Abstract
We previously reported that the STAM family members STAM1 and STAM2 are phosphorylated on tyrosine upon stimulation with cytokines through the γc-Jak3 signaling pathway, which is essential for T-cell development. Mice with targeted mutations in either STAM1 or STAM2 show no abnormality in T-cell development, and mice with double mutations for STAM1 and STAM2 are embryonically lethal; therefore, here we generated mice with T-cell-specific double mutations for STAM1 and STAM2 using the Cre/loxP system. These STAM1−/− STAM2−/− mice showed a significant reduction in thymocytes and a profound reduction in peripheral mature T cells. In proliferation assays, thymocytes derived from the double mutant mice showed a defective response to T-cell-receptor (TCR) stimulation by antibodies and/or cytokines, interleukin-2 (IL-2) and IL-7. However, signaling events downstream of receptors for IL-2 and IL-7, such as activations of STAT5, extracellular signal-regulated kinase (ERK), and protein kinase B (PKB)/Akt, and c-myc induction, were normal in the double mutant thymocytes. Upon TCR-mediated stimulation, prolonged activations of p38 mitogen-activated protein kinase and Jun N-terminal protein kinase were seen, but activations of ERK, PKB/Akt, and intracellular calcium flux were normal in the double mutant thymocytes. When the cell viability of cultured thymocytes was assessed, the double mutant thymocytes died more quickly than controls. These results demonstrate that the STAMs are indispensably involved in T-cell development and survival in the thymus through the prevention of apoptosis but are dispensable for the proximal signaling of TCR and cytokine receptors.
We previously identified a new family of phosphotyrosine proteins named signal-transducing adaptor molecule 1 (STAM1) and STAM2 (6, 34). STAM2 homologues have also been isolated, as EAST and Hbp in chickens and mice, respectively (14, 32). The amino acid sequence homology is about 50% between STAM1 and STAM2, both of which have a unique structure containing an Src homology 3 domain and a tyrosine cluster region that includes an immunoreceptor tyrosine-based activation motif (6, 34). The STAMs are phosphorylated on tyrosine upon stimulation with a variety of cytokines and growth factors, such as interleukin-2 (IL-2), IL-4, IL-7, granulocyte-macrophage colony-stimulating factor (GM-CSF), human growth factor, epidermal growth factor (EGF), and platelet-derived growth factor (6, 34). Our previous study indicated that the STAMs are associated with Jak2 and Jak3 tyrosine kinases and possibly are involved in the regulation of intracellular signal transduction for DNA synthesis and c-myc induction mediated by IL-2 and GM-CSF in the in vitro overexpression system (6, 35). The STAMs have also been shown to be associated with Hrs/Hgs (1), AMSH (36), and UBPY (11). Recently, the STAMs, like Hrs, were found to contain a ubiquitin-interacting motif (UIM) and a Vps27p-Hrs-STAM (VHS) domain (10, 15). The UIM of Hrs is implicated in the endosomal sorting of ubiquitinated proteins (13, 25, 28).
The cytoplasmic domain of the cytokine receptor γc chain is associated with a tyrosine kinase, Jak3, and both the γc chain and Jak3 are indispensable for early T-cell development (3, 5, 20, 21, 23, 37; reviewed in reference 29). Because the STAMs are also associated with Jak3, we generated mice with targeted mutations in the STAM1 and/or STAM2 genes to investigate whether the STAMs are involved in T-cell development. Using the STAM1−/− mice, we demonstrated that STAM1 is indispensable for the survival of CA3 pyramidal cells of the hippocampus but dispensable for the development of lymphocytes and their cytokine-mediated intracellular signal transduction (40). We also generated STAM2 knockout mice, which show no abnormality in any of the tissues tested, including the thymus, spleen, muscle, heart, brain, kidney, lung, and testis; their lymphocytes respond normally to cytokines such as IL-2 and GM-CSF (unpublished data). We then obtained double-knockout mice for both STAM1 and STAM2 by crossing STAM1−/− and STAM2−/− mice and found that the double-knockout mice are embryonically lethal by embryonic day 11.5 (E11.5), with a defect in ventral folding morphogenesis (unpublished data). These observations suggest compensatory functions between STAM1 and STAM2 in T-cell development. To investigate this possibility, we generated mice with T-cell-specific disruption of both STAM1 and STAM2 using the Cre/loxP system, which is effective for the conditional targeting of genes (8, 31, 33). Here, we observed a significant reduction in T-cell development in the thymus and a profound reduction in the peripheral mature T cells in STAM1−/− STAM2−/− mice.
MATERIALS AND METHODS
Construction of the targeting vector.
The STAM1 genomic locus was isolated from the λFixII mouse 129/Sv genomic library (Stratagene) using a 5′ region of STAM1 cDNA. This targeting construct replaces a 0.6-kb PstI-PstI genomic fragment encompassing exons 3 and 4, flanked by 6.5-kb (XhoI-PstI) and 1.4-kb (PstI-PstI) genomic sequences derived from the 129/Sv genomic library (see Fig. 2A). A conditional targeting vector was constructed to delete exons 3 and 4 of the STAM1 gene. A HindIII-XbaI-digested 0.5-kb fragment of the STAM1 genomic DNA containing exons 3 and 4 was inserted between the loxP site and the pGK-neo of the vector (ploxpNEO), which bears a pGK-neo gene cassette flanked by a pair of loxP sequences for positive selections and a diphtheria toxin A-chain gene cassette lacking a polyadenylation site for negative selection. The 6.5-kb (XhoI-PstI) genomic fragment 5′ upstream of exon 3 was blunted and ligated into the XhoI site of the ploxpNEO. The 3′ downstream 1.4-kb (PstI-PstI) genomic fragment was blunted and ligated into the EcoRI site of the ploxpNEO (Fig. 1A).
FIG. 2.
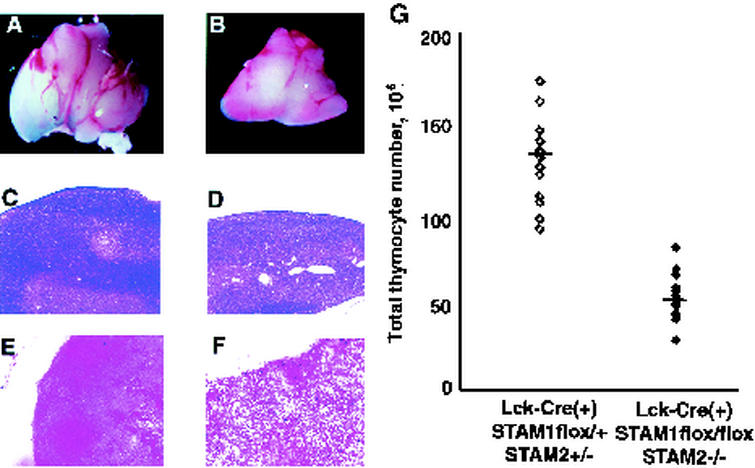
Comparison of histological analysis of thymus and thymocyte counts. Size of thymus (A and B), hematoxylin-eosin-stained sections of thymus (C and D), and TUNEL-stained sections of thymus (E and F) from 4-week-old Lck-Cre (+) STAM1flox/+ STAM2+/− (A, C, and E) and Lck-Cre (+) STAM1flox/flox STAM2−/− (B, D, and F) mice. Magnifications: approximately ×5 (A and B), ×20 (C and D), and ×40 (E and F). (G) Thymocyte counts in 4-week-old Lck-Cre (+) STAM1flox/+ STAM2+/− and Lck-Cre (+) STAM1flox/flox STAM2−/− mice. The bar indicates the average of thymocyte counts.
FIG. 1.
