Abstract
The acquisition of bipedal locomotion is an important aspect of gross motor development that ultimately impacts the cognition of young children. Evidence for associations between nutrition-related variables and walking acquisition exist; however, questions about the importance of weight-for-length and dietary factors and the independent contribution of anemia and growth on walking remain. We examined the effect of nutritional factors on the acquisition of walking in a cross-sectional cohort of 485 4- to 17-mo Nepali children adjusting for age, sex, caste, and socio-economic status (SES). Participants were identified from census data collected in one village development committee (VDC) in Sarlahi District and enrolled in a cross-sectional, community-based study between January and March 2002. Hemoglobin and erythrocyte protoporphyrin (EP) were measured at baseline using a heel prick technique. The mean hemoglobin concentration was 101 ± 12.5 g/L. 57 % were anemic (hemoglobin < 105 g/L), 2.1% were severely anemic (hemoglobin < 7.0 g/L), and 43% of the children had iron deficiency anemia (hemoglobin < 105 g/L; EP > 90 μmol/mol heme). Growth was delayed; 33.5% were stunted and 20.4% were wasted. Multivariate logistic models that controlled for age, sex, caste, and SES revealed that children with higher length-for-age and weight-for-length Z-scores, no anemia, and meat consumption walked at an earlier age than children with lower scores, anemia, and no meat consumption. We conclude that growth, anemia, and diet are independently associated with delays in the onset of bipedal locomotion among young Nepali children.
Index words: motor milestones, infant, anemia, growth, Nepal
INTRODUCTION
Gross motor development is an important aspect of the development of young children. Through the attainment of specific motor skills, children begin to explore their environments and engage in new experiences that promote learning and development of other component processes (1). Tracking motor milestone acquisition has informed researchers about the motor development proficiency of young children. A natural progression of motor milestone development has been documented (2, 3). The time that children spend in any given milestone or take to achieve some of the more advanced milestones (e.g., walking without support) is variable and often depends on whether they achieved a preliminary milestone (4). Advancement to locomotion and beyond is dependent upon the young child’s musculoskeletal system, body size, mass, and proportions (5–9).
Growth faltering is prevalent in developing countries (10) where children are susceptible to infection and malnutrition (11,12). Globally, an estimated 43% of children less than 4 years of age have been found to be anemic (13). Children less than 2 years of age are particularly vulnerable due to their increased demand for nutrients as they transition from breastfeeding exclusively to consuming complementary foods (14). Limited variety of complementary foods has been associated with low nutrient density adequacy of the foods, which may cause malnutrition and developmental delays (15). Animal protein intake was positively associated with earlier walking acquisition among Guatemalan infants (16). Anemia and iron deficiency were independently associated with walking in a cohort of Zanzibari infants (17). Delays in motor milestone acquisition, including walking, were found among cohorts of stunted, underweight Indonesian (18), Zanzibari (17), and Guatemalan infants (16), and among stunted and wasted Pakistani infants (19).
Evidence for an ordering of motor milestones and for clear associations between nutrition-related variables and infant motor development exist; however, several controversies remain: 1) Is there an ordinal relationship between motor milestones?; 2) Is linear growth the salient variable or is wasting as important in walking acquisition?; 3) Do anemia and growth have independent effects on walking in populations of children outside of Zanzibar (17)?; 4) Are dietary factors merely a proxy for anemia?
We identified factors that were associated with the motor milestone acquisition of a nutritionally-at-risk population-based sample of 4- to 17-mo children living in the South Central Terai region of Nepal and attempted to address the questions above. We hypothesized that anthropometric indicators, hemoglobin concentration, and diet would be associated with walking in our Nepali cohort, after controlling for age.
SUBJECTS AND METHODS
Study population
This study was a cross-sectional, community-based investigation of baseline characteristics of children enrolled in a sub-study of a randomized, placebo-controlled clinical trial of zinc and/or iron-folic acid supplementation on childhood mortality, morbidity, growth, and development (trial registered at www.clinicaltrials.gov NCT0109551) between January and March 2002 that occurred in the lowland Terai region of south central Nepal in Sarlahi District, which borders Northern India. 569 eligible children 4- to 17-mo of age were identified in one village development committee (VDC) from census data that were collected by study personnel between December 2000 and March 2001. 494 of these children had their blood drawn and growth indices measured. 485 of the 494 children had complete motor milestones data. 315 of the 485 children were between 8- and 17- mo of age and were therefore eligible for the walking analysis. One child with paralysis was excluded from participation. The study was approved by both the Johns Hopkins University Committee on Human Research and the Nepal Health Research Council.
