Abstract
The “protein only” hypothesis states that the infectious agent causing transmissible spongiform encephalopathies is a conformational isomer of PrP, a host protein predominantly expressed in brain, and is strongly supported by many lines of evidence. Prion diseases are so far unique among conformational diseases in that they are transmissible, not only experimentally but also by natural routes, mainly by ingestion. A striking feature of prions is their extraordinary resistance to conventional sterilization procedures, and their capacity to bind to surfaces of metal and plastic without losing infectivity. This property, first observed in a clinical setting, is now being investigated in experimental settings, both in animals and in cell culture.
Transmissible spongiform encephalopathies (TSEs), or prion diseases, are degenerative disorders of the central nervous system leading to motor dysfunction, dementia, and death. Prion diseases include scrapie of sheep, bovine spongiform encephalopathy (BSE) in cattle, and human diseases such as Creutzfeldt-Jakob disease (CJD), Gerstmann-Sträussler-Scheinker syndrome (GSS) and fatal familial insomnia (FFI). More recently, variant CJD (vCJD), ascribed to consumption of BSE-contaminated products (1), has claimed over 120 victims. Neither humoral nor cellular immunological responses have been detected in prion diseases.
Transmissibility of scrapie was first demonstrated in 1939 (2). The remarkable resistance of the causative agent, later designated prion, was revealed early on when 10% of a flock of Scottish sheep came down with scrapie after having been injected with a vaccine against looping ill prepared from formaldehyde-treated sheep brain extract (3). The agent's unusual resistance to UV irradiation suggested that it might be devoid of nucleic acid (4). The “protein only” hypothesis (5) in its updated version (6) proposes that the prion is a conformational isoform of the normal host protein PrPC (7, 8), which is found predominantly on the outer surface of neurons, attached by a glycosylphosphatidylinositol (GPI) anchor. The abnormal conformer, when introduced into the organism, is thought to cause the conversion of PrPC into a likeness of itself.
In prion disease, a largely protease-resistant, aggregated form of PrP designated PrPSc, accumulates, mainly in brain. It is believed to be the principal or only constituent of the prion (6). No differences in the primary structure of PrPC and PrPSc were detected, suggesting that they differ in their conformation (9). The tertiary structure of PrPC has been elucidated (10), whereas that of PrPSc has not; however, the β-sheet content of PrPSc was shown to be high whereas that of PrPC is low (11, 12). The conclusion that some form of PrP is the essential, perhaps only, constituent of the infectious agent is based on compelling biochemical and genetic evidence (13, 14). The finding that PrP knockout (Prnpo/o) mice are completely protected against scrapie disease and fail to propagate prions (15, 16) and that introduction of murine Prnp transgenes into these mice restores susceptibility to prions (17) is one of the main supports for the “protein only” hypothesis.
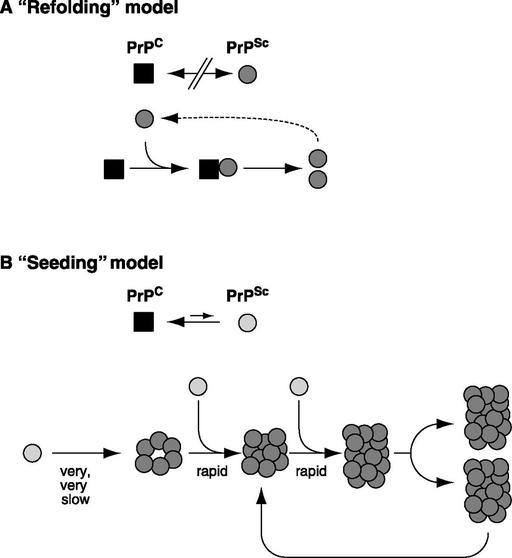
Within the framework of the “protein only” hypothesis, the “refolding model” (Fig. 1A) proposes that PrPC unfolds to some extent and refolds under the influence of a PrPSc molecule and that the two states are separated by an activation energy barrier (18). The “nucleation model” (Fig. 1B) postulates that PrPC is in equilibrium with PrPSc (or a precursor thereof), that the equilibrium is largely in favor of PrPC, and that PrPSc is stable only when it forms a multimer. Once such a multimer or seed is present, monomer addition ensues rapidly (19). “Breakage” of aggregates must be postulated to explain the exponential increase of PrPSc during infection (20). Conversion in vitro of PrPC to a PrPSc-like product has been achieved by incubating 35S-labeled PrPC with PrPSc and demonstrating the appearance of a protease-resistant radioactive product with the mobility of protease-treated authentic PrPSc (21). This in vitro conversion exhibits the species specificity (22) and strain-specificity (23) observed in vivo. However, because the yield is less than stoichiometric with regard to the PrPSc used as seed, it has not been possible to determine whether or not there was an increase in infectivity. Perhaps the “cyclic amplification” procedure reported recently will lead to this goal (24). Although it has been possible to convert recombinant PrPC into a β-sheet-rich, partially protease-resistant structure by physico-chemical procedures (25, 26), there have so far been no reports that such material gives rise to transmissible prion disease (27–29). Also, it has so far not been possible to renature completely denatured prion preparation to an infectious state (30, 31) although the infectivity of partially inactivated material can be increased by renaturation under certain conditions (32, 33). Prusiner and his colleagues have reported that intracerebral injection of a synthetic 55-residue peptide corresponding to region 89–143 of mouse PrP with a P101L substitution can induce neurological, prion-like disease, however this is achieved only in transgenic mice expressing PrP with the same mutation (29). The caveats here are that these transgenic mice show spontaneous disease even without inoculation, albeit only much later, and that transmissibility has yet to be demonstrated.
