Abstract
Misregulation of the evolutionarily conserved GTPase Ran in fission yeast results in defects in several cellular processes in cells that are competent for nucleocytoplasmic protein transport. These results suggest that transport is neither the only nor the primary Ran-dependent process in living cells. The ability of Ran to independently regulate multiple cellular processes in vivo is demonstrated by showing that (i) eight different transport-competent RanGEF (guanine nucleotide exchange factor) mutants have defects in mitotic spindle formation; (ii) the RanGEF temperature-sensitive mutant pim1-d1 has abnormal actin ring structures at the septum. Overexpression of Imp2p, which specifically destabilizes these structures, restores viability. (iii) Ran-dependent processes differ in their requirements for active Ran in vivo. Microtubule function, cytokinesis, and nuclear envelope structure are the Ran-dependent processes most sensitive to the amount of Ran protein in the cell, whereas nucleocytoplasmic protein transport is the most robust. Therefore, the ability of Ran from Schizosaccharomyces pombe to independently regulate multiple cellular processes may reflect differences in its interactions with the binding proteins that mediate these functions and explain the complex phenotypic consequences of its misregulation in vivo.
The evolutionarily conserved small GTPase Ran and its regulators are asymmetrically distributed with respect to the nuclear envelope (NE). The GTPase is predominantly, but not exclusively, nuclear, the guanine nucleotide exchange factor (RanGEF) is a chromatin-associated nuclear protein, and the GTPase-activating protein (RanGAP) is cytoplasmic (reviewed in references 19 and 39). It is therefore presumed that nuclear Ran is mostly GTP bound and cytoplasmic Ran is GDP bound. This Ran-GTP gradient across the NE is essential for Ran-dependent nucleocytoplasmic transport (reviewed in reference 32) and has recently been visualized by fluorescence resonance energy transformer analysis (17). The ability of Ran to stabilize or destabilize transport cargo-receptor complexes, depending upon its nucleotide-bound state and therefore reflecting its intracellular localization, may also explain the role of Ran in regulating other cellular processes such as nucleocytoplasmic transport, NE reassembly, and mitotic spindle formation (reviewed in references 2 and 40). Data, largely generated from in vitro studies using Xenopus laevis extracts, have suggested that Ran's role in spindle aster formation appears to depend on the transport receptors importin α and importin β (reviewed in reference 3). At high concentrations, these receptors inhibit spindle formation in vitro by binding to and sequestering TPX2 and NuMA, which are required for spindle assembly (11, 31, 47). Transport receptor proteins also participate in NE reformation in vitro by an as-yet-unknown mechanism (52).
In fission yeast, budding yeast, and animal cells Ran influences a variety of cellular processes, including nucleocytoplasmic transport, cell cycle progression, DNA replication, chromosome segregation, and NE structure (8; reviewed in references 3, 39, and 40). When Ran from Schizosaccharomyces pombe (RanSp) is misregulated in fission yeast, cells progress normally through the G1, S, and G2 phases of the cell cycle, but after mitosis the chromosomes remain unreplicated and hypercondensed, the septum is abnormally wide (23, 41), and the NE, which normally remains intact throughout the yeast cell cycle (45), fragments at mitosis (4). Because these abnormalities and the loss of viability occur simultaneously at mitosis and depend on the passage through mitosis (4), the interdependency of these defects cannot be easily determined by morphological observations of synchronous cultures. Fission yeast cells in which the RanSp GTPase system is misregulated by inactivating or overexpressing RanGEFSp, RanGAPSp, or RanBP1Sp all have this same complex terminal phenotype, indicating that both Ran-GTP and Ran-GDP, and/or the appropriate balance or cycling between these two forms of RanSp are essential for its function (13, 23, 41).
A point mutant of RanSp, called spi1-25, revealed a transport-independent role of the GTPase system in the regulation of microtubule (MT) stability in vivo in fission yeast (8). We proposed that these spi1-25 defects might be the result of a decrease in the amount of active protein, as only ∼30% of the mutant Spi1-25p protein can bind nucleotide in vitro (8). Here, this hypothesis is addressed by lowering the amount of RanSp in wild-type cells and finding defects in mitotic spindle formation and chromosome segregation. Furthermore, RanGEFSp mutants, which are competent for nucleocytoplasmic transport, also have MT abnormalities and an abnormal stabilization of actin at the medial ring, causing defects in cytokinesis. Taken together, these data provide evidence that RanSp independently regulates cellular processes in the nucleus and the cytoplasm and suggest that these roles may be mediated by factors that differ in their abundance, accessibility, or affinity for Ran or Ran bound to transport receptors.
MATERIALS AND METHODS
Strains, media, and genetic methods.
Strains used in this study are listed in Table 1. Standard cell culture, media, and genetic techniques were used (7, 30, 37). Cells were grown in either yeast extract (YE) or Edinburgh minimal medium (EMM). Ectopic gene expression was from the high-, medium-, or low-strength thiamine-repressible nmt1 gene promoter in plasmids pREP31X, pREP41X, and pREP81X, respectively (9, 26). Expression was fully repressed by addition of 5 μg of thiamine/ml or was partially repressed by the addition of 0.08 μg of thiamine/ml to EMM and induced by washing in EMM lacking thiamine.
TABLE 1.
Strains used in this study
| Strain | Genotype |
|---|---|
| SS482 | h−int::pREP4X-GFP-NLS-LacZ-ura4 ura4-D18 leu1-32 ade6-M216 |
| SS1076 | h−int::pREP4X-GFP-NLS-LacZ-ura4 ura4-D18 leu1-32 ade6-M216 |
| SS777 | h+pim1-d1 int::pREP4X-GFP-NLS-LacZ-ura4 ura4-D18 leu1-32 |
| SS452 | h− JD61 int::pREP4X-GFP-NLS-LacZ-ura4 ura4-D18 leu1-32 ade6-M216 |
| SS453 | h− JD62 int::pREP4X-GFP-NLS-LacZ-ura4 ura4-D18 leu1-32 ade6-M216 |
| SS451 | h+ JD60 int::pREP4X-GFP-NLS-LacZ-ura4 ura4-D18 leu1-32 ade6-M216 |
| SS442 | h−slg51 int::pREP4X-GFP-NLS-LacZ-ura4 ura4-D18 leu1-32 ade6-M216 |
| SS450 | h− JD59 int::pREP4X-GFP-NLS-LacZ-ura4 ura4-D18 leu1-32 ade6-M216 |
| SS440 | h−snsA5 int::pREP4X-GFP-NLS-LacZ-ura4 ura4-D18 leu1-32 ade6-M216 |
| SS481 | h−snsA3 int::pREP4X-GFP-NLS-LacZ-ura4 ura4-D18 leu1-32 ade6-M216 |
| SS767 | h−int::pREP41-GFPpap1-LEU2 ura4-D18 leu1-32 ade6-M210 |
| SS779 | h−pim1-d1 int::pREP41-GFPpap1-LEU2 ura4-D18 leu1-32 ade6-M210 |
| SS1160 | h− JD61 int::pREP41-GFPpap1-LEU2 ura4-D18 leu1-32 ade6-M210 |
| SS1176 | h+ JD62 int::pREP41-GFPpap1-LEU2 ura4-D18 leu1-32 ade6-M216 |
| SS1157 | h+ JD60 int::pREP41-GFPpap1-LEU2 ura4-D18 leu1-32 ade6-M210 |
| SS1175 | h−slg51 int::pREP41-GFPpap1-LEU2 ura4-D18 leu1-32 |
| SS1156 | h− JD59 int::pREP41-GFPpap1-LEU2 ura4-D18 leu1-32 ade6-M216 |
| SS1161 | h−snsA5 int::pREP41-GFPpap1-LEU2 ura4-D18 leu1-32 ade6-M210 |
| SS1162 | h+snsA3 int::pREP41-GFPpap1-LEU2 ura4-D18 leu1-32 ade6-M210 |
| SS446 | h−ura4-D18 leu1-32 ade6-M210 |
| SS749 | h−pim1-d1 ura4-D18 leu1-32 ade6-M210 |
| SS1178 | h− JD61 ura4-D18 leu1-32 ade6-M210 |
| SS1172 | h− JD62 ura4-D18 leu1-32 ade6-M210 |
| SS1148 | h+ JD60 ura4-D18 leu1-32 ade6-M216 |
| SS1179 | h−slg51 ura4-D18 leu1-32 ade6-M216 |
| SS1177 | h+ JD59 ura4-D18 leu1-32 ade6-M216 |
| SS1153 | h−snsA3 ura4-D18 leu1-32 ade6-M210 |
| SS1152 | h−snsA5 ura4-D18 leu1-32 ade6-M210 |
| SS104 | h+leu1-32 ade6-M216 |
| SS872 | h+pim1-d1 leu1-32 ade6-M216 |
| SS849 | h+pim1-d1 mph1::ura4 ura4-D18 leu1-32 ade6-M216 |
| SS772 | h−pim1-d1 mad2::ura4 ura4-D18 leu1-32 ade6-M210 |
| SS562 | h+mph1::ura4 ura4-D18 leu1-32 ade6-M210 |
| SS521 | h+mad2::ura4 ura4-D18 leu1-32 ade6-M216 |
| SS1182 | h−int::pREP3X-spil:LEU2 ura4-D18 leu1-32 |
| SS554 | h−spi1::ura4 int::pREP3X-spi1-LEU2 ura4-D18 leu1-32 |
| SS1036 | h+/90spi1::ura4/spi1::ura4 int::pREP3X-spi1-LEU2 mat2/mat2-102 ura4-D18/ura4-D18 leu1-32/leu1-32 ade6-M210/ade6-M216 |
| SS530 | h−/h+ura4-D18/ura4-D18 leu1-32/leu1-32 ade6-M210/ade6-M216 |
| SS84 | h−pim1-d1 ura4-D18 leu1-32 |
To delete the spi1 gene, approximately 800 bp of genomic DNA 5′ and 3′ of the spi1 locus was cloned into pBS-ura4 (25) flanking the ura4 gene to create pBS-spi1::ura4. This construct was digested with SpeI and BamHI, and the 3.4-kb fragment was transformed into SS1182, leu1-32 ura4-D18 [int-pREP3X-spi1] h−, containing an integrated copy of pREP3X-spi1, a LEU2-containing plasmid in which spi1 is expressed from the nmt1 gene promoter (8). Gene replacement was confirmed by Southern blot analysis, and SS554 was chosen for further study.