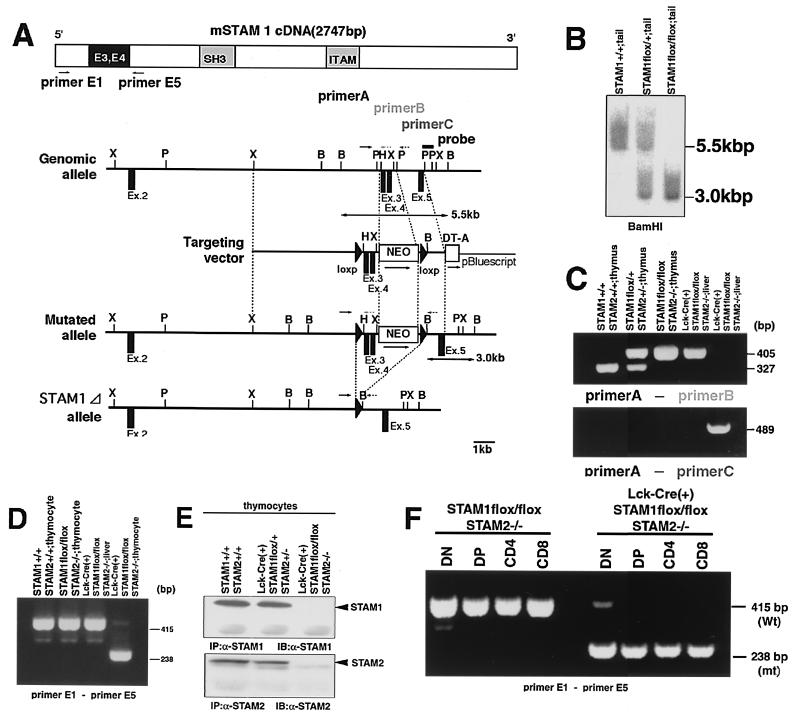
Generation of T-cell-specific disruption of both STAM1 and STAM2 genes. (A) Schematic representation of the mSTAM1 cDNA, stam1 genomic locus, targeting vector, and mutated stam1 locus. The positions of stam1 axons are shown as boxes. Restriction sites: B, BamHI; H, HindIII; P, PstI; X, XbaI. (B) Southern blot analysis of the stam1 mutation in mice. Arrows indicate the position of the DNA fragments corresponding to the wild-type (5.5-kb) and mutated (3.0-kb) alleles. (C) PCR analysis of genomic DNA from thymus and tail. PCR primers used are shown in panel A. (D) RT-PCR analysis of total RNA from thymocytes and liver. The primers used are primers E1 and E5, shown in panel A. Note that a shorter PCR product (238-bp) was amplified from Lck-Cre (+) STAM1 lox/lox thymocytes. (E) Immunoprecipitation analysis for STAM1 and STAM2. Lysates of thymocytes from Lck-Cre (+) STAM1+/+ STAM2+/+, Lck-Cre (+) STAM1flox/+ STAM2+/−, and Lck-Cre (+) STAM1flox/flox STAM2−/− mice were immunoprecipitated and then immunoblotted with anti-STAM1 Ab or anti-STAM2 Ab. (F) RT-PCR analysis of total RNA from each thymocyte fraction.
Generation of “floxed” STAM1 mice.
The linearized targeting vector was electroporated into J1 embryonic stem (ES) cells. Homologous recombination events were assessed by Southern blot hybridization. The targeted STAM1 loxP-flanked (“floxed”) ES clone was then injected into C57BL/6 blastocysts and transferred to foster mothers to obtain chimeric mice. The F1 heterozygous mice carrying the STAM1 floxed mutation were identified by Southern blot hybridization and intercrossed to produce F2 homozygous offspring. The F2 mice were genotyped by Southern blot hybridization and by PCR with DNA from tail biopsy specimens (Fig. 1B).
Generation of Lck-Cre STAM1flox/flox STAM2−/− mice.
STAM2−/− mice were generated by gene targeting (M. Yamada, N. Ishii, K. Murata, H. Sasaki, and K. Sugamura, submitted for publication). STAM1flox/flox mice were mated with STAM2−/− mice to generate STAM1flox/+ STAM2+/− mice. STAM1flox/+ STAM2+/− mice were then intercrossed to generate STAM1flox/flox STAM2−/− mice. Lck-Cre transgenic mice (31, 33) were then bred with the STAM1flox/flox STAM2−/− mice to generate Lck-Cre(+) STAM1flox/+STAM2+/− mice. These mice were mated with STAM1flox/flox STAM2−/− mice. Offspring carrying Lck-Cre (+) STAM1flox/flox STAM2−/− and Lck-Cre (+) STAM1flox/+ STAM2+/− were used for further analysis. These mice were genotyped by PCR with DNA from mouse tail and thymus (Fig. 1C).
The following oligonucleotide primers were used: primer A, CGGGACCAGAGGAAAAGCACCTGTCAC; primer B, ATCAGTGTACAAATGGGAAGGTATTAT; and primer C, AACCCCCAAAATTTACCAGAGAACTTC. PCR conditions were as follows: denaturation at 94°C for 5 min, followed by 40 cycles of 1 min at 94°C, 1 min at 60°C, and 1 min at 72°C. The wild-type and lox alleles gave rise to PCR-amplified fragments of 325 and 405 bp, respectively.
RT-PCR.
Reverse transcription-PCRs (RT-PCRs) were carried out with total RNA derived from mice thymocytes or liver as the template. The total RNA was prepared using TRIzol (Gibco-BRL). The first-strand synthesis was performed using the Superscript Preamplification System (Gibco-BRL). PCRs were performed in a 50-μl mixture consisting of 10 mM Tris-HCl (pH 8.3), 1.5 mM MgCl2, 50 mM KCl, 0.2 mM deoxynucleoside triphosphate mixture, a 1 μM concentration of each primer, 1.25 U of Taq DNA polymerase (TAKARA SHUZO, Kyoto, Japan), and 2 μl of the RT reaction mixture as a template. PCR conditions were as follows: denaturation at 94°C for 2 min, followed by 35 cycles of 30 s at 94°C, 30 s at 57°C, and 1 min at 72°C. The following oligonucleotide primers were used: STAMex1F (primer A), CCCTTCGACCAGGATGTTGAGAAAGCA; STAMex5R (primer B), CCCTTCGACCAGGATGTTGAGAAAGCA.
Immunoprecipitation and immunoblotting.
Immunoprecipitation and immunoblotting were performed according to a modification of a previously described method (20). Briefly, thymocytes from mice were lysed in NP-40 lysis buffer (1% Nonidet P-40, 25 mM Tris-HCl [pH 7.5], 140 mM NaCl, 10 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, aprotinin [20 μg/ml], 1 mM Na3VO4). The supernatants of the lysates were subjected to immunoblotting, or immunoprecipitated with a monoclonal antibody (MAb) specific for STAM1 (33). The lysates or immunoprecipitates were separated by sodium dodecyl sulfate (SDS)-10% polyacrylamide gel electrophoresis and then transferred to polyvinylidene difluoride membranes (Millipore). After blocking with 5% nonfat milk in phosphate-buffered saline (PBS) containing 0.1% Tween 20, the filters were incubated with the primary antibodies (Abs), followed by incubation with anti-mouse immunoglobulin G (IgG) or anti-rabbit IgG coupled with horseradish peroxidase, and visualized using the enhanced chemiluminescence detection system (Amersham Pharmacia Biotech). The Abs for extracellular signal-regulated kinase 1/2 (ERK1/2), p-ERK1/2, Jun N-terminal protein kinase 1/2 (JNK1/2), p-JNK1/2, p38 mitogen-activated protein kinase (p38MAPK), phospho-p38MAPK (p-p38MAPK), p-STAT5, and p-Akt were purchased from Cell Signaling Technology. The Abs for p-Tyr and Akt were purchased from Upstate Biotechnology. The Ab for STAT5a was purchased from Santa Cruz Biotechnology.
Histological analyses of thymus.
Mice were perfused with PBS followed by 4% paraformaldehyde-PBS. The thymuses were removed for processing, embedded in paraffin, and then sliced into 10-μm-thick sections with a microtome. For histological analyses, the sections were stained with hematoxylin-eosin. For terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assays, fresh frozen thymuses of mice were sliced into 10-μm-thick sections with a cryostat. Terminal transferase labeling of fragmented DNA in the sections was then performed with a TACS 2 TdT Kit, HRP-Blue Label (TREVIGEN) according to the manufacturer's protocol.
Flow cytometry.
Thymocytes were suspended in PBS supplemented with 3% fetal bovine serum (FBS). They were preincubated in normal mouse serum to prevent the nonspecific binding of labeled MAbs to the cell surface. They were then stained with MAbs conjugated with fluorescein isothiocyanate, phycoerythrin, or biotin for 30 min at 4°C. The cells were washed with PBS-3% FBS, and the biotinylated Abs were developed with streptavidin-allophycocyanin (PharMingen). All the MAbs were purchased from PharMingen. The surface stainings with MAbs were analyzed with a FACSCalibur flow cytometer (Becton Dickinson) in two- or three-color mode using CellQuest software.
Preparation of thymocyte subpopulations.
