Abstract
The epidermis is a stratified squamous epithelium forming the barrier that excludes harmful microbes and retains body fluids. To perform these functions, proliferative basal cells in the innermost layer periodically detach from an underlying basement membrane of extracellular matrix, move outward and eventually die. Once suprabasal, cells stop dividing and enter a differentiation programme to form the barrier1. The mechanism of stratification is poorly understood. Although studies in vitro have led to the view that stratification occurs through the delamination and subsequent movement of epidermal cells2–4, most culture conditions favour keratinocytes that lack the polarity and cuboidal morphology of basal keratinocytes in tissue. These features could be important in considering an alternative mechanism, that stratification occurs through asymmetric cell divisions in which the mitotic spindle orients perpendicularly to the basement membrane5–7. Here we show that basal epidermal cells use their polarity to divide asymmetrically, generating a committed suprabasal cell and a proliferative basal cell. We further demonstrate that integrins and cadherins are essential for the apical localization of atypical protein kinase C, the Par3–LGN–Inscuteable complex and NuMA–dynactin to align the spindle.
We first addressed whether oriented cell divisions participate in stratification during epidermal development. At embryonic day 12.5 (E12.5), most of the epidermis was single-layered, and most divisions occurred laterally, within the plane of the epithelium. Unexpectedly, however, a few mitotic cells seemed to be dividing perpendicularly to the basement membrane, as judged by staining with 4′,6-diamidino-2-phenylindole (DAPI) and with anti-tubulin (arrow in Fig. 1a, b). Closer inspection revealed the presence of some suprabasal cells within the E12.5 single-layered epithelium. In these regions, the occasional suprabasal cell was often positioned directly over a basal cell, as would be expected for a perpendicular division.
Figure 1. Asymmetric cell divisions govern stratification and differentiation during epidermal development.
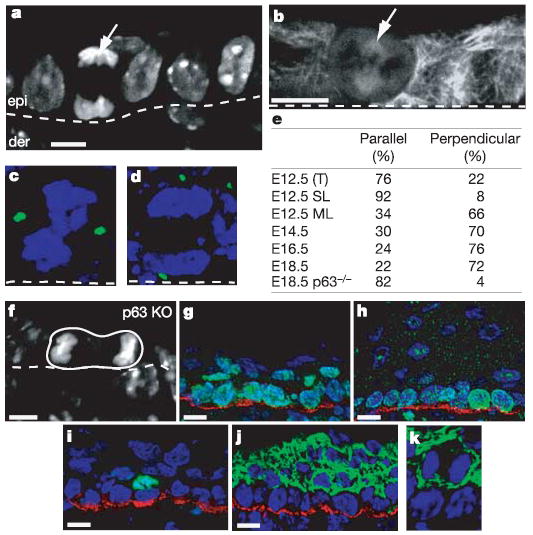
a–d, Images of embryonic skin showing the orientation of mitoses relative to the basement membrane (white dotted line), separating epidermis (epi in a) from dermis (der). Arrows indicate the mitotic cells in E12.5 epidermis; a (anaphase) and b (metaphase). DAPI marks the DNA in a. Indirect immunofluorescence with tubulin antibodies marks the spindle in b. Transgenic Centrin-GFP (green) marks spindle poles in c (metaphase, parallel spindle orientation), and d (anaphase, perpendicular spindle orientation). e, Quantification of division planes in epidermis during development. SL, single-layered E12.5 epidermis, ML, multi-layered E12.5 epidermis, T, total E12.5 epidermis. f, Parallel spindle orientation of an anaphase cell in p63 KO epidermis at E18.5; DAPI staining. g, h, Antibodies against β4 integrin (red) label the base of the basal cells. Antibodies against Ki67 (green) show that not all suprabasal cells at E15.5 (g) have withdrawn from a proliferative state; however, Ki67 is confined to basal cells at E18.5 (h). i, Antibodies against phosphohistone H3 (green) reveal the presence of mitotic suprabasal keratinocytes at E15.5. Red staining shows β4 integrin. j, Keratin 1 immunofluorescence (green) shows that suprabasal cells have turned on this differentiation marker at E15.5. Red staining shows β4 integrin. k, Magnified view of a keratin-1-positive (green) suprabasal mitotic cell in anaphase. Scale bars are 10 μm.
To facilitate quantification, we engineered mice expressing keratin 14 (K14)–centrin coupled to green fluorescent protein (GFP) and examined embryos at later stages of development, as the epidermis became multi-layered. It was now easy to distinguish parallel from perpendicular spindle alignments (Fig. 1c, d). In predominantly single-layered areas of E12.5 epithelium, 92% of divisions occurred parallel to the basement membrane. By contrast, in E12.5 areas that showed early signs of stratification, most mitotic cells had an alignment that was clearly perpendicular. At E12.5, perpendicularly aligned spindles accounted for about 22% of all mitoses, but as stratification progressed across the epithelium, this number rose markedly (Fig. 1e). From E15.5 onwards through postnatal development, more than 70% of spindles were oriented perpendicularly to the basement membrane.
