Abstract
The covalent attachment of heme cofactors to the apo-polypeptides via thioether bonds is unique to the maturation of c-type cytochromes. A number of thiol-disulfide oxidoreductases prepare the apocytochrome for heme insertion in system I and II cytochrome c maturation. Although most thiol-disulfide oxidoreductases are nonspecific, the less common, specific thiol-disulfide oxidoreductases may be key to directing the usage of electrons. Here we demonstrate that unlike other thiol-disulfide oxidoreductases, the protein responsible for reducing oxidized apocytochrome c in Bacillus subtilis, ResA, is specific for cytochrome c550 and utilizes alternate conformations to recognize redox partners. We report solution NMR evidence that ResA undergoes a redox-dependent conformational change between oxidation states, as well as data showing that ResA utilizes a surface cavity present only in the reduced state to recognize a peptide derived from cytochrome c550. Finally, we confirm that ResA is a specific thiol-disulfide oxidoreductase by comparing its reactivity to our mimetic peptide with its reactivity to oxidized glutathione, a nonspecific substrate. This study biochemically demonstrates the specificity of this thiol-disulfide oxidoreductase and enables us to outline a structural mechanism of regulating the usage of electrons in a thiol-disulfide oxidoreductase system.
Keywords: NMR, thiol-disulfide oxidoreductase, x-ray crystallography
The c-type cytochromes are involved in a number of cellular processes including aerobic and anaerobic respiration, plant and bacterial photosynthesis, detoxification via electron transport (1), apoptosis (2, 3), redox metabolism (4), and biosynthesis of heme intermediates (5, 6). Maturation of these important cytochromes involves the posttranslational covalent attachment of a heme cofactor to the C-x-x-C-H motif of the apocytochrome polypeptide. This attachment is distinguished from that in other cytochromes by the presence of two thioether bonds to the vinyl groups of the heme with the histidine residue providing an axial ligand to the heme iron.
Bacteria and plants use two similar but distinct pathways, known as system I and system II, for the maturation of c-type cytochromes whereas animals and fungi rely on an unrelated pathway, known as system III (4). Central to cytochrome c maturation (CCM) by systems I and II are several thiol-disulfide oxidoreductase proteins (4, 7, 8). Some of these are also shared with other disulfide bond-forming pathways of the periplasm and extracellular space where they are involved in disulfide bond formation, isomerization, and reduction (9–12).
Disulfide reduction and oxidation drives CCM in both systems I and II. In both of these systems reduced apocytochrome c is first translocated across the inner membrane. Free thiol group reactivity is prevented by oxidation of the two heme ligating cysteines to a disulfide by the nonspecific thiol-disulfide oxidoreductases, DsbA and BdbD in systems I and II, respectively (4, 13). Oxidized apocytochrome c is then reduced by the DsbE/CcmG–CcmH pair in system I or by ResA in system II before heme insertion (4, 14). Electrons transferred through these redox cycles are obtained from cytoplasmic thioredoxin via an integral membrane protein, DsbD/DipZ (system I) (15) or CcdA (system II) (16, 17).
Thiol-disulfide oxidoreductases typically are nonspecific in their reactivity to substrates, as exemplified by thioredoxin, which catalyzes disulfide reduction and interacts with a multitude of proteins. This is also true for the thiol-disulfide oxidoreductases responsible for oxidizing reduced apocytochrome c and for shuttling electrons from intracellular thioredoxin through the membrane, thus maintaining the oxidizing environment of the periplasm and extracellular space for protein folding and sporulation (15–17). However, genetic evidence suggests that the thioredoxin-like proteins DsbE/CcmG–CcmH or ResA involved in the reverse reaction are specific for CCM (4, 14). The mechanistic basis of substrate specificity by which such thiol-disulfide oxidoreductases recognize their reaction partner is not understood. Previously, it was believed that regions for specific protein–protein interaction were located on sequences inserted in the core thioredoxin fold (18–20). More recently, based on evidence from crystal structures it has been suggested that subtle conformational changes may be responsible for substrate selection by ResA (21).
In this study we present the x-ray crystal structure of the soluble domain of oxidized ResA from Bacillus subtilis at 1.4 Å resolution. Additionally, we use solution NMR spectroscopy to investigate the differences between the reduced and oxidized forms of ResA and provide data showing extensive chemical shift changes indicative of a conformation change between the two states. We also report direct evidence for the involvement of a cavity located on the surface of reduced ResA for histidine recognition of oxidized apocytochrome c. Finally, our biophysical and biochemical results demonstrate that ResA preferentially reduces an oxidized C-x-x-C-H motif contained within a mimetic peptide derived from cytochrome c550, as compared with oxidized glutathione (GSSG), making ResA a confirmed example of a specific thiol-disulfide oxidoreductase. Thus, using this combination of approaches, we show that ResA preferentially recognizes specific binding partners with opposing redox states, using an, as yet, incompletely characterized mechanism of conformational change. Finally, the experimental approach described here provides not only a reliable assay for identifying similar mechanisms in other maturation systems, but also an in vitro assay to determine activity of thiol-disulfide oxidoreductase membrane proteins.
