Abstract
Partial acid/enzymatic hydrolysis of the β-(1→3, 1→6)-glucan from the cell walls of the rice blast disease fungus Pyricularia oryzae (Magnaporthe grisea) released elicitor-active fragments that induced phytoalexin biosynthesis in suspension-cultured rice cells. From the digestion of the glucan by an endo-β-(1→3)-glucanase, one highly elicitor-active glucopentaose was purified as a reduced compound, tetraglucosyl glucitol. The structure of this tetraglucosyl glucitol as well as two other related tetraglucosyl glucitols was elucidated as follows: (1) Glcβ(1→3)Glcβ(1→3)(Glcβ(1→6)) Glcβ(1→3)Glucitol (most active fragment); (2) Glcβ(1→3)(Glcβ(1→6))Glcβ(1→3)Glcβ(1→3)Glucitol; and (3) Glcβ(1→6) Glcβ(1→3)Glcβ(1→3)Glcβ(1→3)Glucitol. However, a synthetic hexa-β-glucoside, known as a minimal structural element for the phytoalexin elicitor for soybean cotyledon cells, did not induce phytoalexin biosynthesis in the rice cells. Conversely, the β-glucan fragment from P. oryzae did not induce phytoalexin biosynthesis in the soybean cotyledon cells, indicating differences in the recognition of glucooligosaccharide elicitor signals in these two plants. Because rice cells have been shown to recognize chitin fragments larger than pentamers as potent elicitors, these results also indicate that the rice cells can recognize at least two types of oligosaccharides from fungal cell walls as signal molecules to initiate defense response.
INTRODUCTION
When attacked by pathogens such as fungi, bacteria, and viruses, higher plants initiate various defense responses, including the following: production of phytoalexins, enzymes such as chitinase and β-glucanase, proteinase inhibitors, and hydroxyproline-rich glycoproteins; generation of reactive oxygen species; and lignification (Dixon and Harrison, 1990; Ryan and Farmer, 1991). These responses can be triggered by the elicitor molecules derived from the cell surfaces of various fungi, bacteria, and host plants or from the proteins/glycoproteins secreted by microorganisms (Darvill and Albersheim, 1984; Côté and Hahn, 1994; Ebel and Cosio, 1994). Because the interaction of the elicitor molecules with putative receptor(s) should be the first step in the initiation of the defense responses, it is critically important to characterize both the structural elements required for the recognition and the receptor molecules to understand the molecular basis for the perception and transduction of elicitor signals in plants.
Fragments of the β-glucan from the pathogenic fungus Phytophthora sojae are among the best-characterized elicitor molecules, eliciting the biosynthesis of a phytoalexin, glyceollin, in soybean cotyledon cells (Sharp et al., 1984a, 1984b). The structure of a highly elicitor-active heptaglucoside was elucidated, and critical glucosyl residues for the recognition were clarified by comparing the activities of various synthetic oligosaccharides (Cheong et al., 1991). This heptaglucoside was shown to elicit the phytoalexin biosynthesis even at a low nanomolar concentration. Although the mixture of fragments from this β-glucan preparation also induced defense responses in several other plants (Cline et al., 1978; Brady et al., 1993; Côté and Hahn, 1994), it is not clear whether the heptaglucoside structure is generally important for recognition by other plants or whether they recognize some other structural unit.
In this article, we report that suspension-cultured rice cells can recognize specific fragments of the β-glucan derived from the cell walls of the rice blast disease fungus Pyricularia oryzae (Magnaporthe grisea) as a potent elicitor for phytoalexin biosynthesis. We show the structure of one highly elicitor-active tetraglucosyl glucitol, the minimal elicitor-active oligosaccharide, and two related tetraglucosyl glucitols derived from the β-glucan. We also show the results of a comparison of the elicitor activity of the glucan fragment with that of the hepta-β-glucoside for rice and soybean cells. These results indicate that the glucooligosaccharide elicitors recognized by rice and soybean cells are quite different, probably reflecting differences in specificity of the receptors in these two plants.
RESULTS
Generation of Elicitor-Active Fragments from the β-Glucan Preparations from Cell Walls of P. oryzae
Cell walls of the rice blast disease fungus P. oryzae are composed of three major constituents: chitin, β-(1→3, 1→6)-glucan, and a proteoheteroglycan rich in mannose (Nakajima et al., 1970). A preliminary survey of the elicitor activity of the partial acid hydrolysates of these polysaccharides in suspension-cultured rice cells showed marked activity in the β-glucan fraction, thus prompting us to isolate active components from this fraction. Also, the addition of β-glucosidase inhibitors such as thio-β-d-glucose and glucono-δ-lactone to the reaction mixture was found to be very important for obtaining reproducible results, suggesting the presence of endogenous glucosidases that inactivate the elicitor-active fragments in the rice cells or medium. After extraction of the proteoheteroglycan from the cell wall preparation of P. oryzae, a β-glucan–rich fraction was extracted with 1 N NaOH and fractionated into two fractions, glucan I and glucan II, by fractional precipitation. Each glucan fraction consisted of 95 to 98% glucose and contained small amounts of mannose and galactose. Methylation analysis of both glucan preparations indicated the presence of typical (1→3, 1→6)–linked glucan structures as reported by Nakajima et al. (1972), although the degree of branching of these two fractions was very different (Table 1). Enzymatic digestion of these glucan preparations by a purified endo-β-(1→3)-glucanase at a concentration of 1 μg/mL released products that could elicit the production of phytoalexins in suspension-cultured rice cells (Table 2). Momilactones A and B were the major phytoalexins produced, along with a small amount of oryzalexins, similar to the results with chitin fragments (Yamada et al., 1993). Accumulation of momilactone A reached a maximum ∼72 hr after the addition of the elicitor. The amount of induced phytoalexins varied from experiment to experiment, depending mainly on the conditions of the cultured cells, but typically ranged from 50 to 200 μg/g cells for momilactone A. All of the experiments were performed using the same batch of cultured cells to ensure the validity of the comparison.
Table 1.
Methylation Analysis of Glucan Preparations from P. oryzae
| Molar Ratio
|
|||
|---|---|---|---|
| Compound | Deduced Linkage | Glucan I | Glucan II |
| 2,3,4,6-Me4-Glc | t-Glc | 0.9 | 0.9 |
| 2,4,6-Me3-Glc | 3-Glc | 46 | 2.0 |
| 2,4-Me2-Glc | 3,6-Glc | 1.0 | 1.0 |
Table 2.
