Abstract
Chemotherapeutic drugs exhibit their cytotoxic effect by inducing apoptosis in tumor cells. Because the serine/threonine kinase Akt is involved in apoptosis suppression, we investigated the relationship between Akt activity and drug sensitivity. We discovered that certain chemotherapeutic drugs induced apoptosis with caspase activation only when Akt was inactivated after drug treatment, while inactivation of Akt was not observed when tumor cells showed resistance to the drug-induced caspase activation. So, turn-off of the Akt-mediated survival signal is correlated with the sensitivity of the cells to chemotherapy. With a cDNA microarray, we revealed that tumor necrosis factor receptor-associated death domain (tradd) gene expression was elevated in response to Akt inactivation. Reportedly, Forkhead family transcription factors are phosphorylated by Akt, which results in their nuclear exit and inactivation. Analysis of the tradd promoter revealed that it contains at least one potential Forkhead family transcription factor-responsive element, and we confirmed that this element was involved in chemotherapeutic drug-induced TRADD expression. Overexpression of mutant TRADD proteins to block its apoptosis-inducing capability attenuated chemotherapeutic drug-induced apoptosis. Thus, chemotherapeutic drugs exhibited their cytotoxic effects in part by down-regulating Akt signaling following TRADD expression. These results indicate that Akt kinase activity after drug treatment is a hallmark of sensitivity of the cells to chemotherapeutic drugs.
Many growth factors and cytokines have been reported to promote cell survival. The characterization of survival signal transduction pathways stimulated by these factors has revealed that phosphatidylinositide-3-OH kinase (PI3K) is involved in apoptosis suppression (16). PI3K is a heterodimeric lipid kinase consisting of an 85-kDa regulatory subunit and a 110-kDa catalytic subunit. After growth factor stimulation, PI3K is activated and generates phospholipid second-messenger molecules, phosphatidylinositol-3,4,5-trisphosphate [PtdIns(3,4,5)P3] and phosphatidylinositol-3,4-bisphosphate [PtdIns(3,4)P2], that raise a diverse set of cellular responses. The major targets of PtdIns(3,4,5)P3 and PtdIns(3,4)P2 are pleckstrin homology domain-containing proteins.
One target of PI3K is the pleckstrin homology domain-containing serine/threonine kinase Akt (also known as protein kinase B) (16), and some pharmacological PI3K inhibitors, such as wortmannin and LY294002, suppress Akt kinase activity. Akt was originally identified as the cellular homolog of the viral oncogene v-akt (45). Akt mediates many PI3K-regulated biological responses, including glucose uptake, protein synthesis, cell cycle progression, and inhibition of apoptosis. Catalytically inactive Akt resides within the cytosol. By stimulation with growth factors and cytokines, the PtdIns(3,4,5)P3 and PtdIns(3,4)P2 proteins generated lead to the recruitment of Akt to the plasma membrane. Akt is then phosphorylated at two specific phosphorylation sites, one in the T loop of the kinase domain (Thr308) and the other in the COOH terminus of the catalytic domain (Ser473), in a region termed the hydrophobic motif (51). Phosphorylation at both residues is necessary for full activation of Akt and the subsequent regulation of multiple cellular processes. Phosphorylation of Akt at Thr308 is catalyzed by ubiquitously expressed 3-phosphoinositide-dependent protein kinase 1 (PDK1), and the kinase responsible for phosphorylation of Akt at Ser473 was called PDK2 (2, 46, 47). Some investigators have reported that Ser473 of Akt was phosphorylated by Akt itself (50) or by PDK1 bound to the COOH-terminal 77-amino-acid fragment of protein kinase C-related kinase 2 (4) or that it was phosphorylated in an integrin-linked kinase-dependent manner (13).
By phosphorylating the proapoptotic Bcl-2 family member Bad, caspase family member caspase 9, and IκB kinase, Akt mediates cell survival (8, 12, 41). The observation that Akt translocated to the nucleus after stimulation with growth factors within 30 min (1) suggested that suppression and up-regulation of gene expressions could be another potential antiapoptotic signaling mechanism mediated by Akt (3, 35). The best-characterized nuclear substrates of Akt are Forkhead family transcription factors, such as FKHR, FKHRL1, and AFX, which are human homologues of the Caenorhabditis elegans protein encoded by the gene daf-16 (26). According to the new nomenclature for FKHR transcription factors, these three proteins have been assigned to the FOXO (named for Forkhead box, group O) subfamily of Forkhead transcription factors (28). Akt-dependent phosphorylation of FOXO subfamily members results in their cytoplasmic localization, possibly by binding to 14-3-3, and inability to exhibit their gene transcription activity (6).
The sensitivity of cells to chemotherapeutic drug-induced apoptosis appears to depend on the balance between proapoptotic and antiapoptotic signals. Therefore, it is possible that chemotherapeutic agents may induce apoptosis not only by increasing the proapoptotic signals but also by decreasing the antiapoptotic signals, such as the PI3K-Akt survival-signaling pathway. We thus investigated the relationship between Akt kinase activity and drug sensitivity. We found that some drugs down-regulated Akt kinase activity only when they could induce caspase activation and apoptotic morphological change, while other drugs that could not induce caspase activation did not affect Akt kinase activity. Because the overexpression of constitutively active Akt overcame the cytotoxic effects of apoptosis-inducing chemotherapeutic drugs, Akt inactivation might play an important role in the sensitization of the cells to some chemotherapeutic drugs. So, by using a cDNA microarray technique, we further investigated the gene expression that is critical for chemotherapeutic drug-induced apoptosis. We discovered that expression of the tumor necrosis factor (TNF) receptor-associated death domain (tradd) gene was promoted in response to Akt inactivation. TRADD is one of the first identified TNF-R1-associated proteins. The predicted 312-amino-acid TRADD protein contains a 111-amino-acid death domain with sequence similarity to that of TNFR1 (25). Analysis of the tradd gene promoter revealed that it contains one potential Forkhead binding motif. Electrophoretic mobility shift assay (EMSA) and promoter-luciferase assays revealed that tradd gene expression is regulated in an FKHR-dependent manner. Moreover, overexpression of the mutant form of TRADD that lacks the death domain attenuated chemotherapeutic drug-induced apoptosis. Therefore, TRADD expression induced by Akt inactivation following FKHR activation participates in chemotherapeutic drug-induced apoptosis.
MATERIALS AND METHODS
Cell culture conditions.
Human lung cancer A549 cells were cultured at 37°C in a humidified atmosphere of 5% CO2 and 95% air in RPMI 1640 medium (Nissui, Tokyo, Japan) supplemented with 10% heat-inactivated fetal bovine serum (Biocell, Carson, Calif.), 2 mM l-glutamine (Gibco Laboratories, Grand Island, N.Y.), and 100 μg of kanamycin per ml (53). Human embryonic kidney 293T cells and human fibrosarcoma HT1080 cells were cultured in Dulbecco's modified Eagle's medium (Nissui) supplemented with 10% fetal bovine serum (40).
Reagents.
Cisplatin (cDDP) and etoposide (VP-16) were kindly provided by Bristol-Myers Squibb Co., Ltd. (Tokyo, Japan). Adriamycin (ADR) was kindly provided by Meiji Seika Co., Ltd. (Tokyo, Japan). Camptothecin (CPT) was kindly provided by Yakult Co., Ltd. (Tokyo, Japan). Z-Asp (Z-Asp-CH2-DCB; benzyloxycarbonyl-Asp-CH2OCO-2,6,-dichlorobenzene) was purchased from Funakoshi (Tokyo, Japan). The fluorogenically labeled tetrapeptide acetyl-l-aspartyl-l-glutamyl-l-valyl-l-aspart-7-amino-4-methylcoumarin (DEVD-AMC) was obtained from the Peptide Institute (Osaka, Japan). LY294002 was purchased from Sigma (St. Louis, Mo.).
Plasmids.
Human wild-type and dominant-negative (K179M) akt1 cDNAs (WT-Akt and DN-Akt, respectively) in a pFLAG-CMV-2 vector (Sigma) were previously reported (40). The NH2-terminal myristoylated active mouse akt1 cDNA (Myr-Akt) in a pUSEamp vector was purchased from Upstate Biotechnology (Lake Placid, N.Y.). Human FLAG-tagged WT-FKHR (wild-type FKHR), AAA-FKHR (in which Akt phosphorylation sites Thr24, Ser256, and Ser319 were converted to Ala), and H215R-FKHR (which loses DNA-binding ability) in a pcDNA3 vector were kindly provided by Eric D. Tang and Frederic G. Barr (49). The pcDNA3 vector encoding enhanced green fluorescent protein (EGFP) has been described previously (33). Human WT-tradd cDNA was generated by reverse transcription (RT)-PCR with human embryonic kidney 293 cDNA as the template. The sense and antisense primers used for the PCR were 5′-AGCTTGAGATCGCAGCTGGGCAA-3′ and 5′-AGCTTCTAAGGCCATCCAGGTTCTC-3′, respectively. The PCR-amplified tradd fragment was digested and cloned into the HindIII site of the FLAG-tagged pcDNA3 vector. The Δ306-312 (Δ306-TRADD; amino acids 1 to 305), Δ301-312 (Δ301-TRADD; amino acids 1 to 300), and Δ296-312 (Δ296-TRADD; amino acids 1 to 295) deletion mutant forms of human tradd cDNA were also generated by PCR with WT-tradd cDNA as the template. The antisense primers used for Δ306-TRADD, Δ301-TRADD, and Δ296-TRADD were 5′-AGCTTCTAGGTCAGGCCCAGCAAGTCC-3′, 5′-AGCTTCTAGTCCTCTGCCAGGCTGGTGAG-3′, and 5′-AGCTTCTAGGTGAGCTCGTTCTCCTCGAGTG-3′, respectively. All PCR products were cloned into a FLAG-tagged pcDNA3 vector.
Transient transfection.
293T cells and HT1080 cells were transfected with a pcDNA3 vector containing EGFP cDNA (23) together with a pUSEamp vector containing mock or Myr-akt cDNA (Upstate Biotechnology) with Superfect reagent (Qiagen, Chatsworth, Calif.). After a 24-h recovery period, cells were treated with VP-16 for an additional 36 h. In some experiments, WT-akt, DN-akt, WT-FKHR, or H215R-FKHR cDNA was transfected with Superfect reagent into HT1080 cells. After transfection for 24 h, we performed a Western blot analysis or a semiquantitative RT-PCR as described below. To estimate the effects of TRADD mutant expression, HT1080 cells were transfected with a pcDNA3 vector containing EGFP cDNA together with FLAG-tagged WT or mutant tradd cDNA. After a 24-h recovery period, transfectants were treated with VP-16 for an additional 36 h.
