Abstract
Many receptor and nonreceptor tyrosine kinases activate phosphoinositide 3-kinases (PI3Ks). To assess the role of the δ isoform of the p110 catalytic subunit of PI3Ks, we derived enzyme-deficient mice. The mice are viable but have decreased numbers of mature B cells, a block in pro-B-cell differentiation, and a B1 B-cell deficiency. Both immunoglobulin M receptor-induced Ca2+ flux and proliferation in response to B-cell mitogens are attenuated. Immunoglobulin levels are decreased substantially. The ability to respond to T-cell-independent antigens is markedly reduced, and the ability to respond to T-cell-dependent antigens is completely eliminated. Germinal center formation in the spleen in response to antigen stimulation is disrupted. These results define a nonredundant signaling pathway(s) utilizing the δ isoform of p110 PI3K for the development and function of B cells.
Lymphocyte development and function are regulated through the coordinated action of receptors of the cytokine receptor superfamily and the B-cell antigen-specific receptor (BCR) or T-cell antigen-specific receptors (TCR). Engagement of either receptor complex initiates tyrosine phosphorylation of a variety of intracellular substrates, including receptor chains, resulting in the initiation of cellular responses. Members of the cytokine receptor superfamily utilize JAK family cytoplasmic kinases (14), while the BCR and TCR complexes utilize members of the Src, Tec, and Zap70/Syk families of tyrosine kinases. In BCR signal transduction, the Tec family kinase Btk plays a critical role as evidenced by the loss of a proliferative response to BCR engagement in Btk-deficient B cells (15, 16). Among the substrates typically phosphorylated and recruited to hematopoietic receptor complexes are the regulatory subunits for phosphoinositide 3-kinases (PI3Ks) (9, 10, 30). In mammals there are three genes that encode adapter proteins for PI3K catalytic subunits, including p85α, p85β, and p55γ. The adapter proteins facilitate association of the catalytic subunits with the receptor complex and are proposed to regulate enzyme activity. The disruption of the p85α gene, in a manner that deletes the p85 isoform as well as two splice variants of p55 and p50, results in defective BCR signaling comparable to that seen with Btk deficiency (11, 26). This strongly suggests that PI3K activity is critical in BCR signal transduction.
Three of the known PI3Ks, i.e., PI3Kα, PI3Kβ, and PI3Kδ, are regulated through their interaction with regulatory subunits (30). The fourth PI3K, PI3Kγ, functions in the context of heterotrimeric G-protein-coupled receptors and is essential for leukocyte function (12). The critical, nonredundant role that PI3Kα plays in cellular responses has been demonstrated through the derivation of mice lacking the gene. This deficiency results in an embryonic lethality at E9.5 to E10.5 due to a severe proliferative defect in many tissues (2). Similarly, deletion of the PI3Kβ gene alone results in a very early embryonic lethality (1, 2). In contrast to PI3Kα and PI3Kβ, PI3Kδ is expressed primarily in hematopoietic cells (32). To identify proteins that are recruited to cytokine receptor complexes, we have used affinity columns containing phosphopeptides corresponding to the major sites of tyrosine phosphorylation of JAK2. Two complexes which bound to a phosphopeptide affinity column derived from the amino acid sequence surrounding Jak2 Y966 were p85α/p110δ and p85β/p110δ. In order to determine the potential role of PI3Kδ in signaling, we derived a strain of mice in which the gene was disrupted in a manner to yield a protein null mutant. As demonstrated here, a deficiency in PI3Kδ results in a very specific loss of function of the BCR complex, while signaling through the cytokine receptor complexes is unaffected.
MATERIALS AND METHODS
Construction of p110δ targeting vector.
Approximately 9 kb of the p110d gene was isolated from a 129/J mouse genomic library in pBeloBacII (Incyte Genomics, St. Louis, Mo.) and subcloned into pBluescript. A neomycin resistance cassette was inserted into the StuI fragment encompassing exons 2 through 6. A diphtheria toxin A cassette mediating negative selection was inserted in the 5′ end of the p110δ-neo construct.
Culture of ES cells and generation of p110δ-deficient mice.
E14 (129/Ola mouse strain) ES cells were cultured in Dulbecco's modified Eagle medium containing 15% fetal calf serum, 1 mM sodium pyruvate, 2 mM l-glutamine, 0.1 mM nonessential amino acids, 55 mM β-mercaptoethanol, 10 ng of gentamicin per ml, and 1,000 U of leukemia inhibitory factor/ml (all from GIBCO-BRL, Rockville, Md.). Irradiated SNLH9 fibroblasts were used as feeders. Twenty-five micrograms of SalI-linearized p110δ targeting construct was electroporated into the ES cells by using a gene pulser (Bio-Rad, Hercules, Calif.) set at 0.23 kV and 500 μF. Selection at 24 h after electroporation was in 350 μg of G418 (GIBCO-BRL) per ml. Drug-resistant ES clones were picked and expanded 7 to 10 days after electroporation. Four correctly targeted and karyotypically normal ES clones were injected into C57BL/6 blastocysts, of which two gave germ line transmission. The mice were maintained under specific-pathogen-free conditions according to institutional guidelines. Genotyping of mice was performed by Southern blot analysis or by PCR.
Flow cytometry.
Single-cell suspensions were depleted of red blood cells by using a hypotonic lysis buffer (pH 7.3) containing 150 mM NH4Cl, 1 mM KHCO3, and 0.1 mM Na2EDTA as described previously (19). Monoclonal antibodies (MAbs) conjugated to either fluorescein isothiocyanate (FITC), phycoerythrin (PE), or CyChrome and specific to murine B220/CD45R, immunoglobulin M (IgM), IgD, CD5, CD43, Thy1.2, interleukin-2 (IL-2) receptor (CD25), CD44, CD4, CD8, Mac-1 (CD11b), and Gr-1 (all from PharMingen) were used to label 106 cells for 30 min on ice. Fc receptors were blocked with anti-FcγIII/II R antibody (Ab) (PharMingen). Cells were washed twice and analyzed on a FACSCalibur with CellQuest software (Becton Dickinson).
Immunoprecipitation, sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and Western blot analysis.
Immunoprecipitation, sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and Western blot analysis were performed as described previously (36). The polyclonal anti-p110δ antiserum was generated by immunizing rabbits with a keyhole limpet hemocyanin (KLH)-conjugated peptide derived from residues 1028 to 1043 of murine p110δ. Anti-phospholipase Cγ2 (anti-PLCγ2) was from Santa Cruz Biotechnologies, anti-Bruton's tyrosine kinase (BTK) was from PharMingen, and anti-phosphotyrosine 4G10 was from Upstate Biotechnology.
Immunizations.
To measure the thymus-dependent immune response, wild-type and mutant mice (five per group) were immunized intraperitoneally with 100 μg of trinitrophenyl (TNP)-KLH (Biosearch Technologies) in a 1:1 emulsion with complete Freund's adjuvant (Sigma), followed by a second immunization 15 days later with 100 μg of TNP-KLH in a 1:1 emulsion with incomplete Freund's adjuvant (Sigma). Sera were collected 21 days after the first immunization and analyzed for TNP-specific Abs by enzyme-linked immunosorbent assay (ELISA). To measure the TI immune response, p110δ−/− mice and wild-type littermates (five per group) were immunized intraperitoneally with 50 μg of TNP-lipopolysaccharide (LPS) (TI-I antigen) (Sigma) or 25 μg of TNP-Ficoll (TI-II antigen) (Biosearch Technologies) in phosphate-buffered saline (PBS). Sera collected 12 days after immunization were analyzed for TNP-specific Abs by ELISA.
ELISAs.
Specific concentrations of IgM, IgG1, IgG2a, IgG2b, IgG3, and IgA in serum were measured by coating Nunc Maxisorp 96-well plates (Thomas Scientific, Swedesboro, N.J.) with isotype-specific goat anti-mouse Abs (Southern Biotechnology Associates, Birmingham, Ala.). Bound immunoglobulin from test sera was detected by using alkaline phosphatase-conjugated goat anti-mouse isotype-specific Abs (Southern Biotechnology), followed by alkaline phosphatase substrate (p-nitrophenyl phosphate; Sigma). The absorbance of the reaction mixture at 405 nm was quantitated with an ELISA plate reader (model 3550; Bio-Rad Laboratories). Each assay included the titration of affinity-purified mouse IgM, IgG1, IgG2a, IgG2b, IgG3, and IgA Abs (Southern Biotechnology), which was used to construct a standard curve. The concentrations of serum immunoglobulin were calculated by comparing the optical density obtained to a standard curve of titrated Abs by using linear regression analysis. Anti-TNP specific immunoglobulin isotype levels were measured by coating plates with TNP-bovine serum albumin (Biosearch Technologies).