Figure 1.
Models for the conformational conversion of PrPC to PrPSc. (A) The “refolding” model. The conformational change is kinetically controlled, a high activation energy barrier preventing spontaneous conversion at detectable rates. Interaction with exogenously introduced PrPSc causes PrPC to undergo an induced conformational change to yield PrPSc. This reaction could be facilitated by an enzyme or chaperone. In the case of certain mutations in PrPC, spontaneous conversion to PrPSc may occur as a rare event, explaining why familial CJD or GSS arise spontaneously, albeit late in life. Sporadic CJD may come about when an extremely rare event (occurring in about one in a million individuals per year) leads to spontaneous conversion of PrPC to PrPSc. (B) The “seeding” model. PrPC and PrPSc (or a PrPSc-like molecule, light) are in equilibrium, with PrPC strongly favored. PrPSc is stabilized only when it adds onto a crystal-like seed or aggregate of PrPSc (dark). Seed formation is rare; however, once a seed is present, monomer addition ensues rapidly. To explain exponential conversion rates, aggregates must be continuously fragmented, generating increasing surfaces for accretion.
“Natural” Transmission of Prions
Although prion diseases are not contagious in the strict sense, i.e., by direct contact, they are transmissible perorally and parenterally. The BSE epidemic that emerged in the mid-eighties and led to about 180,000 clinically diagnosed cases (and likely to many times more nondiagnosed ones) was fueled by the feeding of BSE-prion-contaminated bone-and-meat meal to cattle (34). The kuru epidemic that developed in the first half of the 20th century in Papua New Guinea was caused by ritualistic cannibalism (35) and is believed to have originated from a case of sporadic CJD. Variant CJD is thought to come about by ingestion of BSE-prion-contaminated foodstuff, and certainly mice (36), sheep (37), calves (38), and non-human primates (39, 40) can be experimentally infected with the BSE agent by the oral route. It appears quite likely that sheep scrapie spreads by ingestion of the infectious agent, although the source has not been established; infected placenta has been suggested (41), but scrapie-prion-contaminated feces are a likely possibility that merits investigation. Perhaps the appearance of vCJD in predominantly young individuals is due to infection by contaminated foodstuff through wounds resulting from teething and tooth loss between early infancy and adolescence. Experimental transmission by the dental route has been shown in hamster (42).
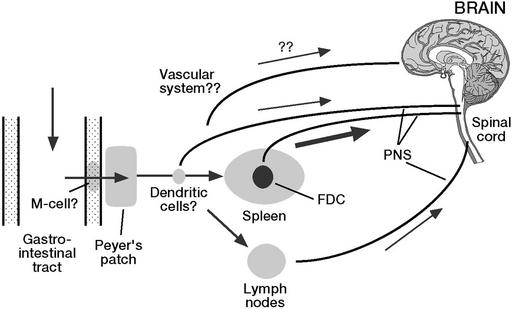
Somehow prions make their way from the digestive tract to the central nervous system (Fig. 2). The relative resistance of prion infectivity to protease digestion (43) probably allows a significant proportion of the infectious agent to survive passage through the digestive tract (36). It is not clear how prions pass through the intestinal mucosa. M cells, which are portals for antigens and pathogens (44–46), are able to mediate transport of prions, at least in an experimental setting (47). Thus, after oral uptake, the infectious agent may penetrate the mucosa through M cells and reach Peyer's patches, where they are found early on (36) as well as the enteric nervous system (48). Depending on the host, other tissues of the lymphoreticular system (LRS), in particular the spleen but also lymph nodes (49), are sites in which prions replicate and accumulate; this result is the case in sheep scrapie, experimental BSE in sheep, vCJD in man, and experimental mouse scrapie, but not BSE in cattle (50). Recent reports suggest that myeloid dendritic cells mediate transport within the lymphoreticular system (51, 52). Interestingly, mature B cells (with or without PrPC expression) are required for amplification of prions in spleen (53), not, however, because they themselves harbor or multiply prions (54), but because they are required for the maturation of follicular dendritic cells, the cells in which prion amplification and PrPSc accumulation occur (55, 56). Nonetheless, neuroinvasion is possible even in the absence of follicular dendritic cells, suggesting that other cell types in the periphery also can amplify prions (49, 57). From the LRS and likely from other sites, prions proceed along the peripheral nervous system to finally reach the brain, either directly via the vagus nerve (58) or via the spinal cord, under involvement of the sympathetic nervous system (59). If a sufficiently high dose of prions is administered i.p., neuroinvasion can occur without participation of the LRS (60). Although prions have not been detected in muscle of scrapie or BSE-infected animals, infectivity has been found in some, but not all skeletal muscles of mice experimentally infected with ME7 or RML prions (61).
Figure 2.
Possible routes of propagation of ingested prions. After oral uptake, prions may penetrate the intestinal mucosa through M cells and reach Peyer's patches as well as the enteric nervous system. Depending on the host, prions may replicate and accumulate in spleen and lymph nodes. Myeloid dendritic cells are thought to mediate transport within the lymphoreticular system. From the lymphoreticular system and likely from other sites, prions proceed along the peripheral nervous system to finally reach the brain, either directly via the vagus nerve or via the spinal cord, under involvement of the sympathetic nervous system.
