Abstract
ASC-2, a recently isolated transcriptional coactivator molecule, stimulates transactivation by multiple transcription factors, including nuclear receptors. We generated a potent dominant negative fragment of ASC-2, encompassing the N-terminal LXXLL motif that binds a broad range of nuclear receptors. This fragment, termed DN1, specifically inhibited endogenous ASC-2 from binding these receptors in vivo, whereas DN1/m, in which the LXXLL motif was mutated to LXXAA to abolish the receptor interactions, was inert. Interestingly, DN1 transgenic mice but not DN1/m transgenic mice exhibited severe microphthalmia and posterior lenticonus with cataract as well as a variety of pathophysiological phenotypes in many other organs. Our results provide a novel insight into the molecular and histopathological mechanism of posterior lenticonus with cataract and attest to the importance of ASC-2 as a pivotal transcriptional coactivator of nuclear receptors in vivo.
The nuclear receptor superfamily is a group of proteins that regulate, in a ligand-dependent manner, transcriptional initiation of target genes by binding to specific DNA sequences named hormone response elements (for a review, see reference 15). Genetic studies indicated that transcription coactivators without specific DNA-binding activity are essential for transcriptional activation, which led to the identification of many proteins interacting with the C-terminal ligand-dependent transactivation domain of nuclear receptors (for reviews, see references 1 and 19). These coactivators, including the p160 family, CBP/p300, p/CAF, TRAP/DRIP, and many others, bridge transcription factors and the basal transcription apparatus and/or remodel the chromatin structures.
ASC-2, also named AIB3, TRBP, RAP250, NRC, and PRIP, is a recently isolated transcriptional coactivator molecule which is gene amplified and overexpressed in human cancers and stimulates transactivation by nuclear receptors, AP-1, NF-κB, SRF, and numerous other transcription factors (1, 10-12, 14, 24). In particular, the single-cell microinjection results with ASC-2 antibody demonstrated that endogenous ASC-2 is required for transactivation by nuclear receptors and AP-1 (11, 12). More recently, ASC-2 was found to exist in a steady-state complex of approximately 2 MDa that contains histone H3-lysine 4-specific methyltransferase enzymes, and chromatin immunoprecipitation experiments demonstrated that this complex specifically binds to the retinoid-responsive β-retinoic acid response element and p21WAF1 promoter regions in a ligand-dependent manner (Goo et al., unpublished data).
Interestingly, ASC-2 contains two nuclear receptor interaction domains (12), both of which are dependent on the integrity of their core LXXLL sequences (16, 19). The C-terminal LXXLL motif specifically interacts with the liver X receptors (LXRα and LXRβ), whereas the N-terminal motif binds a broad range of nuclear receptors (12).
In this report, we show that inhibition of ASC-2 recruitment to nuclear receptors by using a dominant negative fragment of ASC-2 encompassing the N-terminal LXXLL motif results in a plethora of developmental and phenotypic abnormalities in mice, including problems with eye, heart, motor activities, and fat metabolism in liver. In particular, the ocular anomaly provides a genetic and histopathological basis for posterior lenticonus with cataract. In addition, our results clearly establish the importance of ASC-2 in nuclear receptor function in vivo.
MATERIALS AND METHODS
Generation of transgenic mice.
For generation of transgenic mice, hemagglutinin (HA)-tagged DN1, a potent dominant negative fragment of ASC-2 encompassing the N-terminal LXXLL motif, and DN1/m, in which the LXXLL motif was mutated to LXXAA to abolish the receptor interactions, were cloned into mammalian expression vector pCAGGS (18) containing the chicken β-actin promoter linked to a human cytomegalovirus immediate-early enhancer. These plasmids were microinjected into fertilized mouse eggs of strain FVB. The genotype was determined by PCR and Southern analysis of genomic DNA of tail biopsies. The primers used for genotyping were 5′-CTCGAGATGGCCTCCTACCCTTATG-3′ and 5′-CTAATCTTGCTGACTATTTTTCTTC-3′. Five and four lines of transgenic founders expressing DN1 and DN1/m, respectively, in various different tissues were obtained, as demonstrated for transgenic expression by Western blot analysis or immunohistochemistry with anti-HA antibody.
Single-cell microinjection.
Single-cell microinjection was executed as described previously (8). The reporter construct consisted of the β-retinoic acid response element promoter driving the expression of green fluorescent protein (β-RARE-GFP). As an indicator construct for microinjection, cytomegalovirus-driven expression vector for red fluorescent protein (CMV-RFP) was used.
Chromatin immunoprecipitation.
