Abstract
During photosynthesis, part of the fixed carbon is directed into the synthesis of transitory starch, which serves as an intermediate carbon storage facility in chloroplasts. This transitory starch is mobilized during the night. Increasing evidence indicates that the main route of starch breakdown proceeds by way of hydrolytic enzymes and results in glucose formation. This pathway requires a glucose translocator to mediate the export of glucose from the chloroplasts. We have reexamined the kinetic properties of the plastidic glucose translocator and, using a differential labeling procedure, have identified the glucose translocator as a component of the inner envelope membrane. Peptide sequence information derived from this protein was used to isolate cDNA clones encoding a putative plastidic glucose translocator from spinach, potato, tobacco, Arabidopsis, and maize. We also present the molecular characterization of a candidate for a hexose transporter of the plastid envelope membrane. This transporter, initially characterized more than 20 years ago, is closely related to the mammalian glucose transporter GLUT family and differs from all other plant hexose transporters that have been characterized to date.
INTRODUCTION
In plants, carbon fixed during the day is exported from the chloroplasts in the form of triose phosphate (trioseP), which is converted in the cytosol to sucrose. Sucrose often serves as the predominant photoassimilate being allocated to sink tissues. The export of trioseP from the chloroplasts is mediated by the trioseP/3-phosphoglycerate/phosphate translocator (TPT; Fliege et al., 1978; Flügge et al., 1989). Rather than being exported, a considerable amount of the fixed carbon is maintained within the chloroplasts and is involved in the biosynthesis of transitory starch, which could amount to approximately one-half of the carbon assimilated by photosynthesis during the day. During the next dark period, transitory starch is mobilized to sustain a continuous supply of carbon (i.e., sucrose) for export to growing sinks as well as for energy metabolism in leaves. Mutants lacking the ability to synthesize (Caspar et al., 1985; Hanson and McHale, 1988; Huber and Hanson, 1992; Geiger et al., 1995) or degrade transitory starch (Zeeman et al., 1998a, 1998b; Caspar et al., 1991) show reduced growth under conditions in which photosynthesis is restricted.
Starch degradation could follow either the phosphorolytic pathway, yielding trioseP, or the amylolytic pathway, leading to free sugars, glucose (Glc), and maltose. There is evidence that the dominant pathway for the degradation of transitory starch is the amylolytic one. First, trioseP, the end product of the phosphorolytic pathway, must be exported from the chloroplasts and subsequently be converted to hexose phosphate (hexoseP) in the cytosol. This reaction is controlled by the regulatory metabolite fructose 2,6-bisphosphate, which is a strong inhibitor of the cytosolic fructose–bisphosphate phosphatase (Stitt, 1990). During the transition from light to dark, the fructose 2,6-bisphosphate concentration in leaves increases markedly (Servaites et al., 1989a, 1989b), and cytosolic and stromal concentrations of fructose 1,6-bisphosphate and trioseP decrease to zero (Gerhardt et al., 1987), indicating that the synthesis of trioseP and its conversion to hexoseP in the cytosol have ceased. In the dark, however, sucrose synthesis continues, and cytosolic concentrations of hexoseP remain high (Gerhardt et al., 1987) because of starch degradation, transport of these degradation products to the cytosol, and conversion of these products into sucrose.
Second, evidence has been presented that transgenic potato and tobacco plants that have decreased TPT activity direct most of the fixed carbon into the biosynthesis of starch at the expense of sucrose synthesis. The diminished TPT activity in these transformants is compensated for by mobilizing transitory starch by way of the amylolytic pathway and exporting the products of starch breakdown in the form of Glc (Heineke et al., 1994; Häusler et al., 1998).
Third, a study of a high-starch mutant of Arabidopsis, designated sex1 (for starch excess; Caspar et al., 1991), which is unable to degrade starch, supports the notion that the products of starch breakdown are exported from the chloroplast by a translocator different from the TPT. Chloroplasts isolated from the sex1 mutant were shown to possess a functional TPT but were unable to transport Glc (Trethewey and ap Rees, 1994a). Finally, using nuclear magnetic resonance to determine the intramolecular distribution of deuterium-labeled Glc liberated during the breakdown of transitory starch showed that most carbon was exported as hexoses at night (Gleixner et al., 1998; Schleucher et al., 1998).
The amylolytic pathway of starch breakdown requires the presence of a transport capacity for the resulting hexoses. Herold et al. (1981), Beck (1985), and Rost et al. (1996) reported that the chloroplast envelope is permeable for maltose but not for maltodextrins. Rost et al. (1996) demonstrated that the uptake of d-Glc does not compete with maltose transport. Schäfer et al. (1977) reported that several pentoses and hexoses, including Glc, were taken up by isolated chloroplasts by way of facilitated diffusion. The significance of this discovery was obscured largely by the fact that the reported rate of Glc transport was much lower than the rate of Pi transport mediated by the TPT. This made it seem unlikely that, during nighttime, the plastidic Glc translocator (pGlcT) could export all the Glc made from starch synthesized during the day.
Our recent investigations of Glc transport into chloroplasts, using a differential affinity labeling strategy, have allowed us to identify the pGlcT as a component of the inner envelope membrane. We obtained peptide sequence information for this protein and isolated the corresponding cDNA. Here, we identify a candidate for the Glc transporter of the plastid inner envelope membrane and report the results of cloning the corresponding cDNAs from spinach, tobacco, potato, Arabidopsis, and maize.
RESULTS
Measurements of the Transport Capacity of pGlcT and Its Substrate Specificity
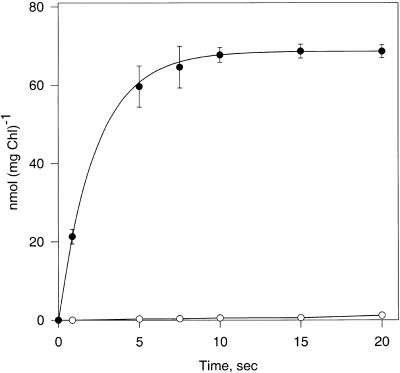
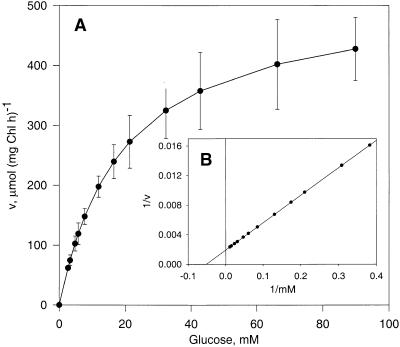
We reexamined the uptake of labeled Glc into intact chloroplasts, using the silicone oil filtration centrifugation method (Heldt, 1980; Gross et al., 1990). Uptake of 4 mM Glc into spinach chloroplasts reached approximately a third of the equilibrium concentration in 1 sec and was nearly complete in 10 sec (Figure 1). Over time, a small amount of this Glc was converted into acidic products. The uptake showed characteristics of a first-order rate curve, having a rate constant of 0.37 sec−1. Using 1-sec uptake times, we measured a Km for Glc uptake of 19.3 mM and a Vmax of 519 μmol mg−1 of chlorophyll hr−1 (Figures 2A and 2B). These data are similar to those of Schäfer et al. (1977), but the Vmax value is ∼10-fold higher than theirs. The higher Vmax value measured by us can be explained by the application of the double-layer silicone–oil–filtration–centrifugation technique, which allows assay times of ∼1 sec or less. Short assay times are required for transport systems that attain equilibrium rapidly. The shortest assay times used by Schäfer et al. (1977) were in the range of 10 sec; consequently, the transport rates were largely underestimated.
Figure 1.
Time Course of Uptake of 4 mM d-14C-Glc into Spinach Chloroplasts.
Shown is the radioactivity present in neutral (closed circles) and acidic (open circles) products. Error bars indicate standard deviation. Chl, chlorophyll.
Figure 2.
Determination of the Kinetic Constants of pGlcT.
(A) Substrate dependency of uptake of d-14C-Glc into spinach chloroplasts. Chl, chlorophyll; h, hour.
(B) Double-reciprocal representation (Lineweaver–Burk plot) of data shown in (A).
Error bars indicate standard deviation. 1/v, reciprocal value of the Glc uptake rate; 1/mM, reciprocal value of the substrate concentration.
To determine the specificity of d-Glc uptake by chloroplasts, we conducted competition experiments. The ability of various sugars at 10-fold higher concentrations to competitively lessen uptake of 1 mM D-14C-Glc into chloroplasts is shown in Table 1. For these experiments, we measured uptake at two times, 1 and 5 sec, and determined the first-order rate constants. 2-Deoxy-d-Glc, d-mannose, and d-Glc, in order, had the greatest effect on inhibiting the uptake of d-14C-Glc; d-sorbitol and l-Glc had the least effect. The other C-2 analog, 2-deoxy-2-amino-d-Glc, and the C-4 analog, d-galactose, were also effective. The effect of the remaining sugars was small, comparable to that of l-Glc.
Table 1.