Screen for suppressors of pim1-d1.
pim1-d1 cells (SS84) were transformed with a cDNA library expressed from the nmt1 promoter in the pREP3X vector (gift of Bruce Edgar, Fred Hutchinson Cancer Research Center, Seattle, Wash., and Chris Norbury, University of Oxford, United Kingdom). Approximately 104 transformants were grown at 25°C on media without thiamine to allow transcription for 24 h and then shifted to 34°C. In order to eliminate transformants carrying plasmid-borne copies of either pim1 or spi1, only colonies that could grow at 34°C but not at 36°C were pursued. To verify the specificity of suppression, colonies were tested for plasmid-dependent growth by replica plating on EMM at 34°C in the presence of thiamine to repress the expression of the cDNAs. Colonies that grew better when the cDNA was transcribed were retested by streaking on EMM plates with or without thiamine at 34°C. Three transformants, each of which carried a different cDNA, met the criteria of the screen. This report describes experiments with one of these genes, named imp2 (increased maximal permissive temperature for pim1).
Chromosome loss assay.
SS1036 homozygous spi1-null cells were grown at 25°C to mid-log phase in EMM. Cells were then transferred to fully supplemented EMM, to remove the nutritional selection, containing 0.08 μg of thiamine/ml to partially repress expression of spi1. Cells were plated on EMM agar containing low adenine (10 μg/ml) to distinguish diploid white colonies from haploid pink colonies or they were serially diluted and spotted on fully supplemented EMM to assess viability.
Microscopy.
For actin staining, cells were fixed in 3.3% formaldehyde in phosphate-buffered saline (PBS) for 30 min (1), and 1 ml of 100-μg/ml tetramethyl rhodamine isothiocyanate (TRITC)-phalloidin (Sigma) was added to 50 ml of fixed cell suspension. After 30 min at room temperature, cells were washed with PBS, dried on coverslips treated with poly-l-lysine (Sigma), and stained with 4,6-diamidino-2-phenylindole (DAPI) dissolved in PBS to visualize the DNA (30). For Arp3 immunolocalization, cells were fixed in methanol (28) and incubated overnight with a 1:200 dilution of anti-Arp3p antibody, a generous gift of Kathy Gould (Howard Hughes Medical Institute, Vanderbilt University, Nashville, Tenn.) and then with Texas Red-labeled goat anti-rabbit secondary antibody (Jackson Laboratory) diluted 1:200 for 2 h. Calcofluor White (CCF) (Sigma) staining of the septum was performed either by adding an equal volume of 100-mg/ml dye in 50% glycerol to the live cell suspension or by mounting fixed cells dried on coverslips in 100-mg/ml CCF solution. Cells fixed with 3.7% formaldehyde-0.2% glutaraldehyde for 90 min were washed three times with PEM [100 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (pH 6.9), 1 mM EGTA, 1 mM MgSO4] and processed for anti-tubulin immunofluorescence as previously described (12). The anti-tubulin antibody TAT-1 (provided by K. Gull [49]) was utilized at a 1:10 dilution, followed by a 1:100 dilution of the secondary antibody Alexa Fluor 488 goat anti-mouse immunoglobulin G (H and L) conjugate (Molecular Probes). In living cells DNA was visualized with Hoechst 33342 and membranes were visualized with DiOC6 previously described (4). Digitized images were captured with a DVC 1300 charge-coupled device camera and QED software, using a Zeiss Axioskop fluorescence microscope.
Nuclear protein transport assays.
Nuclear protein transport was tested in live cells as previously described (8) by monitoring the localization of green fluorescent protein (GFP)-tagged Pap1p expressed from an integrated copy of the gene (46), which was crossed in from the previously described strain SS767 (8).
NE fragmentation assays.
Cells containing an integrated copy of pREP4X-GFP-NLS-LacZ crossed from wild-type strain SS482 (8) were grown in supplemented EMM liquid to mid-log phase at 25°C and then shifted to the stated experimental temperature. Interphase cells or septated cells with a uniform cellular fluorescence in one or both daughter cells were scored as fragmented.
Western analysis.
Total soluble protein extracts were prepared as described elsewhere (30) and quantified by Bradford assay (Bio-Rad). Fifteen micrograms of total soluble protein was separated by sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis and transferred to an Immobilon-P nylon membrane (Millipore). Spi1 protein levels were detected by Western blot analysis using an affinity-purified anti-Spi1p antibody (prepared as described elsewhere [23]) at a 1:1,000 dilution and an anti-rabbit, horseradish peroxidase (HRP)-conjugated secondary antibody at a 1:10,000 dilution, using the ECL detection system (Amersham Life Science). GFP-Pap1 protein levels were detected using an anti-GFP rabbit polyclonal antibody (Molecular Probes) at a 1:500 dilution and an anti-rabbit, HRP-conjugated secondary antibody as above. Levels of Spi1 or GFP-Pap1 were determined by quantifying the intensity of bands from Western analyses using a DC-129 digital camera and Digital Science Analysis software (Eastman Kodak Co.). In the case of Spi1p, a minimum of three experiments were performed to approximate the percent reduction. For each experiment, a range of exposures was analyzed in order to confine the data to a linear range of intensity. Equal loading was confirmed by Coomassie blue staining of the membrane.
TBZ hypersensitivity assays.
Sensitivity to thiabendazole (TBZ) (Sigma) was monitored by growing cells in YE at 25°C, spotting 106 cells and fivefold serial dilutions on YE plates containing either 0 or 15 μg of TBZ/ml, and incubating them at the indicated temperature for 2 to 3 days.
RESULTS
Cells depleted of Spi1p elongate and then arrest with a phenotype similar to pim1-d1 mutant cells.
RanSp spi1-25 mutant cells are viable but have MT defects that cause chromosome missegregation and abnormal cell length and shape (8). This phenotype is very different from that of cells in which the levels of RanGEF, RanGAP, or RanBP1 are altered by mutation or overexpression (8, 13, 23, 41). To better understand the nature of the spi1-25 defect, a spi1-null strain was constructed (see Materials and Methods) and its phenotype was compared to that of the point mutant. spi1 is an essential gene, but the null mutant could be kept alive by expression of the wild-type spi1 gene from the high-strength nmt1 promoter, contained on an integrated copy of pREP3X-spi1. Transcriptional repression by the addition of thiamine caused lethality.
The terminal phenotype of spi1Δ cells (Fig. 1A, panel d) differed significantly from that of either wild-type cells (Fig. 1A, panel a) or spi1-25 mutant cells (Fig. 1A, panel c). After 36 h of transcriptional repression, the terminal phenotype of the spi1Δ cells (Fig. 1A, panel d, and B, panels i, j, k, and l) resembled that of pim1-d1 cells incubated at the restrictive temperature for 4 h (Fig. 1A, panel b) and cells with other perturbations to the RanSp system (4, 23, 41). In all cases, cells undergo a catastrophic mitosis in which the NE fragments, the chromosomes remain condensed, and cells have a wide medial septum (Fig. 1B, panels i, j, k, and l). The difference in phenotype between the spi1-null and spi1 point mutant demonstrates that spi1-25 is not a complete loss-of-function mutation.
FIG. 1.
The terminal arrest phenotype of spi1 null cells is different from the phenotype of spi1-25 mutant cells. (A) Wild-type (a), pim1-d1 (b), and spi1-25 (c) cells grown at 36°C for 4 h, or spi1Δ null cells grown in 5 μg of thiamine/ml for 36 h to repress spi1 expression (d), were fixed in ethanol and stained with DAPI to view the DNA. (B) spi1Δ null cells grown either without thiamine, to allow expression of spi1 (a, b, c, and d), or for 24 h (e, f, g, and h) or 36 h (i, j, k, and l) in the presence of thiamine to repress spi1 expression were stained with either DiOC6, to view the NE (a, c, e, g, i, and k), or Hoechst, to view the DNA (b, d, f, h, j, and l). Septated cells with condensed DNA and no discernible NE are indicated with arrows. Bar = 10 μm.