Not only the biosynthesis of prions, but also their spread depends on PrP-containing cells. This result was demonstrated by the finding that a PrP-expressing neuroectodermal graft in the brain of a Prnpo/o mouse could be infected by intracerebral injection of mouse prions but not by intraocular (62) or i.p. inoculation (63). Even after irradiation and reconstitution with a PrP-expressing lymphohemopoietic system, prions failed to reach the graft after i.p. or i.v. inoculation, showing that neuroinvasion, at least in the mouse, was not mediated by prion transport through the circulation (63) and underlining the requirement of an interposed PrP-expressing compartment, later shown to be the peripheral nervous system (60). In the case of experimental mouse scrapie, prion infectivity could not be detected in leukocytes (64), nor was infectivity detected in the blood of BSE-infected cattle (50) or scrapie-infected sheep (65). However, a low but reproducible titer of prions was detected in blood of scrapie-infected hamsters (66). Also, 1 of 19 sheep transfused with blood from experimentally, orally BSE-infected sheep came down with prion disease (67). The level of prions in blood, which in all cases examined appears to be low or undetectable by the mostly not very sensitive methods used, may vary in different species and/or with different prion strains.
Iatrogenic Transmission of Prions
Almost 300 cases of involuntary transmission of CJD by medical interventions have been reported (68). Most cases are due to injection of cadaveric human growth hormone or transplantation of dura mater; however, a few incidents associated with cornea transplantation have been reported. Four instances of CJD after neurosurgical intervention have been attributed to surgical instruments that had previously been used on CJD patients (69); however, causality was proven only in one case. An electrode that had been inserted into the cortex of an unrecognized CJD patient was subjected to a decontamination procedure involving treatment with benzene, 70% ethanol, and formaldehyde vapor. It was then used in succession on two young patients and cleaned as above after each use. Within 2 yr, both patients came down with CJD. After these events, the tip of the electrode was implanted into the brain of a chimpanzee where it too caused lethal spongiform encephalopathy, proving that the electrode had retained infectious prions over several years and despite repeated attempts at sterilization (70, 71).
Experimental Transmission of Surface-Bound Prions
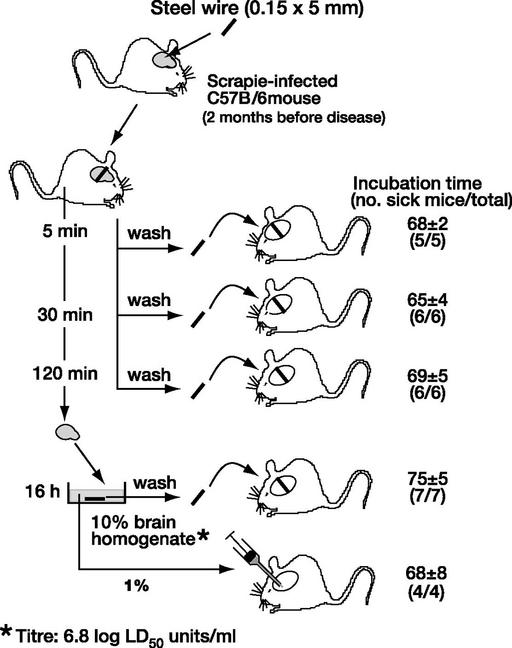
The electrode described above had a complex structure: a steel shaft of about 6 mm diameter, with multiple silver contacts separated by rings of insulating plastic allowing for the existence of crevices into which infectious material might have penetrated. To clarify whether prions would bind to a homogeneous surface, we used fine stainless steel wires as model for a surgical instrument. In a first experiment, wires were incubated overnight with brain homogenate from a terminally sick, murine-scrapie-infected mouse, washed exhaustively with PBS, and permanently implanted into brains of indicator mice. This procedure resulted in scrapie disease within about 70 days, an incubation time only slightly longer than that obtained by injecting 30 μl of 1% brain homogenate (72). To mimic more closely real-life conditions, stainless steel wires were inserted directly into the brains of scrapie-infected, clinically still healthy mice for various periods of time, washed exhaustively, and assayed by permanent insertion into brains of indicator mice. Surprisingly, 5 min of contact sufficed for the wire to acquire a maximum load of infectivity, equivalent to the injection of 30 μl of 1% homogenate of the same brain (Fig. 3). A second important question regards the length of time an infectious wire must remain in contact with brain tissue to initiate disease. Rather than leave the infectious wires permanently in the indicator mouse, they were inserted transiently, for 30 or 120 min, to mimic the conditions that might obtain during a surgical operation. As shown in Table 1, a contact time of 30 min was sufficient to elicit disease, albeit with lower efficiency than was obtained after permanent insertion, as evidenced by the longer incubation time. The wires that had been inserted transiently into indicator mice remained fully infectious when introduced permanently into a further set of indicator mice (Table 1; ref. 73), reflecting the persistence of infectivity, as in the incident with the intracerebral electrode described above.
Figure 3.
Transmission of mouse scrapie prions by stainless steel wire. Stainless steel wires were inserted into the brains of scrapie-infected mice for 5, 30, or 120 min, washed exhaustively, and introduced permanently into brains of indicator mice. Five minutes of contact sufficed for the wire to acquire a maximum load of infectivity, equivalent to the injection of 30 μl of 1% homogenate of the same brain. Data from ref. 73.
Table 1.
Transient insertion of infectious wires into brains of indicator mice
| Inoculation | Sick/ total | Incubation time ± SD, days |
|---|---|---|
| Wires infected by exposure to scrapie brain | ||
| Transient insertion into indicator mice | ||
| 30 min | 4/4* | 94 ± 10 |
| 120 min | 2/2† | 87 ± 113 |
| Permanent insertion into indicator mice | ||
| Wires not previously inserted | 3/3 | 71 ± 2 |
| Wires after transient insertion for: | ||
| 30 min | 4/4 | 71 ± 3 |
| 120 min | 5/5 | 68 ± 1 |
| Controls | ||
| Wires exposed to brain homogenate | 6/6 | 76 ± 3 |
| Brain homogenate (1%, 0.03 ml) | 3/3 | 69 ± 3 |
Infectious wires were prepared by insertion for 5 min into scrapie-infected mouse brain. After washing, they were inserted into brains of six deeply anesthetised Tga20 indicator mice for the times indicated. The recovered wires were washed and implanted into Tga20 mice. As controls, wires incubated with 10% homogenate (6.8 log LD50 units/ml) of the same brain and the homogenate itself were introduced into indicator mice. Modified from ref. 73.