Motor development was evaluated using a 14-item, prospective, ordinal pictorial motor milestones scale. Fieldworkers visited the study children’s homes and asked each child’s mother whether the child had achieved any of the 14 motor milestones prior to the visit. They recorded the most advanced milestone reported. The measure was adapted from a 17-item scale that was used in Indonesia to pinpoint the acquisition of gross motor skills in 12- to 30-mo children (20,21), and modeled after an earlier instrument that proposed a sequence for the development of bipedal locomotion (8). The 17-item scale was expanded downwards to include appropriate items for our younger sample and upwards to avoid a ceiling effect using items from the Bayley Psychomotor Index (22). Pilot testing verified the appropriate ordering of the milestones. Inter-rater reliability and internal validity measures conducted in the field resulted in high correlations between: 1) the mothers’ report to the fieldworker and to a supervisor who repeated the questioning within two weeks of the initial report (Cronbach’s α = 0.97), 2) the mothers’ report to the fieldworker and the supervisor’s observation of the child performing the reported milestone during the supervisor’s visit (Cronbach’s α = 0.98), and 3) the mothers’ report to the supervisor and the supervisor’s observation of the child performing the reported milestone (Cronbach’s α = 0.99). The 14 items used for this study and their corresponding definitions are displayed elsewhere (17).
Blood was collected using a heel prick method to extract three drops of blood from each child. The first was wiped away, the second and third were used for testing zinc protoporphyrin and hemoglobin respectively with a Hemoglobin Photometer (HemoCue AB; Andelholm, Sweden) and a Hematofluorometer (AVIV Biomedical Inc.; New Jersey). Fieldworkers performed daily quality control assessments with a standardized microcuvette and cover glass to test the reliability of the HemoCue and AVIV machines.
Anthropometric measures included weight, recumbent length, mid-upper arm circumference (MUAC)7, and head circumference. Trained fieldworkers measured the infants in the presence of their family members. Each indicator was measured in triplicate. Weight was measured to the nearest 0.1 kilogram using a SECA floor scale (Seca Corporation; Hanover, Maryland). Recumbent length, MUAC, and head circumference were measured to the 0.1 centimeter using a Shorr Board for length (Shorr Productions; Olney, Maryland), a MUAC tape, and a Ross Head Circumference Tape (Holtain Ltd.; United Kingdom).
Questions about infant feeding were asked of the child’s mother by fieldworkers in the child’s home. The fieldworkers read a list of foods and asked whether the mother had fed her child the foods in the previous 7 days. The most common foods and food categories fed to young children were included in the list: non-human milk, rice, bread, biscuits, greens and vegetables, lentils, fruit, egg, and meat. The questionnaire was developed from formative research where mothers were asked to list all foods they fed to infants. Foods that were listed by more than one mother were included in the questionnaire.
Fieldworkers recorded the presence of material assets, house construction, and water source in the child’s home prior to the start of data collection. Basic demographic information including the child’s sex, caste, and birth date were also recorded. When caregivers were unable to remember the exact mo and day of the child’s birth, local calendars with the lunar cycle and a list of local festivals were used to assist in recall.
Data analysis
Data were entered into SQL Server 7.0 and analyzed using SPSS 12.0. Z-scores were calculated for the length-for-age, weight-for-height, and weight-for-age anthropometric measures using the 1978 CDC/WHO growth reference in EpiInfo Version 6.0 (Centers for Disease Control; Atlanta, GA). Stunting, wasting, and underweight were defined respectively as length-for-age, length-for-weight, and weight-for-age < -2 Z-scores. Mean head circumference and MUAC were calculated for the analysis. Eight of the 494 children who had motor milestone and hemoglobin data were excluded from the analysis because their reported motor milestones were found to be implausible for children their age. One child had missing data.
The definition of anemia used in this analysis was a hemoglobin concentration < 105 g/L. While this number is less than 110 g/L, the cut-off proposed by the World Health Organization (WHO) and the Centers for Disease Control (CDC) for children 6 mo to 5 years, it reflects the only reference value derived from iron supplementation data that were collected from breast-fed infants (23). Iron-deficiency anemia was defined as anemia with an erythrocyte protoporphyrin (EP) value > 90 qmol/mol heme (23).