To prepare double-negative (DN), double-positive (DP), CD4-single-positive (CD4 SP), and CD8-single-positive (CD8 SP) thymocytes, we used the magnetic cell sorting system with autoMACS (Miltenyi Biotec). Briefly, total thymocytes were labeled with CD8 microbeads and separated by autoMACS. CD8-negative fractions were labeled with CD4 microbeads and separated. Negative fractions were enriched in DN thymocytes, and positive fractions were enriched in CD4 SP thymocytes. CD8-positive fractions were also labeled with CD4 microbeads and separated. Positive fractions were enriched in DP thymocytes and negative fractions were enriched in CD8 SP thymocytes. The purity of each thymocyte subpopulation was confirmed by flow-cytometric analysis and was 90 to 95%.
Proliferation assay.
Single-cell suspensions of thymocytes in RPMI 1640 medium supplemented with 10% FBS, 50 μM 2-mercaptomethanol, penicillin, and streptomycin were plated in 96-well plates at a density of 2 × 105 cells (total thymocytes) or 5 × 104 cells (CD4 SP thymocytes) per well in 100 μl of medium. Stimuli were added as indicated, and cells were cultured for 42 h. The stimuli were recombinant murine IL-2 (20 ng/ml; PeproTech), recombinant murine IL-7 (10 ng/ml; PeproTech), plate-coated anti-CD3 MAb (3 μg/ml; PharMingen), phorbol myristate acetate (10 ng/ml; Sigma), and ionomycin (1 μg/ml; Sigma). The cells were then pulsed with [3H]thymidine and harvested after 6 h. The incorporated [3H]thymidine was counted with a MicroBeta liquid scintillation counter (Amersham Pharmacia Biotech).
Cell survival assay.
To assess the spontaneous death of DP or CD4 SP thymocyte subsets, cells were cultured for 12, 24, or 48 h in RPMI 1640 medium supplemented with 10% FBS, 50 μM 2-mercaptomethanol, penicillin, and streptomycin. Cells were harvested, washed, and stained with annexin V and propidium iodide, according to the manufacturer's instructions (PharMingen). The cells were then analyzed on a FACSCalibur device. Dead cells were defined as annexin-V-positive cells.
Intracellular staining.
Each subpopulation of thymocytes was stained with Cy-chrome-conjugated anti-CD4 MAb and phycoerythrin-conjugated anti-CD8 MAb (PharMingen) and washed. The cells were resuspended in 100 μl of PBS containing 4% paraformaldehyde, incubated for 20 min on ice, and washed. The cells were then resuspended in 50 μl of PBS containing 1% FBS, 0.1% sodium azide, 0.1% saponin (Sigma), and hamster anti-Bcl-2 MAb or isotype control Ab (5 μg/ml; PharMingen). After a 30-min incubation on ice, the cells were washed and further stained with fluorescein isothiocyanate-conjugated anti-hamster Ig (PharMingen) for 30 min on ice. The cells were then analyzed on a FACSCalibur device.
In vitro kinase assay.
To measure the p38MAPK activity, we used a p38MAPK kit (Cell Signaling Technology) according to the assay protocol of the kit. Briefly, DP thymocytes were stimulated for the indicated times and then lysed in lysis buffer. The supernatants of the lysates were immunoprecipitated with an immobilized p-p38MAPK MAb. The immunoprecipitated pellets were incubated in kinase buffer containing ATF-2 fusion protein and cold ATP. The reaction was terminated with SDS sample buffer. The samples were separated by SDS-polyacrylamide gel electrophoresis and analyzed for ATF-2 phosphorylation on Thr71 by Western blotting using a p-ATF-2 Ab and chemiluminescence detection.
Northern blot analysis.
Total RNA was extracted from thymocytes using TRIzol (Molecular Research Center, Inc.). For Northern blot analysis, 10 μg of total RNA was electrophoresed on a 1% agarose gel containing formaldehyde and then transferred to a Gene Screen membrane (NEN). Probe was labeled with [α-32P]dCTP using a random primed DNA labeling kit (TAKARA SHUZO Co.). Prehybridization and hybridization were performed at 42°C in a solution containing 50% formamide, 5× SSPE (1× SSPE is 0.18 M NaCl, 10 mM NaH2PO4, and 1 mM EDTA [pH 7.7]), 5× Denhardt's solution, 0.1% SDS, and salmon sperm DNA (20 ng/ml). Membrane was washed and subjected to autoradiography with a Bio-Image Analyzer BAS 1500 (Fuji Film). The probe DNAs for c-myc and glyceraldehyde-3-phosphate dehydrogenase cDNA were described previously (40).
RESULTS
Generation of STAM1flox/flox STAM2−/− mice.
Using the STAM1−/− mice, we demonstrated previously that STAM1 is indispensable for the survival of CA3 pyramidal cells of the hippocampus but dispensable for the development of lymphocytes and that their intracellular signal transduction is mediated by cytokines such as IL-2, IL-4, IL-7, and GM-CSF (40). To further investigate the in vivo function of the STAMs, we generated mice with a targeted mutation of the STAM2 gene. STAM2−/− mice were morphologically indistinguishable from wild-type mice, and their lymphocytes showed normal responses to cytokines such as IL-2 and GM-CSF (unpublished data). We then attempted to knock out both the STAM1 and STAM2 genes by crossing STAM1+/− and STAM2−/− mice and found that STAM1−/− STAM2−/− mice are embryonically lethal within E11.5, suggesting that STAM1 and STAM2 can compensate for each other in lymphocyte development (unpublished data).
To investigate the functional significance of the STAMs in T-cell development, we used the Cre/loxP system for conditional disruption of the stam1 gene. A target vector was constructed to delete exons 3 and 4 of the stam1 gene upon the expression of Cre recombinase (Fig. 1A). ES cells were electroporated with this vector and selected in the presence of G418 and ganciclovir. Homologous recombinant clones were identified by Southern blot analysis (data not shown). The STAM1flox ES clones were used to generate chimeric mice in which the floxed STAM1 gene was successfully transmitted to the germ line. Mice homozygous for the floxed STAM1 gene (STAM1flox/flox) were identified by Southern blot and PCR analyses (Fig. 1B and C). STAM1flox/flox mice were born at the expected Mendelian ratios and presented no obvious abnormalities. We then mated STAM1flox/flox mice to STAM2−/− mice to generate STAM1flox/+ STAM2+/− mice. STAM1flox/+ STAM2+/− mice were intercrossed to generate STAM1flox/flox STAM2−/− mice. STAM1flox/flox STAM2−/− mice were born at the expected Mendelian ratios and presented no obvious abnormalities.
Generation of Lck-Cre STAM1flox/flox STAM2−/− mice.
To generate mice in which STAM1 and STAM2 were deficient in a T-cell-specific manner, STAM1flox/flox STAM2−/− mice were mated to mice carrying the Cre transgene under the control of the lck promoter to generate Lck-Cre(+) STAM1flox/+ STAM2+/− mice. These mice were mated with STAM1flox/flox STAM2−/− mice. Mice carrying Lck-Cre, the floxed-STAM1 gene, and the mutated STAM2 gene [Lck-Cre(+) STAM1flox/+ STAM2+/−, Lck-Cre(+) STAM1flox/+ STAM2−/−, Lck-Cre(+) STAM1flox/flox STAM2+/−, Lck-Cre(+) STAM1flox/flox STAM2−/−] were born alive and grew normally under specific-pathogen-free conditions. To evaluate the efficiency of the Cre-mediated deletion of the floxed gene, we performed PCR analysis (Fig. 1C). In the thymocytes from nontransgenic STAM1flox/flox STAM2−/− mice and tails from Lck-Cre(+) STAM1flox/flox STAM2−/− mice, we detected only a 405-bp band by PCR analysis with primers A and B but not a 489-bp band by PCR analysis with primers A and C. Conversely, in thymocytes from Lck-Cre(+) STAM1flox/flox STAM2−/− mice, we detected only a 489-bp band with primers A and C but not a 405-bp band with primers A and B. We also performed RT-PCR analysis (Fig. 1D). A pair of primers spanning exons 3 and 4 amplified a 415-bp fragment in thymocytes from nontransgenic STAM1flox/flox STAM2−/− mice and the livers from Lck-Cre(+) STAM1flox/flox STAM2−/− mice. On the other hand, this pair of primers amplified a 238-bp fragment in the thymocytes from Lck-Cre(+) STAM1flox/flox STAM2−/− mice. These findings confirmed that complete Cre-mediated deletion occurred in the thymocytes of Lck-Cre(+) STAM1flox/flox STAM2−/− mice at both the DNA and mRNA levels. We next analyzed the Cre-mediated deletion at the protein level (Fig. 1E). Thymocytes were lysed and incubated with the anti-STAM1 or anti-STAM2 Ab. The immunoprecipitates were further immunoblotted with the anti-STAM1 or anti-STAM2 Ab. STAM1 and STAM2 proteins were readily detected in the thymocytes from Lck-Cre(+) STAM1+/+ STAM2+/+ and Lck-Cre(+) STAM1+/− STAM2+/− mice. However, STAM1 and STAM2 proteins could not be detected in the thymocytes from Lck-Cre(+) STAM1flox/flox STAM2−/− mice. Thus, the Cre-mediated deletion eliminated the expression of the STAM1 protein. We then performed RT-PCR in each thymocyte fraction to find out when the Cre-mediated deletion appeared during thymocyte development (Fig. 1F). Both the mutant 238-bp band and the wild-type 415-bp band appeared in DN thymocytes. We could detect the mutant 238-bp band but not the wild-type band in DP and SP thymocytes. Thus, the Cre-mediated deletion started in DN thymocytes and was completed in DP thymocytes.