Two of six mice died on the day of the intervention.
Four of six mice died within a day of the intervention.
Why are wires exposed to infected brain or brain homogenates at least as infectious as injected homogenates, which contain far more protein than can be bound to a wire? The surfaces of steel and other metals tightly bind what appears to be a monolayer of protein (74–76). The unexpected high infectivity of steel wires could be due to selective binding of infectious particles or a higher potency of surface bound infectivity. It has been shown that, despite the resistance of PrPSc and scrapie infectivity to treatment in vitro with proteinase K, prion titers in brain after intracerebral inoculation decrease below the level of detectability within 4 days or less (15). On the other hand, infectious wires left for 5 days in brain still retained infectivity (73). Perhaps metal-bound prions may be protected against rapid degradation in the brain, and their apparently high specific infectivity may therefore be due to the long persistence of relatively low levels of infectivity. It can be mentioned in passing that prion-coated gold wires exhibit similar infectivity intracerebrally as steel wires (73), and that plastic surfaces, such as polystyrene (Fig. 4), polypropylene, or polyethylene also tightly bind prions and transmit scrapie infectivity to adherent susceptible cultured cells (M.E., D.R., P.-C.K. and C.W., unpublished data).
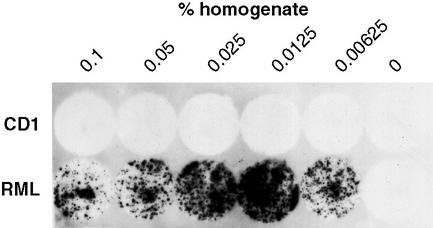
Figure 4.
Infection of mouse neuroblastoma cells by plastic-bound prions. Wells of a polystyrene 96-well microtiter plate were exposed to various dilutions of a homogenate of scrapie-prion-infected mouse brain, washed exhaustively, and dried. Ten thousand N2a/Bos2 mouse neuroblastoma cells (77) were cultured in the wells for 3 days, then transferred to 24-well plates and cultured 4 wk, splitting 1:10 twice a week. The cells were then transferred to coverslips and assayed for the presence of PrPSc (78). Optimal infectivity resulted when plates were coated with 0.0125% homogenate. High concentrations of brain proteins bound to the plastic appear inhibitory for cell infection (P.-C.K. and C.W., unpublished results).
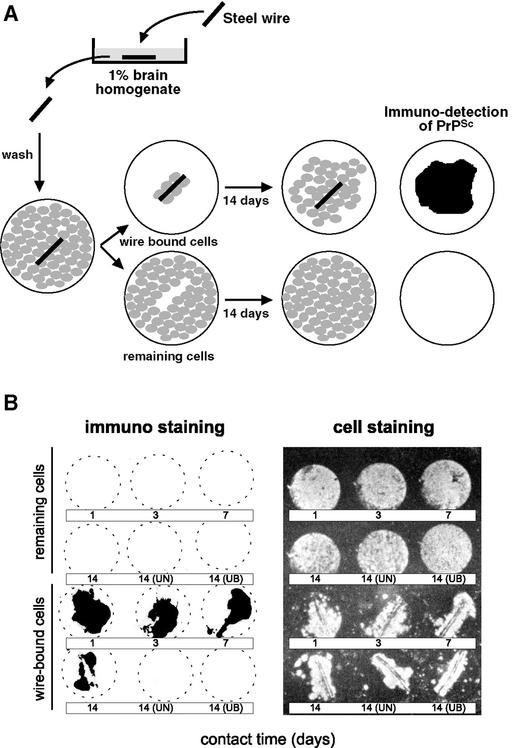
We attempted to elute PrP from infectious steel wires with 2M NaOH, but failed to detect either protein (detection limit, 50 ng per wire) or PrP (detection limit 15 pg per wire). On the other hand, PrP immunoreactivity could be detected at the surface of prion-coated wires by chemiluminescence (73). This finding raises the question as to whether infection of brain tissue elicited by infected wires comes about by direct contact with irreversibly surface-bound prions or whether it is due to a slow, so far undetected release of prions. This question is difficult to answer experimentally; however, it would seem that intimate contact between the prion-loaded surface and target cells is a prerequisite for transmission of infectivity. Prion-coated wires were placed on monolayers of mouse neuroblastoma cells highly susceptible to mouse prions (77). After 1 to 14 days, the wires, to which some cells had adhered, were transferred onto coverslips in the wells of a tissue culture plate and incubated for 14 days, allowing the cells to migrate off the wire and multiply. Cells derived from both the residual monolayer and the wire were blotted onto nitrocellulose membranes and assayed for the presence of protease-resistant PrP, the surrogate marker of prion infection (78). Only the cells derived from the infected wire, but not from the residual monolayer, were PrPSc positive (Fig. 5) and contained infectivity (M.E., E.F. and C.W., unpublished data). This experiment shows that intimate contact between the prion-carrying surface and susceptible cells greatly promotes infection or is prerequisite. Similarly, cell-to-cell transmission of infectivity in cell culture is orders of magnitude more efficient than transmission by a prion preparation (79).
Figure 5.
