Abstract
Protective protein/cathepsin A (PPCA) has a serine carboxypeptidase activity of unknown physiological function. We now demonstrate that this protease activity triggers the degradation of the lysosome-associated membrane protein type 2a (lamp2a), a receptor for chaperone-mediated autophagy (CMA). Degrada tion of lamp2a is important because its level in the lysosomal membrane is a rate-limiting step of CMA. Cells defective in PPCA show reduced rates of lamp2a degradation, higher levels of lamp2a and higher rates of CMA. Restoration of PPCA protease activity increases rates of lamp2a degradation, reduces levels of lysosomal lamp2a and reduces rates of CMA. PPCA associates with lamp2a on the lysosomal membrane and cleaves lamp2a near the boundary between the luminal and transmembrane domains. In addition to the well-studied role of PPCA in targeting and protecting two lysosomal glycosidases, we have defined a role for the proteolytic activity of this multifunctional protein.
Keywords: autophagy/galactosialidosis/lysosomes/proteases/protein degradation
Introduction
Protective protein/cathepsin A (PPCA) is an acidic serine carboxypeptidase (Iodice, 1967) with deamidase and esterase activities at neutral pH (Jackman et al., 1990). In addition, PPCA has a protective function towards two lysosomal glycosidases, β-d-galactosidase and N-acetyl-α-neuraminidase (d’Azzo et al., 1982). PPCA regulates their lysosomal activity and stability and, in the case of α-neuraminidase, its proper targeting to lysosomes (van der Spoel et al., 1998). A primary defect of PPCA affects the activities of both glycosidases and is the cause of galactosialidosis (GS), an autosomal recessive lysosomal storage disease (d’Azzo et al., 1995). The mouse model of GS closely mimics the severe form of the human disease and results in nephropathy, progressive neurodegeneration and premature death (Zhou et al., 1995). Normal levels of PPCA can be restored in cultured GS cells by supplementing the culture medium with the PPCA precursor (Galjart et al., 1990) and in the mouse model by transplantation of bone marrow cells overexpressing the human PPCA (Zhou et al., 1995).
In contrast to the growing knowledge regarding the protective function of PPCA, the physiological relevance of the serine protease activity has remained unclear. The catalytic activity is not required for the protective function, since the lysosomal storage can be corrected with carboxypeptidase-defective mutants of PPCA (Galjart et al., 1991). Hydrolysis of some regulatory peptides by PPCA in vitro has been reported (Hanna et al., 1994; Itoh et al., 1995), but a physiological role has not been deduced. Our current results show that PPCA triggers the degradation of the lysosome-associated membrane protein type 2a (lamp2a), a receptor for chaperone-mediated autophagy (CMA). The amount of receptor at the lyso somal membrane is a rate-limiting step in CMA (Cuervo and Dice, 2000a), so lamp2a cleavage regulates the activity of this lysosomal pathway of protein degradation.
Approximately 30% of cytosolic proteins can be selectively degraded in lysosomes by CMA (Cuervo and Dice, 1998; Dice, 2000). Substrate proteins contain in their sequence a targeting motif, biochemically related to the pentapeptide KFERQ, which is recognized by a cytosolic chaperone, the heat shock cognate protein of 73 kDa (hsc73) (Chiang et al., 1989). The hsc73–substrate protein complex binds to the lysosomal membrane (Cuervo and Dice, 1996), and the substrate protein is then transported through the lysosomal membrane assisted by a form of hsc73 in the lysosomal matrix (Agarraberes et al., 1997; Cuervo et al., 1997). CMA is mainly activated in response to stresses such as nutrient deprivation (Auteri et al., 1983) or exposure to some toxins (Cuervo et al., 1999).
We have previously identified lamp2a as a receptor for CMA substrates (Cuervo and Dice, 1996). Lamp2a is one of three forms of lamp2 that originate by alternative splicing of mRNA from a single gene (Licheter-Konecki et al., 1999). All lamp2s have identical luminal regions but different transmembrane and cytosolic tails (Licheter-Konecki et al., 1999). Knocking out the lamp2 gene results in massive accumulation of autophagic vacuoles in many tissues due to impaired macroautophagy (Tanaka et al., 2000). The identity of the lamp2 isoform required for macroautophagy is unclear. For CMA, we have previously demonstrated that lamp2a is the only isoform involved (Cuervo and Dice, 2000a).
In all the conditions where changes in the activity of CMA have been described (serum deprivation or starvation, aging, overexpression of lamp2a and toxin-induced nephropathies) there is a strong direct correlation between CMA activity and levels of lamp2a at the lysosomal membrane, suggesting that binding of substrate proteins to lamp2a is a rate-limiting step (Cuervo and Dice, 2000a). Interestingly, levels of lamp2a at the lysosomal membrane do not depend on the expression of the protein, but instead on its degradation in the lysosomal compartment (Cuervo and Dice, 2000b). In all conditions that lead to increased CMA there is a decrease in the degradation rate of the receptor at the lysosomal membrane and, conversely, decreased CMA is accompanied by faster degradation of lamp2a (Cuervo and Dice, 2000b). Lamp2a degradation starts with its cleavage at the lysosomal membrane by a metalloprotease and a serine protease, followed by its release as a truncated form into the lysosomal matrix, where it is then completely degraded (Cuervo and Dice, 2000b). In addition to alterations in degradation rate, redistribution of part of the lamp2a from the lysosomal lumen to the membrane can also increase membrane levels of lamp2a (Cuervo and Dice, 2000b).
To identify the protease(s) responsible for the initial cleavages of lamp2a in lysosomes, we have isolated proteins associated with lamp2a that exhibit proteolytic activity. We found that lamp2a interacts with PPCA, a lysosomal serine carboxypetidase, and PPCA triggers lamp2a degradation in lysosomes.
Results
PPCA interacts with lamp2a at the lysosomal membrane
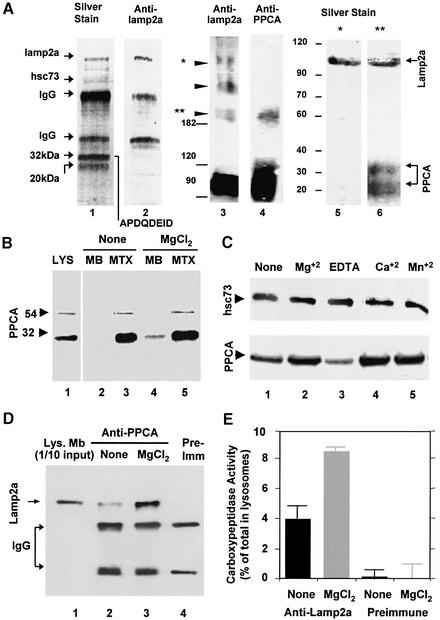
Using a specific antibody against the cytosolic tail of rat and mouse lamp2a as an affinity matrix, we found several proteins that consistently co-purified with lamp2a from reversibly cross-linked and solubilized rat liver lysosomes (Figure 1A, lanes 1 and 2). Two major proteins of 32 and 20 kDa associated with lamp2a. The 32 kDa protein sequence matched the N-terminus of the mature 32 kDa chain of rat PPCA (APDQDEID). Both the 32 and 20 kDa proteins were recognized by a polyclonal antibody against the 54 kDa precursor of PPCA (Galjart et al., 1991) and therefore corresponded to the two subunits of the mature protein. In agreement with our previous observations (Cuervo and Dice, 1996), immunoblot analysis of the eluted proteins revealed that hsc73 also co-purified with lamp2a (Figure 1A, lane 1).
