Abstract
AtATM3, an ATP-binding cassette transporter of Arabidopsis (Arabidopsis thaliana), is a mitochondrial protein involved in the biogenesis of iron-sulfur clusters and iron homeostasis in plants. Our gene expression analysis showed that AtATM3 is up-regulated in roots of plants treated with cadmium [Cd(II)] or lead (II); hence, we investigated whether this gene is involved in heavy metal tolerance. We found that AtATM3-overexpressing plants were enhanced in resistance to Cd, whereas atatm3 mutant plants were more sensitive to Cd than their wild-type controls. Moreover, atatm3 mutant plants expressing 35S promoter-driven AtATM3 were more resistant to Cd than wild-type plants. Since previous reports often showed that the cytosolic glutathione level is positively correlated with heavy metal resistance, we measured nonprotein thiols (NPSH) in these mutant plants. Surprisingly, we found that atatm3 contained more NPSH than the wild type under normal conditions. AtATM3-overexpressing plants did not differ under normal conditions, but contained less NPSH than wild-type plants when exposed to Cd(II). These results suggest a role for AtATM3 in regulating cellular NPSH level, a hypothesis that was further supported by our gene expression study. Genetic or pharmacological inhibition of glutathione biosynthesis led to the elevated expression of AtATM3, whereas expression of the glutathione synthase gene GSH1 was increased under Cd(II) stress and in the atatm3 mutant. Because the closest homolog of AtATM3 in fission yeast (Schizosaccharomyces pombe), HMT1, is a vacuolar membrane-localized phytochelatin-Cd transporter, it is tempting to speculate that glutathione-Cd(II) complexes formed in the mitochondria are exported by AtATM3. In conclusion, our data show that AtATM3 contributes to Cd resistance and suggest that it may mediate transport of glutamine synthetase-conjugated Cd(II) across the mitochondrial membrane.
The ATP-binding cassette (ABC) family is one of the largest protein families in living organisms and occurs in species ranging from bacteria to humans (Higgins, 1992). These transporter proteins have various substrates, including ions, carbohydrates, lipids, xenobiotics, antibiotics, drugs, and heavy metals (Linton and Higgins, 1998; Theodoulou, 2000; Rogers et al., 2001; Martinoia et al., 2002). Arabidopsis (Arabidopsis thaliana) contains approximately 130 ABC proteins, but the precise functions and substrate specificities of most of these transporters still remain obscure (Sanchez-Fernandez et al., 2001; Jasinski et al., 2003). Recently, some Arabidopsis ABC transporters were found to participate in detoxification processes as well as in plant growth and development (Noh et al., 2001; Campbell et al., 2003; Geisler et al., 2005). Their expression is differentially regulated by hormonal, environmental, and chemical factors (Martinoia et al., 2002; van den Brule and Smart, 2002).
The ABC transporter of the mitochondria (ATM) subfamily of Arabidopsis ABC proteins belongs to a group termed half-transporters, which comprise one transmembrane and one ATP-binding domain. There are three known ATM family members in Arabidopsis: two are located immediately adjacent to each other on chromosome IV (AtATM1 and AtATM2) and one on chromosome V (AtATM3). All three encode proteins possessing putative N-terminal mitochondrial targeting sequences and, therefore, are most likely to be mitochondrial proteins. The closest known homolog of AtATM3 is a yeast (Saccharomyces cerevisiae) ABC transporter, Atm1p, which has been shown to localize to the mitochondrial inner membrane with its ABC domain facing the mitochondrial matrix (Leighton and Schatz, 1995). Atm1p has been suggested to function as an exporter of iron (Fe)-sulfur (S) clusters because a mutant strain defective in Atm1p accumulates mitochondrial Fe (Kispal et al., 1997, 1999; Chloupkova et al., 2004). In accordance with their sequence similarity (59% similarity and 44% identity), the functions of Atm1p and AtATM3 also appear to be very similar (Kushnir et al., 2001). Arabidopsis AtATM3 (alias STA1), whose deficiency causes pronounced dwarfism and chlorosis, restores the maturation of cytosolic Fe-S proteins and suppresses the Fe hyperaccumulation phenotype of atm1 yeast (Kushnir et al., 2001). However, since many ABC transporters are known to transport multiple substrates (Rea et al., 1998), it is possible that AtATM3 transports substrates other than Fe-S complexes. Recent literature showed that proteins with close similarity to AtATM3 transport cadmium (Cd) conjugates (Hanikenne et al., 2005). A close homolog of AtATM3 in Caenorhabdtis elegans, CeHMT-1 (47% similarity and 33% identity), has been shown to be required for Cd tolerance in this organism (Vatamaniuk et al., 2005). Another close homolog to AtATM3, the fission yeast (Schizosaccharomyces pombe) HMT1 ABC transporter (43% similarity and 28% identity), has been shown to be localized in the vacuolar membrane and, in this case, it has been unequivocally shown that SpHMT1 transports Cd-phytochelatin complexes. The overall sequence and structural similarity of AtATM3 with these half-size ABC transporters (forward orientation; single transmembrane domain contiguous with a single nucleotide-binding domain) that are involved in Cd tolerance suggests a role of AtATM3 in heavy metal transport. However, further studies are necessary to fully understand the role of AtATM3 in Cd transport of plant systems.
Glutathione is the major constituent of nonprotein thiols (NPSH) in most plant cells. It is generally present at concentrations of 2 to 3 mm in various plant tissues, primarily in its reduced form (GSH; Noctor et al., 2002). Because GSH is a major cellular antioxidant, it is regarded as a determinant/indicator of cellular redox state and may indirectly influence many fundamental cellular processes. Glutathione also seems to be important in defending against environmental stress, including heavy metals (Marrs, 1996; Ball et al., 2004). Increased biosynthesis of GSH enhances Cd and nickel (Ni) tolerance and increases Cd accumulation in the shoots of various plants (Liang Zhu et al., 1999; Zhu et al., 1999; Freeman et al., 2004). An Arabidopsis mutant with a reduced capacity to produce glutathione, cad2, is hypersensitive to both Cd and copper (Cu; Howden et al., 1995; Cobbett et al., 1998). However, elevation of GSH does not always correlate with enhanced tolerance to heavy metals (Arisi et al., 2000; Xiang et al., 2001; Creissen et al., 2004), perhaps because GSH alone is not always sufficient to support the complex mechanisms of resistance to heavy metal-induced stress (Noctor et al., 1998). Resistance to heavy metals involves heavy metals being pumped out at the plasma membrane, chelated, or bound to various thiol compounds in the cytosol and sequestered into vacuoles. Other antioxidant and repair mechanisms may also participate in the process (Clemens, 2001).
Here we demonstrate that expression of the Arabidopsis ATM3 is increased by Cd(II) and lead [Pb(II)] exposure. Moreover, AtATM3-overexpressing plants show enhanced Cd(II) and Pb(II) resistance compared to wild-type controls, whereas AtATM3 knockout plants (atatm3) show Cd(II)-sensitive phenotypes. Finally, the Cd(II) sensitivity of atatm3 plants was rescued when the wild-type ATM3 was expressed in the mutant. These results imply that AtATM3 plays a role in heavy metal resistance mechanisms in Arabidopsis. We also present evidence that AtATM3 is involved in the regulation of cellular glutathione levels.
