Abstract
Inhibition of replicon initiation is a stereotypic DNA damage response mediated through S checkpoint mechanisms not yet fully understood. Studies were undertaken to elucidate the function of checkpoint proteins in the inhibition of replicon initiation following irradiation with 254 nm UV light (UVC) of diploid human fibroblasts immortalized by the ectopic expression of telomerase. Velocity sedimentation analysis of nascent DNA molecules revealed a 50% inhibition of replicon initiation when normal human fibroblasts were treated with a low dose of UVC (1 J/m2). Ataxia telangiectasia (AT), Nijmegen breakage syndrome (NBS), and AT-like disorder fibroblasts, which lack an S checkpoint response when exposed to ionizing radiation, responded normally when exposed to UVC and inhibited replicon initiation. Pretreatment of normal and AT fibroblasts with caffeine or UCN-01, inhibitors of ATR (AT mutated and Rad3 related) and Chk1, respectively, abolished the S checkpoint response to UVC. Moreover, overexpression of kinase-inactive ATR in U2OS cells severely attenuated UVC-induced Chk1 phosphorylation and reversed the UVC-induced inhibition of replicon initiation, as did overexpression of kinase-inactive Chk1. Taken together, these data suggest that the UVC-induced S checkpoint response of inhibition of replicon initiation is mediated by ATR signaling through Chk-1 and is independent of ATM, Nbs1, and Mre11.
Accurate replication and segregation of the human genome depends on interactions between cell cycle checkpoints and pathways of DNA repair. Cell cycle checkpoints are biochemical surveillance pathways that slow or arrest progression through the cell cycle, pending completion of essential events and/or repair of DNA damage. DNA damage checkpoints minimize the probability of replicating and segregating damaged DNA and therefore reduce the frequencies of mutations and chromosomal aberrations that are induced by genotoxic stress. Defects in cell cycle checkpoint function and DNA repair result in genetic instability, which may fuel cancer development (30, 38, 54).
DNA damage checkpoints operate in the G1, S, and G2 phases of the cell cycle, transducing signals from sensors of DNA damage to effector proteins that mediate the appropriate response (1, 21). Essential transducers of DNA damage checkpoint responses are the protein kinases ATM (ataxia telangiectasia [AT] mutated) and ATR (ATM and Rad3 related). Cells from AT patients are hypersensitive to the genotoxic effects of DNA damage induced by ionizing radiation (IR) and are defective in DNA damage checkpoint responses to IR (1, 21, 38). ATR, on the other hand, seems to be required for checkpoint responses to replication blocks caused by hydroxyurea (HU) and UV-induced DNA damage (13, 61, 64). Although the roles of ATM and ATR in DNA damage checkpoint responses in G1 and G2 are comparatively well characterized, the S checkpoint remains a topic of intense investigation (1).
The S checkpoint is activated in human cells in response to diverse forms of DNA damage (33-35, 53). The IR-induced S checkpoint response is inhibited or attenuated by mutations in ATM, Nbs1, and Mre11 (11, 53). Nbs1 and Mre11 are the gene products mutated in the familial genetic instability disorders Nijmegen breakage syndrome (NBS) and AT-like disorder (AT-LD), respectively (55). AT, NBS, and AT-LD are clinically distinct cancer-predisposing disorders, but they are phenotypically similar at the cellular level (55). Cells from these patients are hypersensitive to cell killing following exposure to IR, display increased risk of induced chromosomal aberrations, and fail to repress DNA synthesis in the presence of IR-induced DNA damage, a phenomenon termed radioresistant DNA synthesis (RDS) (11, 53). ATM has been shown to phosphorylate Nbs1 on several serine residues in response to IR. Changing any of these serines to alanine produces mutant Nbs1 proteins that fail to correct RDS in NBS cells (26, 42, 65, 67). The IR-induced S checkpoint has recently been reported to include two parallel ATM-dependent pathways (23). It appears that one pathway involves ATM signaling through Chk2, ultimately resulting in the ubiquitin-mediated proteolysis of Cdc25A (22). Loss of Cdc25A phosphatase activity results in a dramatic inhibition of cyclin E/Cdk2 activity and Cdc45 binding to origins of DNA replication (23). The second pathway is dependent on ATM signaling to the Nbs1/Mre11/Rad50 complex, which when activated localizes to sites of DNA double-strand breaks following exposure to IR (45, 49), and by unknown signaling pathways it also effects inhibition of replicon initiation (23). Although the role of ATM in the IR-induced S checkpoint is indisputable, the checkpoint pathway that mediates the inhibition of replicon initiation following UVC-induced DNA damage is less clear.
Normal S-phase cells respond to UVC-induced DNA damage by reducing the rate of DNA synthesis (34, 35). This inhibition is manifested at the level of chain elongation and replicon initiation (34, 35) and is the result of both passive and active cellular responses to DNA lesions. Passive inhibition of DNA replication is attributed to the physical obstruction of the DNA replication apparatus at sites of DNA damage. An example is the inability of the replicative DNA polymerases α and δ to copy through UVC-induced template lesions such as cyclobutane pyrimidine dimers and 6-4 photoproducts (46, 47). Active inhibition is a trans effect mediated through checkpoint signals that emanate from sites of DNA damage and ultimately inhibit the initiation of distant replicons (56). This S checkpoint response imposes transient delays in S-phase progression and provides more time for DNA repair to remove lesions from unreplicated chromatin. A previous study suggested that AT cells display a reduced S checkpoint response to UVC (52). AT cells, while not hypersensitive to inactivation of colony formation by UVC, are nevertheless hypersensitive to S-dependent UV-induced chromosomal aberrations (20, 40). Less is known about the function of ATR, since it is an essential gene and its deletion results in embryonic lethality in mice (6, 16). However, overexpression of kinase-inactive ATR (ATRki) renders cells hypersensitive to cell killing by DNA damaging agents, including IR, HU, and UV (13, 64). Moreover, phosphorylation of checkpoint-associated proteins p53 and Brca1 has been shown to be ATM dependent following exposure to IR, but ATR dependent following exposure to HU and UV (3, 10, 15, 41, 60, 61).
Cellular responses to UVC-induced DNA damage are dose dependent. The inhibition of replicon initiation in response to UVC is best observed after exposure to 1 J/m2, a dose which produces little reduction in cell colony-forming efficiency and very little inhibition of DNA chain elongation or mitotic entry. Cytotoxic doses between 5 and 10 J/m2 UVC, which have been shown to saturate nucleotide excision repair (NER) (39), significantly inhibit DNA chain elongation and mitotic entry (7, 36) and typically reduce colony formation by 50 to 70% (5). UVC doses of >40 J/m2, which have been used to study cellular stress responses to UVC, inhibit DNA chain elongation severely and inactivate colony formation by >95% of normal human fibroblasts (5). This study examined the role of checkpoint proteins in the response of cells to 1 J/m2 UVC, thereby negating potential contributions associated with saturation of NER, induction of stress responses, and cytotoxicity. The results strongly implicate ATR and Chk1 as important signaling molecules for the UVC-induced inhibition of replicon initiation in human cells.
MATERIALS AND METHODS
Cell lines and culture conditions.
Normal human fibroblasts (NHF1), and AT fibroblasts (GM02052A) were maintained in Dulbecco's modified Eagle's medium (Gibco BRL) supplemented with 2 mM glutamine (Gibco BRL) and 10% fetal bovine serum (HyClone). Fibroblasts containing mutations in Nbs1 (W1799) and Mre11 (AT-LD2) (provided by John Petrini, Memorial Sloan-Kettering Cancer Center) were grown in the same medium supplemented with 16% fetal bovine serum. Doxycycline-inducible U2OS cells (provided by Stuart Schreiber and Paul Nghiem, Harvard University) containing inducible wild-type (wt) or kinase-inactive (Ks) ATR (50) were maintained in the same medium as normal human fibroblasts (NHFs) but supplemented with 200 μg of Geneticin (Sigma)/ml, and 50 μg of Hygromycin (Calbiochem)/ml. All cell lines were maintained at 37°C in a humidified atmosphere of 5% CO2.
