Abstract
Endothelial nitric oxide synthase (eNOS) is an important regulator of cardiovascular homeostasis by production of nitric oxide (NO) from vascular endothelial cells. It can be activated by protein kinase B (PKB)/Akt via phosphorylation at Ser-1177. We are interested in the role of Rho GTPase/Rho kinase (ROCK) pathway in regulation of eNOS expression and activation. Using adenovirus-mediated gene transfer in human umbilical vein endothelial cells (HUVECs), we show here that both active RhoA and ROCK not only downregulate eNOS gene expression as reported previously but also inhibit eNOS phosphorylation at Ser-1177 and cellular NO production with concomitant suppression of PKB activation. Moreover, coexpression of a constitutive active form of PKB restores the phosphorylation but not gene expression of eNOS in the presence of active RhoA. Furthermore, we show that thrombin inhibits eNOS phosphorylation, as well as expression via Rho/ROCK pathway. Expression of the active PKB reverses eNOS phosphorylation but has no effect on downregulation of eNOS expression induced by thrombin. Taken together, these data demonstrate that Rho/ROCK pathway negatively regulates eNOS phosphorylation through inhibition of PKB, whereas it downregulates eNOS expression independent of PKB.
Endothelium-derived nitric oxide (NO), synthesized from l-arginine by endothelial nitric oxide synthase (eNOS), plays a critical role in the regulation of vascular tone and the maintenance of vascular integrity by modulating various processes. It promotes vasodilatation, inhibits platelet activation and prevents vascular smooth muscle cell proliferation and/or migration (4). Abnormalities in eNOS gene expression or activation, which result in decreased NO production, are thought to contribute to the pathogenesis of various cardiovascular disorders such as atherosclerosis and hypertension (49).
eNOS-mediated NO generation is a highly regulated cellular event. The mechanisms controlling eNOS activity involve multiple regulatory steps including gene expression, co- and posttranslational modification, intracellular localization, cofactors and phosphorylation (20). Although eNOS was originally described as a constitutive enzyme with enzyme activation achieved via calmodulin (CaM) binding in response to increased Ca2+, recent studies indicate that a variety of stimuli can modulate its expression at the transcriptional (15) and/or posttranscriptional level (6). Moreover, it is evidenced that phosphorylation of eNOS also modulates eNOS activity independent of Ca2+/CaM. Studies showed that Ser-1177 of human eNOS (Ser-1179 of bovine sequence) is phosphorylated directly by protein kinase B (PKB)/Akt (10, 18, 37), which results in an increase in electron flux through the reductase domain and increase in NO production (36). Shear stress and insulin have been shown to activate eNOS through the activation of PKB (14, 41). eNOS phosphorylation at Ser-1177 by PKB therefore represents another important regulatory mechanism of eNOS activation in addition to Ca2+/CaM-dependent pathway.
The Rho GTPase is a member of the Ras superfamily of small GTP-binding proteins (48). It cycles between inactive GDP-bound and active GTP-bound forms, and exerts the function through its effectors (25). Rho was initially identified as a signaling molecule that regulates cytoskeletal rearrangement and was considered to be primarily involved in cellular functions that are associated with cytoskeleton organization, such as platelet aggregation, smooth muscle cell (SMC) contraction, and cell migration. More recently, additional roles, including the regulation of gene expression and cell proliferation, have been reported (5, 45). All of these Rho-regulated cellular functions are important for the pathogenesis of vascular disease and have been gaining increasing attention in the cardiovascular research (17, 32). Moreover, studies from our own and other laboratories demonstrated that Rho via its downstream effector Rho kinase (ROCK) is also involved in eNOS gene expression by affecting its mRNA stability (12, 30, 31). However, the mechanism by which Rho/ROCK downregulates eNOS mRNA stability remains elusive, and a role of Rho/ROCK in regulation of eNOS enzyme activation is not known. Recently, simvastatin, an inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A reductase that blocks the synthesis of isoprenoids and thus inhibits lipid attachment for Rho and its subsequent membrane translocation and activation (32) was shown to activate PKB (29), which induce eNOS activation. This prompted us to investigate whether there is a cross talk between Rho/ROCK and the PKB pathway, and if so, whether this cross talk regulates eNOS phosphorylation and is responsible for the downregulation of eNOS expression by Rho.
MATERIALS AND METHODS
Materials.
Reagents were purchased or obtained from the following sources: thrombin was from Calbiochem; l-α-phosphatidyl-l-serine, l-α-phosphatidylinositol-4,5-diphosphate sodium salt and platelet-derived growth factor-BB (PDGF-BB) were from Sigma; Redivue [γ-32P]ATP and l-[U-14C]arginine monohydrochloride were from Amersham; thin-layer chromatography plates (silica-60; WF2545) from Merck; endothelial cell growth supplement (ECGS) was from PromoCell GmbH; anti-eNOS monoclonal antibody was from BD Transduction Laboratory; anti-RhoA monoclonal antibody, protein A/G Plus-agarose, and glutathione-agarose were from Santa Cruz Biotechnology, Inc.; anti-Akt, anti-phospho-Akt (Ser-473 and Thr-308) antibodies, anti-phospho-eNOS (Ser-1177) antibody, and the Akt kinase assay kit were from Cell Signaling Technology; anti-phosphotyrosine (4G10) agarose conjugate was from Upstate Biotechnology; anti-mouse and anti-rabbit IgG (H+L) alkaline phosphatase (AP) conjugate and Western Blue stabilized substrate for AP from Promega. The recombinant glutathione S-transferase (GST)-TRBD (Rhotekin Rho binding domain 7-89 amino acid) fusion protein was purified as described previously (39). All of the cell culture materials were purchased from Gibco.
Cultivation of human umbilical vein endothelial cells (HUVECs) and hSMCs.
Endothelial cells were isolated from human umbilical veins as described previously (12). Briefly, fresh blood vessels were harvested in cold sterile phosphate-buffered saline (PBS) with antibiotics (100 U of penicillin and 100 μg of streptomycin/ml). The vessels were incubated with collagenase type II 75 U/ml for 20 min in PBS. Cell pellets were then collected by centrifugation, seeded in culture dishes coated with 1% gelatin and cultured in RPMI 1640 supplemented with 20 mmol of l-glutamine/liter, HEPES buffer solution, 100 U of penicillin and 100 μg of streptomycin/ml, 50 μg of ECGS/ml, 25 μg of heparin/ml, and 5% fetal calf serum (FCS). Endothelial cells are characterized by typical cobblestone and nonoverlapping appearance and indirect immunofluorescence staining by using specific antibodies against von Willebrand factor. Cultivation of human SMCs (hSMCs) isolated from human saphenous veins has been described prevously (51).
Generation of recombinant adenoviruses (rAd).
Expression plasmid encoding an active RhoA (pcDNA3.1-Rho63L) and a dominant-negative RhoA (pcDNA3.1-Rho19N) were kindly provided by Joseph J. Baldassare (24). pCMV constructs encoding a hemagglutinin (HA) epitope-tagged active PKB (pCMV-m/p-HA-PKBα) and an inactive PKB (pCMV-HA-PKBα-K179A) were from B. Hemmings (3). pEF-BOS-myc constructs encoding a constitutively active ROCK (pEF-BOS-myc-CAT) and a dominant-negative form of ROCK [pEF-BOS-myc-RB/PH (TT)] have been described previously (1).
