Abstract
Up-regulation of the high-affinity transport system (HATS) for NO3− and stimulation of lateral root (LR) growth are two important adaptive responses of the root system to nitrogen limitation. Up-regulation of the NO3− HATS by nitrogen starvation is suppressed in the atnrt2.1-1 mutant of Arabidopsis (Arabidopsis thaliana), deleted for both NRT2.1 and NRT2.2 nitrate transporter genes. We then used this mutant to determine whether lack of HATS stimulation affected the response of the root system architecture (RSA) to low NO3− availability. In Wassilewskija (Ws) wild-type plants, transfer from high to low NO3− medium resulted in contrasting responses of RSA, depending on the level of nitrogen limitation. Moderate nitrogen limitation (transfer from 10 mm to 1 or 0.5 mm NO3−) mostly led to an increase in the number of visible laterals, while severe nitrogen stress (transfer from 10 mm to 0.1 or 0.05 mm NO3−) promoted mean LR length. The RSA response of the atnrt2.1-1 mutant to low NO3− was markedly different. After transfer from 10 to 0.5 mm NO3−, the stimulated appearance of LRs was abolished in atnrt2.1-1 plants, whereas the increase in mean LR length was much more pronounced than in Ws. These modifications of RSA mimicked those of Ws plants subjected to severe nitrogen stress and could be fully explained by the lowered NO3− uptake measured in the mutant. This suggests that the uptake rate of NO3−, rather than its external concentration, is the key factor triggering the observed changes in RSA. However, the mutation of NRT2.1 was also found to inhibit initiation of LR primordia in plants subjected to nitrogen limitation independently of the rate of NO3− uptake by the whole root system and even of the presence of added NO3− in the external medium. This indicates a direct stimulatory role for NRT2.1 in this particular step of LR development. Thus, it is concluded that NRT2.1 has a key dual function in coordinating root development with external NO3− availability, both indirectly through its role as a major NO3− uptake system that determines the nitrogen uptake-dependent RSA responses, and directly through a specific action on LR initiation under nitrogen-limited conditions.
To acquire adequate amounts of nitrogen needed to maintain optimal growth, higher plants have to cope with marked spatial and temporal changes in the availability of nitrogen sources (mainly NO3− and NH4+) in the soil (Robinson, 1994). To face this constraint, plants have evolved adaptive mechanisms allowing them to enhance their nitrogen capture efficiency in situations of nitrogen limitation (Clarkson, 1985). In the roots, two of these mechanisms are widely documented. The first one relates to the up-regulation of the high-affinity transport system (HATS) for NO3− or NH4+ (Crawford and Glass, 1998; Forde, 2000; von Wirén et al., 2000). For both ions, this results in an increased uptake rate per unit root length or surface at low external concentrations. The second response is a stimulation of root growth, relative to shoot growth, associated with a strong modification of the root system architecture (RSA). This mainly favors lateral root (LR) growth for exploration of a larger soil volume (Robinson, 1994; Zhang and Forde, 2000).
The molecular bases of both types of responses are beginning to be unraveled. Concerning NO3− uptake systems, compelling evidence indicates that those mediating high-affinity transport are encoded by members of the NRT2 gene family (Crawford and Glass, 1998; Daniel-Vedele et al., 1998; Forde, 2000; Williams and Miller, 2001). In Arabidopsis (Arabidopsis thaliana), several NRT2 genes (out of a total of seven in the family) are significantly expressed in the roots and up-regulated at the transcript level by nitrogen starvation, suggesting that they may be responsible for the stimulation of the NO3− HATS under nitrogen-limiting conditions (Lejay et al., 1999; Zhuo et al., 1999; Gansel et al., 2001; Orsel et al., 2002; Okamoto et al., 2003). However, the functional characterization in planta of the NRT2 transporters remains to be done for the large majority of them. The only available data relate to the phenotype of the atnrt2.1-1 mutant of Arabidopsis, where NRT2.1 and NRT2.2 are deleted, which shows strongly reduced HATS activity (down to only 27% of that in the wild type) in various experimental conditions (Cerezo et al., 2001; Filleur et al., 2001). Furthermore, in agreement with the finding that NRT2.1 expression is up-regulated by nitrogen starvation, the stimulation of the NO3− HATS activity following transfer of the plants to nitrogen-deprived medium was shown to be lost in atnrt2.1-1 plants (Cerezo et al., 2001). This indicates that NRT2.1 and/or NRT2.2 (most probably NRT2.1) encode a major nitrogen starvation-inducible component of the NO3− HATS (Cerezo et al., 2001; Filleur et al., 2001).
Recent findings have also been reported on the molecular mechanisms involved in the response of RSA to nitrogen limitation, mostly with NO3− as the nitrogen source. In Arabidopsis, NO3− availability exerts a stringent control on LR growth through dual regulation involving local induction by NO3− and systemic repression by high NO3− or reduced nitrogen status of the plant (Zhang and Forde, 1998, 2000). These two mechanisms do not act at the same stages of LR development because induction by NO3− promoted elongation of existing LRs, while repression by high nitrogen status prevented meristematic activation in short LRs just after their emergence (Zhang et al., 1999). The MADS box gene ANR1, encoding a NO3−-inducible putative transcription factor, was found to play a key role in mediating the local induction of LR elongation by NO3− (Zhang and Forde, 1998). Furthermore, several mutants affected in hormone biosynthesis or response were shown to have an altered RSA response to changes in NO3− availability. This includes the axr4 mutant for local stimulation by NO3− (Zhang et al., 1999) and various abi and aba mutants for repression by high nitrogen status (Signora et al., 2001).
To date, the regulation of nitrogen uptake systems or RSA by nitrogen availability has been mostly investigated separately, and their interaction and coordination in the integrated response of the root system to nitrogen limitation have received much less attention. Very recently, the importance of this aspect has been highlighted by the observation that NRT2.1 plays a crucial role in repressing LR initiation, possibly acting as a sensor or signal transducer to coordinate root development with changes in external NO3− availability (Little et al., 2005). This role of NRT2.1 has been reported under very specific conditions (i.e. high Suc-to-NO3− ratio in the nutrient medium supplied to the roots), and it is not known whether this is illustrative of a more general involvement of this transporter in the RSA response to nitrogen deficiency. Despite this major breakthrough, central questions concerning nitrogen transporters and LR growth remain without a definite answer. (1) Are they coregulated by common transduction pathways (such as repression by the same nitrogen metabolites)? (2) Are their responses to nitrogen limitation interdependent (lack of one response modifies the other one) or, on the contrary, unrelated? (3) What is their relative importance in the overall adaptive response to unfavorable conditions of nitrogen availability?
To gain further insight on these aspects, we used the atnrt2.1-1 NO3− transport mutant in which one of the responses to low nitrogen availability (i.e. the up-regulation of NO3− HATS activity) is suppressed. The HATS deficiency in atnrt2.1-1 plants had a marked negative impact on growth of the mutant on low NO3− medium (<1 mm), but not when supplied with a high NO3− concentration in the range of several millimolars (Orsel et al., 2004). Thus, atnrt2.1-1 plants offer a unique opportunity to determine the effects of the absence of key NO3− transporters on root development specifically under nitrogen-limiting conditions. To precisely unravel the alterations of the RSA response to low NO3− in this mutant, this response was also thoroughly investigated in wild-type plants, which revealed yet unknown contrasting changes as a function of the level of nitrogen limitation.
RESULTS
Nitrogen Limitation Transiently Promotes LR Growth through Different Mechanisms Depending on the Level of Nitrogen Limitation
To investigate the adaptive responses of RSA to nitrogen limitation in wild-type Arabidopsis, seedlings (ecotype Wassilewskija [Ws]) were first grown for 6 d on 10 mm NO3− medium and then transferred for six additional days to either the same medium (controls) or medium with markedly lower NO3− concentrations (from 0.05–1 mm). In our conditions, visible LR development started at day 1 after transfer (age of 7 d), with the appearance of the first visible lateral (length >0.5 mm). At day 6 after transfer (age of 12 d), the plants had between 18 and 25 LRs, depending on the treatment (data not shown).