Immortalization of the NHF1, GM02052A, W1799, and AT-LD2 cell strains with telomerase was accomplished by infection with a replication-defective retrovirus containing hTERT cDNA in a Moloney murine leukemia virus-based retroviral backbone containing the puromycin resistance gene (hTERT cDNA was provided by Robert Weinberg, MIT) (17). Puromycin-resistant cells expressed telomerase and displayed an indefinite in vitro proliferative life span. NHF1, GM02052A, W1799, and AT-LD2 fibroblasts were transduced with the hTERT retrovirus at population doubling levels (PDL) of 23.5, 31.6, 23.6, and 30, respectively. NHF1-hTERT, GM02052A-hTERT, W1799-hTERT, and AT-LD2-hTERT fibroblasts were maintained in continuous culture up to PDL of 186, 80, 75, and 64, respectively, without reduction in their proliferation rate. Throughout this report, hTERT-expressing normal and mutant fibroblasts are designated NHF1 (the NHF1-hTERT fibroblasts), ATM−/− (GM02052A-hTERT fibroblasts), Nbs1−/− (W1799-hTERT fibroblasts), and Mre11−/− (AT-LD2-hTERT fibroblasts).
Cytogenetics.
Metaphase spreads were prepared from hTERT-immortalized human fibroblasts as previously described (25). Twenty-five metaphases were G-banded according to standard protocols (62) and scored for chromosome number and structure.
Cell irradiation.
Prior to treatment with UVC, culture medium was removed and reserved. Cultures were washed once with warm Hank's balanced salt solution (Gibco BRL) and then placed uncovered under a UV lamp emitting primarily 254 nm radiation at a fluency rate of 0.5 J/m2/s. Following irradiation, reserved medium was replaced and the cultures were incubated for the indicated periods of time. Sham-treated cultures were handled exactly the same way, except that they were not exposed to UVC. Cells treated with gamma rays were maintained in their culture medium and exposed to a 137Cs source at a dose rate of 0.86 Gy/min.
Western analyses.
Logarithmically growing cells were seeded at 106 per 100-mm dish and incubated for 40 h. Cultures were irradiated as described above and incubated in reserved medium for 30 min at 37°C. Cells were harvested by trypsinization, washed once in phosphate-buffered saline, and resuspended in lysis buffer (10 mM sodium phosphate buffer [pH 7.2], 1 mM EDTA, 1 mM EGTA, 150 mM NaCl, and 1% NP-40, supplemented with 10 mM 4-(2-aminoethyl) benzenesulfonyl fluoride, 10 mM β-glycerophosphate, 10 mM sodium orthovanadate, and 10-μg/ml concentrations of leupeptin and aprotinin). Protein concentrations were determined using the Bio-Rad DC protein assay (Bio-Rad Laboratories). Samples containing equal amounts of protein were mixed with an equal volume of 2× Laemmli sample buffer (125 mM Tris-HCl [pH 6.8], 4% sodium dodecyl sulfate [SDS], 20% glycerol) containing 5% β-mercaptoethanol, boiled, and separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were transferred to nitrocellulose and probed with antibodies against ATR (PA1-450 [ABR] or Ab-2 [Oncogene]), Mre11 (NB 100-142 [Novus]), Nbs1 (NB 100-143 [Novus]), Chk1 (G4 [Santa Cruz]), and phospho-serine 317 and phospho-serine 345 Chk1 (Cell Signaling). Production of affinity-purified anti-ATM antibody was previously described (58).
RDS assay.
Logarithmically growing cells were plated at a density of 2.5 × 105 cells per 60-mm dish and grown at 37°C for 30 to 40 h in medium containing 10 nCi of [14C]thymidine (ICN Radiochemicals) per ml to uniformly label DNA. Radioactive medium was replaced with fresh medium to chase [14C]-labeled precursors into DNA for at least 3 h. To determine the ability of cells to repress DNA synthesis in the presence of DNA damage, cells were either sham treated or exposed to gamma rays (2.5, 5.0, and 7.5 Gy). Cells were incubated at 37°C for 1 h and then for 30 min with 20 μCi of [3H]thymidine/ml. Radioactive medium was removed and cells were washed twice in cold phosphate-buffered saline and harvested by scraping with a rubber policeman into 0.5 ml of 0.1 M NaCl containing 0.01 M EDTA (pH 8) per plate. An aliquot (200 μl) was added to a separate tube containing 200 μl of lysis buffer (1 M NaOH, 0.02 M EDTA), and acid-insoluble DNA was collected on GF/C microfiber glass filters (34). Net 3H radioactivity corrected for 14C spillover was normalized for cell number (total 14C radioactivity).
Alkaline sucrose gradient centrifugation.
The methodology used to determine the steady-state distribution of sizes of nascent DNA 30 to 45 min after irradiation of log-phase cultures with either 1 J/m2 UVC or 1.5 Gy IR has been described previously (12, 14). When indicated, caffeine (Sigma) and UCN-01 (provided by the Drug Synthesis and Chemistry Branch, National Cancer Institute [NCI]) were added to the culture medium 30 min prior to irradiation at concentrations of 2 to 3 mM and 100 nM, respectively.
Adenovirus infection.
Recombinant adenoviruses encoding wild-type murine Chk1 or kinase-inactive Chk1 were generously provided by Cyrus Vaziri (28). Adenovirus encoding green fluorescent protein (GFP) was obtained from the UNC Adenovirus Vector Core Facility. Cells were infected with a multiplicity of infection (MOI) of 50 for 15 h, and adenovirus-encoded protein expression was measured by Western immunoblot analysis (Chk1) or fluorescence microscopy (GFP).
RESULTS
Fibroblasts lacking ATM, Nbs1, or Mre11 display the RDS phenotype.
It has been reported previously that immortalization with hTERT provides a cell with enhanced replicative potential while maintaining the genotypic and phenotypic characteristics of the cell of origin (32, 48, 51, 63). Preliminary analyses of metaphase spreads indicated that the NHF1 and ATM−/− fibroblasts have an apparently normal complement of 46 chromosomes. The Nbs1−/− fibroblasts have 45 chromosomes, including a derivative chromosome, presumably originated by a translocation. Mre11−/− fibroblasts have 46 chromosomes, but a derivative chromosome was detected in some of the metaphase spreads. Cytogenetic analysis to complete the description of the karyotype of these cell lines is now in progress.
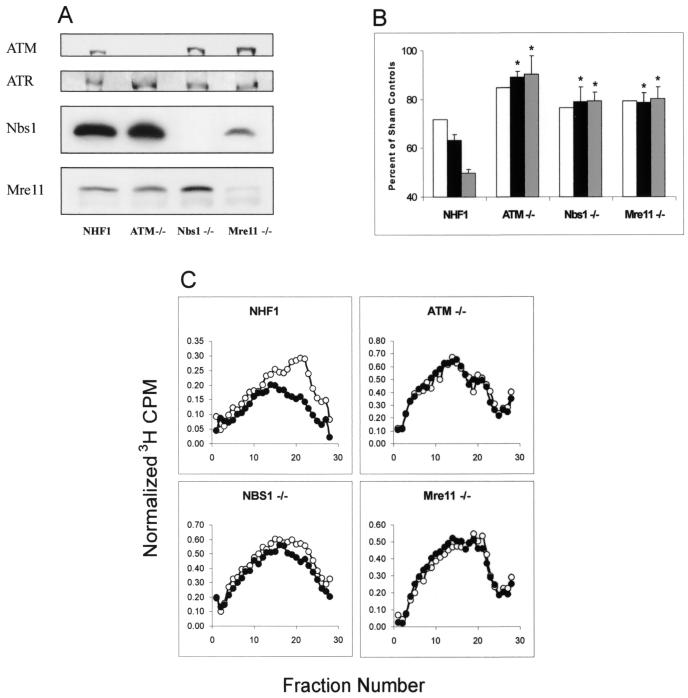
The phenotypes of normal and mutant diploid fibroblasts used in this study were verified by Western immunoblot analysis and DNA synthesis assays following treatment with IR (Fig. 1). Cells from AT, NBS, and AT-LD patients lacked expression of ATM, Nbs1, and Mre11 proteins, respectively. However, all of these cell lines expressed ATR (Fig. 1A). Diploid fibroblasts containing mutations in ATM, Nbs1, or Mre11 are defective in the IR-induced S checkpoint (11, 23, 53). We confirmed the RDS phenotype of these mutant cell lines by measuring the degree of inhibition of [3H]thymidine incorporation into DNA after exposing the cultures to gamma rays (2.5, 5, and 7.5 Gy). Whereas the NHFs inhibited DNA synthesis in a dose-dependent manner, this response was severely compromised and not dose dependent in fibroblasts lacking ATM, Nbs1, or Mre11 (Fig. 1B).