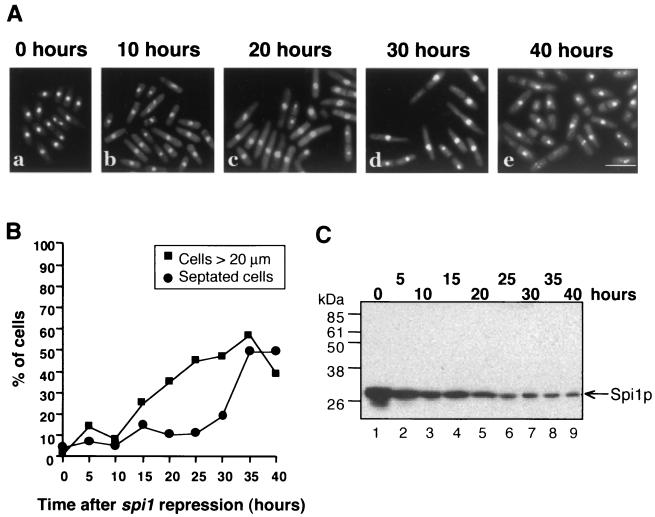
To test the hypothesis that the MT defects in spi1-25 mutant cells are caused by a decrease in the level of active RanSp (Spi1) protein, we first examined spi1Δ cells periodically after nmt1 promoter repression to monitor morphological phenotypes that might arise prior to the terminal arrest (Fig. 2A). After 15 h, when the level of Spi1p was approximately 30% of the initial level (Fig. 2C), 20% of the cells were elongated, and by 25 h 40% of the cells were more than 20 μm in length (Fig. 2B). At 24 h, the majority of spi1Δ cells still contained intact NEs (Fig. 1B, panels, e, f, g, and h). After 40 h, when Spi1p was less than 10% of the initial level (Fig. 2C), the number of septated cells with condensed chromosomes increased from less than 10% to more than 45% (Fig. 2A, panel e, and B). By this point the NEs were already fragmented (Fig. 1B, panels i, j, k, and l) in >40% of cells. The phenotype of spi1Δ cells continuously grown without thiamine is the same as that seen at the zero-hour time point in this experiment (data not shown). These data indicate that cells with very low levels of Spi1p exhibit a terminal arrest phenotype similar to that seen in the pim1-d1 RanGEFSp mutant, but at intermediate Spi1p levels the cells resemble spi1-25 (8).
FIG. 2.
Haploid spi1Δ null cells with low Spi1p levels elongate prior to arresting as septated cells with condensed chromatin. (A) spi1Δ null cells grown in the presence of 5 μg of thiamine/ml, to repress spi1 expression, were fixed and stained with DAPI at the indicated times. Bar = 10 μm. (B) The spi1Δ null cells were grown as for panel A and the percentage of elongated (>20 μm) or septated cells was determined at 5-h intervals. (C) Western blot of equal amounts of total soluble protein from cells grown as for panel A and probed with an anti-Spi1p antibody. Spi1p levels decreased over the first 20 h (lanes 1 to 5; arrow) and then reached a steady state (lanes 6 to 9; arrow).
Lowering the level of Spi1p leads to an increase in chromosome loss and abnormal spindle MTs.
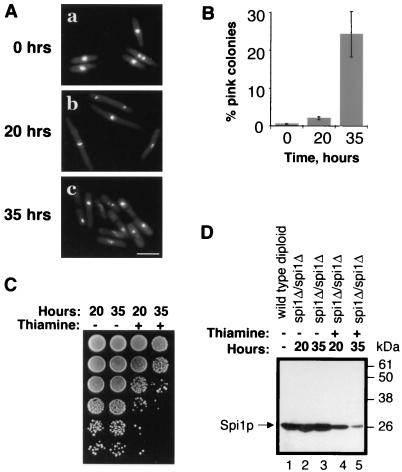
Although the amount of Spi1 protein is similar in wild-type and spi1-25 cells, only a portion of the Spi1-25 protein is competent to bind nucleotide (8). These results suggested the possibility that a decrease in the amount of Spi1 protein might specifically affect MT function. Consistent with this hypothesis is the observation that heterozygous spi1-null diploid cells are unstable and rapidly haploidize (22), a phenomenon that could be caused by abnormal spindle MTs. Because it is not possible to examine these cells as they haploidize, a meiotic-deficient, homozygous spi1-null diploid strain called SS1036 was constructed in which spi1 is expressed from the thiamine-repressible nmt1 gene promoter (see Materials and Methods). Through intragenic complementation, these diploid ade6-M210/ade6-M216 cells grow into white colonies on a plate containing low adenine, while haploid ade6-M210 or ade6-M216 cells grow into pink colonies. The mat2-102 mutation (6) renders the strain meiotic deficient, insuring that haploids can arise only as the result of chromosome loss.
Thirty-five hours after the addition of 0.08 μg of thiamine/ml, when the level of Spi1p had dropped to less than 10% of untreated cells (Fig. 3D, lane 5), 24% ± 6% of colonies were pink, compared to 0.3% ± 0.1% in the untreated population (Fig. 3B). Flow cytometric analysis of the DNA content of representative white and pink colonies confirmed that they contained diploid and haploid cells, respectively (data not shown). At that time, most of the cells were septated (Fig. 3A, panel c), and cell viability was low (Fig. 3C). The observation that 35 h after transcriptional repression of spi1 many cells had already reached their terminal lethal phenotype indicated that any aberrant mitoses that might be responsible for the observed chromosome loss would have occurred at an earlier time.
FIG. 3.
Mitotic spi1Δ/Δspi1Δ null cells with low Spi1p levels undergo chromosome loss. (A) spi1Δ/spi1Δ homozygous null diploid cells grown in 0.08 μg of thiamine/ml for 0, 20, or 35 h were ethanol fixed and stained with DAPI. At 0 h (a), cells appeared normal; at 20 h (b), they were elongated; and at 35 h (c), a majority were septated and had condensed chromosomes. (B) The spi1Δ/spi1Δ homozygous null cells grown as for panel A were plated on low adenine and the number of pink (haploid) and white (diploid) colonies was determined. Data represent the average of five independent experiments. (C) A total of 106, and 10-fold serial dilutions of, spi1Δ/spi1Δ homozygous null cells grown as for panel A were spotted on fully supplemented EMM to test viability. (D) Spi1p levels decrease in Δspi1/Δspi1 homozygous null cells grown in 0.08 μg of thiamine/ml (arrow). Equal amounts of total protein extracts from wild-type diploid cells and spi1Δ/spi1Δ homozygous null cells were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and probed with an anti-Spi1p antibody. Without thiamine, protein levels remain unchanged in spi1Δ/spi1Δ homozygous null cells and were comparable to levels in wild-type diploid cells (lanes 2 and 3, compared to lane 1). Spi1p expression in the sample incubated in 0.08 μg of thiamine/ml for 20 h (lane 4) was reduced to approximately 30% that of untreated cells (lane 2), and that in cells incubated for 35 h (lane 5) was reduced to less than 10% (lane 3).
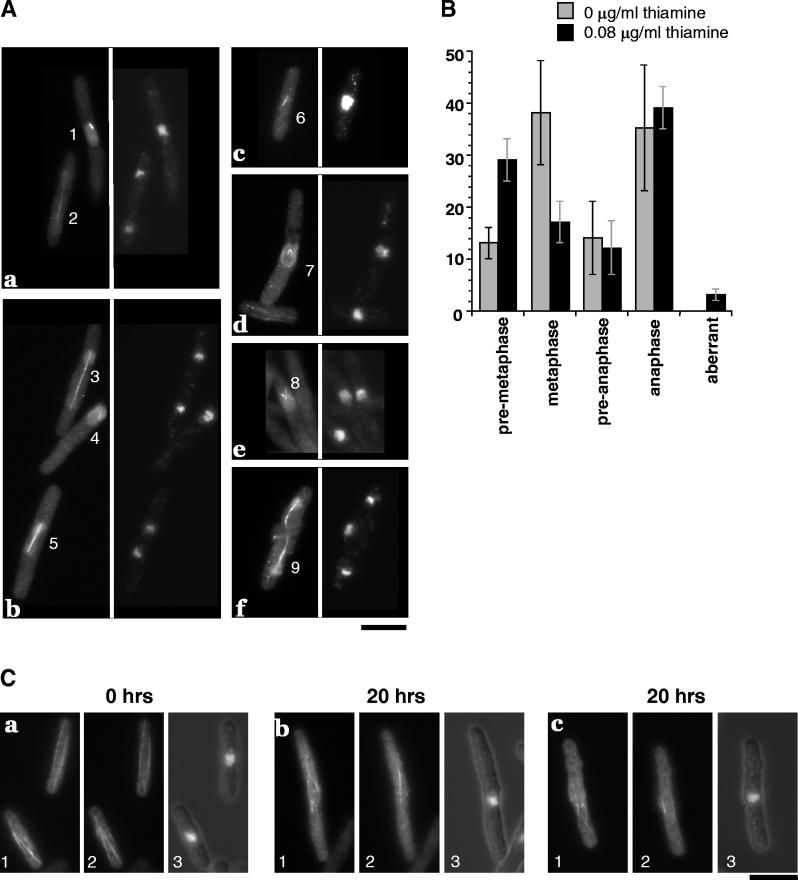
MT structure was therefore examined 20 h after transcriptional repression, when the Spi1 protein level was reduced to approximately 30% that of untreated cells (Fig. 3D) and the cells were elongated (Fig. 3A) and were nearly as viable as untreated cells (Fig. 3C) but had not yet undergone haploidization (Fig. 3B). An examination of mitotic cells by anti-tubulin immunolocalization revealed that 29% ± 4% contained short, preanaphase spindles (Fig. 4A, cells 1 and 4) compared to only 13% ± 3% of control cells (Fig. 4B), indicating a delay in early mitosis. Furthermore, 3% ± 1% of the cells had aberrant spindle structures never seen in untreated control cells (Fig. 4B) or wild-type cells (8). These aberrant structures included V- and star-shaped spindles and elongated but misshapen spindles that had not properly separated the DNA (Fig. 4A, cells 7, 8, and 9). These data indicate that a moderate decrease in the level of Spi1p results in spindle assembly defects similar to those seen in the spi1-25 mutant (8). These defects result in a subsequent loss of chromosome segregation fidelity that causes haploidization.
FIG. 4.