Results and Discussion
Overall Structure Comparison and NMR Chemical Shift Assignments.
A high-quality electron-density map of the oxidized His-tagged soluble domain of ResA (htsResAox) at 1.8 Å resolution was obtained by means of multiwavelength anomalous diffraction phasing using the anomalous signal of the incorporated Se atoms. Refinement of the final atomic model was completed at 1.4 Å resolution with Rwork = 16.2% and Rfree = 17.5%. There are two copies of oxidized htsResA in the asymmetric unit. Although crystallization conditions were different, the molecules in the crystal structures presented here and in a recent report (ref. 21; PDB entry 1ST9) are packed in the same lattice arrangement. Both structures are in close agreement as demonstrated by the pairwise superposition of monomers (Table 2, which is published as supporting information on the PNAS web site). Because of the low rms deviation values for backbone atoms, all models of ResAox are considered equivalent (Fig. 5a, which is published as supporting information on the PNAS web site).
Backbone chemical shifts of both reduced and oxidized states of htsResA (htsResAred and htsResAox, respectively) were assigned from standard double and triple resonance NMR experiments using uniformly 15N- and 15N/13C-labeled protein samples. Chemical shifts were assigned for the entire polypeptide backbone except the His-tag for both states of htsResA; also not assigned were chemical shifts for residues S37, E75, E80, and F81 for htsResAox. Secondary structure elements were derived from an analysis of the backbone chemical shift assignments by using the program talos (22). For htsResAox the secondary structure elements assigned by NMR and from the x-ray structures are consistent. However, for htsResAred there are differences in the secondary structures assigned by NMR and x-ray crystallography, which are discussed in the next section.
Redox-State-Dependent Conformational Changes in ResA.
The solution properties of htsResAred and htsResAox were characterized by measuring 2D 1H/15N heteronuclear single quantum coherence (HSQC) spectra in the presence of DTT (htsResAred) and after full oxidation by either mimetic peptide or GSSG (htsResAox). Differences between the spectra of htsResAred and htsResAox clearly indicate significant backbone chemical shift changes distributed throughout the protein (Fig. 1). These indicate significant changes in the local environment around many backbone amides in htsResA, suggestive of widespread conformational changes.
Fig. 1.
Superimposed 1H/15N HSQC spectra of 1 mM 15N/13C-labeled htsResAred (black contours) and htsResAox (red contours) recorded in the presence of 10 mM DTT and after treatment with 20 mM GSSG, respectively, indicate widespread chemical shift changes.
Redox-coupled conformational changes have been proposed to be the mechanism by which ResA identifies its binding partner during its redox cycle (21). This proposal is based on conformational variations between the oxidized and reduced forms (Fig. 5b) localized to active-site helix (α1), the β7–α4 loop, and a cavity near the active site present on the surface of ResAred that is discussed later. However, significant differences are also observed in α3 and the N-terminal β hairpin (Fig. 5b). With an overall rms deviation of 0.73 Å between 135 Cα atoms of ResAred and ResAox all of these observed backbone differences may justifiably be viewed as an artifact related to crystallization, as previously suggested by Crow et al. (21) for the variations at α3 and the N-terminal β hairpin. However, the other changes are likely to be biologically relevant because the differences at α1 are a consequence of the formation of the disulfide bond, whereas the rearrangement of residues on the β7–α4 loop closes the cavity on the surface of ResAox (21). In addition to the biologically relevant, redox-coupled changes seen in the x-ray structures, NMR analysis suggests that upon oxidation of ResA some secondary structure elements might be shortened by one to three residues. In comparison, the hydrogen bonding pattern and length of secondary structure elements in the x-ray structures of both htsResAred and htsResAox are identical with one notable exception: the first four residues in helix 1 (α1) containing the active site C77 are in a 310 hydrogen bonding pattern (Fig. 5b) consistent with the unwinding found in the reduced form of human thioredoxin (23–25). It is possible that crystal packing prevents the variations in hydrogen bonding patterns consistent with the secondary and tertiary structural changes indicated by the substantial chemical shift changes observed by NMR analysis.