RESULTS
AtATM3 Is Up-Regulated by Cd(II) or Pb(II)
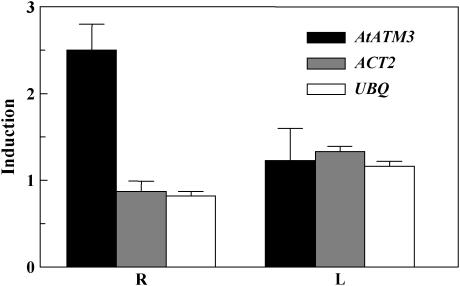
We investigated gene expression patterns following Cd treatment using a cDNA microarray glass slide containing genes encoding putative ABC proteins as well as some housekeeping genes. This chip is described in detail in Bovet et al. (2003, 2005). After a 24-h treatment with 50 μm Cd(II), AtATM3 expression was up-regulated 2.5-fold in the roots of 3-week-old Arabidopsis seedlings, whereas the two housekeeping genes, Actin2 (Act2) and Ubiquitin (Ubq), were unaffected (Fig. 1). Notably, none of these three genes were induced in the leaves. Identical cDNA microarray slides were also hybridized using mRNA isolated from the roots of 2-week-old Arabidopsis seedlings treated with 0.5 mm Pb(NO3)2 for 2 and 10 h. AtATM3 was also up-regulated by Pb(II), with comparable levels of induction observed as for Cd(II) (data not shown). Reverse transcription (RT)-PCR conducted on roots or shoots of 2-week-old Arabidopsis plants treated with Cd(II) and Pb(II) confirmed the microarray data (data not shown). Additional microarray data (data not shown) indicated that AtATM3 mRNA expression was not modulated by short-term (2 h) exposure to 250 μm zinc [Zn(II); heavy metal not complexing with thiol groups] or 6-h exposure to 0.8 μg/mL methyl-jasmonic acid (pathogen and wounding response), 100 μm menadione (oxidative stress), or 200 nm pro- and primisulfuron (inhibitors of acetolactate synthase, first-step branched-chain amino acids).
Figure 1.
Cd(II)- and Pb(II)-induced elevation of AtATM3 transcript levels in roots and leaves of Arabidopsis. Microarray analysis of transcript levels of AtATM3 and two housekeeping genes, Act2 and Ubq, in roots and leaves after a 24-h treatment with 50 μm CdCl2 is presented. Results from two distinct hybridization experiments on the basis of triplicate AtATM3 array spots (n = 3) are combined (mean ± sd). R, Root; L, leaf.
Arabidopsis Plants Overexpressing AtATM3 Exhibit Enhanced Cd(II) and Pb(II) Resistance
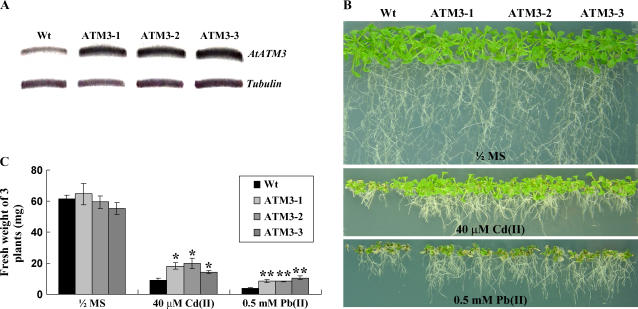
To test whether AtATM3 can improve heavy metal resistance in planta, we generated Arabidopsis plants carrying a transgene in which expression of AtATM3 was driven by the cauliflower mosaic virus 35S promoter. AtATM3 transcript levels determined by RT-PCR were higher in transgenic plants than in wild-type controls (Fig. 2A). To test their Cd(II) resistance phenotype, seeds of three different T3 homozygous lines were germinated and grown on 0.5× Murashige and Skoog (MS) agar plates, with or without 40 μm CdCl2 or 0.5 mm Pb(NO3)2, for 2 to 3 weeks. In control 0.5× MS plates, the growth of wild-type and AtATM3 transgenic plants was similar (Fig. 2B, top). However, in 0.5× MS plates containing Cd(II) or Pb(II), transgenic plants grew better than wild-type plants (Fig. 2B, middle and bottom). Quantitative analyses confirmed that the fresh weights of AtATM3-overexpressing and wild-type plants were similar in the 0.5× MS medium but, in the Cd(II)- or Pb(II)-containing medium, fresh weights of all three lines of 35S∷AtATM3 plants were 1.5- to 2-fold higher than those of wild-type plants (Fig. 2C). This indicates that AtATM3 overexpression enhances Cd(II) and Pb(II) resistance in Arabidopsis and suggests that this protein may be functionally implicated in heavy metal resistance in vivo. We tested the 35S∷AtATM3 plant lines for resistance to hydrogen peroxide, but found no difference compared to the wild type (data not shown).
Figure 2.
Phenotypes of AtATM3-overexpressing Arabidopsis grown on Cd(II)- or Pb(II)-containing plates. A, AtATM3 transcript levels in wild-type and transgenic plants determined by RT-PCR. B, Representative photographs of wild-type and three independent AtATM3 transgenic T3 homozygous plants (ATM3-1, 3-2, and 3-3) germinated and grown on 0.5× MS agar plates, with or without 40 μm CdCl2 or 0.5 mm Pb(NO3)2, for 2 weeks. C, Fresh weight of wild-type and AtATM3 transgenic plants is shown in B. Bar = se. Significant differences from the wild type as determined by Student's t test are indicated. *, P < 0.01; **, P < 0.03.
AtATM3-Overexpressing Transgenic Arabidopsis Plants Have Increased Cd(II) Content
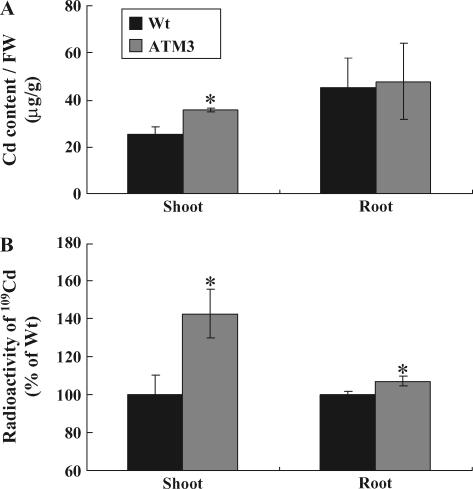
We measured Cd(II) content in both wild-type and 35S∷AtATM3 plants grown for 10 d on 0.5× MS medium and then treated on the root with 100 μm CdCl2 for 24 h. In the shoot, AtATM3-overexpressing plants had consistently higher Cd content than wild-type plants (Fig. 3A). Specifically, the average Cd(II) level in AtATM3 shoots was 53% higher than wild-type shoots (39.2 μg/g shoot fresh weight for ATM3 plants, 25.6 μg/g for wild type). When the 2-fold increase in fresh weight of AtATM3-overexpressing plants in Cd(II)-containing medium (Fig. 2, B and C is taken into account, it implies that total Cd content in AtATM3 transgenic shoots is more than 2-fold that of wild-type plants upon exposure to Cd(II). In roots, there was no significant difference in the level of Cd observed between wild-type and AtATM3 plants (Fig. 3A). These results suggest that AtATM3 plants transport more Cd(II) to the shoot than wild-type plants.
Figure 3.
Cd content of wild-type and AtATM3-overexpressing plants. A, Cd content of AtATM3-overexpressing (ATM3-1) and wild-type plants. FW, Fresh weight. B, 109Cd radioactivity from shoots and roots of Arabidopsis plants treated with 0.1 μm 109CdCl2 for 24 h. Arabidopsis plants were grown vertically for 10 d on 0.5× MS agar plates and then treated for 24 h with 100 μm nonradioactive CdCl2 (A) or 0.1 μm109CdCl2 (B). Shoots and roots were harvested separately before measuring Cd content using an atomic absorption spectrometer (A) or liquid scintillation counter (B). See “Materials and Methods” for further details about the experiment. Significant differences from wild type as determined by Student's t test are indicated (*, P < 0.05). Bars = se.