Neuroblastoma cells are infected by contact with prion-coated stainless steel wires. (A) Stainless steel wires were exposed to scrapie-infected brain homogenates, washed, and placed on a confluent layer of neuroblastoma cells. After periods ranging from 1 to 14 days, the wire, to which a few cells had attached, were placed on a coverslip in a separate well and cultured for further 14 days. Both the cells remaining in the original dish (“remaining cells”) and those derived from the cells clinging to the wire were assayed for PrPSc by the cell blot assay (78) and the mouse bioassay. (B Left) The cultures derived from wire-bound cells have been infected, as evidenced by the accumulation of PrPSc, whereas residual cells remain uninfected. (Right) The location of cells as revealed by ethidium bromide staining. UN, Blank wire; UB, wire treated with uninfected brain homogenate (M.E. and C.W., unpublished results).
The availability of prion-coated steel wires mimicking contaminated surgical instruments makes it possible to assess the efficacy of sterilization conditions on surface-bound prions. Preliminary results (Table 2) confirm that treatment with formaldehyde is insufficient to sterilize infectious wires, whereas treatment with sodium hydroxide, guanidinium thiocyanate (73), or autoclaving at 121°C for 20 min is efficacious (E.F. and C.W., unpublished results). It is, however, not appropriate to derive from these experiments recommendations for the sterilization of surgical instruments; it will first be necessary to validate the procedures scaling up the contact surface between metal and brain tissue and, importantly, using vCJD prions in a susceptible host, preferably a non-human primate.
Table 2.
Effect of various treatments on the infectivity of wire-bound prions
| Sick/total | Incubation time ± SD, days | |
|---|---|---|
| Uninfected wires | ||
| Untreated | 0/3 | >260 |
| Infected wires | ||
| Untreated | 6/6 | 76 ± 5 |
| NaOH (1 M, 1 h, 25°C) | 0/6 | >260 |
| Formaldehyde (10%, 1 h, 25°C) | 6/6 | 92 ± 8 |
| Guanidinium thiocyanate (4 M, 16 h, 25°C) | 0/6 | >260 |
| Autoclaving (121°C, 20 min) | 0/6 | >170 |
Modified from ref. 73 and unpublished results (E.F. and C.W.).
Concluding Remarks
Twenty or more diseases of humans are associated with the deposition of β-sheet-rich protein aggregates, or amyloid (80, 81). They are frequently designated “conformational diseases” although it is not in all cases clear whether, or to what extent, the misfolded proteins are the cause of the disease rather than the consequence. Prion diseases are so far unique conformational diseases, because they are transmissible by misfolded protein, not only under experimental conditions but also naturally, predominantly by ingestion. Although in certain cases the inception of an experimental amyloidosis can be accelerated by the injection of amyloid into a predisposed host (82), prions are exceptional in that they are able to enter their hosts by natural portals and make their way from the gut to the brain, utilizing intermediate tissues for amplification. In the case of microbes and viruses, such sophisticated behavior is attributed to evolutionary processes, that is, genomic mutations and selection of mutants that most readily enter their host and find a suitable niche in which to replicate and/or perpetuate themselves; however, prion protein is encoded by the genome of its host. So what drives the prion to become more efficient in the destruction of its parent? We can only speculate. For example, the “misfolded” form of PrP may have originated as a “messenger” protein that on the one hand has or had a physiological function but on the other has a malignant potential that is rarely realized and was not selected against because evolutionary pressure does not operate efficiently at postreproductive age. It has been proposed that in yeast a “prion-like” phenomenon involving Sup35 may confer selective advantage on yeast growing under fluctuating environmental conditions (83). Another possibility is that PrP/PrPSc is derived from an ancient pathogen whose genetic material was integrated into the genome of its host and harnessed to fulfill a useful function while its pathogenic potential was minimized. More trivially, mammalian prion disease could be the result of the natural propensity of proteins to assume a β-sheet-rich conformation (84), a failure of the organism to prevent their formation and accumulation in some cases, and the coincidental ability of the conformational isomer to penetrate organisms and cells through natural portals.
Abbreviations
- BSE
bovine spongiform encephalopathy
- CJD
Creutzfeldt-Jakob disease
- vCJD
variant CJD
Footnotes
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “Self-Perpetuating Structural States in Biology, Disease, and Genetics,” held March 22–24, 2002, at the National Academy of Sciences in Washington, DC.
