Abstract
Homologous genes and gene products often have redundant physiological functions. Members of the tumor necrosis factor (TNF) family of cytokines can signal activation, proliferation, differentiation, costimulation, inhibition, or cell death, depending on the type and status of the target cell. TNF, lymphotoxin α (LTα), and LTβ form a subfamily of a larger family of TNF-related ligands with their genes being linked within a compact 12-kb cluster inside the major histocompatibility complex locus. Singly TNF-, LTα-, and LTβ-deficient mice share several phenotypic features, suggesting that TNF/LT signaling pathways may regulate overlapping sets of target genes. In order to directly address the issue of redundancy of TNF/LT signaling, we used the Cre-loxP recombination system to create mice with a deletion of the entire TNF/LT locus. Mice with a triple LTβ/TNF/LTα deficiency essentially manifest a combination of LT and TNF single-knockout phenotypes, except for microarchitecture of the spleen, where the disorder of lymphoid cell positioning and functional T- and B-cell compartmentalization is severer than that found in TNF or LT single-knockout mice. Thus, our data support the notion that TNF and LT have largely nonredundant functions in vivo.
The cytokines lymphotoxin α (LTα), LTβ, and tumor necrosis factor (TNF) are structurally related members of the TNF ligand family (37) encoded by genes tightly linked within the major histocompatibility complex gene complex (7, 36, 44, 52).
TNF is produced by a variety of lymphoid and nonlymphoid cells as either a membrane-bound or soluble homotrimer, either of which interacts with the two TNF receptors, TNRFp55 and TNRFp75 (37). LTα and LTβ expression is restricted to activated lymphocytes, NK cells (67), and a subset of CD4+ CD3− cells involved in organogenesis of lymph nodes (LN) and Peyer's patches (PP) (42, 70). LTα lacks the transmembrane domain and is secreted as a homotrimer, yet it can be retained on the cell surface in heterotrimeric complexes with LTβ, a type II transmembrane protein (7). While LTα3 shares receptors with TNF, the predominant surface LTα1β2 heterotrimer signals through a distinct receptor, LTβR (9).
Studies with gene-targeted mice deficient for TNRFp55 (51, 56), TNRFp75 (15), LTα (5, 12), TNF (33, 38, 49), or TNF/LTα (2, 16) have highlighted the distinct functions of TNF and LTα in vivo. TNF is involved in host defense against invading pathogens and in regulation of both proinflammatory and anti-inflammatory responses (38). TNF is also essential for the generation of adaptive B-cell immune responses (49, 51, 56). LTα together with LTβ (acting through the LTβ receptor) is crucial for the development of LN and PP and for the organization of the white pulp of the spleen (1, 5, 12, 19, 32), as well as for expression of lymphoid tissue chemokines (48, 60) and for NK and dendritic cell (DC) recruitment to lymphoid organs (26, 69). The function of LTβR signaling in maintaining the structural integrity of the spleen was also shown to be important for efficient antiviral response (6, 30). Full development of PP, in addition to LTβR signaling, requires signaling via TNRFp55 (47, 53). Each of the three cytokines appears to be indispensable for the formation of B-cell follicles, germinal centers (GCs), and follicular DC (FDC) networks (1, 19, 32, 40, 49).
At least four signaling pathways should be considered in relation to disrupted TNF and LT signaling: TNF-TNRFp55, TNF-TNRFp75, LTα1LTβ2-LTβR, and LTα3-TNRFp55. Additional pathways, suggested by in vitro studies, are LTα2LTβ1-TNRFp55 and LTα3-TNRFp75, although their in vivo contributions remain to be demonstrated. LTβ3 has never been observed under physiological conditions, and no receptor has been described for this hypothetical ligand. Recently, a limited functional role of LIGHT-LTβR interaction was reported in studies utilizing LIGHT transgenic (66) and LTβ/LIGHT double-knockout mice (59).
In order to address the possibility of gene redundancy common for multigene families and of potential cross talk between TNF and LT signaling pathways, we created mice with a deletion of the entire TNF/LT locus (TNF/LTΔ3 mice). Grossly, these mice do not show additional major defects compared to the expected combination of the previously reported LTα, LTβ, and TNF knockout phenotypes. Nevertheless, the independent effects of single LTα, LTβ, and TNF deficiencies add up in the spleens of TNF/LTΔ3 mice, resulting in quantitative differences in spleen morphology and gene expression.
MATERIALS AND METHODS
Gene targeting.
Genomic clones and construction of the multipurpose targeting vector pTV2-TK, embryonic stem (ES) cell transfection, selection, and screening of homologous recombinants have been described previously (1, 45). One ES clone (out of 400) that contained integration of all four loxP sites was expanded and transiently transfected in vitro with the pIC-Cre expression vector (24). After transfection 380 ES colonies were picked and expanded and were then screened for the loss of the neo marker by growing them in the presence of G418. The structure of the deletion in G418-sensitive ES clones was determined by Southern blotting using BamHI digestion and hybridization with the SphI-PstI fragment from the LTβ promoter (Fig. 1). Out of 14 ES cell clones analyzed, seven clones had the complete deletion in the targeted TNF/LT locus and two of them were injected into C57BL/6 blastocysts (29) to obtain chimeric mice and subsequently the germ line transmission of the targeted allele. The following oligonucleotides were used for routine genotyping of TNF/LTΔ3 mice by PCR: gtype1, 5′-CGG GTC TCC GAC CTA GAG ATC; gtype2, 5′-CCA CAA CAG GTG TGA CTG TCT C; and gtype4, 5′-CCA CTT GTC CAG TGC CTG CTC.
FIG. 1.
Generation of TNF/LTΔ3 mice. (A) Targeting strategy used for deletion of the entire TNF-LT locus. (B) Southern blot analysis of genomic DNA from mouse tail biopsies: lane 1, wild type; lane 2, heterozygote; and lane 3, mutant. (C) PCR genotyping of TNF/LTΔ3 mice. wt, wild type; mut, mutant. (D) RT-PCR assay of total RNA extracted from concanavalin A-activated splenocytes derived from TNF/LTΔ3 (lanes 1 to 4) or wild-type (lanes 6 to 9) mice; lane 5, molecular weight standard.
Mice.
Heterozygous (LTβ/TNF/LTα)Δ/+ mice were bred to obtain homozygous, triply deficient (TNF/LTΔ3) mice. The mice were then backcrossed to the C57BL/6 background for 6 generations, subjected to embryo rederivation at Taconic, Inc. (Germantown, N.Y.), and further backcrossed to N12 (overall number of backcrosses to the C57BL/6 background). LTα−/− (N12), TNF−/− (N2), TNF/LTα−/−(N6), and LTβΔ/Δ (N12) mice have been described previously (1, 12, 16, 38, 49). All mice were housed under specific-pathogen-free conditions.