FIG. 1.
Telomerase-expressing fibroblasts from patients with familial cancer syndromes display the RDS phenotype. (A) Protein extracts were prepared from NHF1, ATM−/−, Nbs1−/−, and Mre11−/− fibroblasts, and 100 μg of total protein was analyzed by western immunoblot analysis. (B) RDS results. Cells were grown in the presence of [14C]thymidine for ∼40 h to label DNA uniformly and then in nonradioactive medium overnight. Cells were sham treated or exposed to gamma rays, incubated at 37°C for 30 min, and then labeled for 15 min in medium containing [3H]thymidine. Net 3H radioactivity corrected for 14C spillover was normalized to cell number (total 14C radioactivity). The normalized 3H counts per minute was graphed as a percent of sham controls (n = 3). White bar, 2.5 Gy; black bar, 5.0 Gy; gray bar, 7.5 Gy. *, significant difference from NHF1 (P < 0.01). (C) Cells were uniformly labeled as described above and either sham treated or irradiated with 1.5 Gy IR. Following irradiation, cells were incubated for 30 min at 37°C and then pulse-labeled with [3H]thymidine for 15 min. Cells were harvested, and nascent DNA was separated by velocity sedimentation (see Materials and Methods). Net 3H radioactivity corrected for 14C spillover was normalized to cell number (total 14C radioactivity). ○, nascent DNA distributions from sham-treated cells; •, distributions from cultures irradiated with 1.5 Gy.
Exposure to DNA damaging agents reduces the rate of DNA synthesis through passive inhibition of DNA chain elongation and active inhibition of replicon initiation (34, 35, 53). Velocity sedimentation analysis of pulse-labeled DNA reveals the steady-state distribution of nascent DNA intermediates in S-phase cells. Figure 1C demonstrates the effect of low-dose IR on normal and mutant human fibroblasts. Normal cells treated with 1.5 Gy displayed a selective reduction in labeling of nascent DNA banding in gradient fractions 14 to 24 (Mr, 3 × 106 to 9 × 107), reflecting an inhibition of replicon initiation. DNA in fractions 5 to 13 of the sedimentation profiles (Mr, 1 × 108 to 4 × 108) represents products of DNA chain elongation and replicon merging from replication sites that initiated prior to irradiation. Low-dose IR had little effect on DNA chain elongation in active replicons. The selective inhibition of DNA synthesis in low-molecular-weight replication intermediates was not apparent in cells with ATM, Nbs1, or Mre11 mutations (Fig. 1C). Therefore, inhibition of replicon initiation is an ATM-, Nbs1-, and Mre11-dependent response to IR-induced DNA damage.
UVC-induced DNA damage inhibits replicon initiation.
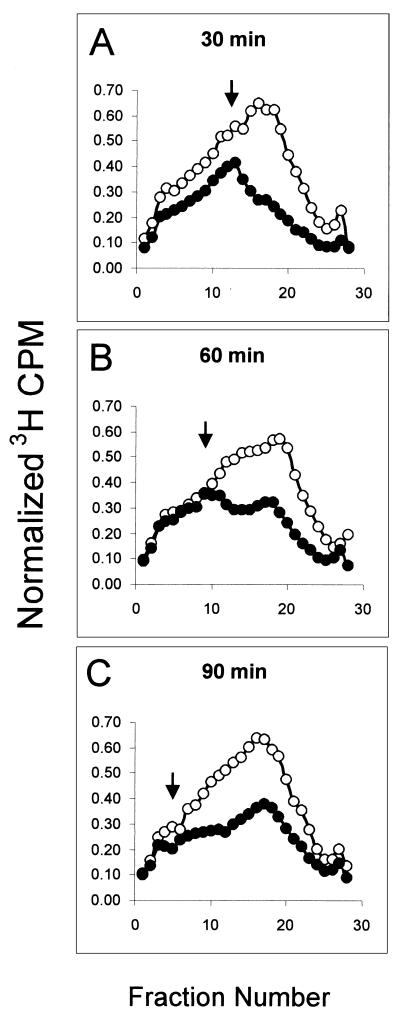
A low dose (1 J/m2) of UVC (254 nm), causing very little cytotoxicity in NHFs, also inhibits DNA synthesis by reducing the rate of initiation of new replicons (34, 35). The experiment illustrated in Fig. 2 shows how the inhibition of synthesis of low-molecular-weight intermediates of DNA replication, observed 30 to 45 min after irradiation, spread progressively to higher-molecular-weight intermediates, as the incubation time prior to the [3H]thymidine pulse was extended from 30 to 90 min. Note that the point of divergence between irradiated and sham profiles moved progressively to higher molecular weights as the length of incubation postirradiation was increased. These results illustrate both the transient nature of the inhibition of DNA synthesis by low-dose UVC and the precursor-product relationship of newly synthesized DNA from single replicons to multireplicon size. This phenomenon of inhibition of low-molecular-weight DNA (Fig. 2A) that sweeps out towards higher-molecular-weight intermediates (Fig. 2B and C) lends support to the interpretation that low doses of IR (Fig. 1C) and UVC (Fig. 2) induced an inhibition of initiation of those replicons that were programmed to be activated shortly after irradiation. By 90 min after irradiation, some recovery of replicon initiation was apparent as increased synthesis of low-molecular-weight DNA (Fig. 2C). As previously published (34), recovery of replicon initiation rate was nearly complete 3 h after exposing NHFs to low-dose UVC.
FIG. 2.
UVC-induced DNA damage inhibits replicon initiation. NHF1 cells were uniformly labeled as described above and sham treated or irradiated with 1 J/m2 UVC. Following treatment, cells were incubated in reserved medium for either 30 (A), 60 (B), or 90 (C) min prior to a 15-min pulse with [3H]thymidine. Velocity sedimentation analysis of nascent DNA was done as described in the legend to Fig. 1C. Open circles represent profiles from sham-treated cells, while closed circles represent those from UVC-irradiated cultures. Arrows point to the position in which the nascent DNA distribution from irradiated cells diverges noticeably from that observed with sham-treated cells.
The UVC-induced inhibition of replicon initiation is independent of ATM, Nbs1, and Mre11.
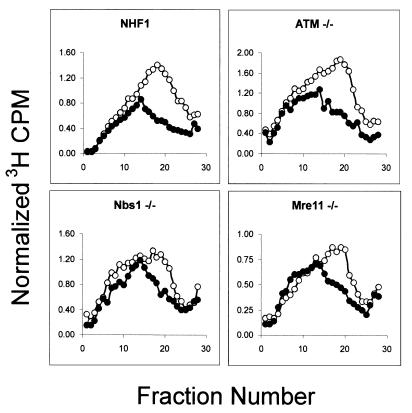
After establishing that our ATM−/−, Nbs1−/−, and Mre11−/− cell lines were defective in the IR-induced S checkpoint, we tested their response to UVC. hTERT-expressing normal and mutant fibroblasts were treated with 1 J/m2 of UVC and the steady-state distribution of sizes of nascent DNA was analyzed by velocity sedimentation. Whereas the AT, NBS, and AT-LD mutant lines were defective in their response to IR, all of these cell lines responded to UVC by inhibiting replicon initiation within the range (39 to 57%) seen in NHF1 (Fig. 3; compare to Fig. 4, below). Thus, the UVC-induced inhibition of replicon initiation is independent of ATM, Nbs1, and Mre11.
FIG. 3.