Western blot analysis.
Western blot analysis was performed as described previously (40). We used antibodies to Akt, phospho-Akt (Ser473), phospho-FKHR (Ser256), and FKHR (New England Biolabs, Inc., Beverly, Mass.); an antibody to β-actin (Sigma); an antibody to caspase 9 (Pharmingen, San Diego, Calif.); antibodies to caspase 3 and PDK1 (Transduction Laboratories, Lexington, Ky.); an antibody to mitogen-activated protein kinase (MAPK; Santa Cruz Biotechnology, Santa Cruz, Calif.); an antibody to phospho-MAPK (Promega, Madison, Wis.); or an antibody to TRADD (MBL, Nagoya, Japan).
Apoptosis assay.
For flow cytometric analysis, cells were first fixed with 70% ethanol and then incubated with RNase A at 37°C for 1 h. The cells were then stained in propidium iodide solution (50 μg of propidium iodide per ml, 0.1% sodium citrate, 0.1% NP-40) for 30 min at 4°C. The cells were analyzed with a FACScan flow cytometer (Becton Dickinson, Braintree, Mass.) with Cell Quest software. To measure caspase activity in the cell lysates, we incubated them with DEVD-AMC as described previously (18). To measure the survival of transfected cells, we counted at least 100 EGFP-positive cells that did not show nuclear condensation and fragmentation as described previously (23).
Measurement of Akt and PDK1 kinase activities.
293T cells were treated with or without VP-16 for 36 h. The cells were then solubilized with lysis buffer containing 50 mM Tris-HCl (pH 7.5), 1% Triton X-100, 1 mM EDTA, 1 mM EGTA, 10 mM β-glycerophosphate, 50 mM sodium fluoride, 0.5 mM sodium vanadate, 5 mM sodium pyrophosphate, 1 μM microcystin, 0.1% 2-mercaptoethanol, 0.1 mM phenylmethylsulfonyl fluoride, and 1 μg each of aprotinin, pepstatin, and leupeptin per ml. Cell lysates were reacted with protein G-Sepharose that had been conjugated with a sheep anti-Akt antibody (Upstate Biotechnology) or a sheep anti-PDK1 antibody (Upstate Biotechnology) for 2 h at 4°C (36, 43). The beads were washed three times with lysis buffer. Akt or PDK1 kinase activity was estimated with an Akt kinase assay kit or a PDK1 kinase assay kit, respectively, in accordance with the manufacturer's (Upstate Biotechnology) instructions.
PI3K assay.
PI3K activity was determined as previously described (15). Briefly, cells were harvested and solubilized with lysis buffer containing 20 mM Tris-HCl (pH 7.5), 145 mM NaCl, 10% glycerol, 5 mM EDTA, 1% Triton X-100, 0.5% Nonidet P-40, 100 mM sodium fluoride, 0.5 mM sodium vanadate, and 10 μg each of aprotinin and leupeptin per ml. Cell lysates were incubated with agarose-conjugated antiphosphotyrosine (PY20) antibody (Transduction Laboratories) for 2 h at 4°C. The beads were then washed sequentially three times with wash buffer A (Tris-HCl [pH 7.5], 150 mM NaCl, 0.01% Nonidet P-40, 100 μM sodium vanadate), wash buffer B (100 mM Tris-HCl [pH 7.5], 500 mM LiCl, 100 μM sodium vanadate), and wash buffer C (10 mM Tris-HCl [pH 7.5], 100 mM NaCl, 1 mM EDTA, 100 μM sodium vanadate). The beads were then resuspended in 50 μl of kinase assay buffer (10 mM Tris-HCl [pH 7.5], 100 mM NaCl, 1 mM EDTA, 20 mM MgCl2), and the kinase reaction was initiated by adding 20 μg of phosphatidylinositol and 50 μM ATP containing 20 μCi of [γ-32P]ATP. The samples were incubated for 20 min at 23°C, and the reactions were terminated by adding 20 μl of 8 N HCl. The samples were then extracted with 160 μl of chloroform-methanol (1:1), and the organic phase was concentrated by evaporation. The resultant lipid fractions were resolved by thin-layer chromatography in chloroform-methanol-water-ammonium hydroxide (60:47:11.3:2). The phosphorylated products were then visualized by autoradiography.
Preparation of probes for microarrays.
A549 cells were treated with or without 50 μM LY294002 for 3 h. The cells were washed with phosphate-buffered saline, and total RNAs were extracted with an RNeasy Midi kit (Qiagen). Total RNAs were purified twice with spin columns containing oligodeoxythymidylic acid cellulose in the presence of 1 U of DNase I (mRNA Purification Kit; Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom). The purified mRNAs (2.5 μg) were incubated with 3 μg of random hexamers at 70°C for 10 min and cooled on ice. We then added 10 μl of 5× first-strand buffer (5 μl of 0.1 M dithiothreitol, 1.5 μl of RNase inhibitor, 1 μl of 25 mM dGTP, 1 μl of 25 mM dATP, 1 μl of 25 mM dTTP, 2 μl of 1 mM dCTP, 2 μl of 1 mM Cy-3-dCTP [for mRNA from control cells] or Cy-5-dCTP [for mRNA from LY294002-treated cells] [Amersham Pharmacia Biotech], 2.5 μl of Superscript II). After incubation at 37°C for 2 h and denaturation of enzymes in boiling water, template mRNAs were degraded at 37°C for 10 min with 2 μl of 2.5 N NaOH. The samples were then neutralized by addition of 2 μl of 2.5 N HCl and 10 μl of 1 M Tris-HCl (pH 6.8). The labeled probes were purified with a Qiagen PCR purification kit, and their volumes were adjusted to 5 μl. The labeled probes were boiled and then incubated at 70°C for 40 min with 1 mg of oligo(dA)80. Finally, 10 μl of microarray hybridization solution buffer version 2 (Amersham Pharmacia Biotech) and 20 μl of formamide were added.
Hybridization and scanning of microarrays.
After loading the probes, we covered the slides with coverslips, sealed them with adhesive, and then incubated the microarrays at 60°C for 14 h in humid boxes. Hybridized slides were washed for 10 min with 1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)-0.2% sodium dodecyl sulfate (SDS) at 55°C, twice for 10 min each time with 0.1× SSC-0.2% SDS at 55°C, twice for 1 min each time with 0.1× SSC at 23°C, and finally for 1 min with water at 23°C. Washed slides were air dried and scanned with a Microarray Scanner Generation III (Amersham Pharmacia Biotech) (34).
Quantification of data.
Signal intensities of Cy-3 (green) and Cy-5 (red) were obtained from the microarray images with data extraction software (Array Vision; Imaging Research Inc., Amersham Pharmacia Biotech). Average intensities from duplicate spots were calculated, and then we normalized the results for differential efficiencies of labeling and detection on the basis of signals from 52 preselected internal-control genes that are stable in most experiments.
Semiquantitative RT-PCR analysis.
We extracted total RNA from cells with TRIZOL reagent in accordance with the manufacturer's (GIBCO-BRL) instructions. RT-PCR experiments were carried out with cDNAs generated from 2 μg of total RNA with a GeneAmp RNA PCR kit (Applied Biosystems, Foster City, Calif.). The RT-PCR exponential phase was determined on 25 cycles to allow semiquantitative comparisons of cDNAs developed from identical reactions. All reactions involved initial denaturation at 94°C for 30 s, 60°C for 30 s, and 72°C for 1 min on a GeneAmp PCR system 9700 (Applied Biosystems).
EMSA.
293T cells were transfected with WT-FKHR or H215R-FKHR. Nuclear extracts from all samples were obtained by using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Pierce, Rockford, Ill.). Biotin end-labeled double-stranded oligonucleotides 5′-biotin-TGAAAACAAATTTATTTTGGGTAC-3′ and 5′-biotin-CCAAAATAAATTTGTTTTCAGTAC-3′ (−2027 to −2036 of TRADD), 5′-biotin-TGAAACCAAAGTCATTTTGGGTAC-3′ and 5′-biotin-CCAAAATGACTTTGGTTTCAGTAC-3′ (mutant TRADD), and 5′-biotin-TGCAAAACAAACTTATTTTGGGTAC-3′ and 5′-biotin-CCAAAATAAGTTTGTTTTGCAGTAC-3′ (IGF-BP1-IRS), as well as nonlabeled oligonucleotides containing FKHR consensus tradd gene sequences (5′-TGAAAACAAATTTATTTTGGGTAC-3′ and 5′-CCAAAATAAATTTGTTTTCAGTAC-3′) were generated with an oligonucleotide synthesizer (Forkhead binding sites are underlined). Binding reactions were performed in a buffer containing 5 μg of nuclear extract protein, buffer (10 mM Tris, [pH 7.5], 50 mM KCl, 5 mM MgCl2, 1 mM dithiothreitol, 0.05% NP-40, 2.5% glycerol), 1 μg of poly(dI-dC), and 2 mM biotin-labeled DNA. Reaction mixtures were incubated at 23°C for 20 min. Competition reactions were performed by adding a 200-fold excess of unlabeled double-stranded FKHR consensus oligonucleotide to the reaction mixture. Reaction mixtures were electrophoresed on a 6% precast TBE gel (Invitrogen) at 100 V for 1 h in 100 mM Tris-borate-EDTA buffer. Reaction mixtures were transfected onto a nylon membrane. The biotin-labeled DNA was detected with a LightShift chemiluminescent EMSA kit in accordance with the manufacturer's (Pierce) instructions.
Luciferase assay.
A 582-bp DNA fragment of tradd intron 1, −1663 to −2214, containing a potential Forkhead binding sequence (AAAACAAATT) was amplified by a PCR with forward primer 5′-CTAGCCAAGGGGCCAGAAAGACATG-3′ and reverse primer 5′-CTAGCGTAGCTGGGACTTTAGGCA-3′. The fragment was then cloned into a pGL3-promoter vector (Promega) (intron 1) at the NheI site. Double-stranded oligonucleotides containing a potential Forkhead binding sequence (sense, 5′-TGAAAACAAATTTATTTTGGGTAC-3′; antisense, 5′-CCAAAATAAATTTGTTTTCAGTAC-3′) were ligated into a pGL3-promoter vector at the KpnI site (pGL3-×1). Double-stranded oligonucleotides containing a mutant Forkhead binding sequence (sense, 5′-TGAAACCAAAGTCATTTTGGGTAC-3′; antisense, 5′-CCAAAATGACTTTGGTTTCAGTAC-3′) were also ligated into a pGL3-promoter vector at the KpnI site (pGL3-Mutant). Double-stranded oligonucleotides contained in IGF-BP1-IRS (sense, 5′-TGCAAAACAAACTTATTTTGGGTAC-3′; antisense, 5′-CCAAAATAAGTTTGTTTTGCAGTAC-3′) were also ligated into a pGL3-promoter vector at the KpnI site (pGL3-1×IRS). Double-stranded oligonucleotides were further ligated tandemly into the pGL3-promoter vector to generate pGL3-×2 or pGL3-3×IRS. Quantification of all luciferase activities was performed with a dual-luciferase reporter assay system in accordance with the manufacturer's (Promega) instructions. Luciferase activity was measured with a MicroLumat LB96P luminometer (Berthold, Bad Wildbad, Germany). Activities were normalized by cotransfection of control thymidine kinase-driven Renilla luciferase plasmid pRL-TK (Promega).