RNA analysis.
Total cellular RNA was extracted using Trizol reagent (Life Technologies, Bethesda, Md.) according to the manufacturer's instructions. One microgram of RNA was used for reverse transcriptase (RT)-PCR analysis with a Superscript II kit (BRL/Life Technologies, Rockville, Md.) and gene-specific primers to LTβ (5′-TCG GGT TGA GAA GAT CAT TGG and 5′-GCT CGT GTA CCA TAA CGA CC), LTα (5′-AAC CTG CTG CTC ACC TTG TT and 5′-CAG TGC AAA GGC TCC AAA GA), TNF (5′-CTC AGA TCA TCT TCT CAA AA and 5′-TGA CTC CAA AGT AGA CCT G), and β-actin (5′-CCA AGG TGT GAT GGT GGG AAT G and 5′-CCA GAG GCA TAC AGG GAC AGC). For Northern analysis, 10 μg of total RNA was separated on a 1.5% formaldehyde agarose gel, blotted to a Hybond N nylon membrane (Amersham, Little Chalfont, Buckinghamshire, England), and hybridized to specific probes for SPLASH, MARCO, MPO, and glyeraldehyde-3-phosphate dehydrogenase (60).
Immunohistochemistry.
Immunohistochemistry was performed as described earlier (1, 47). The following rat anti-mouse monoclonal antibodies were used: anti-CD3, anti-immunoglobulin D (IgD), anti-B220 (PharMingen), ER-TR7 (Biogenesis, Poole, United Kingdom), MOMA1 (Research Diagnostics, Flanders, N.J.), and FDC-M1 (generously provided by M. Kosco-Vilbois [Serono Pharmaceutical Research Institute, Geneva, Switzerland]). Mouse anti-rat IgG conjugated with horseradish peroxidase was obtained from Jackson ImmunoResearch; streptavidin conjugated with alkaline phosphatase was from Sigma. Horseradish peroxidase was developed with a peroxidase substrate kit, and alkaline phosphatase (AP) was developed with the Vector Blue substrate kit (both from Vector Laboratories, Burlingame, Calif.); sections were counterstained with Mayer's hematoxylin if indicated, mounted with glycerol-gelatin, and documented with the Axioskop 2 microscope system (Carl Zeiss, Thornwood, N.Y.) equipped with a charge-coupled device camera.
Immunizations.
Mice were immunized intraperitoneally (i.p.) with 200 μl of phosphate-buffered saline (PBS) containing 108 sheep red blood cells (SRBC). Eight to 10 days after immunization mice were sacrificed and spleens were prepared for immunohistochemical staining.
Infections.
Mice were injected either i.p. or in the back skin with the indicated number of live Listeria monocytogenes bacteria (strain EGD Sv 1/2a) in 0.2 ml of PBS. Indicated numbers of Salmonella enterica serovar Typhimurium bacteria (strain TMLR 66) were administered per os in 0.3 ml of PBS to mice that were deprived of food and maintained on 1% sodium bicarbonate-water for 24 h. Animals were monitored twice a day and euthanatized when moribund.
Specific antibody responses.
Blood was taken from the eye on days 8, 14, and 23 postimmunization. Specific antibodies were measured and analyzed as previously described (18). In brief, Maxisorp microtiter plates (Nunc, Roskilde, Denmark) were coated overnight with SRBC (100 μl at 5 × 107/ml) that were suspended in 0.25% glutaraldehyde in PBS. Thereafter, the plates were blocked with 2% bovine serum albumin in PBS for 2 h at room temperature. Diluted mouse sera were then added and incubated overnight at 4°C. AP-conjugated goat anti-mouse IgG (Southern Biotechnology Associates Inc., Birmingham, Ala.) was diluted 1:2,000, and 100 μl was added and incubated at 4°C for 1 h. Plates were washed three times with PBS after the step. The final washing was followed by addition of the AP substrate p-nitrophenyl phosphate (Sigma Chemical Co.) at 1 mg/ml. The reaction was stopped with 1.5 M NaOH. Absorbance was read at 405 nm.
Flow cytometry.
Flow cytometry analysis of erythrocyte-depleted, single-cell suspensions from thymus, spleen, peripheral blood, bone marrow, or peritoneal cavity lavage was performed as described earlier (19).
RESULTS AND DISCUSSION
Genetic inactivation of the entire TNF/LT locus in mice.
The murine genes for LTα, LTβ, and TNF are tightly clustered within the 12-kb TNF/LT locus (34, 52). A Cre-loxP-mediated gene-targeting approach (24, 58) was used to create an 8.4-kb deletion across the locus to disrupt all three genes. To do so, we transfected ES cells with the targeting vector pTV2-TK (1) and examined 43 positive clones that contained a correct single-copy insertion of the neo cassette and also contained LoxP site 1 according to Southern analysis (Fig. 1A). The following distribution of partial recombination events was found. Twelve clones did not contain any additional LoxP sites and thus could not be used for Cre-mediated gene inactivation. Fifteen clones contained LoxP site 2 but not sites 3 and 4; these clones were subsequently used to generate LTβΔ/Δ mice (1). Additionally, 15 clones contained LoxP sites 2 and 3 but not site 4; these clones were subsequently used to generate (LTβ/TNF)Δ/Δ double-knockout mice (35). Finally, a single clone (no. 129) containing all four loxP motifs from the targeted construct (see Fig. 1A and data not shown) was subjected to Cre-mediated recombination in vitro. Two clones with the desired deletion between the two most distal loxP sites were identified by Southern blot analysis (data not shown) and were used to generate chimeric mice, which then transmitted the targeted allele into the germ line. As a result, we created an 8.4-kb deletion in an allele of the TNF/LT locus that encompassed the entire tnf gene, as well as the last coding exons of both ltα and ltβ genes (Fig. 1A). The remaining portions of ltα and ltβ genes could not encode functional proteins. Upon heterozygous breeding the homozygous TNF/LTΔ3 mice were born at the expected Mendelian frequency and appeared healthy. However, when the mutation was transferred to the C57BL/6 background (n = 12) and when a homozygous breeding colony was established, a lower breeding efficiency was consistently noted at three different animal facilities (data not shown), suggesting an as-yet-unidentified deficiency in reproduction and/or behavior.
Deletion in the TNF/LT locus was confirmed by Southern analysis (Fig. 1B). Mice were routinely genotyped using allele-specific PCR (Fig. 1C). RT-PCR analysis confirmed the absence of transcripts for TNF, LTα, and LTβ in concanavalin A-activated splenocytes from TNF/LTΔ3 mice (Fig. 1D).
Leukocyte numbers in periphery of TNF/LTΔ3 mice.
