Abstract
Thyroid hormone receptors (TR) act as activators of transcription in the presence of the thyroid hormone (T3) and as repressors in its absence. While many in vitro approaches have been used to study the molecular mechanisms of TR action, their physiological relevance has not been addressed. Here we investigate how TR regulates gene expression during vertebrate postembryonic development by using T3-dependent amphibian metamorphosis as a model. Earlier studies suggest that TR acts as a repressor during premetamorphosis when T3 is absent. We hypothesize that corepressor complexes containing the nuclear receptor corepressor (N-CoR) are key factors in this TR-dependent gene repression, which is important for premetamorphic tadpole growth. To test this hypothesis, we isolated Xenopus laevis N-CoR (xN-CoR) and showed that it was present in pre- and metamorphic tadpoles. Using a chromatin immunoprecipitation assay, we demonstrated that xN-CoR was recruited to the promoters of T3 response genes during premetamorphosis and released upon T3 treatment, accompanied by a local increase in histone acetylation. Furthermore, overexpression of a dominant-negative N-CoR in tadpole tail muscle led to increased transcription from a T3-dependent promoter. Our data indicate that N-CoR is recruited by unliganded TR to repress target gene expression during premetamorphic animal growth, an important process that prepares the tadpole for metamorphosis.
Combinatorial actions of transcription factors are critical for coordinating development, cell homeostasis, and physiology (31). Nuclear receptors represent a large class of transcription factors implicated in the control of many important biological processes (29, 31). This superfamily includes receptors for thyroid hormone (T3, or 3,5,3′-triiodothyronine), vitamin D, retinoids, steroids, and components of lipid metabolism, as well as a number of orphan receptors (29). One of the most striking developmental processes involving nuclear receptors is amphibian metamorphosis.
The metamorphic transformation involves degeneration of larval tissues through apoptosis with concurrent proliferation and differentiation of adult cell types (6, 48). Metamorphosis requires the induction of a number of gene cascades at a precise developmental time point (47, 49). In Xenopus laevis, the switch to the metamorphic program is entirely T3 dependent (6, 48) but only occurs when the tadpole has grown to a suitable size and stage. This suggests that a molecular repressor mechanism must be in place during the growth period to repress metamorphosis-inducing genes.
Our working hypothesis was that this repressor mechanism, vital to correct physiological development, would involve certain corepressors. This hypothesis was derived from the results of a series of elegant in vitro and ex vivo experiments that show liganded as well as unliganded thyroid hormone receptors (TRs) to be capable of binding to target genes through cis-acting DNA sequences known as thyroid hormone response elements (T3REs). In the absence of T3, TR represses basal transcription and the addition of T3 relieves this repression and further enhances the promoter activity (21, 29, 30, 54). As in higher vertebrates, both TRα and TRβ exist in amphibians such as X. laevis (63). Interestingly, TRα and TRβ are differentially regulated in X. laevis (26, 64). The TRβ genes have little expression prior to metamorphosis and are upregulated by the rising concentration of T3 during metamorphosis as direct T3 response genes (28, 39). In contrast, TRα genes are activated shortly after the completion of embryogenesis but well before maturation of the thyroid gland and production of endogenous T3. These findings suggest a dual-function model for TRs during development (38). During premetamorphosis, TRs would act to repress the T3 response genes to ensure proper tadpole growth. Later, as T3 synthesis and secretion occur, TRs would then activate T3 response genes, thus inducing metamorphosis.
In vitro and tissue culture cell studies on the mechanisms underlying gene regulation by TR have led to the identification of coactivators and corepressors that bind TR in the presence and absence, respectively, of T3. These cofactors form multiprotein complexes (3, 14, 32). Two of the best studied, highly related corepressors that may mediate the repressive effects of TR are the nuclear receptor corepressor (N-CoR) (19) and silencing mediator for retinoid and thyroid hormone receptors (SMRT) (4). They appear to do so through mechanisms involving histone deacetylase (HDAC)-containing complexes (1, 16, 18, 22, 24, 25, 27, 52, 53, 55, 57).
Despite the extensive studies on N-CoR and TR in cell cultures and in vitro, few studies have investigated the physiological roles of N-CoR and TR in vivo, due in part to the lack of appropriate model systems. Here we make use of the total dependence of amphibian metamorphosis on T3 (6) to analyze the potentially critical physiological role of N-CoR in TR function during vertebrate development. We cloned X. laevis N-CoR (xN-CoR) and analyzed its expression and function in developing tadpoles. We show that xN-CoR is expressed and recruited to T3 response genes in premetamorphic tissues when T3 is absent. T3 treatment releases xN-CoR, accompanying increased local histone acetylation. In addition, we provide in vivo evidence to show that xN-CoR is involved in gene repression by unliganded TR in premetamorphic tadpoles.
MATERIALS AND METHODS
Animals and treatments.
Tadpoles of the South African clawed frog (X. laevis) were obtained from Nasco (Fort Atkinson, Wis.). Developmental stages were determined according to the method of Nieuwkopp and Faber (NF) (34). When indicated, 10 stage NF55 tadpoles were treated, for 1 to 7 days, in 4 liters of dechlorinated tap water with 5 or 10 nM T3 (Sigma). Tadpoles were sacrificed by decapitation after anesthesia for tissue isolation.
Cloning xN-CoR.
Total RNAs were extracted from stage 6 oocytes by use of RNAzol (Tel-Test) and were used for reverse transcription (RT) followed by PCR with degenerate primers to amplify regions of xN-CoR. The primers were selected from regions that were likely conserved among mammalian and amphibian N-CoR genes, such as the receptor interaction and repression domains (ID and RD, respectively). We thus obtained a C-terminal clone corresponding to a part of the ID with forward primer 5′CAAGGCCCTGCTGTGCAYGARAARCARGAY3′ and reverse primer 5′AACACCATGATCYTCMACYTTRTCRTCRAA3′ (where Y indicates a mixture of T and C at that position, R indicates a mixture of A and G, and M indicates a mixture of all four nucleotides) and another clone of the N-terminal part corresponding to the first RD with forward primer 5′GACATCAGCARGARTTYGCH3′ and reverse primer 5′GTTCTCRTCCCADATDATYGT3′. The RT reaction was done with SuperScriptII (GIBCO-BRL) and the reverse primer following the recommendations of the manufacturer. The PCR was done using ExTaq (Takara Shuzo, Kyoto, Japan) as previously described (40), with 40 cycles of 94°C for 1 min, 42°C for 1 min, and 72°C for 1 min. The PCR fragments were cloned in the pCR2-1 vector by use of the TA-Cloning kit from Invitrogen used according to the recommendations of the manufacturer.
The sequence between the N- and C-terminal clones was obtained by using RT-PCR. The RT reaction was done at 58°C with Thermoscript (GIBCO-BRL) following the recommendations of the manufacturer. PCR was done with a Perkin-Elmer Kit (GeneAmp XL PCR kit) and performed in 100 μl (total volume) composed of 30 μl of buffer, 8 μl of deoxynucleoside triphosphate mix (25 mM each), 1 μl of rTth, 3 μl of cDNA, 5 μl of magnesium acetate (25 mM), and 2.5 μl of both forward and reverse primer (25 pmol of each/reaction). PCR was done for 40 cycles of 93°C for 1 min and 60°C for 9 min. The forward and reverse primers were, respectively, 5′AGCCTCTAGATGATTATCGATCTTCTCACTTGGAAGCATC3′ and 5′AACCTCTAGATAGCTCCTCTACATAGGGTCAC3′. The PCR fragment was cloned into the XbaI site of pBluescript II (SK) vector (Stratagene) through the XbaI site introduced into the PCR primers.
The 5′ and 3′ ends of xN-CoR were obtained by anchor PCR according to the method of Frohman et al. (10), with modifications as previously described (61). The reverse primer used to clone the 5′end was 5′TAGCCTGATCTTCTGTCTTGGGGTCTG3′ and the forward primer used to clone the 3′end was 5′ATACGTCCCCAATGGTGATGTATAAGAAGC3′. The PCR fragments were cloned in the pCR2-1 vector using the TA-Cloning kit (Invitrogen).