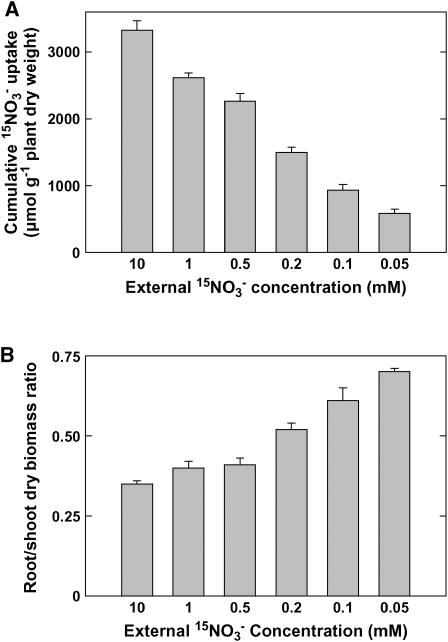
The level of nitrogen limitation experienced by the seedlings was quantified by measuring the cumulative uptake of 15NO3− during the whole 6-d period following transfer to lower NO3− medium (Fig. 1A). Compared with controls left on 10 mm NO3−, plants transferred to lower NO3− concentrations had a reduced NO3− uptake, with a decrease ranging from 21% to 82% between 1 and 0.05 mm external NO3−, respectively. This was associated with various degrees of morphological adaptation at the whole plant level. For instance, a well-known response to nitrogen limitation is the increase in root-to-shoot biomass ratio. This ratio, measured at the end of the experiments, was found to increase gradually from 0.35 to 0.71 in plants supplied with decreasing NO3− concentrations from 10 to 0.05 mm (Fig. 1B). Thus, this protocol succeeded in generating a wide and almost uniform range of different levels of nitrogen limitation and of adaptive developmental responses.
Figure 1.
Cumulative 15NO3− uptake (A) and root-to-shoot dry biomass ratio (B) in wild-type (Ws) Arabidopsis seedlings during 6 d following transfer from 10 mm NO3− to media containing 15NO3− at the indicated concentrations. Seedlings were grown on vertical agar plates and supplied with 10 mm NO3− until the age of 6 d prior to the transfer. Cumulative uptake was expressed on a total plant biomass basis to avoid bias due to changes in the root-to-shoot biomass ratio after growth on different NO3− concentrations. Average values (± se) of five repeats of two seedlings each.
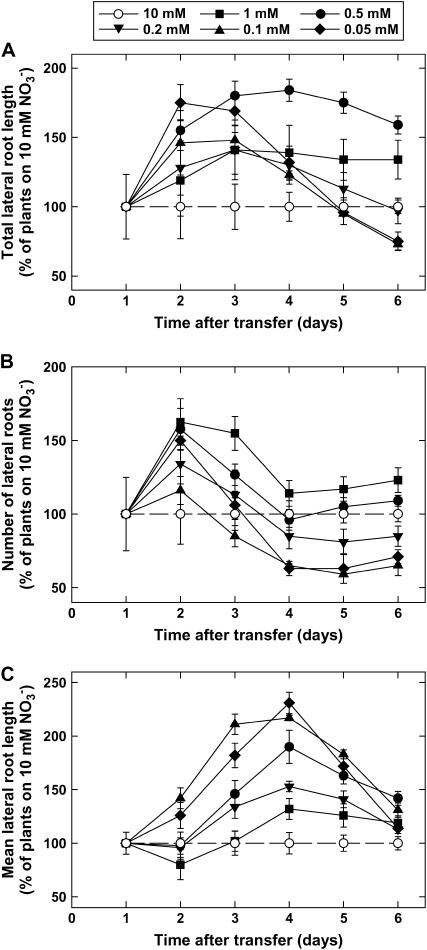
Concerning root growth, transfer of the seedlings to low NO3− medium had little effect on the elongation of the primary root (data not shown) or on the appearance of the first visible lateral, which was always recorded at day 1 after transfer (i.e. at the age of 7 d). However, subsequent development of LRs was strongly affected by the external NO3− concentration. As soon as day 2 after transfer, LR growth was significantly promoted by nitrogen limitation (Fig. 2A) because of both an accelerated appearance of visible LRs (Fig. 2B) and an increase in the mean length of individual LRs (Fig. 2C).
Figure 2.
Effect of lowered NO3− availability on LR growth in wild-type (Ws) Arabidopsis seedlings. The plants were grown on vertical agar plates at 10 mm NO3− and were transferred at the age of 6 d to different media containing NO3− at the indicated concentrations. Total length of LRs (A), number of LRs (B), and mean length of LRs (C) were determined by image analysis. Only LRs >0.5 mm, visible without the use of a microscope, were taken into account. Data are normalized with those obtained in control plants transferred to a 10 mm NO3− medium to allow visualization of changes occurring during the first days after transfer when all three parameters were at low absolute values. Average values (± se) of 12 seedlings.
The positive effect of low NO3− availability on the appearance of new LRs (Fig. 2B) was fast (recorded already at day 2; i.e. the day after the first LRs appeared), but only transient (it almost disappeared after day 3). Furthermore, it was much more pronounced for plants experiencing moderate nitrogen limitation (1 or 0.5 mm NO3−) than for plants subjected to high nitrogen stress (0.2, 0.1, or 0.05 mm NO3−). In the latter, the initial increase in LR number compared with controls was soon followed by a slowing down of the appearance of new laterals. From day 4 onward, plants transferred to 0.2, 0.1, or 0.05 mm NO3− had significantly less visible LRs than controls, which mostly explains their decrease in total LR length relative to controls during the same period (Fig. 2A).
In nitrogen-limited plants, the mean length of individual LRs increased faster than in controls from day 1 or day 2 after transfer to day 4, suggesting stimulation of growth of these roots by nitrogen limitation (Fig. 2C). This stimulation peaked at day 4 and thus appeared to be a more delayed response than the increase in LR number. From day 4 to day 6 after transfer, the tendency was reversed, with a higher increase in mean LR length in controls than in nitrogen-limited plants. Interestingly, mean length of individual LRs was generally higher in plants experiencing the strongest nitrogen limitation (0.1 or 0.05 mm external NO3−), which is the reverse of what was noticed for LR number (Fig. 2B).
A common feature of the response of both LR number and mean LR length to nitrogen limitation is that they are only transiently increased in nitrogen-limited plants compared with controls. Concerning LR number, this is partly explained by the fact that LR appearance in the portion of the primary root generated after transfer was delayed in nitrogen-limited plants compared with controls (Table I). In this portion of the primary root, LRs started to be visible at day 3 after transfer, and during day 3 and day 4, their number increased at a slower rate in nitrogen-limited plants than in controls. This counterbalanced after day 3 the initial increase in visible LR number occurring in the preexisting zone of the primary root (Fig. 2B). A detailed investigation of LR development in the newly formed portion of the primary root showed that, at day 4 after transfer, the delayed generation of visible LRs in nitrogen-limited plants was due not to lower initiation of LR primordia, but to both a reduced emergence of the initiated primordia and a slower activation of the emerged primordia (Table I). Later on (i.e. during day 5 and day 6 after transfer), visible LR appearance in the apical part of the primary root occurred at a similar rate in both nitrogen-limited and control plants, thus resulting in an only slightly reduced LR number in nitrogen-limited plants at the end of the experiments (Table I).
Table I.
Microscopic observations at day 4 and day 6 after transfer from 10 mm nitrate of LR initiation events and development in the most basal 3 cm (day 4) or 4 cm (day 6) of the primary root zone that developed after transfer in wild-type plants (Ws)
Values are the average of six to 10 plants ± se. Stages of LR development were defined according to Malamy and Benfey (1997), with stages I to VII grouped here as unemerged primordia.