In vitro B- and T-cell proliferative responses.
For B-cell proliferation assays, B cells purified by fluorescence-activated cell sorting (FACS) (105/well; >95% pure) were cultured in triplicate in 96-well U-bottom plates. Purified goat anti-mouse IgM (μ chain) (Jackson ImmunoResearch), anti-CD40 MAb (PharMingen), LPS (Sigma), or phorbol myristate acetate (PMA) and ionomycin (Sigma) were added as mitogens at the indicated concentrations. Cells were cultured for 48 h, after which they were labeled with 1 μCi of [3H]thymidine per well for 18 h and harvested with a TOMTECH Harvester 96 MachII to measure incorporated radioactivity. T-cell proliferation assays were conducted similarly. Percentages of Thy1.2+ cells within splenocyte suspensions were identified by FACS analysis, and splenocytes were cultured in triplicate U-bottom 96-well plates such that a constant number of T cells (1.5 × 105/well) were used per assay. Anti-CD3 MAb (145-2C11; Pharmingen), phytohemagglutinin (PHA), concanavalin A (ConA), and PMA plus ionomycin (Sigma) were added at the indicated concentrations.
Ca+2 measurement and phosphotyrosine blots.
Splenocytes were suspended in Dulbecco's modified Eagle medium plus 10% fetal calf serum, loaded with Indo-1 (10 μM; Molecular Probes, Eugene, Oreg.) for 30 min at room temperature, and stained with either PE-anti-B220 or PE-anti-CD4 and FITC-anti-CD8 for an additional 20 min at 4°C. Cells were stimulated with anti-mouse IgM (Jackson ImmunoResearch) for B cells or rat anti-mouse CD3 Ab (145-2C11) followed by anti-hamster IgG Ab (Jackson ImmunoResearch) for T cells. Ionomycin (Calbiochem) was used as a positive control. To induce tyrosine phosphorylation, splenic B cells were purified by complement lysis with anti-Thy1.2 Ab (AT83) and rabbit and guinea pig complement (Cedarlane Laboratory). Purified B cells (90%) were stimulated with anti-IgM (20 μg/ml) for 3 min and then lysed as indicated above.
Immunohistochemistry.
For detection of a germinal center formation response, p110δ−/− mice and their wild-type littermates were immunized intraperitoneally with TNP-KLH (Biosearch Technologies) as described above or with 150 μl of a 10% suspension of sheep red blood cells (Colorado Serum, Denver, Colo.). Spleens were snap frozen in liquid nitrogen and sectioned. Frozen sections 8 nm thick were fixed with acetone for 15 min at −20°C and rehydrated with PBS. Sections were double stained with biotinylated B220 (1:200) and FITC-Thy1.2 (1:50) or FITC-B220 (1:200) (all from PharMingen) or biotinylated peanut agglutinin (PNA) (10 μg/ml; Vector, Burlingame, Calif.) in PBS with 3% bovine serum albumin for 2 h at room temperature. Sections were washed and subsequently counterstained with streptavidin-tetramethyl rhodamine isocyanate (1:1,000) (Southern Biotechnology) for 1 h at room temperature. After being washed in PBS, the sections were mounted in Fluoromount G (Southern Biotechnology). All slides were evaluated and photographed with a Leica TCS confocal laser scanning microscope.
Bone marrow colony assays.
Cells were prepared from bone marrow in α-MEM medium (Life Technologies) containing 2% fetal bovine serum (StemCell Technologies) and counted in the presence of 3% acetic acid to lyse red blood cells. Cells were cultured in 0.9% MethoCult M3230 (StemCell Technologies) with recombinant cytokines at the following concentrations: BFU-E, 3 U of recombinant human erythropoietin (rhEpo) (Amgen) per ml plus 10 ng of recombinant murine IL-3 (rmIL-3) (R&D Systems) per ml; CFU-Mix, 10 ng of rmIL-3 (R&D) per ml; CFU-Eos, 20 ng of rmIL-5 (R&D) per ml; CFU-Meg, 50 ng of rcombinant human thrombopoietin (Genzyme) per ml; CFU-G, 10 ng of recombinant human granulocyte colony-stimulating factor (rhG-CSF) (Amgen) per ml; CFU-GM, 10 ng of recombinant murine granulocyte-macrophage CSF (rmGM-CSF) (R&D) per ml; and CFU-M, 10 ng of rmCSF-1 (R&D) per ml. Pre-B-cell assays were conducted with MethoCult M3630 by using 10 ng of rhIL-7 (StemCell Technologies) per ml. Assay mixtures were plated in 35-mm-diameter culture dishes in duplicate and scored as previously described (28).
RESULTS
Generation of p110δ mice.
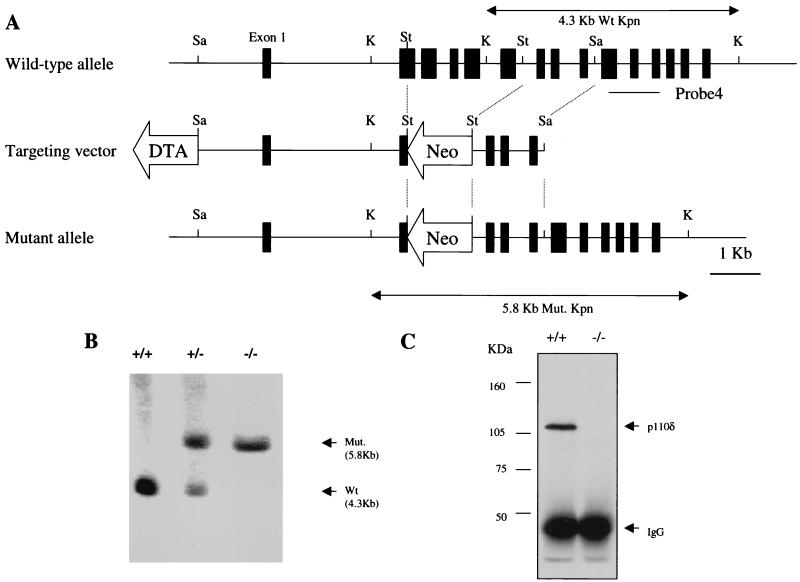
The p110δ gene was disrupted in murine E14 ES cells by the replacement of a 2.6-kb fragment containing exons 2 through 6 with a neo gene expression cassette (Fig. 1). The design of the targeting construct relied on the finding that deletion of >150 amino acids from the N-terminal region of p110δ eliminated kinase activity (32). Four correctly targeted ES clones were injected into C57BL/6 blastocysts, two of which were transmitted to the germ line. The two mouse lines had comparable phenotypes. Southern blot analysis confirmed the presence of the targeted locus (Fig. 1B). Heterozygous mice were bred to obtain mice homozygous for the targeted allele. Homozygous offspring were viable, were obtained with the expected Mendelian ratio, and lacked p110δ protein as assessed by immunoprecipitation and Western blot analysis of splenic extracts (Fig. 1C). The absence of p110δ had no effect on the levels of p110α or p110β in splenic extracts (data not shown).
FIG. 1.
Targeted disruption of murine p110δ gene. (A) A targeting construct that would replace the indicated exons with a neomycin resistance cassette was developed. The locations of the 3′ external probe and PCR primers (P1, P2, and P3) for genotyping are indicated. Restriction enzyme sites: Sa, SacI; K, KpnI; St, StuI. DTA, diphtheria toxin A; Wt, wild type. (B) Southern blot analysis of KpnI-digested genomic DNA from p110δ heterozygous mice probed with the indicated probe. The positions of the mutant 5.8-kb and wild-type 4.3-kb KpnI fragments are indicated. (C) Absence of p110δ protein expression in p110δ−/− mice. Total splenic lysate (2 mg) was used for immunoprecipitation and Western blot analysis with rabbit polyclonal anti-p110δ antibodies (see Materials and Methods).