Perpendicular alignments yielded one basal and one suprabasal cell, and their temporal appearance during skin development correlated well with stratification. A functional link was provided by examining p63-null embryos, defective in the transcription factor p63 but also in stratification8,9. Divisions in E18.5 p63-null epidermis were lateral to the basement membrane (an example is shown in Fig. 1f). In normal adults, homeostasis of stratification also seemed to be dependent on perpendicular divisions, because most divisions in both adult epidermis (about 85%) and tongue (about 65%) still occurred in this fashion, even though overall mitoses waned considerably once animals reached full size.
A feature of these unexpected perpendicular spindle orientations is that one cell maintains and the other loses contact with the underlying basement membrane. Many key functions including extracellular matrix protein secretion, integrin-mediated focal adhesion and growth factor signalling are already known to be basally localized, and perpendicular divisions provide a natural mechanism for their unequal partitioning to two daughter cells1,10,11. Perpendicular spindle orientations in the basal epidermal layer therefore automatically result not only in stratification but also in asymmetric cell divisions.
Using K14–histone H2B mice to label the epidermis uniformly12, we determined that the population of basal cells increased about threefold between E13.5 and E15.5. This expansion reflected the increasing size of the embryo and the ability of basal cells to divide symmetrically and remain attached to the basement membrane. By contrast, the increase in suprabasal cells during this time was much too large to be explained by the concomitant increase in asymmetric cell divisions (see Fig. 1e). This conundrum was solved by discovering that, in contrast to fully stratified E18.5 and postnatal epidermis, many E15.5 suprabasal cells were proliferative. They expressed the proliferation marker Ki67, and in mitoses they possessed phosphorylated histone H3 (Fig. 1g–i). E15.5 suprabasal cells, including mitotic cells, were nevertheless positive for the differentiation marker keratin-1 (Fig. 1j, k). The mitotic potential of these cells was shown by using monastrol or nocodazole to trigger the spindle checkpoint, arresting 15–20% of suprabasal cells in mitosis within 5 h (data not shown). By retaining mitotic activity at this age, the suprabasal population could expand more rapidly to form the multilayered epidermis. Nevertheless, the proliferative potential of suprabasal cells was short-lived and they were positive for differentiation markers.
How do epidermal cells control their spindle orientation and divide asymmetrically? In Drosophila neuroblasts, a protein complex containing Inscuteable and Partner of Inscuteable (Pins) captures a spindle pole at the apical cortex, aligning the spindle with the apical–basal axis7,13–16. Although mammalian LGN is a genuine Pins homologue, no Inscuteable counterpart has been cloned. Reports of asymmetric divisions in mammals are rare, and it remains unknown whether this mechanism has been conserved.
We therefore cloned a distant mouse homologue of Drosophila Inscuteable, naming it mInsc. Expression of the mInsc gene in embryonic, newborn and adult skin was confirmed by northern blot and reverse transcriptase polymerase chain reaction analyses (Supplementary Fig. S1a, b). Because mInsc and Inscuteable share only about 20% sequence identity (Supplementary Fig. S1c), we first examined whether mInsc interacts with LGN and Par3, the two known binding partners for Inscuteable. When transiently expressed together in cultured keratinocytes, epitope-tagged mInsc and LGN formed a complex that could be immunoprecipitated by either anti-mInsc or anti-LGN (Fig. 2a). The endogenous mInsc, LGN and Par3 proteins also existed as a complex. Thus, when extracts of E14.5 skin epidermis were immunoprecipitated with a newly raised anti-mInsc antibody, immunoblot analyses detected the three proteins in the complex (Fig. 2b). Similarly, immunoprecipitates of Par3 contained mInsc. The total levels of these proteins were always significantly greater in embryonic skin enriched for mitotic keratinocytes (Fig. 2c). This coordinate mitotic regulation of mInsc and LGN is consistent with their putative role in governing spindle alignment.
Figure 2. Mitotic apical localization of a mInsc–LGN–Par3 complex.
