. 8.
Prolonged JNK activation affects the susceptibility of cells to TNF-α-induced apoptosis. (A) TNF-α-sensitive LM cells were treated with TNF-α (20 ng/ml) and harvested at the time points indicated. JNK activity and expression were measured as described in the legend to Fig. 4. (B) LM cells were cotransfected with expression vectors encoding M2-JNK (1.5 μg) and either the JNKK2(K149M) mutant or an empty vector (6.0 μg of each). Cells were treated with or without TNF-α (20 ng/ml) for 15 min. The activity of M2-JNK and the expression of transfected constructs were determined as previously described (49). (C) LM cells were cotransfected with expression vectors encoding GFP (1.5 μg) and either the JNKK2(K149M) mutant or an empty vector (6.0 μg of each). Cells were treated with or without TNF-α (20 ng/ml) for 8 h, stained with Hoechst dye, and visualized by fluorescence microscopy. The death rate of transfected (GFP-positive) cells was calculated by counting six randomly selected areas (total, 30 to 40 cells per area). Results are presented as means ± standard errors and represent two individual experiments. (D) TNF-α-insensitive HT-1080 cells were treated with TNF-α (20 ng/ml) for various times as indicated. JNK activity and expression were measured as described in the legend to Fig. 4. (E) HT-1080 cells were infected with Ad/IκBα(A32/36) (MOI, 500). After 24 h, cells were treated with TNF-α (20 ng/ml) for various times as indicated. JNK activity and expression were measured as described in the legend to Fig. 4. (F) HT-1080 cells infected with Ad/IκBα(A32/36) (MOI, 500) were stimulated with TNF-α (20 ng/ml). After 30 min, cells were treated with SP600125 (20 μM) and harvested at the time points indicated. JNK activity and expression were measured as described in the legend to Fig. 4. (G) HT-1080 cells were infected with Ad/IκBα(A32/36) or Ad/GFP (MOI, 500) and treated with TNF-α (20 ng/ml). After 30 min, cells were treated with or without SP600125 (20 μM) and cell death was calculated 9 h later as described in the legend to Fig. 2C. The results shown represent three separate experiments.
DISCUSSION
In this report, we show that decoupling of NF-κB activation from its inhibition of JNK activation sensitizes cells to TNF-α killing.
Typically, TNF-α does not induce apoptosis unless NF-κB activation is impaired (49). In the presence of CHX, which blocks or reduces synthesis of proteins including NF-κB-induced antiapoptotic gene products, TNF-α does kill various types of cells (30, 50). An exception to this scenario is MCF-7 cells, which undergo TNF-α-induced apoptosis despite activation of NF-κB (30, 50). Overexpression of RelA or the constitutively active IKKβ(EE) mutant in MCF-7 cells was unable to suppress TNF-α killing (Fig. 1C). However, RelA or IKKβ(EE) did inhibit TNF-α-CHX-induced apoptosis in TNF-α-insensitive MCF-7-R cells (Fig. 1D). Although both TPA and IL-1 are activators of the IKK/NF-κB pathway (Fig. 2A and B), pretreatment with TPA, but not IL-1, suppressed TNF-α killing of MCF-7 cells (Fig. 2C). In contrast, IL-1 was able to suppress TNF-α killing of MCF-7-R cells (Fig. 2D). Since IL-1 induces similar levels of NF-κB activation in MCF-7 and MCF-7-R cells (Tang and Lin, unpublished), it is unlikely that the inability of IL-1 to suppress TNF-α killing of MCF-7 cells is due to weaker induction of NF-κB. Interestingly, inactivation of the IKK/NF-κB pathway promoted TNF-α killing of MCF-7 cells (Fig. 3B and E). Thus, activation of NF-κB is necessary but not sufficient for inhibition of TNF-α killing of cells sensitive to it.