| Nitrate Concentration | No. of LRs (a) | No. of Emerged Primordia (b) | No. of Unemerged Primordia (c) | Total No. of Initiated Primordia (a + b + c) | Proportion of Emerged Primordia (a + b/a + b + c) | Proportion of Emerged Primordia Developed into LRs (a/a + b) | Proportion of Initiated Primordia Developed into LRs (a/a + b + c) |
|---|---|---|---|---|---|---|---|
| Day 4 | |||||||
| 0.1 mm | 3.10 ± 0.28 | 3.60 ± 0.43 | 6.60 ± 0.70 | 13.31 ± 0.62 | 0.51 ± 0.03 | 0.47 ± 0.05 | 0.24 ± 0.03 |
| 0.5 mm | 3.10 ± 0.28 | 3.70 ± 0.56 | 5.00 ± 0.61 | 13.30 ± 0.70 | 0.63 ± 0.04 | 0.56 ± 0.04 | 0.35 ± 0.04 |
| 10 mm | 5.75 ± 0.45 | 3.25 ± 0.37 | 4.13 ± 0.40 | 13.13 ± 0.44 | 0.69 ± 0.03 | 0.64 ± 0.04 | 0.44 ± 0.04 |
| Day 6 | |||||||
| 0.1 mm | 13.83 ± 2.02 | 3.00 ± 0.51 | 2.83 ± 0.79 | 19.67 ± 1.08 | 0.85 ± 0.04 | 0.80 ± 0.06 | 0.69 ± 0.08 |
| 0.5 mm | 13.00 ± 0.41 | 2.33 ± 0.62 | 2.33 ± 0.23 | 17.67 ± 0.84 | 0.87 ± 0.01 | 0.85 ± 0.03 | 0.74 ± 0.04 |
| 10 mm | 15.00 ± 1.24 | 1.50 ± 0.49 | 1.67 ± 0.42 | 18.17 ± 0.94 | 0.91 ± 0.02 | 0.90 ± 0.04 | 0.82 ± 0.05 |
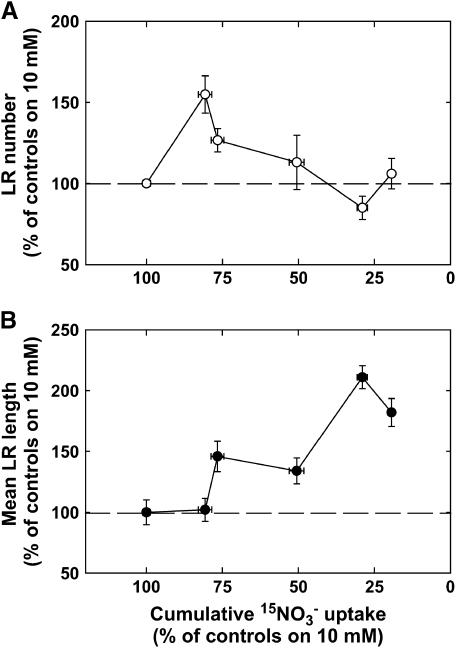
In summary, two different phases could be distinguished in the RSA response to a marked step down in external NO3− availability during the 6 d after transfer. First, until day 3, low NO3− quickly triggers stimulation of LR growth, which specifically occurred in the portion of the primary root that had developed before transfer and involved both accelerated LR appearance and increased LR elongation. When relating these two components of the RSA response (Fig. 2, B and C) to the cumulative NO3− uptake after the transfer (Fig. 1), it appears that, during the first 3 d after transfer, the stimulation of LR growth by low NO3− relied for a large part on increased production of visible laterals in plants submitted to low nitrogen stress, whereas it was mostly due to faster elongation of laterals in plants experiencing a high level of nitrogen deficiency (Fig. 3). Thereafter, between day 4 and day 6 after transfer, LR growth was no more stimulated in nitrogen-limited plants as compared to controls, partly because of delayed generation of LRs in the portion of the primary root that developed after transfer.
Figure 3.
Effect of lowered NO3− uptake on LR growth in wild-type (Ws) Arabidopsis seedlings. The data are from Figures 1 and 2, B and C, to plot the number of LRs (A) and the mean length of LRs (B) as a function of the cumulative 15NO3− uptake in wild-type (Ws) Arabidopsis seedlings during 6 d following transfer from 10 mm NO3− to media containing 15NO3− at the concentrations indicated in Figure 1. Data are normalized with those obtained in control plants transferred to a 10 mm NO3− medium. Average values (± se) of 12 seedlings.
Lack of Up-Regulation of the NO3− HATS in the atnrt2.1-1 Mutant Results in Strongly Altered RSA Responses to Nitrogen Limitation When Compared with Ws
To determine whether the root morphological responses to nitrogen limitation described above in Ws are dependent on the physiological responses at the NO3− uptake system level, we investigated the RSA phenotype of the atnrt2.1-1 mutant after transfer from 10 to 0.5 mm NO3−. This particular treatment was selected among the range investigated previously, first because it corresponded to the highest relative increase in total LR length as compared with control plants (Fig. 2A), and second because it triggered both types of RSA responses (accelerated appearance of new LRs and increase in mean LR length; see Fig. 2, B and C). For this series of experiments, plants were transferred to nitrogen-limited medium after 8 d of growth on 10 mm NO3− and studied for another 3 d after transfer, which corresponded to the stimulatory phase of LR growth in the previously formed portion of the primary root (Fig. 2A). Postponing the transfer to day 8 allowed for a better investigation of the adaptive response during a period of quantitatively more important LR growth than at day 6 after germination.
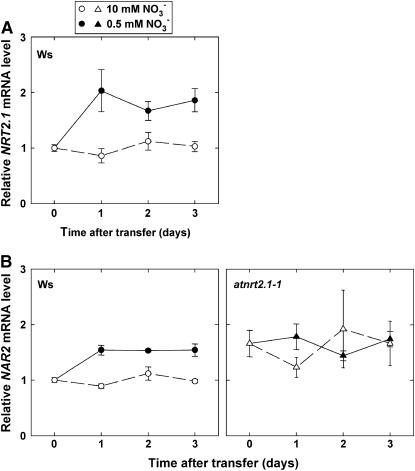
In wild-type plants, transfer from 10 to 0.5 mm NO3− markedly stimulated NRT2.1 mRNA accumulation in the roots as soon as 1 d after the transfer (Fig. 4A). In addition to NRT2.1, a homolog of the NAR2 gene of Chlamydomonas reinhardtii (At5g50200) was also investigated. In both C. reinhardtii and barley (Hordeum vulgare), NAR2 genes encode a putative partner of NRT2 proteins required for functionality of the HATS (Quesada et al., 1994; Zhou et al., 2000; Tong et al., 2005). Interestingly, the pattern of expression of At5g50200 was very similar to that of NRT2.1 (Fig. 4B), suggesting coregulation of these two genes. In the atnrt2.1-1 mutant, the At5g50200 transcript level was higher than in Ws before the transfer and was not significantly affected by nitrogen limitation afterward (Fig. 4B).
Figure 4.
Effect of lowered NO3− availability on the accumulation of NRT2.1 (A) and NAR2 (B) homolog transcripts in the roots of wild-type (Ws) or atnrt2.1-1 mutant Arabidopsis seedlings. The seedlings were grown on vertical agar plates at 10 mm NO3− and were transferred at the age of 8 d to medium containing either 0.5 or 10 mm NO3−. Steady-state mRNA levels were assayed by real-time reverse transcription-PCR. Average values (± se) of three independent repeats.
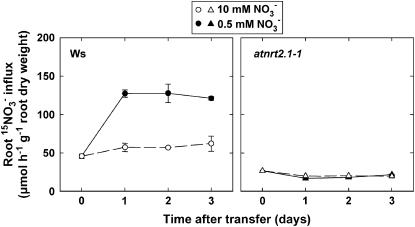
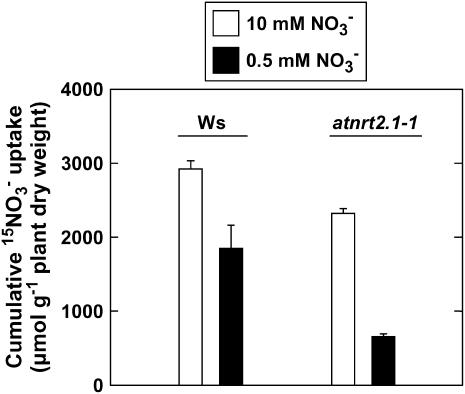
Changes in root 15NO3− influx by the HATS (assayed at 0.2 mm external 15NO3−) in Ws plants (Fig. 5) resembled those of both the NRT2.1 and the NAR2 homolog transcript levels (Fig. 4, B and C), with a strong stimulation in nitrogen-limited Ws plants compared with controls. In the atnrt2.1-1 mutant, root 15NO3− influx remained at a low and similar level in both groups of plants either transferred to 0.5 mm or left on 10 mm NO3− (Fig. 5). Accordingly, cumulative 15NO3− uptake during the 3-d period after transfer (assayed in a different batch of plants than those used for influx measurement) was much more restricted by the lowering of the external NO3− concentration in the atnrt2.1-1 mutant (−72%) than in the wild type (−36%; Fig. 6). These data confirm that the usual up-regulation of the NO3− HATS activity in response to nitrogen limitation is lost in atnrt2.1-1 plants.
Figure 5.
Effect of lowered NO3− availability on root 15NO3− influx by the HATS in wild-type (Ws) or atnrt2.1-1 mutant Arabidopsis seedlings. The seedlings were grown on vertical agar plates at 10 mm NO3− and were transferred at the age of 8 d to medium containing either 0.5 or 10 mm NO3−. Root 15NO3− influx was assayed at 0.2 mm external 15NO3− concentration to be representative of the Vmax of the HATS. Average values (± se) of five repeats of two seedlings each.
Figure 6.
Cumulative 15NO3− uptake in wild-type (Ws) or atnrt2.1-1 mutant Arabidopsis seedlings during 3 d following transfer from 10 mm NO3− to medium containing either 0.5 or 10 mm 15NO3−. Seedlings were grown on vertical agar plates and supplied with 10 mm NO3− until the age of 8 d prior to the transfer. Cumulative uptake was expressed on a total plant biomass basis to avoid bias due to changes in the root-to-shoot biomass ratio after growth on different NO3− concentrations. Average values (± se) of five repeats of two seedlings each.