Fig. 1. PPCA associates with lamp2a in rat liver lysosomes. (A) Solubilized lysosomes cross-linked with DSP were subjected to affinity chromato graphy with the antibody against the cytosolic tail of lamp2a (lanes 1 and 2). Eluted proteins were subjected to SDS–PAGE and silver stained (lane 1), or immunoblotted for lamp2a (lane 2). The sequence of the N-terminus of the 32 kDa band is shown. Part of the solubilized lysosomes were directly subjected to non-reducing SDS–PAGE (5%) and immunoblotted for lamp2a (lane 3) or PPCA (lane 4). Arrowheads indicate lamp2a multimeric complexes. The 600 kDa (*) and 200 kDa (**) molecular weight complexes of lamp2a were excised and subjected to reducing SDS–PAGE (12%) and silver staining (lanes 5 and 6). Molecular weight (kDa) is indicated on the left. (B) Immunoblot analysis of PPCA in rat liver lysosomes, their membranes (MB) and matrices (MTX) (100 µg of protein) incubated alone (None) or with 5 mM MgCl2. Arrowheads indicate the precursor (54 kDa) and heavy chain (32 kDa) of the mature PPCA. (C) Immunoblot analysis of hsc73 (upper) and PPCA (lower) in membranes from rat liver lysosomes incubated alone (None) or with 5 mM MgCl2, CaCl2, MnCl2 or 2 mM EDTA. (D) Immunoblot analysis of lamp2a co-immunoprecipitated with PPCA in the absence of chemical cross-linkers from membranes of liver lysosomes incubated as in (B). Light and heavy chains of IgG are shown. Lane 1 contains 1/10 of the total amount of lysosomal membranes used for immunoprecipitation. (E) Membranes from lysosomes treated as in (D) were subjected to immunoprecipitation with an antibody specific for lamp2a or with a pre-immune serum. Carboxypeptidase activity in total lysosomes and in the immunoprecipitates was measured as described under Materials and methods. Values are expressed as percentage of total carboxypeptidase activity in lysosomes present in the immunoprecipitates and are the mean ± SE of three different experiments.
Non-reducing electrophoresis of rat liver lysosomes revealed that monomers of lamp2a preferentially associated with dimers of PPCA. Lamp2a formed different high molecular weight complexes in the lysosomal membrane (Figure 1A, lane 3) (Cuervo and Dice, 2000a), but the antibody against PPCA only recognized the 200 kDa complex (Figure 1A, lane 4) and a 100 kDa band previously identified as mature PPCA homodimers (Galjart et al., 1991). The two subunits of PPCA and lamp2a were the only components detected in the 200 kDa complex after reducing electrophoresis (Figure 1A, lane 6). None of the PPCA subunits was detected as part of the 600 kDa lamp2a complexes (Figure 1A, lane 5).
Most PPCA is located in the lysosomal lumen, but a small percentage (∼10%) associates with the lysosomal membrane in a cation-dependent manner (Figure 1B and C). The membrane-bound PPCA was resistant to washing with buffers containing 1 M NaCl, but could be released by alkali extraction (data not shown). Lamp2a co-immunoprecipitated with the membrane-associated PPCA in rat liver lysosomes even in the absence of cross-linkers (Figure 1D), indicating that the lamp2a–PPCA complexes are normally present in the lysosomal membrane. The amount of lamp2a in the PPCA immunoprecipitate increased in the presence of MgCl2 (Figure 1D).
PPCA associated with lamp2a is catalytically active. Approximately 4% of the total carboxypeptidase activity in lysosomes could be co-immunoprecipitated with lamp2a (Figure 1E). The amount of carboxypeptidase activity recovered in the lamp2a immunoprecipitate increased to 9% in the presence of MgCl2 (Figure 1E). No carboxypeptidase activity was recovered using pre-immune serum in the same protocol (Figure 1E).
Association of PPCA with the lysosomal membrane correlates with increased lamp2a degradation
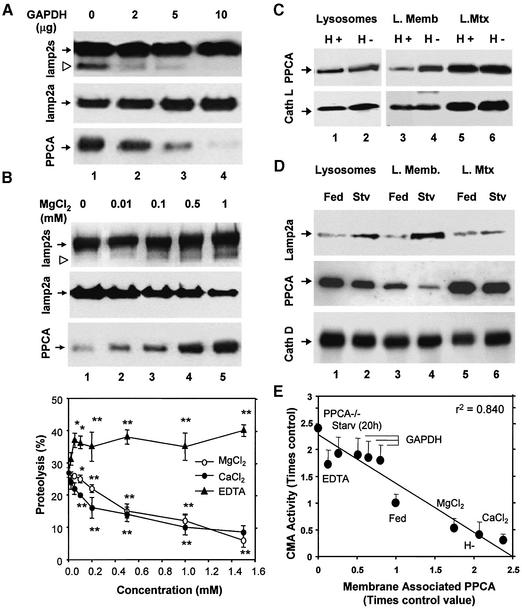
Degradation of lamp2a in lysosomal membranes can be followed in vitro as the accumulation of a truncated form of lamp2a lacking the cytosolic and transmembrane regions, and detected with an antibody against the luminal region of lamp2 (Cuervo and Dice, 2000b). Although this antibody recognizes all forms of lamp2, we have previously demonstrated that the truncated form primarily originates from cleavage of lamp2a (Cuervo and Dice, 2000b). Incubation of rat liver lysosomes with substrate proteins for CMA, such as glyceraldehyde-3-phosphate dehydrogenase (GAPDH), reduced lamp2a cleavage (Cuervo and Dice, 2000b; Figure 2A). The amount of membrane-associated PPCA decreased progressively with the increase in the concentration of GAPDH (Figure 2A, lower blot). In contrast, increasing concentrations of MgCl2, even in the presence of substrate proteins, increased the amount of PPCA associated with the membrane (Figure 2B, lower) and stimulated lamp2a degradation (Figure 2B, upper and middle blots).
Fig. 2. Levels of PPCA associated with the lysosomal membrane correlate with rates of CMA. Membranes of lysosomes incubated with increasing concentrations of GAPDH (A) or MgCl2 (B, top panel) as labeled, were analyzed by immunoblot for all forms of lamp2 (upper), lamp2a (middle) or PPCA (lower). The arrowhead indicates a previously identified truncated form of lamp2 lacking the cytosolic/transmembrane region (Cuervo and Dice, 2000b). (B, bottom panel) Chart showing the effect of increasing concentrations of MgCl2, CaCl2 or EDTA on the degradation of [14C]GAPDH by intact rat liver lysosomes. Values are means ± SE of six different experiments. Differences from the control value were significant at P < 0.001 (**) or P < 0.01 (*). (C) Immunoblot analysis for PPCA (upper) and cathepsin L (lower) of rat liver lysosomes (100 µg of protein) with high (H+) and low (H–) activity for CMA and of the lysosomal membranes (L.Memb) and matrices (L.Mtx) from both groups of lysosomes (200 µg of protein). (D) Immunoblot analysis for lamp2a, PPCA and cathepsin D, as labeled, of lysosomes (100 µg of protein) from fed or 48 h starved rats and the membranes (L. Memb) and matrices (L. Mtx) of both goups of lysosomes (200 µg of protein). Only mature forms of the cathepsins are shown. (E) Correlation between CMA activity, measured as in (B, bottom panel) and levels of PPCA at the lysosomal membrane determined by denstometric quantification of immunoblots similar to those shown in Figures 1 and 2 in lysosomes from fed or 20 h starved rats (Starv 20h), highly active (H+) or less active (H–) lysosomes, or lysosomes incubated with 1 mM CaCl2, MgCl2, EDTA, or increasing amounts (2, 5, 10 µg) of GAPDH. Values are expressed as times the value in fed rats (control) and are means ± SE of 3–6 different experiments.
As expected from the cation-dependent association of PPCA with the lysosomal membrane, divalent cations had an inhibitory effect, reversed by EDTA, on the uptake and degradation of GAPDH by intact lysosomes (Figure 2B, bottom chart). Significant inhibition was achieved by adding CaCl2 at concentrations previously shown to cause intralysosomal levels of Ca2+ in the physiological range (0.5–10 µM) (Haller et al., 1996). None of the cations had a direct effect on the degradation of GAPDH by broken lysosomes (data not shown), suggesting that their effect was mainly on substrate binding and uptake rather than on degradation in the lysosomal matrix.