Inhibition of 1 mM d-14C-Glc Uptake into Spinach Chloroplasts by 10 mM d-Glc Analogs, Pentoses, and Other Sugars
| d-14C-Glc Uptakea | kb (sec−1) | % of Control |
|---|---|---|
| Control | ||
| +d-Glc | 0.27 | 66 |
| +l-Glc | 0.41 | 100 |
| C-1 analogs | ||
| +Methyl-α-d-glucopyranoside | 0.40 | 98 |
| +Methyl-β-d-glucopyranoside | —c | — |
| C-2 analogs | ||
| +2-deoxy-d-Glc | 0.26 | 63 |
| +d-Man | 0.27 | 65 |
| +2-deoxy-2-d-Glc | 0.34 | 81 |
| C-3 analogs | ||
| +3-O-Methyl-d-Glc | — | — |
| C-4 analog | ||
| +d-Gal | 0.36 | 87 |
| Other hexoses and sugars | ||
| +l-Gal | — | — |
| +d-Fru | 0.40 | 98 |
| +Maltose | — | — |
| +Maltotriose | — | — |
| +Suc | — | — |
| Pentoses | ||
| +d-Xyl | — | — |
| +l-Ara | — | — |
| +d-Rib | — | — |
| +d-Ara | — | — |
(+) indicates the addition of 10 mM Glc analogs, pentoses, and other sugars.
k stands for the first-order rate constant.
⩽l-Glc.
Effect of Inhibitors on the Uptake of Glc into Intact Chloroplasts
We next examined whether molecules that had been shown to bind tightly to the human erythrocyte Glc transporter (GLUT1) also inhibited Glc uptake by chloroplasts. Cytochalasin B (Shanahan, 1982; Klip et al., 1983), derivatives of phlorizin (Hosang et al., 1981), and N-(azidosalicyl)-6-amido-6-deoxyglucopyranose (ASA-Glc; Shanahan et al., 1985) are potent inhibitors of GLUT1 and have been used as affinity probes for the purification of GLUT1 (Baldwin and Lienhard, 1989). In general, these inhibitors had only a small effect on Glc uptake into chloroplasts; for example, uptake of 2 mM d-Glc was inhibited only ∼30% by 10 μM cytochalasin B, 40% by 250 μM phlorizin, and not at all by 10 μM ASA-Glc. 4,4′-Diisothiocyanatostilbene-2,2′-disulfonic acid, a specific and potent inhibitor of anionic membrane translocators, at 10 μM completely inhibited the TPT activity (Rumpho and Edwards, 1985) but had no effect on Glc uptake (data not shown).
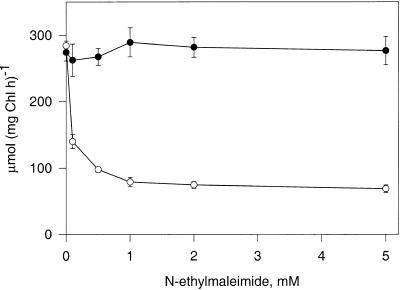
On the other hand, pGlcT activity could be inhibited by incubating chloroplasts with sulfhydryl reagents, for example, N-ethylmaleimide (NEM). Initial attempts to inhibit Glc influx by treating chloroplasts with 1 mM NEM at pH values of 5.6, 6.6, and 7.6 had no effect on Glc transport. However, reduction of chloroplasts with 1 mM DTT before incubation with NEM resulted in ∼70% inhibition at 1 mM NEM (Figure 3).
Figure 3.
Effect of NEM Treatment on the Rate of Glc Transport into Spinach Chloroplasts.
The effect of pretreatment of spinach chloroplasts with NEM in the presence of sorbitol or Glc on the resulting rate of 20 mM D-14C-Glc uptake is shown. Chloroplasts were incubated in buffer B and 1 mM DTT for 15 min at 20°C, collected, and resuspended three times to remove DTT. Aliquots were resuspended in an osmoticum containing either 330 mM sorbitol (open circles) or 330 mM Glc (closed circles) for 15 min at 20°C. NEM was added at the concentration shown, and incubation continued for another 30 min. Chloroplasts were collected and resuspended three times to remove NEM and Glc. Uptake of 4 mM D-14C-Glc was measured in 1-sec assays. Error bars indicate standard deviation. Chl, chlorophyll; h, hour.
Identification of the pGlcT by Differential Labeling with NEM
Successful use of NEM as an affinity label to identify a single protein among a large pool of proteins requires the presence of reduced sulfhydryl groups that can be protected by substrate or ligand. For maximal protection of sulfhydryl groups, substrate concentrations must be ∼10 to 20 times the Km value (Henderson and Macpherson, 1986). For the pGlcT, with a Km (Glc) of ∼20 mM, maximum protection requires Glc concentrations of 200 to 400 mM. Fortunately, although low concentrations of Glc easily permeate the chloroplast envelope and equilibrate, high concentrations of Glc do not equilibrate across the chloroplast envelope (Schäfer et al., 1977). By replacing the 0.33 M sorbitol osmoticum with 0.33 M Glc, Glc transport activity was completely protected at NEM concentrations as great as 5 mM (Figure 3).
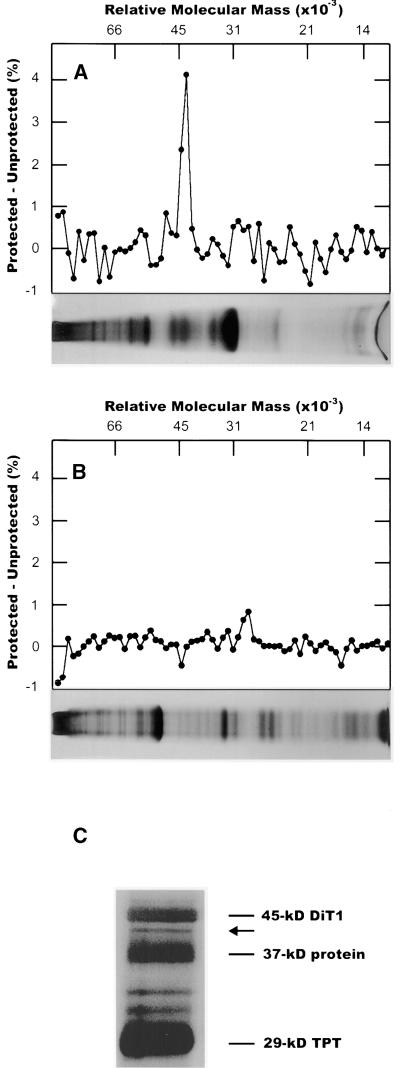
To differentially label sulfhydryl groups of Glc-protected proteins on the chloroplast envelope, we exposed protected chloroplasts to 14C-NEM and unprotected chloroplasts to 3H-NEM. Inner envelope membranes were isolated from these chloroplasts by sucrose density centrifugation and subsequently analyzed by SDS-PAGE. After differential labeling, membrane proteins having sulfhydryl groups protected from unlabeled NEM should bind proportionately more labeled NEM and contain a greater percentage of the total radioactivity than when unprotected. We consistently observed a large increase in the percentage of total 14C radioactivity compared with the percentage of the total tritium radioactivity in an area of the gel corresponding to a molecular mass of ∼43 kD. In some preparations, other smaller peaks and troughs were seen on the gel, but they were not consistently present in all preparations.
The identification of the specific 43-kD protein band was difficult because of the large number of proteins present on the gel. We tried to fractionate envelopes with chloroform–methanol (2:1 [v/v]) according to Piccioni et al. (1982) to separate peripheral from integral membrane proteins. In our hands, fractionation by chloroform and methanol was not discrete; that is, the same proteins were present in both fractions to various degrees. In contrast, partitioning membrane proteins between n-butanol and water gave far superior and more reproducible results (Lockshin and Burris, 1966). Fractionation of membranes in n-butanol:water consistently resulted in approximately one-third of the total protein entering the butanol fraction. Lipids present in the butanol fraction were removed by extracting the lyophilized butanol fraction with n-hexane.
Figures 4A and 4B show the differential labeling pattern of the Glc-protected and unprotected envelope membrane proteins when using 3H- and 14C-NEM as labeling reagents. The Glc-protected 43-kD protein is present in the butanol fraction (Figure 4A) but was not detectable in the aqueous fraction (Figure 4B). To identify this protein precisely as a component of the butanol fraction, we extracted envelope membranes by a 20-fold excess of n-butanol, and the butanol-soluble proteins subsequently were analyzed by SDS-PAGE. Figure 4C displays butanol-soluble proteins in the 30- to 50-kD range. Amino acid sequence analyses revealed the presence of the 29-kD TPT (Flügge et al., 1989), the 37-kD protein (Dreses-Weringloer et al., 1990), and the 45-kD 2-oxoglutarate/malate translocator (DiT1; Weber et al., 1995).
Figure 4.
Differential Labeling of pGlcT Using 3H- and 14C-NEM for Glc-Protected and Glc-Unprotected Envelope Proteins and Their Separation on SDS–Polyacrylamide Gels.
Labeling of Glc-protected or Glc-unprotected spinach chloroplasts was performed as described in the text. Chloroplast envelopes were prepared, and membrane proteins were partitioned between n-butanol and water. Fractions were dried, taken up in sample buffer, and subjected to SDS-PAGE. After staining and drying, the gel was cut into 1-mm sections, and the tritium and 14C present in each section were determined. Data are plotted as the difference in percentage of total radioactivity present in each section (14Csection/14Ctotal − 3Hsection/3Htotal). At the bottom of each plot are the gels, showing the positions of proteins with respect to the sections cut from the gel.
(A) n-Butanol fraction.
(B) Aqueous fraction.
(C) Envelope membranes were extracted by 20-fold excess n-butanol. The butanol fraction was dried, washed with n-hexane, and analyzed by SDS-PAGE. The 43-kD GlcT protein is indicated by an arrow.