All the clones were sequenced on both strands. The sequences of the overlapping clones belonging to the same N-CoR gene (with homology of more than 95% in the overlapped regions) were compiled to generate the final full-length xN-CoR sequence and the deduced amino acid sequence (Fig. 1A).
FIG. 1.
Sequence and domain structure of xN-CoR. (A) Amino acid sequence of xN-CoR as predicted from the full-length cDNA sequence. The three RDs, located in the N-terminal part of the protein, are underlined. The receptor IDs, represented in bold, are located in the C-terminal part. The CoRNRs within the ID are underlined. (B) Schematic diagram of xN-CoR showing the various conserved domains: RDs I to III, SNOR (for present in SMRTER, SMRT, and N-CoR), SANT 1 and 2 (for present in SWI3, ADA2, N-CoR, and TFIIIB-B′), ITS (motif of isoleucine-threonine-serine), IDs (N and C terminal), CoRNR (1 to 3), and LSD (motif of leucine-serine-aspartic acid).
RNA extraction, Northern blot, and RT-PCR analysis.
All the tissues (intestine, tail, and hindlimb) from 2 to 10 tadpoles at different stages of development or following T3 treatment were collected, flash frozen, and stored at −80°C. RNA extraction and Northern blotting were performed as previously described (58). The xN-CoR probe used corresponds to amino acids (aa) 1992 to 2265. The expression of the ribosomal protein L8 (rpl8), which is constant in development and not affected by T3 treatment (45), was used as a loading control.
For RT-PCR analysis, the RT reactions were performed as previously described (40). One microliter of the resulting cDNA solution was used for PCR in 50 μl of reaction containing 10× ExTaq buffer (5 μl; Takara Shuzo), deoxynucleoside triphosphates (8 μl; 2.5 mM each; Takara Shuzo), four primers (reverse and forward primers for the gene of interest and for rpl8; 2 μl of 10 μM solution for each; Invitrogen), ExTaq polymerase (0.5 μl; 5 U/μl; Takara Shuzo), and [α-32P]dCTP (0.1 μl; 1 μCi). PCRs were done for the indicated number of cycles, each consisting of 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min. The primers used were as follows: for xN-CoR, forward, 5′-AGACTGCCAGTGACTCG-3′, and reverse, 5′-CTGTGCTCTGACTAG-3′; for xSMRT, forward, 5′-ACAGTCACCGTGCCATTCTCAGCC-3′, and reverse, 5′-TGGAGATATGGGGTCCATACCATC-3′; and for rpl8, forward, 5′-AAAGAGAAACTGCTGGC-3′, and reverse, 5′-GACGACCAGTACGACGA-3′. PCR products were resolved on a 6% acrylamide-Tris-borate-EDTA gel and visualized by autoradiography. PhosphorImager scanning (Molecular Dynamics) was used to quantify the signals of the Northern blot and RT-PCR.
Antibody generation and coimmunoprecipitation.
Rabbit polyclonal antibody specific for xN-CoR was raised against bacterially expressed N-terminal polypeptide (aa 34 to 272) after cloning it in the pET21 vector (Novagen). Bacterial expression and purification of the polypeptide was performed according to the pET manual system (Novagen).
Groups of 20 mature oocytes were obtained as previously described (2) and were microinjected with 2 ng of in vitro-transcribed RNA encoding Xenopus TRβ or RXRα or a no RNA control. Oocytes were incubated overnight at 18°C, with or without T3 (50 nM), as indicated. Protein extracts were made by homogenization of the oocytes in buffer A (20 mM HEPES, 150 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA, 0.5% NP-40, 1 mM phenylmethylsulfonyl fluoride, 0.5 mM dithiothreitol, 1 μg of leupeptin per ml, 1 μg of pepstatin per ml, and 1 μg of aprotinin per ml) followed by centrifugation. The soluble fraction was removed and incubated with antibodies against the indicated protein for 1 h at 4°C. Protein A-Sepharose beads were added to collect the immune complexes and the beads were washed five times in buffer A. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis buffer was added to the beads, and the eluted proteins were analyzed by standard sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting.
ChIP assay.
The chromatin immunoprecipitation (ChIP) assay and the materials used, except the xN-CoR antibody, were as previously described (40). Preimmune serum was used as the control for antibody specificity.
In vivo gene transfer.
In vivo gene transfer was done as previously described (5), with the following plasmids. T3RE-tk-fLuciferase expresses the firefly (Photinus pyralis) luciferase reporter gene under the control of a promoter containing a T3RE made of two direct repeats of AGGTCA separated by four base pairs and of the minimal thymidine kinase (tk) promoter (kind gift of T. Collingwood, Sangamo, Richmond, Ga.). The plasmid phRL-SV40 expresses the humanized Renilla (Renilla reniformis) luciferase reporter gene under the control of the simian virus 40 (SV40) promoter (Promega). The CoRNR expression vector, corresponding to pCMX-nls-Gal4-CoRNRbox1, expresses the dominant-negative N-CoR fusion protein made of a nuclear localization signal from the cytomegalovirus (CMV) promoter and the Gal4 DNA binding domain fused to 20 amino acids of the mouse CoRNRbox1 (PASNLGLEDIIRKALMGSFD) (a kind gift of Mitchell A. Lazar, University of Pennsylvania, Philadelphia [20]). The related control expression vector is identical to the CoRNR vector with the exception that the CoRNRbox1 peptide was replaced by an irrelevant sequence (AASIPLARS). This construct was obtained by removing the CoRNRbox1 of pCMX-nls-Gal4-CoRNRbox1 using BamHI and NotI restriction enzymes. pCMV-TRα expresses the X. laevis TRα under the control of the CMV promoter. pcDNA3-fLuciferase, kindly provided by M. Schleef (Qiagen), expresses the firefly luciferase under the control of the CMV promoter of pcDNA3 vector (Invitrogen). pcDNA3 plasmid was also used as an empty vector to equalize the amount of injected DNA. All plasmids were purified using the Qiagen procedure.
For somatic gene transfer, 1 μl of solution containing the indicated amounts of purified plasmid DNA (between 0.7 and 2.1 μg) in 0.07 M NaCl colored with fast green (Sigma) was injected into the dorsal muscle of stage NF55 tadpoles. Transfection efficiency was normalized by coinjecting the construct phRL-SV40. Two days after injection, the tadpoles were sacrificed. The dorsal muscles were collected and flash frozen. Homogenates were obtained by sonication in 500 μl of passive lysis buffer (Promega). The dual luciferase assay was performed according to the manufacturer's recommendations (Promega) on 20 μl of homogenate. Student's t test was used to assess statistical differences between means.
Nucleotide sequence accession number.
The full-length xN-CoR sequence has been deposited in GenBank (GenBank accession no. AF495886).
RESULTS
Cloning of xN-CoR.
To isolate cDNA encoding xN-CoR, we used RT-PCR to amplify the conserved domains of N-CoR from stage 6 oocyte mRNA, as traditional screening of a cDNA library was not successful. Two clones were isolated that encoded, respectively, part of the first RD corresponding to aa 34 to 272 and part of the nuclear receptor ID corresponding to aa 1992 to 2265 (Fig. 1). The sequence between these NH2- and COOH-terminal regions was obtained by RT-PCR. Finally, the 5′ and 3′ends of xN-CoR were obtained by rapid amplification of cDNA ends-PCR.
Sequence alignment of multiple overlapping cDNA clones yielded a full-length sequence encoding a large protein of 2,498 aa (Fig. 1A). xN-CoR has a 68% overall identity (80% similarity) in amino acid sequence with human and mouse N-CoR (human and mouse N-CoR share 91% identity and 94% similarity) and contains conserved domains found in mammalian N-CoR (20, 56) and SMRT (4, 35, 36) and the Drosophila-related protein SMRTER (51) (Fig. 1B and 2). These domains in xN-CoR share 42 to 100% identity with their mammalian counterparts and up to 75% identity with those in Drosophila SMRTER (Table 1).
FIG. 2.