Impairment of T-cell development in Lck-Cre(+) STAM1flox/flox STAM2−/− mice.
We examined whether the deficiency of both STAM1 and STAM2 affects T-cell development. The total thymocyte numbers of the Lck-Cre(+) STAM1flox/flox STAM2−/− mice were smaller than those of control [Lck-Cre(+) STAM1flox/+ STAM2+/−] mice (Fig. 2A and B). Histological examinations revealed a remarkable decrease in nucleated cells in the cortex area of the Lck-Cre(+) STAM1flox/flox STAM2−/− mice (Fig. 2C and D). We then performed TUNEL staining, which detects DNA fragmentation in dying cells. Many TUNEL-positive cells were detected in the thymuses of Lck-Cre(+) STAM1flox/flox STAM2−/− mice, whereas only a few were seen in those of control mice (Fig. 2E and F). The total number of nucleated cells in the thymus of Lck-Cre(+) STAM1flox/flox STAM2−/− mice was almost one-third that in the thymuses of control mice (Fig. 2G). The total numbers of nucleated cells in the spleen or lymph nodes of Lck-Cre(+) STAM1flox/flox STAM2−/− mice were almost the same as those in control mice (data not shown).
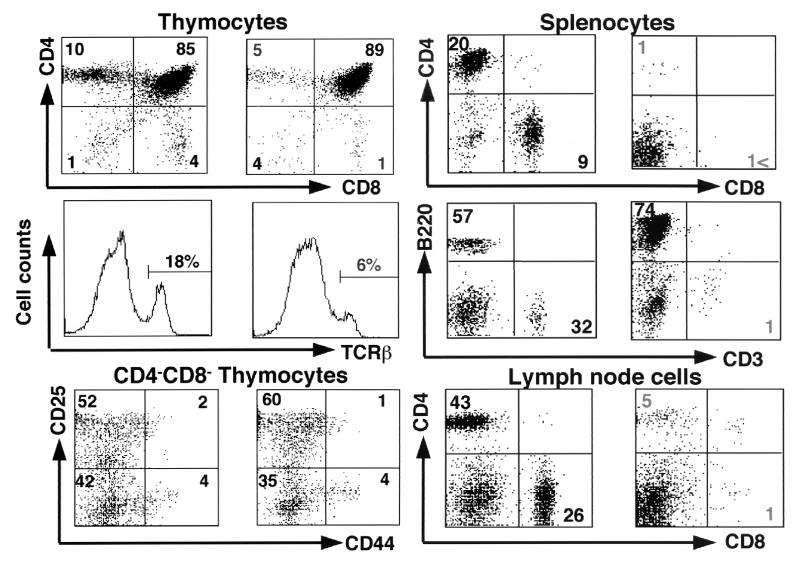
We next performed flow-cytometric analyses of the lymphoid organs, such as the thymus, spleen, and lymph nodes. Flow-cytometric analyses of thymocytes revealed that CD4 SP or CD8 SP thymocytes expressing high levels of T-cell receptor (TCR) were significantly reduced in the Lck-Cre(+) STAM1flox/flox STAM2−/− mice (Fig. 3). The DN compartment was further analyzed for the expression of CD44 and CD25, which are used to define four subsets of DN thymocytes (7). There was no significant difference in the four subsets between Lck-Cre(+) STAM1flox/flox STAM2−/− and control mice (Fig. 3). Moreover, flow-cytometric analyses of splenocytes and lymph node cells showed that peripheral T cells were remarkably reduced in the Lck-Cre(+) STAM1flox/flox STAM2−/− mice (Fig. 3). These results indicate that the double deficiency of STAM1 and STAM2 affects thymocyte development, especially the mature single-positive thymocyte development, and that the STAMs are indispensable for the development and intrinsic maintenance of peripheral T cells.
FIG. 3.
Flow-cytometric analysis. Total thymocytes, DN thymocytes, splenocytes, and lymph node cells derived from 4-week-old Lck-Cre (+) STAM1flox/+ STAM2+/− (n = 8) (left) and Lck-Cre (+) STAM1flox/flox STAM2−/− (right) (n = 8) mice were stained with Abs for CD4 and CD8, TCRβ, CD25 and CD44, or CD3 and B220. Numbers indicate the average percentages of the gated cellular subpopulations within the lymphocyte population.
Proliferative responses of STAM1- and STAM2-deficient T cells upon stimulation with cytokines.
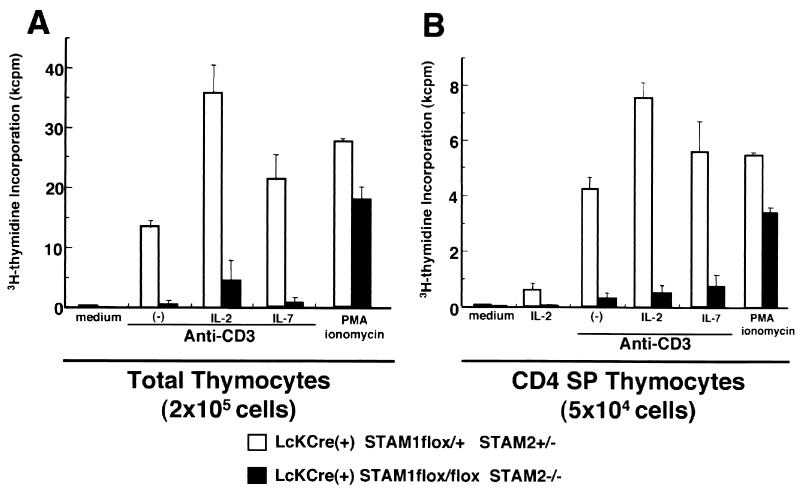
We previously reported that STAM1 and STAM2 are phosphorylated on tyrosine upon stimulation with IL-2 or IL-7, which share the common γ chain as a receptor subunit (6, 34). We also confirmed that TCR stimulation also induced the tyrosine phosphorylation of STAM1 and STAM2 (unpublished data). We then examined the proliferation of T cells in response to TCR stimulation with or without IL-2 or IL-7 in the Lck-Cre(+) STAM1flox/flox STAM2−/− mice. We could only examine the proliferation of thymocytes, because there were too few peripheral T cells in the Lck-Cre(+) STAM1flox/flox STAM2−/− mice to isolate. The total thymocytes and CD4 SP thymocytes of the Lck-Cre(+) STAM1flox/flox STAM2−/− mice showed profoundly reduced proliferation responses upon stimulation with the anti-CD3 Ab alone or anti-CD3 Ab plus IL-2 or IL-7 compared with control mice (Fig. 4A and B). These results indicate that the responses to both TCR stimulation and cytokines are severely impaired in the T cells derived from Lck-Cre(+) STAM1flox/flox STAM2−/− mice.
FIG. 4.
Proliferative responses of thymocytes. Total thymocytes (2 × 105 per well) (A) and CD4 SP thymocytes (5 × 104 per well) (B) were stimulated with indicated ligands: plate-coated anti-CD3 MAb (10 mg/ml), plate-coated anti-CD28 MAb (1 mg/ml), murine IL-2 (10 nM), murine IL-7 (10 ng/ml), phorbol myristate acetate (10 ng/ml), and ionomycin (1 mg/ml). They were cultured for 42 h and were then pulsed with [3H]thymidine and harvested after 6 h.