An analysis of oxidation-induced changes in 15N, 13C, and 1H chemical shifts allowed us to detail the structural changes in the htsResA backbone (Fig. 2a–d). Effects on tertiary structure are revealed by the Δδ(HN,N) analysis (Fig. 2 a and b) whereas influences on secondary structure are demonstrated by the ΔΔδox-red analysis (Fig. 2 c and d). There are substantial chemical shift changes to the htsResA backbone at regions removed by up to 29 Å from the active site, including residues on β1, β2, and β4 within the protein core. In contrast, a similar analysis of human thioredoxin indicated that conformational changes between the oxidized and reduced forms are localized to residues 27–39, which include the active site cysteines (C32 and C35) (23). Thus, upon oxidation, ResA exhibits more extensive chemical shift changes throughout the protein relative to those observed in human thioredoxin.
Fig. 2.
Residues affected by the redox state of htsResA. (a) Chemical shift changes of the assigned 1H/15N values for the backbone amide groups are plotted versus residue number. The color-coded bar denotes shift changes of ≤0.21 ppm (blue), 0.21–0.42 ppm (cyan), 0.42–0.63 ppm (lime-green), 0.63–0.84 ppm (green), 0.84–1.05 ppm (yellow), and ≥1.05 ppm (red) with the colored lines corresponding to the lower boundaries of each range. (b) The ribbon diagrams of htsResAox are color-coded by 1H/15N chemical shift changes according to the color-coded bar in a. Black denotes locations of unassigned or proline residues. (c) Chemical shift changes between htsResAred and htsResAox of the assigned 13C values for Cα and Cβ carbons are plotted versus residue number. The color-coded bar denotes shift changes of ≤0.51 ppm (blue), 0.51–1.02 ppm (cyan), 1.02–1.53 ppm (green), 1.53–2.04 ppm (yellow), and ≥2.04 ppm (red) with the colored lines corresponding to the lower boundaries of each range. (d) The ribbon diagrams of htsResAox are color-coded by 13C chemical shift changes according to the color-coded bar in c. Black denotes unassigned residues.
The largest backbone chemical shifts map to htsResA residues T72, W73, C74, E75, K79, Y117, D136, P139, L140, P141, T142, and T159, which line a groove found on the surface of ResAred, supporting the proposal that this is the binding surface for oxidized apocytochrome c (21). This surface of htsResA is structurally analogous to the substrate-interacting surface of thioredoxin (24–27).
Residues Involved in Substrate Specificity.
Because ResA exhibits conformational changes reflecting its redox state, it is important to identify residues responsible for regulating substrate interaction in both the reduced and oxidized states. These residues were identified from 1H/15N HSQC spectra measured while titrating increasing amounts of mimetic peptide, such that the observed chemical shift represented a population-weighted average of the chemical shifts of the free and complexed states. In physiological conditions, ResAred reduces the C-x-x-C-H motif disulfide of oxidized apocytochrome c to free cysteines. However, in vitro, it is technically impossible to simultaneously maintain the two interacting components in contrasting redox states. Therefore, the titration was performed either with both htsResA and the mimetic peptide reduced by 20 mM DTT or with both htsResA and the mimetic peptide in their oxidized forms. These complexes exhibited fast exchange on the NMR time scale. From these experiments, residues whose chemical shifts were affected by interaction with the mimetic peptide were identified (Fig. 6 a and b, which is published as supporting information on the PNAS web site) (28, 29). Notably, several peaks have hooked or bent trajectories, which is strongly suggestive of a three-state binding mechanism where each of two sites interacts with substrate at different affinities. Because these curved trajectories complicated the standard Δδ analysis for titrations, we used a cumulative shift analysis for these residues by summing the Δδ for subsequent points in the titration to obtain an overall Δδ. Although there is substantial overlap between the residues most influenced in the reduced and oxidized conditions, these residues map to two distinct areas of htsResA (Fig. 3a–d). With both peptide and htsResA in the reduced state the residues map to the proposed binding groove leading to the active site cysteines (Fig. 3b), whereas in the oxidized state the most highly perturbed residues localized toward the C termini of the strands constituting the central β-sheet on the opposite side of α1 toward α2, as well as along α3, adjacent to the active site (Fig. 3d). This pattern is consistent with the theory that conformational changes influence binding partner affinity.
Fig. 3.