Various functional motifs are conserved among N-CoR, SMRT, and SMRTER. Alignments of xN-CoR, human N-CoR (hN-CoR; GenBank accession no. AF044209), mouse N-CoR (mN-CoR; GenBank accession no. U35312), human SMRT (hSMRT; GenBank accession no. AF125672), and Drosophila SMRTER (GenBank accession no. AF175223) are given for the SNOR, SANT, ITS, LSD, and CoRNR motifs. Dashes indicate sequences identical to that of xN-CoR, while blank spaces are gaps introduced for better alignments. Amino acid positions of each motif in the respective protein are also indicated.
TABLE 1.
Identities between xN-CoR and other nuclear corepressors
| Domain | % Identity with xN-CoRa
|
|||
|---|---|---|---|---|
| hN-CoR | mN-CoR | hSMRT | SMRTER | |
| RDI (aa 86-385) | 84 | 85 | ||
| RDII (aa 743-1006) | 43 | 42 | ||
| RDIII (aa 1082-1507) | 70 | 68 | ||
| ID-N (aa 2007-2224) | 60 | 60 | ||
| ID-C (aa 2287-2349) | 93 | 93 | ||
| SNOR (aa 170-431) | 97 | 97 | 83 | 28 |
| SANT 1 (aa 432-479) | 87 | 87 | 75 | 65 |
| SANT 2 (aa 620-667) | 67 | 67 | 60 | 23 |
| DAD (aa 395-479) | 91 | 91 | 73 | 49 |
| ITS (aa 1664-1679) | 100 | 100 | 81 | 75 |
| CoRNR box 3 (aa 2007-2014) | 95 | 95 | 65 | |
| CoRNR box 2 (aa 2117-2135) | 95 | 95 | 53 | |
| CoRNR box 1 (aa 2320-2339) | 95 | 95 | ||
Blank spaces indicate that the corresponding region is absent or does not show significant homology.
Several regions that are probably critical for N-CoR-mediated repression by unliganded nuclear receptors are of particular interest. These include the three RDs, RDI, RDII, and RDIII, in the N-terminal half and two receptor IDs, ID-N and ID-C, in the C-terminal part of mammalian N-CoR (1, 18, 19, 43). These RDs and IDs are not conserved in Drosophila SMRTER (51). Each ID contains a conserved hydrophobic core (I/LXXII) referred to as the CoRNR (corepressor-nuclear receptor) box (19, 33, 37, 55). There are three identified CoRNR boxes in N-CoR and two in SMRT but only one in SMRTER. xN-CoR has the corresponding three conserved CoRNR boxes (Fig. 1 and Table 1). In addition, a high degree of identity was found in the xN-CoR sequence corresponding to the deacetylase-activating domain (DAD). DAD encompasses the C-terminal part of the SNOR (present in SMRTER, SMRT, and N-CoR) and SANT1 (present in SWI3, ADA2, N-CoR, and TFIIIB-B′) domains and has been shown recently to be necessary and sufficient for HDAC3 activation by SMRT or N-CoR (17). This sequence corresponds to aa 395 to 479 in xN-CoR. These high levels of homology between xN-CoR and its related mammalian corepressors suggest similar properties for xN-CoR.
xN-CoR is expressed in premetamorphic tadpoles and upregulated during metamorphosis.
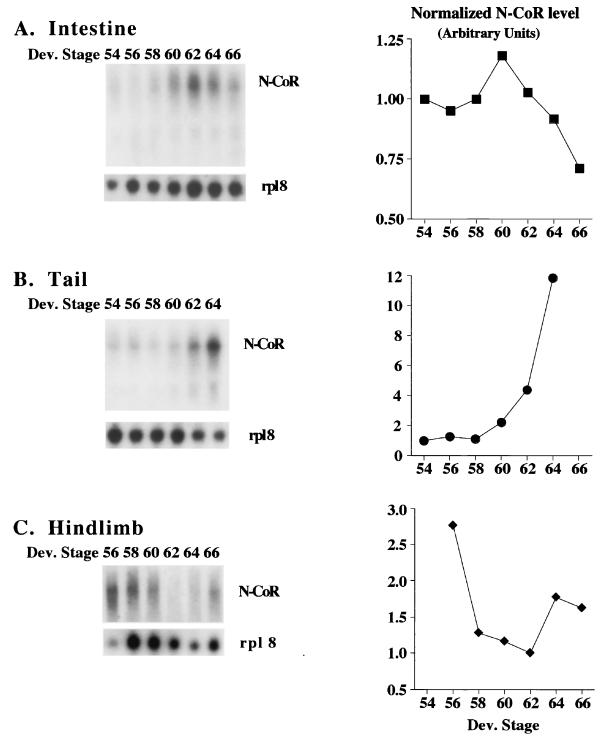
To investigate the potential roles of N-CoR in frog development, we analyzed its developmental expression profiles. We focused on three organs that undergo contrasting changes during metamorphosis. These are the intestine, which undergoes extensive remodeling involving both the degeneration of the vast majority of the larval cells and development of the adult tissues; the tail, which resorbs completely; and the hindlimbs, which develop de novo (6). Northern blot analyses were carried out on total RNA isolated from these organs at different developmental stages. As a loading control, we analyzed the expression of the ribosomal protein L8 (rpl8), which remains constant during natural or induced metamorphosis (44, 45, 58). The probe against xN-CoR mainly recognized a transcript of around 9 kb (Fig. 3), consistent with the expected size for the encoded protein. xN-CoR mRNA was found to be present prior to and throughout metamorphosis in all tissues analyzed (Fig. 3). In the intestine (Fig. 3A), xN-CoR was found to be expressed both prior to (stages NF54 to -58) and during (stages NF58 to -66) intestinal remodeling (46). Specifically, xN-CoR mRNA levels increased slightly at stage NF60 and then decreased to lower levels by the end of metamorphosis (stage NF66). In the tail (Fig. 3B), xN-CoR mRNA levels were upregulated dramatically by stages NF62 and NF64, which correspond to the period when tail regression takes place (6, 47). In the hindlimbs (Fig. 3C), xN-CoR mRNA was present at higher levels around stage NF56. As metamorphosis progressed, the mRNA decreased to lower levels and then increased slightly by the end of metamorphosis. Interestingly, limb morphogenesis takes place at stages NF54 to NF58, and after stage NF60, the hindlimb undergoes mainly growth with little morphological changes (34). Thus, higher levels of xN-CoR correlate with metamorphic changes in these organs, especially the tail, although the significance of the slight increase in the hindlimb by the end of metamorphosis is unclear.
FIG. 3.
High levels of xN-CoR gene transcripts are present during intestinal remodeling (A), tail resorption (B), and hindlimb morphogenesis (C). Each lane had 10 μg of total RNA, except for stage NF64 tail and stage NF56 hindlimb, which contained 5 μg. The same blot was probed with either an 800-bp xN-CoR cDNA fragment corresponding to aa 1992 to 2265 or the loading control gene rpl8, whose expression remains constant during development (44, 45, 58). The signals from the blots were quantified with a PhosphorImager and plotted on the right after normalization against the control rpl8 signals. The data represent one of two independent experiments with similar conclusions.