Elicitor Activity of the β-(1→3)-Glucanase Digests of Glucan Preparations from P. oryzae
| Compound | Concentration (μg/mL) |
Elicitor Activity Momilactone A (μg/g) |
|---|---|---|
| Glucan I | 10 | 162 (±15) |
| 1 | 56 (±8) | |
| 0.1 | 0 | |
| Glucan II | 10 | 127 (±20) |
| 1 | 35 (±6) | |
| 0.1 | 0 |
Isolation of Elicitor-Active β-Glucan Fragments
The partial enzymatic hydrolysate of the less-branched glucan preparation, glucan I, was used to isolate elicitor-active fragments. Gel filtration of the hydrolysate on Biogel P-4 (data not shown; see Methods) showed detectable elicitor activity in the oligosaccharide fractions larger than tetraose.
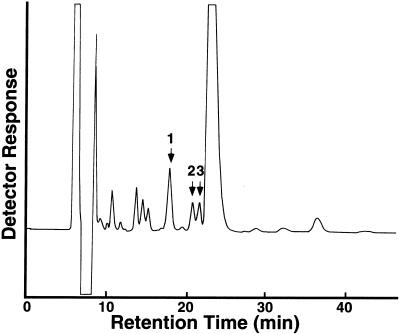
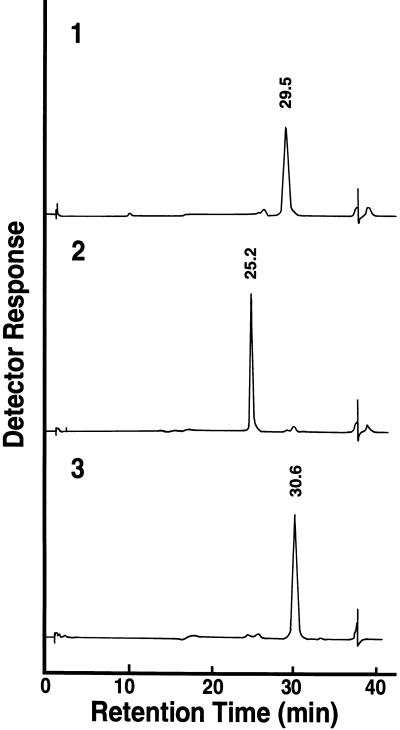
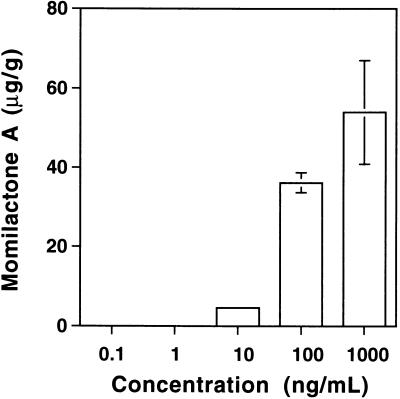
The smallest elicitor-active fraction, the pentaose fraction, was further fractionated by reverse-phase HPLC after the oligosaccharides in this fraction had been converted to the corresponding alditols with sodium borohydride to avoid separation of the corresponding anomers (Figure 1). The elicitor activity was little affected by this treatment (data not shown). Elicitor assay of each oligosaccharide recovered from HPLC indicated that only a few oligosaccharides carried detectable elicitor activity (Figure 1). These oligosaccharides were rechromatographed and purified to apparent homogeneity (data not shown). The purity of each oligosaccharide was further confirmed by high-performance anion-exchange chromatography (HPAEC) (Figure 2). Figure 3 shows the dose dependency of the elicitor activity of the peak 1 oligosaccharide, which showed the greatest and most reproducible activity of the three fractions. Elicitor activity was detected with as little peak 1 oligosaccharide as 10 ng/mL. Similar quantitative experiments could not be performed for the other two oligosaccharides because of their much weaker activities (at least 100-fold less than that of the peak 1 oligosaccharide) as well as their lower yield.
Figure 1.
Purification of Elicitor-Active Tetraglucosyl Glucitols by HPLC.
Fragments of the β-glucan generated by the enzymatic digestion were fractionated on Biogel P-4. All fractions larger than tetramers showed a potent elicitor activity. The pentaose fraction was reduced by NaBH4, and 5 mg of the reduced product was further fractionated by reverse-phase HPLC on an Inertsil ODS 3 column. The column was equilibrated with 8% CH3OH at 0.5 mL/min for 2 hr before the addition of the sample and was eluted with the same buffer. Peaks indicated by the arrows showed detectable elicitor activity.
Figure 2.
Analysis of the Purity of Each Tetraglucosyl Glucitol Fraction by HPAEC.
Each of the three peak fractions indicated in Figure 1 was rechromatographed on the same column and analyzed for purity by HPAEC with an HPLC (Dionex, model D-300 BioLC) equipped with a CarboPac PA1 column and a pulsed amperometric detector. The elution profiles 1, 2, and 3 correspond to those of purified peaks 1, 2, and 3 shown in Figure 1, respectively.
Figure 3.
Dose Response of the Purified Peak 1 Oligosaccharide for Momilactone A Biosynthesis.
The values represent the average of triplicate experiments. The error bars indicate ±sd.
Structure of Elicitor-Active Tetraglucosyl Glucitols
All three oligosaccharides gave only glucose and glucitol by acid hydrolysis. Electrospray mass spectrometry (MS) indicated that they are all tetraglucosyl glucitols. The anomeric configuration of the tetraglucosyl glucitol was determined by 1H–nuclear magnetic resonance spectroscopy (data not shown). The three anomeric protons at δ 4.66 (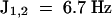 ) were assigned to the anomeric protons of the three β-(1→ 3)–linked residues. The one proton signal at δ 4.59 (
) were assigned to the anomeric protons of the three β-(1→ 3)–linked residues. The one proton signal at δ 4.59 (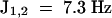 ) was assigned to the β-(1→6)–linked glycosyl residue, given that the anomeric proton of β-(1→3)–linked residues generally resonates at a lower field than H-1 of the same residues in a β-(1→6) linkage (Ló et al., 1993). Methylation analysis was performed only for peak 1 oligosaccharide, the most elicitor-active and abundant oligosaccharide. Methylation analysis indicated the presence of a nonreducing end and 3-linked and 3,6-linked glucose residues in an approximate ratio of 2:1:1 in the original tetraglucosyl glucitol (Table 3). The presence of 1,2,4,5,6-penta-O-methylglucitol in the hydrolysate showed that the reducing-end glucose residue was substituted only at the O-3 position (Table 3), although the yield of this very volatile compound was considerably less than the expected value for a tetraglucosyl glucitol.