References
- 1.Will R G, Ironside J W, Zeidler M, Cousens S N, Estibeiro K, Alperovitch A, Poser S, Pocchiari M, Hofman A, Smith P G. Lancet. 1996;347:921–925. doi: 10.1016/s0140-6736(96)91412-9. [DOI] [PubMed] [Google Scholar]
- 2.Cuille J, Chelle P L. C R Seances Acad Sci. 1939;208:1058–1160. [Google Scholar]
- 3.Gordon W S. Vet Rec. 1946;58:516–520. [PubMed] [Google Scholar]
- 4.Alper T, Cramp W A, Haig D A, Clarke M C. Nature (London) 1967;214:764–766. doi: 10.1038/214764a0. [DOI] [PubMed] [Google Scholar]
- 5.Griffith J S. Nature (London) 1967;215:1043–1044. doi: 10.1038/2151043a0. [DOI] [PubMed] [Google Scholar]
- 6.Prusiner S B. Annu Rev Microbiol. 1989;43:345–374. doi: 10.1146/annurev.mi.43.100189.002021. [DOI] [PubMed] [Google Scholar]
- 7.Basler K, Oesch B, Scott M, Westaway D, Walchli M, Groth D F, McKinley M P, Prusiner S B, Weissmann C. Cell. 1986;46:417–428. doi: 10.1016/0092-8674(86)90662-8. [DOI] [PubMed] [Google Scholar]
- 8.Oesch B, Westaway D, Wälchli M, McKinley M P, Kent S B, Aebersold R, Barry R A, Tempst P, Teplow D B, Hood L E, Prusiner S B, Weissmann C. Cell. 1985;40:735–746. doi: 10.1016/0092-8674(85)90333-2. [DOI] [PubMed] [Google Scholar]
- 9.Stahl N, Baldwin M A, Teplow D B, Hood L, Gibson B W, Burlingame A L, Prusiner S B. Biochemistry. 1993;32:1991–2002. doi: 10.1021/bi00059a016. [DOI] [PubMed] [Google Scholar]
- 10.Riek R, Hornemann S, Wider G, Glockshuber R, Wüthrich K. FEBS Lett. 1997;413:282–288. doi: 10.1016/s0014-5793(97)00920-4. [DOI] [PubMed] [Google Scholar]
- 11.Pan K M, Baldwin M, Nguyen J, Gasset M, Serban A, Groth D, Mehlhorn I, Huang Z, Fletterick R J, Cohen F E, Prusiner S B. Proc Natl Acad Sci USA. 1993;90:10962–10966. doi: 10.1073/pnas.90.23.10962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caughey B W, Dong A, Bhat K S, Ernst D, Hayes S F, Caughey W S. Biochemistry. 1991;30:7672–7680. doi: 10.1021/bi00245a003. [DOI] [PubMed] [Google Scholar]
- 13.Weissmann C. J Biol Chem. 1999;274:3–6. doi: 10.1074/jbc.274.1.3. [DOI] [PubMed] [Google Scholar]
- 14.Prusiner S B. Proc Natl Acad Sci USA. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Büeler H, Aguzzi A, Sailer A, Greiner R A, Autenried P, Aguet M, Weissmann C. Cell. 1993;73:1339–1347. doi: 10.1016/0092-8674(93)90360-3. [DOI] [PubMed] [Google Scholar]
- 16.Sailer A, Büeler H, Fischer M, Aguzzi A, Weissmann C. Cell. 1994;77:967–968. doi: 10.1016/0092-8674(94)90436-7. [DOI] [PubMed] [Google Scholar]
- 17.Fischer M, Rülicke T, Raeber A, Sailer A, Moser M, Oesch B, Brandner S, Aguzzi A, Weissmann C. EMBO J. 1996;15:1255–1264. [PMC free article] [PubMed] [Google Scholar]
- 18.Prusiner S B. Science. 1991;252:1515–1522. doi: 10.1126/science.1675487. [DOI] [PubMed] [Google Scholar]
- 19.Jarrett J T, Lansbury P J. Cell. 1993;73:1055–1058. doi: 10.1016/0092-8674(93)90635-4. [DOI] [PubMed] [Google Scholar]
- 20.Orgel L E. Chem Biol. 1996;3:413–414. doi: 10.1016/s1074-5521(96)90087-3. [DOI] [PubMed] [Google Scholar]
- 21.Kocisko D A, Come J H, Priola S A, Chesebro B, Raymond G J, Lansbury P T, Caughey B. Nature (London) 1994;370:471–474. doi: 10.1038/370471a0. [DOI] [PubMed] [Google Scholar]
- 22.Raymond G J, Hope J, Kocisko D A, Priola S A, Raymond L D, Bossers A, Ironside J, Will R G, Chen S G, Petersen R B, et al. Nature (London) 1997;388:285–288. doi: 10.1038/40876. [DOI] [PubMed] [Google Scholar]
- 23.Bessen R A, Kocisko D A, Raymond G J, Nandan S, Lansbury P T, Caughey B. Nature (London) 1995;375:698–700. doi: 10.1038/375698a0. [DOI] [PubMed] [Google Scholar]
- 24.Saborio G P, Permanne B, Soto C. Nature (London) 2001;411:810–813. doi: 10.1038/35081095. [DOI] [PubMed] [Google Scholar]
- 25.Lu B Y, Chang J Y. Biochemistry. 2001;40:13390–13396. doi: 10.1021/bi011111t. [DOI] [PubMed] [Google Scholar]
- 26.Jackson G S, Hosszu L L, Power A, Hill A F, Kenney J, Saibil H, Craven C J, Waltho J P, Clarke A R, Collinge J. Science. 1999;283:1935–1937. doi: 10.1126/science.283.5409.1935. [DOI] [PubMed] [Google Scholar]
- 27.Hill A F, Antoniou M, Collinge J. J Gen Virol. 1999;80:11–14. doi: 10.1099/0022-1317-80-1-11. [DOI] [PubMed] [Google Scholar]
- 28.Shaked G M, Fridlander G, Meiner Z, Taraboulos A, Gabizon R. J Biol Chem. 1999;274:17981–17986. doi: 10.1074/jbc.274.25.17981. [DOI] [PubMed] [Google Scholar]
- 29.Kaneko K, Ball H L, Wille H, Zhang H, Groth D, Torchia M, Tremblay P, Safar J, Prusiner S B, DeArmond S J, et al. J Mol Biol. 2000;295:997–1007. doi: 10.1006/jmbi.1999.3386. [DOI] [PubMed] [Google Scholar]