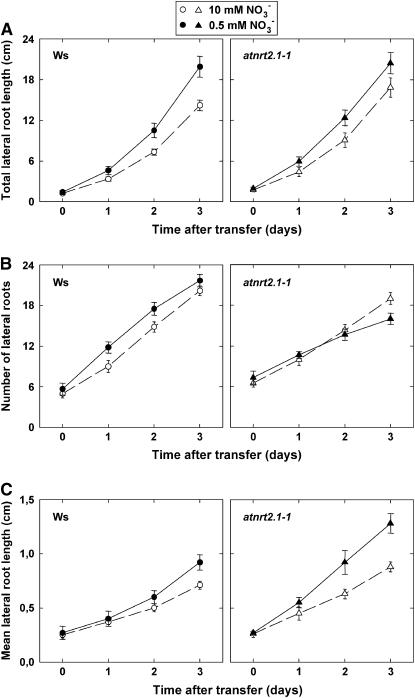
In control plants left on 10 mm NO3−, primary and LR growth during the 3 d following transfer was very similar between the two genotypes (Fig. 7; data not shown), although some variability could be observed, depending on the experiment. Our data thus confirm that mutation of NRT2.1 and NRT2.2 had no significant consequence on the growth of the root system under nonlimiting nitrogen supply (Orsel et al., 2004; Little et al., 2005).
Figure 7.
Effect of lowered NO3− availability on LR growth in wild-type (Ws) or atnrt2.1-1 mutant Arabidopsis seedlings. Plants were grown on vertical agar plates at 10 mm NO3− and transferred at the age of 8 d to medium containing either 0.5 or 10 mm NO3−. Total length of LRs (A), number of LRs (B), and mean length of LRs (C) were determined by image analysis. Only LRs >0.5 mm, visible without the use of a microscope, were taken into account. Average values (± se) of 12 seedlings.
Ws plants transferred to 0.5 mm NO3− after 8 d of growth on 10 mm medium showed the same responses of RSA to nitrogen limitation (Fig. 7) as those described previously for transfer after 6 d (Fig. 2). These include no change in the primary root growth (data not shown) and an increase in total LR length (Fig. 7A) because of both an early stimulation of the appearance of new laterals (Fig. 7B) and a more delayed increase in the mean length of individual LRs (Fig. 7C). The enhancement of LR appearance in nitrogen-limited Ws plants occurred very fast and was mostly observed during the first 24 h after transfer to 0.5 mm NO3− (6.17 ± 0.93 new visible LRs scored in nitrogen-limited plants as opposed to 3.83 ± 0.74 in controls). The stimulation of mean LR length in Ws by nitrogen limitation became visible only during day 2 and day 3 (Fig. 7C), when the mean elongation rate of individual LRs was 20% and 25% higher in nitrogen-limited plants than in controls, respectively.
In atnrt2.1-1 plants, total LR length was increased by low NO3− supply as in Ws plants (Fig. 7A). However, this apparent similarity between the two genotypes hid markedly different responses when considering the components of LR growth. Indeed, after transfer to low NO3− medium, the immediate stimulation of visible LR appearance was lost in the atnrt2.1-1 mutant and total LR number after 3 d was even reduced as compared with controls (Fig. 7B). On the other hand, the increase in mean LR length occurred earlier and was more pronounced in the mutant than in Ws (Fig. 7C). The elongation rate of existing laterals was increased by 40% during day 1 after transfer and by 34% during day 2 after transfer in nitrogen-limited atnrt2.1-1 plants when compared with atnrt2.1-1 controls. These characteristics of the RSA response in atnrt2.1-1 plants transferred from 10 to 0.5 mm NO3−, favoring higher LR length over increased LR number, resemble those observed in Ws plants experiencing a high level of nitrogen limitation (i.e. transferred to 0.1 or 0.05 mm NO3−; see Figs. 2 and 3). It is also noteworthy that, because of the impaired HATS, transfer of atnrt2.1-1 plants from 10 to 0.5 mm NO3− resulted in roughly the same relative decrease in NO3− uptake as that recorded in Ws plants transferred from 10 to 0.1 mm NO3− (−70% in both cases; compare Figs. 1A and 6). Accordingly, the phenotype of atnrt2.1-1 plants transferred from 10 to 0.5 mm NO3− (i.e. 70% decrease in NO3− uptake rate [Fig. 6], no increase in LR number, and 50% increase in mean LR length [Fig. 7]), matches very well the quantitative pattern depicted for Ws plants submitted to a range of various levels of nitrogen limitation (Fig. 3). Thus, it is tempting to suggest that the altered RSA phenotype of the atnrt2.1-1 mutant on 0.5 mm NO3− is simply because of the reduced NO3− uptake in the mutant, which generated a shift toward more pronounced nitrogen deficiency than in Ws. Furthermore, the increase in root-to-shoot biomass ratio after 3 d on 0.5 mm NO3− compared with 10 mm NO3− was much higher in atnrt2.1-1 than in Ws (from 0.44–0.75 in atnrt2.1-1 and from 0.34–0.43 in Ws). This also could be explained by lowered NO3− uptake and stronger nitrogen limitation in the mutant (see Fig. 1B).
The Mutation of NRT2.1 Strongly Affects LR Initiation Independently of Root NO3− Uptake
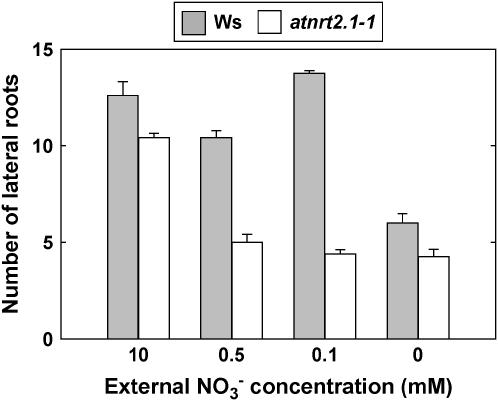
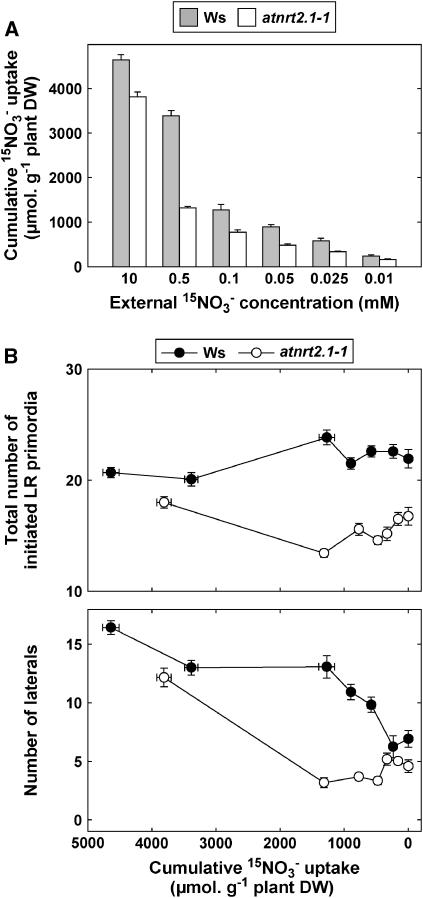
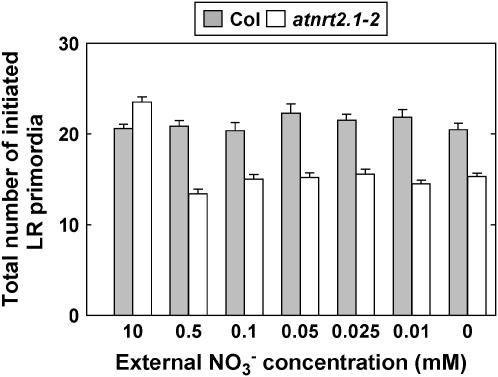
When considering an extended period of nitrogen limitation (6 d instead of 3 d), a particularly striking aspect of the RSA phenotype of nitrogen-limited atnrt2.1-1 plants was the strongly reduced number of visible laterals in the portion of the primary root developing after transfer to the 0.5 mm medium (Fig. 8). Transfer of atnrt2.1-1 plants either to lower NO3− concentration (0.1 mm) or to nitrogen-free medium did not amplify this inhibition of LR appearance. These observations clearly contrast with the results obtained in Ws, where transfer of the plants to 0.1 mm did not result in a decrease in total LR number. Only total nitrogen deprivation succeeded in markedly reducing LR number in the newly formed portion of the primary root in wild-type plants (Fig. 8). Contrary to the other RSA responses investigated above, this suggests that the reduced branching of the newly formed portion of the primary root in the atnrt2.1-1 mutant cannot simply be explained by lowered NO3− uptake rate. To further investigate this point, a series of experiments was performed to determine how NO3− uptake rate affects both LR initiation and appearance in Ws and atnrt2.1-1 plants. Therefore, cumulative 15NO3− uptake was measured in plants of both genotypes after transfer from 10 mm NO3− to various concentrations of 15NO3−, ranging from 10 to 0 mm, and total numbers of initiated LR primordia and visible LRs were scored in the newly formed portion of the primary root. As expected, cumulative 15NO3− uptake was strongly reduced in the mutant as compared to the wild type when the external 15NO3− concentration was below 1 mm (Fig. 9A). When both numbers of initiated primordia and visible LRs were plotted against cumulative 15NO3− uptake, it clearly appeared that in all low NO3− media (from 0.5–0.01 mm), the atnrt2.1-1 mutant had a strong defect in branching of the newly formed portion of the primary root (Fig. 9B). This cannot be explained by the lowered NO3− uptake in the mutant because, with similar cumulative 15NO3− uptake in the range of 500 to 1,500 μmol g−1 plant dry weight for both genotypes, atnrt2.1-1 plants had 3 to 4 times less visible laterals and 30% to 40% less LR primordia than Ws plants. Interestingly, the defect in LR primordia initiation in the mutant was also observed in the absence of added NO3− in the medium. To determine whether this phenotype was specifically due to NRT2.1 mutation, we investigated a second atnrt2.1 allele, the atnrt2.1-2 mutant in the Columbia (Col-0) background carrying a T-DNA insertion located in the first intron of the gene. As for atnrt2.1-1, atnrt2.1-2 plants also displayed a lowered number of LR primordia in the portion of the primary root that developed after transfer to low NO3− medium (Fig. 10). In both atnrt2.1-1 and atnrt2.1-2 mutants, however, LR primordia initiation and new LR appearance were not altered at high NO3− uptake rate or availability when compared to the wild type (Figs. 9B and 10).