Primary mouse embryonic fibroblasts (MEF) from wild-type, DN1-transgenic, and DN1/m-transgenic mice were prepared from embryos and cultured in Dulbecco's modified Eagle's medium with 10% fetal bovine serum. These cells or 293T cells transfected with the DN1 or DN1/m expression vector were treated with 0.1 μM 9-cis-retinoic acid for 40 min. Soluble chromatin from these cells was prepared and immunoprecipitated with the indicated antibodies, as recently described (22). The final DNA extractions were amplified by using a pair of primers that encompass the β-retinoic acid response element region and generate a 288-bp PCR product. The primers used were 5′-AAGCTCTGTGAGAATCCTG-3′ and 5′-GGATCCTACCCCGACGGTG-3′.
Histological examination and immunohistochemistry.
Organs were excised, fixed with 10% formaldehyde, embedded in paraffin, sectioned in 4-μm slices and stained with hematoxylin and eosin as described previously (21). The organs examined were eyes, liver, kidney, heart, lung, spleen, stomach, brain, pituitary gland, gall bladder, and adrenal gland from mouse embryos and postnatal animals.
To measure cellular proliferation in embryonic eyes, pregnant mice were injected intraperitoneally with bromodeoxyuridine (BrdU; Roche) 2 h before necropsy. Embryos were isolated and fixed in 10% formalin. Then 4-μm paraffin sections were blocked in 2.5% bovine serum albumin and incubated with mouse monoclonal antibody against BrdU. After washing in phosphate-buffered saline, sections were incubated with fluorescein isothiocyanate-conjugated anti-mouse immunoglobulin secondary antibody (Pierce) and washed with phosphate-buffered saline containing 4′,6′-diamidino-2-phenylindole (DAPI) (Sigma).
RESULTS AND DISCUSSION
DN1, a specific inhibitor of ASC-2.
DN1 encodes ASC-2 residues 849 to 929 and contains the N-terminal LXXLL motif. DN1 exerted a potent dominant negative effect on transactivation by retinoid and other nuclear receptors, whereas DN1/m, in which the LXXLL motif was mutated to LXXAA to disable the receptor interactions, was inert (Fig. 1A and data not shown). It is important to note that DN1 had no significant effect on the basal level of transactivation by the retinoic acid receptor. In addition, DN1 did not significantly repress transactivation mediated by Gal4 alone or the Gal4-VP16 fusion protein (Fig. 1B). Similarly, DN1 had no dominant negative effects on transactivation by other transcription factors, including AP-1 and NF-κB (data not shown). Importantly, DN1-mediated repression was recovered by overexpressed ASC-2 but not by two well-characterized LXXLL-type coactivators, SRC-1 and TRAP220 (16, 19) (Fig. 1A). Similar results were also obtained with single-cell microinjections (Fig. 1C).
FIG. 1.
DN1 specifically impairs the interactions of nuclear receptors with ASC-2. (A and B) A retinoid-responsive β-retinoic acid response element (RARE)-thymidine kinase (TK)-luciferase (Luc) or Gal4-TK-Luc reporter construct was cotransfected into HeLa cells along with a lacZ expression vector (100 ng) and expression vectors for DN1, DN1/m, ASC-2, SRC-1, TRAP220, Gal4/N, and Gal4/VP16, as indicated. Solid and shaded bars indicate the absence and presence of 0.1 μM 9-cis-retinoic acid (RA), respectively. Normalized luciferase expression from triplicate samples was calculated relative to LacZ expression. (C) Photographs of red fluorescent protein (RFP)-positive injected cells and the corresponding pattern of GFP-positive cells microinjected either with vector alone or with expression vectors for DN1, ASC-2, and SRC-1. The presence or absence of 0.1 μM 9-cis-retinoic acid (RA) is indicated. The number of cells that expressed GFP was counted relative to the total number of RFP-positive cells and expressed as a percentage of the green-fluorescing cells, as indicated in the right panel. Experiments were repeated twice with similar results, with >200 cells injected; error bars indicate 2 standard errors.
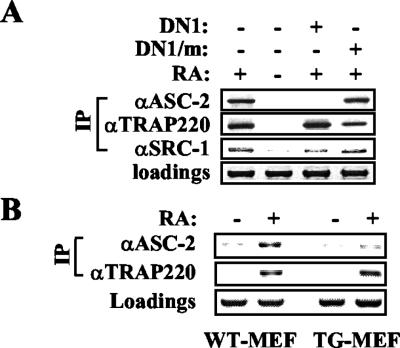
In chromatin immunoprecipitation experiments, DN1 but not DN1/m inhibited retinoid-dependent recruitment of ASC-2 to the retinoid-responsive retinoic acid receptor β2 and p21WAF1 promoters (Fig. 2A and data not shown). In contrast, both DN1 and DN1/m had no effect on the retinoid-dependent recruitment of SRC-1 and TRAP220. These results, along with our recent finding that DN1 but not DN1/m blocks retinoid-dependent recruitment of the whole ASC-2 complex (Goo et al., unpublished data), strongly suggest that DN1 exerts its repressive effects by specifically blocking the interactions of the endogenous ASC-2 with nuclear receptors.