The cellular composition of thymus, bone marrow, spleen, blood, and peritoneal cavity was analyzed by hemocytometry and flow cytometry. A two- to threefold increase in the number of leukocytes was detected in the spleen and blood and peritoneal cavity of TNF/LTΔ3 and LTβ- and LTα-deficient mice, while differentials in blood and the T- to B-cell ratio in spleen were not altered (data not shown). Cell numbers in the spleen, blood, and peritoneal cavity of TNF-deficient mice were not different from those found in wild-type mice, indicating that this phenomenon is related to LT and not to TNF signaling and that it possibly reflects the absence of peripheral LN in LT-deficient mice. Although TNF, LTβ, and LTα were shown to be expressed in both embryonic and adult murine thymus (20, 52), no change in main thymocyte populations was detected in TNF/LTΔ3 mice by flow cytometry (data not shown). Thus, with regard to major leukocyte populations, TNF/LTΔ3 mice appear to closely resemble mice with disrupted LT signaling.
Peripheral lymphoid organs in TNF/LTΔ3 mice.
In view of the profound defects in the development and organization of peripheral lymphoid organs found in LTα- and LTβ-deficient mice (1, 5, 12, 32) and to a lesser extent in TNF-deficient mice (49), the development of lymphoid organs in TNF/LTΔ3 mice was analyzed in parallel with that in LTα- and LTβ-deficient and singly TNF-deficient mice. As expected, no PP and no mesenteric, brachial, axillary, inguinal, or popliteal LN could be detected upon morphological and histological inspection. Thus, this phenotype was similar to the phenotype of LTα-deficient mice (5, 12) and LTβR-deficient mice (19) but was different from that of LTβ-deficient mice (1, 32) or doubly LTβ- and TNF-deficient mice (35) (which usually develop mucosal LN) and that of TNF-deficient mice (which develop all LN).
Additional disruption of spleen architecture in TNF/LTΔ3 mice compared to that in singly deficient mice.
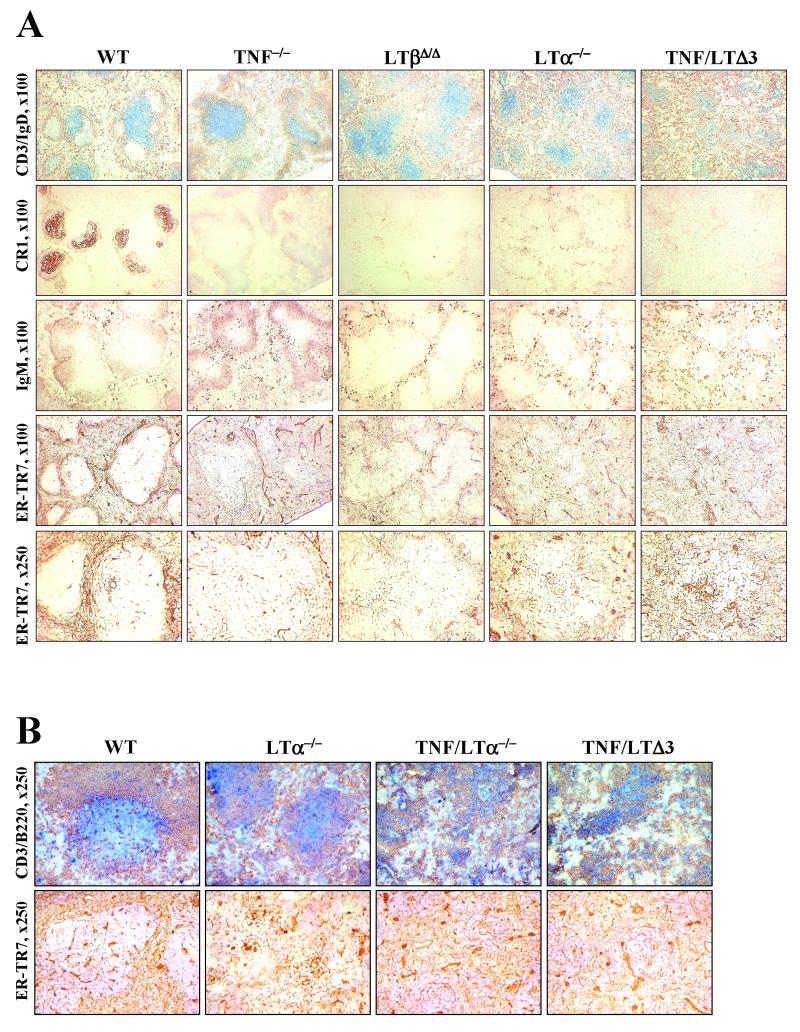
Immunohistochemical analysis of spleens of TNF/LTΔ3 mice revealed profound alterations of the splenic architecture, which appeared to be more severely disturbed than in any of three mouse strains with single deficiency, including LTα deficiency. Detailed immunohistochemical comparison of immune reactions to a T-cell-dependent antigen in TNF/LTΔ3, TNF−/−, LTβΔ/Δ, and LTα−/− mice was performed on spleens from mice immunized with SRBC. Consistent with previous studies (40, 49), LTα−/− and TNF−/− mice did not form GCs, while the clusters of peanut agglutinin-positive cells around central arterioles could be found in spleens of LTβΔ/Δ mice (35; data not shown). There was no GC formation in TNF/LTΔ3 mice, as determined by peanut agglutinin and IgD staining (Fig. 2A). Staining for CR1 (Fig. 2A) or FDC-M1 (data not shown) did not reveal any FDC clusters in the triply mutant or any of the singly mutant mice. The absence of the marginal zone in the spleen of TNF/LTΔ3, LTβΔ/Δ, and LTα−/− mice was demonstrated by negative MOMA-1 and MAdCAM-1 labeling (data not shown). Double staining for CD3+ T cells and IgD+ B cells (Fig. 2A, top row) revealed the absence of polarized B-cell follicles in all mutant mice analyzed and a gradual reduction of the size of white pulp in the following order: wild type > LTβΔ/Δ ≈ TNF−/− > LTα−/− > TNF/LTΔ3. Spatial segregation of T and B cells was reduced in the same order (Fig. 2A). T- and B-cell areas were relatively distinct in TNF−/− mice; in LTβΔ/Δ mice, T-cell zones appeared distinct, while B cells were significantly scattered; LTα−/− mice showed mixed T- and B-cell areas and the boundary between white and red pulp was less defined. The distribution of lymphocytes and their functional compartmentalization were most severely disorganized in the spleen of TNF/LTΔ3 mice, with IgD+ B cells scattered along red and white pulp and T cells mostly condensed around central arterioles (Fig. 2A).
FIG. 2.