Figure 8.
Number of LRs in the most basal 4 cm of the primary root portion that developed after transfer of Ws and atnrt2.1-1 plants to low NO3− medium. The plants were transferred at day 6 from 10 mm NO3− to NO3− medium at the concentrations indicated, and visible LRs (length >0.5 mm) were scored at day 12. Average values (± se) of 12 seedlings.
Figure 9.
Cumulative 15NO3− uptake (A) and LR development in the newly formed portion of the primary root (B) of Ws and atnrt2.1-1 plants transferred to low NO3− medium. Plants were transferred at day 6 from 10 mm NO3− to either nitrogen-free medium or to 15NO3− medium at the concentrations indicated in A. Total 15N content of the plants and total numbers of visible LRs (length >0.5 mm) and LR primordia were determined at day 12. Average values (± se) of five repeats of two seedlings each for cumulative uptake or of 12 seedlings for root architecture analysis.
Figure 10.
Total number of initiated LR primordia in the most basal 4 cm of the primary root portion that developed after transfer of Col-0 and atnrt2.1-2 plants to low NO3− medium. Plants were transferred at day 6 from 10 mm NO3− to NO3− medium at the concentrations indicated. Total number of initiated LR primordia was determined at day 12. Average values (± se) of 12 seedlings.
NRT2.1 Is Not Expressed in the New LR Primordia or Young LRs Generated in Response to Nitrogen Limitation
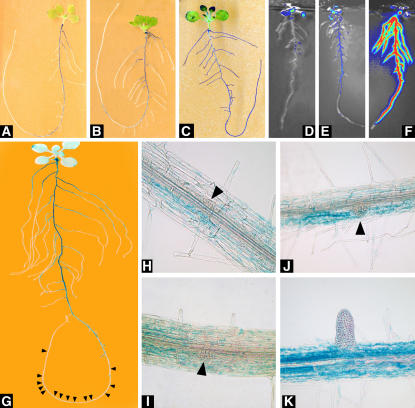
The RSA phenotype of atnrt2.1 mutants is characterized by an inhibition of the generation of young LRs (Figs. 7 and 9B) or of initiation of LR primordia (Figs. 9B and 10) in response to low NO3−. Transgenic lines expressing β-glucuronidase (GUS) or luciferase (LUC) reporter genes under the control of the NRT2.1 promoter were used to determine whether this is associated with the fact that NRT2.1 is expressed in these organs. Previous experiments suggested that, in nitrogen-sufficient plants, NRT2.1 expression is restricted to oldest portions of the roots (Nazoa et al., 2003). In agreement with this conclusion, histochemical staining in pNRT2.1∷GUS transformants showed GUS activity limited to the basal part of the primary root (corresponding roughly to the branched zone where LRs are visible) and to the very base of old laterals, regardless of the external NO3− concentration (Fig. 11, A and B). This pattern of expression was confirmed by bioluminescence imaging in pNRT2.1∷LUC transformants (Fig. 11, D and E). The LUC gene also provides a better reporter than the GUS gene to observe the stimulation of pNRT2.1 activity at 0.5 mm NO3− (Fig. 11, D and E), most probably because of the shorter half life of the LUC protein and mRNA (Van Leeuwen et al., 2000). With both reporter genes, no pNRT2.1 activity could be detected in the young laterals or in the apex of older ones. To determine whether pNRT2.1 is active in the primary root zone where LR primordia are initiated and emerge, GUS activity was investigated at the microscopic level, using a modified protocol for improved staining (see “Materials and Methods”). After increased penetration of the substrate (vacuum infiltration and treatment with detergent), and 16 h instead of 6 h of incubation, much stronger GUS staining was observed in the basal portions of the primary root and of all laterals (compare Fig. 11, A and G). Faint staining also became visible in patches below the branched zone of the primary root (see Fig. 11G, arrowheads). In these areas, very low pNRT2.1 activity could be detected in the vicinity of LR primordia at various stages of their development (Fig. 11, H–K). However, neither these primordia nor the young emerged LRs were stained.
Figure 11.
Tissue localization of NRT2.1 expression. Histochemical localization of GUS activity and bioluminescence imaging of LUC activity in transgenic Arabidopsis plants expressing pNRT2.1∷GUS (A, B, and G–K), p35S∷GUS (C), pNRT2.1∷LUC (D and E), and p35S∷LUC (F). Plants were grown for 7 d on vertical agar plates at 10 mm NO3− and transferred for 3 d (A–F) or 4 d (G–K) to medium containing either 0.5 mm NO3− (B, E, and G–K) or 10 mm NO3− (A, C, D, and F). Arrowheads in G indicate patches of faint staining. Arrowheads in I and J indicate LR primordia at various stages of development before emergence.
DISCUSSION
The Strength of the Nitrogen Stress Experienced by the Plant Determines the Morphological Response of the Root System
In this study, we specifically focused on the short-term adaptive responses of the root system of Arabidopsis plants to a sudden decrease in the external NO3− availability. The treatments applied (transfer from 10 mm NO3− to 0.05–1 mm NO3−) are mostly expected to relieve the systemic repression exerted by high NO3− on LR development (seen at NO3− concentration ≥10 mm), whereas the local stimulatory effect of NO3− on LR elongation (seen at NO3− concentration as low as 50 μm) should not be strongly affected (Zhang and Forde, 1998; Zhang et al., 1999). Repression of root branching by high NO3− has already been thoroughly investigated and our data on Ws plants is illustrative of classical effects of nitrogen limitation (i.e. that decreasing external NO3− concentration had no effect on primary root elongation, but stimulates LR growth, in particular by increasing the number of visible LRs; Zhang and Forde, 1998, 2000; Signora et al., 2001). However, several original findings are reported here that give a much more detailed picture of the overall RSA response to low NO3−. A major outcome is that different levels of nitrogen limitation did not result in a unique, more or less pronounced RSA response, but induced markedly different morphological changes (Figs. 2 and 3). Whereas a shift from high to moderate concentrations of NO3− resulted in a fast increase in the number of visible laterals, but had only a limited effect on mean LR length, transfer of the plants to the lowest NO3− levels predominantly stimulated mean LR length (Fig. 2, B and C) through an increased elongation rate of existing laterals. To our knowledge, such differences in the way RSA responds to various levels of nitrogen limitation have not been previously reported. However, careful analysis of the literature provides some support to our conclusions. Indeed, a positive effect of very low NO3− availability (0.1 or 0.164 mm versus 1 mm) on LR length, but not on LR density, has been reported by Linkohr et al. (2002). Reciprocally, when comparing moderate versus high NO3− concentration (0.5 or 1 mm versus 10 or 50 mm), Zhang et al. (1999) and Tranbarger et al. (2003) found an effect mostly on the total number or density of visible laterals, but not on the mean length or elongation rate of these LRs. This suggests that different strategies for modifying RSA are employed by the plant to react to different strengths of a specific nutritional stress, as is now well documented to be the case in reaction to different types of nutrient stress (e.g. nitrogen, phosphorus, sulfur, or iron deprivation; Forde and Lorenzo, 2001; Lopez-Bucio et al., 2003).