RESULTS
Relationship between cell sensitivity to chemotherapeutic drugs and Akt activity.
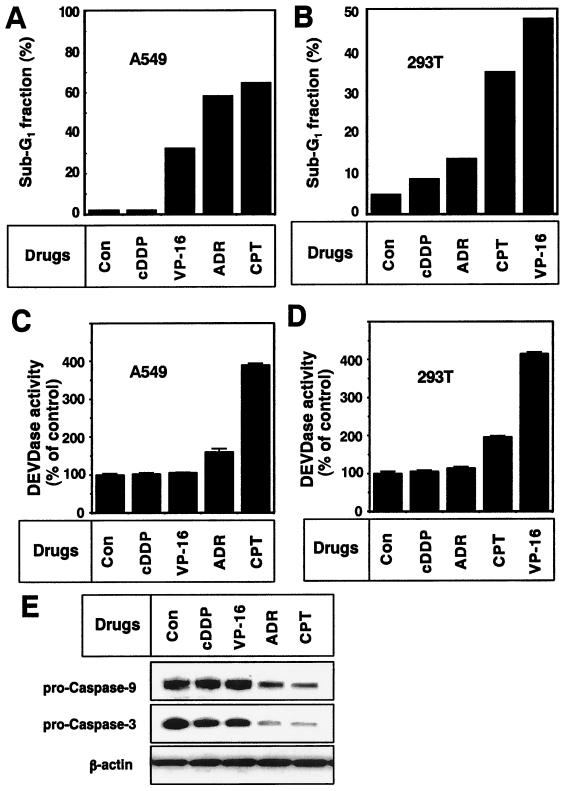
First, we examined the sensitivity of A549 and 293T cells to cDDP, VP-16, ADR, and CPT. VP-16, ADR, and CPT increased the sub-G1 fraction of the population of A549 cells (Fig. 1A), and cDDP resulted in no increase. Similarly, ADR, CPT, and VP-16, but not cDDP, increased the apoptotic sub-G1 fraction of 293T cells (Fig. 1B). We observed caspase activation (Fig. 1C) and a decrease in latent forms of caspases 3 and 9 (Fig. 1E) in ADR- and CPT-treated A549 cells. Caspase activation was not observed in cDDP- or VP-16-treated A549 cells (Fig. 1C and E), although VP-16 induced an apoptotic morphological change in A549 cells. Moreover, caspase activation was observed in CPT- and VP-16-treated 293T cells but not in those treated with cDDP or ADR (Fig. 1D). Therefore, A549 and 293T cells showed different sensitivities to chemotherapeutic drugs.
FIG. 1.
Chemotherapeutic drug-induced apoptosis with caspase activation in A549 and 293T cells. A549 (A and B) or 293T (C and D) cells were treated with 10 μM cDDP, VP-16, ADR, or CPT for 48 h. (A and C) The apoptotic sub-G1 fraction of the population was determined by FACScan analyses as described in Materials and Methods. (B and D) Cell lysates were incubated with 10 μM fluorogenically labeled tetrapeptide DEVD-AMC for 1 h at 37°C. The increase in caspase 3-like protease (DEVDase) activity in the cell lysates was determined as described in Materials and Methods. Each vertical bar represents the mean ± the standard deviation of three independent experiments. (E) After treatment with the indicated drugs (10 μM) for 48 h, A549 cell lysates were subjected to Western blot analysis with antibodies to procaspase 9, procaspase 3, and β-actin. Con, no-treatment control.
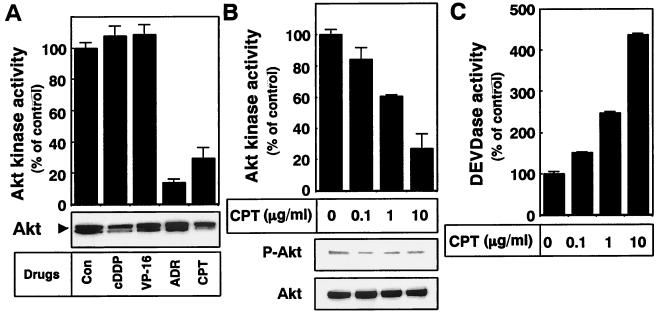
Because inhibition of the Akt survival pathway is linked with the cytotoxic effects of some drugs (24, 36), we next investigated the change in Akt kinase activity after drug treatment in A549 cells. As shown in Fig. 2A, Akt kinase activity was down-regulated only when A549 cells were incubated with ADR and CPT. By contrast, cDDP and VP-16, neither of which could induce caspase activation in A549 cells (Fig. 1C), had no effect on Akt kinase activity (Fig. 2A). CPT decreased Akt kinase activity in a dose-dependent manner (Fig. 2B). Since activation of Akt correlates with phosphorylation of the kinase on the Thr308 and Ser473 residues, we used an anti-phospho-Akt (Ser473) antibody to examine the amount of the phosphorylated form of Akt after CPT treatment. Consistent with the decrease in Akt kinase activity, CPT treatment decreased the amount of phospho-Akt (Fig. 2B). Under these conditions, we found that CPT promoted the activation of caspase 3-like proteases in a dose-dependent manner (Fig. 2C). These results indicate that caspases were activated in inverse proportion to Akt inactivation.
FIG. 2.
Down-regulation of Akt kinase activity by chemotherapeutic drug treatment. (A) A549 cells were treated with medium alone (control [Con]) or 10 μM cDDP, VP-16, ADR, or CPT for 36 h. Endogenous Akt was then immunoprecipitated from each cell lysate and subjected to Akt kinase activity assay as described in Materials and Methods. Each vertical bar represents the mean ± the standard deviation of three independent experiments. The amount of immunoprecipitated Akt was confirmed by Western blot analysis with an anti-Akt antibody (bottom). (B and C) A549 cells were treated with the indicated concentrations of CPT for 36 h. (B) Akt was immunoprecipitated from each cell lysate and subjected to Akt kinase activity assay as described in Materials and Methods. Each vertical bar represents the mean ± the standard deviation of three independent experiments. Cell lysates were subjected to Western blot analysis with an anti-phospho-Akt (Ser473) antibody or an anti-Akt antibody. (C) Each cell lysate was incubated with DEVD-AMC for 1 h at 37°C. The increase in caspase 3-like protease (DEVDase) activity in the cell lysates was determined as described in Materials and Methods. Each vertical bar represents the mean ± the standard deviation of three independent experiments.
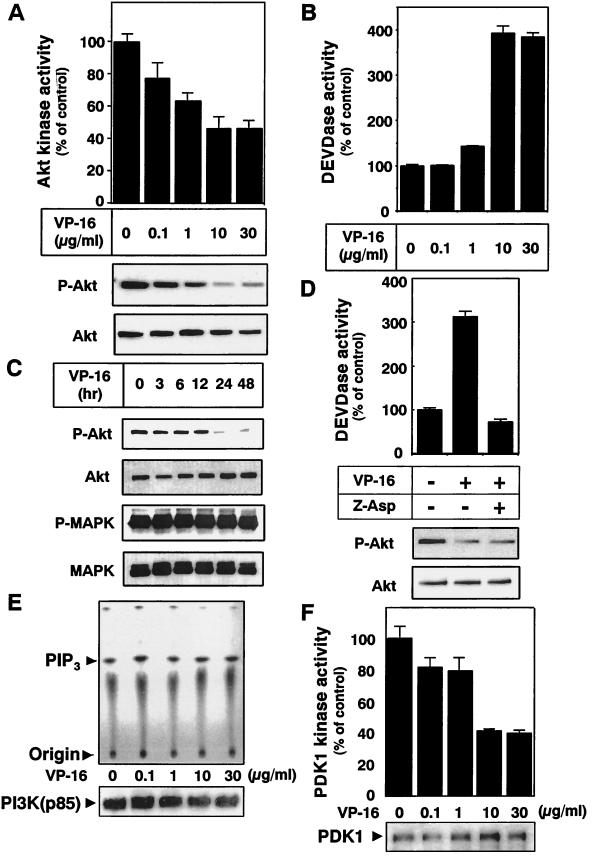
We also examined the phospho-Akt level and Akt kinase activity after drug treatment in 293T cells and found that VP-16 could induce apoptosis with caspase activation (Fig. 1B and D). VP-16 decreased Akt kinase activity with Akt dephosphorylation in a dose-dependent fashion in 293T cells (Fig. 3A). Like CPT-treated A549 cells, caspase 3-like proteases were activated in inverse proportion to the Akt inactivation (Fig. 3B). Time course analysis revealed that down-regulation of the phospho-Akt level occurred 24 h after VP-16 addition. We also investigated whether VP-16 resulted in a general inactivation of growth factor-induced signaling pathways. We examined the change in the amount of phospho-MAPK/extracellular signal-regulated kinase by Western blot analysis. No change was found after VP-16 treatment (Fig. 3C). These results indicate that the cytotoxic effect of VP-16 in 293T cells might be mediated via inhibition of the Akt survival-signaling pathway.
FIG. 3.