Lack of an effect of p110δ deficiency on hematopoietic tissues.
In contrast to the widely distributed p110α and p110β proteins, p110δ is expressed primarily in circulating neutrophils, monocytes, and lymphocytes as well as in lymphoid tissues, including spleen, lymph nodes, and thymus (32). Therefore, we examined a number of hematopoietic parameters in the mutant mice. There were no significant differences between wild-type and mutant mice in the number of red cells (Table 1) or in hemoglobin levels or hematocrits (data not shown). Similarly, there were no significant effects of p110δ deficiency on the numbers and morphology of peripheral blood leukocytes, including neutrophils, eosinophils, basophils, monocytes, and lymphocytes (Table 1). The recovery of bone marrow cells was normal, and in colony assays of bone marrow progenitors, there were no significant alterations in the frequency and morphology of colonies in response to a variety of cytokines (Table 2).
TABLE 1.
Peripheral blood cell numbers in wild-type and p110δ-deficient mice (n = 11)a
| Mice | Total no. of white blood cells μl | Neutrophils (%) | Lymphocytes (%) | Monocytes (%) | Eosinophils (%) | Basophils (%) | Erythrocytes (106/μl) | Platelets (103/μl) |
|---|---|---|---|---|---|---|---|---|
| p110δ+/+ | 9,093 ± 2,849 | 23.7 ± 12.6 | 71.8 ± 12.6 | 3.7 ± 1.2 | 0.6 ± 0.3 | 0.2 ± 0.2 | 8.38 ± 0.78 | 1,245.8 ± 143.4 |
| p110δ−/− | 11,206 ± 2,729 | 23.8 ± 9.4 | 70.9 ± 9.3 | 4.2 ± 2.4 | 0.8 ± 0.5 | 0.3 ± 0.2 | 8.54 ± 0.64 | 1,589.2 ± 562.2 |
The phenotype was determined by flow cytometry. The numbers and percentages of each group of cells were determined for each mouse, and the mean value and standard deviation were calculated.
TABLE 2.
In vitro colony formation by bone marrow hematopoietic progenitors from wild-type and p110δ-deficient mice in response to various cytokines
| Mice | Colony formationa in response to:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Epo + IL-3 (BFU-E) | IL-3 (CFU-Mix) | IL-9 (CFU-Eos) | IL-7 (pre-B) | Tpo (CFU-Meg) | G-CSF (CFU-G) | GM-CSF (CFU-GM) | CSF-1 (CFU-M) | |
| p110δ+/+ | 3.3 ± 0.8 | 24 ± 10 | 12.2 ± 2.5 | 52 ± 11 | 2.6 ± 0.6 | 74 ± 15 | 347 ± 30 | 351 ± 63 |
| p110δ−/− | 2.6 ± 0.7 | 20 ± 11 | 7.3 ± 1.6 | 47 ± 13 | 2.7 ± 0.7 | 72 ± 8 | 361 ± 21 | 406 ± 39 |
Mean number of colonies ± standard deviation per 2 × 105 cells (BFU-E and CFU-Eos), 1.5 × 104 cells (CFU-Mix and CFU-GM), 5 × 104 cells (pre-B, CFU-G, and CFU-M), or 4 × 105 cells (CFU-Meg).
Defective B-cell development in p110δ−/− mice.
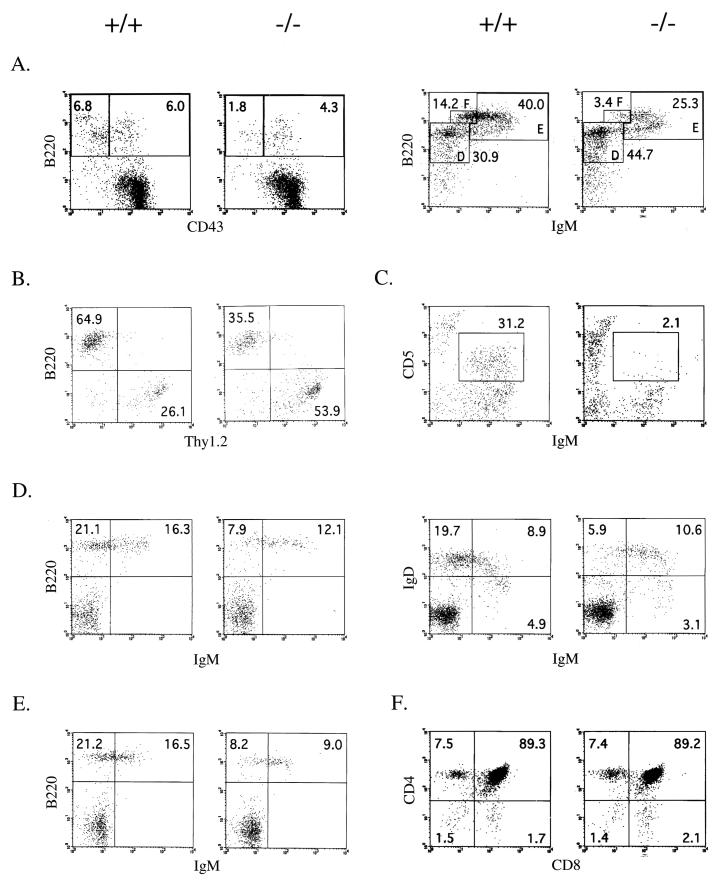
Analysis of B-cell populations indicated that the deficiency of p110δ had a significant effect on the numbers and the various subpopulations of differentiating B cells. Although the total numbers of bone marrow cells were comparable to those in wild-type mice (54.1 ± 5.3 [× 106] versus 54.3 ± 2.3 [× 106]; n = 8), there was an approximately 25% decrease in the percentage of bone marrow B-lineage cells (21.1 ± 5.2 versus 15.6 ± 5.8). Among the bone marrow B-cell subpopulations, the percentages of pro-B cells (CD43+ B220+) were similar for wild-type and p110δ-deficient bone marrow (Fig. 2A). However, the more mature B-cell subpopulation of B220+ CD43− cells was reduced (Fig. 2). Of the more mature populations, there were significant reductions in the percentages of both the B220+ IgM+ cells and the more mature B220++ IgMlow cells.
FIG. 2.
Flow cytometric analysis of B- and T-cell development in the primary and secondary lymphoid organs. Total cells isolated from 3- to 4-month-old mice were stained with the indicated antibodies and analyzed by FACS. The numbers in each region indicate the percentage of the total cells plotted. The data shown are representative of those from a minimum of six pairs of mice examined. (A) Early and late stages (left and right, respectively) of B-cell maturation in the bone marrow. Left panel, B220 versus CD43 profiles of IgM−-gated cells; right panels, B220 versus IgM profiles of CD43−-gated cells (fractions D, E, and F). (B) Analysis of splenic B and T cells, showing profile of B220 versus Thy1.2 gated lymphocytes. (C) Analysis of peritoneal B1a cells, displayed as the profile of IgM+ CD5+ cells within the lymphocyte gate. (D) Maturation stages of splenic B cells. Left panels, B220 versus IgM profiles of gated lymphocytes; right panels, IgD versus IgM profiles of gated lymphocytes. (E) Analysis of lymph node B cells, showing B220 IgM profiles of gated lymphocytes. (F) Maturation of thymocytes is unimpaired (CD4 versus CD8 profile of gated thymocytes).
Gross macroscopic examination of lymphoid organs revealed a consistent reduction in the sizes of spleens and lymph nodes isolated from p110δ-deficient animals compared to wild-type littermate controls. In preparations of splenocytes, the percentages of total B220+ lymphocytes was reduced from 42.3% ± 5.0% to 32.1% ± 11.4% (Table 3 and Fig. 2B). As shown in Fig. 2D, there was a marked reduction in the IgMlow IgDhigh population and in the most mature B220+ IgM− population. Similar to the case for the spleen populations, the percentage of B220+ lymphocytes in lymph nodes was reduced from 31.6% ± 4.5% to 19.0% ± 3.2% (n = 6) (Table 3).
TABLE 3.