htsResA residues influenced by titration of mimetic peptide in reducing (a and b) and oxidizing (c and d) conditions. (a) Chemical shift changes under reducing conditions from the 1H/15N HSQC spectra plotted versus residue position. The color-coded bar denotes shift changes of ≤0.0417 ppm (blue), 0.0417–0.0834 ppm (cyan), 0.0834–0.125 ppm (lime-green), 0.125–0.167 ppm (green), 0.167–0.208 ppm (yellow), and ≥0.208 ppm (red) with the dashed colored lines corresponding to the lower boundaries of each range. (b) The surface and ribbon representations of ResAred (PDB ID code 1SU9) are color-coded by chemical shift ranges according to the color-coded bar in a. The active-site cysteines and the proposed histidine recognition pocket are indicated. The carbonyl group of Val-156 (red) lines the histidine pocket and is clearly influenced during the titration. (c) Chemical shift changes from the 1H/15N HSQC spectra recorded under oxidizing conditions plotted versus residue position. The color-coded bar denotes shift changes ≤0.0417 ppm (blue), 0.0417–0.0834 ppm (cyan), 0.0834–0.125 ppm (lime-green), 0.125–0.167 ppm (green), 0.167–0.208 ppm (yellow), and ≥0.208 ppm (red) with the dashed colored lines denoting the lower boundaries of each range. (d) The surface and ribbon diagrams of htsResAox are color-coded by chemical shift ranges according to the color-coded bar in c. The data measured under oxidizing conditions indicate a separate binding surface toward the top of htsResA as compared with the data measured under reducing conditions.
The structures of human thioredoxin complexed with peptides from NFκB clearly show that the orientation of the interacting sequence is not important for a nonspecific thiol-disulfide oxidoreductase (24, 25). In contrast, it has been suggested that a deeper pocket within the proposed binding groove on the surface of ResAred recognizes the histidine of the C-x-x-C-H motif (21). Our titration data indicate that the environment of residue V156, whose carbonyl oxygen lines the bottom of this pocket, is dramatically influenced by the presence of peptide in reducing conditions (Fig. 3 a and b) but is significantly less affected in the oxidized conditions (Fig. 3 c and d), experimentally verifying that this pocket is involved in apocytochrome c recognition. Thus, ResAred appears to use this redox-state-dependent surface feature to recognize and bind oxidized apocytochrome c in a specific orientation.
Substrate Specificity Dictates Rate of Redox Changes in ResA.
The specificity of htsResA was estimated from a comparison of the rates of conversion of htsResAred to htsResAox, measured by 1H/15N HSQC experiments using the oxidized mimetic peptide and GSSG. Although the conversion from the reduced to the oxidized state of htsResA was easily observed in all of the 1H/15N HSQC experiments, technical limitations prevented a rate constant analysis of every peak. A common subset of 31 1H/15N HSQC peaks were analyzed for their rates of conversion by plotting each peak’s normalized intensity versus time (Fig. 7, which is published as supporting information on the PNAS web site). Rate constants for each peak were derived by fitting the decay of intensity for peaks corresponding to the reduced state to a first order exponential equation. Overall mean rates for each reaction condition were obtained by inputting the rate constants for each of the 31 peaks into a 10% trimmed mean calculation (Table 1). A comparison of these mean rates indicated that fully reduced 15N-labeled htsResA was converted readily by both substrates, and the conversion was limited by the concentration of substrate. At all concentrations the rate of conversion by the mimetic peptide was greater than that for GSSG (Table 1). This rate enhancement could be a consequence of either of two possibilities. First, the midpoint potential of the mimetic peptide may be significantly more positive than that of glutathione, and the larger difference in midpoint potentials may increase the rate of the reaction. The second possibility is that ResA may use the C-x-x-C-H motif for substrate recognition, which enhances the rate of conversion by either enhancing binding affinity or imposing a preferred orientation for electron transfer.
Table 1.
Mean rate constants for conversion of htsResAred to htsResAox
| Concentration | Peptide rate, min−1 | GSSG rate, min−1 |
|---|---|---|
| 1 mM | 0.0108 ± 0.0012 | 0.00725 ± 0.0005 |
| 250 μM | 0.0036 ± 0.0002 | 0.0015 ± 0.0003 |
| 62.5 μM | 0.00186 ± 0.0003 | N.D. |
N.D., not determined.
To distinguish between these two possibilities, we compared the midpoint potential (Em) of the mimetic peptide with that of glutathione and DTT, all of which oxidize ResA (Em = −345 mV). Strikingly, the midpoint potential of the mimetic peptide (Em = −332 mV), determined from the fluorescence of the two tyrosines in the peptide (Fig. 4), was more negative than the established midpoint potential of glutathione (Em = −245 mV) (30). Furthermore, the rate of conversion of ResA by oxidized DTT, whose midpoint potential (Em = −330 mV) is similar to that of the mimetic peptide, was dramatically slowed, and a clear rate constant was not obtained (data not shown). This analysis indicates that substrate-specific recognition, rather than a difference in midpoint potentials, dictates the peptide-mediated, enhanced rate of conversion between htsResAred and htsResAox. It is likely that enhanced binding affinity between ResA and substrate, analogous to KM for enzyme reactions, or binding in a preferred conformation that accelerates the electron transfer, similar to kcat, is the primary accelerating factor for this conversion. Despite the nonspecific nature of most thioredoxin-like proteins, our data finally confirm ResA as a substrate-specific thiol-disulfide oxidoreductase by biochemical characterization.