Inactivation of the PDK1-Akt signaling pathway during VP-16-induced 293T-cell apoptosis. (A and B) 293T cells were treated with the indicated concentrations of VP-16 for 36 h. (A) Endogenous Akt was immunoprecipitated from each cell lysate and subjected to Akt kinase activity assay as described in Materials and Methods. Each vertical bar represents the mean ± the standard deviation of three independent experiments. Cell lysates were subjected to SDS-polyacrylamide gel electrophoresis, followed by Western blot analysis with an anti-phospho-Akt (Ser473) antibody or an anti-Akt antibody (bottom). The results shown are representative of at least two independent experiments. (B) Cell lysates were incubated with DEVD-AMC for 1 h at 37°C. The increase in caspase 3-like protease (DEVDase) activity in the cell lysates was determined as described in Materials and Methods. Each vertical bar represents the mean ± the standard deviation of three independent experiments. (C) 293T cells were incubated with 10 μg of VP-16 per ml. At the indicated time points, cells were harvested and subjected to Western blot analysis with an anti-phospho-Akt (Ser473) antibody, an anti-Akt antibody, an anti-phospho-MAPK antibody, or an anti-MAPK antibody. (D) 293T cells were incubated with medium alone, 10 μg of VP-16 per ml, or 10 μg of VP-16 per ml plus 100 μg of Z-Asp per ml for 36 h. The increase in caspase 3-like protease (DEVDase) activity in the cell lysates was determined as described in Materials and Methods. Each vertical bar represents the mean ± the standard deviation of three independent experiments. Cell lysates were subjected to Western blot analysis with an anti-phospho-Akt (Ser473) antibody or an anti-Akt antibody (bottom). (E and F) 293T cells were incubated with the indicated concentration of VP-16 for 36 h. (E) Phosphotyrosine-containing proteins were immunoprecipitated with an antiphosphotyrosine antibody (PY20) and then incubated with phosphatidylinositol in the presence of 50 μM ATP containing 20 μCi of [γ-32P]ATP. PI3K kinase activity was assessed by resolving the lipid fractions on a thin-layer chromatography plate following autoradiography. The amount of immunoprecipitated PI3K was confirmed by Western blot analysis with an anti-p85α subunit of PI3K (bottom). (F) PDK1 kinase activity was evaluated as described in Materials and Methods. The PDK1 kinase activity in untreated 293T cell lysates was normalized as 100%. Each vertical bar represents the mean ± the standard deviation of three independent experiments. The amount of immunoprecipitated PDK1 was confirmed by Western blot analysis with an anti-PDK1 antibody (bottom).
Akt inactivation occurs prior to caspase activation.
Akt has been shown to suppress caspase activation both directly and indirectly (51). In order to exclude the possibility that Akt inactivation is an event that occurs downstream of caspase activation, we examined the effect of caspase inhibitor Z-Asp (32) on VP-16-induced Akt dephosphorylation in 293T cells. Addition of Z-Asp suppressed VP-16-induced activation of caspase 3-like proteases in these cells (Fig. 3D). However, Z-Asp could not suppress Akt dephosphorylation (Fig. 3D). We also found that Z-Asp could not inhibit the Akt dephosphorylation induced by CPT in A549 cells (data not shown). These results indicate that inhibition of Akt is an event that occurs upstream of caspase activation in chemotherapeutic drug-induced apoptosis.
Suppression of PDK1 is one of the mechanisms of chemotherapeutic drug-mediated Akt inactivation.
To clarify the mechanisms of Akt dephosphorylation after treatment of cells with apoptosis-promoting chemotherapeutic drugs, we estimated the activities of the upstream Akt kinases PI3K and PDK1. PI3K plays an important role in the activation of Akt by producing PtdIns(3,4,5)P3 and PtdIns(3,4)P2, which recruit Akt to the plasma membrane (1). PDK1 was known to phosphorylate Akt at the Thr308 residue (2, 46, 47). We first examined the activities of PI3K by incubating phosphatidylinositol with the immunoprecipitated phosphotyrosine-containing proteins. As shown in Fig. 3E, VP-16 treatment had no effect on PI3K activity in 293T cells. Treatment of VP-16 also did not affect the level of PI3K expression. In contrast, VP-16 suppressed PDK1 kinase activity in 293T cells in a dose-dependent manner without affecting the PDK1 expression level (Fig. 3F). To examine whether VP-16 directly inhibits PDK1 kinase activity, recombinant active PDK1 was incubated with VP-16 in vitro before measurement of its kinase activity. VP-16 had no direct effect on PDK1 kinase activity in vitro (data not shown). Therefore, VP-16 indirectly suppressed PDK1 kinase activity in cells, resulting in Akt inactivation. Although we could not rule out other possibilities (e.g., activation of phosphatases or an effect on PI3K substrate availability in the cells), PDK1 inactivation might be one of the mechanisms of VP-16-mediated Akt inactivation.
Overexpression of constitutively active Akt overcomes chemotherapeutic drug-induced apoptosis.
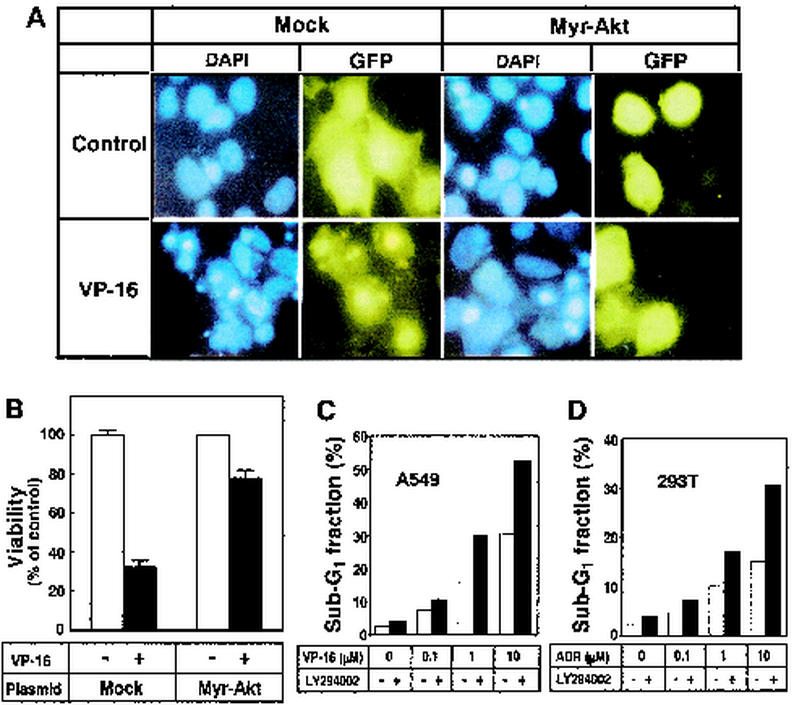
To confirm that Akt inactivation is involved in chemotherapeutic drug-induced apoptosis, we transfected an active form of akt cDNA (Myr-akt) together with pcDNA3-EGFP into HT1080 cells. Transfection of Myr-akt alone had no effect on the cells' steady-state survival. After cultivation for 36 h in medium containing 10 μg of VP-16 per ml, cells were stained with 4′,6′-diamidino-2-phenylindole (DAPI) to detect apoptotic cells with condensed and fragmented nuclei. As shown in Fig. 4A, VP-16 induced the formation of condensed and fragmented nuclei in HT1080 cells and transfection of the active form of Akt suppressed apoptotic morphological changes in VP-16-treated cells. We then determined the number of surviving cells without nuclear condensation and fragmentation among 100 EGFP-positive (transfected) cells (Fig. 4B). Treatment of VP-16 decreased the viable HT1080 cell proportion to about 30%. Overexpression of Myr-Akt overcame the cytotoxic effects of VP-16 and increased the proportion of surviving cells to about 80%. These results indicate that Akt inactivation participates in enhancement of the sensitivity of HT1080 cells to VP-16.
FIG. 4.
Overexpression of the active form of Akt overcomes the cytotoxic effect of VP-16. (A and B) HT1080 cells were transfected with the pcDNA3-EGFP plasmid together with the empty vector pUSEamp (Mock) or the pUSEamp vector encoding the active form of Akt (Myr-Akt). After transfection for 24 h, HT1080 cells were treated with 10 μg of VP-16 per ml for an additional 36 h. After fixation, cells were stained with DAPI. Nuclear fragmentation was visualized with a fluorescence microscope. (B) The number of surviving cells was determined by counting 100 EGFP-positive (transfected) cells that did not show nuclear condensation and fragmentation. Each vertical bar represents the mean ± the standard deviation of three independent experiments. (C) A549 cells were treated with the indicated concentrations of VP-16 in the presence (solid columns) or absence (open columns) of 20 μM LY294002 for 48 h. (D) 293T cells were treated with the indicated concentrations of ADR in the presence (solid columns) or absence (open columns) of 20 μM LY294002 for 48 h. The apoptotic sub-G1 fraction of the population was determined by FACScan analysis as described in Materials and Methods.
To further support the notion that Akt inhibition is a prerequisite for chemotherapeutic drugs to induce apoptosis, we examined the effect of the PI3K inhibitor LY294002 on the apoptotic responses of A549 cells to VP-16 and 293T cells to ADR. As shown in Fig. 1 and 2, VP-16 could not inhibit Akt and could not induce caspase activation in A549 cells. However, cotreatment of A549 cells with 20 μM LY294002 increased the apoptotic sub-G1 fractions (Fig. 4C). ADR was not a strong apoptotic agent in 293T cells (Fig. 1). Inhibition of Akt by LY294002 sensitized 293T cells to the apoptotic effect of ADR (Fig. 4D). These results rule out the possibility that cells are resistant to the drugs simply because they do not respond to them. Moreover, Akt inactivation is required for chemotherapeutic drugs to induce apoptosis with caspase activation.
Inhibition of the PI3K-Akt pathway increases tradd gene expression by activating Forkhead transcription factors.
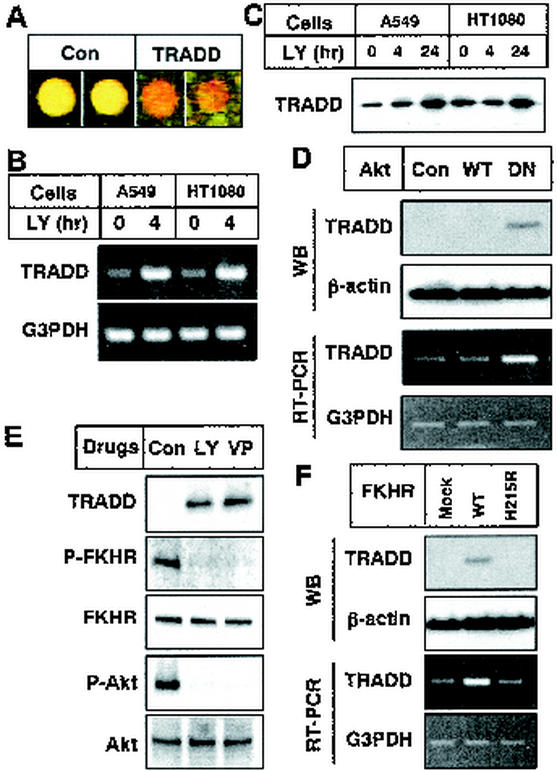
To identify genes critical for chemotherapeutic drug-induced apoptosis, we compared mRNA expression in nontreated cells with that in LY294002-treated cells by using cDNA microarrays containing 4,009 genes. Although chemotherapeutic drugs had no effects on PI3K (Fig. 3E and data not shown), we used the PI3K inhibitor LY294002 because Akt-specific inhibitors had not yet been reported and LY294002 completely suppressed Akt kinase activity in cells. After normalization to correct for differential labeling efficiencies, we found increased tradd gene expression in LY294002-treated cells with increased Cy-5 (red) signals in two independent spots (Fig. 5A). Semiquantitative RT-PCR experiments confirmed that LY294002 treatment stimulated tradd gene expression in A549 and HT1080 cells (Fig. 5B). As shown in Fig. 5C, this treatment certainly enhanced TRADD protein expression in both cell types. Because Akt was the main factor downstream of PI3K, we examined whether Akt is involved in tradd gene expression by transfecting WT- or DN-akt. We detected the increase in tradd mRNA and protein expression only in the DN-akt transfectants (Fig. 5D). Therefore, Akt inactivation is linked to tradd expression.