Low NO3− Has Contrasting Effects on Root Branching Depending on the NO3− Treatment History along the Primary Root Axis
Another unexpected finding was that NO3− limitation has opposite effects on root branching depending on whether the primary root had experienced the high NO3− pretreatment or whether it had grown after transfer to low NO3−. Indeed, appearance of new visible LRs could be stimulated by low NO3− in the basal preexisting primary root zone (Figs. 2B and 7), whereas it was delayed in the apical newly formed portion of the primary root because of reduction of both emergence and activation of LR primordia (Table I). These data show that the effect of NO3− on LR development not only depends on the current local NO3− availability, but also on the prior nutrition regime of the primary axis. Delayed appearance of LRs in the newly formed portion of the primary root of nitrogen-limited plants counterbalanced the initial stimulation occurring in the more basal part of the root system (Fig. 2B). This explains why enhancement of total LR growth was only transient in our experiments (Fig. 2). The reason for the delayed LR development in the newly formed portion of the primary root is unknown, but it is unlikely to be due to nutrient shortage. Indeed, this hypothesis is difficult to reconcile with the fact that, in this portion, both emergence and activation of LR primordia recovered after 6 d on low NO3− (Table I). Furthermore, except at 0.05 mm, both the increase in biomass of the whole root system and the elongation rate of the primary root were unaffected by external NO3− concentration in our experiments (data not shown). Finally, in plants continuously grown on low NO3−, where nitrogen deficiency effects should be most pronounced, activation of emerged primordia was shown to be stimulated rather than reduced, resulting in a marked increase in total LR length when compared to nitrogen-sufficient plants (Zhang and Forde, 1998; Tranbarger et al., 2003).
The Altered RSA Phenotype of the atnrt2.1-1 Mutant: Both the Consequence of Modified NO3− Uptake and of a Direct Role of NRT2.1 in LR Development
The atnrt2.1-1 mutant displayed a markedly altered RSA response to nitrogen limitation when compared with Ws plants (Figs. 7–9). This demonstrates a strong interaction between physiological and developmental processes involved in the adaptive responses of plants to nitrogen stress. However, the overall phenotype of the atnrt2.1-1 mutant indicates that, in our system, the physiological response corresponding to the up-regulation of the HATS had a predominant quantitative role over the morphological response of the root system in stimulating the NO3− uptake capacity of the plant. After transfer to 0.5 mm NO3−, the HATS activity per gram root dry weight was 6-fold higher in Ws than in atnrt2.1-1 (Fig. 5). This was clearly not compensated for by a 6-fold stimulation of root growth in the atnrt2.1-1 mutant compared with Ws (Fig. 7). As a consequence, atnrt2.1-1 plants were unable to react as efficiently as the wild type to a 20-fold decrease in external NO3− availability (Fig. 6). As was the case in Ws plants transferred to very low NO3− concentrations (0.1 or 0.05 mm), the RSA response of the mutant was qualitatively modified, but the main quantitative morphological adjustment in atnrt2.1-1 plants resulted from reduced shoot growth, leading to a markedly increased root-to-shoot ratio (see also Orsel et al., 2004).
Concerning LR development in the preexisting portion of the primary root (i.e. during the first 3 d after transfer, before any LR emergence in the newly formed apical part of the primary root), lowered NO3− availability did not result in stimulated LR appearance in atnrt2.1-1 plants, contrary to what was observed in Ws (Fig. 7B). However, the increase in mean LR length induced by low NO3− was much more pronounced in atnrt2.1-1 than in Ws (Fig. 7C). These alterations of the RSA response to low NO3− in the mutant can simply be explained by the lowered NO3− uptake rate, because they can be predicted from the data obtained in wild-type plants submitted to a range of various levels of nitrogen limitation (Fig. 3). This suggests that the atnrt2.1-1 mutant has the same pattern of RSA response to nitrogen limitation as the wild type, when the actual level of nitrogen constraint is taken into account. This hypothesis highlights the previous conclusion that regulation of LR growth by NO3− is not triggered by direct sensing of the external NO3− concentration, but is dependent on the amount of NO3− taken up by the plant (Zhang et al., 1999; Zhang and Forde, 2000). The same rationale can be applied to the effect of low NO3− on the root-to-shoot biomass ratio, which was found to be much more increased in atnrt2.1-1 plants (up to 0.75) than in Ws plants (up to 0.43) after transfer to 0.5 mm NO3−. This could also be explained by the stronger nitrogen stress experienced by the atnrt2.1-1 plants in this situation (see Figs. 1 and 6). Taken together, these considerations mean that quantitative changes in nutrient uptake should be taken into account very precisely when assessing the effects of mutations on the response of RSA to nutrient cues. This also means that NRT2.1 expression, through its major impact on root NO3− uptake rate (Cerezo et al., 2001; Filleur et al., 2001), is a key factor indirectly governing RSA.
However, not all the aspects of the RSA response of atnrt2.1 mutants to low NO3− can be explained by reduced NO3− uptake. In particular, the inhibition of LR primordia initiation in atnrt2.1 plants supplied with low NO3− concentrations (Figs. 9 and 10) is intriguing for at least two reasons. First, initiation of LR primordia is a process not repressed by nitrogen limitation in wild-type plants (Table I; Figs. 9 and 10; see also Forde and Lorenzo, 2001). Second, the inhibition of LR primordia initiation was observed only in atnrt2.1 plants transferred to low NO3− (Figs. 9B and 10) and appeared to be independent from the rate of NO3− uptake by the whole root system (Fig. 9B). Similar phenotypes were found in two independent atnrt2.1 mutants (Figs. 9B and 10) and this points to a direct and specific role of NRT2.1 in the initiation of LR primordia. Such a role has recently been proposed by Little et al. (2005) on the basis that NRT2.1 mutation alleviates the repression of LR primordia initiation by a high carbon-to-nitrogen ratio in the external medium (7.5% Suc with 0.1 mm NO3−) independently of the role of NRT2.1 in NO3− uptake. Even more surprising was the finding that the phenotype of the atnrt2.1 mutants was observed in the absence of added NO3− in the external medium (Little et al., 2005). Our own data fully confirm this main conclusion of an important direct role of NRT2.1 in the process of LR primordia initiation, which is not explained by NRT2.1 NO3− uptake function and which could also be observed in the absence of added NO3− in the medium (Fig. 9B). However, in our conditions, it is difficult to be sure that the absence of added NO3− in the external medium prevented any NO3− uptake activity by the roots. Indeed, because the plants were grown previously on 10 mm, it is possible that, upon transfer to nitrogen-free medium, NO3− may have been effluxed out of the root cells before being taken up again by the influx systems. Such a futile cycle would mostly be restricted to the root apoplast, making it difficult to detect, but might have been sufficient to maintain a low level of NRT2.1 expression and activity in the wild type. The very surprising point is that, in our hands, atnrt2.1 mutants display exactly the reverse phenotype than that reported by Little et al. (2005; i.e. an inhibition, rather than a stimulation, of LR primordia initiation). Whether this discrepancy is because of the absence of high Suc concentration in our growth medium or to other differences in the experimental conditions between our study and that of Little et al. (2005) is not known. It shows, however, that NRT2.1 is not always a repressor of LR initiation and that its sensing or signaling role on root branching is probably more complex than previously envisaged. The observation that pNRT2.1 was active (although at a very low level) in the primary root portion where LR primordia are initiated and emerge (Fig. 7, H–K) is consistent with the hypothesis that NRT2.1 plays a role in LR primordia initiation. It may indicate that the presence and/or activity of NRT2.1 in these areas contribute to local signaling that stimulates LR primordia development.
Further stages of LR development beyond primordia initiation also seemed to be affected by the mutation of NRT2.1. For the intermediate range of cumulative NO3− uptake values (500–1,500 μmol g−1 plant dry weight), the total number of visible laterals in the apical part of the primary root of the mutant was even more strongly reduced than the number of LR primordia (Fig. 9B), whereas there was almost no effect on the number of LRs in plants with either high or very low NO3− uptake rates. This shows that, besides primordia initiation, other steps of LR development, such as emergence or activation of primordia, are also affected in a complex way both by the external NO3− concentration (see Table I) and the presence and/or activity of NRT2.1.
An additional question raised by our results is to know whether other proteins putatively involved in NO3− HATS activity also participate to the signaling governing LR development. One interesting candidate would then be the At5g50200 gene, encoding a homolog of the C. reinhardtii CrNAR2 gene (Quesada et al., 1994). The product of CrNAR2 has been shown to be strictly required for high-affinity NO3− uptake in C. reinhardtii (Quesada et al., 1994; Zhou et al., 2000), leading to the hypothesis that the CrNAR2 protein could be a component of the active HATS in this species, together with CrNRT2 proteins. Recently, a barley homolog of CrNAR2 was shown to be required for functional expression of HvNRT2.1 in Xenopus oocytes (Tong et al., 2005). Our expression data show that the Arabidopsis NAR2 homolog appears to be coregulated with NRT2.1 in response to nitrogen limitation (Fig. 4). The recent report that this NAR2 homolog is also inducible by NO3−, as is NRT2.1 (Scheible et al., 2004), provides indirect evidence that the product of this gene may play a role in the HATS in Arabidopsis. Future investigation on knockout mutants of At5g50200 will allow the study of this hypothesis and will also provide an opportunity to determine whether or not this gene is also involved in the NO3− regulation of LR development.