Fig. 4.
Determination of the midpoint potential of the mimetic peptide at pH 6.9. The solid line represents a fit to the Nernst equation, n = 2. This gave an Em value of −332 mV.
Theory of an Electron Control Point.
The use of thioredoxin-derived reducing equivalents is a fundamental requirement for a variety of bacterial extracellular and periplasmic activities, such as the activities of the disulfide bond forming (Dsb) system, the crosslinking of spore coat proteins, and CCM. These processes cannot be controlled by concentration dependence alone; and, in the case of CCM, genetic elimination of the terminal thioredoxin-like protein has been shown to specifically abolish maturation of c-type cytochromes for a number of systems (5, 14, 31, 32). Crow et al. (21) presented a structure-based theory that ResA (system II CCM) uses redox-coupled conformational changes to select its substrate. Here we have presented further evidence showing that ResA does indeed undergo significant conformational shifts between the reduced and oxidized states, and these changes may be more extensive than what is observed in the x-ray structures. Additionally, we have shown that ResAred specifically recognizes a model peptide designed to mimic the oxidized form of apocytochrome c. These results suggest a redox cycle in which ResA, the terminal thioredoxin-like protein in B. subtilis CCM, acts as a control point that siphons off the electrons needed for CCM only in response to the direct availability of apocytochrome c. Presumably, ResAox is regenerated to the reduced state by nonspecific interactions with the integral membrane thiol-disulfide oxidoreductase, CcdA. This idea is further supported by the observation that CcdA regenerates not only ResA, but also StoA, a protein involved in spore coat protein crosslinking (13, 16, 17, 33). ResAred then specifically binds oxidized apocytochrome c, ensuring that electrons are not lost to random disulfides in the extracellular environment. This redox-state-dependent selection of interaction partners by ResA may be a general mechanism by which specific thiol-disulfide oxidoreductases act as the control point for directing electrons into protein maturation pathways. Finally, the experiments presented here provide a reliable assay to explore this possibility in other systems that exhibit thioredoxin partner specificity.
Materials and Methods
Protein Expression and Purification.
The His-tagged, soluble domain, comprising residues 37–179, of B. subtilis ResA (htsResA) was cloned, expressed, and purified as described in ref. 14 with the following modifications. Escherichia coli BL21(DE3) or B834(DE3) cells were grown at 37°C before overnight induction at 20°C. For structure determination, selenomethionine htsResA was expressed in B834(DE3) cells grown in minimal media supplemented with l-selenomethionine. Uniformly isotopically labeled htsResA used for NMR was expressed in BL21(DE3) cells grown in M9 minimal media supplemented with 1 g/liter U-15N ammonium chloride and 3 g/liter U-13C glucose as needed. To fully oxidize the active site cysteines, htsResA was incubated with 20 mM GSSG for 8–12 h before desalting by using a HiPrep 26/10 Desalting column (Amersham Pharmacia Biotech).
Crystallization and Structure Determination.
Crystals were grown by using the hanging drop vapor diffusion method. A drop containing a 1:1 ratio of protein solution (13 mg/ml htsResA/25 mM Hepes, pH 7.2/150 mM NaCl/1 mM EDTA) to reservoir solution [100 mM Mes, pH 6.8/2.2 M (NH4)2SO4/2–5 mM l-Cys] was suspended over a 1-ml reservoir. The crystals belong to space group P65 with cell dimensions a = b = 61.1 Å and c = 166.8 Å, and two htsResA monomers per asymmetric unit (VM = 2.64 Å3/Da).
Diffraction data were collected from crystals frozen in 20% vol/vol glycerol at cryogenic conditions (100 K). High-resolution diffraction data were collected at the Advanced Photon Source (Argonne National Laboratory, Argonne, IL) synchrotron SBC beamline BM19. For structure solution, a modified inverse-beam procedure was used. A single pass of 120° of diffraction data were collected at each of three wavelengths corresponding to 0.9807 Å (absorption peak), 0.9808 Å (inflection point), and 0.9537 Å (high-energy remote). This was repeated after a 180° rotation about the oscillation axis for a total of 720° of data. For model building and refinement, a two-pass high-resolution data set was collected from another crystal at a wavelength of 0.9641 Å. The data were analyzed and reduced to averaged intensities by using hkl2000 (34, 35). Intensities were converted to structure factor amplitudes by using programs from the ccp4 suite (36, 37). Data collection statistics are summarized in Table 3, which is published as supporting information on the PNAS web site.