The protein band corresponding to an apparent molecular mass of 43 kD (Figure 4C; indicated by an arrow) was excised from preparative gels and digested in situ with the endoproteinase Lys-C to identify the amino acid sequence of internal peptides. We obtained one peptide sequence (KGRSLEEIELALSPAV; P1).
Molecular Cloning of the Glc Translocator from Spinach Chloroplast Envelope Membranes
On the basis of the P1 peptide sequence, we designed two degenerate oligonucleotides (gene-specific primers) that were used for rapid amplification of cDNA ends (RACE; Schaefer, 1995). Single-stranded DNA was reverse transcribed from poly(A)+ RNA that was isolated from spinach leaves harvested in the middle of light and in the middle of dark periods. An adapter oligo(dT)15 oligonucleotide (RACE adapter primer) was used to prime cDNA synthesis. Two consecutive polymerase chain reactions using nested gene-specific primers and nested primers derived from the RACE adapter primer were able to amplify a 300-bp DNA fragment that then was used to screen a cDNA library from spinach leaves. Several identical clones and one clone encoding the complete pGlcT open reading frame were isolated (GenBank accession number AF215851). This last clone was used to screen cDNA libraries from potato leaves (GenBank accession number AF215853), tobacco leaves (GenBank accession number AF215852), maize leaves (GenBank accession number AF215854), and a genomic library from Arabidopsis (GenBank accession number AF215855). We were able to isolate corresponding clones from all of the screened libraries, but none of these cDNA clones encoded the full-length pGlcT protein.
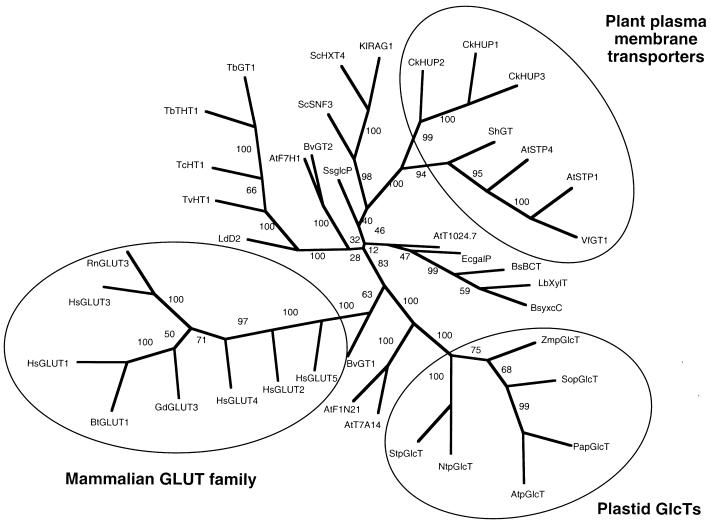
The cDNA from spinach was 1861 bp long, corresponding to 551 amino acid residues, with a predicted molecular mass of 57.6 kD. The sequence of the P1 peptide was present right at the C terminus of the precursor protein (amino acid residues 536 to 551). A comparison of the protein sequence with entries in GenBank revealed strong similarity (∼80% amino acid identities) to a putative sugar transporter from apricot (GenBank accession number AF000952) and weaker but definite similarities to hexose transporters from mammals and bacteria and to hexose transporters of the plant plasma membrane (Figures 5 and 6).
Figure 5.
Evolutionary Relationships of Hexose Transporters Represented as an Unrooted Phylogenetic Tree.
Transporters belonging to the families of mammalian hexose transporters (GLUT family), plant plasma membrane transporters, and the plastidic GlcTs are encircled. The first two letters of the acronyms indicate the species (At, Arabidopsis thaliana; Bs, Bacillus subtilis; Bt, Bos taurus; Bv, Beta vulgaris; Ck, Chlorella kessleri; Ec, Escherichia coli; Gd, Gallus domesticus; Hs, Homo sapiens; Kl, Kluyveromyces lactis; Lb, Lactobacillus brevis; Ld, Leishmania donovani; Nt, Nicotiana tabacum; Pa, Prunus armeniaca; Rn, Rattus norwegicus; Sc, Saccharomyces cerevisiae; Sh, Saccharum hybrid cultivar; So, Spinacia oleracea; Ss, Synechocystis sp (PCC6803); St, Solanum tuberosum; Tb, Trypanosoma brucei; Tc, T. cruzi; Tv, T. vivax; Vf, Vicia faba; Zm, Zea mays. Bootstrap values are depicted on the respective branches.
Figure 6.
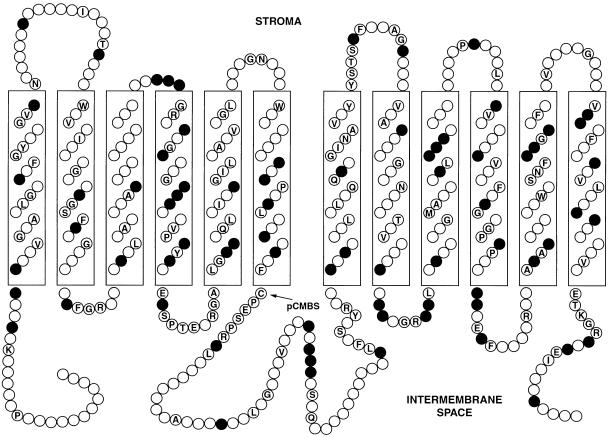
Schematic Representation of the Proposed Transmembrane Topology of the Putative Mature pGlcT Protein from Spinach Chloroplast Envelope Membranes.
The 12 transmembrane helices are shown as boxes. Identical amino acids in the human GLUT1 and the spinach chloroplast envelope membrane pGlcT are indicated by their single-letter abbreviations, and chemically similar residues (D,E; F,Y,W; I,L,V,M; K,R; N,Q; and S,T) are noted by shaded circles. The cysteine residue that is protected by Glc from labeling with pCMBS is indicated by an arrow. Adapted from Gould and Bell (1990).
Import of the pGlcT Precursor Protein into Isolated Chloroplasts
The N terminus of pGlcT shared no regions of homology with other hexose transporters but did have characteristics of a plastid-targeting sequence, as analyzed by ChloroP V1.1 (http://www.cbs.dtu.dk/services/ChloroP/; Emanuelsson et al., 1999). The transit peptide cleavage site was predicted to be located between residues 85 and 86 of the precursor protein. Similarities between the plant pGlcTs (spinach, apricot, tobacco, potato, maize, and Arabidopsis) and members of the human GLUT family start ∼20 amino acid residues downstream of the putative processing site. Analysis of the cDNA sequence of the pGlcT homolog from apricot revealed that the deduced protein sequence submitted to GenBank with the cDNA sequence does not represent the full-length protein because the cDNA sequence was not translated from the first ATG within this sequence. Translation of this cDNA from the first ATG revealed the presence of a putative plastid targeting sequence in this protein also.
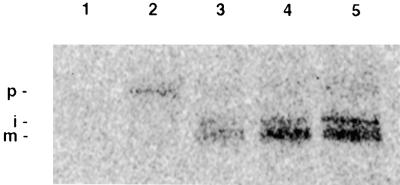
To determine whether the predicted chloroplast-targeting sequence in the spinach pGlcT directs the precursor protein to the chloroplast inner envelope membrane, we performed in vitro protein-importing experiments. The pGlcT cDNA was synthesized in vitro in the presence of 35S-methionine and added to isolated intact spinach chloroplasts, which subsequently were fractionated into a soluble fraction and fractions containing thylakoids and envelope membranes (Figure 7). The imported protein was completely absent from the soluble fraction (data not shown), and the processed mature protein (apparent molecular mass of 43 kD) was found in the envelope fraction. Pretreatment of chloroplasts with thermolysin led to a complete loss of binding and importation of pGlcT, indicating the requirement for proteinaceous surface receptors (Figure 7, lane 1). Importation was strictly dependent on ATP (Figure 7, lanes 2 to 4). Subsequent treatment of the chloroplasts with thermolysin revealed that the processed mature form of pGlcT was inserted into the envelope membrane and was resistant to protease (Figure 7, lane 5). As with importing the Glc 6-phosphate/phosphate translocator (Kammerer et al., 1998) and the putative plastidic adenylate translocator of maize amyloplasts (Bt1; Li et al., 1992), processing of the spinach pGlcT precursor protein obviously proceeded through an intermediate (apparent molecular mass of 46 kD; Figure 7). In summary, the in vitro protein-importing experiments demonstrate that the N terminus of pGlcT has the characteristics of a plastid-targeting sequence and is able to direct the spinach pGlcT protein correctly to the plastid inner envelope membrane where it is inserted, resistant to protease treatment, and processed to its mature form.
Figure 7.
Importation of the 35S-Labeled pGlcT Precursor Protein into Isolated Spinach Chloroplasts.
Importation was performed at 25°C either in the dark (lane 2) or in the light (lane 1 and lanes 3 to 5) and in the presence of 2 mM ATP (lanes 4 and 5) or 5 μM carbonylcyanide m-chlorophenylhydrazone (lane 2). Sample 1 (lane 1) contained plastids that were pretreated with thermolysin (30 μg mL−1). After importation, sample 5 (lane 5) was further treated with thermolysin (50 μg mL−1) in the presence of 1 mM CaCl2 for 30 min. Envelope membranes were isolated as described earlier (Flügge et al., 1989) and analyzed by SDS-PAGE and phosphoimaging. p, i, and m represent the precursor protein, the intermediate form, and the mature form, respectively.