Mitotic cells with low Spi1p levels have aberrant spindles. (A) spi1Δ/spi1Δ homozygous null cells grown for 20 h after the addition of 0.08 μg of thiamine/ml to reduce the level of Spi1p. (Panels a to f) Shown in each panel is tubulin, visualized with the TAT-1 antibody (left), or DNA morphology, visualized with DAPI (right). (a, b, and c) Normal spindles with short, premetaphase spindle (cells 1 and 4); metaphase spindle (cell 6); preanaphase spindle (cell 5); elongated late anaphase spindle (cells 2 and 3). (d, e, and f) Aberrant spindle structures with monopolar spindle (cells 7 and 8); aberrantly elongated spindle with missegregated DNA (cell 9). Bar = 10 μm. (B) spi1Δ/spi1Δ homozygous null cells with mitotic spindle structures were classified by their stage in mitosis or the presence of abnormalities from three independent experiments. A minimum of 1,000 cells were examined in each experiment, of which 5 to 7% of cells were mitotic. (C) spi1Δ/spi1Δ homozygous null cells were cultured and processed as for panel A to visualize MTs (panels 1 and 2, different focal planes of the same cells) or DNA (panel 3). Compared to cells grown without thiamine (a), elongated cells from cultures grown in the presence of thiamine for 20 h (b and c) contain cytoplasmic MTs that fail to reach the cell tips. Bar = 10 μm.
To determine whether reducing the level of Spi1p also affects cytoplasmic MTs, cell shape and cytoplasmic MT distribution were examined. In cells in which spi1 transcription was not repressed (Fig. 4C, panel a) and in wild-type cells (8), the cytoplasmic MTs extend the entire length of the cell. Twenty hours after transcriptional repression, >30% of cells were elongated or misshaped, and in these cells the cytoplasmic MTs failed to reach the tips of the cells (Fig. 4C, panels b and c). Therefore, cells with low Spi1p protein levels are elongated and misshapen due to structural defects in the cytoplasmic MTs, as is the case in spi1-25 cells (8).
RanGEFSp pim1-d1 mutant cells are competent for nuclear protein import and export.
Nucleocytoplasmic transport is the best-characterized Ran-dependent process (18), but studying the effects of the Ran on this process in S. pombe is complicated by the fact that RanSp misregulation causes NE fragmentation at mitosis (4). Nuclear import was initially monitored using the GFP-simian virus 40 NLS-β-gal reporter (8), which is imported in a Ran-dependent manner in other organisms (20, 29) and is commonly used to detect import defects. In wild-type and pim1-d1 mutant cells, GFP-β-gal was constitutively localized to the nucleus at both the permissive and restrictive temperatures prior to NE fragmentation (data not shown). However, using this type of reporter, a defect in import can only be detected by the cytoplasmic accumulation of newly synthesized GFP-fusion proteins. A more sensitive assay of nucleocytoplasmic protein import and export utilizes the GFP-Pap1p reporter (46). Pap1p is an S. pombe protein that shuttles between the nucleus and the cytoplasm but appears cytoplasmic at steady state. In response to cellular stress, which can be mimicked by the addition of hydrogen peroxide, Pap1p rapidly accumulates in the nucleus. Upon removal of hydrogen peroxide, the protein relocalizes to the cytoplasm.
pim1-d1 and wild-type control cells containing the integrated pREP41-GFP-pap1 plasmid were grown to mid-log phase at 25°C and then incubated at either the permissive (25°C) or restrictive (36°C) temperature for 2 h. At this time, the mutant Pim1-d1 protein is inactivated (41), but the majority of the cells still have intact NEs (Table 2) (4). None of the cells in either sample contained GFP-Pap1p fluorescence in the nucleus (Fig. 5A, panels a and d), indicating that both strains are able to export GFP-Pap1p to the cytoplasm at steady state. Cells were then treated with hydrogen peroxide for 15 min, and the localization of GFP-Pap1p was determined (Fig. 5A, panels b and e). At 25°C, 92% ± 12% of wild-type and 86% ± 1% pim1-d1 cells had nuclear GFP-Pap1p fluorescence. At 36°C, 99% ± 3% of wild-type and 93% ± 5% of pim1-d1 cells contained nuclear GFP-Pap1p fluorescence (Fig. 5B). Therefore, pim1-d1 mutant cells are competent for nuclear protein import at 36°C. Both strains were also examined at earlier time points to compare the kinetics of import. As early as 5 min after the addition of hydrogen peroxide, both wild-type and pim1-d1 cells had accumulated nuclear fluorescence (data not shown), indicating that the rate of import in these two strains is similar.
TABLE 2.
pim1 mutants contain intact NEs at their HPT
| Strain | HPT (°C) | % Fragmented NE at:
|
% Septated at:
|
||||
|---|---|---|---|---|---|---|---|
| HPT | 36°C, 2 h | 36°C, 4 h | HPT | 36°C, 2 h | 36°C, 4 h | ||
| pim1-d1 | 32 | 17 ± 5 | 14 ± 4 | 46 ± 13 | 20 ± 1 | 13 ± 6 | 44 ± 13 |
| JD59 | 25 | 5 ± 3 | 15 ± 12 | 51 ± 16 | 13 ± 9 | 13 ± 11 | 43 ± 14 |
| JD60 | 25 | 1 ± 1 | 20 ± 6 | 41 ± 12 | 15 ± 3 | 16 ± 4 | 40 ± 9 |
| JD61 | 29 | 6 ± 4 | 21 ± 3 | 49 ± 3 | 16 ± 2 | 17 ± 1 | 45 ± 1 |
| JD62 | 25 | 1 ± 1 | 20 ± 1 | 52 ± 2 | 15 ± 5 | 18 ± 4 | 49 ± 0 |
| slg51 | 29 | 1 ± 1 | 4 ± 1 | 29 ± 5 | 10 ± 1 | 8 ± 1 | 23 ± 11 |
| sns-A3 | 29 | 0 ± 0 | 15 ± 3 | 65 ± 1 | 14 ± 2 | 15 ± 1 | 60 ± 1 |
| sns-A5 | 32 | 2 ± 1 | 12 ± 3 | 37 ± 1 | 9 ± 6 | 16 ± 1 | 26 ± 4 |
| Wild type | NAa | NA | 0.5 ± 1 | 0.5 ± 1 | NA | 10 ± 1 | 12 ± 1 |
NA, not applicable.
FIG. 5.
The RanGEFSp pim1-d1 mutant is competent for nucleocytoplasmic transport. (A) The localization of GFP-Pap1p was monitored in living pim1-d1 or wild-type cells incubated for 2 h at 36°C. Wild-type (a) and pim1-d1 (d) cells grown without hydrogen peroxide exported GFP-Pap1p to the cytoplasm. When treated with hydrogen peroxide for 15 min, wild-type (b) and pim1-d1 (e) cells imported GFP-Pap1p to the nucleus. Fifteen minutes after washing, wild-type (c) and pim1-d1 (f) cells exported GFP-Pap1p back to the cytoplasm. Bar = 10 μm. (B) The percentage of cells with nuclear GFP-Pap1p fluorescence prior to hydrogen peroxide treatment (−H2O2), 15 min after hydrogen peroxide addition (+H2O2), and 15 min after removal (wash). The standard deviation was calculated from a minimum of three experiments for each sample.
To test the ability of pim1-d1 cells to export GFP-Pap1p, cells were grown for 2 h at 36°C and incubated for 15 min in the presence of hydrogen peroxide to allow GFP-Pap1p to accumulate in the nucleus. Fifteen minutes after removal of hydrogen peroxide at 25°C, 0.3% ± 0.4% of wild-type and 0.5% ± 0.6% of pim1-d1 cells had nuclear fluorescence. At 36°C, 0% ± 0% of wild-type and 0.1% ± 0.2% of pim1-d1 cells had nuclear fluorescence (Fig. 5A, panels c and f, and B), indicating that these RanGEFSp mutant cells are competent for nuclear protein export. An analysis of seven other previously characterized pim1 mutants (24) yielded similar results (data not shown). Although the percentage of pim1-d1 and wild-type cells with nuclear GFP-Pap1p was similar after hydrogen peroxide treatment, GFP-Pap1p fluorescence (Fig. 5A, compare panels a, b, and c with panels d, e, and f) and the GFP-Pap1p protein level (data not shown) were lower in the mutant cells, suggesting that the lower GFP fluorescence intensity in pim1-d1 cells does not represent a defect in import efficiency.
RanGEFSp mutants are sensitive to TBZ in the absence of protein transport defects.
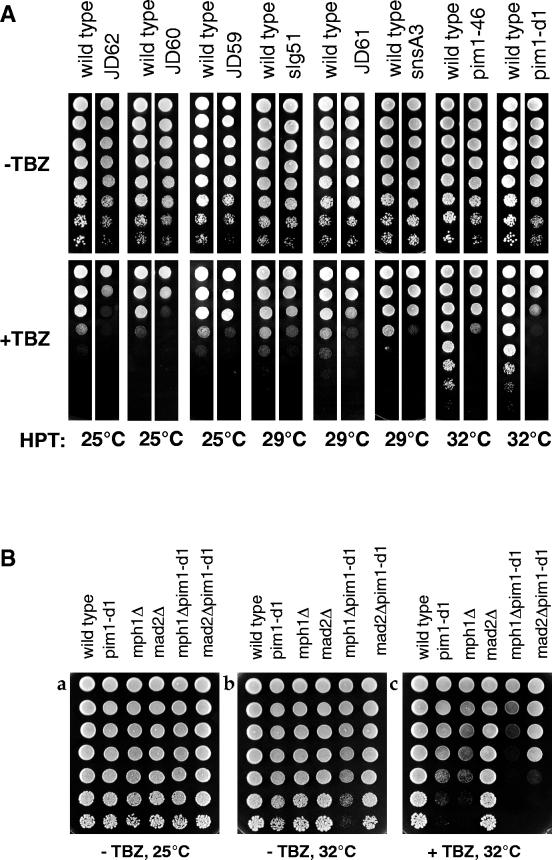
That MT abnormalities are seen both in spi1-25 mutant cells and in wild-type cells with a lower than normal amount of RanSp suggested that other perturbations to the RanSp GTPase system might also cause MT defects. Although it has been reported that the MTs in pim1-46 cells are morphologically normal (22), RanGEFSp mutant cells may contain subtle defects in MT structure that cannot be observed morphologically. To test this possibility, eight previously characterized strains, each containing a different mutation in RanGEFSp (pim1) (24), were assessed for hypersensitivity to the MT-destabilizing drug TBZ. The highest permissive temperature (HPT), at which the mutants grew as well as wild-type cells and had normal septa and intact NEs, was found to range between 25 and 32°C (Table 3). To test for MT defects, each strain was grown in rich medium at 25°C, spotted onto TBZ-containing plates, and incubated at its HPT. Assessing sensitivity at the HPT addresses the question of whether MT defects exist in viable cells. All eight strains were hypersensitive to TBZ at their HPT (Fig. 6A). pim1-d1 and pim1-46 were most sensitive, and JD59 was the least sensitive. These data suggest that mutations in RanGEFSp result in MT defects.