UVC-induced inhibition of replicon initiation is independent of ATM, Nbs1, and Mre11. Indicated cell lines were uniformly labeled and sham treated or irradiated with 1 J/m2 UVC. Nascent DNA distribution profiles were determined as described in the legend to Fig. 1C. Cultures were incubated for 30 min and then pulse-labeled with [3H]thymidine. ○, sham-treated cells; •, UVC-irradiated cells. The average inhibition of replicon initiation in cells exposed to 1 J/m2 UVC was 50% (n = 19; range, 39 to 57%) in NHF1; 49% (n = 7) in ATM−/−; 40% (n = 2) in NBS1−/−; and 42% (n = 2) in Mre11−/− cells.
FIG. 4.
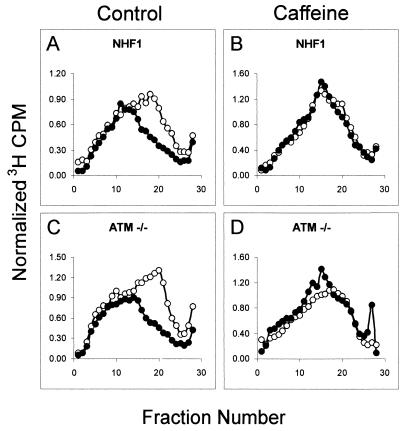
UVC-induced inhibition of replicon initiation is sensitive to caffeine. NHF1 and ATM−/− cells were uniformly labeled as described above and incubated for 30 min in medium alone (A and C) or medium containing 3 mM caffeine (B and D) for 30 min. Cells were sham treated or irradiated with 1 J/m2 UVC, incubated in reserved medium for 30 min, and then pulse-labeled with [3H]thymidine for 15 min. Cells treated with caffeine remained in the presence of this inhibitor throughout the treatment period. Cells were harvested, and nascent DNA was separated by velocity sedimentation as described in the legend to Fig. 1C. ○, sham-treated cells; •, UVC-irradiated cells.
The response to UVC-induced DNA damage depends on ATR.
Recent reports have suggested a role for ATR as the transducer kinase of UVC-induced DNA damage in mammalian, Xenopus laevis, and yeast systems. Overexpression of a kinase-inactive ATR (ATRki) rendered human fibroblasts more sensitive to killing by DNA damaging agents and defective in the IR-induced G2 DNA damage checkpoint (13, 64). ATR has been shown to be critical in the replication checkpoint that prevents premature chromatin condensation following exposure to UV and HU (50). In yeast, ATR homologues rad3 and mec1 play an important role in S-phase surveillance, as mutants are defective in the replication checkpoint as well as the ability to suppress firing of late replicating origins following exposure to HU (56). These studies, among others, have demonstrated a crucial role of ATR in regulating the transition through the S phase of the cell cycle. Nonetheless, confirmation of the potential role of ATR as the sensor kinase of UVC-induced DNA damage is hampered by the fact that it is an essential gene in mammals and null mutants are not viable (6, 16). To test the hypothesis that ATR mediates the UVC-induced S checkpoint, we used caffeine as an inhibitor of ATM and ATR kinases (4, 57, 68). Pretreatment of NHF1 and ATM−/− fibroblasts with 3 mM caffeine, 30 min prior to irradiation, completely abolished the UVC-induced inhibition of replicon initiation (Fig. 4).
We overexpressed either ATRwt or ATRki in U2OS cells to test the role of ATR in the UVC-induced S checkpoint directly. As seen in Fig. 5A, addition of doxycycline for 48 h resulted in a ∼5-fold increase in ATRwt or ATRki expression over endogenous levels. Additionally, velocity sedimentation analyses revealed that U2OS cells responded to low-dose UVC by inhibiting replicon initiation (Fig. 5B). However, overexpression of ATRki or pretreatment with 2 mM caffeine abolished this response, suggesting that ATR signaling is required to inhibit replicon initiation following UVC-induced DNA damage (Fig. 5B). U2OS cells induced to overexpress ATRwt inhibited replicon initiation to the same extent as uninduced controls following exposure to 1 J/m2 UVC. Figure 5C illustrates the average degree of inhibition of replicon initiation in U2OS cells, as determined from several velocity sedimentation profiles (Fig. 5B, fractions 14 to 23). Whereas uninduced U2OS cells inhibited replicon initiation by ∼36% 30 min after irradiation with 1 J/m2, overexpression of ATRki resulted in a significant reversal of this response (∼9% inhibition). Further, overexpression of ATRwt did not affect the response to UVC, and [3H]thymidine incorporation was inhibited to the same extent as in uninduced cells (Fig. 5C).
FIG. 5.
The UVC-induced S checkpoint is ATR dependent. (A) U2OS cells were grown in medium containing 1 μg of doxycycline (Dox)/ml for 48 h to induce the expression of either ATRwt or ATRki. Protein extracts were prepared, and 100 μg of extract was analyzed by Western immunoblot analysis. (B) U2OS cells were uniformly labeled in the absence (uninduced) or the presence (48 h) of 1 μg of doxycycline/ml and pretreated with either medium or 2 mM caffeine 30 min prior to a sham treatment or irradiation with 1 J/m2 UVC. Following irradiation, cells were incubated for 30 min at 37°C and then pulse-labeled with [3H]thymidine for 15 min in their respective medium (with or without doxycycline; with or without caffeine). Cells were harvested, and nascent DNA was separated by velocity sedimentation as described in the legend to Fig. 1C. ○, sham-treated cells; •, UVC-irradiated cells. (C) The degree of inhibition of replicon initiation was calculated from velocity sedimentation profiles of separate experiments and graphed as the average percentage of control (n = 6). *, significant difference from uninduced controls (P < 0.0001).
Activation of Chk1 is required for the UVC-induced S checkpoint response.
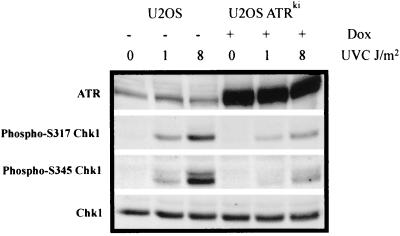
In response to UVC-induced DNA damage and replication blocks, ATR mediates checkpoint signaling through its downstream effector kinase Chk1 (29, 43, 66). Immunodepletion of XATR from a Xenopus cell-free system not only abolished UV-induced Chk1 phosphorylation but also severely compromised checkpoint responses to replication blocks and UV-induced DNA damage (29, 31). Using antibodies specific for Chk1 phosphopeptides, we analyzed Chk1 phosphorylation at serine 317 and serine 345, following exposure of U2OS cells to UVC. Chk1 was phosphorylated on both serine 317 and serine 345 within 30 min following irradiation with 1 J/m2 UVC (Fig. 6). A more lethal dose of UVC (8 J/m2), which has been shown to severely inhibit DNA chain elongation (12), resulted in a more significant degree of Chk1 phosphorylation. Furthermore, overexpression of ATRki severely attenuated Chk1 phosphorylation (∼70%). These findings suggest that not only high doses of UVC but also doses as low as 1 J/m2 result in ATR-dependent Chk1 phosphorylation. Evidence for activation of Chk1 after 1 and 8 J/m2 was also obtained in NHF1 and HeLa cells (results not shown).
FIG. 6.
ATR-dependent Chk1 phosphorylation following UVC-induced DNA damage. U2OS cells were grown for 48 h in the presence or absence of 1 μg of doxycycline (Dox)/ml to induce the expression of ATRki. Cells were either sham treated (0) or irradiated with 1 or 8 J/m2 UVC. Cells were incubated for 30 min in reserved medium and then harvested. Protein extracts were prepared, and 100 μg of extract was analyzed by Western immunoblot analysis.
The role of Chk1 in the UVC-induced inhibition of replicon initiation was tested using the chemical inhibitor UCN-01. According to several reports, treatment with UCN-01 abolished checkpoint responses to DNA damage via Chk-1 inhibition (9, 24, 27, 44). As seen in Fig. 7, treatment of NHF1 and ATM−/− fibroblasts with 100 nM UCN-01, 30 min prior to irradiation, completely reversed the UVC-induced inhibition of replicon initiation. To test the role of Chk1 in the UVC-induced S checkpoint further, wild-type Chk1 (Chk1wt) or kinase-inactive Chk1 (Chk1ki) was expressed in U2OS cells. Infection of cells for 15 h resulted in a ∼10-fold increase in Chk1 expression over endogenous levels (Fig. 7E). Analysis of adenovirus-GFP-infected cells with fluorescence microscopy revealed that ∼90% were GFP positive. Whereas uninfected and adenovirus-GFP-infected cells responded to UVC by inhibiting DNA synthesis, overexpression of Chk1ki abolished this response (Fig. 7F), suggesting that Chk1 is the effector kinase in the UVC-induced S checkpoint. Interestingly, overexpression of Chk1wt inhibited global DNA synthesis by nearly 95%, while overexpression of Chk1ki stimulated DNA synthesis above levels detected in the uninfected controls. These observations suggest that Chk1 may function at some level in the regulation of DNA synthesis, even in the absence of induced DNA damage.