Levels of PPCA at the lysosomal membrane inversely correlate with CMA activity also under several conditions in vivo. In rat liver, we could isolate two groups of lysosomes with very different activities for CMA (Cuervo et al., 1997). The less active group of lysosomes, which has higher rates of lamp2a degradation (Cuervo and Dice, 2000b), had 2.5-fold higher amounts of PPCA associated with the lysosomal membrane than the active group (Figure 2C, upper). The amount of cathepsin L in the lysosomal membrane and matrix was similar in both groups of lysosomes (Figure 2C, lower).
Nutrient deprivation activates CMA and increases levels of lamp2a at the lysosomal membrane mostly due to a decrease in rates of lamp2a degradation (Cuervo and Dice, 2000b). In lysosomes from starved rats, total levels of PPCA and matrix levels declined 2-fold compared with fed rats, but the amount of PPCA associated with the lysosomal membrane decreased 3- to 4-fold (Figure 2D, middle). We found no significant changes in the lysosomal levels of cathepsin D after starvation (Figure 2D, lower).
Interaction of PPCA with lamp2a at the lysosomal membrane occurs under conditions in which lamp2a is rapidly degraded. Furthermore, as shown in Figure 2E, levels of PPCA associated with the lysosomal membrane were strongly inversely correlated with CMA activity.
PPCA-defective cells have increased levels of lamp2a and higher rates of CMA
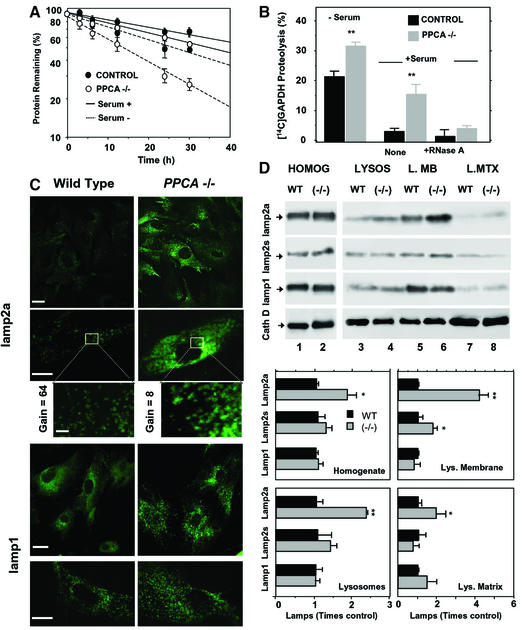
We used cultured fibroblasts from PPCA(–/–) mice (Zhou et al., 1995) to analyze the effect of PPCA absence on CMA. When maintained in the presence of serum, the rate of total protein degradation in PPCA(–/–) fibroblasts was consistently slightly higher than in controls (Figure 3A). These differences became more pronounced after serum removal when CMA is preferentially activated (Cuervo and Dice, 1998; Dice, 2000) (Figure 3A). The ability to take up and degrade [14C]GAPDH was 1.5-fold higher than the controls in lysosomes isolated from serum-deprived PPCA(–/–) cells, and 3- to 4-fold higher than the controls in lysosomes from serum-supplemented PPCA(–/–) (Figure 3B). Degradation of [14C]GAPDH by these PPCA(–/–) lysosomes was competed by RNase A, another substrate for CMA, confirming that the degradation was by CMA (Figure 3B). We observed a similar increase in CMA activity with lysosomes isolated from skin fibroblasts of three different GS patients (data not shown). These results indicate that CMA is active even under normal nutritional conditions in PPCA-deficient cells.
Fig. 3. CMA is abnormally activated in PPCA-deficient fibroblasts. (A) Rates of protein degradation in skin fibroblasts from wild-type (control) or PPCA(–/–) mice maintained in the presence (serum +) or absence of serum (serum –). Values are expressed as the percentage of initially radiolabeled proteins remaining at each time and are means ± SE of four different experiments. (B) Degradation of [14C]GAPDH after 30 min incubation with lysosomes isolated from wild-type and PPCA(–/–) mice skin fibroblasts supplemented or deprived of serum was measured as described in Material and methods. Where indicated, 25 µg of RNase A were added. Values are means ± SE of four different experiments. Differences from the control value were significant to P < 0.001 (**). (C) Immunofluorescent staining for lamp2a and lamp1 of skin fibroblasts from wild-type and PPCA(–/–) mice. Gain for lamp2a images (top) was 1/8 of the one for lamp1 (bottom) to visualize the punctate pattern of lamp2a in PPCA(–/–) mice. Inset: detail of the vesicular distribution of lamp2a. The gain in wild type was eight times the gain in PPCA(–/–) mice). Bars: 100 µm (top), 50 µm (bottom), 10 µm (inset). (D) Immunoblot analysis for lamp2a, all lamp2s, lamp1 or cathepsin D (cath D) of homogenates (75 µg of protein), lysosomes (10 µg of protein) and lysosomal membranes (L.MB) and matrices (L.MTX) (5 µg of protein) isolated from wild-type (WT) and PPCA(–/–) skin fibroblasts. The bottom chart shows the densitometric quantification of the membrane proteins (means + SE) from four immunoblots similar to those shown here. Differences from the control value were significant to P < 0.001 (**) and P < 0.05 (*).
Immunofluorescence analysis of lamp2a in serum-deprived mouse skin fibroblasts revealed similar vesicular distribution patterns for lamp2a in wild-type and PPCA(–/–) cells, but stronger immunoreactivity in cells lacking PPCA (Figure 3C). Quantification of immunoblots of cellular homogenates (Figure 3D, bottom panel) revealed 1.5–2 times higher levels of lamp2a in fibroblasts from PPCA(–/–) mice than from wild-type mice (Figure 3D, top blot). After subcellular fractionation, we found that the increase in lamp2a content in PPCA(–/–) cells was mostly at the lysosomal membrane and was 4-fold above controls (Figure 3D, top blot). In contrast, the antibody that recognizes all forms of lamp2 revealed an increase in total lamp2 at the lysosomal membrane of PPCA(–/–) cells of only 1.5- to 2-fold (Figure 3D, middle blot). Lack of PPCA did not significantly change levels of other lysosomal membrane (lamp1; Figure 3C and D) or lysosomal matrix proteins (cathepsin D; Figure 3D, bottom blot).
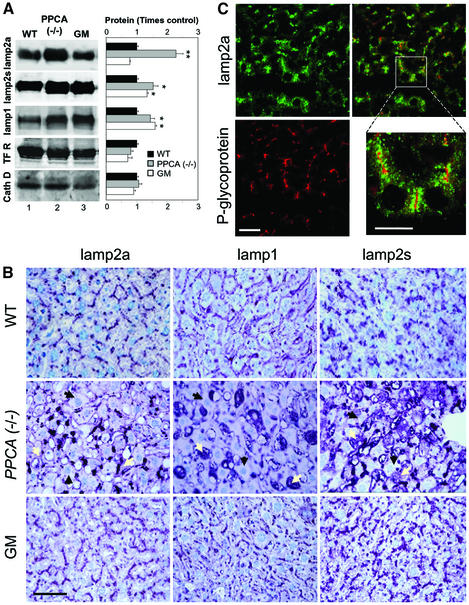
Similarly to skin fibroblasts, we found higher levels of lamp2a in the liver of PPCA(–/–) mice than in wild-type or β-galactosidase(–/–) mice (Figure 4). Immunoblot analysis revealed a 2.5-fold increase in the liver content of lamp2a, but no significant change in the levels of other lysosomal and non-lysosomal proteins such as cathepsin D and the transferrin receptor, respectively (Figure 4A). Although the levels of other forms of lamp2 and of lamp1 were also higher in PPCA(–/–) livers than in wild type (1.5-fold increase; Figure 4A), immunological staining of liver sections revealed clear differences in the distribution pattern for lamp2a when compared with the other lysosomal proteins (Figure 4B). As described for other lysosomal storage diseases (Meikle et al., 1999; Zimmer et al., 1999), livers of PPCA(–/–) mice showed a marked increase of lamp1 mainly in abnormally enlarged Kupffer cells (Figure 4B, middle), which also stained with the antibody that recognizes the luminal region common to all isoforms of lamp2 (Figure 4B, right). In contrast, lamp2a was mainly detected in the periphery of hepatocytes, and to a lesser extent in Kupffer cells. Double immunostaining for lamp2a and for canalicular ABC-type proteins revealed that most of the lamp2a in hepatocytes was located in granular structures, presumably lysosomes, adjacent to the apical plasma membrane (Figure 4C). The differences in levels and cell type distribution between lamp2a and other forms of lamps in liver seem to be unique for PPCA(–/–) mice; in β-galactosidase(–/–) mice livers, we found no preferential increase in lamp2a levels when compared with other isoforms of lamp2 or to lamp1 (Figure 4A and B).