Lymphocyte populations in wild-type and p110δ-deficient micea
| Mice | Spleen (n = 8)
|
Peritoneum (n = 8)
|
Lymph nodes (n = 6)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total cells (106) | Total B cells (%) (B220+) | IgM+ cells (%) | CD4+ T cells (%) | CD8+ T cells (%) | Total cells (106) | B-lineage cells (%) (IgM+) | B1a cells (%) (CD5+ IgM+) | Total cells (106) | Total B cells (%) (B220+) | CD4+ T cells (%) | CD8+ T cells (%) | |
| p110δ+/+ | 47.8 ± 43.0 | 42.3 ± 5.0 | 27.1 ± 4.6 | 19.1 ± 6.1 | 11.6 ± 1.1 | 3.56 ± 0.85 | 31.7 ± 6.2 | 14.0 ± 3.6 | 9.80 ± 3.59 | 31.6 ± 4.5 | 38.7 ± 10.2 | 25.5 ± 3.7 |
| p110δ−/− | 30.4 ± 11.5 | 32.1 ± 11.4 | 20.3 ± 7.3 | 21.7 ± 5.7 | 12.5 ± 3.3 | 1.11 ± 0.28 | 2.4 ± 0.6 | 0.4 ± 0.1 | 6.07 ± 2.14 | 19.0 ± 3.2 | 45.6 ± 4.4 | 27.7 ± 0.8 |
See Table 1, footnote a.
Both strains had comparable proportions of CD4+ and CD8+ peripheral T cells in their spleen and lymph node T-cell compartments (Table 3), and the T-cell subpopulations in the thymus were normal (Fig. 2F). Lastly, there was a complete absence of CD5+ B1 cells in peritoneal lymphocytes (Fig. 2C). Collectively, the phenotypic changes in the B-cell subpopulations are similar to those observed in mice in which the BCR signaling pathway is disrupted by deficiencies in Btk (15, 16), BLNK (21), protein kinase C (PKC) β (17), PLCγ2 (35), 85α (26), Vav-1/Vav-2 (11, 27), and CD19 (7, 23).
Decreased serum immunoglobulin and defective humoral response in p110δ−/− mice.
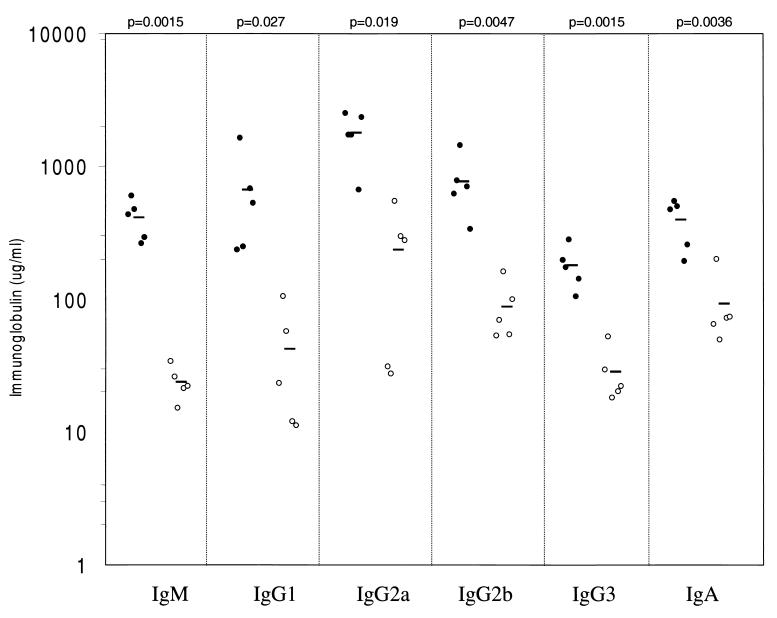
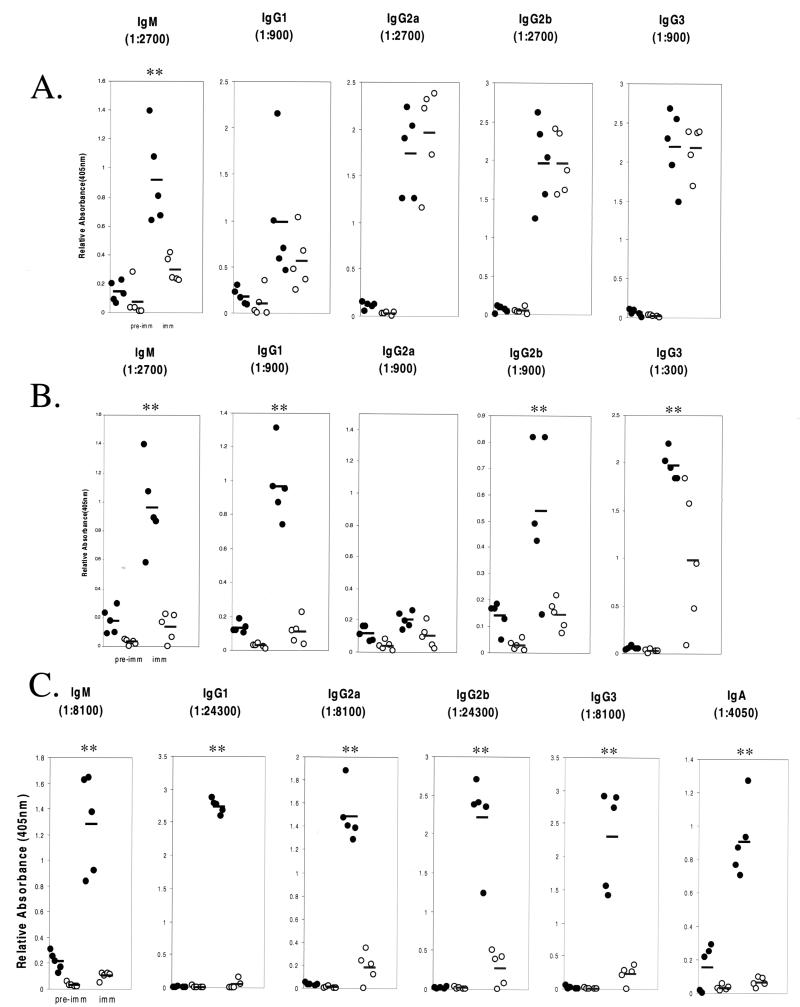
The reduction in the number of peripheral B cells in p110δ knockout mice suggested that B-cell function might be disrupted in the absence of p110δ. To further assess this, serum immunoglobulin levels were measured by ELISA. As illustrated in Fig. 3, unimmunized p110δ-deficient mice had lower levels of serum immunoglobulin of all isotypes examined, with IgM and IgG1 levels being the most dramatically lowered. The requirement for p110δ in the humoral response was examined by immunizing mice with T-independent and T-dependent antigens. Administration of TNP-LPS (a T-independent antigen) resulted in hapten-specific IgM and IgG1 responses in the p110δ-deficient mice that were 30 to 50% lower than those in wild-type mice (Fig. 4A). In contrast, the levels of hapten-specific IgG2a, IgG2b, and IgG3 in the two strains were equivalent. p110δ-deficient mice immunized with the type II antigen TNP-Ficoll had significantly decreased production of hapten-specific IgM, IgG1, IgG2b, and IgG3, whereas IgG2a responses were low in all mice (Fig. 4B). Lastly, p110δ−/− mice immunized with the T-cell-dependent antigen TNP-KLH failed to mount any detectable response (Fig. 4C).
FIG. 3.
Decreased serum immunoglobulin concentrations in p110δ−/− mice. Levels of immunoglobulin isotypes in serum in unimmunized wild type or p110δ-deficient mice were determined by ELISA. The values for each individual mouse tested are plotted on a logarithmic graph. Mean values are indicated by boldface lines. P values as determined by Student's t test are indicated.
FIG. 4.
Defective humoral response to thymus-independent antigens and lack of a humoral response to a thymus-dependent antigen in p110δ-deficient mice. Wild-type (filled circles) or p110δ-deficient (open circles) mice were immunized with either TNP-LPS, a TI-I antigen (A); TNP-Ficoll, a TI-II antigen (B); or TNP-KLH, a T-dependent antigen (C). The serum TNP-specific immunoglobulin isotype response was measured by ELISA. The relative absorbance values at 405 nm for the indicated dilution are plotted for each individual animal. Mean values are indicated by boldface lines. Statistically significant differences (P < 0.05) are indicated (**).