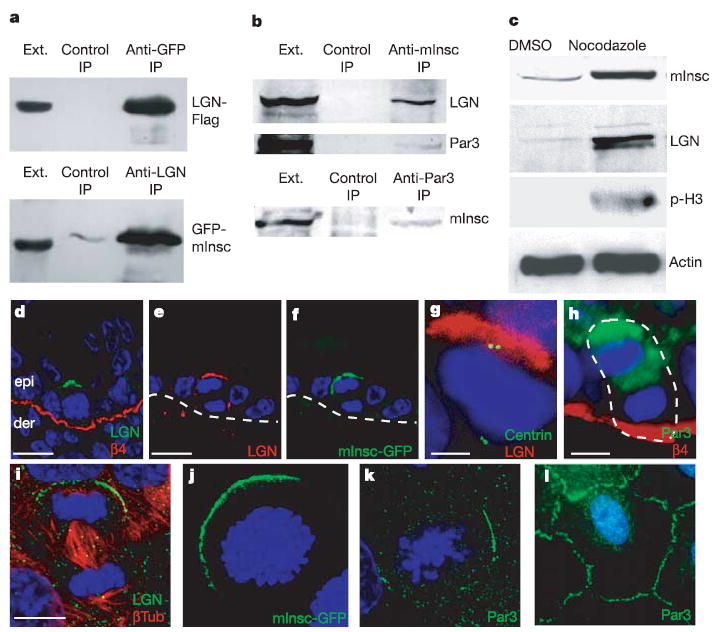
a, mInsc interacts with LGN in cultured cells. Keratinocytes were transfected with K14 promoter driven full-length LGN (LGN-Flag) and mInsc (GFP-mInsc). Reciprocal co-immunoprecipitations (IPs) were performed. The extract (Ext) lane contains 10% of the input protein for the co-immunoprecipitation. b, Endogenous mInsc, LGN and Par3 form a complex in epidermis from E14.5 embryos. Protein extracts were prepared from embryos treated with nocodazole for 5 h to enrich for mitotic cells. Extracts were immunoprecipitated with anti-mInsc or anti-Par3 antibody as indicated, and immunoblots of the IPs were then performed with LGN, Par3 and mInsc antibodies. c, Epidermal protein lysates were prepared from E14.5 embryos treated with dimethylsulphoxide (DMSO; vehicle) or nocodazole for 5 h and probed with the indicated antibodies. d–l, Epifluorescence (GFP) or indirect immunofluorescence with antibodies colour-coded as indicated. DAPI (blue) was used to label chromatin. d–f, Apical crescents of LGN (d, e) and mInsc-GFP (f) are present in most mitotic basal cells of E15.5 embryonic epidermis (d) and adult (e, f). epi, epidermis; der, dermis. Note that e and f show co-localization of LGN and mInsc-GFP to the apical cortex of an anaphase cell. g, One E14.5 spindle pole was always located directly underneath the LGN crescent. h, Par3 localizes at E15.5 to the apical cortex of mitotic basal keratinocytes. The white dashed line demarcates basement membrane in e and f and borders of the anaphase cell in h. i, Mouse skin keratinocytes were stained with anti-LGN and β-tubulin antibodies. j, Keratinocytes were transfected with K14-GFP-mInsc and labelled simultaneously with DAPI to localize the DNA and identify mitotic cells. The image is a compressed Z-stack of confocal sections to view the entire cell. k, l, Keratinocytes were stained for DAPI and Par3 in mitotic (k) and interphase (l) cells. Scale bars are 10 μm, except in g, h, in which they are 5 μm.
Immunofluorescence microscopy revealed further insights into the role of LGN–mInsc complexes in developing epidermis. Cells in interphase or undergoing lateral divisions, either in single-layered or multilayered epidermis, had a diffuse localization of LGN (data not shown). From E15.5 onwards, however, an apical crescent of anti-LGN labelling was seen at the cortex of most mitotic cells (Fig. 2d). Closer inspection revealed that in about 90% of cells with an apical crescent of LGN, one of the spindle poles was positioned directly below it, indicative of a perpendicular alignment of the spindle (Fig. 2g; adult). Co-localization of mInsc and LGN was best revealed in vivo by engineering a transgenic mouse expressing mInsc-GFP in the epidermis. An apical crescent of mInsc-GFP, LGN and Par3 was seen in mitotic basal cells within the stratified epidermis (examples are shown in Fig. 2e–h). Thus, a strict correlation existed between asymmetric cell divisions, stratification and the crescent-shaped apical organization of LGN, mInsc and Par3. Moreover, the localization of the LGN–mInsc–Par3 crescent was always opposite to that of the integrins and basement membrane (Fig. 2d, h).
LGN, mInsc-GFP and Par3 had a mitosis-specific polarized distribution in cultured cells, often localizing asymmetrically to the cortex near one of the two spindle poles (Fig. 2i–k). Although polarization in vitro was more variable, it was observed in at least 15% and sometimes as many as 40% of mitotic keratinocytes. In non-dividing cells, these proteins were distributed at sites of cell–cell contact (Fig. 2l) or were diffuse (data not shown). mInsc–Par3–LGN polarization was unexpected given the flatter morphology of keratinocytes in vitro. However, the distribution occurred under conditions where a rich extracellular matrix was deposited, and where intercellular adhesion was present. We return to these issues below.