Fig. 4. Lamps levels in livers from PPCA(–/–) mice. (A) Immunoblot analysis for lamp2a, all lamp2s, lamp1, transferrin receptor (TFR) or cathepsin D (cath D) of liver homogenates (100 µg of protein) from wild-type (WT), PPCA(–/–) and β-galactosidase(–/–) (GM) mice. Right: densitometric quantification of three immunoblots (means + SE) similar to the ones shown here. Differences from the control value were significant to P < 0.001 (**) and P < 0.05 (*). (B) Immunocytochemistry for lamp2a (left), lamp1 (center) or lamp2s (right) in hematoxylin and eosin-stained sections from the same livers. Bar: 50 µm. Black arrows: hepatocytes; white arrows: Kupffer cells. (C) Immunofluorescence for lamp2a (top) and P-glycoprotein (bottom) in mouse liver sections. The right panel shows merged images. Bar: 100 µm. Inset shows detailed area at higher magnification. Bar: 50 µm.
Degradation of lamp2a is reduced in PPCA-defective cells
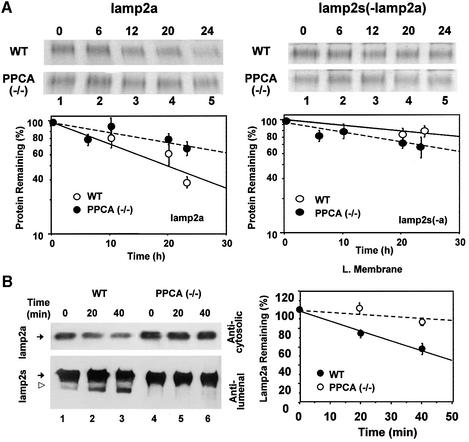
Changes in intracellular levels of lamp2a are regulated in part by changes in its degradation rate (Cuervo and Dice, 2000b). We found that the degradation rate of lamp2a in PPCA(–/–) fibroblasts was slower than in normal fibroblasts (Figure 5A, left). There were no significant differences between null and control mice in the degradation rate of the other isoforms of lamp2 left in the extract after immunoprecipitating lamp2a (Figure 5A, right).
Fig. 5. Impaired lamp2a degradation in PPCA-deficient fibroblasts. (A) Autofluorography of the sequential immunoprecipitation of lamp2a first (left) and all the remaining isoforms of lamp2 second (right) from metabolically labeled wild-type and PPCA(–/–) mouse skin fibroblasts. Values are means ± SE of three different experiments similar to the ones shown in the upper panels. (B) Immunoblot analysis for lamp2a (top) and all lamp2 isoforms (bottom) in lysosomal membranes from wild-type (WT) or PPCA(–/–) mouse skin fibroblasts at different times after incubation at 37°C. The open arrowhead indicates the truncated form of lamp2 lacking the cytosolic/transmembrane region (Cuervo and Dice, 2000b). Right shows the densitometric quantification (mean ± SE) of four different experiments similar to the one shown here. Values are expressed as percentage of remaining lamp2a, and are means ± 1 SE of three different experiments.
The rates of lamp2a degradation in isolated lysosomes incubated in an isotonic medium were also significantly slower in cells from PPCA(–/–) mice than from control mice (Figure 5B), and we could not detect the typical proteolytic fragment of lamp2a normally found in lysosomes (Figure 5B, left). Quantification of four different experiments similar to that shown in Figure 5B (left) is shown in Figure 5B (right).
PPCA serine protease activity restores normal CMA activity in GS cells
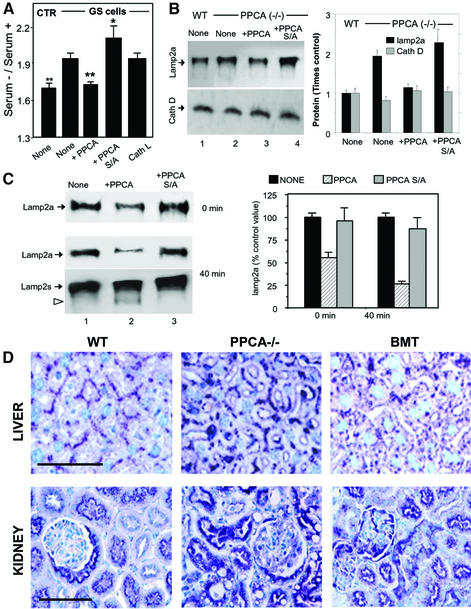
The addition of the PPCA precursor to the culture medium of GS cells restores cathepsin A activity to 60–80% of control values (Galjart et al., 1990). This treatment normalized total protein degradation rates in response to serum removal (Figure 6A). However, when we supplemented GS cells with a PPCA precursor mutated in the serine at the active site (PPCA-S/A) (Galjart et al., 1991), rates of CMA remained higher than in the control cells (Figure 6A). Addition of a precursor of cathepsin L in the culture medium of GS cells did not have any effect on their proteolytic activity (Figure 6A).
Fig. 6. Restoration of PPCA protease activity in GS cells normalizes CMA. (A) Rates of total protein degradation in the presence and absence of serum of normal skin fibroblasts (CTR), GS cells (None) and GS cells supplemented with the precursor forms of native PPCA (+PPCA), a catalytic mutant of PPCA (+PPCA S/A) or cathepsin L (Cath L). Values are means ± SE of six different experiments. Differences from the control value were significant for P < 0.001 (**) and P < 0.05 (*). (B) Immunoblot analysis for lamp2a and cathepsin D (Cath D) of homogenates of wild-type and PPCA(–/–) cells supplemented as in (A). Right: densitometric quantification (means + range of values) of two experiments similar to the one shown. Values are expressed as times the value in wild-type cells. (C) Immunoblot analysis for lamp2a at time 0 or after 40 min incubation at 37°C of lysosomes isolated from PPCA(–/–) cells (None) and PPCA(–/–) cells supplemented as in (A). At 40 min immunoblot with the antibody against the luminal region of lamp2a is depicted to show the truncated form of lamp2a (open arrowhead). Right: densitometric quantification for lamp2a (means + range of values) of two different experiments similar to the one shown. Values are expressed as percentage of the lamp2a present in non-supplemented cells. (D) Immunocytochemistry for lamp2a of liver (top) and kidney (bottom) sections of age-matched wild-type (WT), PPCA(–/–) and bone marrow transplanted (BMT) mice. Bar, 50 µm (liver) or 100 µm (kidney).
As shown in Figure 6B, supplementation of PPCA(–/–) cells with the catalytically active protein, but not with the mutant, decreased intracellular lamp2a to levels similar to that in wild-type cells. This treatment did not change levels of other lysosomal proteins such as cathepsin D (Figure 6B). Lysosomes isolated from PPCA-deficient cells treated with wild-type PPCA showed lower rates of substrate uptake compared with untreated cells or cells treated with PPCA-S/A (data not shown). In addition, they showed lower levels of lamp2a and higher rates of lamp2a degradation (Figure 6C). The lysosomal lamp2a fragment absent in PPCA defective cells was again detected after PPCA supplementation (Figure 6C).