Generation of recombinant adenovirus expressing hemagglutinin (HA)-tagged RhoA, ROCK, and PKBα mutants driven by the cytomegalovirus (CMV) promoter was carried out through homologous recombination between cotransformed virusmid and the corresponding adenoviral transfer plasmid in Escherichia coli as described previously (21). The corresponding cDNAs were first subcloned into the adenoviral transfer vector pSTCO-HA.mcs for Rho and ROCK mutants or pcDNA3.1 for PKBα mutants, allowing the insertion of the transgene into the E1 region of a cloned ΔE1ΔE3 adenoviral backbone (vmRL-CMV1 or vmAdcDNA3, respectively) bearing an empty expression cassette homologous to the one of the pSTCO-HA.mcs or pcDNA3.1, respectively. The transfer plasmid pSTCO-HA-Rho63L, -Rho19N, -CAT, and -RB/PH (TT) was then digested with AclI and XhoI and cotransformed with SwaI-linearized vmRL-CMV1 into a recombination-proficient E. coli strain (BJ5183) by electroporation to allow homologous recombination in BJ5183. The plasmid pcDNA3.1-m/p-HA-PKBα and pcDNA3.1-HA-PKBα-K179A were digested with StuI and SspI and cotransformed with SwaI-linearized vmAdcDNA3 into BJ5183. The candidate recombinants were subsequently screened by colony PCR, followed by retransformation of positive clones into another strain HB101, allowing higher plasmid yield. The resultant recombinant virusmids were digested with PacI and transfected into E1-complementing packaging cells (HER911) to generate recombinant adenovirus. The primary crude lysates of the recombinant adenoviruses were prepared as viral stocks and used to infect HER911 cells for scale up of the viral preparation. The viral titer was determined by plaque assay. The recombinant adenovirus expressing HA-tagged LacZ and green fluorescent protein (rAd-HA-LacZ and rAd-GFP) was described previously (21).
Adenoviral infection.
HUVECs were maintained in complete RPMI 1640 supplemented with 5% FCS and ECGS at 37°C in an atmosphere of 5% CO2 and were passaged when they reached confluence. Cells were seeded at a density of 2 × 105/6-cm dish in complete RPMI 1640 supplemented with 5% FCS and ECGS. 2 days later, cells were infected with the recombinant adenovirus at titers ranging from a multiplicity of infection (MOI) of 100 to 150 and further incubated in 0.2% FCS RPMI 1640 supplemented with ECGS for 18 h or 48 h before treatment and extraction.
Western blot.
Cell extracts were prepared by lysing cells in extraction buffer (120 mM NaCl, 50 mM Tris [pH 8.0], 20 mM NaF, 1 mM benzamidine, 1 mM EDTA, 1 mM EGTA, 1 mM sodium pyrophosphate, 30 mM 4-nitrophenyl phosphate disodium salt hexahydrate, 1% NP-40, and 0.1 M phenylmethylsulfonyl fluoride [PMSF]) as described previously (38). Next, 40-μg extracts were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electrophoretically transferred to an Immobilon-P membrane (Millipore), and the resultant membrane was incubated overnight with the corresponding first antibody at 4°C with gentle agitation after being blocked with 5% skimmed milk. The protein was decorated with a corresponding anti-mouse or anti-rabbit AP-conjugated secondary antibody and detected by using Western Blue stabilized substrate. Quantification of the signals was performed by using NIH Image 1.62 software. In all of the plotted graphics, each point represents the average value from three to five independent experiments.
PKB/Akt kinase assay.
PKB/Akt kinase activity was analyzed by nonradioactive immunoprecipitation-kinase assay using the Akt kinase assay kit from Cell Signaling Technology. Then, 30-μg cell extracts (200 μl) were incubated 2 h with immobilized Akt 1G1 monoclonal antibody. After an extensive washing, the kinase reaction was performed at 30°C for 30 min in the presence of 200 μM cold ATP and GSK-3 substrate. Phosphorylation of GSK-3 was measured by Western blot by using phospho-GSK-3α/β (Ser-21/9) antibody.
Phosphatidylinositol 3-kinase (PI 3-kinase) activity assay.
Cell extracts were prepared by lysing cells on ice for 15 min by the addition of 250 μl of lysis buffer containing 20 mM Tris-HCl, 138 mM NaCl, 2.7 mM KCl (pH 8.0) supplemented with 5% glycerol, 1 mM MgCl2, 1 mM CaCl2, 1 mM sodium-o-vanadate, 20 μM leupeptin, 18 μM pepstatin, 1% NP-40, 5 mM EDTA, and 20 mM NaF. Cell debris and nuclei were removed by centrifugation at 10,000 × g for 10 min at 4°C, and 100 μg of the cell lysate was then immunoprecipitated with anti-phosphotyrosine (4G10) agarose conjugate for 3 h at 4°C. The agarose beads containing the immune complexes were next washed twice with wash buffer (0.1 M Tris-HCl, pH 7.4; 0.5 M LiCl) and twice with kinase buffer (20 mM HEPES, pH 7.4; 5 mM MgCl2) and were then resuspended in 60 μl of kinase buffer (50) containing 10 μCi of [γ-32P]ATP, 60 μM ATP, and 10 μg each of phosphatidylserine and PI 4,5-diphosphate (PIP2). The kinase reaction was allowed to continue for 10 min at 30°C. The reaction was then stopped by the addition of 200 μl of 1 N HCl. The lipid reaction products were extracted with 400 μl of CH3Cl-methanol (1:1), dried, and then resuspended in 20 μl of spotting solution (CH3Cl-methanol [2:1]) and analyzed on oxalate-treated thin-layer chromatography silica gel plate by using CH3Cl-acetone-methanol-acetic acid-H2O (80:30:26:24:14 [vol/vol/vol/vol/vol]) as the separation solvent. The product of PIP3 was quantified by phosphorimager densitometry (Bio-Rad) using ImageQuant software Quantity One (Bio-Rad).
Measurement of eNOS activity (l-citrulline assay).
eNOS activity was measured by l-citrulline assay as previously described (22). Adenovirus-infected semiconfluent HUVECs were incubated at 37°C for 30 min in HEPES buffer (pH 7.4): 10 mM HEPES, 145 mM NaCl, 5 mM KCl, 1 mM MgSO4, 10 mM glucose, and 1.5 mM CaCl2 containing 0.25% bovine serum albumin. Then, 0.5 μCi of l-[14C]arginine/ml was added to each dish for 1 h before the reaction was stopped with cold phosphate-buffered saline (PBS) containing 5 mM l-arginine and 4 mM EDTA. The cells were then denatured in 96% ethanol. After evaporation the soluble cellular components were dissolved in 20 mM HEPES-Na (pH 5.5) and applied to well-equilibrated Dowex (Na+ form) colum. The pellets were dissolved in Western blot extraction buffer for protein concentration determination. The [14C]citrulline content of the eluate was quantified by liquid scintillation counting. Citrulline production by the different infections was expressed in picomoles per microgram of cellular protein obtained after subtracting the basal citrulline release. The basal release was determined from the L-NAME (100 μM), 30-min preincubation-inhibitable radioactivity in noninfected cells.
Pull-down assay of GTP-RhoA.
The activation of RhoA was assessed by a pull-down assay as described previously (44). In brief, HUVECs were washed with ice-cold Tris-buffered saline and lysed in radioimmunoprecipitation assay buffer (50 mM Tris, pH 7.2; 1% Triton X-100; 0.5% sodium deoxycholate; 0.1% sodium dodecyl sulfate; 500 mM NaCl; 10 mM MgCl2; 10 μg each of leupeptin and aprotinin/ml; 1 mM PMSF). Next, 200 μg of cell lysates was incubated with 10 μg of GST-TRBD beads at 4°C for 60 min. The beads were washed four times with buffer B (Tris buffer containing 1% Triton X-100, 150 mM NaCl, 10 mM MgCl2, 10 μg each of leupeptin and aprotinin/ml, and 0.1 mM PMSF). Bound Rho proteins were then detected by Western blot by using a monoclonal antibody against RhoA (Santa Cruz Biotechnology). The total amount of RhoA in cell lysates was used as a control for the cross-comparison of Rho activity (level of GTP-bound Rho).
RESULTS
Effects of Rho/ROCK on eNOS gene expression.