- 30.Post K, Brown D R, Groschup M, Kretzschmar H A, Riesner D. Arch Virol. 2000;Suppl.:265–273. doi: 10.1007/978-3-7091-6308-5_25. [DOI] [PubMed] [Google Scholar]
- 31.Prusiner S B, Groth D, Serban A, Stahl N, Gabizon R. Proc Natl Acad Sci USA. 1993;90:2793–2797. doi: 10.1073/pnas.90.7.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shaked G M, Meiner Z, Avraham I, Taraboulos A, Gabizon R. J Biol Chem. 2001;276:14324–14328. doi: 10.1074/jbc.M007815200. [DOI] [PubMed] [Google Scholar]
- 33.McKenzie D, Bartz J, Mirwald J, Olander D, Marsh R, Aiken J. J Biol Chem. 1998;273:25545–25547. doi: 10.1074/jbc.273.40.25545. [DOI] [PubMed] [Google Scholar]
- 34.Kimberlin R H, Wilesmith J W. Ann N Y Acad Sci. 1994;724:210–220. doi: 10.1111/j.1749-6632.1994.tb38911.x. [DOI] [PubMed] [Google Scholar]
- 35.Alpers M. In: Slow Transmissible Diseases of the Nervous System. Prusiner S B, Hadlow W J, editors. Vol. 1. New York: Academic; 1979. p. 67. [Google Scholar]
- 36.Maignien T, Lasmezas C I, Beringue V, Dormont D, Deslys J P. J Gen Virol. 1999;80:3035–3042. doi: 10.1099/0022-1317-80-11-3035. [DOI] [PubMed] [Google Scholar]
- 37.Jeffrey M, Ryder S, Martin S, Hawkins S A, Terry L, Berthelin-Baker C, Bellworthy S J. J Comp Pathol. 2001;124:280–289. doi: 10.1053/jcpa.2001.0465. [DOI] [PubMed] [Google Scholar]
- 38.Bradley R. In: Bovine Spongiform Encephalopathy: The BSE Dilemma. Gibbs C J Jr, editor. New York: Springer; 1996. p. 11. [Google Scholar]
- 39.Bons N, Mestre-Frances N, Belli P, Cathala F, Gajdusek D C, Brown P. Proc Natl Acad Sci USA. 1999;96:4046–4051. doi: 10.1073/pnas.96.7.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ridley R M, Baker H F. Lancet. 1996;348:1174. doi: 10.1016/s0140-6736(05)65312-3. (lett.). [DOI] [PubMed] [Google Scholar]
- 41.Race R, Jenny A, Sutton D. J Infect Dis. 1998;178:949–953. doi: 10.1086/515669. [DOI] [PubMed] [Google Scholar]
- 42.Ingrosso L, Pisani F, Pocchiari M. J Gen Virol. 1999;80:3043–3047. doi: 10.1099/0022-1317-80-11-3043. [DOI] [PubMed] [Google Scholar]
- 43.Bolton D C, McKinley M P, Prusiner S B. Science. 1982;218:1309–1311. doi: 10.1126/science.6815801. [DOI] [PubMed] [Google Scholar]
- 44.Sansonetti P J, Phalipon A. Semin Immunol. 1999;11:193–203. doi: 10.1006/smim.1999.0175. [DOI] [PubMed] [Google Scholar]
- 45.Kaiserlian D, Etchart N. Semin Immunol. 1999;11:217–224. doi: 10.1006/smim.1999.0177. [DOI] [PubMed] [Google Scholar]
- 46.Hathaway L J, Kraehenbuhl J P. Cell Mol Life Sci. 2000;57:323–232. doi: 10.1007/PL00000693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heppner F L, Christ A D, Klein M A, Prinz M, Fried M, Kraehenbuhl J P, Aguzzi A. Nat Med. 2001;7:976–977. doi: 10.1038/nm0901-976. [DOI] [PubMed] [Google Scholar]
- 48.Beekes M, McBride P A. Neurosci Lett. 2000;278:181–184. doi: 10.1016/s0304-3940(99)00934-9. [DOI] [PubMed] [Google Scholar]
- 49.Prinz M, Montrasio F, Klein M A, Schwarz P, Priller J, Odermatt B, Pfeffer K, Aguzzi A. Proc Natl Acad Sci USA. 2002;99:919–924. doi: 10.1073/pnas.022626399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bradley R. Dev Biol Stand. 1999;99:35–40. [PubMed] [Google Scholar]
- 51.Aucouturier P, Geissmann F, Damotte D, Saborio G P, Meeker H C, Kascsak R, Carp R I, Wisniewski T. J Clin Invest. 2001;108:703–708. doi: 10.1172/JCI13155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang F P, Farquhar C F, Mabbott N A, Bruce M E, MacPherson G G. J Gen Virol. 2002;83:267–271. doi: 10.1099/0022-1317-83-1-267. [DOI] [PubMed] [Google Scholar]
- 53.Klein M A, Frigg R, Raeber A J, Flechsig E, Hegyi I, Zinkernagel R M, Weissmann C, Aguzzi A. Nat Med. 1998;4:1429–1433. doi: 10.1038/4022. [DOI] [PubMed] [Google Scholar]
- 54.Montrasio F, Cozzio A, Flechsig E, Rossi D, Klein M A, Rulicke T, Raeber A J, Vosshenrich C A, Proft J, Aguzzi A, Weissmann C. Proc Natl Acad Sci USA. 2001;98:4034–4037. doi: 10.1073/pnas.051609398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Montrasio F, Frigg R, Glatzel M, Klein M A, Mackay F, Aguzzi A, Weissmann C. Science. 2000;288:1257–1259. doi: 10.1126/science.288.5469.1257. [DOI] [PubMed] [Google Scholar]
- 56.Mabbott N A, Mackay F, Minns F, Bruce M E. Nat Med. 2000;6:719–720. doi: 10.1038/77401. [DOI] [PubMed] [Google Scholar]