A scale was created as a means of summarizing socio-economic status (SES). Twelve of the 17 related items were retained for the scale. These included the presence of the following: having a household latrine, servant, cattle, bicycle, radio, farmable land, home garden plot, second floor on the house, roof, TV, electricity in the house, and bullock cart. Principal components analysis was used to extract the factors for the scale (24). The most comprehensive factor with the largest eigenvalue > 1 was selected for reliability analysis. Cronbach’s α (25) was used to assess the internal consistency of the selected items. The reliability of the SES scale was good (α = 0.78). A higher score on the SES scale reflected a greater number of possessions.
Age of attainment for each milestone was calculated from maternal report and presented as a whole number (i.e., children between 8.0 and 8.11 mo of age were considered to be 8 mo). Children 8- to 17-mo of age (n=485) were classified as walkers or non-walkers for the multivariate analysis (17). The ages reflect the range of children reported to be able to walk without support and include all children within each age mo. Walkers had a highest attained motor milestone of walk without support (walk 2), run, jump, or stand on 1 foot. Non-walkers were able to stand with or without support (stand 1 and stand 2) or walk with support (walk 1), but not without support.
An exploratory analysis was performed on descriptive characteristics of the study sample at baseline. Dichotomous relations between non-walkers and walkers and sex, age, caste, SES, several anthropometric indices, dietary diversity, animal source foods, hemoglobin, anemia, and EP were examined. Means of continuous variables and proportions of categorical variables were analyzed using t- and Chi-Square tests.
Multivariate models were created to test the predictive effect of (1) length-for-age, weight-for-length, and anemia (hemoglobin < 105 g/L) and (2) length-for-age, weight-for-length, anemia, and meat intake on walking among the Nepali cohort. The independent and joint effects of the anthropometric indicators, anemia, and diet on walking acquisition were examined.
RESULTS
Among the 485 children, there were as many boys as there were girls who ranged in age from 4- to 17-mo (Table 1). They were predominately Hindu (84%), with the remaining being Muslim (16%). They owned few possessions. A third were stunted (33.7%), a fifth were wasted (20.6%), and over half were underweight (52.7%). Hemoglobin concentration was below both the recommended cutoffs of 105 (23) and 110 g/L (14) for this age group. Anemia was prevalent, but not very severe: 2.1% of the children had a hemoglobin concentration < 70 g/L.
Table 1.
Characteristics of the study sample1
| Characteristics | n = 485 | |
|---|---|---|
| Female | 241 (49.7) | |
| Age (mo) | 10.8 ± 4.0 | |
| High-caste Hindu : Brahmins and Chhetris | 45 (9.3) | |
| Low-caste Hindu: | ||
| Vaiysha | 300 (61.9) | |
| Shudra | 63 (13.0) | |
| Muslim | 77 (15.9) | |
| Low SES (< 4 possessions)2 | 228 (47.5) | |
| Length-for-age Z-score3 | −1.6 ± 1.1 | |
| Weight-for-length Z-score3 | −1.2 ± 0.9 | |
| Weight-for-age Z-score | −2.0 ± 1.0 | |
| Hemoglobin (g/L) | 101.3 ± 12.5 | |
| Anemia (Hemoglobin < 105 g/L) | 293 (60.4) | |
| Erythrocyte protoporphyrin (umol/mole heme)4 | 129.5 ± 90.0 | |
| Iron deficiency anemia45 | 207 (43.0) | |
| Consumed animal source foods4 | 244 (50.7) | |
| Consumed meat4 | 124 (25.8) | |
Values are n (%) or means ± SD;
SES=socio-economic status, n=480;
n=494;
n=481;
Hemoglobin<105 g/L & Erythrocyte protoporphyrin>90 Qmol/mole heme
Half the sample could sit without support by 6 mo, crawl by 9 mo, walk with support by 11 mo, and walk without support by 14 mo (Figure 1). When the median age in mo for attainment of specific motor milestones for our Nepali sample was plotted against data from a British (26), an American (27), an Indonesian (18), and a Zanzibari sample (17), Nepali children were found to attain milestones about 1.5 mo later than children from Western populations and 1 mo earlier than children from Indonesia and Zanzibar. The Nepali data revealed a developmental progression of motor milestone acquisition that was present in the other samples (Figure 1).
Figure 1.