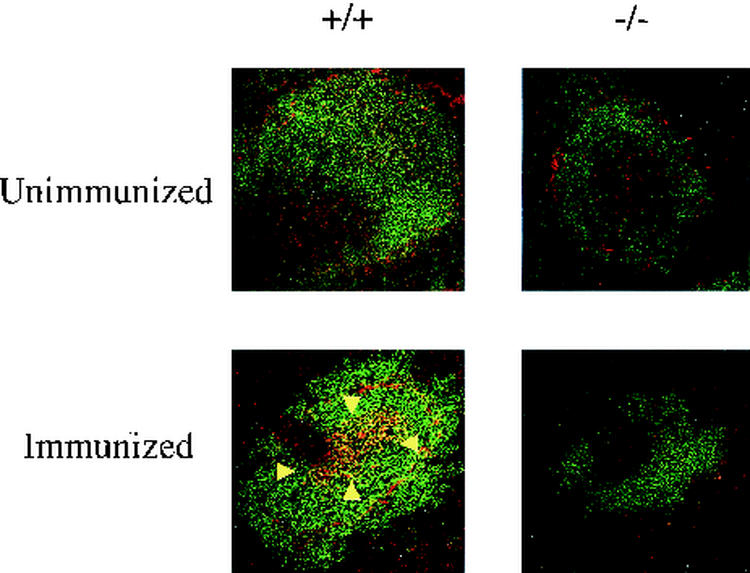
The lack of a response to TNP-KLH suggested that the deficiency of p110δ might also be associated with a defect in germinal center formation. As illustrated in Fig. 5, immunocytochemical staining of spleen cryosections demonstrated that whereas wild-type mice developed germinal centers, detected by PNA staining, mutant mice failed to generate germinal centers. To further confirm that p110δ is required for the process of germinal center formation, wild-type and mutant mice were immunized with sheep red blood cells. Ten days later, at the peak of germinal center formation, spleens were analyzed for germinal centers by PNA staining. In all p110δ−/− mice examined, there was a complete absence of germinal center formation (data not shown). In contrast to the effect of p110δ deficiency on peripheral B cells, we detected no difference between wild-type and mutant mice in the T-cell populations (Thy 1-positive cells) surrounding the splenic follicles (data not shown).
FIG. 5.
Germinal center formation in TNP-KLH-stimulated 4-month-old mice. Germinal centers (arrowheads) were stained with biotinylated PNA followed by streptavidin-tetramethyl rhodamine isocyanate (red). B cells were stained with FITC-B220 (green).
Impairment of in vitro B-cell proliferation with p110δ deficiency.
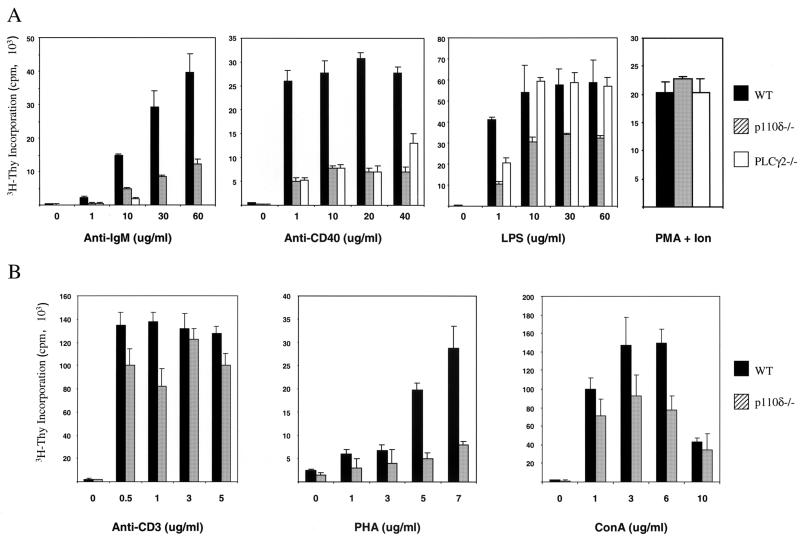
To establish whether the observed in vivo defects in p110δ deficient B cells were due to an intrinsic defect in signal transduction, in vitro B-cell proliferation was examined. The abilities of splenocytes to respond to various concentrations of known activators are shown in Fig. 6. As members of the PI3K family have been demonstrated to lie in the B-cell receptor signaling pathway upstream of PLCγ2, we compared the mitogenic response of p110δ-deficient B cells with that of B cells isolated from PLCγ2−/− mice (35). As illustrated, the proliferation of p110δ-deficient B cells in response to anti-IgM was markedly reduced. However, this reduction was not as severe as that associated with a deficiency of PLCγ2. There was also a decrease in the proliferative response of p110δ-deficient B cells relative to wild-type B cells after stimulation with anti-CD40, similar to the reductions observed with PLCγ2-deficient B cells. In addition, the response of p110δ−/− B cells to LPS was reduced relative to that in the wild type, while the response to phorbol ester and ionomycin was not significantly affected (Fig. 6). Curiously, the response of p110δ−/− B cells to CD40 plus IL-4 was unaffected (data not shown). The response of splenic T lymphocytes was also examined (Fig. 6B). The proliferative response to stimulation through the TCR complex with anti-CD3 was not significantly affected. There was some reduction in the response to ConA and a more striking reduction in the response of lymphocytes to PHA. It should be noted, however, that the response to PHA is only approximately 1/10 that seen in response to anti-CD3. Lastly, the response of T cells to PMA and ionomycin was not altered (Fig. 6), as was the response to stimulation with anti-CD3 plus anti-CD28, with or without addition of exogenous IL-2 (data not shown).
FIG. 6.
Impaired in vitro proliferative response of p110δ-deficient lymphocytes. (A). Purified splenic B cells from 3- to 4-month-old wild-type mice (WT), p110δ−/− mice, and PLCγ2−/− mice were incubated in medium alone or in medium containing the indicated concentrations of various stimuli. Proliferative responses are expressed as mean counts of triplicates from a single [3H]thymidine incorporation assay; error bars indicate standard deviations. Results are representative of those from four to six experiments. Ion, ionomycin. (B) Splenic T cells were incubated in medium alone or in medium containing the indicated concentrations of various stimuli. Proliferative responses are expressed as mean counts of triplicates from a single [3H]thymidine incorporation assay; error bars indicate standard deviations. Results are representative of those from four to six experiments.
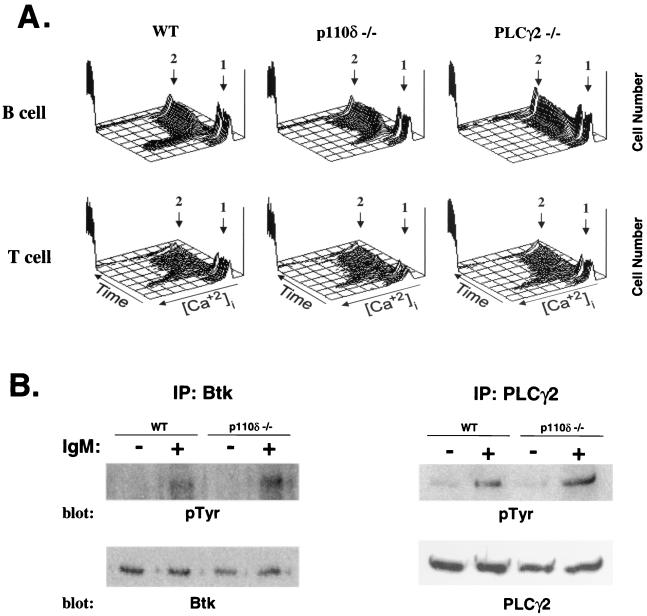
The induction of an intracellular calcium flux is an early signal transduction event that regulates many downstream cellular responses and is a sensitive marker for activation of PLCγ2 and its upstream activators (35). Therefore, we analyzed the ability of anti-IgM to induce an increase in intracellular Ca2+ in p110δ−/− B cells. In contrast to the absence of detectable Ca2+ mobilization in PLCγ2−/− B cells, p110δ−/− B cells induced an increase in intracellular Ca2+ that was approximately 25% of that observed in wild-type cells (Fig. 7A). Lastly, mobilization of intracellular Ca2+ upon anti-CD3 stimulation was unimpaired in p110δ−/− T cells (Fig. 7A).