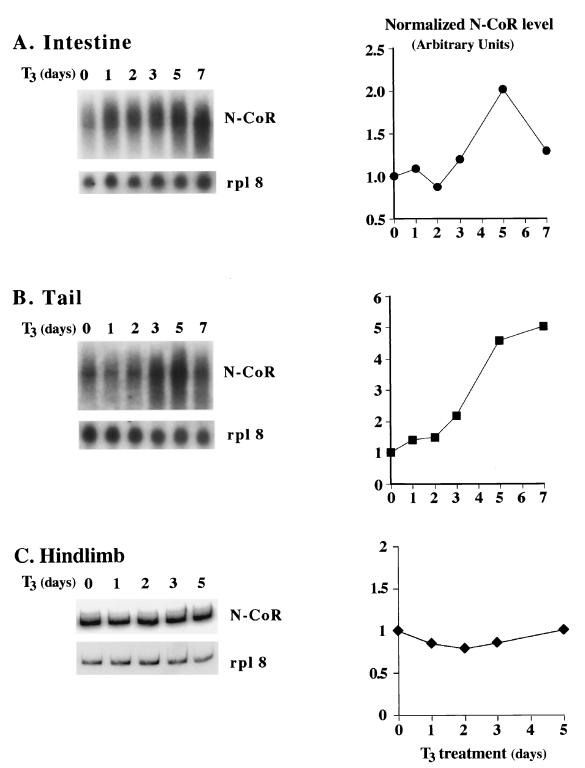
The changes in xN-CoR mRNA levels in these organs during development raised the possibility that xN-CoR might be regulated by T3 since metamorphosis is absolutely dependent upon T3. To test this possibility, stage NF55 tadpoles were treated with 5 nM T3 for up to 7 days, followed by isolation of total RNA from the intestine, tail, and hindlimb. Analyses of the RNA showed that in the intestine, xN-CoR mRNA levels increased after 4 days of T3 treatment and then decreased after 7 days of treatment (Fig. 4A). In the tail, xN-CoR mRNA levels increased dramatically after three or more days of T3 treatment (Fig. 4B). On the other hand, xN-CoR expression was not affected by T3 in the hindlimb (Fig. 4C). These patterns are similar to those during natural metamorphosis (Fig. 3) and suggest that xN-CoR is a late T3 response gene (as reflected by the slow induction kinetics), at least in the tail.
FIG. 4.
T3 treatment of premetamorphic tadpoles increases xN-CoR mRNA levels in the intestine (A) and tail (B) but not in the hindlimb (C). NF55 tadpoles were treated with 5 nM T3 for the indicated number of days. Total RNA was isolated and subjected to Northern blot analysis (10 μg/lane for tail and intestinal RNA) or RT-PCR analysis (for hindlimb RNA, due to the limited amount of RNA isolated from the small organ) for xN-CoR RNA levels. The signals were quantified with a PhosphorImager and plotted on the right after normalization against the control rpl8 signals. All the experiments were done at least twice with similar results.
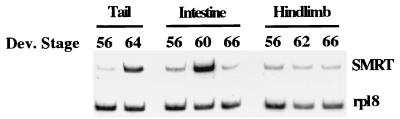
In vitro and in tissue culture cells, both N-CoR and a highly related corepressor, SMRT, have been shown to participate in repression by unliganded TR. We have recently cloned a 2-kb cDNA fragment corresponding to the C-terminal part and encompassing the TR-binding domain of X. laevis SMRT (xSMRT) (T. Amano and Y.-B. Shi, unpublished results). To investigate if xSMRT is also involved in frog development, we carried out RT-PCR analysis of xSMRT expression. The mRNA levels of xSMRT were found to be upregulated during metamorphosis in both the intestine and tail but changed little in the hindlimb (Fig. 5), suggesting that like xN-CoR, xSMRT may participate in gene repression during development.
FIG. 5.
The expression of xSMRT is similar to that of xN-CoR during development. Total RNA was isolated from intestine, tail, and hindlimb from tadpoles at the indicated stages. RT-PCR analysis was carried out to determine the mRNA levels of xSMRT and the internal control gene rpl8. Note that similar to xN-CoR (Fig. 3), xSMRT was upregulated during intestinal remodeling (stage NF60) and tail resorption (stage NF64). The results are from one of two independent experiments with similar results.
xN-CoR interacts with TR in a T3-dependent manner.
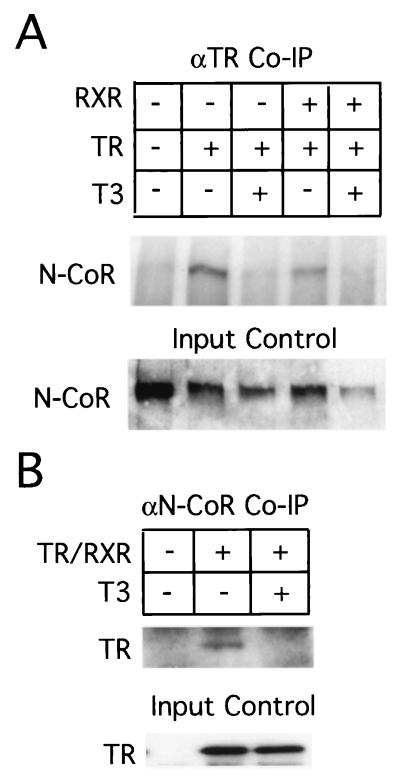
It was shown previously that unliganded TR represses target promoters in Xenopus oocytes and that this repression involves HDAC activity (60). To investigate if xN-CoR interacts with TR to participate in this repression, we overexpressed X. laevis TRβ and RXRα by microinjecting in vitro-transcribed mRNAs into frog oocytes. We analyzed associations of overexpressed TR-RXR with endogenous xN-CoR by immunoprecipitating the extracts from the oocytes with a polyclonal antibody against TR. Western blot analysis of the precipitate with a polyclonal antibody against xN-CoR indicated that unliganded TRβ interacted with endogenous N-CoR and the addition of T3 eliminated this interaction (Fig. 6A). This was also confirmed by immunoprecipitation with the antibody against xN-CoR followed by Western blot analysis for TR (Fig. 6B).
FIG. 6.
xN-CoR interacts in vivo with TR only in the absence of T3. Xenopus mature oocytes were microinjected (+) with in vitro-transcribed mRNA encoding Xenopus TRβ with or without Xenopus RXRα. The oocytes were incubated with or without T3 (50 nM) as indicated. The oocyte extracts were precipitated with antibodies specific for either TRβ (A) or xN-CoR (B). The precipitation products were assayed by Western blotting for xN-CoR (A) and TRβ (B). A part of the oocyte extracts was also analyzed directly by Western blotting for the expression of xN-CoR (A) or TRβ (B) proteins (input control). Uninjected oocytes were used as controls because they lack any detectable level of TRβ (B) or RXRα (data not shown) protein.
xN-CoR is recruited to T3REs of TR target promoters in premetamorphic tadpoles when T3 is absent.
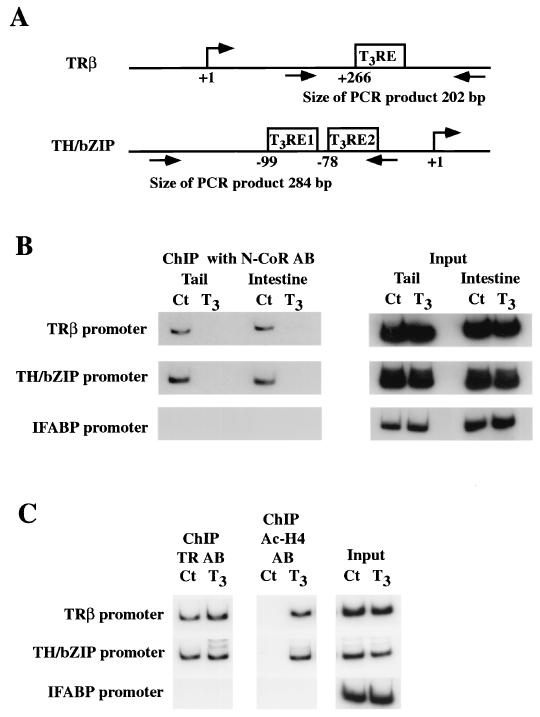
Having demonstrated that xN-CoR is capable of binding to unliganded TR in vivo and is expressed before metamorphosis, we investigated whether xN-CoR is involved in the regulation of T3 response genes in premetamorphic tadpoles. We analyzed the association of xN-CoR with the promoters of T3 target genes by ChIP assay with an antibody against xN-CoR on nuclei isolated from the tail or intestine. The DNA fragments immunoprecipitated by xN-CoR antibodies were analyzed by semiquantitative PCR for the presence of the T3RE regions of T3 target genes. Our analyses were focused on the Xenopus TRβA and TH/bZIP genes, the only two Xenopus direct T3 response genes that have been shown to contain T3REs (11, 28, 39). The regions selected for ChIP analysis corresponded to the sequences containing the functional T3REs (Fig. 7A). As a control, we used the promoter region of the intestinal fatty acid binding protein (IFABP) gene, the expression of which is restricted to intestinal epithelial cells and is not regulated by TR (12, 44).