To test whether the mutant plants change their Cd(II) content when treated with a much lower concentration of Cd(II), we measured Cd(II) flux in the wild-type and AtATM3-overexpressing plants using 109CdCl2. The root parts of the 2-week-old intact Arabidopsis plants were soaked in 0.1 μm 109CdCl2 solution for 24 h and the radioactivity of the separated shoots and roots was measured by scintillation counting. The radioactive Cd content of the shoots and roots of AtATM3-overexpressing plants was 142% ± 13% and 107% ± 3% of those of the wild type, respectively (Fig. 3B).
Because AtATM3 plants were resistant to both Cd(II) and Pb(II), we also measured Pb(II) content, but found no significant difference between wild-type and AtATM3 plants in either the shoot or the root (data not shown). However, again when the 2-fold increase in transgenic fresh weight in Pb(II)-containing medium (Fig. 2B) is factored in, total Pb content in AtATM3 transgenic shoots is more than 2-fold that of wild-type plants when they are grown in the presence of Pb(II).
AtATM3-Mediated Cd(II) or Pb(II) Resistance Is Glutathione Dependent
Glutathione protects plant cells from oxidative stress (Alscher, 1989; Mallick and Mohn, 2000) and is essential for Cd detoxification (Zhu et al., 1999; Xiang et al., 2001; Cobbett and Goldsbrough, 2002). It is also a major reservoir of NPSH and a precursor of phytochelatins, which are also important for heavy metal detoxification (Cobbett, 2000; Gong et al., 2003).
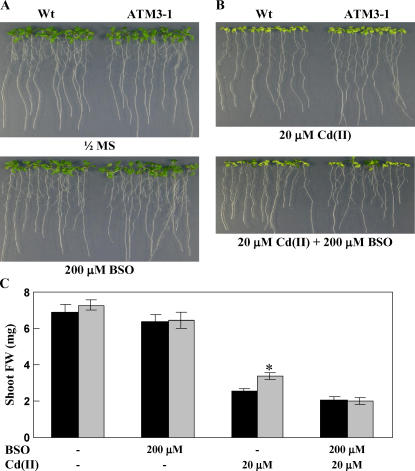
To test whether the Cd(II)-resistance mechanism of AtATM3 is related to glutathione, we compared the growth of wild-type and AtATM3-overexpressing plants in medium containing l-buthionine-(S,R)-sulfoximine (BSO), an inhibitor of γ-glutamyl-cysteine synthetase. In 0.5× MS medium, 200 μm BSO alone did not inhibit the growth of either wild-type or AtATM3-overexpressing (ATM3-1) plants (Fig. 4A). However, when BSO was added together with 20 μm CdCl2, the improved shoot growth of AtATM3-overexpressing plants over wild type to Cd(II) (Fig. 4B, top) disappeared (Fig. 4B, bottom). These results imply that the mechanism of Cd(II) resistance mediated by AtATM3 requires GSH.
Figure 4.
Phenotypes of AtATM3-overexpressing Arabidopsis plants grown on BSO-containing plates. A, Growth of wild-type and AtATM3-overexpressing (ATM3-1) plants on 0.5× MS medium with or without BSO. B, Growth of wild-type and ATM3-1 plants on 0.5× MS + 20 μm Cd(II) medium with or without 200 μm BSO. C, Shoot fresh weight of wild-type and AtATM3-1 plants shown in A and B. The wild-type and ATM3-1 plants were germinated and grown vertically for 2 weeks. A representative result of three independent experiments that gave similar results is shown.
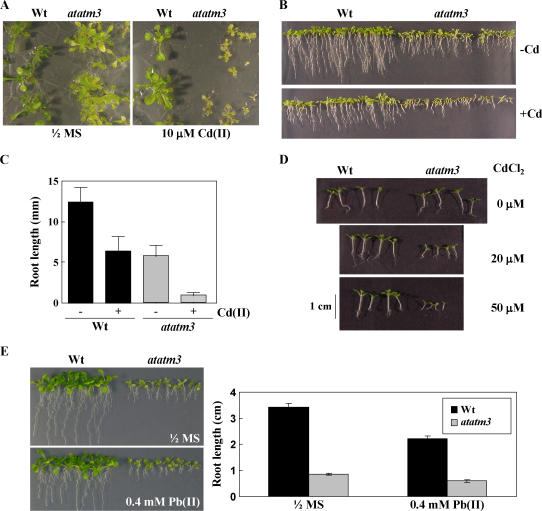
The atatm3 Mutant of Arabidopsis Is More Sensitive to Cd Than Wild-Type Plants
If AtATM3 is involved in heavy metal resistance in Arabidopsis, atatm3 mutants should be more sensitive to heavy metals than wild-type plants. To test this possibility, wild type (ecotype C24) and atatm3 mutants (sta1; Kushnir et al., 2001) were placed on 0.5× MS horizontal agar plates in the presence of 10 μm CdCl2 and grown for 20 d with a daily 16-h light cycle. This treatment did not significantly reduce shoot growth in wild-type plants, but severely reduced that of the mutant plants (Fig. 5A). We then compared root sensitivity to Cd(II) in wild-type and atatm3 mutant plants. Figure 5B shows the effect of 15 μm Cd(II) on root growth after cultivation of wild-type and atatm3 mutants under 16-h light per day on vertical plates. Although the root length was already affected in the atatm3 mutant in the absence of Cd, the presence of the metal further inhibited growth and the extent of root growth inhibition was more severe in the mutant than in the wild type. Specifically, wild-type and mutant roots showed 49% and 85% growth reduction, respectively, as a consequence of Cd(II) treatment (Fig. 5C).
Figure 5.
Phenotypes of wild-type and atatm3 mutant plants grown on Cd(II)-containing plates. A, Effect of Cd(II) on the shoot growth of the AtATM3 knockout mutant (atatm3). Seeds were germinated on horizontally placed 0.5× MS Suc plates (0.6% agar) containing 10 μm CdCl2 and grown for 20 d under 16-h light. B, Effects of Cd(II) on root growth of atatm3 and wild-type plants. Seeds were germinated and grown vertically on KH2PO4 (200 mg/L), MgSO4·7H2O (187.5 mg/L), Ca(NO3)·4H2O (79.25 mg/L), KNO3 (22 mg/L), Fe-EDTA (17.5 mg/L), MnCl2·4H2O (48.75 μg/L), H3BO3 (76.25 μg/L), ZnSO4·7H2O (12.25 μg/L), CuSO4·5H2O (6.875 μg/L), NaNoO4·2H2O (12.5 μg/L), and Ni(NO3)2·6H2O (3.75 μg/L) agar plates in the absence or presence of 15 μm CdCl2 for 8 d with 16-h light. C, Root length of atatm3 and wild-type seedlings shown in B. Bar = sd. D, Effects of Cd(II) on growth of atatm3 and wild-type plants under 8-h-light/16-h-dark conditions. Seeds were germinated and grown horizontally on 0.5× MS Suc agar plates in the presence of increasing Cd(II) concentrations (0, 20, and 50 μm CdCl2) for 13 d. E, Effect of Pb(II) on the growth of the atatm3 mutant. Seeds were germinated and grown vertically on 0.5× MS medium with or without 0.4 mm Pb(NO3)2 for 2 weeks under a 16-h light cycle.