FIG. 5.
Involvement of Akt and FKHR in TRADD expression. (A) Cy-3-labeled cDNA (green; derived from control A549 mRNA) and Cy-5-labeled cDNA (red; derived from LY294002-treated A549 mRNA) were hybridized to a cDNA microarray. The tradd gene recognized by cDNA from LY294002-treated cells more than that from control cells is red. Equal reactivities are yellow. The results of duplicate spots of a ribosomal gene (control [Con]; left side) and the tradd gene (right side) are shown. (B) A549 and HT1080 cells were treated with 50 μM LY294002 for 0 or 4 h. Total RNAs were isolated with Trizol reagent. Equal amounts of total RNAs were reverse transcribed and amplified by PCR with gene-specific primers as described in Materials and Methods. Amplified products were analyzed by electrophoresis on 1% agarose gels. (C) A549 and HT1080 cells were treated with 50 μM LY294002 for the indicated times. Cell lysates were subjected to SDS-polyacrylamide gel electrophoresis, followed by Western blot (WB) analysis with an anti-TRADD antibody. (D) HT1080 cells were transfected with a pcDNA3 vector encoding nothing (control [Con]), WT-Akt (WT), or DN-Akt (DN). After transfection for 24 h, Cell lysates were subjected to SDS-polyacrylamide gel electrophoresis, followed by Western blot analysis with an anti-TRADD antibody or an anti-β-actin antibody (top and second parts, respectively). Equal amounts of total RNAs were reverse transcribed and amplified by PCR with gene-specific primers as described in Materials and Methods. Amplified products were analyzed by electrophoresis on 1% agarose gels (third and bottom parts). (E) HT1080 cells were treated with 50 μM LY294002 for 12 h or 10 μg of VP-16 per ml for 36 h. Cell lysates were subjected to SDS-polyacrylamide gel electrophoresis, followed by Western blot analysis with an anti-TRADD antibody, an anti-phospho-FKHR (Ser256) antibody, an anti-FKHR antibody, an anti-phospho-Akt (Ser473) antibody, or an anti-Akt antibody. (F) HT1080 cells were transfected with a pcDNA3 vector encoding nothing (Mock), WT-FKHR (WT), or H215R-FKHR (H215R). Cell lysates were subjected to SDS-polyacrylamide gel electrophoresis, followed by Western blot analysis with an anti-TRADD antibody and an anti-β-actin antibody (top and second parts, respectively). Equal amounts of total RNAs were reverse transcribed and amplified by PCR with gene-specific primers as described in Materials and Methods. Amplified products were analyzed by electrophoresis on 1% agarose gels (third and bottom parts). The results shown are representative of at least two independent experiments.
The observation that phosphorylated Akt translocated to the nucleus within 30 min suggested that Akt-mediated control of gene expression is another potential antiapoptotic mechanism of Akt (3, 35). The best-characterized nuclear substrates of Akt are the members of the FOXO subfamily of Forkhead transcription factors (e.g., FKHR, FKHRL1, and AFX) (26). To ascertain whether FKHR is involved in tradd gene expression, we first examined the phosphorylation state of FKHR after treatment with VP-16 in HT1080 cells. Western blot analysis revealed that VP-16 decreased the phosphorylated form of FKHR, as well as the phospho-Akt level, without altering the expression of FKHR and Akt, as LY294002 did (Fig. 5E). Thus, we estimated the tradd gene and protein expression levels after transfection of WT-FKHR and the mutant FKHR (H215R-FKHR) that lacks DNA-binding ability. Expression of the tradd gene and the TRADD protein was induced by overexpression of WT-FKHR but not by H215R-FKHR transfection (Fig. 5F), indicating that TRADD expression is dependent on FKHR activity. These results indicate that chemotherapeutic drugs induce TRADD expression by Akt inactivation following FKHR activation.
FKHR directly regulates tradd gene expression.
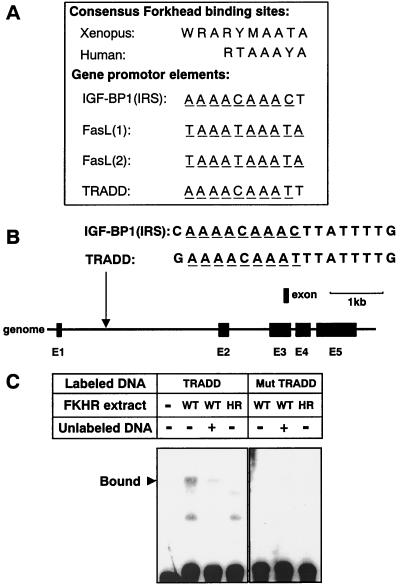
To prove that FKHR regulates tradd gene expression, we searched the consensus Forkhead transcription factor-responsive element (FHRE) in the promoter region of the tradd gene. Forkhead transcription factors are known to recognize the consensus DNA sequences (Fig. 6A). Insulin-like growth factor-binding protein 1 (IGF-BP1) and Fas ligand (FasL) gene transcription is regulated by Forkhead transcription factors. In their promoters, there are one (for IGF-BP1) or two (for FasL) FHRE-like domains (Fig. 6A). We discovered at least one high-homology FHRE-like domain in intron 1 of the tradd gene (90% homology to the IGF-BP1 and FasL genes, as shown in Fig. 6B).
FIG. 6.
Identification of a Forkhead-responsive element in the tradd gene promoter. (A) Comparison of the Xenopus and human consensus Forkhead-responsive element and the Forkhead-responsive element found in the IGF-BP1 (IRS), FasL, or tradd promoter (R = A/G, Y = T/C, W = A/T, M = A/C). The underlined nucleotides matched the predicted Forkhead consensus sequence. (B) Genomic structure of the tradd gene. Black boxes indicate the locations and relative sizes of the five exons. The location of a potential FKHR-binding sequence is indicated in intron 1 and is compared with the consensus FKHR-binding sequence of IGF-BP1 (IRS). (C) Nuclear extracts of HT1080 cells that had been transfected with vector pcDNA3 encoding WT-FKHR (WT) or H215R-FKHR (HR) were incubated with biotin-labeled double-stranded oligonucleotides 5′-biotin-TGAAAACAAATTTATTTTGGGTAC-3′ (TRADD) and 5′-biotin-TGAAACCAAAGTCATTTTGGGTAC-3′ (Mut TRADD) (Forkhead binding sites are underlined). In some experiments, reactions were performed in the presence (+) or absence (−) of a 100-fold excess of unlabeled tradd oligonucleotide (5′-TGAAAACAAATTTATTTTGGGTAC-3′). DNA-protein complexes were fractionated by polyacrylamide gel electrophoresis and visualized by horseradish peroxidase-conjugated streptavidin. The specific DNA-protein complex is indicated on the left.
We then employed an EMSA with double-stranded oligonucleotides containing 10 bp of the identified FHRE-like domain in the tradd gene (Fig. 6A). By incubating the FHRE-like domain with WT-FKHR-overexpressing HT1080 nuclear extracts (Fig. 6C), we observed the formation of a single complex. Formation of the DNA-protein complex was not observed when an H215R-FKHR-containing nuclear extract was incubated with the FHRE-like domain. Moreover, the DNA-protein complex formation was efficiently competed with a 200-fold excess of the unlabeled FHRE-like domain. Neither WT- nor H215R-FKHR could form a complex with the domain with two mutated nucleotides (Mut tradd). Thus, FKHR binds to the identified FHRE-like domain in the tradd gene.
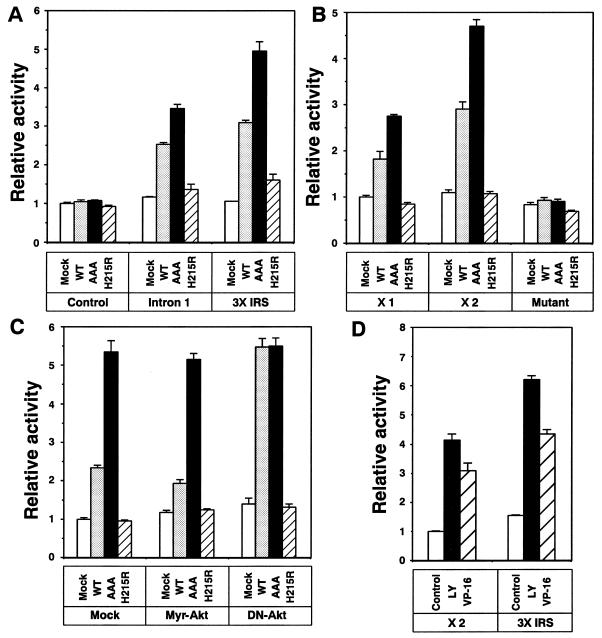
To determine whether FKHR is involved in tradd gene expression by binding to the identified FHRE-like domain, we generated a pGL3 reporter construct containing a 582-bp segment of intron 1 of the tradd gene that contained the domain of interest and that was fused upstream of the luciferase gene. The pGL3-intron 1 reporter construct was then cotransfected into 293T cells with a pcDNA3 vector encoding WT-FKHR, AAA-FKHR (in which Akt phosphorylation sites Thr24, Ser256, and Ser319 were converted to Ala), or H215R-FKHR (lacking DNA-binding ability). As shown in Fig. 7A, cotransfection of WT- or AAA-FKHR resulted in an almost twofold increase in luciferase activity. However, no reporter activity was observed in H215R-FKHR-transfected cells, strongly suggesting the involvement of FKHR in tradd gene regulation. Similar results were obtained with the pGL3 reporter construct containing three copies of the 10-bp FHRE-like domain of the IGF-BP1 gene fused upstream of the luciferase gene (pGL3-3×IRS). Moreover, no reporter activity was observed in the empty pGL3 reporter construct (Mock). We further confirmed the role of FKHR in tradd gene expression by generating three pGL3 reporter vectors in which one or two copies or a mutant form of the 10-bp FHRE-like domain of the tradd gene was fused upstream of the luciferase gene (pGL3-×1, pGL3-×2, or pGL3-Mutant, respectively). Cotransfection of WT- and AAA-FKHR, but not H215R-FKHR, increased luciferase activity in pGL3-×1 and pGL3-×2 but not in pGL3-Mutant (Fig. 7B). Therefore, FKHR was involved in tradd gene transcription by binding to the identified FHRE-like domain.