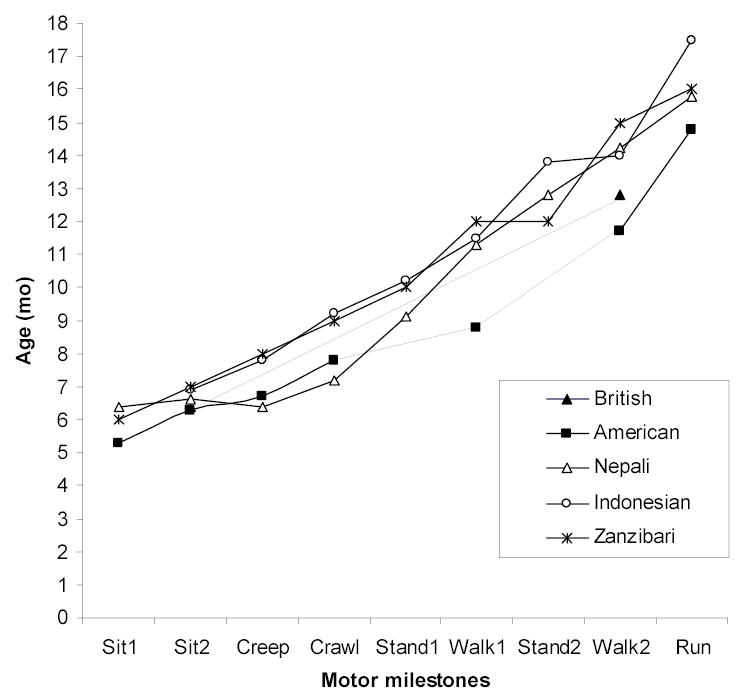
Motor milestone attainment by age among different cohorts of children. Age values are means for the American sample (27) and medians for the Nepali (present study), Zanzabari (17), Indonesian (18), and British (26) samples.
In bivariate analyses, walkers (n=162) and non-walkers (n=153) differed significantly in age, weight-for-age, and diet (Table 2). Compared with non-walkers, walkers were older, less underweight, and ate more animal source foods. Walkers and non-walkers did not differ in the other characteristics.
Table 2.
Walking status of Nepali children by age, weight, and diet (n=315)
| Characteristics | Walkers (n= 162) | Non-walkers (n= 153) |
|---|---|---|
| Age (mo)1** | 14.4 ± 2.1 | 11.9 ± 2.3 |
| Weight-for-age Z-score1* | −2.2 ± 0.9 | −2.4 ± 0.9 |
| Diet2 | ||
| Consume animal source foods3** | 123 (76.4) | 85 (55.6) |
| Consume meat** | 83 (53.4) | 35 (22.9) |
Values represent means ± SD;
Values represent n (%), n=314 overall, n=161 walkers;
non-human breastmilk, eggs, and/or meat;
p<0.05;
p<0.001
In multivariate regression models, age, length-for-age, weight-for-length, anemia (dichotomous variable; hemoglobin < 105 g/L), and meat consumption were significant predictors of walking in the Nepali cohort (Table 3). Models with hemoglobin concentration inserted as a continuous variable and iron deficiency anemia (hemoglobin concentration < 105 g/L and EP > 90 μmol/mol heme) inserted as a dichotomous variable were tested independently in place of anemia, but were not found to be significant. An alternate model with animal source foods as a dichotomous variable was tested and found to be significant, but is not presented because model 2 with meat was found to be stronger. Caste was significant in Model 1, but not in Model 2. The odds that a low caste Hindu and a Muslim child compared with a high caste Hindu -- Brahmin or Chhetri–child had achieved walking were over 3 times greater. The length-for-age, weight-for-length, and anemia odds ratios were similar for each of the two models, revealing an independent association between the anthropometric indicators, anemia, and walking. The adjusted log odds that a child would be able to walk without assistance increased by 70% for every unit increase in length-for-age Z-score, by 65% for every unit increase in weight-for-length Z-score, by 100% when anemia was absent, by 200% when the child was reported to have eaten meat.
Table 3.