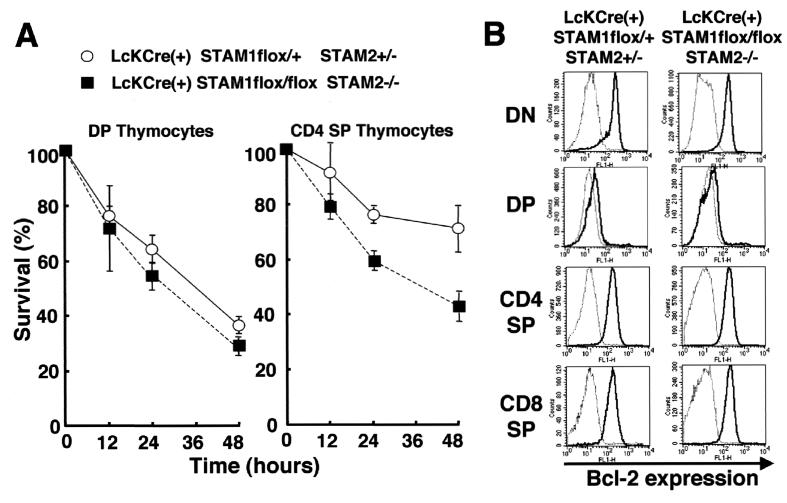
Cell survival and Bcl-2 expression in thymocytes deficient for both STAM1 and STAM2.
We next asked whether the double mutation of STAM1 and STAM2 affects the survival of thymocytes, because a defect in the common γ chain reduces thymocyte survival (3, 4, 5, 21, 22). DP and CD4 SP thymocytes were cultured for 12 to 48 h, and cell viability was assessed by staining with both propidium iodide and annexin V. Both types of thymocytes, and especially the CD4 SP thymocytes, from the Lck-Cre(+) STAM1flox/flox STAM2−/− mice died more quickly than those from control mice (Fig. 5A). This finding indicates that the deficiency of both STAM1 and STAM2 results in the reduction of mature CD4 SP thymocyte survival. Because Bcl-2 expression has been suggested to be important for T-cell survival mediated by signaling through the common γ chain (4, 12, 18, 19, 22), we compared the expression levels of Bcl-2 in the thymocytes of Lck-Cre(+) STAM1flox/flox STAM2−/− versus control mice by flow cytometry. However, there were no significant differences in Bcl-2 expression in the thymocyte subsets, including DN, DP, CD4 SP, and CD8 SP (Fig. 5B). Another Bcl-2-related protein, Bcl-xl, has also been shown to be responsible for preventing the apoptosis of lymphocytes (2, 17). Therefore, we also examined Bcl-xl expression by Western blotting and demonstrated that the expression of Bcl-xl in the STAM1- and STAM2-deficient thymocytes was almost the same as in control thymocytes (data not shown). Taken together, our results indicate that the STAMs are responsible for the survival of thymocytes by a mechanism that is independent of Bcl-2 or Bcl-xl.
FIG. 5.
In vitro cell survival of thymocytes and Bcl-2 expression in thymocytes. (A) In vitro cell survival experiment using DP thymocytes and CD4 SP thymocytes. After the indicated culture time, cells were stained with annexin V. Viable cells were defined as annexin-V-negative cells in each fraction of thymocytes. Error bars, standard deviations. (B) Bcl-2 expression levels in thymocyte fractions. Each fraction of thymocytes was stained with either isotopic control MAb (dotted line) or anti-Bcl-2 mouse MAb (solid line). The results, analyzed by gating CD4−/CD8− (DN) cells, CD4+/CD8+(DP), CD4+ (CD4 SP), or CD8+ (CD8 SP) cells, are shown. Representative results of two independent experiments are presented.
TCR-mediated signaling in thymocytes deficient in both STAM1 and STAM2.
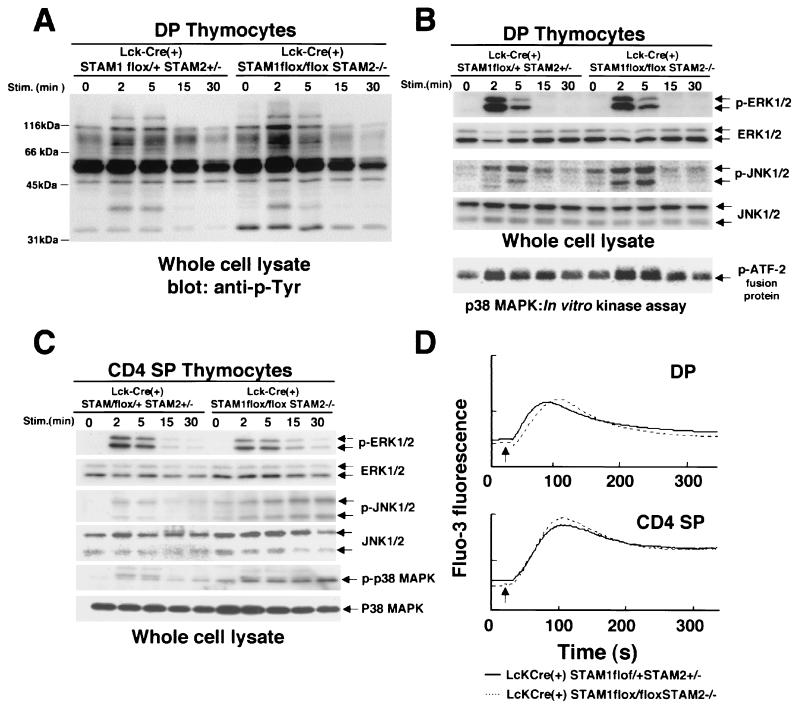
The proliferative response to TCR stimulation was significantly impaired in Lck-Cre(+) STAM1flox/flox STAM2−/− thymocytes. Therefore, to elucidate the molecular basis of the impairment in TCR signaling in the absence of STAMs, we analyzed the signaling pathways in thymocytes activated by TCR engagement. DP and CD4 SP thymocytes were separated, and the activation of signaling molecules downstream of the TCR was examined. The proximal signaling events induced by TCR stimulation are initiated by TCR/CD3-associated protein tyrosine kinases. Immunoblotting with an antiphosphotyrosine Ab showed similar phosphorylation patterns in control and Lck-Cre(+) STAM1flox/flox STAM2−/− DP thymocytes (Fig. 6A). The proximal signaling by the TCR activates ERK, JNK, p38MAPK, and phospholipase C. Phospholipase C generates second messengers that lead to an increase in free cytoplasmic calcium (Ca2+) and the activation of protein kinase C (PKC). Western blotting using phospho-specific ERK and JNK1/2 Abs showed that both ERK and JNK were activated with similar kinetics after TCR cross-linking in control and Lck-Cre(+) STAM1flox/flox STAM2−/− DP thymocytes (Fig. 6B). In vitro kinase assays revealed that the p38MAPK activity was also similar in control and Lck-Cre(+) STAM1flox/flox STAM2−/− DP thymocytes (Fig. 6B).
FIG. 6.
TCR-induced tyrosine phosphorylation, activation of ERK, JNK, and p38MAPKs, and intracellular calcium fluxes in thymocytes. (A and B) Double-positive thymocytes were incubated with anti-CD3 Ab for the indicated times. Induction of tyrosine phosphorylations of total proteins (A), ERK (B) and JNK (B) in whole-cell lysates was analyzed by immunoblotting. p38MAPK activity was measured using an immunocomplex kinase assay (B). (C) CD4 single-positive thymocytes were incubated with anti-CD3 Ab for the indicated times. Induction of ERK, JNK, and p38MAPK phosphorylation was analyzed by immunoblotting. (D) Intracellular calcium fluxes were measured in double-positive thymocytes or CD4 single-positive thymocytes using Fluo-3. Biotinylated anti-CD3 Ab was first added at 0 s, and then streptavidin was added at 20 s (arrow indicated) for cross-linking.
We next examined the TCR signaling in CD4 SP thymocytes. There was no significant difference in ERK1/2 activation after TCR cross-linking between control and Lck-Cre(+) STAM1flox/flox STAM2−/− CD4 SP thymocytes (Fig. 6C). However, Western blotting using anti-phospho-specific JNK and -p38MAPK Abs showed that after TCR cross-linking, both the JNK and p38MAPK activities were more greatly increased and were prolonged in the STAM-deficient CD4 SP thymocytes (Fig. 6C).