Restoration of normal cathepsin A activity in PPCA(–/–) mice also reduced tissue levels of lamp2a. As shown in Figure 6D, lamp2a immunostaining in PPCA(–/–) mice liver (top) and kidney (bottom), where CMA is also normally active, was more similar to controls after transplantation with bone marrow from wild-type mice.
These results suggest that the restoration of normal cathepsin A activity in defective cells promotes degradation of lamp2a. In turn, the decrease in the lysosomal levels of this protein results in reduced CMA.
PPCA cleavage of lamp2a
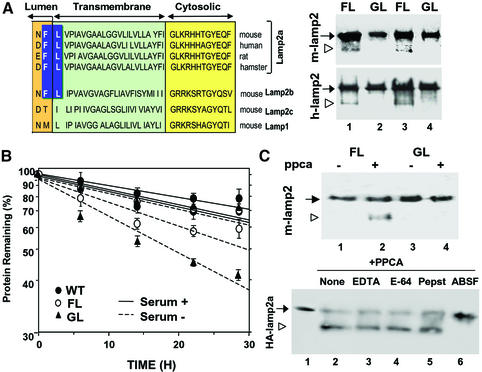
We have previously shown that degradation of lamp2a in lysosomes begins with cleavage of the protein to a truncated form lacking the transmembrane and cytosolic regions by the actions of a metalloprotease and a serine protease (Cuervo and Dice, 2000b). As depicted in Figure 7A (left), close to the junction between the transmembrane and luminal regions of lamp2a and lamp2b, but not of lamp2c or lamp1, there is a conserved FL dipeptide previously described as one of the most preferred cleavage sequences for the carboxypeptidase activity of PPCA in vitro (Galjart et al., 1990; Pshezhetsky and Potier, 1994). To determine whether that motif was relevant for lamp2a cleavage in lysosomes, we mutated the F residue of hemagglutinin-tagged lamp2a (HA-lamp2a) to G. Lysosomal membranes from cells transfected with the cDNAs for mouse (Figure 7A, right panel, top) or human HA-lamp2a (Figure 7A, right panel, bottom) showed obvious degradation products of lamp2a (lanes 1 and 3) not detected in cells transfected with the F→G forms (lanes 2 and 4). Consistent with this lack of lamp2a cleavage, rates of protein degradation in the absence of serum were significantly higher in cells transfected with the F→G form than with wild-type lamp2a (Figure 7B).
Fig. 7. Cleavage site of PPCA in lamp2a. (A) Left: amino acid sequences of the C-terminal regions of lamp2a from different species and of other lamp2 isoforms and lamp1 from mouse. The proposed cleavage site for PPCA in lamp2a is highlighted. Right: immunoblot analysis for HA of lysosomal membranes isolated from two different clones of HEK293 (mouse; upper panel) and NIH 3T3 cells (human; lower panel) stably transfected with wild-type (FL) and mutated (GL) HA-lamp2a. The arrowhead indicates the truncated form of lamp2 lacking the cytosolic/transmembrane region. (B) Total rates of protein degradation in the presence and absence of serum in non-transfected HEK293 cells (WT) and in the transfected HEK293 clones described in (A). Values are expressed as the percentage of initially radiolabeled proteins remaining at each time and are means ± SE of three different experiments. (C) Top: immunoblot analysis with an anti-HA antibody of purified wild-type (FL) and mutated (GL) mouse HA-lamp2a incubated in an acidic pH buffer alone, or with PPCA. Bottom: wild-type HA-lamp2a was incubated as in the top panel without additions (None) or in the presence of 2 mM EDTA, 10 µM l-trans-epoxysuccinyl-leucylamide-(4-guanido)-butane (E-64), 2 µM pepstatin A (Pepst) or 10 µM ABSF. The open arrowhead indicates the truncated form of lamp2a.
To further test direct cleavage of lamp2a by PPCA we incubated purified wild-type and F→G mutant HA-lamp2a with PPCA under conditions shown to maximally activate PPCA carboxypeptidase activity (Iodice, 1967). In the presence of PPCA, we detected a single truncated form of lamp2a of an estimated molecular weight similar to the one observed with isolated lysosomal membranes in the wild-type lamp2a, but not in the F→G mutant (Figure 7C, top, lane 2). Lamp2a cleavage was no longer observed when the serine catalytic site of PPCA was blocked by the specific inhibitor 4-(2-aminoethyl)benzenesulfonyl fluoride (ABSF) (Figure 7C, bottom, lane 6). Inhibitors of the other kinds of proteolytic activities did not have any effect on lamp2a cleavage. These results, together with the inability of the serine catalytic mutant of PPCA to restore normal rates of lamp2a degradation in PPCA(–/–) cells (Figure 6C), indicate that the serine active site of PPCA is required for cleavage of lamp2a.
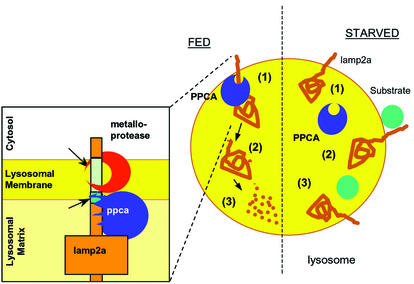
We conclude that under normal conditions a portion of PPCA associates with the lamp2a at the lysosomal membrane and triggers its cleavage, membrane de-insertion and degradation in the lysosomal lumen (Figure 8, FED). PPCA cuts lamp2a in the intersection between the transmembrane and luminal region, and is also required to initiate the cleavage of the C-terminal region of lamp2a by an unidentified metalloprotease (Figure 8, insert). PPCA might act directly, activating the metalloprotease, or indirectly, inducing a conformational change on lamp2a favorable to metalloprotease cleavage. After nutrient deprivation, by unknown mechanisms, but perhaps involving changes in the intralysosomal content of divalent cations, the interaction of PPCA with the lysosomal membrane is reduced and lamp2a is less able to be de graded at the lysosomal membrane (Figure 8, STARVED). This results in an increase in the amount of receptor available for substrate binding and in higher rates of substrate uptake and degradation.
Fig. 8. Hypothetical model for the regulation of CMA by PPCA. Under normal nutritional conditions (FED, left), binding of PPCA to the lysosomal membrane might trigger cleavage of lamp2a by an unidentified metalloprotease (at the transmembrane region) (1) and by PPCA (at the intersection between the transmembrane and luminal regions) (see inset). That cleavage releases a truncated lamp2a into the matrix (2), where it is completely degraded by the lysosomal proteases (3). During starvation (right), PPCA dissociates from the lysosomal membrane (1) so that lamp2a is not longer degraded and remains in the membrane, accessible for substrate binding (2) and uptake into the lysosomes (3).
Discussion
Our results provide new insights into the physiological function of the carboxypeptidase activity of PPCA in vivo. Several lines of evidence support the requirement for PPCA in the degradation of lamp2a at the lysosomal membrane: (i) PPCA associates with lamp2a at the lysosomal membrane, and this association is inhibited in the presence of protein substrates for CMA (Figures 1 and 2); (ii) levels of PPCA at the lysosomal membrane increase under conditions that result in increased rates of lamp2a degradation (Figure 2); (iii) cells defective in PPCA have higher levels of lamp2a in the lysosomal membrane (Figures 3 and 4) and lower rates of lamp2a degradation (Figure 5); (iv) restoration of normal PPCA carboxypeptidase activity in defective cells corrects the reduced degradation of lamp2a (Figure 6); (v) mutation of the putative cleavage site of PPCA in lamp2a prevents lamp2a degradation (Figure 7A); and (vi) PPCA cuts lamp2a in vitro to a truncated form of similar size as the intermediate fragment of lamp2a detected in lysosomes (Figure 7C).