FIG. 7.
Regulation of tradd gene transcription by FKHR. (A) 293T cells were transfected with either the pGL3-promoter vector containing nothing (Control), an FHRE-containing 582-bp segment of intron 1 of the tradd gene (Intron 1), three copies of a 10-bp FHRE-like domain of IGF-BP1 gene (3× IRS) together with the pcDNA3 vector encoding nothing (Mock), WT-FKHR (WT), AAA-FKHR (AAA), or H215R-FKHR (H215R). The pRL-TK plasmid was also cotransfected as a transfection efficiency control. After 24 h of transfection, luciferase activities were calculated with the dual-luciferase reporter assay system as described in Materials and Methods. (B) 293T cells were transfected with vector pGL3 containing one (×1) or two (×2) copies of the 10-bp potential FKHR-binding sequence in the tradd gene or one mutant copy of the potential FKHR-binding sequence (Mutant) together with vector pcDNA3 encoding nothing (Mock), WT-FKHR (WT), AAA-FKHR (AAA), or H215R-FKHR (H215R). The pRL-TK plasmid was also transfected as a transfection efficiency control. After 24 h of transfection, luciferase activities were calculated with the Dual-luciferase reporter assay system as described in Materials and Methods. (C) HT1080 cells were cotransfected with vector pGL3 containing two copies of the 10-bp potential FKHR-binding sequence in the tradd gene (pGL3-×2) and the pRL-TK plasmid as a transfection efficiency control. The pcDNA3 vector encoding nothing (Mock), WT-FKHR (WT), AAA-FKHR (AAA), or H215R-FKHR (H215R) was also cotransfected with a pUSEamp vector encoding nothing (Mock), Myr-Akt, or DN-Akt. After 24 h of transfection, luciferase activities were calculated with the dual-luciferase reporter assay system as described in Materials and Methods. (D) HT1080 cells were transfected with either two copies of the 10-bp potential FKHR-binding sequence in the tradd gene (×2) or three copies of the 10-bp potential FKHR-binding sequence in IGF-BP1 (3×IRS) together with the pRL-TK plasmid as a transfection efficiency control. After 24 h of transfection, cells were treated with 50 μM LY294002 for 12 h or 10 μg of VP-16 per ml for 36 h. Luciferase activities were calculated with the dual-luciferase reporter assay system as described in Materials and Methods.
To check the role of Akt in tradd gene expression, the pGL3-×2 vector and WT-, AAA-, or H215R-FKHR cDNA was cotransfected into HT1080 cells together with the pUSEamp vector encoding nothing (Mock), the active form of Akt (Myr-Akt), or DN-Akt. As shown in Fig. 7C, overexpression of the active form of Akt slightly suppressed the WT-FKHR-induced activation of luciferase. By contrast, DN-Akt clearly enhanced luciferase activity in WT-FKHR-transfected HT1080 cells. Overexpression of active Akt or DN-Akt did not affect the AAA-FKHR-induced activation of luciferase. Therefore, Akt suppresses FKHR-mediated tradd gene transcription by phosphorylation. Since we have shown that VP-16 suppressed Akt kinase activity (Fig. 3A) and FKHR phosphorylation (Fig. 5E), we checked the effect of VP-16 on reporter activity. After transfection of pGL3-×2, cells were treated with 10 μg of VP-16 per ml for 36 h. We found the increase in luciferase activity after VP-16 treatment (Fig. 7D). Similar results were obtained with cells transfected with pGL3-3×IRS. Because treatment of cells with 50 μM LY294002 for 12 h also increased luciferase activity, we saw that tradd gene expression was regulated by FOXO subfamily members through binding to the identified FHRE-like domain present in intron 1.
Involvement of TRADD expression in chemotherapeutic drug-induced apoptosis.
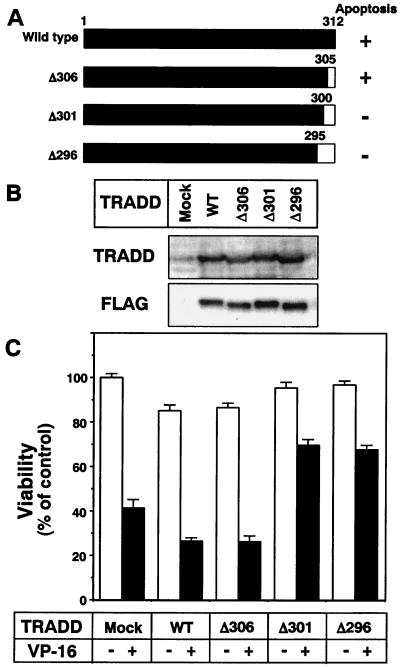
To examine the role of TRADD expression during chemotherapeutic drug-induced apoptosis, we generated a pcDNA3 vector encoding FLAG-tagged WT-TRADD cDNA and carboxy-terminal deletion-containing mutant forms thereof (Fig. 8A). Deletion of 12 or more amino acids was reported to abolish its apoptosis-inducing ability (25). We transfected these plasmids together with an EGFP-expressing plasmid into HT1080 cells (Fig. 8B), following treatment with 10 μg of VP-16 per ml for 24 h. Apoptotic cells were evaluated by counting the EGFP-positive cells that did not show nuclear condensation and fragmentation. As reported previously, overexpression of WT-TRADD and TRADD with seven amino acids deleted (Δ306)-TRADD, but not with TRADD with 12 or 17 amino acids deleted (Δ301- or Δ296-TRADD), slightly decreased the viable cell number without VP-16 treatment (Fig. 8C). The VP-16-induced apoptosis was suppressed by overexpression of Δ301- and Δ296-TRADD. By contrast, WT- and Δ306-TRADD did not exhibit such activity. These results indicate that chemotherapeutic drugs induce apoptosis, as one pathway, by enhancing TRADD expression through Akt inactivation following activation of Forkhead transcription factors.
FIG. 8.
Elimination of chemotherapeutic drug-induced apoptosis by overexpression of mutant TRADD proteins. (A) Schematic representation of the TRADD deletion mutants used in this study. The horizontal bars represent the sequence of TRADD, with black regions corresponding to intact sequences and white region indicating the deleted regions. The apoptosis-inducing ability of each construct was reported previously (25). (B) HT1080 cells were transfected with vector pcDNA3 encoding FLAG-tagged WT and mutant tradd cDNAs. After 24 h of transfection, cell lysates were subjected to Western blot analysis with an anti-TRADD antibody or an anti-FLAG antibody. (C) HT1080 cells were transfected with plasmid pcDNA3-EGFP together with the generated pcDNA3 plasmid containing FLAG-tagged tradd cDNAs. After transfection for 24 h, HT1080 cells were treated with 10 μg of VP-16 per ml for an additional 36 h. After fixation, cells were stained with DAPI. Nuclear fragmentation was visualized with a fluorescence microscope. The number of surviving cells was determined by counting 100 EGFP-positive (transfected) cells not showing nuclear condensation and fragmentation. Each vertical bar represents the mean ± the standard deviation of three independent experiments.
DISCUSSION
The mechanisms by which chemotherapeutic agents induce apoptosis are not fully understood. The actual pharmacological effects of chemotherapy and their biochemical mechanisms are better understood than the downstream events, which trigger the apoptotic cascade. Apoptosis is a major form of cell death in response to chemotherapeutic drug treatment (22, 27), and resistance to apoptosis has been implicated as a critical mechanism of drug resistance.
The PI3K-Akt signaling pathway is known to transmit survival-promoting signals and to protect a variety of cells from apoptosis. By phosphorylating the proapoptotic Bcl-2 family member Bad, the caspase family member caspase 9, and the IκB kinase, Akt also protects cells from apoptosis (8, 12, 41). Therefore, PI3K-Akt might be involved in tumor formation and progression. In fact, Akt is abnormally activated in several human tumors because of its amplification (30, 45) and loss of the tumor suppressor PTEN (phosphatase and tensin homolog deleted on chromosome 10)/MMAC (mutated in multiple advanced cancers) (14, 44). Recent studies documented that PTEN appears to negatively regulate PI3K-Akt survival signaling by removing the D-3 phosphate from PtdIns(3,4,5)P3, which is a primary activator of Akt (31). Loss of PTEN results in high basal activity of Akt in a variety of tumors, while introduction of PTEN suppresses its activity. Recent reports indicate that the Akt survival pathway inhibition is linked with the cytotoxic effects of some drugs (19, 24, 36, 42). Furthermore, inhibition of PI3K could enhance chemotherapeutic drug-induced apoptosis (37). We thus tried to clarify which drugs exhibit their cytotoxicity by inhibiting the Akt-mediated survival-signaling pathway.
We estimated the relationship between drug resistance and Akt activity in A549 and 293T cells (Fig. 1). We found that CPT could induce apoptosis with caspase activation in A549 cells, and under these conditions, Akt was inactivated in the cells. When 293T cells were treated with VP-16, they underwent apoptosis with caspase activation (Fig. 1) and Akt kinase activity was decreased (Fig. 3). Because the caspase inhibitor Z-Asp had no effect on Akt inactivation after drug treatment (Fig. 3D), Akt inactivation was not the result of caspase activation. By contrast, noncytotoxic drugs did not affect the Akt kinase activity, although some drugs (e.g., VP-16 [A549 cells] and ADR [293T cells]) induced apoptotic morphological changes without affecting Akt kinase activity. These results indicate that some chemotherapeutic drugs could induce apoptosis with caspase activation only when the Akt signaling pathway was turned off. The fact that overexpression of active Akt reduced apoptosis (Fig. 4) emphasized the importance of Akt inactivation in chemotherapeutic drug-induced apoptosis. Downregulation of the PI3K-Akt pathway has also been observed in apoptosis induced by hyperosmotic stress, gamma irradiation, UV irradiation, and cell-permeable ceramide (54). Therefore, loss of Akt-mediated survival signals might trigger an apoptotic program after treatment of the cells with some chemotherapeutic drugs. Because inhibition of Akt signaling by LY294002 sensitized cells to the apoptotic effect of chemotherapeutic cells (Fig. 4C and D), combination of chemotherapeutic drugs with inhibitors of Akt signaling would effectively promote apoptosis of tumor cells.