We also examined the TCR-induced Ca2+ fluxes in control and Lck-Cre(+) STAM1flox/flox STAM2−/− thymocytes. In the DP or CD4 SP thymocytes from control and Lck-Cre(+) STAM1flox/flox STAM2−/− mice, the TCR-induced Ca2+ fluxes showed similar activation and inactivation kinetics (Fig. 6D).
These data demonstrate that eliminating the expression of the STAMs affects the JNK and p38MAPK activations that result from TCR stimulation in CD4 SP thymocytes.
Cytokine-mediated signaling in thymocytes deficient in both STAM1 and STAM2.
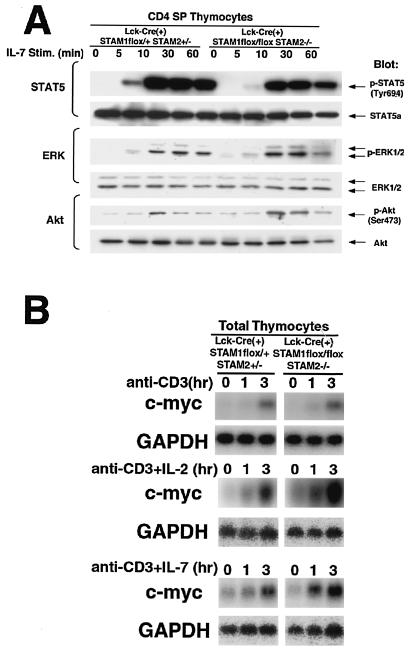
Our previous study of the STAMs and their mutants using an in vitro overexpression system suggested that STAM1 and STAM2 contribute to the signaling downstream of the γc chain, which is shared by the receptors for various cytokines, including IL-2 and IL-7 (6, 35). Therefore, we examined the effect of the deficiency in both STAMs on IL-7-mediated signaling in CD4 SP thymocytes. We first confirmed that the expression levels of the α chain of the IL-7 receptor and the γc chain on CD4 SP thymocytes were not significantly different between the STAM-deficient mice and control mice (data not shown). The proximal signal of IL-7 activates STAT5, ERK, and PKB/Akt. Western blotting analyses showed that STAT5, ERK, and PKB/Akt were phosphorylated with similar kinetics after IL-7 treatment in the CD4 SP thymocytes of control and Lck-Cre(+) STAM1flox/flox STAM2−/− mice (Fig. 7A). These data demonstrate that a deficiency of both STAMs does not affect the IL-7-induced activations of STAT5, ERK, and PKB/Akt in CD4 SP thymocytes. We also examined the effect of the deficiency in both STAMs on IL-2-induced activations of STAT5, ERK, and PKB/Akt, but their significant activations were not observed in both control and Lck-Cre(+) STAM1flox/flox STAM2−/− thymocytes (data not shown).
FIG. 7.
Cytokine-mediated signaling in thymocytes. (A) CD4 SP thymocytes were incubated with murine IL-7 for the indicated times and analyzed for phosphorylation of STAT5, ERK, and Akt by immunoblotting. (B) Total thymocytes were incubated with plate-coated anti-CD3 MAb alone (10 mg/ml) or plate-coated anti-CD3 MAb (10 mg/ml) plus murine IL-2 (10 nM) or murine IL-7 (10 ng/ml) for the indicated times. Total RNA prepared from the cells was first hybridized with the c-myc probe and then rehybridized with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) probe.
c-myc induction in thymocytes deficient in both STAM1 and STAM2 upon stimulation with TCR plus IL-2 or IL-7.
We previously reported that overexpression of either STAM1 or STAM2 enhances c-myc induction upon IL-2 stimulation (6, 35). So, we investigated whether c-myc induction upon TCR stimulation and/or cytokines is impaired in STAM1−/− STAM2−/− thymocytes. When thymocytes were stimulated with IL-2 or IL-7 alone, c-myc induction could not be observed in both control and Lck-Cre(+) STAM1flox/flox STAM2−/− thymocytes (data not shown). However, c-myc inductions were detectable and not significantly different in thymocytes between the T-cell-specific STAM1−/− STAM2−/− mice and wild-type mice upon stimulation with CD3 alone or CD3 plus IL-2 or IL-7 (Fig. 7B). These data suggest that STAMs are dispensable for c-myc induction mediated by the cytokines.
DISCUSSION
STAM1 and STAM2, which are associated with Jak2 and Jak3, were previously suggested to be involved in the signaling for cell growth and c-myc induction mediated by IL-2 and GM-CSF (6, 35). We previously generated STAM1 knockout mice by gene targeting and demonstrated that the targeted disruption of STAM1 had little effect on the development of hematopoietic cells, including those of the T, B, myeloid, and erythroid lineages, or on the proliferative responses of bone marrow cells and splenocytes to IL-2 and GM-CSF (40). Because STAM2 is homologous to STAM1, it was thought to have a similar function (6), and it seemed possible that STAM2 could compensate for STAM1 in the intracellular signaling pathways mediated by cytokines in STAM1−/− mice. To test this possibility, we generated mice with a targeted mutation in the STAM2 gene and mice that were doubly deficient for STAM1 and STAM2 by crossing the STAM2−/− mice and STAM1−/− mice. The STAM2−/− mice did not show any abnormal phenotype even in lymphocyte development, but the mice that were doubly deficient for STAM1 and STAM2 became embryonically lethal by E11.5, with a defect in ventral folding morphogenesis (unpublished data). These results indicated that the STAMs play critical roles in mouse embryonic development. Thus, to begin to elucidate the roles of the STAMs, here we induced a T-cell-specific disruption of both the STAM1 and STAM2 genes in mice using the Cre/loxP system. The resulting mice, which were doubly deficient for STAM1 and STAM2, showed a significant reduction in thymocytes and a profound reduction in peripheral mature T cells. These results suggested that the STAMs are compensatory for each other and play important roles in the regulation of T-cell development.
STAMs are indispensable for T-cell development and survival but dispensable for the proximal signaling of cytokine receptors.
Lck-Cre(+) STAM1flox/flox STAM2−/− mice showed a significant reduction in the total number of thymocytes and in the proportion of CD4 SP thymocytes. Moreover, the peripheral T cells were almost completely absent in the Lck-Cre(+) STAM1flox/flox STAM2−/− mice. These results indicate that the deficiency in both STAM1 and STAM2 affects thymocyte development, especially mature SP thymocyte development, and that the STAMs are indispensable for the development and intrinsic maintenance of peripheral T cells. In proliferation assays, thymocytes from the mutant mice showed defective responses to TCR stimulation by Abs and/or cytokines such as IL-2 and IL-7. Furthermore, in cell viability assays, cultured STAM1−/− STAM2−/− CD4 SP thymocytes died more quickly than controls. Mice deficient in the γc chain were previously demonstrated to carry significantly decreased numbers of lymphocytes, including T and NK cells, in which Bcl-2 expression was reduced (4, 12, 18, 22), and the introduction of Bcl-2 resulted in a recovery of T cells in these γc-chain-deficient mice (12). Therefore, we examined whether the double mutation of the STAMs affected expression of Bcl-2 and its homologous molecule, Bcl-xl, in thymocytes and found that their expressions were normal in the thymocyte subsets from the STAM1−/− STAM2−/− mice, such as DN, DP, CD4 SP, and CD8 SP. These data suggest that the STAMs are necessary for the proliferation of thymocytes in response to TCR and cytokine stimulations and for the survival of mature T cells in a manner that is independent of Bcl-2 and Bcl-xl expression.
IL-7 signaling via the γc chain-Jak3 complex is essential for T-cell survival (3, 5, 20, 21, 37). Because STAM1 and STAM2 are known to be tyrosine phosphorylated in response to IL-7 (6, 34), we analyzed the IL-7 signaling pathway in STAM1- and STAM2-deficient CD4 SP thymocytes. Our data demonstrate that the loss of STAM expression does not affect the IL-7-induced activations of proximal signaling molecules such as STAT5, ERK, and PKB/Akt. Furthermore, although our previous study showed the possible involvement of STAM1 and STAM2 in IL-2-mediated c-myc induction in BAF/B03 cells overexpressing either STAM1 or STAM2 (6, 35), the present study clearly demonstrated that the STAMs are dispensable for c-myc induction in thymocytes upon stimulation with anti-CD3 plus IL-2 or IL-7.