FIG. 2.
(A) 293 cells were cotransfected with the β-retinoic acid response element-thymidine kinase-luciferase reporter construct and expression vectors for DN1 and DN1/m in either the absence or presence of 0.1 μM 9-cis-retinoic acid (RA), as indicated. Chromatin from these cells was isolated and immunoprecipitated (IP) with anti-ASC-2, anti-TRAP220, and anti-SRC-1 antibodies. The β-retinoic acid response element region of the reporter gene present in the immunoprecipitated samples was amplified by PCR, and input PCR is shown for loading controls. (B) Chromatin from MEF cells isolated from wild-type (WT) and DN1-transgenic (TG) mice was subjected to chromatin immunoprecipitation experiments. Results for DN1/m-transgenic MEF cells were identical to those for wild-type MEF cells (data not shown).
Coping with expected embryonic lethality with transgenic mice expressing DN1.
Gene targeting approaches to elucidate the role of many coactivators in mice have often been hampered by early embryonic lethality or functional redundancy. From the multiplicity of the ASC-2 target transcription factors, the lack of homologues, and its abundant expression in early embryos (1, 10, 11, 12, 14, 24), deletion of the ASC-2 gene in mice was predicted to be lethal at the early embryonic stage.
To circumvent this potential lethality problem as well as to unravel the nuclear receptor-specific function of ASC-2 among its multitude of in vivo functions, we took an alternative approach to expressing DN1, a dominant negative fragment of ASC-2 in mice. Thus, any phenotype in DN1-expressing transgenic mice not observed with DN1/m-expressing mice should reflect the in vivo function of ASC-2 specific to DN1-interacting nuclear receptors. The ubiquitously active β-actin promoter was exploited to obtain five independent transgenic founder mouse lines that expressed DN1 in various tissues examined (designated DN1-transgenic). Consistent with the cell-based results, retinoid-dependent recruitment of ASC-2 to the retinoic acid response element from the retinoic acid receptor β2 promoter was significantly decreased in MEF from DN1-transgenic mice relative to controls, whereas no such effect was observed with TRAP220 (Fig. 2B).
These transgenic mice showed a variety of pathological anomalies in many different organs (Table 1). Importantly, these transgenes were not lethal except for the early death of DN1-transgenic mouse 87 with heart failure (within 2 months after birth). The defects included incomplete septum closure as well as thrombosis and hypertrophy of the left atrium of the heart, unusually swollen pituitary and adrenal glands, impaired motor activities, much smaller spleen, fat-loaded liver, congested and inflammatory lung in which COX-2 expression was strongly induced (data not shown), and eyes with microphthalmia and posterior lenticonus with cataract.
TABLE 1.
Summary of multiple phenotypes observed with DN1-transgenic micea
| Abnormality | Expression in transgenic mouse line
|
||||
|---|---|---|---|---|---|
| 54 | 71 | 84 | 87 | 104 | |
| Eye | |||||
| Embryonic eye | |||||
| Microphthalmia | +* | − | +* | NA | − |
| Retinal dysplasia: presence of retinal folds | +* | − | +* | NA | − |
| Sclera locally thinner or missing | +* | − | +* | NA | − |
| Retrolenticular membrane | +* | − | +* | NA | − |
| PHPV | +* | − | +* | NA | − |
| Cataracts | +* | − | +* | NA | − |
| Adult eye (DN1 expression) | ++ | + | ++ | ++ | + |
| Posterior lenticonus | +* | − | +* | NA | − |
| Cataracts | +* | +¶ | +* | +§ | +¶ |
| Microphthalmia | +* | +¶ | +* | +§ | +¶ |
| Retinal dysplasia | +* | − | +* | NA | − |
| Sclera locally thinner or missing | +* | − | +* | NA | − |
| Retrolenticular membrane | +* | − | +* | NA | − |
| PHPV | +* | − | +* | NA | − |
| Heart | |||||
| Embryonic heart | |||||
| Ventricular septal defects | ND | ND | + | ND | ND |
| Adult heart (DN1 expression) | − | ++ | + | +++ | ++ |
| Atrial thrombosis | − | + | − | + | + |
| Hypertrophy | − | + | − | + | + |
| Conduction defects | − | + | − | ND | + |
| Respiratory tract (DN1 expression) | − | + | − | + | + |
| Lung hypoplasia | − | + | − | + | + |
| Lung congestion | − | + | − | + | + |
| Lung inflammation | − | + | − | + | + |
| Liver (DN1 expression) | − | + | − | + | + |
| Fatty liver | − | + | − | + | + |
| Thymus (DN1 expression) | − | + | − | + | + |
| Thymic atrophy; agenesis | − | + | − | + | + |
| Spleen (DN1 expression) | − | + | − | + | + |
| Spleen atrophy | − | + | − | + | + |
| Gall bladder (DN1 expression) | ND | + | ND | + | + |
| Gall bladder hydrops | − | + | − | + | + |
| Kidney (DN1 expression) | − | + | − | + | + |
| Kidney hypoplasia | − | + | − | + | + |
| Adrenal gland (DN1 expression) | ND | + | ND | ND | + |
| Adrenal gland redness | − | + | − | + | + |
| Brain (DN1 expression) | + | + | + | ND | − |
| Motor defects: rota-rod activity defect | + | ND | + | ND | ND |
| Pituitary gland redness; swelling | − | + | − | + | + |
The relative level of DN1 expression is indicated (−, no expression detected). Founder lines 54, 84, and 87 exhibited complete penetrance of eye phenotype, though 71 and 104 were incomplete. The severity of the phenotypes in each line was proportional to the expression level of DN1. The founder mouse from line 87, which developed bilateral cataract and microphthalmia (§), produced only three transgene-positive pups out of 68 total progeny, possibly due to partial embryonic lethality in this line. These pups had no eyes and died from heart failure. ND, not determined. NA, not applicable due to the absence of eye. ✻, unilateral or bilateral defects; ¶, unilateral defects; PHPV, persistent hyperproliferation of primary vitreous.