FIG. 7.
Chk1 activation is required for the UVC-induced S checkpoint. (A) NHF1 and ATM−/− cells were uniformly labeled as described and incubated in medium containing dimethyl sulfoxide (A and C) or 100 nM UCN-01 (B and D) for 30 min. Cells were either sham treated or irradiated with 1 J/m2 UVC and incubated in reserved medium for 30 min prior to a 15-min pulse with [3H]thymidine in their respective media. Cells were harvested, and nascent DNA was separated by velocity sedimentation as described in the legend to Fig. 1C. ○, sham-treated cells; •, UVC-irradiated cells. (E) U2OS cells were infected with adenovirus encoding Chk1wt, Chk1ki, or GFP at an MOI of 50 for 15 h. Protein extracts were prepared, and Chk1 expression was measured by Western immunoblot analysis. (F) U2OS cells were grown in the presence of [14C]thymidine for ∼40 h to label DNA uniformly and then in nonradioactive medium containing adenovirus at an MOI of 50 for 15 h. Cells were either sham treated (black bars) or exposed to 1 J/m2 UVC (white bars), incubated for 30 min at 37°C, and then labeled for 15 min in medium containing [3H]thymidine. Net 3H radioactivity corrected for 14C spillover was normalized to cell number (total 14C radioactivity). The normalized 3H counts per minute was graphed as a percentage of the sham control value (n = 4 to 7). *, significant difference from sham controls (P < 0.0001).
DISCUSSION
The S checkpoint is an active response to DNA damage that inhibits the initiation of licensed replicons. Here we report the first genetic evidence of ATR- and Chk1-dependent inhibition of replicon initiation in human cells treated with low-dose UVC. The S checkpoint response to UVC was examined in diploid human fibroblasts and an osteogenic sarcoma cell line by using a low dose that does not saturate DNA excision repair or induce mitogen-activated protein kinase-associated stress responses. Velocity sedimentation was used to analyze nascent DNA molecules that were labeled within 30 to 45 min after exposure of cells to UVC. This technique demonstrates the selective inhibition of synthesis of low-molecular-weight DNA intermediates, which are proximal to origins of replication in cells exposed to genotoxic agents. Telomerase-expressing NHFs responded to 1 J/m2 UVC by inhibiting replicon initiation by 50%. The results with the telomerase-expressing NHFs mirrored those published for other diploid strains of fibroblasts derived from healthy individuals (12, 34, 35, 53). Furthermore, whereas the S checkpoint response to IR is ATM, Nbs1, and Mre11 dependent, fibroblasts with either null or hypomorphic mutations at these loci were proficient in their response to UVC and inhibited replicon initiation within the same range as seen in NHFs. These data indicate that ATM, Nbs1, and Mre11 are not involved in mediating the response of inhibition of replicon initiation following UVC irradiation. A previous report suggesting that ATM was involved in the S checkpoint response to low-dose UVC (52) was done with SV40-transformed fibroblasts. Human fibroblasts transformed with the SV40 large T antigen were recently reported to be defective in S checkpoint responses to both IR and UVC (8). SV40-transformed fibroblasts also display defective G1 and G2 checkpoint responses to IR (37). Due to their aneuploidy and genetic instability, SV40-transformed cells should be used with caution in the analysis of cell cycle checkpoint responses. Fibroblasts immortalized by ectopic expression of telomerase appear to represent a more useful model for studies of human checkpoint function.
Since ATR is an essential gene and embryos lacking expression of this protein are not viable (6, 16), we examined the contribution of ATR to the UVC-induced inhibition of replicon initiation by pretreating cells with caffeine, an inhibitor of ATM and ATR kinases (4, 57). Treatment of normal and ATM−/− fibroblasts with caffeine fully reversed the UVC-induced inhibition of replicon initiation. Moreover, overexpression of ATRki in U2OS cells reversed the S checkpoint response to low-dose UVC, as revealed by velocity sedimentation. Taken together, these data indicate that the UVC-induced S checkpoint response of inhibition of replicon initiation is mediated by ATR.
Like ATR, Chk1 is an essential gene, and null mutations result in embryonic lethality (43, 59). Nonetheless, blastocytes or embryonic stem cells from Chk1−/− mice are deficient in the IR-induced G2 DNA damage checkpoint, as well as the UV- and aphidicolin-induced replication checkpoint (43, 59). Because ATR−/− and Chk1−/− mice have the same phenotype and are equally deficient in checkpoint responses to DNA damage, an association between the two has been hypothesized. Mammalian Chk1 has recently been shown to be phosphorylated on serines 317 and 345 following exposure to UV and HU (43, 66). Additionally, overexpression of ATRki completely abolishes Chk1 phosphorylation in 293T cells (43). Nghiem et al. (50) recently demonstrated the requirement of ATR and Chk1 in the replication checkpoint. Overexpression of ATRki, or Chk1ki, reversed the inhibition of mitosis and produced premature chromatin condensation in U2OS cells treated with HU or UVB (50). Immunodepletion of ATR from Xenopus egg extracts also abolished XChk1 phosphorylation induced by UV-damaged DNA (29, 31). Further, XChk1 containing nonphosphorylatable residues at conserved ATR phosphorylation sites was deficient in the DNA replication checkpoint (29). As a group, these studies suggest that ATR and Chk1 are required for checkpoint responses to UV-induced DNA damage and incomplete DNA synthesis.
Consistent with the findings mentioned above, Chk1 was phosphorylated on serine 317 and serine 345 in response to a low fluency of UVC in U2OS cells. At a dose that inhibited replicon initiation but not chain elongation (1 J/m2), modest Chk1 phosphorylation was observed. A toxic dose of UVC (8 J/m2) that caused a more severe inhibition of DNA chain elongation induced a significant increase in Chk1 phosphorylation. These findings suggest that the phosphorylation status of Chk-1 and/or the effective concentration of activated Chk1 might determine the checkpoint-signaling pathway that is triggered. The UVC-induced S checkpoint response of inhibition of replicon initiation may only require a low degree of Chk1 phosphorylation to inhibit origin firing. Lethal doses of UVC that severely block DNA chain elongation and activate the replication checkpoint may need a higher concentration of active Chk1 to signal to a more diverse group of downstream effectors, such as those that block premature chromosome condensation. Additionally, pretreatment of cells with UCN-01 or overexpression of Chk1ki abolished the inhibition of DNA synthesis by a low dose of UVC. Since the UVC-induced S checkpoint response is caffeine and UCN-01 sensitive, independent of ATM, and can be reversed by overexpressing ATRki or Chk1ki, our data strongly suggest that the upstream transducers that respond to low levels of UVC-induced DNA damage are ATR and Chk1. The chemical carcinogen BPDE produces the same stereotypic inhibition of replicon initiation as UVC and IR (33). Consistent with the data reported here, Guo et al. (28) recently demonstrated that a caffeine-sensitive, Chk1-dependent pathway mediates the BPDE-induced inhibition of replicon initiation.