FIG. 7.
ChIP assays show that T3 treatment leads to the release of xN-CoR from and increase of histone acetylation at T3 response gene promoters. (A) Schematic diagrams showing the regions that control T3-dependent transcription of the two T3-responsive genes TRβ and TH/bZIP. The positions of the transcription start site, T3REs, and primers used for PCR are indicated. (B) xN-CoR is associated with T3 response genes and released upon T3 treatment. Stage NF55 tadpole tail and intestinal nuclei were isolated after treatment with 10 nM T3 for 48 h. Chromatin was cross-linked with formaldehyde, fragmented, immunoprecipitated with antibody against xN-CoR, and analyzed by PCR for the presence of a T3RE-containing fragment of TRβA and TH/bZIP promoters or for the presence of IFABP promoter, which is not regulated by T3 and thus served as a negative control. (C) In the tail, TR binds to the T3REs constitutively while local histone acetylation is upregulated by T3 treatment. The experiments were done as for panel B except for the use of the antibody against TR or acetylated histone H4 for the ChIP assay. Aliquots of the chromatin before immunoprecipitation were used for PCR as the control for DNA quantity (input). The data represent one out of several independent experiments with identical results. Ct, control; AB, antibody.
As shown in Fig. 7B, ChIP analysis with the antibody against xN-CoR revealed xN-CoR recruitment to the TRβA and TH/bZIP promoters, but not to the IFABP promoter, in both the tails and intestines of premetamorphic tadpoles (stage NF55). On the other hand, no xN-CoR recruitment was observed for any promoter when preimmune serum was used for ChIP (data not shown).
The association of xN-CoR with the T3RE regions suggests that xN-CoR was recruited by unliganded TR in premetamorphic tadpoles. This conclusion is supported by the ability of T3 treatment (10 nM, 2 days) to release xN-CoR from both promoters in the tail and intestine (Fig. 7B). Furthermore, ChIP assays with TR and acetylated histone H4 antibodies showed that in the tail TR was bound to both promoters constitutively while histone acetylation was increased by T3 treatment (Fig. 7C) (40). No binding of TR or change in H4 acetylation was observed on the IFABP promoter (Fig. 7C).
xN-CoR is involved in the repression of T3 response genes in premetamorphic tadpoles.
To investigate directly whether xN-CoR participates in the regulation of T3 response genes in premetamorphic tadpoles, we employed an in vivo somatic gene transfer approach (5). We adapted this approach instead of transgenesis in order to avoid affecting embryogenesis, which would prevent the study of gene function during metamorphosis, as N-CoR is highly expressed during early development (data not shown). Thus, we microinjected into Xenopus dorsal tail muscle a plasmid with the constitutive CMV promoter driving the expression of the mouse N-CoR CoRNR box1, which differs from its Xenopus counterpart by only 1 aa (Fig. 2 and Table 1). This N-CoR fragment has been previously shown to interact strongly with unliganded TR and to function as a dominant-negative N-CoR by competing efficiently with full-length N-CoR for binding to TR (20). For a reporter, we used the dual luciferase system, consisting of the firefly luciferase gene driven by a T3-dependent promoter (T3RE-tk-fLuciferase) and the Renilla luciferase gene driven by the constitutive SV40 promoter (phRL-SV40). This allowed us to assay the activities of the firefly and Renilla luciferases in the same sample. When the two constructs were injected into the tadpole tail, the activities of the two enzymes were highly correlated (r = 0.97) (data not shown). Thus, to control for variations in injection among different animals, we coinjected the reporter plasmid and the control Renilla luciferase constructs and measured firefly luciferase activities normalized against Renilla luciferase activities.
By using this system, we found that overexpression of the dominant-negative CoRNR resulted in a dramatic upregulation of expression from the T3-dependent promoter (Fig. 8A). The control peptide (Ct) had no effect. The upregulation by CoRNR was dependent on the presence of the T3RE in the promoter, as no effect by CoRNR was observed when the firefly luciferase was under the control of the constitutive CMV promoter (Fig. 8B). As expected, the CoRNR box did not enhance the activity of the constitutively expressed Renilla luciferase from the SV40 promoter (Fig. 8C).
FIG. 8.
Dominant-negative N-CoR (CoRNR) abolishes the repression of T3RE-containing promoter by unliganded TR. (A) Overexpression of CoRNR, but not the control peptide (Ct), upregulates the expression of T3RE-tk-fLuciferase. An aliquot (0.5 μg) of the CoRNR vector, expressing the dominant-negative N-CoR, or the control vector, expressing a nonspecific peptide, was coinjected with 0.5 μg of the T3RE-tk-fLuciferase reporter and 0.1 μg of the control reporter phRL-SV40 into Xenopus dorsal tail muscle. Two days later, the luciferase activities were assayed from tail muscle homogenates. The ratio of the activity of the firefly luciferase (fLuciferase) to that of the control Renilla luciferase (rLuciferease) was plotted together with standard errors. (B) CoRNR had no effect on the firefly luciferase expression under the control of the CMV promoter, which is not regulated by T3. The experiments were done as for panel A except that T3RE-tk-fLuciferase was replaced by CMV-fLuciferase. (C) The dominant-negative N-CoR does not affect the expression of Renilla luciferase reporter under the control of a constitutive promoter. The experiments were done as for panel A except that T3RE-tk-fLuciferase was omitted. (D) Overexpression of TRα represses T3RE-tk-fLuciferase reporter while high levels of CoRNR overexpression alleviate this repression. The T3RE-tk-fLuciferase reporter (0.5 μg) and the control reporter plasmid phRL-SV40 (0.1 μg) were coinjected with or without the indicated amounts of pCMV-TRα, which expressed TRα, and the N-CoRNR vector or the Ct vector into Xenopus dorsal tail muscle. The activities of the luciferases were measured and plotted as for panel A. The data are given as means ± standard errors of the means. Each points represents at least seven animals. **, significant changes (P < 0.01) between the two sets of data. All the experiments were done at least twice with similar results.
To determine whether the CoRNR effect on expression from the T3-dependent promoter involved TR, we overexpressed TRα using a CMV promoter construct. TRα overexpression repressed firefly luciferase expression driven by the T3-dependent promoter (Fig. 8D, compare the Ct column with 1.5 μg of empty vector to the Ct column with 0.5 μg of TRα vector). This could be due to insufficient levels of endogenous TRα in tail muscle cells (26) to completely repress transcription from the T3-dependent promoter. More importantly, in the presence of this overexpressed TRα, coinjection of 0.5 μg of CoRNR vector failed to upregulate the T3-dependent promoter in comparison to the control vector (compare the Ct and CoRNR columns with 0.5 μg of each vector in the middle of Fig. 8D), even though it could do so in the absence of the overexpressed TRα (Fig. 8A). On the other hand, increasing the amount of CoRNR but not the control peptide, i.e., injecting 1 μg of the respective vectors, could still upregulate the T3-dependent promoter even in the presence of overexpressed TRα (Fig. 8D, compare the Ct and CoRNR columns with 0.5 μg of TRα and 1 μg of Ct or CoRNR vectors). In addition, substituting TRβ for TRα yielded the same result (data not shown). Thus, the CoRNR relieves the repression of the reporter specifically by competing for binding to the unliganded TR at the T3RE.
DISCUSSION
The roles of active transcriptional repression in developmental and pathological processes have received ever-increasing attention in recent years. This has led to the identification and characterization of many corepressors. N-CoR is one of the best-studied such corepressors. However, few physiological data from appropriate in vivo models are available. Here, taking advantage of the total dependence of amphibian metamorphosis on T3, we provide strong in vivo evidence to show that xN-CoR participates in the repression of T3 response genes by unliganded TR during premetamorphic tadpole development.
N-CoR is highly conserved throughout evolution and plays multiple roles in vertebrate development.