Defective spleen organization in TNF/LTΔ3 mice. Magnifications are given on left. (A) Splenic cryosections of 6- to 8-week-old mice immunized with SRBC were stained to detect the distribution of T cells (CD3, blue) and B cells (IgD [brown], IgM), FDC (CR1), or stromal elements (ER-TR7) in TNF/LTΔ3 and single-knockout mice. WT, wild type. (B) Side-by-side comparison with TNF/LTα−/− mice. Splenic cryosections of 6- to 8-week-old nonimmunized mice were stained with anti-CD3 (blue), anti-B220 (brown), or anti-ER-TR7.
The stromal components of the spleen that support spatial organization of the lymphoid tissues were characterized by labeling with the ER-TR7 antibody, which detects reticular fibroblasts and blood vessel walls (65). The extent of spatial organization of the ER-TR7-positive stromal elements in the spleens of various knockout mice was progressively reduced in ways that correlated with the effects of these gene deletions on segregation of T and B cells (Fig. 2A, two bottom rows). This observation highlights a close correlation between functional compartmentalization of lymphocytes and the distribution of stromal elements in the maintenance of microarchitecture of the spleen. Disruption of this microarchitecture was most evident in the spleen of TNF/LTΔ3 mice, where the reduced white pulp area contained disordered coarse stromal elements, especially condensed around central arterioles (Fig. 2A).
Thus, disruption of both TNF and LT signaling in TNF/LTΔ3 mice results in more severe defects in spleen architecture than in singly TNF- or LT-deficient mice. Additionally, side-by-side comparison of spleens from wild-type, LTα−/−, TNF/LTα−/−, and TNF/LTΔ3 mice did not reveal noticeable differences between TNF/LTα−/− and TNF/LTΔ3 mice (Fig. 2B and data not shown), suggesting the lack of separate function for LTβ (independent of LTα) in the maintenance of splenic microarchitecture.
Gene expression in spleen.
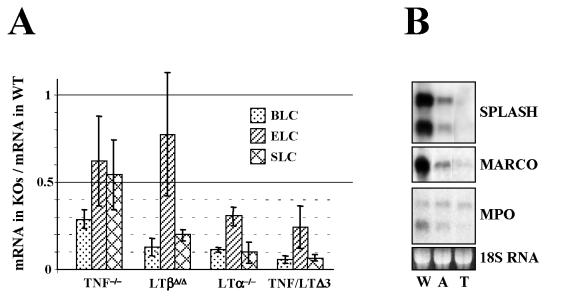
Recently, by employing gene expression profiling, we have identified several genes with significantly lower levels of expression in the spleens of LTα- and TNF/LTα-deficient mice than in wild-type controls (60, 61), among them the genes encoding B-cell chemoattractant BLC (CXCL13) and CCR7 ligands SLC and ELC (reviewed in reference 10). Previously Ngo et al. (48) linked altered chemokine expression by stromal cells to the LTβR signaling pathway and, therefore, to LT deficiency. To determine whether the additional disruption of spleen microarchitecture in TNF/LTΔ3 mice may be correlated with further downregulation of chemokine expression, we performed Northern analysis of total splenic RNA from TNF/LTΔ3 and single-knockout mice. Indeed, the level of BLC transcript was somewhat lower in the spleen of TNF/LTΔ3 mice than in that of singly LTα-deficient mice. Our chemokine data for TNF/LTΔ3 mice are similar to those reported earlier for TNF/LTα−/− mice (48) and consistent with a similar disruption of splenic microarchitecture in these two strains (Fig. 2B).
We also compared levels of expression for several selected genes and found them to be further decreased in the spleens of TNF/LTΔ3 mice compared to those of LTα-deficient mice (Fig. 3B). Examples include SPLASH, MARCO, and MPO (60, 61). SPLASH is a secretory-type phospholipase A2 (64) expressed in spleen by stromal cells of white pulp (62); expression of the SPLASH homologue sPLA2-II was previously shown to be regulated by TNF (3). MARCO is a scavenger receptor expressed by marginal zone macrophages (13), implicated in clearance of bacteria and environmental dust particles (21). MPO is one of the major components of neutrophil granules (4) and plays a role in innate immunity, although the significance of MPO expression in spleen is not clear. All three genes showed further reduction in expression in TNF/LTΔ3 mice compared to that in any single-knockout mice, although the major drop in the expression from the wild-type level can be attributed to an LTα deficiency (Fig. 3).
FIG. 3.
Reduced gene expression in spleens of LT and TNF mutant mice. (A) Comparison of chemokine expression (quantification of Northern analysis). WT, wild type; KO, knockout. (B) Expression of SPLASH, MARCO, and MPO. W, wild type; A, LTα−/−; T, “triple” TNF/LTΔ3.
Impaired antibody responses.
In order to assess antibody responses to a T-cell-dependent antigen, mice were immunized with SRBC and specific immunoglobulin titers in serum were measured at days 8 and 23. Specific IgG antibodies were almost undetectable in TNF/LTΔ3, LTβΔ/Δ, LTα, and TNRFp55−/− mice, consistent with defective class switching in all these strains (data not shown). Accordingly, total IgM levels were increased in LTα−/−, LTβ−/−,and TNF/LTΔ3 mice to about two times that of wild-type controls, with no statistically significant difference between individual knockout strains. This effect appears to be related to the increased number of IgM-producing plasma cells in spleen (Fig. 2A, note increased number of IgMbright cells in the red pulp of all knockout mice compared to that of the wild type).
Host defense functions.
We next tested whether the disruption of both TNF and LT signaling in TNF/LTΔ3 mice led to increased susceptibility to infections, compared to single TNF deficiency. Wild-type and mutant mice were first infected with low-dose S. enterica serovar Typhimurium (105 CFU/mouse per os), an intracellular parasite that interacts with a host via a complex mechanism (reviewed in references 25 and 27), involving both TNF signaling (17, 39, 43) and gut-associated lymphoid tissues (11). All mice survived the low-dose challenge with mild symptoms of illness. In order to assess the development of protective immunity, high-dose S. enterica serovar Typhimurium (108 CFU/mouse per os) was administered 30 days after the primary infection. As indicated by the survival data, wild-type mice were able to mount a partially protective response, while all knockout mice died with similar kinetics (Fig. 4A), with no significant difference between TNF−/−, LTβΔ/Δ, and TNF/LTΔ3 mice. Salmonella is known to utilize both M cells (28) and DC (54) located in organized mucosal lymphoid tissues to invade the host. Therefore, we were surprised that LTβ-deficient mice that lack PP (1, 32) and have deficient migration of DC (69) were as susceptible to secondary Salmonella challenge as were TNF-deficient mice. On the other hand, recent studies (41) revealed an important role for Salmonella-specific CD4+ T cells located in the PP and in LN (but not in other organs) in protective immune response against Salmonella infection. Our results with LTβΔ/Δ mice suggest that LT-mediated organization of mucosal lymphoid tissue plays an essential role in this process and are in agreement with another recent study on leishmaniasis in LTβ−/− mice (68). Thus, both TNF- and LT-mediated pathways play an important role in protection against S. enterica serovar Typhimurium but do not appear to be redundant, as the TNF/LTΔ3 mice do not show a severer phenotype in this model.