FIG. 7.
Induction of Ca2+ mobilization and tyrosine phosphorylation in p110δ-deficient mice. (A) Induction of Ca2+ was measured by flow cytometry following stimulation of Indo-1-labeled splenic B cells with anti-IgM or of splenic T cells with anti-CD3 followed by anti-IgG. Arrow 1, time point of specific stimulation; arrow 2, time point of ionomycin addition. The results are representative of those from four independent experiments. WT, wild type. (B) Purified splenic B cells from wild-type and p110δ−/− mice were stimulated with anti-IgM (20 μg/ml) for 3 min and analyzed for the induction of tyrosine phosphorylation (pTyr) of Btk and PLCγ2. IP, immunoprecipitation.
A deficiency in p110δ could potentially affect the recruitment and activation of components of the BCR complex that utilize pleckstrin homology domains to be localized in the complex, including Btk and PLCγ2. We therefore assessed the recruitment of these proteins into the complex by examining their extent of tyrosine phosphorylation following receptor engagement. As illustrated in Fig. 7B, both Btk and PLCγ2 were inducibly tyrosine phosphorylated in p110δ-deficient cells at levels comparable to those in wild-type cells.
DISCUSSION
The results demonstrate the essential, nonredundant role that p110δ plays in BCR signal transduction. The remarkable specificity of function was unexpected, since p110δ is expressed in a variety of hematopoietic lineages. Indeed, our interest in p110δ arose from studies of cytokine signaling in myeloid-lineage cells. In particular, the cytoplasmic protein tyrosine kinase Jak2 is associated with many cytokine receptors (13), and with receptor engagement it is activated, resulting in phosphorylation of at least 10 major autophosphorylation sites (unpublished data). When one of these sites, Y966, was used to develop phosphopeptide affinity columns, it was found to bind a number of signal-transducing proteins, including a complex of p85α/p85β with p110δ. Importantly, in the myeloid cell line used, complexes of p85α/p85β with p110α or p110β were not detected, although both are expressed in these cells. The basis for this specificity is not known but could reside in the characteristics of p85α/p85β when bound to the Y966 phosphopeptide. In spite of the ability of Y966 to uniquely recruit the p85α/p110δ or p85β/p110δ complex, the absence of p110δ has no detectable affect on the responses of a number of cytokines that utilize Jak2, including IL-3 and Epo. The possibility exists that the recruitment of PI3K activity to these receptor complexes is not critical for their physiological function. Consistent with this, mutant mice that contain a truncated Epo receptor that lacks receptor domains required for optimal activation of PI3K activity (38) have normal erythropoiesis. Alternatively, in the absence of p110δ, p110α or p110β may replace the requirement for p110δ. It should be noted, however, that there was no obvious evidence for altered Epo signaling in mice that are deficient in p85α, and thus a different regulatory subunit would also be involved.
Perhaps one of the most striking features is the similarity of the effect of p110δ deficiency to those of deficiencies of a number of other genes on BCR signaling. First, the similarities seen with deficiencies of p85α and p110δ would indicate that p110δ uniquely associates with p85α in the context of the BCR. This is again surprising, since both p110α and p110β associate with p85α and have previously been shown to be present in B cells (32). Moreover, deficiency of p110δ does not detectably alter the levels of p110α or p110β in splenic lymphocytes (data not shown). The specificity may reside in the BCR complex and its ability to recruit specific p85α/p110 complexes. For example, CD19 is uniquely required for B-cell responses to mitogens, generation of phosphatidylinositol triphosphate, PLC activation, and Ca2+ mobilization following BCR engagement, and the deficiency of CD19 results in a very similar in vivo phenotype to that seen with a deficiency of p110δ (7, 23). Given the lack of a significant effect of p110δ deficiency on TCR signaling pathways (see below), it can by hypothesized that the lack of T-dependent antibody responses in p110δ−/− mice is directly related to impaired CD19 signaling. Interestingly, the properties of CD19-deficient B cells have led to the proposal that tyrosine phosphorylation of CD19 on Y484 and Y515 is uniquely required for activation of PI3K activity, which, in turn, is required for phosphoinositide hydrolysis and Ca2+ mobilization (3).
The deficiency in p110δ also has a consequence very similar to that of a deficiency in PLCγ2 (35). It should be noted, however, that the deficiency in PLCγ2 affects a number of receptors of the Fc family of receptors that are not detectably affected by the p110δ deficiency. Thus, only within the context of the BCR is p110δ required for PLCγ2 function. It is possible that in the context of other receptor complexes, which do not require CD19, the p85/p110 complexes may function redundantly or have different specificities. The results demonstrate that in the absence of p110δ, PLCγ2 is recruited to the receptor complex based on its induced tyrosine phosphorylation. However, functionally it is not fully activated, as evidenced by the greatly reduced Ca2+ mobilization. It can be proposed that the engagement of the pleckstrin homology domain of PLCγ2 may be required for its appropriate localization or activation. In this regard it should also be noted that a deficiency in the PIP3 phosphatase PTEN results in B-cell hyperactivity (6). In addition to potentially affecting PLCγ2 activity in the complex, p110δ may also affect Btk function. As shown here, Btk is recruited to the complex and is inducibly tyrosine phosphorylated in the absence of p110δ. However, the naturally occurring Xid mutation in mice involves a single amino acid change in the pleckstrin homology domain (22, 29) and creates a phenotype that is similar to that seen with Btk deficiency (15, 16, 31, 33) and deficiencies in other components of the complex.
Based on the above observations, it can be proposed that the p85α/p110δ is uniquely recruited to the BCR complex, most likely through a specificity involving CD19. Once recruited, PIP3 generation is required to form binding sites for both Btk and PLCγ2 that may stabilize the complex or contribute to the overall activation of the complex. The essential function of the complex is the hydrolysis of membrane phosphoinositides and the generation of IP3 and diacylglyerol. These mediators, in turn, are essential for Ca2+ mobilization and the activation of PKC β. Again, the critical role for PKC β is evident in the phenotype of PKC β-deficient mice, which have BCR defects identical to the deficiencies of the other components (17). The other possibility is that PI3K activity is required for the activation of Akt, with the major isoform being Akt1. However, mice deficient in neither Akt1 nor Akt2 have been reported to have B-cell defects comparable to those seen in PKC β-deficient mice (4, 5).
The other essential components of the BCR complex, based on the phenotypes of deficient mice, are the adapter protein Blnk (18, 21), the PI3K binding protein BCAP (37), and members of the Vav family of GTP exchange factors. The adapter protein Blnk is critical for the recruitment and activation of PLCγ2 through direct interaction of the SH2 domains of PLCγ2 with tyrosine-phosphorylated Blnk, and Blnk deficiency results in the elimination of the Ca2+ signal in BCR signaling. A deficiency in BCAP also creates a very similar phenotype and, biochemically, is also associated with loss of PLCγ2 function but retention of the recruitment and tyrosine phosphorylation of PLCγ2 in the BCR complex. Based on these observations it can be postulated that BCAP is required to recruit, activate, and/or stabilize the p85/p110δ complex in the context of the BCR signalsome. In the case of Vav, a deficiency of Vav-1 has a severe consequence for T-cell development and a lesser consequence for B-cell development (8, 39). However, the absence of both Vav-1 and Vav-2 dramatically affects BCR signaling and results in a phenotype comparable to that seen in p110δ-deficient mice (27). Importantly, the absence of Vav-1 and Vav-2 eliminates the Ca2+ flux induced by BCR engagement, and the activation of Vav-1 and Vav-2 has been proposed to require binding of PIP3 by their pleckstrin homology domains. The precise nature by which Vav-1 and Vav-2 contribute to the BCR complex signaling is not known.