LGN has recently been shown to interact directly with NuMA, a large coiled-coil protein that is nuclear in interphase and organizes the spindle poles during mitosis in cultured cells17–20. This and a possible relation to a Caenorhabditis elegans spindle protein Lin-5 (ref. 21) led us to investigate whether NuMA participates in aligning the spindle poles with LGN–Inscutable–Par3 complexes to govern asymmetric cell divisions. Both cultured keratinocytes and cells within the basal layer of embryonic epidermis possessed the expected nuclear localization of NuMA during interphase (not shown) and spindle-pole localization of NuMA during mitosis (Fig. 3a, b). However, asymmetrically dividing basal cells frequently exhibited more intense and expanded anti-NuMA staining at their apical surface (examples are shown in Fig. 3a, b).
Figure 3. Polarized mitotic localization of NuMA and dynactin.
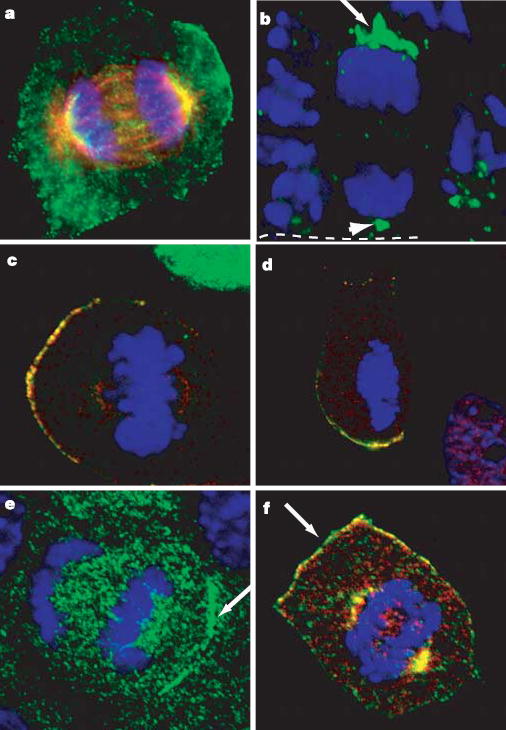
a, Mitotic keratinocyte stained simultaneously for NuMA (green) and microtubules (β-tubulin; red). b, E15.5 skin section stained with anti-NuMA antibodies reveals localization of NuMA (green) to spindle poles (arrowhead) and a cortical apical crescent (arrow). White dotted line denotes basement membrane. c, LGN (green) and NuMA (red) localize to the same crescent. K14-GFP-LGN-transfected keratinocytes were stained with antibodies against NuMA. d, Keratinocytes were treated with nocodazole to disrupt microtubules and then stained as in c. e, An anaphase keratinocyte stained for the p150glued subunit (green) of the dynactin complex. f, Co-localization of NuMA (green) and p150glued (red) in a mitotic keratinocyte. DNA is stained with DAPI (blue).
The crescent-shaped apical distribution of NuMA resembled that of the LGN complex, and immunofluorescence microscopy of K14-GFP-LGN-transfected keratinocytes confirmed their colocalization (Fig. 3c). Neither NuMA nor LGN required microtubules for their localization to the cortex, as judged by treatment with nocodazole (Fig. 3d). As reported earlier22, however, NuMA’s localization to the centrosome was microtubule-dependent.
Asymmetric forces pulling on microtubules at the cell cortex seem to be responsible for spindle reorientation in asymmetrically dividing cells within the C. elegans embryo23. Although dynein–dynactin motor complexes at the cortex are believed to provide a pulling force on astral microtubules, they have not been observed to be asymmetrically localized in Drosophila neuroblast or in one-cell C. elegans embryos. Interestingly, in mitotic keratinocytes, polarized cortical immunolocalization was observed with antibodies against p150glued, a subunit of the dynactin complex (Fig. 3e). Moreover, dynactin colocalized with NuMA both at spindles/spindle poles and in the polarized cortical crescent (Fig. 3f). These findings indicate that NuMA and dynein–dynactin-dependent pulling forces at the apical cortex might function in asymmetric divisions.
Asymmetric LGN–mInsc polarization in epidermis differed from all previous examples in that it occurred in cells attached to an underlying basement membrane, a process known to be dependent on β4 integrin (essential for hemidesmosomes)24,25 and β1 integrin (essential for focal adhesions and basement membrane assembly)26,27. Using mouse genetics, we uncovered the functional importance of the basement membrane in LGN–mInsc polarization. Thus, although proper apical complex localization was maintained in β4-null epidermis, it was aberrant in β1-null epidermis, which is unable to assemble a basement membrane (Fig. 4a, b). The LGN complex still formed a crescent in β1-null epidermis, but it was no longer restricted to the apical domain (Fig. 4b). Sometimes the crescent was even basal. The data were quantified and are presented in the diagrams in Fig. 4i, j. Each dot represents the centre of an LGN crescent in a mitotic cell.
Figure 4. Cadherin and integrin requirements for LGN–NuMA localization and spindle orientation.