FIG. 4.
TNF/LTΔ3 and TNF-deficient mice are equally susceptible to bacterial infections. WT, wild type. (A) Mice were infected with 108 S. enterica serovar Typhimurium bacteria 30 days after primary infection with 105 bacteria. (B) Mice were injected i.p. with 5 × 103 L. monocytogenes bacteria. (C) Mice were injected subcutaneously with 2 × 104 L. monocytogenes bacteria.
We then tested the ability of TNF/LTΔ3 mice to withstand infection with another bacterial pathogen, L. monocytogenes (14, 31, 63). Wild-type, TNF−/−, LTα−/−, LTβΔ/Δ, and TNF/LTΔ3 mice were injected i.p. with 5 × 103 live L. monocytogenes bacteria. All wild-type controls survived the infection, while TNF−/− and TNF/LTΔ3 mice died by day 8 (Fig. 4B) without significant difference in survival between TNF−/− and TNF/LTΔ3 mice. All LTα−/− and LTβΔ/Δ mice survived this challenge (data not shown). The route of infection did not appear to play a role in this model, since, after subcutaneous infection with 2 × 104 L. monocytogenes bacteria, all TNF−/− and TNF/LTΔ3 mice died with indistinguishable kinetics (Fig. 4C) while all LTβΔ/Δ mice survived. Prior immunization of mice with heat-killed Listeria followed by infection with live bacteria also revealed no difference between TNF−/− and TNF/LTΔ3 mice (data not shown), indicating that TNF, but not LT signaling, is critical for protection against Listeria.
Additionally, TNF/LTΔ3 mice and TNF−/− mice were equally resistant to lipopolysaccharide toxicity after d-galactosamine priming (data not shown), in accordance with the primary role of TNF in this model (38, 49, 51, 56).
These results indicate distinct roles for TNF and LT in immunity against pathogens and provide no evidence for redundancy in TNF and LT signaling in host defense functions.
Conclusion: essentially distinct TNF and LT signaling in vivo.
TNF and LT have been initially defined as distinct and unrelated biological activities (8, 22, 57). It was discovered later that TNF and LT are closely related cytokines (23) encoded by highly homologous genes that apparently evolved from a common ancestor (46). Soluble TNF and LT engage the same receptors in vitro and manifest similar functions in many in vitro assays (23, 50). However, the unifying concept for TNF and LT (which even resulted in temporary renaming of LT to TNF beta) was later challenged by the discovery of LTβ (7) and LTβR (9) and by the unexpected phenotype of LTα-deficient mice (12). Analysis of lymphoid tissues in LTα-, LTβ-, and TNF-deficient mice revealed some significant overlaps in phenotypes, as exemplified by the absence of splenic GCs and defective antibody responses in these mice. Additionally, recent data suggested that LTα plays a distinct role in the control of Mycobacterium tuberculosis infection (55), an example of a function previously believed to be associated exclusively with TNF.
We anticipated that cytokine redundancy and overlap in signaling pathways could have resulted in a severer phenotype in TNF/LTΔ3 mice than in singly deficient mice, including the LTα knockout mice. However, these mice show an additive combination of phenotypes of LT and TNF deficiencies, comprising the defects in the lymphoid development and maintenance of lymphoid organs due to disrupted LTα/LTβ→LTβR signaling and defects in host defense functions due to disrupted TNF→TNRFp55 (and possibly LTα→TNRFp55) signaling. We did not detect yet any additional deficiencies in the immune system of TNF/LTΔ3 mice, except in the architecture of the spleen, where the defects caused by abolition of the two signaling pathways added together result in quantitative, rather than qualitative, phenotypic changes. Therefore, LTα/LTβ and TNF manifest themselves in vivo predominantly as distinct cytokines with small but significant overlap in biological function schematically depicted in Fig. 5. These additional changes in TNF/LTΔ3 mice can be interpreted based on disruption of redundant action of TNF and LT signaling pathways on the same target gene(s).
FIG. 5.
Distinct and overlapping physiological functions of the TNF/LT family. Areas on the diagram symbolize subsets of functions mediated by single molecules or by their combinations.
Acknowledgments
D.V.K., M.B.A., and A.V.T. contributed equally to this work.
The TNF/LTΔ3 strain was generated as a collaboration among the laboratories of S.A.N., K.P., and K.R. We are greatly indebted to A. Tarakhovsky for his guidance and advice. We thank B. I. Marakusha for expert help in infection experiments; B. Ryffel for TNF/LTα−/− mice; R. Kühn for E14.1 cells; H. Gu for the pIC-Cre expression vector; U. Huffstadt, K. Mink, and T. Stull for technical assistance; and the personnel of the Laboratory Animal Breeding Center, Branch of Schemyakin and Ovchinnikov Institute of Bioorganic Chemistry, for expert help with mouse breeding and production. We are grateful to J. J. Oppenheim and A. Rudensky for critical reading of the manuscript.