The role of JNK activation in apoptosis is highly controversial, as it has been reported to have a proapoptotic role, an antiapoptotic role, or no role in this process (21, 30, 40, 49). Our results suggest that, in the absence of NF-κB-mediated inhibition, JNK activation may contribute to TNF-α killing. Pretreatment of MCF-7 cells with the specific JNK inhibitor SP600125 blocked JNK activation (Fig. 4A) and TNF-α killing (Fig. 4B). Under the same conditions, SP600125 has no detectable inhibitory effect on activation of p38, ERK (Fig. 4A), or IKK (Tang and Lin, unpublished). Although we cannot formally exclude the possibility that SP600125 can block TNF-α-induced apoptosis by inhibiting a target(s) other than JNK, it is likely that JNK activation is involved in TNF-α killing. This notion is supported by the findings that TNF-α killing was suppressed by expression of the dominant negative JNKK2(K149M) mutant (Fig. 4C) but promoted by the JNKK2-JNK1 fusion protein that has constitutive JNK activity (Fig. 4C). It is important to note that expression of the JNKK2-JNK1 fusion protein alone did not induce apoptosis in MCF-7 cells (Tang and Lin, unpublished) (49), suggesting that JNK activation may promote, but is unable to initiate, the apoptotic process. Although both IL-1 and TPA activate NF-κB in MCF-7 cells, pretreatment with TPA, but not IL-1, suppressed TNF-α killing and JNK activation (Fig. 2C and 5A and B). Thus, regulation of JNK, but not NF-κB, may be a critical event that determines whether MCF-7 cells exposed to TNF-α live or die. Consistently, the inhibition by TPA of TNF-α-induced JNK activation and apoptosis is, in part, mediated by an OV-sensitive JNK phosphatase(s) (Fig. 5C and D). In contrast, inactivation of NF-κB by infection of MCF-7 cells with adenovirus encoding the superrepressor IκBα(A32/36) only slightly affected the inhibitory effect of TPA on TNF-α-induced JNK activation and apoptosis (Tang and Lin, unpublished).
The duration of activation appears to be critical for whether JNK affects the susceptibility of cells to TNF-α killing. Restoration of transient JNK activation in MCF-7 cells with JNK inhibitor SP600125 treatment (Fig. 7A) suppressed TNF-α killing (Fig. 7B). Inhibition of prolonged JNK activation in LM cells also suppressed TNF-α killing (Fig. 8G). Furthermore, expression of IκBα(A32/36) in cells that are originally resistant to TNF-α killing, such as MCF-7-R and HT-1080 cells, converted JNK activation from transient to prolonged (Fig. 6B and 8E) and sensitized cells to TNF-α-induced apoptosis (Fig. 7D and 8G). Interestingly, treatment of IκBα(A32/36) expressing MCF-7-R or HT-1080 cells with SP600125 for 30 min following TNF-α stimulation restored transient JNK activation (Fig. 7C and 8F) and resistance to TNF-α killing (Fig. 7D and 8G). These data are consistent with our previous finding (49) that, in addition to inhibition of caspases, NF-κB inhibits prolonged JNK activation by TNF-α, thereby suppressing apoptosis.
The failure of NF-κB activation to suppress TNF-α-induced apoptosis in MCF-7 cells is likely due, at least in part, to its inability to prevent prolonged JNK activation. Activation of JNK by TNF-α was transient in TNF-α-insensitive MCF-7-R cells (Fig. 6A). This is due to NF-κB-mediated inhibition since suppression of NF-κB activation by IκBα(A32/36) expression or CHX treatment converted JNK activation from transient to prolonged (Fig. 6B; Tang and Lin, unpublished). In contrast, JNK activation by TNF-α was prolonged in MCF-7 and LM cells (Fig. 6E and 8A), suggesting that TNF-α activation of the IKK/NF-κB pathway in MCF-7 cells (Fig. 2A and B) and in LM cells (Tang and Lin, unpublished) may be incapable, for reasons unknown, of inhibiting prolonged JNK activation by TNF-α. In support of this notion, overexpression of RelA is unable to prevent prolonged JNK activation by TNF-α (Fig. 6G). Consistently, expression of IκBα(A32/36) had little effect on JNK activation by TNF-α (Fig. 6F). Since NF-κB can still be activated by TNF-α (Fig. 2B; Tang and Lin, unpublished), it is likely that there is a decoupling of NF-κB activation from inhibition of JNK activation.