Predictors of walking among 8- to 17-mo Nepali infants (n=301)1
| Characteristics | Model 12 | Model 22 |
|---|---|---|
| Age (mo) | 1.79 ± 0.07*** | 1.74 ± 0.07*** |
| Low caste Hindu3 | 3.52 ± 0.58* | 2.99 ± 0.60 |
| Muslim | 3.87 ± 0.65* | 3.06 ± 0.67 |
| Low SES4 | 1.43 ± 0.29 | 1.37 ± 0.30 |
| Female | 1.11 ± 0.28 | 1.07 ± 0.29 |
| Length-for-Age Z-score | 1.66 ± 0.14*** | 1.69 ± 0.15** |
| Weight-for-Length Z-score | 1.62 ± 0.18** | 1.65 ± 0.18** |
| No anemia (hemoglobin≥105g/L) | 2.09 ± 0.31* | 2.02 ± 0.32* |
| Meat | 2.93 ± 0.30*** |
301 of the 485 children were age-eligible and had complete data for the walking analysis;
logistic regression; Odds ratios ± standard error;
Vaiyshas, Shudras vs. Brahmins and Chhetris;
Socio-economic status, less than median of 4 possessions;
p< 0.05,
p< 0.01,
p<0.001
DISCUSSION
The data offer confirmatory evidence of an ordinal relationship between the milestones and reveal a range of timing of motor milestone acquisition. We conclude that, although there are occasional individual children that show some transpositions in the ordering of motor milestones, the acquisition of these milestones is ordered across different cultures and levels of nutritional risk in different populations. Further, this ordering holds despite the fact that the timing of the acquisition varies among well and poorly nourished infants and toddlers.
Predictors of walking
Walking without assistance in our Nepali sample was associated with age, length-for-age, weight-for-length, anemia, and diet. The study reveals an independent effect of two anthropometric indicators, anemia, and meat intake on walking acquisition and contributes to prior evidence of motor development being sensitive to nutritional factors. This is the first study in the known literature that was able to examine the combined effects of growth, anemia, and diet on walking acquisition.
Consistent with previous research, we found children with higher length-for-age Z-scores and no anemia walked at an earlier age than children with lower Z-scores and anemia. In our sample, weight-for-length also was associated with walking acquisition, which was a finding that supported two studies (17,19), but contradicted a third (18). Results from a Pakistani sample revealed a linear, inverse association between thinness at birth, postnatal stunting and wasting and observed motor milestone acquisition (19). A study conducted in Tanzania (17) that examined physical growth and different iron-status indicators found length-for-age, weight-for-length, anemia and/or iron-deficiency to be associated with walking. Results from a study conducted among socially deprived Indonesian pre-school children found motor development was strongly associated with length-for-age, moderately associated with weight-for-age, and not associated with weight-for-length (18). The combined results of these studies show an association between children’s length and weight-for-length and the attainment of bipedal locomotion in several populations on different continents.
Our finding that growth and anemia were independently associated with walking replicate the findings from Zanzibar (17) that examined the association of physical growth and iron-status indicators on the same motor milestones that were used in the present Nepal study. Prior to the Zanzibar study, associations between anemia and motor development were characterized by results from standardized tests of infant development that measure global gross motor development indices (28–31). Anemic and/or iron-deficient children were found to perform poorer in comparison with their non-anemic peers on the Bayley Scales of Infant Development motor scale and school-aged tests of motor development (30, 32–34). The literature reveals a robust relation between anemia and motor development. This study, in conjunction with the Zanzibar one, reveals an independent effect of anemia on walking, a specific motor skill.
Our finding that diet is associated with walking is consistent with research conducted in Guatemala (16). In two longitudinal studies, length-for-age, weight-for-age, and animal protein intake from complementary foods were associated with earlier age of walking. Animal source foods both contain bio-available iron and enhance iron absorption from non-animal foods (35); however, anemia was not measured in the Guatemala study. Thus, the question arises, was animal protein intake a proxy for anemia? Our study reveals that in Nepal anemia and meat intake were independently associated with walking.
Our finding that animal protein was associated with development supported the Guatemala study (16) and longitudinal research conducted in Egypt, Mexico, and Kenya where positive associations were found between animal source foods and growth, cognitive development, and physical activity among school-aged children (35–38). A more recent study conducted in rural Kenya found supplementation with animal source foods had positive effects on children’s cognitive performance; however, different types of animal source foods were not found to produce the same effects among the study children (39). Children who consumed meat performed significantly better on general problem-solving ability measured by the Raven’s Progressive Matrices than children in any of the other groups (39). This finding paralleled ours where the odds of walking for our Nepali children who consumed meat were higher than the odds of walking for children who consumed animal source foods, including meat, eggs, and non-human milk. Our data emphasize the importance of meat intake in a young child’s diet, and suggest that these dietary variables are not simply proxies for anemia status or better growth. Further research is needed to clarify whether meat intake influences motor development through other biological pathways, or whether this strong association is caused by residual confounding by other unmeasured social or health variables.