This project has been funded in whole or in part with U.S. federal funds from the National Cancer Institute, National Institutes of Health (contract N01-CO-12400); by the Deutsche Forschungsgemeinschaft (grant Pf259/2-4); and by grants 98-04-49103 and 02-04-49105 from the Russian Foundation for Basic Research. During this project R.L.T. and S.A.N. were International Research Scholars of the Howard Hughes Medical Institute, and A.V.T. was supported by a research training fellowship from the International Agency for Research on Cancer.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
REFERENCES
- 1.Alimzhanov, M. B., D. V. Kuprash, M. H. Kosco-Vilbois, A. Luz, R. L. Turetskaya, A. Tarakhovsky, K. Rajewsky, S. A. Nedospasov, and K. Pfeffer. 1997. Abnormal development of secondary lymphoid tissues in lymphotoxin beta-deficient mice. Proc. Natl. Acad. Sci. USA 94:9302-9307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amiot, F., Y. Bellkaid, M. Lebastard, P. Ave, F. Dautry, and G. Milon. 1996. Abnormal organisation of the splenic marginal zone and the correlated leukocytosis in lymphotoxin-alpha and tumor necrosis factor alpha double deficient mice. Eur. Cytokine Netw. 7:733-739. [PubMed] [Google Scholar]
- 3.Arbibe, L., D. Vial, I. Rosinski-Chupin, N. Havet, M. Huerre, B. B. Vargaftig, and L. Touqui. 1997. Endotoxin induces expression of type II phospholipase A2 in macrophages during acute lung injury in guinea pigs: involvement of TNF-alpha in lipopolysaccharide-induced type II phospholipase A2 synthesis. J. Immunol. 159:391-400. [PubMed] [Google Scholar]
- 4.Badwey, J. A., and M. L. Karnovsky. 1980. Active oxygen species and the functions of phagocytic leukocytes. Annu. Rev. Biochem. 49:695-726. [DOI] [PubMed] [Google Scholar]
- 5.Banks, T. A., B. T. Rouse, M. K. Kerley, P. J. Blair, V. L. Godfrey, N. A. Kuklin, D. M. Bouley, J. Thomas, S. Kanangat, and M. L. Mucenski. 1995. Lymphotoxin-alpha-deficient mice. Effects on secondary lymphoid organ development and humoral immune responsiveness. J. Immunol. 155:1685-1693. [PubMed] [Google Scholar]
- 6.Berger, D. P., D. Naniche, M. T. Crowley, P. A. Koni, R. A. Flavell, and M. B. Oldstone. 1999. Lymphotoxin-beta-deficient mice show defective antiviral immunity. Virology 260:136-147. [DOI] [PubMed] [Google Scholar]
- 7.Browning, J. L., A. Ngam-ek, P. Lawton, J. DeMarinis, R. Tizard, E. P. Chow, C. Hession, B. O'Brine-Greco, S. F. Foley, and C. F. Ware. 1993. Lymphotoxin beta, a novel member of the TNF family that forms a heteromeric complex with lymphotoxin on the cell surface. Cell 72:847-856. [DOI] [PubMed] [Google Scholar]
- 8.Carswell, E. A., L. J. Old, R. L. Kassel, S. Green, N. Fiore, and B. Williamson. 1975. An endotoxin-induced serum factor that causes necrosis of tumors. Proc. Natl. Acad. Sci. USA 72:3666-3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crowe, P. D., T. L. VanArsdale, B. N. Walter, C. F. Ware, C. Hession, B. Ehrenfels, J. L. Browning, W. S. Din, R. G. Goodwin, and C. A. Smith. 1994. A lymphotoxin-beta-specific receptor. Science 264:707-710. [DOI] [PubMed] [Google Scholar]
- 10.Cyster, J. G. 1999. Chemokines and cell migration in secondary lymphoid organs. Science 286:2098-2102. [DOI] [PubMed] [Google Scholar]
- 11.Davis, I. A., and B. T. Rouse. 1998. Immune responsiveness of lymphotoxin-alpha-deficient mice: two reconstitution models. Cell. Immunol. 189:116-124. [DOI] [PubMed] [Google Scholar]
- 12.De Togni, P., J. Goellner, N. H. Ruddle, P. R. Streeter, A. Fick, S. Mariathasan, S. C. Smith, R. Carlson, L. P. Shornick, J. Strauss-Schoenberger, J. H. Russell, R. Karr, and D. D. Chaplin. 1994. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science 264:703-707. [DOI] [PubMed] [Google Scholar]
- 13.Elomaa, O., M. Kangas, C. Sahlberg, J. Tuukkanen, R. Sormunen, A. Liakka, I. Thesleff, G. Kraal, and K. Tryggvason. 1995. Cloning of a novel bacteria-binding receptor structurally related to scavenger receptors and expressed in a subset of macrophages. Cell 80:603-609. [DOI] [PubMed] [Google Scholar]
- 14.Endres, R., A. Luz, H. Schulze, H. Neubauer, A. Futterer, S. M. Holland, H. Wagner, and K. Pfeffer. 1997. Listeriosis in p47(phox−/−) and TRp55−/− mice: protection despite absence of ROI and susceptibility despite presence of RNI. Immunity 7:419-432. [DOI] [PubMed] [Google Scholar]
- 15.Erickson, S. L., F. J. de Sauvage, K. Kikly, K. Carver-Moore, S. Pitts-Meek, N. Gillett, K. C. Sheehan, R. D. Schreiber, D. V. Goeddel, and M. W. Moore. 1994. Decreased sensitivity to tumour-necrosis factor but normal T-cell development in TNF receptor-2-deficient mice. Nature 372:560-563. [DOI] [PubMed] [Google Scholar]
- 16.Eugster, H. P., M. Muller, U. Karrer, B. D. Car, B. Schnyder, V. M. Eng, G. Woerly, M. Le Hir, F. di Padova, M. Aguet, R. Zinkernagel, H. Bluethmann, and B. Ryffel. 1996. Multiple immune abnormalities in tumor necrosis factor and lymphotoxin-alpha double-deficient mice. Int. Immunol. 8:23-36. [DOI] [PubMed] [Google Scholar]
- 17.Everest, P., M. Roberts, and G. Dougan. 1998. Susceptibility to Salmonella typhimurium infection and effectiveness of vaccination in mice deficient in the tumor necrosis factor alpha p55 receptor. Infect. Immun. 66:3355-3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu, Y. X., H. Molina, M. Matsumoto, G. Huang, J. Min, and D. D. Chaplin. 1997. Lymphotoxin-alpha (LTalpha) supports development of splenic follicular structure that is required for IgG responses. J. Exp. Med. 185:2111-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Futterer, A., K. Mink, A. Luz, M. H. Kosco-Vilbois, and K. Pfeffer. 1998. The lymphotoxin beta receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity 9:59-70. [DOI] [PubMed] [Google Scholar]
- 20.Giroir, B. P., T. Brown, and B. Beutler. 1992. Constitutive synthesis of tumor necrosis factor in the thymus. Proc. Natl. Acad. Sci. USA 89:4864-4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gough, P. J., and S. Gordon. 2000. The role of scavenger receptors in the innate immune system. Microbes Infect. 2:305-311. [DOI] [PubMed] [Google Scholar]
- 22.Granger, G. A., and T. W. Williams. 1968. Lymphocyte cytotoxicity in vitro: activation and release of a cytotoxic factor. Nature 218:1253-1254. [DOI] [PubMed] [Google Scholar]
- 23.Gray, P. W., B. B. Aggarwal, C. V. Benton, T. S. Bringman, W. J. Henzel, J. A. Jarrett, D. W. Leung, B. Moffat, P. Ng, and L. P. Svedersky. 1984. Cloning and expression of cDNA for human lymphotoxin, a lymphokine with tumour necrosis activity. Nature 312:721-724. [DOI] [PubMed] [Google Scholar]
- 24.Gu, H., Y. R. Zou, and K. Rajewsky. 1993. Independent control of immunoglobulin switch recombination at individual switch regions evidenced through Cre-loxP-mediated gene targeting. Cell 73:1155-1164. [DOI] [PubMed] [Google Scholar]
- 25.Hughes, E. A., and J. E. Galan. 2002. Immune response to Salmonella: location, location, location? Immunity 16:325-328. [DOI] [PubMed] [Google Scholar]
- 26.Ito, D., T. C. Back, A. N. Shakhov, R. H. Wiltrout, and S. A. Nedospasov. 1999. Mice with a targeted mutation in lymphotoxin-alpha exhibit enhanced tumor growth and metastasis: impaired NK cell development and recruitment. J. Immunol. 163:2809-2815. [PubMed] [Google Scholar]
- 27.Jones, B. D., and S. Falkow. 1996. Salmonellosis: host immune responses and bacterial virulence determinants. Annu. Rev. Immunol. 14:533-561. [DOI] [PubMed] [Google Scholar]
- 28.Jones, B. D., N. Ghori, and S. Falkow. 1994. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer's patches. J. Exp. Med. 180:15-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joyner, A. L. 1993. Gene targeting: a practical approach. IRL Press, New York, N.Y.