TABLE 3.
Percent pim1-d1 cells with fragmented NEs over a range of temperatures
| Temp (h) | % Fragmented NE | % Septated |
|---|---|---|
| 25°C | 0 ± 0 | 8 ± 4 |
| 32°C (4) | 17 ± 5 | 20 ± 1 |
| 34°C (4) | 22 ± 5 | 20 ± 4 |
| 36°C (4) | 46 ± 13 | 44 ± 13 |
FIG. 6.
Mutations in pim1 cause hypersensitivity to TBZ and require an intact spindle assembly checkpoint for maximum viability. (A) RanGEFSp mutant strains representing eight different mutations in pim1 were assessed for TBZ hypersensitivity at their HPT. Fivefold serial dilutions of an equal number of wild-type and mutant cells were spotted on YE plates containing 0 (−TBZ) or 15 μg of TBZ/ml (+TBZ). All RanGEFSp mutant strains were more sensitive to TBZ than wild-type cells grown at the same temperature. (B) To determine whether pim1-d1 RanGEFSp cells required an intact spindle checkpoint, fivefold serial dilutions of an equal number of wild-type, pim1-d1, Δmph1, Δmad2, Δmph1 pim1-d1, and Δmad2 pim1-d1 mutant cells were spotted on YE plates containing either 0 (−TBZ) or 15 μg of TBZ/ml (+TBZ). Plates were incubated at either 25°C for 3 days or 32°C for 2 days.
To ask whether unstable MTs exist in the absence of TBZ, double-mutant strains of pim1-d1 and null alleles of the essential spindle checkpoint genes mph1 or mad2 (14, 15) were constructed. These genes ensure cell survival in the presence of spindle damage by halting cell cycle progression until all chromosomes are properly attached to the spindle MTs (reviewed in reference 10). A comparison of the growth of single and double mutants grown at 32°C, the HPT for pim1-d1, shows that the pim1-d1 mph1Δ double mutant has growth defects even in the absence of TBZ (Fig. 6B, compare panels a and b), suggesting that there are endogenous spindle defects in pim1-d1 cells that are normally detected by the spindle checkpoint. pim1-d1 is also synthetically lethal with both checkpoint mutations in the presence of TBZ (Fig. 6B, panel c), presumably because the endogenous MT defects are enhanced in the presence of this drug. These data indicate that pim1-d1 cells require an intact spindle checkpoint pathway for optimal viability.
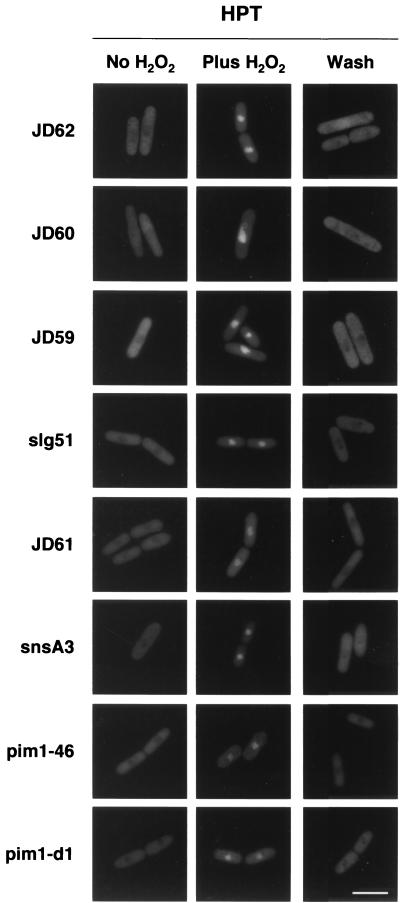
To test whether these MT defects are independent of nucleocytoplasmic transport defects, the localization of GFP-Pap1p (46) was assessed in RanGEFSp cells incubated for 18 h at either 25°C or the appropriate HPT (Fig. 7). Cells were incubated without hydrogen peroxide (to monitor steady-state localization), after incubation in hydrogen peroxide (to monitor import), and after hydrogen peroxide was washed out (to monitor export), as previously described. For all strains, the majority of the cells (>95%) were able to export GFP-Pap1p at steady state, import GFP-Pap1p to the nucleus in the presence of hydrogen peroxide, and export GFP-Pap1p back to the cytoplasm when the hydrogen peroxide was removed at 25°C (data not shown) or the HPT (Fig. 7). These data indicate that under conditions in which RanGEFSp strains have defects in MTs they are competent for nucleocytoplasmic transport.
FIG. 7.
RanGEFSp mutants can import and export GFP-Pap1p. Eight temperature-sensitive RanGEFSp strains, mutated in pim1, were tested for their ability to properly localize GFP-Pap1p when incubated at their HPT overnight. All strains grown without hydrogen peroxide (No H2O2) were able to export GFP-Pap1p to the cytoplasm. When treated with hydrogen peroxide (Plus H2O2), all were able to import GFP-Pap1p into the nucleus. After H2O2 removal (Wash), all strains were able to export GFP-Pap1p back to the cytoplasm. Shown are representative cells for each condition. Bar = 10 μm.
Rescue of the septation defect in the RanGEFSp mutant pim1-d1 at its lowest restrictive temperature increases viability.
Characterization of fission yeast strains in which Ran function is impaired by mutation or overexpression of its regulators indicated that this GTPase independently regulates multiple cellular processes (13, 23). Consistent with this conclusion, previous screens to find suppressors of the temperature-sensitive lethality of the RanGEFSp mutants pim1-d1 or pim1-46 at 36°C identified more than 50 rescuing plasmids that represented only two genes: pim1 itself and the high-copy suppressor spi1, which encodes RanSp (22, 35, 41). If the lethality of RanGEFts mutants were caused by misregulation of multiple independent downstream pathways, then only restoration of the function of the GTPase system itself would be expected to restore viability to the strain. However, it might be possible to identify individual effector pathways by finding suppressors of pim1-d1 that are able to rescue viability at the lowest restrictive temperature of 34°C but not at 36°C (see Materials and Methods). One cDNA clone that met these criteria contained the imp2 gene, which encodes a protein that colocalizes with and is required for destabilization of the medial ring after septation (5).
The effect of ectopic overexpression of imp2 in pim1-d1 cells was tested to determine how Imp2p rescues the viability of this strain. pim1-d1 mutant cells were cotransformed with pREP82X-GFP-cdc4 and either pREP41X-imp2 or a vector control, grown at 25°C in EMM for 20 h to induce expression, and then shifted to either 34 or 36°C for 8 h. In the absence of ectopic imp2 overexpression, the frequency of pim1-d1 cells that had both condensed chromosomes and abnormal actin ring structures at the septum was 19% lower at 34°C than at 36°C (Table 4), indicating that the penetrance of these defects is lower at 34°C (Table 4). However, when imp2 was overexpressed in pim1-d1 cells, this difference doubled to 37%, indicating that imp2 expression destabilized the actin ring structures and thereby partially rescued the viability.
TABLE 4.
Presence of actin ring structures in pim1-d1 cells overexpressing imp2
| Strain | % Septated cells with condensed chromosomes at:
|
|
|---|---|---|
| 34°C | 36°C | |
| pim1-d1 + pREP82X-GFPcdc4 | 77 ± 6 | 96 ± 5 |
| + pREP81X | ||
| pim1-d1 + pREP82X-GFPcdc4 | 55 ± 7 | 92 ± 4 |
| + pREP41X-imp2 | ||
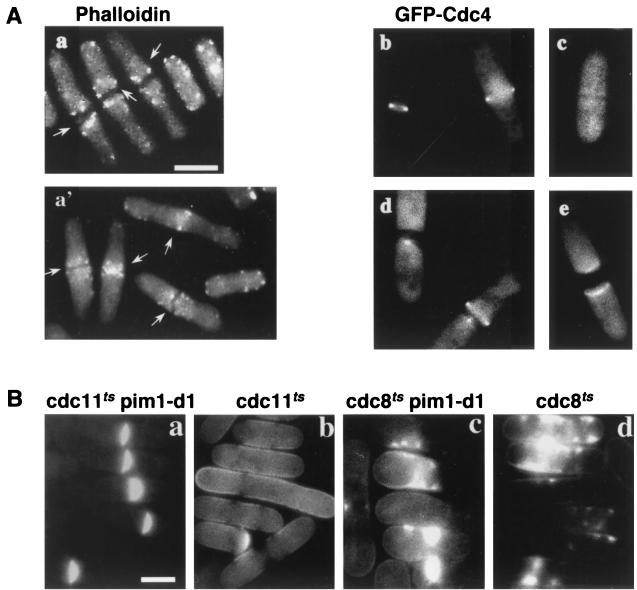
Cytokinesis in fission yeast is accomplished by construction of an actin- and myosin-containing ring in the middle of the cell. Contraction of this ring is required for the centripetal deposition of a specialized cell wall called the septum that physically separates the two daughter cells. After septation, actin relocalizes from the site of septation back to what will become the growing tips of the cell (1). To understand the mechanism by which the actin-destabilizing protein Imp2p rescues the viability of pim1-d1, which is known to arrest with an abnormally wide medial septum (23), actin structures were examined in mutant cells by using TRITC-labeled phalloidin. At the restrictive temperature, septated pim1-d1 cells contained actin at the septum, as wild-type cells do (21), but the structures in pim1-d1 cells were aberrant and included abnormally large aggregates and ring structures (Fig. 8A, compare panels a and a′). As has been previously described, the GFP-tagged myosin light-chain protein Cdc4p (1) localizes specifically to the medial ring (27) in septating wild-type cells (Fig. 8B, panel b) but disappears once the primary septum is formed (Fig. 8B, panel c). However, in pim1-d1 cells, GFP-Cdc4p was still present at the septum in 70% of septated cells (Fig. 8A, panels d and e). GFP-Cdc4p-containing structures were heterogeneous and included abnormal spots, rings, and filaments, similar to the structures seen with phalloidin staining. In contrast, Arp3p, which is found exclusively in actin patches (28), localized normally to the septum in both pim1-d1 and wild-type cells undergoing septation (data not shown). Therefore, actin accumulates at the septum in pim1-d1 cells in a structure which is normally absent in wild-type cells once the primary septum is formed and may be responsible for development of the abnormally wide septum in these cells.