) was assigned to the β-(1→6)–linked glycosyl residue, given that the anomeric proton of β-(1→3)–linked residues generally resonates at a lower field than H-1 of the same residues in a β-(1→6) linkage (Ló et al., 1993). Methylation analysis was performed only for peak 1 oligosaccharide, the most elicitor-active and abundant oligosaccharide. Methylation analysis indicated the presence of a nonreducing end and 3-linked and 3,6-linked glucose residues in an approximate ratio of 2:1:1 in the original tetraglucosyl glucitol (Table 3). The presence of 1,2,4,5,6-penta-O-methylglucitol in the hydrolysate showed that the reducing-end glucose residue was substituted only at the O-3 position (Table 3), although the yield of this very volatile compound was considerably less than the expected value for a tetraglucosyl glucitol.
Table 3.
Methylation Analysis of Peak 1 Oligosaccharide
| Compound | Deduced Linkage | Molar Ratio |
|---|---|---|
| 1,2,4,5,6-Me5-Glc | 3-Glucitol | 0.4 |
| 2,3,4,6-Me4-Glc | t-Glc | 2.0 |
| 2,4,6-Me3-Glc | 3-Glc | 1.2 |
| 2,4-Me2-Glc | 3,6-Glc | 1.0 |
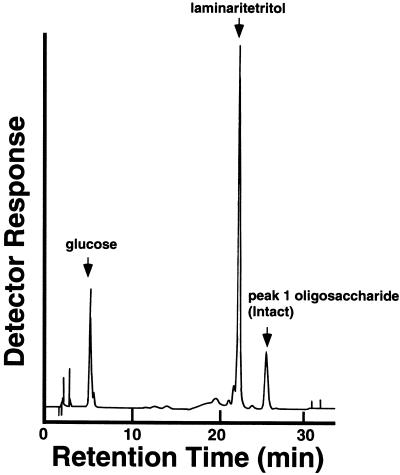
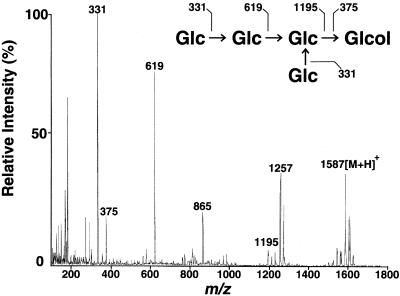
Acetolysis, which is known to cleave (1→6) linkages much faster than (1→3) linkages (Rosenfeld and Ballou, 1974), yielded only laminaritetritol and glucose from all three oligosaccharides (Figure 4). These results indicated that the original tetraglucosyl glucitols have common structural features in which a glucosyl residue is attached to the backbone of laminaritetraose through (1→6) linkage. Because the reducing-end glucosyl residue is not branched, as discussed above, possible structures for these oligosaccharides are restricted to those listed in Figure 5. The fragmentation pattern of the acetylated tetraglucosyl glucitols (Figure 6) obtained by electrospray MS provided a useful basis for the identification of the corresponding structure for each tetraglucosyl glucitol, as shown in Figure 5 for peak 1 oligosaccharide. Signals corresponding to A+-type (non-reducing-end) fragment ions at mass-to-charge ratios (m/z) of 1195, 619, and 331 and to reducing-end fragment ions at m/z 375 were also observed. Electrospray MS–MS daughter-ion spectra of the fragment ions at m/z 1257 and 865 contained daughter ions at m/z 619 and 331, corresponding to [Glc-Glc]+ and [Glc]+ ions, respectively (data not shown). Therefore, both of the ions at m/z 1257 and 865 can be generated by the β-cleavage–type loss of a side-chain glucosyl unit from the parent ion (m/z 1587) or from the ion at m/z 1195 (“double-cleavage”; Dell, 1987). Final assignment of the structure of each tetraglucosyl glucitol is shown in Figure 5.
Figure 4.
Analysis of the Fragments Obtained by Acetolysis of the Purified Peak 1 Oligosaccharide.
The fragments obtained by acetolysis were analyzed by HPAEC. The three major peaks corresponded to glucose, laminaritetritol, and peak 1 oligosaccharide, respectively.
Figure 5.
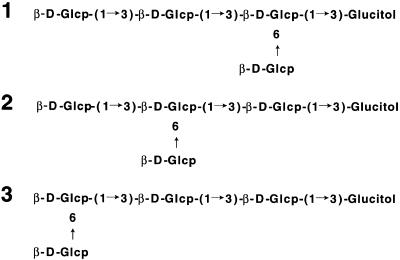
Structure of the Elicitor-Active Tetraglucosyl Glucitol Obtained from the β-Glucan of P. Oryzae.
Structures 1 to 3 correspond to those of purified peaks 1, 2, and 3 shown in Figure 1, respectively. Glcp, glucopyranose.
Figure 6.
Electrospray Mass Spectrum of the Per-O-Acetylated Peak 1 Oligosaccharide.
Assignment of major fragments (numbered peaks) is also shown. The mass spectrum was obtained with a biomolecular mass analyzer. Glc, glucopyranosyl residue; Glcol, glucitol; [M + H], protonated parent ion.
Differences between Rice and Soybean in Recognition of Glucooligosaccharide Elicitors
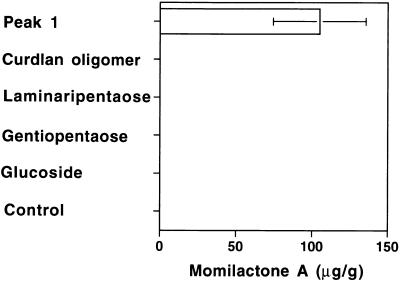
Based on the structures of the isolated elicitor-active tetraglucosyl glucitols shown in Figure 5, the elicitor activity of several structurally related oligosaccharides was tested (Figure 7). Linear glucopentaoses consisting of 3-linked or 6-linked residues, laminaripentaose and gentiopentaose, respectively, showed no detectable elicitor activity in rice cells. A partial hydrolysate of curdlan, a mixture of β-(1→3)–linked glucooligosaccharides, was also not active. These results suggested the importance of the branched structure in the peak 1 oligosaccharide for the elicitor activity. A synthetic hexa-β-glucoside (Hong and Ogawa, 1990), already shown to be a minimal structural element for the phytoalexin elicitor for soybean cotyledon (Cheong et al., 1991), did not show any activity, even at 10 μg/mL—a 1000-fold higher concentration than that required for peak 1 oligosaccharide to show detectable activity in rice cells and also >1000-fold than required for the hexaglucoside itself to show detectable activity in the soybean cells. The result clearly showed that the rice cells cannot recognize this compound as a phytoalexin elicitor.
Figure 7.
Comparison of the Elicitor Activities of Peak 1 Oligosaccharide, Curdran Oligomer, Laminaripentaose, Gentiopentaose, Laminaripentaose, and Synthetic Hexa-(1→3, 1→6)-β-Glucoside.