- 30.Karrer, U., A. Althage, B. Odermatt, C. W. M. Roberts, S. J. Korsmeyer, S. Miyawaki, H. Hengartner, and R. M. Zinkernagel. 1997. On the key role of secondary lymphoid organs in antiviral immune responses studied in alymphoplastic (aly/aly) and spleenless (Hox11−/−) mutant mice. J. Exp. Med. 185:2157-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaufmann, S. H. 1993. Immunity to intracellular bacteria. Annu. Rev. Immunol. 11:129-163. [DOI] [PubMed] [Google Scholar]
- 32.Koni, P. A., R. Sacca, P. Lawton, J. L. Browning, N. H. Ruddle, and R. A. Flavell. 1997. Distinct roles in lymphoid organogenesis for lymphotoxins alpha and beta revealed in lymphotoxin beta-deficient mice. Immunity 6:491-500. [DOI] [PubMed] [Google Scholar]
- 33.Korner, H., M. Cook, D. S. Riminton, F. A. Lemckert, R. M. Hoek, B. Ledermann, F. Kontgen, B. Fazekas de St. Groth, and J. D. Sedgwick. 1997. Distinct roles for lymphotoxin-alpha and tumor necrosis factor in organogenesis and spatial organization of lymphoid tissue. Eur J. Immunol. 27:2600-2609. [DOI] [PubMed] [Google Scholar]
- 34.Kuprash, D. V., M. B. Alimzhanov, D. K. Pokholok, S. V. Kozlov, T. I. Novobrantseva, R. L. Turetskaia, and S. A. Nedospasov. 1994. Characteristics of murine chromosome 17 containing three tumor necrosis factor family genes, including the gene of the transmembrane subunit of lymphotoxin. Dokl. Akad. Nauk 337:683-686. (In Russian.) [PubMed] [Google Scholar]
- 35.Kuprash, D. V., M. B. Alimzhanov, A. Tumanov, A. O. Anderson, K. Pfeffer, and S. A. Nedospasov. 1999. TNF and lymphotoxin beta cooperate in the maintenance of secondary lymphoid tissue microarchitecture but not in the development of lymph nodes. J. Immunol. 163:6575-6580. [PubMed] [Google Scholar]
- 36.Lawton, P., J. Nelson, R. Tizard, and J. L. Browning. 1995. Characterization of the mouse lymphotoxin-beta gene. J. Immunol. 154:239-246. [PubMed] [Google Scholar]
- 37.Locksley, R. M., N. Killeen, and M. J. Lenardo. 2001. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104:487-501. [DOI] [PubMed] [Google Scholar]
- 38.Marino, M. W., A. Dunn, D. Grail, M. Inglese, Y. Noguchi, E. Richards, A. Jungbluth, H. Wada, M. Moore, B. Williamson, S. Basu, and L. J. Old. 1997. Characterization of tumor necrosis factor-deficient mice. Proc. Natl. Acad. Sci. USA 94:8093-8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mastroeni, P., J. N. Skepper, and C. E. Hormaeche. 1995. Effect of anti-tumor necrosis factor alpha antibodies on histopathology of primary Salmonella infections. Infect. Immun. 63:3674-3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsumoto, M., S. Mariathasan, M. H. Nahm, F. Baranyay, J. J. Peschon, and D. D. Chaplin. 1996. Role of lymphotoxin and the type I TNF receptor in the formation of germinal centers. Science 271:1289-1291. [DOI] [PubMed] [Google Scholar]
- 41.McSorley, S. J., S. Asch, M. Costalonga, R. L. Reinhardt, and M. K. Jenkins. 2002. Tracking salmonella-specific CD4 T cells in vivo reveals a local mucosal response to a disseminated infection. Immunity 16:365-377. [DOI] [PubMed] [Google Scholar]
- 42.Mebius, R. E., P. Rennert, and I. L. Weissman. 1997. Developing lymph nodes collect CD4+CD3− LTbeta+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity 7:493-504. [DOI] [PubMed] [Google Scholar]
- 43.Morrissey, P. J., K. Charrier, and S. N. Vogel. 1995. Exogenous tumor necrosis factor alpha and interleukin-1α increase resistance to Salmonella typhimurium: efficacy is influenced by the Ity and Lps loci. Infect. Immun. 63:3196-3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muller, U., C. V. Jongeneel, S. A. Nedospasov, K. F. Lindahl, and M. Steinmetz. 1987. Tumour necrosis factor and lymphotoxin genes map close to H-2D in the mouse major histocompatibility complex. Nature 325:265-267. [DOI] [PubMed] [Google Scholar]
- 45.Nedospasov, S. A., B. Hirt, A. N. Shakhov, V. N. Dobrynin, E. Kawashima, R. S. Accolla, and C. V. Jongeneel. 1986. The genes for tumor necrosis factor (TNF-alpha) and lymphotoxin (TNF-beta) are tandemly arranged on chromosome 17 of the mouse. Nucleic Acids Res. 14:7713-7725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nedospasov, S. A., A. N. Shakhov, R. L. Turetskaya, V. A. Mett, M. M. Azizov, G. P. Georgiev, V. G. Korobko, V. N. Dobrynin, S. A. Filippov, N. S. Bystrov, E. F. Boldyreva, S. A. Chuvpilo, A. M. Chumakov, L. N. Shingarova, and Y. A. Ovchinnikov. 1986. Tandem arrangement of genes coding for tumor necrosis factor (TNF-alpha) and lymphotoxin (TNF-beta) in the human genome. Cold Spring Harbor Symp. Quant. Biol. 51:611-624. [DOI] [PubMed] [Google Scholar]
- 47.Neumann, B., A. Luz, K. Pfeffer, and B. Holzmann. 1996. Defective Peyer's patch organogenesis in mice lacking the 55-kD receptor for tumor necrosis factor. J. Exp. Med. 184:259-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ngo, V. N., H. Korner, M. D. Gunn, K. N. Schmidt, R. D. Sean, M. D. Cooper, J. L. Browning, J. D. Sedgwick, and J. G. Cyster. 1999. Lymphotoxin alpha/beta and tumor necrosis factor are required for stromal cell expression of homing chemokines in B and T cell areas of the spleen. J. Exp. Med. 189:403-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pasparakis, M., L. Alexopoulou, V. Episkopou, and G. Kollias. 1996. Immune and inflammatory responses in TNF alpha-deficient mice: a critical requirement for TNF alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J. Exp. Med. 184:1397-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pennica, D., G. E. Nedwin, J. S. Hayflick, P. H. Seeburg, R. Derynck, M. A. Palladino, W. J. Kohr, B. B. Aggarwal, and D. V. Goeddel. 1984. Human tumour necrosis factor: precursor structure, expression and homology to lymphotoxin. Nature 312:724-729. [DOI] [PubMed] [Google Scholar]