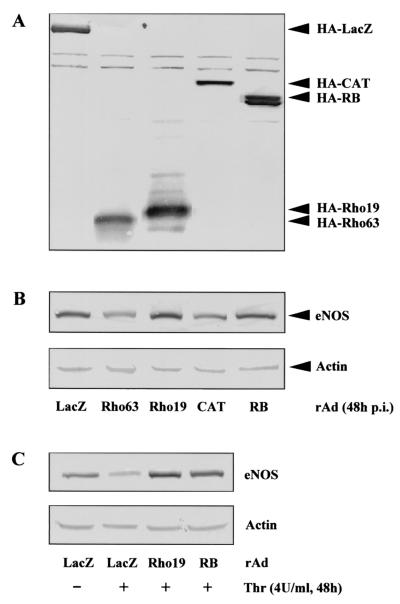
Our previous study demonstrated that thrombin-mediated downregulation of eNOS gene expression via decreasing the eNOS mRNA stability could be prevented either by the Rho GTPase inhibitor C3 exoenzyme or by the ROCK inhibitor Y-27632 in cultured HUVECs (12). This suggests that Rho/ROCK pathway plays an important role in eNOS gene expression. To further investigate the role of Rho/ROCK in the regulation of eNOS gene expression and the underlying mechanism, effects of an active or a dominant-negative form of Rho or ROCK on eNOS expression in HUVECs were analyzed by using adenovirus-mediated gene transfer. For this purpose, the infectivity of HUVECs was first determined by infecting the cells with rAd-GFP. The expression of GFP was detected in ca. 100% of the HUVECs (data not shown). Next, the HUVECs were infected with recombinant adenovirus expressing various transgenes and their effects on eNOS gene expression were analyzed by Western blot. All of the transgenes were expressed as detected by Western blot by using monoclonal anti-HA antibody 12CA5 (Fig. 1A). The effect of the transgenes on eNOS expression was analyzed by Western blotting with anti-eNOS antibody. Figure 1B shows that ectopic expression of either active Rho (Rho63) or active ROCK (CAT) led to a decrease in eNOS expression, whereas ectopic expression of dominant-negative mutant of Rho (Rho19) or ROCK (RB) abrogated downregulation of eNOS by thrombin (4 U/ml, 48 h) (Fig. 1C). These data support our previous observation that the Rho/ROCK pathway plays an important role in eNOS expression.
FIG. 1.
Effects of Rho/ROCK on eNOS gene expression. (A) HUVECs were infected with recombinant adenovirus (rAd) expressing HA-tagged LacZ, active Rho (Rho63), dominant-negative Rho (Rho19), active ROCK (CAT), and dominant-negative ROCK (RB) as indicated, at titers ranging from 100 to 150 MOI, and incubated in 0.2% FCS-RPMI 1640 supplemented with ECGS for 48 h. Cells were then extracted without any treatment, and the expression of the transgenes were analyzed by Western blotting with anti-HA antibody 12CA5. (B) The effect of the various transgenes on eNOS expression was examined by Western blotting with anti-eNOS antibody. (C) HUVECs were infected with recombinant adenovirus as indicated and serum-starved in 0.2% FCS culture medium for 24 h, followed by treatment with thrombin (4 U/ml) for 48 h. The expression of eNOS was detected by Western blotting. Actin in lower panels served as control for loading.
Inactivation of PKB by Rho/ROCK.
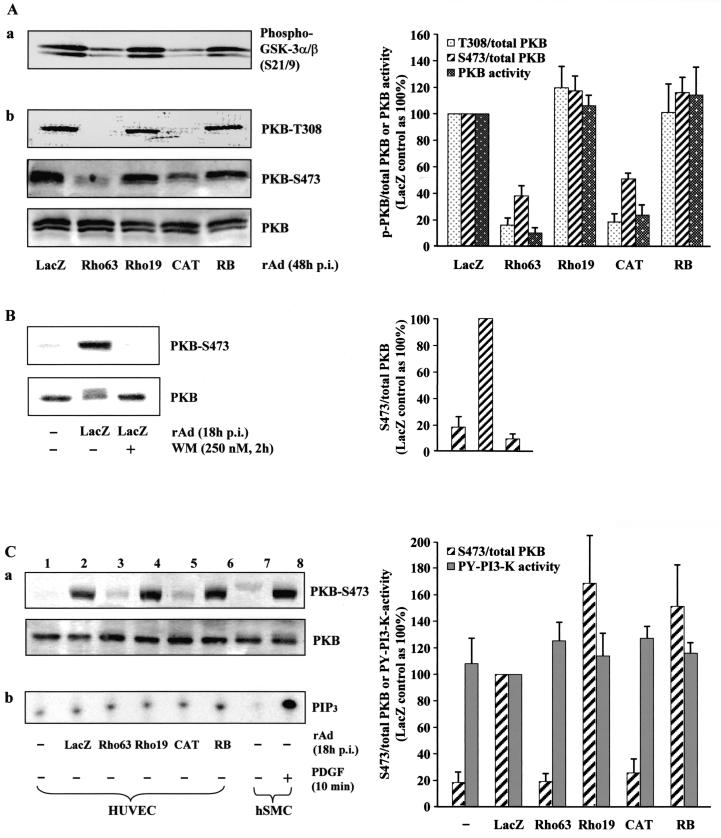
As a next step, we intended to investigate the mechanism by which the Rho/ROCK pathway downregulates expression of eNOS gene. Recently, simvastatin, an indirect inhibitor of Rho (32), was shown to activate PKB (29), implicating a possible cross talk between Rho and PKB pathway. We were therefore interested to examine whether Rho/ROCK can inactivate PKB, resulting in downregulation of eNOS expression. To do so, cultured HUVECs were infected with recombinant adenovirus expressing active or dominant-negative mutant of Rho or ROCK, and their effects on PKB activity were assessed by in vitro nonradioactive immunoprecipitation-kinase assay (Fig. 2Aa), as well as by monitoring the phosphorylation of PKB at Thr-308 and Ser-473 (Fig. 2Ab). Quantification of the data is shown in the right panel. The extent of PKB phosphorylation was detected with an anti-phospho-Akt (Thr-308 or Ser-473) antibody. Ectopic expression of active Rho or ROCK decreased both PKB activity (Fig. 2Aa) and PKB phosphorylation at Thr-308 and Ser-473 without affecting PKB protein level (Fig. 2Ab), whereas the dominant-negative mutant of Rho or ROCK did not significantly affect PKB activity or phosphorylation. These data provide evidence that there is indeed a cross talk between Rho/ROCK and PKB pathway and that Rho/ROCK pathway negatively regulates PKB activation. As monitoring Ser-473 reflect PKB activity (Fig. 2A), the effects on PKB activity were therefore assessed by only monitoring Ser-473 for further studies.
FIG. 2.
Active Rho or ROCK inhibits phosphorylation and activity of PKB without affecting p85-PI 3-kinase activity. (A) HUVECs were infected and extracted as described in Fig. 1A. Panel a shows the effect of Rho or ROCK mutants on PKB activity. A total of 30 μg of lysates was used for immunoprecipitation with immobilized Akt 1G1 monoclonal antibody and subjected to in vitro kinase assay with GSK-3 as substrate. Phosphorylation of GSK-3 was measured by Western blot with phospho-GSK-3α/β (Ser-21/9) antibody. In panel b, the extent of PKB phosphorylation was analyzed by Western blotting with antibodies specific for phosphorylated Thr-308 or Ser-473 of PKB as indicated, whereas total PKB expression was detected by Western blotting with antibodies that recognize PKB regardless of their phosphorylation status (lower panel). Shown are representative Western blots of five independent experiments. (B) HUVECs were either uninfected or infected, as indicated, and extracted at 18 h p.i. To inhibit PI 3-kinase, cells were treated with 250 nM wortmannin (WM) for 2 h before extraction. Cell lysates were assayed for PKB phosphorylation at Ser-473. (C) Lanes 1 to 6, HUVECs were either left uninfected or were infected as indicated and extracted at 18 h p.i. Cell lysates were assayed for PKB phosphorylation at Ser-473 (panel a) and in vitro PI 3-kinase activity in anti-phosphotyrosine (PY) immunoprecipitates (panel b) as described in Materials and Methods. Quantification of signal intensities by using the average of five independent experiments is shown in the right panels. Lanes 7 and 8, cell lysates prepared from untreated and PDGF-treated (50 ng/ml of PDGF-BB for 10 min) hSMCs were included as a positive control for PI 3-kinase activation.