The molecular mechanism underlying the decoupling of NF-κB activation from inhibition of JNK activation by TNF-α in MCF-7 cells remains to be determined. Although NF-κB activation is unable to prevent JNK activation in MCF-7 cells (Fig. 6G), overexpression of XIAP, an NF-κB-induced JNK inhibitor, converted JNK activation from prolonged to transient (Fig. 6H). Note that overexpression of XIAP in MCF-7 cells enhances expression levels of cotransfected JNK, resulting in higher basal JNK activity (Tang and Lin, unpublished), in agreement with our previous finding (49). However, XIAP is unlikely to be responsible for the different sensitivities of MCF-7 and MCF-7-R cells to TNF-α-induced apoptosis since they have similar levels of endogenous XIAP (Tang and Lin, unpublished). Thus, overexpression of XIAP in MCF-7 cells may compensate for the need of other yet-to-be identified JNK inhibitors. Nevertheless, it is possible that NF-κB may fail to induce, for reasons yet to be identified, a certain inhibitor(s) of JNK activation (49). Another possibility is that proteolysis of IKKβ by caspase 3-related caspases, such as caspase 7 (MCF-7 cells have no detectable caspase 3 activity) (19), may blunt NF-κB activation during apoptosis (50). This could reduce the production of NF-κB-induced antiapoptotic gene products, which otherwise inhibit caspases and JNK. Consequently, in addition to caspase activation, proteolysis of IKKβ may contribute to apoptosis by allowing prolonged JNK activation. Further studies are needed to explore these possibilities.
Acknowledgments
We thank Geoffrey L. Greene for TNF-α-resistant MCF-7-R cells, David A. Brenner for Ad/IκBα(A32/36), and Chenfei Yu for helping with adenovirus preparation.
This work was supported by National Institutes of Health grants CA73740 and CA92650 and American Cancer Society grant CCG-98471 (A.L.).
REFERENCES
- 1.Ashkenazi, A., and V. M. Dixit. 1998. Death receptors: signaling and modulation. Science 281:1305-1308. [DOI] [PubMed] [Google Scholar]
- 2.Baeuerle, P. A., and D. Baltimore. 1996. NF-κ B: ten years after. Cell 87:13-20. [DOI] [PubMed] [Google Scholar]
- 3.Baldwin, A. S., Jr. 1996. The NF-κ B and IκB proteins: new discoveries and insights. Annu. Rev. Immunol. 14:649-683. [DOI] [PubMed] [Google Scholar]
- 4.Barnes, P. J., and M. Karin. 1997. Nuclear factor-κB: a pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 336:1066-1071. [DOI] [PubMed] [Google Scholar]
- 5.Baud, V., and M. Karin. 2001. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 11:372-377. [DOI] [PubMed] [Google Scholar]
- 6.Beg, A. A., and D. Baltimore. 1996. An essential role for NF-κB in preventing TNF-α-induced cell death. Science 274:782-784. [DOI] [PubMed] [Google Scholar]
- 7.Chang, L., and M. Karin. 2001. Mammalian MAP kinase signalling cascades. Nature 410:37-40. [DOI] [PubMed] [Google Scholar]