Expression Analysis of pGlcT
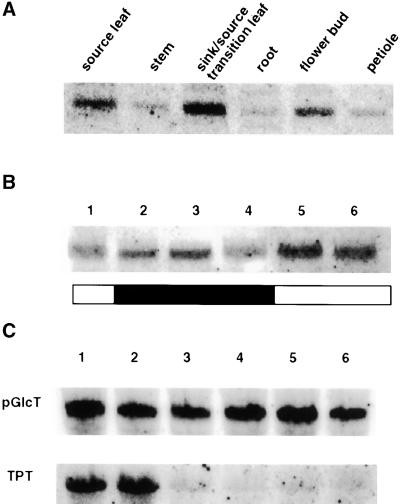
We analyzed the steady state amounts of pGlcT mRNA in different tissues and during a light/dark cycle in tobacco plants. The greatest amounts of pGlcT mRNA were found in source/sink transition leaves, followed by source leaves and green flower buds. Expression was low in sink tissues such as stems, roots, and petioles (Figure 8A); no strong variations in the pGlcT mRNA amounts were observed throughout cycles of light and dark. Expression was lowest at the ends of the dark and light periods and greatest at the beginnings of the dark and light periods (Figure 8B). Using Arabidopsis leaves, we analyzed the amounts of pGlcT mRNA in the middle of the light and dark periods by semiquantitative reverse transcription–polymerase chain reaction. No significant differences in the steady state mRNA levels could be detected (data not shown).
Figure 8.
Expression Analysis of the Gene Encoding pGlcT.
(A) RNA gel blot analysis of pGlcT mRNA in different tissues of tobacco. Thirty micrograms of total RNA isolated from source leaves, stems, sink leaves, roots, flower buds, and petioles was hybridized with the pGlcT cDNA probe from spinach after gel electrophoresis and subsequent transfer of the RNA to a nylon membrane.
(B) Diurnal variation of the steady state concentration of pGlcT mRNA in source leaves of tobacco. Total RNA was isolated from source leaves of tobacco plants after 10 hr of light (lane 1), 1 hr of dark (lane 2), 4 hr of dark (lane 3), 12 hr of dark (lane 4), 1 hr of light (lane 5), and 3 hr of light (lane 6) during a 12-hr-light/12-hr-dark cycle. Analysis (30 μg per lane) was performed as indicated in (A).
(C) RNA gel blot analysis of pGlcT and TPT mRNA of wild-type and transgenic tobacco plants. Total RNA was isolated from wild-type plants (lanes 1 and 2) and from plants showing an antisense repression of the chloroplastic TPT (lanes 3 to 6; Häusler et al., 1998). Analysis (50 μg per lane) was performed as in (A).
In tobacco lines that show a strong antisense repression of the plastidic TPT (Häusler et al., 1998), we were not able to detect an increase in the pGlcT mRNA (Figure 8C), although Häusler et al. (1998) have demonstrated an increased transport capacity for Glc in plastids isolated from these plants. That might indicate a control on plastidic Glc transport capacity at the post-transcriptional or post-translational level.
Expression of the Spinach pGlcT in the Arabidopsis Mutant sex1 Does Not Complement the Mutant Phenotype
Trethewey and ap Rees (1994a)(1994b) have analyzed in more detail the starch excess mutant sex1 that originally was isolated by Caspar et al. (1991). Their results indicate that sex1 is deficient in the pGlcT. To determine whether the mutation in sex1 can be complemented by the pGlcT cDNA, we transformed the Arabidopsis mutant with the corresponding cDNA from spinach by an in-the-plant transformation protocol (Bechtold et al., 1993). Having obtained 70 independent transgenic plant lines, we tested all transformants for their ability to mobilize transitory starch during the dark period. In contrast to the wild type, none of the transformants was able to mobilize starch, even after prolonged incubation in the dark. Obviously, the cDNA that encodes the pGlcT from spinach is not able to complement the mutant phenotype of sex1.
To further determine whether sex1 actually carries a mutation in the pGlcT, we mapped the Arabidopsis pGlcT gene with the help of yeast artificial chromosome (YAC) contigs immobilized on nylon filters. We were able to localize the Arabidopsis pGlcT gene on YACs CIC3F11, CIC8E12, and CIC8H6. This places the gene between markers ve029 and nga106 on chromosome 5 (Schmidt et al., 1997; http://nasc.nott.ac.uk/new_ri_map.html).
Using cleaved amplified polymorphic sequence markers (Konieczny and Ausubel, 1993), we localized the sex1 mutation on chromosome 1 between the markers phyA and NCC1. No homology with sugar transporters was found in this region. From the complementation assay described above and the genetic data, sex1 appears unlikely to be deficient in the pGlcT. A detailed description of the genetic location of the sex1 mutation will be given in a forthcoming paper (H. Kofler et al., manuscript in preparation).
DISCUSSION
Using a differential labeling strategy, we identified a 43-kD protein of the chloroplast inner envelope membrane as a candidate for the pGlcT. A peptide sequence derived from this protein was used to isolate the corresponding cDNA from spinach. This cDNA then was used to clone the homologous cDNAs from different plant species. In vitro protein importation experiments demonstrated that pGlcT is efficiently imported into the chloroplast inner envelope membrane, where it is processed to its mature form.
The mature proteins of the pGlcTs show a strong amino acid sequence identity (80 to 86%). They share ∼40% identical amino acid residues with two uncharacterized putative sugar transporters from Arabidopsis that have been identified on bacterial artificial chromosome sequences in the Arabidopsis genome project (F1N21 and T7A41). The putative sugar transporter found on bacterial artificial chromosome F1N21 also possesses an N-terminal extension that might represent a targeting signal; however, this sequence was not classified as a chloroplast targeting signal by ChloroP.
A weaker but definite similarity was observed to hexose transporters from mammals and bacteria (amino acid identities of ∼34%). Approximately 30% of the amino acids were identical to those of hexose transporters of the plant plasma membrane (Figure 5).
Analysis of the pGlcT Sequence
Henderson (1991) has reported that a repeated [RK]XGR[RK] motif in the hydrophilic loops connecting helices 2 and 3 as well as helices 8 and 9 is a common feature shared by sugar facilitators of eukaryotes and bacteria. In the pGlcTs, this motif is conserved in the loop connecting helices 8 and 9, whereas the second motif includes a threonine residue inserted in front of the last basic residue: [RK]XGRT[RK] (Figure 6).
The binding of cytochalasin B to the mammalian GLUT1 transporter requires the presence of two tryptophan residues (Trp-388 and Trp-412) and an Asn-Trp motif in helix 11 (reviewed in Walmsley et al., 1998). In the plant pGlcT family, the residue corresponding to Trp-388 is replaced by an alanine (Ala-467; spinach), and a histidine residue (His-490; spinach) replaces the asparagine of the GLUT1 Asn-Trp motif. These modifications are probably the reason why cytochalasin B exerts little inhibition on the uptake of Glc into isolated chloroplasts (see above).
The selectivity filter in all Glc-specific members of the human GLUT family (GLUT1, GLUT3, and GLUT4) was shown to reside in helix 7, which contains a highly conserved QLSQQLS motif (residues 279 to 281; human GLUT1). In the plant pGlcT family, this motif is FLFQQ[LMF]A (residues 358 to 364; spinach pGlcT). Hexose transporters that are missing this QLS motif also can transport fructose (reviewed in Walmsley et al., 1998). In contrast, we found that pGlcT does not transport fructose (Table 1). A detailed analysis of pGlcT using purified recombinant protein is needed to determine its substrate specificity.
According to the nomenclature suggested by Saier (1998), we group the pGlcT into the sugar porter family (2.1.1) that is part of the large major facilitator superfamily (Saier, 1998; http://www-biology.ucsd.edu/~msaier/transport/titlepage.html).
Transmembrane Topology of the Spinach pGlcT
The pGlcT protein is very hydrophobic, with an overall deduced polarity index of the precursor protein of 33% (Capaldi and Vanderkooi, 1972). The hydrophobicity distribution analysis (Kyte and Doolittle, 1982) revealed the presence of ∼12 transmembrane-spanning regions, arranged in two pairs of six membrane-spanning segments separated by a relatively long (∼60 amino acid residues) hydrophilic loop (Figure 6).
As described above, the pGlcT could be protected from binding the sulfhydryl reagent NEM by application of high amounts of Glc, which were not transported into the chloro-plast. Therefore, the amino acid residue protected from binding of NEM presumably is exposed to the cytosolic side of the inner envelope membrane. Another sulfhydryl reagent, p-chloromercuribenzenesulfonic acid (pCMBS), also was an effective inhibitor of Glc uptake into chloroplasts, leading to an almost complete inhibition of the pGlcT at 40 μM pCMBS (data not shown). Analysis of the distribution of the six cysteine residues in the mature pGlcT revealed that only one is located in the soluble parts of the protein (as predicted by hydropathy analysis).
This cysteine residue, found in the long hydrophilic loop that connects transmembrane segments 6 and 7 directly adjacent to the PESPR motif found in many Glc transporters, is not found in the corresponding pGlcTs from tobacco, potato, Arabidopsis, apricot, and maize. This might explain why we have not been able to differentially label a 43-kD protein with NEM in the chloroplast envelope membrane of Arabidopsis (data not shown).