The degradation of lamp2a by PPCA is regulated by the ability of PPCA to interact with the lysosomal membrane (Figure 2C and D). Binding of PPCA with lamp2a is promoted by divalent cations, such as Ca2+, which largely accumulate in lysosomal vesicles in the form of a rapidly mobilizable pool (Haller et al., 1996). Changes in the intralysosomal levels of calcium in different physiological conditions could regulate the amount of PPCA bound to the lysosomal membrane. Perhaps starvation reduces intralysosomal levels of cations, favoring the release of PPCA from the membrane and preserving lamp2a. This could result in higher levels of receptor at the lysosomal membrane accessible for binding and uptake of substrate proteins.
PPCA directly cleaves lamp2a in vitro, but that cleavage is prevented if a critical F in lamp2a is changed to G (Figure 7C). PPCA is the only enzyme found in mammals that at acidic pH selectively hydrolyzes between hydrophobic amino acids, such as the FL dipeptide in lamp2a (Galjart et al., 1990; Pshezhetsky and Potier, 1994). In cells lacking PPCA, degradation of lamp2a is severely impaired. The resulting higher levels of lamp2a at the lysosomal membrane lead to the activation of CMA in these cells even under normal nutritional conditions (Figure 3B). The additional increase in CMA observed after removal of serum in PPCA(–/–) cells (Figure 3A and B) is probably due to the starvation-induced recruitment of the luminal lamp2a to the lysosomal membrane reported previously (Cuervo and Dice, 2000b).
Eliminating the expression of all lamp2a isoforms results in accumulation of autophagic vacuoles in the cytoplasm of different cells (Tanaka et al., 2000). Interestingly, an excess of lamp2a does not significantly modify this process. Thus, we did not find significant changes in the content of autophagic vacuoles in the PPCA(–/–) cells that contain abnormally high levels of lamp2a in their lysosomal compartment (Figure 4). These results are in agreement with our previous studies in which overexpression of lamp2a in CHO cells did not significantly modify degradation of proteins by macroautophagy (Cuervo and Dice, 1996).
Our immunohistochemical studies in liver and kidney of PPCA(–/–) mice reveal cell type-dependent differences in the amount of intracellular lamp2a and other lamps accumulated (Figure 4). Those differences may be due to differences in the percentage of each of the lamp2 isoforms expressed in different cell types (Licheter-Konecki et al., 1999). Lamp2a seems to be preferentially expressed in hepatocytes, but poorly expressed in Kupffer cells. The observed enrichment of lamp1 and other forms of lamp2 in Kupffer cells is common in lysosomal storage diseases (Zimmer et al., 1999). In contrast, the preferential accumulation of lamp2a in lysosomes of fibroblasts and hepatocytes (Figures 3D and 4B) seems directly associated with the lack of PPCA.
The role of PPCA in the cleavage of lamp2a is rather specific. Only the degradation rate of lamp2a, but not that of the other lamp2 isoforms, decreases in PPCA-deficient fibroblasts (Figure 5A). In addition, in PPCA(–/–) cells we found only a slight increase in total lamp2 (Figures 3D and 4A), of which lamp2a is only 25% (Cuervo and Dice, 2000a). Interestingly, despite the normal degradation rates observed for the other isoforms of lamp2 in PPCA(–/–) cells, the putative cleavage site for PPCA in lamp2a (FL) is also present in lamp2b (Figure 7A). The degradation of lamps in other lysosomal storage diseases has been reported to be normal (Zimmer et al., 1999), supporting the idea that the impaired degradation of lamp2a in GS cells results directly from the lack of PPCA, rather than as a consequence of general lysosomal dysfunction.
The degradation of integral membrane proteins by specific membrane-bound proteases has been described in other organelles and at the plasma membrane (Akiyama et al., 1996; Kihara et al., 1996; Ozols, 1997). In addition, regulated degradation has been proven to modulate the levels of several membrane receptors (Hooper et al., 1997). Thus, the identification of PPCA as the protease that initiates lamp2a degradation will lead to a better understanding of the regulation of CMA, and in turn might be useful to correct the abnormal activity of this pathway during aging (Cuervo and Dice, 2000c) or specific kidney pathologies (Cuervo et al., 1999). Furthermore, changes in the intracellular levels of lamp2a could also affect some of the functions that lamp2a shares with other types of lamp2, such as autophagic vacuole maturation (Tanaka et al., 2000) or intercellular adhesion (Licheter-Konecki et al., 1999).
In conclusion, we have identified for the first time a physiological role for the serine protease activity of PPCA in initiating the degradation of lamp2a. In GS patients and PPCA(–/–) mice, the impaired degradation of lamp2a results in elevated CMA, and that may contribute to certain symptoms of disease, including the lower weight of affected individuals (Zhou et al., 1995).
Materials and methods
Animals and cells
Male Wistar rats (200–250 g) fasted for 20 h before killing were used. Mice with the null mutation at the PPCA locus and primary cultures of their skin fibroblasts were generated as described previously (Zhou et al., 1995). Skin fibroblasts from normal individuals and from a GS affected patient (Kleijer et al., 1979) and COS-1 cells were obtained from the Rotterdam Cell Repository (Dr M.F.Niermeijer). Human embryo kidney cells (HEK293) and mouse fibroblasts (NIH 3T3) were from the American Type Culture Collection (Manassas, VA). All cells were maintained in Dulbecco’s modified Eagle’s medium (Sigma, St Louis, MO) in the presence of 10% newborn calf serum (NCS) or 5% in the case of COS-1 cells.
Chemicals
Sources of chemicals and antibodies were as described previously (Galjart et al., 1991; Bonten et al., 1995; Cuervo and Dice, 1996, 2000b; Cuervo et al., 1997). The antibodies against the precursor (anti 54-kDa) and the 32 kDa subunit (anti 32-kDa) of PPCA, and against the cytosolic tail of rat and mouse lamp2a, were prepared in our laboratories (Galjart et al., 1991; Cuervo and Dice, 1996). The antibodies against human and mouse lamp1 and lamp2 were from the Developmental Studies Hybridoma Bank (Iowa University, Iowa City, IA), against cathepsin D from Santa Cruz Biotechnology (Santa Cruz, CA), against ABC-type P-glycoproteins (c219) from Signet Pathologies (Dedham, MA) and against transferrin receptor from Zymed Laboratories (South San Francisco, CA). The polyclonal antibody and the cDNA for cathepsin L were a generous gift from Dr Gary Sahagian (Tufts University, Boston, MA). The cross-linking agent dithiobis[succinimidyl propionate] (DSP) was from Pierce (Rockford, IL), and the protease inhibitors were from Sigma.
Isolation of subcellular fractions
Rat liver lysosomes were isolated from a light mitochondrial–lysosomal fraction in a discontinuous metrizamide density gradient (Cuervo et al., 1997). Lysosomal fractions with different activities for CMA were separated by differential centrifugation (Cuervo et al., 1997). Lysosomes from cultured cells were isolated as described previously (Storrie and Madden, 1990). Preparations with >10% broken lysosomes, measured as β-hexosaminidase latency, were discarded. Lysosomal matrices and membranes were isolated after hypotonic shock (Ohsumi et al., 1983).
Purification of lamp2a-associated proteins
Rat liver lysosomes were solubilized in lysis buffer (50 mM Tris–HCl pH 8, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 1 mM DTT) for 2 h at 0°C and then centrifuged at 100 000 g for 30 min. Solubilized proteins were subjected to affinity chromatography with anti-lamp2a immobilized in Aminolink Plus gel (Pierce). After extensive washing, retained proteins were eluted with 100 mM glycine pH 2.3, and collected in neutralizing trizma buffer.