The programs solve and resolve (38, 39) were used for merging the three wavelength multiwavelength anomalous diffraction data sets, phase determination, phase improvement, and automated model building, using the data acquired at 0.9537 Å as the reference set. All 10 Se sites expected were located and their positional and scattering parameters refined before initial phase calculation (overall figure of merit of 0.73). These phases and structure factor amplitudes of the reference data set were used to calculate an initial electron density map to 1.8 Å resolution.
Model Building and Refinement.
The program o (40) was used to display electron density maps and to construct, revise, and analyze atomic models. An initial model was constructed with reference maps to 1.8 Å resolution. Model residue numbers are consistent with the N-terminal start methionine of the full-length protein being residue one. refmac5 (41) from the ccp4 suite (36, 37) was used with a maximum-likelihood target function for automated refinement against the 1.4 Å resolution data acquired at 0.9641 Å. The addition of ordered solvent molecules, and protein components in alternative conformations, completed the refinement with R = 16.2% and Rfree = 17.5%. Additional model statistics are provided in Table 4, which is published as supporting information on the PNAS web site. The refined model of htsResAox and the structure factor amplitudes are available in the Protein Data Bank (PDB ID code 2F9S).
NMR Spectroscopy.
All NMR data were recorded at 35°C with Varian Inova 500- and 600-MHz spectrometers and the programs nmrpipe for data processing (42) and nmrview for data analysis (43). Backbone 15N, 13C, and 1H chemical shift assignments were made by using standard methods and 3D HNCACB, CBCA(CO)NH, HNCO, and HN(CA)CO data sets (44). To study htsResA–substrate interactions, a peptide corresponding to residues 53–73 of B. subtilis cytochrome c550 was chemically synthesized. The reaction kinetics of htsResA with various substrates were determined by recording 1H/15N HSQC spectra sequentially obtained while 250 μM htsResA reacted with either the mimetic peptide (62.5 μM, 250 μM, and 1 mM), GSSG (250 μM and 1 mM), or oxidized DTT (1 mM). Peak intensities for 31 selected peaks were followed and plotted versus time. These intensities were fit to a first-order exponential equation (y = A·exp(−xB) + C) in nmrview to obtain rate constants for individual sites, and these were subjected to a 10% trimmed mean analysis to obtain a final global rate constant. Titrations of fully oxidized and reduced samples were recorded as 1H/15N HSQC spectra after stepwise addition of the mimetic peptide substrate to U-15N-labeled htsResA. Titration analysis was performed by using nmrview to monitor the effect of substrate addition on the change of chemical shift for various peaks (Eq. 1):
A cumulative chemical shift was calculated based on the sum of the distances between successive peaks while a total chemical shift was calculated by the distance between peaks at the starting peptide concentration and the positions of the corresponding peaks at the final peptide concentration. Secondary structure elements for the oxidized and reduced species were predicted by using the φ and ψ dihedral angles generated by an analysis of 15N, 13C, and 1H chemical shifts using talos (22). Additionally, oxidation-induced tertiary structure changes were estimated by evaluating chemical shift changes in the 1H/15N HSQC spectra using Eq. 1, whereas changes in secondary structure were estimated by evaluating the deviation of backbone 13Cα and 13Cβ chemical shifts from random coil values (Eq. 2):
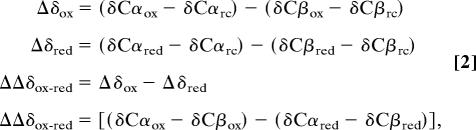 |
where δCαox is the observed chemical shift value for a given Cα in oxidized htsResA, δCαred is the observed chemical shift for the same atom in reduced htsResA, and δCαrc is the literature value for the equivalent atom in a random coil conformation (45). Thus, Δδox and Δδred provide information similar to the well established 13C chemical shift index method for determining secondary structure (46), and ΔΔδox-red corresponds to the effect that oxidation has on these values.
Determination of Redox Potentials.
The redox potential of the mimetic peptide was determined by using a 50 μM solution of fully oxidized peptide in deoxygenated buffer (25 mM PBS, pH 6.9). The peptide was incubated for 3 h at 25°C at a range of redox potentials generated by varying ratios of oxidized and reduced DTT, at a total concentration of 5 mM. Fluorescence emission intensity was measured by exciting at 280 nm and monitoring emission at 300–420 nm by using a PerkinElmer LS55 fluorimeter with excitation and emission slits set to 2.5 nm. Data were fit to the Nernst equation described by Erlendsson et al. (14).
Supplementary Material
Acknowledgments
We thank S. Sinha for critical reading of the manuscript. Use of the Argonne National Laboratory Structural Biology Center beamlines at the Advanced Photon Source was supported by the U.S. Department of Energy, Office of Energy Research, under Contract W-31-109-ENG-38. Q.W. and P.J.A.E. were supported by Robert A. Welch Foundation Grant I-1424 (to K.H.G.). J.D. is an Investigator of the Howard Hughes Medical Institute. Additional support was provided by Robert A. Welch Foundation Grant I-1185 (to J.D.).