For human GLUT1, Cys-207 and Cys-429 are the targets of pCMBS (Wellner et al., 1994). Cys-260 of the spinach pGlcT corresponds to Cys-207 of the human Glc transporter GLUT1. The second cysteine residue, Cys-429 in human GLUT1, is replaced by a phenylalanine residue in all plant pGlcTs analyzed to date (Phe-500; spinach). By analogy with human GLUT1, we propose that Cys-260 of the spinach pGlcT represents the residue labeled by sulfhydryl reagents in intact spinach chloroplasts.
On the basis of the predicted arrangement of the transmembrane segments of the pGlcT and taking into account that Cys-260 is accessible from the intermembrane space, we suggest that both the N and C termini of the spinach chloroplast pGlct are exposed to the intermembrane space of the chloroplast envelope membrane. This membrane topology of the pGlcT resembles that of the plasma membrane hexose transporters. Both N and C termini of the pGlcT are exposed to the cytosolic side of the inner envelope membrane. From an evolutionary point of view, this could mean that the endosymbiotic host cell that took up a photosynthetic organism might regard the starch present in the newly acquired plastid as an extracellular resource. Secretion of amylases from the host cell to the plastid would require an uptake system for Glc. In fact, bacterial amylases and maltases always are found to be secreted to the external medium and followed by the uptake of breakdown products, whereas intercellular polysaccharides (glycogen) are mobilized by phosphorolytic activities.
On the Physiologic Function of the pGlcT during Starch Mobilization
To establish that the chloroplast GlcT functions during starch mobilization, it is important to demonstrate that it has the capacity to transport Glc at rates that are comparable to the rates of starch mobilization measured in intact leaves. Typical rates of starch mobilization for sugar beet leaves are ∼50 microatoms of carbon mg−1 of chlorophyll hr−1 (Fondy et al., 1989). Using the Km and Vmax values measured for zero-trans influx in this study and assuming that 1 mg of chlorophyll is equivalent to a chloroplast volume of 25 μL and a steady state stromal Glc concentration of 0.3 μmol mg−1 of chlorophyll (Stitt et al., 1985; Trethewey and ap Rees, 1994a, 1994b), which corresponds to 12 mM, we calculate that the maximum Glc flux across the chloroplast envelope would be ∼1200 microatoms of carbon mg−1 of chlorophyll hr−1 (200 μmol of Glc mg−1 of chlorophyll hr−1). Although these values were calculated from zero-trans influx experiments, we assume that the pGlcT has the capacity for high export rates; factors controlling the Glc concentrations on either side of the inner membrane most likely determine the direction and rate of net flux (for a detailed discussion of zero-trans influx and efflux kinetics, see Geiger et al., 2000). The assumption is that the outer chloroplast membrane is freely permeable to solutes of molecular masses up to several kilodaltons; however, the recent discovery of channel-like proteins in the outer envelope membrane that are specific for certain metabolites opens the possibility that the outer membrane may have some control over the types of molecules that are moving into and out of the chloroplast (Pohlmeyer et al., 1997). The Glc concentration is maintained at ∼100 μM in the cytosol of numerous plants during daytime (Moore et al., 1997) and might be low also during the night, whereas the stromal Glc concentration at night is much higher (∼12 mM; Stitt et al., 1985; Trethewey and ap Rees, 1994a, 1994b). Under such conditions, Glc flux from the chloroplast to the cytosol would be favored.
The probable physiologic function of the spinach pGlcT is to catalyze the efflux of Glc derived from the amylolytic breakdown of transitory starch. A steep concentration gradient between the chloroplast stroma and the cytosol would be required to obtain high rates of Glc transport. After being exported from the chloroplasts, Glc has to be converted into Glc 6-phosphate by the action of a hexokinase. It recently has been reported that such a hexokinase activity is localized to the outer membrane of chloroplasts (Wiese et al., 1999). We propose that the main function of this envelope-bound hexokinase is to phosphorylate Glc directly when leaving the chloroplasts, thereby maintaining a high concentration gradient for Glc between the stroma and the cytosol (Figure 9).
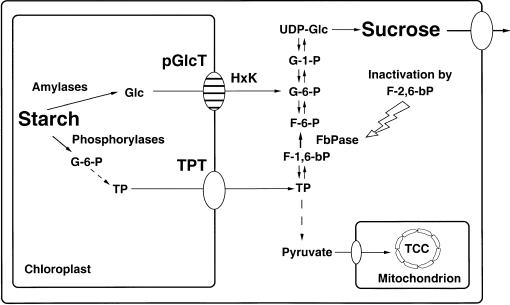
Figure 9.
Proposed Function of the pGlcT Protein in Starch Mobilization.
Glc from hydrolytic breakdown of transitory starch is exported from the chloroplasts by pGlcT and subsequently converted to Glc 6-phosphate (G-6-P) by a hexokinase (HxK) that is located in the chloroplast outer envelope membrane. Glucose 6-phosphate is shuttled to the site of sucrose biosynthesis, whereas trioseP that results from phosphorolytic starch breakdown is subjected to glycolysis. F-6-P, fructose 6-phosphate; F-1,6-bP, fructose 1,6-bisphosphate; F-2,6-bP, fructose 2,6-bisphosphate; FbPase, fructose 1,6-bisphosphate phosphatase; G-1-P, Glc 1-phosphate; TCC, tricarboxylic acid cycle; TP, trioseP.
This idea is corroborated by the recent findings of Veramendi et al. (1999), who created transgenic potato plants with much less hexokinase 1 activity. These transformants did not show substantial changes in tuber carbohydrate metabolism, but transitory starch in the chloroplasts was markedly accumulated. This increase in starch content at dawn was accompanied by a Glc content that approximately doubled and a decrease in sucrose. These findings confirm the assumption that Glc leaves the plastid passively by way of the pGlcT, moving along a concentration gradient that is maintained by subsequent phosphorylation of the exported Glc by the action of an envelope-bound hexokinase (Wiese et al., 1999). The observed decreased ability to mobilize transitory starch during the dark period resembles that of the Arabidopsis sex1 mutant (Caspar et al., 1991), which some have assumed to be defective in the pGlcT (Trethewey and ap Rees, 1994a, 1994b). However, we demonstrated that sex1 does not carry a defect in the pGlcT.
Antisense repression of the gene encoding pGlcT or identification of a knockout mutant by means of reverse genetics is needed to characterize further the role of pGlcT in plant metabolism. Transgenic tobacco plants with reduced TPT activity have been demonstrated to show a transient increase in the amount of transitory starch. The accumulated starch is mobilized already during ongoing photosynthesis, and this starch mobilization is accompanied by increased activities of amylases, the pGlcT, and hexokinase (Häusler et al., 1998). Thus, both the pGlcT and the envelope-bound hexokinase are key players in the process of amylolytic breakdown of starch.
METHODS
Chemicals
14C-3-phosphoglycerate was synthesized from NaH14CO3, ribulose 1,5-bisphosphate, and ribulose 1,5-bisphosphate/carboxylase and was purified by ion exchange chromatography with an AG50X8-Cl− column (Bio-Rad) and a 0 to 400 mM LiCl gradient; its specific activity was determined by enzymatic analysis (Lowry and Passonneau, 1972) and scintillation counting. Other radiochemicals were purchased from New England Nuclear Life Science (Boston, MA) and ICN (Eschwege, Germany). ASA-Glc (N-[azidosalicyl]-6-amido-6-deoxyglucopyranose) was a gift of W. Hitz (DuPont, Wilmington, DE). Sorbitol and other chemicals were purchased from Sigma. Sorbitol (S-7547) was found by enzymatic analysis (Lowry and Passonneau, 1972) to contain 0.5 μg/g glucose (Glc) and was used without further purification.
Reagents and enzymes for recombinant DNA techniques were obtained from Promega. U. Sonnewald (Institut für Pflanzengenetik und Kulturpflanzenforschung, Gatersleben, Germany) kindly provided the cDNA libraries from potato and tobacco leaves. The cDNA libraries from spinach, Arabidopsis, and maize leaves were obtained from Stratagene.
Plant Material
Spinach (Spinacia oleracea cv Melody) plants were grown in 6-liter containers in a mixture of Metromix 360 (Scotts Sierra, Marysville, OH):sand (1:1 [v/v]) under a 10-hr photoperiod (25°C day and 20°C night) and a photosynthetic photon flux of 600 μmol m−2 sec−1 provided by sodium vapor and metal halide lamps. Plants were irrigated at 6-hr intervals with a balanced nutrient solution.
Chloroplast Isolation
Spinach leaves from 6-week-old plants were harvested 1 hr before the beginning of the light period. Chloroplasts were isolated and purified according to Robinson (1983) and resuspended in buffer B (330 mM sorbitol, 50 mM Hepes-KOH, pH 7.6, 1 mM MgCl2, 1 mM MnCl2, and 2 mM EDTA).