Uptake and degradation of substrate proteins by isolated lysosomes
[14C]GAPDH was incubated in MOPS buffer pH 7.3, 0.3 M sucrose, 1 mM DTT with lysosomes for 30 min (Cuervo et al., 1997). Degradation of [14C]GAPDH was measured after precipitation in trichloroacetic acid (TCA) as described previously (Cuervo et al., 1997). Proteolysis was expressed as the percentage of the initial acid-insoluble radioactivity (protein) transformed into acid-soluble radioactivity (amino acids and small peptides) at the end of the incubation.
Cellular protein degradation
Confluent cells were labeled with [3H]leucine (2 µCi/ml) for 48 h at 37°C and then extensively washed and maintained in complete (10% NCS) or serum-deprived medium containing an excess of unlabeled leucine. Aliquots of the medium taken at different times were precipitated in TCA and proteolysis was measured as above (Auteri et al., 1983). Total radioactivity incorporated into cellular proteins was determined in duplicate samples as the amount of acid-precipitable radioactivity in labeled cells immediately after washing.
Degradation of lamp2s
In cultured cells, degradation of lamp2a or the other isoforms of lamp2 together was followed after [35S]methionine/cysteine radiolabeling by sequential immunoprecipitation, first with a specific antibody against lamp2a and then with an antibody against all lamp2s as described (Cuervo and Dice, 2000a). The degradation of lamp2s in isolated lysosomes was analyzed by incubating them in an isotonic medium for 15 min at 37°C, except were indicated. Samples were then immunoblotted for lamp2a or all forms of lamp2 together. Degradation of lamp2a is linear with time for the first 20 min of incubation (Cuervo and Dice, 2000b).
Immunocytochemical and histological staining
For immunofluorescence studies, cells grown on coverslips until confluent or tissue sections (6–8 µm) preserved frozen were fixed with a 3% formaldehyde solution, blocked, and then incubated with the primary and corresponding fluorescein isothiocyanate- or Texas Red-conjugated secondary antibodies. Images were acquired on a Bio-Rad MRC-1024 confocal microscope (Bio-Rad Laboratories, Hercules, CA) equipped with an argon laser. Co-localization was determined using MetaMorph (Universal Imaging, Downingtown, PA). Mouse tissue sections were incubated with the desired antibodies and developed using the Vector ABC horseradish peroxidase system (Vector Labs, Burlingame, CA) (Rottier et al., 1998). Paraffin sections were stained with hematoxylin and eosin by standard methods (Rottier et al., 1998). All digital microscopic images were prepared using Adobe Photoshop 5.0 software (Adobe Systems Inc., Mountain View, CA).
PPCA uptake by GS cells
Human PPCA uptake by GS cells was performed as described previously (Galjart et al., 1991) using the wild-type cDNAs coding for the human PPCA or for the catalytic mutant (PPCA-S/A), in which the Ser150 of the 32 kDa chain of PPCA was replaced by alanine (Galjart et al., 1991). The medium of COS-1, 48 h after transfection with these cDNAs, was added to confluent GS human fibroblasts in 35 mm dishes (Galjart et al., 1990). When total protein degradation was analyzed, radiolabeling was initiated the second day after PPCA supplementation by adding [3H]leucine (2 µCi/ml) and then incubating the cells at 37°C for two additional days. PPCA supplementation did not modify the amount of radioactivity incorporated into proteins. We also found similar PPCA carboxypeptidase activity in GS cells maintained in the presence or absence of serum after supplementation.
Bone marrow transplantation
Bone marrow (BM) transplantation was performed as described previously (Hahn et al., 1998). Recipient GS mice (C57BL/6 × 129/J × FVB/NJ) were lethally irradiated 24 h before transplantation. BM cells obtained from wild-type FVB/NJ mice were subjected to complement lysis to achieve T-cell depletion and then injected in the recipient via the tail vein.
Cleavage of lamp2a by PPCA
HA-tagged lamp2a was constructed and stably transfected in HEK293 cells as described previously (Cuervo and Dice, 2000a). The F→G point mutation on HA-lamp2a was performed with the QuikChange™ kit (Stratagene, La Jolla, CA) according to the manufacturer’s indications. Wild-type and mutated HA-lamp2a were purified from stably transfected HEK293 cells by affinity chromatography through an anti-HA matrix as described previously (Cuervo and Dice, 2000a). PPCA was purified from insect cells infected with baculovirus constructs encoding human PPCA as described previously (Bonten et al., 1995). The PPCA preparation contains both precursor and mature forms in a ratio 10:1. Equimolar amounts of lamp2a and PPCA were incubated in 100 mM sodium acetate pH 5.2, 100 mM NaCl for 3 h at 37°C.
General methods
Protein was determined by the Lowry method (Lowry et al., 1951) using bovine serum albumin as a standard. Lysosomal enzymatic activities were measured as reported previously (Storrie and Madden, 1990). After SDS–PAGE (Laemmli, 1970) and immunoblotting (Towbin et al., 1979), the proteins recognized by the specific antibodies were visualized by chemiluminescence methods (RenaissanceR; NEN-Life Science Products). Carboxypeptidase activity was measured using the substrate CBZ-Phe-Leu as reported previously (Galjart et al., 1990; Pshezhetsky and Potier, 1994). Chemical cross-linking of lysosomal samples was performed following the manufacturer’s instructions (Pierce). Cultured cells were transfected by the calcium phosphate method, and selected for stable transfectants by resistance to geneticin (Cuervo and Dice, 1996). Densitometric quantification of the immunoblotted membranes and stained gels was performed with an Image Analyzer System (Inotech S-100; Sunnyvale, CA). Student’s t-test was used for statistical analyses.
Acknowledgments
Acknowledgements
We gratefully acknowledge the help of Maria del Pilar Martin, Thasia Leimig and Daniel Ortiz with different technical aspects of this work, and of Mike Berne for the peptide sequencing. This work was supported by the National Institutes of Health grants AG00829 (A.M.C.), AG06116 (J.F.D.) and DK52025 (A.d’A.), and by Research Grants from the American Federation of Aging Research and the Howard Hughes Medical Institute (A.M.C.).
References
- Agarraberes F., Terlecky,S.R. and Dice,J.F. (1997) An intralysosomal hsp70 is required for a selective pathway of lysosomal protein degradation. J. Cell Biol., 137, 825–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T., Kihara,A., Tokuda,H. and Ito,K. (1996) FtsH (HflB) is an ATP-dependent protease selectively acting on SecY and some other membrane proteins. J. Biol. Chem., 271, 31196–31201. [DOI] [PubMed] [Google Scholar]
- Auteri J.S. et al. (1983) Regulation of intracellular protein degradation in IMR-90 human diploid fibroblasts. J. Cell Physiol., 115, 159–166. [DOI] [PubMed] [Google Scholar]
- Bonten E.J., Galjart,N.J., Willemsen,R., Usmany,M., Vlak,J.M. and d’Azzo,A. (1995) Lysosomal protective protein/cathepsin A. Role of the ‘linker’ domain in catalytic activation. J. Biol. Chem., 270, 26441–26445. [DOI] [PubMed] [Google Scholar]
- Chiang H.L., Terlecky,S.R., Plant,C.P. and Dice,J.F. (1989) A role for a 70-kilodalton heat shock protein in lysosomal degradation of intracellular proteins. Science, 246, 382–385. [DOI] [PubMed] [Google Scholar]
- Cuervo A.M. and Dice,J.F. (1996) A receptor for the selective uptake and degradation of proteins by lysosomes. Science, 273, 501–503. [DOI] [PubMed] [Google Scholar]
- Cuervo A.M. and Dice,J.F. (1998) Lysosomes, a meeting point of proteins, chaperones and proteases. J. Mol. Med., 76, 6–12. [DOI] [PubMed] [Google Scholar]
- Cuervo A.M. and Dice,J.F. (2000a) Unique properties of lamp2a compared to other lamp2 isoforms. J. Cell Sci., 113, 4441–4450. [DOI] [PubMed] [Google Scholar]
- Cuervo A.M. and Dice,J.F. (2000b) Regulation of lamp2a levels in the lysosomal membrane. Traffic, 1, 570–583. [DOI] [PubMed] [Google Scholar]
- Cuervo A.M. and Dice,J.F. (2000c) Age-related decline in chaperone-mediated autophagy. J. Biol. Chem., 275, 31505–31513. [DOI] [PubMed] [Google Scholar]
- Cuervo A.M., Dice,J.F. and Knecht,E. (1997) A population of rat liver lysosomes responsible for the selective uptake and degradation of cytosolic proteins. J. Biol. Chem., 272, 5606–5615. [DOI] [PubMed] [Google Scholar]
- Cuervo A.M., Hildebrand,H., Bomhard,E.M. and Dice,J.F. (1999) Direct lysosomal uptake of α2-microglobulin contributes to chemically induced nephropathy. Kidney Int., 55, 100–200. [DOI] [PubMed] [Google Scholar]
- d’Azzo A., Hoogeveen,A., Reuser,A.J., Robinson,D. and Galjaard,H. (1982) Molecular defect in combined β-galactosidase and neuraminidase deficiency in man. Proc. Natl Acad. Sci. USA, 79, 4535–4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Azzo A., Andria,G., Strisciuglio,P. and Galijaard,H. (1995) Galactosialidosis. In Scriver,C., Beaudet,A., Sly,W. and Valle,D. (eds), Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill, New York, NY, pp. 2835–2837.