The effect of Cd(II) was also investigated on seedlings grown horizontally under short-day conditions (8-h light/d). Under such photoperiodic conditions, wild-type and atatm3 mutant seedlings exhibited similar root and shoot growth during the first 14 d from the onset of germination (Fig. 5D). At the same developmental stage, however, mutant seedlings showed marked inhibition of shoot and root growth compared to wild-type controls when treated with 20 or 50 μm Cd(II) (Fig. 5D). It appears that, in Arabidopsis, loss of AtATM3 renders plants extremely sensitive to Cd(II).
To test whether the atatm3 mutant is also sensitive to Pb(II), seeds of the wild type and the atatm3 mutant were germinated and grown on vertically positioned 0.5× MS agar plates, with or without 0.4 mm Pb(NO3)2, for 2 weeks. Surprisingly, the extent of growth inhibition by Pb(II) was nearly the same for the atatm3 mutant as for the wild type (Fig. 5E). Specifically, wild-type and mutant roots showed 35% ± 3% and 31% ± 5% growth reduction, respectively, after Pb(II) treatment (Fig. 5E). These results indicate that the atatm3 mutant is more sensitive to Cd(II), but not to Pb(II), than wild type.
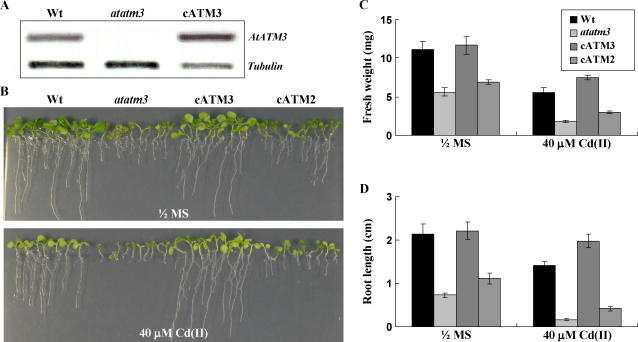
Reversion of the Arabidopsis atatm3 Mutant Phenotype after Complementation with AtATM3
To confirm the functional significance of AtATM3 in Cd(II) resistance, we used genetically complemented atatm3 mutant plants (cATM3 transgenic plants) that expressed the full-length AtATM3 under the control of the 35S promoter (Kushnir et al., 2001). We used these plants to see whether the Cd(II) sensitivity conferred by the atatm3 mutation could be complemented by the presence of wild-type AtATM3. AtATM3 transcript levels as determined by RT-PCR, which were undetectable in the atatm3 mutant, were much higher in cATM3 transgenic plants than in wild-type control mutants (Fig. 6A). Seeds of wild type, the atatm3 mutant, and the atatm3 mutant complemented with AtATM3 (cATM3 transgenic plants) were germinated and grown on 0.5× MS agar plates, with or without 40 μm CdCl2 for 2 to 3 weeks. In control 0.5× MS plates, the stunted growth of the atatm3 mutant was completely reversed in the cATM3 transgenic plants (Fig. 6B, left). In plates containing 40 μm CdCl2 (Fig. 6B, right), cATM3 transgenic plants showed enhanced Cd(II) resistance compared to wild-type plants (Fig. 6, C and D). This improved Cd(II) resistance of the cATM3 transgenic plants is likely to be related to the higher level of the AtATM3 transcript in cATM3 transgenic plants.
Figure 6.
Complementation of the atatm3 mutant by overexpression of AtATM3. A, AtATM3 transcript levels in wild-type, atatm3, and cATM3 transgenic plants as determined by RT-PCR. B, Growth of wild-type atatm3 mutant, AtATM3-complemented atatm3 plants (cATM3), and AtATM2-complemented atatm3 plants (cATM2) on 0.5× MS medium or 40 μm Cd(II)-containing 0.5× MS agar plates. C and D, Fresh weight and root length of wild-type and AtATM3 mutant plants shown in B.
To test whether AtATM2 and AtATM3 have overlapping functions in Cd(II) resistance, we further analyzed transgenic atatm3 plants that expressed AtATM2 under the control of the 35S promoter (cATM2; Kushnir et al., 2001). Although the 35S∷AtATM2 plant (cATM2) partially reversed the stunted growth of the atatm3 mutant, it did not complement the Cd(II)-sensitive phenotype of the atatm3 mutant (Fig. 6), indicating that AtATM2 (alias STA2), which is the closest homolog of AtATM3 in Arabidopsis, has a very minor role, if any, in Cd(II) detoxification.
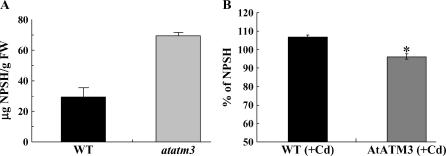
Level of NPSH Is Elevated in atatm3
Increased sensitivity of the atatm3 mutant to Cd(II) may reflect a reduced glutathione level in cytosol because the mutant cells experience oxidative stress (Kushnir et al., 2001). GSH is known to protect green plant cells from oxidative stress (Alscher, 1989; Mallick and Mohn, 2000) and GSH and phytochelatins are essential for Cd detoxification (Zhu et al., 1999; Xiang et al., 2001; Cobbett and Goldsbrough, 2002). Because NPSH levels closely reflect glutathione content in plant cells (Noctor et al., 1998; Meyer and Fricker, 2002), we quantified them in the atatm3 mutant and AtATM3-overexpressing seedlings. Surprisingly, NPSH levels were more than 2-fold higher in atatm3 mutant seedlings than in the wild-type controls (Fig. 7A). In AtATM3-overexpressing plants, NPSH levels were not different under control conditions (data not shown), but they were slightly, albeit significantly, lower than wild-type levels when plants were grown in Cd(II)-containing medium (Fig. 7B). This indicates that AtATM3 affects intracellular NPSH levels and suggests that either lack of or excess AtATM3 in the mitochondrial membrane causes abnormal compartmentalization of NPSH, thereby distorting the normal synthesis and/or intracellular distribution of NPSH.
Figure 7.
NPSH levels in wild-type, atatm3 mutant, and AtATM3-overexpressing plants. A, NPSH in 1-week-old wild-type and atatm3 mutant seedlings. B, Relative levels of NPSH in 2-week-old wild-type and AtATM3-overexpressing plants compared to NPSH level of non-Cd-treated wild type, determined as described in “Materials and Methods.”
AtATM3 Plays a Role in the Control of GSH Level
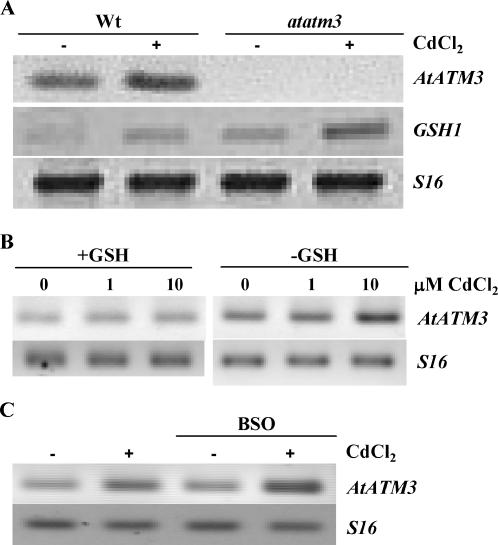
To determine whether AtATM3 plays a role in regulating intracellular GSH, we compared wild-type plants and the atatm3 mutant for their expression of an enzyme involved in GSH synthesis, GSH1 (γ-glutamyl-cysteine synthase; Xiang et al., 2001). Plants were grown for 1 week in 0.5× MS agar plates, with or without 10 μm CdCl2 under 16-h light per day and AtGSH1 transcript levels were assayed by semiquantitative RT-PCR. Even prior to Cd(II) treatment, AtGSH1 transcripts were elevated in the atatm3 mutant compared to the wild-type control (compare the first and the third lanes of the second gel in Fig. 8A), which is consistent with its higher NPSH content. After exposure to Cd(II), AtGSH1 transcript levels were elevated in both wild-type and mutant plants, with the mutant having a higher mRNA level than the wild type (compare the second and the fourth lanes of the second gel in Fig. 8A). Notably, Cd stress and AtATM3 knockout increased the expression of AtGSH1 in an additive manner.