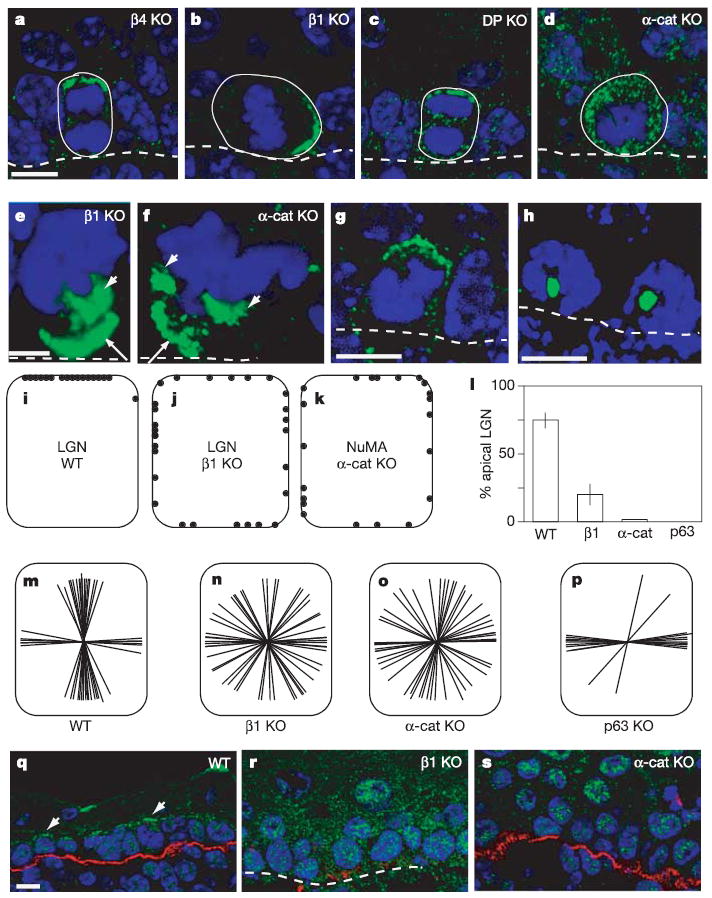
a–d, LGN localization (green staining) was determined in embryonic skin from knockout (KO) mice lacking β4 integrin (a), β1 integrin (b), desmoplakin (c) and α-catenin (d). e, f, NuMA localization (green) in β1-integrin KO (e) and α-catenin KO (f) epidermis. g, LGN (green) and h, NuMA (green) localization in sections from embryos treated with monastrol for 5 h. i, j, Schematic representation of LGN localization in WT (i) and β1 KO (j) epidermis. Each dot represents the centre of an LGN crescent in a single mitotic cell. k, Schematic representation of NuMA localization in α-catenin KO epidermis. l, Quantification of cells with apical cortical localization of LGN. Note that α-catenin (α-cat) and p63 KOs do not show cortical localization of LGN. Error bars represent standard deviation. m–p, Schematic representation of spindle orientation in embryo skin from WT mice (m) and in β1 KO (n), α-catenin KO (o) and p63 KO (p) mice. Each line represents the spindle axis of a single late metaphase or anaphase cell. q–s, Immunolocalization of PKC-ζ (green) in WT (q), β1-integrin KO (r) and α-catenin KO epidermis (s). Red staining shows β4 integrin. Scale bars are 10 μm, except in e, f, in which they are 5 μm.
The localization of NuMA was similarly aberrant in the β1-null epidermis (Fig. 4e). This was accompanied by abnormalities in spindle orientation and cell divisions. Figure 4m, n illustrates the spindle orientations measured in 25 mitotic cells. Taken together, these findings establish a role for β1 and basement membrane in LGN complex distribution and in proper spindle orientation.
Next we examined the role of cell–cell adhesion in promoting LGN complex localization. Gene targeting in mice has revealed essential roles for desmoplakin (a component of desmosomes)28 and α-catenin (a component of adherens junctions)29 in intercellular adhesion within the epidermis. LGN complexes and NuMA had a normal localization in epidermis that lacked desmoplakin (Fig. 4c). However, in the absence of α-catenin LGN no longer localized to the cell cortex, with a resulting randomization of spindle alignment and misoriented cell divisions (Fig. 4d, o). These findings revealed an essential role for α-catenin in the targeting and/or maintenance of the polarized LGN crescent.
Although LGN (shown) and Par3 (not shown) lost their cortical association, NuMA showed merely a randomization of polarization in the α-catenin knockout (KO), more similar to β1-null epidermis (Fig. 4f, k). However, in about 90% of cases, the spindle was aligned with the randomly positioned NuMA crescent. The loss of polarity and control of spindle orientation explains many of the architectural and biochemical defects in the α-catenin KO epidermis, including the absence of organized columns of stratified cells, the presence of suprabasal mitoses, the increased suprabasal inheritence and expression of integrins, the patchy keratin-1 expression and the formation of internalized masses of disorganized epidermal cells (see Supplementary Fig. S3 and ref. 29).