Remarkably, at least four independent transgenic lines that expressed DN1/m (designated DN1/m-transgenic) had no such defects and were phenotypically indistinguishable from wild-type mice, allowing us to use both DN1/m-transgenic and wild-type mice as controls. Thus, the defects observed with DN1-transgenic mice must have resulted from the DN1-mediated impairment of the ASC-2-nuclear receptor interactions, faithfully reflecting the in vitro results (Fig. 1 and 2A). These results also demonstrate that ASC-2 is pivotal for a variety of developmental functions of nuclear receptors in vivo. Deciphering the specific nuclear receptor(s) responsible for each defect will be a major challenge due to the multiplicity of nuclear receptors that can be modulated by DN1. For instance, among the heart defects that we observed with DN1-transgenic mice, only incomplete septum closure was previously ascribed to deletion of the retinoid X receptor α gene (9), suggesting that the other heart defects may involve additional nuclear receptors.
Interestingly, the phenotypes observed were nicely correlated with the expression patterns and levels of DN1. For instance, the expression level of DN1 in the eye was significantly greater in DN1-transgenic mice 54, 84, and 87 than in DN1-transgenic mice 71 and 104 (Table 1 and Fig. 3A). Accordingly, the former group had either bilateral or unilateral eye defects as well as severe embryonic eye development problems, whereas the latter group exhibited only milder, unilateral defects and no embryonic eye anomalies (Table 1). Similarly, DN1-transgenic mouse 87, which showed the highest expression level of DN1 in the heart, died with heart failure. In contrast, DN1-transgenic mouse 54 showed no defective heart phenotype at all, consistent with the lack of DN1 expression in the heart.
FIG. 3.
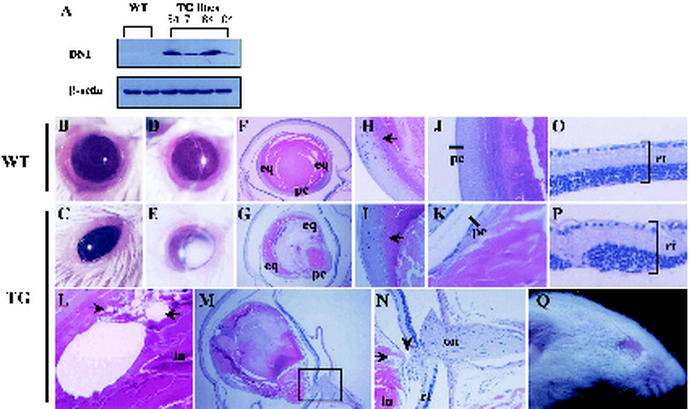
DN1-transgenic mice exhibit severe ocular defects. Expression of DN1 in the eyes of DN1-transgenic mouse lines was determined by Western blot analysis with anti-HA antibody (A). (B and C) Ocular photographs of adult wild-type (WT) mouse eye (B) and DN1-transgenic (TG) mouse with microphthalmia (C). (D and E) Slit lamp ocular photographs of the wild-type mouse eye (D) and the DN1-transgenic mouse with cataract (E). (F to P) Hematoxylin and eosin staining of sections of wild-type and DN1-transgenic mice. Compared with the wild-type mouse (F), the DN1-transgenic mouse exhibited posterior lenticonus with numerous vacuoles (L, arrow), higher cellularity of the posterior region (N, arrow), and posterior projection of lens (G). The equatorial region of the lens of the wild-type mouse (H) and DN1-transgenic mouse (I) was intact, suggesting that higher cellularity of the posterior segment is not attributed to abnormal migration of epithelial cells from the equatorial to the posterior region. Relative to the intact posterior capsule in the wild-type mouse (J, bar), however, ambiguous and defective posterior capsule was observed with the DN1-transgenic mouse (K, bar). A different plane from the DN1-transgenic mouse eye showed persistent fusion between the posterior region of the lens and the optic nerve (M and N, arrowhead). (N) Higher magnification of the boxed area in M. (O and P) Retinas from the wild-type mouse (O) and a DN1-transgenic mouse with retinal dysplasia (P). In some severe cases, mice were born without eyes (Q, line 87). eq, equatorial region; ln, lens; on, optic nerve; pc, posterior lens capsule; rt, retina.