Uptake Assays
Uptake assays were initiated by exposing chloroplasts to d-14C-Glc at 20°C or 14C-3-phosphoglycerate at 4°C in room light; they were terminated by centrifugation of chloroplasts through silicone oil (Heldt, 1980). In addition to labeled substrates, l-3H-Glc or d-3H sorbitol were included to measure the sorbitol-permeable space. For assay times >5 sec, uptake was measured by using assay tubes containing a single oil layer (Heldt, 1980). One-second uptake assays were measured by modification of the double oil-layer method of Gross et al. (1990). Microcentrifuge tubes contained five layers: 50 μL of glycerol–methanol–water (2:1:1 [v/v]), 50 μL of polyphenylmethylsiloxane (silicone oil, AR200; Fluka, Deisenhofen, Germany), 100 μL of assay buffer (buffer B containing 0.22 M sorbitol, 0.11 M sucrose, and radiolabeled substrates), 50 μL of polyphenylmethylsiloxane, and 100 μL of chloroplast suspension in buffer B containing 50 μg of chlorophyll. From extrapolation of time-course measurements of 14C-3-phosphoglycerate uptake at 4°C by using a combination of single and double oil-layer tubes, we measured an uptake time of 0.85 sec for the double oil-layer method used. After centrifugation, the sample-containing tubes were frozen in liquid N2, and the lower part of the tubes containing the chloroplast pellet and a small part of the oil layer were removed with a tubing cutter and placed in 1.5-mL tubes containing 450 μL of 80% (v/v) methanol and 450 μL of Tris-HCl–washed CHCl3. The chloroplast pellet was dispersed, and the cut tip was discarded. Water (450 μL) then was added, and the sample was mixed on a vortex-type mixer. The two phases were separated by centrifugation for 1 min at 12,000g. The bottom organic layer was removed and diluted to 5 mL with ethanol. A649 and A665 were measured, from which the chlorophyll concentration was determined (Wintermans and DeMots, 1965). The aqueous phase then was diluted to 1 mL with water. Radioactivity was separated into basic, acidic, and neutral fractions by filtering the aqueous extract sequentially through 0.5-mL columns containing ion exchange resin forms AG50X8-H+ and AG1X8-formate (Bio-Rad). The radioactivity in each fraction was determined by scintillation counting.
Analysis of Radiolabeled Products
Chloroplasts were incubated in tubes containing 3H2O and 4 mM either d-14C-Glc or 14C-sorbitol for various times. Uptake was terminated by centrifugation through silicone oil. Tubes containing d-14C-Glc were analyzed to identify the nature of the radioactive products. The lower HClO4 layer was removed and neutralized with KOH. The chloroplast pellet was extracted in 0.2 mL of chloroform–methanol (1:3 [v/v]), and the extract was separated into organic and aqueous phases by adding 0.2 mL of water. Extracts were combined and diluted to 1 mL with water. An aliquot was removed for determining total radioactivity, and the remainder was passed successively through 0.5-mL ion exchange columns containing AG50X8-H+ and AG1X8-formate to bind basic and acidic products, respectively. Ion exchange columns were washed twice with 1 mL of water. Radioactivity passing through both columns was considered to represent label in neutral products. The columns were eluted with 2 mL of 2 N HCl, and aliquots were removed for determining radioactivity. The concentrations of labeled Glc and acidic products present in the sorbitol-impermeable space were calculated from measurements of 14C-sorbitol and 3H2O at the same times.
Differential Labeling and Membrane Isolation
Differential labeling of chloroplasts with 3H-N-ethylmaleimide (3H-NEM) and 14C-NEM was based on the procedure of Henderson and Macpherson (1986). Chloroplasts containing ∼20 mg of chlorophyll were incubated in 10 mL of buffer B and 1 mM DTT for 15 min at 20°C, divided equally between two tubes, and collected by centrifugation for 5 min at 2000g. Chloroplasts in one tube were resuspended in 10 mL of buffer B (unprotected); those in the other were resuspended in buffer B in which Glc replaced sorbitol (protected). After 15 min at 20°C, freshly prepared 5 mM NEM was added to each. After 30 min, chloroplasts were collected by centrifugation and resuspended in buffer B three times to remove traces of NEM and Glc. Unprotected chloroplasts were incubated for 30 min at 20°C with 0.5 mM 3H-NEM (500 Ci/mol) and protected chloroplasts with 0.5 mM 14C-NEM (100 Ci/mol). Both sets of chloroplasts were collected by centrifugation, and envelope membranes were prepared as described by Keegstra and Yousif (1986). Purified inner envelope vesicles were removed from the 1 and 0.8 M sucrose interface, diluted to 0.2 M sucrose, collected by ultracentrifugation at 90,000g for 1 hr, and resuspended in 0.1 mL of 0.2 M sucrose, 10 mM Hepes, and 1 mM MgCl2.
Sample Preparation and SDS-PAGE
Inner membrane vesicles from protected and unprotected chloroplasts were mixed and placed on ice. An equal volume of ice-cold n-butanol was added and mixed by vortex mixing for 10 sec. Layers were separated by centrifugation at 20,000g for 1 min in a microcentrifuge. The butanol and aqueous layers were removed, leaving the insoluble residue that appeared at the interface. The insoluble residue was extracted two more times with equal amounts of water and n-butanol, and these were added to the initial fractions and dried in a rotary evaporator (Bachhofer, Reutlingen, Germany). The small amount of insoluble residue remaining was added to the aqueous fraction. Lipids were removed from the dried butanol fraction by extraction with n-hexane. The hexane-insoluble residue was collected by centrifugation at 20,000g in a microcentrifuge. The hexane was removed, and the pellet was dried under N2. Proteins were solubilized in sample buffer (50 mM Tris-HCl, pH 6.8, 2% [w/v] SDS, 20% [v/v] glycerol, and 5 mM DTT), heated at 60°C for 30 min, and subjected to SDS-PAGE on a 12.5% acrylamide running gel and a 5% stacking gel at 10°C (Henderson and Macpherson, 1986). From gels that had been stained with Coomassie Brilliant Blue R 250, the lane containing the sample of interest was removed from the gel. Sections 1 mm thick were removed in order, and each was digested in 0.1 mL of 80% H2O2 and 70% HClO4 (6:1 [v/v]) for 1 hr at 70°C. After decay of chemiluminescence, the radioactivity present was determined by scintillation counting using a dual-labeling format. The percentage of total 14C and tritium radioactivity present in each section was determined. Data are plotted as the difference in percentage of total radioactivity present in each section (14Csection/14Ctotal − 3Hsection/3Htotal).
Peptide Sequencing of the Glc Translocator and Cloning of Its cDNA
Envelope membranes (5 mg/mL) were extracted by a 20-fold excess of n-butanol at pH 3.0. The butanol fraction was dried, washed with n-hexane, and analyzed by SDS-PAGE (Laemmli, 1970). The protein with an apparent molecular mass of 43 kD was excised from the Coomassie blue–stained gel and digested with endoproteinase Lys-C while still in the polyacrylamide matrix. The resulting peptides were eluted, purified by reverse phase HPLC (Eckerskorn and Lottspeich, 1989), and sequenced in a gas-phase sequencer (Eckerskorn et al., 1988).
From the 43-kD protein, we obtained one peptide sequence: KGRSLEEIELALSPAV (P1). Two degenerate oligonucleotides were designed on the basis of the peptide sequence: 5′-AARGGIMGIWIIYTIGARGARATIGA-3′ (01) and 5′-GARATIGARYTIGCIYTIWIICCIGC-3′ (02), where R is an A or G residue, I is inosine, Y is C or T, W is A or T, and M is A or C.
A first-strand cDNA was prepared by using poly(A)+ RNA from light- and dark-harvested spinach leaves, an oligo(dT)15 anchor primer (rapid amplification of cDNA ends [RACE] adapter primer) designated 5′-d(CCACGAGTCGACTCTAGAGCTCGGATCCTT[T]15)-3′, and a cDNA preamplification system, according to the instructions given by the manufacturer (Gibco BRL Life Technologies, Gaithersburg, MD). The resulting single-stranded cDNA was the template for the RACE procedure as described previously (Kammerer et al., 1998). The generated DNA fragment (210 bp) was subcloned into the pGEM-Teasy vector according to the instructions given by the manufacturer (Promega) and sequenced. The fragment was used for a plaque hybridization screening of a cDNA library from spinach leaves. Positive plaques were purified, and the pBluescript SK+ phagemid containing the cDNA insert was excised from the purified phages in vivo according to the instructions given by the manufacturer (Stratagene). The full-length cDNA was sequenced on both strands and then used for plaque hybridization screenings of cDNA libraries from maize, tobacco, potato, and Arabidopsis. Phagemids were prepared as described above, and the longest inserts were sequenced on both strands.
Protein Import Assays
Spinach chloroplasts were isolated from spinach leaves as described previously (Douce and Joyard, 1982). The cloned cDNA of the plastidic Glc translocator (pGlcT) protein from spinach leaves was transcribed and translated by the TNT lysate system, according to the instructions of the manufacturer (Promega); 35S-methionine was the labeled amino acid. Protein import assays were performed in import buffer (250 mM sorbitol, 10 mM methionine, 25 mM potassium gluconate, 2 mM MgSO4, 50 mM Hepes-KOH, pH 8.0, and 0.2% BSA) containing radiolabel, in vitro–synthesized precursor protein, and purified organelles equivalent to 200 μg of chlorophyll in a final volume of 300 μL. Other additions are indicated in the legend to Figure 7. The import reaction was allowed to proceed for 20 min. Envelope membranes were isolated as previously described (Flügge et al., 1989). All fractions were analyzed by SDS-PAGE and a PhosphoImager (Molecular Dynamics, Krefeld, Germany).
Isolation of RNA and RNA Gel Blot Hybridization
Total RNA was isolated from different tissues of tobacco according to Logemann et al. (1987). Equal amounts of RNA were size-fractionated on agarose–formaldehyde gels, and RNA was transferred to Pall Biodyne B nylon membranes (Pall GmbH, Dreieich, Germany) by downward capillary blot in 10 × SSC (1 × SSC is 0.15 M NaCl and 0.015 M sodium citrate). Hybridization of immobilized RNA to cDNA probes was performed overnight in 7% SDS (w/v), 500 mM NaPi, pH 7.2, and 1 mM EDTA (Church and Gilbert, 1984) at 62°C. Nonspecifically bound probe was removed by washing the filter with 6 × SSC and 0.2% SDS for 5 min at room temperature, with 4 × SSC and 0.2% SDS for 5 min at room temperature, and with 2 × SSC and 0.2% SDS at room temperature for 5 min, followed by two subsequent stringent washes with 1 × SSC and 0.2% SDS at 65°C for 5 min each.