- 8.Chu, Z. L., T. A. McKinsey, L. Liu, J. J. Gentry, M. H. Malim, and D. W. Ballard. 1997. Suppression of tumor necrosis factor-induced cell death by inhibitor of apoptosis c-IAP2 is under NF-κB control. Proc. Natl. Acad. Sci. USA 94:10057-10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cryns, V., and J. Yuan. 1998. Proteases to die for. Genes Dev. 12:1551-1570. [DOI] [PubMed] [Google Scholar]
- 10.Davis, R. J. 2000. Signal transduction by the JNK group of MAP kinases. Cell 103:239-252. [DOI] [PubMed] [Google Scholar]
- 11.Derijard, B., M. Hibi, I. H. Wu, T. Barrett, B. Su, T. Deng, M. Karin, and R. J. Davis. 1994. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell 76:1025-1037. [DOI] [PubMed] [Google Scholar]
- 12.DiDonato, J. A., M. Hayakawa, D. M. Rothwarf, E. Zandi, and M. Karin. 1997. A cytokine-responsive IκB kinase that activates the transcription factor NF-κB. Nature 388:548-554. [DOI] [PubMed] [Google Scholar]
- 13.Ghosh, S., M. J. May, and E. B. Kopp. 1998. NF-κB and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 16:225-260. [DOI] [PubMed] [Google Scholar]
- 14.Gschwendt, M., H. J. Muller, K. Kielbassa, R. Zang, W. Kittstein, G. Rincke, and F. Marks. 1994. Rottlerin, a novel protein kinase inhibitor. Biochem. Biophys. Res. Commun. 199:93-98. [DOI] [PubMed] [Google Scholar]
- 15.Han, Z., D. L. Boyle, L. Chang, B. Bennett, M. Karin, L. Yang, A. M. Manning, and G. S. Firestein. 2001. c-Jun N-terminal kinase is required for metalloproteinase expression and joint destruction in inflammatory arthritis. J. Clin. Investig. 108:73-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hibi, M., A. Lin, T. Smeal, A. Minden, and M. Karin. 1993. Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 7:2135-2148. [DOI] [PubMed] [Google Scholar]
- 17.Hu, Y., V. Baud, M. Delhase, P. Zhang, T. Deerinck, M. Ellisman, R. Johnson, and M. Karin. 1999. Abnormal morphogenesis but intact IKK activation in mice lacking the IKKα subunit of IκB kinase. Science 284:316-320. [DOI] [PubMed] [Google Scholar]
- 18.Irmler, M., M. Thome, M. Hahne, P. Schneider, K. Hofmann, V. Steiner, J. L. Bodmer, M. Schroter, K. Burns, C. Mattmann, D. Rimoldi, L. E. French, and J. Tschopp. 1997. Inhibition of death receptor signals by cellular FLIP. Nature 388:190-195. [DOI] [PubMed] [Google Scholar]
- 19.Janicke, R. U., M. L. Sprengart, M. R. Wati, and A. G. Porter. 1998. Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J. Biol. Chem. 273:9357-9360. [DOI] [PubMed] [Google Scholar]
- 20.Karin, M., and Y. Ben-Neriah. 2000. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu. Rev. Immunol. 18:621-663. [DOI] [PubMed] [Google Scholar]
- 21.Karin, M., and A. Lin. 2002. NF-κB at the crossroad of life and death. Nat. Immunol. 3:221-227. [DOI] [PubMed] [Google Scholar]
- 22.Kyriakis, J. M., P. Banerjee, E. Nikolakaki, T. Dai, E. A. Rubie, M. F. Ahmad, J. Avruch, and J. R. Woodgett. 1994. The stress-activated protein kinase subfamily of c-Jun kinases. Nature 369:156-160. [DOI] [PubMed] [Google Scholar]
- 23.Lee, F. S., J. Hagler, Z. J. Chen, and T. Maniatis. 1997. Activation of the IκBα kinase complex by MEKK1, a kinase of the JNK pathway. Cell 88:213-222. [DOI] [PubMed] [Google Scholar]