- Dice J.F. (2000) Lysosomal Pathways of Protein Degradation. Landes Bioscience, Austin, TX.
- Galjart N.J., Gillemans,N., Meijer,D. and d’Azzo,A. (1990) Mouse ‘protective protein’. cDNA cloning, sequence comparison and expression. J. Biol. Chem., 265, 4678–4684. [PubMed] [Google Scholar]
- Galjart N.J., Morreau,H., Willemsen,R., Gillemans,N., Bonten,E.J. and d’Azzo,A. (1991) Human lysosomal protective protein has cathepsin A-like activity distinct from its protective function. J. Biol. Chem., 266, 14754–14762. [PubMed] [Google Scholar]
- Hahn C.N., Martin,M.P., Zhou,X.Y., Mann,L.W. and d’Azzo,A. (1998) Correction of murine galactosialidosis mice: indirect evidence for spatial requirement of the catalytic rather than the protective function of PPCA. Hum. Mol. Genet., 7, 1787–1794. [DOI] [PubMed] [Google Scholar]
- Haller T., Dietl,P., Deetjen,P. and Volkl,H. (1996) The lysosomal compartment as intracellular calcium store in MDCK cells: a possible involvement in InsP3-mediated Ca2+ release. Cell Calcium, 19, 157–165. [DOI] [PubMed] [Google Scholar]
- Hanna W.L., Turbov,J.M., Jackman,H.L., Tan,F. and Froelich,C. (1994) Dominant chymotrypsin-like esterase activity in human lymphocyte granules is mediated by the serine carboxypeptidase called cathepsin A-like protective protein. J. Immunol., 153, 4663–4672. [PubMed] [Google Scholar]
- Hooper N.M., Karran,E.H. and Turner,A.J. (1997) Membrane protein secretases. Biochem. J., 321, 265–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iodice A., (1967) The carboxypeptidase nature of cathepsin A. Arch. Biochem. Biophys., 121, 241–242. [DOI] [PubMed] [Google Scholar]
- Itoh K., Kase,R., Shimmoto,M., Satake,H. and Suzuki,Y. (1995) Protective protein as an endogenous endothelin degradation enzyme in human tissues. J. Biol. Chem., 270, 515–518. [DOI] [PubMed] [Google Scholar]
- Jackman H.L., Tan,F.L., Tamei,H., Beurling-Harbury,C., Li,X.Y., Skidgel,R.A. and Erdös,E.G. (1990) A peptidase in human platelets that deamidates tachykinins. Probable identity with the lysosomal ‘protective protein’. J. Biol. Chem., 265, 11265–11272. [PubMed] [Google Scholar]
- Kihara A., Akiyama,Y. and Ito,K. (1996) A protease complex in the Escherichia coli plasma membrane: HflKC (HflA) forms a complex with FtsH (HflB), regulating its proteolytic activity against SecY. EMBO J., 15, 6122–6131. [PMC free article] [PubMed] [Google Scholar]
- Kleijer W.J., Hoogeveen,A.T., Verheijen,F.W., Niermeijer,M.F., Galjaard,H., O’Brien,J.S. and Wanner,T.G. (1979) Prenatal diag nosis of sialidosis with combined neuraminidase and β-galactosidase deficiency. Clin. Genet., 16, 60–61. [DOI] [PubMed] [Google Scholar]
- Laemmli U.K. (1970) Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature, 227, 680–685. [DOI] [PubMed] [Google Scholar]
- Licheter-Konecki U., Moter,S.E., Krawisz,B.R., Schlotter,M., Hipke,C. and Konecki,D.S. (1999) Expression patterns of murine lysosome-associated membrane protein 2 (Lamp-2) transcripts during morphogenesis. Differentiation, 65, 43–58. [DOI] [PubMed] [Google Scholar]
- Lowry O.H., Rosebrough,N.J., Farr,A.L. and Randall,R.J. (1951) Protein measurement with the Folin phenol reagent. J. Biol. Chem., 193, 265–275. [PubMed] [Google Scholar]
- Meikle P.J., Yan,M., Ravenscroft,E.M., Isaac,E.L., Hopwood,J.J. and Brooks,D.A. (1999) Altered trafficking and turnover of LAMP-1 in Pompe disease-affected cells. Mol. Genet. Metab., 66, 179–188. [DOI] [PubMed] [Google Scholar]
- Ohsumi Y., Ishikawa,T. and Kato,K. (1983) A rapid and simplified method for the preparation of lysosomal membranes from rat liver. J. Biochem., 93, 547–556. [PubMed] [Google Scholar]
- Ozols J. (1997) Degradation of hepatic stearyl coA delta 9-desaturase. Mol. Biol. Cell, 8, 2281–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pshezhetsky A.V. and Potier,M. (1994) Direct affinity purification and supramolecular organization of human lysosomal cathepsin A. Arch. Biochem. Biophys., 313, 64–70. [DOI] [PubMed] [Google Scholar]
- Rottier R.J., Hahn,C.N., Mann,L.W., Martin,M.P., Smeyne,R.J., Suzuki,K. and d’Azzo,A. (1998) Lack of PPCA expression only partially coincides with lysosomal storage in galactosialidosis mice: indirect evidence for spatial requirement of the catalytic rather than the protective function of PPCA. Hum. Mol. Genet., 7, 1787–1794. [DOI] [PubMed] [Google Scholar]
- Storrie B. and Madden,E.A. (1990) Isolation of subcellular organelles. Methods Enzymol., 182, 203–225. [DOI] [PubMed] [Google Scholar]
- Tanaka Y. et al. (2000) Accumulation of autophagic vacuoles and cardiomyopathy in Lamp-2-deficient mice. Nature, 406, 902–906. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin,T. and Gordon,J. (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedures and some applications. Proc. Natl Acad. Sci. USA, 76, 4350–4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Spoel A., Bonten,E. and d’Azzo,A. (1998) Transport of human lysosomal neuraminidase to mature lysosomes requires protective protein/cathepsin A. EMBO J., 17, 1588–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X.Y. et al. (1995) Mouse model for the lysosomal disorder galactosialidosis and correction of the phenotype with overexpressing erythroid precursor cells. Genes Dev., 9, 2623–2634. [DOI] [PubMed] [Google Scholar]
- Zimmer K.P., Le Coutre,P., Aerts,H.M., Harzer,K., Fukuda,M., O’Brien,J.S. and Naim,H.Y. (1999) Intracellular transport of acid β-glucosidase and lysosome-associated membrane proteins is affected in Gaucher’s disease (G202R mutation). J. Pathol., 188, 407–414. [DOI] [PubMed] [Google Scholar]