N-CoR was initially identified as a corepressor for nuclear hormone receptors and subsequently found to interact with a number of other transcription factors as well. Thus, it is not surprising that Xenopus N-CoR is highly conserved with its mammalian homologue. The sequence homologies imply similar properties of N-CoRs in different species. Thus, the biological functions of N-CoR will probably be determined by its spatial and temporal expression profiles as well as by those factors interacting with N-CoR.
The broad tissue expression profiles and the existence of many N-CoR-interacting transcription factors suggest multiple roles for N-CoR during development. Consistent with this idea, N-CoR knockout mice show multiple defects during embryogenesis, including decreased erythroblast proliferation, leading to embryonic lethality (23). This makes it difficult to use N-CoR knockout mice to analyze the role of N-CoR in nuclear receptor function in development. Similarly, targeting a dominant-negative corepressor to mouse liver leads to increased cell proliferation and upregulation of T3 response genes in hypothyroid animals (8). It is unclear, however, how N-CoR affects such diverse processes, as many transcription factors that are capable of interacting with N-CoR are likely involved. Frog metamorphosis, on the other hand, is basically controlled by a single transcriptional factor, the TR. The presence of xN-CoR in premetamorphic tadpole tissues suggests that it is involved in transcriptional repression by unliganded TRs (see below).
In addition, xN-CoR also appears to have a role in organ transformation during metamorphosis. Our expression analysis shows that the expression of xN-CoR is upregulated during both natural and T3-induced metamorphosis, especially in the tail. It is very likely that xN-CoR exerts its metamorphic roles through transcription factors other than TRs, as the presence of T3 during metamorphosis will dissociate N-CoR from TR. Our earlier studies have shown that the N-CoR-binding corepressor Sin3 and its associated HDAC rpd3, or HDAC1/2, have similar expression profiles to those of xN-CoR (41, 42). The biochemical purification of N-CoR complexes has led to the identification of an N-CoR-Sin3-rpd3 complex in X. laevis (24). In addition, direct support for the involvement of an HDAC complex in metamorphosis has come from studies using a specific inhibitor of HDACs, trichostatin A, which blocks metamorphosis by inhibiting the regulation of late T3 response genes without affecting activation of direct T3 response genes (41). These results suggest that the xN-CoR-Sin3-rpd3 complex may be actively involved in a step downstream of gene activation by liganded TR in most, if not all, organs during metamorphosis.
The involvement of corepressors both in the repression by unliganded TR during premetamorphosis and at a later, TR-independent step during metamorphosis is also supported by the similar expression profiles of the related corepressor xSMRT during development. Whether N-CoR and SMRT are functionally redundant during development remains to be determined. On the other hand, N-CoR null cells, which retain SMRT expression, lose their ability to repress TR target genes (23). Clearly, further studies with different model systems, including amphibian development, are needed to clarify this important issue in development.
N-CoR participates in the repression of T3 response genes in premetamorphic tadpoles.
N-CoR has been shown to bind to TR in the absence of T3, and inhibiting N-CoR function is known to increase the expression of T3 response genes in the absence of T3 (8, 19, 23). However, there has been little in vivo evidence demonstrating the physiological role of N-CoR in TR function. As in amphibian development, unliganded TR has been suggested to play a role during development of fish (7, 62) and mammals (13, 15). The physiological importance of repression by unliganded TR is also supported by the fact that mice lacking TRs (both α and β) and T3 exhibit a milder phenotype than mice lacking only T3 (9). An interesting parallel to frog development is found in some insects that undergo metamorphosis during their life cycle. There, the ecdysone receptor, which, like TR, mediates the metamorphic signal, also functions as a transcriptional repressor in the absence of its ligand (50) and requires the N-CoR-related protein SMRTER to repress genes (51). All of these processes that involve nuclear receptor-mediated gene repression are characterized by the presence of a growth period. The total dependence of amphibian metamorphosis provides a rare, well-defined system to investigate the developmental function of gene repression by unliganded TR and the involvement of N-CoR in such processes in vivo.
Earlier work using the frog oocyte system has demonstrated the dual functions of TR even in the context of chromatin (59, 60). In addition, several studies support a role for unliganded TR in gene repression during Xenopus development. First, TRα genes are activated after tadpole hatching (stage NF35) but well before the synthesis of endogenous T3, although TRβ genes are repressed until metamorphosis (64). Second, we have shown that TRs are bound to T3REs of endogenous T3 response genes prior to metamorphosis (40). Finally, overexpression of TRs and RXRs together in Xenopus embryos leads to repression of known T3 response genes in the absence of T3 (38).
Our present data indicate that a potential mechanism for the repression by unliganded TR is through xN-CoR. First, xN-CoR is expressed in premetamorphic tadpoles. Second, xN-CoR only binds TR in the absence of T3. More importantly, our ChIP analyses clearly demonstrate for the first time that xN-CoR is specifically recruited to endogenous T3 response genes in the absence of ligand in developing animals. Moreover, T3 treatment of premetamorphic tadpoles leads to the release of xN-CoR from the T3 response genes, arguing that xN-CoR is recruited by unliganded TR. Finally, in vivo expression of a dominant-negative N-CoR in premetamorphic tadpole tail muscle reverses the repression of a T3-dependent reporter by unliganded TR. Due to the low percentages of the tail cells transfected by the in vivo gene transfer approach, it is difficult to directly demonstrate that the dominant-negative N-CoR was recruited by TR to the reporter gene in the transfected tadpole tail. Existing in vitro and in vivo studies argue that this is the underlying mechanism. Taking all our findings together, it is most likely that during premetamorphic development, unliganded TRs recruit xN-CoR to repress target gene transcription. As the expression of the T3 response genes is believed to initiate metamorphosis, their repression by the unliganded TR through xN-CoR would serve to prevent premature tissue transformation, thus ensuring proper tadpole growth. As T3 becomes available later in development, this repression is relieved to activate the metamorphic tissue transformation processes.
Acknowledgments
We thank A. P. Wolffe, F. Urnov, T. Collingwood, and P. Wade for helpful discussions. Alan Wolffe died in an accident recently. His encouragement and suggestions are remembered with gratitude. We are indebted to M. A. Lazar and T. Collingwood for providing plasmids. We also thank Gérard Benisti and Etienne LeGoff (UMR 8572, Paris, France) for taking care of animals.
This work was supported in part by the Association pour la Recherche contre le Cancer, the Centre Nationale de la Recherche Scientifique, and the Muséum National d'Histoire Naturelle.
P.L.J. and E.H. contributed equally to this study.
REFERENCES
- 1.Alland, L., R. Muhle, Jr., H. Hou, J. Potes, L. Chin, N. Schreiber-Agus, and R. A. DePinho. 1997. Role for N-CoR and histone deacetylase in Sin3-mediated transcriptional repression. Nature 387:49-55. [DOI] [PubMed] [Google Scholar]
- 2.Almouzni, G., and A. P. Wolffe. 1993. Replication coupled chromatin assembly is required for the repression of basal transcription in vivo. Genes Dev. 7:2033-2047. [DOI] [PubMed] [Google Scholar]
- 3.Burke, L. J., and A. Baniahmad. 2000. Co-repressors 2000: FASEB J. 14:1876-1888. [DOI] [PubMed] [Google Scholar]
- 4.Chen, J. D., and R. M. Evans. 1995. A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature 377:454-457. [DOI] [PubMed] [Google Scholar]
- 5.de Luze, A., L. Sachs, and B. Demeneix. 1993. Thyroid hormone-dependent transcriptional regulation of exogenous genes transferred into Xenopus tadpole muscle in vivo. Proc. Natl. Acad. Sci. USA 90:7322-7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dodd, M. H. I., and J. M. Dodd. 1976. The biology of metamorphosis, p. 467-599. In B. Lofts (ed.), Physiology of the amphibia. Academic Press, New York, N.Y.