FIG. 8.
Overexpression of imp2 destabilizes abnormal actin ring structures in RanGEFSp pim1-d1 mutant cells at 34°C. (A) pim1-d1 cells grown at 36°C were stained with TRITC-labeled phalloidin to detect filamentous actin in order to visualize actin structures in septating cells (a, arrows), which are aberrant in comparison to wild-type cells undergoing normal septation when grown at 36°C (a′, arrows). GFP-Cdc4p localization is shown in live wild-type (b and c) and pim1-d1 (d and e) cells cultured at 36°C. Bar = 5 μm. (B) pim1-d1 suppresses the septation defect in cdc11ts but not cdc8ts. pim1-d1 cdc11-119 (a), pim1-d1 cdc8-27 (c), and the corresponding single mutants cdc11-119 (b) and cdc8-27 (d) were incubated at 36°C for 4 h and stained with CCF to visualize septal material. Bar = 5 μm.
To confirm the hypothesis that actin structures are abnormally stabilized in the absence of RanGEF function, genetic interactions between pim1 and two classes of previously characterized septation genes (34) were tested. One class, represented by temperature-sensitive mutations in cdc11, cdc14, and cdc15, is proficient in forming an actin ring but the ring is unstable and the cells fail to septate. The lack of septation caused by the cdc11-119 mutation (Fig. 8B) and by cdc14-118 and cdc15-140 (data not shown) was suppressed by pim1-d1. Double-mutant cells (Fig. 8B, panel a), but not cdc11-119 single-mutant cells (Fig. 8B, panel b), arrested with a medial septum which was visualized with the fluorescent dye CCF. The other class, represented by cdc3, cdc4, and cdc8, does not form an actin ring and deposits septal material in a disorganized manner. No suppression was observed in the double mutants pim1-d1 cdc8-27 (Fig. 8B, panel c), pim1-d1 cdc3-124, or pim1-d1 cdc4-8 (data not shown). Septal material was deposited in a disorganized fashion in both the pim1-d1 cdc8-27 double mutant (Fig. 8C, panel c) and the cdc8-27 single mutant (Fig. 8B, panel d), but septa were not formed. Taken together with the failure to disassemble actin ring structures at the septum of pim1-d1 mutant cells, these results suggest that the pim1-d1 mutation suppresses the septation defect caused by mutations in cdc11, cdc14, and cdc15 by stabilizing the medial actin ring.
DISCUSSION
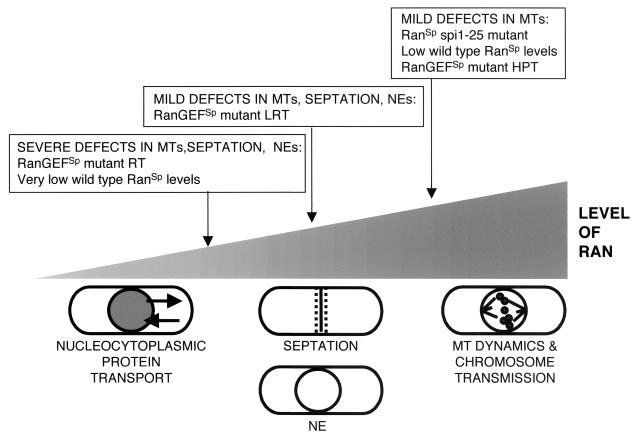
When the RanSp (Spi1p) GTPase system is misregulated by overexpressing or mutating RanGEFSp, RanGAPSp, or RanBP1Sp, cells arrest after mitosis, with condensed chromosomes, fragmented NEs, and a wide medial septum (4, 13, 23, 41). We report here that spi1-null cells arrest with this same complex terminal phenotype. In contrast, spi1-25 cells, carrying a point mutation in the RanSp GTPase, have normal NE and septa and are competent for nucleocytoplasmic protein transport but contain both abnormal cytoplasmic MTs, which affect cell shape, and abnormal spindle MT structures, which cause chromosome loss (8). These MT-specific defects raised the possibility that spi1-25 was a separation-of-function mutation. However, its ability to interact with its known binding partners and to rescue the phenotypes of pim1-d1 (8) and spi1-25 (S. S. Salus and S. Sazer, unpublished data) when overexpressed suggested that the Spi1-25 mutant protein could carry out all of its normal functions, albeit with a lower efficiency than the wild-type protein. This conclusion was bolstered by the finding that only 30% of Spi1-25p could bind nucleotide in vitro (8). These results led to the hypothesis that in spi1-25 cells only a portion of the GTPase is active and that this decrease adversely affects one but not all Ran-dependent processes. Here we test this hypothesis, describe Ran's influence on both the tubulin and actin cytoskeletons, and show that RanSp-dependent processes require different amounts of active RanSp (Fig. 9).
FIG. 9.
Model of RanSp multiple functions. RanSp-dependent cellular processes are differentially sensitive to the level of active RanSp. Cells with relatively small decreases in active RanSp have abnormalities in MTs but not nucleocytoplasmic transport, NE structure, or septation, suggesting that the MT cytoskeleton is most sensitive to the level of functional RanSp. At moderate levels of active RanSp, cells have mild defects in MTs, NE, and septation. Viability can be increased by specifically suppressing the septation defect, indicating that it is the next-most-sensitive Ran-dependent process. Only when Ran function is severely reduced do most cells undergo fragmentation of the NE. Although it is likely that nucleocytoplasmic protein transport is regulated by the RanSp system in fission yeast, defects in MT, NE, and medial ring structures occur in cells that are competent for transport, making it the most robust Ran-dependent process.
The RanGEFSp pim1-d1 mutant has defects in the actin cytoskeleton.
It was known that pim1-d1 mutant cells arrest with an aberrantly wide medial septum (23), but the reason for this defect was unknown. Here, microscopic examination of pim1-d1 cells revealed the persistence of actin- and Cdc4p-containing ring structures at the septum, which are normally disassembled and relocalized to the growing tips of the cells once septation is complete. These data provide the first demonstration of a role for the RanSp system in a cytoplasmic process, the disassembly of the actin ring, and reestablishment of the interphase actin organization. They also explain why cells in which Ran function is compromised do not elongate at the restrictive temperature, as do the classical cdc (cell division cycle) mutants (34). Overexpression of the imp2 gene (5), which encodes a protein that destabilizes actin ring structures in wild-type cells, rescues this defect and partially restores viability to the strain at its lowest restrictive temperature of 34°C. Presumably because it cannot rescue other cellular defects, overexpression of imp2 cannot suppress the temperature-sensitive lethality of pim1-d1 cells at 36°C.
The RanGEFSp mutants are competent for nucleocytoplasmic protein transport.
Several characteristics of pim1-d1 mutant cells indicate that they are competent for nucleocytoplasmic transport (4, 41). At the restrictive temperature they (i) grow and elongate during interphase; (ii) activate the Cdc2p/Cdc13 CDK complex and enter mitosis with normal kinetics; (iii) reenter the cell cycle from G0 and replicate DNA during S phase; (iv) condense chromatin in preparation for mitosis; and (v) build a mitotic spindle from a nuclear pool of tubulin. These processes all require the nuclear localization of tubulin and regulatory proteins such as DNA origin recognition complex components (33) and condensin complex subunits (44). However, the central role of Ran in nucleocytoplasmic protein transport in other systems prompted a direct examination of the transport competence of RanGEFSp mutant cells. Eight different RanGEFSp mutant strains are competent for nucleocytoplasmic protein transport after 2 h at the restrictive temperature when the majority of cells still contain intact NEs (Table 2). Evolutionary conservation of Ran and its regulators, the identification in the S. pombe genome (48) of structural homologues of import receptors and nucleoporins with which Ran is known to interact in other organisms, and the interaction of RanSp with some of these factors in a yeast two-hybrid screen (N. Ong and S. Sazer, unpublished results) make it likely that Ran does participate in nucleocytoplasmic transport in S. pombe. Yet, mutations in a core component of the RanSp GTPase system do not perturb nucleocytoplasmic protein transport prior to the development of the terminal lethal phenotype. This unexpected finding may be the result of the NE fragmentation defect that causes cell lethality prior to the development of a transport defect severe enough to be detected. The data reported here demonstrate that in living fission yeast cells, Ran-dependent processes differ in their requirements for the Ran GTPase and that nucleocytoplasmic transport is the most robust of Ran's functions. That nuclear protein import can continue despite fluctuations in the relative abundance of transport factors is supported by a recent mathematical model and supporting experimental data (43).