As we reported previously, Hsp90 can protect Akt from dephosphorylation (43). We thus investigated Akt-Hsp90 binding during chemotherapeutic drug-induced apoptosis. However, CPT and VP-16 did not interfere with Akt-Hsp90 binding (unpublished observation). We then examined the change in the activities of upstream Akt kinases PI3K and PDK1. We did not observe a PI3K activity decrease after treating 293T cells with the apoptosis-inducing drug VP-16 (Fig. 3E). Upon examination of the change in PDK1 kinase activity, we found PDK1 inactivation in VP-16-treated 293T cells (Fig. 3F). Because PDK1 kinase activity was not inhibited by in vitro treatment with VP-16 (data not shown), this drug might indirectly inhibit PDK1 kinase activity. PDK1 itself is a member of the AGC subfamily of protein kinases and was phosphorylated by itself at the activation loop (Ser241), which led to its own activation (9). Since Ser241 residues in PDK1 were resistant to dephosphorylation by phosphatases (2, 9), the phosphatases might not be involved in PDK1 inactivation. Recently, Frodin et al. identified Ser386 in the hydrophobic motif of RSK2 as a phosphorylation-dependent docking site and activator of PDK1 (17). We reported that PDK1-Hsp90 binding prevented PDK1 from proteasome-dependent degradation and keeps PDK1 in a soluble and active conformational state (19). Moreover, PDK1 kinase activity is promoted by phosphorylation at tyrosine residues, presumably by a member of the Src kinase family (20, 38). Thus, it might be possible that chemotherapeutic drugs inhibit PDK1 autophosphorylation and autoactivation by indirectly suppressing such interacting proteins. We also could not rule out the possibility that treatment of cells with chemotherapeutic drugs induced phosphatase activation and made the substrate of PI3K unavailable to the enzyme in the cells. This possibility is a subject for future studies.
Because chemotherapeutic drugs induced Akt inactivation (Fig. 2 and 3), we hypothesized that some genes were upregulated after drug treatment to promote apoptosis. We then performed a cDNA microarray analysis and discovered tradd gene expression after chemotherapy (Fig. 5A). TRADD is one of several TNF-R1-associated proteins and contains a 111-amino-acid death domain with sequence similarity to that of TNFR1. The experiments described thus far have identified four distinct properties of TRADD: interaction with TNFR1, self-association, induction of apoptosis, and activation of NF-κB (25). TRADD expression was promoted after irradiation in a radiosensitive glioblastoma multiforme cell line (52). Overexpression of TRADD led to two major TNF- or TRAIL-induced responses, activation of NF-κB and induction of apoptosis. Recently, a direct correlation between Akt activation and resistance of TRAIL in some prostate cancer cells has been reported (10). Inhibition of the Akt signaling pathway by PI3K inhibitors, DN-Akt overexpression, or PTEN transfection renders prostate cancer cells sensitive to TRAIL (10). We also observed tradd mRNA and protein expression after treatment with LY294002 or after DN-akt transfection (Fig. 5). Moreover, we confirmed that overexpression of TRADD deletion mutants that lack apoptosis-inducing capability suppressed chemotherapeutic drug-induced apoptosis (Fig. 8). Therefore, we found TRADD expression to be critical for apoptosis induction after chemotherapy. Because inhibition of the Akt signaling pathway promoted TRADD expression, it is reasonable to see the increase in TNF sensitivity after inhibition of the PI3K-Akt pathway.
Upon growth factor stimulation, Akt is activated and phosphorylates FKHR, FKHRL1, and AFX (FOXO subfamily members) at three key regulatory sites (Thr24, Ser256, and Ser319 in FKHR). Akt has been reported to phosphorylate the second regulatory site of FOXO subfamily members (Ser256 for FKHR) with higher affinity than the first and third sites (21). However, it is not clear what kinds of protein kinases can phosphorylate the first and third FOXO subfamily member regulatory sites and what the contribution of each site is to the control of FOXO subfamily member-dependent gene expression and biological functions. Phosphorylation of the FOXO subfamily members induced their cytoplasmic localization, which resulted in the inhibition of FOXO subfamily member-dependent transcription (5, 7, 21, 29, 39, 48, 49). Since Akt inactivation led to dephosphorylation of FKHR (Fig. 5E), we investigated the role of FOXO subfamily members in tradd gene expression. Although FKHR contains a Forkhead domain that has been shown to be involved in DNA binding, it had not been determined whether FKHR or DAF16 interacts directly with DNA. Several reports indicate that the FHREs in the IGF-BP1 (11) and FasL (7) promoter regions are associated with the insulin-mediated repression of gene transcription. We searched the FHRE domain in the tradd gene and discovered the presence of one region 90% homologous and three regions 80% homologous to the FHRE-like domain in the IGF-BP1 promoter (Fig. 6B and data not shown). An EMSA and promoter-luciferase analysis revealed that the 90% homologous region identified is critical for FKHR-dependent tradd gene expression (Fig. 6 and 7). These results indicate that chemotherapeutic drugs induce tradd gene expression in a FOXO subfamily member-dependent manner.
In conclusion, we found a relationship between Akt inactivation and drug sensitivity. Because Akt inactivation was found only when the cells were sensitive to the chemotherapeutic drugs tested, it could be a hallmark in examining the sensitivity of cells to some chemotherapeutic agents. Moreover, we discovered that Akt inactivation resulted in TRADD expression by activating Forkhead transcription factors.
Acknowledgments
We are grateful to E. D. Tang and F. G. Barr for providing WT-, AAA-, and H215R-FKHR plasmids. We thank M. Naito and A. Tomida for helpful discussions.
This work was supported in part by a special grant for Advanced Research on Cancer from the Ministry of Education, Culture, Sports, Science and Technology, Japan (T.T.), by the Foundation for Promotion of Cancer Research in Japan (N.F.), and by a grant for Research Fellowships of the Japanese Society for the Promotion of Science for Young Scientists (S.R.).
REFERENCES
- 1.Alessi, D. R., and P. Cohen. 1998. Mechanism of activation and function of protein kinase B. Curr. Opin. Genet. Dev. 8:55-62. [DOI] [PubMed] [Google Scholar]
- 2.Alessi, D. R., S. R. James, C. P. Downes, A. B. Holmes, P. R. Gaffney, C. B. Reese, and P. Cohen. 1997. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Ba. Curr. Biol. 7:261-269. [DOI] [PubMed] [Google Scholar]
- 3.Andjelkovic, M., D. R. Alessi, R. Meier, A. Fernandez, N. J. Lamb, M. Frech, P. Cron, P. Cohen, J. M. Lucocq, and B. A. Hemmings. 1997. Role of translocation in the activation and function of protein kinase B. J. Biol. Chem. 272:31515-31524. [DOI] [PubMed] [Google Scholar]
- 4.Balendran, A., A. Casamayor, M. Deak, A. Paterson, P. Gaffney, R. Currie, C. P. Downes, and D. R. Alessi. 1999. PDK1 acquires PDK2 activity in the presence of a synthetic peptide derived from the carboxyl terminus of PRK2. Curr. Biol. 9:393-404. [DOI] [PubMed] [Google Scholar]
- 5.Biggs, W. H., III, J. Meisenhelder, T. Hunter, W. K. Cavenee, and K. C. Arden. 1999. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc. Natl. Acad. Sci. USA 96:7421-7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brownawell, A. M., G. J. Kops, I. G. Macara, and B. M. Burgering. 2001. Inhibition of nuclear import by protein kinase B (Akt) regulates the subcellular distribution and activity of the Forkhead transcription factor AFX. Mol. Cell. Biol. 21:3534-3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunet, A., A. Bonni, M. J. Zigmond, M. Z. Lin, P. Juo, L. S. Hu, M. J. Anderson, K. C. Arden, J. Blenis, and M. E. Greenberg. 1999. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96:857-868. [DOI] [PubMed] [Google Scholar]
- 8.Cardone, M. H., N. Roy, H. R. Stennicke, G. S. Salvesen, T. F. Franke, E. Stanbridge, S. Frisch, and J. C. Reed. 1998. Regulation of cell death protease caspase-9 by phosphorylation. Science 282:1318-1321. [DOI] [PubMed] [Google Scholar]
- 9.Casamayor, A., N. A. Morrice, and D. R. Alessi. 1999. Phosphorylation of Ser-241 is essential for the activity of 3-phosphoinositide-dependent protein kinase-1: identification of five sites of phosphorylation in vivo. Biochem. J. 342:287-292. [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, X., H. Thakkar, F. Tyan, S. Gim, H. Robinson, C. Lee, S. K. Pandey, C. Nwokorie, N. Onwudiwe, and R. K. Srivastava. 2001. Constitutively active Akt is an important regulator of TRAIL sensitivity in prostate cancer. Oncogene 20:6073-6083. [DOI] [PubMed] [Google Scholar]
- 11.Cichy, S. B., S. Uddin, A. Danilkovich, S. Guo, A. Klippel, and T. G. Unterman. 1998. Protein kinase B/Akt mediates effects of insulin on hepatic insulin-like growth factor-binding protein-1 gene expression through a conserved insulin response sequence. J. Biol. Chem. 273:6482-6487. [DOI] [PubMed] [Google Scholar]
- 12.Datta, S. R., H. Dudek, X. Tao, S. Masters, H. Fu, Y. Gotoh, and M. E. Greenberg. 1997. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91:231-241. [DOI] [PubMed] [Google Scholar]