If LGN and NuMA interact directly, why do LGN complexes fail to polarize in the absence of α-catenin, whereas NuMA still forms a cortical crescent? Probing more deeply into this issue, we discovered that in wild-type (WT) epidermis, the kinesin Eg5 inhibitor monastrol resulted in the collapse of NuMA to the centre of the single microtubule monoaster, but LGN remained localized to the apical cortex (Fig. 4g, h). Taken together, these data indicate that the cortical localization of NuMA and LGN might be independent of each other, with LGN dependent on, and NuMA independent of, Par3 polarization.
Atypical protein kinase C (PKC-ζ) is part of a protein complex that is important for specifying the apical domain of polarized epithelia29. Consistent with this notion was the observation that PKC-ζ was expressed in most if not all basal cells in WT epidermis, where it had a marked apical localization (Fig. 4q). This seemed to be independent of cell cycle status. However, PKC-ζ’s apical localization was lost in both β1-null and α-catenin-null epidermis (Fig. 4r, s).
Finally, if apical localization of LGN–mInsc complexes is required for asymmetric cell divisions, then genetic models in which these divisions do not occur should lack this apical localization. We therefore turned again to the p63-null embryo. Unlike in their control littermates, apical accumulation of LGN was not detected in the p63-null epidermal keratinocytes (Fig. 4l). Concomitantly, and as shown in Fig. 4p, spindle orientations were overwhelmingly lateral. These data underscore the importance of the LGN–mInsc complexes in the governance of asymmetric cell divisions and in the organization and homeostasis of the epidermis as columnar units of stratified cells. The maintenance of lateral divisions in the p63-null epidermis is similar to that which occurs in simple epithelial tissues and contrasts with the random divisions that occur in the α-catenin-null epidermis. Although beyond the scope of the present study, the difference indicates that α-catenin might be involved in governing not only asymmetric divisions, dependent on LGN–mInsc, but also in symmetric divisions, which did not seem to depend on LGN–mInsc. This would be in agreement with genetic studies in Drosophila30.
Few examples of asymmetric cell divisions have been documented in mammals, but we have shown that in epidermis, proper columnar stratification and tissue organization are driven at least in part by oriented, asymmetric cell divisions. In contrast to previous examples of asymmetric divisions, here the epidermis controls polarity by making intercellular connections with its neighbours as well as attaching to an underlying basement membrane. We found that α-catenin is essential not only for the apical recruitment of PKC-ζ but also for the assembly of the apical LGN–mInsc–Par3 crescent. Although the assemblies of the LGN–mInsc–Par3 and NuMA crescents are independent of one another, their apical localization and involvement in achieving the asymmetric cell division seem to be intimately linked. Given the role of α-cateninin in the association of LGN with the cortex, it is noteworthy that adherens junctions (and focal adhesions) participate in orchestrating actin cytoarchitecture in epithelial tissues. It has been previously reported that disruption of F-actin causes a loss of cortical association of LGN31.
In addition, the basement membrane provides a natural mechanism for concentrating cell determinants at the base of the keratinocyte, and although β1 integrin is not essential for the assembly of the Par3–LGN–mInsc–NuMA–dynactin crescent, it does have a function in apically orienting PKC-ζ and the crescent and hence dictating the directionality of the ensuing cell division. In so doing, this newly formed apical crescent could become a molecular centre for the association of key cell commitment or differentiation factors, just as the basally oriented integrins are known to form a localization site for growth-promoting factors. In this regard, we showed that in the absence of p63, localization of the LGN–mInsc complex and perpendicular angling of the spindle plane do not occur and partitioning of determinants becomes symmetric, concomitant with impaired stratification and differentiation. Our findings now provide a framework for future studies in this area.
METHODS
Generation and immunoprecipitation of mInsc antibody
An antibody was raised in rabbits immunized with a peptide CGDKQRVDTPYTRDQ. By immunoblot analysis, affinity-purified antibody recognized a single band corresponding to the expected size of mInsc.
Protein extracts were prepared from E15.5 epidermis or cultured cells in buffer containing 50 mM Hepes pH 7.4, 100 mM KCl, 2 mM MgCl2, 0.5% Triton X-100, supplemented with 1 mM dithiothreitol and protease inhibitors. Extracts were incubated with 2 μg of control or specific antibody bound to protein G–Sepharose, then washed extensively with buffer; bound proteins were eluted with SDS sample buffer and subjected to immunoblot analysis. Other antibodies used included anti-LGN (S. Bahria), anti-Par3 (I. Macara), anti-NuMA (D. Compton), anti-Flag (Sigma), anti-GFP (Abcam), anti-β-tubulin (Sigma), anti-PKC-ζ (Santa Cruz), anti-Ki67 (Novocastro), anti-pH3 (Upstate) and anti-K1 (Fuchs laboratory).