Prolonged activations of JNK and p38MAPK in STAM-deficient thymocytes upon stimulation through CD3.
TCR signaling is critically involved in the positive and negative selection of T cells during cell development in the thymus (27, 29). Therefore, to elucidate the molecular basis of the impaired TCR signaling in the absence of STAMs, we analyzed the pathways activated by TCR engagement in the STAM1- and STAM2-deficient thymocytes. There were no significant differences in ERK1/2 activation and Ca2+ influx after TCR cross-linking between control and Lck-Cre(+) STAM1flox/flox STAM2−/− thymocytes. However, both the JNK and p38MAPK activities after TCR cross-linking were more greatly increased and prolonged in the doubly deficient CD4 SP thymocytes. JNK and p38MAPK are thought to be involved in the apoptosis of various cells (9, 39). Moreover, several studies have indicated that p38MAPK is involved in the negative selection of T cells in the thymus (30). Therefore, an increased and prolonged activation of JNK and p38MAPK might cause the profound reduction of CD4 SP thymocytes and peripheral T cells in the Lck-Cre(+) STAM1flox/flox STAM2−/− mice. The molecular basis of the increased activation of JNK and p38MAPK in the STAM-deficient mature T cells is still obscure. However, it is worth noting that we previously found that STAM1 is associated with Hrs/Hgs and that Hrs interacts with TAK1 and PAK1 (26), because TAK1 and PAK1 are known to be involved in JNK and p38MAPK cascades (38, 41).
The STAMs may play critical roles in the fundamental programs involved in lymphocyte development and survival rather than the intracellular signaling for lymphocyte proliferation. Hrs, which is associated with the STAMs, has recently been found to play a critical role in the endosomal sorting of ubiquitinated membrane proteins, such as EGF receptors (13, 25, 28). Like Hrs, the STAMs contain a VHS domain, which is also found in other proteins, most of which are involved in vesicular trafficking (15, 16, 42), and a UIM identified in a wide variety of proteins, most of which are involved in the ubiquitination and endosomal sorting of ubiquitinated membrane proteins (10, 24). Although we have observed that eliminating the expression of the STAMs barely affects EGF receptor degradation in embryonic fibroblasts, for which the endosomal sorting of ubiquitinated EGF receptors is prerequisite (unpublished data), we should further examine the possible involvement of the STAMs in endosomal sorting and the degradation of cytokine receptors and their associated proteins in T cells. These possible roles of STAMs for endosomal sorting might affect lymphocyte development and survival. We have not yet confirmed this possibility, because the present Lck-Cre(+) STAM1flox/flox STAM2−/− mice do not provide an adequate supply of T cells for these experiments.
Acknowledgments
We thank Tetsuo Noda for providing the J1 ES cell line, a pGK-neo cassette (ploxpNEO) flanked by a pair of loxP sequences, and a diphtheria toxin A-chain gene cassette without a polyadenylation site, and we thank Junji Takeda for providing Lck-Cre transgenic mice.
This work was supported in part by CREST (Core Research for Evolutional Science and Technology) of the Japan Science and Technology Corporation (JST), a grant-in-aid for scientific research on priority areas from the Ministry of Education, Science, Sports and Culture of Japan.
REFERENCES
- 1.Asao, H., Y. Sasaki, T. Arita, N. Tanaka, K. Endo, H. Kasai, T. Takeshita, Y. Endo, T. Fujita, and K. Sugamura. 1997. Hrs is associated with STAM, a signal-transducing adaptor molecule. J. Biol. Chem. 272:32785-32791. [DOI] [PubMed] [Google Scholar]
- 2.Boise, L. H., M. Gonzalez-Garcia, C. E. Postema, L. Ding, T. Lindsten, L. A. Turka, X. Mao, G. Nunez, and C. B. Thompson. 1993. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell 74:597-608. [DOI] [PubMed] [Google Scholar]
- 3.Cao, X., E. W. Shores, J. Hu-Li, M. R. Anver, B. L. Kelsall, S. M. Russell, J. Drago, M. Noguchi, A. Grinberg, E. T. Bloom, et al. 1995. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity 2:223-238. [DOI] [PubMed] [Google Scholar]
- 4.Di Santo, J. P., I. Aifantis, E. Rosmaraki, C. Garcia, J. Feinberg, H. J. Fehling, A. Fischer, H. Boehmer, and B. Rocha. 1999. The common cytokine receptor chain and the pre-T cell receptor provide independent but critically overlapping signals in early α/β T cell development. J. Exp. Med. 189:563-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DiSanto, J. P., W. Muller, D. Guy-Grand, A. Fischer and K. Rajewsky. 1995. Lymphoid development in mice with a targeted deletion of the interleukin 2 receptor gamma chain. Proc. Natl. Acad. Sci. USA 92:377-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Endo, K., T. Takeshita, H. Kasai, Y. Sasaki, N. Tanaka, H. Asao, K. Kikuchi, M. Yamada, M. Chen, J. J. O'Shea, and K. Sugamura. 2000. STAM2, a new member of the STAM family, binding to the Janus kinases. FEBS Lett. 477:55-61. [DOI] [PubMed] [Google Scholar]
- 7.Godfrey, D. I., J. Kennedy, T. Suda, and A. Zlotnik. 1993. A developmental pathway involving four phenotypically and functionally distinct subsets of CD3-CD4-CD8- triple-negative adult mouse thymocytes defined by CD44 and CD25 expression. J. Immunol. 150:4244-4252. [PubMed] [Google Scholar]
- 8.Gu, H., J. D. Marth, P. C. Orban, H. Mossmann, and K. Rajewsky. 1994. Deletion of a DNA polymerase β gene segment in T cells using cell type-specific gene targeting. Science 265:103-106. [DOI] [PubMed] [Google Scholar]
- 9.Hochedlinger, K., E. F. Wagner, and K. Sabapathy. 2002. Differential effects of JNK1 and JNK2 on signal specific induction of apoptosis. Oncogene 21:2441-2445. [DOI] [PubMed] [Google Scholar]
- 10.Hofmann, K., and L. Falquet. 2001. A ubiquitin-interacting motif conserved in components of the proteosomal and lysosomal protein degradation systems. Trends Biochem. Sci. 26:347-349. [DOI] [PubMed] [Google Scholar]
- 11.Kato, M., K. Miyazawa, and N. Kitamura. 2000. A deubiquitinating enzyme UBPY interacts with the Src homology 3 domain of Hrs-binding protein via a novel binding motif PX(V/I)(D/N)RXXKP. J. Biol. Chem. 275:37481-37487. [DOI] [PubMed] [Google Scholar]
- 12.Kondo, M., K. Akashi, J. Domen, K. Sugamura, and I. L. Weissman. 1997. Bcl-2 rescues T lymphopoiesis, but not B or NK cell development, in common γ-chain-deficient mice. Immunity 7:155-162. [DOI] [PubMed] [Google Scholar]
- 13.Lloyd, T. E., R. Atkinson, M. N. Wu, Y. Zhou, G. Pennetta, and H. J. Bellen. 2002. Hrs regulates endosome membrane invagination and tyrosine kinase receptor signaling in Drosophila. Cell 108:261-269. [DOI] [PubMed] [Google Scholar]
- 14.Lohi, O., A. Poussu, J. Meriläinen, S. Kellokumpu, V. M. Wasenius, and V. P. Lehto. 1998. EAST, an epidermal growth factor receptor- and Eps15-associated protein with Src homology 3 and tyrosine-based activation motif domains. J. Biol. Chem. 273:21408-21415. [DOI] [PubMed] [Google Scholar]
- 15.Lohi, O., and V. P. Lehto. 1998. VHS domain marks a group of proteins involved in endocytosis and vesicular trafficking. FEBS Lett. 440:255-257. [DOI] [PubMed] [Google Scholar]