Cellular responses to UVC are significantly affected by dose. The low dose of 1 J/m2 used in these studies, which does not saturate NER and inhibits DNA chain elongation minimally, produces about 1 pyrimidine dimer per 4 × 107-Da replicon (5). Nucleotide excision and postreplication repair activities, in concert with inhibition of replicon initiation, can largely ameliorate the genotoxic effects of such a low dose. The protective influence of NER is seen in the greater inhibition of DNA chain elongation 30 min after irradiation of repair-defective xeroderma pigmentosum (XP) cells (34). Pol η-dependent replicative bypass is also evident as XP variant cells display significant inhibition of DNA chain elongation after 1 J/m2 (14, 35). Chk1 is activated modestly by low-dose UVC to induce the transient inhibition of replicon initiation. Higher doses of UVC in the range of 5 to 10 J/m2 saturate NER (39), severely inhibit DNA chain elongation due to the large numbers of photoproducts blocking DNA polymerases (5), and activate Chk1 more strongly. This greater level of activation might reflect the larger number of blocked growing points and may be required to sustain the inhibition of replicon initiation for a longer period and to block premature chromosome condensation as progression through S phase is significantly impeded. Survival is less certain after higher damage levels, and mutations and chromosomal aberrations are measurably increased in the survivors (40). Still-higher doses, in the range of 25 to 50 J/m2, can cross-link receptor tyrosine kinases and activate cytoplasmic kinase cascades (18, 19), in addition to the above-mentioned effects deriving from nuclear DNA damage. Activation of JNK appears to be a high-dose phenomenon, with a threshold of no response evident at UVC doses lower than 20 J/m2 (2). The severe inhibition of DNA chain elongation after such high fluencies of UVC produces a state of replicative arrest similar to that induced by depletion of DNA precursors using HU or aphidicolin. Under these conditions, ATR and Chk1 appear to be required to prevent activation of late origins (24, 56) and premature chromosome condensation (50) as elements of replication checkpoint function. It is evident from our studies that inhibition of replicon initiation is phenomenologically equivalent to inhibition of late origins. Because the inhibition of replicon initiation is triggered rapidly after UVC, the effect may not be limited to those replicons that initiate late in S phase but could include any replicon or replicon cluster that is scheduled to initiate replication after the DNA damage is incurred.
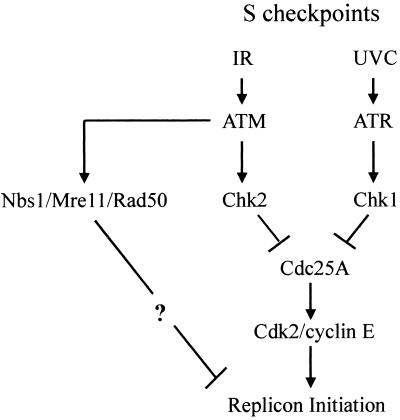
In summary, the S checkpoint that inhibits replicon initiation following exposure to low-dose UVC is distinct from the IR-induced S checkpoint (Fig. 8). ATM, Nbs1, and Mre11 are required for the S checkpoint response to IR. ATM signals to activate Chk2 and thereby to inhibit Cdc25A and cyclin E/Cdk2 kinase activity. The contribution of Nbs1 and Mre11 to inhibition of replicon initiation by IR is independent of Chk2 and Cdc25A signaling, through a still-undetermined pathway (23). ATR and Chk1 are required for the S checkpoint response to UVC, and these transducers may also inactivate Cdc25A (44). Thus, human cells express at least two independent signaling mechanisms that regulate the replicon initiation rate in response to environmental stress.
FIG. 8.
Schematic model of ATM- and ATR-dependent S checkpoint signaling pathways.
ADDENDUM IN PROOF
A report recently appeared showing results similar to those presented here (H. Miao, J. Seiler, and W. C. Burhans, J. Biol. Chem., electronic manuscript 04264, 2002).
Acknowledgments
We thank Paula Deming for technical assistance and helpful discussion, Fei Zou for biostatistical analyses, and Miriam Bryant for assistance with the cytogenetic analyses. We also thank John Petrini (Memorial Sloan Kettering Cancer Center) for providing the parental W1799 and AT-LD2 cell strains, Stuart Schreiber and Paul Nghiem (Harvard University) for providing the ATR-inducible U2OS cells, Robert Weinberg (MIT) for providing the hTERT cDNA, Cyrus Vaziri (Boston University) for providing Chk1-expressing adenoviruses, and Robert Schultz (NCI Drug Synthesis and Chemistry Branch) for the UCN-01.
This work was supported primarily by PHS grant CA55065 (M.C.S.). This study was also supported by PHS grant CA81343 (W.K.K.) and Center grants P30-CA16086 and P30-ES10126. T.P.H. is supported by Environmental Pathology Training Grant ES07017.
REFERENCES
- 1.Abraham, R. T. 2001. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 15:2177-2196. [DOI] [PubMed] [Google Scholar]
- 2.Adler, V., S. Y. Fuchs, J. Kim, A. Kraft, M. P. King, J. Pelling, and Z. Ronai. 1995. jun-NH2-terminal kinase activation mediated by UV-induced DNA lesions in melanoma and fibroblast cells. Cell Growth Differ. 6:1437-1446. [PubMed] [Google Scholar]
- 3.Banin, S., L. Moyal, S. Shieh, Y. Taya, C. W. Anderson, L. Chessa, N. I. Smorodinsky, C. Prives, Y. Reiss, Y. Shiloh, and Y. Ziv. 1998. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science 281:1674-1677. [DOI] [PubMed] [Google Scholar]
- 4.Blasina, A., B. D. Price, G. A. Turenne, and C. H. McGowan. 1999. Caffeine inhibits the checkpoint kinase ATM. Curr. Biol. 9:1135-1138. [DOI] [PubMed] [Google Scholar]
- 5.Boyer, J. C., W. K. Kaufmann, B. P. Brylawski, and M. Cordeiro-Stone. 1990. Defective postreplication repair in xeroderma pigmentosum variant fibroblasts. Cancer Res. 50:2593-2598. [PubMed] [Google Scholar]
- 6.Brown, E. J., and D. Baltimore. 2000. ATR disruption leads to chromosomal fragmentation and early embryonic lethality. Genes Dev. 14:397-402. [PMC free article] [PubMed] [Google Scholar]
- 7.Bulavin, D. V., Y. Higashimoto, I. J. Popoff, W. A. Gaarde, V. Basrur, O. Potapova, E. Appella, and A. J. Fornace, Jr. 2001. Initiation of a G2/M checkpoint after ultraviolet radiation requires p38 kinase. Nature 411:102-107. [DOI] [PubMed] [Google Scholar]
- 8.Bullock, S. K., W. K. Kaufmann, and M. Cordeiro-Stone. 2001. Enhanced S phase delay and inhibition of replication of an undamaged shuttle vector in UVC-irradiated xeroderma pigmentosum variant. Carcinogenesis 22:233-241. [DOI] [PubMed] [Google Scholar]
- 9.Busby, E. C., D. F. Leistritz, R. T. Abraham, L. M. Karnitz, and J. N. Sarkaria. 2000. The radiosensitizing agent 7-hydroxystaurosporine (UCN-01) inhibits the DNA damage checkpoint kinase hChk1. Cancer Res. 60:2108-2112. [PubMed] [Google Scholar]
- 10.Canman, C. E., D. S. Lim, K. A. Cimprich, Y. Taya, K. Tamai, K. Sakaguchi, E. Appella, M. B. Kastan, and J. D. Siliciano. 1998. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science 281:1677-1679. [DOI] [PubMed] [Google Scholar]