The concentration of each sugar was 1 μg/mL for peak 1 oligosaccharide (Peak 1) and 10 μg/mL for other sugars. Each reaction mixture contained 10 μg/mL thio-β-glucose to prevent degradation of the sugars by endogenous glucosidases. Thio-β-glucose solution without the tested sugars was used as a control. The values represent the average of duplicate experiments. The error bar indicates ±sd. Glucoside, hexa-(1→3, 1→6)-β-glucoside.
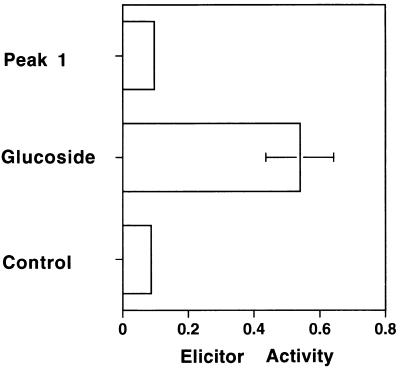
On the other hand, peak 1 oligosaccharide, at a sufficiently high concentration to induce phytoalexin biosynthesis in the rice cells, could not induce phytoalexin biosynthesis in the soybean cotyledon cells (Figure 8), indicating that the soybean cells cannot recognize the glucan fragment that is a potent elicitor for the rice cells.
Figure 8.
Comparison of the Elicitor Activity of Peak 1 Oligosaccharide and Synthetic Hexa-(1→3, 1→6)-β-Glucoside for Soybean Cotyledon Cells.
One microgram per milliliter of each sugar was used for the assay. Thio-β-glucose (10 μg/mL) was used as a control. The values represent the average of duplicate experiments. Elicitor activity was determined at A285 nm. The error bar indicates ±sd. Glucoside, hexa-(1→3, 1→6)-β-glucoside; Peak 1, peak 1 oligosaccharide.
DISCUSSION
In this article, we show that the fragments of the (1→3, 1→6)–linked β-glucans from the cell walls of the rice blast disease fungus P. oryzae are a potent elicitor of phytoalexin biosynthesis in suspension-cultured rice cells; we also report the structure of one highly elicitor-active and two related less active tetraglucosyl glucitols isolated from this preparation. Little information has been available on β-glucan fragments that act as an elicitor signal for the production of phytoalexins in a monocot plant. Although Inui et al. (1997) reported that laminarioligosaccharides induced chitinase activity in suspension-cultured rice cells at a fairly high concentration (1 to 100 μg/mL), the activity of linear laminarioligosaccharides to induce phytoalexin biosynthesis seems to be very low, insofar as we could not detect any activity for laminaripentaose or a partial hydrolysate of curdlan. These results suggest that the branched structure in the peak 1 oligosaccharide is critical for the elicitor activity. On the other hand, the very low elicitor activity of the two closely related tetraglucosyl glucitols (peaks 2 and 3) indicated that the extended laminaribiose unit in the peak 1 oligosaccharide may also contribute to the interaction of this oligosaccharide with a putative receptor molecule.
An interesting feature of the peak 1 oligosaccharide is the contrast between its structure and that of the hepta-β-glucoside elicitor, which is known as a potent phytoalexin elicitor in soybean (Sharp et al., 1984b). Instead of the 6-linked backbone and 3-linked glucosyl stubs in the heptaglucoside structure, the peak 1 oligosaccharide has a 3-linked backbone and a 6-linked glucosyl stub. This difference in structure probably explains the different elicitor activities of these oligosaccharides in each plant. Although the different structural requirement for the elicitor-active glucan fragments in these two plants is obvious, precise characterization of the structural unit recognized by the rice cells awaits further experiments with various structurally related oligosaccharides. As already described, several plant species other than soybean are responsive to the mixture of β-glucan fragments from P. sojae (Cline et al., 1978; Brady et al., 1993; Côté and Hahn, 1994), although whether they recognize the heptaglucoside structure or other oligosaccharide units is not clear. It should be interesting to see whether various plants can be grouped depending on the structure of the oligoglucan elicitors they recognize. Such studies may increase our understanding of the evolutional relationships of the receptor molecules for this class of elicitors.
The fact that these fragments were obtained by the enzymatic digestion of a cell wall component of a pathogenic fungus of rice plant increases their biological relevance. Many plants are known to produce endo-β-(1→3)-glucanases (Stone and Clarke, 1993; Høj and Fincher, 1995), including rice plant (Akiyama et al., 1996, 1997); these enzymes might be involved in the defense mechanism by generating elicitor-active glucan fragments as well as by directly attacking pathogens. An endo-β-(1→3)-glucanase from soybean has been shown to generate elicitor-active fragments from the cell walls of Phytophthora megasperma, although the sizes of these fragments were much larger than that of the heptaglucoside elicitor, and their structure was not fully understood (Okinaka et al., 1995). Induction of the gene expression of some endo-β-(1→3)-glucanases by fungal infection or elicitor treatment has also been reported (Kauffman et al., 1987; Jutidamorongphan et al., 1991; Kaku et al., 1997), indicating that these enzymes might have a function in both generation and amplification of the oligoglucan elicitor signals.
The fact that the rice and soybean cells recognize the specific glucan fragments from the cell surface of the corresponding pathogens may suggest involvement of the glucan elicitors in the recognition of a restricted group of fungi, including the pathogens for these plants, although this speculation requires a more careful examination. The situation seems different from that of other oligosaccharide elicitors, such as the fragments of chitin/chitosan or oligogalacturonides, which have a linear structure and show little structural variation specific to their origin other than chain length. Thus, recognition of the latter group of compounds has been thought to play a role in recognition of a broader “potential pathogen/predator” or “self/non-self” rather than recognition of a specific pathogen.
Concerning the recognition of chitin fragments by rice cells, we had previously reported that N-acetylchitooligosaccharides larger than pentamers act as very potent phytoalexin elicitors for suspension-cultured rice cells (Yamada et al., 1993). N-acetylchitooligosaccharides also induce many other cellular responses, such as changes of membrane potential (Kuchitsu et al., 1993; Kikuyama et al., 1997), ion flux (Kuchitsu et al., 1997), reactive oxygen generation (Kuchitsu et al., 1995), biosynthesis of jasmonic acid (Nojiri et al., 1996), and expression of several defense-related genes in rice cells (Minami et al., 1996). We found that at least some of these responses are also induced by the glucan fragment elicitor (T. Yamaguchi, Y. Maehara, and N. Shibuya, unpublished data). Thus, the results reported here clearly demonstrate that suspension-cultured rice cells can recognize both N-acetylchitooligosaccharides and specific β-glucan fragments as potent elicitor signals.