Caste, but not sex or SES was predictive of walking acquisition in Model 1 with growth and anemia. In a mixed Nigerian and white sample, girls attained milestones earlier than boys (40). For SES, an American sample (27) found children with a higher SES to have slower motor development. Researchers found class differences for walking in a British sample (26). As far as we know caste has not been a factor in previous motor development analyses; however, racial differences have been addressed. Research conducted among Nigerian children reports that the African children attained milestones earlier than the white children with whom they were compared (40). The American sample had similar findings; African-American children developed faster than Caucasian children (27). In our sample, low caste Hindus and Muslims walked earlier than high caste Hindu children. It is possible that caste is a proxy for SES and that our findings parallel those found in the American sample (27), as evidenced by our finding that low caste children were at significantly greater odds of walking earlier than high caste children and that low SES children tended to be at greater odds (p = 0.29) of walking earlier than high SES children. It is also possible that caste is a unique social construct, similar if not equal to race. Caste laws were originally based on race.
This cross-sectional study is limited in its ability to address causation and provide exact timing of walking acquisition. Having collected data at only one time point does not allow us to specify when the Nepali children achieved walking without support nor does it allow us to compare the effect of the different predictors among children who have achieved walking. Nevertheless, this study strengthens existing knowledge by confirming an association between and revealing the independent effect of the two growth indices, anemia, and diet on bipedal locomotion.
Longitudinal and randomized, placebo-controlled clinical trial research is needed to delineate how differences in iron and other nutritional factors influence bipedal locomotion and ultimately the cognitive development of young children. The present data indicate that the variability of nutritional status among infants and toddlers accounts for part of the variance in gross motor development. As each of the motor milestones involves different body parts and biomechanics that result in different kinds of behaviors (5–9), we conclude that nutrition factors affect a developmental system (41). This, in turn, is likely to influence other developmental systems as well.
Footnotes
Funded by the National Institutes of Health, Bethesda, Maryland (HD 38753), the Bill and Melinda Gates Foundation, Seattle, Washington (810-2054), and a Cooperative Agreement between JHU and the Office of Health and Nutrition, US Agency for International Development, Washington DC (HRN-A-00-97-00015-00).
The data were presented with the same authors in a poster titled “Growth indices and hemoglobin concentration independently predict motor milestone acquisition of 4- to 17-month children living in south central Nepal.” at the Society for Research on Child Development (SRCD) Biennial Meeting; 2005 April 7–10; Atlanta, GA.
Abbreviations used: CDC, Centers for Disease Control; EP, erythrocyte protoporphyrin; MUAC, mid-upper arm circumference; SES, socio-economic status; VDC, village development committee; WHO, World Health Organization
References
- 1.Bertenthal BI, Campos JJ. A systems approach to the organizing effect of self-produced locomotion during infancy. Adv Infancy Res. 1990;6:1–60. [Google Scholar]
- 2.Tracey F. The Psychology of Childhood. Boston: D.C. Health; 1909.
- 3.Burnside LH. Co-ordination of locomotion of infants. Gen Psychol Monogr. 1927;2:279–372. [Google Scholar]
- 4.Robson P. Prewalking locomotor movements and their use in predicting standing and walking. Child: Care Health Dev. 1984;10:317–330. doi: 10.1111/j.1365-2214.1984.tb00189.x. [DOI] [PubMed] [Google Scholar]
- 5.Pollitt E, Oh S-Y. Early supplementary feeding, infant development and health policy. Food Nutr Bull. 1994;15:208–214. [Google Scholar]
- 6.Adolph KE, Vereijken B, Denny MA. Learning to crawl. Child Dev. 1998;69(5):1299–1312. [PubMed] [Google Scholar]
- 7.Thelen E. Learning to Walk is Still an “Old” Problem: A Reply to Zelazo (1983) J Mot Beh. 1983;15(2):139–61. doi: 10.1080/00222895.1983.10735293. [DOI] [PubMed] [Google Scholar]
- 8.McGraw MB. The Neuromuscular Maturation of the Human Infant. New York: Columbia University Press; 1943.