MATERIALS AND METHODS
Plant Material
The Arabidopsis (Arabidopsis thaliana) genotypes used in this study were the wild-type Ws and Col-0 ecotypes; the atnrt2.1-1 mutant in the Ws background (formerly atnrt2a) obtained from the collection of the Insitut National de la Recherche Agronomique, Versailles, and deleted for the NRT2.1 (At1g08090) and NRT2.2 (At1g08100) nitrate transporter genes (Filleur et al., 2001); the atnrt2.1-2 mutant in the Col-0 background, obtained from the Salk Institute (Salk_035429) and carrying a T-DNA insertion in the first intron of NRT2.1 (these two mutants were renamed according to the nomenclature proposed by Little et al., 2005); and transformants carrying the following promoter-reporter gene fusions: pNRT2.1∷GUS (Nazoa et al., 2003), pNRT2.1∷LUC, p35S∷GUS (Lagarde et al., 1996), and p35S∷LUC (Dorbe et al., 1998). For the pNRT2.1∷LUC construct, 1,202 bp located upstream of the translation initiation codon of NRT2.1 were fused to the LUC coding fragment (pSP-luc+; Promega) followed by the 35S cauliflower mosaic virus termination sequence. This cassette was inserted in the binary vector pBIB-HYG (Becker, 1990) and Arabidopsis ecotype Col-0 was transformed using Agrobacterium tumefaciens GV3101 according to Clough and Bent (1998).
Growth Medium and Growth Conditions
Basic medium contained 0.5 mm CaSO4, 0.5 mm MgCl2, 1 mm KH2PO4, 2.5 mm MES (Sigma), pH 5.8, 50 μm NaFe EDTA, 50 μm H3BO3, 12 μm MnCl2, 1 μm CuCl2, 1 μm ZnCl2, and 0.03 μm NH4Mo. This basic medium was complemented with KNO3 as a sole nitrogen source at the concentrations indicated for each individual experiment. The K+ concentration was adjusted to 10 mm by the addition of K2SO4 in all media with KNO3 concentrations lower than 10 mm. Arabidopsis seeds were surface sterilized for 10 min in 1 mL of 50% (v/v) ethanol containing 2% (w/v) Bayrochlor (Bayrol), followed by five washes with 100% ethanol and drying in a laminar air flow. Sterilized seeds were planted with a sterile toothpick in 12- × 12-cm transparent plates on 40 mL of solid medium (1% Difco Bacto agar; BD Biosciences) containing 10 mm nitrate. After storing for 2 d at 4°C in the dark, plates were incubated vertically in a growth chamber at 22°C, with a 16-h light/8-h dark regime and a light intensity of 150 μmol m−2 s−1. Plantlets growing on the surface of the agar were transferred at various time points, as indicated, to fresh growth media (at five plants per plate) containing various nitrate concentrations.
Net NO3− Uptake and Influx Studies
Root influx and net uptake of NO3− were determined by 15N labeling. For measurement of cumulative NO3− uptake during the period from beginning of the treatment to the end of the experiment, the treatment medium was supplemented with K15NO3 (atom % 15N: 1.0%). Liquid media for influx studies contained basic nitrogen-free medium supplemented with 0.2 mm K15NO3 (atom % 15N: 99%). For influx assays, the plants were transferred to a 5-cm-diameter petri dish containing 0.1 mm CaSO4, with the roots in the solution and the aerial parts outside. This solution was replaced after 1 min with the 0.2 mm 15NO3− solution for 5 min. Plant roots were then rinsed again for 1 min in 0.1 mm CaSO4 before being harvested, dried at 70°C for 48 h, and weighed. Influx was calculated as μmol 15NO3− h−1 g−1 root dry weight after determination of total 15N in roots, and net uptake was calculated as μmol 15NO3− per plant dry weight after determination of total 15N in both roots and shoots. The 15N analyses were performed using an integrated system for continuous flow isotope ratio mass spectrometry (Euro-EA elemental analyzer; EuroVector S.P.A.; and Isoprime mass spectrometer; GV Instruments).
RNA Extraction and Gene Expression Analysis
Frozen (−80°C) root samples (50–100 mg) were homogenized for 1 min at 30 s−1 (Retch mixer mill MM301) in 2-mL tubes containing two tungsten beads (2.5 mm diameter). Total RNA was extracted from homogenized tissues using TRIzol reagent (Invitrogen) according to the manufacturer's protocol. Three micrograms of RQ-DNase (Promega) digested total RNA were used to prepare cDNA by reverse transcription using Moloney murine leukemia virus reverse transcriptase (Promega) and oligo dT(18) primers, according to the manufacturer's protocol. Gene expression was determined by quantitative real-time PCR (LightCycler; Roche Diagnostics) using gene-specific primers (see sequences below) and LightCycler FastStart DNA Master SYBR Green I (Roche Diagnostics). Expression levels of tested genes were normalized to expression levels of the EF1α (At1g07920, At1g07930, and At1g07940) or CLATHRIN (At4g24550) gene. Gene-specific primer sequences were (5′ to 3′ and corresponding to forward [F] or reverse [R] gene sequence), for NRT2.1: F, aacaagggctaacgtggatg; R, ctgcttctcctgctcattcc; for CrNAR2 homolog (At5g50200): F, ggccatgaagttgcctatg; R, tcttggccttcctcttctca; for EF1α: F, gtcgattctggaaagtcgacc; R, aatgtcaatggtgataccacgc; for CLATHRIN: F, agcatacactgcgtgcaaag; R, tcgcctgtgtcacatatctc. A second homolog of CrNAR2 is present in the Arabidopsis genome (At4g24720), but has not been investigated in this study because no evidence for its expression (expressed sequence tags, cDNA, or microarray data) was found in the databases.
Analysis of Root Growth
The root systems in vertical agar plates were scanned daily at 300 dpi (Epson perfection 2450 photo; Seiko Epson). Root growth parameters were determined after analysis of scanned images using the Optimas image analysis software (MediaCybernetics). For each plant, the precise coordinates of the primary root apex were determined after the transfer initiating the treatment. This allowed the identification of the portion of the primary root that had developed before and during the treatment, respectively. LR primordia were counted using a conventional light microscope (Olympus BH-2) at 160× magnification following a protocol adapted from Malamy and Ryan (2001). LR initiation was defined as the processes occurring between stages I and VII, LR emergence, the process occurring after stage VII and corresponding to the appearance of the primordia tip outside the root epidermis (according to the nomenclature proposed by Malamy and Benfey (1997). Total number of initiated LR primordia represents the sum of LRs and emerged and nonemerged primordia. Statistical comparisons of means between treatments or genotypes were performed using the pooled Student's t test (Montgomery and Runger, 1994).
GUS and LUC Expression Analysis
Histochemical analysis of the GUS reporter enzyme activity was adapted from Jefferson (1987). Plantlets were incubated for 6 h in reaction buffer without detergent, containing 1 mm 5-bromo-4-chloro-3-indolyl-β-d-glucuronid acid as the substrate. For improved staining, samples were vacuum infiltrated for 30 min, incubated for 16 h in reaction buffer containing 0.05% Triton X-100 and 2 mm 5-bromo-4-chloro-3-indolyl-β-d-glucuronid acid, and plant pigments were cleared with ethanol. GUS-stained plants were embedded in Isomount mounting medium (Labonord) on a glass slide and scanned at 1,200 dpi (Epson perfection 2450 photo; Seiko Epson). For LUC assays, plants were sprayed with 1 mm luciferin (Promega) in 0.01% Triton X-100 and incubated for 10 min in the dark at room temperature before acquisition of emitted light for 5 min with a cooled CCD camera (Hamamatsu Photonics). The acquired image was superimposed using the HiPiC software (Hamamatsu Photonics,) on a morphological picture of the plant taken in normal light.
Note Added in Proof
In an article published in this same issue of Plant Physiology, Okamoto et al. (Okamoto M, Kumar A, Li W, Wang Y, Siddiqi MY, Crawford NM, Glass ADM [2006] High-affinity nitrate transport in roots of Arabidopsis depends on expression of the NAR2-like gene AtNRT3.1. Plant Physiol 140: ▪▪▪) analyzed the function of NRT3.1 in Arabidopsis. This gene turned out to be the one we investigated as “the homolog of the NAR2 gene of Chlamydomonas reinhardtii (At5g50200).” The results reported by Okamoto et al. establish the role of NRT3.1 in the high-affinity nitrate transport in Arabidopsis and confirm most of the hypotheses we raised in the “Discussion” section concerning the function of this gene.