This raises interesting questions about the relationships between the receptors and signal transduction cascades for these two oligosaccharide elicitors in rice. Concerning the putative receptor molecule for the N-acetylchitooligosaccharide elicitor, we previously reported the presence of a high-affinity binding site for this elicitor in the plasma membrane preparation from suspension-cultured rice cells (Shibuya et al., 1993, 1996). Binding characteristics of the binding site corresponded well with the response of the rice cells to the elicitor, suggesting that the site functions as the receptor for the N-acetylchitooligosaccharide elicitor. Recently, using photoaffinity labeling as well as affinity cross-linking, we identified a plasma membrane protein corresponding to this binding site (Ito et al., 1997). Concerning the putative receptor molecule for elicitor-active β-glucan fragments, only the high-affinity binding protein for the hepta-β-glucoside elicitor in soybean membrane preparations has been studied extensively (Yoshikawa et al., 1983; Cosio et al., 1988, 1990a, 1990b, 1992; Cheong and Hahn, 1991; Cheong et al., 1993; Mithöfer et al., 1996). A cDNA encoding a soybean glucan-elicitor binding protein was recently cloned (Umemoto et al., 1997). Because the hepta-β-glucoside did not show elicitor activity for rice cells, the structure and binding characteristics of the receptor molecule for the β-glucan elicitor of rice cells should be markedly different from those of soybean. Identification and characterization of putative receptor molecules for the β-glucan elicitor in rice cells as well as elucidation of the relationships between the signal transduction cascades downstream of these elicitor signals should be studied further.
METHODS
Synthetic Aryl-Hexa-β-Glucoside
Synthetic aryl-hexa-β-glucoside, which shows very high elicitor activity on soybean (Glycine max) cotyledon cells, was synthesized as reported previously (Hong and Ogawa, 1990).
Suspension Culture of Rice Cells
The suspension culture of Oryzae sativa cv BL-1 was maintained as described previously (Kuchitsu et al., 1993; Yamada et al., 1993) in N6 medium containing 1 mg/L 2,4-D. The suspension culture was incubated on a rotary shaker at 25°C and 120 rpm in the dark. Every 2 weeks, a 6-mL aliquot of the loosely packed cells was transferred to a new flask containing 90 mL of the same medium and incubated further. After every other transfer to the new medium, the cell clusters were filtered through a 20-mesh filter to make fine aggregates and were used for the next culture.
Elicitor Assay
Samples to be tested were dissolved in 100 μL of redistilled water and autoclaved at 121°C for 20 min. A 900-μL sample of the suspension-cultured cells (∼20 to 30 mg of fresh cells) was added aseptically to this solution and incubated at 25°C with reciprocal shaking. Ten micrograms of thio-β-d-glucose per milliliter was added to the incubation medium to prevent the action of endogenous β-glucosidases. At the end of the incubation period, the reaction mixture was centrifuged for 10 min at 1700g, and the recovered cells were washed again with 1 mL of water. The amount of the cells used for each reaction was determined separately by measuring the fresh weight of the recovered pellet. The supernatant and washings were combined and applied to a Bond Elut C18 column (Varian Analytical Instruments, Harbor City, CA) equilibrated with water. The column was eluted successively with 10 mL of water and 1 mL of 80% methanol. The methanol fraction was concentrated to dryness at 40°C under vacuum, dissolved in 50 μL of methanol, and used for gas–liquid chromatographic analysis of phytoalexins. Because of the variability of the amount of phytoalexins produced in each experiment, each set of experiments was performed with the same batch of cultured cells. All of the experiments were performed in duplicate or triplicate, and the results were evaluated statistically. The soybean cotyledon assay was performed using the method of Sharp et al. (1984a).
Analysis of Phytoalexins
Phytoalexins were analyzed routinely using a gas chromatograph (model GC 14A; Shimadzu, Kyoto, Japan) with a moving needle system for sample injection and a flame ionization detector. A fused silica glass capillary column coated with DB-5 (0.25 mm × 25 m; J and W Scientific, Folsom, CA) or Neutrabond-1 (0.25 mm × 30 m; GL Science, Tokyo, Japan) was used for the analysis. An HP-5972 gas chromatograph–mass spectrometer (Hewlett Packard, Palo Alto, CA) was also used to identify each phytoalexin.
Cultivation of Pyricularia oryzae
Stock culture of P. oryzae Ken-60-19 was kindly supplied by Dr. Masako Katagiri of the National Institute of Agro-Environmental Sciences (Tsukuba, Ibaraki, Japan) and maintained on potato-agar-dextrose media. For preculture, a part of the stock culture was transferred to a 500-mL Sakaguchi flask containing 100 mL of a culture medium that contained 50 mL of soy sauce and 50 g of sucrose per liter and was incubated for 72 hr on a rotary shaker at 28°C and 120 rpm. The culture was prepared by transferring each 10 mL of the mycelial suspension to the new media in the same flasks and incubating for another 24 hr. For larger scale culture, a jar fermentor (consisting of two 30-liter vessels) was used. The mycelial suspension from the culture flasks was added to the same medium in the fermentor, diluting the mycelial suspension by 5% (total volume of 20 liters per vessel). Incubation was performed for 24 hr at 28°C with an aeration rate of 20 L/min and agitation at 250 rpm. The cultured mycelia were harvested on filter paper and kept frozen before use.
Preparation of β-Glucan Fractions
The mycelia of P. oryzae were extracted successively at 4°C with distilled water and 0.1 M phosphate buffer, pH 7.2, that contained 0.5 M NaCl. The residual mycelia were homogenized with a Waring blender and the same phosphate buffer containing NaCl at 4°C and centrifuged. This homogenization process was repeated twice to thoroughly extract the soluble materials. The insoluble residues were further washed with distilled water and then delipidated by successive treatment with acetone, 1:1 ratio of chloroform/methanol (v/v), chloroform, and acetone. The yield of the cell wall preparation thus obtained was <2% of the wet mycelia.
The cell wall preparation was suspended with 50 volumes of distilled water and autoclaved for 15 min at 121°C. After this process had been repeated three times, the residual walls were extracted overnight at room temperature with 25 to 50 volumes of 1 N NaOH containing 0.01% NaBH4, and the supernatant was recovered by centrifugation. This was repeated twice with 1 N NaOH and once with distilled water. These alkaline extracts were combined and used for further fractionation of β-glucans. The alkaline extract was neutralized to pH 6.0 with acetic acid. The precipitate formed by this neutralization was recovered by centrifugation at 4°C and named the glucan I fraction. Three volumes of ethanol were added to the remaining supernatant to precipitate another glucan fraction, glucan II. Both glucan preparations were dialyzed against distilled water and lyophilized.