- 24.Li, H., H. Zhu, C. J. Xu, and J. Yuan. 1998. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94:491-501. [DOI] [PubMed] [Google Scholar]
- 25.Li, Q., Q. Lu, J. Y. Hwang, D. Buscher, K. F. Lee, J. C. Izpisua-Belmonte, and I. M. Verma. 1999. IKK1-deficient mice exhibit abnormal development of skin and skeleton. Genes Dev. 13:1322-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li, Q., D. Van Antwerp, F. Mercurio, K. F. Lee, and I. M. Verma. 1999. Severe liver degeneration in mice lacking the IκB kinase 2 gene. Science 284:321-325. [DOI] [PubMed] [Google Scholar]
- 27.Li, Z. W., W. Chu, Y. Hu, M. Delhase, T. Deerinck, M. Ellisman, R. Johnson, and M. Karin. 1999. The IKKβ subunit of IκB kinase (IKK) is essential for nuclear factor κB activation and prevention of apoptosis. J. Exp. Med. 189:1839-1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin, A., A. Minden, H. Martinetto, F. X. Claret, C. Lange-Carter, F. Mercurio, G. L. Johnson, and M. Karin. 1995. Identification of a dual specificity kinase that activates the Jun kinases and p38-Mpk2. Science 268:286-290. [DOI] [PubMed] [Google Scholar]
- 29.Liston, P., N. Roy, K. Tamai, C. Lefebvre, S. Baird, G. Cherton-Horvat, R. Farahani, M. McLean, J. E. Ikeda, A. MacKenzie, and R. G. Korneluk. 1996. Suppression of apoptosis in mammalian cells by NAIP and a related family of IAP genes. Nature 379:349-353. [DOI] [PubMed] [Google Scholar]
- 30.Liu, Z. G., H. Hsu, D. V. Goeddel, and M. Karin. 1996. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-κB activation prevents cell death. Cell 87:565-576. [DOI] [PubMed] [Google Scholar]
- 31.Locksley, R. M., N. Killeen, and M. J. Lenardo. 2001. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104:487-501. [DOI] [PubMed] [Google Scholar]
- 32.Luo, X., I. Budihardjo, H. Zou, C. Slaughter, and X. Wang. 1998. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94:481-490. [DOI] [PubMed] [Google Scholar]
- 33.Makris, C., V. L. Godfrey, G. Krahn-Senftleben, T. Takahashi, J. L. Roberts, T. Schwarz, L. Feng, R. S. Johnson, and M. Karin. 2000. Female mice heterozygous for IKKγ/NEMO deficiencies develop a dermatopathy similar to the human X-linked disorder incontinentia pigmenti. Mol. Cell 5:969-979. [DOI] [PubMed] [Google Scholar]
- 34.May, M. J., F. D'Acquisto, L. A. Madge, J. Glockner, J. S. Pober, and S. Ghosh. 2000. Selective inhibition of NF-κB activation by a peptide that blocks the interaction of NEMO with the IκB kinase complex. Science 289:1550-1554. [DOI] [PubMed] [Google Scholar]
- 35.Mayne, G. C., and A. W. Murray. 1998. Evidence that protein kinase Cε mediates phorbol ester inhibition of calphostin C- and tumor necrosis factor-alpha-induced apoptosis in U937 histiocytic lymphoma cells. J. Biol. Chem. 273:24115-24121. [DOI] [PubMed] [Google Scholar]