RanGEFSp mutant cells have MT defects.
The finding that decreasing the levels of RanSp perturbs MT function prompted the reassessment of the RanGEFSp mutant phenotype, although chromosome segregation and spindles appear morphologically normal in these cells (22, 41). We have previously characterized eight different mutations in Pim1p and mapped them onto the crystal structure of human RanGEF, a seven-bladed, β-propeller structure. These Pim1p mutant residues, most of which lie in the beta strands of the propeller blades, are likely to perturb protein structure at high temperatures (38). All eight pim1 mutants were hypersensitive to the MT-destabilizing drug TBZ at their HPT. The synthetic lethality of pim1-d1 and null alleles of the spindle checkpoint genes mad2 and mph1 indicate that this mutant has MT defects even in the absence of TBZ that are normally detected by the spindle checkpoint system. The facts that the mutants grow normally at their respective HPT, are competent for nucleocytoplasmic transport, and have intact NEs suggest that these MT defects are independent of other Ran-dependent processes. This is consistent with the model that MT function requires higher levels of active RanSp protein than other Ran-dependent processes (Fig. 9).
Cellular processes have different requirements for the amount of RanSp.
Mutant spi1-25 cells have aberrant spindle and cytoplasmic MTs (8) but a normal NE and septum, a phenotype that differs from the terminal phenotype of cells in which regulators of RanSp are mutated or overexpressed (13, 23, 41) or the spi1 gene is deleted (Fig. 2A). However, the spi1-25 MT defects can be phenocopied by moderately reducing the amount of Spi1p in otherwise wild-type cells. These data support our proposal that the spi1-25 phenotype results not from a separation-of-function mutation but from a decrease in the amount of active RanSp (8). Cells with a moderate level of Spi1p contained abnormal mitotic spindles that correlated with an increase in chromosome loss and abnormal cytoplasmic MTs that did not reach the tips of these elongated and misshaped cells. When Spi1p levels were further decreased, cells arrested with fragmented NEs and abnormal septa (Fig. 9). While technical limitations prevented us from directly testing nuclear transport in cells in which a reduction in the level of wild-type Spi1p caused microtubule defects, both the spi1-25 mutant and the pim1 temperature-sensitive mutants were transport competent yet had abnormal MTs. These data indicate that RanSp-dependent processes can be separated in terms of their requirements for RanSp and that MT function is especially sensitive to decreased Ran function.
Ran functions independently in multiple cellular pathways.
When Ran was first shown to be required for nuclear protein transport, it was proposed that this was its primary role and that all other cellular defects caused by misregulation of the GTPase were secondary consequences of transport defects (reviewed in reference 39). However, our finding that RanGEFSp mutants are competent for nucleocytoplasmic transport at the restrictive temperature prior to NE fragmentation suggests that these two processes are independent. This conclusion is corroborated by data from nuclear-free in vitro extract systems, in which there can be no nucleocytoplasmic transport, showing that NE reformation requires both RanGTP hydrolysis and exchange, and the transport receptor protein importin β (16, 50-52). The mechanism by which RanSp maintains the structural integrity of the NE during mitosis in fission yeast is unknown. While the NE in yeast does not break down in mitosis, it grows and undergoes a dramatic change in shape before being resolved into two daughter nuclei (45). Like NE assembly in higher eukaryotes, NE maintenance in fission yeast is Ran dependent and requires both RanSp-GDP and RanSp-GTP, since reducing the level of the GTPase, GEF, or GAP results in NE fragmentation (4, 23).
In vitro studies in Xenopus egg extracts demonstrated Ran's ability to stimulate the formation of spindle asters in the absence of active protein import or export (reviewed in reference 3). The mechanism by which Ran could promote aster formation in vivo may also be mediated by transport receptors. Increasing the concentration of importin α or importin β inhibits spindle aster formation in vitro by sequestering the aster-promoting activity (APA) of the MT-associated proteins TPX2 and NuMA (11, 31, 47). A model based on these studies (reviewed in reference 3) proposes that the high concentration of Ran-GTP near the chromatin-associated RanGEF (17) liberates these APA factors from their receptors, thereby promoting spindle formation. However, the necessity of chromosomes to signal their location to the forming spindle may be specific to the very large eggs from which the extracts used in these studies are made. In budding and fission yeast, the chromosomes are always confined to the nucleus within which the spindle forms. As shown here, when the level of wild-type RanSp protein was lowered in fission yeast, the number of mitotic cells containing premetaphase spindles or aberrantly formed spindles increased. Whether a chromatin-localized Ran-GTP gradient is present in and required for spindle formation in vivo in higher and lower eukaryotic cells remains to be demonstrated.
The data presented here demonstrate that RanSp is important in the mitotic-specific reorganization of the actin cytoskeleton, the MT cytoskeleton, and the nucleus in fission yeast. We propose that these processes require higher levels of RanSp than does nucleocytoplasmic protein transport (Fig. 9). If nuclear Ran-GTP stabilizes export receptor-cargo complexes, destabilizes import receptor-cargo complexes, and destabilizes receptor-APA complexes in the vicinity of chromosomes at mitosis, it is not clear how transport-competent cells could have mitotic spindle defects. One possibility is that factors other than Ran-GTP that differ in concentration, accessibility, or binding affinity affect the stability of these nuclear complexes. A recent systems analysis of Ran-dependent transport (43) predicts that, in addition to Ran-GTP, other factors are required to efficiently dissociate transport receptor-cargo complexes in the nucleus, since the estimated free Ran-GTP concentration is much lower than the dissociation constant for the binding of Ran to import receptors. Previously described factors that influence the stability of import cargo-transport receptor complexes specifically in the nucleus (36, 42) may be found to play a more general role in regulating nuclear-specific Ran functions. In the budding yeast Saccharomyces cerevisiae, the dissociation of the TATA binding protein from its import receptor Kap114 (36) depends on Ran-GTP but is stimulated by double-stranded TATA containing DNA. Of particular relevance to the fission yeast studies described here, this DNA becomes essential for complex dissociation when the concentration of Ran-GTP is low.
Acknowledgments
We thank Ngoctuyen Ong and Cristina Lopez for technical assistance; Cristina Lopez and Sheila Kadura for the data shown in Fig. 6; Michiya Nishino and Alberto Ribes-Zamora for initial studies on nucleocytoplasmic transport in the pim1 mutant collection; Kathy Gould for the GFP-cdc4 fusion constructs and the anti-Arp3 antibody; Keith Gull for the TAT-1 antibody; and Ursula Fleig, Xiangwei He, and Richard Atkinson for critical reading of the manuscript.
The National Institutes of Health (GM49119) supported this work.