Purification of Endo-β-(1→3)-Glucanase
Purification of an endo-β-(1→3)-glucanase from Zymolyase-100T from Arthrobacter luteus (Seikagaku Corp., Tokyo, Japan) was performed using a modification of the method of Kitamura (1982). The extract of Zymolyase-100T (5 mg/0.1 mL) in 20 mM Tris-HCl buffer, pH 8.0, was applied to a column of TSK-gel SW-3000 (7.8 × 300 mm; Toso, Tokyo, Japan) and eluted with the same buffer by HPLC (model 625 LC system; Waters Corporation, Milford, MA). The active fractions were collected and further purified by anion-exchange chromatography on a Mono-Q column (10 × 100 mm; model HR 10/10; Pharmacia, Uppsala, Sweden) that had been equilibrated with 20 mM Tris-HCl buffer and was eluted with the same buffer containing a linear gradient of 0 to 1 M NaCl. The enzyme activity was determined by analyzing the reducing sugars generated from laminaran by the Somogyi–Nelson method (Somogyi, 1952).
Generation and Purification of Elicitor-Active β-Glucan Fragments
For partial acid hydrolysis, each 100-mg β-glucan preparation was treated with 20 mL of 90% formic acid for 10 min at 100°C and then with 20 mL of 0.1 N trifluoroacetic acid (TFA) for 1 hr at 100°C. After removal of the TFA by evaporation, the residue was extracted with 20 mL of distilled water three times. The extract was concentrated and centrifuged to remove insoluble materials. For enzymatic degradation and purification of elicitor-active fragments, 100 mg of the less-branched β-glucan preparation (glucan I) was digested with 10 μg of the purified endo-β-1,3-glucanase, pH 5.8, for 6 hr at 40°C. After the reaction was stopped by boiling the reaction mixture, the insoluble substrate was precipitated by adding 4 volumes of ethanol. The supernatant was concentrated and dissolved with a small amount of distilled water, applied to a superfine Biogel P-4 column (2.5 × 90 cm; Bio-Rad, Hercules, CA) and eluted with distilled water at 50°C using an HPLC pump. A Superdex peptide column (10 × 300 mm; model V; Pharmacia) was also used for the gel filtration with HPLC. The elution was followed by a refractive index monitor (model 830-RI; JASCO, Tokyo, Japan) and also a phenol–sulfuric acid assay (Dubois et al., 1956). The pentamer fraction was recovered and reduced with sodium borohydride. The resulting glucopentitol fraction was concentrated and applied to an ODS column (4.6 × 250 mm; model Inertsil ODS 3; GL Science) and eluted with 8% CH3OH at 0.5 mL/min. The elicitor-active fractions were recovered and lyophilized for further analyses.
Analysis of Sugars by High-Performance Anion Exchange Chromatography
High-performance anion exchange chromatography (HPAEC) of monooligosaccharides and oligosaccharides was performed with an HPLC (model D-300 BioLC; Dionex, Sunnyvale, CA) equipped with a Carbo Pac PA1 column (4 × 250 mm). The column was equilibrated with 0.1 N NaOH for 30 min before each analysis. For analysis of oligosaccharides, the column was eluted with 0.1 N NaOH for 5 min, followed by a linear gradient of 0 to 0.1 M sodium acetate in 0.1 N NaOH for 30 min. For monosaccharides, the column was eluted with 0.005 N NaOH for 5 min and then with water for 30 min. A pulsed-amperometric detector was used for the detection of sugars.
Structural Analysis
Methylation analysis of glucan I, glucan II, and purified oligosaccharides was performed using the method of Hakomori (1964) as described previously (Shibuya et al., 1991; Kawaguchi et al., 1996). Fully methylated polysaccharides were hydrolyzed by heating with 90% formic acid for 1 hr at 90°C and then with 1 N TFA for 12 hr at 100°C. Methylated oligosaccharides were hydrolyzed by heating with 2 N TFA for 1 hr at 121°C. Partially methylated sugars were converted to the corresponding alditol acetates and analyzed with a gas chromatograph (model GC 14A; Shimadzu) equipped with a capillary column (0.25 mm × 30 m; model SP2330; Supelco, Bellefonte, PA) and a flame ionization detector. The spectrum of each sugar was analyzed with a gas chromatograph (model HP 5890; Hewlett Packard) and a molecular mass analyzer (model DX 303 HF; Jeol Ltd., Tokyo, Japan). Acetolysis of oligosaccharides was performed using the method of Rosenfeld and Ballou (1974). Fully acetylated oligosaccharides were hydrolyzed with a 10:10:1 ratio of acetic anhydride–acetic acid–sulfuric acid for 24 hr at room temperature. The fragments were then analyzed by HPAEC. The column was eluted with a linear gradient of 0 to 0.1 M sodium acetate in 0.1 N NaOH for 30 min. The per-O-acetylated glucopentaose was analyzed by electrospray mass spectrometry (MS) with a biomolecular mass analyzer (model API III; PE-Sciex, Thornhill, Canada) interfaced to a Macintosh II Fx data station. The mass spectrometer was operated in the positive-ion mode with an ion spray voltage of 4500 V and an orifice potential of 20 V. The 0.1 μg/μL carbohydrate solution in aqueous 50% methanol containing 1% TFA was applied at 2 μL/min. The mass range was scanned from 100 to 1800 amu. Fifteen scans were collected and averaged. Electrospray MS–MS was performed by selecting ions with mass-to-charge ratios (m/z) of 1257 and 865 in the first quadrupole, which was then collisionally activated with argon gas (collision gas thickness of 200 × 1012 to 300 × 1012 molecules cm−2) in a high-pressure quadrupole collision cell. The fragment ions generated on collision with the neutral gas molecules were separated in the third quadrupole to obtain the daughter-ion mass spectra.
1H–Nuclear Magnetic Resonance Spectroscopy
The ∼0.3-mg sample of tetraglucosyl glucitol was dissolved in D2O (99.6%) to replace exchangeable protons with deuterons and then was freeze-dried. Before use, the sample was redissolved in D2O (99.96%). 1H–nuclear magnetic resonance spectra were recorded at 500 MHz with a spectrometer (model Alpha 500; Jeol) at 20°C. Chemical shifts were referenced to external acetone (δ 2.23).
Acknowledgments
We thank Dr. Kiyoshi Morikawa of Seikagaku Corp. for the cultivation of P. oryzae. This work was supported in part by the Program for Promotion of Basic Research Activities for Innovative Biosciences (Tokyo, Japan).