- 13.Delcommenne, M., C. Tan, V. Gray, L. Rue, J. Woodgett, and S. Dedhar. 1998. Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc. Natl. Acad. Sci. USA 95:11211-11216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Cristofano, A., and P. P. Pandolfi. 2000. The multiple roles of PTEN in tumor suppression. Cell 100:387-390. [DOI] [PubMed] [Google Scholar]
- 15.Didichenko, S. A., B. Tilton, B. A. Hemmings, K. Ballmer-Hofer, and M. Thelen. 1996. Constitutive activation of protein kinase B and phosphorylation of p47phox by a membrane-targeted phosphoinositide 3-kinase. Curr. Biol. 6:1271-1278. [DOI] [PubMed] [Google Scholar]
- 16.Franke, T. F., D. R. Kaplan, and L. C. Cantley. 1997. PI3K: downstream AKTion blocks apoptosis. Cell 88:435-437. [DOI] [PubMed] [Google Scholar]
- 17.Frodin, M., C. J. Jensen, K. Merienne, and S. Gammeltoft. 2000. A phosphoserine-regulated docking site in the protein kinase RSK2 that recruits and activates PDK1. EMBO J. 19:2924-2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujita, N., A. Nagahashi, K. Nagashima, S. Rokudai, and T. Tsuruo. 1998. Acceleration of apoptotic cell death after the cleavage of Bcl-XL protein by caspase-3-like proteases. Oncogene 17:1295-1304. [DOI] [PubMed] [Google Scholar]
- 19.Fujita, N., S. Sato, A. Ishida, and T. Tsuruo. 2002. Involvement of Hsp90 in signaling and stability of 3-phosphoinositide-dependent kinase-1. J. Biol. Chem. 277:10346-10353. [DOI] [PubMed] [Google Scholar]
- 20.Grillo, S., T. Gremeaux, A. Casamayor, D. R. Alessi, Y. Le Marchand-Brustel, and J. F. Tanti. 2000. Peroxovanadate induces tyrosine phosphorylation of phosphoinositide-dependent protein kinase-1 potential involvement of src kinase. Eur. J. Biochem. 267:6642-6649. [DOI] [PubMed] [Google Scholar]
- 21.Guo, S., G. Rena, S. Cichy, X. He, P. Cohen, and T. Unterman. 1999. Phosphorylation of serine 256 by protein kinase B disrupts transactivation by FKHR and mediates effects of insulin on insulin-like growth factor-binding protein-1 promoter activity through a conserved insulin response sequence. J. Biol. Chem. 274:17184-17192. [DOI] [PubMed] [Google Scholar]
- 22.Hickman, J. A. 1992. Apoptosis induced by anticancer drugs. Cancer Metastasis Rev. 11:121-139. [DOI] [PubMed] [Google Scholar]
- 23.Hirotani, M., Y. Zhang, N. Fujita, M. Naito, and T. Tsuruo. 1999. NH2-terminal BH4 domain of Bcl-2 is functional for heterodimerization with Bax and inhibition of apoptosis. J. Biol. Chem. 274:20415-20420. [DOI] [PubMed] [Google Scholar]
- 24.Hsu, A. L., T. T. Ching, D. S. Wang, X. Song, V. M. Rangnekar, and C. S. Chen. 2000. The cyclooxygenase-2 inhibitor celecoxib induces apoptosis by blocking Akt activation in human prostate cancer cells independently of Bcl-2. J. Biol. Chem. 275:11397-11403. [DOI] [PubMed] [Google Scholar]
- 25.Hsu, H., J. Xiong, and D. V. Goeddel. 1995. The TNF receptor 1-associated protein TRADD signals cell death and NF-κB activation. Cell 81:495-504. [DOI] [PubMed] [Google Scholar]
- 26.Kaestner, K. H., W. Knochel, and D. E. Martinez. 2000. Unified nomenclature for the winged helix/forkhead transcription factors. Genes Dev. 14:142-146. [PubMed] [Google Scholar]
- 27.Kerr, J. F., C. M. Winterford, and B. V. Harmon. 1994. Apoptosis: its significance in cancer and cancer therapy. Cancer 73:2013-2026. [DOI] [PubMed] [Google Scholar]
- 28.Kops, G. J., and B. M. Burgering. 1999. Forkhead transcription factors: new insights into protein kinase B (c-akt) signaling. J. Mol. Med. 77:656-665. [DOI] [PubMed] [Google Scholar]
- 29.Kops, G. J., N. D. de Ruiter, A. M. De Vries-Smits, D. R. Powell, J. L. Bos, and B. M. Burgering. 1999. Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature 398:630-634. [DOI] [PubMed] [Google Scholar]
- 30.Liu, A. X., J. R. Testa, T. C. Hamilton, R. Jove, S. V. Nicosia, and J. Q. Cheng. 1998. AKT2, a member of the protein kinase B family, is activated by growth factors, v-Ha-ras, and v-src through phosphatidylinositol 3-kinase in human ovarian epithelial cancer cells. Cancer Res. 58:2973-2977. [PubMed] [Google Scholar]
- 31.Maehama, T., and J. E. Dixon. 1998. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem. 273:13375-13378. [DOI] [PubMed] [Google Scholar]
- 32.Mashima, T., M. Naito, S. Kataoka, H. Kawai, and T. Tsuruo. 1995. Aspartate-based inhibitor of interleukin-1β-converting enzyme prevents antitumor agent-induced apoptosis in human myeloid leukemia U937 cells. Biochem. Biophys. Res. Commun. 209:907-915. [DOI] [PubMed] [Google Scholar]
- 33.Mashima, T., M. Naito, and T. Tsuruo. 1999. Caspase-mediated cleavage of cytoskeletal actin plays a positive role in the process of morphological apoptosis. Oncogene 18:2423-2430. [DOI] [PubMed] [Google Scholar]
- 34.Matsushima-Nishiu, M., M. Unoki, K. Ono, T. Tsunoda, T. Minaguchi, H. Kuramoto, M. Nishida, T. Satoh, T. Tanaka, and Y. Nakamura. 2001. Growth and gene expression profile analyses of endometrial cancer cells expressing exogenous PTEN. Cancer Res. 61:3741-3749. [PubMed] [Google Scholar]
- 35.Meier, R., D. R. Alessi, P. Cron, M. Andjelkovic, and B. A. Hemmings. 1997. Mitogenic activation, phosphorylation, and nuclear translocation of protein kinase Bβ. J. Biol. Chem. 272:30491-30497. [DOI] [PubMed] [Google Scholar]
- 36.Nakashio, A., N. Fujita, S. Rokudai, S. Sato, and T. Tsuruo. 2000. Prevention of phosphatidylinositol 3′-kinase-Akt survival signaling pathway during topotecan-induced apoptosis. Cancer Res. 60:5303-5309. [PubMed] [Google Scholar]
- 37.Ng, S. S. W., M. S. Tsao, S. Chow, and D. W. Hedley. 2000. Inhibition of phosphatidylinositide 3-kinase enhances gemcitabine-induced apoptosis in human pancreatic cancer cells. Cancer Res. 60:5451-5455. [PubMed] [Google Scholar]
- 38.Park, J., M. M. Hill, D. Hess, D. P. Brazil, J. Hofsteenge, and B. A. Hemmings. 2001. Identification of tyrosine phosphorylation sites on 3-phosphoinositide-dependent protein kinase-1 and their role in regulating kinase activity. J. Biol. Chem. 276:37459-37471. [DOI] [PubMed] [Google Scholar]
- 39.Rena, G., S. Guo, S. C. Cichy, T. G. Unterman, and P. Cohen. 1999. Phosphorylation of the transcription factor Forkhead family member FKHR by protein kinase B. J. Biol. Chem. 274:17179-17183. [DOI] [PubMed] [Google Scholar]
- 40.Rokudai, S., N. Fujita, Y. Hashimoto, and T. Tsuruo. 2000. Cleavage and inactivation of antiapoptotic Akt/PKB by caspases during apoptosis. J. Cell. Physiol. 182:290-296. [DOI] [PubMed] [Google Scholar]
- 41.Romashkova, J. A., and S. S. Makarov. 1999. NF-κB is a target of AKT in anti-apoptotic PDGF signalling. Nature 401:86-90. [DOI] [PubMed] [Google Scholar]
- 42.Sato, S., N. Fujita, and T. Tsuruo. 2002. Interference with PDK1-Akt survival signaling pathway by UCN-01 (7-hydroxystaurosporine). Oncogene 21:1727-1738. [DOI] [PubMed] [Google Scholar]
- 43.Sato, S., N. Fujita, and T. Tsuruo. 2000. Modulation of akt kinase activity by binding to hsp90. Proc. Natl. Acad. Sci. USA 97:10832-10837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simpson, L., and R. Parsons. 2001. PTEN: life as a tumor suppressor. Exp. Cell Res. 264:29-41. [DOI] [PubMed] [Google Scholar]
- 45.Staal, S. P. 1987. Molecular cloning of the akt oncogene and its human homologues AKT1 and AKT2: amplification of AKT1 in a primary human gastric adenocarcinoma. Proc. Natl. Acad. Sci. USA 84:5034-5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stephens, L., K. Anderson, D. Stokoe, H. Erdjument-Bromage, G. F. Painter, A. B. Holmes, P. R. Gaffney, C. B. Reese, F. McCormick, P. Tempst, J. Coadwell, and P. T. Hawkins. 1998. Protein kinase B kinases that mediate phosphatidylinositol-3,4,5-trisphosphate-dependent activation of protein kinase B. Science 279:710-714. [DOI] [PubMed] [Google Scholar]
- 47.Stokoe, D., L. R. Stephens, T. Copeland, P. R. Gaffney, C. B. Reese, G. F. Painter, A. B. Holmes, F. McCormick, and P. T. Hawkins. 1997. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science 277:567-570. [DOI] [PubMed] [Google Scholar]
- 48.Takaishi, H., H. Konishi, H. Matsuzaki, Y. Ono, Y. Shirai, N. Saito, T. Kitamura, W. Ogawa, M. Kasuga, U. Kikkawa, and Y. Nishizuka. 1999. Regulation of nuclear translocation of Forkhead transcription factor AFX by protein kinase B. Proc. Natl. Acad. Sci. USA 96:11836-11841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang, E. D., G. Nunez, F. G. Barr, and K. L. Guan. 1999. Negative regulation of the Forkhead transcription factor FKHR by Akt. J. Biol. Chem. 274:16741-16746. [DOI] [PubMed] [Google Scholar]
- 50.Toker, A., and A. C. Newton. 2000. Akt/protein kinase B is regulated by autophosphorylation at the hypothetical PDK-2 site. J. Biol. Chem. 275:8271-8274. [DOI] [PubMed] [Google Scholar]
- 51.Vanhaesebroeck, B., and D. R. Alessi. 2000. The PI3K-PDK1 connection: more than just a road to PKB. Biochem. J. 346:561-576. [PMC free article] [PubMed] [Google Scholar]
- 52.Yount, G. L., G. Afshar, S. Ries, M. Korn, N. Shalev, D. Basila, F. McCormick, and D. A. Haas-Kogan. 2001. Transcriptional activation of TRADD mediates p53-independent radiation-induced apoptosis of glioma cells. Oncogene 20:2826-2835. [DOI] [PubMed] [Google Scholar]
- 53.Zhang, Y., N. Fujita, and T. Tsuruo. 1999. Caspase-mediated cleavage of p21Waf1/Cip1 converts cancer cells from growth arrest to undergoing apoptosis. Oncogene 18:1131-1138. [DOI] [PubMed] [Google Scholar]
- 54.Zundel, W., and A. Giaccia. 1998. Inhibition of the anti-apoptotic PI(3)K/Akt/Bad pathway by stress. Genes Dev. 12:1941-1946. [DOI] [PMC free article] [PubMed] [Google Scholar]