DN1-transgenic mice as a novel animal model for posterior lenticonus with cataract.
Cataract, a disease in which the crystalline lens becomes opaque, is the leading cause of blindness (7). Posterior lenticonus, a rare congenital abnormality (1 out of 100,000) with a conical projection of the posterior lens capsule, is usually accompanied by cataract (4). However, posterior lenticonus is a common cause of unilateral infantile cataract and is responsible for a significant proportion of childhood cataracts (approximately 14% of unilateral cases and approximately 9% of bilateral cases) (20).
Interestingly, all five transgenic lines expressed DN1 protein in their eyes (Table 1 and Fig. 3A) and developed microphthalmia and cataracts (Fig. 3B to E). In addition, histological examination of affected lenses from at least two independent mice (54 and 84 in Table 1) indicated posterior lenticonus with numerous vacuolations (Fig. 3G and L), covered by relatively intact migration of equatorial epithelial cells (Fig. 3I) but defective posterior lens capsule (Fig. 3K) in 2-month-old adult mice. Posterior lenticonus is known to occur as a sporadic condition, and its etiology has been widely debated due to the rare occurrence of this condition and the difficulties in obtaining clinical samples (4). However, its possible pathogenic causes have been speculated to include overgrowth of posterior lens fibers, uneven pull on the zonules during embryogenesis, and hyaloid artery traction.
In our DN1-transgenic animals, detailed serial sections of the eyes revealed a persistent connection between the optic nerve and projected posterior segment (Fig. 3M and N). Furthermore, DN1-transgenic mouse retinas exhibited marked disorganization of cellular layer patterns of the neural retina (Fig. 3O and P). From these results, we suggest that posterior lenticonus with cataract derives from persistent traction between the optic nerve and posterior segment of the lens, resulting in rupture or protrusion of a weak and thin part of the posterior lens capsule.
It should be noted that posterior lenticonus is responsible for a significant portion of childhood cataracts, implying congenital or early-onset defects (20). With our DN1-transgenic animals, ocular abnormalities were also observed when newborns opened their eyes, which prompted us to examine their embryonic eye development. Gross anatomical examination of embryonic eyes (Fig. 4A and B) and hematoxylin and eosin staining of eyes from embryonic day 13.5 (E13.5) to E17.5 (Fig. 4C to H) revealed that eyes from DN1-transgenic mice had abnormal lens development. The size of the palpebral fissure was reduced in DN1-transgenic mouse embryo eyes (Fig. 4B), which may explain the absence of eyes in a severe case (Fig. 3Q).
FIG. 4.
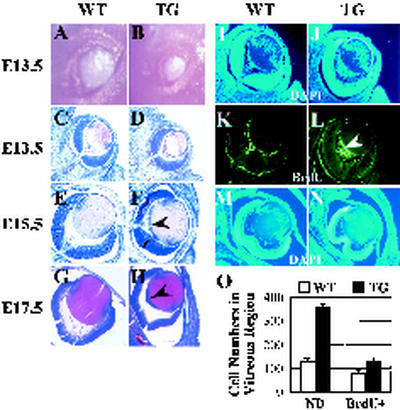
Ocular defects of DN1-transgenic mice in embryonic stage. (A and B) Embryonic eyes of a wild-type (WT) mouse (A) and a DN1-transgenic (TG) mouse with microphthalmia (B). (C to H) Hematoxylin and eosin staining of wild-type (C, E, and G) and DN1-transgenic (D, F, and H) mice at different embryonic stages. Persistent hyperproliferation of primary vitreous was evident at E13.5 in the eyes of the DN1 transgenic mouse (D) compared to the wild type (C). Primary vitreous was fully regressed from E15.5 (E and M) to E17.5 (G) in eyes from the wild-type but not the DN1-transgenic mouse (F and H, arrowhead). DAPI staining revealed abnormal nuclear density in eyes from E13.5 and E15.5 DN1-transgenic mice (J and N) compared to the wild type (I and M). Furthermore, abnormal retinal fold fused with the posterior region of the lens was observed (N), suggesting the cause of retinal dysplasia in adults. (K and L) BrdU incorporation was increased in eyes from DN1-transgenic mice (L, arrowhead). (O) Quantitative representation of DAPI (I and J) and BrdU (K and L) staining. ND, nuclear density; BrdU+, positive BrdU staining.