Evidence has been presented that adenovirus infection triggers activation of the PI 3-kinase pathway via interaction of the adenoviral penton base caspid with αv integrins (34, 35, 42). To demonstrate that the PKB activation upon adenoviral infection shown in Fig. 2A is PI 3-kinase dependent, PI 3-kinase inhibitor wortmannin was used to block PI 3-kinase activation. In agreement with the established role of PI 3-kinase in PKB activation, integrin-mediated PKB activation upon control adenoviral infection was effectively inhibited by wortmannin treatment (Fig. 2B). Next, we sought to determine whether activation of Rho/ROCK pathway inhibits PI 3-kinase activation by analyzing the effect of active Rho or ROCK on PI 3-kinase activity in an in vitro PI 3-kinase assay in anti-phosphotyrosine immunoprecipitates (Fig. 2Cb). Surprisingly, in contrast to the strong phosphorylation or activation of PKB upon adenoviral infection (Fig. 2Ca, compare lane 1 with lane 2) and inhibition of PKB phosphorylation or activation by active Rho or ROCK (Fig. 2Ca, compare lanes 3 and 5 with lane 2), no obvious corresponding changes in PI 3-kinase activity could be observed, only a low basal PI 3-kinase activity was detected (Fig. 2Cb, lanes 1 to 6). To ensure that the whole procedures from cell extraction to immunoprecipitation with anti-phosphotyrosine antibody and lipid kinase reaction work properly to allow a detection of an PI 3-kinase activation, cell lysates were prepared from PDGF-stimulated hSMCs and included as a positive control for PI 3-kinase activation (Fig. 2Cb, lanes 7 and 8). Given that the inhibition of PI 3-kinase with wortmannin resulted in inhibition of PKB phosphorylation or activation (Fig. 2B), these data suggest that, in HUVECs and under our experimental condition, the basal PI 3-kinase activity is required for the PKB activity; however, PKB activation is unlikely to result from an activation of PI 3-kinase but may be from other mechanism such as inhibition of PTEN, inhibition of phosphatase 2A or even other unknown novel mechanism. The yet-to-be-clarified mechanism responsible for PKB activation would be therefore the target of Rho/ROCK to negatively regulate PKB activation shown here.
Effect of Rho/ROCK on eNOS phosphorylation.
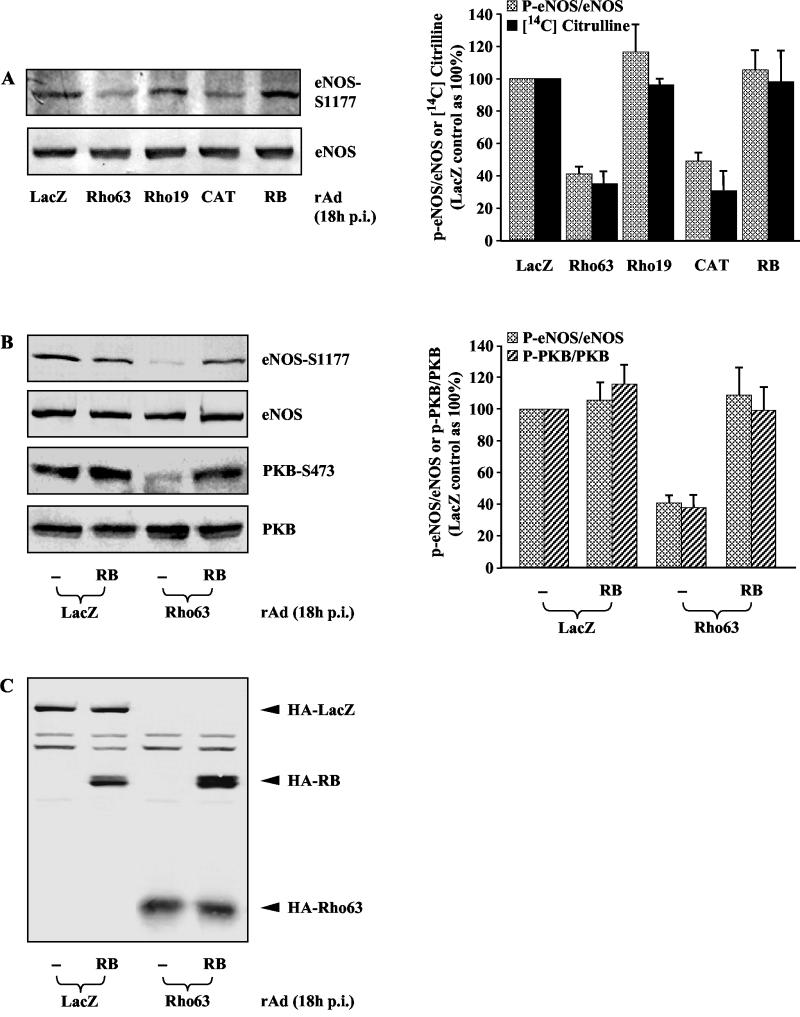
The fact that PKB activates eNOS by phosphorylating the enzyme at Ser-1177 (10, 18, 37), and that Rho/ROCK suppresses PKB activation (Fig. 2), suggested that Rho/ROCK might negatively regulate eNOS activation, besides having suppression of its gene expression. To test this hypothesis, the effect of Rho or ROCK on eNOS phosphorylation at Ser-1177 was analyzed. Indeed, in parallel with the inhibition on PKB phosphorylation and activation (Fig. 2), eNOS phosphorylation was also suppressed upon expression of active Rho or ROCK (Fig. 3A). To verify that eNOS enzyme activity parallels its phosphorylation, eNOS activity was measured in parallel under the same experimental conditions by in vivo l-citrulline assay. In agreement with the previously demonstrated role of eNOS phosphorylation at Ser-1177 in activation of eNOS enzyme activity (10, 18, 37), eNOS activity correlates well with the eNOS phosphorylation (Fig. 3A, right panel), indicating that the modulation of the Rho/ROCK on eNOS phosphorylation indeed reflects their effects on eNOS activity. Of note, in contrast to experiments shown in Fig. 1B where cell lysates were prepared at 48 h postinfection (p.i) and eNOS expression was clearly downregulated upon active Rho or ROCK expression, in these experiments the cell lysates were prepared at 18 h p.i. to ensure that the suppression of eNOS phosphorylation is not a consequence of downregulation of eNOS expression by Rho or ROCK. As shown in Fig. 3A, lower panel, there was no significant effect on eNOS expression yet at this time point. Moreover, coexpression of the dominant-negative ROCK (RB) prevented Rho63-mediated decrease in both PKB phosphorylation and eNOS phosphorylation (Fig. 3B). To rule out the possibility that the reversal effect of RB on phosphorylation of PKB and eNOS is due to unexpected transcriptional effects on Rho63, the expression of Rho63 in the absence or presence of RB was determined by Western blot. No obvious difference was observed under this condition (Fig. 3C). We conclude that Rho not only downregulates eNOS expression (Fig. 1) (12, 30, 31) but also inhibits eNOS phosphorylation and activity via its downstream target ROCK.
FIG. 3.
Rho inhibits phosphorylation of PKB and eNOS in parallel via its downstream target ROCK. Cells were infected as indicated and extracted at 18 h p.i. A representative Western blot from five (A) or three (B) independent experiments is shown under various conditions as indicated. Quantification of the data is shown in the corresponding right panels. (A) The extent of eNOS phosphorylation was analyzed by Western blotting with antibodies specific for phosphorylated Ser-1177 of eNOS (upper panel), whereas eNOS expression was analyzed by Western blotting with antibodies that recognize eNOS regardless of its phosphorylation status (lower panel). In parallel, under the same experimental conditions, eNOS activity was monitored by analyzing the cellular NO production measured as the formation of its coproduct [14C]citrulline. (B) The extent of eNOS and PKB phosphorylation was analyzed as described in Fig. 3A and 2, respectively. (C) The expression of HA-tagged LacZ, RB, and Rho63 was detected by Western blotting with monoclonal anti-HA antibody 12CA5.
Role of PKB in Rho-mediated downregulation of eNOS phosphorylation and expression.