- 36.Mercurio, F., B. W. Murray, A. Shevchenko, B. L. Bennett, D. B. Young, J. W. Li, G. Pascual, A. Motiwala, H. Zhu, M. Mann, and A. M. Manning. 1999. IκB kinase (IKK)-associated protein 1, a common component of the heterogeneous IKK complex. Mol. Cell. Biol. 19:1526-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mercurio, F., H. Zhu, B. W. Murray, A. Shevchenko, B. L. Bennett, J. Li, D. B. Young, M. Barbosa, M. Mann, A. Manning, and A. Rao. 1997. IKK-1 and IKK-2: cytokine-activated IκB kinases essential for NF-κB activation. Science 278:860-866. [DOI] [PubMed] [Google Scholar]
- 38.Minden, A., A. Lin, M. McMahon, C. Lange-Carter, B. Derijard, R. J. Davis, G. L. Johnson, and M. Karin. 1994. Differential activation of ERK and JNK mitogen-activated protein kinases by Raf-1 and MEKK. Science 266:1719-1723. [DOI] [PubMed] [Google Scholar]
- 39.Muzio, M., A. M. Chinnaiyan, F. C. Kischkel, K. O'Rourke, A. Shevchenko, J. Ni, C. Scaffidi, J. D. Bretz, M. Zhang, R. Gentz, M. Mann, P. H. Krammer, M. E. Peter, and V. M. Dixit. 1996. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell 85:817-827. [DOI] [PubMed] [Google Scholar]
- 40.Natoli, G., A. Costanzo, A. Ianni, D. J. Templeton, J. R. Woodgett, C. Balsano, and M. Levrero. 1997. Activation of SAPK/JNK by TNF receptor 1 through a noncytotoxic TRAF2-dependent pathway. Science 275:200-203. [DOI] [PubMed] [Google Scholar]
- 41.Nemoto, S., J. A. DiDonato, and A. Lin. 1998. Coordinate regulation of IκB kinases by mitogen-activated protein kinase kinase kinase 1 and NF-κB-inducing kinase. Mol. Cell. Biol. 18:7336-7343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Regnier, C. H., H. Y. Song, X. Gao, D. V. Goeddel, Z. Cao, and M. Rothe. 1997. Identification and characterization of an IκB kinase. Cell 90:373-383. [DOI] [PubMed] [Google Scholar]
- 43.Rothwarf, D. M., E. Zandi, G. Natoli, and M. Karin. 1998. IKK-γ is an essential regulatory subunit of the IκB kinase complex. Nature 395:297-300. [DOI] [PubMed] [Google Scholar]
- 44.Rudolph, D., W. C. Yeh, A. Wakeham, B. Rudolph, D. Nallainathan, J. Potter, A. J. Elia, and T. W. Mak. 2000. Severe liver degeneration and lack of NF-κB activation in NEMO/IKKγ-deficient mice. Genes Dev. 14:854-862. [PMC free article] [PubMed] [Google Scholar]
- 45.Schmidt-Supprian, M., W. Bloch, G. Courtois, K. Addicks, A. Israel, K. Rajewsky, and M. Pasparakis. 2000. NEMO/IKKγ-deficient mice model incontinentia pigmenti. Mol. Cell 5:981-992. [DOI] [PubMed] [Google Scholar]
- 46.Siebenlist, U., G. Franzoso, and K. Brown. 1994. Structure, regulation and function of NF-κ B. Annu. Rev. Cell Biol. 10:405-455. [DOI] [PubMed] [Google Scholar]
- 47.Takeda, K., O. Takeuchi, T. Tsujimura, S. Itami, O. Adachi, T. Kawai, H. Sanjo, K. Yoshikawa, N. Terada, and S. Akira. 1999. Limb and skin abnormalities in mice lacking IKKα. Science 284:313-316. [DOI] [PubMed] [Google Scholar]
- 48.Tanaka, M., M. E. Fuentes, K. Yamaguchi, M. H. Durnin, S. A. Dalrymple, K. L. Hardy, and D. V. Goeddel. 1999. Embryonic lethality, liver degeneration, and impaired NF-κB activation in IKK-β-deficient mice. Immunity 10:421-429. [DOI] [PubMed] [Google Scholar]