- 9.Thelen E. Development origins of motor coordination: leg movements in human infants. Dev Psych. 1985;18:1–22. doi: 10.1002/dev.420180102. [DOI] [PubMed] [Google Scholar]
- 10.DeOnis M. Measuring nutritional status in relation to mortality. Bull World Health Organ. 2000;78:1271–1274. [PMC free article] [PubMed] [Google Scholar]
- 11.Rivera J, Martorell R. Nutrition, infection and growth. Part I: Effects of infection on growth. Clin Nutr. 1988;7:156–162. [Google Scholar]
- 12.Habicht JP, Martorell R, Rivera JA. Nutritional impact of supplementation in the INCAP longitudinal study: analytic strategies and inferences. J Nutr. 1995;125:1042S–1050S. doi: 10.1093/jn/125.suppl_4.1042S. [DOI] [PubMed] [Google Scholar]
- 13.Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJ. Selected major risk factors and global and regional burden of disease. Lancet. 2002;360(9343):1347–60. doi: 10.1016/S0140-6736(02)11403-6. the Comparative Risk Assessment Collaborating Group. [DOI] [PubMed] [Google Scholar]
- 14.WHO/UNICEF. Complementary feeding of young children in developing countries: a review of current scientific knowledge. Geneva: World Health Organization; 1998.
- 15.Dewey KG, Cohen RJ, Arimond M, Ruel MT. Dietary diversity is predictive of the nutrient density of complementary foods for breastfed children in developing countries. Experimental Biology and XXXV International Congress of Physiological Sciences meeting abstracts [accessed at http://select.biosis.org/faseb] FASEB J. 2005;19 Abstract #575.3. [Google Scholar]
- 16.Kuklina EV, Ramakrishnan U, Stein AD, Barnhart HH, Martorell R. Growth and diet quality are associated with the attainment of walking in rural Guatemalan infants. J Nutr. 2004;134:3296–3300. doi: 10.1093/jn/134.12.3296. [DOI] [PubMed] [Google Scholar]
- 17.Kariger PK, Stoltzfus RJ, Olney D, Sazawal S, Black RE, Tielsch JM, et al. Iron deficiency and physical growth predict attainment of walking but not crawling in a cross-sectional sample of poorly nourished Zanzibari infants. J Nutr. 2005;135:814–819. doi: 10.1093/jn/135.4.814. [DOI] [PubMed] [Google Scholar]
- 18.Pollitt E, Husaini MA, Harahap H, Halati S, Nugraheni A, Sherlock AO. Stunting and delayed motor development in rural West Java. Am J Hum Biol. 1994;6:627–635. doi: 10.1002/ajhb.1310060511. [DOI] [PubMed] [Google Scholar]
- 19.Cheung YB, Yip PSF, Karlberg JPE. Fetal growth, early postnatal growth and motor development in Pakistani infants. Int J Epidemiol. 2001;30:66–74. doi: 10.1093/ije/30.1.66. [DOI] [PubMed] [Google Scholar]
- 20.Pollitt E, Jahari A, Walka H. A developmental view of he effects of an energy and micronurient supplement in undernourished children in Indonesia. Eur J Clin Nutr. 2000;54(Supp 2):S107–113. doi: 10.1038/sj.ejcn.1601012. [DOI] [PubMed] [Google Scholar]
- 21.Jahari AB, Saco-Pollitt C, Husaini MA, Pollitt E. Effects of an energy and micronutrient supplement on motor development and motor activity in undernourished children in Indonesia. Eur J Clin Nutr. 2000;54(2):S60–S68. doi: 10.1038/sj.ejcn.1601006. [DOI] [PubMed] [Google Scholar]
- 22.Bayley N. Bayley Scales of Infant Development. New York: Psychological Corporation; 1969.