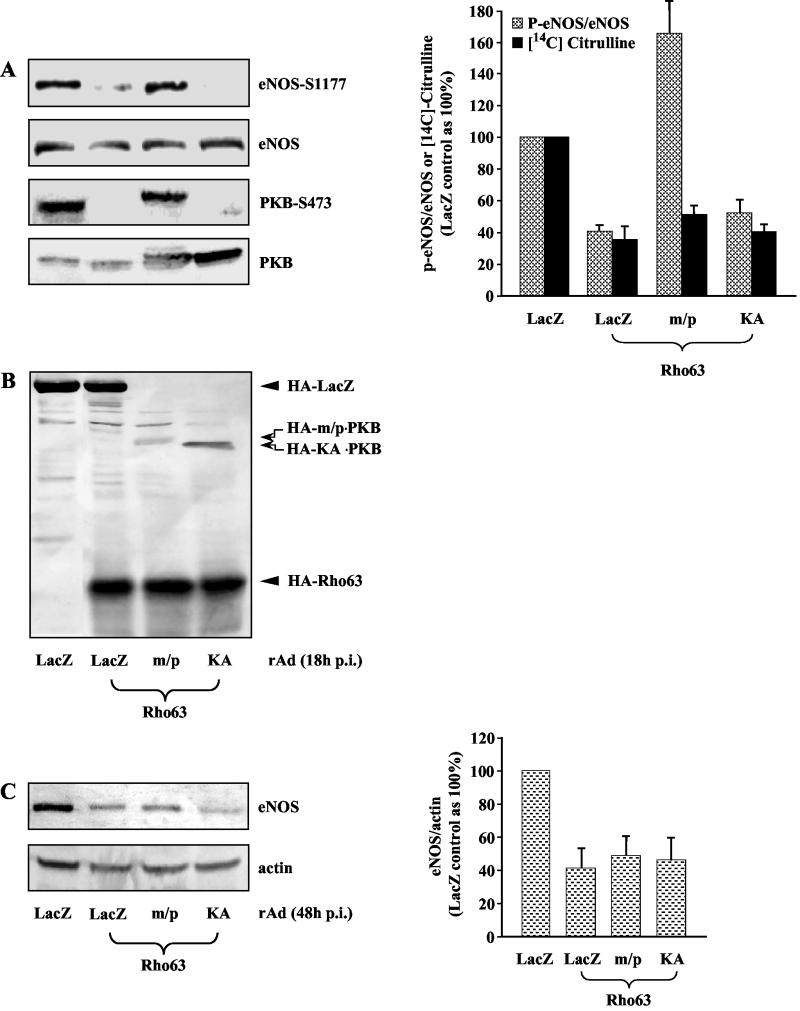
After determining the results described above, it was of interest to determine whether Rho/ROCK inhibits eNOS gene expression and phosphorylation via inhibition of PKB. For this purpose, cells were coinfected with recombinant adenoviruses expressing a constitutively active or inactive mutant of PKB, and their effects on Rho63-mediated downregulation of eNOS phosphorylation and cellular NO production reflected by l-citrulline production (extracts prepared at 18 h p.i., Fig. 4A), as well as eNOS expression (extracts prepared at 48 h p.i, Fig. 4C) were assessed. Ectopic expression of the active PKB (m/p), but not the inactive PKB (KA) restored eNOS phosphorylation in the presence of Rho63 (Fig. 4A). To ensure that the rescuing effect of m/p-PKB on eNOS phosphorylation in the presence of Rho63 is not due to unexpected transcriptional effects on Rho63, we performed control Western blots. As shown in Fig. 4B, no inhibitory effect of m/p-PKB on the expression of Rho63 was observed, suggesting that inactivation of PKB is indeed responsible for negative regulation of eNOS phosphorylation by Rho/ROCK pathway. Of note, in contrast to the effect of Rho/ROCK constructs on eNOS phosphorylation and cellular NO production, which parallel each other as shown in Fig. 3A, right panel, Fig. 4A reveals that active PKB restored eNOS phsphorylation without restoring cellular NO production. As eNOS activity was monitored by measuring l-citrulline production in living cells with l-[14C]arginine, these data suggest that Rho/ROCK pathway may also downregulate other factors that affect cellular NO production such as the availability of the substrate arginine or eNOS cofactor through PKB-independent pathway. Moreover, in contrast to its rescuing effect on eNOS phosphorylation, m/p-PKB could not restore eNOS gene expression in the presence of Rho63 (Fig. 4C). These observations indicate that Rho/ROCK pathway negatively regulates eNOS phosphorylation through inhibition of PKB pathway, whereas it downregulates eNOS gene expression via another yet to be identified pathway.
FIG. 4.
Active PKB reversed Rho63-mediated inhibition of phosphorylation but not of the expression of eNOS. Cells were infected as indicated, and the cell lysates were prepared 18 h p.i. (A and B) or 48 h p.i. (C). A representative Western blot from five independent experiments is shown under various conditions as indicated. Quantification of the data by using an average of five independent experiments is shown in the corresponding right panels. (A) Effect of PKB on Rho63-mediated dephosphorylation of eNOS and PKB and on cellular NO production measured as the formation of [14C]citrulline. (B) The expression of HA-tagged LacZ, active PKB (m/p), inactive PKB (KA), and Rho63 detected by Western blot with monoclonal anti-HA antibody 12CA5. (C) Effect of PKB on Rho63-mediated downregulation of eNOS gene expression.
Role of Rho and PKB pathways in thrombin-mediated downregulation of eNOS phosphorylation and expression.
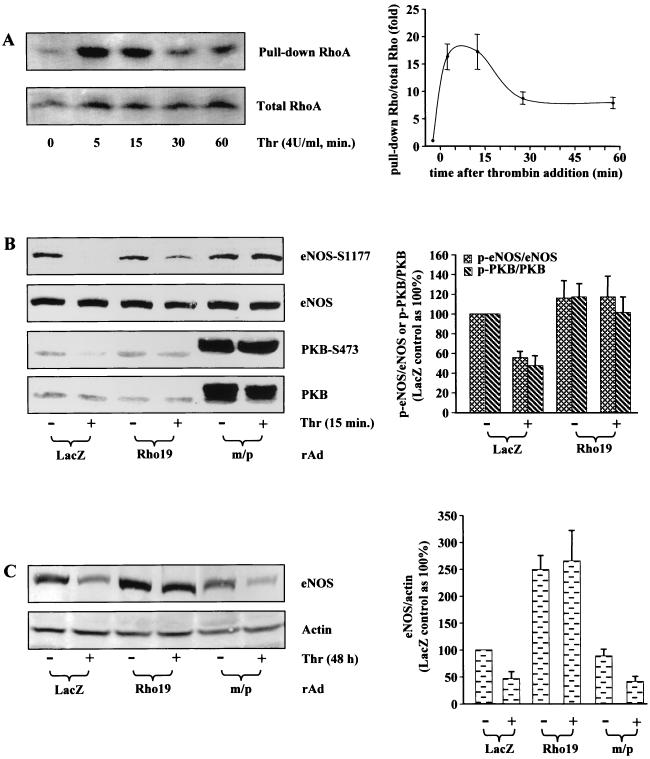
Finally, we sought to examine whether the conclusions drawn above would also hold true for thrombin, a risk factor in the pathogenesis of cardiovascular disease that activates the Rho/ROCK pathway (12) (Fig. 5A). Treatment of HUVECs with thrombin (4 U/ml) for 15 min decreased the phosphorylation of eNOS and PKB in parallel without significantly affecting the protein level of eNOS or PKB (Fig. 5B). Moreover, inhibition of phosphorylation of eNOS or PKB by thrombin was reversed by ectopic expression of the dominant-negative Rho (Rho19) or the active PKB (m/p) (Fig. 5B), suggesting that thrombin inhibits eNOS phosphorylation via Rho, which in turn inactivates the PKB pathway. Furthermore, in agreement with previous observations and our conclusion above, the downregulation of eNOS expression by thrombin (4 U/ml, 48 h) was prevented by dominant-negative Rho but not by the active PKB (Fig. 5C). Taken together, these results further confirm our observation obtained with Rho mutants shown above and demonstrate that thrombin downregulates both eNOS expression and phosphorylation via Rho pathway, which inhibits PKB and subsequently eNOS phosphorylation but not eNOS expression.