In normal eye development, the primary vitreous (retrolenticular membrane) disappears by E14 (Fig. 4C, E, and G) (9). However, the primary vitreous membrane of DN1-transgenic mice did not regress (Fig. 4D, F, and H) and even developed to fuse to the posterior capsule of the lens in adulthood (Fig. 3M and N). To further examine the proliferation and differentiation status of lens cells, DAPI staining and BrdU incorporation were performed. At E15.5 and E17.5, most of the lens cell nuclei had migrated from the periphery region towards the center in wild-type animals (Fig. 4E, G, and M). In contrast, higher nucleus density near the posterior surface of the lens and vitreous cavity was observed with the lens of E13.5 DN1-transgenic mice (Fig. 4D and J), which persisted until E17.5 in the lenses of DN1-transgenic mice (Fig. 4F, H, and N). These results suggest that the normal proliferation and differentiation process of the embryonic lens cells from DN1-transgenic animals are profoundly impaired.
Consistent with these results, the near posterior surface of the lens and vitreous cavity region of DN1-transgenic mice showed higher DNA replication rates, as indicated by BrdU incorporation (Fig. 4L and O). Interestingly, this phenotype closely resembles that of persistent hyperplastic primary vitreous (5), a congenital developmental anomaly of human eyes. Taken together, these results further support the hypothesis that traction on the posterior capsule of the lens by remnants of the primary vitreous and the hyaloid vascular system at embryonic stages may cause posterior lenticonus with cataract after birth.
Defect in retinoic acid receptor signaling in DN1-transgenic mice.
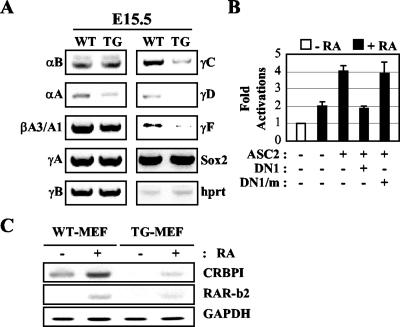
Cataract in childhood is caused not only by posterior lenticonus (20) but also by dysregulation or mutation of various crystallin genes, the hallmark of normal lens differentiation (7). The αB and γF crystallin genes were previously shown to contain functional retinoic acid response elements (6, 23). Other γ-crystallin genes were also suggested to have putative retinoic acid response elements (13). Indeed, reverse transcription-PCR analyses of RNAs from the eyes of E15.5 wild-type and DN1-transgenic mice (17) showed that the mRNA levels of the αB, γC, γD, and γF crystallin genes were significantly downregulated in DN1-transgenic mice (Fig. 5A). These results newly identified the γC and γD-crystallin genes as functional retinoid-responsive genes. In contrast, expression of other crystallin genes that are not retinoid responsive, notably βA3/A1, was unaffected. Similarly, transcription of luciferase reporter constructs driven by the retinoid-responsive promoters of the αB and γF crystallin genes (6, 23) was activated by retinoids in cotransfections and was repressed by coexpressed DN1 but not DN1/m (Fig. 5B and data not shown). Reverse transcription-PCR analyses of RNAs from MEF from wild-type and DN1-transgenic mice revealed that the mRNA levels of the retinoid-responsive genes CRBPI and retinoic acid receptor β2 (3) were compromised in DN1-transgenic mice (Fig. 5C).
FIG. 5.
Defect in retinoid signaling in DN1-transgenic mice. (A) Reverse transcription-PCR analyses of RNAs from the eyes of E15.5 wild-type (WT) and DN1-transgenic (TG) mice were executed as previously described (17). hprt, hypoxanthine-guanine phosphoribosyl transferase. (B) The luciferase reporter construct driven by the retinoid-responsive γF-crystallin gene promoter was cotransfected into CV1 cells along with the LacZ expression vector (100 ng) and expression vectors for DN1, DN1/m, and ASC-2. Open and solid bars indicate the absence and presence of 0.1 μM 9-cis-retinoic acid (RA), respectively. Normalized luciferase expression from triplicate samples was calculated relative to LacZ expressions. (C) Reverse transcription-PCR analyses of RNAs from MEF of wild-type and DN1-transgenic mice treated with either vehicle or 0.1 μM 9-cis-retinoic acid were executed as described previously (17). RAR-b2, retinoic acid receptor β2; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Interestingly, deletion of the retinoid X receptor α gene was previously shown to result in development of cataracts and incomplete septum closure in the embryonic heart (9), phenocopying some of the defects observed with our DN1-transgenic mice. In addition, a phenotypic synergy was observed with ocular defects when the retinoid X receptor α mutation was introduced into retinoic acid receptor α or retinoic acid receptor γ mutant backgrounds (9). However, it should be noted that the embryonic lethality and functional redundancy in these retinoid X receptor α-deficient mice made it impossible to evaluate ocular defects of the adult stage, such as posterior lenticonus, as described in this report. Overall, our findings strongly suggest that retinoic acid receptor signaling is impaired in DN1-transgenic mice and that the nuclear receptors targeted by DN1 in eye development likely include retinoid receptors.