References
- Akiyama, T., Kaku, H., and Shibuya, N. (1996). Purification and properties of a basic endo-1,3-β-glucanase from rice (Oryzae sativa). Plant Cell Physiol. 37, 702–705. [DOI] [PubMed] [Google Scholar]
- Akiyama, T., Shibuya, N., Hrmova, M., and Fincher, G.B. (1997). Purification and characterization of a (1→3)-β-d-glucan endohydrolase from rice (Oryzae sativa) bran. Carbohydr. Res. 297, 365–374. [DOI] [PubMed] [Google Scholar]
- Brady, K.P., Darvill, A.G., and Albersheim, P. (1993). Activation of a tobacco glycine-rich protein gene by a fungal glucan preparation. Plant J. 4, 517–524. [DOI] [PubMed] [Google Scholar]
- Cheong, J.J., and Hahn, M.G. (1991). A specific, high-affinity binding site for the hepta-β-glucoside elicitor exists in soybean membranes. Plant Cell 3, 137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong, J.J., Birberg, W., Fugedi, P., Pilotti, A., Garegg, P.J., Hong, N., Ogawa, T., and Hahn, M.G. (1991). Structure–activity relationships of oligo-β-glucoside elicitors of phytoalexin accumulation in soybean. Plant Cell 3, 127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong, J.J., Alba, R., Côté, F., Enkerli, J., and Hahn, M.G. (1993). Solubilization of functional plasma membrane–localized hepta-β-glucoside elicitor-binding proteins from soybean. Plant Physiol. 103, 1173–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline, K., Wade, M., and Albersheim, P. (1978). Host–pathogen interactions. XV. Fungal glucans which elicit phytoalexin accumulation in soybean also elicit the accumulation of phytoalexins in other plants. Plant Physiol. 62, 918–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosio, E.G., Pöpperl, H., Schmidt, W.E., and Ebel, J. (1988). High-affinity binding of fungal β-glucan fragments to soybean (Glycine max L.) microsomal fractions and protoplasts. Eur. J. Biochem. 175, 309–315. [DOI] [PubMed] [Google Scholar]
- Cosio, E.G., Frey, T., and Ebel, J. (1990. a). Solubilization of soybean membrane binding sites for fungal β-glucans that elicit phytoalexin accumulation. FEBS Lett. 264, 235–238. [DOI] [PubMed] [Google Scholar]
- Cosio, E.G., Frey, T., Verduyn, R., Boom, J., and Ebel, J. (1990. b). High-affinity binding of a synthetic heptaglucoside and fungal glucan phytoalexin elicitors to soybean membranes. FEBS Lett. 271, 223–226. [DOI] [PubMed] [Google Scholar]
- Cosio, E.G., Frey, T., and Ebel, J. (1992). Identification of a high-affinity binding protein for a hepta-β-glucoside phytoalexin elicitor in soybean. Eur. J. Biochem. 204, 1115–1123. [DOI] [PubMed] [Google Scholar]
- Côté, F., and Hahn, M.G. (1994). Oligosaccharins: Stuctures and signal transduction. Plant Mol. Biol. 26, 1379–1411. [DOI] [PubMed] [Google Scholar]
- Darvill, A.G., and Albersheim, P. (1984). Phytoalexins and their elicitor—A defense against microbial infection in plants. Annu. Rev. Plant Physiol. 35, 243–275. [Google Scholar]
- Dell, A. (1987). F.A.B.–mass spectrometry of carbohydrates. Adv. Carbohydr. Chem. Biochem. 45, 19–71. [DOI] [PubMed] [Google Scholar]
- Dixon, R.A., and Harrison, M.J. (1990). Activation, structure, and organization of genes involved in microbial defense in plants. Adv. Genet. 28, 165–234. [DOI] [PubMed] [Google Scholar]
- Dubois, M., Gilles, K.A., Hamilton, J.K., Rebers, P.A., and Smith, F. (1956). Colorimetric method for determination of sugars and related substances. Anal. Chem. 28, 350–356. [Google Scholar]
- Ebel, J., and Cosio, E.G. (1994). Elicitor of plant defense responses. Int. Rev. Cytol. 148, 1–36. [Google Scholar]
- Hakomori, S. (1964). A rapid permethylation of glycolipid, and polysaccharide catalyzed by methylsulfinyl carbanion in dimethyl sulfoxide. J. Biochem. 55, 205–208. [PubMed] [Google Scholar]
- Høj, P.B., and Fincher, G.B. (1995). Molecular evolution of plant β-glucan endohydrolases. Plant J. 7, 367–379. [DOI] [PubMed] [Google Scholar]
- Hong, N., and Ogawa, T. (1990). Stereocontrolled syntheses of phytoalexin elicitor-active β-d-glucohexaosides and β-d-glucononaoside. Tetrahedron Lett. 31, 3179–3182. [Google Scholar]
- Inui, H., Yamaguchi, Y., and Hirano, S. (1997). Elicitor actions of N-acetylchitooligosaccharides and laminarioligosaccharides for chitinase and l-phenylalanine ammonia-lyase induction in rice suspension culture. Biosci. Biotechnol. Biochem. 61, 975–978. [DOI] [PubMed] [Google Scholar]
- Ito, Y., Kaku, H., and Shibuya, N. (1997). Identification of a high-affinity binding protein for N-acetylchitooligosaccharide elicitor in the plasma membrane of suspension-cultured rice cells by affinity labeling. Plant J. 12, 347–356. [DOI] [PubMed] [Google Scholar]
- Jutidamorongphan, W., Anderson, J.B., MacKinnon, G., Manners, J.M., Simpson, R.S., and Scott, K.J. (1991). Induction of β-1,3-glucanase in barley in response to infection by fungal pathogens. Mol. Plant-Microbe Interact. 4, 234–238. [PubMed] [Google Scholar]
- Kaku, H., Shibuya, N., Xu, P., Aryan, A.P., and Fincher, G.B. (1997). N-acetylchitooligosaccharides elicit expression of a single (1→3)-β-glucanase gene in suspension-cultured cells from barley (Hordeum vulgare). Physiol. Plant. 100, 111–118. [Google Scholar]
- Kauffman, S., Legrand, M., Geoffrey, P., and Fritig, B. (1987). Biological function of pathogenesis-related proteins of tobacco have 1,3-β-glucanase activity. EMBO J. 6, 3209–3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi, K., Shibuya, N., and Ishii, T. (1996). A novel tetrasaccharide, with a structure similar to the terminal sequence of an arabinogalactactan-protein, accumulates in rice anthers in a stage-specific manner. Plant J. 9, 777–785. [DOI] [PubMed] [Google Scholar]