FIG. 5.
Role of Rho and PKB in thrombin-mediated downregulation of eNOS phosphorylation and expression. (A) Time course of Rho activation by thrombin. HUVECs were serum starved for 24 h and then treated with 4 U of thrombin/ml for the indicated time. The cell lysates were subjected to pull-down assay of GTP-Rho as described in Materials and Methods. (B) Effect of dominant-negative Rho (Rho19) and active PKB (m/p) on thrombin-induced dephosphorylation of eNOS and PKB. Cells were infected as indicated and serum starved in 0.2% FCS culture medium without ECGS for 24 h. The cells were then either left untreated (−) or were treated with 4 U of thrombin/ml (+) for 15 min. (C) Effect of Rho19 and m/p-PKB on thrombin-mediated downregulation of eNOS gene expression. Cells were infected and serum starved as in panel B, except that cells were treated with thrombin for 48 h instead of 15 min. Shown are representative blots from three (panel A) or five (panels B and C) independent experiments. Quantification of the data under each condition is shown in the corresponding right panels.
DISCUSSION
In the present study we demonstrate that Rho/ROCK not only downregulates eNOS expression but it also inhibits eNOS phosphorylation and its activity. Moreover, we provide evidence that inactivation of PKB by Rho/ROCK is responsible for the inhibition of eNOS phosphorylation but not the downregulation of eNOS gene expression. Furthermore, these conclusions are reinforced by the results obtained with thrombin. Concomitant with its ability to activate Rho/ROCK, thrombin suppressed the phosphorylation of PKB and eNOS, in addition to its downregulatory effect on eNOS expression. Although the inhibition of both expression and phosphorylation of eNOS by thrombin is mediated by Rho/ROCK pathway, only the suppression of eNOS phosphorylation could be attributed to inactivation of PKB by thrombin.
Rho GTPase has been shown to regulate a wide spectrum of cellular function (48). Increasing evidence suggests a role of Rho-regulated cellular function in mediating cardiovascular pathologies, such as smooth muscle hypercontraction (47), SMC growth and migration that account for neointimal formation of stenosis (13, 46), and platelet aggregation (40, 43). Moreover, in recent years, much has been learned about the role of Rho in modulating endothelial function. In the context of endothelial dysfunction, Rho has been reported to downregulate eNOS expression (12, 30, 31) and is essential for the basal production of endothelin-1 (23), a potent vasoconstrictor and mitogen that regulates vascular tone and remodeling (33). In agreement with previous reports, we demonstrate here that activation of Rho is sufficient to downregulate eNOS expression and is required for thrombin-mediated decrease in eNOS expression (Fig. 1). Moreover, in the present study, we provide additional evidence for an important role of Rho in modulating PKB/eNOS pathway. We show that the activation of Rho, either by ectopic expression of active mutant of Rho or by a pathological stimulus thrombin dramatically inhibited phosphorylation of eNOS in parallel to that of PKB (Fig. 2 and 3). Of interest, whereas thrombin-induced dephosphorylation of eNOS occurs within 15 min (Fig. 5B), it takes hours to days to achieve downregulation of eNOS expression (Fig. 5C) (12, 30, 31). The same is true with the constructs expressing active Rho or ROCK. Due to the fact that it takes at least more than 8 h to express the transgenes, we do not know exactly how rapidly the dephosphorylation was induced upon Rho63 expression. However, eNOS dephosphorylation could be already observed at 18 h p.i., at a time point when eNOS expression was not yet significantly affected (Fig. 3 and 4A). In this respect, signals such as thrombin would cause endothelial dysfunction via the activation of Rho that in turn leads to dephosphorylation or inactivation of eNOS and suppression of its gene expression. This mechanism may play an important role in acute coronary syndromes in patients with heart disease.
To date, a number of downstream effector proteins of Rho have been identified (5). Among them, ROCK is the most extensively studied. Many of the Rho-regulated cellular functions have been shown to be mediated by ROCK, such as smooth muscle contraction (2, 26, 28), cytoskeletal rearrangement and c-fos expression (7), and cytokinesis (52). We show here that Rho-induced dephosphorylation of PKB and eNOS is also mediated by ROCK. This conclusion is based on the observations that the active ROCK led to dephosphorylation of both PKB and eNOS (Fig. 2 and 3A), the dominant-negative ROCK prevented Rho63-mediated inhibition of PKB and eNOS phosphorylation (Fig. 3B).
Another important evidence we provide in this report is the cross talk between Rho/ROCK and PKB. The importance of this finding should be underscored by the fact that PKB serves as a multifunctional regulator of cell survival, growth, and glucose metabolism (9). With respect to its cardiovascular functions, PKB plays a critical role in endothelial cell survival (16, 19) and promotes angiogenesis (11, 29) mediated by vascular endothelial growth factor, in addition to its involvement in controlling vasomotor activity via regulating eNOS pathway as mentioned above. Here we demonstrate that the cross talk between Rho/ROCK and PKB pathways is responsible for Rho-mediated eNOS dephosphorylation. Two lines of evidence supported the involvement of PKB in Rho/ROCK-mediated inhibition of eNOS phosphorylation. The first evidence came from experiments with constitutively active mutants. The data in Fig. 2 and 3 show that dephosphorylation of PKB and eNOS by active Rho or ROCK closely parallel one another. The second piece of evidence is provided by the rescue experiment in which an active PKB, but not an inactive PKB, restored phosphorylation of eNOS in the presence of active Rho (Fig. 4A). Since evidence has been presented that eNOS phosphorylation by PKB led to increased NO production, we wish to point out that active PKB restored eNOS phosphorylation in the presence of active Rho mutant without restoring cellular NO production (Fig. 4A). Taking into account that eNOS activity was monitored by measuring the cellular NO production in living cells with l-[14C]arginine and that the effects of Rho and ROCK mutants on eNOS phosphorylation and cellular NO production parallel each other (Fig. 3A), a probable explanation would be that Rho/ROCK pathway may also downregulate other factors such as the availability of the substrate arginine or eNOS cofactor that are necessary for a normal cellular NO production but through other pathway(s) independent of PKB.
Although the role of PKB in eNOS phosphorylation leading to increased NO production is well documented and established, little is know about an involvement of PKB in eNOS gene expression. In view of the fact that PI 3-kinase was reported to be involved in eNOS gene expression (8, 27), and PKB is a well-characterized downstream signaling of PI 3-kinase, it is important to point out that active PKB did not rescue Rho63- or thrombin-mediated eNOS gene downregulation (Fig. 4C and 5C), indicating that PKB is not required for Rho63 or thrombin to downregulate eNOS expression. However, this does not rule out a role of PKB in the regulation of eNOS gene expression. One possibility could be that PKB does not play a role in the regulation of eNOS mRNA stability by thrombin or Rho and yet is involved in eNOS transcriptional regulation as reported in one study regarding regulation of eNOS gene expression by PI 3-kinase (8).
Regarding a role of PI 3-kinase in Rho/ROCK-mediated downregulation of PKB phosphorylation and activity by active mutants of Rho/ROCK, our data suggest that the inhibition of PKB activity by Rho/ROCK is unlikely to result from inhibition of PI 3-kinase activity since the PKB activation upon adenoviral infection or the inhibition of PKB activation by active Rho/ROCK is not accompanied by corresponding changes in PI 3-kinase activity (Fig. 2C). This is noteworthy in view of the fact that PI 3-kinase is strongly activated upon adenoviral infection in the human colon carcinoma cell line SW480 (34, 35). Thus, although the basal PI 3-kinase activity is required for PKB phosphorylation and activity in HUVECs under our experimental conditions (Fig. 2B), the PKB activation probably did not result from PI 3-kinase activation but may be from other mechanism such as inactivation of PTEN, inactivation of phosphatase 2A, or even some other unknown novel mechanism. What mechanism accounts for the PKB activation and would be therefore the target of Rho/ROCK to negatively regulate PKB activation reported in this study remains to be shown.