Our strategy of expressing a specific dominant negative fragment in mice is an excellent alternative to gene targeting approaches, which often result in functional redundancy or early embryonic lethality. More importantly, this approach should facilitate the dissection of a specific function of multifunctional coactivators such as ASC-2, CBP/p300, and SRC-1. As exemplified in this report, DN1-transgenic and DN1/m-transgenic animals enabled us to study the physiological role of ASC-2 with a subset of nuclear receptors that bind the N-terminal LXXLL motif of ASC-2 without complications from a multitude of other nuclear receptors and transcription factors. Similarly, transgenic mouse lines that express another dominant negative fragment of ASC-2 containing the second LXXLL motif, which specifically interacts with the orphan nuclear receptors LXRα and LXRβ (12), rapidly accumulated a large amount of cholesterol in the liver (our unpublished results), as expected from the known function of LXRs to stimulate the transcription of genes encoding transporters involved in cholesterol efflux (2).
We conclude that ASC-2 is likely an essential transcriptional coactivator of many nuclear receptors in vivo. Importantly, our studies provide novel insights into the molecular mechanism for the pathogeneses of human posterior lenticonus with cataract (4, 5, 7). Likewise, these mice will prove to be useful as a unique in vivo model for a variety of pathogeneses and diseases that involve ASC-2-interacting nuclear receptors.
Acknowledgments
We thank David Moore, Ronald A. DePinho, Jung-Eun Jang, and Yu-Jin Kim for critical reading of the manuscript and excellent technical contributions.
This work was supported by grants from Postech Biotech Center (3PD02002) and GenoCheck, Inc. (to J.W.L.) and 21C Frontier Functional Human Genome Project from MOST, NRL (M1-0203-00-0108), KOSEF (SRC-MTRC), and Samsung Biomedical Research Institute (to H.W.L.). C.C. is the recipient of a BK21 Research Fellowship from KME.
The first two authors contributed equally.
REFERENCES
- 1.Caira, F., P. Antonson, M. Pelto-Huikko, E. Treuter, and J. A. Gustafsson. 2000. Cloning and characterization of RAP250, a novel nuclear receptor coactivator. J. Biol. Chem. 275:5308-5317. [DOI] [PubMed] [Google Scholar]
- 2.Chawla, A., J. J. Repa, R. M. Evans, and D. J. Mangelsdorf. 2001. Nuclear receptors and lipid physiology: opening the X-files. Science 294:1866-1870. [DOI] [PubMed] [Google Scholar]
- 3.Chiva, H., J. Clifford, D. Metzger, and P. Chambon. 1997. Distinct retinoid X receptor-retinoic acid receptor heterodimers are differentially involved in the control of expression of retinoid target genes in F9 embryonal carcinoma cells. Mol. Cell. Biol. 17:3013-3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gibbs, M. L., M. Jacobs, A. O. Wilkie, and D. Taylor. 1993. Posterior lenticonus: clinical patterns and genetics. J. Pediatr. Ophthalmol. Strabismus 30:171-175. [DOI] [PubMed] [Google Scholar]
- 5.Goldberg, M. F. 1997. Persistent fetal vasculature (PFV): an integrated interpretation of signs and symptoms associated with persistent hyperplastic primary vitreous (PHPV). LIV Edward Jackson Memorial Lecture. Am. J. Ophthalmol. 124:587-626. [DOI] [PubMed] [Google Scholar]
- 6.Gopal-Srivastava, R., A. Cvekl, and J. Piatigorsky. 1998. Involvement of retinoic acid/retinoid receptors in the regulation of murine αB-crystallin/small heat shock protein gene expression in the lens. J. Biol. Chem. 273:17954-17961. [DOI] [PubMed] [Google Scholar]
- 7.Javitt, J. C., F. Wang, and S. K. West. 1996. Blindness due to cataract: epidemiology and prevention. Annu. Rev. Public Health 17:159-177. [DOI] [PubMed] [Google Scholar]