Figure 8.
Correlation of GSH with AtATM3 expression. A, Analysis of GSH1 transcript level by RT-PCR in wild-type and atatm3 seedlings exposed to Cd(II). Total RNA was isolated from seedlings cultivated for 1 week in 0.5× MS agar plates in the absence or presence of 10 μm Cd(II) after seed sterilization. B, Transcript levels of AtATM3 in Arabidopsis GSH1 sense (+GSH) and antisense (−GSH) seedlings (Xiang et al., 2001) exposed to 0, 1, or 10 μm CdCl2 in 0.5× MS agar plates for 1 week following seed sterilization. C, AtATM3 transcript levels in Arabidopsis wild-type seedlings grown in 0.5× MS agar plates in the presence or absence of 0.5 mm BSO and 10 μm CdCl2 for 1 week after seed sterilization. S16 was used as a control, housekeeping gene.
To test whether the converse is also true (i.e. absence of AtGSH1 expression or reduced level of GSH induces AtATM3 expression), we used two different approaches. First, we reduced the level of GSH in plants by adding a chemical compound (BSO) that is known to inhibit the activity of GSH1 (Griffith, 1982). Second, we analyzed GSH1 antisense plants that have a low GSH level due to reduced expression of the AtGSH1 gene (Xiang et al., 2001). Expression of AtATM3 was significantly higher in GSH1 antisense plants with reduced expression of GSH1 (−GSH) than in GSH1 sense plants with increased expression of GSH1 (+GSH; Fig. 8B). Treatment with Cd(II) further increased the expression of AtATM3, both in low GSH mutants and in wild-type plants exposed to BSO (Fig. 8, B and C). Because Cd(II) treatment provides a strong sink for free cellular GSH (Meyer and Fricker, 2002) by binding to GSH and phytochelatin in the cytoplasm, we conclude from Figure 8, B and C, that under conditions of decreased GSH, expression of AtATM3 increases. Together with the observation that AtGSH1 is induced in the atatm3 mutant, these data suggest that AtATM3 and AtGSH1 have a close functional link in controlling GSH levels.
DISCUSSION
AtATM3 is a mitochondrial transporter that is essential for Fe homeostasis in Arabidopsis (Kushnir et al., 2001). In this study, we provide several lines of evidence to implicate AtATM3 in heavy metal resistance in Arabidopsis. First, using cDNA microarray and RT-PCR technology, we show that AtATM3 is induced by Cd(II) or Pb(II) (Fig. 1). Second, we demonstrate that AtATM3-overexpressing plants grow better than wild-type controls on either Cd(II)- or Pb(II)-containing medium (Fig. 2). Third, we provide evidence that atatm3 plants are more sensitive to Cd(II) than wild-type plants (Fig. 5) and that expressing the AtATM3 gene under the 35S promoter complements the atatm3 phenotype and renders the mutant even more resistant to Cd(II) than the wild type (Fig. 6).
The atatm3 mutant plants are stunted in growth under normal conditions. Because it is deficient in the mechanism whereby the Fe-S cluster is transported from mitochondria to the cytosol (Kushnir et al., 2001), this mutant cannot assemble sufficient levels of Fe-S cluster-containing enzymes for the many metabolic steps that require these complexes in the cytosol. Moreover, atatm3 has an elevated level of mitochondrial free Fe, which results in increased oxidative stress (Kushnir et al., 2001). Based on these results and the fact that Cd(II) causes a high level of oxidative stress, we predicted that this mutant plant may be more sensitive to Cd(II) than a wild-type plant. Our results support this hypothesis (Fig. 5). We also predicted that the glutathione level would be reduced in this mutant plant, which experiences oxidative stress, but, in contrast to our expectation, the atatm3 mutant plant exhibited an increased level of NPSH (Fig. 7A). The observed increase in GSH1 expression in this mutant (Fig. 8A) was consistent with its high NPSH level. However, this did not explain why this mutant with elevated NPSH levels is more sensitive to Cd(II) than wild-type plants. We propose that the atatm3 mutant's hypersensitivity to Cd may occur because most of its GSH is trapped inside mitochondria, resulting in depletion of cytosolic GSH. If cytosolic GSH concentration is reduced, Cd(II) cannot be complexed efficiently by this compound and can therefore induce serious damage. It is also possible that Cd transported to the mitochondria cannot be exported in this mutant because glutathione-Cd(II) complexes formed in the mitochondria may rely on AtATM3 for export. It should be mentioned that, in fission yeast, HMT1, the closest known homolog of AtATM3 in this organism, is a phytochelatin-Cd transporter (Ortiz et al., 1995). In the same study, low-Mr and high-Mr Cd-S complexes were described. Whether all these complexes are formed within the vacuole or whether some are formed in the mitochondria remains to be resolved. Therefore, oxidative stress in mitochondria may result from both free Fe and Cd trapped within this organelle. Whether AtATM3 indeed transports glutamine synthetase-conjugated Cd(II) across the mitochondrial membrane or only has an indirect effect on this process is an important question to be addressed in the future.
It is noteworthy that AtATM3-overexpressing plants were not different from wild-type plants with respect to their resistance to hydrogen peroxide (data not shown). Only AtATM3-overexpressing plants (Fig. 2) and not knockout plants (Fig. 5) showed a level of resistance to Pb(II) that was different from that of the wild-type plants. Neither could we detect any difference in growth of AtATM3-overexpressing plants from that of wild type in media containing two to three different concentrations of Cu(II), Fe(III), and Zn(II), which reduced the growth of the plants to less than 60% compared to normal 0.5× MS medium (data not shown). These results indicate that AtATM3 may be more specifically involved in Cd(II) resistance than in the Pb(II) or other heavy metal resistance or the general oxidative stress response, and that it may have higher affinity for Cd(II) or Cd(II)-conjugated compounds than for Pb(II) or Pb(II)-conjugated compounds.
AtATM3-overexpressing plants did not differ from wild-type controls in terms of growth or NPSH levels under normal conditions, but they did possess enhanced Cd(II) tolerance. Their NPSH level did not increase under Cd stress, resulting in a lower NPSH level under Cd(II) stress compared to wild-type plants (Fig. 7B). Normally, cellular reactive oxygen species levels increase under Cd(II) stress (Schutzendubel and Polle, 2002), damaging the Fe-S clusters in basic metabolic enzymes (Imsande, 1999). With more AtATM3 in the mitochondrial membrane, it is feasible that overexpressing plants may more efficiently supply Fe-S clusters to the cytosol, thereby reducing the free Fe concentration in the mitochondria and the basal oxidative stress level. This would suggest that export of Fe-S clusters from the mitochondrial matrix is a key regulatory step for the incorporation of Fe into Fe-S complexes. Glutathione is required for the export of Fe-S clusters across the mitochondrial membrane (Sipos et al., 2002). Thus, excess AtATM3 may result in reduced free GSH levels because increased transport of glutamine synthetase-conjugated Fe-S clusters consumes more GSH. If this explanation is correct, then the effect of AtATM3 on Cd(II) resistance should depend on GSH concentration. This possibility was tested using BSO, an inhibitor of GSH synthesis. BSO abolished Cd(II) resistance of AtATM3-overexpressing plants (Fig. 4), indicating the essential role of GSH in AtATM3-mediated resistance to Cd(II).