Mitotic arrests were performed by incubating embryos in medium containing 10 μM nocodazole or 100 μM monastrol for 5 h at 37°C.
Quantification of LGN–NuMA localization and spindle orientation
Late metaphase and anphase spindles were used for quantification. Spindles that were oriented at 90 ± 30° to the basement membrane were classed as perpendicular; those that were oriented at 0 ± 30° were classed as parallel. LGN–NuMA apical localization was determined by dividing the cell into four quadrants, namely apical, basal and two lateral surfaces. We then determined the quadrant in which the centre of the LGN crescent lay. At embryonic stages, 100–200 mitoses were counted. In adults, because of the low mitotic index we found very few cells at a cell-cycle stage that could be quantified. For the epidermis we found 17 of 20 cells dividing asymmetrically, and 13 of 20 in the tongue. To determine the relationship between the NuMA cortical crescent and the spindle in α-catenin KO, we stained with NuMA and examined late metaphase and anaphase cells in which a clear cortical crescent, as well as one or more separated spindle poles was obvious. In 22 of 25 cases, the spindle was aligned with the cortical crescent.
Image acquisition
After fixation and staining, imaging was performed on a Zeiss Axioplan 2 microscope with Apotome attachment, using an Axiocam MRm camera, and Axiovision software. Quantification of basal cell numbers was performed by flow cytometry of cells isolated from full skin of K14–histone 2B mice labelled with β4-integrin antibodies. Basal-to-suprabasal ratios were determined by manually counting basal (β4-integrin-positive) and suprabasal (β4-integrin-negative) cells in the microscope.
Supplementary Material
Acknowledgments
We thank M. Bornens and A. Mills for reagents; J. Fan for transgenic injections; L. Polak, E. Gonzales and LARC staff for care of the mice; H. Rhee for assistance with flow cytometry; members of the Fuchs laboratory for criticisms; and T. Kapoor for reagents and discussions. E.F. is an Investigator of the Howard Hughes Medical Institute. T.L. is a Jane Coffin Child postdoctoral fellow. This work was supported by a grant from the National Institutes of Health.
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
References
- 1.Fuchs E, Raghavan S. Getting under the skin of epidermal morphogenesis. Nature Rev Genet. 2002;3:199–209. doi: 10.1038/nrg758. [DOI] [PubMed] [Google Scholar]
- 2.Vaezi A, Bauer C, Vasioukhin V, Fuchs E. Actin cable dynamics and Rho/Rock orchestrate a polarized cytoskeletal architecture in the early steps of assembling a stratified epithelium. Dev Cell. 2002;3:367–381. doi: 10.1016/s1534-5807(02)00259-9. [DOI] [PubMed] [Google Scholar]
- 3.Watt FM. Selective migration of terminally differentiating cells from the basal layer of cultured human epidermis. J Cell Biol. 1984;98:16–21. doi: 10.1083/jcb.98.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watt FM, Green H. Stratification and terminal differentiation of cultured epidermal cells. Nature. 1982;295:434–436. doi: 10.1038/295434a0. [DOI] [PubMed] [Google Scholar]
- 5.Betschinger J, Knoblich JA. Dare to be different: asymmetric cell division in Drosophila, C. elegans and vertebrates. Curr Biol. 2004;14:R674–R685. doi: 10.1016/j.cub.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 6.Cowan CR, Hyman AA. Asymmetric cell division in C. elegans: cortical polarity and spindle positioning. Annu Rev Cell Dev Biol. 2004;20:427–453. doi: 10.1146/annurev.cellbio.19.111301.113823. [DOI] [PubMed] [Google Scholar]
- 7.Roegiers F, Jan YN. Asymmetric cell division. Curr Opin Cell Biol. 2004;16:195–205. doi: 10.1016/j.ceb.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Mills AA, et al. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature. 1999;398:708–713. doi: 10.1038/19531. [DOI] [PubMed] [Google Scholar]
- 9.Yang A, et al. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature. 1999;398:714–718. doi: 10.1038/19539. [DOI] [PubMed] [Google Scholar]
- 10.Mariotti A, et al. EGF-R signalling through Fyn kinase disrupts the function of integrin α6β4 at hemidesmosomes: role in epithelial cell migration and carcinoma invasion. J Cell Biol. 2001;155:447–458. doi: 10.1083/jcb.200105017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schlaepfer DD, Hunter T. Evidence for in vivo phosphorylation of the Grb2 SH2-domain binding site on focal adhesion kinase by Src-family protein-tyrosine kinases. Mol Cell Biol. 1996;16:5623–5633. doi: 10.1128/mcb.16.10.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tumbar T, et al. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kraut R, Chia W, Jan LY, Jan YN, Knoblich JA. Role of inscuteable in orienting asymmetric cell divisions in Drosophila. Nature. 1996;383:50–55. doi: 10.1038/383050a0. [DOI] [PubMed] [Google Scholar]