- 57.Oldstone M B, Race R, Thomas D, Lewicki H, Homann D, Smelt S, Holz A, Koni P, Lo D, Chesebro B, Flavell R. J Virol. 2002;76:4357–4363. doi: 10.1128/JVI.76.9.4357-4363.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beekes M, McBride P A, Baldauf E. J Gen Virol. 1998;79:601–607. doi: 10.1099/0022-1317-79-3-601. [DOI] [PubMed] [Google Scholar]
- 59.Bencsik A, Lezmi S, Hunsmann G, Baron T. Dev Immunol. 2001;8:235–241. doi: 10.1155/2001/40871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Race R, Oldstone M, Chesebro B. J Virol. 2000;74:828–833. doi: 10.1128/jvi.74.2.828-833.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bosque P J, Ryou C, Telling G, Peretz D, Legname G, DeArmond S J, Prusiner S B. Proc Natl Acad Sci USA. 2002;99:3812–3817. doi: 10.1073/pnas.052707499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brandner S, Raeber A, Sailer A, Blattler T, Fischer M, Weissmann C, Aguzzi A. Proc Natl Acad Sci USA. 1996;93:13148–13151. doi: 10.1073/pnas.93.23.13148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blättler T, Brandner S, Raeber A J, Klein M A, Voigtlander T, Weissmann C, Aguzzi A. Nature (London) 1997;389:69–73. doi: 10.1038/37981. [DOI] [PubMed] [Google Scholar]
- 64.Raeber A J, Klein M A, Frigg R, Flechsig E, Aguzzi A, Weissmann C. EMBO J. 1999;18:2702–2706. doi: 10.1093/emboj/18.10.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hadlow W J, Kennedy R C, Race R E. J Infect Dis. 1982;146:657–664. doi: 10.1093/infdis/146.5.657. [DOI] [PubMed] [Google Scholar]
- 66.Holada K, Vostal J G, Theisen P W, MacAuley C, Gregori L, Rohwer R G. J Virol. 2002;76:4649–4650. doi: 10.1128/JVI.76.9.4649-4650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Houston F, Foster J D, Chong A, Hunter N, Bostock C J. Lancet. 2000;356:999–1000. doi: 10.1016/s0140-6736(00)02719-7. [DOI] [PubMed] [Google Scholar]
- 68.Brown P, Preece M, Brandel J P, Sato T, McShane L, Zerr I, Fletcher A, Will R G, Pocchiari M, Cashman N R, et al. Neurology. 2000;55:1075–1081. doi: 10.1212/wnl.55.8.1075. [DOI] [PubMed] [Google Scholar]
- 69.Brown P. In: Prion Diseases. Baker H, Ridley R M, editors. Totowa, NJ: Humana; 1996. pp. 139–154. [Google Scholar]
- 70.Bernoulli C, Siegfried J, Baumgartner G, Regli F, Rabinowicz T, Gajdusek D C, Gibbs C J., Jr Lancet. 1977;1:478–479. doi: 10.1016/s0140-6736(77)91958-4. [DOI] [PubMed] [Google Scholar]
- 71.Gibbs C J, Jr, Asher D M, Kobrine A, Amyx H L, Sulima M P, Gajdusek D C. J Neurol Neurosurg Psychiatry. 1994;57:757–758. doi: 10.1136/jnnp.57.6.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zobeley E, Flechsig E, Cozzio A, Enari M, Weissmann C. Mol Med. 1999;5:240–243. [PMC free article] [PubMed] [Google Scholar]
- 73.Flechsig E, Hegyi I, Enari M, Schwarz P, Collinge J, Weissmann C. Mol Med. 2001;7:679–684. [PMC free article] [PubMed] [Google Scholar]
- 74.Williams D F, Askill I N, Smith R. J Biomed Mater Res. 1985;19:313–320. doi: 10.1002/jbm.820190312. [DOI] [PubMed] [Google Scholar]
- 75.Williams R L, Williams D F. Biomaterials. 1988;9:206–212. doi: 10.1016/0142-9612(88)90085-3. [DOI] [PubMed] [Google Scholar]
- 76.Eckert R, Jeney S, Horber J K. Cell Biol Int. 1997;21:707–713. doi: 10.1006/cbir.1997.0215. [DOI] [PubMed] [Google Scholar]
- 77.Enari M, Flechsig E, Weissmann C. Proc Natl Acad Sci USA. 2001;98:9295–9299. doi: 10.1073/pnas.151242598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bosque P J, Prusiner S B. J Virol. 2000;74:4377–4386. doi: 10.1128/jvi.74.9.4377-4386.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kanu N, Imokawa Y, Drechsel D N, Williamson R A, Birkett C R, Bostock C J, Brockes J P. Curr Biol. 2002;12:523–530. doi: 10.1016/s0960-9822(02)00722-4. [DOI] [PubMed] [Google Scholar]
- 80.Carrell R W, Lomas D A. Lancet. 1997;350:134–138. doi: 10.1016/S0140-6736(97)02073-4. [DOI] [PubMed] [Google Scholar]
- 81.Lansbury P T., Jr Proc Natl Acad Sci USA. 1999;96:3342–3344. doi: 10.1073/pnas.96.7.3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Johan K, Westermark G, Engstrom U, Gustavsson A, Hultman P, Westermark P. Proc Natl Acad Sci USA. 1998;95:2558–2563. doi: 10.1073/pnas.95.5.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.True H L, Lindquist S L. Nature (London) 2000;407:477–483. doi: 10.1038/35035005. [DOI] [PubMed] [Google Scholar]
- 84.Chiti F, Webster P, Taddei N, Clark A, Stefani M, Ramponi G, Dobson C M. Proc Natl Acad Sci USA. 1999;96:3590–3594. doi: 10.1073/pnas.96.7.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]