A role for AtATM3 in transporting Fe-S clusters has previously been suggested in atatm3 mutants (Kushnir et al., 2001). We propose that AtATM3 is also important for regulating cellular GSH levels based on two lines of evidence. First, NPSH levels are increased in atatm3 knockout plants relative to the wild type under normal growth conditions and reduced in AtATM3-overexpressing plants treated with Cd(II). Consistent with these results, yeast cells lacking functional Atm1p, a close homolog of AtATM3, accumulate high amounts of cellular glutathione (Kispal et al., 1997). Second, expression of AtATM3 and AtGSH1 are correlated. Expression of AtGSH1, a gene encoding a key enzyme important for glutathione synthesis, was increased in the atatm3 mutant (Fig. 8A) and AtATM3 expression was elevated under low GSH conditions (Fig. 8, B and C). These results suggest that, similar to AtGSH1, ATATM3 may be involved in controlling intracellular GSH levels such that AtATM3 gene expression is activated when the cytosolic GSH level is low. The precise biochemical mechanisms that AtATM3 employs to regulate cytosolic GSH remain to be elucidated. If AtATM3 indeed transports glutathione-conjugated molecules, such as Fe-S clusters, across the mitochondrial membrane, it may constitute an exit pathway for moving glutathione to the cytosol. Once there, glutathione can be dissociated from the conjugated molecule, reduced, and reused as an antioxidant. In other words, AtATM3 may significantly influence cytosolic GSH levels by providing a pathway for glutathione recycling.
Interestingly, AtATM1 and AtATM2 transcript levels also increased after Cd exposure, but to a lesser extent than AtATM3 (data not shown). In addition, the expression of AtATM1 and AtATM2 in the atatm3 mutant was not affected by the absence or presence of Cd(II) (data not shown). Finally, atatm3 mutant plants expressing AtATM2 did not recover the same level of Cd(II) resistance as wild-type plants. These results indicate that neither of these genes compensates for the lack of AtATM3 in the mutant.
Increased Cd content in the shoot of the overexpressing plants (Fig. 3) has two plausible explanations. First, the level of Cd binding to glutathione or other thiol compounds may be increased in the cytoplasm of AtATM3-overexpressing cells simply because the cytosolic glutathione level is elevated due to increased mitochondrial transport of glutathionated Cd(II) and other glutathione complexes by AtATM3. Although the roots of AtATM3-overexpressing plants did not show altered Cd content following long-term (longer than 24 h) exposure to a high concentration (100 μm) of Cd(II) (Fig. 3A), they did show higher Cd content following short-term (5 h) incubation in medium containing the same concentration of Cd(II) (data not shown) or after 24-h incubation in medium containing a very low concentration (0.1 μm) Cd(II) (Fig. 3B). Second, long-distance transport of Cd from the root to the shoot increased. Although we are not certain about the mechanism of increased translocation in the AtATM3-overexpressing plants, higher cytosolic concentrations of thiolated Cd(II) would result in increased Cd release to the apoplast, thereby enhancing translocation from the root to the shoot. This increased translocation of Cd(II) to the shoot is a favorable characteristic for plants used in phytoremediation because it is normal practice to harvest shoots, and not roots, for this purpose. The AtATM3 gene confers another useful characteristic for phytoremediation to overexpressing plants: an increase in total Cd and Pb content. The increase reflects both the higher fresh weights of the plants (Cd and Pb) and an increased concentration of Cd.
In summary, our results show that AtATM3 is important for Cd(II) and Pb(II) resistance in Arabidopsis, possibly by functioning as a transporter of GS-conjugated Cd(II) and/or Fe-S clusters across the mitochondrial membrane. Moreover, we show that overexpression of this gene is likely to be useful for phytoremediation because it increases shoot Cd content and enhances Cd and Pb tolerance of the whole plant.
MATERIALS AND METHODS
Plant Material and Heavy Metal Resistance Test
Arabidopsis (Arabidopsis thaliana) seeds (ecotypes Columbia [Col-0] and C24) were surface sterilized, placed in the dark at 4°C for 2 d, and then sown on Murashige and Skoog (1962) agar plates with 3% Suc. Plants were grown in a controlled environment with 16-h light/8-h dark cycles at 22°C/18°C. For heavy metal resistance tests, seeds were surface sterilized, germinated, and grown vertically on 0.5× MS agar plates, with or without heavy metals, for 2 weeks in a controlled environment with 16-h-light/8-h-dark cycles at 22°C/18°C.
cDNA Microarray and Semiquantitative RT-PCR
DNA fragments of the AtATM3 (At5g58270), Act2 (At5g09810), and Ubq (At2g17200) genes, obtained from PCR amplification with AV549794, 202F3T7, and 113G15T7 expressed sequence tag templates, respectively, were spotted onto chips among other coding sequences. The low-density cDNA microarray glass slides, mRNA isolation, and hybridization procedures used in this experiment are detailed in Bovet et al. (2003, 2005).
To analyze the expression of AtATM3, total RNA was extracted using TRIzol reagent and cDNA was synthesized using an RT-PCR kit (Invitrogen) employing the SuperScript first-strand synthesis system (Invitrogen). To amplify AtATM3 cDNA, PCR was performed using the following specific primers: ATM3-2 (5′-GCTGGCTTGGCGTGCTGCAATTCATG-3′) and ATM3-3 (5′-GGTTCACTATTCCAATTTGATAGC-3′). β-Tubulin (At5g23860) was amplified using primers Tub-F (5′-GCTGACGTTTTCTGTATTCC-3′) and Tub-R (5′-AGGCTCTGTATTGCTGTGAT-3′) and used as expressional control.
For semiquantitative RT-PCR (two-step), the housekeeping gene, Act2, and S16 were amplified using the following primers: actin2-S (5′-TGGAATCCACGAGACAACCTA-3′) and actin2-AS (5′-TTCTGTGAACGATTCCTGGAC-3′) and S16-S (5′-GGCGACTCAACCAGCTACTGA-3′) and S16-AS (5′-GTCCATAGCTGCGCATATCTC-3′). The primers for AtATM3 and GSH1 were ATM3-S (5′-TGCTCGGACATTTTTGAAATC-3′) and ATM3-AS (5′-GTCCATAGCTGCGCATATCTC-3′), GSH1-S (5′-ATCTACGCTTTGTCCCCATTC-3′) and GSH1-AS (5′-ATATTCCCAGAGGTTCGGTG-3′), respectively. cDNAs were prepared using Moloney murine leukemia virus reverse transcriptase (Promega), as indicated by the manufacturer, and then diluted 10 times for the PCR reaction.
Cloning and Generation of AtATM3 Overexpressing Arabidopsis
AtATM3 full-length cDNA was amplified by PCR using primers ATM3F (5′-ATGTCGAGAGGATCTCGATTCGTTAGG-3′) and ATM3R (5′-CTATTCCAATTTGATAGCTGCATCAAG-3′). PCR products were ligated into the pGEM-T easy vector (Promega) using T4 DNA ligase and the fidelity of the AtATM3 sequence was confirmed by automated DNA sequencing (ABI 3100; Perkin-Elmer). AtATM3-T constructs were digested with SpeI and PmlI restriction enzymes and then inserted into the pCambia1302 plant binary vector, which contains the cauliflower mosaic virus 35S promoter. The constructs were introduced into the Agrobacterium GV3101 strain, which was then used to transform Arabidopsis (Col-0) using the floral-dip method (Clough and Bent., 1998). Transformed seeds were selected on 30 μg/mL hygromycin-containing 0.5× MS agar plates. For heavy metal resistance tests, seeds were surface sterilized, placed in the dark at 4°C for 2 d, and then sown on 0.5× MS (with 1.5% Suc) agar plates, with or without heavy metal, and then plants were grown vertically in a controlled environment with 16-h-light/8-h-dark cycles at 22°C/18°C, respectively, for 2 weeks. To detect the expression of the AtATM3 in the transgenic plants, RT-PCR was performed using the ATM3-2 and ATM3-3 primers.