- 7.Essner, J. J., J. J. Breuer, R. D. Essner, S. C. Fahrenkrug, and P. B. Hackett, Jr. 1997. The zebra fish thyroid hormone receptor α1 is expressed during early embryogenesis and can function in transcriptional repression. Differentiation 62:107-117. [DOI] [PubMed] [Google Scholar]
- 8.Feng, X., Y. Jiang, P. Meltzer, and P. M. Yen. 2001. Transgenic targeting of a dominant negative corepressor to liver blocks basal repression by thyroid hormone receptor and increases cell proliferation. J. Biol. Chem. 276:15066-15072. [DOI] [PubMed] [Google Scholar]
- 9.Flamant, F., A.-L. Poguet, M. Plateroti, O. Chassande, K. Gauthier, N. Streichnberger, A. Mansouri, and J. Samarut. 2001. Congenital hypothyroid Pas8−/− mutant mice can be rescued by inactivating the TRα gene. Mol. Endocrinol. 16:24-32. [DOI] [PubMed] [Google Scholar]
- 10.Frohman, M. A., M. K. Dush, and G. R. Martin. 1988. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc. Natl. Acad. Sci. USA 85:8998-9002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furlow, J. D., and D. D. Brown. 1999. In vitro and in vivo analysis of the regulation of a transcription factor gene by thyroid hormone during Xenopus laevis metamorphosis. Mol. Endocrinol. 13:2076-2089. [DOI] [PubMed] [Google Scholar]
- 12.Gao, X., T. Sedgwick, Y.-B. Shi, and T. Evans. 1998. Distinct functions are implicated for the GATA-4, -5, and -6 transcription factors in the regulation of intestine epithelial cell differentiation. Mol. Cell. Biol. 18:2901-2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gauthier, K., O. Chassande, M. Plateroti, J. P. Roux, C. Legrand, B. Pain, B. Rousset, R. Weiss, J. Trouillas, and J. Samarut. 1999. Different functions for the thyroid hormone receptors TRα and TRβ in the control of thyroid hormone production and post-natal development. EMBO J. 18:623-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glass, C. K., and M. G. Rosenfeld. 2000. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 14:121-141. [PubMed] [Google Scholar]
- 15.Gothe, S., Z. Wang, L. Ng, J. M. Kindblom, A. Campos Barros, C. Ohlsson, B. Vennström, and D. Forrest. 1999. Mice devoid of all known thyroid hormone receptors are viable but exhibit disorders of the pituitary-thyroid axis, growth, and bone maturation. Genes Dev. 13:1329-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guenther, M. G., W. S. Lane, W. Fischle, E. Verdin, M. A. Lazar, and R. Shiekhatter. 2000. A core SMRT corepressor complex containing HDAC3 and TGL1, a WD40-repeat protein linked to deafness. Genes Dev. 14:1048-1057. [PMC free article] [PubMed] [Google Scholar]
- 17.Guenther, M. G., O. Barak, and M. A. Lazar. 2001. The SMRT and N-CoR corepressors are activating cofactors for histone deacetylase 3. Mol. Cell. Biol. 21:6091-6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heinzel, T., R. M. Lavinsky, T.-M. Mullen, M. Soderstrom, C. D. Laherty, J. Torchia, W. M. Yang, G. Brard, S. D. Ngo, J. R. Davie, E. Seto, R. N. Eisenman, D. W. Rose, C. K. Glass, and M. G. Rosenfeld. 1997. A complex containing N-CoR, mSin3 and histone deacetylase mediates transcriptional repression. Nature 387:43-48. [DOI] [PubMed] [Google Scholar]
- 19.Hörlein, A. J., A. M. Näär, T. Heinzel, J. Torchia, B. Gloss, R. Kurokawa, A. Ryan, Y. Kamei, M. Söderström, C. K. Glass, and M. G. Rosenfeld. 1995. Ligand-independent repression by the thyroid hormone receptor mediated by a nuclear receptor co-repressor. Nature 377:397-404. [DOI] [PubMed] [Google Scholar]
- 20.Hu, I., and M. A. Lazar. 1999. The CoRNR motif controls the recruitment of corepressors by nuclear hormone receptors. Nature 402:93-96. [DOI] [PubMed] [Google Scholar]
- 21.Hu, I., and M. A. Lazar. 2000. Transcriptional repression by nuclear hormone receptors. Trends Endocrinol. Metab. 11:6-10. [DOI] [PubMed] [Google Scholar]
- 22.Huang, E. Y., J. Zhang, E. A. Miska, M. G. Guenther, T. Kouzarides, and M. A. Lazar. 2000. Nuclear receptor corepressors partner with class II histone deacetylases in a Sin3-independent pathway. Genes Dev. 14:45-54. [PMC free article] [PubMed] [Google Scholar]
- 23.Jepsen, K., O. Hermanson, T. M. Onami, A. S. Gleiberman, V. Lunyak, R. J. McEvilly, R. Kurokawa, V. Kumar, F. Liu, E. Seto, S. M. Hedrick, G. Mandel, C. K. Glass, D. W. Rose, and M. G. Rosenfeld. 2000. Combinatorial roles of the nuclear receptor corepressor in transcription and development. Cell 102:753-763. [DOI] [PubMed] [Google Scholar]
- 24.Jones, P. L., L. M. Sachs, N. Rouse, P. A. Wade, and Y.-B. Shi. 2001. Multiple N-CoR complexes contain distinct histone deacetylases. J. Biol. Chem. 276:8807-8811. [DOI] [PubMed] [Google Scholar]
- 25.Kao, H.-Y., M. Downes, P. Ordentlich, and R. M. Evans. 2000. Isolation of a novel histone deacetylase reveals that class I and II deacetylases promote SMRT-mediated repression. Genes Dev. 14:55-66. [PMC free article] [PubMed] [Google Scholar]
- 26.Kawahara, A., B. S. Baker, and J. R. Tata. 1991. Developmental and regional expression of thyroid hormone receptor genes during Xenopus metamorphosis. Development 112:933-943. [DOI] [PubMed] [Google Scholar]
- 27.Li, J., J. Wang, J. Wang, Z. Nawaz, J. M. Liu, J. Qin, and J. Wong. 2000. Both corepressor proteins SMRT and C-CoR exist in large protein complexes containing HDAC3. EMBO J. 19:4342-4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Machuca, I., G. Esslemont, L. Fairclough, and J. R. Tata. 1995. Analysis of structure and expression of the Xenopus thyroid hormone receptor β gene to explain its autoregulation. Mol. Endocrinol. 9:96-107. [DOI] [PubMed] [Google Scholar]
- 29.Mangelsdorf, D. J., C. Thummel, M. Beato, P. Herrlich, G. Schutz, K. Umesono, B. Blumberg, P. Kastner, M. Mark, P. Chambon, and R. M. Evans. 1995. The nuclear receptor superfamily: the second decade. Cell 83:835-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mangelsdorf, D. J., and R. M. Evans. 1995. The RXR heterodimers and orphan receptors. Cell 83:841-850. [DOI] [PubMed] [Google Scholar]
- 31.Mannervik, M., Y. Nibu, H. Zhang, and M. Levine. 1999. Transcriptional coregulators in development. Science 284:606-609. [DOI] [PubMed] [Google Scholar]
- 32.McKenna, N. J., R. B. Lanz, and B. W. O'Malley. 1999. Nuclear receptor coregulators: cellular and molecular biology. Endocr. Rev. 20:321-344. [DOI] [PubMed] [Google Scholar]
- 33.Nagy, L., H.-Y. Kao, J. D. Love, C. Li, E. Banayo, J. T. Gooch, V. Krishna, K. Chatterjee, R. M. Evans, and J. W. R. Schwabe. 1999. Mechanism of corepressor binding and release from nuclear hormone receptors. Genes Dev. 13:3209-3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nieuwkopp, P., and J. Faber. 1956. Normal table of Xenopus laevis. North Holland Publishing, Amsterdam, The Netherlands.