In contrast to the case for the BCR complex, the deficiency in p110δ did not affect signaling through aggregation of the TCR complex with anti-CD3 either in terms of a mitogenic response or in the induction of a Ca2+ flux. However, there was a reduction in the much weaker responses of T cells to PHA and ConA, the significance of which is not known. Although the absolute numbers of splenic and lymph node T cells are slightly reduced, it is likely that this reduction is related to the overall reduced size of the spleen and lymph nodes. In addition to there being no differences in peripheral blood lymphocyte numbers between wild-type and p110δ-deficient mice, no significant differences in thymic size or cellularity was observed. Importantly, many of the components of the BCR complex are distinct from those of the TCR complex, although often family members are. For example, PLCγ2 is not required for a Ca2+ flux following TCR aggregation, although PLCγ1 likely is essential. Similarly, the TCR complex requires Rlk and Itk (24) and not Btk. As noted above, it has been suggested that Vav-1 is critical to TCR signaling, while Vav-1 and Vav-2 redundantly are required for BCR signaling. In the TCR complex the adapter protein Blnk is replaced by the family member SLP-76. Further downstream, the critical role of PKC β in B-cell responses is replaced by PKC θ in T cells (25). Thus, although the ultimate function of the receptor complex might be comparable, the functional components of the complex are different and are likely to have evolved to function synergistically.
Hypogammaglobulenima in humans is associated with mutations in the BCR complex components Btk and BLNK. It might be anticipated that other components, identified through gene disruptions in mice, would contribute to those cases in which an etiology is not known. Recent studies (34), however, failed to identify causative mutations in PLCγ2 in 32 such cases. It should be noted, however, that the deficiency of PLCγ2 in mice affects many receptors of the immunoglobulin superfamily, including the collagen receptor complex on platelets, and therefore a spectrum of phenotypic changes would be expected in individuals with a deficiency in PLCγ2. In contrast, as described here, p110δ deficiency results in a very selective defect in B cells and consequently is a potential candidate for hypogammaglobulenima in humans.
Following submission of this paper, an article appeared which described the properties of a mouse strain in which homologous recombination was used to create a dominant negative form of p110γ (20). Similar to the properties of mice in which a null mutation exists, signaling through the BCR complex was affected, although the degree of loss of the calcium response was less. This may due to a role for p110δ in forming a stable BCR complex in addition to its enzymatic role, possibly through its interaction with BCAP. A significant difference existed, however, in the conclusions regarding T-cell responses. As noted above, we have not detected any consistent differences in the response of T cells to TCR engagement in numerous experiments. In mice expressing the dominant negative form of p110δ, the responses to anti-CD3 were reduced 30 to 50% while the responses to anti-CD3 plus anti-CD28 were either not decreased or increased. It was concluded that the reduction in the T-cell responses was responsible for the dramatic reduction in T-dependent antibody responses, a phenotype also seen in our null mutants. As noted above, however, the absence of CD19 also results in a loss of T-dependent responses, and its role in the BCR complex might equally explain the loss of T-dependent antibody responses, independent of a functional role for p110δ in T-cell responses. Lastly, we have not observed an inflammatory bowel disease in mice containing a null mutation similar to that described for mutant mice containing the dominant negative mutation. This difference could potentially be due to differences in animal housing conditions coupled with the B-cell defects. Alternatively, the dominant negative protein may create a phenotype in other lineages of cells.
Acknowledgments
We acknowledge Ken Sato, Jean-Christophe Marine, Evan Parganas, Catriona McKay, Richard Moriggl, Mei-Hwei Chang, and Kai-Hsin Lin for valuable discussions. We also thank John Raucci for the injection of ES cells into blastocysts and the staff of the Animal Resources facility for help in animal maintenance. The FACS sorting and Ca2+ flux studies were performed by Richard Ashmun and Mahnaz Paktinat, and general technical support was provided by Linda Synder and Kristen Rothammer. The ES cells were kindly provided by Jan van Deursen.
S.-T.J was supported by the National Taiwan University Hospital and Taiwan National Science Council (grants NSC89-2314-B-002-399 and NSC90-2314-B-002-181). This work is supported by Cancer Center CORE grant CA21765, by grant RO1 DK42932 to J.N.I., by grant PO1 HL53749, and by the American Lebanese Syrian Associated Charities (ALSAC).
REFERENCES
- 1.Bi, L., I. Okabe, D. J. Bernard, and R. L. Nussbaum. 2002. Early embryonic lethality in mice deficient in the p110beta catalytic subunit of PI 3-kinase. Mamm. Genome 13:169-172. [DOI] [PubMed] [Google Scholar]
- 2.Bi, L., I. Okabe, D. J. Bernard, A. Wynshaw-Boris, and R. L. Nussbaum. 1999. Proliferative defect and embryonic lethality in mice homozygous for a deletion in the p110alpha subunit of phosphoinositide 3-kinase. J. Biol. Chem. 274:10963-10968. [DOI] [PubMed] [Google Scholar]
- 3.Buhl, A. M., C. M. Pleiman, R. C. Rickert, and J. C. Cambier. 1997. Qualitative regulation of B cell antigen receptor signaling by CD19: selective requirement for PI3-kinase activation, inositol-1,4,5-trisphosphate production and Ca2+ mobilization. J. Exp. Med. 186:1897-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, W. S., P. Z. Xu, K. Gottlob, M. L. Chen, K. Sokol, T. Shiyanova, I. Roninson, W. Weng, R. Suzuki, K. Tobe, T. Kadowaki, and N. Hay. 2001. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 15:2203-2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho, H., J. Mu, J. K. Kim, J. L. Thorvaldsen, Q. Chu, E. B. Crenshaw III, K. H. Kaestner, M. S. Bartolomei, G. I. Shulman, and M. J. Birnbaum. 2001. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta). Science 292:1728-1731. [DOI] [PubMed] [Google Scholar]
- 6.Di Cristofano, A., P. Kotsi, Y. F. Peng, C. Cordon-Cardo, K. B. Elkon, and P. P. Pandolfi. 1999. Impaired Fas response and autoimmunity in Pten+/− mice. Science 285:2122-2125. [DOI] [PubMed] [Google Scholar]
- 7.Engel, P., L. J. Zhou, D. C. Ord, S. Sato, B. Koller, and T. F. Tedder. 1995. Abnormal B lymphocyte development, activation, and differentiation in mice that lack or overexpress the CD19 signal transduction molecule. Immunity 3:39-50. [DOI] [PubMed] [Google Scholar]
- 8.Fischer, K.-D., A. Zmuidzinas, S. Gardner, M. Barbacid, A. Bernstein, and C. Guidos. 1995. Defective T-cell receptor signalling and positive selection of Vav-deficient CD4+CD8+ thymoctyes. Nature 374:474-477. [DOI] [PubMed] [Google Scholar]
- 9.Fruman, D. A., and L. C. Cantley. 2002. Phosphoinositide 3-kinase in immunological systems. Semin. Immunol. 14:7-18. [DOI] [PubMed] [Google Scholar]
- 10.Fruman, D. A., R. E. Meyers, and L. C. Cantley. 1998. Phosphoinositide kinases. Annu. Rev. Biochem. 67:481-507. [DOI] [PubMed] [Google Scholar]