Measurement of Heavy Metal Content in Transgenic Plants
To measure heavy metal content in plants, wild-type and transgenic plants were grown on 0.5× MS agar medium supplemented with 1.5% Suc for 10 d and then the roots of wild-type and AtATM3 transgenic plants were soaked in 100 μm CdCl2 or 1 mm Pb(NO3)2 solutions for 24 h. Shoots and roots were harvested separately, washed three times with ice-cold water, and then digested with 11 n HNO3 at 200°C overnight. Digested samples were diluted with 0.1 n HNO3 and metal content was analyzed using an atomic absorption spectrometer (SpectrAA-800; Varian). To measure 109Cd content, wild-type and AtATM3-overexpressing plants were grown vertically on 0.5× MS agar plates for 2 weeks and then each plant was transferred into a microplate well containing 0.1 μm 109CdCl2 and 3H2O and then incubated for a further 24 h. Shoots and roots were harvested separately, washed three times with ice-cold water, and then soaked in scintillation cocktail solution for 24 h. 109Cd content was measured using a liquid scintillation counter (Tri-CARB2100TR; Packard Bioscience) and normalized by 3H2O.
Assay of Cd(II) Sensitivity in atatm3
After sterilization, seeds (approximately 20) were placed on 0.8% agar plates containing as nutrient mixture either 0.5× MS or KH2PO4 (200 mg/L), MgSO4·7H2O (187.5 mg/L), Ca(NO3)·4H2O (79.25 mg/L), KNO3 (22 mg/L), Fe-EDTA (17.5 mg/L), MnCl2·4H2O (48.75 μg/L), H3BO3 (76.25 μg/L), ZnSO4·7H2O (12.25 μg/L), CuSO4·5H2O (6.875 μg/L), NaNoO4·2H2O (12.5 μg/L), Ni(NO3)2·6H2O (3.75 μg/L), and 1% (w/v) Suc. The plates were stored at 4°C for 16 h for synchronization of seed germination and then placed vertically in the phytotron (25°C, 16-h light, and 70% humidity). After 4 d, each root apex was pointed with a marker at the back of the plates. Finally, the lengths of the roots were measured after 24 h under the same growth conditions. For shoot growth observation, sterilized seeds were deposited on similar agar plates (see above) and kept horizontally for 13 d (22°C, 8-h light, and 70% humidity).
Determination of NPSH
To determine the NPSH level, wild-type and atatm3 mutant seedlings were grown for 1 week on 0.5× MS agar plates and whole seedlings were harvested. GSH (for the standard curve) or soluble protein extracts obtained after grinding seedlings at 4°C and two successive centrifugation steps at 14,000g for 15 min were diluted in 0.1 m phosphate buffer (pH 7.4) supplemented with 10% sulfosalicylic acid. After 30-min incubation at 4°C, soluble proteins (2.48 mL) were centrifuged and mixed with 20 μL of 10 mm 5′,5′-dithiobis(2-nitrobenzoic acid). After 5 min, absorbance was measured at 412 nm and NPSH content extrapolated from the GSH standard curve. To determine total sulfhydryls and NPSH in the wild-type and AtATM3-overexpressing plants, plants were grown on 0.5× MS agar plates for 2 weeks and their roots were submerged in water or 100 μm CdCl2 solution for 16 h. Whole plants were harvested, ground with a mortar and pestle, and suspended in 20 mm HEPES buffer (pH 7.5) containing 0.2 m sorbitol and 5 mm EDTA. Total sulfhydryls and NPSH were measured by Ellman assay (Sedlak and Lindsay, 1968) and normalized using protein quantity.
Acknowledgments
We thank Dr. D.J. Oliver for gsh1 mutant plants, Prof. M. Van Montagu for useful discussion and continuous support, and DongHwan Shim for excellent technical assistance.
This work was supported by grants from the National Research Laboratory program (awarded to Y.L.) and from the Bundesamt fuer Bildung und Wissenschaft (Switzerland) under European Cooperation in the Field of Scientific and Technical Research Action E28 (Genosilva: European Forest Genomic Network, to L.B.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Youngsook Lee (ylee@postech.ac.kr).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.074146.
References
- Alscher RG (1989) Biosynthesis and antioxidant function of glutathione in plants. Physiol Plant 77: 457–464 [Google Scholar]
- Arisi AC, Monquo B, Lagriffioul A, Mench M, Foyer CH, Jouanin L (2000) Responses to cadmium in leaves of transformed poplars overexpressing γ-glutamylcysteine synthetase. Physiol Plant 109: 143–149 [Google Scholar]
- Ball L, Accotto GP, Bechtold U, Creissen G, Funck D, Jimenez A, Kular B, Leyland N, Mejia-Carranza J, Reynolds H, et al (2004) Evidence for a direct link between glutathione biosynthesis and stress defense gene expression in Arabidopsis. Plant Cell 16: 2448–2462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovet L, Eggmann T, Meylan-Bettex M, Polier J, Kammer P, Marin E, Feller U, Martinoia E (2003) Transcript levels of AtMRPs after cadmium treatment: induction of AtMRP3. Plant Cell Environ 26: 371–381 [Google Scholar]
- Bovet L, Feller U, Martinoia E (2005) Possible involvement of plant ABC transporters in cadmium detoxification: a cDNA sub-microarray approach. Environ Int 31: 263–267 [DOI] [PubMed] [Google Scholar]
- Campbell EJ, Schenk PM, Kazan K, Penninckx IA, Anderson JP, Maclean DJ, Cammue BP, Ebert PR, Manners JM (2003) Pathogen-responsive expression of a putative ATP-binding cassette transporter gene conferring resistance to the diterpenoid sclareol is regulated by multiple defense signaling pathways in Arabidopsis. Plant Physiol 133: 1272–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chloupkova M, Reaves SK, LeBard LM, Koeller DM (2004) The mitochondrial ABC transporter Atm1p functions as a homodimer. FEBS Lett 569: 65–69 [DOI] [PubMed] [Google Scholar]
- Clemens S (2001) Molecular mechanisms of plant metal tolerance and homeostasis. Planta 212: 475–486 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Cobbett CS (2000) Phytochelatins and their roles in heavy metal detoxification. Plant Physiol 123: 825–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbett CS, Goldsbrough P (2002) Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annu Rev Plant Biol 53: 159–182 [DOI] [PubMed] [Google Scholar]
- Cobbett CS, May MJ, Howden R, Rolls B (1998) The glutathione-deficient, cadmium-sensitive mutant, cad2-1, of Arabidopsis thaliana is deficient in gamma-glutamylcysteine synthetase. Plant J 16: 73–78 [DOI] [PubMed] [Google Scholar]
- Creissen G, Firmin J, Fryer M, Kular B, Leyland N, Reynolds H, Pastori G, Wellburn F, Baker N, Wellburn A, et al (2004) Elevated glutathione biosynthetic capacity in the chloroplasts of transgenic tobacco plants paradoxically causes increased oxidative stress. Plant Cell 11: 1277–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JL, Persans MW, Nieman K, Albrecht C, Peer W, Pickering IJ, Slat DE (2004) Increased glutathione biosynthesis plays a role in nickel tolerance in thlaspi nickel hyperaccumulators. Plant Cell 16: 2176–2179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler M, Blakeslee JJ, Bouchard R, Lee OR, Vincenzetti V, Bandyopadhyay A, Titapiwatanakun B, Peer WA, Bailly A, Richards EL, et al (2005) Cellular efflux of auxin catalyzed by the Arabidopsis MDR/PGP transporter AtPGP1. Plant J 44: 179–194 [DOI] [PubMed] [Google Scholar]