Abbreviations
- CCM
cytochrome c maturation
- GSSG
oxidized glutathione
- HSQC
heteronuclear single quantum coherence.
Footnotes
Conflict of interest statement: No conflicts declared.
Data deposition: The x-ray crystallographic structure factors and coordinates for the refined model have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 2F9S).
References
- 1.Pettigrew G. W., Moore G. R. Cytochromes c: Biological Aspects. Berlin: Springer; 1987. [Google Scholar]
- 2.Yang J., Liu X., Bhalla K., Kim C. N., Ibrado A. M., Cai J., Peng T. I., Jones D. P., Wang X. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 3.Kluck R. M., Bossy-Wetzel E., Green D. R., Newmeyer D. D. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 4.Kranz R., Lill R., Goldman B., Bonnard G., Merchant S. Mol. Microbiol. 1998;29:383–396. doi: 10.1046/j.1365-2958.1998.00869.x. [DOI] [PubMed] [Google Scholar]
- 5.Biel S. W., Biel A. J. J. Bacteriol. 1990;172:1321–1326. doi: 10.1128/jb.172.3.1321-1326.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldman B. S., Beckman D. L., Bali A., Monika E. M., Gabbert K. K., Kranz R. G. J. Mol. Biol. 1997;268:724–738. doi: 10.1006/jmbi.1997.0992. [DOI] [PubMed] [Google Scholar]
- 7.Thöny-Meyer L. Biochem. Soc. Trans. 2002;30:633–638. doi: 10.1042/bst0300633. [DOI] [PubMed] [Google Scholar]
- 8.Page M. D., Sambongi Y., Ferguson S. J. Trends Biochem. Sci. 1998;23:103–108. doi: 10.1016/s0968-0004(98)01173-6. [DOI] [PubMed] [Google Scholar]
- 9.Kadokura H., Katzen F., Beckwith J. Annu. Rev. Biochem. 2003;72:111–135. doi: 10.1146/annurev.biochem.72.121801.161459. [DOI] [PubMed] [Google Scholar]
- 10.Collet J. F., Bardwell J. C. Mol. Microbiol. 2002;44:1–8. doi: 10.1046/j.1365-2958.2002.02851.x. [DOI] [PubMed] [Google Scholar]
- 11.Fabianek R. A., Hennecke H., Thöny-Meyer L. FEMS Microbiol. Rev. 2000;24:303–316. doi: 10.1111/j.1574-6976.2000.tb00544.x. [DOI] [PubMed] [Google Scholar]
- 12.Frand A. R., Cuozzo J. W., Kaiser C. A. Trends Cell Biol. 2000;10:203–210. doi: 10.1016/s0962-8924(00)01745-1. [DOI] [PubMed] [Google Scholar]
- 13.Erlendsson L. S., Hederstedt L. J. Bacteriol. 2002;184:1423–1429. doi: 10.1128/JB.184.5.1423-1429.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erlendsson L. S., Acheson R. M., Hederstedt L., Le Brun N. E. J. Biol. Chem. 2003;278:17852–17858. doi: 10.1074/jbc.M300103200. [DOI] [PubMed] [Google Scholar]
- 15.Katzen F., Beckwith J. Cell. 2000;103:769–779. doi: 10.1016/s0092-8674(00)00180-x. [DOI] [PubMed] [Google Scholar]
- 16.Schiött T., Throne-Holst M., Hederstedt L. J. Bacteriol. 1997;179:4523–4529. doi: 10.1128/jb.179.14.4523-4529.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schiött T., von Wachenfeldt C., Hederstedt L. J. Bacteriol. 1997;179:1962–1973. doi: 10.1128/jb.179.6.1962-1973.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Capitani G., Rossmann R., Sargent D. F., Grutter M. G., Richmond T. J., Hennecke H. J. Mol. Biol. 2001;311:1037–1048. doi: 10.1006/jmbi.2001.4913. [DOI] [PubMed] [Google Scholar]
- 19.Edeling M. A., Guddat L. W., Fabianek R. A., Thöny-Meyer L., Martin J. L. Structure (Cambridge, Mass.) 2002;10:973–979. doi: 10.1016/s0969-2126(02)00794-3. [DOI] [PubMed] [Google Scholar]
- 20.Goulding C. W., Apostol M. I., Gleiter S., Parseghian A., Bardwell J., Gennaro M., Eisenberg D. J. Biol. Chem. 2004;279:3516–3524. doi: 10.1074/jbc.M311833200. [DOI] [PubMed] [Google Scholar]
- 21.Crow A., Acheson R. M., Le Brun N. E., Oubrie A. J. Biol. Chem. 2004;279:23654–23660. doi: 10.1074/jbc.M402823200. [DOI] [PubMed] [Google Scholar]