Hybridization Analysis of Yeast Artificial Chromosome Clones
Mapping of pGlcT in the Arabidopsis genome with the help of yeast artificial chromosome (YAC) contigs immobilized on nylon filters was performed as described by Schmidt and Dean (1995).
Construction of Sequence Alignments and Phylogenetic Trees
Multiple alignment of protein sequences was performed with the help of the ClustalX program (Higgins et al., 1996; Thompson et al., 1997). From all sequences that showed N- or C-terminal extensions (e.g., targeting signals) in comparison with the related hexose transporter proteins, we removed these extensions to improve the quality of the alignments. We then constructed an unrooted phylogenetic tree from the aligned sequences, using the distance matrix neighbor-joining method (Saitou and Nei, 1987) from within the ClustalX program. The tree was displayed with the help of the TREEVIEW program (Page, 1996).
Acknowledgments
This work was supported in part by grants from the Monsanto Company (to D.R.G); Grant No. 9501219 from the U.S. Department of Agriculture, National Research Initiative Competitive Grants Program (to J.C.S); Grant No. IBN-9205966 from the National Science Foundation (to D.R.G); and grants from the Deutsche Forschungs-gemeinschaft (to U.-I.F. and A.W.) and the Fonds der Chemischen Industrie (to U.-I.F.). We are grateful to Dr. Renate Schmidt (Max Delbrück Labor, Cologne, Germany) for help with YAC filter hybridizations and mapping the pGlcT.
References
- Baldwin, S.A., and Lienhard, G.E. (1989). Purification and reconstitution of glucose transporter from human erythrocytes. Methods Enzymol. 174, 39–50. [DOI] [PubMed] [Google Scholar]
- Bechtold, N., Ellis, G., and Pelletier, G. (1993). In planta Agrobacterium-mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. C. R. Acad. Sci. 316, 1194–1199. [DOI] [PubMed] [Google Scholar]
- Beck, E. (1985). The degradation of transitory starch in chloroplasts. In Regulation of Carbon Partitioning in Photosynthetic Tissue, R.L. Heath and J. Preiss, eds (Baltimore, MD: Waverly), pp. 27–44.
- Capaldi, R.A., and Vanderkooi, G. (1972). The low polarity of many membrane proteins. Proc. Natl. Acad. Sci. USA 69, 930–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspar, T., Huber, S.C., and Somerville, C. (1985). Alterations in growth, photosynthesis, and respiration in a starchless mutant of Arabidopsis thaliana (L) deficient in chloroplast phosphoglucomutase activity. Plant Physiol. 79, 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspar, T., Lin, T.-P., Kakefuda, G., Benbow, L., Preiss, J., and Somerville, C. (1991). Mutants of Arabidopsis with altered regulation of starch degradation. Plant Physiol. 95, 1181–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church, G.M., and Gilbert, W. (1984). Genomic sequencing. Proc. Natl. Acad. Sci. USA 81, 1991–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douce, R., and Joyard, J. (1982). Purification of the chloroplast envelope. In Methods of Chloroplast Molecular Biology, M. Edelman, R.B. Hallick, and N.-H. Chua, eds (Amsterdam, The Netherlands: Elsevier), pp. 239–256.
- Dreses-Weringloer, U., Fischer, K., Wachter, E., Link, T.A., and Flügge, U.I. (1990). cDNA sequence of the precursor of the 37 kDa inner envelope membrane polypeptide from spinach chloroplasts: Its transit peptide contains an amphiphilic α-helix as the only detectable structural element. Eur. J. Biochem. 195, 361–368. [DOI] [PubMed] [Google Scholar]
- Eckerskorn, C., and Lottspeich, F. (1989). Internal amino acid sequence analysis of proteins separated by gel electrophoresis after tryptic digestion in polyacrylamide matrix. Chromatographia 28, 92–94. [Google Scholar]
- Eckerskorn, C., Mewes, W., Goretzki, H., and Lottspeich, F. (1988). A new siliconized-glass fiber as support for protein chemical analysis of electroblotted proteins. Eur. J. Biochem. 176, 509–519. [DOI] [PubMed] [Google Scholar]
- Emanuelsson, O., Nielsen, H., and von Heijne, G. (1999). ChloroP, a neural network-based method for predicting chloroplast transit peptides and their cleavage sites. Protein Sci. 8, 978–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliege, R., Flügge, U.-I., Werdan, K., and Heldt, H.W. (1978). Specific transport of inorganic phosphate, 3-phosphoglycerate and triosephosphates across the inner membrane of the envelope in spinach chloroplasts. Biochim. Biophys. Acta 502, 232–247. [DOI] [PubMed] [Google Scholar]
- Flügge, U.-I., Fischer, K., Gross, A., Sebald, W., Lottspeich, F., and Eckerskorn, C. (1989). The triose phosphate-3-phosphoglycerate-phosphate translocator from spinach chloroplasts: Nucleotide sequence of a full-length cDNA clone and import of the in vitro synthesized precursor protein into chloroplasts. EMBO J. 8, 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fondy, B.R., Geiger, D.R., and Servaites, J.C. (1989). Photosynthesis, carbohydrate metabolism and export in Beta vulgaris L. and Phaseolus vulgaris L. during square and sinusoidal light regimes. Plant Physiol. 89, 396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger, D.R., Shieh, W.-J., and Yu, X.-M. (1995). Photosynthetic carbon metabolism and translocation in wild-type and starch-deficient mutant Nicotiana sylvestris L. Plant Physiol. 107, 507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger, D.R., Servaites, J.C., and Fuchs, M.A. (2000). Role of starch in carbon translocation and partitioning at the plant level. Aust. J. Plant Physiol., in press.
- Gerhardt, R., Stitt, M., and Heldt, H.W. (1987). Subcellular metabolite levels in spinach leaves. Plant Physiol. 83, 399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleixner, G., Scrimgeour, C., Schmidt, H.-L., and Viola, R. (1998). Stable isotope distribution in the major metabolites of source and sink organs of Solanum tuberosum L.: A powerful tool in the study of metabolite partitioning in intact plants. Planta 207, 241–245. [Google Scholar]
- Gould, G.W., and Bell, G.I. (1990). Facilitative glucose transporters: An expanding family. Trends Biochem. Sci. 15, 18–23. [DOI] [PubMed] [Google Scholar]
- Gross, A., Brückner, G., Heldt, H.W., and Flügge, U.-I. (1990). Comparison of the kinetic properties, inhibition and labelling of the phosphate translocators from maize and spinach mesophyll chloroplasts. Planta 180, 262–271. [DOI] [PubMed] [Google Scholar]
- Hanson, K.R., and McHale, N.A. (1988). A starchless mutant of Nicotiana sylvestris containing a modified plastid phosphoglucomutase. Plant Physiol. 88, 838–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häusler, R.E., Schlieben, N.H., Schulz, B., and Flügge, U.-I. (1998). Compensation of decreased triose phosphate/phosphate translocator activity by accelerated starch turnover and glucose transport in transgenic tobacco. Planta 204, 366–376. [DOI] [PubMed] [Google Scholar]
- Heineke, D., Kruse, A., Flügge, U.I., Frommer, W.B., Riesmeier, J.W., Willmitzer, L., and Heldt, H.W. (1994). Effect of antisense repression of the chloroplast triose-phosphate translocator on photosynthetic metabolism in transgenic potato plants. Planta 193, 174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt, H.W. (1980). Measurement of metabolite movement across the envelope and of the pH in the stroma and the thylakoid space in intact chloroplasts. Methods Enzymol. 69, 604–613. [Google Scholar]
- Henderson, P.J.F. (1991). Sugar transport proteins. Curr. Opin. Struct. Biol. 1, 590–601. [Google Scholar]
- Henderson, P.J.F., and Macpherson, A.J.S. (1986). Assay, genetics, proteins, and reconstitution of proton-linked galactose, arabinose, and xylose transport systems of Escherichia coli. Methods Enzymol. 125, 387–429. [DOI] [PubMed] [Google Scholar]
- Herold, A., Leegood, R.C., McNeil, P.H., and Robinson, S.P. (1981). Accumulation of maltose during photosynthesis in protoplasts isolated from spinach leaves treated with mannose. Plant Physiol. 67, 85–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins, D.G., Thompson, J.D., and Gibson, T.J. (1996). Using CLUSTAL for multiple sequence alignments. Methods Enzymol. 266, 383–402. [DOI] [PubMed] [Google Scholar]
- Hosang, M., Gibbs, E.M., Deidrich, D.F., and Semenza, G. (1981). Photoaffinity labeling and identification of (a component of) the small-intestinal Na+, d-glucose transporter using 4-azidophlorizin. FEBS Lett. 130, 244–248. [DOI] [PubMed] [Google Scholar]
- Huber, S.C., and Hanson, K.R. (1992). Carbon partitioning and growth of a starchless mutant of Nicotiana sylvestris. Plant Physiol. 99, 1449–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammerer, B., Fischer, K., Hilpert, B., Schubert, S., Gutensohn, M., Weber, A., and Flügge, U.-I. (1998). Molecular characterization of a carbon transporter in plastids of heterotrophic tissues: The glucose 6-phosphate/phosphate antiporter. Plant Cell 10, 105–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keegstra, K., and Yousif, A.E. (1986). Isolation and characterization of chloroplast envelope membranes. Methods Enzymol. 118, 316–325. [Google Scholar]
- Klip, A., Walker, D., Ransome, K.J., Schroer, D.W., and Lienhard, G.E. (1983). Identification of the glucose transporter in rate skeletal muscle. Arch. Biochem. Biophys. 226, 198–205. [DOI] [PubMed] [Google Scholar]
- Konieczny, A., and Ausubel, F.M. (1993). A procedure for mapping Arabidopsis mutations using co-dominant ecotype-specific PCR-based markers. Plant J. 4, 403–410. [DOI] [PubMed] [Google Scholar]
- Kyte, J., and Doolittle, R.F. (1982). A simple method for displaying the hydrophobic character of a protein. J. Mol. Biol. 157, 105–132. [DOI] [PubMed] [Google Scholar]
- Laemmli, U.K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685. [DOI] [PubMed] [Google Scholar]
- Li, H., Sullivan, T.D., and Keegstra, K. (1992). Information for targeting to the chloroplastic inner envelope membrane is contained in the mature region of the maize Bt1-encoded protein. J. Biol. Chem. 267, 18999–19004. [PubMed] [Google Scholar]
- Lockshin, A., and Burris, R.H. (1966). Solubilization and properties of chloroplast lamellar protein. Proc. Natl. Acad. Sci. USA 56, 1564–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logemann, J., Schell, J., and Willmitzer, L. (1987). Improved method for the isolation of RNA from plant tissues. Anal. Biochem. 163, 16–20. [DOI] [PubMed] [Google Scholar]
- Lowry, O.H., and Passonneau, J.V. (1972). A Flexible System of Enzymatic Analysis. (New York: Academic Press).