- Gong JM, Lee DA, Schroeder JI (2003) Long-distance root-to-shoot transport of phytochelatins and cadmium in Arabidopsis. Proc Natl Acad Sci USA 100: 10118–10123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith OW (1982) Mechanism of action, metabolism, and toxicity of buthionine sulfoximine and its higher homologs, potent inhibitors of glutathione synthesis. J Biol Chem 257: 13704–13712 [PubMed] [Google Scholar]
- Hanikenne M, Motte P, Wu MCS, Wang T, Loppes R, Matagne RF (2005) A mitochondrial half-size ABC transporter is involved in cadmium tolerance in Chlamydomonas reinhardtii. Plant Cell Environ 28: 863–873 [Google Scholar]
- Higgins CF (1992) ABC transporters: from microorganisms to man. Annu Rev Cell Biol 8: 67–113 [DOI] [PubMed] [Google Scholar]
- Howden R, Andersen CR, Glodsbrough PB, Cobbett CS (1995) A cadmium-sensitive, glutathione-deficient mutant of Arabidopsis thaliana. Plant Physiol 107: 1067–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imsande J (1999) Iron-sulfur clusters: formation, perturbation, and physiological functions. Plant Physiol Biochem 37: 87–97 [Google Scholar]
- Jasinski M, Ducos E, Martinoia E, Boutry M (2003) The ATP-binding cassette transporters: structure, function and gene family comparison between rice and Arabidopsis. Plant Physiol 131: 1169–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kispal G, Csere P, Guiard B, Lill R (1997) The ABC transporter Atm1p is required for mitochondrial iron homeostasis. FEBS Lett 418: 346–350 [DOI] [PubMed] [Google Scholar]
- Kispal G, Csere P, Prohl C, Lill R (1999) The mitochondrial proteins Atm1p and Nfs1p are essential for biogenesis of cytosolic Fe/S proteins. EMBO J 18: 3981–3989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushnir S, Babiychuk E, Storozhenko S, Davey MW, Papenbrock J, De Rycke R, Engler G, Stephan UW, Lange H, Kispal G, et al (2001) A mutation of the mitochondrial ABC transporter Sta1 leads to dwarfism and chlorosis in the Arabidopsis mutant starik. Plant Cell 13: 89–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton J, Schatz G (1995) An ABC transporter in the mitochondrial inner membrane is required for normal growth of yeast. EMBO J 14: 188–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Zhu Y, Pilon-Smits EA, Jouanin L, Terry N (1999) Overexpression of glutathione synthetase in Indian mustard enhances cadmium accumulation and tolerance. Plant Physiol 119: 73–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linton KJ, Higgins CF (1998) The Escherichia coli ATP-binding cassette (ABC) proteins. Mol Microbiol 28: 5–13 [DOI] [PubMed] [Google Scholar]
- Mallick N, Mohn FH (2000) Reactive oxygen species: response of alga cells. J Plant Physiol 157: 183–193 [Google Scholar]
- Marrs K (1996) The functions and regulation of glutathione S-transferases in plants. Annu Rev Plant Physiol Plant Mol Biol 47: 127–158 [DOI] [PubMed] [Google Scholar]
- Martinoia E, Klein M, Geisler M, Bovet L, Forestier C, Kolukisaoglu U, Muller-Rober B, Schulz B (2002) Multifunctionality of plant ABC transporters—more than just detoxifiers. Planta 214: 345–355 [DOI] [PubMed] [Google Scholar]
- Meyer AJ, Fricker MD (2002) Control of demand-driven biosynthesis of glutathione in green Arabidopsis suspension culture cells. Plant Physiol 130: 1927–1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473–497 [Google Scholar]
- Noctor G, Arisi AC, Jouanin L, Kunert KJ, Rennenberg H, Foyer CH (1998) Glutathione: biosynthesis, metabolism and relationship to stress tolerance in transformed plants. J Exp Bot 49: 623–647 [Google Scholar]
- Noctor G, Gornez L, Vanacker H, Foyer CH (2002) Interactions between biosynthesis, compartmentation and transport in the control of glutathione homeostasis and signaling. J Exp Bot 53: 1283–1304 [DOI] [PubMed] [Google Scholar]
- Noh B, Murphy AS, Spalding EP (2001) Multidrug resistance-like genes of Arabidopsis required for auxin transport and auxin-mediated development. Plant Cell 13: 2441–2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz DF, Ruscitti T, McCue KF, Ow DW (1995) Transport of metal-binding peptides by HMT1, a fission yeast ABC-type vacuolar membrane protein. J Biol Chem 270: 4721–4728 [DOI] [PubMed] [Google Scholar]
- Rea PA, Li ZS, Lu YP, Drozdowicz YM, Martinoia E (1998) From vacuolar GS-X pumps to multispecific ABC transporters. Annu Rev Plant Physiol Plant Mol Biol 49: 727–760 [DOI] [PubMed] [Google Scholar]
- Rogers B, Decottignies A, Kolaczkowski M, Carvajal E, Balzi E, Goffeau A (2001) The pleiotropic drug ABC transporters from Saccharomyces cerevisiae. J Mol Microbiol Biotechnol 3: 207–214 [PubMed] [Google Scholar]
- Sanchez-Fernandez R, Davis TG, Coleman JO, Rea PA (2001) The Arabidopsis thaliana ABC protein superfamily, a complete inventory. J Biol Chem 276: 30231–30244 [DOI] [PubMed] [Google Scholar]
- Schutzendubel A, Polle A (2002) Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization. J Exp Bot 53: 1351–1365 [PubMed] [Google Scholar]
- Sedlak J, Lindsay RH (1968) Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem 25: 192–205 [DOI] [PubMed] [Google Scholar]
- Sipos K, Lange H, Fekete Z, Ullmann P, Lill R, Kispal G (2002) Maturation of cytosolic iron-sulfur proteins requires glutathione. J Biol Chem 277: 26944–26949 [DOI] [PubMed] [Google Scholar]
- Theodoulou FL (2000) Plant ABC transporters. Biochim Biophys Acta 1465: 79–103 [DOI] [PubMed] [Google Scholar]
- van den Brule S, Smart CC (2002) The plant PDR family ABC transporters. Planta 216: 95–106 [DOI] [PubMed] [Google Scholar]
- Vatamaniuk OK, Bucher EA, Sundaram MV, Rea PA (2005) CeHMT-1, a putative phytochelatin transporter, is required for cadmium tolerance in Caenorhabdtis elegans. J Biol Chem 280: 23684–23690 [DOI] [PubMed] [Google Scholar]
- Xiang C, Werner BL, Christensen EM, Oliver DJ (2001) The biological functions of glutathione revisited in Arabidopsis transgenic plants with altered glutathione levels. Plant Physiol 126: 564–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu YL, Pilon-Smits EA, Tarun AS, Weber SU, Jouanin L, Terry N (1999) Cadmium tolerance and accumulation in Indian mustard is enhanced by overexpressing γ-glutamylcysteine synthetase. Plant Physiol 121: 1169–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]