The data presented here are summarized as follows: a stimulus that activates Rho/ROCK pathway would lead to dephosphorylation of eNOS while simultaneously suppressing its expression by decreasing its mRNA stability to achieve rapid and long-term decrease in NO production. Whereas inhibition of PKB by Rho/ROCK is responsible for the negative regulation of eNOS phosphorylation, the downregulation of eNOS expression is attributed to another yet-to-be-identified pathway. An important task will be to further elucidate the mechanism by which Rho/ROCK inactivates PKB. In addition, the impact of the cross talk between the two pathways on other PKB-regulated cellular functions should be a worthwhile goal for future research.
Acknowledgments
We thank S. Brenz and P. Matthey for valuable help in generation of the recombinant adenoviruses, M. P. Wymann and G. Bulgarelli-Leva for advice in performing the PI 3-kinase activity assay, J. J. Baldassare for pcDNA3.1-Rho63L and pCDNA3.1-Rho19N, B. Hemmings for pCMV-m/p-HA-PKBα and pCMV-HA-PKBα-K179A, and M. A. Schwartz for pGEX-2T-TRBD.
This work was supported by the Swiss National Research Foundation (Nr 31-63811.00), the Stanley Thomas Johnson foundation, Roche Research Foundation (63-2000), and the Swiss Heart Foundation. X.-F.M. was a recipient of MHV-fellowship of Swiss National Research Foundation (Nr 32-63170.00).
REFERENCES
- 1.Amano, M., K. Chihara, N. Nakamura, Y. Fukata, T. Yano, M. Shibata, M. Ikebe, and K. Kaibuchi. 1998. Myosin II activation promotes neurite retraction during the action of Rho and Rho-kinase. Genes Cells 3:177-188. [DOI] [PubMed] [Google Scholar]
- 2.Amano, M., M. Ito, K. Kimura, Y. Fukata, K. Chihara, T. Nakano, Y. Matsuura, and K. Kaibuchi. 1996. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J. Biol. Chem. 271:20246-20249. [DOI] [PubMed] [Google Scholar]
- 3.Andjelkovic, M., D. R. Alessi, R. Meier, A. Fernandez, N. J. Lamb, M. Frech, P. Cron, P. Cohen, J. M. Lucocq, and B. A. Hemmings. 1997. Role of translocation in the activation and function of protein kinase B. J. Biol. Chem. 272:31515-31524. [DOI] [PubMed] [Google Scholar]
- 4.Arnal, J. F., A. T. Dinh-Xuan, M. Pueyo, B. Darblade, and J. Rami. 1999. Endothelium-derived nitric oxide and vascular physiology and pathology. Cell. Mol. Life Sci. 55:1078-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aspenstrom, P. 1999. Effectors for the Rho GTPases. Curr. Opin. Cell Biol. 11:95-102. [DOI] [PubMed] [Google Scholar]
- 6.Bloch, K. D. 1999. Regulation of endothelial NO synthase mRNA stability: RNA-binding proteins crowd on the 3′-untranslated region. Circ. Res. 85:653-655. [DOI] [PubMed] [Google Scholar]
- 7.Chihara, K., M. Amano, N. Nakamura, T. Yano, M. Shibata, T. Tokui, H. Ichikawa, R. Ikebe, M. Ikebe, and K. Kaibuchi. 1997. Cytoskeletal rearrangements and transcriptional activation of c-fos serum response element by Rho-kinase. J. Biol. Chem. 272:25121-25127. [DOI] [PubMed] [Google Scholar]
- 8.Cieslik, K., C. S. Abrams, and K. K. Wu. 2001. Up-regulation of endothelial nitric-oxide synthase promoter by the phosphatidylinositol 3-kinase gamma/Janus kinase 2/MEK-1-dependent pathway. J. Biol. Chem. 276:1211-1219. [DOI] [PubMed] [Google Scholar]
- 9.Datta, S. R., A. Brunet, and M. E. Greenberg. 1999. Cellular survival: a play in three Akts. Genes Dev. 13:2905-2927. [DOI] [PubMed] [Google Scholar]
- 10.Dimmeler, S., I. Fleming, B. Fisslthaler, C. Hermann, R. Busse, and A. M. Zeiher. 1999. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399:601-605. [DOI] [PubMed] [Google Scholar]
- 11.Dimmeler, S., and A. M. Zeiher. 2000. Akt takes center stage in angiogenesis signaling. Circ. Res. 86:4-5. [DOI] [PubMed] [Google Scholar]
- 12.Eto, M., C. Barandier, L. Rathgeb, T. Kozai, H. Joch, Z. Yang, and T. F. Luscher. 2001. Thrombin suppresses endothelial nitric oxide synthase and upregulates endothelin-converting enzyme-1 expression by distinct pathways: role of Rho/ROCK and mitogen-activated protein kinase. Circ. Res. 89:583-590. [DOI] [PubMed] [Google Scholar]
- 13.Eto, Y., H. Shimokawa, J. Hiroki, K. Morishige, T. Kandabashi, Y. Matsumoto, M. Amano, M. Hoshijima, K. Kaibuchi, and A. Takeshita. 2000. Gene transfer of dominant negative Rho kinase suppresses neointimal formation after balloon injury in pigs. Am. J. Physiol. Heart Circ. Physiol. 278:H1744-H1750. [DOI] [PubMed] [Google Scholar]
- 14.Fisslthaler, B., S. Dimmeler, C. Hermann, R. Busse, and I. Fleming. 2000. Phosphorylation and activation of the endothelial nitric oxide synthase by fluid shear stress. Acta Physiol. Scand. 168:81-88. [DOI] [PubMed] [Google Scholar]
- 15.Forstermann, U., J. P. Boissel, and H. Kleinert. 1998. Expressional control of the “constitutive” isoforms of nitric oxide synthase (NOS I and NOS III). FASEB J. 12:773-790. [PubMed] [Google Scholar]
- 16.Fujio, Y., and K. Walsh. 1999. Akt mediates cytoprotection of endothelial cells by vascular endothelial growth factor in an anchorage-dependent manner. J. Biol. Chem. 274:16349-16354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukata, Y., M. Amano, and K. Kaibuchi. 2001. Rho-Rho-kinase pathway in smooth muscle contraction and cytoskeletal reorganization of non-muscle cells. Trends Pharmacol. Sci. 22:32-39. [DOI] [PubMed] [Google Scholar]
- 18.Fulton, D., J. P. Gratton, T. J. McCabe, J. Fontana, Y. Fujio, K. Walsh, T. F. Franke, A. Papapetropoulos, and W. C. Sessa. 1999. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature 399:597-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerber, H. P., A. McMurtrey, J. Kowalski, M. Yan, B. A. Keyt, V. Dixit, and N. Ferrara. 1998. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J. Biol. Chem. 273:30336-30343. [DOI] [PubMed] [Google Scholar]
- 20.Govers, R., and T. J. Rabelink. 2001. Cellular regulation of endothelial nitric oxide synthase. Am. J. Physiol. Renal Physiol. 280:F193-F206. [DOI] [PubMed] [Google Scholar]
- 21.Heider, H., S. B. Verca, S. Rusconi, and R. Asmis. 2000. Comparison of lipid-mediated and adenoviral gene transfer in human monocyte-derived macrophages and COS-7 cells. BioTechniques 28:260-265, 268-270. [DOI] [PubMed] [Google Scholar]
- 22.Heller, R., F. Munscher-Paulig, R. Grabner, and U. Till. 1999. l-Ascorbic acid potentiates nitric oxide synthesis in endothelial cells. J. Biol. Chem. 274:8254-8260. [DOI] [PubMed] [Google Scholar]
- 23.Hernandez-Perera, O., D. Perez-Sala, E. Soria, and S. Lamas. 2000. Involvement of Rho GTPases in the transcriptional inhibition of preproendothelin-1 gene expression by simvastatin in vascular endothelial cells. Circ. Res. 87:616-622. [DOI] [PubMed] [Google Scholar]