- 35.Ordentlich, P., M. Downes, W. Xie, A. Genin, N. B. Spinner, and R. M. Evans. 1999. Unique forms of human and mouse nuclear receptor corepressor SMRT. Proc. Natl. Acad. Sci. USA 96:2639-2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park, E.-J., D. J. Schroen, M. Yang, H. Li, L. Li, and J. D. Chen. 1999. SMRTe, a silencing mediator for retinoid and thyroid hormone receptors—extended isoform that is more related to the nuclear receptor corepressor. Proc. Natl. Acad. Sci. USA 96:3519-3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perissi, V., L. M. Staszewski, E. M. McInerney, R. Kurokawa, A. Krones, D. W. Rose, M. H. Lambert, M. V. Milburn, C. K. Glass, and M. G. Rosenfeld. 1999. Molecular determinants of nuclear receptor-corepressor interaction. Genes Dev. 13:3198-3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Puzianowska-Kuznicka, M., S. Damjanovski, and Y.-B. Shi. 1997. Both TR and RXR are required to efficiently mediate the effects of thyroid hormone on embryonic development and specific gene regulation in Xenopus laevis. Mol. Cell. Biol. 17:4738-4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ranjan, M., J. Wong, and Y.-B. Shi. 1994. Transcriptional repression of Xenopus TRβ gene is mediated by a thyroid hormone response element located near the start site. J. Biol. Chem. 269:24699-24705. [PubMed] [Google Scholar]
- 40.Sachs, L. M., and Y.-B. Shi. 2000. Targeted chromatin binding and histone acetylation in vivo by thyroid hormone receptor during amphibian development. Proc. Natl. Acad. Sci. USA 97:13138-13143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sachs, L. M., T. Amano, N. Rouse, and Y.-B. Shi. 2001. Requirement of histone deacetylase at two distinct steps in thyroid hormone receptor mediated gene regulation during amphibian development. Dev. Dyn. 222:280-291. [DOI] [PubMed] [Google Scholar]
- 42.Sachs, L. M., T. Amano, and Y.-B. Shi. 2001. An essential role of histone deacetylases in postembryonic organ transformations in Xenopus laevis. Int. J. Mol. Med. 8:595-601. [DOI] [PubMed] [Google Scholar]
- 43.Seol, W., M. J. Mahon, Y. K. Lee, and D. D. Moore. 1996. Two receptor interacting domains in the nuclear hormone receptor corepressor RIP13/N-CoR. Mol. Endocrinol. 10:1646-1655. [DOI] [PubMed] [Google Scholar]
- 44.Shi, Y.-B., and W. P. Hayes. 1994. Thyroid hormone-dependent regulation of the intestinal fatty acid-binding protein gene during amphibian metamorphosis. Dev. Biol. 161:48-58. [DOI] [PubMed] [Google Scholar]
- 45.Shi, Y.-B., and V. C.-T. Liang. 1994. Cloning and characterization of the ribosomal protein L8 gene from Xenopus laevis. Biochem. Biophys. Acta 1217:227-228. [DOI] [PubMed] [Google Scholar]
- 46.Shi, Y.-B., and A. Ishizuya-Oka. 1996. Biphasic intestinal development in amphibian: embryogenesis and remodeling during metamorphosis. Curr. Top. Dev. Biol. 32:205-235. [DOI] [PubMed] [Google Scholar]
- 47.Shi, Y.-B. 1996. Thyroid hormone-regulated early and late genes during amphibian metamorphosis, p. 505-538. In L. I. Gilbert, J. R. Tata, and B. G. Atkinson (ed.), Metamorphosis: post-embryonic reprogramming of gene expression in amphibian and insect cells. Academic Press, New York, N.Y.
- 48.Shi, Y.-B. 1999. Amphibian metamorphosis. From morphology to molecular biology. John Wiley & Sons, Inc., New York, N.Y.
- 49.Tata, J. R. 1993. Gene expression during metamorphosis: an ideal model for post-embryonic development. BioEssays 15:239-248. [DOI] [PubMed] [Google Scholar]
- 50.Thornmeyer, D., S. P. Tenbaum, R. Renkawitz, and A. Baniahmad. 1999. EcR interacts with corepressors and harbours an autonomous silencing domain functional in both Drosophila and vertebrate cells. J. Steroid Biochem. Mol. Biol. 68:163-169. [DOI] [PubMed] [Google Scholar]
- 51.Tsai, C.-C., H.-Y. Kao, T.-P. Yao, M. McKeown, and R. M. Evans. 1999. SMRTER, a Drosophila nuclear receptor coregulator, reveals that EcR-mediated repression is critical for development. Mol. Cell 4:175-186. [DOI] [PubMed] [Google Scholar]
- 52.Uderhill, C., M. S. Qutob, S. P. Yee, and J. Torchia. 2000. A novel nuclear receptor corepressor complex, N-CoR, contains components of the mammalian SWI/SNF complex and corepressor KAP-1. J. Biol. Chem. 275:40463-40470. [DOI] [PubMed] [Google Scholar]
- 53.Urnov, F. D., J. Yee, L. Sachs, T. N. Collingwood, A. Bauer, H. Beug, Y.-B. Shi, and A. P. Wolffe. 2000. Targeting of N-CoR and histone deacetylase 3 by the oncoprotein v-ErbA yields a chomatin infrastructure-dependent transcriptional repression pathway. EMBO J. 19:4074-4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Urnov, F. D., and A. P. Wolffe. 2001. A necessary good: nuclear hormone receptors and their chromatin templates. Mol. Endocrinol. 15:1-16. [DOI] [PubMed] [Google Scholar]
- 55.Wang, J., T. Hoshino, R. L. Redner, S. Kajigaya, and J. M. Liu. 1998. ETO, fusion partner in t(8;21) acute myeloid leukemia, represses transcription by interaction with the human N-CoR/mSin3/HDAC1 complex. Proc. Natl. Acad. Sci. USA 95:10860-10865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Webb, P., C. M. Anderson, C. Valentine, P. Nguyen, A. Marimuthu, B. L. West, J. D. Baxter, and P. J. Kushner. 2000. The nuclear receptor corepressor (N-CoR) contains three isoleucine motifs (I/LXXII) that serve as receptor interaction domains (IDs). Mol. Endocrinol. 14:1976-1985. [DOI] [PubMed] [Google Scholar]
- 57.Wen, Y.-D., V. Perissi, L. M. Staszewski, W.-M. Yang, A. Krones, C. K. Glass, M. G. Rosenfeld, and E. Seto. 2000. The histone deacetylase-3 complex contains nuclear receptor corepressors. Proc. Natl. Acad. Sci. USA 97:7202-7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wong, J., and Y.-B. Shi. 1995. Coordinated regulation of and transcriptional activation by Xenopus thyroid hormone and retinoid X receptors. J. Biol. Chem. 270:18479-18483. [DOI] [PubMed] [Google Scholar]
- 59.Wong, J., Y.-B. Shi, and A. P. Wolffe. 1995. A role for nucleosome assembly in both silencing and activation of the Xenopus TRβA gene by thyroid hormone receptor. Genes Dev. 9:2696-2711. [DOI] [PubMed] [Google Scholar]
- 60.Wong, J., D. Patterton, A. Imhof, D. Guschin, Y.-B. Shi, and A. P. Wolffe. 1998. Distinct requirements for chromatin assembly in transcriptional repression by thyroid hormone receptor and histone deacetylase. EMBO J. 17:520-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wong, J., V.C.-T. Liang, L. M. Sachs, and Y.-B. Shi. 1998. Transcription from the thyroid hormone-dependent promoter of the Xenopus laevis thyroid hormone receptor βA gene requires a novel upstream element and the initiator, but not a TATA box. J. Biol. Chem. 273:14186-14193. [DOI] [PubMed] [Google Scholar]
- 62.Yamano, K., and S. Miwa. 1998. Differential gene expression of thyroid hormone receptor α and β in fish development. Gen. Compar. Endocrinol. 109:75-85. [DOI] [PubMed] [Google Scholar]
- 63.Yaoita, Y., Y.-B. Shi, and D. D. Brown. 1990. Xenopus laevis α and β thyroid hormone receptors. Proc. Natl. Acad. Sci. USA 87:7090-7094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yaoita, Y., and D. D. Brown. 1990. A correlation of thyroid hormone receptor gene expression with amphibian metamorphosis. Genes Dev. 4:1917-1924. [DOI] [PubMed] [Google Scholar]