Acknowledgments
We are grateful to Hoai-Nam Truong for supplying seeds of p35S∷LUC transformants and to Gaëlle Viennois for assistance with microscopic procedures.
This work was supported by the European Union Research Training Network, “Plant Use of Nitrate” (HPRN–CT–2002–00247; http://www.plusn.org) and by a French government research program, “Action Concertée Incitative—Biologie du Développement et Physiologie Intégrative.”
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Philippe Nacry (nacry@ensam.inra.fr).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.075721.
References
- Becker D (1990) Binary vectors which allow the exchange of plant selectable markers and reporter genes. Nucleic Acids Res 18: 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerezo M, Tillard P, Filleur S, Muños S, Daniel-Vedele F, Gojon A (2001) Major alterations of the regulation of root NO3− uptake are associated with the mutation of NRT2.1 and NRT2.2 genes in Arabidopsis. Plant Physiol 127: 262–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson DT (1985) Factors affecting mineral nutrient acquisition by plants. Annu Rev Plant Physiol 36: 77–115 [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Crawford NM, Glass ADM (1998) Molecular and physiological aspects of nitrate uptake in plants. Trends Plant Sci 3: 389–395 [Google Scholar]
- Daniel-Vedele F, Filleur S, Caboche M (1998) Nitrate transport: a key step in nitrate assimilation. Curr Opin Plant Biol 1: 235–239 [DOI] [PubMed] [Google Scholar]
- Dorbe M-F, Truong H-N, Crété P, Daniel-Vedele F (1998) Deletion analysis of the tobacco Nii1 promoter in Arabidopsis thaliana. Plant Sci 139: 71–82 [Google Scholar]
- Filleur S, Dorbe MF, Cerezo M, Orsel M, Granier F, Gojon A, Daniel-Vedele F (2001) An Arabidopsis T-DNA mutant affected in NRT2 genes is impaired in nitrate uptake. FEBS Lett 489: 220–224 [DOI] [PubMed] [Google Scholar]
- Forde B, Lorenzo H (2001) The nutritional control of root development. Plant Soil 232: 51–68 [Google Scholar]
- Forde BG (2000) Nitrate transporters in plants: structure, function and regulation. Biochim Biophys Acta 1465: 219–235 [DOI] [PubMed] [Google Scholar]
- Gansel X, Muños S, Tillard P, Gojon A (2001) Differential regulation of the NO3− and NH4+ transporter genes AtNRT2.1 and AtAMT1.1 in Arabidopsis: relation with long-distance and local controls by N status of the plant. Plant J 26: 143–155 [DOI] [PubMed] [Google Scholar]
- Jefferson RA (1987) Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Rep 5: 387–405 [Google Scholar]
- Lagarde D, Basset M, Lepetit M, Conejero G, Gaymard F, Astruc S, Grignon C (1996) Tissue-specific expression of Arabidopsis AKT1 gene is consistent with a role in K+ nutrition. Plant J 9: 195–203 [DOI] [PubMed] [Google Scholar]
- Lejay L, Tillard P, Lepetit M, Olive F, Filleur S, Daniel-Vedele F, Gojon A (1999) Molecular and functional regulation of two NO3− uptake systems by N- and C-status of Arabidopsis plants. Plant J 18: 509–519 [DOI] [PubMed] [Google Scholar]
- Linkohr BI, Williamson LC, Fitter AH, Leyser HM (2002) Nitrate and phosphate availability and distribution have different effects on root system architecture of Arabidopsis. Plant J 29: 751–760 [DOI] [PubMed] [Google Scholar]
- Little DY, Rao H, Oliva S, Daniel-Vedele F, Krapp A, Malamy JE (2005) The putative high-affinity nitrate transporter NRT2.1 represses lateral root initiation in response to nutrient cues. Proc Natl Acad Sci USA 102: 13693–13698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Bucio J, Cruz-Ramirez A, Herrera-Estrella L (2003) The role of nutrient availability in regulating root architecture. Curr Opin Plant Biol 6: 280–287 [DOI] [PubMed] [Google Scholar]
- Malamy JE, Benfey PN (1997) Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124: 33–44 [DOI] [PubMed] [Google Scholar]
- Malamy JE, Ryan KS (2001) Environmental regulation of lateral root initiation in Arabidopsis. Plant Physiol 127: 899–909 [PMC free article] [PubMed] [Google Scholar]
- Montgomery DC, Runger GC (1994) Applied Statistics and Probability for Engineers. John Wiley & Sons, New York
- Nazoa P, Vidmar JJ, Tranbarger TJ, Mouline K, Damiani I, Tillard P, Zhuo D, Glass AD, Touraine B (2003) Regulation of the nitrate transporter gene AtNRT2.1 in Arabidopsis thaliana: responses to nitrate, amino acids and developmental stage. Plant Mol Biol 52: 689–703 [DOI] [PubMed] [Google Scholar]
- Okamoto M, Vidmar JJ, Glass AD (2003) Regulation of NRT1 and NRT2 gene families of Arabidopsis thaliana: responses to nitrate provision. Plant Cell Physiol 44: 304–317 [DOI] [PubMed] [Google Scholar]
- Orsel M, Eulenburg K, Krapp A, Daniel-Vedele F (2004) Disruption of the nitrate transporter genes AtNRT2.1 and AtNRT2.2 restricts growth at low external nitrate concentration. Planta 219: 714–721 [DOI] [PubMed] [Google Scholar]
- Orsel M, Krapp A, Daniel-Vedele F (2002) Analysis of the NRT2 nitrate transporter family in Arabidopsis: structure and gene expression. Plant Physiol 129: 886–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada A, Galván A, Fernández E (1994) Identification of nitrate transporter genes in Chlamydomonas reinhardtii. Plant J 5: 407–419 [DOI] [PubMed] [Google Scholar]
- Robinson D (1994) The responses of plants to non-uniform supplies of nutrients. New Phytol 127: 635–674 [DOI] [PubMed] [Google Scholar]
- Scheible WR, Morcuende R, Czechowski T, Fritz C, Osuna D, Palacios-Rojas N, Schindelasch D, Thimm O, Udvardi MK, Stitt M (2004) Genome-wide reprogramming of primary and secondary metabolism, protein synthesis, cellular growth processes, and the regulatory infrastructure of Arabidopsis in response to nitrogen. Plant Physiol 136: 2483–2499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signora L, De Smet I, Foyer CH, Zhang H (2001) ABA plays a central role in mediating the regulatory effects of nitrate on root branching in Arabidopsis. Plant J 28: 655–662 [DOI] [PubMed] [Google Scholar]
- Tong Y, Zhou JJ, Li Z, Miller AJ (2005) A two-component high-affinity nitrate uptake system in barley. Plant J 41: 441–450 [DOI] [PubMed] [Google Scholar]
- Tranbarger TJ, Al-Ghazi Y, Muller B, Teyssendier de la Serve B, Doumas P, Touraine B (2003) Transcription factor genes with expression correlated to nitrate-related root plasticity of Arabidopsis thaliana. Plant Cell Envir 26: 459–469 [Google Scholar]
- Van Leeuwen W, Hagendoorn MJM, Ruttink T, Van Poecke R, Van der Plas LHW, Van der Krol AR (2000) The use of the luciferase reporter system for in planta gene expression studies. Plant Mol Biol Rep 18: 143a–143t [Google Scholar]
- von Wirén N, Gazzarrini S, Gojon A, Frommer WB (2000) The molecular physiology of ammonium uptake and retrieval. Curr Opin Plant Biol 3: 254–261 [PubMed] [Google Scholar]
- Williams LE, Miller AJ (2001) Transporters responsible for the uptake and partitioning of nitrogenous solutes. Annu Rev Plant Physiol Plant Mol Biol 52: 659–688 [DOI] [PubMed] [Google Scholar]
- Zhang H, Forde BG (1998) An Arabidopsis MADS box gene that controls nutrient-induced changes in root architecture. Science 279: 407–409 [DOI] [PubMed] [Google Scholar]
- Zhang H, Forde BG (2000) Regulation of Arabidopsis root development by nitrate availability. J Exp Bot 51: 51–59 [PubMed] [Google Scholar]
- Zhang H, Jennings A, Barlow PW, Forde BG (1999) Dual pathways for regulation of root branching by nitrate. Proc Natl Acad Sci USA 96: 6529–6534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J-J, Fernández E, Galván A, Miller AJ (2000) A high affinity nitrate transport system from Chlamydomonas requires two gene products. FEBS Lett 466: 225–227 [DOI] [PubMed] [Google Scholar]
- Zhuo D, Okamoto M, Vidmar JJ, Glass AD (1999) Regulation of a putative high-affinity nitrate transporter (Nrt2;1At) in roots of Arabidopsis thaliana. Plant J 17: 563–568 [DOI] [PubMed] [Google Scholar]