- 22.Cornilescu G., Delaglio F., Bax A. J. Biomol. NMR. 1999;13:289–302. doi: 10.1023/a:1008392405740. [DOI] [PubMed] [Google Scholar]
- 23.Qin J., Clore G. M., Gronenborn A. M. Structure. 1994;2:503–522. doi: 10.1016/s0969-2126(00)00051-4. [DOI] [PubMed] [Google Scholar]
- 24.Qin J., Clore G. M., Kennedy W. M., Huth J. R., Gronenborn A. M. Structure. 1995;3:289–297. doi: 10.1016/s0969-2126(01)00159-9. [DOI] [PubMed] [Google Scholar]
- 25.Qin J., Clore G. M., Gronenborn A. M. Biochemistry. 1996;35:7–13. doi: 10.1021/bi952299h. [DOI] [PubMed] [Google Scholar]
- 26.Doublie S., Tabor S., Long A. M., Richardson C. C., Ellenberger T. Nature. 1998;391:251–258. doi: 10.1038/34593. [DOI] [PubMed] [Google Scholar]
- 27.Lennon B. W., Williams C. H., Jr, Ludwig M. L. Science. 2000;289:1190–1194. doi: 10.1126/science.289.5482.1190. [DOI] [PubMed] [Google Scholar]
- 28.Amezcua C. A., Harper S. M., Rutter J., Gardner K. H. Structure (Cambridge, Mass.) 2002;10:1349–1361. doi: 10.1016/s0969-2126(02)00857-2. [DOI] [PubMed] [Google Scholar]
- 29.Erbel P. J., Card P. B., Karakuzu O., Bruick R. K., Gardner K. H. Proc. Natl. Acad. Sci. USA. 2003;100:15504–15509. doi: 10.1073/pnas.2533374100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salamon Z., Gleason F. K., Tollin G. Arch. Biochem. Biophys. 1992;299:193–198. doi: 10.1016/0003-9861(92)90262-u. [DOI] [PubMed] [Google Scholar]
- 31.Monika E. M., Goldman B. S., Beckman D. L., Kranz R. G. J. Mol. Biol. 1997;271:679–692. doi: 10.1006/jmbi.1997.1227. [DOI] [PubMed] [Google Scholar]
- 32.Beckman D. L., Trawick D. R., Kranz R. G. Genes Dev. 1992;6:268–283. doi: 10.1101/gad.6.2.268. [DOI] [PubMed] [Google Scholar]
- 33.Schiött T., Hederstedt L. J. Bacteriol. 2000;182:2845–2854. doi: 10.1128/jb.182.10.2845-2854.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Otwinowski Z., Minor W. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 35.Otwinowski Z., Minor W. In: International Tables for Crystallography. Rossmann M. G., editor. Vol. F. Dordrecht, The Netherlands: Kluwer Academic; 2001. pp. 226–235. [Google Scholar]
- 36.Collaborative Computational Project, Number 4. Acta Crystallogr. D. 1994;50:760–763. [Google Scholar]
- 37.Potterton E., Briggs P., Turkenburg M., Dodson E. Acta Crystallogr. D. 2003;59:1131–1137. doi: 10.1107/s0907444903008126. [DOI] [PubMed] [Google Scholar]
- 38.Terwilliger T. C., Berendzen J. Acta Crystallogr. D. 1999;55:849–861. doi: 10.1107/S0907444999000839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Terwilliger T. C. Acta Crystallogr. D. 2000;56:965–972. doi: 10.1107/S0907444900005072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones T. A., Zou J. Y., Cowan S. W., Kjeldgaard Acta Crystallogr. A. 1991;47:110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- 41.Murshudov G. N., Vagin A. A., Dodson E. J. Acta Crystallogr. D. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 42.Delaglio F., Grzesiek S., Vuister G. W., Zhu G., Pfeifer J., Bax A. J. Biomol. NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 43.Johnson B. A., Blevins R. A. J. Biomol. NMR. 1994;4:603–614. doi: 10.1007/BF00404272. [DOI] [PubMed] [Google Scholar]
- 44.Cavanagh J., Fairbrother W. J., Palmer A. G., Skelton N. J. Protein NMR Spectroscopy: Principles and Practice. San Diego: Academic; 1995. [Google Scholar]
- 45.Wishart D. S., Sykes B. D. J. Biomol. NMR. 1994;4:171–180. doi: 10.1007/BF00175245. [DOI] [PubMed] [Google Scholar]
- 46.Spera S., Bax A. J. Am. Chem. Soc. 1991;113:5490–5492. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