- 23.Domellof M, Dewey KG, Lonnerdal B, Cohen RJ, Hernell O. The diagnostic criteria for iron deficiency in infants should be reevaluated. J Nutr. 2002;132(12):3680–3686. doi: 10.1093/jn/132.12.3680. [DOI] [PubMed] [Google Scholar]
- 24.Mahoney CA, Thombs DL, Howe CZ. The art and science of scale development in health education research. Health Educ Res. 1995;10:1–10. doi: 10.1093/her/10.1.1-a. [DOI] [PubMed] [Google Scholar]
- 25.Cronbach LJ. Coefficient alpha and the internal structure of tests. Psychometrika. 1951;16:297–334. [Google Scholar]
- 26.Neligan G, Prudham D. Norms for four standard developmental milestones by sex, social class and place in family. Dev Med Child Neurol. 1969;11:413–422. doi: 10.1111/j.1469-8749.1969.tb01459.x. [DOI] [PubMed] [Google Scholar]
- 27.Capute AJ, Shapiro BK, Palmer FE, Ross A, Wachtel RC. Normal gross motor development: the influences of race, sex and socio-economic status. Dev Med Child Neurol. 1985;27(5):635–43. doi: 10.1111/j.1469-8749.1985.tb14136.x. [DOI] [PubMed] [Google Scholar]
- 28.Lozoff B, Brittenham GM, Viteri FE, Wolf AW, Urrutia JJ. The effects of short-term oral iron therapy on developmental deficits in iron-deficient anemic infants. J Pediatr. 1982;100(3):351–357. doi: 10.1016/s0022-3476(82)80428-9. [DOI] [PubMed] [Google Scholar]
- 29.Lozoff B, Brittenham GM, Wolf AW, McClish DK, Kuhnert PM, Jimenez E, et al. Iron deficiency anemia and iron therapy effects on infant developmental test performance. Pediatr. 1987;79(6):981–995. [PubMed] [Google Scholar]
- 30.Walter T, De Andraca I, Chadud P, Perales CG. Iron deficiency anemia: adverse effects on infant psychomotor development. Pediatr. 1989;84(1):7–17. [PubMed] [Google Scholar]
- 31.Stoltzfus RJ, Kvalsvig JD, Chwaya HM, Montresor A, Albonico M, Tielsch JM, et al. Effects of iron supplementation and anthelminthic treatment on motor and language development of preschool children in Zanzibar: double blind, placebo controlled study. Br Med J. 2001;323:1389–1393. doi: 10.1136/bmj.323.7326.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sherriff A, Emond A, Bell JC, Golding J. Should infants be screened for anaemia? A prospective study investigating the relation between haemoglobin at 8, 12, and 18 months and development at 18 months. Arch Disabil Chil. 2001;84(6):480–485. doi: 10.1136/adc.84.6.480. ALSPAC Study Team. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Idjradinata P, Pollitt E. Reversal of developmental delays in iron-deficient anaemic infants treated with iron. Lancet. 1993;341(8836):1–4. doi: 10.1016/0140-6736(93)92477-b. [DOI] [PubMed] [Google Scholar]
- 34.Lozoff B, Jimenez E, Wolf AW. Long-term developmental outcome of infants with iron deficiency. New Engl J Med. 1991;325:687–694. doi: 10.1056/NEJM199109053251004. [DOI] [PubMed] [Google Scholar]
- 35.Neumann CG, Harris DM, Rogers LM. Contribution of animal source foods in improving diet quality and function in children in the developing world. Nutr Res. 2002;22:193–220. [Google Scholar]
- 36.Allen LH, Backstrand J, Chavez A, Pelto GH. People cannot live by tortillas alone. Final report Phase II: Nutrition CRSP. Storrs, CT: University of Connecticut; 1992.
- 37.Neumann CG, Bwibo NO, Sigman M. Functional Implications of Malnutrition, Kenya. Final Report Phase II: Nutrition CRSP. Los Angeles, CA: University of California; 1992.
- 38.Kirksey A, Harrison GG, Galal OM, McCabe GA, Wachs TD, Rahmanifar. The human cost of moderate malnutrition in an Egyptian village. Final Report Phase II: Nutrition CRSP. Lafayette, IN: Purdue University; 1992.
- 39.Whaley SE, Sigman M, Neumann C, Bwibo NO, Guthrie D, Weiss RE, et al. The impact of dietary intervention on the cognitive development of Kenyan school children. J Nutr. 2003;133:3965S–3971S. doi: 10.1093/jn/133.11.3965S. [DOI] [PubMed] [Google Scholar]
- 40.Iloejo SO, Obiekwe VU, Kaine WN. Gross motor development of Nigerian children. Ann Trop Paediatr. 1991;11(1):33–39. doi: 10.1080/02724936.1991.11747475. [DOI] [PubMed] [Google Scholar]
- 41.Smith LB, Thelen E. Development as a dynamic system. Trends Cogn Sci. 2003;7(8):343–348. doi: 10.1016/s1364-6613(03)00156-6. [DOI] [PubMed] [Google Scholar]