- 11.Carney, J. P., R. S. Maser, H. Olivares, E. M. Davis, M. Le Beau, J. R. Yates, I. I. I., L. Hays, W. F. Morgan, and J. H. Petrini. 1998. The hMre11/hRad50 protein complex and Nijmegen breakage syndrome: linkage of double-strand break repair to the cellular DNA damage response. Cell 93:477-486. [DOI] [PubMed] [Google Scholar]
- 12.Cistulli, C. A., and W. K. Kaufmann. 1998. p53-dependent signaling sustains DNA replication and enhances clonogenic survival in 254 nm ultraviolet-irradiated human fibroblasts. Cancer Res. 58:1993-2002. [PubMed] [Google Scholar]
- 13.Cliby, W. A., C. J. Roberts, K. A. Cimprich, C. M. Stringer, J. R. Lamb, S. L. Schreiber, and S. H. Friend. 1998. Overexpression of a kinase-inactive ATR protein causes sensitivity to DNA-damaging agents and defects in cell cycle checkpoints. EMBO J. 17:159-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cordeiro-Stone, M., A. Frank, M. Bryant, I. Oguejiofor, S. B. Hatch, L. D. McDaniel, and W. K. Kaufmann. 2002. DNA damage responses protect xeroderma pigmentosum variant from UVC-induced clastogenesis. Carcinogenesis 23:959-965. [DOI] [PubMed] [Google Scholar]
- 15.Cortez, D., Y. Wang, J. Qin, and S. J. Elledge. 1999. Requirement of ATM-dependent phosphorylation of Brca1 in the DNA damage response to double-strand breaks. Science 286:1162-1166. [DOI] [PubMed] [Google Scholar]
- 16.de Klein, A., M. Muijtjens, R. van Os, Y. Verhoeven, B. Smit, A. M. Carr, A. R. Lehmann, and J. H. Hoeijmakers. 2000. Targeted disruption of the cell-cycle checkpoint gene ATR leads to early embryonic lethality in mice. Curr. Biol. 10:479-482. [DOI] [PubMed] [Google Scholar]
- 17.Deming, P. B., C. A. Cistulli, H. Zhao, P. R. Graves, H. Piwnica-Worms, R. S. Paules, C. S. Downes, and W. K. Kaufmann. 2001. The human decatenation checkpoint. Proc. Natl. Acad. Sci. USA 98:12044-12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Derijard, B., M. Hibi, I. H. Wu, T. Barrett, B. Su, T. Deng, M. Karin, and R. J. Davis. 1994. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell 76:1025-1037. [DOI] [PubMed] [Google Scholar]
- 19.Devary, Y., R. A. Gottlieb, T. Smeal, and M. Karin. 1992. The mammalian ultraviolet response is triggered by activation of Src tyrosine kinases. Cell 71:1081-1091. [DOI] [PubMed] [Google Scholar]
- 20.Ejima, Y., and M. S. Sasaki. 1986. Enhanced expression of X-ray- and UV-induced chromosome aberrations by cytosine arabinoside in ataxia telangiectasia cells. Mutat. Res. 159:117-123. [DOI] [PubMed] [Google Scholar]
- 21.Elledge, S. J. 1996. Cell cycle checkpoints: preventing an identity crisis. Science 274:1664-1672. [DOI] [PubMed] [Google Scholar]
- 22.Falck, J., N. Mailand, R. G. Syljuasen, J. Bartek, and J. Lukas. 2001. The ATM-Chk2-Cdc25A checkpoint pathway guards against radioresistant DNA synthesis. Nature 410:842-847. [DOI] [PubMed] [Google Scholar]
- 23.Falck, J., J. H. Petrini, B. R. Williams, J. Lukas, and J. Bartek. 2002. The DNA damage-dependent intra-S phase checkpoint is regulated by parallel pathways. Nat. Genet. 30:290-294. [DOI] [PubMed] [Google Scholar]
- 24.Feijoo, C., C. Hall-Jackson, R. Wu, D. Jenkins, J. Leitch, D. M. Gilbert, and C. Smythe. 2001. Activation of mammalian Chk1 during DNA replication arrest: a role for Chk1 in the intra-S phase checkpoint monitoring replication origin firing. J. Cell Biol. 154:913-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Filatov, L., V. Golubovskaya, J. C. Hurt, L. L. Byrd, J. M. Phillips, and W. K. Kaufmann. 1998. Chromosomal instability is correlated with telomere erosion and inactivation of G2 checkpoint function in human fibroblasts expressing human papillomavirus type 16 E6 oncoprotein. Oncogene 16:1825-1838. [DOI] [PubMed] [Google Scholar]
- 26.Gatei, M., D. Young, K. M. Cerosaletti, A. Desai-Mehta, K. Spring, S. Kozlov, M. F. Lavin, R. A. Gatti, P. Concannon, and K. Khanna. 2000. ATM-dependent phosphorylation of nibrin in response to radiation exposure. Nat. Genet. 25:115-119. [DOI] [PubMed] [Google Scholar]
- 27.Graves, P. R., L. Yu, J. K. Schwarz, J. Gales, E. A. Sausville, P. M. O'Connor, and H. Piwnica-Worms. 2000. The Chk1 protein kinase and the Cdc25C regulatory pathways are targets of the anticancer agent UCN-01. J. Biol. Chem. 275:5600-5605. [DOI] [PubMed] [Google Scholar]
- 28.Guo, N., D. V. Faller, and C. Vaziri. 2002. Carcinogen-induced S-phase arrest is Chk1 mediated and caffeine sensitive. Cell Growth Differ. 13:77-86. [PubMed] [Google Scholar]
- 29.Guo, Z., A. Kumagai, S. X. Wang, and W. G. Dunphy. 2000. Requirement for Atr in phosphorylation of Chk1 and cell cycle regulation in response to DNA replication blocks and UV-damaged DNA in Xenopus egg extracts. Genes Dev. 14:2745-2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hartwell, L. H., and M. B. Kastan. 1994. Cell cycle control and cancer. Science 266:1821-1828. [DOI] [PubMed] [Google Scholar]
- 31.Hekmat-Nejad, M., Z. You, M. C. Yee, J. W. Newport, and K. A. Cimprich. 2000. Xenopus ATR is a replication-dependent chromatin-binding protein required for the DNA replication checkpoint. Curr. Biol. 10:1565-1573. [DOI] [PubMed] [Google Scholar]
- 32.Jiang, X. R., G. Jimenez, E. Chang, M. Frolkis, B. Kusler, M. Sage, M. Beeche, A. G. Bodnar, G. M. Wahl, T. D. Tlsty, and C. P. Chiu. 1999. Telomerase expression in human somatic cells does not induce changes associated with a transformed phenotype. Nat. Genet. 21:111-114. [DOI] [PubMed] [Google Scholar]
- 33.Kaufmann, W. K., J. C. Boyer, B. A. Smith, and M. Cordeiro-Stone. 1985. DNA repair and replication in human fibroblasts treated with (+/−)-R- 7,t-8-dihydroxy-t-9,10-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene. Biochim. Biophys. Acta 824:146-151. [DOI] [PubMed] [Google Scholar]
- 34.Kaufmann, W. K., and J. E. Cleaver. 1981. Mechanisms of inhibition of DNA replication by ultraviolet light in normal human and xeroderma pigmentosum fibroblasts. J. Mol. Biol. 149:171-187. [DOI] [PubMed] [Google Scholar]
- 35.Kaufmann, W. K., J. E. Cleaver, and R. B. Painter. 1980. Ultraviolet radiation inhibits replicon initiation in S phase human cells. Biochim. Biophys. Acta 608:191-195. [DOI] [PubMed] [Google Scholar]
- 36.Kaufmann, W. K., and P. E. Kies. 1998. DNA signals for G2 checkpoint response in diploid human fibroblasts. Mutat. Res. 400:153-167. [DOI] [PubMed] [Google Scholar]
- 37.Kaufmann, W. K., E. N. Levedakou, H. L. Grady, R. S. Paules, and G. H. Stein. 1995. Attenuation of G2 checkpoint function precedes human cell immortalization. Cancer Res. 55:7-11. [PubMed] [Google Scholar]
- 38.Kaufmann, W. K., and R. S. Paules. 1996. DNA damage and cell cycle checkpoints. FASEB J. 10:238-247. [DOI] [PubMed] [Google Scholar]
- 39.Kaufmann, W. K., and S. J. Wilson. 1990. DNA repair endonuclease activity during synchronous growth of diploid human fibroblasts. Mutat. Res. 236:107-117. [DOI] [PubMed] [Google Scholar]
- 40.Kaufmann, W. K., and S. J. Wilson. 1994. G1 arrest and cell-cycle-dependent clastogenesis in UV-irradiated human fibroblasts. Mutat. Res. 314:67-76. [DOI] [PubMed] [Google Scholar]