- 16.Lohi, O., and V. P. Lehto. 2001. STAM/EAST/Hbp adapter proteins—integrators of signalling pathways. FEBS Lett. 508:287-290. [DOI] [PubMed] [Google Scholar]
- 17.Motoyama, N., F. Wang, K. A. Roth, H. Sawa, K.-I. Nakayama, K. Nakayama, I. Negishi, S. Senju, Q. Zhang, S. Fujii, and D. Y. Loh. 1995. Massive cell death of immature hematopoietic cells and neurons in Bcl-x-deficient mice. Science 267:1506-1510. [DOI] [PubMed] [Google Scholar]
- 18.Nakajima, H., and W. J. Leonard. 1999. Role of Bcl-2 in αβ T cell development in mice deficient in the common cytokine receptor γ-chain: the requirement for Bcl-2 differs depending on the TCR/MHC affinity. J. Immunol. 162:782-790. [PubMed] [Google Scholar]
- 19.Nakayama, K., K. Nakayama, I. Negishi, K. Kuida, H. Sawa, and D. Y. Loh. 1994. Targeted disruption of Bcl-2 alpha beta in mice: occurrence of gray hair, polycystic kidney disease, and lymphocytopenia. Proc. Natl. Acad. Sci. USA 91:3700-3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nosaka, T., J. M. van Deursen, R. A. Tripp, W. E. Thierfelder, B. A. Witthuhn, A. P. McMickle, P. C. Doherty, G. C. Grosveld, and J. N. Ihle. 1995. Defective lymphoid development in mice lacking Jak3. Science 270:800-802. [DOI] [PubMed] [Google Scholar]
- 21.Ohbo, K., T. Suda, M. Hashiyama, A. Mantani, M. Ikebe, K. Miyakawa, M. Moriyama, M. Nakamura, M. Katsuki, K. Takahashi, K. Yamamura, and K. Sugamura. 1996. Modulation of hematopoiesis in mice with a truncated mutant of the interleukin-2 receptor gamma chain. Blood 87:956-967. [PubMed] [Google Scholar]
- 22.Otsu, M., K Sugamura, and F. Candotti. 2001. Lack of dominant-negative effects of a truncated γc on retroviral-mediated gene correction of immunodeficient mice. Blood 97:1618-1624. [DOI] [PubMed] [Google Scholar]
- 23.Park, S. Y., K. Saijo, T. Takahashi, M. Osawa, H. Arase, N. Hirayama, K. Miyake, H. Nakauchi, T. Shirasawa, and T. Saito. 1995. Developmental defects of lymphoid cells in Jak3 kinase-deficient mice. Immunity 3:771-782. [DOI] [PubMed] [Google Scholar]
- 24.Polo, S., S. Sigismund, M. Faretta, M. Guidi, M. R. Capua, G. Bossi, H. Chen, P. De Camilli, and P. P. Di Fiore. 2002. Single motif responsible for ubiquitin recognition and monoubiquitination in endocytic proteins. Nature 416:451-455. [DOI] [PubMed] [Google Scholar]
- 25.Raiborg, C., K. G. Bache, D. J. Gillooly, I. H. Madshus, E. Stang, and H. Stenmark. 2002. Hrs sorts ubiquitinated proteins into clathrin-coated microdomains of early endosomes Nat. Cell Biol. 4:394-398. [DOI] [PubMed] [Google Scholar]
- 26.Sasaki, Y., and K. Sugamura. 2001. Involvement of Hgs/Hrs in signaling for cytokine-mediated c-fos induction through interaction with TAK1 and Pak1. J. Biol. Chem. 276:29943-29952. [DOI] [PubMed] [Google Scholar]
- 27.Sebzda, E., S. Mariathasan, T. Ohteki, R. Jones, M. F. Bachmann, and P. S. Ohashi. 1999. Selection of the T cell repertoire. Annu. Rev. Immunol. 17:829-874. [DOI] [PubMed] [Google Scholar]
- 28.Shih, S. C., D. J. Katzmann, J. D. Schnell, M. Sutanto, S. D. Emr, and L. Hicke. 2002. Epsins and Vps27p/Hrs contain ubiquitin-binding domains that function in receptor endocytosis. Nat. Cell Biol. 4:389-393. [DOI] [PubMed] [Google Scholar]
- 29.Sugamura, K., H. Asao, M. Kondo, N. Tanaka, N. Ishii, K. Ohbo, M. Nakamura, and T. Takeshita. 1996. The interleukin 2 receptor γ chain: its role in the multiple cytokine receptor complexes and T cell development in XSCID. Annu. Rev. Immunol. 14:179-205. [DOI] [PubMed] [Google Scholar]
- 30.Sugawara, T., T. Moriguchi, E. Nishida, and Y. Takahama. 1998. Differential roles of ERK and p38 MAP kinase pathways in positive and negative selection of T lymphocytes. Immunity 9:565-574. [DOI] [PubMed] [Google Scholar]
- 31.Takahama, Y., K. Ohishi, Y. Tokoro, T. Sugawara, Y. Yoshimura, M. Okabe, T. Kinoshita, and J. Takeda. 1998. Functional competence of T cells in the absence of glycosylphosphatidylinositol-anchored proteins caused by T cell-specific disruption of the Pig-a gene. Eur. J. Immunol. 28:2159-2166. [DOI] [PubMed] [Google Scholar]
- 32.Takata, H., M. Kato, K. Denda, and N. Kitamura. 2000. A Hrs binding protein having a Src homology 3 domain is involved in intracellular degradation of growth factors and their receptors. Gene Cells 5:57-69. [DOI] [PubMed] [Google Scholar]
- 33.Takeda, K., T. Kaisho, N. Yoshida, J. Takeda, T. Kishimoto, and S. Akira. 1998. Stat3 activation is responsible for IL-6-dependent T cell proliferation through preventing apoptosis: generation and characterization of T cell-specific Stat3-deficient mice. J. Immunol. 161:4652-4660. [PubMed] [Google Scholar]
- 34.Takeshita, T., T. Arita, H. Asao, N. Tanaka, M. Higuchi, H. Kuroda, K. Kaneko, H. Munakata, Y. Endo, T. Fujita, and K. Sugamura. 1996. Cloning of a novel signal-transducing adaptor molecule containing an SH3 domain and ITAM. Biochem. Biophys. Res. Commun. 225:1035-1039. [DOI] [PubMed] [Google Scholar]
- 35.Takeshita, T., T. Arita, M. Higuchi, H. Asao, K. Endo, H. Kuroda, N. Tanaka, K.Murata, N. Ishii, and K. Sugamura. 1997. STAM, signal transducing adaptor molecule, is associated with Janus kinases and involved in signaling for cell growth and c-myc induction. Immunity 6:449-457. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka, N., K. Kaneko, H. Asao, H. Kasai, Y. Endo, T. Fujita, T. Takeshita, and K. Sugamura. 1999. Possible involvement of a novel STAM-associated molecule “AMSH” in intracellular signal transduction mediated by cytokines. J. Biol. Chem. 274:19129-19135. [DOI] [PubMed] [Google Scholar]
- 37.Thomis, D. C., C. B. Gurniak, E. Tivol, A. H. Sharpe, and L. J. Berg. 1995. Defects in B lymphocyte maturation and T lymphocyte activation in mice lacking Jak3. Science 270:794-797. [DOI] [PubMed] [Google Scholar]
- 38.Wang, W., G. Zhou, M. C. Hu, Z. Yao, and T. H. Tan. 1997. Activation of the hematopoietic progenitor kinase-1 (HPK1)-dependent, stress-activated c-Jun N-terminal kinase (JNK) pathway by transforming growth factor beta (TGF-beta)-activated kinase (TAK1), a kinase mediator of TGF beta signal transduction. J. Biol. Chem. 272:22771-22775. [DOI] [PubMed] [Google Scholar]
- 39.Xia, Z., M. Dickens, J. Raingeaud, R. J. Davis, and M. E. Greenberg. 1995. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270:1326-1331. [DOI] [PubMed] [Google Scholar]
- 40.Yamada, M., T. Takeshita, S. Miura, K. Murata, Y. Kimura, N. Ishii, M. Nose, H. Sakagami, H. Kondo, F. Tashiro, J.-I. Miyazaki, H. Sasaki, and K. Sugamura. 2001. Loss of hippocampal CA3 pyramidal neurons in mice lacking STAM1. Mol. Cell. Biol. 21:3807-3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang, S., J. Han, M. A. Sells, J. Chernoff, U. G. Knaus, R. J. Ulevitch, and G. M. Bokoch. 1995. Rho family GTPases regulate p38 mitogen-activated protein kinase through the downstream mediator Pak1. J. Biol. Chem. 270:23934-23936. [DOI] [PubMed] [Google Scholar]
- 42.Zhu, Y., B. Doray, A. Poussu, V.-P. Lehto, and S. Kornfeld. 2001. Binding of GGA2 to the lysosomal enzyme sorting motif of the mannose 6-phosphate receptor. Science 292:1716-1718. [DOI] [PubMed] [Google Scholar]