- 51.Pfeffer, K., T. Matsuyama, T. M. Kundig, A. Wakeham, K. Kishihara, A. Shahinian, K. Wiegmann, P. S. Ohashi, M. Kronke, and T. W. Mak. 1993. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell 73:457-467. [DOI] [PubMed] [Google Scholar]
- 52.Pokholok, D. K., I. G. Maroulakou, D. V. Kuprash, M. B. Alimzhanov, S. V. Kozlov, T. I. Novobrantseva, R. L. Turetskaya, J. E. Green, and S. A. Nedospasov. 1995. Cloning and expression analysis of the murine lymphotoxin beta gene. Proc. Natl. Acad. Sci. USA 92:674-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rennert, P. D., J. L. Browning, R. Mebius, F. Mackay, and P. S. Hochman. 1996. Surface lymphotoxin alpha/beta complex is required for the development of peripheral lymphoid organs. J. Exp. Med. 184:1999-2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rescigno, M., M. Urbano, B. Valzasina, M. Francolini, G. Rotta, R. Bonasio, F. Granucci, J. P. Kraehenbuhl, and P. Ricciardi-Castagnoli. 2001. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol. 2:361-367. [DOI] [PubMed] [Google Scholar]
- 55.Roach, D. R., H. Briscoe, B. Saunders, M. P. France, S. Riminton, and W. J. Britton. 2001. Secreted lymphotoxin-alpha is essential for the control of an intracellular bacterial infection. J. Exp. Med. 193:239-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rothe, J., W. Lesslauer, H. Lotscher, Y. Lang, P. Koebel, F. Kontgen, A. Althage, R. Zinkernagel, M. Steinmetz, and H. Bluethmann. 1993. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature 364:798-802. [DOI] [PubMed] [Google Scholar]
- 57.Ruddle, N. H., and B. H. Waksman. 1967. Cytotoxic effect of lymphocyte-antigen interaction in delayed hypersensitivity. Science 157:1060-1062. [DOI] [PubMed] [Google Scholar]
- 58.Sauer, B. 1993. Manipulation of transgenes by site-specific recombination: use of Cre recombinase. Methods Enzymol. 225:890-900. [DOI] [PubMed] [Google Scholar]
- 59.Scheu, S., J. Alferink, T. Potzel, W. Barchet, U. Kalinke, and K. Pfeffer. 2002. Targeted disruption of LIGHT causes defects in costimulatory T cell activation and reveals cooperation with lymphotoxin beta in mesenteric lymph node genesis. J. Exp. Med. 195:1613-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shakhov, A. N., I. G. Lyakhov, A. V. Tumanov, A. V. Rubtsov, L. N. Drutskaya, M. W. Marino, and S. A. Nedospasov. 2000. Gene profiling approach in the analysis of lymphotoxin and TNF deficiencies. J. Leukoc. Biol. 68:151-157. [PubMed] [Google Scholar]
- 61.Shakhov, A. N., and S. A. Nedospasov. 2001. Expression profiling in knockout mice: lymphotoxin versus tumor necrosis factor in the maintenance of splenic microarchitecture. Cytokine Growth Factor Rev. 12:107-119. [DOI] [PubMed] [Google Scholar]
- 62.Shakhov, A. N., A. V. Rubtsov, I. G. Lyakhov, A. V. Tumanov, and S. A. Nedospasov. 2000. SPLASH (PLA211D), a novel member of phospholipase A2 family, is associated with lymphotoxin deficiency. Genes Immunity 1:191-199. [DOI] [PubMed] [Google Scholar]
- 63.Unanue, E. R. 1997. Inter-relationship among macrophages, natural killer cells and neutrophils in early stages of Listeria resistance. Curr. Opin. Immunol. 9:35-43. [DOI] [PubMed] [Google Scholar]
- 64.Valentin, E., R. S. Koduri, J. C. Scimeca, G. Carle, M. H. Gelb, M. Lazdunski, and G. Lambeau. 1999. Cloning and recombinant expression of a novel mouse-secreted phospholipase A2. J. Biol. Chem. 274:19152-19160. [DOI] [PubMed] [Google Scholar]
- 65.Van Vliet, E., M. Melis, J. M. Foidart, and W. van Ewijk. 1986. Reticular fibroblasts in peripheral lymphoid organs identified by a monoclonal antibody. J. Histochem. Cytochem. 34:883-890. [DOI] [PubMed] [Google Scholar]
- 66.Wang, J., A. Foster, R. Chin, P. Yu, Y. Sun, Y. Wang, K. Pfeffer, and Y. X. Fu. 2002. The complementation of lymphotoxin deficiency with LIGHT, a newly discovered TNF family member, for the restoration of secondary lymphoid structure and function. Eur. J. Immunol. 32:1969-1979. [DOI] [PubMed] [Google Scholar]
- 67.Ware, C. F., P. D. Crowe, M. H. Grayson, M. J. Androlewicz, and J. L. Browning. 1992. Expression of surface lymphotoxin and tumor necrosis factor on activated T, B, and natural killer cells. J. Immunol. 149:3881-3888. [PubMed] [Google Scholar]
- 68.Wilhelm, P., D. S. Riminton, U. Ritter, F. A. Lemckert, C. Scheidig, R. Hoek, J. D. Sedgwick, and H. Korner. 2002. Membrane lymphotoxin contributes to anti-leishmanial immunity by controlling structural integrity of lymphoid organs. Eur. J. Immunol. 32:1993-2003. [DOI] [PubMed] [Google Scholar]
- 69.Wu, Q., Y. Wang, J. Wang, E. O. Hedgeman, J. L. Browning, and Y. X. Fu. 1999. The requirement of membrane lymphotoxin for the presence of dendritic cells in lymphoid tissues. J. Exp. Med. 190:629-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yoshida, H., H. Kawamoto, S. M. Santee, H. Hashi, K. Honda, S. Nishikawa, C. F. Ware, Y. Katsura, and S. I. Nishikawa. 2001. Expression of alpha(4)beta(7) integrin defines a distinct pathway of lymphoid progenitors committed to T cells, fetal intestinal lymphotoxin producer, NK, and dendritic cells. J. Immunol. 167:2511-2521. [DOI] [PubMed] [Google Scholar]