- 24.Hu, W., C. J. Bellone, and J. J. Baldassare. 1999. RhoA stimulates p27Kip degradation through its regulation of cyclin E/CDK2 activity. J. Biol. Chem. 274:3396-3401. [DOI] [PubMed] [Google Scholar]
- 25.Kaibuchi, K., S. Kuroda, and M. Amano. 1999. Regulation of the cytoskeleton and cell adhesion by the Rho family GTPases in mammalian cells. Annu. Rev. Biochem. 68:459-486. [DOI] [PubMed] [Google Scholar]
- 26.Kimura, K., M. Ito, M. Amano, K. Chihara, Y. Fukata, M. Nakafuku, B. Yamamori, J. Feng, T. Nakano, K. Okawa, A. Iwamatsu, and K. Kaibuchi. 1996. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 273:245-248. [DOI] [PubMed] [Google Scholar]
- 27.Kuboki, K., Z. Y. Jiang, N. Takahara, S. W. Ha, M. Igarashi, T. Yamauchi, E. P. Feener, T. P. Herbert, C. J. Rhodes, and G. L. King. 2000. Regulation of endothelial constitutive nitric oxide synthase gene expression in endothelial cells and in vivo: a specific vascular action of insulin. Circulation 101:676-681. [DOI] [PubMed] [Google Scholar]
- 28.Kureishi, Y., S. Kobayashi, M. Amano, K. Kimura, H. Kanaide, T. Nakano, K. Kaibuchi, and M. Ito. 1997. Rho-associated kinase directly induces smooth muscle contraction through myosin light chain phosphorylation. J. Biol. Chem. 272:12257-12260. [DOI] [PubMed] [Google Scholar]
- 29.Kureishi, Y., Z. Luo, I. Shiojima, A. Bialik, D. Fulton, D. J. Lefer, W. C. Sessa, and K. Walsh. 2000. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat. Med. 6:1004-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laufs, U., M. Endres, and J. K. Liao. 1999. Regulation of endothelial NO production by Rho GTPase. Med. Klin. 94:211-218. [DOI] [PubMed] [Google Scholar]
- 31.Laufs, U., and J. K. Liao. 1998. Post-transcriptional regulation of endothelial nitric oxide synthase mRNA stability by Rho GTPase. J. Biol. Chem. 273:24266-24271. [DOI] [PubMed] [Google Scholar]
- 32.Laufs, U., and J. K. Liao. 2000. Targeting Rho in cardiovascular disease. Circ. Res. 87:526-528. [DOI] [PubMed] [Google Scholar]
- 33.Levin, E. R. 1995. Endothelins. N. Engl. J. Med. 333:356-363. [DOI] [PubMed] [Google Scholar]
- 34.Li, E., D. Stupack, R. Klemke, D. A. Cheresh, and G. R. Nemerow. 1998. Adenovirus endocytosis via alpha(v) integrins requires phosphoinositide-3-OH kinase. J. Virol. 72:2055-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li, E., D. G. Stupack, S. L. Brown, R. Klemke, D. D. Schlaepfer, and G. R. Nemerow. 2000. Association of p130CAS with phosphatidylinositol-3-OH kinase mediates adenovirus cell entry. J. Biol. Chem. 275:14729-14735. [DOI] [PubMed] [Google Scholar]
- 36.McCabe, T. J., D. Fulton, L. J. Roman, and W. C. Sessa. 2000. Enhanced electron flux and reduced calmodulin dissociation may explain “calcium-independent” eNOS activation by phosphorylation. J. Biol. Chem. 275:6123-6128. [DOI] [PubMed] [Google Scholar]
- 37.Michell, B. J., J. E. Griffiths, K. I. Mitchelhill, I. Rodriguez-Crespo, T. Tiganis, S. Bozinovski, P. R. de Montellano, B. E. Kemp, and R. B. Pearson. 1999. The Akt kinase signals directly to endothelial nitric oxide synthase. Curr. Biol. 9:845-848. [DOI] [PubMed] [Google Scholar]
- 38.Ming, X. F., B. M. Burgering, S. Wennstrom, L. Claesson-Welsh, C. H. Heldin, J. L. Bos, S. C. Kozma, and G. Thomas. 1994. Activation of p70/p85 S6 kinase by a pathway independent of p21ras. Nature 371:426-429. [DOI] [PubMed] [Google Scholar]
- 39.Ming, X. F., M. Kaiser, and C. Moroni. 1998. c-jun N-terminal kinase is involved in AUUUA-mediated interleukin-3 mRNA turnover in mast cells. EMBO J. 17:6039-6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Missy, K., M. Plantavid, P. Pacaud, C. Viala, H. Chap, and B. Payrastre. 2001. Rho-kinase is involved in the sustained phosphorylation of myosin and the irreversible platelet aggregation induced by PAR1 activating peptide. Thromb. Haemost. 85:514-520. [PubMed] [Google Scholar]
- 41.Montagnani, M., H. Chen, V. A. Barr, and M. J. Quon. 2001. Insulin-stimulated activation of eNOS is independent of Ca2+ but requires phosphorylation by Akt at Ser1179. J. Biol. Chem. 276:30392-30398. [DOI] [PubMed] [Google Scholar]
- 42.Nemerow, G. R., and P. L. Stewart. 1999. Role of alpha(v) integrins in adenovirus cell entry and gene delivery. Microbiol. Mol. Biol. Rev. 63:725-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nishioka, H., H. Horiuchi, A. Tabuchi, A. Yoshioka, R. Shirakawa, and T. Kita. 2001. Small GTPase Rho regulates thrombin-induced platelet aggregation. Biochem. Biophys. Res. Commun. 280:970-975. [DOI] [PubMed] [Google Scholar]
- 44.Ren, X. D., W. B. Kiosses, and M. A. Schwartz. 1999. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 18:578-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seasholtz, T. M., M. Majumdar, and J. H. Brown. 1999. Rho as a mediator of G protein-coupled receptor signaling. Mol. Pharmacol. 55:949-956. [DOI] [PubMed] [Google Scholar]
- 46.Seasholtz, T. M., M. Majumdar, D. D. Kaplan, and J. H. Brown. 1999. Rho and Rho kinase mediate thrombin-stimulated vascular smooth muscle cell DNA synthesis and migration. Circ. Res. 84:1186-1193. [DOI] [PubMed] [Google Scholar]
- 47.Shimokawa, H. 2000. Cellular and molecular mechanisms of coronary artery spasm: lessons from animal models. Jpn. Circ. J. 64:1-12. [DOI] [PubMed] [Google Scholar]
- 48.Van Aelst, L., and C. D'Souza-Schorey. 1997. Rho GTPases and signaling networks. Genes Dev. 11:2295-2322. [DOI] [PubMed] [Google Scholar]
- 49.Wever, R. M., T. F. Luscher, F. Cosentino, and T. J. Rabelink. 1998. Atherosclerosis and the two faces of endothelial nitric oxide synthase. Circulation 97:108-112. [DOI] [PubMed] [Google Scholar]
- 50.Wymann, M. P., G. Bulgarelli-Leva, M. J. Zvelebil, L. Pirola, B. Vanhaesebroeck, M. D. Waterfield, and G. Panayotou. 1996. Wortmannin inactivates phosphoinositide 3-kinase by covalent modification of Lys-802, a residue involved in the phosphate transfer reaction. Mol. Cell. Biol. 16:1722-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang, Z., B. S. Oemar, T. Carrel, B. Kipfer, F. Julmy, and T. F. Lüscher. 1998. Different proliferative properties of smooth muscle cells of human arterial and venous bypass vessels: role of PDGF receptors, mitogen-activated protein kinase and cyclin-dependent kinase inhibitors. Circulation 97:181-187. [DOI] [PubMed] [Google Scholar]
- 52.Yasui, Y., M. Amano, K. Nagata, N. Inagaki, H. Nakamura, H. Saya, K. Kaibuchi, and M. Inagaki. 1998. Roles of Rho-associated kinase in cytokinesis; mutations in Rho-associated kinase phosphorylation sites impair cytokinetic segregation of glial filaments. J. Cell Biol. 143:1249-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]