- 41.Lakin, N. D., B. C. Hann, and S. P. Jackson. 1999. The ataxia-telangiectasia related protein ATR mediates DNA-dependent phosphorylation of p53. Oncogene 18:3989-3995. [DOI] [PubMed] [Google Scholar]
- 42.Lim, D. S., S. T. Kim, B. Xu, R. S. Maser, J. Lin, J. H. Petrini, and M. B. Kastan. 2000. ATM phosphorylates p95/nbs1 in an S-phase checkpoint pathway. Nature 404:613-617. [DOI] [PubMed] [Google Scholar]
- 43.Liu, Q., S. Guntuku, X. S. Cui, S. Matsuoka, D. Cortez, K. Tamai, G. Luo, S. Carattini-Rivera, F. DeMayo, A. Bradley, L. A. Donehower, and S. J. Elledge. 2000. Chk1 is an essential kinase that is regulated by Atr and required for the G2/M DNA damage checkpoint. Genes Dev. 14:1448-1459. [PMC free article] [PubMed] [Google Scholar]
- 44.Mailand, N., J. Falck, C. Lukas, R. G. Syljuasen, M. Welcker, J. Bartek, and J. Lukas. 2000. Rapid destruction of human Cdc25A in response to DNA damage. Science 288:1425-1429. [DOI] [PubMed] [Google Scholar]
- 45.Maser, R. S., K. J. Monsen, B. E. Nelms, and J. H. Petrini. 1997. hMre11 and hRad50 nuclear foci are induced during the normal cellular response to DNA double-strand breaks. Mol. Cell. Biol. 17:6087-6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moore, P., and B. S. Strauss. 1979. Sites of inhibition of in vitro DNA synthesis in carcinogen- and UV-treated phi X174 DNA. Nature 278:664-666. [DOI] [PubMed] [Google Scholar]
- 47.Moore, P. D., K. K. Bose, S. D. Rabkin, and B. S. Strauss. 1981. Sites of termination of in vitro DNA synthesis on ultraviolet- and N-acetylaminofluorene-treated phi X174 templates by prokaryotic and eukaryotic DNA polymerases. Proc. Natl. Acad. Sci. USA 78:110-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morales, C. P., S. E. Holt, M. Ouellette, K. J. Kaur, Y. Yan, K. S. Wilson, M. A. White, W. E. Wright, and J. W. Shay. 1999. Absence of cancer-associated changes in human fibroblasts immortalized with telomerase. Nat. Genet. 21:115-118. [DOI] [PubMed] [Google Scholar]
- 49.Nelms, B. E., R. S. Maser, J. F. Mackay, M. G. Lagally, and J. H. Petrini. 1998. In situ visualization of DNA double-strand break repair in human fibroblasts. Science 280:590-592. [DOI] [PubMed] [Google Scholar]
- 50.Nghiem, P., P. K. Park, Y. Kim, C. Vaziri, and S. L. Schreiber. 2001. ATR inhibition selectively sensitizes G1 checkpoint-deficient cells to lethal premature chromatin condensation. Proc. Natl. Acad. Sci. USA 98:9092-9097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ouellette, M. M., L. D. McDaniel, W. E. Wright, J. W. Shay, and R. A. Schultz. 2000. The establishment of telomerase-immortalized cell lines representing human chromosome instability syndromes. Hum. Mol. Genet. 9:403-411. [DOI] [PubMed] [Google Scholar]
- 52.Painter, R. B. 1980. Inhibition and recovery of DNA synthesis in human cells after exposure to ultraviolet light. Mutat. Res. 145:63-69. [DOI] [PubMed] [Google Scholar]
- 53.Painter, R. B., and B. R. Young. 1980. Radiosensitivity in ataxia-telangiectasia: a new explanation. Proc. Natl. Acad. Sci. USA 77:7315-7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paulovich, A. G., D. P. Toczyski, and L. H. Hartwell. 1997. When checkpoints fail. Cell 88:315-321. [DOI] [PubMed] [Google Scholar]
- 55.Petrini, J. H. 2000. The Mre11 complex and ATM: collaborating to navigate S phase. Curr. Opin. Cell Biol. 12:293-296. [DOI] [PubMed] [Google Scholar]
- 56.Santocanale, C., and J. F. Diffley. 1998. A Mec1- and Rad53-dependent checkpoint controls late-firing origins of DNA replication. Nature 395:615-618. [DOI] [PubMed] [Google Scholar]
- 57.Sarkaria, J. N., E. C. Busby, R. S. Tibbetts, P. Roos, Y. Taya, L. M. Karnitz, and R. T. Abraham. 1999. Inhibition of ATM and ATR kinase activities by the radiosensitizing agent, caffeine. Cancer Res. 59:4375-4382. [PubMed] [Google Scholar]
- 58.Shackelford, R. E., C. L. Innes, S. O. Sieber, A. N. Heinloth, S. A. Leadon, and R. S. Paules. 2001. The ataxia telangiectasia gene product is required for oxidative stress-induced G1 and G2 checkpoint function in human fibroblasts. J. Biol. Chem. 276:21951-21952. [DOI] [PubMed] [Google Scholar]
- 59.Takai, H., K. Tominaga, N. Motoyama, Y. A. Minamishima, H. Nagahama, T. Tsukiyama, K. Ikeda, K. Nakayama, M. Nakanishi, and K. Nakayama. 2000. Aberrant cell cycle checkpoint function and early embryonic death in Chk1−/− mice. Genes Dev. 14:1439-1447. [PMC free article] [PubMed] [Google Scholar]
- 60.Tibbetts, R. S., K. M. Brumbaugh, J. M. Williams, J. N. Sarkaria, W. A. Cliby, S. Y. Shieh, Y. Taya, C. Prives, and R. T. Abraham. 1999. A role for ATR in the DNA damage-induced phosphorylation of p53. Genes Dev. 13:152-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tibbetts, R. S., D. Cortez, K. M. Brumbaugh, R. Scully, D. Livingston, S. J. Elledge, and R. T. Abraham. 2000. Functional interactions between BRCA1 and the checkpoint kinase ATR during genotoxic stress. Genes Dev. 14:2989-3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang, H. C., and S. Fedoroff. 1972. Banding in human chromosomes treated with trypsin. Nat. New Biol. 235:52-54. [DOI] [PubMed] [Google Scholar]
- 63.Wood, L. D., T. L. Halvorsen, S. Dhar, J. A. Baur, R. K. Pandita, W. E. Wright, M. P. Hande, G. Calaf, T. K. Hei, F. Levine, J. W. Shay, J. J. Wang, and T. K. Pandita. 2001. Characterization of ataxia telangiectasia fibroblasts with extended life-span through telomerase expression. Oncogene 20:278-288. [DOI] [PubMed] [Google Scholar]
- 64.Wright, J. A., K. S. Keegan, D. R. Herendeen, N. J. Bentley, A. M. Carr, M. F. Hoekstra, and P. Concannon. 1998. Protein kinase mutants of human ATR increase sensitivity to UV and ionizing radiation and abrogate cell cycle checkpoint control. Proc. Natl. Acad. Sci. USA 95:7445-7450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu, X., V. Ranganathan, D. S. Weisman, W. F. Heine, D. N. Ciccone, T. B. O'Neill, K. E. Crick, K. A. Pierce, W. S. Lane, G. Rathbun, D. M. Livingston, and D. T. Weaver. 2000. ATM phosphorylation of Nijmegen breakage syndrome protein is required in a DNA damage response. Nature 405:477-482. [DOI] [PubMed] [Google Scholar]
- 66.Zhao, H., and H. Piwnica-Worms. 2001. ATR-mediated checkpoint pathways regulate phosphorylation and activation of human Chk1. Mol. Cell. Biol. 21:4129-4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhao, S., Y. C. Weng, S. S. Yuan, Y. T. Lin, H. C. Hsu, S. C. Lin, E. Gerbino, M. H. Song, M. Z. Zdzienicka, R. A. Gatti, J. W. Shay, Y. Ziv, Y. Shiloh, and E. Y. Lee. 2000. Functional link between ataxia-telangiectasia and Nijmegen breakage syndrome gene products. Nature 405:473-477. [DOI] [PubMed] [Google Scholar]
- 68.Zhou, B. B., P. Chaturvedi, K. Spring, S. P. Scott, R. A. Johanson, R. Mishra, M. R. Mattern, J. D. Winkler, and K. K. Khanna. 2000. Caffeine abolishes the mammalian G2/M DNA damage checkpoint by inhibiting ataxia-telangiectasia-mutated kinase activity. J. Biol. Chem. 275:10342-10348. [DOI] [PubMed] [Google Scholar]