- 14.Parmentier ML, et al. Rapsynoid/partner of inscuteable controls asymmetric division of larval neuroblasts in Drosophila. J Neurosci. 2000;20:RC84 . doi: 10.1523/JNEUROSCI.20-14-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schober M, Schaefer M, Knoblich JA. Bazooka recruits Inscuteable to orient asymmetric cell divisions in Drosophila neuroblasts. Nature. 1999;402:548–551. doi: 10.1038/990135. [DOI] [PubMed] [Google Scholar]
- 16.Yu F, Morin X, Cai Y, Yang X, Chia W. Analysis of partner of inscuteable, a novel player of Drosophila asymmetric divisions, reveals two distinct steps in inscuteable apical localization. Cell. 2000;100:399–409. doi: 10.1016/s0092-8674(00)80676-5. [DOI] [PubMed] [Google Scholar]
- 17.Du Q, Macara IG. Mammalian Pins is a conformational switch that links NuMA to heterotrimeric G proteins. Cell. 2004;119:503–516. doi: 10.1016/j.cell.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 18.Du Q, Stukenberg PT, Macara IG. A mammalian Partner of inscuteable binds NuMA and regulates mitotic spindle organization. Nature Cell Biol. 2001;3:1069–1075. doi: 10.1038/ncb1201-1069. [DOI] [PubMed] [Google Scholar]
- 19.Gaglio T, Saredi A, Compton DA. NuMA is required for the organization of microtubules into aster-like mitotic arrays. J Cell Biol. 1995;131:693–708. doi: 10.1083/jcb.131.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kisurina-Evgenieva O, et al. Multiple mechanisms regulate NuMA dynamics at spindle poles. J Cell Sci. 2004;117:6391–6400. doi: 10.1242/jcs.01568. [DOI] [PubMed] [Google Scholar]
- 21.Srinivasan DG, Fisk RM, Xu H, vam den Heuvel S. A complex of Lin-5 and GPR proteins regulates G-protein signalling and spindle function in C. elegans. Genes Dev. 2003;17:1225–1239. doi: 10.1101/gad.1081203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Price CM, Pettijohn DE. Redistribution of the nuclear mitotic apparatus protein (NuMA) during mitosis and nuclear assembly. Properties of purified NuMA protein. Exp Cell Res. 1986;166:95–317. doi: 10.1016/0014-4827(86)90478-7. [DOI] [PubMed] [Google Scholar]
- 23.Grill SW, Howard J, Schaffer E, Stelzer EH, Hyman AA. The distribution of active force generators controls mitotic spindle position. Science. 2003;301:518–521. doi: 10.1126/science.1086560. [DOI] [PubMed] [Google Scholar]
- 24.Dowling J, Yu QC, Fuchs E. Beta4 integrin is required for hemidesmosome formation, cell adhesion and cell survival. J Cell Biol. 1996;134:559–572. doi: 10.1083/jcb.134.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Neut R, Krimpenfort P, Calafat J, Niessen CM, Sonnenberg A. Epithelial detachment due to absence of hemidesmosomes in integrin β4 null mice. Nature Genet. 1996;13:366–369. doi: 10.1038/ng0796-366. [DOI] [PubMed] [Google Scholar]
- 26.Brakebusch C, et al. Skin and hair follicle integrity is crucially dependent on beta 1 integrin expression on keratinocytes. EMBO J. 2000;19:3990–4003. doi: 10.1093/emboj/19.15.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raghavan S, Bauer C, Mundschau G, Li Q, Fuchs E. Conditional ablation of β1 integrin in skin. Severe defects in epidermal proliferation, basement membrane formation, and hair follicle invagination. J Cell Biol. 2000;150:1149–1160. doi: 10.1083/jcb.150.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vasioukhin V, Bowers E, Bauer C, Degenstein L, Fuchs E. Desmoplakin is essential in epidermal sheet formation. Nature Cell Biol. 2001;3:1076–1085. doi: 10.1038/ncb1201-1076. [DOI] [PubMed] [Google Scholar]
- 29.Vasioukhin V, Bauer C, Degenstein L, Wise B, Fuchs E. Hyperproliferation and defects in epithelial polarity upon conditional ablation of alpha-catenin in skin. Cell. 2001;104:605–617. doi: 10.1016/s0092-8674(01)00246-x. [DOI] [PubMed] [Google Scholar]
- 30.Lu B, Roegiers F, Jan LY, Jan YN. Adherens junctions inhibit asymmetric division in the Drosophila epithelium. Nature. 2001;409:522–525. doi: 10.1038/35054077. [DOI] [PubMed] [Google Scholar]
- 31.Kaushik R, Yu F, Chia W, Yang X, Bahri S. Subcellular localization of LGN during mitosis: evidence for its cortical localization in mitotic cell culture systems and its requirement for normal cell cycle progression. Mol Biol Cell. 2003;14:3144–3155. doi: 10.1091/mbc.E03-04-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