REFERENCES
- 1.Balasubramanian, M. K., D. McCollum, and K. L. Gould. 1997. Cytokinesis in fission yeast Schizosaccharomyces pombe. Methods Enzymol. 283:494-506. [DOI] [PubMed] [Google Scholar]
- 2.Clarke, P. R., and C. Zhang. 2001. Ran GTPase: a master regulator of nuclear structure and function during the eukaryotic cell division cycle? Trends Cell Biol. 11:366-371. [DOI] [PubMed] [Google Scholar]
- 3.Dasso, M. 2001. Running on Ran: nuclear transport and the mitotic spindle. Cell 104:321-324. [DOI] [PubMed] [Google Scholar]
- 4.Demeter, J., M. Morphew, and S. Sazer. 1995. A mutation in the RCC1-related protein Pim1 results in nuclear envelope fragmentation in fission yeast. Proc. Natl. Acad. Sci. USA 92:1436-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demeter, J., and S. Sazer. 1998. imp2, a new component of the actin ring in the fission yeast Schizosaccharomyces pombe. J. Cell Biol. 143:415-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Egel, R. 1973. Commitment to meiosis in fission yeast. Mol. Gen. Genet. 121:277-284. [Google Scholar]
- 7.Elble, R. 1992. A simple and efficient procedure for transformation of yeasts. BioTechniques 13:18-20. [PubMed] [Google Scholar]
- 8.Fleig, U., S. S. Salus, I. Karig, and S. Sazer. 2000. The fission yeast ran GTPase is required for microtubule integrity. J. Cell Biol. 151:1101-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forsburg, S. L. 1993. Comparison of Schizosaccharomyces pombe expression systems. Nucleic Acids Res. 21:2955-2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gardner, R. D., and D. J. Burke. 2000. The spindle checkpoint: two transitions, two pathways. Trends Cell Biol. 10:154-158. [DOI] [PubMed] [Google Scholar]
- 11.Gruss, O. J., R. E. Carazo-Salas, C. A. Schatz, G. Guarguaglini, J. Kast, M. Wilm, N. Le Bot, I. Vernos, E. Karsenti, and I. W. Mattaj. 2001. Ran induces spindle assembly by reversing the inhibitory effect of importin alpha on TPX2 activity. Cell 104:83-93. [DOI] [PubMed] [Google Scholar]
- 12.Hagan, I. M., and J. S. Hyams. 1988. The use of cell division cycle mutants to investigate the control of microtubule distribution in the fission yeast Schizosaccharomyces pombe. J. Cell Sci. 89:343-357. [DOI] [PubMed] [Google Scholar]
- 13.He, X., N. Hayashi, N. G. Walcott, Y. Azuma, T. E. Patterson, F. R. Bischoff, T. Nishimoto, and S. Sazer. 1998. The identification of cDNAs that affect the mitosis-to-interphase transition in Schizosaccharomyces pombe, including sbp1, which encodes a Spi1p-GTP-binding protein. Genetics 148:645-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He, X., M. H. Jones, M. Winey, and S. Sazer. 1998. Mph1, a member of the Mps1-like family of dual specificity protein kinases, is required for the spindle checkpoint in S. pombe. J. Cell Sci. 111:1635-1647. [DOI] [PubMed] [Google Scholar]
- 15.He, X., T. E. Patterson, and S. Sazer. 1997. The Schizosaccharomyces pombe spindle checkpoint protein Mad2 blocks anaphase initiation and interacts genetically with the APC. Proc. Natl. Acad. Sci. USA 94:7965-7970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hetzer, M., D. Bilbao-Cortes, T. C. Walther, O. J. Gruss, and I. W. Mattaj. 2000. GTP hydrolysis by Ran is required for nuclear envelope assembly. Mol. Cell 5:1013-1024. [DOI] [PubMed] [Google Scholar]
- 17.Kalab, P., K. Weis, and R. Heald. 2002. Visualization of a Ran-GTP gradient in interphase and mitotic Xenopus egg extracts. Science 295:2452-2456. [DOI] [PubMed] [Google Scholar]
- 18.Kuersten, S., M. Ohno, and I. W. Mattaj. 2001. Nucleocytoplasmic transport: Ran, beta and beyond. Trends Cell Biol. 11:497-503. [DOI] [PubMed] [Google Scholar]
- 19.Kunzler, M., and E. Hurt. 2001. Targeting of Ran: variation on a common theme? J. Cell Sci. 114:3233-3241. [DOI] [PubMed] [Google Scholar]
- 20.Lee, M. S., M. Henry, and P. A. Silver. 1996. A protein that shuttles between the nucleus and the cytoplasm is an important mediator of RNA export. Genes Dev. 10:1233-1246. [DOI] [PubMed] [Google Scholar]
- 21.Marks, J., I. M. Hagan, and J. S. Hyams. 1986. Growth polarity and cytokinesis in fission yeast: the role of the cytoskeleton. J. Cell Sci. Suppl. 5:229-241. [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto, T., and D. Beach. 1991. Premature initiation of mitosis in yeast lacking RCC1 or an interacting GTPase. Cell 66:347-360. [DOI] [PubMed] [Google Scholar]
- 23.Matynia, A., K. Dimitrov, U. Mueller, X. He, and S. Sazer. 1996. Perturbations in the Spi1 GTPase cycle of Schizosaccharomyces pombe through its GAP and GEF components result in similar phenotypic consequences. Mol. Cell. Biol. 16:6352-6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matynia, A., U. Mueller, N. Ong, J. Demeter, A. L. Granger, K. Hinata, and S. Sazer. 1998. Isolation and characterization of fission yeast sns mutants defective at the mitosis to interphase transition. Genetics 148:1799-1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matynia, A., S. S. Salus, and S. Sazer. 2002. Three proteins required for early steps in the protein secretory pathway also affect nuclear envelope structure and cell cycle progression in fission yeast. J. Cell Sci. 115:421-431. [DOI] [PubMed] [Google Scholar]
- 26.Maundrell, K. 1993. Thiamine-repressible expression vectors pREP and pRIP for fission yeast. Gene 123:127-130. [DOI] [PubMed] [Google Scholar]
- 27.McCollum, D., M. K. Balasubramanian, L. E. Pelcher, S. M. Hemmingsen, and K. L. Gould. 1995. Schizosaccharomyces pombe cdc4 gene encodes a novel EF-hand protein essential for cytokinesis. J. Cell Biol. 130:651-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCollum, D., A. Feoktistova, M. Morphew, M. Balasubramanian, and K. L. Gould. 1996. The Schizosaccharomyces pombe actin-related protein, Arp3, is a component of the cortical actin cytoskeleton and interacts with profilin. EMBO J. 15:6438-6446. [PMC free article] [PubMed] [Google Scholar]
- 29.Melchior, F., B. Paschal, J. Evans, and L. Grace. 1993. Inhibition of nuclear protein import by nonhydrolyzable analogues of GTP and identification of the small GTPase Ran/TC4 as an essential transport factor. J. Cell Biol. 123:1649-1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moreno, S., A. Klar, and P. Nurse. 1991. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194:795-823. [DOI] [PubMed] [Google Scholar]
- 31.Nachury, M. V., T. J. Maresca, W. C. Salmon, C. M. Waterman-Storer, R. Heald, and K. Weis. 2001. Importin beta is a mitotic target of the small GTPase Ran in spindle assembly. Cell 104:95-106. [DOI] [PubMed] [Google Scholar]
- 32.Nakielny, S., and G. Dreyfuss. 1999. Transport of proteins and RNAs in and out of the nucleus. Cell 99:677-690. [DOI] [PubMed] [Google Scholar]
- 33.Nishitani, H., and P. Nurse. 1995. p65cdc18 plays a major role controlling the initiation of DNA replication in fission yeast. Cell 83:397-405. [DOI] [PubMed] [Google Scholar]
- 34.Nurse, P., P. Thuriaux, and K. Nasmyth. 1976. Genetic control of the cell division cycle in the fission yeast Schizosaccharomyces pombe. Mol. Gen. Genet. 146:167-178. [DOI] [PubMed] [Google Scholar]
- 35.Patterson, T. E., G. R. Stark, and S. Sazer. 1995. A strategy for quickly identifying all unique two-hybrid or library plasmids within a pool of yeast transformants. Nucleic Acids Res. 23:4222-4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pemberton, L. F., J. S. Rosenblum, and G. Blobel. 1999. Nuclear import of the TATA-binding protein: mediation by the karyopherin Kap114p and a possible mechanism for intranuclear targeting. J. Cell Biol. 145:1407-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prentice, H. L. 1992. High efficiency transformation of Schizosaccharomyces pombe by electroporation. Nucleic Acids Res. 20:621.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Renault, L., N. Nassar, I. Vetter, J. Becker, C. Klebe, M. Roth, and A. Wittinghofer. 1998. The 1.7 Å crystal structure of the regulator of chromosome condensation (RCC1) reveals a seven-bladed propeller. Nature 392:97-101. [DOI] [PubMed] [Google Scholar]
- 39.Sazer, S. 1996. The search for the primary function of the Ran GTPase continues. Trends Cell Biol. 6:81-85. [DOI] [PubMed] [Google Scholar]
- 40.Sazer, S., and M. Dasso. 2000. The Ran decathlon: multiple roles of Ran. J. Cell Sci. 113:1111-1118. [DOI] [PubMed] [Google Scholar]
- 41.Sazer, S., and P. Nurse. 1994. A fission yeast RCC1-related protein is required for the mitosis to interphase transition. EMBO J. 13:606-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Senger, B., G. Simos, F. R. Bischoff, A. Podtelejnikov, M. Mann, and E. Hurt. 1998. Mtr10p functions as a nuclear import receptor for the mRNA-binding protein Npl3p. EMBO J. 17:2196-2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith, A. E., B. M. Slepchenko, J. C. Schaff, L. M. Loew, and I. G. Macara. 2002. Systems analysis of Ran transport. Science 295:488-491. [DOI] [PubMed] [Google Scholar]
- 44.Sutani, T., T. Yuasa, T. Tomonaga, N. Dohmae, K. Takio, and M. Yanagida. 1999. Fission yeast condensin complex: essential roles of non-SMC subunits for condensation and Cdc2 phosphorylation of Cut3/SMC4. Genes Dev. 13:2271-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tanaka, K., and T. Kanbe. 1986. Mitosis in the fission yeast Schizosacchar omyces pombe as revealed by freeze-substitution electron microscopy. J. Cell Sci. 80:253-268. [DOI] [PubMed] [Google Scholar]
- 46.Toone, W. M., S. Kuge, M. Samuels, B. A. Morgan, T. Toda, and N. Jones. 1998. Regulation of the fission yeast transcription factor Pap1 by oxidative stress: requirement for the nuclear export factor Crm1 (exportin) and the stress-activated MAP kinase sty1/Spc1. Genes Dev. 12:1453-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wiese, C., A. Wilde, M. S. Moore, S. A. Adam, A. Merdes, and Y. Zheng. 2001. Role of importin-beta in coupling Ran to downstream targets in microtubule assembly. Science 291:653-656. [DOI] [PubMed] [Google Scholar]
- 48.Wood, V., R. Gwilliam, M. A. Rajandream, M. Lyne, et al. 2002. The genome sequence of Schizosaccharomyces pombe. Nature 415:871-880. [DOI] [PubMed] [Google Scholar]
- 49.Woods, A., T. Sherwin, R. Sasse, T. H. MacRae, A. J. Baines, and K. Gull. 1989. Definition of individual components within the cytoskeleton of Trypanosoma brucei by a library of monoclonal antibodies. J. Cell Sci. 93:491-500. [DOI] [PubMed] [Google Scholar]
- 50.Zhang, C., and P. R. Clarke. 2000. Chromatin-independent nuclear envelope assembly induced by Ran GTPase in Xenopus egg extracts. Science 288:1429-1432. [DOI] [PubMed] [Google Scholar]
- 51.Zhang, C., and P. R. Clarke. 2001. Roles of Ran-GTP and Ran-GDP in precursor vesicle recruitment and fusion during nuclear envelope assembly in a human cell-free system. Curr. Biol. 11:208-212. [DOI] [PubMed] [Google Scholar]
- 52.Zhang, C., J. R. Hutchins, P. Muhlhausser, U. Kutay, and P. R. Clarke. 2002. Role of importin-beta in the control of nuclear envelope assembly by Ran. Curr. Biol. 12:498-502. [DOI] [PubMed] [Google Scholar]