- 8.Jhun, B. H., D. W. Rose, B. L. Seely, L. Rameh, L. Cantley, A. R. Saltiel, and J. M. Olefsky. 1994. Microinjection of the SH2 domain of the 85-kilodalton subunit of phosphatidylinositol 3-kinase inhibits insulin-induced DNA synthesis and c-fos expression. Mol. Cell. Biol. 14:7466-7475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kastner, P., J. M. Grondona, M. Mark, A. Gansmuller, M. LeMeur, D. Decimo, J. L. Vonesch, P. Dolle, and P. Chambon. 1994. Genetic analysis of retinoid X receptor alpha developmental function: convergence of retinoid X receptor and retinoic acid receptor signaling pathways in heart and eye morphogenesis. Cell 78:987-1003. [DOI] [PubMed] [Google Scholar]
- 10.Ko, L., G. R. Cardona, and W. W. Chin. 2000. Thyroid hormone receptor-binding protein, an LXXLL motif-containing protein, functions as a general coactivator. Proc. Natl. Acad. Sci. USA 97:6212-6217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee, S. K., S. L. Anzick, J. E. Choi, L. Bubendorf, X. Y. Guan, Y. K. Jung, O. P. Kallioniemi, J. Kononen, J. M. Trent, D. Azorsa, B. H. Jhun, J. H. Cheong, Y. C. Lee, P. S. Meltzer, and J. W. Lee. 1999. A nuclear factor, ASC-2, as a cancer-amplified transcriptional coactivator essential for ligand-dependent transactivation by nuclear receptors in vivo. J. Biol. Chem. 274:34283-34293. [DOI] [PubMed] [Google Scholar]
- 12.Lee, S. K., S. Y. Jung, Y. S. Kim, S. Y. Na, Y. C. Lee, and J. W. Lee. 2001. Two distinct nuclear receptor-interaction domains and CREB-binding protein-dependent transactivation function of activating signal cointegrator-2. Mol. Endocrinol. 15:241-254. [DOI] [PubMed] [Google Scholar]
- 13.Lengler, J., E. Krausz, S. Tomarev, A. Prescott, R. A. Quinlan, and J. Graw. 2001. Antagonistic action of Six3 and Prox1 at the gamma-crystallin promoter. Nucleic Acids Res. 29:515-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahajan, M. A., and H. H. Samuels. 2000. A new family of nuclear receptor coregulators that integrate nuclear receptor signaling through CREB-binding protein. Mol. Cell. Biol. 20:5048-5063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mangelsdorf, D. J., C. Thummel, M. Beato, P. Herrlich, G. Schutz, K. Umesono, B. Blumberg, P. Kastner, M. Mark, P. Chambon, and R. M. Evans. 1995. The nuclear receptor superfamily: the second decade. Cell 83:835-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKenna, N. J., and B. W. O'Malley. 2000. From ligand to response: generating diversity in nuclear receptor coregulator function. J. Steroid Biochem. Mol. Biol. 74:351-356. [DOI] [PubMed] [Google Scholar]
- 17.Nishiguchi, S., H. Wood, H. Kondoh, R. Lovell-Badge, and V. Episkopou. 1998. Sox1 directly regulates the gamma-crystallin genes and is essential for lens development in mice. Genes Dev. 12:776-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niwa, H., K. Yamamura, and J. Miyazaki. 1991. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108:193-199. [DOI] [PubMed] [Google Scholar]
- 19.Rosenfeld, M. G., and C. K. Glass. 2001. Coregulator codes of transcriptional regulation by nuclear receptors. J. Biol. Chem. 276:36865-36868. [DOI] [PubMed] [Google Scholar]
- 20.Russell-Eggitt, I. M. 2000. Non-syndromic posterior lenticonus a cause of childhood cataract: evidence for X-linked inheritance. Eye 14:861-863. [DOI] [PubMed] [Google Scholar]
- 21.Serrano, M., H. Lee, L. Chin, C. Cordon-Cardo, D. Beach, and R. A. DePinho. 1996. Role of the INK4a locus in tumor suppression and cell mortality. Cell 85:27-37. [DOI] [PubMed] [Google Scholar]
- 22.Shang, Y., X. Hu, J. DiRenzo, M. A. Lazar, and M. Brown. 2000. Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell 103:843-852. [DOI] [PubMed] [Google Scholar]
- 23.Tini, M., G. Otulakowski, M. L. Breitman, L. C. Tsui, and V. Giguere. 1993. An everted repeat mediates retinoic acid induction of the gamma F-crystallin gene: evidence of a direct role for retinoids in lens development. Genes Dev. 7:295-307. [DOI] [PubMed] [Google Scholar]
- 24.Zhu, Y., L. Kan, C. Qi, Y. S. Kanwar, A. V. Yeldandi, M. S. Rao, and J. K. Reddy. 2000. Isolation and characterization of peroxisome proliferator-activated receptor (PPAR) interacting protein (PRIP) as a coactivator for PPAR. J. Biol. Chem. 275:13510-13516. [DOI] [PubMed] [Google Scholar]