- 49.Tang, G., Y. Minemoto, B. Dibling, N. H. Purcell, Z. Li, M. Karin, and A. Lin. 2001. Inhibition of JNK activation through NF-κB target genes. Nature 414:313-317. [DOI] [PubMed] [Google Scholar]
- 50.Tang, G., J. Yang, Y. Minemoto, and A. Lin. 2001. Blocking caspase-3-mediated proteolysis of IKKβ suppresses TNFα-induced apoptosis. Mol. Cell 8:1005-1016. [DOI] [PubMed] [Google Scholar]
- 51.Tartaglia, L. A., and D. V. Goeddel. 1992. Two TNF receptors. Immunol. Today 13:151-153. [DOI] [PubMed] [Google Scholar]
- 52.Thanos, D., and T. Maniatis. 1995. NF-κB: a lesson in family values. Cell 80:529-532. [DOI] [PubMed] [Google Scholar]
- 53.Tracey, K. J., and A. Cerami. 1993. Tumor necrosis factor: an updated review of its biology. Crit. Care Med. 21:S415-S422. [PubMed] [Google Scholar]
- 54.Van Antwerp, D. J., S. J. Martin, T. Kafri, D. R. Green, and I. M. Verma. 1996. Suppression of TNFα-induced apoptosis by NF-κB. Science 274:787-789. [DOI] [PubMed] [Google Scholar]
- 55.Verma, I. M., J. K. Stevenson, E. M. Schwarz, D. Van Antwerp, and S. Miyamoto. 1995. Rel/NF-κB/IκB family: intimate tales of association and dissociation. Genes Dev. 9:2723-2735. [DOI] [PubMed] [Google Scholar]
- 56.Wang, C. Y., D. C. Guttridge, M. W. Mayo, and A. S. Baldwin, Jr. 1999. NF-κB induces expression of the Bcl-2 homologue A1/Bfl-1 to preferentially suppress chemotherapy-induced apoptosis. Mol. Cell. Biol. 19:5923-5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang, C. Y., M. W. Mayo, R. G. Korneluk, D. V. Goeddel, and A. S. Baldwin, Jr. 1998. NF-κB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 281:1680-1683. [DOI] [PubMed] [Google Scholar]
- 58.Wang, K., X. M. Yin, D. T. Chao, C. L. Milliman, and S. J. Korsmeyer. 1996. BID: a novel BH3 domain-only death agonist. Genes Dev. 10:2859-2869. [DOI] [PubMed] [Google Scholar]
- 59.Woronicz, J. D., X. Gao, Z. Cao, M. Rothe, and D. V. Goeddel. 1997. IκB kinase-beta: NF-κB activation and complex formation with IκB kinase-α and NIK. Science 278:866-869. [DOI] [PubMed] [Google Scholar]
- 60.Yamaoka, S., G. Courtois, C. Bessia, S. T. Whiteside, R. Weil, F. Agou, H. E. Kirk, R. J. Kay, and A. Israel. 1998. Complementation cloning of NEMO, a component of the IκB kinase complex essential for NF-κB activation. Cell 93:1231-1240. [DOI] [PubMed] [Google Scholar]
- 61.Zandi, E., D. M. Rothwarf, M. Delhase, M. Hayakawa, and M. Karin. 1997. The IκB kinase complex (IKK) contains two kinase subunits, IKKα and IKKβ, necessary for IκB phosphorylation and NF-κB activation. Cell 91:243-252. [DOI] [PubMed] [Google Scholar]
- 62.Zha, J., S. Weiler, K. J. Oh, M. C. Wei, and S. J. Korsmeyer. 2000. Posttranslational N-myristoylation of BID as a molecular switch for targeting mitochondria and apoptosis. Science 290:1761-1765. [DOI] [PubMed] [Google Scholar]
- 63.Zheng, C., J. Xiang, T. Hunter, and A. Lin. 1999. The JNKK2-JNK1 fusion protein acts as a constitutively active c-Jun kinase that stimulates c-Jun transcription activity. J. Biol. Chem. 274:28966-28971. [DOI] [PubMed] [Google Scholar]