- Kikuyama, M., Kuchitsu, K., and Shibuya, N. (1997). Membrane depolarization induced by N-acetylchitooligosaccharide elicitor in suspension-cultured rice cells. Plant Cell Physiol. 38, 902–909. [Google Scholar]
- Kitamura, K. (1982). Re-examination of Zymolyase purification. Agric. Biol. Chem. 46, 963–969. [Google Scholar]
- Kuchitsu, K., Kikuyama, M., and Shibuya, N. (1993). N-acetylchitooligosaccharides, biotic elicitor for phytoalexin production, induce transient membrane depolarization in suspension-cultured rice cells. Protoplasma 174, 79–81. [Google Scholar]
- Kuchitsu, K., Kosaka, T., Shiga, T., and Shibuya, N. (1995). EPR evidence for generation of hydroxyl radical triggered by N-acetylchitooligosaccharide elicitor and a protein phosphatase inhibitor in suspension-cultured rice cells. Protoplasma 188, 138–142. [Google Scholar]
- Kuchitsu, K., Yazaki, Y., Sakano, K., and Shibuya, N. (1997). Transient cytoplasmic pH change and ion fluxes through the plasma membrane in suspension-cultured rice cells triggered by N-acetylchitooligosaccharide elicitor. Plant Cell Physiol. 38, 1012–1028. [Google Scholar]
- Ló, V.M., Hahn, M.G., Hong, N., Ogawa, T., and van Halbeek, H. (1993). Complete assignment of the 1H-NMR spectra of phytoalexin-elicitor-active oligosaccharides. Carbohydr. Res. 215, 333–345. [DOI] [PubMed] [Google Scholar]
- Minami, E., Kuchitsu, K., He, D.Y., Kouchi, H., Midoh, N., Ohtsuki, Y., and Shibuya, N. (1996). Two novel genes rapidly and transiently activated in suspension-cultured rice cells by treatment with N-acetylchitoheptaose, a biotic elicitor for phytoalexin production. Plant Cell Physiol. 37, 563–567. [DOI] [PubMed] [Google Scholar]
- Mithöfer, A., Lottspeich, F., and Ebel, J. (1996). One-step purification of the β-glucan elicitor-binding protein from soybean (Glycine max L.) roots and characterization of an anti-peptide antiserum. FEBS Lett. 381, 203–207. [DOI] [PubMed] [Google Scholar]
- Nakajima, T., Tamari, K., Matsuda, K., Tanaka, H., and Ogasawara, N. (1970). Studies on the cell wall of Piricularia oryzae. II. The chemical constitutions of the cell wall. Agric. Biol. Chem. 34, 553–560. [Google Scholar]
- Nakajima, T., Tamari, K., Matsuda, K., Tanaka, H., and Ogasawara, N. (1972). Studies on the cell wall of Piricularia oryzae. III. The chemical structure of the β-glucan. Agric. Biol. Chem. 36, 11–17. [Google Scholar]
- Nojiri, H., Sugimori, M., Yamane, H., Nishimura, Y., Yamada, A., Shibuya, N., Kodama, O., Murofushi, N., and Ohmori, T. (1996). Involvement of jasmonic acid in elicitor-induced phytoalexin production in suspension-cultured rice cells. Plant Physiol. 110, 387–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okinaka, Y., Mimori, K., Takeo, K., Kitamura, S., Takeuchi, Y., Yamaoka, N., and Yoshikawa, M. (1995). A structural model for the mechanisms of elicitor release from fungal cell wall by plant β-1,3-endoglucanase. Plant Physiol. 109, 839–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld, L., and Ballou, C.E. (1974). Acetolysis of disaccharides: Compariative kinetics and mechanism. Carbohydr. Res. 8, 287–298. [Google Scholar]
- Ryan, C.A., and Farmer, E.E. (1991). Oligosaccharide signals in plants: A current assessment. Annu. Rev. Plant Physiol. 42, 651–674. [Google Scholar]
- Sharp, J.K., Valent, B., and Albersheim, P. (1984. a). Purification and partial characterization of a β-glucan fragment that elicits phytoalexin accumulation in soybean. J. Biol. Chem. 259, 11312–11320. [PubMed] [Google Scholar]
- Sharp, J.K., McNeil, M., and Albersheim, P. (1984. b). The primary structures of one elicitor-active and seven elicitor-inactive hexa(β-d-glucopyranosyl)-d-glucitols isolated from the mycelial walls of Phytophthora megasperma f. sp. glycinera. J. Biol. Chem. 259, 11321–11336. [PubMed] [Google Scholar]
- Shibuya, N., Amano, K., Azuma, J., Nishihara, T., Kitamura, Y., Noguchi, T., and Koga, T. (1991). 6-Deoxy-d-talan and 6-deoxy-l-talan. Novel serotype-specific polysaccharide antigens from Actinobacillus actinomycetemcomitans. J. Biol. Chem. 266, 16318–16323. [PubMed] [Google Scholar]
- Shibuya, N., Kaku, H., Kuchitsu, K., and Maliarik, M.J. (1993). Identification of a novel high-affinity binding site for N-acetylchitooligosaccharide elicitor in the membrane fraction from suspension-cultured rice cells. FEBS Lett. 329, 75–78. [DOI] [PubMed] [Google Scholar]
- Shibuya, N., Ebisu, N., Kamada, Y., Kaku, H., Cohn, J., and Ito, Y. (1996). Localization and binding characteristics of a high-affinity binding site for N-acetylchitooligosaccharide elicitor in the plasma membrane from suspension-cultured rice cells suggest a role as a receptor for the elicitor signal at the cell surface. Plant Cell Physiol. 37, 894–898. [Google Scholar]
- Somogyi, M. (1952). Notes on sugar determination. J. Biol. Chem. 195, 19–23. [PubMed] [Google Scholar]
- Stone, B.A., and Clarke, A.E. (1993). The Chemistry and Biology of (1-3)-β-Glucans. (Bundoora, Victoria, Australia: La Trobe University Press).
- Umemoto, N., Kakitani, M., Iwamatsu, A., Yoshikawa, M., Yamaoka, N., and Ishida, I. (1997). The structure and function of a soybean β-glucan-elicitor binding protein. Proc. Natl. Acad. Sci. USA 94, 1029–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada, A., Shibuya, N., Kodama, O., and Akatsuka, T. (1993). Induction of phytoalexin formation in suspension-cultured rice cells by N-acetylchitooligosaccharides. Biosci. Biotechnol. Biochem. 57, 405–409. [Google Scholar]
- Yoshikawa, M., Keen, N.T., and Wang, M.C. (1983). A receptor on soybean membranes for a fungal elicitor of phytoalexin accumulation. Plant Physiol. 73, 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]