- Moore, B.D., Palmquist, D.E., and Seemann, J.R. (1997). Influence of plant growth at high CO2 concentrations on leaf content of ribulose-1,5-bisphosphate carboxylase/oxygenase and intracellular distribution of soluble carbohydrates in tobacco, snapdragon, and parsley. Plant Physiol. 115, 241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page, R.D.M. (1996). TREEVIEW: An application to display phylogenetic trees on personal computers. Comp. Appl. Biosci. 12, 357–358. [DOI] [PubMed] [Google Scholar]
- Piccioni, R., Bellemare, G., and Chua, N.-H. (1982). Methods of polyacrylamide gel electrophoresis in the analysis and preparation of plant polypeptides. In Methods of Chloroplast Molecular Biology, M. Edelman, R.B. Hallick, and N.-H. Chua, eds (Amsterdam, The Netherlands: Elsevier), pp. 985–1014.
- Pohlmeyer, K., Soll, J., Steinkamp, T., Hinnah, S., and Wagner, R. (1997). Isolation and characterization of an amino acid–selective channel protein present in the chloroplastic outer envelope membrane. Proc. Natl. Acad. Sci. USA 94, 9504–9509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, S.P. (1983). Isolation of intact chloroplasts with high CO2 fixation from sugar beet leaves containing calcium oxalate. Photosynth. Res. 4, 281–287. [DOI] [PubMed] [Google Scholar]
- Rost, S., Frank, C., and Beck, E. (1996). The chloroplast envelope is permeable for maltose but not for maltodextrins. Biochim. Biophys. Acta 1291, 221–227. [DOI] [PubMed] [Google Scholar]
- Rumpho, M.E., and Edwards, G.E. (1985). Characterization of 4,4′-diisothiocyano-2,2′-disulfonic acid stilbene inhibition of 3-phosphoglycerate–dependent O2 evolution in isolated chloroplasts. Plant Physiol. 78, 537–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier, M.H., Jr. (1998). Molecular phylogeny as a basis for the classification of transport proteins from bacteria, archaea and eukarya. Adv. Microbiol. Physiol. 40, 81–136. [DOI] [PubMed] [Google Scholar]
- Saitou, N., and Nei, M. (1987). The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4, 406–425. [DOI] [PubMed] [Google Scholar]
- Schaefer, B.C. (1995). Revolutions in the rapid amplification of cDNA ends: New strategies for polymerase chain reaction cloning of full-length cDNA ends. Anal. Biochem. 227, 255–273. [DOI] [PubMed] [Google Scholar]
- Schäfer, G., Heber, U., and Heldt, H.W. (1977). Glucose transport into spinach chloroplasts. Plant Physiol. 60, 286–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleucher, J., Vanderveer, P.J., and Sharkey, T.D. (1998). Export of carbon from chloroplasts at night. Plant Physiol. 118, 1439–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, R., and Dean, C. (1995). Hybridization analysis of YAC clones. Methods Mol. Cell. Biol. 5, 309–318. [Google Scholar]
- Schmidt, R., Love, K., West, J., Lenehan, Z., and Dean, C. (1997). Description of 31 YAC contigs spanning the majority of Arabidopsis thaliana chromosome 5. Plant J. 11, 563–572. [DOI] [PubMed] [Google Scholar]
- Servaites, J.C., Geiger, D.R., Tucci, M.A., and Fondy, B.R. (1989. a). Leaf carbon metabolism and metabolite levels during a period of sinusoidal light. Plant Physiol. 89, 403–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servaites, J.C., Fondy, B.R., Li, B., and Geiger, D.R. (1989. b). Sources of carbon for export from spinach leaves throughout the day. Plant Physiol. 90, 1168–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanahan, M.F. (1982). Cytochalasin B. A natural photoaffinity ligand for labeling the human erythrocyte glucose transporter. J. Biol. Chem. 257, 7290–7293. [PubMed] [Google Scholar]
- Shanahan, M.F., Wadzinski, B.E., Lowndes, J.M., and Ruoho, A.E. (1985). Photoaffinity labeling of the human erythrocyte monosaccharide transporter with an aryl azide derivative of d-glucose. J. Biol. Chem. 260, 10897–10900. [PubMed] [Google Scholar]
- Stitt, M. (1990). Fructose-2,6-bisphosphate as a regulatory metabolite in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 41, 153–185. [Google Scholar]
- Stitt, M., Wirtz, W., Gerhardt, R., Heldt, H.W., Spencer, C., Walker, D., and Foyer, C. (1985). A comparative study of metabolite levels in plant leaf material in the dark. Planta 166, 354–364. [DOI] [PubMed] [Google Scholar]
- Thompson, J.D., Gibson, T.J., Plewniak, F., Jeanmougin, F., and Higgins, D.G. (1997). The ClustalX windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25, 4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trethewey, R.N., and ap Rees, T. (1994. a). A mutant of Arabidopsis thaliana lacking the ability to transport glucose across the chloroplast envelope. Biochem J. 301, 449–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trethewey, R.N., and ap Rees, T. (1994. b). The role of the hexose transporter in the chloroplasts of Arabidopsis thaliana L. Planta 195, 168.–174. [Google Scholar]
- Veramendi, J., Roessner, U., Renz, A., Willmitzer, L., and Trethewey, R.N. (1999). Antisense repression of hexokinase 1 leads to an overaccumulation of starch in leaves of transgenic potato plants but not to significant changes in tuber carbohydrate metabolism. Plant Physiol. 121, 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmsley, A.R., Barett, M.P., Bringaud, F., and Gould, G.W. (1998). Sugar transporters from bacteria, parasites and mammals: Structure–activity relationships. Trends Biochem. Sci. 23, 476–481. [DOI] [PubMed] [Google Scholar]
- Weber, A., Menzlaff, E., Arbinger, B., Gutensohn, M., Eckerskorn, C., and Flügge, U.-I. (1995). The 2-oxoglutarate/malate translocator of chloroplast envelope membranes: Molecular cloning of a transporter protein containing a 12-helix motif and expression of the functional protein in yeast cells. Biochemistry 34, 2621–2627. [DOI] [PubMed] [Google Scholar]
- Wellner, M., Monden, I., and Keller, K. (1994). The role of cysteine residues in glucose-transporter-GLUT1–mediated transport and transport inhibition. Biochem. J. 299, 813–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese, A., Gröner, F., Sonnewald, U., Deppner, H., Lerchl, J., Hebbeker, U., Flügge, U.I., and Weber, A. (1999). Spinach hexokinase I is located in the outer envelope membrane of plastids. FEBS Lett. 461, 13–18. [DOI] [PubMed] [Google Scholar]
- Wintermans, J.F., and DeMots, A. (1965). Spectrophotometric characteristics of chlorophyll a and b and their pheophytins in ethanol. Biochim. Biophys. Acta 109, 448–453. [DOI] [PubMed] [Google Scholar]
- Zeeman, S.C., Northorp, F., Smith, A.M., and ap Rees, T. (1998. a). A starch-accumulating mutant of Arabidopsis thaliana deficient in a chloroplastic starch-hydrolysing enzyme. Plant J. 15, 357–365. [DOI] [PubMed] [Google Scholar]
- Zeeman, S.C., Umemoto, T., Lue, W.L., Au-Yeung, P., Martin, C., Smith, A.M., and Chen, J. (1998. b). A mutant of Arabidopsis lacking a chloroplastic isoamylase accumulates both starch and phytoglycogen. Plant Cell 10, 1699–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]