- 11.Fruman, D. A., S. B. Snapper, C. M. Yballe, L. Davidson, J. Y. Yu, F. W. Alt, and L. C. Cantley. 1999. Impaired B cell development and proliferation in absence of phosphoinositide 3-kinase p85alpha. Science 283:393-397. [DOI] [PubMed] [Google Scholar]
- 12.Hirsch, E., V. L. Katanaev, C. Garlanda, O. Azzolino, L. Pirola, L. Silengo, S. Sozzani, A. Mantovani, F. Altruda, and M. P. Wymann. 2000. Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science 287:1049-1053. [DOI] [PubMed] [Google Scholar]
- 13.Ihle, J. N. 1994. The Janus kinase family and signaling through members of the cytokine receptor superfamily. Proc. Soc. Exp. Biol. Med. 206:268-272. [DOI] [PubMed] [Google Scholar]
- 14.Ihle, J. N. 1996. Janus kinases in cytokine signalling. Philos. Trans. R. Soc. London B 351:159-166. [DOI] [PubMed] [Google Scholar]
- 15.Kerner, J. D., M. W. Appleby, R. N. Mohr, S. Chien, D. J. Rawlings, C. R. Maliszewski, O. N. Witte, and R. M. Perlmutter. 1995. Impaired expansion of mouse B cell progenitors lacking Btk. Immunity 3:301-312. [DOI] [PubMed] [Google Scholar]
- 16.Khan, W. N., F. W. Alt, R. M. Gerstein, B. A. Malynn, I. Larsson, G. Rathbun, L. Davidson, S. Muller, A. B. Kantor, and L. A. Herzenberg. 1995. Defective B cell development and function in Btk-deficient mice. Immunity 3:283-299. [DOI] [PubMed] [Google Scholar]
- 17.Leitges, M., C. Schmedt, R. Guinamard, J. Davoust, S. Schaal, S. Stabel, and A. Tarakhovsky. 1996. Immunodeficiency in protein kinase cbeta-deficient mice. Science 273:788-791. [DOI] [PubMed] [Google Scholar]
- 18.Minegishi, Y., J. Rohrer, E. Coustan-Smith, H. M. Lederman, R. Pappu, D. Campana, A. C. Chan, and M. E. Conley. 1999. An essential role for BLNK in human B cell development. Science 286:1954-1957. [DOI] [PubMed] [Google Scholar]
- 19.Moriggl, R., D. J. Topham, S. Teglund, V. Sexl, C. McKay, D. Wang, A. Hoffmeyer, J. van Deursen, M. Y. Sangster, K. D. Bunting, G. C. Grosveld, and J. N. Ihle. 1999. Stat5 is required for IL-2 induced cell cycle progression of peripheral T cells. Immunity 10:249-259. [DOI] [PubMed] [Google Scholar]
- 20.Okkenhaug, K., A. Bilancio, G. Farjot, H. Priddle, S. Sancho, E. Peskett, W. Pearce, S. E. Meek, A. Salpekar, M. D. Waterfield, A. J. Smith, and B. Vanhaesebroeck. 2002. Impaired B and T cell antigen receptor signaling in p110delta PI 3-kinase mutant mice. Science 297:1031-1034. [DOI] [PubMed] [Google Scholar]
- 21.Pappu, R., A. M. Cheng, B. Li, Q. Gong, C. Chiu, N. Griffin, M. White, B. P. Sleckman, and A. C. Chan. 1999. Requirement for B cell linker protein (BLNK) in B cell development. Science 286:1949-1954. [DOI] [PubMed] [Google Scholar]
- 22.Rawlings, D. J., D. C. Saffran, S. Tsukada, D. A. Largaespada, J. C. Grimaldi, L. Cohen, R. N. Mohr, J. F. Bazan, M. Howard, N. G. Copeland, N. A. Jenkins, and O. N. Witte. 1993. Mutation of unique region of Bruton's tyrosine kinase in immunodeficient XID mice. Science 261:358-361. [DOI] [PubMed] [Google Scholar]
- 23.Rickert, R. C., K. Rajewsky, and J. Roes. 1995. Impairment of T-cell-dependent B-cell responses and B-1 cell development in CD19-deficient mice. Nature 376:352-355. [DOI] [PubMed] [Google Scholar]
- 24.Schaeffer, E. M., J. Debnath, G. Yap, D. McVicar, X. C. Liao, D. R. Littman, A. Sher, H. E. Varmus, M. J. Lenardo, and P. L. Schwartzberg. 1999. Requirement for Tec kinases Rlk and Itk in T cell receptor signaling and immunity. Science 284:638-641. [DOI] [PubMed] [Google Scholar]
- 25.Sun, Z., C. W. Arendt, W. Ellmeier, E. M. Schaeffer, M. J. Sunshine, L. Gandhi, J. Annes, D. Petrzilka, A. Kupfer, P. L. Schwartzberg, and D. R. Littman. 2000. PKC-theta is required for TCR-induced NF-kappaB activation in mature but not immature T lymphocytes. Nature 404:402-407. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki, H., Y. Terauchi, M. Fujiwara, S. Aizawa, Y. Yazaki, T. Kadowaki, and S. Koyasu. 1999. Xid-like immunodeficiency in mice with disruption of the p85alpha subunit of phosphoinositide 3-kinase. Science 283:390-392. [DOI] [PubMed] [Google Scholar]
- 27.Tedford, K., L. Nitschke, I. Girkontaite, A. Charlesworth, G. Chan, V. Sakk, M. Barbacid, and K. D. Fischer. 2001. Compensation between Vav-1 and Vav-2 in B cell development and antigen receptor signaling. Nat. Immunol. 2:548-555. [DOI] [PubMed] [Google Scholar]
- 28.Teglund, S., C. McKay, E. Schuetz, J. van Deursen, D. Stravopodis, D. Wang, M. Brown, S. Bodner, G. Grosveld, and J. N. Ihle. 1998. Stat5a and Stat5b proteins have essential and non-essential, or redundant, roles in cytokine responses. Cell 93:841-850. [DOI] [PubMed] [Google Scholar]
- 29.Thomas, J. D., P. Sideras, C. I. Smith, I. Vorechovsky, V. Chapman, and W. E. Paul. 1993. Colocalization of X-linked agammaglobulinemia and X-linked immunodeficiency genes. Science 261:355-358. [DOI] [PubMed] [Google Scholar]
- 30.Toker, A., and L. Cantley. 1997. Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature 387:673-676. [DOI] [PubMed] [Google Scholar]
- 31.Tsukada, S., D. C. Saffran, D. J. Rawlings, O. Parolini, R. C. Allen, I. Klisak, H. Kubagawa, T. Mohandas, S. Quan, J. W. Belmont, M. D. Cooper, M. E. Conley, and O. N. Witte. 1993. Deficient expression of a B-cell cytoplasmic tyrosine kinase in human X-linked agammaglobulinemia. Cell 72:279-290. [DOI] [PubMed] [Google Scholar]
- 32.Vanhaesebroeck, B., M. J. Welham, K. Kotani, R. Stein, P. H. Warne, M. J. Zvelebil, K. Higashi, S. Volinia, J. Downward, and M. D. Waterfield. 1997. P110delta, a novel phosphoinositide 3-kinase in leukocytes. Proc. Natl. Acad. Sci. USA 94:4330-4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vetrie, D., I. Vorechovsky, P. Sideras, J. Holland, A. Davies, F. Flinter, L. Hammarstrom, C. Kinnon, R. Levinsky, M. Bobrow, C. I. E. Smith, and D. R. Bentley. 1993. The gene involved in X-linked agammaglobulinaemia is a member of the src family of protein-tyrosine kinases. Nature 361:226-233. (Erratum, 364:362.) [DOI] [PubMed] [Google Scholar]
- 34.Wang, D., E. C. Boylin, Y. Minegishi, R. Wen, C. I. Smith, J. N. Ihle, and M. E. Conley. 2001. Variations in the human phospholipase Cgamma2 gene in patients with B-cell defects of unknown etiology. Immunogenetics 53:550-556. [DOI] [PubMed] [Google Scholar]
- 35.Wang, D., J. Feng, R. Wen, J. C. Marine, M. Y. Sangster, E. Parganas, A. Hoffmeyer, C. W. Jackson, J. L. Cleveland, P. J. Murray, and J. N. Ihle. 2000. Phospholipase Cgamma2 is essential in the functions of B cell and several Fc receptors. Immunity 13:25-35. [DOI] [PubMed] [Google Scholar]
- 36.Wang, D., D. Stravopodis, S. Teglund, J. Kitazawa, and J. N. Ihle. 1996. Naturally occurring dominant negative variants of Stat5. Mol. Cell. Biol. 16:6141-6148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamazaki, T., K. Takeda, K. Gotoh, H. Takeshima, S. Akira, and T. Kurosaki. 2002. Essential immunoregulatory role for BCAP in B cell development and function. J. Exp. Med. 195:535-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zang, H., K. Sato, H. Nakajima, C. McKay, P. A. Ney, and J. N. Ihle. 2001. The distal region and receptor tyrosines of the Epo receptor are non-essential for in vivo erythropoiesis. EMBO J. 20:3156-3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang, R., F. W. Alt, L. Davidson, S. H. Orkin, and W. Swat. 1995. Defective signalling through the T- and B-cell antigen receptors in lymphoid cells lacking the vav proto-oncogene. Nature 374:470-473. [DOI] [PubMed] [Google Scholar]